Abstract
Background:
Courtship behavior plays a critical role in attracting females and reproduction success. However, the effects of exposure to a ubiquitous contaminant di(2-ethylhexyl) phthalate (DEHP) on these behaviors and, in particular, on courtship vocalizations have not been examined.
Objective:
The effects of adult exposure to DEHP on courtship and mating behaviors and gonadotropic axis and neural mechanisms involved in DEHP-induced effects were analyzed in male mice.
Methods:
Adult C57BL/6J males were orally exposed to DEHP (0, 0.5, 5, and ) for 4 wk. Olfactory preference, ultrasonic vocalizations (USVs), partner preference and mating, as well as locomotor activity and motor coordination, were measured. The kisspeptin system and testosterone levels were analyzed. Proteomic and molecular studies were conducted on the hypothalamic preoptic nucleus, the key region involved in sexual motivation to vocalize and mate.
Results:
DEHP at reduced the emission of USVs, whereas lower doses changed the ratio of syllable categories. This was associated with diminished sexual interest of female partners toward males exposed to 5 or and increased latency to mate, despite normal olfactory preference. The kisspeptin system and circulating testosterone levels were unaffected. In DEHP-exposed males, proteomic analysis of the preoptic nucleus identified differentially expressed proteins connected to the androgen receptor (AR). Indeed, exposure to 5 or of DEHP induced selective AR downregulation in this nucleus and upstream chemosensory regions. The involvement of AR changes in the observed alterations was further supported by the reduced emission of courtship vocalizations in males with disrupted neural AR expression.
Conclusions:
These data demonstrate the critical role of neural AR in courtship vocalizations and raises the possibility that the vulnerability of this signaling pathway to exposure to endocrine disrupters may be detrimental for courtship communication and mating in several species. https://doi.org/10.1289/EHP1443
Introduction
In the last decades, a decline in male reproductive health of wildlife and humans has been documented in several locations worldwide (WHO 2012). This decline parallels the massive increase in chemical contamination due to the use of natural or man-made synthetic compounds with hormone-disrupting activities. Among these chemicals, phthalates are the most frequently detected organic pollutants in the environment in both urban and rural areas, due to their extensive use in the manufacture and processing of plastic products (Gao and Wen 2016). In rodents, malformations of the male genital tract have been reported for developmental exposure to di(2-ethylhexyl) phthalate (DEHP) (Christiansen et al. 2010; Moore et al. 2001). DEHP effects on sexual behavior were also examined for developmental exposure (Andrade et al. 2006; Dalsenter et al. 2006; Moore et al. 2001). Whether and how adult exposure to DEHP affects the male brain, particularly the neural structures underlying courtship behaviors, is yet to be studied. It is now largely established that the adult brain retains plasticity, as several factors, such as environmental stimulation, drugs of abuse, or aging, affect neural structure and function.
Courtship behaviors are essential for sexual reproduction and constitute a chain of behavioral responses leading to mating. In rodents, the courting male displays a chemoinvestigating behavior towards females with an olfactory preference for those that are receptive. In response to females or their odors, the male produces ultrasonic vocalizations (USVs) (Bean 1982; Dizinno and Whitney 1977; Nyby et al. 1977), which display the characteristics of songs observed in songbirds (Holy and Guo 2005). The emission of courtship vocalizations conveys information about the male’s motivational state and plays a role in attracting the female partner. These precopulatory behaviors precede the copulatory phase, during which the male exhibits mounting, thrusting, and intromitting behaviors before reaching ejaculation.
Here, we characterized the effects of chronic exposure to DEHP during adulthood on courtship behaviors and the mechanisms underlying DEHP-induced effects in the male brain. For this purpose, adult C57BL/6J mice were orally exposed for 4 wk to the vehicle or DEHP at the tolerable daily intake dose () established by the European Food Safety Authority, and two lower doses of 0.5 and , which frame the environmental exposure reported worldwide (Martine et al. 2013; Dewalque et al. 2014; Koch et al. 2006; Lorber et al. 2010). Courtship behavior was analyzed by testing olfactory preference for receptive females, emission of courtship vocalizations, and ability to attract receptive females and initiate mating.
To understand how DEHP exposure affects male behaviors, we first measured circulating levels of testosterone and analyzed the integrity of the hypothalamic–pituitary–gonadal (HPG) axis through analysis of the kisspeptin system. Indeed, these behaviors are tightly controlled by testosterone, and DEHP exposure could indirectly induce behavioral modifications through changes in this system. Kisspeptin acts as a central regulator of the HPG axis and is currently considered to be a key central target of testosterone feedback regulation for gonadotropin-releasing hormone/luteinizing hormone release (Navarro et al. 2011; Raskin et al. 2009; Ruka et al. 2016; Smith et al. 2005). A mouse line expressing green fluorescent protein (GFP) reporter gene under the control of the Kiss1 promoter (Gottsch et al. 2011) was also used in order to determine DEHP-induced effects on Kiss1 expression.
We next asked whether exposure to DEHP directly triggers modifications in neural structures involved in sexual behavior. The medial preoptic nucleus (MPN), a key region underlying the motivation to vocalize and mate (Alward et al. 2013; Balthazart et al. 2004; Riters and Ball 1999), was subjected to a proteomic analysis in order to identify the targets of DEHP exposure. Chemosignals transmitted from the olfactory bulb through the medial amygdala and bed nucleus of stria terminalis are processed into behavioral responses in this nucleus. The choice of the proteomic approach strategy was motivated by the fact that proteins are the principal biological effector molecules of a cell. The obtained data were validated by Western blotting and verified by immunohistochemical and molecular analyses, in parallel to assessment of courtship vocalizations in a mouse line lacking the androgen receptor (AR) in the nervous system (Raskin et al. 2009).
Methods
Animals and Treatments
Studies were performed in accordance with the French and European legal requirements (Decree 2010/63/UE) and were approved by the Charles Darwin Ethical Committee (project number 01,490-01). All animals were treated humanely and with regard for alleviation of suffering.
Mice were housed in nest-enriched polysulfone cages under a 12:12h light–dark cycle, maintained at 22°C, and fed a standard diet with free access to food and water. The number of experimental and control groups are given in the figure legends.
In order to mimic the major route of exposure, DEHP (Sigma-Aldrich) was dissolved in ethanol and water and incorporated into food. Eight-week-old C57BL/6J males (Janvier) were fed ad libitum with chow containing the vehicle (controls) or DEHP so that the exposure was equivalent to 50 (DEHP-50 group), 5 (DEHP-5 group) or (DEHP-0.5 group). DEHP doses were calculated for a daily intake food of per animal. Mice were weighed weekly and DEHP doses adjusted to their body weight. Body weight followed throughout the exposure period was similar in the four treatment groups (mean of the first day of exposure and the last day of exposure). Analyses started after 4 wk of exposure and were maintained during this period.
Kiss1-creGFP transgenic mice [Kiss-1tm1.1(cre/EGFP)Stei/J; The Jackson Laboratory] were bred on a C57BL/6J background for over 10 generations. The mouse line lacking the neural AR was obtained on a C57BL/6J background, as previously shown (Raskin et al. 2009, 2012). As mutant males () exhibit higher hormonal levels compared to their control littermates (), testosterone levels were normalized between the two genotypes by gonadectomy and supplementation with subcutaneous implants containing testosterone (Sigma-Aldrich), as previously described (Raskin et al. 2012).
Behavioral Tests
Tests were conducted under red light illumination 2 h after lights off and videotaped for further analyses. Four weeks after DEHP exposure or testosterone supplementation, naïve males were individually housed for 3 d. Each male was paired in its home cage with a sexually receptive female and let to reach ejaculation. This first sexual experience was performed in order to increase the expression of behaviors such as olfactory preference, USVs, and sexual interest of studied males toward receptive C57BL/6J females (Dizinno et al. 1978; Marie-Luce et al. 2013; Picot et al. 2014). Behavioral analyses were conducted 2 wk later.
Receptive females were ovariectomized, supplemented with implants containing estradiol benzoate (Sigma-Aldrich) and primed with progesterone (Sigma-Aldrich) 4–5 h before the test, as previously described (Raskin et al. 2009).
Olfactory preference.
Olfactory preference was assessed in an enclosed Plexiglas Y-maze, as previously described (Picot et al. 2014). On the day of the test, mice were offered the choice between an anesthetized sexually receptive female and an anesthetized gonadally intact male. The time spent in chemoinvestigation of each stimulus and the number of entries in each arm were scored during the 5-min test.
Ultrasonic vocalizations.
Each male was tested in its home cage. The vocalizations were recorded, after the introduction of a receptive female, for 4 min with an Ultrasound gate microphone CM16/CMPA (Avisoft Bioacoustics), which was connected to an Ultrasound recording interface plugged into a computer equipped with the recording software Avisoft-SASLab Pro 5.2.09, and then analyzed using SASLab Pro (Avisoft Bioacoustics). Spectrograms were generated for each detected call (frequency resolution: fast Fourrier transform length: 512; frame size: 100%; overlap: 50%). The parameters used for the automatic quantification of the USVs were: cutoff frequency of and element separation based on an automatic single threshold with a hold time of 15 ms. Syllables were identified and grouped into three main categories (Figure S1A), as previously described (Ey et al. 2013). The total number and duration of USVs, the number and duration of each syllable, and the percentage of each category (number of syllables of each category/total number) were analyzed.
Partner preference.
Females were allowed to become familiar with the testing arena (Figure S2A) for 10 min during two consecutive days. The day of the test, each sexually receptive female was placed in the neutral chamber and allowed to freely explore each chamber of the testing arena for 10 min. A DEHP-treated male was placed inside a corral and randomly assigned to the left or right chamber, while a vehicle-treated male was placed inside a corral in the other chamber. The number of entries in each compartment and time spent sniffing each male over the 10-min test by the female were also scored.
Mating.
Each male was tested in its home cage for 10 h after the introduction of the receptive female, as previously described (Picot et al. 2014). The latency to the first anogenital chemoinvestigation and intromission and latency to ejaculation (time from female introduction into the cage until the behavior), the mating duration (time from the first intromission until ejaculation), the frequency of mounts with and without intromissions, the intromission ratio, and genital grooming were scored.
Locomotor activity and motor function.
Locomotor activity was analyzed in a computed circular corridor made of two concentric cylinders and crossed by four diametrically opposite infrared beams, as previously described (Raskin et al. 2009). The locomotor activity was counted when animals interrupted two successive beams and thus had traveled a quarter of the circular corridor. Activity of another group of males was recorded for 10 h in experimental conditions similar to those used for sexual behavioral tests, as previously shown (Raskin et al. 2009). Males were also tested in the rotarod task assessing balance and coordination over three consecutive days, and the latency to fall was recorded, as previously described (Boussicault et al. 2016).
Urogenital Tract Weight and Hormone Measurements
At the end of behavioral experiments, animals were sacrificed to collect blood and to weigh testis and seminal vesicles. Sera were extracted, and levels of testosterone were measured by radioimmunoassay at the hormonal assay platform of the Unité Mixte de Recherche (UMR) 7247/Institut National de la Recherche Agronomique (INRA)/Centre National de la Recherche Scientifique (CNRS) laboratory using 3H-T, as previously described (Picot et al. 2014). The mean intra-assay coefficient of variation was 7%, and assay sensitivity was .
Proteomic Analysis
Proteins were extracted from punches of the MPN (four to five males per group) in buffer A ( urea, thiourea, 1% CHAPS (G-Biosciences), 10% isobutanol, 0.5% Triton X-100 (GE Healthcare), 0.5% SB 3-10). Protein concentrations were established using the Quick Start Bradford Protein Assay (Bio-Rad) using bovine serum albumin as standard.
Protein extracts were labeled with the 3Dye Cy2/3/5 fluor Labeling Pack (Fluoprobes, Interchim) following manufacturer’s instructions. Each gel contained of internal standard labeled with Cy2 and of two different samples labeled with Cy3 and Cy5, respectively. For isoelectrofocalization, strips (pH 5–8, 24 cm, Bio-Rad) were rehydrated for 16 h with the samples diluted in buffer A supplemented with dithiothreitol (DTT) and 0.5% immobilized pH gradient buffer. Isoelectric focusing was run up to 90,000 Vh (Ettan II IPGphor system, GE Healthcare). Strips were soaked for 15 min in buffer B [ urea, Tris, 30% glycerol, 3% sodium dodecyl sulfate (SDS)] with 1% DTT, and then 20 min in buffer B with 2.5% iodoacetamide. Strips were loaded onto a 14% SDS polyacrylamide gels for overnight separation (Ettan DALTsix, GE Healthcare). 2-D gels were scanned using an Ettan DIGE Imager (GE Healthcare). After scanning, gels were fixed and stained with silver nitrate: incubated for 1 min in 0.02% sodium thiosulfate, rinsed in ultrapure water, incubated for 30 min in 0.2% silver nitrate solution, washed in ultrapure water, and developed in 3% sodium carbonate, 0.00,125% sodium thiosulfate, and 0.03% formalin.
Image analysis was performed using Progenesis SameSpots (version 3.2; TotalLab). Analysis of variance (ANOVA) analyses were performed among the three treatment groups and on each pair of groups. Significant spots with a ratio higher than or equal to 1.3 were selected for protein identification.
Spots of interest were excised and destained with potassium ferricyanide and sodium thiosulfate. Gel pieces were washed in ultrapure water and then in acetonitrile (ACN) and air dried. Gel pieces were rehydrated on ice with ammonium bicarbonate, 5% ACN, and of trypsin (G-Biosciences). Digestion was performed overnight at 37°C. Supernatants were collected, and gel pieces were rinsed twice with 0.1% trifluoroacetic acid and 60% ACN in an ultrasonic bath to extract residual peptides. Peptides were dried out in a vacuum centrifuge, resuspended in 0.1% formic acid and 3% CAN, and stored at .
For liquid chromotagraphy tandem-mass spectrometry (LC-MS/MS) analyses, digests were injected into an UltiMate 3,000 Nano LC system (Thermo Fisher Scientific) coupled to an Esquire HCTultra nESI IT-MS (Bruker Daltonics). Peptides were desalted for 5 min on a C18 RP precolumn (, ID, , ) then separated on a C18 RP column (, identifier, , , Thermo Fisher Scientific) with a 30-min gradient from 2% to 35% ACN in 0.1% formic acid. MS/MS acquisition cycles were performed on the top eight most abundant eluting peptides. Protein identification was carried out with MASCOT (2.2.07, Matrix Science): one missed cleavage, carbamidomethylation of cysteine (fixed modification), oxidation of methionine (variable modification), 0.5 Da MS and MS/MS tolerances, database UniProt mouse reference proteome (22,215 entries) (UniProt Consortium 2017). Proteins with at least two unique peptides with a p-value were validated.
Western Blotting
Validation was performed on punches dissected from the MPN. Protein extracts (), prepared as previously described (Picot et al. 2016), were separated on 10% polyacrylamide gel and transferred onto a nitrocellulose membrane. Blots were probed with monoclonal anti-glial fibrillary acidic protein (GFAP) (1:500, Sigma-Aldrich), , and anti-glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) (both at 1:1,000, Santa Cruz Biotechnology), and anti-N-myc downstream-regulated gene 2 (NDRG2) (1:1,000, Cell Signaling) overnight and then peroxidase-conjugated second antibodies (Amersham Biosciences) diluted at 1:5,000. Signals were visualized with SuperSignal detection kit (Thermo Fisher Scientific), quantified with ImageJ software (version 2.0.0-rc-15/1.49j; National Institutes of Health), and normalized with respect to the housekeeping reference GAPDH.
Immunohistochemistry
Animals were sacrificed and transcardially perfused with a solution of 4% paraformaldhehyde (PFA) in phosphate buffer. Brains were postfixed overnight in 4% PFA, cryoprotected in sucrose, and stored until analyses. Brains were sliced into coronal sections of and processed for single and double immunolabeling.
Kisspeptin immunolabeling was processed with anti-kisspeptin AC053, and sections were counterstained with Hoechst, as previously shown (Naulé et al. 2015). The number of labeled cell soma and fiber density in the rostral periventricular area of the third ventricle (RP3V, Clarkson and Herbison 2006) and arcuate nucleus were measured. Quantifications were performed on three sections sampled at three anteroposterior levels of the RP3V (plates 29, 30, and 31–32 of Paxinos and Franklin Atlas) and on three sections of the arcuate nucleus sampled at the level of the anterior, median, and caudal arcuate nucleus (plates 43, 47, and 50).
For and EGFP/AR double immunolabelling, sections were blocked for 30 min with 2% normal donkey serum and incubated with polyclonal chicken anti-GFP (1:10,000, Aves Lab) and either rabbit anti-AR or anti- (1:250, Santa Cruz Biotechnology) for 3 d. A cocktail of Alexa Fluor 488-conjugated donkey anti-chicken and Cyanin 3-conjugated donkey anti-rabbit antibodies (1:500, Jackson ImmunoResearch) was applied to the sections for 2 h. Sections were counterstained with Hoechst and mounted on slides with Fluoromount (Sigma-Aldrich). Quantifications of the proportion of GFP cells coexpressing either AR or were performed on two sections sampled at two levels of the RP3V (plates 30 and 31–32) and from 12 equidistant () hemisections encompassing the whole rostrocaudal extent of the arcuate nucleus (plates 41 and 53). Data are displayed as average numbers of GFP-positive nuclear counts per section or hemisection and per group.
AR- and was processed, as previously shown (Naulé et al. 2016). The number of labeled cells per sections counted in anatomically matched sections identified using the Mouse Brain Atlas of Paxinos and Franklin (2001).
Quantitative Reverse Transcription Polymerase Chain Reaction
Fresh tissues were rapidly frozen in a isopentane solution and stored at until use. Total RNAs were extracted using TRIzol reagent (Invitrogen). RNA () was reverse transcribed into cDNA using the Superscript III First-Strand Synthesis System (Invitrogen) and random hexamers. Each DNA sample was analyzed in triplicate, along with standard and no-template controls. The incubation media contained of each reverse transcription reaction in of Mastermix, along with forward and reverse primers (AR: ggcggtccttcactaatgtcaact and tggagccatccaaactcttgagac; glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH; Naulé et al. 2016). Polymerase chain reaction (PCR) conditions were 95°C for 5 min, 42 cycles at 95°C for 15 s, 61°C for 15 s, and 72°C for 10 s. PCR specificity was verified by melting curve analysis, and product purity was confirmed by agarose gel electrophoresis. All experiments had efficiencies between 90% and 101% and were analyzed with the LCS 480 software (version 1.5, Roche). Each sample was run in triplicate to obtain an average cycle threshold value (Ct), and relative gene expression was determined using the comparative Ct method (Pfaffl 2001). The data were normalized to GAPDH expression level.
Statistics.
Data were expressed as means Student’s t-tests were used to determine the effect of neural AR invalidation on the number and duration of vocalizations (unpaired samples) and for the time spent in investigation for partner preference (paired samples). Two-way ANOVA was used to analyze the main effects of exposure and stimulus on olfactory preference, and exposure and time on locomotor activity. One-way ANOVA was used to analyze the effect of DEHP exposure on the remaining data. Tukey post hoc tests were used to determine group differences. p-Values were considered to be significant.
Results
Effect of Di(2-ethylhexyl) Phthalate Exposure on Courtship Behavior
We compared the olfactory investigation and emission of courtship vocalizations in the presence of sexually receptive females between males exposed to the vehicle or DEHP.
Olfactory preference.
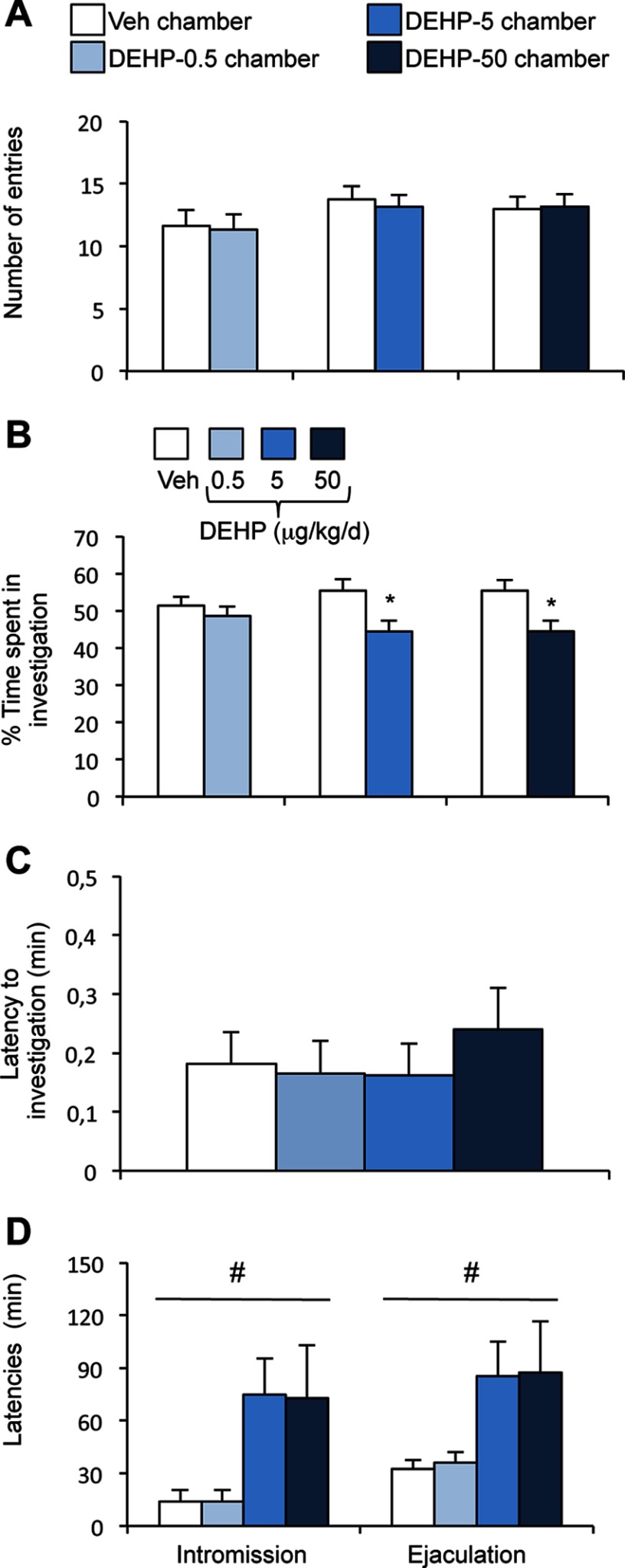
We tested the ability of males to discriminate between male and female odors in preference tests using gonadally intact males vs. sexually receptive females. In this Y-maze paradigm, the total time spent sniffing the stimuli was equivalent between males from the four exposure groups (Figure 1A). Two-way ANOVA of the number of entries into each arm (Figure 1B) and the percentage of time spent sniffing each stimulus (Figure 1C) showed an effect of stimulus [, , and , , respectively], but not of DEHP exposure on both parameters [, , and , , respectively]. Males displayed a preference for female cues, regardless of their exposure to DEHP.
Figure 1.
Effects of phthalate di(2-ethylhexyl) phthalate (DEHP) on olfactory preference and courtship vocalizations in male mice. (A) Total time spent in the chemoinvestigation of male (♂) and sexually receptive female (♀) stimuli by males exposed to the vehicle (Veh) or DEHP (0.5, 5, or ). (B) Number of entries into the male or female arm of the Y-maze. (C) Percentage of time spent in investigating males (♂) vs. sexually receptive females (♀). Data are expressed as the means of 11–12 males per treatment group, * vs. the male arm. (D–E) Representative sound waveform for males exposed to vehicle (D) or DEHP at (E) in the presence of a sexually receptive female. (F–G) Total number (F) and duration (G) of ultrasonic vocalizations (USVs) produced during the 4-min test by males exposed to the vehicle or DEHP at 0.5, 5, or . Data are expressed as the means of 11–12 males per treatment group; *, **.
Courtship vocalizations.
In the presence of a sexually receptive female, male mice vocalized mainly at a frequency of (see Figure S1A). Figure 1D–E illustrates typical sound waveforms of vocalizations produced by a vehicle and a DEHP-50 exposed male. One-way ANOVA showed an effect of DEHP exposure on the total number (; Figure 1F) and duration (, Figure 1G) of emitted USVs. Post hoc analyses revealed a significant reduction of USV frequency () and duration () in the DEHP-50 group. Detailed analyses of courtship vocalizations showed the presence of nine major syllables, grouped into three main categories identified as simple (short, upward, downward, flat), complex (modulated, complex), and with frequency jumps (mixed, one jump, with frequency jumps) as illustrated (see Figure S1A). Comparison of the frequency of each syllable during the 4-min recording showed an effect of DEHP treatment on eight of the nine emitted vocalizations (Figure 2A). The frequency of all eight syllables was reduced after chronic exposure to DEHP-50, whereas the number of short and flat USVs decreased in the DEHP-0.5 and DEHP-5 groups (Figure 2A). The percentage of emitted USVs per category between the four groups was conserved in the DEHP-50 group, but was altered in the DEHP-0.5 and DEHP-5 groups ( of simple syllables and of USVs with frequency jumps) relative to the vehicle group (Figure 2B).
Figure 2.
Quantitative and qualitative analyses of syllables emitted by males exposed to di(2-ethylhexyl) phthalate (DEHP). (A) Total number of each syllable type produced in the presence of a sexually receptive female during the 4-min recording for males exposed to the vehicle (Veh) or DEHP at 0.5, 5, or . One-way analysis of variance (ANOVA) showed an effect of DEHP treatment (#) on short (), flat (), downward (), modulated (), complex (), mixed (), one-jump (), and frequency jump (0.019) syllables. Post hoc comparisons are indicated: *, **, ***. Data are expressed as the means of 11–12 males per treatment group. (B) Percentage of courtship vocalizations per category (simple, complex, and with frequency jumps). (C) Total duration of each syllable type. One-way ANOVA showed an effect of DEHP treatment (#) on short (), modulated (), complex (), one-jump (), and frequency jump (0.05) syllables. Post hoc comparisons are indicated: *, **, ***.
DEHP exposure also affected the total duration of emitted syllables, with a significant effect on short, modulated, complex, one-jump, and frequency jump syllables (Figure 2C). However, DEHP exposure had no effect on the mean duration of any syllable in the four groups (see Figure S1B). The observed changes in the total duration of courtship vocalizations following chronic exposure to DEHP were, therefore, mainly due to differences in the number of emitted USVs.
Partner Preference and Mating
Previous studies have suggested that courtship vocalizations attract the female to approach the vocalizing males, which may then facilitate copulation (Pomerantz et al. 1983). We thus analyzed vehicle- and DEHP-exposed males for their ability to attract and mate with a receptive female.
Partner preference.
A sexually experienced receptive female was presented with a pair of males, one from the vehicle group and the other from the DEHP-exposed group. Each male was placed in one of two opposite compartments, separated by a neutral one, in a three-chamber paradigm (see Figure S2A). The total time spent by the female to investigate both stimuli (see Figure S2A) and the number of entries in each compartment (vehicle vs. DEHP-0.5, vehicle vs. DEHP-5, and vehicle vs. DEHP-50) were similar for the three experimental conditions (Figure 3A). The percentage of time spent investigating each male show that the females exhibited comparable interest for vehicle and DEHP-0.5 males (Figure 3B), but spent more time investigating vehicle males than DEHP-5 or DEHP-50 males ( and 0.035 vs. vehicle males, respectively).
Figure 3.
Effects of di(2-ethylhexyl) phthalate (DEHP) treatment on female interest in males and latency to intromission and ejaculation. (A–B) The partner preference test was performed in a three-chamber paradigm. (A) The number of entries of sexually receptive females into the chamber of the vehicle (Veh) vs. the chamber of the DEHP-exposed male. (B) The percentage of time spent investigating the vehicle (Veh) vs. the DEHP-exposed male. Data are expressed as the means of 11–12 males per treatment group, * vs. the vehicle male. (C–D) In the mating test, one-way analysis of variance (ANOVA) showed no effect of DEHP treatment on the latency to the first anogenital chemoinvestigation towards sexually receptive females (C), but an effect (#) on the latencies to first intromission and ejaculation (D). Data are expressed as the means of 11–12 males per treatment.
Mating.
We compared the latency and frequency of mating events of vehicle and DEHP-treated males. One-way ANOVA showed no effect of DEHP exposure on the latency to the first anogenital chemoinvestigation towards receptive females (; Figure 3C). By contrast, there was a significant effect of DEHP exposure on the latency to the first intromission () and to ejaculation (), with the DEHP-5 and DEHP-50 groups exhibiting these behaviors later than the vehicle group (Figure 3D). The time from the first intromission to ejaculation (mating duration) was comparable between the four groups (Table 1), indicating that the increased latency to ejaculation exhibited by DEHP-5 and DEHP-50 groups was mainly due to delayed intromission. Once copulation was initiated, the frequency of mounts, intromissions, and thrusts were comparable between the treatment groups (Table 1). The intromission ratio, generally used to evaluate the efficiency of erection in copula (Ågmo 1997; Cruz et al. 1999), and time spent in genital grooming were also unchanged by DEHP exposure ( and , respectively).
Table 1.
Quantification of sexual behavior and endocrine analysis.
| Exposure | Vehicle | DEHP-0.5 | DEHP-5 | DEHP-50 |
|---|---|---|---|---|
| Mating behavior componentsa | ||||
| Number of M | ||||
| Number of MI | ||||
| Number of Th | ||||
| MI ratio | ||||
| Mating duration (min) | ||||
| Grooming duration (%) | ||||
| Urogenital tract weight and testosterone levelsb | ||||
| Body weight (g) | ||||
| Testis weight (% bw) | ||||
| SV weight (% bw) | ||||
| Testosterone (ng/ml) |
The number of mounts without intromissions (M), mounts with intromissions (MI) and thrusts (Th), mounting index ratio (), mating duration (time from the first intromission to ejaculation), and genital grooming expressed as percentage of mating duration, were determined in males exposed to the vehicle or di(2-ethylhexyl) phthalate (DEHP) at 0.5, 5, or . Data are expressed as the means for 11–12 males per treatment group.
Body weight (bw), testicular and seminal vesicle (SV) weights, and testosterone levels of vehicle and DEHP-exposed males subjected to behavioral tests. Data are expressed as the means for 11–12 males per treatment group. The data for the testis and SV are expressed as the percentages of the corresponding body weight.
Locomotor activity and motor function.
The altered courtship behavior was associated with normal activity and motor function of DEHP-exposed mice. Locomotor response recorded immediately after male introduction in the corridor did not show any difference between the four groups (Figure S2B). Locomotor activity was also measured for 10 h in the same experimental conditions showing DEHP effects on courtship behaviors. Activity was maximal during the first 6 h of the dark phase before decreasing progressively for all treatment groups (Figure S2C). In the rotarod, the latency to fall was not significantly different between the three treatment groups (Figure S2D).
Analysis of the Hypothalamic–Pituitary–Gonadal Axis
We tested whether the behavioral alterations induced by DEHP exposure were caused by changes in kisspeptin system and testosterone levels. Male behaviors are, indeed, tightly regulated by gonadal testosterone. Furthermore, developmental exposure to DEHP doses has been shown to reduce testosterone production in mice (Barakat et al. 2017; Pocar et al. 2012). We compared the number of kisspeptin-immunoreactive cells in the RP3V and the density of kisspeptin-immunoreactive fibers in the RP3V and arcuate nucleus between vehicle- and DEHP-exposed males. Kisspeptin neurons are localized in two hypothalamic regions. The RP3V, which is involved in the positive feedback and ovulatory surge of LH in females, contains fewer cells in males due its perinatal masculinization by testosterone (Clarkson and Herbison 2006, Clarkson and Herbison 2009). The arcuate nucleus integrates the negative feedback exerted by sex steroid hormones in both sexes.
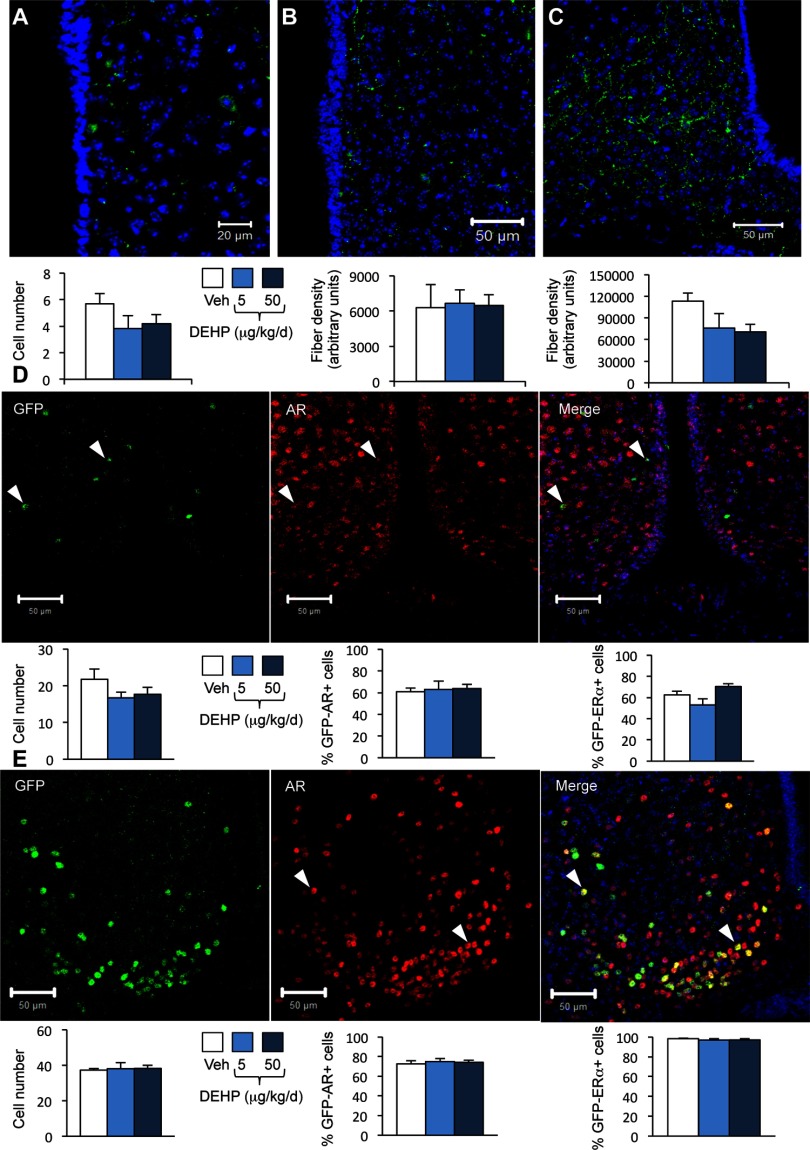
One-way ANOVA showed no significant effects of DEHP treatment on kisspeptin immunoreactivity in these two brain regions (Figure 4A–C). We further investigated possible alterations of Kiss1 gene expression levels upon DEHP exposure by quantifying the number of GFP-positive cells in mice exposed to vehicle or DEHP from a line expressing GFP under the control of the Kiss1 promoter. One-way ANOVA showed no significant effects of DEHP treatment on the number of GFP-positive cells in the RP3V or the arcuate nuclei (Figure 4D–E). The proportion of GFP-positive cells coexpressing the AR or was also unchanged by DEHP exposure (Figure 4D–E; Figure S3).
Figure 4.
Effects of di(2-ethylhexyl) phthalate (DEHP) on kisspeptin, green fluorescent protein (GFP)/androgen receptor (AR) and immunoreactivity in wild type and Kiss1-creGFP male mice. Mice were treated with DEHP (0.5, 5, or ) or the vehicle (Veh). Analyses were performed in the rostral periventricular area of the third ventricle (RP3V) and arcuate nucleus. (A–C) Representative immunolabelling (upper panels) and quantification (lower panels) of the number of kisspeptin-immunoreactive neurons in the RP3V (A) and fiber density in the RP3V (B) and arcuate nucleus (C) in wild type mice. Data are expressed as the means of six males per treatment group. (D) Upper panels: Representative immunolabelling of GFP- (left), AR-immunoreactivity (middle), and the merge (right) in the RP3V of Kiss1-creGFP males. Lower panels: quantification of the number of GFP-cells (left), GFP/AR- (middle), and -coexpressing cells (right). Data are expressed as the means of five to six males per treatment group. (E) Upper panels: Representative immunolabelling of GFP- (left), AR-immunoreactivity (middle), and the merge (right) in the arcuate nucleus of Kiss1-creGFP males. Lower panels: quantification of the number of GFP-cells (left), GFP/AR-(middle), and -coexpressing cells (right). Data are expressed as the means of five to six males per treatment group.
The weight of the androgen-dependent seminal vesicles and testis, as well as circulating levels of testosterone, were comparable between the four treatment groups (Table 1). Altogether, these data indicate that the observed behavioral alterations were not mediated by changes in the HPG axis.
Proteome-Based Analyses of the Medial Preoptic Nucleus
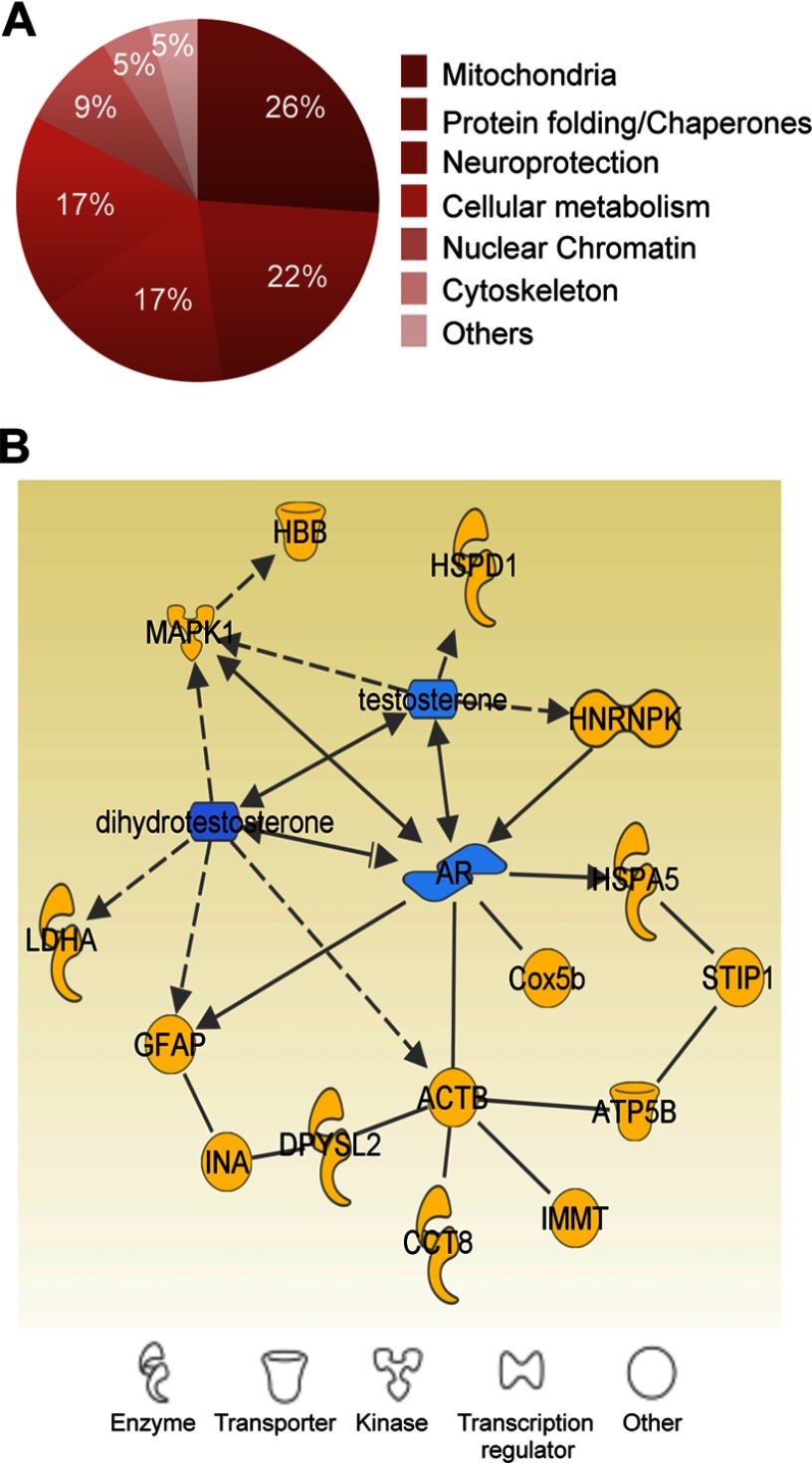
We conducted a proteomic analysis in the MPN in order to test whether DEHP exposure triggered modifications in this key region underlying the motivation to vocalize and copulate (Alward et al. 2013; Balthazart et al. 2004). Punches collected from males exposed to the vehicle or 5 or DEHP were subjected to 2-D fluorescence difference gel electrophoresis followed by protein identification by LC-MS/MS. Table 2 summarizes the paired changes of the levels of the proteins identified in the exposed mice. We classified them into six main categories based on their localization and function: mitochondrial function, protein folding, cellular stress, neuroprotection, transcription, and cytoskeletal plasticity (Figure 5A). We further analyzed these 22 significantly differentially expressed proteins by protein–protein interaction network analysis using Ingenuity Pathway (version 9.0; QIAGEN) software. Fifteen of these proteins were either directly or indirectly connected to androgens and their receptors (Figure 5B).
Table 2.
List of differentially expressed proteins identified by the proteomic analysis.
| Spot ID | All groups | DEHP-5/Vehicle | DEHP-50/Vehicle | DEHP-50/DEPH-5 | Accession number | Protein name | Gene name | Mascot score | Number of unique peptides | Sequence coverage (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | Ratio | p-Value | Ratio | p-Value | Ratio | |||||||
| e0035 | 0 .0013 | 0.0153 | 1.6 | 0.2748 | 0.0003 | P56480 | ATP synthase subunit beta mitochondrial | Atp5b | 464.9 | 7 | 9.3 | ||
| e0011 | 0.0155 | 0.0407 | 1.4 | 0.2496 | 0.0107 | A8DUK4 | Beta-globin | Hbbt1 | 153.4 | 2 | 17.0 | ||
| 0292 | 0.0115 | 0.1072 | 0.0963 | 1.2 | 0.0090 | 1.6 | Q8CAQ8 | Mitochondrial inner membrane protein | Immt | 469.4 | 8 | 12.8 | |
| 1226 | 0.0371 | 0.2880 | 1.1 | 0.1185 | 0.0211 | P56480 | ATP synthase subunit beta mitochondrial | Atp5b | 93.3 | 2 | 4.3 | ||
| 0348 | 0.0275 | 0.1685 | 0.1579 | 1.2 | 0.0143 | 1.4 | P20029 | 78 kDa glucose-regulated protein | Hspa5 | 1134.6 | 17 | 31.5 | |
| 0484 | 0.0079 | 0.0822 | 0.0634 | 1.1 | 0.0083 | 1.3 | P63038 | 60 kDa heat shock protein mitochondrial | Hspd1 | 612.4 | 10 | 19.9 | |
| P46660 | Alpha-internexin | Ina | 492.0 | 8 | 13.6 | ||||||||
| P42932 | T-complex protein 1 subunit theta | Cct8 | 490.0 | 7 | 15.0 | ||||||||
| 1273 | 0.0329 | 0.9353 | 1.0 | 0.0134 | 1.3 | 0.0390 | 1.3 | P17742 | Peptidyl-prolyl cis-trans isomerase A | Ppia | 433.6 | 10 | 39.0 |
| e0571 | 0.0257 | 0.1106 | 0.2886 | 1.1 | 0.0103 | 1.3 | Q60864 | Stress-induced-phosphoprotein 1 | Stip1 | 604.3 | 14 | 27.1 | |
| e0051 | 0.0691 | 0.1661 | 0.0302 | 0.3220 | P14152 | Malate dehydrogenase cytoplasmic | Mdh1 | 147.7 | 3 | 10.8 | |||
| 0656 | 0.0467 | 0.3558 | 1.3 | 0.0075 | 1.7 | 0.1530 | 1.3 | P03995 | Glial fibrillary acidic protein | Gfap | 1175.2 | 18 | 37.9 |
| 1328 | 0.2000 | 0.0486 | 1.4 | 0.3277 | 1.2 | 0.4250 | Q9ER97 | Neuroglobin | Ngb | 160.5 | 3 | 22.5 | |
| 0743 | 0.0746 | 0.0801 | 1.4 | 0.8369 | 1.0 | 0.0242 | P60710 | Actin cytoplasmic 1 | Actb | 262.0 | 7 | 16.8 | |
| e0562 | 0.1622 | 0.2589 | 1.4 | 0.0379 | 1.5 | 0.6510 | 1.1 | P03995 | Glial fibrillary acidic protein | Gfap | 677.6 | 10 | 24.7 |
| e0603 | 0.1293 | 0.5013 | 1.2 | 0.0113 | 1.4 | 0.2740 | 1.2 | Q9Z1N5 | Spliceosome RNA helicase Ddx39b | Ddx39b | 273.6 | 5 | 10.0 |
| 0434 | 0.0161 | 0.0142 | 0.9955 | 1.0 | 0.0121 | 1.3 | O08553 | Dihydropyrimidinase-related protein 2 | Dpysl2 | 671.9 | 12 | 21.0 | |
| 0476 | 0.0197 | 0.3031 | 1.1 | 0.0141 | 1.3 | 0.0596 | 1.2 | P61979 | Heterogeneous nuclear ribonucleoprotein K | Hnrnpk | 442.8 | 7 | 18.6 |
| 0771 | 0.0125 | 0.0221 | 0.2263 | 0.0325 | 1.2 | Q9JHI5 | Isovaleryl-CoA dehydrogenase mitochondrial | Ivd | 533.7 | 10 | 23.3 | ||
| 0798 | 0.0298 | 0.0166 | 1.3 | 0.4885 | 1.1 | 0.0452 | P05063 | Fructose-bisphosphate aldolase C | Aldoc | 362.2 | 6 | 16.8 | |
| P63085 | Mitogen-activated protein kinase 1 | Mapk1 | 302.4 | 7 | 16.8 | ||||||||
| 0921 | 0.0127 | 0.5274 | 1.1 | 0.0183 | 0.0100 | P06151 | L-lactate dehydrogenase A chain | Ldha | 332.2 | 6 | 18.4 | ||
| 1355 | 0.0124 | 0.0217 | 1.3 | 0.4772 | 0.0123 | P19536 | Cytochrome c oxidase subunit 5B mitochondrial | Cox5b | 72.0 | 2 | 8.6 | ||
Note: Statistical tests and protein identification results from the 2D-DIGE analysis. The accession numbers are the protein identifiers in the UniProt database (http://www.uniprot.org). The Mascot score threshold for protein identification by liquid chromotagraphy tandem-mass spectrometry (LC-MS/MS) was 31 per peptide (), and a minimum of two significant peptides was required to consider the protein identified. APT, adenosine triphosphate; ID, identifier.
Figure 5.
Proteome analysis of the medial preoptic nucleus (MPN) in di(2-ethylhexyl) phthalate (DEHP)–exposed males. (A) Classification of differentially expressed proteins between the exposed groups into six major categories based on cellular localization and function. The percentages of proteins in each category are indicated. (B) Androgen-related protein interaction network generated by Ingenuity Pathway Analysis.
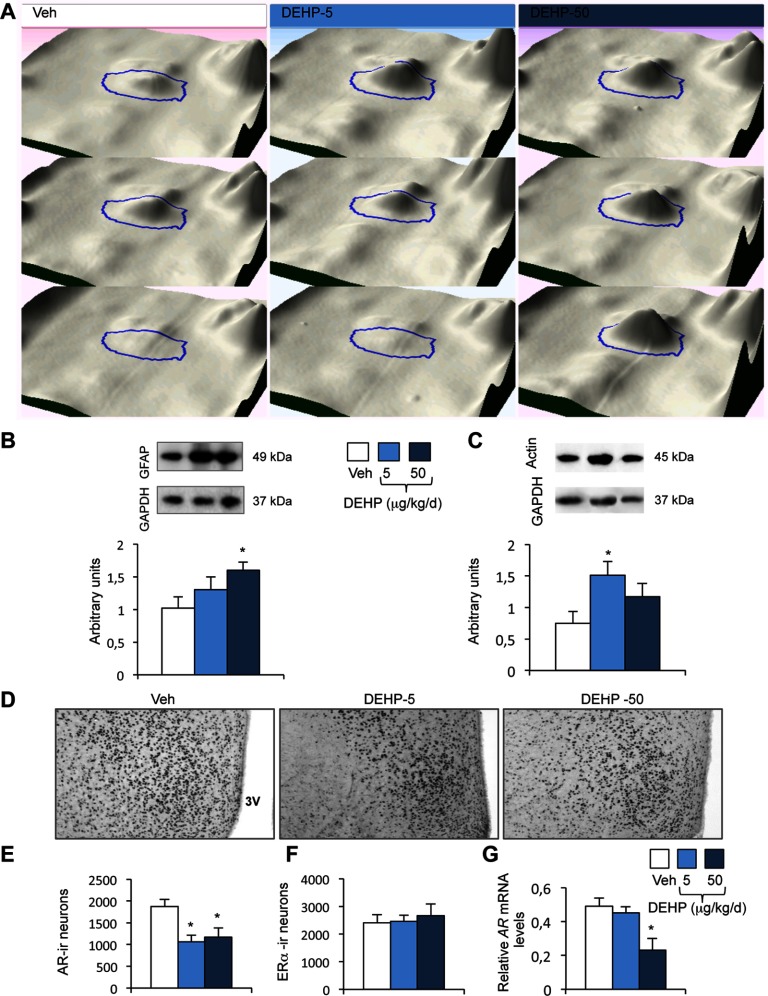
We confirmed the proteomic data by Western blot validation by focusing on GFAP and . Both cytoskeletal proteins underlie the structural and organizational changes induced by androgens in glial and neuronal cells to regulate neural functions, including sexual behavior (Day et al. 1998; Garcia-Estrada et al. 1993; Hong et al. 2008; Matsumoto et al. 1994; Will et al. 2015). The proteomic analysis showed higher levels of GFAP protein in the DEHP-50 group than in the vehicle group (Table 2 and Figure 6A). We confirmed this result by Western blotting, showing the progressive upregulation of GFAP, which was significant in the DEHP-50 group (Figure 6B). Upregulation of GFAP is a hallmark of astrocyte activation, which is induced in response to insults (Filous and Silver 2016), but also by androgen depletion (Attallah et al. 2016). In order to confirm a potential astrocyte activation induced by DEHP exposure, we quantified the protein levels of another astrocyte protein i.e., NDRG2. This member of the NDRG gene family is induced in the early phase of astrocyte activation in the mouse brain (Flügge et al. 2014; Takarada-Iemata et al. 2014). Data illustrated in Figure S4 show that NDRG2 levels were increased in the MPN of DEHP-5 and DEHP-50 groups ().
Figure 6.
Validation of proteomic data and characterization of androgen receptor (AR) and expression in the medial preoptic nucleus (MPN). (A) 3-D view of the spot ID:0656, corresponding to GFAP, shown for three animals exposed to the vehicle (Veh) or DEHP at 5 or . (B–C) Upper panels: Representative Western blots of GFAP (B), (C), and GAPDH used as a protein reference, in the MPN of the Veh, DEHP-5, and DEHP-50 groups. Lower panels: quantification of the protein levels normalized to GAPDH. Data are expressed as the means of four males per treatment group, * compared to the vehicle group. (D–F) Representative AR-immunolabeling of the medial preoptic nucleus of males exposed to the vehicle (Veh) and DEHP at 5 or (D) and quantitative data for AR- (E) and cells (F). 3V: Third ventricle. Data are expressed as the means of four to six males per treatment group, * vs. the vehicle group. (G) AR gene expression in males exposed to the vehicle and DEHP at 5 or . Data are expressed as the means of six to eight males per treatment group, * compared to the vehicle group.
In agreement with proteomic data (Table 2), amount quantified by Western blotting was significantly higher in the DEHP-5 group than in males exposed to the vehicle, and unchanged in the DEHP-50 group (Figure 6C).
Effect of Di(2-ethylhexyl) Phthalate Exposure on Neural Androgen Receptor Expression
We asked whether DEHP exposure affected AR expression, since a significant number of differentially expressed proteins, identified by the proteomic approach, were connected to this receptor. We therefore assessed the effects of DEHP exposure on the number of neurons that express the AR in the MPN, and performed a similar analysis for . Indeed, testosterone-induced regulation of male sexual behavior involves both activation of the AR and stimulation of following neural aromatization of testosterone into estradiol (Naulé et al. 2016; Ogawa et al. 1997, 1998; Raskin et al. 2009; Wersinger et al. 1997). There were significantly fewer AR-immunoreactive cells in the MPNs of DEHP-5 and DEHP-50 exposed mice ( and , respectively) than in vehicle treated mice (Figure 6D–E). In contrast, the number of neurons were unaffected by DEHP exposure (Figure 6F). The changes in AR protein levels induced by DEHP treatment appeared to be due to changes in transcription, at least for the DEHP-50 group, as AR mRNA levels were 53% lower in the DEHP-50 group than in vehicle-exposed mice (Figure 6G). The MPN receives chemosignals transmitted from the olfactory bulb through the medial amygdala and bed nucleus of stria terminalis. These brain areas showed a similar downregulation of AR expression (see Figure S5).
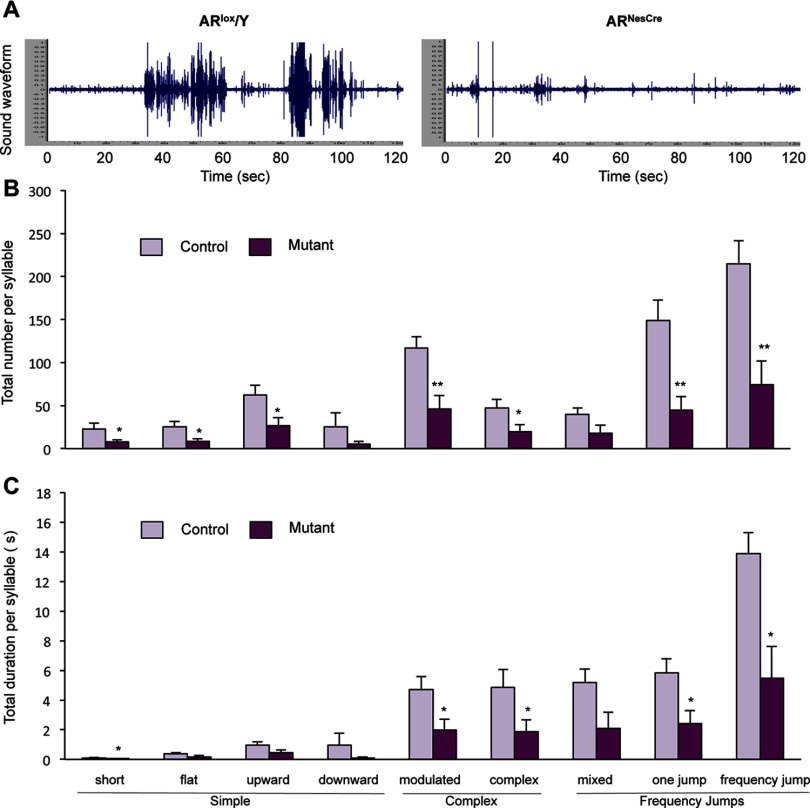
Effect of Neural Androgen Receptor Knockout on Courtship Vocalizations
The behavioral alterations induced by DEHP exposure are associated with neural downregulation of the AR. In order to test whether neural AR decrease triggers behavioral alterations similar to those observed for DEHP exposure, we used male mice lacking the neural AR. We have previously shown that neural AR invalidation alters mating without affecting olfactory preference (Raskin et al. 2009). Here, we addressed whether neural AR knockout also affects the emission of USVs during courtship, for the first time. Representative sound waveforms of vocalizations produced by a control () and mutant male () are illustrated in Figure 7A. males displayed a significantly diminished total number ( vs. in controls) and duration ( s vs. s in controls) of USVs in the presence of receptive females. The neural AR knockout mice exhibited a significantly lower number of emitted syllables ( to ) for seven of the nine syllables than their control littermates (Figure 7B). Analysis of the total duration of each syllable showed reduced times ( to ) for short, modulated, complex, one-jump, and frequency-jump syllables (Figure 7C). Among the emitted syllables, only the mean duration of mixed USVs was reduced in the mutant mice (see Figure S6A). These alterations were associated with increased latencies to intromit and ejaculate (see Figure S6B), as previously reported (Raskin et al. 2009; Raskin et al. 2012). Therefore, male neural AR knockout mice display disrupted courtship vocalizations similar to those of the DEHP exposure model. This finding, together with the proteomic and immunohistochemical analyses, suggests that this signaling pathway represents one of the main targets for endocrine disruption of courtship behavior in adult mice by DEHP.
Figure 7.
Effect of neural androgen receptor (AR) invalidation on ultrasonic vocalizations (USVs). (A) Representative sound waveform for a control (, left) and mutant male (, right) in the presence of a sexually receptive female. (B–C) Number (B) and duration (C) of syllable types by category produced during the 4-min recording in the presence of a sexually receptive female. Data are expressed as the means of nine males per treatment group, *, ** compared to control littermates.
Discussion
We demonstrate that adult exposure of male mice to environmental doses of a phthalate plasticizer, DEHP, lowers the emission of courtship vocalizations and delays mating. This was associated with cellular changes in the MPN, the key region involved in the motivation to vocalize and mate, and AR downregulation in the MPN and upstream chemosensory regions of the neural circuitry responsible for sexual behavior. Therefore, exposure to low doses of DEHP targets neural AR signaling, thereby altering neural structures and functions of the adult brain.
Courtship vocalizations have been extensively studied in songbirds and have been shown to play a key role in female attraction and the success of sexual reproduction. Only recently has the emission of USVs during courtship gained new interest in mice (Chabout et al. 2015; Holy and Guo 2005), although it has been known since the 1970s (Nyby et al. 1977; Whitney et al. 1973; Whitney et al. 1974). Here, we show that exposure of adult male mice to DEHP disrupts courtship by altering the production of USVs. DEHP at reduced both the number and duration of syllables emitted during the 4-min test. Exposure to DEHP doses of 0.5 and resulted in a significant reduction of simple syllables, whereas those of the frequency jump category seemed to increase, changing the ratio of both categories. The alterations induced by DEHP were mainly due to a lower frequency of the emitted USVs, as the mean duration of each syllable did not change.
DEHP exposure of 5 and also increased the latency to the first intromission, whereas the mating duration and parameters of sexual performance (frequency of mounts with intromissions and thrusts, intromission ratio, genital grooming) remained unchanged. These behavioral alterations were not due to a loss of discrimination of olfactory cues because the olfactory preference of the males and latency to the first anogenital chemoinvestigation of females were normal. They were also not due to changes in locomotor activity or motor function. Altogether, these data indicate that DEHP-exposed males exhibit altered emission of USVs and delayed initiation of mating, suggesting a potential effect of DEHP on male sexual arousal (Dizinno and Whitney 1977; Hamson et al. 2009). In our sexual tests, cohabitation of males and females was forced, triggering the occurrence of mating, despite the behavioral alterations seen during the precopulatory phase of sexual behavior. When receptive females were given a choice between exposed and unexposed males, they displayed more interest towards unexposed males than males exposed to DEHP at 5 or . This could be explained, at least partly, by the altered production or ratio of USVs by DEHP-exposed males. Indeed, in addition to the olfactory cues used to discriminate between males (Bowers and Alexander 1967), female mice also exhibit an interest in male USVs (Pomerantz et al. 1983). In two-partner preference tests, the female mice responded preferentially to vocalizing males. This preference, which is also observed in rats and hamsters (Floody et al. 1979; Thomas et al. 1981), is believed to keep the female near the male and facilitate copulation.
It is interesting to note that DEHP at doses of 5 and had similar effects on the initiation of mating and female attraction, whereas they differentially affected the emission of USVs. The effects induced by DEHP-5 on the ratio of syllable categories may be sufficient to reduce female attraction and delay mating. Alternatively, it is possible that DEHP-5 may differently affect these neural structures, as the emission of vocalizations involve, not only the MPN for motivation, but also a motor system for USV production.
Androgens are required for mating and also influence the production of courtship vocalizations in male mice (Dizinno and Whitney 1977; Nunez et al. 1978; Warburton et al. 1989). In the present exposed mouse model, the observed behavioral alterations were not due to changes at the level of the gonadotropic axis. Hence, exposure to DEHP did not alter the number of kisspeptin-immunoreactive neurons or fiber density expression, nor did it alter activity of the kiss1 promoter, shown using a GFP reporter in the two hypothalamic nuclei involved in the regulation of the gonadotropic axis. These results are consistent with the unchanged levels of circulating testosterone and weight of the androgen-dependent seminal vesicle. Thus, the low DEHP doses used in this study did not impact the adult HPG axis. These results are in agreement with previous data showing that oral exposure of adult rats to DEHP (0.1 to ) for 28 d induced no detectable changes in androgen biosynthesis (Akingbemi et al. 2001). In another rat study, adult exposure to DEHP (320 to 20,000 ppm) for 60 d did not significantly change testosterone and LH levels (Agarwal et al. 1986). The HPG axis seems more vulnerable to DEHP exposure during development, as shown by altered testosterone levels and expression of pituitary gonadotropins in both mice (Barakat et al. 2017; Carbone et al. 2013; Pocar et al. 2012) and rat (Akingbemi et al. 2001; Noriega et al. 2009).
The behavioral alterations induced by adult exposure to DEHP were not due to changes in androgen levels, but were probably due to modifications of their signaling pathway in the neural regions underlying sexual behavior. Proteomic analysis of the MPN identified differentially expressed proteins involved in cellular processes ranging from mitochondrial functions, metabolic processes, and cytoskeleton plasticity to transcription and neuroprotection. Ingenuity Pathway Analysis revealed a link between androgens and several proteins identified by the proteomic approach and validated for GFAP and actin, two androgen-sensitive proteins (Day et al. 1998; Garcia-Estrada et al. 1993; Hong et al. 2008). DEHP exposure may thus induce changes in androgen sensitivity, which then trigger modifications in neuronal and glial structure and plasticity. This was illustrated by astrocyte activation, which is generally induced by injury and disease (Filous and Silver 2016) or following androgen depletion (Attallah et al. 2016), and identified in the present study by increased protein levels of GFAP and NDRG2. In agreement with our hypothesis, we found AR expression to be reduced in the MPN and upstream chemosensory regions (bed nucleus of stria terminalis and medial amygdala) of DEHP-exposed males. DEHP exposure did not affect the number of neurons or protein levels in these brain areas. Therefore, chronic exposure to DEHP changes neural sensitivity to androgens through selective downregulation of AR expression. Given the key role of this signaling pathway in the activation of male sexual behavior by testosterone (Raskin et al. 2009, 2012; Picot et al. 2014), we suggest that DEHP exposure alters courtship behavior at least partly through AR decrease.
The direct link between reduced AR expression and altered courtship vocalizations was further analyzed in male mice where the neural AR gene was selectively disrupted. The obtained data show reduced emission of vocalizations and confirm delayed mating in mutant males. This indicates, for the first time, that the neural AR is critical for the androgen-induced regulation of courtship vocalizations. Further studies should address the link between AR downregulation, cellular changes including astrocyte activation, and reduced behaviors, since a neuroprotective role has been suggested for androgens (Gracia-Estrada et al. 1993; Attallah et al. 2016). The molecular mechanisms through which DEHP acts to induce AR downregulation also need to be studied. In songbirds, humans, and mice, the methylation state of the AR gene appears to be tightly associated with its expression (Keil et al. 2014; Kinoshita et al. 2000; Movérare-Skrtic et al. 2009; Wada et al. 2013). It is thus possible that low doses of DEHP trigger such epigenetic modifications, as has been shown in other models (Kuo et al. 2013; Manikkam et al. 2013; Rajesh and Balasubramanian 2014), thereby reducing AR expression and related behaviors.
The data presented here indicate that the adult brain is highly sensitive to DEHP exposure. Studies on DEHP have mostly focused on exposure during development and puberty, whereas exposure during adulthood has been underevaluated and the risk perhaps underestimated. Indeed, among the rodent studies addressing the effects of DEHP exposure in males, only four works were dedicated to adult exposure and its potential impact on the rat testis (Agarwal et al. 1986; Dostal et al. 1988; Guo et al. 2013; Li et al. 2012), while no data are available on brain and behavior in rat or mice. Our results on the impact of low doses of DEHP may be also informative, as most studies on DEHP-induced effects on the nervous system used higher doses of this molecule in mice (Komada et al. 2016; Tanaka 2002; Tanaka 2005; Tanida et al. 2009; Xu et al. 2015) and rat (Carbone et al. 2010; Carbone et al. 2013; Smith et al. 2011, 2015; Smith and Holahan 2014; Lin et al. 2015).
The presently described effects of DEHP remind our previous study showing that adult exposure to low-dose bisphenol A (BPA) increases the latency to ejaculation without affecting testosterone levels (Picot et al. 2014). However, BPA did not affect the expression level of the neural AR, although antiandrogenic activity was suggested for this compound. This suggests that endocrine disrupters may target the AR signaling pathway through different mechanisms (Picot et al. 2014).
The present demonstration of the involvement of the neural AR signaling pathway in courtship vocalizations, and its vulnerability to environmental exposure to DEHP in mice, is highly relevant for several vertebrate species. Vocalizations produced during courtship are androgen-dependent in fish, amphibians, birds, and mammals (Bass and Remage-Healey 2008). Indeed, AR levels within brain regions involved in sexual motivation and vocal production increase in association with seasonal increases in courtship singing and mating behavior in songbirds (Gahr and Metzdorf 1997; Soma et al. 1999; Wacker et al. 2010). Abundant AR mRNAs have also been found in central nuclei involved in male vocalizations in fish (Forlano et al. 2010). In humans, a normal androgen state is required for libido and sexual desire. It is thus possible that chronic exposure to DEHP during adulthood may also be detrimental for courtship behavior and sexual motivation in these species, as our data show effects of DEHP at doses close to those of environmental exposure. In this context, a recent human study reported an association between environmental exposure to DEHP and low interest in sexual activity (Barrett et al. 2014).
Conclusion
In summary, this study shows for the first time that chronic exposure during adulthood to low doses of a phthalate plasticizer impairs courtship vocalizations and initiation of mating in male mice. This reduced sexual arousal may occur at least in part through alteration of the neural AR signaling pathway, as shown by proteomic and genetic analyses. These data are relevant for several species, including humans. The present exposure and genetic models are pertinent to deciphering the mechanisms underlying neural disruption of the AR.
Supplemental Material
Acknowledgments
This work was supported by the French Agency for Food, Environmental and Occupational Health & Safety (Anses, Project No 2012-2-077). We thank the Institut National de la Recherche Agronomique (INRA) hormonal assay platform for testosterone assay and the UPMC and INRA (Unité Expérimentale de Physiologie Animale de l'Orfrasière) platforms for taking care of the animals. We are grateful to V. Robert (INRA UMR85) for his expert assistance in PCR genotyping of the Kiss1-creGFP mouse line and RT-qPCR experiments. We also thank M. Le Gall (Plateforme Protéomique 3P5, Institut Cochin, U1016) for Ingenuity Pathway Analysis.
References
- Agarwal DK, Eustis S, Lamb JC, Reel JR, Kluwe WM. 1986. Effects of di(2-ethylhexyl) phthalate on the gonadal pathophysiology, sperm morphology, and reproductive performance of male rats. Environ Health Perspect 65:343–350, PMID: 3709461, 10.1289/ehp.8665343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågmo A. 1997. Male rat sexual behavior. Brain Res Protoc 1(2):203–209, 10.1016/S1385-299X(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Youker RT, Sottas CM, Ge R, Katz E, Klinefelter GR, et al. 2001. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol Reprod 65(4):1252–1259, PMID: 11566751, 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. 2013. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc Natl Acad Sci USA 110(48):19573–19578, PMID: 24218603, 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AJM, Grande SW, Talsness CE, Gericke C, Grote K, Golombiewski A, et al. 2006. A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology 228(1):85–97, PMID: 16996189, 10.1016/j.tox.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Atallah A, Mhaouty-Kodja S, Grange-Messent V. 2016. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab Dec; PMID: 28256950, 10.1177/0271678X16683961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. 2004. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav 83(2):247–270, PMID: 15488543, 10.1016/j.physbeh.2004.08.025.g. [DOI] [PubMed] [Google Scholar]
- Barakat R, Lin PP, Rattan S, Brehm E, Canisso IF, Abosalum ME, et al. 2017. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol Sci 156(1):96–108, PMID: 28082598, 10.1093/toxsci/kfw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Wang C, Drobnis EZ, Bruce Redmon J, Swan SH. 2014. Environmental exposure to di-2-ethylhexyl phthalate is associated with low interest in sexual activity in premenopausal women. Horm Behav 66(5):787–792, PMID: 25448532, 10.1016/j.yhbeh.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. 2008. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav 53(5):659–672, PMID: 18262186, 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean NJ. 1982. Olfactory and vomeronasal mediation of ultrasonic vocalizations in male mice. Physiol Behav 28(1):31–37, PMID: 7079320. [DOI] [PubMed] [Google Scholar]
- Boussicault L, Alves S, Lamazière A, Planques A, Heck N, Moumné L, et al. 2016. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 139(Pt 3):953–970, PMID: 26912634, 10.1093/brain/awv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Alexander BK. 1967. Mice: Individual recognition by olfactory cues. Science 158(3805):1208–1210, PMID: 6057298, 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Scacchi P, et al. 2013. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav 63(5):692–699, PMID: 23399322, 10.1016/j.yhbeh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Carbone S, Szwarcfarb B, Ponzo O, Reynoso R, Cardoso N, Deguiz L, et al. 2010. Impact of gestational and lactational phthalate exposure on hypothalamic content of amino acid neurotransmitters and FSH secretion in peripubertal male rats. Neurotoxicology 31(6):747–751, PMID: 20600290, 10.1016/j.neuro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Chabout J, Sarkar A, Dunson DB, Jarvis ED. 2015. Male mice song syntax depends on social contexts and influences female preferences. Front Behav Neurosci 9:76, PMID: 25883559, 10.3389/fnbeh.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, et al. 2010. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol 30(2):313–321, PMID: 20420898, 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147(12):5817–5825, PMID: 16959837, 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. 2009. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 21(4):305–311, PMID: 19207812, 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- Cruz MR, Liu YC, Manzo J, Pacheco P, Sachs BD. 1999. Peripheral nerves mediating penile erection in the rat. J Auton Nerv Syst 76(1):15–27, PMID: 10323303, 10.1016/S0165-1838(98)00191-X. [DOI] [PubMed] [Google Scholar]
- Dalsenter PR, Santana GM, Grande SW, Andrade AJ, Araújo SL. 2006. Phthalate affect the reproductive function and sexual behavior of male Wistar rats. Hum Exp Toxicol 25(6):297–303, PMID: 16866186, 10.1191/0960327105ht624oa. [DOI] [PubMed] [Google Scholar]
- Day JR, Frank AT, O’Callaghan JP, Jones BC, Anderson JE. 1998. The effect of age and testosterone on the expression of glial fibrillary acidic protein in the rat cerebellum. Exp Neurol 151(2):343–346, PMID: 9628769, 10.1006/exnr.1998.6801. [DOI] [PubMed] [Google Scholar]
- Dewalque L, Charlier C, Pirard C. 2014. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol Lett 231(2):161–168, PMID: 24968065, 10.1016/j.toxlet.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Dizinno G, Whitney G. 1977. Androgen influence on male mouse ultrasounds during courtship. Horm Behav 8(2):188–192, PMID: 863399. [DOI] [PubMed] [Google Scholar]
- Dizinno G, Whitney G, Nyby J. 1978. Ultrasonic vocalizations by male mice (Mus musculus) to female sex pheromone: Experiential determinants 1. Behav Biol 22(1):104–113, 10.1016/S0091-6773(78)92094-1. [DOI] [Google Scholar]
- Dostal LA, Chapin RE, Stefanski SA, Harris MW, Schwetz BA. 1988. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol Appl Pharmacol 95(1):104–121, PMID: 3413790. [DOI] [PubMed] [Google Scholar]
- Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, et al. 2013. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res 256:677–689, PMID: 23994547, 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Filous AR, Silver J. 2016. Targeting astrocytes in CNS injury and disease: A translational research approach. Prog Neurobiol 144:173–187, PMID: 27026202, 10.1016/j.pneurobio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR, Walsh C, Flanagan MT. 1979. Testosterone stimulates ultrasound production by male hamsters. Horm Behav 12(2):164–171, PMID: 488926, 10.1016/0018-506X(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Flügge G, Araya-Callis C, Garea-Rodriguez E, Stadelmann-Nessler C, Fuchs E. 2014. NDRG2 as a marker protein for brain astrocytes. Cell Tissue Res 357(1):31–41, PMID: 24816982, 10.1007/s00441-014-1837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre M, Deitcher DL, Bass AH. 2010. Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J Comp Neurol 518(4):493–512, PMID: 20020540, 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. 1997. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull 44(4):509–517, PMID: 9370218. [DOI] [PubMed] [Google Scholar]
- Gao DW, Wen ZD. 2016. Phthalate esters in the environment: a critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ 541:986–1001, PMID: 26473701, 10.1016/j.scitotenv.2015.09.148. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. 1993. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res 628(1-2):271–278, PMID: 8313156, 10.1016/0006-8993(93)90964-O. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, et al. 2011. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152(11):4298–4309, PMID: 21933870, 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Li XW, Liang Y, Ge Y, Chen X, Lian QQ, et al. 2013. The increased number of Leydig cells by di(2-ethylhexyl) phthalate comes from the differentiation of stem cells into Leydig cell lineage in the adult rat testis. Toxicology 306:9–15, PMID: 23391632, 10.1016/j.tox.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Hamson DK, Csupity AS, Ali FM, Watson NV. 2009. Partner preference and mount latency are masculinized in androgen insensitive rats. Physiol Behav 98(1-2):25–30, PMID: 19375435, 10.1016/j.physbeh.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. 2005. Ultrasonic songs of male mice. PLoS Biol 3(12):e386, PMID: 16248680, 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MH, Sun H, Jin CH, Chapman M, Hu J, Chang W, et al. 2008. Cell-specific activation of the human skeletal alpha-actin by androgens. Endocrinology 149(3):1103–1112, PMID: 18063690, 10.1210/en.2007-0530. [DOI] [PubMed] [Google Scholar]
- Keil KP, Abler LL, Laporta J, Altmann HM, Yang B, Jarrard DF, et al. 2014. Androgen receptor DNA methylation regulates the timing and androgen sensitivity of mouse prostate ductal development. Dev Biol 396(2):237–245, PMID: 25446526, 10.1016/j.ydbio.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, et al. 2000. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res 60(13):3623–3630, PMID: 10910077. [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. 2006. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure–an update and latest results. Int J Androl 29(1):155–165. discussion: 181–185, PMID: 16466535, 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- Komada M, Gendai Y, Kagawa N, Nagao T. 2016. Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicol Lett 259:69–79, PMID: 27472966, 10.1016/j.toxlet.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, et al. 2013. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy 68(7):870–879, PMID: 23738920, 10.1111/all.12162. [DOI] [PubMed] [Google Scholar]
- Li XW, Liang Y, Su Y, Deng H, Li XH, Guo J, et al. 2012. Adverse effects of di-(2-ethylhexyl) phthalate on Leydig cell regeneration in the adult rat testis. Toxicol Lett 215(2):84–91, PMID: 23064086, 10.1016/j.toxlet.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Lin H, Yuan K, Li L, Liu S, Li S, Hu G, et al. 2015. In Utero exposure to diethylhexyl phthalate affects rat brain development: A behavioral and genomic approach. Int J Environ Res Public Health 12(11):13696–13710, PMID: 26516888, 10.3390/ijerph121113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M, Angerer J, Koch HM. 2010. A simple pharmacokinetic model to characterize exposure of Americans to di-2-ethylhexyl phthalate. J Expo Sci Environ Epidemiol 20(1):38–53, PMID: 19127283, 10.1038/jes.2008.74. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. 2013. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8(1):e55387, PMID: 23359474, 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie-Luce C, Raskin K, Bolborea M, Monin M, Picot M, Mhaouty-Kodja S. 2013. Effects of neural androgen receptor disruption on aggressive behavior, arginine vasopressin and galanin systems in the bed nucleus of stria terminalis and lateral septum. Gen Comp Endocrinol 188:218–225, PMID: 23583766, 10.1016/j.ygcen.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Martine B, Marie-Jeanne T, Cendrine D, Fabrice A, Marc C. 2013. Assessment of adult human exposure to phthalate esters in the urban centre of Paris (France). Bull Environ Contam Toxicol 90(1):91–96, 10.1007/s00128-012-0859-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. 1994. Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm Behav 28(4):357–366, PMID: 7729804, 10.1006/hbeh.1994.1032. [DOI] [PubMed] [Google Scholar]
- Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. 2001. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environ Health Perspect 109(3):229–237, PMID: 11333183, 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movérare-Skrtic S, Mellström D, Vandenput L, Ehrich M, Ohlsson C. 2009. Peripheral blood leukocyte distribution and body mass index are associated with the methylation pattern of the androgen receptor promoter. Endocrine 35(2):204–210, PMID: 19199084, 10.1007/s12020-009-9153-7. [DOI] [PubMed] [Google Scholar]
- Naulé L, Marie-Luce C, Parmentier C, Martini M, Albac C, Trouillet AC, et al. 2016. Revisiting the neural role of estrogen receptor beta in male sexual behavior by conditional mutagenesis. Horm Behav 80:1–9, PMID: 26836767, 10.1016/j.yhbeh.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Naulé L, Robert V, Parmentier C, Martini M, Keller M, Cohen-Solal M, et al. 2015. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum Mol Genet 24(25):7326–7338, PMID: 26464488, 10.1093/hmg/ddv430. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, et al. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152(11):4265–4275, PMID: 21914775, 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE Jr. 2009. Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci 111(1):163–178, PMID: 19528224, 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Nyby J, Whitney G. 1978. The effects of testosterone, estradiol, and dihydrotestosterone on male mouse (Mus musculus) ultrasonic vocalizations. Horm Behav 11(3):264–272, PMID: 753695. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. 1977. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus). Anim Behav 25(2):333–341, PMID: 889149. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. 1997. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94(4):1476–1481, PMID: 9037078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. 1998. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology 139(12):5058–5069, PMID: 9832445, 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. 2001. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. San Diego, CA:Academic Press. [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45, PMID: 11328886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Billard JM, Dombret C, Albac C, Karameh N, Daumas S, et al. 2016. Neural androgen receptor deletion impairs the temporal processing of objects and hippocampal CA1-dependent mechanisms. PLoS One 11(2):e0148328, 10.1371/journal.pone.0148328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Naulé L, Marie-Luce C, Martini M, Raskin K, Grange-Messent V, et al. 2014. Vulnerability of the neural circuitry underlying sexual behavior to chronic adult exposure to oral bisphenol a in male mice. Endocrinology 155(2):502–512, PMID: 24265451, 10.1210/en.2013-1639. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, et al. 2012. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 153(2):937–948, PMID: 22147016, 10.1210/en.2011-1450. [DOI] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. 1983. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav 31(1):91–96, PMID: 6685321. [DOI] [PubMed] [Google Scholar]
- Rajesh P, Balasubramanian K. 2014. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol 223(1):47–66, PMID: 25232145, 10.1530/JOE-14-0111. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, et al. 2009. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci 29(14):4461–4470, PMID: 19357272, 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin K, Marie-Luce C, Picot M, Bernard V, Mailly P, Hardin-Pouzet H, et al. 2012. Characterization of the spinal nucleus of the bulbocavernosus neuromuscular system in male mice lacking androgen receptor in the nervous system. Endocrinology 153(7):3376–3385, PMID: 22585832, 10.1210/en.2012-1001. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. 1999. Lesions to the medial preoptic area affect singing in the male European Starling (Sturnus vulgaris). Horm Behav 36(3):276–286, PMID: 10603291, 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Ruka KA, Burger LL, Moenter SM. 2016. Both estrogen and androgen modify the response to activation of neurokinin-3 and κ-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology 157:752–763, PMID: 26562263, 10.1210/en.2015-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Farmer K, Lee H, Holahan MR, Smith JC. 2015. Altered hippocampal lipid profile following acute postnatal exposure to di(2-ethylhexyl) phthalate in rats. IJERPH 12:13542–13559, 10.3390/ijerph121013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Holahan MR. 2014. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male Long Evans rats. PLoS One 9(10):e109522, PMID: 25295592, 10.1371/journal.pone.0109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, MacDonald A, Holahan MR. 2011. Acute postnatal exposure to di(2-ethylhexyl) phthalate adversely impacts hippocampal development in the male rat. Neuroscience 193:100–108, PMID: 21782900, 10.1016/j.neuroscience.2011.06.082. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146(7):2976–2984, PMID: 15831567, 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. 1999. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol 409(2):224–236, PMID: 10379916, . [DOI] [PubMed] [Google Scholar]
- Takarada-Iemata M, Kezuka D, Takeichi T, Ikawa M, Hattori T, Kitao Y, et al. 2014. Deletion of N-myc downstream-regulated gene 2 attenuates reactive astrogliosis and inflammatory response in a mouse model of cortical stab injury. J Neurochem 130(3):374–387, PMID: 24697507, 10.1111/jnc.12729. [DOI] [PubMed] [Google Scholar]
- Tanaka T. 2002. Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (DEHP) administered to mice in the diet. Food Chem Toxicol 40(10):1499–1506, PMID: 12387315, 10.1016/S0278-6915(02)00073-X. [DOI] [PubMed] [Google Scholar]
- Tanaka T. 2005. Reproductive and neurobehavioural effects of bis(2-ethylhexyl) phthalate (DEHP) in a cross-mating toxicity study of mice. Food Chem Toxicol 43(4):581–589, PMID: 15721206, 10.1016/j.fct.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, et al. 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett 189(1):40–47, PMID: 19481886, 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Talalas L, Barfield RJ. 1981. Effect of devocalization of the male on mating behavior in rats. J Comp Physiol Psychol 95(4):630–637, 10.1037/h0077803. [DOI] [Google Scholar]
- UniProt Consortium. 2017. Nucleic Acids Res, 45(D1):D158–D169, 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DW, Wingfield JC, Davis JE, Meddle SL. 2010. Seasonal changes in aromatase and androgen receptor, but not estrogen receptor mRNA expression in the brain of the free-living male song sparrow, Melospiza melodia morphna. J Comp Neurol 518(18):3819–3835, PMID: 20653036, 10.1002/cne.22426. [DOI] [PubMed] [Google Scholar]
- Wada K, Hayase S, Imai R, Mori C, Kobayashi M, Liu WC, et al. 2013. Differential androgen receptor expression and DNA methylation state in striatum song nucleus Area X between wild and domesticated songbird strains. Eur J Neurosci 38(4):2600–2610, PMID: 23701473, 10.1111/ejn.12258. [DOI] [PubMed] [Google Scholar]
- Warburton VL, Sales GD, Milligan SR. 1989. The emission and elicitation of mouse ultrasonic vocalizations: the effects of age, sex and gonadal status. Physiol Behav 45(1):41–47, PMID: 2727141, 10.1016/0031-9384(89)90164-9. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. 1997. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav 32(3):176–183, PMID: 9454668, 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whitney G, Alpern M, Dizinno G, Horowitz G. 1974. Female odors evoke ultrasounds from male mice. Anim Learn Behav 2(1):13–18, PMID: 4468889. [DOI] [PubMed] [Google Scholar]
- Whitney G, Coble JR, Stockton MD, Tilson EF. 1973. Ultrasonic emissions: do they facilitate courtship of mice. J Comp Physiol Psychol 84(3):445–452, PMID: 4745813. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2012. “State of the Science of Endocrine Disrupting Chemicals.” http://www.who.int/ceh/publications/endocrine/en/ [accessed 24 October 2016].
- Will RG, Nutsch VL, Turner JM, Hattori T, Tobiansky DJ, Dominguez JM. 2015. Astrocytes in the medial preoptic area modulate ejaculation latency in an experience-dependent fashion. Behav Neurosci 129(1):68–73, 10.1037/bne0000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. 2015. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere 124:22–31, PMID: 25441928, 10.1016/j.chemosphere.2014.10.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



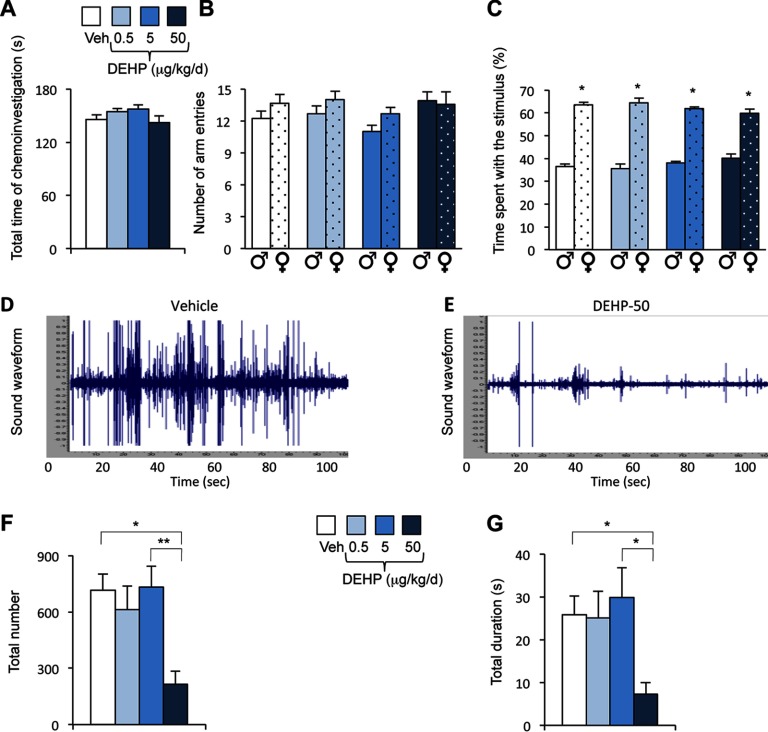
![Figure 2A is a bar graph with standard errors of mean plotting total number (y-axis) of short, flat, upward, and downward (simple group); modulated and complex (complex group); and mixed, one jump, and frequency jump (frequency jump group) syllables (x-axis) for the Veh and 0.5, 5, and 50 micrograms per kilogram per day DEHP exposure groups. Figure 2B comprises four pie charts showing the proportion of simple, complex, and frequency jump syllables in the Veh (29, 24, and 47 percent) and DEHP 0.5 (18, 25, 57 and percent), DEHP 5 (18, 28, and 54 percent), and DEHP 50 (29.5, 26, and 44.5 percent) exposure groups. Figure 2C is a bar graph with standard errors of mean plotting total duration (y-axis) of short, flat, upward, and downward (simple group); modulated and complex (complex group); and mixed, one jump, and frequency jump (frequency jump group) syllables (x-axis) for the Veh and 0.5, 5, and 50 micrograms per kilogram per day DEHP exposure groups].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/07da/5915199/343b1723e73c/EHP1443_f2.jpg)