Abstract
Background:
Exposure to the environmental endocrine disruptor bisphenol A (BPA) is ubiquitous and associated with the increased risk of diabetes and obesity. However, the underlying mechanisms remain unknown. We recently demonstrated that perinatal BPA exposure is associated with higher body fat, impaired glucose tolerance, and reduced insulin secretion in first- (F1) and second-generation (F2) C57BL/6J male mice offspring.
Objective:
We sought to determine the multigenerational effects of maternal bisphenol A exposure on mouse pancreatic islets.
Methods:
Cellular and molecular mechanisms underlying these persistent changes were determined in F1 and F2 adult offspring of F0 mothers exposed to two relevant human exposure levels of BPA ( and ).
Results:
Both doses of BPA significantly impaired insulin secretion in male but not female F1 and F2 offspring. Surprisingly, LowerB and UpperB induced islet inflammation in male F1 offspring that persisted into the next generation. We also observed dose-specific effects of BPA on islets in males. UpperB exposure impaired mitochondrial function, whereas LowerB exposure significantly reduced mass and increased death that persisted in the F2 generation. Transcriptome analyses supported these physiologic findings and there were significant dose-specific changes in the expression of genes regulating inflammation and mitochondrial function. Previously we observed increased expression of the critically important gene, Igf2 in whole F1 embryos. Surprisingly, increased Igf2 expression persisted in the islets of male F1 and F2 offspring and was associated with altered DNA methylation.
Conclusion:
These findings demonstrate that maternal BPA exposure has dose- and sex-specific effects on pancreatic islets of adult F1 and F2 mice offspring. The transmission of these changes across multiple generations may involve either mitochondrial dysfunction and/or epigenetic modifications. https://doi.org/10.1289/EHP1674
Introduction
Over the past decade, the incidence of diabetes has increased dramatically. The WHO estimates mortality due to diabetes to increase by 50% in the next 10 y, making it a major cause of death by 2030 and placing a substantial economic burden on the health care system globally (Mathers and Loncar 2006; American Diabetes Association 2013). Therefore, understanding the underlying factors of this global epidemic is critical. It has been well established that factors such as food intake and sedentary lifestyle significantly influence the incidence of diabetes. Although these are important factors, changes in our lifestyle have also increased our exposure to a variety of synthetic chemicals that are linked to various diseases such as diabetes and obesity in experimental models and human epidemiological studies (Alonso-Magdalena et al. 2015b; Jašarević et al. 2011; Lang et al. 2008; Newbold et al. 2008).
Bisphenol A (BPA) is one such synthetic chemical to which we are ubiquitously exposed through food, drink, and skin contact (Stahlhut et al. 2009). Detectable amounts of BPA are found in urine samples of populations from around the world (Nahar et al. 2012; Stahlhut et al. 2009; Zhang et al. 2011). Exposure to BPA has been associated with prediabetes and type 2 diabetes in human cross sectional (Aekplakorn et al. 2015; Ahmadkhaniha et al. 2014; Sabanayagam et al. 2013; Shankar and Teppala 2011) and longitudinal studies (Sun et al. 2014). Such associations have been further confirmed using animal models of exposure during adulthood (Alonso-Magdalena et al. 2006; Batista et al. 2012).
The pancreas undergoes substantial remodeling during late gestation and early neonatal life. This involves formation of new by differentiation of precursor cells during late gestation (Hellerstrom et al. 1988) followed by substantial remodeling, including high rates of replication and cell death in the neonatal period (Finegood et al. 1995; Scaglia et al. 1995, 1997; Swenne 1983). The replication rate gradually declines (Finegood et al. 1995; Swenne 1983), and post-weaning turnover is regulated by death and very slow rates of replication (Bouwens and Rooman 2005; Scaglia et al. 1995). An exposure to synthetic chemicals such as BPA during this vulnerable period is even more concerning because of the potential to not only alter normal fetal development, but also to impact later life health and disease. Furthermore, the fetus is particularly vulnerable to the effects of BPA because the degradation pathways are immature, leading to accumulation and high levels of BPA (Takahashi and Oishi 2000; Zalko et al. 2003). Consistent with this finding, our previous studies and studies by others have shown that perinatal exposure to BPA leads to metabolic defects in offspring (Alonso-Magdalena et al. 2010; Angle et al. 2013; Mackay et al. 2013; van Esterik et al. 2014; Wei et al. 2011) and in subsequent generation offspring (Li et al. 2014; Susiarjo et al. 2015). We recently demonstrated that maternal exposure via maternal transmission to relevant human exposure levels of BPA, (LowerB) or (UpperB) prior to conception and through lactation is associated with higher body fat, impaired glucose tolerance, and reduced glucose-stimulated insulin secretion (GSIS) across two generations in adult male mice offspring (Susiarjo et al. 2015). Despite our expanding knowledge on the association of BPA with increased risk of diabetes, the mechanisms underlying this link remain to be elucidated.
In the current study, we tested the hypothesis that early developmental exposure to BPA alters key aspects of function leading to impaired insulin secretion in the offspring and that these changes would persist across two generations.
Materials and Methods
Experimental Paradigm
The animal work was conducted with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee. All animals were treated humanely and with regard for alleviation of suffering. The animals used in this study were generated using the same procedure as described previously (Susiarjo et al. 2015). (See Figure S1 for the experimental design.) C57BL/6 virgin female mice (F0) were randomly assigned to the following three diets from 2 wk prior to mating until weaning: (LowerB), (UpperB) BPA, and 7% corn oil (Control). These doses were selected based on our previous study (Susiarjo et al. 2013), where we reported that the levels of unconjugated BPA in serum from pregnant mice were representative of human exposure levels (Susiarjo et al. 2013). The offspring after weaning, and the subsequent generations, were maintained on the control diet. All terminal tests were performed in 16- to 21-wk-old first- (F1) and second-generation (F2) adult offspring, other than cell viability assays that were performed in 8- to 10-wk-old offspring. The experiments were blinded, where possible (e.g., immunofluorescence staining and Luminex assay). To account for robustness of the studied outcomes and cohort consistency in our paradigm, the end points measured were from animals generated across multiple cohorts studied in two different animal care facilities, with a control group included in all cohorts (see Figure S2). For most assessments, litters per group were used other than for extensive cytokine assays, RNA seq, islet perifusion ramps, and islet cytosolic calcium determination where mice from litters per group were used. Metabolic studies including GSIS and ramp studies were performed in both male and female offspring. However, because no metabolic phenotype was observed in female offspring across two generations, all mechanistic studies were performed on males only. Females were only additionally assessed for transcriptomic differences via RNA seq to determine if there were additional pathways we did not anticipate that might be disrupted in the islet.
Islet Isolation
F1 and F2 adult mice pancreata were perfused with Hanks’ balanced salt solution (Life Technologies) supplemented with 2.5% bovine serum albumin (BSA) (wt/vol; Sigma), (Sigma), and Collagenase P (Roche); excised; and incubated at 37°C for 10–15 min. After digestion, islets were washed and then purified using a Histopaque 10771 and Histopaque 11191 (Sigma) gradient. To provide sufficient starting material for Oroboros, RNA and DNA extraction, islets had to be pooled from 2–3 male mice (either male or female for RNA) from within the same litter. This helped minimize the within-litter variability, which is not uncommon in endocrine-disruptor models. For remaining evaluations, one mouse per litter was randomly selected as a litter representative. More than one islet-specific end point was determined for each litter, where possible (see Figure S2). A combination of studied outcomes from pooled as well as from an individual animal from each litter helped in assessing both nonrandom and stochastic biological changes.
Insulin Measurement and Islet Perifusion Study
Isolation of pancreatic islets, perifusion, and insulin assays were performed as previously described (Li et al. 2010). In brief, islets were isolated by collagenase digestion and cultured with glucose in RPMI 1640 medium (Sigma) for 2 d. Islets were perifused with a Krebs-Ringer bicarbonate buffer containing 0.25% BSA at a flow rate of . Ramps at increments of for glucose, and for were performed. Thirty millimolar potassium chloride was used to determine maximum insulin release. Insulin was measured in the perfusates following the ramp studies, as well as in the lysates from freshly isolated islets by homogeneous time-resolved fluorescence technology (Cisbio Kit).
Islet Mitochondrial Function by High-Resolution Respirometry
A high-resolution respirometry was performed in overnight-cultured intact islets using (Oroboros Instruments, Innsbruck, Austria). The intact islets were diluted with RPMI and cells/chamber were added to each chamber of the in volume per chamber. For each experiment, two Oroboros machines were used at a time, and in each machine islets from one of the BPA-exposed groups and Control were run in parallel. The use of chambers for a particular treatment and control was switched between the experiments to avoid any bias due to chamber differences. The experiment was performed at 37°C with constant magnetic stirring set at 750 rpm. After the addition of intact islets into the oxygraph chambers, the basal respiration referred to as the ROUTINE state was measured when a stable oxygen flux was reached. Following this step, inhibitors for the different mitochondrial respiratory complexes were added and oxygen consumption of mitochondria was measured according to established protocol published previously (Zhang et al. 2013). The inhibitors were added in the following order: oligomycin () (Sigma), to inhibit complex V (to assess nonmitochondrial respiratory capacity or proton LEAK rate), carbonyl cyanide-p-trifluoro-methoxyphenylhydrazone (FCCP) (Sigma) uncoupler with stepwise titration in increments [to assess maximal electron transport system respiratory capacity rate (ETS)]. Rotenone (Sigma) was added in a final concentration to inhibit complex I, and antimycin A (Sigma) in a final concentration was added last to inhibit complex III. Data was analyzed using DatLab4 and DatLab6 (Oroboros, Austria) software, and normalized to the total DNA concentration.
Islet Cytosolic Calcium Levels
In isolated islets, cytosolic calcium responses were measured by dual-wavelength fluorescence microscopy using Fura-2AM (Life Technologies) as a calcium indicator, and calcium dynamics were measured with the Zeiss AxioVision system (Carl Zeiss Microscopy, Thornwood, NY) (Li et al. 2013).
Histology
Pancreata were excised, and fixed in 10% formalin (pH 7.0) for 48 h at room temperature, and embedded in paraffin (Tissue-Tek, 4,583). Six sections of thickness, apart were selected for immunohistochemical (IHC) and immunofluorescence (IF) staining per animal. Prior to any antigen-specific staining, subsets of sections from each animal were H & E stained, and assessed by an independent, blinded pathologist for section quality (see Figure S3). All slides were pretreated in an antigen retriever with either citrate buffer pH 7.6 (Vector Labs), or pH9 Retrieval Solution (DAKO), and blocked using 2% fetal bovine serum.
For immunohistochemical staining, slides were incubated in primary F4/80 antibody (Invitrogen) 1:50 overnight at 4°C, or CD3 antibody (Santa Cruz) 1:250 for 2 h at room temperature. Secondary anti-rat IgG (Vector Lab) and anti-goat IgG (Vector Lab) antibodies were used in a 1:200 dilution for 30 min at room temperature.
For immunofluorescence staining, slides were incubated with antibodies against insulin (Abcam) 1:50 for 16 h at 4°C, glucagon (Biogenix) 1:50 for 1 h at room temperature, and somatostatin (Biorad) 1:50 for 1 h at room temperature. Secondary antibodies Alexa Fluor® 594 anti-guinea pig (Invitrogen), Alexa Fluor® 647 anti-rabbit (Invitrogen), and Alexa Fluor® 488 anti-rat (Invitrogen) 1:200 were applied for 1 h at room temperature in the dark. The nuclei were stained using DAPI (Sigma). To quantify staining, slides were digitally scanned at magnification on an Aperio Scanscope CS-O (brightfield) and Scanscope IF (fluorescent) (Leica Biosystems). Nonfluorescent staining was analyzed by ImageScope v12.2.2.5015 (Leica Biosystems) using Aperio-Color Deconvolution v9.1 algorithm. The area of CD3 and F4/80 staining was calculated within islets, pancreatic lymph nodes, and non-islet tissue by multiplying percent positive staining with total stained area and the product was normalized to total analysis area. Fluorescent staining was analyzed by HALO v2.0.1018 (Indica Lab) using Indica Labs Area Quantification FL v1.0 algorithm. Islets were identified as insulin surrounded by glucagon and somatostatin staining. Beta-, alpha-, and delta-cell mass was calculated by multiplying the average insulin, glucagon, and somatostatin stained area, respectively, within each islet relative to the total area scanned with the pancreatic weight and then corrected for body weight measured at tissue harvest.
Cytokine Measurement
The cytokine and chemokine panels were measured in pancreatic lysates by mouse Cytokine/Chemokine Luminex 25plex Assay with a detection limit of to (EMD Millipore), as described previously (Jaeckle Santos et al. 2014). Interleukin-6 (IL6) and monocyte chemoattractant protein-1 (MCP1) levels were confirmed using mouse ELISA kits (Thermo Fisher). Cytokine levels were normalized to total protein concentration measured by BCA (bicinchoninic acid) assay (Pierce).
RNA Sequencing
Total RNA was extracted from pooled islets using the Trizol and Qiagen RNeasy mini kit following the manufacturer’s instructions. RNA integrity was determined using Agilent RNA 6000 Nano Kit and samples with an RNA integrity number of were used to make RNA libraries. TruSeq Illumina RNA libraries were sequenced using the high-throughput whole transcriptome next generation sequencing to generate 60–80 million reads per sample. Fastq data was processed with HTSeq-count v0.6.1, and aligned using STAR v2.4.0 software. The edgeR v3.12.1 was used to find differentially expressed genes via and false discovery rate values. Functional analyses were conducted using Ingenuity Pathway Analysis (Ingenuity Systems; http://www.ingenuity.com).
Islet mRNA Expression Analysis
RNA extracted from pooled islets with an RNA integrity number of were used for gene expression analysis. Using Biorad iScript kit, cDNA was subsequently generated. Real-time polymerase chain reaction (PCR) was performed using TaqMan Gene expression assay in Applied Biosystems 7900HT Fast Real-Time PCR System. F1 data was normalized to Rpl19 and Beta actin, and F2 data was normalized to Rpl19 and Cyclophilin A (Ppia). The primers and probes are listed in Table S1.
Islet Protein Expression Analysis
The levels of estrogen receptor alpha (ESR1) and uncoupling protein 2 (UCP2) proteins were measured in lysates by using mouse ESR1 (Aviva Systems Biology), and mouse UCP2-Ab (MyBioSource) ELISA kits, following the manufacturer’s instructions. Target protein levels were normalized to total protein concentration measured by BCA assay (Pierce).
Islet DNA Methylation Analysis
DNA was extracted from pooled islets using the QIAmp DNA Mini Kit (Qiagen), and of DNA was bisulfite treated using the EpiTect Bisulfite Kit (Qiagen) following the manufacturer’s protocol. Pyrosequencing was conducted to measure the methylation status of the promoter region of the Igf2 DMR1 (chr7: 149,851,180–149,851,655; NCBI37/MM9) and H19/Igf2 ICR (chr7: 149,767,599–149,767,819; NCBI37/MM9), and exon A and exon C of the Esr1 gene as described previously by Susiarjo et al. (2013) and Kundakovic et al. (2013), respectively. Briefly, of purified bisulfite-treated DNA was amplified using a PyroMark PCR kit (Qiagen) and primers specific for the Igf2 DMR1, H19/Igf2 ICR, and exon A and exon C of the Esr1gene (see Table S2). Ten microliters of the biotinylated PCR product was used for each sequencing assay and sequenced using a PyroMark Q96MD (Qiagen) pyrosequencer and PyroMark Gold 96 reagents kit (Qiagen) and specific pyrosequencing primers (see Table S2). Percent methylation levels of CpG sites were quantified using Qiagen’s Pyro Q-CpG software.
Islet Viability Assays
Lysates from freshly isolated islets from 8- to 10-wk-old F1 and F2 mice were assessed for apoptosis using the caspase 3 activity fluorometric assay (Abcam), BCL2 levels using the mouse BCL2 ELISA kit (LS Bio), and phospho-AKT (pSer473) and total AKT levels using the AKT (pSer473) + total AKT ELISA kit (Abcam) following the manufacturer’s instructions.
Statistical Analysis
For comparison between LowerB with Control, and UpperB with Control, we used Dunnett’s test, which accounts for multiple testing. For pyrosequencing data, using Dunnett’s test, we statistically analyzed methylation levels at individual CpG sites as well as mean methylation across all CpG sites for our two BPA treatment groups relative to Control. In addition, we performed multivariate analyses of variance followed by repeated measures response design to determine a) methylation levels differ between the groups, b) methylation levels differ between CpG sites, and c) differences in methylation levels across CpG sites interact with study groups. Nonparametric data were log-transformed to approximate a standard distribution, where required. All values are presented as . A was considered significant. All data were analyzed using JMP and Prism analysis software.
Results
Dose- and Sex-Specific Effects of Maternal BPA Exposure on Islet Mitochondrial Function in F1 and F2 Adult Offspring
Using our mouse model of BPA exposure, we previously observed impaired GSIS in the F1 and F2 generation adult male offspring of mothers (F0) exposed to (LowerB) or (UpperB) relative to Controls (7% corn oil diet) (Susiarjo et al. 2015). Nutrient-induced insulin secretion requires ATP production from mitochondria (Antinozzi et al. 2002; Ortsäter et al. 2002). Mitochondrial dysfunction has been associated with failure in islets of intrauterine growth-restricted rats (Simmons et al. 2005). To assess whether impaired GSIS in F1 and F2 adult male offspring is due to altered mitochondrial driven insulin secretion, we measured insulin secretion in response to , a substrate metabolized in mitochondria. Surprisingly, –stimulated insulin secretion in the islets of F1 and F2 LowerB males was comparable to levels in Controls. However, it trended to be reduced in F1 and F2 UpperB males relative to Controls (Figure 1), thus reflecting an abnormal mitochondrial phenotype in islets of UpperB males. We saw no differences in glucose or –stimulated insulin secretion in LowerB or UpperB F1 or F2 females compared with Control females (Figure 2). Therefore, all further studies were conducted exclusively in adult male offspring.
Figure 1.
Mitochondrial driven insulin secretion in islets of F1 and F2 adult male offspring: (A, C) F1 and (B, D) F2 adult male offspring. The maximal KIC-stimulated insulin release was calculated as the insulin area under the curve for KIC. Data are individual litter data (one animal per litter), presented as , and analyzed using Dunnett’s test. p-Values are relative to Control. Note: KCL, potassium chloride; KIC, .
Figure 2.
Glucose-stimulated and mitochondrial associated insulin secretion in islets of F1 and F2 adult female offspring: (A–B, E–F) F1, and (C–D, G–H) F2 adult female offspring. The maximal glucose- and KIC-stimulated insulin release was calculated as the insulin area under the curve for glucose or KIC, respectively. Data are individual litter data (one animal per litter), presented as , and analyzed using Dunnett’s test. are relative to Control. Note: KCL, potassium chloride; KIC, .
To determine whether the phenotype in males was associated with mitochondrial dysfunction, we performed high-resolution respirometry to assess oxygen consumption in intact islet mitochondria using Oroboros. Consistent with the above results, we did not see any difference in oxygen consumption in the intact islets of F1 and F2 LowerB males, but did observe reduced basal and maximal oxygen consumption in the intact islets of F1 and F2 UpperB males relative to Controls (Figure 3). These results are consistent with abnormal mitochondrial function in the islets of the UpperB males, and the reduction in GSIS in F1 and F2 LowerB males may be due to a factor other than a mitochondrial defect.
Figure 3.
Basal and maximal mitochondrial oxygen consumption in islets of F1 and F2 adult male offspring: (A–B) F1, and (C–D) F2 adult male offspring. Data are individual litter data (islets pooled from 2–3 males from the same litter) with mean superimposed. Data were analyzed using Dunnett’s test. p-values are relative to Control. Oxygen flow per cell at: ROUTINE, i.e., basal state of cell respiration; electron transport system [ETS; uncoupled; carbonyl cyanide-p-trifluoro-methoxyphenylhydrazone (FCCP)]; and residual oxygen consumption (rox; rotenone, antimycin A).
Others have shown that BPA exposure alters expression of key mitochondrial genes such as oxoglutarate dehydrogenase (Ogdh) and uncoupling protein 2 (Ucp2) in rodent islets (Wei et al. 2011; Song et al. 2012). Because we observed mitochondrial dysfunction in the current study, we sought to determine whether these previously demonstrated mitochondrial targets of BPA are affected. OGDH is the rate-limiting enzyme in the tricarboxylic acid (TCA) cycle, which plays a key role in mitochondrial function. UCP2 is a member of the mitochondrial carrier family whose loss of function mutation can cause hyperinsulinism, whereas its overexpression is associated with the efflux of TCA cycle intermediates out of the mitochondria, thereby reducing acetyl-CoA–oxidizing substrates in the mitochondria (González-Barroso et al. 2008; Vozza et al. 2014). Ogdh expression was only mildly increased in LowerB F2 males compared with Controls (Table 1). In contrast, Ucp2 mRNA levels were significantly increased in LowerB and UpperB F1 and F2 males compared with Controls (Table 1). The changes in mitochondrial gene expression are consistent with UpperB F1 and F2 mitochondrial impairment, although these changes did not translate to statistically significant differences in protein expression levels. F1 and F2 UpperB males (F1 , ; F2 , ; ) and LowerB males (F1 , ; F2 , ; ) had increased UCP2 protein levels relative to Controls (F1 ; F2 ; ); however, these findings were not statistically significant.
Table 1.
mRNA Levels in islets of F1 and F2 Lower BPA and Upper BPA male mice.
| Gene | Lower BPA (95% CI) | Upper BPA (95% CI) |
|---|---|---|
| Igf2 | ||
| F1 | 1.67 (1.20, 2.31)* | 2.19 (1.71, 2.79)* |
| F2 | 1.48 (1.03, 2.30)* | 1.23 (1.13, 1.34)* |
| Ucp2 | ||
| F1 | 1.51 (1.03, 2.21)* | 2.54 (1.52, 3.24)* |
| F2 | 2.05 (1.78, 2.36)* | 1.74 (1.05, 3.08)* |
| Ogdh | ||
| F1 | 0.97 (0.72, 1.29) | 1.40 (0.96, 2.03) |
| F2 | 1.20 (1.18, 1.22)* | 1.32 (0.97, 1.80) |
| Esr1 | ||
| F1 | 0.70 (0.53, 0.93)* | 0.88 (0.82, 0.94)* |
| F2 | 0.38 (0.18, 0.84)* | 0.48 (0.24, 0.97)* |
| Pdx1 | ||
| F1 | 1.32 (0.82, 2.11) | 1.72 (1.11, 2.64)* |
| F2 | 1.31 (0.86, 2.00) | 1.21 (0.48, 3.03) |
| Igf1 | ||
| F1 | 1.05 (0.95, 1.15) | 1.55 (1.10, 2.19)* |
| F2 | 1.08 (0.94, 1.25) | 1.23 (1.13, 1.34)* |
| F1 | 1.06 (0.89, 1.27) | 1.43 (1.30, 1.59)* |
| F2 | 1.00 (0.85, 1.17) | 1.21 (0.94, 1.57) |
| Kcnj11 | ||
| F1 | 1.54 (0.70, 3.41) | 0.94 (0.45, 1.99) |
| F2 | 1.56 (0.92, 2.64) | 1.06 (0.36, 3.12) |
| Abcc8 | ||
| F1 | 1.48 (0.75, 2.92) | 0.99 (0.55, 1.78) |
| F2 | 1.94 (1.48, 2.54)* | 1.46 (0.63, 3.37) |
| Snap25 | ||
| F1 | 1.02 (0.43, 2.38) | 0.71 (0.30, 1.65) |
| F2 | 1.46 (0.66, 3.26) | 1.17 (0.38, 3.61) |
Note: Data were normalized to Rpl19 and Beta actin or Ppia mRNA levels and expressed as fold change relative to Controls (where Control is set at 1.0) with 95% confidence intervals (CI) in islets of F1 and F2 Lower BPA () and Upper BPA () male mice. litters per group; islets pooled from 2–3 male mice per litter. * vs. Controls.
Dose-Specific Effects of Maternal BPA Exposure on Mass in F1 and F2 Adult Male Offspring
An alternative hypothesis for reduced insulin secretion is that BPA exposure is associated with a reduction in the number of insulin-secreting cells. To determine whether reduced GSIS in BPA-exposed F1 and F2 male offspring is linked to reduced mass, we stained pancreatic sections with anti-insulin, glucagon and somatostatin antibodies to quantify , alpha-, and delta-cell mass, respectively. F1 and F2 LowerB males had reduced mass adjusted for body weight relative to Controls (Figure 4). F1 and F2 UpperB and Control males had comparable mass. Alpha-cell mass was similar among the F1 males, but F2 LowerB males had a modest increase in alpha-cell mass compared with Controls (see Figure S4). F1 and F2 LowerB and UpperB had comparable delta mass as Controls (see Figure S4). Thus, LowerB exposure specifically reduced mass across two generations. We also measured pancreatic insulin content in F1 and F2 males. UpperB had similar (F1 , ; F2 , ; ), whereas LowerB tended to have reduced insulin content (F1 , ; F2 , ; ) relative to Controls (F1 ; F2 ; ).
Figure 4.
Beta cell mass of F1 and F2 adult male offspring. Representative photomicrographs of pancreatic immunofluorescent staining in (A–C) F1 and (D–F) F2 Control, LowerB, UpperB male mice. All images have insulin (red), glucagon (green), somatostatin (yellow), and DAPI (blue). Image magnified . Data are individual litter data (one animal per litter) with mean superimposed from (G) F1 and (H) F2 males, analyzed by Dunnett’s test. p-Values are relative to Control.
We then examined possible mechanisms underlying the reduction in mass. Caspase 3 activity, a marker of cell death, was significantly increased in islets of F1 and F2 LowerB males but not in islets from UpperB males (Figure 5). BCL2, an anti-apoptotic protein, did not differ in F1or F2 LowerB compared with Controls, but levels were increased in F1 UpperB males relative to Controls (; Figure 5). These changes were associated with increased AKT phosphorylation in F1 and F2 UpperB males relative to Controls. F1 and F2 LowerB males had similar AKT phosphorylation as Controls (Figure 5). AKT phosphorylation mediates the cell survival response by inhibiting programed cell death (Reed and Paternostro 1999; Yao and Cooper 1995). Thus, the UpperB group had increased AKT phosphorylation and no difference in islet cell death and mass, whereas the LowerB group has increased islet cell death and decreased mass across two generations.
Figure 5.
Cell viability in islets of (A–C) F1 and (D–F) F2 male offspring. Data are individual litter data (one animal per litter) with mean superimposed. Each parameter is normalized to total protein concentration. Data were analyzed using Dunnett’s test performed on log-transformed data, where required (BCL2). Note: RFU: relative fluorescence unit. p-Values are relative to Control.
Effects of Maternal BPA Exposure on Cytokine/Chemokine Levels/Expression in Pancreata of F1 and F2 Adult Male Offspring
Several studies indicate that immune cells such as macrophages, dendritic cells, and lymphocytes are present in islets and may play an important role in normal islet development (Criscimanna et al. 2014; Geutskens et al. 2005; Jansen et al. 1993, 1994). In fact, immune cell defects are linked to abnormal pancreatic development and glucose homeostasis observed in athymic BALB/c nude mice (Zeidler et al. 1991). To explore whether –specific effects of BPA are associated with a perturbed immune response, we assessed the cytokine/chemokine profile in pancreatic lysates of F1 and F2 mice via a Luminex assay. Because different immune cell types produce and respond to different cytokines/chemokines, we have provided a list of these cell types in Table 2; see also Tables S3 and S4. In F1 and F2 BPA-exposed versus Control mice levels of the cytokine IL4 was reduced, but IL5 was elevated, and pro-inflammatory cytokines IL6 and MCP1 were also elevated (Table 2). Compared with Controls, F1 UpperB males also had increased levels of G-CSF (granulocyte colony stimulating factor) and IL9 (see Table S3). IL17 was increased in F2 LowerB and UpperB males compared with Controls (Table 2). The levels of other cytokines measured were comparable among the three groups (see Tables S3 and S4). We validated the changes in levels of key cytokines via ELISA and saw a similar trend of increased cytokine levels of IL6 as well as significantly increased MCP1 levels in F1 and F2 LowerB and UpperB males relative to Controls (Figure 6). This indicated that both doses of BPA increased levels of pro-inflammatory cytokines in islets.
Table 2.
Cytokine/chemokine levels on a LUMINEX assay in pancreatic lysates of F1 and F2 adult male offspring.
| Cytokine/Chemokine | Inflammatory action | Produced by | Target cell/action | F1 Offspring | F2 Offspring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control () | LowerB () | UpperB () | LowerB (p-value) | UpperB (p-value) | Control () | LowerB () | UpperB () | LowerB (p-value) | UpperB (p-value) | ||||
| IL4 | pro/anti | Macrophages, eosinophills, basophills | Macrophages, T cells, | 0.60 | 0.75 | 0.23 | 0.04 | ||||||
| IL5 | pro/anti | cells, mast Cells | B-cell growth, eosinophills | 0.08 | 0.01 | 0.43 | 0.09 | ||||||
| IL6 | pro/anti | T-cells, macrophages | B-cells, naïve T cells , | 0.89 | 0.08 | 0.15 | 0.04 | ||||||
| IL17 | pro | cells | Recruits macrophages and neutrophils | 0.20 | 0.33 | 0.14 | 0.03 | ||||||
| MCP1 | pro | Chemoattractant produced by injured tissue, or infection | Macrophages, memory T cells, dendritic cells, basophils | 0.18 | 0.02 | 0.27 | 0.09 | ||||||
| pro | Macrophages , natural killer and mast cells | Macrophages, IL1- and IL6-producing cells | 0.59 | 0.53 | 0.62 | 0.28 | |||||||
| pro | Natural killer cells, natural killer T-cells, macrophages, , | Macrophages, MHCII | 0.45 | 0.59 | 0.99 | 0.85 | |||||||
Note: Data are normalized to total protein concentration as pg of cytokine or chemokine per of total protein, and presented as . Decimal places are represented by E notation, where E represents base 10, followed by the power of 10 (example, , , ). Information in columns 2, 3, and 4 is derived from Cavaillon 2001; Commins et al. 2010; Kimura and Kishimoto 2010; Kourilsky and Truffa-Bachi 2001; Turner et al. 2014. Control, 7% corn oil diet; LowerB, Lower BPA (); UpperB, Upper BPA (). to per group. p-Values are from Dunnett’s test performed on log-transformed data, where required (F1 IL5, IL6, MCP1, and ; F2 IL4, IL5 IL6, IL17, , and ).
Figure 6.
IL6, MCP1 levels in (A–B) F1 and (C–D) F2 adult male offspring. Note: IL6, interleukin 6; MCPm: monocyte chemoattractant protein-1. Cytokine levels for each sample were normalized to total protein concentration. Data are individual litter data (one animal per litter) with mean superimposed. Data were analyzed by Dunnett’s test performed on log-transformed data, where required (F1 IL6, MCP1; F2 IL6). p-Values are relative to Control.
To verify the increased inflammatory phenotype in the pancreas of F1 and F2 BPA-exposed male mice, we immunostained pancreatic sections with CD3, a marker for T lymphocytes, and with F4/80, a marker for macrophages. We consistently observed increased immunostaining of CD3 and F4/80 in the islets of BPA-exposed F1 and F2 male mice compared with Controls (Figure 7; Table 3). Thus, lower- and upper-dose BPA exposure increased islet inflammation across two generations in males. Taken together these findings indicate the recruitment of macrophages and cells and, possibly, the recruitment of neutrophils and activation of eosinophils in the pancreas.
Figure 7.
Representative photomicrographs of pancreatic immunohistochemical staining in F1 and F2 adult male offspring: (A–H) F1 and (I–P) F2 Control, LowerB, UpperB mice, and Positive Ctrl (internal Control—lymph node). Image magnified . Note: CD3, cluster of differentiation 3 (marker for T lymphocytes); F4/80, marker for macrophages. F1, litters per group (one animal per litter); F2, litters Control and UpperB, litters LowerB (one animal per litter).
Table 3.
Quantification of CD3 and F4/80 staining area in pancreatic sections of F1 and F2 adult male offspring.
| Staining area | Control () | LowerB () | UpperB () | LowerB vs. Control (p-value) | UpperB vs Control (p-value) |
|---|---|---|---|---|---|
| Area of percent positive CD3 staining in islets/total analysis area | |||||
| F1 | 0.04 | 0.02 | |||
| F2 | 0.51 | 0.06 | |||
| Area of percent positive CD3 staining in pancreas excluding islets and lymph nodes/total analysis area | |||||
| F1 | 0.42 | 0.04 | |||
| F2 | 0.04 | 0.18 | |||
| Area of percent positive CD3 staining in pancreatic lymph nodes/total analysis area | |||||
| F1 | 0.08 | 0.05 | |||
| F2 | 0.78 | 0.84 | |||
| Area of percent positive F4/80 staining in islets/total analysis area | |||||
| F1 | 0.12 | 0.02 | |||
| F2 | 0.15 | 0.04 | |||
| Area of percent positive F4/80 staining in pancreas excluding islets and lymph nodes/total analysis area | |||||
| F1 | 0.09 | 0.01 | |||
| F2 | 0.10 | 0.03 | |||
| Area of percent positive F4/80 staining in pancreatic lymph nodes/total analysis area | |||||
| F1 | 0.60 | 0.10 | |||
| F2 | 0.94 | 0.81 |
Note: Data are normalized to total analysis area and presented as . Control, 7% corn oil diet; LowerB, Lower BPA (); UpperB, Upper BPA (). litters per group (one animal per litter). p-Values are from Dunnett’s test performed on log-transformed data, where required (F1 CD3 islets, F1 CD3 excluding islets and lymph nodes, F2 CD3 islets, F2 F4/80 lymph nodes).
Effects of Maternal BPA Exposure on Estrogen Receptor Alpha Gene Expression in Islets of F1 and F2 Adult Male Offspring
The endocrine-disrupting actions of BPA are mediated by both estrogen receptor dependent and independent pathways (Yoon et al. 2014). Estrogen receptors (ESR1, or Esr1) are critically important to function and development (Tiano et al. 2011; Yuchi et al. 2015) and have anti-inflammatory effects in other organs (Miller et al. 2012). We observed a significant reduction in mRNA expression of Esr1 in F1 and F2 LowerB and UpperB males compared with Controls (Table 1). The Esr1 mRNA changes in the LowerB group correlated with its protein expression, such that F1 and F2 LowerB males (F1 , ; F2 , ; ) trended toward reduced ESR1 protein levels relative to Controls (F1 ; F2 ; ); however, F1 and F2 UpperB males (F1 , ; F2 , ; ) had ESR1 protein levels that were similar to those of Controls. BPA also acts via the estrogen receptor beta (ESR2, or Esr2) and alters stimulus-secretion coupling through its effects on channels and increasing glucose-stimulated levels (Soriano et al. 2009; Soriano et al. 2012). We did not observe any difference in either mRNA levels of Esr2, channels subunits Kcnj11 and Abcc8, and Snap25, which mediate the calcium signaling necessary for insulin secretion (Tables 1, 4, and 5), or in cytosolic levels in isolated islets of F1 and F2 LowerB and UpperB adult males relative to Controls (see Figure S5). These results demonstrate that the effects of BPA exposure are mediated preferentially by Esr1 and not Esr2 or targets of Esr2 in our study.
Table 4.
Genes with in F1 Lower BPA male vs. Control male from RNA seq analysis. The genes that were assessed via qPCR are included in the table (rows 10–20) for completeness.
| Serial no. | Gene name | logFC | logCPM | p-Value | QValue.BH |
|---|---|---|---|---|---|
| 1 | Lars2 | 9.43 | 1.49E-06 | 0.04 | |
| 2 | Cth | 2.43 | 1.60E-06 | 0.04 | |
| 3 | Klk1b22 | 8.85E-06 | 0.08 | ||
| 4 | Cyp2c70 | 1.32E-05 | 0.08 | ||
| 5 | Cpa5 | 5.70 | 1.41E-05 | 0.08 | |
| 6 | Klk1b24 | 1.56 | 1.65E-05 | 0.08 | |
| 7 | Tinag | 1.01 | 2.22E-05 | 0.09 | |
| 8 | Reg3a | 3.38 | 2.83E-05 | 0.09 | |
| 9 | Fgf21 | 0.34 | 2.88E-05 | 0.09 | |
| 10 | Igf2 | 4.27 | 1.26 | 0.02 | 0.80 |
| 11 | Esr1 | 1.56 | 0.18 | 0.80 | |
| 12 | Esr2 | 0.87 | 0.18 | 0.80 | |
| 13 | Snap25 | 1.58 | 0.31 | 0.98 | |
| 14 | Kcnj11 | 6.35 | 0.31 | 0.98 | |
| 15 | Ogdh | 0.31 | 2.77 | 0.70 | 1.00 |
| 16 | Abcc8 | 0.18 | 5.18 | 0.77 | 1.00 |
| 17 | Pdx1 | 0.25 | 2.62 | 0.78 | 1.00 |
| 18 | Igf1 | 0.18 | 2.41 | 0.83 | 1.00 |
| 19 | Ucp2 | 1.15 | 0.84 | 1.00 | |
| 20 | Hnf1a | 6.06 | 0.86 | 1.00 |
Note: CPM, counts per million; FC, fold change. litters per group (islets pooled from 2–3 male mice per litter).
Table 5.
Genes with in F1 Upper BPA male vs. Control male from RNA seq analysis. The genes that were assessed via qPCR are included in the table (rows 42–52) for completeness.
| Serial no. | Gene name | logFC | logCPM | p-Value | QValue.BH |
|---|---|---|---|---|---|
| 1 | Lars2 | 9.43 | 1.99E-14 | 4.87E-10 | |
| 2 | Rn45s | 13.57 | 7.84E-10 | 9.59E-06 | |
| 3 | Dbt | 3.19 | 3.93E-07 | 0.003 | |
| 4 | Klk1b5 | 2.25 | 5.21E-06 | 0.03 | |
| 5 | Matn4 | 9.06 | 7.90E-06 | 0.004 | |
| 6 | Ctnnbip1 | 6.67 | 1.75E-05 | 0.007 | |
| 7 | Sdhc | 4.94 | 6.00E-05 | 0.02 | |
| 8 | Spryd4 | 4.13 | 6.11E-05 | 0.02 | |
| 9 | Chst2 | 5.92 | 8.48E-05 | 0.02 | |
| 10 | Nccrp1 | 4.16 | 0.0002 | 0.03 | |
| 11 | 2610203C22Rik | 4.51 | 0.0003 | 0.04 | |
| 12 | Gm5148 | 10.35 | 0.0002 | 0.04 | |
| 13 | Zfhx2os | 1.45 | 6.56 | 0.0003 | 0.05 |
| 14 | Fam69a | 8.97 | 0.0003 | 0.05 | |
| 15 | 0610039K10Rik | 9.10 | 0.0006 | 0.06 | |
| 16 | 9530027J09Rik | 7.67 | 0.0008 | 0.06 | |
| 17 | Casc1 | 4.96 | 0.0005 | 0.06 | |
| 18 | Ccdc176 | 7.85 | 0.0007 | 0.06 | |
| 19 | Ifi27l2a | 4.68 | 0.0006 | 0.06 | |
| 20 | Loxl3 | 7.96 | 0.0007 | 0.06 | |
| 21 | Lrrc75b | 4.31 | 0.0007 | 0.06 | |
| 22 | Pigf | 8.57 | 0.0007 | 0.06 | |
| 23 | 4930429F24Rik | 1.29 | 5.68 | 0.0009 | 0.06 |
| 24 | Nphs1os | 7.69 | 0.0009 | 0.06 | |
| 25 | 1700028J19Rik | 4.77 | 0.001 | 0.07 | |
| 26 | A230070E04Rik | 1.45 | 5.51 | 0.001 | 0.07 |
| 27 | Adam17 | 7.30 | 0.002 | 0.08 | |
| 28 | Arhgap26 | 4.72 | 0.002 | 0.08 | |
| 29 | C330022C24Rik | 5.73 | 0.002 | 0.08 | |
| 30 | Cand2 | 11.15 | 0.001 | 0.08 | |
| 31 | Casp9 | 18.49 | 0.002 | 0.08 | |
| 32 | Snx22 | 12.90 | 0.002 | 0.08 | |
| 33 | Spg11 | 6.16 | 0.001 | 0.08 | |
| 34 | Tap2 | 5.53 | 0.002 | 0.08 | |
| 35 | Vmn2r9 | 6.12 | 0.002 | 0.08 | |
| 36 | Gm5088 | 10.41 | 0.002 | 0.08 | |
| 37 | Gtf2i | 4.38 | 0.002 | 0.08 | |
| 38 | Txnip | 5.21 | 0.002 | 0.08 | |
| 39 | Prss57 | 6.75 | 0.002 | 0.09 | |
| 40 | Tspan4 | 6.53 | 0.002 | 0.09 | |
| 41 | Slc12a4 | 4.98 | 0.002 | 0.09 | |
| 42 | Esr1 | 1.62 | 1.56 | 0.20 | 0.94 |
| 43 | Abcc8 | 0.78 | 5.18 | 0.19 | 0.94 |
| 44 | Igf2 | 1.24 | 1.26 | 0.28 | 0.94 |
| 45 | Esr2 | 0.87 | 0.35 | 0.94 | |
| 46 | Pdx1 | 0.67 | 2.62 | 0.39 | 0.97 |
| 47 | Igf1 | 2.41 | 0.56 | 1 | |
| 48 | Hnf1a | 6.06 | 0.71 | 1 | |
| 49 | Snap25 | 0.34 | 1.58 | 0.76 | 1 |
| 50 | Ogdh | 0.16 | 2.77 | 0.83 | 1 |
| 51 | Kcnj11 | 0.07 | 6.35 | 0.90 | 1 |
| 52 | Ucp2 | 0.07 | 1.15 | 0.97 | 1 |
Note: CPM, counts per million; FC, fold change. litters per group (islets pooled from 2–3 male mice per litter).
To determine whether BPA mediates change in islets Esr1 gene expression via DNA methylation as shown by others in mouse (Kundakovic et al. 2013) and rat (Chang et al. 2016b) brain, we assessed methylation status of 16 CpG sites across untranslated exon A and exon C of Esr1 gene in islets. We observed similar percent methylation at all five CpG sites of exon C among the three groups (see Figure S6). We observed minimal (4–5%), yet dose-specific, changes in the DNA methylation levels of exon A in islets (Figure 8). F1 LowerB males trended toward increased methylation levels at CpG sites 1 and 4, but F2 LowerB males had similar percent methylation level at all CpG sites examined relative to Controls (Figure 8). The mean methylation across all CpG sites was also similar in F1 and F2 LowerB males compared with Controls. In contrast, F2 UpperB males trend toward increased methylation at CpG sites 3, 4, 5, and 10 and mean methylation across all CpG sites relative to Controls (Figure 8). F1 UpperB males had similar methylation at individual CpG sites and mean methylation across all CpG sites relative to Controls (Figure 8). On repeated measures analysis, we observed significant differences in methylation levels between CpG sites (); however, we saw no statistical difference in methylation levels between the groups () or groups*CpG site interaction () in islets of F1 and F2 males. Overall these findings indicate that BPA had minimal effects on Esr1 methylation in islets. It is possible that in addition to these minimal differences in methylation levels, BPA may be mediating its effect on Esr1 via other regulatory mechanisms such as changes in chromatin acetylation/methylation levels. This requires further investigation.
Figure 8.
Percent DNA methylation at Igf2 DMR1 and Esr1 Exon A in islets of F1 and F2 adult male offspring: (A–B) F1 and (C–D) F2 adult male offspring. Data are percent CpG methylation values from individual litter (islets pooled from 2–3 males from the same litter) and presented as . Data were analyzed using Dunnett’s test; p-Values are relative to Control. ** , * and .
Effects of Maternal BPA Exposure on Igf2 Expression and DNA Methylation at Igf2 DMR1 in Islets of F1 and F2 Adult Male Offspring
Insulin-like growth factor 2 (Igf2) plays a critical role in function, proliferation, and apoptosis. We and others have reported that Igf2 overexpression is associated with impaired glucose tolerance (Höög et al. 1996; Susiarjo et al. 2015) and impaired insulin secretion (Höög et al. 1996; Petrik et al. 1999). Overexpression of Igf2 has also been reported to lead to dysfunction and marked morphological abnormalities of the islet (Casellas et al. 2015). In previous studies, we observed an increased expression of Igf2 in F1 and F2 upper dose BPA-exposed embryos (Susiarjo et al. 2013). Thus, to determine whether Igf2 expression is also increased postnatally in islets and whether these changes persist into the next generation, we measured mRNA levels in islets from F1 and F2 LowerB and UpperB groups by quantitative PCR (qPCR). We found that Igf2 mRNA levels were significantly increased in F1 and F2 LowerB and UpperB males compared with Controls (Table 1).
Previously, we determined that the increase in expression of Igf2 in F1 and F2 islets in UpperB males was associated with increased methylation at the Igf2 DMR1 (Susiarjo et al. 2013, 2015). Thus, we measured DNA methylation at H19/Igf2 imprinting control region (ICR) and at Igf2 DMR1 in islets. F1 and F2 LowerB males had DNA methylation levels at Igf2 DMR1 and H19/Igf2 ICR comparable with Controls (Figure 8; see also Figure S6). In contrast, F1 UpperB males had increased methylation at CpG site 1 and increased mean methylation, and F2 UpperB males had increased methylation at CpG sites 1 and 2 as well as increased mean methylation at Igf2 DMR1 relative to Controls (Figure 8). Interestingly, on repeated measures, we observed differences in methylation levels across CpG sites (F1 ; F2 ) and between groups (F1 ; F2 ), but no statistical difference in groups*CpG site interaction (F1 ; F2 ) at Igf2 DMR1. This analysis indicated that BPA affects methylation level at CpG sites but does not account for the methylation differences between CpG sites at Igf2 DMR1. However, we saw no difference in methylation at H19/Igf2 ICR (see Figure S6), suggesting that the increase in Igf2 expression in the islet results from increased expression of the normally expressed paternal allele and not loss of imprinting.
Effects of Maternal BPA Exposure on the Transcriptome of F1 Adult Male and Female Offspring
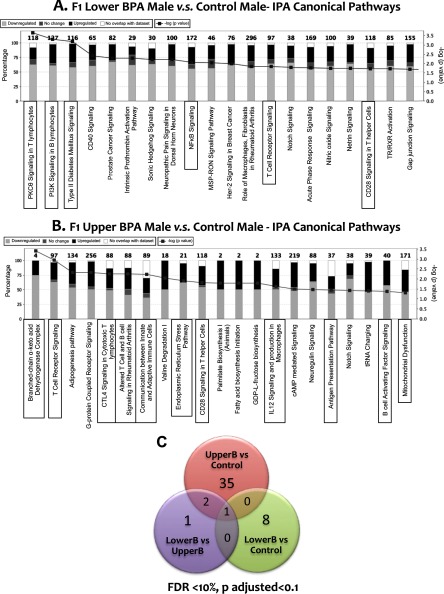
To identify novel pathways and to determine whether changes in islet phenotypes in BPA-exposed mice are regulated at the gene expression level, we conducted whole transcriptome analysis via RNA sequencing in males and females. Consistent with our phenotypic data, we saw no differentially expressed genes in females (Tables 6 and 7). Because of interanimal variability and the small sample size, there were very few differentially expressed genes in males when the analysis was corrected for multiple testing (). Interestingly, however, of the few differentially expressed genes, there was minimal overlap between the LowerB and UpperB males (Figure 9), consistent with the observed dose-specific phenotypes. Moreover, qPCR of several key islet genes demonstrated robust differences between the groups despite higher q-values in the RNAseq analysis (Tables 1, 4, 5). Thus, we performed ingenuity pathway analysis on the RNAseq data using a and a fold change of 1.5. Multiple genes associated with inflammation in LowerB males, and both inflammation and mitochondrial function including branched-chain acid dehydrogenase complex in UpperB males were altered compared with Controls, which is consistent with the phenotypic data (Figure 9). Other pathways that are critical for normal pancreatic development and regulation of insulin secretion such as sonic hedgehog (Thomas et al. 2000) and gap-junction signaling (Bavamian et al. 2007; Klee et al. 2011) were altered in LowerB males, whereas pathways involved in inflammation and mitochondrial dysfunction such as G-protein couple receptor (Patial et al. 2009; Sorriento et al. 2013) and endoplasmic reticulum stress (Rocha et al. 2016) and signaling were altered in UpperB males. Unexpectedly, there were minimal changes in mRNA expression levels as determined by RNA Seq and qPCR of other regulatory genes such as Pdx1, Igf1, and Hnf1a in BPA groups compared with Controls (Tables 1, 4, and 5).
Table 6.
Top 25 genes, by p-values, in F1 Lower BPA female vs. Control female from RNA seq analysis.
| Serial no. | Gene name | logFC | logCPM | p-Value | QValue.BH |
|---|---|---|---|---|---|
| 1 | Creb3l3 | 2.61 | 9.51E-05 | 1 | |
| 2 | 4833419F23Rik | 5.26 | 2.44 | 2.03E-04 | 1 |
| 3 | Gm10012 | 2.66 | 3.88E-04 | 1 | |
| 4 | Mpzl2 | 5.01 | 1.96 | 3.88E-04 | 1 |
| 5 | Fam204a | 5.13 | 2.23 | 7.35E-04 | 1 |
| 6 | Krt19 | 2.51 | 7.40E-04 | 1 | |
| 7 | Nedd9 | 2.14 | 7.86E-04 | 1 | |
| 8 | 4930470O06Rik | 2.03 | 8.05E-04 | 1 | |
| 9 | Egr1 | 2.23 | 9.15E-04 | 1 | |
| 10 | 1700019G17Rik | 2.32 | 0.001 | 1 | |
| 11 | Trmt5 | 4.89 | 2.24 | 0.001 | 1 |
| 12 | Espl1 | 2.04 | 0.001 | 1 | |
| 13 | St18 | 2.65 | 0.001 | 1 | |
| 14 | Tspan2os | 2.74 | 0.001 | 1 | |
| 15 | Drg1 | 2.25 | 0.001 | 1 | |
| 16 | Ggta1 | 5.51 | 2.06 | 0.001 | 1 |
| 17 | Fbxw7 | 2.28 | 0.001 | 1 | |
| 18 | Serf2 | 2.29 | 0.001 | 1 | |
| 19 | 4930509J09Rik | 2.40 | 0.001 | 1 | |
| 20 | Dhrs1 | 2.21 | 0.001 | 1 | |
| 21 | 8430437L04Rik | 1.96 | 0.001 | 1 | |
| 22 | Rragb | 4.71 | 2.38 | 0.002 | 1 |
| 23 | Sh3bgr | 4.66 | 1.80 | 0.002 | 1 |
| 24 | Taf11 | 2.12 | 0.002 | 1 | |
| 25 | 4931431B13Rik | 4.75 | 0.002 | 1 |
Note: CPM, counts per million; FC, fold change. litters per group (islets pooled from 2–3 female mice per litter).
Table 7.
Top 25 genes, by p-values, in F1 Upper BPA female vs. Control female from RNA seq analysis.
| Serial no. | Gene name | logFC | logCPM | p-Value | QValue.BH |
|---|---|---|---|---|---|
| 1 | Fabp1 | 5.54 | 4.93E-05 | 0.83 | |
| 2 | Spr | 5.41 | 2.14 | 8.67E-05 | 0.83 |
| 3 | Gda | 3.71 | 1.82E-04 | 0.83 | |
| 4 | BC051537 | 3.19 | 5.86 | 2.27E-04 | 0.83 |
| 5 | St3gal4 | 3.01 | 2.86E-04 | 0.83 | |
| 6 | Rragb | 5.26 | 2.38 | 3.79E-04 | 0.83 |
| 7 | Wdr74 | 4.98 | 2.01 | 4.44E-04 | 0.83 |
| 8 | Myo1a | 4.63 | 4.77E-04 | 0.83 | |
| 9 | Rprm | 5.64 | 2.26 | 5.47E-04 | 0.83 |
| 10 | Pcsk5 | 2.50 | 0.0006 | 0.83 | |
| 11 | Psmg3 | 3.18 | 0.0006 | 0.83 | |
| 12 | Smim24 | 5.48 | 0.0007 | 0.83 | |
| 13 | Apoa1 | 5.39 | 0.0007 | 0.83 | |
| 14 | Rab40b | 5.35 | 2.17 | 0.0007 | 0.83 |
| 15 | Rbp2 | 4.70 | 0.0008 | 0.83 | |
| 16 | Ephx2 | 3.12 | 0.0009 | 0.83 | |
| 17 | Ccs | 2.77 | 0.0009 | 0.83 | |
| 18 | Ano3 | 2.56 | 0.0009 | 0.83 | |
| 19 | Tctn1 | 2.22 | 8.44 | 0.0009 | 0.83 |
| 20 | 4833419F23Rik | 4.78 | 2.44 | 0.001 | 0.83 |
| 21 | Adipor2 | 3.21 | 0.001 | 0.83 | |
| 22 | Enpp3 | 2.33 | 0.001 | 0.83 | |
| 23 | Slc15a1 | 3.33 | 0.001 | 0.83 | |
| 24 | Pkdrej | 2.69 | 0.001 | 0.83 | |
| 25 | Sis | 6.32 | 0.001 | 0.83 |
Note: CPM, counts per million; FC, fold change. litters per group (islets pooled from 2–3 female mice per litter).
Figure 9.
RNA Seq data in islets of F1 male mice. (A) F1 Lower BPA male vs. Control male canonical pathways from Ingenuity Pathway Analysis (IPA); IPA parameters: 962 genes; ; inflammatory pathways highlighted by the rectangular box. (B) F1 Upper BPA male vs. Control male canonical Pathways from IPA; IPA parameters: 1,275 genes; ; inflammatory and mitochondrial dysfunction pathways highlighted by the rectangular box. (C) Venn diagram illustrating number of differentially expressed genes when comparing F1 Lower BPA (LowerB), Upper BPA (UpperB), and Control males; parameters: false discovery rate (FDR) , which is equivalent to adjusted (also known as q-value) ; litters (islets pooled from 2–3 males from the same litter) per group.
Discussion
We recently reported that early-life exposure to the endocrine-disrupting chemical BPA in the mouse is associated with sex-specific metabolic health defects across multiple generations (Susiarjo et al. 2015). In the current study, we provide further evidence that BPA has sex-specific effects, such that the islet function of only males is affected. Additionally, we observed dose-specific effects in male offspring of dams fed a lower-dose versus a higher-dose BPA diet. We show here that the insulin secretory defect in male offspring is associated with impaired mitochondrial function in UpperB mice and reduced mass in the LowerB mice (Table 8). It is not uncommon to find dose-specific effects following exposure to endocrine-disrupting chemicals (Vandenberg et al. 2012). Various studies in the mouse have shown that BPA affects metabolism (Alonso-Magdalena et al. 2010), DNA methylation levels (Kim et al. 2014), and behavior (Jašarević et al. 2013) in a dose-specific manner. The dose-specific findings of the current study are consistent with these previous studies. Uniquely, our current study shows that the dose-specific effects of BPA on the pancreas are not limited to the first, but are transmitted to the second generation. These persistent phenotypic changes into at least the second generation are not due to an altered metabolic milieu in the pregnant F1 female, and thus may be transmitted through epigenetic mechanisms or via mitochondria (Susiarjo et al. 2015). Our current findings of persistent changes in DNA methylation at Igf2 and altered mitochondria function in in F1 and F2 offspring suggest that both mechanisms are involved.
Table 8.
Dose-specific effects of bisphenol A on pancreatic islets of male offspring.
| Group | Insulin content | Cytosolic calcium | Mitochondria | Beta cell mass, and islet viability | Gene expression and methylation | Inflammation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KIC ramp | Oroboros | mRNA/protein levels | Histology | Viability assay | mRNA/protein levels | Igf2/Esr1 methylation | Luminex | ELISA | Histology | ||||
| F1 GENERATION | |||||||||||||
| LowerB | insulin content# | NS | NS | NS | Ucp2 UCP2 protein levels NS | mass | Cell death | Igf2, Esr1 ESR1# | Esr1# | IL5# | IL6# MCP1 NS | CD3, F4/80 | |
| UpperB | NS | NS | KIC-stimulated insulin release | Basal and maximal respiration | Ucp2 UCP2 protein levels NS | NS | Akt-phos, Bcl2# | Igf2, Esr1 ESR1 NS | Igf2 | IL5, IL6#, MCP1, G-CSF, IL9# | IL6, NS MCP1 | CD3, F4/80 | |
| F2 GENERATION | |||||||||||||
| LowerB | insulin content# | NS | NS | NS | Ucp2, Ogdh UCP2 protein levels NS | mass | Cell death# | Igf2, Esr1 ESR1# | NS | IL17 | IL6# MCP1 | CD3, F4/80# | |
| UpperB | NS | NS | KIC-stimulated insulin release | Basal and maximal respiration | Ucp2 UCP2 protein levels NS | NS | Akt-phos | Igf2, Esr1 ESR1 NS | Igf2Esr1# | IL4, IL5#, IL6, IL17, MCP1# | IL6, NS MCP1 | CD3, F4/80 | |
Note: Arrows indicate levels are statistically () increased or decreased relative to Controls. LowerB, Lower BPA (); NS, not significant (); UpperB, Upper BPA (). # and .
Mitochondrial impairment, and altered expression levels of key mitochondrial genes such as Ucp2 and Ogdh have been previously reported in the pancreas of first-generation adult male offspring of BPA-exposed rat dams (Wei et al. 2011), in isolated rat islets (Song et al. 2012), and in BPA-exposed INS1 cells (Lin et al. 2013). Importantly, others have shown that increased Ucp2 expression increases efflux of acetyl-CoA–oxidizing substrates such as of the TCA cycle out of the mitochondria (Vozza et al. 2014). It is known that promotes glutamate oxidation to produce (Zielke et al. 1997). Therefore, the increased Ucp2 expression with an efflux of TCA intermediates from the mitochondria as previously shown (Vozza et al. 2014) suggests altered metabolism. Although in the current study we observed increased Ucp2 mRNA expression levels in BPA-exposed groups, we did not find statistically increased UCP2 protein levels. However, our transcriptome analysis revealed differential expression of other genes such as Ucp2 that are also involved in the branched-chain dehydrogenase complex, which catalyzes the metabolism of branched-chain amino acids such as leucine and its derivative, . This is consistent with a reduction in –stimulated insulin secretion in the UpperB group. Therefore, it may be that genes other than Ucp2 impair metabolism, consequently affecting –stimulated insulin secretion.
Reduced –stimulated insulin secretion and mitochondrial dysfunction persisting until the second generation in islets of the UpperB group indicate that the higher dose of BPA is associated with mitochondrial impairment. Mitochondrial impairment is associated with the production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals as seen in different type 2 diabetes models and IUGR rats (Bindokas et al. 2003; Ihara et al. 1999; Simmons et al. 2005). ROS can lead to the activation of , which in turn increases pro-inflammatory cytokine production (Kepp et al. 2011), eventually leading to increased death and dysfunction. Our study demonstrates that UpperB exposure is associated with impaired mitochondrial function and increased pro-inflammatory cytokine production. Whether this is due to oxidative stress remains to be determined. Elevated pro-inflammatory cytokine levels have been shown to induce death (Sauter et al. 2015), which in turn reduces mass. AKT phosphorylation inhibits programed cell death (Reed and Paternostro 1999; Yao and Cooper 1995); in the current study, increased AKT phosphorylation levels and the lack of changes in cell death observed in UpperB islets are consistent with these previous findings. The mechanism underlying this change remains to be determined.
Despite the lack of changes in mitochondrial function in the LowerB group, the first- and second-generation offspring of this group had significantly reduced function as defined by impaired insulin secretion, which suggested that a lower dose of BPA may act via an alternate pathway in , such as a reduction in mass. Impaired insulin secretion is known to be secondary to reduced mass in humans (Butler et al. 2003; Rahier et al. 2008; Yoon et al. 2003) and animals (Bansal et al. 2015; Kjems et al. 2001; Movassat et al. 1997; Simmons et al. 2001). Indeed, we observed reduced mass adjusted for body weight across two generations in adult male offspring of LowerB exposed dams, and these changes were associated with increased cell death. These finding are consistent with previous studies that reported reduced mass at birth in the offspring of rats exposed to BPA/kg body weight per day throughout gestation and lactation (Chang et al. 2016a) and increased cell death in BPA-exposed INS1 cells (Gong et al. 2013; Lin et al. 2013). mass was also reduced and cell death was increased at 6 mo after delivery in female mice that were exposed to BPA during pregnancy (Alonso-Magdalena et al. 2015a). Interestingly, when C57BL/6 mice dams were subcutaneously injected from either day 6 of pregnancy (P6) until birth (PND0), PND0 until weaning (PND21), or P6-PND21 with BPA/kg body weight per day, mass was increased in 3-mo-old male offspring (Liu et al. 2013). Differences in timing, dose, route of exposure, and methods of measurement of mass that were not corrected for body weight may explain the different results in our study compared with those of Liu et al. (2013). In rodents, ( mass has a linear correlation with body weight such that mass increases with body weight (Montanya et al. 2000). Therefore, we corrected mass for body weight in our study, so that any changes due to difference in body weight per se would be accounted for. We chose oral BPA exposure via diet as it has been shown to be effective and a representative model of human exposures because humans are chronically exposed to BPA primarily through ingestion, and our doses are well within the human exposure limits (Susiarjo et al. 2013; Sieli et al. 2011). Importantly, end points measured in this study were from animals generated across multiple cohorts studied in two different animal care facilities with a control group included in each cohort. This reflects robustness of the studied outcomes and cohort consistency in our paradigm.
Our study is the first to show that developmental BPA exposure induces inflammation in the islet in both F1 and F2 generations. Although immune cells are present in the pancreas and play an important role in normal pancreatic development (Criscimanna et al. 2014; Geutskens et al. 2005; Jansen et al. 1993, 1994), several studies have reported that increased cytokine levels, and increased immune cell infiltration in islets, is associated with dysfunction in type 2 diabetes (Böni-Schnetzler et al. 2008; Ehses et al. 2007; Homo-Delarche et al. 2006; Kim et al. 2005; Lin et al. 2012; Maedler et al. 2002; Richardson et al. 2009). Increased levels of pro-inflammatory cytokines such as IL6 and MCP1 reduce insulin secretion in human and mouse islets (Sauter et al. 2015). Inflammatory cytokines also impair mitochondria function in the , which in turn blunts insulin secretion (Gao et al. 2015). Thus, it is likely that BPA-induced islet inflammation plays a key role in the abnormal phenotype observed in F1 and F2 mice.
BPA is an estrogen mimic, and therefore, it is plausible that BPA could alter Esr1 expression. In fact, maternal BPA exposure has been shown to down-regulate Esr1 mRNA and protein expression at P7 and P21 in the hippocampus of male rat offspring (Xu et al. 2014). Two injections of ICI 182,780, a brain-permeable endoplasmic reticulum (ER) antagonist, in rat pups were able to reverse this change, demonstrating that BPA is causal in reducing Esr1 expression (Xu et al. 2014). Consistent with these findings, we observed reduced Esr1 gene expression in islets of both BPA exposure groups, and reduced ESR1 protein levels in lower-dose exposure group. However, because BPA has weak binding affinity for estrogen receptors relative to estrogens, it is likely that BPA mediates its effect on estrogen receptor expression without directly binding to the receptor. Other mechanisms, such as a direct effect on DNA methylation, may instead be responsible for altering Esr1 gene expression as shown in the mouse (Kundakovic et al. 2013) and rat brain (Chang et al. 2016b). We assessed DNA methylation status at 16 CpG sites in exon A and exon C of Esr1 gene in islets, and found minimal differences. It is possible that other gene regulatory mechanisms such as a change in chromatin conformation may be involved. This remains to be determined.
Estrogen receptors are widely distributed in the thymus (Kawashima et al. 1992), bone marrow (Smithson et al. 1998), and spleen (Samy et al. 2003), suggesting that they play a role in regulating normal immune response. In fact, loss of Esr1 leads to perturbed development of the thymus and spleen (Erlandsson et al. 2001) and to altered immune responses (Douin-Echinard et al. 2008; Lambert et al. 2005; Maret et al. 2003). Altered Esr1 expression could therefore influence the effect of developmental exposure to BPA on the pancreatic immune response, leading to the increased inflammation that we observed in the islets of the BPA-exposed animals in this study.
It is remarkable that the changes we reported in Igf2 expression and DNA methylation in the embryo persist in the islet in adulthood and into the next generation. Further, in previous studies, the metabolic phenotypes in the F1 and F2 BPA male offspring were linked to Igf2 overexpression (Susiarjo et al. 2015). Although Igf2 is protective in the adult and increased expression prevents death (Cornu et al. 2009; Hughes et al. 2013), overexpression of Igf2 in the fetus results in marked abnormalities in fetal and adult life (Casellas et al. 2015; Höög et al. 1996; Petrik et al. 1999). These abnormalities may be secondary to increased islet inflammation because Igf2 overexpression has been reported to be associated with increased immune cell infiltration in the rodent pancreas (Casellas et al. 2015). Therefore, it is plausible to suggest that Igf2 in combination with reduced Esr1 mediates the increased inflammatory response that we observed in islets of the BPA-exposed animals.
Finally, ingenuity pathway analysis of the RNAseq data revealed multiple pathways that were altered and could contribute to the abnormal pancreatic phenotype in the offspring of BPA-exposed mice, including sonic hedgehog (Shh) and gap-junction signaling in the LowerB dose group and ER stress in the UpperB dose group. Shh is known to play a critical role in pancreatic development (Thomas et al. 2000), and gap junctions help establish cross-talk within islet cells thereby regulating normal insulin secretion and mass (Bavamian et al. 2007; Klee et al. 2011). ER stress is associated with mitochondrial dysfunction (Rocha et al. 2016). Although we did not further interrogate these pathways in our study, they remain important targets for future research.
Conclusion
The current study elucidates the underlying mechanisms responsible for impaired insulin secretion in mice exposed to relevant human exposure levels of BPA during development. The persistent changes in insulin secretion, mitochondria function, and mass across multiple generations are consistent with an epigenetically mediated mechanism. These findings have public health ramifications and identify targets of BPA that could be used to develop potential therapeutic strategies to prevent environment-induced diabetes.
Supplemental Material
Acknowledgments
The authors would like to acknowledge D. Condon, Z. (Paul) Wang, T. Kim, and M. Li for their bioinformatics and biostatistics input. We are also grateful for the services of the University of Pennsylvania Next Generation Sequencing Core and the Radioimmunoassay and Biomarkers Core (P30 DK19525), and the Children’s Hospital of Philadelphia Pathology Core.
This work is supported by the National Institute of Environmental Health Sciences/National Institutes of Health (ES023284 and ES013508 [M.S.B., R.A.S.]); March of Dimes (M.S.B.); the Perelman School of Medicine Center of Excellence in Environmental Toxicology (CEET-ES-013508-05 [R.A.S.], RO1DK098517 [C.L.], and T32 ES019851 [F.X.]), the Bioenergetics Core of the Children’s Hospital of Philadelphia and Development Disabilities Research Center (IDDRC; U54-HD086984), and the Islet Cell Biology Core of the Penn Diabetes Institute for Diabetes, Obesity & Metabolism (P30DK19525).
References
- Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B. 2015. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes 7(2):240–249, PMID: 24720399, 10.1111/1753-0407.12159. [DOI] [PubMed] [Google Scholar]
- Ahmadkhaniha R, Mansouri M, Yunesian M, Omidfar K, Jeddi MZ, Larijani B, et al. 2014. Association of urinary bisphenol A concentration with type-2 diabetes mellitus. J Environ Health Sci Eng 12(1):64, PMID: 24625016, 10.1186/2052-336X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, García-Arévalo M, Quesada I, Nadal Á. 2015a. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 156:1659–1670, PMID: 25830705, 10.1210/en.2014-1952. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. 2006. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 114(1):106–112, PMID: 16393666, 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal Á. 2015b. Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose Response 13(2):1559325815590395, PMID: 26676280, 10.1177/1559325815590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, et al. 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118(9):1243–1250, PMID: 20488778, 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. 2013. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 36:1033–1046, PMID: 23468086, 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, et al. 2013. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol 42:256–268, PMID: 23892310, 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinozzi PA, Ishihara H, Newgard CB, Wollheim CB. 2002. Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic beta cell: a survey of four fuel secretagogues. J Biol Chem 277(14):11746–11755, PMID: 11821387, 10.1074/jbc.M108462200. [DOI] [PubMed] [Google Scholar]
- Bansal A, Bloomfield FH, Connor KL, Dragunow M, Thorstensen EB, Oliver MH, et al. 2015. Glucocorticoid-induced preterm birth and neonatal hyperglycemia alter ovine β-cell development. Endocrinology 156(10):3763–3776, PMID: 26204462, 10.1210/en.2015-1095. [DOI] [PubMed] [Google Scholar]
- Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, et al. 2012. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One 7(3):e33814, PMID: 22470480, 10.31371/journal.pone.0033814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavamian S, Klee P, Britan A, Populaire C, Caille D, Cancela J, et al. 2007. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes Metab 9(suppl 2):118–132, PMID: 17919186, 10.1111/j.1463-1326.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. 2003. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 278(11):9796–9801, PMID: 12514170, 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, et al. 2008. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J Clin Endocrinol Metab 93(10):4065–4074, PMID: 18664535, 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L, Rooman I. 2005. Regulation of pancreatic beta-cell mass. Physiol Rev 85(4):1255–1270, PMID: 16183912, 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. 2003. Beta-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52(1):102–110, PMID: 12502499. [DOI] [PubMed] [Google Scholar]
- Casellas A, Mallol C, Salavert A, Jimenez V, Garcia M, Agudo J, et al. 2015. Insulin-like growth factor 2 overexpression induces β-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem 290(27):16772–16785, PMID: 25971976, 10.1074/jbc.M115.642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM. 2001. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 47(4):695–702, PMID: 11502077. [PubMed] [Google Scholar]
- Chang H, Wang M, Xia W, Chen T, Huo W, Mao Z, et al. 2016b. Perinatal exposure to low-dose bisphenol A disrupts learning/memory and DNA methylation of estrogen receptor alpha in the hippocampus. Toxicol Res 5:828–835, 10.1039/C5TX00449G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Wang D, Xia W, Pan X, Huo W, Xu S, et al. 2016a. Epigenetic disruption and glucose homeostasis changes following low-dose maternal bisphenol A exposure. Toxicol Res 5:1400–1409, 10.1039/C6TX00047A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins SP, Borish L, Steinke JW. 2010. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol 125(2 suppl 2):S53–S72, PMID: 19932918, 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. 2009. Glucagon-like peptide-1 protects β-cells against apoptosis by increasing the activity of an Igf-2/Igf-1 receptor autocrine loop. Diabetes 58(8):1816–1825, PMID: 19401425, 10.2337/db09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F. 2014. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and β-cell regeneration in mice. Gastroenterology 147(5):1106–1118.e11, PMID: 25128759, 10.1053/j.gastro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, Chambon P, et al. 2008. Estrogen receptor α, but not β, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol 180(6):3661–3669, PMID: 18322171. [DOI] [PubMed] [Google Scholar]
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, et al. 2007. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56(9):2356–2370, PMID: 17579207, 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. 2001. Role of oestrogen receptors α and β in immune organ development and in oestrogen-mediated effects on thymus. Immunology 103(1):17–25, PMID: 11380688, 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner-Weir S. 1995. Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44(3):249–256, PMID: 7883109, 10.2337/diabetes.44.3.249. [DOI] [PubMed] [Google Scholar]
- Gao J, Sang M, Zhang X, Zheng T, Pan J, Dai M, et al. 2015. Miro1-mediated mitochondrial dysfunction under high nutrient stress is linked to NOD-like receptor 3 (NLRP3)-dependent inflammatory responses in rat pancreatic beta cells. Free Radic Biol Med 89:322–332, PMID: 26427885, 10.1016/j.freeradbiomed.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. 2005. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol 78(4):845–852, PMID: 16037409, 10.1189/jlb.1004624. [DOI] [PubMed] [Google Scholar]
- Gong H, Zhang X, Cheng B, Sun Y, Li C, Li T, et al. 2013. Bisphenol A accelerates toxic amyloid formation of human islet amyloid polypeptide: a possible link between bisphenol A exposure and type 2 diabetes. PLoS One 8(1):e54198, PMID: 23372685, 10.1371/journal.pone.0054198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Barroso MM, Giurgea I, Bouillaud F, Anedda A, Bellanne-Chantelot C, Hubert L, et al. 2008. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One 3(12):e3850, PMID: 19065272, 10.1371/journal.pone.0003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstrom C, Swenne I, Andersson A. 1988. Islet replication and diabetes. In: The Pathology of the Endocrine Pancreas in Diabetes. Lefebvre PJ, Pipeleers DG, eds. Berlin, Germany:Springer-Verlag, 141–170. [Google Scholar]
- Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, et al. 2006. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes 55(6):1625–1633, PMID: 16731824, 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- Höög A, Sandberg-Nordqvist AC, Abdel-Halim SM, Carlsson-Skwirut C, Guenifi A, Tally M, et al. 1996. Increased amounts of a high molecular weight insulin-like growth factor II (IGF-II) peptide and IGF-II messenger ribonucleic acid in pancreatic islets of diabetic Goto-Kakizaki rats. Endocrinology 137(6):2415–2423, PMID: 8641194, 10.1210/endo.137.6.8641194. [DOI] [PubMed] [Google Scholar]
- Hughes A, Mohanasundaram D, Kireta S, Jessup CF, Drogemuller CJ, Coates PT. 2013. Insulin-like growth factor-II (IGF-II) prevents proinflammatory cytokine-induced apoptosis and significantly improves islet survival after transplantation. Transplantation 95(5):671–678, PMID: 23364485, 10.1097/TP.0b013e31827fa453. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. 1999. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 48(4):927–932, PMID: 10102716, 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- Jaeckle Santos LJ, Li C, Doulias PT, Ischiropoulos H, Worthen GS, Simmons RA. 2014. Neutralizing Th2 inflammation in neonatal islets prevents β-cell failure in adult IUGR rats. Diabetes 63(5):1672–1684, PMID: 24408314, 10.2337/db13-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. 1994. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43(5):667–675, PMID: 8168644, 10.2337/diabetes.43.5.667. [DOI] [PubMed] [Google Scholar]
- Jansen A, Voorbij HA, Jeucken PH, Bruining GJ, Hooijkaas H, Drexhage HA. 1993. An immunohistochemical study on organized lymphoid cell infiltrates in fetal and neonatal pancreases. A comparison with similar infiltrates found in the pancreas of a diabetic infant. Autoimmunity 15(1):31–38, PMID: 8218828, 10.3109/08916939309004836. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Sieli PT, Twellman EE, Welsh TH Jr, Schachtman TR, Roberts RM, et al. 2011. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A 108(28):11715–11720, PMID: 21709224, 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Williams SA, Vandas GM, Ellersieck MR, Liao C, Kannan K, et al. 2013. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm Behav 63(1):180–189, PMID: 23051835, 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima I, Seiki K, Sakabe K, Ihara S, Akatsuka A, Katsumata Y. 1992. Localization of estrogen receptors and estrogen receptor-mRNA in female mouse thymus. Thymus 20(2):115–121, PMID: 1519316. [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Kroemer G. 2011. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol 12(3):199–200, PMID: 21321591, 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sartor MA, Rozek LS, Faulk C, Anderson OS, Jones TR, et al. 2014. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics 15(1):30, PMID: 24433282, 10.1186/1471-2164-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Lee JW, Gao B, Jung MH. 2005. Synergistic activation of JNK/SAPK induced by TNF-α and IFN-γ: apoptosis of pancreatic β-cells via the p53 and ROS pathway. Cell Signal 17(12):1516–1532, PMID: 15908180, 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kimura A, Kishimoto T. 2010. Il-6: Regulator of Treg/Th17 balance. Eur J Immunol 40(7):1830–1835, PMID: 20583029, 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, et al. 2001. Decrease in β-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes 50(9):2001–2012, PMID: 11522665. [DOI] [PubMed] [Google Scholar]
- Klee P, Lamprianou S, Charollais A, Caille D, Sarro R, Cederroth M, et al. 2011. Connexin implication in the control of the murine beta-cell mass. Pediatr Res 70(2):142–147, PMID: 21527868, 10.1203/PDR.0b013e318220f106. [DOI] [PubMed] [Google Scholar]
- Kourilsky P, Truffa-Bachi P. 2001. Cytokine fields and the polarization of the immune response. Trends Immunol 22(9):502–509, PMID: 11525941, 10.1016/S1471-4906(01)02012-9. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. 2013. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A 110(24):9956–9961, PMID: 23716699, 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. 2005. Estrogen receptor alpha (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17β-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol 175(9):5716–5723, PMID: 16237062. [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300(11):1303–1310, PMID: 18799442, 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Palladino A, Narayan S, Russell LK, Sayed S, et al. 2010. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem 285(41):31806–31818, PMID: 20670938, 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. 2013. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J Biol Chem 288(6):3938–3951, PMID: 23266825, 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chang H, Xia W, Mao Z, Li Y, Xu S. 2014. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol Lett 228(3):192–199, PMID: 24793715, 10.1016/j.toxlet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Lin CY, Ni CC, Yin MC, Lii CK. 2012. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine 59(1):65–71, PMID: 22579112, 10.1016/j.cyto.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, et al. 2013. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 4:e460, PMID: 23328667, 10.1038/cddis.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, et al. 2013. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS One 8(5):e64143, PMID: 23675523, 10.1371/journal.pone.0064143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay H, Patterson ZR, Khazall R, Patel S, Tsirlin D, Abizaid A. 2013. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology 154(4):1465–1475, PMID: 23493373, 10.1210/en.2012-2044. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. 2002. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110(6):851–860, PMID: 12235117, 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, et al. 2003. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol 33(2):512–521, PMID: 12645950, 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. 2006. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3(11):e442, PMID: 17132052, 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Brown LM, Rayalam S, Della-Fera MA, Baile CA. 2012. Estrogens, inflammation and obesity: an overview. Front Biol 7(1):40–47, PMID: 26811110, 10.1007/s11515-011-1174-y.26811110 [DOI] [Google Scholar]
- Montanya E, Nacher V, Biarnés M, Soler J. 2000. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes 49(8):1341–1346, PMID: 10923635, 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- Movassat J, Saulnier C, Serradas P, Portha B. 1997. Impaired development of pancreatic beta-cell mass is a primary event during the progression to diabetes in the GK rat. Diabetologia 40(8):916–925, PMID: 9267986, 10.1007/s001250050768. [DOI] [PubMed] [Google Scholar]
- Nahar MS, Soliman AS, Colacino JA, Calafat AM, Battige K, Hablas A, et al. 2012. Urinary bisphenol A concentrations in girls from rural and urban Egypt: a pilot study. Environ Health 11:20, PMID: 22472083, 10.1186/1476-069X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. 2008. Effects of endocrine disruptors on obesity. Int J Androl 31(2):201–208, PMID: 18315718, 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Ortsäter H, Liss P, Åkerman KE, Bergsten P. 2002. Contribution of glycolytic and mitochondrial pathways in glucose-induced changes in islet respiration and insulin secretion. Pflugers Arch 444(4):506–512, PMID: 12136270, 10.1007/s00424-002-0842-9. [DOI] [PubMed] [Google Scholar]
- Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. 2009. G-protein-coupled-receptor kinases mediate TNFα-induced NFκB signalling via direct interaction with and phosphorylation of IκBα. Biochem J 425(1):169–178, PMID: 19796012, 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik J, Pell JM, Arany E, McDonald TJ, Dean WL, Reik W, et al. 1999. Overexpression of insulin-like growth factor-II in transgenic mice is associated with pancreatic islet cell hyperplasia. Endocrinology 140(5):2353–2363, PMID: 10218989, 10.1210/endo.140.5.6732. [DOI] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. 2008. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 10:32–42, PMID: 18834431, 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Reed JC, Paternostro G. 1999. Postmitochondrial regulation of apoptosis during heart failure. Proc Natl Acad Sci U S A 96(14):7614–7616, PMID: 10393865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. 2009. Islet-associated macrophages in type 2 diabetes. Diabetologia 52(8):1686–1688, PMID: 19504085, 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- Rocha M, Diaz-Morales N, Rovira-Llopis S, Escribano-Lopez I, Bañuls C, Hernandez-Mijares A, et al. 2016. Mitochondrial dysfunction and endoplasmic reticulum stress in diabetes. Curr Pharm Des 22(18):2640–2649, PMID: 26861650, 10.2174/1381612822666160209152033. [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, Teppala S, Shankar A. 2013. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol 50(4):625–631, PMID: 23636267, 10.1007/s00592-013-0472-z. [DOI] [PubMed] [Google Scholar]
- Samy TS, Zheng R, Matsutani T, Rue LW III, Bland KI, Chaudry IH. 2003. Mechanism for normal splenic T lymphocyte functions in proestrus females after trauma: enhanced local synthesis of 17β-estradiol. Am J Physiol Cell Physiol 285(1):C139–C149, PMID: 12660147, 10.1152/ajpcell.00058.2003. [DOI] [PubMed] [Google Scholar]
- Sauter NS, Thienel C, Plutino Y, Kampe K, Dror E, Traub S, et al. 2015. Angiotensin II induces interleukin-1β-mediated islet inflammation and β-cell dysfunction independently of vasoconstrictive effects. Diabetes 64(4):1273–1283, PMID: 25352639, 10.2337/db14-1282. [DOI] [PubMed] [Google Scholar]
- Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. 1997. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138(4):1736–1741, PMID: 9075738, 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- Scaglia L, Smith FE, Bonner-Weir S. 1995. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology 136(12):5461–5468, PMID: 7588296, 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- Shankar A, Teppala S. 2011. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 96(12):3822–3826, PMID: 21956417, 10.1210/jc.2011-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieli PT, Jašarević E, Warzak DA, Mao J, Ellersieck MR, Liao C, et al. 2011. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect 119(9):1260–1265, PMID: 21642047, 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RA, Suponitsky-Kroyter I, Selak MA. 2005. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to β-cell failure. J Biol Chem 280(31):28785–28791, PMID: 15946949, 10.1074/jbc.M505695200. [DOI] [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. 2001. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50(10):2279–2286, PMID: 11574409, 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. 1998. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol 161(1):27–34, PMID: 9647203. [PubMed] [Google Scholar]
- Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, et al. 2012. Low-level phenolic estrogen pollutants impair islet morphology and β-cell function in isolated rat islets. J Endocrinol 215(2):303–311, PMID: 22946080, 10.1530/JOE-12-0219. [DOI] [PubMed] [Google Scholar]
- Soriano S, Alonso-Magdalena P, García-Arévalo M, Novials A, Muhammed SJ, Salehi A, et al. 2012. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One 7(2):e31109, PMID: 22347437, 10.1371/journal.pone.0031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, et al. 2009. Rapid regulation of K(ATP) channel activity by 17β-estradiol in pancreatic β-cells involves the estrogen receptor β and the atrial natriuretic peptide receptor. Mol Endocrinol 23(12):1973–1982, PMID: 19855088, 10.1210/me.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D, Fusco A, Ciccarelli M, Rungi A, Anastasio A, Carillo A, et al. 2013. Mitochondrial G protein coupled receptor kinase 2 regulates proinflammatory responses in macrophages. FEBS Lett 587(21):3487–3494, PMID: 24036448, 10.1016/j.febslet.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. 2009. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect 117(5):784–789, PMID: 19479022, 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, et al. 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 122(6):616–623, PMID: 24633239, 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. 2013. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet 9(4):e1003401, PMID: 23593014, 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, et al. 2015. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 156(6):2049–2058, PMID: 25807043, 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenne I. 1983. Effects of aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes 32(1):14–19, PMID: 6336699, 10.2337/diab.32.1.14. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. 2000. Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 108(10):931–935, PMID: 11049811, 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MK, Rastalsky N, Lee JH, Habener JF. 2000. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49(12):2039–2047, PMID: 11118005, 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, et al. 2011. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest 121(8):3331–3342, PMID: 21747171, 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MD, Nedjai B, Hurst T, Pennington DJ. 2014. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 1843(11):2563–2582, PMID: 24892271, 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, et al. 2014. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology 321:40–52, PMID: 24726836, 10.1016/j.tox.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, et al. 2014. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A 111(3):960–965, PMID: 24395786, 10.1073/pnas.1317400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. 2011. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 152(8):3049–3061, PMID: 21586551, 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Xu XB, He Y, Song C, Ke X, Fan SJ, Peng WJ, et al. 2014. Bisphenol A regulates the estrogen receptor alpha signaling in developing hippocampus of male rats through estrogen receptor. Hippocampus 24(12):1570–1580, PMID: 25074486, 10.1002/hipo.22336. [DOI] [PubMed] [Google Scholar]
- Yao R, Cooper GM. 1995. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267(5206):2003–2006, PMID: 7701324, 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. 2003. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88(5):2300–2308, PMID: 12727989, 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- Yoon K, Kwack SJ, Kim HS, Lee BM. 2014. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev 17(3):127–174, PMID: 24749480, 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- Yuchi Y, Cai Y, Legein B, De Groef S, Leuckx G, Coppens V, et al. 2015. Estrogen receptor α regulates β-cell formation during pancreas development and following injury. Diabetes 64(9):3218–3228, PMID: 26015547, 10.2337/db14-1798. [DOI] [PubMed] [Google Scholar]
- Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, et al. 2003. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111(3):309–319, PMID: 12611660, 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler A, Shargill NS, Chan TM. 1991. Peripheral insulin insensitivity in the hyperglycemic athymic nude mouse: similarity to noninsulin-dependent diabetes mellitus. Proc Soc Exp Biol Med 196(4):457–460, PMID: 2008443, 10.3181/00379727-196-43216. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Alomirah H, Cho HS, Li YF, Liao C, Minh TB, et al. 2011. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol 45(16):7044–7050, PMID: 21732633, 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tsukikawa M, Peng M, Polyak E, Nakamaru-Ogiso E, Ostrovsky J, et al. 2013. Primary respiratory chain disease causes tissue-specific dysregulation of the global transcriptome and nutrient-sensing signaling network. PLoS One 8(7):e69282, PMID: 23894440, 10.1371/journal.pone.0069282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Huang Y, Baab PJ, Collins RM Jr, Zielke CL, Tildon JT. 1997. Effect of α-ketoisocaproate and leucine on the in vivo oxidation of glutamate and glutamine in the rat brain. Neurochem Res 22(9):1159–1164, PMID: 9251107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figures 1A and 1B are line graphs with standard error of the mean plotting KIC ramp. The figures plot insulin release in nanograms per 200 islets per minute (y-axis) across duration of perifusion in minutes (x-axis) in the islets of F1 male mice (0 to 15 millimolar) [Control (n equals 4), Lower BPA (n equals 3), and Upper BPA (n equals 3)] and F2 male mice [Control (n equals 3), Lower BPA (n equals 3), and Upper BPA (n equals 3)], respectively. Figures 1C and 1D are bar graphs with standard error of the mean plotting maximal KIC-stimulated insulin release (y-axis) in islets of F1 mice [Control (n equals 4), Lower BPA (n equals 3, p equals 0.62), and Upper BPA (n equals 3, p equals 0.08)] and F2 mice [Control (n equals 3), Lower BPA (n equals 3, p equals 0.47), and Upper BPA (n equals 3, p equals 0.06)], respectively. p-Values are relative to Control](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/6976b434741c/EHP1674_f1.jpg)
![Figures 2A and 2C are line graphs with standard error of the mean plotting glucose ramp. The figures plot insulin release in nanograms per 200 islets per minute (y-axis) across duration of perifusion in minutes (x-axis) in islets of F1 and F2 female mice (0 to 25 millimolar), respectively. Figures 2B and 2D are line graphs with standard error of the mean plotting KIC ramp. The figures plot same data in the islets of F1 and F2 female mice (0 to 15 millimolar), respectively. Figures 2E, 2F, 2G, and 2H are bar graphs with standard error of the mean plotting maximal KIC-stimulated insulin release (y-axis) in islets of F1 female mice (glucose ramp) [Control (n equals 3), Lower BPA (n equals 3, p equals 0.29), and Upper BPA (n equals 3, p equals 0.48)], F1 female mice (KIC ramp) [Control (n equals 3), Lower BPA (n equals 3, p equals 0.74), and Upper BPA (n equals 3, p equals 0.94)], F2 female mice (glucose ramp) [Control (n equals 3), Lower BPA (n equals 3, p equals 0.99), and Upper BPA (n equals 3, p equals 0.99)], and F2 female mice (KIC ramp) [Control (n equals 3), Lower BPA (n equals 3, p equals 0.57), and Upper BPA (n equals 3, p equals 0.59)], respectively. p-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/10c29683b93b/EHP1674_f2.jpg)
![Figure 3A plots basal respiration in F1 male mice [Control (n equals 6), Lower BPA (n equals 5, p equals 0.17), and Upper BPA (n equals 6, p equals 0.005)]. Figure 3B plots maximal respiration in F1 male mice [Control (n equals 6), Lower BPA (n equals 5, p equals 0.71), and Upper BPA (n equals 6, p equals 0.05)]. Figure 3C plots basal respiration in F2 male mice [Control (n equals 4), Lower BPA (n equals 4, p equals 0.48), and Upper BPA (n equals 4, p equals 0.04)]. Figure 3D plots maximal respiration in F2 male mice [Control (n equals 4), Lower BPA (n equals 4, p equals 0.70), and Upper BPA (n equals 4, p equals 0.03)]. The consumption of islet mitochondrial oxygen per DNA concentration is measured in picomole per seconds times million cells per nanograms per microliter (y-axis) for all the four graphs. p-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/e57567d80c9d/EHP1674_f3.jpg)
![Figures 4A, 4B, 4C, 4D, 4E, and 4F, are photomicrographs. Figure 4G plots beta cell mass per body weight in F1 male mice [Control (n equals 6),] Lower BPA (n equals 6, p equals 0.03), and Upper BPA (n equals 6, p equals 0.99)]. Figure 4H plots beta cell mass per body weight in F2 male mice [Control (n equals 6),] Lower BPA (n equals 4, p equals 0.04), and Upper BPA (n equals 6, p equals 0.21). Beta cell mass per body weight is measured in gram per gram (y-axis). p-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/58d59425c79f/EHP1674_f4.jpg)
![Figures 5A, 5B, and 5C, respectively, plot caspase 3 activity per total protein in RFU per microgram, BCL2 levels per total protein in picograms per microgram, and the ratio of phosphorylated AKT and total AKT per total protein in ratio per microgram (y-axis), for the F1 male caspase 3 activity [Control (n equals 5), Lower BPA (n equals 5, p equals 0.04), and Upper BPA (n equals 4, p equals 0.31)], F1 male BCL2 [Control (n equals 5), Lower BPA (n equals 5, p equals 0.57), and Upper BPA (n equals 4, p equals 0.06)], and F1 male AKT phos [Control (n equals 5), Lower BPA (n equals 5, p equals 0.18), and Upper BPA (n equals 4, p equals 0.05)]. Figures 5D, 5E, and 5F, respectively, plot caspase 3 activity per total protein in RFU per microgram, BCL2 levels in total protein per picograms per microgram, and the ratio of phosphorylated AKT and total AKT per total protein in ratio per microgram (y-axis), for the F2 male caspase 3 activity [Control (n equals 5), Lower BPA (n equals 5, p equals 0.08), and Upper BPA (n equals 5, p equals 0.98)], F2 male BCL2 [Control (n equals 5), Lower BPA (n equals 5, p equals 0.89), and Upper BPA (n equals 5, p equals 0.68)], and F2 male AKT phos [Control (n equals 5), Lower BPA (n equals 5, p equals 0.32), and Upper BPA (n equals 5, p equals 0.01)]. p-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/67baf3b8e23c/EHP1674_f5.jpg)
![Figures 6A and 6C plot IL6 per total protein in picograms per microgram (y-axis) for the IL6 in F1 male mice: [Control (n equals 4), Lower BPA (n equals 3, p equals 0.09), and Upper BPA (n equals 3, p equals 0.10)] and IL6 in F2 male mice [Control (n equals 4), Lower BPA (n equals 3, p equals 0.08), and Upper BPA (n equals 3, p equals 0.11)]. Figures 6B and 6D plot MCP1 per total protein in pictograms per microgram for MCP1 in F1 male mice (Figure 6B) [Control (n equals 4), Lower BPA (n equals 3, p equals 0.16), and Upper BPA (n equals 3, p equals 0.02)] and MCP1 in F2 male mice (Figure 6D) [Control (n equals 4), Lower BPA (n equals 3, p equals 0.001), and Upper BPA (n equals 3, p equals 0.001)]. p-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/f36771f53dc5/EHP1674_f6.jpg)
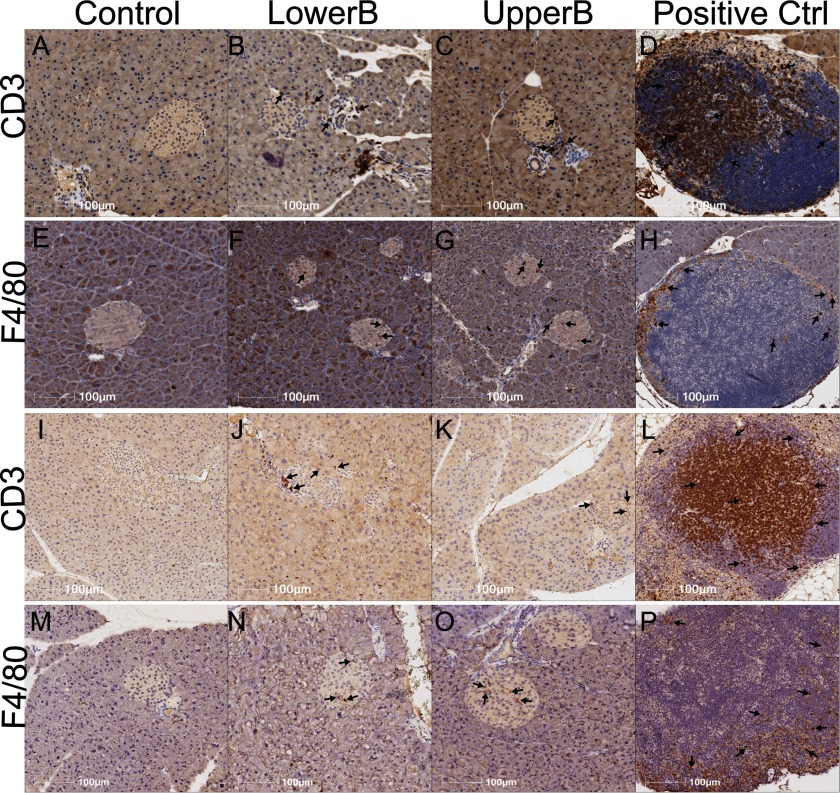
![Figures 8A to 8D are bar graphs with standard error of the mean. Figures 8A and 8B plot percentage of CpG methylation in, respectively, Igf2 DMR1 F1 male islets and Esr1 Exon A F1 male islets (y-axis) [Control (n equals 5), Lower BPA (n equals 6), and Upper BPA (n equals 6) for both]. Figures 8C and 8D plot percentage of CpG methylation in, respectively, Igf2 DMR1 F2 male islets and Esr1 Exon A F2 male islets (y-axis) [Control (n equals 5), Lower BPA (n equals 5), and Upper BPA (n equals 5) for both. Double stars are p-values less than 0.05, while single star is p-value greater than 0.05, but less than 0.09. P-Values are relative to Control.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/25ce/5915189/552f0f22e524/EHP1674_f8.jpg)