Abstract
A coherent set of epidemiological data shows that the Mediterranean diet has beneficial effects capable of preventing a variety of age-related diseases in which low-grade, chronic inflammation/inflammaging plays a major role, but the underpinning mechanism(s) is/are still unclear. It is suggested here that the Mediterranean diet can be conceptualized as a form of chronic hormetic stress, similar to what has been proposed regarding calorie restriction, the most thoroughly studied nutritional intervention. Data on the presence in key Mediterranean foods of a variety of compounds capable of exerting hormetic effects are summarized, and the mechanistic role of the nuclear factor erythroid 2 pathway is highlighted. Within this conceptual framework, particular attention has been devoted to the neurohormetic and neuroprotective properties of the Mediterranean diet, as well as to its ability to maintain an optimal balance between pro- and anti-inflammaging. Finally, the European Commission–funded project NU-AGE is discussed because it addresses a number of variables not commonly taken into consideration, such as age, sex, and ethnicity/genetics, that can modulate the hormetic effect of the Mediterranean diet.
Keywords: hormesis, inflammaging, Mediterranean diet, Nrf2, stress
INTRODUCTION
An enormous variety of stimuli (chemical, physical, biological, psychological) can be sensed as stressors by organisms, leading to physiological reactions through a nonspecific response referred to as a stress response.1 The stress response can spread at the systemic level through the production and secretion of hormones, cytokines, and neurotransmitters aimed at the modulation of the neuroendocrine and immune systems.2,3 During evolution, neuroendocrine and immune functions are managed by a unique cellular protagonist, the macrophage, within an integrated and conserved defense network.4,5 On the basis of this evolutionary perspective, it was conceptualized that immune and stress responses functionally overlap and that classic “antigens” (viruses and bacteria, but also food) can be considered stressors that were likely major selective forces for the evolution of the immune system.6,7
A stressor can prove deleterious or beneficial depending on the reaction it evokes, which, in turn, depends on the intensity and persistence of the stressor itself, as well as on the individual’s capacity to cope with it.1 In this context, it is important to discriminate between acute stress, associated with a quick and short-term response, and chronic prolonged stress. At variance with acute stress, chronic stress is much more difficult to define and quantify because of its relatively lower intensity and longer persistence; its outcome can also be either a progressive adaptation or a progressive deterioration.1,8
Counterintuitively, exposure to very low doses of a substance that is toxic at high doses can elicit beneficial effects. This phenomenon (mithridatism) was conceptualized in 1538 by Paracelsus, who wrote: “[A]ll things are poison and not without poison; only the dose makes a thing not a poison.” In 1887, H. Schulz demonstrated for the first time that a toxic substance may induce opposite effects depending on the dose (Arndt Schulz Law).9 In more recent years, the beneficial effect of exposure to low-grade potentially damaging conditions or very low doses of otherwise toxic compounds has been conceptualized as “preconditioning” and “hormesis.” The term hormesis, coined within the toxicology field, defines adaptive, nonmonotonic, biphasic dose–response relations following an initial disruption in homeostasis (Figure 1).10 In the framework of this conceptualization, the activation and upregulation of cellular and molecular defense pathways due to mild stressors and the subsequent adaptation and protection are named pre- and postconditioning, respectively.11,12 The biological importance of these concepts—particularly of hormesis—is far reaching because they may represent the most satisfactory explanation of a variety of complex phenomena. In this regard, it is important to highlight that the health effects of the most thoroughly studied topic in the aging/nutrition field (ie, calorie restriction) have been conceptualized within the hormesis paradigm.11,13 The main mechanisms involved are stress-activated cellular pathways, such as heat shock protein (HSP) response, unfolded protein response, DNA damage response, sirtuin response, autophagy, antioxidant system activation, and inflammation.14,15
Figure 1.

Biphasic dose-response curve toward a hormetic stimulus. Under a certain threshold, the effect of a stimulus/stress on the measured trait is positive (improvement), whereas over this threshold it becomes detrimental/toxic. The range of hormetic doses is indicated as the “hormesis zone.”
INFLAMMAGING AND THE MEDITERRANEAN DIET
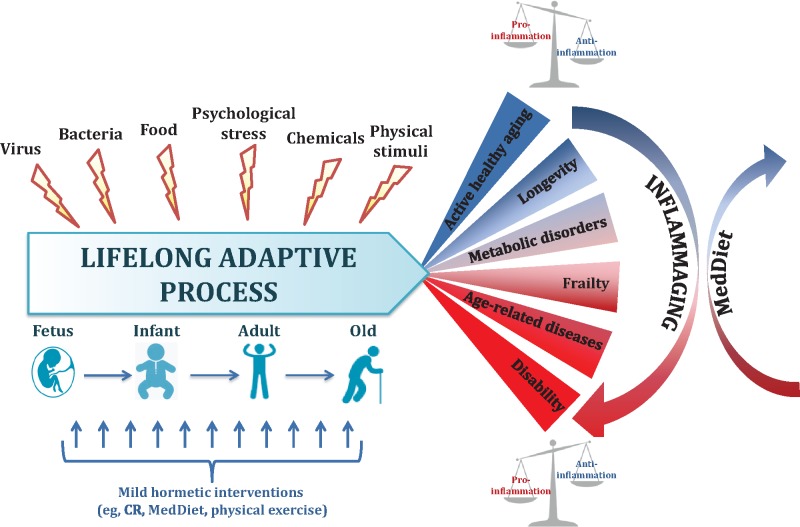
It is widely accepted that old age is characterized by a peculiar low-grade, chronic, and “sterile” inflammatory state, which has been termed inflammaging.7,16,17 Inflammation has likely been selected during evolution for its beneficial role in fighting infections and facilitating tissue repair in the early phases of life. However, it appears that, with aging, the load of exogenous and endogenous stressors capable of eliciting inflammatory responses gradually increases unabated, leading to high levels of proinflammatory mediators, which are believed to substantially contribute to the pathogenesis of many, if not all, age-associated diseases and to the progression of the aging process.16,17 Inflammaging likely results from the imbalance between the production of pro- and anti-inflammatory mediators in a sort of adaptive mechanism to a person’s lifelong exposure to stressors, whereby inflammation continuously triggers anti-inflammatory responses.18 In this context, good nutrition, particularly as provided by the Mediterranean diet (MedDiet), could represent a powerful tool to profoundly remodulate this systemic inflammatory balance on a long-term scale by slowing down the age-related increase in the production of inflammatory molecules and by favoring adaptive anti-inflammatory responses. The MedDiet may, therefore, shift the age at which this ratio (pro-/anti-inflammation) trespasses beyond the threshold that separates physiological inflammation from unbalanced pro-inflammation, which, in turn, favors age-related inflammatory diseases (Figure 2). It can be surmised that the effect of the MedDiet on inflammaging, in a fashion similar to the one proposed to explain the effects of calorie restriction, is hormetic. In other words, specific components of the MedDiet likely minimize the increase of inflammatory stimuli by acting as hormetins.
Figure 2.
Aging as a lifelong adaptation to stressors. From intrauterine life to old age, humans are constantly exposed to a series of stressors that includes physical and chemical pollutants and viral and bacterial antigens, as well as food-derived molecules that are sensed by a series of stress-responding mechanisms, leading to a lifelong adaptation. Depending on the outcome of this adaptation, the balance between pro- and anti-inflammatory parameters may be preserved or not, and, consequently, aging will either be delayed or age-related diseases may emerge. The Mediterranean diet is a powerful tool to counteract inflammaging and its consequences. Abbreviations: CR, calorie restriction; MedDiet, Mediterranean diet.
Inflammaging could, in turn, be considered a sort of hormetic stress, having positive outcomes at low doses (physiological inflammation) at young and adult ages and becoming detrimental during the postreproductive period, especially in people who, as a result of genetic background and/or unhealthy lifestyle, including poor nutrition, are not able to maintain an optimal balance between inflammaging and anti-inflammaging.
NUCLEAR FACTOR ERYTHROID 2–RELATED VITAGENE NETWORK AND HORMESIS
Nuclear factor erythroid 2 (Nrf2) is a key transcription factor that regulates a wide range of genes (>500) with cytoprotective functions. The physiological activation of Nrf2 provokes a moderate stress response, ensuring health benefits and a prolonged lifespan in animal models.19 On the contrary, excessive long-term Nrf2 stimulation may lead to pathophysiological outcomes, classifying Nrf2 signaling as a hormetic-like pathway.20–24 One of the main networks regulated by Nrf2 is the vitagene system, a complex and integrated adaptive defense mechanism whose genes encode for members of the HSP family, such as heme-oxygenase-1 and HSP 72, thioredoxin/thioredoxin reductase, and sirtuins.25,26 The term HSP denotes a highly conserved cytoprotective mechanism that is devoted to protein folding and repair, but under no-stress conditions, is also involved in multiple functions such as transport of macromolecules, cell signaling, transcription, division, and migration.27–29 Heme-oxygenase-1 (Hsp32), thioredoxin (a class of small redox proteins), and thioredoxin reductase can be transcriptionally upregulated by Nrf2 as part of the electrophile counterattack, also called the phase 2 response; this is a cell surveillance strategy to counteract any damaging effects of oxidant and electrophile molecules.26,30,31 The induction of a phase 2 response can lead to the expression of numerous Nrf2-dependent antioxidant genes,19,32,33 thus representing a strong mechanism for maintaining redox homeostasis. Sirtuins are a group of NAD+-dependent deacetylase enzymes involved in the regulation of vital biological processes such as apoptosis, cell differentiation, DNA repair, energy transduction, inflammation, and neuroprotection. They have, therefore, been associated with metabolic control, cell survival, and healthy aging.34–38 The vitagene network is a powerful system for the promotion of long-term health through an increase in cellular stress tolerance.39–41
Among the activators of the vitagene system are several nutrients, including carnosic acid, resveratrol, sulforaphane, dimethyl fumarate, and acetyl-l-carnitine.31,37,42–47 This supports the hypothesis that the beneficial effects of some nutrients are mediated through a hormetic stress response.
HORMESIS AND NUTRITION
The biphasic dose–response characteristic of the hormetic phenomenon can be triggered by multiple stressful conditions or toxic agents, including nutrients.37,41,48–50 Recent emerging evidence shows that vitamins, minerals, and phytochemicals may exert healthy benefits acting in a hormetic-like manner through the modulation of stress-response pathways, rendering the hormesis concept fully applicable to the field of nutrition.51 All of the compounds, natural or synthetic, that display this property are called hormetins.52
Importantly, the activation of hormetic mechanisms in various animal models has appeared to prolong lifespan and delay the onset of age-related functional impairments.53 It has been reported, for example, that calorie-restricted animals, which experience an extension of lifespan, show many signs of stress response and hypothalamic–pituitary–adrenal activation.54 Thus, this type of nutritional intervention can be assumed to present a moderate amount of stress that is capable of inducing a hormetic response, thereby enhancing the ability of the organism to cope with many different noxae.55 Nevertheless, it is interesting to note that the beneficial effect of calorie restriction is not universal but species specific56 and that different mouse strains can have opposite responses, from life extending to life shortening,57 suggesting that genetic background can play a major role in the control of stress response.
The MedDiet, one of the more intensely studied dietary patterns together with the Okinawan diet, is rich in foods containing mild (submicromolar) but significant concentrations of hormetins. Thus, it may be argued that the healthful features attributed to the MedDiet could, at least in part, be ascribed to a moderate and chronic activation of stress-response mechanisms due to the long-term consumption of low doses of these hormetins.
CULTURAL AND HEALTH SIGNIFICANCE OF THE MEDITERRANEAN DIET
The MedDiet is a cultural dietary model typically adhered to by populations living in the Mediterranean region. Some years ago, Keys et al.58 demonstrated that Mediterranean populations, such as Greeks and Italians, showed reduced mortality from cardiovascular diseases compared with people living in northern Europe or the United States. Subsequently, several observational, longitudinal, and randomized controlled trials have demonstrated that the MedDiet may play a role in the prevention of a wide range of chronic diseases, such as cardiovascular diseases, type 2 diabetes, cancer, and dementia, and could thereby reduce all causes of mortality.59 These data were greeted with remarkable support by the international scientific community, fostering support for the diffusion of the MedDiet model as a tool to achieve an overall health condition that improves life expectancy. Thus, the MedDiet became one of the central pillars of public health policy programs in many countries.
The MedDiet has been included by UNESCO on its Representative List of Intangible Cultural Heritage of Humanity, which indicates it has exceptional scientific and cultural value. To be included on this list, it was determined that the diet met the following criteria: “Transmitted from generation to generation, particularly through families, the Mediterranean diet provides a sense of belonging and sharing and constitutes for those who live in the Mediterranean basin a marker of identity and a space for sharing and dialogue.”60 UNESCO also determined that the MedDiet “could contribute to raising awareness of the significance of healthy and sustainable food related practices in other parts of the world, while encouraging intercultural dialogue, testifying to creativity and promoting respect for cultural, environmental, and biological diversity.”60 The MedDiet is thus recognized not only as a diet but as a complete lifestyle based on the social and cultural aspects typical of Mediterranean countries.
To aid understanding of the MedDiet pattern, a dietary pyramid that provides a qualitative and quantitative graphical representation of the main food groups and their optimal consumption frequency was developed. This innovative graphical representation focuses on a global overview of the Mediterranean lifestyle, reflecting not only food selection, cooking, eating, and number of servings but also emphasizing details closely related to social and economic elements, such as the importance of regular physical activity, adequate rest, and conviviality.61 The MedDiet can be considered a recommended dietary pattern and a healthy and rewarding way of thinking, acting, and living.
EFFECT OF AN INTEGRATED STRESS RESPONSE STIMULATED BY THE MEDITERRANEAN DIET
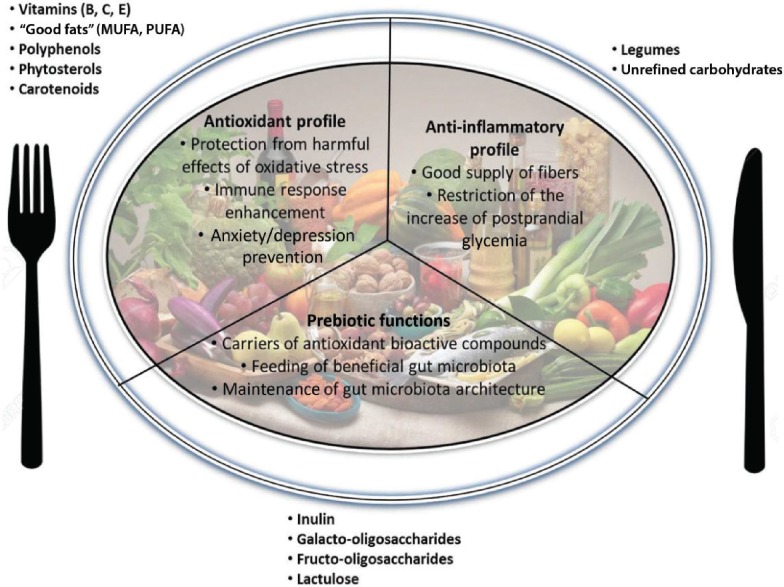
The MedDiet is a well-balanced diet characterized by consistent intake of extra-virgin olive oil, vegetables, fruits, nuts and legumes, whole grains, and fish (especially marine species) and by moderate consumption of eggs, dairy products, lean meats, and alcohol (usually red wine during meals). At the same time, the MedDiet recommends a reduction of saturated fats (butter and other animal fats), red meat, refined carbohydrates, and sweets (Box 1). On the whole, the MedDiet provides an equilibrated mix of nutrients with antioxidant, anti-inflammatory, and prebiotic effects (Figure 3). In addition, emerging experimental evidence, discussed herein, shows that some nutrients typical of the MedDiet, acting as hormetins, may elicit cellular stress responses (Table 1).
Box 1Mediterranean diet in 9 points
High consumption of whole-grain cereals
High consumption of vegetables and fruit
High consumption of legumes
High consumption of fish
High intake of monounsaturated fatty acids (olive oil) and polyunsaturated fatty acids (fish, nuts)
Low intake of saturated fatty acids and hydrogenated oils
Low consumption of meat and meat products
Low-to-moderate consumption of milk and dairy products
Moderate consumption of alcohol (red wine at meals)
Figure 3.
Beneficial components of the Mediterranean diet. Foods, nutrients, and micronutrients (ie, legumes, “good fats,” vitamins) provided by the Mediterranean diet contribute to its beneficial effects (antioxidant and anti-inflammatory profiles, prebiotic functions). Abbreviations: MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
Table 1.
Nutritional hormetins of typical Mediterranean foods able to activate specific stress-response pathways
| Nutritional hormetin | Food item within traditional Mediterranean diet | Stress pathway |
|---|---|---|
| Phytochemicals (phenolic antioxidants, terpenoids, carotenoids, and allium-derived sulfur compounds) | Olives, legumes, leafy green vegetables, tomatoes, eggplant, fruits, garlic, and onion | Activation of nuclear factor erythroid 2 (Nrf2) |
| Resveratrol | Grapes, red wine | Regulation of redox homeostasis |
| Activation of Nrf2 and sirtuin pathway | ||
| Blocking of nuclear factor κB (NF-κB) | ||
| Vitamin E | Dried fruits, herbs, leafy green vegetables | Activation of heat shock response |
| Down-regulation of NF-κB | ||
| n-3 polyunsaturated fatty acids | Fish, nuts | Activation of Nrf2 |
| Blocking of NF-κB | ||
| Fiber | Legumes, unrefined whole-grain cereals, fresh vegetables, fruits | Cooperation with cellular stress pathways (heat shock proteins) |
Phytochemicals
Phytochemicals (polyphenols, phytosterols, and carotenoids) are often considered as products without nutritive value. They constitute a heterogeneous group of bioactive compounds present in fruits, vegetables, nuts, grains, and legumes that display a strong anti-inflammatory function due to their powerful antioxidant profile. The antioxidant properties allow them to also exert an immunomodulatory and chemopreventive effect on cell regulatory activities. However, since the amount of phytochemicals normally consumed through diet is well below the micromolar order, the antioxidant effect of these molecules is not accounted for by a simple scavenging of reactive oxygen species. Recent studies suggest that this antioxidant action may be exerted by the activation of antioxidant stress responses (endogenous antioxidant enzymes, redox systems).25,62,63
Evolutionarily, phytochemicals, which activate defense cellular stress-response pathways, have been developed by plants as a form of protection against pathogens, insects, and animals.26,47–49,64,65 Accordingly, the moderate and continuous consumption of phytochemicals through plant ingestion may induce hormetic responses.37,41
Phytochemicals contained in the foods of a MedDiet and proposed as nutritional hormetins include ferulic acid, luteolin, phenethyl isothiocyanate, and resveratrol.49
Ferulic acid.
This is a phenolic compound abundant in fruit and vegetables such as tomatoes, sweet corn, and rice66 which is able to promote the transcription of antioxidant genes, such as heme-oxygenase-1 and quinone oxidoreductase, through Nrf2 activation.43 This compound also seems have a potential anti-inflammatory function, reducing levels of prostaglandin E2, tumor necrosis factor α, and nitric oxide synthase.67–69 It has also been reported to be efficient in combating colon carcinogenesis in rat,70 skin tumorigenesis,71 and amyloid-beta peptide toxicity.72 Notably, high doses of ferulic acid induce mouse skin tumor promotion and oxidative stress,73 whereas its derivatives display an effective capacity to suppress oxidative stress and inflammation at nanomolar concentrations, but not at the micromolar concentrations at which they become cytotoxic.74
Luteolin.
This is a flavonoid compound found in significant amounts in carrots, fennel, peppers, and celery. It has shown anticancer properties in a mouse model of skin tumor,75 in human melanoma cells, and in human myeloid leukemia cells, where it induces cell cycle arrest or apoptotic cell death in a dose–dependent manner.76,77 In addition, luteolin may protect neuronal cell lines against oxidative damage induced by hydrogen peroxide78 and counteract the toxic effects induced by N-methyl-4-phenyl-pyridinium.79 These protective functions against oxidative stress depend on the activation of Nrf2.79
Phenethyl isothiocyanate.
This compound is found in crucifer vegetables such as turnips, watercress, and radishes, as well as in Chinese cabbage and rutabagas.80,81 The substance has been shown to exert a chemopreventive action in the case of mammary tumors and stomach and pulmonary adenomas, inhibiting the carcinogenesis induced by 7,12-dimethylbenz[a]anthracene in animal models.82 Phenethyl isothiocyanate also seems capable of preventing lung tumors induced by tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in rat and mouse.83,84 It may also induce apoptosis in human glioma cells,85 oral cancer cells,86 and human lung cancer cells.87 Its remarkable chemopreventive action is likely due to the alteration of phase I/ phase II enzymes that affect carcinogen metabolism.88,89 A recent paper reports its ability to protect human skin against ultraviolet radiation-induced oxidative stress and apoptosis through Nrf2 and antioxidant enzymes.90
Resveratrol (trans-3, 5, 4′-trihydroxystilbene).
This is a polyphenol that is present in >70 plant species and is particularly high in grapes (50–100 μg of resveratrol per gram of fresh grape skin) and red wine. In plants such as Vitis vinifera, it is produced in response to infection (especially fungal) and to some environmental stresses (ie, water deprivation and ultraviolet irradiation) and acts as a natural antibiotic as well as an inhibitor of proliferation.91 Given its ability to scavenge intracellular radical oxygen species, resveratrol is considered a potent natural in vivo antioxidant.92 However, like most antioxidants, resveratrol can effectively scavenge free radicals if present at micromolar concentrations. Bioavailability studies have shown that resveratrol is quickly absorbed in the gastrointestinal tract after oral consumption, but it is metabolized in its glucuronide and sulfate derivatives by rapid metabolism in enterocytes. Thus, the blood concentrations of resveratrol are in the nanomolar range, making it almost impossible to reach pharmacologically significant doses.93,94 It has, therefore, been suggested that its positive effects are mediated by a hormetic mechanism.95,96 In this regard, resveratrol has been demonstrated to be a potent allosteric activator of SIRT1,97 a member of the sirtuin family able to mediate the healthy benefits of dietary restriction and physical exercise.98 For this reason, resveratrol has been indicated as an effective mimetic of some of the effects of calorie restriction.99 In addition, resveratrol can induce the activation of the Nrf2 pathway and the inhibition of nuclear factor κB (NF-κB) activity,100–102 exerting a protective effect against neuroinflammation and counteracting the progression of brain aging. The mechanism of action of resveratrol can be represented by a biphasic dose–response curve where low doses of this phytochemical induce mild stress in neural cells, enhancing the ability of the nervous system to cope with stress and promoting its optimal function and longevity.103 Until now, only a few clinical trials evaluating the potential therapeutic outcomes of resveratrol have been performed, with quite discordant results.104 A meta-analysis of 11 studies indicated that resveratrol consumption significantly reduces fasting glucose, insulin, glycated hemoglobin levels, and insulin resistance in diabetic participants, whereas it has no effect on the glycemic profile of nondiabetic persons.105 However, a more recent randomized, double-blind, crossover study has demonstrated that resveratrol supplementation did not improve hepatic or peripheral insulin sensitivity in subjects with well-controlled type 2 diabetes.106 An active grape formulation containing polyphenols that was administered for 6 months to elderly subjects with mild cognitive impairment prevented brain metabolism decline in the right posterior cingulate cortex and in the left superior posterolateral temporal cortex. Even if no significant differences with respect to the placebo group were found in the neuropsychological battery scores, a protective effect of grapes against the early pathologic metabolic decline typical of Alzheimer's disease has been hypothesized.107 In summary, resveratrol can be considered a nutritional phytochemical hormetin able to elicit adaptive stress-response signaling pathways that increase cellular resistance to injuries and diseases.
On the whole, some plant polyphenols, which are abundantly present in the MedDiet, may exert positive modulations on health and lifespan through the activation of metabolic responses and stress-related pathways similar to the responses elicited by calorie restriction and intermitting fasting.108
Besides the above-mentioned specific substances, phytochemicals such as phenolic antioxidants, terpenoids, carotenoids, and allium-derived sulfur compounds, which are commonly present in traditional foods of the MedDiet (olives, legumes, leafy green vegetables, tomatoes, eggplant, fruits, garlic, and onion), are capable of inducing the stress response through Nrf2.109–113
Furthermore, oleuropein, curcumin,114 and quercetin are able to control the oxidative stress load by activating the ubiquitin-proteasome system or Nrf2, which leads to the transcription of proteasome subunit genes.115,116 The ubiquitin-proteasome system is one of the main intracellular protein degradation machineries and is devoted to the clearance of short-lived regulatory protein and the removal of misfolded, damaged, and oxidized proteins,117–119 thus representing a key mechanism in cellular homeostasis maintenance.
Vitamins
Appropriate daily doses of vitamins are essential and beneficial, whereas their excess can lead to hypervitaminosis, following a hormetic U-shaped curve.120,121 Vitamins directly activate cellular and systemic pathways to serve as defense mechanisms against different stressors.122 The A, C, and E vitamins, which display free-radical scavenging features, are discussed here briefly because redox homeostasis is a fundamental goal of stress responses.
Vitamin A.
This vitamin and its active derivatives (retinoids) are key players in many physiological processes such as eyesight, reproduction, embryonic growth and development, immune competence, cell differentiation and proliferation, apoptosis, maintenance of epithelial tissue, and brain functions. Carotenoids, the precursors of vitamin A, are fruit and vegetable pigments with several properties. They are immunostimulants, they exert photo-protection of sunlight-exposed tissues,123 they prevent oxidative tissue damage, and they modulate the Nrf2 signaling pathway. Thus, typical Mediterranean foods rich in carotenoids, such as tomatoes and leafy green vegetables, may also be considered Nrf2 activator foods.19
Vitamin C.
This vitamin is mainly found in fresh fruits (including citrus fruits, berries, kiwi) and vegetables (including brassicas, peppers, leafy greens, tomatoes). Its antioxidant activity is needed for tissue growth and repair and to block the oxidative damage caused by cancer, heart diseases, and other pathological conditions.124 An increase in the daily intake of vitamin C is recommended in cases of infections, surgical procedures, cigarette smoking, other stress-related conditions, and diets poor in fresh plant food and fruit.125
Vitamin E.
This vitamin is contained especially in dried fruits, herbs, and leafy green vegetables. It preserves cell membranes from free-radical activity and is one of the most effective nutrients for strengthening the immune system. Several studies in animal and human models have shown that vitamin E deficiency impairs the humoral and cell-mediated immune function; conversely, its supplementation reinforces the immune response against several pathogens, thus accelerating infection resolution, particularly in elderly people.126 A recent study performed on sheep showed that dietary antioxidant supplementation with vitamin E plus selenium may protect against heat stress by inducing the expression of skeletal muscle HSP70 and HSP90 and the downregulation of NF-κB.127 The HSPs represent a highly conserved cytoprotective mechanism essential for cell survival, whereas NF-κB is the crucial transcription factor responsible for the proinflammatory response to stress.128 In addition, different isoforms of vitamin E (α-, γ-, δ-tocopherol) have been shown to be involved in the activation of Nrf2 and NF-κB pathways, thus modulating the inflammatory response in human intestinal cells.129
Lipids
The so-called “good fats” are the monounsaturated fatty acids and polyunsaturated fatty acids (PUFAs) found in vegetable oils (especially extra-virgin olive oil), nuts, seeds, and fish. The MedDiet provides an excellent dietary fat profile characterized by a low intake of saturated fats and trans-fatty acids130 and an optimal ratio between n-6 and n-3 PUFAs. This fat combination and proportion in the MedDiet provides this dietary pattern with one of the most powerful anti-inflammatory profiles. Animal studies suggest that dietary supplementation with n-3 PUFAs prevents anxiety and depressive behavior, as well as stress-induced learning/memory deficits.131 In humans, n-3 PUFAs have also been found to exert antidepressive and antistress effects.132–134,135 A meta-analysis confirmed that n-3 PUFA levels are lower in depressed patients compared with control subjects, whereas no differences in n-6 PUFAs levels were observed. Of the 2 most prevalent n-3 PUFAs, eicosapentaenoic acid (EPA) seems to be more effective than docosahexaenoic acid (DHA) in treating depression symptoms.133,136 Concerning physiological responses to stress, 3 weeks of supplementation with n-3 PUFAs (fish oil) significantly reduced plasma levels of cortisol, adrenaline, and nonesterified fatty acids, as well as energy consumption elicited by mental stress effects in health conditions.135 Intake of n-3 PUFAs can also influence mood and cognition by blocking the NF-κB pathway,137,138 thus protecting against proinflammatory stress responses.128 Frequent consumption of marine fish, which is typical with a MedDiet, provides high levels of EPA and DHA competing with n-6 PUFAs for the same series of enzymes; this reduces the production of arachidonic acid–derived proinflammatory eicosanoids, such as prostaglandin E2, leukotriene B4, and thromboxane (2-series), with a chemotactic and procoagulant action, and increases the synthesis of anti-inflammatory eicosanoids, such as prostaglandin E3, leukotriene B5, and thromboxane (3-series), with immunomodulatory and neuroprotective effects.139 Furthermore, moderate concentrations of DHA and EPA, alone or in combination, activate the Nrf2 pathway and the mRNA expression of its target genes, achieving optimal antioxidant cellular protection.140
The specific properties of olive oil are described in Box 2. This dietary product is a mix of various chemical compounds and can be considered one of the iconic foods of the MedDiet, together with red wine.
Box 2Properties of olive oil
Olive oil represents the main source of fat in the Mediterranean diet. The health-promoting effects of olive oil have been described relative to the prevention of several conditions, such as cancer, metabolic syndrome, cardiovascular and cerebrovascular events, neurodegenerative diseases, and autoimmune inflammatory diseases.141–144
Extra virgin olive oil (EVOO) is obtained from olives exclusively by mechanical or physical methods, under conditions that do not modify its natural composition. Oleic acid (18:1, n-9) is the most represented fatty acid (FA) (36%–84% of the FA total fraction), whereas linoleic acid is the main essential polyunsaturated fatty acid (3%–21% of the FA total fraction) in EVOO. In particular, oleic acid has the following cardioprotective benefits: affects plasma lipids and lipoprotein patterns; affects blood cell membrane composition and fluidity; inhibits coagulation; improves glucose metabolism; controls blood pressure; and modulates inflammation and oxidative stress.144
Besides its excellent lipid profile, EVOO contains hundreds of bioactive compounds, including a number of triterpens (ie, squalene), biophenols (phenyl alcohols hydroxytyrosol, tyrosol, oleuropein, ligstroside, verbascoside, lignans, flavonoids), pigments (chlorophylls and carotenoids, xanthophylls, lutein), and vitamin E (tocopherols), which several studies have indicated are responsible for the beneficial effects of EVOO.145–147 The antioxidant, antimicrobial, and anti-inflammatory properties of these molecules, as well as their effects on lipid metabolism, were recently reviewed.144 Given that the antioxidant activity of EVOO biophenols in vivo is critically restricted by their low bioavailability, their systemic positive effects are likely due to activation of the endogenous stress-response systems (drug-metabolizing enzymes, antioxidant and phase II enzymes, and transporter proteins).148 Thus, it can be suggested that EVOO biophenols could function as hormetic compounds that are able to trigger adaptive response pathways (parahormesis).149 The low level of oxidized forms of EVOO biophenols in plasma and tissues can transitorily induce a mild state of oxidative stress capable of upregulating the endogenous antioxidant and detoxification enzymes and of rendering the cell more resistant against the long-term damaging effects of more powerful oxidative stress stimuli. In particular, the positive hormetic effect of EVOO biophenols at their low in vivo concentration may be exerted through the activation of the Nrf2-targeted “early warning signal.”148 In fact, a number of recent studies have shown that hydroxytyrosol, oleuropein, and oleacein can activate the Nrf2 pathway both in in vitro cellular models150–152 and in vivo animal models.153 In addition, the anticancer activity of EVOO secoiridoids (oleuropein aglycon and decarboxymethyl oleuropein aglycon) is related to the activation of antiaging/cellular stress-like gene signatures (endoplasmic reticulum stress, sirtuin-1, NRF2 signaling, and the energy-sensing protein AMPK, a critical gerosuppressor of mTOR).154
Carbohydrates and fiber
The most common carbohydrate-rich foods in the MedDiet are nonrefined whole-grain cereals and legumes. Legumes are low-fat, nutrient-rich foods that provide large amounts of vitamins, minerals (iron, selenium, phosphorus, and potassium), protein, and fiber. The consumption of both whole-grain cereals and legumes has the advantage of reducing the increase of postprandial glycemia and ensures a good supply of dietary fiber. Indeed, low glycemic indexes help to keep the postprandial rise of blood glucose levels and, consequently, insulin secretion under control. It has been demonstrated that high insulin levels foster inflammation,155 and the consumption of high-glycemic-index foods is associated with increased cardiovascular risk. On the other hand, low-glycemic-index diets have antiatherogenic effects because they lead to a decrease in the production of atherogenic lipoproteins, oxidized low-density lipoproteins, and inflammatory biomarkers (C-reactive protein, plasminogen activator inhibitor-1).156 In addition, these foods play a very important role in preventing hypercholesterolemia and type 2 diabetes.157 Interestingly, metabolic dysfunctions seem to also be regulated by intracellular stress pathways. For example, HSP90 inhibitors may improve the regulation of glucose metabolism in diabetic mice,158 whereas decreased expression of HSP72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance.159 These and other types of emerging experimental evidence suggest that stress-response mechanisms, metabolic pathways, and nutrients may jointly regulate metabolic functions at cellular and physiological levels.158 Therefore, a dietary pattern generating a low glycemic load, like the MedDiet, may protect against metabolic dysfunctions and also cooperate with stress-response pathways.
In addition, the weekly consumption of whole-grain cereals and legumes suggested by the MedDiet provides a good amount of dietary fiber (β-glucans, arabinoxylans, galactomannans, pectins) with numerous advantages for the consumer. In fact, fiber helps to achieve satiety and control body weight, while exerting a proven anti-inflammatory action that decreases systemic inflammation-associated biomarkers, such as C-reactive protein, interleukin 6, and tumor necrosis factor α,160–162 and inhibits the expression of specific inflammation-related genes such as cyclo-oxygenase-2 and inducible nitric oxide synthase.163 Fiber is also an efficient carrier for antioxidant bioactive substances like vitamins, carotenoids, and polyphenols, which follow a common and synergic physiological process within the gastrointestinal tract.
Specific dietary fibers called prebiotics (eg, inulin, galacto-oligosaccharides, lactulose, fructo-oligosaccharides), which are contained in plants, cereals, fresh and dried fruits (particularly bananas, onions, garlic, asparagus, wheat, rye, Jerusalem artichoke), and legumes,164 have a central role in the maintenance of gut health, as they provide the selective substrate for the growth of “health-promoting bacteria” such as Bifidobacterium and Lactobacillum.165
Overall, these micronutrients (phytochemicals, vitamins, fats, complex carbohydrates, and fibers) may exert multiple positive effects on human health. All of the data reported above seem to indicate that the healthful properties of the MedDiet could be partially accounted for by a hormetic stress response elicited by these compounds (Table 1). Indeed, when consumed in excess, many of these nutritional hormetins become detrimental, exerting a dual effect that fits perfectly with the biphasic curve of the hormetic response.
NEUROHORMESIS AND THE GUT–GUT MICROBIOTA–BRAIN AXIS
Phytochemicals found in fruit and vegetables exhibit several neuroprotective properties. Various interventional trials suggest that a diet rich in phytochemicals may enhance neuroplasticity and resistance to neurodegeneration, thereby postponing or preventing neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases in animal models.25,48,166 The term neurohormesis indicates the ability of the central nervous system to respond to exogenous as well as endogenous (ie, nitric oxide, carbon monoxide, glutamate, Ca2+) toxic agents,167–170 which represent mild stress and a driving force to augment the neuronal resistance toward stronger insults.25 There are several examples of neurohormetic phytochemicals, such as curcumin, sulforaphane in broccoli, and other chemical compounds contained in blueberry, green tea, green leafy vegetables, citrus fruits, coffee, and dark chocolate.25,49
Garlic and onion are rich in volatile organosulfur substances, such as allium and allicin, capable of inducing the Nrf2 pathway.62,171 Among them, diallyl trisulfide has been shown, in rat spinal cord explants, to protect motor neurons against glutamate-induced excitotoxicity by activating Nrf2 and increasing the expression of the antioxidant enzyme quinone oxidoreductase 1.172 Other allyl-containing sulfides might upregulate neuroprotective mitochondrial proteins activating stress-response mechanisms.171
Carnosic acid is abundant in rosemary (Rosmarinus officinalis), which is a very common herb in the Mediterranean basin173 and an acknowledged inducer of Nrf2.174,175 Carnosic acid has been demonstrated to protect against ischemia and reperfusion by activating phase 2 enzymes and reduced glutathione (GSH) metabolism in mouse brain.174 This function has been confirmed by another study performed in murine hippocampal cells.176 In addition, carnosic acid may enhance the expression of neurotrophin nerve growth factor, promoting neurite outgrowth in neuronal culture cells.177 A similar mechanism dependent on Nrf2 has also been detected in normal human astrocytes.175
Caffeine is a well-known psychoactive substance widely consumed worldwide. Recent investigations highlighted the role of caffeine in improving cognitive functions and synaptic plasticity and in counteracting neurodegeneration in Alzheimer’s and Parkinson's diseases.178–180
Ferulic acid ethyl ester, a hydrofobic form of ferulic acid, may increase heme-oxygenase activity in rat astrocytes/neurons181 and may protect against oxidative stress induced by amyloid-beta peptide on isolated synaptosomes (synaptic terminal from neurons).182
Resveratrol also displays neurohormetic functions, as detailed above in the section on phytochemicals.
Finally, in the study of the relationship between diet and the central nervous system, the central role of gut microbiota and its influence on neurological and psychological pathways, cognition, and behavior is emerging.183 The gastrointestinal tract and the gut microbiota establish a strong bidirectional connection with the central nervous system (gut–gut microbiota–brain axis), integrating the neurohumoral signals from/to the central nervous system, the neuroendocrine and immune systems, the autonomic nervous system, and the enteric nervous system.184 A number of experimental observations have shown that even mild alterations of gut microbiota composition are able to provoke modifications of cerebral functions, and, vice versa, the brain can deeply affect intestinal functions through the secretion of hormones, neuropeptides, and neurotransmitters (substance P, neurotensin, corticotropin-releasing factor, 5-hydroxytryptamine, and acetylcholine).184 Microbial subproducts may cross-react with human antigens and stimulate the immune system,185,186 and neurotoxic metabolites (such as D-lactic acid and ammonia) are able to cross the blood–brain barrier and cause neurotoxicity or neuroinflammation.187–189
THE EUROPEAN NU-AGE PROJECT
One of the main goals of the recently concluded European Commission–funded project NU-AGE (“New dietary strategies addressing the specific needs of the elderly population for healthy aging in Europe”; grant agreement 266486, www.nu-age.eu),190,191 whose results are currently being processed, was to test the hypothesis that inflammaging can be counteracted by a complete MedDiet-based nutritional approach suitably customized for different elderly populations in Europe (NU-AGE diet)192 as well as the importance of crucial but neglected aspects impacting MedDiet efficacy (Figure 4). Within this framework, 1250 volunteers, aged 65–79 years and gender-balanced, were enrolled in 5 European countries (Italy, France, Poland, the Netherlands, and the United Kingdom). At the beginning and end of the 1-year NU-AGE diet trial, all subjects underwent a comprehensive evaluation concerning genetics, epigenetics, transcriptomics, metagenomics, and metabolomics. The particular approach of the NU-AGE project might make it possible to not only identify a greater number of molecular processes involved in the dietary response to inflammaging but also to study the possible hormetic effects of the MedDiet components.193 Further studies like NU-AGE are necessary to disentangle the complex network of cellular pathways regulated by the MedDiet and its hormetic properties and to evaluate the impact of individual, population, and environmental variables on different outcomes such as age-related diseases, aging, and longevity.
Figure 4.

Critical and neglected aspects of the Mediterranean diet’s effect across individual, population, and environmental variables within a global perspective.Abbreviation: MedDiet, Mediterranean diet.
CONCLUSION
The finds of this review suggest that, on the basis of available experimental evidence, the well-recognized epidemiological beneficial outcomes of the MedDiet can be likely ascribed to a long-lasting hormetic effect. The data summarized here indicate that a variety of hormetins present in foods, mostly of vegetal origin, are a characteristic and important component of the MedDiet. The conceptualization of the MedDiet as a hormetic intervention fits the emerging general hypothesis that the most effective antiaging interventions, capable of largely preventing and/or postponing age-related pathological conditions, act by promoting hormetic effects. Indeed, the 2 most feasible and effective antiaging pillars, ie, nutritional intervention (calorie restriction and intermittent fasting) and appropriate physical activity, have been interpreted within the hormesis paradigm. Both types of hormetic intervention have been shown to decrease the accumulation of senescent cells, which largely contribute to inflammaging with their proinflammatory secretory phenotype,194 thus attenuating age-related deterioration and preserving the functionality of several organs. The findings of this review support the previously reported suggestion195 that a moderate level of stressors may be determinative in setting the basal tone of stress resistance mechanisms, thereby maintaining and preserving the health status.
Acknowledgments
Funding/support. This study was supported by the European Union’s Seventh Framework Program under grant agreement no. 266486 (“NU-AGE: New dietary strategies addressing the specific needs of the elderly population for healthy aging in Europe”) to C. Franceschi; EU Joint Programming Initiative: A Healthy Diet for a Healthy Life-Project “The Food Biomarkers Alliance (FOODBALL)” ID: 749; the European Union’s Seventh Framework Program under grant agreement no. 613979 (“MyNewGut: Microbiome’s influence on energy balance and brain development/function put into action to tackle diet-related diseases and behavior”); and the CL.USTER “A.GRIFOOD” N.AZIONALE—“CL.A.N.” project, identification code: CTN01_00230_413096 (“Promotion of consumer health: nutritional enhancement of traditional Italian agricultural food products [PROS.IT]”) to S.S.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Selye H. The Stress of Life. New York: McGraw-Hill Book Co; 1978. [Google Scholar]
- 2. Ottaviani E, Franceschi C. The neuroimmunology of stress from invertebrates to man. Prog Neurobiol. 1996;48:421–440. [DOI] [PubMed] [Google Scholar]
- 3. Ottaviani E, Franchini A, Franceschi C. Pro-opiomelanocortin-derived peptides, cytokines, and nitric oxide in immune responses and stress: an evolutionary approach. Int Rev Cytol. 1997;170:79–141. [DOI] [PubMed] [Google Scholar]
- 4. Ottaviani E, Franceschi C. A new theory on the common evolutionary origin of natural immunity, inflammation and stress response: the invertebrate phagocytic immunocyte as an eye-witness. Domest Anim Endocrinol. 1998;15:291–296. [DOI] [PubMed] [Google Scholar]
- 5. Ottaviani E, Malagoli D, Capri M et al. Ecoimmunology: is there any room for the neuroendocrine system? Bioessays. 2008;30:868–874. [DOI] [PubMed] [Google Scholar]
- 6. Franceschi C, Bonafe M, Valensin S et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 7. Ostan R, Bucci L, Capri M et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15 :224–240. [DOI] [PubMed] [Google Scholar]
- 8. Prenderville JA, Kennedy PJ, Dinan TG et al. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38:13–25. [DOI] [PubMed] [Google Scholar]
- 9. Schulz H. Über Hefegifte. Pflugers Arch Ges Physiol Mensch Tiere. 1888;42:517–541. [Google Scholar]
- 10. Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21:91–97. [DOI] [PubMed] [Google Scholar]
- 11. Calabrese EJ, Bachmann KA, Bailer AJ et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. [DOI] [PubMed] [Google Scholar]
- 12. Le Bourg E, Suresh R, eds. Mild Stress and Healthy Aging: Applying Hormesis in Aging Research and Intervention. Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- 13. Calabrese EJ, Dhawan G, Kapoor R et al. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16:693–707. [DOI] [PubMed] [Google Scholar]
- 14. Rattan SI, Demirovic D. Hormesis can and does work in humans. Dose Response. 2009;8:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demirovic D, Rattan SI. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013;48:94–98. [DOI] [PubMed] [Google Scholar]
- 16. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 17. Kennedy BK, Berger SL, Brunet A et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spazzafumo L, Olivieri F, Abbatecola AM et al. Remodelling of biological parameters during human aging: evidence for complex regulation in longevity and in type 2 diabetes. Age (Dordr). 2013;35:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pall ML, Levine S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao. 2015;67:1–18. [PubMed] [Google Scholar]
- 20. Wakabayashi N, Itoh K, Wakabayashi J et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. [DOI] [PubMed] [Google Scholar]
- 21. Rajasekaran NS, Connell P, Christians ES et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maher J, Yamamoto M. The rise of antioxidant signaling—the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. [DOI] [PubMed] [Google Scholar]
- 23. Rajasekaran NS, Varadharaj S, Khanderao GD et al. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal. 2011;14:957–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan NS, Wahli W. The emerging role of Nrf2 in dermatotoxicology. EMBO Mol Med. 2014;6:431–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mattson MP, Cheng A. Neurohormetic phytochemicals: low dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006; 29:632–639. [DOI] [PubMed] [Google Scholar]
- 26. Calabrese V, Cornelius C, Mancuso C et al. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. [DOI] [PubMed] [Google Scholar]
- 27. Calabrese V, Butterfield DA, Giuffrida Stella AM. Aging and oxidative stress response in the CNS. In Lajtha A, Regino P-PJ, Rossner S, eds. Development and Aging Changes in the Nervous System. Handbook of Neurochemistry and Molecular Neurobiology, 3rd ed. New York: Springer; 2008:128–234. [Google Scholar]
- 28. Calabrese V, Cornelius C, Mancuso C et al. Redox homeostasis and cellular stress response in aging and neurodegeneration. In: Uppu RM, Murthy SN, Pryor WA et al., eds. Free Radical and Antioxidant Protocols, 2nd ed.Los Angeles: Humana Press; 2008. [Google Scholar]
- 29. Calabrese V, Cornelius C, Mancuso C et al. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 2010;610:285–308. [DOI] [PubMed] [Google Scholar]
- 30. Mancuso C, Bates TE, Butterfield DA et al. Natural antioxidants in Alzheimer's disease. Expert Opin Investig Drugs. 2007;16:1921–1931. [DOI] [PubMed] [Google Scholar]
- 31. Cornelius C, Perrotta R, Graziano A et al. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a “chi.” Immun Ageing. 2013;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. [DOI] [PubMed] [Google Scholar]
- 33. Kumar H, Kim IS, More SV et al. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep. 2014;31:109–139. [DOI] [PubMed] [Google Scholar]
- 34. Landry J, Sutton A, Tafrov ST et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dali-Youcef N, Lagouge M, Froelich S et al. Sirtuins: the “magnificent seven,” function, metabolism and longevity. Ann Med. 2007;39:335–345. [DOI] [PubMed] [Google Scholar]
- 37. Calabrese V, Cornelius C, Dinkova-Kostova AT et al. Vitagenes, cellular stress response, and acetylcarnitine: relevance to hormesis. Biofactors. 2009;35:146–160. [DOI] [PubMed] [Google Scholar]
- 38. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calabrese EJ, Baldwin LA. Chemical hormesis: its historical foundations as a biological hypothesis. Toxicol Pathol. 1999;27:195–216. [DOI] [PubMed] [Google Scholar]
- 40. Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. [DOI] [PubMed] [Google Scholar]
- 41. Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66:594–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calabrese V, Pierluigi M, Cornelius C et al. Phenolics in aging and neurodegenerative disorders. In: Fraga CG, ed. Phenolic Compounds of Plant Origin and Health: The Biochemistry Behind Their Nutritional and Pharmacological Value. New York: Wiley & Sons; 2009. [Google Scholar]
- 43. Calabrese V, Cornelius C, Mancuso C et al. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci (Landmark Ed). 2009;14:376–397. [DOI] [PubMed] [Google Scholar]
- 44. Bellia F, Calabrese V, Guarino F et al. Carnosinase levels in aging brain: redox state induction and cellular stress response. Antioxid Redox Signal. 2009;11:2759–2775. [DOI] [PubMed] [Google Scholar]
- 45. Calabrese V, Cornelius C, Trovato A et al. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16:877–883. [DOI] [PubMed] [Google Scholar]
- 46. Liu J, Head E, Gharib AM et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Calabrese V, Guagliano E, Sapienza M et al. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res. 2007;32:757–773. [DOI] [PubMed] [Google Scholar]
- 48. Mattson MP, Son TG, Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. [DOI] [PubMed] [Google Scholar]
- 52. Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. [DOI] [PubMed] [Google Scholar]
- 53. Calabrese EJ, Dhawan G, Kapoor R et al. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16:693–707. [DOI] [PubMed] [Google Scholar]
- 54. Grayson BE, Hakala-Finch AP, Kekulawala M et al. Weight loss by calorie restriction versus bariatric surgery differentially regulates the hypothalamo-pituitary-adrenocortical axis in male rats. Stress. 2014;17:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pijl H. Longevity. The allostatic load of dietary restriction. Physiol Behav. 2012;106:51–57. [DOI] [PubMed] [Google Scholar]
- 56. Mockett RJ, Cooper TM, Orr WC et al. Effects of caloric restriction are species-specific. Biogerontology. 2006;7:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liao CY, Rikke BA, Johnson TE et al. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Keys A, Menotti A, Aravanis C et al. The seven countries study: 2,289 deaths in 15 years. Prev Med. 1984;13:141–154. [DOI] [PubMed] [Google Scholar]
- 59. Korre M, Tsoukas MA, Frantzeskou E et al. Mediterranean diet and workplace health promotion. Curr Cardiovasc Risk Rep. 2014;8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Intergovernmental Committee for the Safeguarding of the Intangible Cultural Heritage. Decision of the Intergovernmental Committee: 8.COM 8.10. Published 2013. http://www.unesco.org/culture/ich/en/decisions/8.COM/8.10. Accessed June 15, 2016. [Google Scholar]
- 61. Bach-Faig A, Berry EM, Lairon D et al. Mediterranean Diet Foundation Expert Group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274–2284. [DOI] [PubMed] [Google Scholar]
- 62. Calabrese V, Cornelius C, Dinkova-Kostova AT et al. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee J, Jo DG, Park D et al. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev. 2014;66:815–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao B. Natural antioxidants protect neurons in Alzheimer's disease and Parkinson's disease. Neurochem Res. 2009;34:630–638. [DOI] [PubMed] [Google Scholar]
- 65. Calabrese EJ. Alzheimer’s disease drugs: an application of the hormetic dose-response model. Crit Rev Toxicol. 2008;38:419–451. [DOI] [PubMed] [Google Scholar]
- 66. Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ou L, Kong LY, Zhang XM et al. Oxidation of ferulic acid by Momordica charantia peroxidase and related anti-inflammation activity changes. Biol Pharm Bull. 2003;26:1511–1516. [DOI] [PubMed] [Google Scholar]
- 68. Tetsuka T, Baier LD, Morrison AR. Antioxidants inhibit interleukin-1-induced cyclooxygenase and nitric-oxide synthase expression in rat mesangial cells. Evidence for post-transcriptional regulation. J Biol Chem. 1996;271:11689–11693. [DOI] [PubMed] [Google Scholar]
- 69. Murakami A, Nakamura Y, Koshimizu K et al. FA15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumor promotion: comparison with ferulic acid. Cancer Lett. 2002;180:121–129. [DOI] [PubMed] [Google Scholar]
- 70. Kawabata K, Yamamoto T, Hara A et al. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. [DOI] [PubMed] [Google Scholar]
- 71. Asanoma M, Takahashi K, Miyabe M et al. Inhibitory effect of topical application of polymerized ferulic acid, a synthetic lignin, on tumor promotion in mouse skin two-stage tumorigenesis. Carcinogenesis. 1994;15:2069–2071. [DOI] [PubMed] [Google Scholar]
- 72. Kim HS, Cho JY, Kim DH et al. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull. 2004;27:120–121. [DOI] [PubMed] [Google Scholar]
- 73. Nakamura Y, Torikai K, Ohto Y et al. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose-and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis. 2000;21:1899–1907. [DOI] [PubMed] [Google Scholar]
- 74. Murakami A, Kadota M, Takahashi D et al. Suppressive effects of novel ferulic acid derivatives on cellular responses induced by phorbol ester, and by combined lipopolysaccharide and interferon-gamma. Cancer Lett. 2000;157:77–85. [DOI] [PubMed] [Google Scholar]
- 75. Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;26:560–563. [DOI] [PubMed] [Google Scholar]
- 76. Huang YT, Hwang JJ, Lee PP et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ko WG, Kang TH, Lee SJ et al. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res. 2002;16:295–298. [DOI] [PubMed] [Google Scholar]
- 78. Dajas F, Rivera F, Blasina F et al. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res. 2003;5:425–432. [DOI] [PubMed] [Google Scholar]
- 79. Wruck CJ, Claussen M, Fuhrmann G et al. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl. 2007;72:57–67. [DOI] [PubMed] [Google Scholar]
- 80. Tookey HL, VanEtten CH, Daxenbichler ME. Glucosinolates. In: Liener IE, ed. Toxic Constituents of Plant Staffs. New York: Academic Press; 1980:103–142. [Google Scholar]
- 81. Carlson DG, Daxenbichler ME, VanEtten CH et al. Glucosinolates in crucifer vegetables: turnips and rutabagas. J Agric Food Chem. 1981;29:1235–1239. [DOI] [PubMed] [Google Scholar]
- 82. Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. [DOI] [PubMed] [Google Scholar]
- 83. Morse MA, Eklind KI, Amin SG et al. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989;10:1757–1759. [DOI] [PubMed] [Google Scholar]
- 84. Morse MA, Wang CX, Stoner GD et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–553. [PubMed] [Google Scholar]
- 85. Su JC, Lin K, Wang Y et al. In vitro studies of phenethyl isothiocyanate against the growth of LN229 human glioma cells. Int J Clin Exp Pathol. 2015;8:4269–4276 [PMC free article] [PubMed] [Google Scholar]
- 86. Yeh YT, Yeh H, Su SH et al. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic Biol Med. 2014;74:1–13. [DOI] [PubMed] [Google Scholar]
- 87. Pawlik A, Szczepanski MA, Klimaszewska A et al. Phenethyl isothiocyanate-induced cytoskeletal changes and cell death in lung cancer cells. Food Chem Toxicol. 2012;50:3577–3594. [DOI] [PubMed] [Google Scholar]
- 88. Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. [DOI] [PubMed] [Google Scholar]
- 89. Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. [DOI] [PubMed] [Google Scholar]
- 90. Kleszczyński K, Ernst IM, Wagner AE et al. Sulforaphane and phenylethyl isothiocyanate protect human skin against UVR-induced oxidative stress and apoptosis: role of Nrf2-dependent gene expression and antioxidant enzymes. Pharmacol Res. 2013;78:28–40. [DOI] [PubMed] [Google Scholar]
- 91. Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. [DOI] [PubMed] [Google Scholar]
- 92. Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8:478–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. [DOI] [PubMed] [Google Scholar]
- 94. Pazoki-Toroudi H, Amani H, Ajami M et al. Targeting mTOR signaling by polyphenols: a new therapeutic target for ageing. Ageing Res Rev. 2016;31:55–66. [DOI] [PubMed] [Google Scholar]
- 95. Calabrese EJ, Mattson MP, Calabrese V. Dose response biology: the case of resveratrol. Hum Exp Toxicol. 2010;29:1034–1037. [DOI] [PubMed] [Google Scholar]
- 96. Mark PM, Aiwu C. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. [DOI] [PubMed] [Google Scholar]
- 97. Wood JG, Rogina B, Lavu S et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. [DOI] [PubMed] [Google Scholar]
- 98. Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Testa G, Biasi F, Poli G et al. Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des. 2014;20:2950–2977. [DOI] [PubMed] [Google Scholar]
- 100. Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. [DOI] [PubMed] [Google Scholar]
- 101. Capiralla H, Vingtdeux V, Zhao H et al. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem. 2012;120:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhong LM, Zong Y, Sun L et al. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012;7:e32195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Corbi G, Conti V, Davinelli S et al. Dietary phytochemicals in neuroimmunoaging: a new therapeutic possibility for humans? Front Pharmacol. 2016;7:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Conti V, Izzo V, Corbi G et al. Antioxidant supplementation in the treatment of aging-associated diseases. Front Pharmacol. 2016;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu K, Zhou R, Wang B et al. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014;99:1510–1519. [DOI] [PubMed] [Google Scholar]
- 106. Timmers S, de Ligt M, Phielix E et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: a randomized controlled trial. Diabetes Care. 2016;39:2211–2217. [DOI] [PubMed] [Google Scholar]
- 107. Lee J, Torosyan N, Silverman DH. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: a double-blinded placebo controlled pilot study. Exp Gerontol. 2017(Pt2):121–128. [DOI] [PubMed] [Google Scholar]
- 108. Suresh R, Le Bourg E, eds. Hormesis in Health and Disease. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 109. Kafatos A, Verhagen H, Moschandreas J et al. Mediterranean diet of Crete: foods and nutrient content. J Am Diet Assoc. 2000;100:1487–1493. [DOI] [PubMed] [Google Scholar]
- 110. Simopoulos AP. The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J Nutr. 2001;131(suppl 11):3065S–3073S. [DOI] [PubMed] [Google Scholar]
- 111. Willcox DC, Willcox BJ, Todoriki H et al. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28(suppl):500S–516S. [DOI] [PubMed] [Google Scholar]
- 112. Cipollina C, Salvatore SR, Muldoon MF et al. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS One. 2014;9:e94836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Willett WC, Sacks F, Trichopoulou A et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(suppl 6):1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 114. Salvioli S, Sikora E, Cooper EL et al. Curcumin in cell death processes: a challenge for CAM of age-related pathologies. Evid Based Complement Alternat Med. 2007;4:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bellavista E, Andreoli F, Parenti MD et al. Immunoproteasome in cancer and neuropathologies: a new therapeutic target? Curr Pharm Des. 2013;19:702–718. [PubMed] [Google Scholar]
- 116. Chondrogianni N, Voutetakis K, Kapetanou M et al. Proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res Rev. 2015;23(pt A):37–55. [DOI] [PubMed] [Google Scholar]
- 117. Chondrogianni N, Chinou I, Rivett AJ et al. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10:157–172. [DOI] [PubMed] [Google Scholar]
- 118. Chondrogianni N, Kapeta S, Chinou I et al. Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45:763–771. [DOI] [PubMed] [Google Scholar]
- 119. Höhn TJ, Grune T. The proteasome and the degradation of oxidized proteins: part III—redox regulation of the proteasomal system. Redox Biol. 2014;2:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eaton DL, Klaassen CD. Principles of toxicology. In: Klassen CD, ed. Casarett and Doull’s Toxicology. The Basic Science of Poisons. 6th ed.New York, McGraw-Hill; 2001:11–34. [Google Scholar]
- 121. Hayes DP. Nutritional hormesis. Eur J Clin Nutr. 2007;61:147–159. [DOI] [PubMed] [Google Scholar]
- 122. Kodentsova VM, Vrzhesinskaia OA, Mazo VK. Vitamins and oxidative stress. Vopr Pitan. 2013;82:11–18. [PubMed] [Google Scholar]
- 123. Álvarez R, Vaz B, Gronemeyer H et al. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev. 2014;114:1–125. [DOI] [PubMed] [Google Scholar]
- 124. Amir AB, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–173. [DOI] [PubMed] [Google Scholar]
- 125. Traber MG, Stevens JF. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14:283–289. [DOI] [PubMed] [Google Scholar]
- 127. Chauhan SS, Celi P, Fahri FT et al. Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J Anim Sci. 2014;92:4897–4908. [DOI] [PubMed] [Google Scholar]
- 128. Bierhaus A, Wolf J, Andrassy M et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Elisia I, Kitts DD. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol Cell Biochem. 2015;404:123–131. [DOI] [PubMed] [Google Scholar]
- 130. De Lorgeril M, Salen P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ferraz AC, Delattre AM, Almendra RG et al. Chronic omega- 3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res 2011;219:116–122. [DOI] [PubMed] [Google Scholar]
- 132. Parker G, Gibson NA, Brotchie H et al. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. [DOI] [PubMed] [Google Scholar]
- 133. Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28:525–542. [DOI] [PubMed] [Google Scholar]
- 134. Giles GE, Mahoney CR, Kanarek RB. Omega-3 fatty acids influence mood in healthy and depressed individuals. Nutr Rev. 2013;71:727–741. [DOI] [PubMed] [Google Scholar]
- 135. Delarue J, Matzinger O, Binnert C et al. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–295. [DOI] [PubMed] [Google Scholar]
- 136. Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. [DOI] [PubMed] [Google Scholar]
- 137. Jolly CA, Muthukumar A, Avula CP et al. Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–2760. [DOI] [PubMed] [Google Scholar]
- 138. Zhao Y, Joshi-Barve S, Barve S et al. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. [DOI] [PubMed] [Google Scholar]
- 139. Wall R, Ross RP, Fitzgerald GF et al. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. [DOI] [PubMed] [Google Scholar]
- 140. Saw CL, Yang AY, Guo Y et al. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. 2013;62:869–875. [DOI] [PubMed] [Google Scholar]
- 141. Aparicio-Soto M, Sánchez-Hidalgo M, Rosillo MÁ et al. Extra virgin olive oil: a key functional food for prevention of immune-inflammatory diseases. Food Funct. 2016;7:4492–4505. [DOI] [PubMed] [Google Scholar]
- 142. Estruch R, Ros E, Salas-Salvado J et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 143. Turner R, Etienne N, Alonso MG et al. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int J Vitam Nutr Res. 2005;75:61–70. [DOI] [PubMed] [Google Scholar]
- 144. Piroddi M, Albini A, Fabiani R et al. Nutrigenomics of extra-virgin olive oil: a review. Biofactors. 2017;43:17–41. [DOI] [PubMed] [Google Scholar]
- 145. Galli F, Azzi A, Birringer M et al. Vitamin E: emerging aspects and new directions. Free Radic Biol Med. 2016;102:16–36. [DOI] [PubMed] [Google Scholar]
- 146. Ghanbari R, Anwar F, Alkharfy KM et al. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci. 2012;13:3291–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tsimidou MZ. Virgin olive oil (VOO) and other olive tree products as sources of a-tocopherol. Updating and perspective. In: Catala A, ed. Tocopherol: Sources, Uses and Health Benefits. New York: Nova Science Publisher; 2012:1–21. [Google Scholar]
- 148. Galli F. Interactions of polyphenolic compounds with drug disposition and metabolism. Curr Drug Metab. 2007;8:830–838. [DOI] [PubMed] [Google Scholar]
- 149. Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Martin MA, Ramos S, Granado-Serrano AB et al. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol Nutr Food Res. 2010;54:956–966. [DOI] [PubMed] [Google Scholar]
- 151. Zou X, Feng Z, Li Y et al. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem. 2012;23:994–1006. [DOI] [PubMed] [Google Scholar]
- 152. Parzonko A, Czerwinska ME, Kiss AK et al. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine. 2013;20:1088–1094. [DOI] [PubMed] [Google Scholar]
- 153. Bayram B, Ozcelik B, Grimm S et al. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012;15:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Menendez JA, Joven J, Aragonès G et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: a new family of gerosuppressant agents. Cell Cycle. 2013;12:555–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhang B, Kang M, Xie Q et al. Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J Agric Food Chem. 2011;59:537–545. [DOI] [PubMed] [Google Scholar]
- 156. Mirrahimi A, Chiavaroli L, Srichaikul K et al. The role of glycemic index and glycemic load in cardiovascular disease and its risk factors: a review of the recent literature. Curr Atheroscler Rep. 2014;16:381. [DOI] [PubMed] [Google Scholar]
- 157. Kalogeropoulos N, Chiou A, Ioannou M et al. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Chemistry. 2010;121:682–690. [Google Scholar]
- 158. Lee JH, Gao J, Kosinski PA et al. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem Biophys Res Commun. 2013;430:1109–1113. [DOI] [PubMed] [Google Scholar]
- 159. Kurucz I, Morva A, Vaag A et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. [DOI] [PubMed] [Google Scholar]
- 160. Ma Y, Griffith JA, Chasan-Taber L et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ma Y, Hébert JR, Li W et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008;24:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chuang SC, Vermeulen R, Sharabiani MT et al. The intake of grain fibers modulates cytokine levels in blood. Biomarkers. 2011;16:504–510. [DOI] [PubMed] [Google Scholar]
- 163. Reddy BS, Hirose Y, Cohen LA et al. Preventive potential of wheat bran fractions against experimental colon carcinogenesis: implications for human colon cancer prevention. Cancer Res. 2000;60:4792–4797. [PubMed] [Google Scholar]
- 164. Costa GT, Abreu GC, Guimarães AB et al. Fructo-oligosaccharide effects on serum cholesterol levels. An overview. Acta Cir Bras. 2015;30:366–370. [DOI] [PubMed] [Google Scholar]
- 165. Candela M, Turroni S, Garagnani P et al. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. [DOI] [PubMed] [Google Scholar]
- 166. Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81(suppl 1):313S–316S. [DOI] [PubMed] [Google Scholar]
- 167. Huang PL. Nitric oxide and cerebral ischemic preconditioning. Cell Calcium. 2004;36:323–329. [DOI] [PubMed] [Google Scholar]
- 168. Doré S, Goto S, Sampei K et al. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99:587–592. [DOI] [PubMed] [Google Scholar]
- 169. Jiang X, Tian F, Mearow K et al. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. [DOI] [PubMed] [Google Scholar]
- 170. Sée V, Boutillier AL, Bito H et al. Calcium/calmodulin-dependent protein kinase type IV (CaMKIV) inhibits apoptosis induced by potassium deprivation in cerebellar granule neurons. FASEB J. 2001;15:134–144. [DOI] [PubMed] [Google Scholar]
- 171. Chen C, Pung D, Leong V et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–1590. [DOI] [PubMed] [Google Scholar]
- 172. Sun MM, Bu H, Li B et al. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol Res. 2009;31:23–27. [DOI] [PubMed] [Google Scholar]
- 173. Kosaka K, Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol Pharm Bull. 2003;26:1620–1622. [DOI] [PubMed] [Google Scholar]
- 174. Satoh T, Kosaka K, Itoh K et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mimura J, Kosaka K, Maruyama A et al. Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J Biochem. 2011;150:209–217. [DOI] [PubMed] [Google Scholar]
- 176. Tamaki Y, Tabuchi T, Takahashi T et al. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–688. [DOI] [PubMed] [Google Scholar]
- 177. Kosaka K, Mimura J, Itoh K et al. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem. 2010;147:73–81. [DOI] [PubMed] [Google Scholar]
- 178. Sonsalla PK, Wong LY, Harris SL et al. Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson's disease. Exp Neurol. 2012;234:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sallaberry C, Nunes F, Costa MS et al. Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunocontent in middle-aged rats. Neuropharmacology. 2013;64:153–159. [DOI] [PubMed] [Google Scholar]
- 180. Laurent C, Eddarkaoui S, Derisbourg M et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol Aging. 2014;35:2079–2090. [DOI] [PubMed] [Google Scholar]
- 181. Scapagnini G, Butterfield DA, Colombrita C et al. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 2004;6:811–818. [DOI] [PubMed] [Google Scholar]
- 182. Perluigi M, Joshi G, Sultana R et al. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. J Neurosci Res. 2006;84:418–426. [DOI] [PubMed] [Google Scholar]
- 183. Caracciolo B, Xu W, Collins S et al. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev. 2014;136–137:59–69. [DOI] [PubMed] [Google Scholar]
- 184. Petra AI, Panagiotidou S, Hatziagelaki E et al. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Berer K, Mues M, Koutrolos M et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. [DOI] [PubMed] [Google Scholar]
- 186. Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25:488–795. [DOI] [PubMed] [Google Scholar]
- 187. Sheedy JR, Wettenhall RE, Scanlon D et al. Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo. 2009;23:621–628. [PubMed] [Google Scholar]
- 188. Skowrońska M, Albrecht J. Alterations of blood brain barrier function in hyperammonemia: an overview. Neurotox Res. 2012;21:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Qureshi MO, Khokhar N, Shafqat F. Ammonia levels and the severity of hepatic encephalopathy. J Coll Physicians Surg Pak. 2014;24:160–163. [PubMed] [Google Scholar]
- 190. NU-AGE Consortium. European Project NU-AGE. http://www.nu-age.eu. Accessed January 10, 2017. [Google Scholar]
- 191. Santoro A, Pini E, Scurti M et al. NU-AGE Consortium. Combating inflammaging through a Mediterranean whole diet approach: the NU-AGE project’s conceptual framework and design. Mech Ageing Dev. 2014;136–137:3–13. [DOI] [PubMed] [Google Scholar]
- 192. Berendsen A, Santoro A, Pini E et al. Reprint of: a parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: design of the NU-AGE dietary intervention study. Mech Ageing Dev. 2014;136–137:14–21. [DOI] [PubMed] [Google Scholar]
- 193. Santoro A, Brigidi P, Gonos ES et al. (Eds.) Mediterranean diet and inflammaging in the elderly: the European project NU-AGE. Mech Ageing Dev. 2014;136–137 (Special Issue):1–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Franceschi C, Garagnani P, Vitale G et al. Inflammaging and “garb-aging.” Trends Endocrinol Metab. 2017;28:199–212. [DOI] [PubMed] [Google Scholar]
- 195. Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger.” Cell Metab. 2008;7:200–203. [DOI] [PubMed] [Google Scholar]