Abstract
Objective
Osteoarthritis is a leading cause of disability for which there is no cure. Psychosocial-oriented treatments are underexplored. We developed and tested an intervention to build positive psychological skills (e.g., gratitude) to reduce osteoarthritis symptom severity, including pain and functioning, and to improve psychosocial well-being in patients with knee or hip osteoarthritis.
Design
Two-arm randomized design with six-month follow-up.
Setting
An academic Veterans Affairs Medical Center.
Subjects
Patients aged 50 years or older with knee or hip osteoarthritis and pain ratings of 4 or higher.
Methods
Patients (N = 42) were randomized to a six-week program containing positive skill-building activities or neutral control activities tailored to the patient population. Adherence was assessed by telephone each week. We assessed osteoarthritis symptom severity (WOMAC Osteoarthritis Index) and measures of well-being (positive affect, negative affect, and life satisfaction) at baseline and by telephone one, three, and six months after the program ended. We used linear mixed models to examine changes over time.
Results
The majority (64%) of patients completed more than 80% of their weekly activities. Patients in the positive (vs neutral) program reported significantly more improvement over time in osteoarthritis symptom severity (P = 0.02, Cohen’s d = 0.86), negative affect (P = 0.03, Cohen’s d = 0.50), and life satisfaction (P = 0.02, Cohen’s d = 0.36).
Conclusions
The study successfully engaged patients with knee or hip osteoarthritis in a six-week intervention to build positive psychological skills. Improving osteoarthritis symptom severity and measures of psychosocial well-being, the intervention shows promise as a tool for chronic pain management.
Keywords: Chronic Pain, Mind-Body Therapies, Veterans, Osteoarthritis, Psychosocial factors, Psychology
Introduction
Arthritis affects more than one in five (52.5 million) adults in the United States [1]. Osteoarthritis (OA), the most prevalent form of arthritis, causes substantial disability [2,3]. Current treatments focus on alleviating symptoms, preserving function, and improving quality of life [4–6] as ways to slow or halt disease progression have not been identified. The majority of existing OA treatments yield only small to moderate reductions in pain and disability and can have negative side effects [5–8]. In the context of society-wide efforts to reduce reliance on opioids and other potentially harmful pharmacological pain treatments, safe and effective nonpharmacological techniques for managing OA pain and symptoms are urgently needed.
The biopsychosocial model of pain can inform the development of nonpharmacological OA treatments as it recognizes that one’s pain experience is determined by a complex interaction of biological, psychological, and social factors [9–11]. For example, co-occurring depression and pain-related cognitions such as low self-efficacy and high catastrophizing are associated with worse pain and functioning among patients with OA [12,13]. Social factors such as stress and lack of support also predict worse OA outcomes [10]. The extensive interplay between psychosocial factors and OA outcomes suggests that OA treatments specifically targeting psychosocial well-being should be explored.
Evidence from the burgeoning field of positive psychology, which investigates how and why people flourish, supports the value of targeting psychosocial wellness to reduce pain [14–17]. For example, naturally occurring positive affect, defined as the feelings experienced when one is pleasurably engaged with the environment [14], is associated with less pain in women with OA or fibromyalgia [18] and better functioning following a hip fracture [19]. Optimism induced in a controlled setting also reduces pain intensity ratings [20]. Finally, initial evidence from two randomized trials suggests that engaging in activities explicitly designed to build positive psychological skills (subsequently referred to as “positive activities”) improves pain symptoms [21,22]. In a study of volunteers from a positive psychology website who reported pain at baseline, those randomly assigned to complete four or six positive activities over six weeks reported greater reductions in pain than those assigned to a no-activity control group [21]. A recent pilot study also found preliminary support for feasibility and efficacy of an individually tailored, web-based positive psychological intervention for individuals with disability (e.g., spinal cord injury) and chronic pain [22]. Although these studies support the use of positive activities for pain management, they are limited by relatively weak control conditions, short-term follow-up periods, and demographically homogeneous samples comprising mostly non-Hispanic white, highly educated women. Research is needed to test the long-term effects of positive activities on pain in different patient populations using a strong comparison condition. In this study, we extend the literature by randomizing a demographically diverse sample of military veterans with chronic pain from knee or hip osteoarthritis to complete a six-week program containing positive skill-building activities or neutral control activities. Following patients for six months, we hypothesized that patients randomized to the positive (versus neutral) program would report greater improvements over time in OA symptom severity and measures of psychosocial well-being.
Methods
Participants and Recruitment
The study was conducted at the Veterans Affairs (VA) Pittsburgh Healthcare System and was approved by the medical center’s Institutional Review Board. Patients who were 50 years of age or older, had a diagnosis of OA (International Classification of Diseases-9 code 715), and had been seen in primary care in the past six months were identified from VA electronic health records and mailed invitations to be screened for the study. Study brochures were also distributed throughout the medical center. Eligibility of interested patients was confirmed using telephone screening surveys conducted by study staff prior to enrollment. To be eligible, patients had to be veterans aged 50 years or older; receive primary care from the VA; report having physician-diagnosed OA; have frequent knee or hip pain [23]; and rate their pain level as 4 or greater on a 0–10 scale. Patients were excluded if they reported inflammatory arthritis or low back pain; problems with serious illness, memory, hearing, or eyesight that would prevent them from completing the study; inability to receive study-related telephone calls; or inability to complete simple writing activities without assistance.
Intervention Development
We developed a six-week intervention program consisting of positive psychological skill-building activities drawn from the positive psychology literature and then refined based on qualitative input from patients. Numerous activities have been developed to build positive psychological skills, including activities focused on gratitude, kindness, optimism, mindfulness, self-affirmation, identifying and using personal strengths, reflecting on good things, forgiveness, or some combination thereof [15,24,25]. A meta-analysis found that the most effective positive activity interventions are administered individually (versus in a group setting) and include a variety of activities introduced over multiple weeks [25]. Allowing flexibility in how activities are implemented by individuals also increases adherence [26,27]. With these principles and our target population in mind, we identified activities from the positive psychology literature that were simple to complete, that did not require extensive training or follow-up, that worked well when self-administered, and that could be adapted for use by those with low literacy. These activities were focusing attention on positive events [15,28]; expressing gratitude [15,29,30]; increasing kindness [31]; savoring [32,33]; and increasing engagement in activities that bring enjoyment, provide a sense of achievement, or bring one closer to others [24]. To create a robust control program, we also selected a set of structurally similar but affectively neutral activities that served as comparisons in prior studies testing positive activities (Table 1) [15,30,34,35].
Table 1.
Activities included in a positive (intervention) and neutral (control) program developed for patients with knee or hip osteoarthritis
| Week | Positive Program | Neutral Program |
|---|---|---|
| 1 | Three Good Things: Write down 3 good things that happened each day. | Events that Affect You: Write down 3 things that affected you each day. |
| 2 | Expressing Thanks: Write a letter to someone whose actions affected you but you never thanked. Deliver the letter in person, if possible. | Changing your Circumstances: Identify small ways you could change your life circumstances. Write down one change that you will try to make in the next week. |
| 3 | Acts of Kindness: Do 5 acts of kindness in a single day. Write down what you did, how it made you feel, and how the recipient responded. | Early Memories: Each day, write down an early memory, how it made you feel, and who you were with. |
| 4 | Making Good Moments Last: Spend 2-3 minutes each day focusing on a good moment and making it last. Write down what you focused on, how you made it last, and how it made you feel. | Getting Organized: Create a mental outline of everything you have done in the past 7 days and then make a written list. |
| 5 | Increasing Pleasant Activity: Identify activities you enjoy, give you a sense of achievement, or bring you closer to others. Try to do at least 4 each day. Write down the activities you do each day in the next week. | Planning the Future: Spend time each morning planning what you are going to do that day. Write down your plans for each day. |
| 6 | Practice Your Favorite(s): Pick one of the first 5 activities that you really liked and do it again. | Practice Your Favorite(s): Pick one of the first 5 activities that you really liked and do it again. |
We wrote instructions for each activity at a sixth-grade reading level and assembled them into positive and neutral workbooks. We obtained feedback on the workbooks through qualitative interviews with 10 patients meeting study eligibility criteria. After providing written informed consent, patients silently read the instructions for each activity and were then asked open-ended questions about what they liked or disliked about it, how it could be improved, and the likelihood that they would complete the activity. Responses were documented in written notes taken by trained interviewers. Patients were compensated $25. The lead investigator (LH) reviewed the notes and worked with the study team to modify activity instructions to reflect the feedback received.
The response to the initial draft of activities was overwhelmingly positive, with patients expressing that they thought doing the activities would help them feel good about themselves and would provide motivation and fulfillment. All patients indicated that they would be very likely to complete all the activities. Although patients were able to read and comprehend the original instructions, we increased the readability of the materials based on the interviews. For example, some patients were unfamiliar with the idea of “savoring,” so we modified the activity title and instructions using words that were better understood by the target population. We renamed and revised an activity focused on gratitude for similar reasons.
We compiled the revised activities into positive and neutral workbooks that were written at a 4.5-grade reading level and were designed to be completed over six weeks (Table 1). Both workbooks contained an identical introductory page explaining the rationale for building positive psychological skills to help with arthritis pain. On subsequent pages, the workbooks contained instructions for one new positive or neutral activity each week for the first five weeks and instructions to repeat a favorite activity in week 6.
Study Procedure
Using the previously described eligibility criteria and recruitment procedures, we invited a new cohort of patients who did not participate in the intervention development activities to enroll in a randomized trial of the positive and neutral programs. Patients who met eligibility requirements attended an in-person baseline visit at the medical center, during which one of three study staff members obtained written informed consent, administered baseline measures, and gave participants their workbook for the program to which they were randomly assigned. All staff members were fully trained to administer both programs and to collect all study assessments.
During the consent process, participants were informed that the purpose of the study was to identify activities that people with arthritis can put into practice in their daily lives to help stay positive and increase their overall well-being. They were told that they would be randomly assigned to one of two six-week programs consisting of a series of activities that may help them stay positive. They were told that the two programs contained different activities. Patients who consented to participate then provided responses to a baseline survey administered by the study staff member who obtained their consent.
Upon completion of the baseline survey, the study staff member opened a sealed envelope that contained the workbook for either the positive or neutral program. To maintain blinding of study staff during the collection of baseline measures, the study biostatistician placed positive and neutral program workbooks in sealed envelopes in a sequence determined by a permuted block randomization scheme, stratified by patient race (African American or white), prior to enrollment of the first study participant. For each baseline visit, study staff members took the next sealed envelope in the predetermined sequence but remained unaware of which program was in the envelope until after the consent process and baseline surveys were completed.
After opening the envelope, the study staff member provided the participant with a general orientation to the workbook. By design, the positive and neutral program workbooks had the same cover page and introductory text so that the general orientation would be similar. Both workbooks were titled “Staying Positive with Arthritis” and contained a brief explanation of why building habits that help one to stay positive may be beneficial for people with arthritis. The study staff member then reviewed the instructions for the first week’s activity, which was either positive or neutral depending on the program to which the participant was randomly assigned (Table 1). At this time, the study staff member was no longer blinded to the assignment of that participant due to their familiarity with both programs, but participants were not explicitly told whether they were in the positive or neutral program. Participants were kept blinded to their assigned program to ensure that changes observed in response to the positive program were not due to placebo effects, expectations, or motivation.
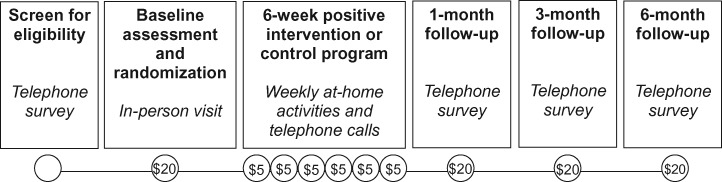
At the end of the baseline visit, participants were instructed to complete the first activity on their own over the next week. They were also asked to confirm their availability for a 10- to 15-minute telephone call from the study staff that would take place seven days from the baseline visit, and every week thereafter for the duration of the six-week program. All contact with participants after the baseline visit was by telephone. For the six weeks following the baseline visit, study staff telephoned participants at the agreed-upon time to assess adherence to the previous week’s activity and to review instructions for the next week’s activity. Study staff then collected follow-up measures by telephone one, three, and six months after the end of the program. Participants were compensated $20 for the baseline visit, $5 for each weekly phone call, and $20 for each follow-up assessment (Figure 1).
Figure 1.
Summary of study events and participant compensation.
Study Measures
Primary Outcome
Our primary outcome was OA symptom severity measured by the Western Ontario MacMaster Osteoarthritis Index (WOMAC) [36] at baseline and at one, three, and six months following completion of the six-week program. The WOMAC includes 24 items that ask participants to rate pain, stiffness, and difficulty with physical functioning ranging from none to extreme (corresponding to values of 0–4). An overall score was computed by summing responses and transforming scores to a scale ranging from 0 to 100, with higher scores indicating more severe OA symptoms [37]. We also calculated scores for subscales that assessed pain (five items), stiffness (two items), and difficulty with physical functioning (17 items), with higher scores indicating worse pain, stiffness, or functioning, respectively.
Secondary Outcomes
We also assessed three psychosocial outcomes at baseline and at one, three, and six months following completion of the program. Positive and negative affect were assessed using the 10-item Positive and Negative Affect Schedule (PANAS), on which participants indicated the extent to which they felt five positive (alert, inspired, determined, attentive, active) and five negative (upset, hostile, ashamed, nervous, afraid) states during the past week using a 1 (never) to 5 (always) scale [38]. Positive and negative affect scores were calculated by summing the responses across items (possible ranges = 5–25). Life satisfaction, a measure of overall subjective well-being, was assessed using the Satisfaction With Life Scale [39]. For this measure, participants indicated the extent to which they agreed with five statements (e.g., “The conditions of your life are excellent”) on a scale of 1 (strongly disagree) to 5 (strongly agree). Scores were calculated by summing responses across items (possible range = 5–25).
Demographic and Clinical Characteristics
Participants’ age, sex, income, education, employment, marital status, and general health status (excellent, very good, good, fair, or poor) were assessed at baseline. Health literacy was also assessed using the item “How confident are you filling out medical forms by yourself?” [40]. Physical and psychological comorbid conditions were assessed using an interviewer-administered version of the Charlson Comorbidity Index [41] and self-reported diagnoses of depression or anxiety [42]. Self-reported pharmacological and nonpharmacological OA treatments being used at baseline were assessed using a list of treatment options from the Osteoarthritis Initiative [23]. Pharmacological treatment categories included acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDS), topical NSAIDS, COX-2 selective inhibitors, opioids, and hyaluronic acid or steroid injections. Nonpharmacological treatment categories included acupuncture/acupressure/massage therapy, chiropractic care, homeo/naturopathy, physical therapy, water- or land-based exercise, health supplements for joint pain, vitamins, herbs, topical creams/oils, copper bracelets or magnets, yoga/tai chi/chi gong/Pilates, relaxation/mind-body activities, and spiritual activities.
Adherence and Engagement
Adherence was assessed in the weekly calls by asking participants whether they completed the previous week’s activity (yes/no) [43]. Participants were also asked to rate how much they felt they benefited from, how much they enjoyed, and how difficult they found each activity (1 = not at all; 7 = extremely) [43].
Statistical Analyses
We tested differences in baseline characteristics between the positive intervention and neutral control programs using chi-square or Fisher exact tests for categorical variables and t tests for continuous variables. We assessed changes in outcomes using linear mixed models that accounted for repeated measures and missing data. Models included fixed effects for program (positive vs neutral), time (baseline, one month, three months, and six months), and the interaction between program and time. Cohen’s d effect sizes were calculated to assess the magnitude of change from baseline to six months across treatment groups for all outcomes. Analyses followed an intent-to-treat principle [44] and were performed using Stata software [45]. The treatment group was not blinded during analysis.
Results
Sample
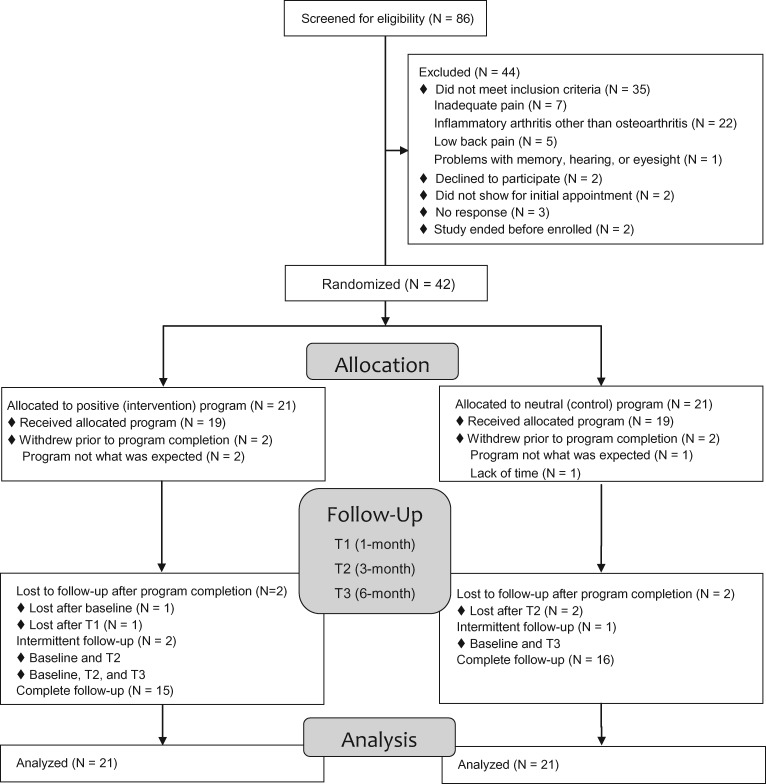
Of 86 patients who were screened for eligibility, 51 met all inclusion criteria and 42 were consented and randomized (Figure 2). On average, participants were 67.5 years old, 16.7% were female, and 42.9% were black or African American. Over half the sample was married (59.5%), retired (52.4%), and had at least some college education (64.3%). Low health literacy was common, with 54.8% reporting that they were not at all confident filling out medical forms without assistance (Table 2). There were no significant differences in any baseline characteristics, including use of pharmacological or nonpharmacological treatments for OA, across those in the positive and neutral programs (Table 2).
Figure 2.
Study flow diagram.
Table 2.
Baseline characteristics of patients with knee or hip osteoarthritis randomized to complete a 6-week positive or neutral program
| Characteristics | Total Sample (N = 42) | Positive Program (N = 21) | Neutral Program (N = 21) | P* |
|---|---|---|---|---|
| Age, mean (SD), y | 67.5 (10.3) | 69.2 (11.3) | 65.7 (9.1) | 0.28 |
| Female, No. (%) | 7 (16.7) | 4 (19.1) | 3 (14.3) | 0.99 |
| Race, No. (%) | 0.53 | |||
| Black/African American | 18 (42.9) | 8 (38.1) | 10 (47.6) | |
| White | 24 (57.1) | 13 (61.9) | 11 (52.4) | |
| Income, No. (%) | 0.72 | |||
| Less than $20,000 | 14 (35.9) | 5 (27.8) | 9 (42.9) | |
| $20,000–$49,999 | 13 (33.3) | 7 (38.9) | 6 (28.6) | |
| $50,000 or more | 12 (30.1) | 6 (33.3) | 6 (28.6) | |
| Education, No. (%) | 0.33 | |||
| High school graduate or less | 15 (35.7) | 6 (28.6) | 9 (42.9) | |
| At least some college | 27 (64.3) | 15 (71.4) | 12 (57.1) | |
| Employment status, No. (%) | 0.28 | |||
| Employed | 7 (16.7) | 4 (19.0) | 3 (14.2) | |
| Retired | 22 (52.4) | 13 (61.9) | 9 (42.9) | |
| Unemployed or disabled | 13 (31.0) | 4 (19.0) | 9 (42.9) | |
| Married or living with partner, No. (%) | 25 (59.5) | 14 (66.7) | 11 (52.4) | 0.35 |
| Excellent of very good health, No. (%) | 15 (35.7) | 6 (28.6) | 9 (42.9) | 0.33 |
| Confident filling out medical forms (health literacy item), No. (%) | ||||
| Not at all | 23 (54.8) | 12 (57.1) | 11 (52.4) | 0.76 |
| Type of osteoarthritis, No. (%) | 0.63 | |||
| Hip | 2 (4.8) | 1 (4.8) | 1 (4.8) | |
| Knee | 23 (54.8) | 10 (47.6) | 13 (61.9) | |
| Hip and knee | 17 (40.5) | 10 (47.6) | 7 (33.3) | |
| Charlson comorbidity index, mean (SD) | 3.7 (2.8) | 3.2 (2.4) | 4.1 (3.1) | 0.27 |
| Anxiety disorder, No. (%) | 14 (33.3) | 8 (38.1) | 6 (28.6) | 0.51 |
| Depressive disorder, No. (%) | 20 (47.6) | 10 (47.6) | 10 (47.6) | 0.99 |
| Pain rating on 0–10 scale, mean (SD) | 7.6 (0.2) | 8.0 (0.3) | 7.3 (0.3) | 0.16 |
| Treatments used for joint pain or arthritis, No. (%)† | ||||
| Acetaminophen | 12 (28.6) | 8 (38.1) | 4 (19.0) | 0.17 |
| NSAIDS | 25 (59.5) | 12 (57.1) | 13 (61.9) | 0.75 |
| Topical NSAIDS | 17 (40.5) | 9 (42.9) | 8 (38.1) | 0.75 |
| COX-2 selective inhibitors | 2 (4.8) | 1 (4.8) | 1 (4.8) | 0.99 |
| Opioids | 12 (28.6) | 6 (28.6) | 6 (28.6) | 0.99 |
| Hyaluronic acid or steroid injections | 6 (15.0) | 2 (33.3) | 4 (66.6) | 0.38 |
| Chiropractic care | 1 (2.4) | 0 (0.0) | 1 (4.8) | 0.31 |
| Physical therapy | 2 (4.8) | 0 (0.0) | 2 (9.5) | 0.15 |
| Water or land-based exercise | 7 (16.7) | 3 (14.3) | 4 (19.1) | 0.68 |
| Health supplements for joint pain | 7 (16.7) | 4 (19.1) | 3 (14.3) | 0.68 |
| Vitamins or minerals | 20 (47.6) | 10 (47.6) | 10 (47.6) | 0.99 |
| Herbs | 2 (4.8) | 1 (4.8) | 1 (4.8) | 0.99 |
| Topical creams/oils | 19 (45.2) | 10 (47.6) | 9 (42.9) | 0.76 |
| Copper bracelets or magnets | 2 (4.8) | 2 (9.52) | 0 (0.0) | 0.15 |
| Yoga, tai chi, chi gong, or Pilates | 2 (4.8) | 1 (4.8) | 1 (4.8) | 0.99 |
| Relaxation or mind-body activities | 12 (28.6) | 7 (58.3) | 5 (23.8) | 0.50 |
| Spiritual activities | 17 (40.5) | 10 (47.6) | 7 (33.3) | 0.35 |
NSAIDS = nonsteroidal anti-inflammatory drugs.
P values are based on Fisher exact tests for gender and employment status, chi-square tests for all other categorical variables, and t tests for all continuous variables.
No participants were using the following treatments at baseline: acupuncture, acupressure, or massage; homeopathy or naturopathy.
Adherence and Engagement
Early on, three participants (two from the positive program, one from the neutral program) withdrew after baseline because the program did not match their expectations. The withdrawal of those patients prompted us to revise the recruitment and consent materials to introduce the biopsychosocial model of pain and clarify the nature of the program being tested. After those changes were made, one additional participant withdrew due to lack of time and another was lost to follow-up after baseline for unknown reasons (Figure 1). All participants were included in the analysis.
Retention through both six-week programs was high, with 78.6% of participants overall completing at least five of six weekly calls and 64.3% reporting that they completed at least five of the six weekly activities (Table 3). Participants rated the activities as highly beneficial (mean = 5.3), highly enjoyable (mean = 5.4), and low in difficulty (mean = 2.0). There were no significant differences in completion rates or activity ratings across the positive and neutral groups (Table 3).
Table 3.
Adherence to and ratings of positive and neutral programs
| Total Sample (N = 42) | Positive Program (N = 21) | Neutral Program (N = 21) | P | |
|---|---|---|---|---|
| Adherence, No. (%) | ||||
| At least 5 of 6 calls completed | 33 (78.6) | 16 (76.2) | 17 (81.0) | 0.99 |
| At least 5 of 6 activities completed | 27 (64.3) | 13 (61.9) | 14 (66.7) | 0.75 |
| Ratings of completed weekly activities, mean (%)* | ||||
| Perceived benefit | 5.3 (1.1) | 5.2 (1.4) | 5.3 (0.9) | 0.75 |
| Perceived enjoyment | 5.4 (0.9) | 5.4 (1.0) | 5.4 (0.8) | 0.96 |
| Perceived difficulty | 2.0 (1.0) | 1. 8 (0.9) | 2.3 (1.0) | 0.13 |
Participants who did not complete a weekly call were counted as not completing that week’s activity.
Activities were rated on seven-point Likert scales (1 = not at all and 7 = extremely).
OA Symptom Severity
Retention remained high for the one- (81.0%), three- (83.3%), and six-month (78.6%) follow-up surveys. Baseline total WOMAC scores were comparable for the positive (mean = 52.4) and neutral (mean = 50.4) groups at baseline (Table 4). Compared with baseline, mean total WOMAC scores in the positive group decreased (i.e., improved) by 14.2, 10.9, and 12.6 points at one, three, and six months, respectively. Mean total WOMAC scores in the neutral group decreased by less than two points at each time point (Table 4). The interaction between program and time was statistically significant (χ2 = 9.83, P = 0.02), indicating greater improvement in total WOMAC scores over time for the positive program. Changes from baseline to six months corresponded to a large effect size (Cohen’s d = 0.86).
Table 4.
Change in total and specific (pain, stiffness, and physical function) osteoarthritis symptoms in patients with knee or hip osteoarthritis after completing a 6-week positive (intervention) or neutral (control) program
| Positive (Intervention) Group |
Neutral (Control) Group |
Group × Time Interaction |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WOMAC Outcomes | Baseline (N = 21) | 1-mo (N = 16) | 3-mo (N = 17) | 6-mo (N = 16) | Baseline (N = 21) | 1-mo (N = 18) | 3-mo (N = 18) | 6-mo (N = 17) | Chi-square | P |
| Total | ||||||||||
| Mean (SD) | 52.4 (13.1) | 38.2 (10.5) | 41.5 (17.1) | 39.8 (15.6) | 50.4 (17.4) | 49.3 (15.0) | 50.1 (10.9) | 48.6 (11.8) | 9.83 | 0.02 |
| Δ from baseline | –14.2 | –10.9 | –12.6 | –1.1 | –0.3 | –1.8 | ||||
| Pain | ||||||||||
| Mean (SD) | 51.4 (15.5) | 39.1 (12.3) | 40.6 (17.8) | 37.2 (14.7) | 51.4 (18.0) | 53.3 (13.4) | 50.6 (10.8) | 46.8 (13.6) | 6.65 | 0.08 |
| Δ from baseline | –12.3 | –10.8 | –14.2 | 1.9 | –0.8 | –4.6 | ||||
| Stiffness | ||||||||||
| Mean (SD) | 56.5 (17.5) | 40.6 (22.1) | 47.8 (22.2) | 43.8 (19.9) | 57.7 (18.7) | 52.8 (18.5) | 55.6 (14.4) | 50.7 (20.9) | 2.99 | 0.39 |
| Δ from baseline | –15.9 | –8.7 | –12.7 | –4.9 | –2.1 | –7.0 | ||||
| Physical function | ||||||||||
| Mean (SD) | 52.2 (13.8) | 37.6 (11.7) | 41.1 (16.9) | 40.1 (16.5) | 49.3 (18.3) | 47.7 (16.4) | 49.3 (12.1) | 49.0 (12.0) | 9.74 | 0.02 |
| Δ from baseline | –14.6 | –11.1 | –12.1 | –1.6 | 0.0 | –0.3 | ||||
Mean changes in the individual WOMAC subscales indicated greater improvement in pain, stiffness, and physical function in the positive vs neutral program, with the largest differences occurring at the one-month time point (Table 4). However, the interaction between program and time was statistically significant only for the physical functioning subscale (χ2 = 9.74, P = 0.02), with changes from baseline to six months corresponding to a large effect size (0.86). Although the interactions between program and time did not reach statistical significance for pain (χ2 = 6.65, P = 0.08) or stiffness (χ2 = 2.99, P = 0.39), changes from baseline to six months corresponded to a moderate effect size for pain (Cohen’s d = 0.66) and a small effect size for stiffness (Cohen’s d = 0.35).
Psychosocial Outcomes
The interaction between program and time was not statistically significant for positive affect (χ2 = 1.67, P = 0.64, Cohen’s d = 0.35). However, there were statistically significant interactions for negative affect (χ2 = 9.14, P = 0.03) and life satisfaction (χ2 = 9.72, P = 0.02) in the expected directions (Table 5). The positive program had a small to medium effect on both outcomes (Cohen’s d = 0.50 and 0.36, respectively).
Table 5.
Change in positive affect, negative affect, and life satisfaction in patients with knee or hip osteoarthritis after completing a 6-week positive (intervention) or neutral (control) program
| Positive (Intervention) Group |
Neutral (Control) Group |
Group × Time Interaction |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Baseline (N = 21) | 1-mo (N = 16) | 3-mo (N = 17) | 6-mo (N = 16) | Baseline (N = 21) | 1-mo (N = 18) | 3-mo (N = 18) | 6-mo (N = 17) | Chi-square | P |
| Positive affect | ||||||||||
| Mean (SD) | 18.7 (4.2) | 20.2 (4.5) | 18.1 (4.2) | 18.8 (3.8) | 17.9 (4.6) | 17.5 (4.6) | 16.1 (4.3) | 16.4 (4.9) | 1.67 | 0.64 |
| Δ from baseline | +1.5 | –0.6 | +0.1 | 0.4 | –1.8 | –1.5 | ||||
| Negative affect | ||||||||||
| Mean (SD) | 11.8 (4.2) | 9.1 (4.1) | 9.8 (4.9) | 9.7 (4.1) | 10.0 (5.1) | 10.1 (5.6) | 10.1 (5.3) | 10.0 (5.1) | 9.14 | 0.03 |
| Δ from baseline | –2.7 | –2.0 | –2.1 | +0.1 | +0.1 | 0.0 | ||||
| Life satisfaction | ||||||||||
| Mean (SD) | 13.9 (4.4) | 16.7 (4.7) | 14.8 (5.1) | 15.1 (4.8) | 16.5 (5.4) | 14.9 (5.7) | 15.2 (5.1) | 16.0 (5.5) | 9.72 | 0.02 |
| Δ from baseline | +2.8 | +0.9 | +1.2 | –1.6 | –1.3 | –0.5 | ||||
Discussion
In this small sample of patients with knee/hip OA who receive care at a major academic VA medical center, we found that a six-week intervention focused on building positive psychological skills such as gratitude and kindness significantly reduced OA symptom severity and improved measures of well-being. In contrast to prior research, this study tested the effects of a positive psychological intervention against a strong, affectively neutral control program; followed patients for six months after the intervention ended; and included a mostly male, racially diverse patient sample with low health literacy. The study not only demonstrated the feasibility of engaging and retaining patients in the use of positive psychology to address pain from knee or hip OA, it showed that a positive psychological intervention yielded substantial, lasting benefits for pain-related and psychosocial outcomes. These results are particularly encouraging in that they indicate the potential of a nonpharmacological therapy to improve symptom management in a patient population with moderate to severe pain.
The benefits of positive psychological interventions for mental health and overall well-being are well established [25,46,47]. The current study adds to existing literature on the use of positive psychological interventions to improve physical health indicators, in addition to overall well-being, in patient populations with chronic or severe health conditions [22,46–55]. To our knowledge, this is the first effort to test the effects of this type of intervention on pain and functioning in patients with OA. The positive activities program tested in this study yielded a statistically significant improvement in overall OA symptom severity and produced a much larger effect size (Cohen’s d = 0.86) than most existing pharmacological and nonpharmacological OA treatments [5–8]. A comprehensive review of OA treatments found that nonpharmacological treatments had a combined effect size of 0.25 on pain; the combined effect size of pharmacological treatments (0.39) was only slightly larger [7]. An updated review of the literature found that effect sizes for most OA treatments have either remained the same or have diminished as higher-quality studies have been published [5]. If the large reduction in OA symptom severity found in this study were to hold in a broader sample, using positive activities as part of an overall treatment program for patients with OA could have tremendous impact.
It is worth noting that the effect of the positive activities program varied across specific OA symptoms. The largest effect size was observed for physical function (0.86), followed by pain severity (0.66), then stiffness (0.35). The physical functioning subscale, which included ratings of how much difficulty patients had completing daily tasks (e.g., going up or down stairs, getting dressed), was the only subscale for which the effect was statistically significant. It is unclear why the effects for the pain and stiffness subscales did not reach statistical significance. One possibility is that the study was underpowered to detect effects on pain and stiffness and that the observed effects would reach significance in a larger sample. A second possibility is that the estimates for pain and stiffness were less precise or sensitive to change than the estimates for physical functioning as they were based on subscales comprising fewer items (five items for pain and two items for stiffness, vs 17 items for physical function).
A third possibility is that the positive activities program reduced perceived difficulty completing daily tasks without actually reducing pain or stiffness. This is a common observation of patients in pain rehabilitation programs, which generally demonstrate functional improvements even when pain scores do not appreciably change. This explanation is highly plausible given the nature of the intervention, which is designed to retrain the brain to focus on positive experiences. By refocusing attention on the positive aspects of daily life, the intervention may also reduce the salience of mild or moderate aggravations, such as the difficulty someone with OA might experience when getting out of a chair to greet a good friend. In this scenario, although the nociceptive pain stimulus from OA has not changed, its perceived impact on functioning could be diminished if the patient is more focused on the positive experience of spending time with a friend than on the difficulty of getting out of the chair. Future studies that include objective measures of physical functioning (e.g., gait speed) are needed to determine whether positive psychological interventions improve actual functioning, self-appraisals of functioning, or both.
The six-month follow-up period in this study is also notable as previous studies of positive psychological interventions have typically assessed change only over the course of treatment or up to three months following program completion [22,48–54]. The positive intervention tested in this study continued to have a large effect on OA symptom severity, as well as moderate to small effects on life satisfaction and negative affect, six months after the intervention ended. The sustained impact of the intervention on these outcomes supports the broaden-and-build model of positive emotions [56,57]. This model asserts that, in the moment, positive emotions broaden attention and increase the range of thoughts and actions one is likely to pursue. This broadened state, in turn, supports the accrual of personal resources such as cognitive flexibility, creativity, and social connections. These increased personal resources produce more opportunities to experience positive emotions, which feed back into a self-reinforcing upward spiral that produces long-term gains in overall well-being.
The positive psychological intervention tested in this study, although only six weeks long, contained activities designed to kick-start the broaden-and-build process. Although our findings suggest that the intervention was successful in producing sustained change in this sample, additional research is needed to determine whether it was in fact due to the iterative broaden-and-build process. A key question that remains is why we did not find a significant change in positive affect in this study. With only 21 participants per group, the study’s small sample size could explain the lack of significant change in positive affect. It is also possible that the abbreviated measure of positive affect that was used in this study, which focused on positive states such as “inspired” and “attentive,” did not capture dimensions of positive affect that were stimulated by the intervention. The timing of the measure could also have been problematic as positive affect was assessed at baseline and during follow-up surveys but not during the six-week intervention when participants were completing the positive activities. The activities may have produced a short-term influx of positive affect, which, according to the broaden-and-build process, would have promoted thoughts and behaviors that foster psychological, physical, and social resources that form the foundation for long-term resilience and overall well-being. Studies that use ecological momentary assessment or other methods to capture moment-to-moment variations in positive affect may be necessary to demonstrate the immediate, intermediate, and long-term sequelae of positive affect.
In the absence of a demonstrable change in positive affect in this study, additional mechanisms that could account for the observed improvements in OA symptom severity should be considered. The benefit of reducing negative affect should not be discounted given the well-established associations of depression with OA-related pain and functional impairment [58,59]. The positive psychological intervention could also reduce OA symptom severity by impacting a number of psychological and social factors that have been shown to be associated with OA-related pain or functioning [10]. For example, building positive psychological skills may reduce pain catastrophizing, increase pain-related self-efficacy, and increase one’s engagement in social activities, all of which could yield reductions in pain and functional difficulty [60–62]. These and other potential mechanisms should be examined in future studies that are powered to test complex multiple mediation pathways between positive psychological interventions and OA-related outcomes.
The importance of including a strong control program in this study cannot be overemphasized as most studies testing similar interventions lacked control groups of any kind or included usual care or wait-list controls [48–54]. Our use of a neutral control program that was similar in structure and length to the positive program ensures that the observed differences in outcomes can be attributed to the positive activities themselves, rather than to motivation, effort, attention, or regression to the mean.
Finally, this study demonstrates the feasibility and acceptability of this type of intervention program in a much more diverse patient population than those targeted in previous studies. While prior studies testing the effects of positive activities on pain have included predominantly female, white, highly educated participants [21,22], our study sample included mostly men, a third of whom had a high school education or less, all of whom were categorized as having inadequate health literacy, and half of whom were African American. This study shows that positive psychological interventions, when designed for maximum readability, can be comprehended and used by a wide range of demographic groups while maintaining their fidelity and impact.
There are important limitations to consider in interpreting our findings. First, we recruited patients from a single VA medical center; therefore, our findings may not generalize to other health care settings or patient populations. Second, the small sample size did not allow for exploration of complex mediation models or subgroup comparisons. Last, as already discussed, the study raises important questions for future studies regarding the mechanisms by which the intervention improved OA symptom severity and the factors that contribute to its sustained impact.
In conclusion, in this study we found that a six-week positive psychological intervention produced large reductions in OA symptom severity compared with a robust control program. The study also demonstrated the feasibility of engaging and retaining a diverse sample of patients with knee/hip OA from a large VA medical center in the use of positive psychology to address pain from knee or hip OA. Future large trials are needed to replicate our findings, identify mechanisms by which positive psychological skills reduce pain, and determine whether the benefits of such an intervention vary across clinical and demographic patient subgroups.
References
- 1. Barbour KE, Helmick CG, Theis KA, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States. MMWR Morb Mortal Wkly Rep 2013;6244:869–73. [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;581:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA.. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum 2009;6012:3546–53. [DOI] [PubMed] [Google Scholar]
- 4. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;162:137–62. [DOI] [PubMed] [Google Scholar]
- 5. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;184:476–99. [DOI] [PubMed] [Google Scholar]
- 6. McAlindon TE, Bannuru RR, Sullivan M, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;223:363–88. [DOI] [PubMed] [Google Scholar]
- 7. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007;159:981–1000. [DOI] [PubMed] [Google Scholar]
- 8. Zou K, Wong J, Abdullah N, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: Meta-analysis of randomised controlled trials. Ann Rheum Dis 2016;75:1964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;1334:581–624. [DOI] [PubMed] [Google Scholar]
- 10. Somers TJ, Keefe FJ, Godiwala N, Hoyler GH.. Psychosocial factors and the pain experience of osteoarthritis patients: New findings and new directions. Curr Opin Rheumatol 2009;215:501–6. [DOI] [PubMed] [Google Scholar]
- 11. Allen KD, Helmick CG, Schwartz TA, et al. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: The Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2009;179:1132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez-Olivo MA, Landon GC, Siff SJ, et al. Psychosocial determinants of outcomes in knee replacement. Ann Rheum Dis 2011;7010:1775–81. [DOI] [PubMed] [Google Scholar]
- 13. Patten SB, Williams JV, Wang J.. Mental disorders in a population sample with musculoskeletal disorders. BMC Musculoskelet Disord 2006;7:37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pressman SD, Cohen S.. Does positive affect influence health? Psychol Bull 2005;1316:925–71. [DOI] [PubMed] [Google Scholar]
- 15. Seligman MEP, Steen TA, Park N, Peterson C.. Positive psychology progress: Empirical validation of interventions. Am Psychol 2005;605:410–21. [DOI] [PubMed] [Google Scholar]
- 16. Seligman MEP, Csikszentmihalyi M.. Positive psychology: An introduction. Am Psychol 2000;551:5–14. [DOI] [PubMed] [Google Scholar]
- 17. Gable SL, Haidt J.. What (and why) is positive psychology? Rev Gen Psychol 2005;92:103. [Google Scholar]
- 18. Zautra AJ, Johnson LM, Davis MC.. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol 2005;732:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fredman L, Hawkes WG, Black S, Bertrand RM, Magaziner J.. Elderly patients with hip fracture with positive affect have better functional recovery over 2 years. J Am Geriatr Soc 2006;547:1074–81. [DOI] [PubMed] [Google Scholar]
- 20. Hanssen MM, Peters ML, Vlaeyen JW, Meevissen YM, Vancleef LM.. Optimism lowers pain: Evidence of the causal status and underlying mechanisms. Pain 2013;1541:53–8. [DOI] [PubMed] [Google Scholar]
- 21. Hausmann LRM, Parks A, Youk AO, Kwoh CK.. Reduction of bodily pain in response to an online positive activities intervention. J Pain 2014;155:560–7. [DOI] [PubMed] [Google Scholar]
- 22. Müller R, Gertz KJ, Molton IR, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability. A Feasibility Trial. Clin J Pain 2016;321:32–44. [DOI] [PubMed] [Google Scholar]
- 23. Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative: Protocol for the cohort study. Available at: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf (accessed October 2012).
- 24. Mazzucchelli TG, Kane RT, Rees CS.. Behavioral activation interventions for well-being: A meta-analysis. J Posit Psychol 2010;52:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sin NL, Lyubomirsky S.. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: A practice-friendly meta-analysis. J Clin Psychol 2009;655:467–87. [DOI] [PubMed] [Google Scholar]
- 26. Schueller SM. Preferences for positive psychology exercises. J Posit Psychol 2010;53:192–203. [Google Scholar]
- 27. Parks AC, Schueller S, Tasimi A.. Increasing happiness in the general population: Empirically supported self-help? In: Boniwell I, David S, Conley Ayers A, eds. Oxford Handbook of Happiness. Oxford: Oxford University Press; 2013:962–77. [Google Scholar]
- 28. Seligman MEP, Rashid T, Parks AC.. Positive psychotherapy. Am Psychol 2006;618:774–88. [DOI] [PubMed] [Google Scholar]
- 29. Emmons RA, Shelton CM.. Gratitude and the science of positive psychology In: Snyder CR, Lopez SJ, eds. Handbook of Positive Psychology. New York: Oxford University Press; 2002:459–71. [Google Scholar]
- 30. Lyubomirsky S, Dickerhoof R, Boehm JK, Sheldon KM.. Becoming happier takes both a will and a proper way: An experimental longitudinal intervention to boost well-being. Emotion 2011;112:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyubomirsky S, Sheldon KM, Schkade D.. Pursuing happiness: The architecture of sustainable change. Rev Gen Psychol 2005;92:111–31. [Google Scholar]
- 32. Brown KW, Ryan RM.. The benefits of being present: Mindfulness and its role in psychological well-being. J Pers Soc Psychol 2003;844:822–48. [DOI] [PubMed] [Google Scholar]
- 33. Grossman P, Niemann L, Schmidt S, Walach H.. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res 2004;571:35–43. [DOI] [PubMed] [Google Scholar]
- 34. Emmons RA, McCullough ME.. Counting blessings versus burdens: An experimental investigation of gratitude and subjective well-being in daily life. J Pers Soc Psychol 2003;842:377–89. [DOI] [PubMed] [Google Scholar]
- 35. Sheldon KM, Abad N, Ferguson Y, et al. Persistent pursuit of need-satisfying goals leads to increased happiness: A 6-month experimental longitudinal study. Motiv Emot 2010;341:39–48. [Google Scholar]
- 36. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW.. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;1512:1833–40. [PubMed] [Google Scholar]
- 37. Bellamy N. WOMAC Osteoarthritis Index: User Guide XI. 2015. [Google Scholar]
- 38. Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross Cult Psychol 2007;382:227–42. [Google Scholar]
- 39. Diener E, Emmons RA, Larsen RJ, Griffin S.. The satisfaction with life scale. J Pers Assess 1985;491:71–5. [DOI] [PubMed] [Google Scholar]
- 40. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;235:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaudhry S, Jin L, Meltzer D.. Use of a self-report-generated Charlson comorbidity index for predicting mortality. Med Care 2005;436:607–15. [DOI] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Questionnaire. 2010. Available at: http://www.cdc.gov/brfss/questionnaires/pdf-ques/2010brfss.pdf (accessed February 2017).
- 43. Schueller SM, Parks AC.. Disseminating self-help: Positive psychology exercises in an online trial. J Med Internet Res 2012;143:e63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moher D, Schulz KF, Altman DG.. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;3579263:1191–4. [PubMed] [Google Scholar]
- 45. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 46. Casellas-Grau A, Font A, Vives J.. Positive psychology interventions in breast cancer: A systematic review. Psychooncology 2014;231:9–19. [DOI] [PubMed] [Google Scholar]
- 47. Macaskill A. Review of positive psychology applications in clinical medical populations. Healthcare 2016;43:66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer PS, Johnson DP, Parks A, Iwanski C, Penn DL.. Positive living: A pilot study of group positive psychotherapy for people with schizophrenia. J Pos Psychol 2012;73:239–48. [Google Scholar]
- 49. Huffman JC, Mastromauro CA, Boehm JK, et al. Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart Int 2011;62:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moskowitz JT, Hult JR, Duncan LG, et al. A positive affect intervention for people experiencing health-related stress: Development and non-randomized pilot test. J Health Psychol 2012;175:676–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Redwine LS, Henry BL, Pung MA, et al. Pilot randomized study of a gratitude journaling intervention on heart rate variability and inflammatory biomarkers in patients with stage B heart failure. Psychosom Med 2016;78:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huffman JC, Millstein RA, Mastromauro CA, et al. A positive psychology intervention for patients with an acute coronary syndrome: Treatment development and proof-of-concept trial. J Happiness Studies 2016;175:1985–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohn MA, Pietrucha ME, Saslow LR, Hult JR, Moskowitz JT.. An online positive affect skills intervention reduces depression in adults with type 2 diabetes. J Pos Psychol 2014;96:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DuBois CM, Millstein RA, Celano CM, Wexler DJ, Huffman JC.. Feasibility and acceptability of a positive psychological intervention for patients with type 2 diabetes. Prim Care Companion CNS Disord 2016;183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mancuso CA, Choi TN, Westermann H, et al. Increasing physical activity in patients with asthma through positive affect and self-affirmation: A randomized trial. Arch Intern Med 2012;1724:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fredrickson BL. What good are positive emotions? Rev Gen Psychol 1998;23:300–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fredrickson BL. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. Am Psychol 2001;563:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Rooij M, van der Leeden M, Heymans MW, et al. Prognosis of pain and physical functioning in patients with knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2016;684:481–92. [DOI] [PubMed] [Google Scholar]
- 59. Phyomaung PP, Dubowitz J, Cicuttini FM, et al. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskelet Disord 2014;15:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jia X, Jackson T.. Pain beliefs and problems in functioning among people with arthritis: A meta-analytic review. J Behav Med 2016;395:735–56. [DOI] [PubMed] [Google Scholar]
- 61. Campbell CM, Edwards RR.. Mind–body interactions in pain: The neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res 2009;1533:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: Role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum 2003;4812:3359–70. [DOI] [PubMed] [Google Scholar]