Abstract
Objective
There is some evidence that lung function and chronic kidney disease (CKD) may be related. We evaluated the impact of lung function on the development of CKD in a large-scale longitudinal study.
Method
Retrospective longitudinal analyses were conducted among subjects who participated in comprehensive health check-ups at least four times during 7 years (between 2006 and 2012). We investigated the development of CKD during the follow-up period according to lung function status.
Results
Ten thousand one hundred and twenty-eight individuals (mean age =51.2 years) without CKD at baseline were enrolled. During the mean follow-up of 5 years (58.5±14.4 months), 167 of the 10 128 subjects (1.6%) developed CKD. Multivariable Cox proportional hazards analyses adjusting for age, sex, body mass index, systolic blood pressure, fasting glucose, estimated glomerular filtration rate, uric acid, triglycerides, serum albumin, and the presence of diabetes and hypertension revealed that a decrease of 10% in the forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) ratio was associated with a 35% increase in the development of CKD during the follow-up. The incidence of CKD was higher in those with an FEV1/FVC ratio <0.8 compared with those with FEV1/FVC ratio ≥0.8 (HR=1.454; 95% CI 1.042 to 2.028, p=0.028).
Conclusions
Limited airflow as measured by the FEV1/FVC ratio was associated with an increased risk of CKD.
Keywords: lung function, chronic renal failure, Fev1/fvc, chronic renal insufficiency
Strengths and limitations of this study.
The strengths of this study are the large sample and longitudinal nature of the study.
This study is one of few studies on the relationship between lung function and chronic kidney disease development.
Limitations of this study are its retrospective nature and failure to note symptoms of any airway disease.
Another limitation of this study is that most patients had normal pulmonary function; thus it needs further study in patients with chronic obstructive pulmonary disease.
Introduction
Chronic kidney disease (CKD) is an increasing public health problem in Korea and is associated with significant morbidity and mortality.1–4 CKD is linked to various other diseases, including hypertension, diabetes, atherosclerosis and metabolic syndrome (MetS).5 6Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease.7 It is known that cardiovascular disease, osteoporosis, diabetes and MetS are systemic effects caused by COPD and also its comorbidities.7 8 Proinflammatory cytokines, including tumour necrosis factor α and interleukin 6, induce insulin resistance by blocking signalling via the insulin receptor, thereby increasing the risk of type 2 diabetes and MetS in patients with COPD.8 However, only a few studies have examined the association between renal function and lung function, and found that kidney dysfunction is more common in patients with COPD.9–13 These studies suggest that CKD is associated with COPD. However, few studies have explored the relationship between lung function and the incidence of CKD. Thus, we examined cross-sectional and longitudinal associations between lung function, measured by calculating forced vital capacity (FVC), forced expiratory volume in 1s (FEV1), or the FEV1/FVC ratio, and the development of CKD after adjusting for other risk factors for CKD.
Methods
Study population and data collection
This was a retrospective longitudinal cohort study. Many people undergo comprehensive health check-ups each year at the Samsung Medical Center. Initial data were obtained from 25 170 individuals who participated in comprehensive health check-ups at least four times over 7 years (between January 2006 and December 2012). The data from the first visits served as baseline data. In total, 15 042 subjects were excluded for the following reasons: (1) missing pulmonary function test (n=3370), (2) age <20 years (n=7), (3) body mass index (BMI) <18.5 kg/m2 (n=456), (4) CKD evident at baseline (n=965), (5) missing urine albumin/Cr ratio (n=6249) and (6) current smoker (n=3995). We excluded current smokers from this analysis because smoking itself has a significant effect on lung function. It would have been difficult to correctly evaluate the association between lung function and the development of CKD if we included current smokers in the study. A total of 10 128 individuals (mean age 51.2 years) without CKD at baseline were enrolled.
Measurements
All subjects fasted overnight, and blood was collected subsequently. All laboratory tests were performed in the central certified laboratory of Samsung Medical Center. Anthropometric and other biochemical variables were measured as described previously.13 We measured serum creatinine using the kinetic alkaline picrate method (Jaffe reaction; Roche Modular DP, Roche, Switzerland).
Definition of development of CKD
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.14 CKD was defined as an eGFR <60 mL/min/1.73 m2 in patients over 40 years old and eGFR <75 mL/min/1.73 m2 in patients younger than 40 years old.15
Statistical analysis
Data expressed as mean±SEM. Metabolic risk factors, the prevalence of diabetes and other clinical characteristics were compared between subjects who did and did not develop CKD. A Student’s t-test was used to compare continuous variables, and the exact χ2 test was used to detect differences between groups. Pearson’s correlation analyses were used to generate correlation coefficients between eGFR and other factors. We used multivariable Cox proportional hazards analyses to estimate HRs with 95% CIs for the development of CKD during follow-up per 10% decrease in the FEV1/FVC ratio. We compared the development of CKD between two groups (FEV1/FVC ratio >0.8 vs FEV1/FVC ratio <0.8) during follow-up using the HRs for incidences of CKD estimated by Cox proportional hazards analysis. All statistical analyses were performed using Predictive Analytics Software (PASW) (V.17.0; SPSS, Chicago, Illinois, USA). A p value <0.05 was considered statistically significant.
Patient and public involvement statement
This study was a retrospective study. Patients were not involved in this study.
Results
During the mean follow-up of 5 years (58.5± 14.4 months), 167 of the 10 128 subjects (1.6%) developed CKD. The baseline characteristics of those with and without de novo CKD are shown in table 1. Those who developed CKD during the follow-up were significantly older and had a higher baseline BMI, higher triglyceride (Tg) levels and higher blood pressure (table 1). These subjects also had a lower baseline eGFR and FEV1/FVC ratio (table 1). In addition, the incidence of hypertension at baseline was higher in subjects who developed CKD (table 1).
Table 1.
Baseline characteristics of all subjects by the development of chronic kidney disease (CKD)
| The subjects who developed CKD (n=167) |
All other subjects (n=9961) |
P values | |
| Age (years) | 60.0±0.8 | 51.0±0.1 | <0.001 |
| Sex (male, %) | 134 (80.2%) | 5749 (57.7%) | <0.001 |
| BMI (kg/m 2) | 2 4.7±0.2 | 23.8±0.1 | <0.001 |
| SBP (mm Hg) | 118.9±1.3 | 114.1±0.2 | <0.001 |
| DBP (mm Hg) | 72.5±0.8 | 70.6±0.1 | 0.022 |
| Total cholesterol (mg/dL) | 191.8±2.7 | 192.7±0.3 | 0.733 |
| Triglyceride (mg/dL) |
142.9±5.7 | 122.4±0.7 | <0.001 |
| HDL cholesterol (mg/dL) | 54.1±1.0 | 57.4±0.2 | <0.001 |
| Fasting glucose (mg/dL) | 93.4±1.2 | 92.0±0.2 | 0.248 |
| Haemoglobin A 1c(%) | 5.5 3±0.05 | 5.42±0.01 | 0.023 |
| Albumin (mg/L) | 4.25±0.02 | 4.31±0.01 | 0.001 |
| Haemoglobin (g/dL) | 14.7±0.1 | 14.4±0.1 | <0.001 |
| Urea (mg/dL) | 15.36±0.29 | 13.4 7±0.04 | < 0.001 |
| Creatinine (mg/dL) | 1.09±0.01 | 0.90±0.01 | < 0.001 |
| Uric acid (mg/dL) | 5.9 2±0.11 | 5.15±0.01 | <0.001 |
| eGFR (ml/min per 1.73m2) | 69.9±0.7 | 88.5±0.1 | <0.001 |
| hs-CRP (mg/L) | 0.14±0.02 | 0.11±0.01 | 0.154 |
| FVC (%) | 93.4±0.9 | 95.1±0.1 | 0.076 |
| FEV1 (%) | 100.7±1.2 | 103.1±0.1 | 0.046 |
| FEV1/FVC ratio | 0.77±0.52 | 0.81±0.06 | <0.001 |
| Diabetes mellitus (%) |
14 (8.4 %) | 583 (5.9%) | 0.182 |
| Hypertension (%) | 66 (40.7%) | 1602 (16.1%) | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; hs-CRP, high-sensitivity C reactive protein; SBP, systolic blood pressure.
The baseline eGFR correlated negatively with age, BMI, systolic blood pressure (SBP), total cholesterol, Hemoglobin A1c (HbA1c), uric acid, and high-sensitivity C reactive protein (hs-CRP). Conversely, eGFR correlated positively with High-density lipoprotein cholesterol (HDL‐C) and the FEV1/FVC ratio (table 2). The hs-CRP level correlated negatively with FVC (r = –0.043, p<0.001), FEV1 (r = –0.041, p<0.001) and FEV1/FVC ratio (r = –0.020, p<0.001).
Table 2.
Correlation between the eGFR and risk factors for chronic kidney disease
| Variable | eGFR (n=10 128) |
|
| Correlation coefficient | P values | |
| Age (years) | −0.417 | <0.001 |
| BMI (kg/m2) | −0.153 | <0.001 |
| SBP (mm Hg) | −0.078 | <0.001 |
| Fasting glucose (mg/dL) | −0.032 | 0.840 |
| Albumin (mg/dL) | −0.093 | <0.001 |
| Total cholesterol (mg/dL) | −0.063 | <0.001 |
| Triglyceride (mg/dL) | −0.053 | <0.001 |
| HDL cholesterol (mg/dL) | 0.073 | <0.001 |
| HbA1c (%) | −0.062 | <0.001 |
| Uric acid (mg/dL) | −0.304 | <0.001 |
| hs-CRP (mg/L) | −0.018 | 0.030 |
| Haemoglobin (g/dL) | − 0.245 | <0.001 |
| Baseline FVC (%) | −0.033 | <0.001 |
| Baseline FEV1 (%) | −0.034 | <0.001 |
| FEV1/FVC ratio | 0.175 | <0.001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; hs-CRP, high-sensitivity C reactive protein; SBP, systolic blood pressure.
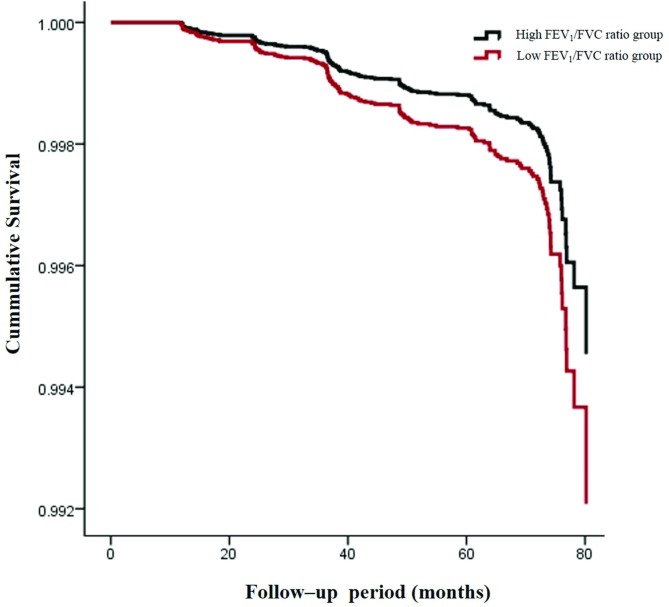
After adjustment for risk factors for CKD, including age, sex, BMI, eGFR, fasting glucose, haemoglobin, uric acid, serum albumin, Tg, SBP and the presence of diabetes and/or hypertension, the risk of developing CKD increased as the FEV1/FVC ratio decreased (table 3). The HR for the incidence of CKD with a 10% decrease in the FEV1/FVC ratio was 1.346. A lower baseline FEV1/FVC ratio was associated with the development of CKD even after adjusting for risk factors for CKD. We divided the subjects into two groups according to FEV1/FVC ratios (<0.8 and ≥0.8). The incidence of CKD was 2.8% and 1.0%, respectively. Age, sex, BMI, eGFR, SBP, levels of haemoglobin, albumin, uric acid, Tg, fasting glucose, the presence of diabetes and the hypertension-adjusted risk of developing CKD were higher in those with an FEV1/FVC ratio less than 0.8 compared with those with an FEV1/FVC ratio greater than 0.8 (HR=1.454; 95% CI 1.042 to 2.028, p=0.028; figure 1).
Table 3.
HR for incident chronic kidney disease by a 10 decrement in the FVC/FEV1 ratio
| Variable | HR (95% CI) |
P values | |
| Crude model (95% CI) |
1 | 2.136 (1.787 to 2.552) | <0.001 |
| Adjusted* (95% CI) |
1 | ||
| Model 1 | 1 | 1.419 (1.126 to 1.788) | 0.003 |
| Model 2 | 1 | 1.384 (1.091 to 1.755) | 0.007 |
| Model 3 | 1 | 1.346 (1.062 to 1.705) | 0.014 |
Multivariate model 1: adjusted for age, sex, body mass index and the estimated glomerular filtration rate.
Multivariate model 2: adjusted for the factors of model 1, systolic blood pressure and levels of haemoglobin, albumin, uric acid, triglyceride and fasting glucose.
Multivariate model 3: adjusted for the factors of model 2 and the presence of diabetes or hypertension at baseline.
*By multivariable Cox’s proportional hazards analysis.
FEV1, forced expiratory volume in 1s; FVC, forced vital capacity.
Figure 1.
Cumulative survival curve for incident chronic renal failure from values of FEV1/FVC ratio. The data are adjusted for age, sex, smoking status, body mass index, estimated glomerular filtration rate, systolic blood pressure, levels of haemoglobin, albumin, uric acid, triglyceride and fasting glucose, and the presence of diabetes and/or hypertension at baseline. Cox’s proportional hazards regression model was employed to draw the curves. FEV1, forced expiratory volume in 1s; FVC, forced vital capacity.
Discussion
We found that lower FEV1/FVC ratio was associated with an increased risk of CKD during the mean follow-up of 5 years. The FEV1/FVC ratio (Tiffeneau Index) is an index of airflow limitation.16 To the best of our knowledge, only a few studies have explored associations between lung function and CKD. Some studies found that kidney dysfunction was more common in patients with COPD.9–12 Incalzi et al found that the prevalence of CKD is increased in patients with COPD, and CKD is a common comorbidity of COPD.9 Van Gestel et al also found that COPD was associated with CKD in patients undergoing vascular surgery.10 However, most of these studies were conducted in patients with COPD and had a cross-sectional design. Even less is known about the relationship between lung function and CKD incidence. Chen et al explored the association between COPD and CKD incidence using hospital records and found that the overall incidence of CKD was higher in patients with COPD than patients without COPD.11 Our study also showed that subjects with a lower FEV1/FVC ratio were at increased risk of developing CKD. This remained true after adjusting for other known risk factors for CKD. Chen et al used insurance research data, which did not include information on the GFR, pulmonary function or laboratory test results.11 Unlike Chen et al, we used the eGFR and pulmonary function test data to define CKD and pulmonary function.
Although our study results may be due to a causal relationship, there are some plausible explanations for this relationship. The lung dysfunction has risk factors in common with CKD including old age, smoking, diabetes, hypertension and obesity.17–19Patients with reduced lung function may have coexisting diabetes, MetS and/or hypertension, which are known risk factors for CKD.20 21 We also found that those who developed CKD were more likely to have hypertension and/or dyslipidaemia at baseline and more likely to be older. Baseline age, BMI, HbA1c level and SBP negatively correlated with eGFR. However, reduced lung function (FEV1/FVC ratio) at baseline was associated with CKD development after adjusting for these risk factors although the HR decreases after adjusting other risk factors, and baseline FEV1/FVC also positively correlated with eGFR. This implies that lung function affects CKD development. Hypoxaemia or increased oxidative stress caused by decreased lung function could contribute to the development of CKD. Accumulating evidence suggests that renal hypoxia is a key player in the progression of CKD.22 Chronic renal hypoxia is closely linked to capillary rarefaction, which affects tubular epithelial cells, fibroblasts and inflammatory cells, resulting in tubulointerstitial fibrosis.23 Increased oxidative stress could also contribute to the onset of CKD. Oxidative stress increases the generation of advanced glycation end products (AGEs), which in turn interact with receptors for AGEs (RAGE).24 AGEs were increased in patients with COPD,24 and one recent study showed that pulmonary and renal endothelial cell injury was linked to increased endothelial AGEs and RAGE.25 These studies were done in patients with COPD or COPD animal models, and lung function in our study group was near normal. The attenuated association between FEV1/FVC and the development of CKD after adjustment for other factors might be explained by the difference in lung function status of subjects in our study and those of other studies.
Systemic inflammation may also be a link between lung dysfunction and CKD. COPD is a systemic inflammatory disease.26 Low-grade systemic inflammation was noted in subjects with airflow obstructions.26 High serum CRP concentrations have been reported in patients with COPD.27 Thus, COPD is a systemic inflammatory disease involving the lungs. Inflammation also plays a role in the development of CKD and is associated with increased levels of inflammatory and procoagulant biomarkers, including CRP and fibrinogen.28 Inflammation may trigger the development of CKD in those with lung dysfunction. To understand why lung function was associated with the development of CKD, we explored the association between hs-CRP levels and lung function. The hs-CRP level was negatively associated with the FEV1/FVC ratio. This indicates that inflammation may play a role in the relationship between lung dysfunction and CKD.
Our study has certain limitations. First, all subjects were volunteers, self-scheduling comprehensive health examinations (sometimes annually) for at least 5 years, implying that they were more concerned about their health and therefore not representative of the general population. Second, we used the CKD-EPI equation to estimate GFR, because the Modification of Diet in renal disease (MDRD) equation was developed in subjects with CKD and underestimated GFR.20 So the incidence of CKD was low, and we did not assess proteinuria. Thus, we did not analyse the relationship between lung function and proteinuria. Third, the work was retrospective in nature and the symptoms of any airway disease were not noted. Prospective studies are needed to further define these relationships.
We confirmed that the incidence of CKD is increased in patients with airflow limitation, as measured by the FEV1/FVC ratio. CKD is associated with mortality and morbidity. Therefore, it is important to recognise the relationship between reduced lung function and CKD development in clinical practice.
Conclusion
Limited airflow as measured by the FEV1/FVC ratio was associated with an increased risk of CKD. This implies that common pathogenic mechanisms exist between a decline in lung function and the development of CKD. The baseline FEV1/FVC ratio could be helpful for identifying individuals at risk for CKD.
Supplementary Material
Footnotes
Contributors: SKK, JHK, JCB: Substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data. J-HB, KYH, M-KL: Drafting the work or revising it critically for important intellectual content. JHK: Final approval of the version published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Ethical Committee of Samsung Medical Center at Sungkyunkwan University, Seoul, Korea.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J 2014;38:252–60. 10.4093/dmj.2014.38.4.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkins RM, Bucaloiu ID, Kirchner HL, et al. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol 2011;6:1879–86. 10.2215/CJN.00470111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–47. 10.1681/ASN.2005101085 [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 5. Webster AC, Nagler EV, Morton RL, et al. Chronic Kidney Disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 6. Prasad GV. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J Nephrol 2014;3:210–9. 10.5527/wjn.v3.i4.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choudhury G, Rabinovich R, MacNee W. Comorbidities and systemic effects of chronic obstructive pulmonary disease. Clin Chest Med 2014;35:101–30. 10.1016/j.ccm.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 8. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165–85. 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 9. Incalzi RA, Corsonello A, Pedone C, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest 2010;137:831–7. 10.1378/chest.09-1710 [DOI] [PubMed] [Google Scholar]
- 10. van Gestel YR, Chonchol M, Hoeks SE, et al. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrol Dial Transplant 2009;24:2763–7. 10.1093/ndt/gfp171 [DOI] [PubMed] [Google Scholar]
- 11. Chen CY, Liao KM. Chronic obstructive pulmonary disease is associated with risk of chronic kidney disease: a nationwide case-cohort study. Sci Rep 2016;6:25855 10.1038/srep25855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshizawa T, Okada K, Furuichi S, et al. Prevalence of chronic kidney diseases in patients with chronic obstructive pulmonary disease: assessment based on glomerular filtration rate estimated from creatinine and cystatin C levels. Int J Chron Obstruct Pulmon Dis 2015;10:1283–9. 10.2147/COPD.S80673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SK, Hur KY, Choi YH, et al. The Relationship between lung function and metabolic syndrome in obese and non-obese korean adult males. Korean Diabetes J 2010;34:253–60. 10.4093/kdj.2010.34.4.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev 2016;37:17–26. [PMC free article] [PubMed] [Google Scholar]
- 16. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 2017;49:1700214 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 17. Ehrlich SF, Quesenberry CP, Van Den Eeden SK, et al. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010;33:55–60. 10.2337/dc09-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baffi CW, Wood L, Winnica D, et al. Metabolic syndrome and the lung. Chest 2016;149:1525–34. 10.1016/j.chest.2015.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucove J, Vupputuri S, Heiss G, et al. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kidney Dis 2008;51:21–8. 10.1053/j.ajkd.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004;140:167–74. 10.7326/0003-4819-140-3-200402030-00007 [DOI] [PubMed] [Google Scholar]
- 21. Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine 2016;95:e3013 10.1097/MD.0000000000003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka S, Tanaka T, Nangaku M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis 2015;1:80–9. 10.1159/000381515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 2014;307:F1187–95. 10.1152/ajprenal.00425.2014 [DOI] [PubMed] [Google Scholar]
- 24. Wu L, Ma L, Nicholson LF, et al. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med 2011;105:329–36. 10.1016/j.rmed.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 25. Polverino F, Laucho-Contreras ME, Petersen H, et al. A pilot study linking endothelial injury in lungs and kidneys in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:1464–76. 10.1164/rccm.201609-1765OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;107:1514–9. 10.1161/01.CIR.0000056767.69054.B3 [DOI] [PubMed] [Google Scholar]
- 27. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–88. 10.1183/09031936.03.00040703 [DOI] [PubMed] [Google Scholar]
- 28. Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003;107:87–92. 10.1161/01.CIR.0000042700.48769.59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.