Abstract
Background:
Maternal folic acid (FA) protects against developmental toxicity from certain environmental chemicals.
Objective:
We examined combined exposures to maternal FA and pesticides in relation to autism spectrum disorder (ASD).
Methods:
Participants were California children born from 2000–2007 who were enrolled in the Childhood Autism Risks from Genetics and the Environment (CHARGE) case–control study at age 2–5 y, were clinically confirmed to have ASD () or typical development (), and had information on maternal supplemental FA and pesticide exposures. Maternal supplemental FA and household pesticide product use were retrospectively collected in telephone interviews from 2003–2011. High vs. low daily FA intake was dichotomized at (median). Mothers’ addresses were linked to a statewide database of commercial applications to estimate agricultural pesticide exposure.
Results:
High FA intake () during the first pregnancy month and no known pesticide exposure was the reference group for all analyses. Compared with this group, ASD was increased in association with and any indoor pesticide exposure {adjusted odds ratio [95% confidence interval (CI): 1.3, 4.7]} compared with low FA [ (95% CI: 0.7, 2.2)] or indoor pesticides [ (95% CI: 1.1, 2.8)] alone. ORs for the combination of low FA and regular pregnancy exposure () to pet pesticides or to outdoor sprays and foggers were 3.9 (95% CI: 1.4, 11.5) and 4.1 (95% CI: 1.7, 10.1), respectively. ORs for low maternal FA and agricultural pesticide exposure 3 mo before or after conception were 2.2 (95% CI: 0.7, 6.5) for chlorpyrifos, 2.3 (95% CI: 0.98, 5.3) for organophosphates, 2.1 (95% CI: 0.9, 4.8) for pyrethroids, and 1.5 (95% CI: 0.5, 4.8) for carbamates. Except for carbamates, these ORs were approximately two times greater than those for either exposure alone or for the expected ORs for combined exposures under multiplicative or additive models.
Conclusions:
In this study population, associations between pesticide exposures and ASD were attenuated among those with high versus low FA intake during the first month of pregnancy. Confirmatory and mechanistic studies are needed. https://doi.org/10.1289/EHP604
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social reciprocity and communication, and repetitive behaviors or restricted interests. ASD prevalence in the United States has increased over the past decade and is currently estimated to affect one in 68 children (Christensen 2016). Several epidemiologic studies have reported a reduced likelihood of ASD and autistic traits in children whose mothers took supplements containing folic acid (FA) near the time of conception (Braun et al. 2014a; Schmidt et al. 2011, 2012; Steenweg-de Graaff et al. 2015; Suren et al. 2013), yet not all studies have observed this association (Virk et al. 2016). Our previous work suggested that only genetically susceptible individuals (mothers and children with less efficient folate-dependent one-carbon metabolism genes) experienced a reduced risk for ASD associated with maternal FA intake (Schmidt et al. 2011, 2012). Under the paradigm that autism etiology is multifactorial, we hypothesized that there are environmentally susceptible individuals that may experience an enhanced benefit from reduced ASD risk in association with maternal periconceptional FA intake; that is to say, that nutrient status can modify risks associated with other environmental agents.
Pesticides are neurotoxic by design (Rosas and Eskenazi 2008), and associations have been reported between ASD diagnoses or symptoms and organochlorine, organophosphate, and pyrethroid pesticide exposures during pregnancy (Braun et al. 2014b; Eskenazi et al. 2007; Keil et al. 2014; McCanlies et al. 2012; Roberts et al. 2007; Roberts and English 2013; Shelton et al. 2014). In animal studies, FA has been shown to protect against effects resulting from developmental exposure to a variety of environmental chemicals, including effects of methomyl insecticide on reproductive outcomes in male rats (Shalaby et al. 2010) and effects of bisphenol A (BPA) on DNA methylation in mice (Dolinoy et al. 2007). To our knowledge, no previous study has examined whether associations between pesticides and neurodevelopmental outcomes in children are modified by maternal FA intake. The goal of the present study was to examine associations between ASD and combined exposures of maternal FA intake and pesticides, with the hypothesis that children with combined exposure to pesticides and low maternal periconceptional FA would have a greater risk of ASD than children with developmental exposure to pesticides and high maternal periconceptional FA or children with low FA and no pesticide exposure.
Methods
Study Design and Population
Interview data and biological specimens for this ongoing study were obtained from participants in the ongoing Childhood Autism Risks from Genetics and the Environment (CHARGE) population-based case–control study who were enrolled as described previously (Hertz-Picciotto et al. 2006). Eligible children include those between 2 and 5 y old, born in California, living with at least one biologic parent who speaks English or Spanish, and residing in the catchment areas of a specified list of California Regional Centers that coordinate services for persons with developmental disabilities. Children with autism are identified through the California Regional Center System, and general-population controls are identified from state birth files and are frequency matched to the expected age, sex and catchment area distribution of the autism cases. Children with confirmed diagnoses were included in the present analyses if their mothers completed the original exposure questionnaire before November 2011, when revisions affecting diet and supplement data collection were implemented. Owing to low enrollment of controls in the beginning of the study (from 1997 until 1999) and only three controls (no cases) born in 2008 who completed the original questionnaire, only children born between 2000 and 2007 were included in analyses. The CHARGE Study protocol was approved by institutional review boards at the University of California, Davis, and the University of California, Los Angeles, and by the State of California Committee for the Protection of Human Subjects. Written informed consent was obtained before participation.
Diagnostic Classification
All children were assessed for cognitive function using the Mullen Scales of Early Learning (MSEL) (Mullen 1995) and for adaptive function using the Vineland Adaptive Behavior Scales (VABS) (Sparrow et al. 1984). The children of families recruited from the general population were screened for evidence of ASD using the Social Communication Questionnaire (SCQ). If they scored , they were evaluated for ASD; if diagnosed, they were included as cases. Children sampled from the general population were defined as typically developing (TD) controls if they received a score on the SCQ and scored in the normal range on the MSEL and the VABS, thereby showing no evidence of other types of cognitive or adaptive delays.
Diagnoses of ASD were confirmed by study personnel using the Autism Diagnostic Interview, Revised (ADI®-R) (Lord et al. 1994, 1997) and the Autism Diagnostic Observation Schedule–Generic (ADOS-G) (Lord et al. 2000, 2003). ASD was defined by the ASD2 criteria of Risi et al. (2006) as meeting criteria a) on Social and Communication domains of the ADI®-R before 36 mo; b) on Social and within 2 points of Communication domain criteria on the ADI®-R; c) on Communication and within 2 points on the Social domain criteria on the ADI®-R; or d) within 1 point on both Social and Communication domains on the ADI®-R before 36 mo, and above the cutoff for ASD on the ADOS (Risi et al. 2006).
Exposure Measurement
Exposures in the CHARGE study were obtained through telephone interviews for the period 3 mo before conception until the time of the interview (when the child was 2–5 y old). This study focuses on exposures during the index period, defined as the 3 mo before conception, and during pregnancy. The date of conception was calculated by subtracting gestational age (reported by mothers) from the child’s date of birth.
Maternal FA intake.
Maternal intake of FA and other nutrients was determined using data collected through telephone interviews on intake of multivitamins, prenatal vitamins, nutrient-specific vitamins, cereals, and other fortified foods or supplements (i.e., breakfast shakes and protein bars), for each month of the index period as described previously (Schmidt et al. 2012, 2014). Data included whether each item was consumed, and if so, the brand, dose, frequency, and months consumed. From this information, we calculated a value of each nutrient for each product and summed these into a total average value for each month for each woman. Nutrient amounts assigned to products were as reported by the manufacturer, or if this was not available, a standard amount was assigned based on the amount most commonly found in similar products. Total supplemental intake was quantified for the following nutrients: FA, vitamin B12, vitamin B6, vitamin D (ergocalciferol or cholecalciferol), calcium, iron, vitamin A (beta-carotene, retinol), vitamin E, and vitamin C. Total intake of choline, betaine, and zinc was quantified from sources with the information available. Total average FA intake (from all supplements and fortified sources) in the first month of pregnancy was the primary variable used for all analyses below given that we previously found that this month was most strongly associated with reduced ASD (Schmidt et al. 2012). Intake of vitamins B6 and B12 in the first month was also explored for interaction with pesticide exposures and confounding effects; the other nutrients were examined as potential confounders. Supplemental nutrient intake was quantified for all participants with interviews through November 2011, when the CHARGE questionnaire was modified.
Household pesticide exposure.
Regarding the 3 mo before pregnancy with the index child until the time of the interview, the CHARGE parental telephone interview asked, “Did you or anyone in your household use…?” Items included flea or tick soaps or shampoos on pets; sprays, dusts, powders, or skin applications for fleas or ticks on pets; professional pest control or extermination; ant-, fly-, or cockroach-control products; and indoor foggers. Further questions addressed product type (e.g., spray, bait); brand name; whether the application was indoors, outdoors, or on a pet; and use of professional pest control services. We also obtained information on the timing of pesticide use and combined product types to assign exposure by time period; however, the numbers of exposed were too small to examine combined exposure associations by specific timing in this study, and exposure during the whole pregnancy period was used. Use of pesticide-containing poisoned bait containers was not included because they have a small surface area of pesticide, which would result in low volatilization and thus limited exposure. Similarly, our primary analyses of indoor pesticides excluded use of flea and tick pet collars because of their limited release of pesticides into the environment; however, additional analyses were conducted that included these items in the “any indoor pesticides” variable.
Commercial agricultural pesticide exposure.
The CHARGE study catchment area includes the northern part of the California Central Valley, a dense agricultural region with heavy pesticide usage, as well as urban and suburban areas surrounding Sacramento and parts of the San Francisco Bay Area. Commercial pesticide applicators in California are required to report to the Department of Pesticide Regulation the type, amount of active ingredient (in pounds), location, and application type (i.e., aerial, ground, ground injection) of every agricultural pesticide used. Pesticide use reports (PURs) are publicly available for download by year (www.cdpr.ca.gov/docs/pur/purmain.htm). The PUR data are available down to 2.59 square km (1 square mile) units known as the meridian township range section (MTRS), a parceling by the U.S. Geological Survey for the whole country. Thereby, each application is linked to each and every MTRS where it is applied.
Compounds recorded in the PUR database are identifiable by unique product codes, which we cross-linked with registration records from the U.S. Environmental Protection Agency (EPA) (http://www.pesticideinfo.org/Search_Chemicals.jsp) to sort into chemical classes (e.g., organophosphate, pyrethroid).
Using the address history data recorded for CHARGE study participants, we geocoded each address from 3 mo before conception, by day, through delivery. Overall, 99% of addresses were successfully geocoded to obtain a longitude and latitude with a match of in ArcMap (version 10.0; Environmental Systems Research Institute, Inc.) using the U.S. Rooftop search algorithm. Unmatched addresses were manually matched to the most likely address. For each day of pregnancy, the home was assigned to the MTRS in which it was located. For example, if a mother moved on day 46 of her pregnancy, the MTRS code would change from that of the previous home to that of the new home on day 47; this allowed correct addresses to be captured for women at each time period for the 1 in 5 participants that moved during pregnancy.
Using a spatial model developed in ArcGIS, for each day, circular buffers were drawn around each home with radii of 1,250, 1,500, and . If the buffer intersected the centroid (centermost point) of an MTRS where pesticides had been applied, the type and amount were linked to the home as a proximal exposure. This model generated an exposure profile by day of pregnancy. All records with no exposure identified were assigned to zero pounds applied. The daily exposure profile was then aggregated into time periods of interest for analysis, such as months and trimesters; for this study, we used the 6-mo period beginning 3 mo before conception through the end of the third month of pregnancy (end of the first trimester) to be consistent with the timing in the first month of pregnancy, when FA intake is most associated with reduced likelihood of ASD and would be most likely to modify the association between pesticides and ASD. In explorative analysis, we also examined exposure during all of pregnancy. Because two thirds of participants experienced no pesticide applied within this proximity to their homes, analyses were conducted using binary variables for “exposed” and “unexposed.”
Occupational pesticide exposure.
Parental occupational history information was collected during the CHARGE telephone interview. Occupational information included the place of employment, month and year of employment, which month(s) of pregnancy (or the postnatal period) the job was held, and the total hours worked at each job. These data were sent to the National Institute for Occupational Safety and Health (NIOSH) for analyses. Each job reported was assigned a North American Industry Classification System (NAICS) and 2000 Standard Occupational Classification (SOC) (http://www.census.gov/people/io/methodology/) code. Two experienced industrial hygienists qualitatively estimated occupational exposures based on the NAICS and SOC codes as well as on parental job history information, duties, tasks, and responsibilities. For each job, the industrial hygienists independently assigned a qualitatively defined ordinal exposure level estimate to a selected list of chemical and physical agents including pesticides (insecticides, fungicides, and rodenticides) (McCanlies et al. 2012). They were blinded to the children’s case status (ASD or TD). After the industrial hygienists independently estimated exposure levels, they compared their estimates; any differences were discussed, and a consensus on the estimated exposure levels was determined. Based on the information provided in the database for each job, a code of 0 (none), 1 (exposure above background levels; no more than a few days per year), 2 (most likely exposed; exposure was unlikely to be daily), or 3 (definitely exposed; frequent or routine exposure) was entered to estimate both the frequency and intensity for each of the agents of interest. We only used the pesticide data for the present study. Few mothers had occupational exposure to pesticides during pregnancy or during the 3 mo before pregnancy. Therefore, we dichotomized occupational pesticide exposure during this period as regular versus none or some, and we only included occupational exposure with household and agricultural pesticide exposures when classifying women as having “any pesticide” exposure rather than analyzing it as a separate exposure.
Statistical Analysis
FA intake and prenatal pesticide exposures were dichotomized and evaluated separately and as combined four-level exposure variables ( and pesticide exposure, and pesticide exposure, and and no pesticide exposure compared with and no pesticide exposure as a common reference group) in logistic regression models with ASD versus TD as the outcome. Several time intervals were considered for pesticide exposures using the information on the period from 3 mo before conception through the end of pregnancy, with the primary time of interest being exposure in the months near conception. Separate models were fitted for each time interval and pesticide class.
Total FA summed from all available sources (vitamins, supplements, cereals, etc.) in the first month of pregnancy [the time period during which FA was most strongly associated with ASD in this population (Schmidt et al. 2012)] was dichotomized as above or below (the amount in most prenatal vitamins and the median for controls). We also examined combined associations when dichotomizing at , the dietary reference intake for pregnancy (Institute of Medicine, Food and Nutrition Board 2000).
Household pesticides were classified as separate binary indicators (no exposure vs. any), and when numbers allowed (with all cell sizes ), we examined exposure by frequency defined as regular use (occurring in mo of pregnancy), some use (in mo of pregnancy), or no exposure (reference group). Regular use was examined separately because it would deliver a greater exposure than sporadic use and would be more likely to include a susceptible time period if the fetus was not susceptible during the entire pregnancy. Additionally, in previous analyses of the association between household pesticides and ASD in CHARGE participants, associations were found primarily for regular users. Thus, for the present study, regular exposure was considered “exposed.” Pesticide exposures included use of any flea products on indoor pets during pregnancy and use of any professional or self-applied sprays or foggers indoors or outdoors during pregnancy. Pet flea and tick products were examined separately from indoor sprays and foggers to assess independent associations in combination with FA intake, but because effect estimates of these different types of pesticides were in the same direction, they were also examined in combination (any vs. no exposure to either type) for increased power.
Carbamate, organochlorine, organophosphate, and pyrethroid agricultural pesticides were measured at buffer distances of 1,250, 1,500, and around the residence. Commercial agricultural pesticide exposures were categorized into two levels representing any versus no pesticide application in the specified area for the chosen prenatal time interval. We chose to use the buffer distance for our primary analyses to reflect the most proximal exposure and conducted sensitivity analyses using the 1,500- and buffers.
Potential confounders were identified by considering elements that may influence exposure to pesticides or to FA supplements and risk for autism, particularly attributes pertaining to socioeconomic variables such as home ownership and mother’s education because these were confounders for associations between FA intake and ASD and between pesticides and ASD when their main effects were examined independently within the same parent study (Schmidt et al. 2012; Shelton et al. 2014). Other variables considered as potential confounders included maternal and paternal age, maximum education of parents, home ownership, type of insurance at delivery, maternal birthplace, education, smoking in the 3 mo before or during pregnancy, intention of getting pregnant when she did, intake of vitamins B6 and B12 from supplements in the first month of pregnancy, and child’s sex, race/ethnicity, and year of birth. Changes of in the beta estimates for the effects of interest (the doubly exposed category) were used as the criteria for confounder inclusion, both when each potential confounder was evaluated by itself and when each was removed from a full model.
For each FA–pesticide exposure combination, we used the Akaike Information Criterion (AIC), a complexity-adjusted goodness-of-fit measure (Burnham et al. 2002), to compare the model with the two binary exposure variables (for pesticides and FA intake) as main effects versus the model with the four-level combined exposure classification, which is equivalent to adding an interaction term to the main effects model. Expected joint effects under an additive model were calculated by adding the ORs of the groups with only one exposure and subtracting 1. Expected joint effects under a multiplicative model were calculated by multiplying the ORs of the groups with only one exposure. In addition, the relative excess risk due to interaction (RERI) and 95% CIs were calculated using the ic package in Stata v.12.0 (StataCorp LLC) (Andersson et al. 2005; Hosmer and Lemeshow 1992). All other statistical analyses were performed using SAS software v.9.3 (SAS Institute Inc.). Associations with vitamin B6–pesticide, vitamin B12–pesticide, and FA/vitamin B6–pesticide exposure combinations were evaluated in the same manner. Complete case analyses were conducted for all associations.
Results
Case and Control Characteristics and Exposure Frequencies
Of the 806 (466 ASD and 340 TD) participants born 2000–2007 and whose mothers were interviewed by November 2011, data on FA intake in the first month were available for 394 (85%) ASD and 282 (83%) TD participants; indoor pesticide exposure was available for 409 (88%) ASD and 303 (89%) TD participants; outdoor household pesticide exposure was available for 402 (86%) ASD and 303 (89%) TD participants; agricultural pesticide exposure was available for 428 (92%) ASD and 310 (91%) TD participants; and occupational pesticide exposure was available for 343 (74%) ASD and 255 (75%) TD participants (Table 1). Participants who had information available on both folic acid intake in the first month of pregnancy and at least one of the pesticides studied included 296 (64%) ASD and 220 (65%) TD participants (Table 1). Regardless of the availability of folic acid and pesticide exposure information, case children were more likely to be born in the first years of the study than controls, and mothers of children with ASD were less likely to own their home than mothers of TD children (Table 1). Parents of children with ASD were less likely to report taking FA in the first month of pregnancy and were more likely to report any exposure to indoor household pesticides during pregnancy and any pesticide exposure (Table 1). For ASD and TD with interviews before November 2011, mothers of children with ASD were more likely to have vitamin B6 intake above the median in the first pregnancy month than mothers of TD children, but this difference did not reach significance in the sample with folic acid and pesticide data. For those with folic acid and pesticide data, household outdoor pesticide exposure was significantly more common among mothers of children with ASD than among mothers of TD children.
Table 1.
Characteristics of children with autism spectrum disorder (ASD) and typical development (TD) and their mothers in the CHARGE case–control study [ (%)].
| Characteristic | Cases and controls with interviews 2011 or before | Cases and controls with information on folic acid and pesticide exposures | ||||
|---|---|---|---|---|---|---|
| ASD () | TD () | p-Valuea | ASD (a) | TD (a) | p-Valuea | |
| Child sex | 0.18 | 0.3663 | ||||
| Male | 400 (85.8) | 280 (82.4) | 256 (86.49) | 184 (83.64) | ||
| Female | 66 (14.2) | 60 (17.6) | 40 (13.51) | 36 (16.36) | ||
| Child’s race/ethnicity | 0.10 | 0.2059 | ||||
| Non-Hispanic white | 241 (51.7) | 175 (51.5) | 146 (49.32) | 110 (50.00) | ||
| Hispanic | 142 (30.5) | 101 (29.7) | 98 (33.11) | 70 (31.82) | ||
| Non-Hispanic black | 9 (1.9) | 8 (2.4) | 8 (2.70) | 4 (1.82) | ||
| Asian | 28 (6.0) | 9 (2.6) | 16 (5.41) | 5 (2.27) | ||
| Mixed and other | 46 (9.9) | 47 (13.8) | 28 (9.46) | 31 (14.09) | ||
| Child’s birth year | ||||||
| 2000–2001 | 191 (41.0) | 59 (17.4) | 119 (40.20) | 37 (16.82) | ||
| 2002–2003 | 121 (26.0) | 133 (39.1) | 86 (29.05) | 92 (41.82) | ||
| 2004–2005 | 115 (24.7) | 92 (27.1) | 71 (23.99) | 57 (25.91) | ||
| 2006–2007 | 39 (8.4) | 56 (16.5) | 20 (6.76) | 34 (15.45) | ||
| Maternal age at child’s birth (years) | 0.19 | 0.2399 | ||||
| 8 (1.7) | 12 (3.5) | 6 (2.03) | 11 (5.00) | |||
| 20–25 | 83 (17.8) | 55 (16.8) | 47 (15.88) | 36 (16.36) | ||
| 26–29 | 106 (22.8) | 66 (19.4) | 73 (24.66) | 43 (19.55) | ||
| 30–34 | 148 (31.8) | 128 (37.7) | 97 (32.77) | 84 (38.18) | ||
| 35–39 | 103 (22.1) | 63 (18.5) | 63 (21.28) | 39 (17.73) | ||
| 18 (3.9) | 16 (4.7) | 10 (3.38) | 7 (3.18) | |||
| Maternal birthplace | 0.14 | 0.4657 | ||||
| United States | 353 (75.8) | 277 (81.5) | 224 (75.68) | 176 (80.00) | ||
| Mexico | 38 (8.2) | 23 (6.8) | 25 (8.45) | 17 (7.73) | ||
| Other | 75 (16.1) | 40 (11.8) | 47 (15.88) | 27 (12.27) | ||
| Maternal education | 0.11 | 0.0671 | ||||
| High school graduate or less | 65 (13.9) | 53 (15.6) | 40 (13.51) | 38 (17.27) | ||
| Some college, vocational, associate degree | 180 (38.6) | 107 (31.5) | 116 (39.19) | 65 (29.55) | ||
| Bachelor or higher degree | 221 (47.4) | 180 (52.9) | 140 (47.30) | 117 (53.18) | ||
| Home ownership | 0.001 | 0.0184 | ||||
| No | 152 (33.4) | 76 (22.8) | 96 (33.10) | 51 (23.50) | ||
| Yes | 303 (66.6) | 258 (77.2) | 194 (66.90) | 166 (76.50) | ||
| Missing information | 11 | 6 | 6 | 3 | ||
| Insurance delivery type | 0.08 | 0.5908 | ||||
| Private | 380 (81.6) | 292 (86.1) | 245 (82.77) | 186 (84.55) | ||
| Government program | 86 (18.5) | 47 (13.9) | 51 (17.23) | 34 (15.45) | ||
| Intention to become pregnant | 0.51 | 0.6725 | ||||
| Intended to become pregnant when they did | 292 (64.3) | 228 (67.9) | 187 (64.71) | 150 (68.81) | ||
| Indifferent about becoming pregnant at that time | 60 (13.2) | 47 (14.0) | 41 (14.19) | 31 (14.22) | ||
| Intended to become pregnant later | 66 (14.5) | 41 (12.2) | 39 (13.49) | 25 (11.47) | ||
| Did not intend to become pregnant at all | 36 (7.9) | 20 (6.0) | 22 (7.61) | 12 (5.50) | ||
| Missing information | 12 | 4 | 7 | 2 | ||
| Maternal cigarette smokingb | 0.06 | 0.1632 | ||||
| No | 395 (86.1) | 305 (90.5) | 255 (86.44) | 199 (90.45) | ||
| Yes | 64 (13.9) | 32 (9.5) | 40 (13.56) | 21 (9.55) | ||
| Missing information | 7 | 3 | 1 | 0 | ||
| Folic acid pregnancy month 1c | 0.01 | 0.0492 | ||||
| 210 (53.3) | 121 (42.9) | 151 (51.01) | 93 (42.27) | |||
| 184 (46.7) | 161 (57.1) | 145 (48.99) | 127 (57.73) | |||
| Missing information | 72 | 58 | ||||
| Folic acid pregnancy month 1c | 0.0146 | 0.0827 | ||||
| 191 (48.48) | 110 (39.01) | 137 (46.28) | 85 (38.64) | |||
| 203 (51.52) | 172 (60.99) | 159 (53.72) | 135 (61.36) | |||
| Missing information | 72 | 58 | ||||
| Vitamin B12 pregnancy month 1c | 0.1622 | 0.1307 | ||||
| 213 (50.71) | 141 (45.48) | 148 (50.17) | 95 (43.18) | |||
| 207 (49.29) | 169 (54.52) | 147 (49.83) | 125 (56.82) | |||
| Missing information | 46 | 30 | 1 | 0 | ||
| Vitamin B6 pregnancy month 1c | 0.0278 | 0.1155 | ||||
| 245 (58.19) | 155 (50.00) | 170 (57.43) | 111 (50.45) | |||
| 176 (41.93) | 155 (50.00) | 126 (42.57) | 109 (49.55) | |||
| Missing information | 45 | 30 | ||||
| Occupational pesticide | 0.8493 | 0.7407 | ||||
| None | 345 (98.29) | 256 (98.08) | 227 (99.13) | 163 (98.79) | ||
| Any | 6 (1.71) | 5 (1.92) | 2 (0.87) | 2 (1.21) | ||
| Missing information | 115 | 79 | 67 | 55 | ||
| Household indoor pesticide exposured | 0.005 | 0.0109 | ||||
| No | 220 (53.8) | 195 (64.4) | 165 (55.74) | 147 (66.82) | ||
| Yes | 189 (46.2) | 108 (35.6) | 131 (44.26) | 73 (33.18) | ||
| Missing information | 57 | 37 | ||||
| Household outdoor pesticide exposuree | 0.07 | 0.0189 | ||||
| No | 248 (61.7) | 207 (68.3) | 179 (60.47) | 155 (70.45) | ||
| Yes | 154 (38.3) | 96 (31.7) | 117 (39.53) | 65 (29.55) | ||
| Missing information | 64 | 37 | ||||
| Agricultural pesticide exposuref | 0.75 | 0.3051 | ||||
| No | 351 (82.0) | 257 (82.9) | 240 (81.08) | 186 (84.55) | ||
| Yes | 77 (18.0) | 53 (17.1) | 56 (18.92) | 34 (15.45) | ||
| Missing information | 38 | 30 | ||||
| Any pesticide exposureg | 0.04 | 0.0579 | ||||
| No | 124 (35.4) | 116 (43.8) | 110 (37.16) | 100 (45.45) | ||
| Yes | 226 (64.6) | 149 (56.2) | 186 (62.84) | 120 (54.55) | ||
| Missing information | 116 | 75 | ||||
Note: Limited to those with information on both maternal folic acid intake and at least one type of pesticide exposure. ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and Environment; TD, typical development.
derived from chi-squared tests comparing category proportions between the ASD group and the TD group.
Mother reported smoking any tobacco product before or during pregnancy.
Average folic acid consumed per day summed from prenatal vitamins, multivitamins, folic acid supplements, other supplements, and breakfast cereals.
Maternally reported exposure to professionally or self-applied pesticide sprays or foggers or pet pesticides (flea/tick shampoos, pouches, not collars) inside the home during pregnancy.
Maternally reported exposure to professionally or self-applied pesticide sprays or foggers outside the home during pregnancy.
Exposure to carbamate, organochlorine, organophosphate, and pyrethroid pesticides applied to agricultural fields within a buffer around the mother’s home during the period from 3 mo before through the 3rd month after conception based on linkage of her address(es) to the California Pesticide Use Report.
Maternal exposure to any indoor or outdoor household pesticides, or agricultural pesticides, as defined above.
Household Pesticide Exposure by Maternal FA Intake
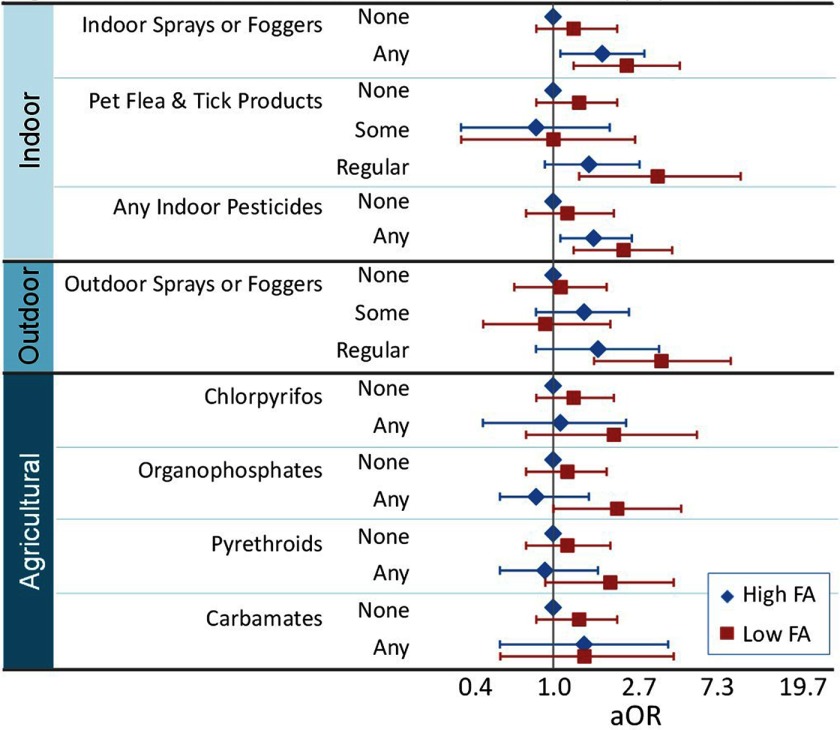
Home ownership, child’s year of birth, and maternal vitamin B6 and vitamin D (natural log) intake in the first pregnancy month met confounder criteria and were thus included as adjustment variables in all models. Overall, adjusted ORs for ASD tended to be highest when mothers were exposed to pesticides and reported taking in the first month of pregnancy compared with all other groups (Figure 1). Compared with women having above-median FA intake () during the first month of pregnancy and no indoor pesticide exposure, women with below-median FA intake and regular exposure to indoor sprays and foggers were more likely to have a child with ASD [ (95% CI: 1.3, 5.2)] than those with either low FA intake [ (95% CI: 0.8, 2.3)] or regular exposure to indoor sprays and foggers alone [ (95% CI: 1.1, 3.3)] (Table 2). Similarly, women with below-median FA intake and regular exposure to pet flea and tick products were associated with higher risk of having a child with ASD [ (95% CI: 1.4, 11.5)] than those with either low FA intake [ (95% CI: 0.8, 2.3)] or regular exposure to pet flea and tick products alone [ (95% CI: 0.9, 3.1)]. Women with the combination of below-median FA intake and exposure to any indoor pesticides were associated with elevated risk of having a child with ASD [ (95% CI: 1.3, 4.7)] compared with those having no exposure and high FA intake, which was greater than those with exposure who had above-median FA intake [ (95% CI: 1.1-2.8)]. Finally, regular exposure to outdoor sprays and foggers in combination with lower FA was associated with elevated estimated risk [ (95% CI: 1.7, 10.1)] that was more than twice that of those with above-median FA intake and regular pesticide exposure, again compared with the lowest risk group [ (95% CI: 0.8, 4.0)]. All ORs for those with double exposure were greater than expected by additive or multiplicative models, with ORs from slightly greater to more than twice as great (Table 2). Inclusion of additional covariates produced similar results with generally increased ORs in all categories and with ORs for the doubly exposed category that were greater than expected for most pesticide types (see Tables S1, S2). Effect estimates were similar but slightly attenuated in additional analyses including flea and tick collars (see Table S3). The results followed similar patterns when dichotomizing FA at (see Tables S4, S5).
Figure 1.
Autism spectrum disorder odds ratios for pesticide and folic acid exposure combinations. Odds ratios and 95% confidence intervals (bars) for the association between Autism spectrum disorder (ASD) and combinations of exposures to pesticides and average maternal folic acid (FA) intake (, ) during the first month of pregnancy were adjusted for home ownership, child’s year of birth, maternal intake of vitamins B6 and D (natural log) in the first month of pregnancy. In all comparisons, the reference group was those with above-median FA intake () during the first pregnancy month and no pesticide exposure.
Table 2.
Combinations of household pesticide exposure and maternal folic acid intake the first month of pregnancy in relation to risk for autism spectrum disorders.
| Pesticide exposure during pregnancy | Maternal folic acid intakea | ASD [ (%)] | TD [ (%)] | Expected joint OR: multiplicative modelb | Expected joint OR: additive modelc | ORd (95% CI) | RERI (95% CI) |
|---|---|---|---|---|---|---|---|
| Indoor sprays or foggers | |||||||
| None | 107 (32.4) | 116 (46.8) | 1.0 | ||||
| 120 (36.4) | 84 (33.9) | 1.3 (0.8, 2.3) | |||||
| Any | 49 (14.9) | 27 (10.9) | 1.9 (1.1, 3.3) | ||||
| 54 (16.4) | 21 (8.5) | 2.6 | 2.2 | 2.6 (1.3, 5.2) | 0.4 (, 2.1) | ||
| Pet flea and tick products | |||||||
| None | 127 (36.3) | 118 (46.1) | 1.0 | ||||
| 149 (42.6) | 95 (37.1) | 1.4 (0.8, 2.3) | |||||
| Somee | 8 (2.3) | 12 (4.7) | 0.8 (0.3, 2.1) | ||||
| 8 (2.3) | 8 (3.1) | 1.1 | 1.2 | 1.0 (0.3, 2.9) | (, 1.1) | ||
| Regularf | 33 (9.4) | 18 (7.0) | 1.6 (0.9, 3.1) | ||||
| 25 (7.1) | 5 (2.0) | 2.3 | 2.0 | 3.9 (1.4, 11.5) | 2.0 (, 6.2) | ||
| Any | 41 (11.7) | 30 (11.7) | 1.3 (0.8, 2.3) | ||||
| 33 (9.4) | 13 (5.1) | 1.8 | 1.7 | 2.1 (0.99, 4.7) | 0.6 (, 2.2) | ||
| Any indoor pesticidesg | |||||||
| None | 81 (24.4) | 90 (36.9) | 1.0 | ||||
| 100 (30.1) | 75 (30.7) | 1.2 (0.7, 2.2) | |||||
| Any | 77 (23.2) | 50 (20.5) | 1.7 (1.1, 2.8) | ||||
| 74 (22.3) | 29 (11.9) | 2.0 | 1.9 | 2.5 (1.3, 4.7) | 0.6 (, 1.9) | ||
| Outdoor sprays or foggers | |||||||
| None | 96 (30.9) | 95 (39.9) | 1.0 | ||||
| 100 (32.2) | 73 (30.7) | 1.1 (0.6, 2.0) | |||||
| Somee | 34 (10.9) | 31 (13) | 1.5 (0.8, 2.7) | ||||
| 18 (5.8) | 19 (8.0) | 1.7 | 1.6 | 0.9 (0.4, 2.1) | (, 0.5) | ||
| Regularf | 23 (7.4) | 12 (5.0) | 1.8 (0.8, 4.0) | ||||
| 40 (12.9) | 8 (3.4) | 2.0 | 1.9 | 4.1 (1.7, 10.1) | 2.0 (, 5.3) | ||
| Any | 57 (18.3) | 43 (18.1) | 1.6 (1.0, 2.7) | ||||
| 58 (18.7) | 27 (11.3) | 1.8 | 1.7 | 2.0 (1.0, 3.8) | 0.2 (, 1.5) | ||
| Any household indoor or outdoor pesticides | |||||||
| None | 62 (20.0) | 67 (28.6) | 1.0 | ||||
| 77 (24.8) | 58 (24.8) | 1.2 (0.6, 2.3) | |||||
| Any | 87 (28.1) | 67 (28.6) | 1.6 (1.0, 2.7) | ||||
| 84 (27.1) | 42 (18.0) | 2.0 | 1.8 | 2.1 (1.1, 3.9) | 0.2 (, 1.3) | ||
| Any household or agricultural pesticidesh | |||||||
| None | 47 (16.5) | 53 (24.8) | 1.0 | ||||
| 60 (21.1) | 45 (21.0) | 1.2 (0.6, 2.5) | |||||
| Any | 94 (33.0) | 70 (32.7) | 1.7 (1.0, 2.9) | ||||
| 84 (29.5) | 46 (21.5) | 2.0 | 1.9 | 2.1 (1.1, 4.1) | 0.2 (, 1.4) |
Note: ASD, autism spectrum disorder; CI, confidence interval; OR, odds ratio; RERI, relative excess risk due to interaction; TD, typical development.
Average daily intake during first month of pregnancy.
Expected combined OR for multiplicative model calculated as the product of the ORs for no pesticide exposure and folic acid , pesticide exposure and folic acid .
Expected combined OR for additive model calculated as .
ORs adjusted for home ownership, child’s birth year, and maternal vitamin B6 and vitamin D (natural log) intake during the first month of pregnancy.
Exposure to pesticides reported for of pregnancy.
Exposure to pesticides reported for of pregnancy.
Maternally reported exposure to professionally or self-applied pesticide sprays or foggers or pet pesticides (flea/tick shampoos, pouches, not collars) inside the home during pregnancy.
Any household indoor or outdoor pesticide exposure during pregnancy, or agricultural pesticide exposure from 3 mo before through the 3rd month of pregnancy.
Agricultural Pesticide Exposure by Maternal FA Intake
The joint OR for low maternal FA intake and exposure to any agricultural pesticides 3 mo before or after conception was 2.0 (95% CI: 0.9, 4.2), which was greater than the OR for low FA intake and no pesticide exposure [1.2 (95% CI: 0.7, 2.1)] and the OR for high FA intake and pesticide exposure [1.0 (95% CI: 0.6, 1.8)]. ORs for the combination of low maternal FA intake and exposure to individual agricultural pesticides 3 mo before or after conception were 2.2 (95% CI: 0.7, 6.5) for chlorpyrifos, 2.3 (95% CI: 0.98, 5.3) for organophosphates, 1.7 (95% CI: 0.8, 3.7) for pyrethroids, and 1.3 (95% CI: 0.4, 4.0) for carbamates (Table 3, Figure 1). Except for carbamates, these nonsignificant ORs were greater than those for agricultural pesticide exposure with higher FA intake or for low FA with no pesticide exposure, and they were greater than expected by additive or multiplicative models. The results were similar when examining agricultural pesticide exposure during pregnancy rather than in the periconceptional months (see Table S6). Results using the buffer showed a similar pattern for greater, but slightly attenuated, ORs in the combined low FA + pesticide category; this pattern was only observed for chlorpyrifos when using the buffer (see Tables S7, S8).
Table 3.
Combinations of agricultural pesticide exposure and maternal folic acid intake the first month of pregnancy in relation to risk for autism spectrum disorders.
| Periconceptional agricultural/commercial pesticide exposurea | Maternal folic acid intakeb | ASD [ (%)] | TD [ (%)] | Expected joint OR: multiplicative modelc | Expected joint OR: additive modeld | Observed ORe (95% CI) | RERI (95% CI) |
|---|---|---|---|---|---|---|---|
| Chlorpyrifos | |||||||
| None | 159 (45.6) | 132 (52.8) | 1.0 | ||||
| 165 (47.3) | 101 (40.4) | 1.3 (0.8, 2.2) | |||||
| Any | 12 (3.4) | 11 (4.4) | 1.1 (0.4, 2.6) | ||||
| 13 (3.7) | 6 (2.4) | 1.4 | 1.4 | 2.2 (0.7, 6.5) | 0.8 (, 3.2) | ||
| Organophosphates | |||||||
| None | 145 (41.6) | 120 (48) | 1.0 | ||||
| 150 (43) | 96 (38.4) | 1.2 (0.7, 2.0) | |||||
| Any | 26 (7.5) | 23 (9.2) | 0.8 (0.5, 1.6) | ||||
| 28 (8.0) | 11 (4.4) | 1.0 | 1.0 | 2.3 (0.98, 5.3) | 1.2 (, 3.0) | ||
| Pyrethroids | |||||||
| None | 149 (42.7) | 124 (49.6) | 1.0 | ||||
| 153 (43.8) | 96 (38.4) | 1.2 (0.7, 2.1) | |||||
| Any | 22 (6.3) | 19 (7.6) | 0.9 (0.5, 1.8) | ||||
| 25 (7.2) | 11 (4.4) | 1.1 | 1.2 | 2.1 (0.9, 4.8) | 0.9 (, 2.6) | ||
| Carbamates | |||||||
| None | 160 (45.9) | 138 (55.2) | 1.0 | ||||
| 169 (48.4) | 102 (40.8) | 1.4 (0.8, 2.3) | |||||
| Any | 11 (3.2) | 5 (2.0) | 1.5 (0.5, 4.5) | ||||
| 9 (2.6) | 5 (2.0) | 2.0 | 1.8 | 1.5 (0.5, 4.8) | (, 2.0) | ||
| Any agricultural pesticides | |||||||
| None | 137 (39.3) | 117 (46.8) | 1.0 | ||||
| 145 (41.6) | 91 (36.4) | 1.2 (0.7, 2.1) | |||||
| Any | 34 (9.7) | 26 (10.4) | 1.0 (0.6, 1.8) | ||||
| 33 (9.5) | 16 (6.4) | 1.2 | 1.2 | 2.0 (0.9, 4.2) | 0.7 (, 2.1) |
Note: ASD, autism spectrum disorder; CI, confidence interval; OR, odds ratio; RERI, relative excess risk due to interaction; TD, typical development.
Any during the period 3 mo before or after conception, using a buffer.
Average daily intake during first month of pregnancy.
Expected combined OR for multiplicative model calculated as the product of the ORs for no pesticide exposure and folic acid , pesticide exposure and folic acid .
Expected combined OR for additive model calculated as .
ORs adjusted for home ownership, child’s birth year, and maternal vitamin B6 and vitamin D (natural log) intake during the first month of pregnancy.
Only for agriculturally applied organophosphate pesticides was the AIC for the model with an interaction term between maternal first-month FA intake and pesticide exposure less than the AIC for the model without an interaction term, indicating a better-fitting model; for all other pesticide exposures, the model without an interaction term was the better-fitting model (see Table S9). Maternal intake of vitamins B12 and B6 was highly correlated with maternal FA intake from supplements, and the results for combinations of high (above median) and low vitamin B12 and B6 in combination with pesticide exposures were relatively similar to those with FA, with greater ORs than expected for doubly exposed participants, but with less consistency across types of pesticides (see Tables S10–S13). Because FA and vitamin B6 intake were correlated and each met criteria as a confounder for the other with similar patterns when combined with pesticide exposure, we also examined joint associations of low (below median) maternal FA and vitamin B6 intake compared with either high maternal FA or vitamin B6 intake in combination with each pesticide; the results were similar with regard to the observed combined exposure category having higher ORs than expected, with consistently higher ORs in all categories (see Tables S14, 15).
Occupational Pesticide Exposure
Five (1.5%) of 343 mothers of children with ASD and 5 (2.0%) of 255 mothers of children with TD had occupational pesticide exposure. Mothers of children with ASD were more likely than mothers of TD children to be classified with frequent/regular occupational exposure to pesticides, with (, 80% of exposed, compared with (20% of exposed) regularly exposed. Because the numbers of exposed participants were so low, we did not examine occupational pesticide exposure separately in combination with FA, but the OR for joint exposure (to low FA and exposure to pesticides) in analyses including regular occupational exposure in combination with household or agricultural pesticide exposure (as any pesticide exposure) of 1.7 (95% CI: 0.8, 3.5) was attenuated compared with the OR of 2.1 (95% CI: 1.1, 4.1) for any pesticide exposure without regular occupational exposure and only slightly greater than expected by multiplicative (1.6) or additive (1.5) models (see Table S16).
Discussion
In this California study population, we found that associations between household and agricultural pesticide exposures and ASD in the child were reduced among women with higher () FA intake near the time of conception compared with associations among women with lower intake. To our knowledge, this study provides the first evidence for attenuation of the association between gestational pesticide exposures and ASD by maternal FA intake. These findings are congruent with both human and animal studies demonstrating the ability of maternal FA to alter effects of environmental toxicants on the developing offspring. In a prospective cohort study of 291 women in China, maternal pre-conception serum folate and B-vitamin sufficiency was shown to protect against adverse reproductive effects of 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane (DDT) exposure (Ouyang et al. 2014). Human studies suggest that FA might reduce the potency of other contaminants, including arsenic, a potent neurotoxicant contained in a few pesticides unlikely to be captured in this study. In a double-blind placebo-controlled randomized trial of 200 adults, FA supplementation in highly arsenic-exposed individuals appeared to enhance arsenic methylation, which may reduce its toxicity (Gamble et al. 2006). Another double-blind placebo-controlled randomized trial of adults in Bangladesh suggested that higher doses of FA () were needed to reduce blood arsenic concentration in populations containing folate-replete individuals (Peters et al. 2015). Notably, a recent study of 57 cases and 55 controls in Bangladesh showed that first-trimester inorganic arsenic exposure also significantly reduced the protective effects of FA supplementation against neural tube defects (Mazumdar et al. 2015), suggesting that higher doses of FA might be needed to provide neuroprotection in those exposed to environmental contaminants.
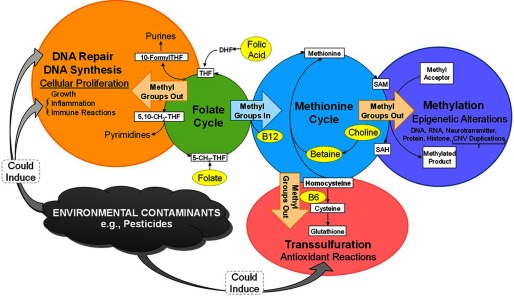
Although noncausal explanations for the reduction of ASD risk in association with pesticide exposures by FA cannot be ruled out, one can speculate that potential mechanisms could involve folate’s antioxidant properties (Joshi et al. 2001), its role in DNA repair (Duthie 1999; Duthie et al. 2004), or its influence on DNA methylation (James et al. 2004; James et al. 2009), as shown in Figure 2. Folate’s role as a major methyl donor could be relevant given that all other proposed pathways could lead to depletion of methyl groups necessary for DNA methylation (Figure 2), which could be critical near conception, when the methylome is demethylated and then reestablished (Reik et al. 2001). Vitamin B6 also contributes to this one-carbon methylation pathway. Methylation pathways were proposed to explain reduced male reproductive effects of exposure to the insecticide methomyl in rats receiving FA (Shalaby et al. 2010), and maternal folate supplementation was shown to prevent the effects of developmental exposure to BPA on DNA methylation in mice (Dolinoy et al. 2007). Evidence in human studies has suggested that FA might alter susceptibility to arsenic toxicity through methylation pathways (Howe et al. 2014; Lambrou et al. 2012). In addition, a recent crossover study of 10 adults reported that changes in DNA methylation following 2 h of controlled exposure to particulate matter with an aerodynamic diameter () were not observed in the same 10 loci when exposure followed 4 wk of B vitamin supplementation, including high doses of FA () and vitamin B6 () (Zhong et al. 2017).
Figure 2.
Pathways connecting folic acid to potential mechanisms of environmental contaminants. Folic acid inputs into the folate cycle through conversion to tetrahydrofolate (THF), which augments folate’s essential role as a donor and acceptor of one-carbon units, important for the biosynthesis of nucleic acids, proteins, and methyl groups (Crider et al. 2012). During development, biosynthesis of nucleic acids is necessary for DNA synthesis, repair, and cell division, and methyl groups are important for regulation of gene expression (Crider et al. 2012). Environmental contaminants such as pesticides can trigger immune responses and inflammation (Voccia et al. 1999) that induce cellular proliferation and DNA synthesis; similarly, pesticides can induce DNA damage (Corsini et al. 2008; Undeğer and Başaran 2005) that requires repair; both of these folate-dependent processes necessitate biosynthesis of nucleic acids, which could deplete folate at a time during early pregnancy when demand is high but could potentially be countered with high folate quantities. Environmental contaminants can also induce oxidative stress (Abdollahi et al. 2004); in response, homocysteine is permanently removed from the methionine cycle through degradation into cysteine in the transsulfuration cycle, where it is converted to cysteine and then to glutathione, a universal antioxidant (Schmidt and LaSalle 2011). This diversion of the methionine cycle towards glutathione antioxidant reactions and away from DNA synthesis, repair, and methylation may be countered by high folate supply, driving conversion of homocysteine to methionine and the biosynthesis of methionine to S-adenosylmethionine (SAM), which serves as a methyl donor for methylation reactions that are particularly critical during key periods of growth and remethylation at the start of development. Note: CNV, copy number variation.
The suggestion of the involvement of methylation pathways is also congruent with studies providing evidence for altered DNA methylation linked to exposure to several types of pesticides [reviewed by Collotta et al. (2013)]. This evidence includes associations between low-dose exposure to organochlorine pesticides and global DNA hypomethylation estimated by the percentage of 5-methyl-cytosine (%5-mC) in Alu and LINE-1 assays in 86 healthy Koreans (Kim et al. 2010) and a significant inverse linear relationship between plasma concentrations of DDT, 1,1-bis-(4-chlorophenyl)-2,2-dichloroethene (DDE), and other persistent organic pollutants (POPs) and blood global DNA methylation estimated in Alu repeated elements in 71 Greenlandic Inuit with high POP levels (Rusiecki et al. 2008). Maternal self-reported pesticide exposure was linked to placental DNA methylation changes using whole-genome bisulfite sequencing in a cohort study of 47 mothers of children with ASD (Schmidt et al. 2016). In rats, DDT exposure altered the methylation pattern in DNA extracted from the hypothalamus of young male rats, with significant hypomethylation of CpG islands in 6 genes compared with controls (Shutoh et al. 2009). Evidence for DNA methylation effects has also been observed for nonpersistent pesticides, such as organophosphates (Zhang et al. 2012a, 2012b). Oxidative stress is another potential mechanism that could be induced by a variety of classes of pesticides and could be attenuated by folic acid through several pathways, as shown in Figure 2. The reasons for a lack of FA attenuation of the association for carbamates are unclear but could result from alternate mechanisms for this particular pesticide class.
Study Limitations and Strengths
A major limitation of this study was the reliance on self-reported FA and household pesticide exposure and the potential for recall bias to explain the observed associations, at least in part. For the higher OR in the group with combined exposure to be explained by recall bias, case mothers would have had to both under-report FA intake and over-report pesticide exposures. However, FA intake that was self-reported during pregnancy was also associated with a reduced risk of ASD () in a prospective cohort of Norwegian women (Suren et al. 2013). In addition, self-reported household pesticide use has been shown to be reliable in a case–control study of cutaneous melanoma in men and women of all ages living in Rome (163 cases and 113 controls) who were given the same pesticide questionnaire apart (Fortes et al. 2009) and valid in 185 older male orchardists in Washington state recalling information 20–25 y later (Engel et al. 2001). Finally, patterns of association with agricultural pesticide exposures, which were not self-reported, were similar to those for self-reported exposures in combination with FA.
For the household pesticide analyses, we combined all pesticide classes together; by not examining interaction effects by each pesticide type (e.g., pyrethroids), it is possible that individual effects of some pesticide types were diluted. Additionally, too few women were exposed to certain classes of agricultural pesticides, including organochlorines (which have previously been linked to ASD), to produce stable estimates. Thus, interactions between FA and some specific pesticides could not be evaluated. However, the classes of pesticides examined—chlorpyrifos, organophosphates, pyrethroids, and carbamates—include several that are among the most widely prevalent exposures in the United States.
Missing data were a limitation of our analyses. Although 88–92% of participants had data available for each pesticide exposure other than occupational pesticide exposure, and 84% had data on folic acid intake, when examining folic acid and pesticide exposure in combination, a high percentage (24–32%) of participants were missing data on one exposure or the other. Missing data were particularly an issue for occupational pesticide exposure, where 36% of cases and 38% of controls were missing data. Although missingness appeared nondifferential across case status, there was potential for bias due to missing data if the missingness was informative.
In addition, very few mothers in our study population reported occupations that were likely to result in regular pesticide exposure in the 3 mo before and during pregnancy. Consequently, we were unable to thoroughly evaluate interactions between maternal FA intake and occupational pesticide exposure independently. Further, the strongest associations between household pesticides and ASD, and where we observed the greatest attenuation of ORs by FA, were in mothers with regular exposure during pregnancy, but we were unable to examine associations by frequency for all pesticide exposures, and estimates for pesticides classified as 3-level exposures were imprecise owing to small numbers of observations.
This study collected information on and evaluated numerous factors as potential confounders of the joint association of FA and pesticide exposures in relation to ASD, including most ASD risk factors identified in previous studies. ORs for the doubly exposed category remained greater than expected for most pesticide types in full models adjusting for additional factors that did not meet the criteria for confounders. However, confounding by other unmeasured factors is possible.
Strengths of this study include its extensive collection of environmental data to allow the examination of exposure combinations. Few other autism studies have collected information on nutrient intake and pesticide exposures, including timing and dose, from a large enough number of participants to allow examination of their combined effects. In addition, this study included clinically confirmed diagnostic classification using gold-standard standardized assessments.
Public Health Implications
The use of indoor and outdoor pesticides around the household was commonly reported in our study. Based on the findings of previous studies linking maternal pesticide exposure to ASD or other adverse neurodevelopmental outcomes (Braun et al. 2014b; Eskenazi et al. 2007; Keil et al. 2014; McCanlies et al. 2012; Roberts et al. 2007; Roberts and English 2013; Shelton et al. 2014) and on our results showing that many maternal pesticide exposures were significantly associated with ASD even among women with high FA intakes, we would recommend that mothers avoid household pesticide use during pregnancy. However, it is more difficult to avoid agricultural pesticide exposures. In our California-based case–control study, children of women who were exposed to pesticides during pregnancy were less likely to be diagnosed with ASD if their mothers had high versus low FA intake. Overall, our findings support the beneficial effects of FA supplementation during pregnancy.
Conclusion
These findings suggest that supplemental FA taken during the first month of pregnancy could potentially reduce, but not eliminate, the increased risk of ASD associated with maternal pesticide exposure before and during pregnancy. Larger studies, exposure measurements or markers that are prospectively collected, and research on potential mechanisms would be helpful in moving the field forward.
Supplemental Material
Acknowledgements
The authors would like to thank the CHARGE investigators, staff, and most of all, the participants for their valuable contributions. V. K. gratefully acknowledges the University of Southern California Rose Hills? Ph.D. Fellowship for its support.
This study was supported by the National Institutes of Health (NIH) (grant numbers R21-ES021330, R01-ES015359, P01-ES11269, 2K12HD051958, R21-ES19002, P30-ES023513, and U54-HD079125), by the U.S. Environmental Protection Agency STAR program (grant numbers: R-829388 and R833292), and by the University of California, Davis MIND (Medical Investigations of Neurodevelopmental Disorders) Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. 2004. Pesticides and oxidative stress: a review. Med Sci Monit 10(6):RA141–RA147, PMID: 15173684. [PubMed] [Google Scholar]
- Braun JM, Froehlich T, Kalkbrenner A, Pfeiffer CM, Fazili Z, Yolton K, et al. 2014a. Brief report: are autistic-behaviors in children related to prenatal vitamin use and maternal whole blood folate concentrations? J Autism Dev Disord 44(10):2602–2607, PMID: 24710813, 10.1007/s10803-014-2114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. 2014b. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children. The HOME study. Environ Health Perspect 122(5):513–520, PMID: 24622245, 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, Burnham KP. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed New York:Springer. [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. 2016. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 65:1–23, PMID: 27031587, 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collotta M, Bertazzi PA, Bollati V. 2013. Epigenetics and pesticides. Toxicology 307:35–41, PMID: 23380243, 10.1016/j.tox.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Corsini E, Liesivuori J, Vergieva T, Van Loveren H, Colosio C. 2008. Effects of pesticide exposure on the human immune system. Hum Exp Toxicol 27(6):671–680, PMID: 19042949, 10.1177/0960327108094509. [DOI] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, Bailey LB. 2012. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr 3(1):21–38, PMID: 22332098, 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 104(32):13056–13061, PMID: 17670942, 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie SJ. 1999. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 55(3):578–592, PMID: 10746348, 10.1258/0007142991902646. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Narayanan S, Sharp L, Little J, Basten G, Powers H. 2004. Folate, DNA stability and colo-rectal neoplasia. Proc Nutr Soc 63(4):571–578, PMID: 15831129, 10.1079/PNS2004. [DOI] [PubMed] [Google Scholar]
- Engel LS, Seixas NS, Keifer MC, Longstreth WT Jr, Checkoway H. 2001. Validity study of self-reported pesticide exposure among orchardists. J Expo Anal Environ Epidemiol 11:359–368, PMID: 11687909, 10.1038/sj.jea.7500176. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115(5):792–798, PMID: 17520070, 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes C, Mastroeni S, Boffetta P, Salvatori V, Melo N, Bolli S, et al. 2009. Reliability of self-reported household pesticide use. Eur J Cancer Prev 18(5):404–406, PMID: 19433981, 10.1097/CEJ.0b013e32832caab5. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. 2006. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr 84(5):1093–1101, PMID: 17093162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 114(7):1119–1125, PMID: 16835068, 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG,Niedzwiecki MM, Hall MN, Liu X, Ilievski V, Slavkovich V, et al. 2014. Folate and cobalamin modify associations between s-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed bangladeshi adults. J Nutr 144(5):690–697, PMID: 24598884, 10.3945/jn.113.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. 2000. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC:National Academic Press. [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. 2004. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617, PMID: 15585776. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. 2009. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr 89(1):425–430, PMID: 19056591, 10.3945/ajcn.2008.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. 2001. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med 30(12):1390–1399, PMID: 11390184, 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- Keil AP, Daniels JL, Hertz-Picciotto I. 2014. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case–control study. Environ Health 13(1):3, PMID: 24456651, 10.1186/1476-069X-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, et al. 2010. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect 118(3):370–374, PMID: 20064773, 10.1289/ehp.0901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrou A, Baccarelli A, Wright RO, Weisskopf M, Bollati V, Amarasiriwardena C, et al. 2012. Arsenic exposure and DNA methylation among elderly men. Epidemiology 23(5):668–676, PMID: 22833016, 10.1097/EDE.0b013e31825afb0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et al. 1997. Diagnosing autism: analyses of data from the autism diagnostic interview. J Autism Dev Disord 27(5):501–517, PMID: 9403369. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. 2000. The Autism Diagnostic Observation Schedule (ADOS). Los Angeles, CA:Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. 2003. Autism Diagnostic Observation Schedule Manual. Los Angeles, CA:Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24(5):659–685, PMID: 7814313, 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Ibne Hasan MO, Hamid R, Valeri L, Paul L, Selhub J, et al. 2015. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: A case control study in Bangladesh. Environ Health 14:34, PMID: 25885259, 10.1186/s12940-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCanlies EC, Fekedulegn D, Mnatsakanova A, Burchfiel CM, Sanderson WT, Charles LE, et al. 2012. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord 42(11):2323–2334, PMID: 22399411, 10.1007/s10803-012-1468-1. [DOI] [PubMed] [Google Scholar]
- Mullen EM. 1995. Scales of Early Learning. Circle Pines, MN:American Guidance Services Inc. [Google Scholar]
- Ouyang F, Longnecker MP, Venners SA, Johnson S, Korrick S, Zhang J, et al. 2014. Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: independent and joint effects on women's reproductive outcomes. Am J Clin Nutr 100(6):1470–1478, PMID: 25411282, 10.3945/ajcn.114.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. 2015. Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect 123(12):1294–1301, PMID: 25978852, 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. 2001. Epigenetic reprogramming in mammalian development. Science 293(5532):1089–1093, PMID: 11498579, 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Szatmari P, et al. 2006. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 45(9):1094–1103, PMID: 16926617, 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB. 2013. Bayesian modeling of time-dependent vulnerability to environmental hazards: an example using autism and pesticide data. Stat Med 32(13):2308–2319, PMID: 22961924, 10.1002/sim.5600. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. 2007. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 115(10):1482–1489, PMID: 17938740, 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas LG, Eskenazi B. 2008. Pesticides and child neurodevelopment. Curr Opin Pediatr 20(2):191–197, PMID: 18332717, 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. 2008. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect 116(11):1547–1552, PMID: 19057709, 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. 2011. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22(4):476–485, PMID: 21610500, 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. 2012. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the charge (childhood autism risks from genetics and environment) case–control study. Am J Clin Nutr 96(1):80–89, PMID: 22648721, 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Schroeder DI, Crary-Dooley FK, Barkoski JM, Trancredi DJ, Walker CK, et al. 2016. Self-reported pregnancy exposures and placental DNA methylation in the MARBLES prospective autism sibling study. Environ Epigenet 2(4):dvw024, 10.1093/eep/dvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, LaSalle JM. 2011. Interactions between folate, other B vitamins, DNA methylation, and neurodevelopmental disorders. In: Nutrition, epigenetic mechanisms, and human disease, (Maulik N, Maulik G, eds). Boca Raton, FL:CRC Press: Taylor & Francis Group. [Google Scholar]
- Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. 2014. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol 180(9):890–900, PMID: 25249546, 10.1093/aje/kwu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby MA, El Zorba HY, Ziada RM. 2010. Reproductive toxicity of methomyl insecticide in male rats and protective effect of folic acid. Food Chem Toxicol 48(11):3221–3226, PMID: 20813150, 10.1016/j.fct.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 122(10):1103–1109, PMID: 24954055, 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh Y, Takeda M, Ohtsuka R, Haishima A, Yamaguchi S, Fujie H, et al. 2009. Low dose effects of dichlorodiphenyltrichloroethane (DDT) on gene transcription and DNA methylation in the hypothalamus of young male rats: implication of hormesis-like effects. J Toxicol Sci 34(5):469–482, PMID: 19797855, 10.2131/jts.34.469. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. 1984. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. Circle Pines, MN:American Guidance Services, Inc. [Google Scholar]
- Steenweg-de Graaff J, Ghassabian A, Jaddoe VW, Tiemeier H, Roza SJ. 2015. Folate concentrations during pregnancy and autistic traits in the offspring. The Generation R Study. Eur J Public Health 25(3):431–433, PMID: 25085472, 10.1093/eurpub/cku126. [DOI] [PubMed] [Google Scholar]
- Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. 2013. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309(6):570–577, PMID: 23403681, 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undeğer U, Başaran N. 2005. Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch Toxicol 79(3):169–176, PMID: 15798889, 10.1007/s00204-004-0616-6. [DOI] [PubMed] [Google Scholar]
- Virk J, Liew Z, Olsen J, Nohr EA, Catov JM, Ritz B. 2016. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism 20(6):710–718, PMID: 26408631, 10.1177/1362361315604076. [DOI] [PubMed] [Google Scholar]
- Voccia I, Blakley B, Brousseau P, Fournier M. 1999. Immunotoxicity of pesticides: a review. Toxicol Ind Health 15(1–2):119–132, PMID: 10188195, 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wallace AD, Du P, Kibbe WA, Jafari N, Xie H, et al. 2012a. DNA methylation alterations in response to pesticide exposure in vitro. Environ Mol Mutagen 53(7):542–549, PMID: 22847954, 10.1002/em.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H, et al. 2012b. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol 34(3):959–968, PMID: 22964155, 10.1016/j.etap.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Karlsson O, Wang G, Li J, Guo Y, Lin X, et al. 2017. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci USA 114(13):3503–3508, PMID: 28289216, 10.1073/pnas.1618545114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.