Abstract
Background:
Household air pollution due to biomass combustion for residential heating adversely affects vulnerable populations. Randomized controlled trials to improve indoor air quality in homes of children with asthma are limited, and no such studies have been conducted in homes using wood for heating.
Objectives:
Our aims were to test the hypothesis that household-level interventions, specifically improved-technology wood-burning appliances or air-filtration devices, would improve health measures, in particular Pediatric Asthma Quality of Life Questionnaire (PAQLQ) scores, relative to placebo, among children living with asthma in homes with wood-burning stoves.
Methods:
A three-arm placebo-controlled randomized trial was conducted in homes with wood-burning stoves among children with asthma. Multiple preintervention and postintervention data included PAQLQ (primary outcome), peak expiratory flow (PEF) monitoring, diurnal peak flow variability (dPFV, an indicator of airway hyperreactivity) and indoor particulate matter (PM) .
Results:
Relative to placebo, neither the air filter nor the woodstove intervention showed improvement in quality-of-life measures. Among the secondary outcomes, dPFV showed a 4.1 percentage point decrease in variability [ to ] for air-filtration use in comparison with placebo. The air-filter intervention showed a 67% (95% CI: 50% to 77%) reduction in indoor , but no change was observed with the improved-technology woodstove intervention.
Conclusions:
Among children with asthma and chronic exposure to woodsmoke, an air-filter intervention that improved indoor air quality did not affect quality-of-life measures. Intent-to-treat analysis did show an improvement in the secondary measure of dPFV.
Trial registration:
ClincialTrials.gov NCT00807183. https://doi.org/10.1289/EHP849
Introduction
Several studies of children with asthma in urban environments have targeted the household environment to improve health outcomes (Bryant-Stephens 2009; Butz et al. 2011; Eggleston et al. 2005; Krieger et al. 2005; Levy et al. 2006; Morgan et al. 2004; Sweet et al. 2014). These intervention studies have targeted asthma triggers by employing education strategies and/or directly mitigating exposure to allergens through pest management and/or use of allergen-impermeable mattress and pillow encasings. Three of these studies incorporated air-filter devices in the intervention strategy to reduce children’s exposure to airborne allergens, dust, or environmental tobacco smoke (Butz et al. 2011; Eggleston et al. 2005; Morgan et al. 2004). The intervention strategies consistently demonstrated declines in asthma-related symptom measures. However, all of these studies were conducted in urban environments, and the nature of these exposures likely differ from that of exposures in rural environments.
Attention to home heating sources in asthma intervention trials has been limited. One randomized trial showed lower frequency of asthma symptoms and health care visits compared with controls when improved heating-source interventions were employed to increase indoor temperature and decrease concentrations (Howden-Chapman et al. 2008). To our knowledge, no randomized controlled trials among children with asthma have been conducted that target indoor particulate matter (PM) exposures in rural-area homes heated by residential woodstoves.
Biomass combustion is known to be an important contributor to household air pollution in developing countries with resultant adverse health effects (Smith et al. 2014). The exposure scenarios and health consequences related to biomass combustion in developing-country settings are distinct from those in high-income countries. Nevertheless, burning wood for residential heating is an important PM exposure source in many developed countries, particularly in rural settings. The U.S. Department of Energy estimates that there are homes that use wood as a primary or secondary heating fuel (U.S. Department of Energy 2009), translating to more than three million children living in woodstove-heated homes (Noonan et al. 2015). Estimates of the contribution of wood-burning to ambient air quality can vary widely (Naeher et al. 2007), but woodsmoke accounts for 80– 90% of the ambient PM concentrations in communities with a high proportion of wood-burning households (Johnston et al. 2013; McGowan et al. 2002; Ward and Lange 2010; Ward et al. 2006). In the European Union, it is estimated that domestic woodstoves will be the dominant source of ambient fine PM (), accounting for 38% of all emissions by 2020 (Sigsgaard et al. 2015).
Indoor concentrations in homes that heat with wood often exceed the World Health Organization daily ambient air-quality standard of (Noonan et al. 2012; Semmens et al. 2015; Ward et al. 2011), likely the result of indoor PM sources and infiltration of ambient PM from local sources (Allen et al. 2003; Barn et al. 2008; Hystad et al. 2009; Semmens et al. 2015). Few exposure-reduction interventions have been tested in such wood-burning households (Allen et al. 2011; Noonan et al. 2012; Ward et al. 2011), and none has been evaluated in the context of impact on children’s health.
We report here results from a randomized controlled trial to evaluate interventions targeting biomass smoke PM from older-model residential woodstoves in homes of children with asthma. Older-model stoves are models defined as not being certified by the U.S. Environmental Protection Agency (EPA), or those that have emissions of of PM for a catalytic stove or for a noncatalytic stove. The Asthma Randomized Trial of Indoor Wood Smoke (ARTIS) took place in rural areas of Montana, Idaho, and Alaska, where residential wood combustion is a major source of ambient and indoor and the primary source of home heating during cold-temperature periods. Our aims were to test the hypothesis that (a) improved-technology wood-burning stoves or (b) air-filtration units would result in improvements, relative to placebo, in asthma measures among children in participating homes.
Methods
Study Overview
The ARTIS study design has been previously described (Noonan and Ward 2012). Briefly, ARTIS was a three-arm randomized placebo-controlled intervention trial with two intervention strategies for reducing in-home woodsmoke PM (Figure 1). Eligible participants were identified by parent-response screening questionnaire (Magzamen et al. 2005) and included children with asthma, age 6–18 y, residing in a non-tobacco-smoking household that used an older-model woodstove as their primary source of heating. If a household had more than one eligible child with asthma, the child with more severe asthma based on screening questions was designated as the primary participant in the household, but all eligible, consented children with asthma were included in the analyses. Overall, ARTIS was conducted over five years. Each household participated in two consecutive winter periods with household interventions occurring between the two winter periods (Figure 2). Homes were randomly assigned to one of three treatments: the woodstove-intervention group receiving improved-technology wood-burning appliances (i.e., EPA-certified woodstoves), the air-filter group receiving functioning air-filtration devices, and the placebo group receiving sham air-filtration devices. Detailed specifications of the intervention treatments can be found in Supplemental Material. This study was approved by the University of Montana Institutional Review Board. Participating children completed documented assent procedures, and a parent or guardian signed parental permission forms.
Figure 1.
Trial profile.
Note: The woodstove changeout arm (W) was discontinued prior to recruiting the final cohort of homes.
Figure 2.
Study locations according to years of participation.
Note: The woodstove changeout arm (W) was discontinued prior to recruiting the final cohort of homes. F, air filter arm; W, woodstove changeout arm; P, placebo arm.
Health Measures
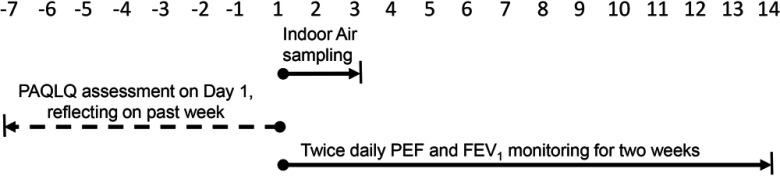
The primary health outcome was change in the score on the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) administered directly to children at each visit. The PAQLQ is a 23-item asthma-specific battery that provides an overall score as well as domain scores for symptoms (10 items), activity limitation (5 items), and emotional function (8 items) (Juniper et al. 1996). The instrument requires the participating children to reflect on the experiences over the prior week; thus, it is a one-week retrospective self-assessment (Figure 3). This instrument has strong correlation, both longitudinal and cross-sectional, with other measures of asthma severity, such as peak flow monitoring, medication use, and symptom diaries, among children with asthma ages 7 through 17 y (Juniper et al. 1996). The score for each domain is the average of the items in it, with scores ranging from 1 to 7 (with 7 as the optimal score), and the overall PAQLQ score was the mean score across the three domains. The primary hypothesis was that the preintervention to postintervention change in PAQLQ scores would be greater in the woodstove changeout or air filter arms relative to placebo. Our a priori power estimates indicated that when comparing two groups, a sample size of 30 subjects per group would provide 80% power to demonstrate a difference in PAQLQ scores of 1.0 unit or greater at the 95% confidence level (CI).
Figure 3.
Schematic of sampling visit. Each visit occurred multiple times during each of two winter periods, a preintervention winter and a postintervention winter. For a given visit, study personnel administered the PAQLQ to participants. The PAQLQ is a self-assessment questionnaire reflecting on the child’s past week of experiences. At the sampling visit, children are trained in the use of the expiratory flow monitor and begin recording twice-daily measures of PEF and for two weeks. Indoor air monitoring was initiated on Day 0 and continued for 48 h. Note: , forced expiratory volume in the 1st second; PAQLQ, pediatric asthma quality of life questionnaire; PEF, peak expiratory flow.
Secondary outcomes included home-based peak flow monitoring. Parents and children were trained to collect twice-daily measures (in the morning and in the evening) of peak expiratory flow (PEF) and forced expiratory volume in the first second () using the PiKo-1 Peak Flow Meter (Ferraris). These measures were collected and logged during a two-week period beginning with the first day of an exposure sampling visit. For each measure, the child performed a peak flow maneuver three times, and the device stored the best result for PEF and . Outcomes from these two-week recordings included average percent predicted morning PEF and , average percent predicted evening PEF and , and average diurnal PEF variability (dPFV). Daily dPFV was the amplitude as a percentage of the mean of consecutive evening to morning readings, or (previous night PEF–morning PEF)/(mean of the two measures) (Lebowitz et al. 1997). Whereas lower percent predicted PEF and are considered worse outcomes, the opposite is true for dPFV, where higher dPFV is considered a worse outcome for children with asthma.
Additional child data were collected to characterize baseline health. During each two-week sampling period, parents of participating children were also asked to record daily health-related events for their child, including daytime cough or wheezing, nighttime cough or wheezing, activity limitations due to asthma symptoms, and use of asthma medications. These data together with peak-flow monitoring data collected during the baseline (preintervention) winter were used to classify asthma severity based on the 2007 National Heart Lung and Blood Institute (NHLBI) guidelines (National Heart Lung and Blood Institute 2007). For methods used to translate these data to the 2007 NHLBI asthma severity classifications and for overall severity classification of participants and severity classification according to specific components, see Supplemental Material and Table S1.
Exposure Assessment
The exposure and health assessments took place on two, 48-h observation periods during each of two winter periods, before and after intervention. A DustTrak® Aerosol Monitor 8520/8530 (TSI) was used to continuously measure concentrations that were corrected for woodsmoke (McNamara et al. 2011). A Lighthouse 3016-IAQ particle counter (Lighthouse Worldwide Solutions) was used to continuously measure particle number concentrations of varying size fractions (0.30–0.49, 0.50–0.99, 1.00–2.49, 2.5–5.0, and 5.0–10.0, ). Particle number concentrations (PNCs), reported as the number of particles per cubic centimeter, were summed across the size fractions and were used as a surrogate for concentrations of coarse PM (PMc). Samplers had 60-s recording intervals, and were zero calibrated prior to each sampling event. We included only those 48-h averages that were generated from data that were at least 80% complete to ensure that the averages were representative of concentrations experienced during the entire sampling events. Instrument malfunctions (e.g., flow errors) or power failures were the primary reasons for sampling events with less than 100% air-sampling data capture.
Prior to sampling, participants completed demographics and home characteristics surveys. These surveys documented information such as household income, education, type/age/size of home, age of woodstove and specific activities that occurred in the home during the 48-h sampling periods.
Statistical Analysis
We used analysis of variance or chi-square tests, as appropriate, to compare differences between treatment groups in descriptive characteristics such as age, sex, race/ethnicity, and baseline health measures. We followed the principle of intent-to-treat and included all participants who were randomized to a treatment arm and had at least one baseline measure. The objective of our statistical analysis was to evaluate whether intervention assignment modified the preintervention-to-postintervention winter change in PAQLQ or peak flow measure. As such, the linear mixed effects model included fixed effects for a) winter (i.e., preintervention or postintervention winter), b) intervention assignment (e.g., air filter), and c) a multiplicative interaction term between these two variables to assess effect modification by intervention assignment. These models also included fixed effects for age and gender. Initially, random effects were included for both the intercept and the slope (i.e., winter), which in effect allowed each participant to have his or her own intercept and slope describing change in asthma measure from the preintervention to postintervention winter. However, there were problems with estimation of the model when we included a random effect for the slope (i.e., winter). As a result, our final model included a random effect only for the intercept. Winters were nested within participants, and participants were nested within homes. The linear mixed-model analysis amounts to adopting likelihood-based available-case analysis for handling missing data. In post-hoc sensitivity analyses, we examined the impact on results when we adjusted for additional covariates, including baseline asthma measures, use of asthma medications, and ambient temperature because these factors could influence both the use of the woodstove and asthma measures. We also performed separate analyses for each level of baseline asthma severity. All intent-to-treat analyses were conducted using SAS (version 9.3; SAS Institute Inc.).
The interventions utilized in this study were designed to improve various measures of childhood asthma by improving indoor air quality. Specifically, the objective was to lower concentrations of in homes. To assess the extent to which PM reductions contributed to intervention efficacy, we added both natural log-transformed and coarse PM as time-dependent variables to our primary models and examined potential attenuation of efficacy of the interventions. In addition, to examine further whether impacts on health were consistent with changes in PM, we used linear mixed models to assess the effect of concentrations on PAQLQ scores and peak flow measures. These post-hoc analyses were no longer based on a randomized design and were adjusted for treatment assignment, gender, and age. concentrations were skewed and were natural log-transformed. Thus, results are presented as the change in PAQLQ or peak flow measure associated with a doubling of .
Results
Almost 7,000 recruitment surveys were administered and follow-up contact with the 408 potentially eligible households resulted in 115 recruited children with asthma living in 98 eligible households (Figure 1). Insufficient baseline data were collected for one participant to classify according to asthma severity, and they were not included in further analysis. Among the 114 remaining participants, health and exposure data were captured at 422 overall visits (226 during the preintervention winter and 196 during the postintervention winter). Prior to enrollment of the final cohort of homes, the woodstove intervention arm was discontinued as interim analyses indicated that the woodstove change-out was not efficacious in reducing indoor (Ward et al. 2017). Thus, the sample size in the air filter and placebo arms were approximately twice that of the woodstove arm.
Descriptive and baseline health characteristics by assigned treatment arm are shown in Table 1. The overall mean [standard deviation (SD)] age was 12.4 y (3.0), and 48.3% of participants were female. Age, gender, and body mass index were similar across treatment arms. Most participants were white (82%) and non-Hispanic (95%), but race and ethnicity were not reported by parents for seven children. Among those with available race data, 4 of the 41 participants assigned to the air-filter arm were American Indian/Alaskan Native (AI/AN), and no participants reported being of AI/AN race in the other two arms. In addition, 101 of the 114 participants (89%) were classified as having moderate to severe persistent asthma (Table 1 and Table S1). A majority of caregivers (57%) reported household incomes greater than per year, and 72% reported having some postsecondary education. The majority of homes (87%) reported having a dog or cat. These factors were similar by treatment arm assignment. During the preintervention winter, overall median concentrations were , and were higher, relative to placebo, in homes assigned to the woodstove changeout arm of the trial. Median PMc was 0.32 particles per and similar in all treatment arms.
Table 1.
Baseline participant and household characteristics.
| Participant characteristics | Woodstove changeout () | Air filter () | Placebo () |
|---|---|---|---|
| Age, mean years (SD) | 12.3 (3.1) | 12.7 (3.3) | 12.2 (2.5) |
| Body mass index percentile, mean (SD) | 67.6 (28.4) | 69.7 (27.5) | 62.4 (30.1) |
| Female sex, (%) | 10 (45.5) | 25 (54.5) | 20 (43.5) |
| Race, (%)a | |||
| American Indian/Alaskan Native | 0 (0.0) | 4 (9.8) | 0 (0.0) |
| White | 16 (76.2) | 32 (78.1) | 40 (88.9) |
| Other | 5 (23.8) | 5 (12.2) | 5 (11.1) |
| Non-Hispanic ethnicity, (%)a | 20 (95.2) | 39 (95.1) | 43 (95.6) |
| Household income , (%)a | 12 (57) | 20 (50) | 28 (62) |
| Household post-secondary education, (%)a | 15 (75) | 29 (74) | 31 (69) |
| Dogs or cats in home, (%) | 17 (77) | 39 (85) | 41 (89) |
| Baseline (preintervention) Health Measures | |||
| Asthma Severity, (%) | |||
| Intermittent or Mild | 2 (9.1) | 5 (10.9) | 6 (13.0) |
| Moderate or Severe | 20 (90.9) | 41 (89.1) | 40 (87.0) |
| PAQLQ, mean (SD) | |||
| Overall | 5.1 (1.0) | 5.4 (1.0) | 5.4 (1.0) |
| Symptoms | 4.9 (1.2) | 5.3 (1.0) | 5.3 (1.1) |
| Limitation of Activity | 4.6 (1.3) | 5.0 (1.1) | 5.0 (1.3) |
| Emotion | 5.7 (0.8) | 5.6 (1.1) | 5.7 (1.0) |
| Two-week spirometry monitoring, mean (SD) | |||
| Evening percent predicted | 75.3 (19.0) | 87.2 (18.1) | 88.3 (24.9) |
| Morning percent predicted | 73.1 (19.4) | 86.2 (18.2) | 87.5 (24.4) |
| Evening PEF percent predicted | 72.4 (25.8) | 84.9 (24.7) | 85.1 (21.9) |
| Morning PEF percent predicted | 70.8 (25.6) | 82.3 (22.8) | 82.9 (23.0) |
| Diurnal PEF variability (dPFV), % | 18.5 (8.5) | 17.0 (10.4) | 14.4 (6.3) |
| Two-week reporting of symptoms and medical usage | |||
| Daytime cough/wheezing, days | |||
| Mild | 5.7 (3.8) | 4.3 (3.2) | 4.2 (3.1) |
| Moderate/Severe | 1.9 (2.9) | 1.0 (1.5) | 0.9 (1.6) |
| Nighttime cough/wheezing, days | |||
| Mild | 3.2 (3.0) | 2.6 (2.7) | 3.3 (3.0) |
| Moderate/Severe | 2.9 (4.6) | 0.8 (1.4) | 0.9 (1.6) |
| Altered activity/behavior, days | |||
| Mild | 3.8 (2.9) | 2.0 (2.3) | 2.1 (2.4) |
| Moderate/Severe | 1.3 (1.9) | 0.8 (1.4) | 1.0 (2.4) |
| Medication usage, doses | |||
| Long-term control medications | 4.4 (6.2) | 7.5 (10.6) | 4.0 (7.4) |
| Quick relief medications | 4.9 (7.1) | 4.1 (7.9) | 3.8 (6.4) |
Note: , forced expiratory volume in the first second; PAQLQ, pediatric asthma quality of life; PEF, peak expiratory flow.
Race/ethnicity, household income, and household education not reported for 7, 8, and 10 participant parents, respectively.
Among the baseline health measures, averaged over preintervention winter sampling visits, asthma severity and PAQLQ did not differ among the treatment arms (Table 1). Children in the woodstove changeout arm had a slightly higher proportion of participants experiencing moderate to severe asthma and lower mean PAQLQ scores. and PEF percent predicted were also lower among participants in the woodstove changeout arm in comparison with the other study arms. Mean (SD) dPFV during the preintervention winter was 16.3% (11.1) across treatment arms.
Within each arm, preintervention to postintervention changes for PAQLQ and spirometry measures are presented in Table 2. For all treatment arms, the overall PAQLQ composite score, as well as domain scores for symptoms, activity limitation, and emotion, increased in magnitude by 0.2–0.7 on the seven-point scale from one winter to the next. For the spirometry measures, increases in and PEF percent predicted measures would indicate improvement in lung function, whereas decreases in dPFV would also indicate improvement in lung function. In general, and PEF percent predicted measures decreased (i.e., worsened) from preintervention to postintervention winters in both the air-filter and placebo arms. In contrast, dPFV change in the air-filter arm showed decreased variation, suggesting improved function with a percentage point change of (95% to 0.7), or a 11.8% (95% CI: 27% to ) improvement in comparison with baseline in this arm.
Table 2.
Preintervention to postintervention mean changes [95% confidence interval (CI)]a within arm and within intervention, relative to placebo ( participants).
| Outcome | Observations | Within group change | Placebo | Change relative to placebo | ||
|---|---|---|---|---|---|---|
| Woodstove changeout | Air filter | Woodstove changeout | Air Filter | |||
| PAQLQ | ||||||
| Overall | 422 | 0.47 (0.04, 0.90) | 0.22 (, 0.52) | 0.29 (0.01, 0.58) | 0.18 (, 0.69) | (, 0.34) |
| Symptoms | 422 | 0.61 (0.13, 1.1) | 0.19 (, 0.52) | 0.23 (, 0.55) | 0.38 (, 0.95) | (, 0.41) |
| Limitation of Activity | 422 | 0.61 (0.09, 1.1) | 0.23 (, 0.59) | 0.48 (0.13, 0.82) | 0.13 (, 0.74) | (, 0.24) |
| Emotion | 422 | 0.18 (, 0.61) | 0.24 (, 0.53) | 0.28 (, 0.56) | (, 0.41) | (, 0.36) |
| Two-week spirometry monitoring | ||||||
| Evening % predicted | 406 | (, 8.5) | (, 3.1) | (, 2.6) | 2.9 (, 13) | 0.24 (, 8.3) |
| Morning % predicted | 408 | 0.96 (, 9.8) | (, 2.7) | (, 3.1) | 3.6 (, 14) | (, 7.5) |
| Evening PEF % predicted | 407 | 0.07 (, 8.0) | (, 0.98) | (, ) | 7.1 (, 16) | 2.4 (, 9.9) |
| Morning PEF % predicted | 409 | 1.1 (, 9.2) | (, 2.3) | (, ) | 7.8 (, 17) | 3.4 (, 11) |
| Diurnal PEF variability, % | 404 | (, 3.0) | (, 0.7) | 2.2 (, 4.7) | (, 1.6) | (, ) |
Note: , forced expiratory volume in the first second; PAQLQ, pediatric asthma quality of life; PEF, peak expiratory flow.
Adjusted for age and gender.
Efficacy analyses of preintervention to postintervention changes in PAQLQ and spirometry measures for treatment arms relative to placebo are presented in Table 2. Relative to the placebo arm, the air-filter intervention and the woodstove changeout showed no improvement in PAQLQ composite or domain scores. Mean change in PEF and were generally better for the air-filter and woodstove changeout arms, in comparison with the placebo arm. For example, change (and 95% CI), relative to placebo, in morning percent predicted PEF were 3.4 ( to 11) and 7.8 ( to 17) for air-filter and woodstove changeout arms, respectively. Observations for dPFV showed lesser variability for the air-filter arm, with a 4.1 percentage point greater reduction in dPFV ( to ) from the preintervention to postintervention winters for the air-filter arm, relative to the placebo arm, suggesting improved airway function (Table 2). The woodstove changeout arm showed a 3.0 percentage point reduction for dPFV relative to placebo ( to 1.6).
To further evaluate the influence of asthma severity status on intervention efficacy, we conducted stratified analysis for intervention changes to dPFV according to baseline asthma severity (Table 3). The highest point estimate for reduction in dPFV among the air-filter group was observed among the children with indications of severe asthma at baseline, but no differential effect in dPFV was evident when stratifying by severe or moderate asthma groups. When combining those children with severe and moderate asthma at baseline, the change in dPFV among the air-filter group relative to placebo was similar to the primary analysis (; 95% CI: to ). When including only primary participants, or the child in a given home with the more severe asthma symptoms, the change in dPFV among the air-filter group relative to placebo was lower (; 95% CI: to 0.80; see Table S3). Additional sensitivity analyses, adjusting for baseline health measure and/or ambient temperature, did not change the effect estimates appreciably (see Table S2).
Table 3.
Intervention effect on diurnal peak flow variability according to baseline asthma severity, relative to placebo, adjusted for age and gender.
| Participant number | Observations | Woodstove changeout | Air filter | |
|---|---|---|---|---|
| Mean change (95% CI) | Mean change (95% CI) | |||
| All participants | 114 | 404 | (, 1.6) | (, ) |
| Severe | 50 | 170 | (, 6.4) | (, 2.6) |
| Moderate | 51 | 190 | (, 3.3) | (, 1.6) |
| Intermittent/mild | 13 | 44 | 0.07 (, 10) | (, 10) |
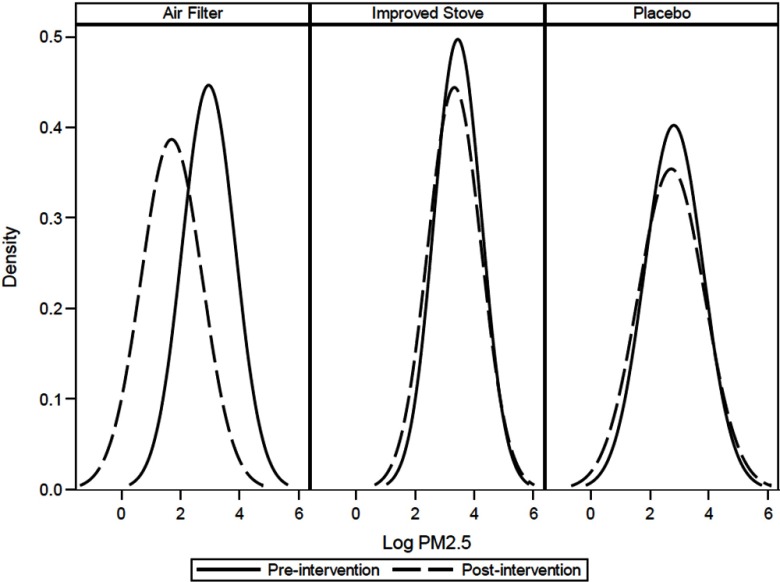
As shown previously, we observed a strong overall reduction in indoor concentrations (; 95% CI: to ) for the air-filter group relative to the placebo group, but we observed no overall change to indoor for the woodstove exchange (0.0%; 95% CI: , 65%), relative to the placebo group (Ward et al. 2017). Figure 4 shows a smoothed distribution of log-transformed measures, indicating strong overlap in preintervention and postintervention observations. Distributions of preintervention and postintervention measures for the woodstove exchange and the placebo arms were similar, consistent with the previously reported findings of no change for each of these arms. We observed strong reductions in PMc in both the filter (72%) and placebo (57%) intervention arms (Ward et al. 2017).
Figure 4.
Kernal density plot of pre- (solid line) and postintervention (dashed line) 48-h indoor fine particulate matter () by treatment arm.
To further explore these changes to indoor air quality and impact on health outcomes, we conducted post hoc analyses. Inclusion of and PMc in our primary models attenuated the estimate of the efficacy of the air filter in improving dPFV, relative to placebo (Table 4). Intervention assignment continued to be unassociated with overall PAQLQ or the PAQLQ domain scores when PM measures were included in analyses. However, despite having no indication of efficacy for air-filter interventions on PAQLQ, our exposure response analysis did indicate that PAQLQ was associated with (Table 5). Because PM measures were natural log-transformed, we report changes in each response variable associated with a doubling of PM exposure. A doubling of concentration was associated with small declines in overall PAQLQ [ (95% CI: , )] and emotion domain [ (95% CI: , )] scores. was not associated with changes in most of our assessed spirometry measures. However, consistent with our intent-to-treat analysis, a doubling was associated with increased (i.e., worsening) dPFV, 0.64 (95% CI: , 1.34).
Table 4.
Preintervention to postintervention mean changes (95% CI) for treatments relative to placebo, adjusted for age, gender and indoor particulate matter ( participants).
| Outcome | Adjusted for age, gender, a | Adjusted for age, gender, PMcb | Adjusted for age, gender, , PMcb | |||
|---|---|---|---|---|---|---|
| Woodstove changeout | Air filter | Woodstove changeout | Air filter | Woodstove changeout | Air filter | |
| PAQLQ | ||||||
| Overall | 0.18 (, 0.71) | (, 0.27) | 0.20 (, 0.77) | (, 0.32) | 0.20 (, 0.77) | (, 0.24) |
| Symptoms | 0.38 (, 0.96) | (, 0.34) | 0.39 (, 1.0) | (, 0.37) | 0.38 (, 1.0) | (, 0.29) |
| Limitation of Activity | 0.13 (, 0.75) | (, 0.24) | 0.16 (, 0.83) | (, 0.24) | 0.16 (, 0.83) | (, 0.24) |
| Emotion | (, 0.44) | (, 0.30) | (, 0.51) | (, 0.38) | (, 0.51) | (, 0.27) |
| Two-week spirometry monitoring | ||||||
| Evening % predicted | 2.6 (, 13) | (, 7.7) | 1.3 (, 13) | (, 8.3) | 1.2 (, 13) | (, 7.8) |
| Morning % predicted | 3.2 (, 14) | (, 6.9) | 2.2 (, 14) | (, 4.2) | 2.2 (, 14) | (, 6.7) |
| Evening PEF % predicted | 6.9 (, 17) | 0.62 (, 8.6) | 6.3 (, 16) | 2.2 (, 10) | 6.2 (, 17) | 1.2 (, 9.7) |
| Morning PEF % predicted | 7.7 (, 17) | 1.7 (, 9.7) | 7.1 (, 17) | 2.5 (, 11) | 7.0 (, 17) | 1.8 (, 10) |
| Diurnal PEF variability, % | (, 2.0) | (, 0.4) | (, 2.3) | (, 1.0) | (, 2.3) | (, 1.7) |
Note: forces expiratory volume in first second; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; PEF, peak expiratory flow.
Missing observations for models with : for PAQLQ measures; for spirometry measures.
Missing observations for models with : for PAQLQ measures; for spirometry measures.
Table 5.
Effects of doubling fine fraction particulate matter () concentrations on quality of life measures and two-week spirometry monitoring, adjusting for age, gender, and intervention assignment.
| Outcome | Obs | Estimate | 95% CI |
|---|---|---|---|
| PAQLQ | |||
| Overall | 415 | (, ) | |
| Symptoms | 415 | (, 0.001) | |
| Limitation of activity activity | 415 | (, 0.03) | |
| Emotion | 415 | (, ) | |
| Spirometry | |||
| Evening | 398 | 0.55 | (, 1.88) |
| Morning | 400 | 0.43 | (, 1.77) |
| Evening PEF | 399 | 0.02 | (, 1.55) |
| Morning PEF | 401 | (, 1.28) | |
| dPFV | 396 | 0.64 | (, 1.34) |
Note: dPFV, diurnal peak flow variability; , forced expiratory volume in first second; Obs, observations; PAQLQ, pediatric asthma quality of life Questionnaire; PEF, peak expiratory flow.
Discussion
To our knowledge, this investigation is the first randomized controlled trial aimed at improving asthma symptoms in children by reducing indoor concentrations generated from woodstoves used for heating. Although the filter intervention resulted in the anticipated reductions in indoor concentrations, we did not observe treatment efficacy according to PAQLQ, our primary health outcome measure (Table 2). Given that this was a subjective metric measured over a two-winter period, it is possible that there was a learning effect due to repeated assessment. Indeed, we observed an improvement in the magnitude of overall PAQLQ score from the preintervention to postintervention winters in all treatment arms. Improvements also were observed in the symptom and activity limitation PAQLQ domains for the woodstove changeout arm and in the activity limitation PAQLQ domains for the placebo arm (Table 2). As in analyses of the overall PAQLQ score, no treatment effect with respect to placebo was observed for these domains. The preintervention to postintervention changes in PAQLQ were modest across all treatment arms with most point estimate changes less than one-half unit, whereas changes in this scale of 0.5 or more points have been translated as a clinically relevant outcome (Juniper et al. 1996).
Assignment to the filter arm was associated with strong reductions in indoor concentrations in this randomized trial (Ward et al. 2017). Reductions in coarse particles also were observed in the filter arm and, unexpectedly, in the placebo arm. This latter observation may explain, in part, why we did not demonstrate efficacy for PAQLQ in the air-filter arm, relative to the placebo arm. The mechanism for the observed improvement in overall PAQLQ score, as well as in scores in the PAQLQ symptom and activity limitation domains, in the woodstove arm is unclear. As demonstrated previously, homes assigned to this intervention experienced no improvement in concentrations or PNCs of any size; they did experience reductions in carbon monoxide concentrations, however (Ward et al. 2017).
Peak flow measures were the secondary health measures for this trial. We observed an improvement in dPFV, specifically a 4.1-percentage point reduction for children in the filter arm, relative to placebo. Of note, the filter arm had higher preintervention dPFV, relative to the placebo, indicating the randomization was not entirely successful in balancing asthma severity between groups. Thus, we cannot rule out completely the possibility that regression to the mean may explain, at least in part, the observed efficacy of the filter in improving dPFV. When analyses were limited to only those with moderate or severe persistent asthma, the improvement in dPFV variability was similar, perhaps even stronger for those with severe asthma. Home-based peak flow monitoring is not well supported as a tool for clinical decision making of asthma therapy (Kamps et al. 2001; Self et al. 2014; Yoos et al. 2002). There also are questions about the validity of dPFV as a clinical tool for asthmatic children, and correlation between measures of peak expiratory flow variation and asthma symptoms may wane over longer periods of observation (i.e., ), suggesting that this measure may be less desirable as a tool for management of asthma in children (Brand et al. 1999). Nonetheless, dPFV does track with hyper-responsiveness, severity of symptoms, unscheduled medical visits, and response to bronchodilators (Brand et al. 1999; Brouwer et al. 2006; Greenberg et al. 2012; Mortimer et al. 2001), and change in dPFV has been used as a measure that reflects the dynamic nature of asthma (Lebowitz et al. 1997). There is also support for using dPFV and similar measures that reflect the natural diurnal variation of airway function and response to exposures and treatments in randomized controlled clinical trials (Frey and Bielicki 2017; Kaminsky et al. 2017). Here we use dPFV in repeated two-week snapshots to indicate short-term change and make no inferences regarding its utility as a self-management tool. Increased dPFV has been shown to correspond to bronchial responsiveness to nonspecific challenges (Cockcroft and Hargreave 1990; Hetzel and Clark 1980). More recently, dPFV among children with asthma was positively associated with indoor measures of specific fungi species (Bundy et al. 2009; Douwes et al. 2000).
Comparisons of PM and other air pollutant exposures with dPFV among children with asthma are limited and have indicated that pollutant effects on dPFV may be stronger among children with mild asthma. A study of children with mild asthma in Korea showed that dPFV increased during Asian dust events in comparison with control days, with corresponding strong correlations observed between concentrations and dPFV (Yoo et al. 2008). A panel study of children in France showed an association between ambient and dPFV, but only among those children with mild asthma; no associations were observed for ambient and dPFV (Segala et al. 1998). In contrast, the present study supports an association between smaller size fraction PM, specifically , and our findings of efficacy with respect to dPFV remained robust, perhaps stronger, when limiting the analysis to children with moderate to severe asthma. Our observed response in dPFV among children with moderate to severe asthma was consistent with studies of medical intervention. Measures of PFV were found to change favorably in response to treatment with inhaled corticosteroids among children with moderate to severe asthma (Simons 1997; van Essen-Zandvliet et al. 1992), but not necessarily among children with mild asthma (Waalkens et al. 1991).
The beneficial impact of the air-filter intervention on dPFV was not corroborated with parallel findings in other pulmonary function measures, i.e., morning and evening percent predicted and PEF. The within-group improvement in dPFV following the air-filter intervention also was fairly small (i.e., 2 percentage point decline). Nevertheless, this change did indicate a greater than 11% improvement in dPFV, relative to the baseline winter mean in this group. Our exposure response analysis also showed an association between elevated concentrations and higher dPFV, consistent with our overall hypothesis that the air-filter intervention improves health by improving air quality.
This study was relatively large, including more than 400 multiday exposure and health sampling visits among 114 children with asthma from various rural regions in western Montana, Idaho, and Alaska. Extensive information on air pollutant exposures, asthma measures, and relevant covariates was ascertained with the randomized, placebo-controlled design providing protection against confounding. However, we note several limitations. First, to the extent possible, participating filter and placebo homes were blinded to their treatment assignment. This blinding was not possible for the homes receiving the woodstove intervention. Moreover, field staff responsible for collecting exposure and health data were not blinded, as study protocol and efficient use of project resources required them to install intervention filter units, replace filters at prespecified intervals, and collect home-based health and exposure data. Quality-control procedures were employed to protect against any potential influence resulting from failures in blinding at the participant and investigator levels, but this circumstance remains a limitation when considered in the context of rigorous randomized controlled trial designs. Second, performance and recording of self-monitored PEF and measures is highly effort dependent. Our procedures included training participants and parents on best-effort procedures. Although within-individual variation in effort is likely, we expect that this error would be random rather than systematic over the two-winter observation period, particularly when considering the most robust finding, change in dPFV, where the smallest unit of measure is percent difference in successive PEF measures in less than 24 h (i.e., evening to morning). Third, despite the randomized design, we observed higher preintervention concentrations and poorer baseline health in participants residing in households assigned to the woodstove changeout arm. This arm of the study was eliminated early due to evidence of failed efficacy with respect to reduction of indoor concentrations. Had this arm continued to full recruitment, it is possible that the baseline exposure and health measures would have balanced with the other arms. This change did result in larger numbers of participants allocated to the air-filter and placebo arms, making comparison between those arms more robust. Nevertheless, observations for the woodstove changeout arm relative to the placebo arm should be interpreted with caution. Fourth, the nature of the intervention strategy being tested in this study called for between-winter, rather than within-winter, evaluations of preintervention-to-postintervention health changes. Asthma health and response to environmental stimuli can change during a one-year period, particularly among adolescents who typically have worsening symptoms during these ages. Given the randomized design, we expect that such intra-individual changes would not be differential with respect to treatment arm. A crossover design was not possible in this study due to the woodstove changeout arm, but this approach may have been a more robust approach for evaluating within-winter filter versus placebo effects for children with asthma. Fifth, the efficacy of the air-filter intervention depended on the home residents operating the unit at the recommended setting. To assess compliance, we measured energy usage with Kilowatt meters attached at the filter units. As demonstrated previously, mean percent compliance (i.e., actual Kilowatt usage as a proportion of predicated Kilowatt usage) was 78%, and exposure reduction was robust to lower levels of compliance (Ward et al. 2017). Sixth, although we conducted a limited number of primary analyses, we performed nine primary statistical tests and might observe an association due to chance alone. As a result, we cannot rule out the possibility that the efficacy of the air filter in improving dPFV is simply due to chance. Finally, unlike patient-level clinical trials where compliant delivery of a drug or procedure accounts for 100% of the intended dose, we must recognize that environmental intervention studies account for some component of dose. Thus, despite substantial reductions in indoor concentration following the introduction of the filter, participating children were still exposed to measurable .
Conclusions
Biomass combustion during residential heating is an important source of household air pollution, particularly in rural communities, and evidence-based strategies to improve indoor air quality and asthma outcomes in such settings are needed. This randomized trial targeted a vulnerable population, children with asthma who were chronically exposed to woodsmoke. The interventions tested did not result in improvements to quality-of-life measures, but the air-filtration device, a simple in-home intervention, was efficacious for improving the secondary measure dPFV, an indirect measure of airway hyper-responsiveness. The attenuation of the effect when including PM in the models as well as the exposure–response analysis provide evidence that filter-based PM reductions contribute, at least partially, to the observed improvements in the dPFV. This trial was conducted across several rural communities in three states, but translation of these findings to other settings with similarly exposed child asthma populations would require further study and inquiry into the challenges associated with dissemination of in-home PM reduction strategies.
Supplemental Material
Acknowledgments
The authors thank J. Balmes and K. Smith for their advice on study design. The authors also thank 3M Company for advice and materials in the design of the placebo filter.
This study was funded by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) 1R01ES016336-01 and 3R01ES016336-02S1. Additional support was provided by NIGMS (1U54GM104944 and P30GM103338) and NICHD (1UG1HD090902).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Allen R, Larson T, Sheppard L, Wallace L, Liu LJ. 2003. Use of real-time light scattering data to estimate the contribution of infiltrated and indoor-generated particles to indoor air. Environ Sci Technol 37(16):3484–3492, PMID: 12953856, 10.1021/es021007e. [DOI] [PubMed] [Google Scholar]
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, et al. 2011. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med 183(9):1222–1230, PMID: 21257787, 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- Barn P, Larson T, Noullett M, Kennedy S, Copes R, Brauer M. 2008. Infiltration of forest fire and residential wood smoke: an evaluation of air cleaner effectiveness. J Expo Sci Environ Epidemiol 18(5):503–511, PMID: 18059421, 10.1038/sj.jes.7500640. [DOI] [PubMed] [Google Scholar]
- Brand PL, Duiverman EJ, Waalkens HJ, van Essen-Zandvliet EE, Kerrebijn KF. 1999. Peak flow variation in childhood asthma: correlation with symptoms, airways obstruction, and hyperresponsiveness during long-term treatment with inhaled corticosteroids. Dutch CNSLD Study Group. Thorax 54(2):103–107, PMID: 10325912, 10.1136/thx.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer AF, Roorda RJ, Brand PL. 2006. Home spirometry and asthma severity in children. Eur Respir J 28(6):1131–1137, PMID: 16870659, 10.1183/09031936.06.00118205. [DOI] [PubMed] [Google Scholar]
- Bryant-Stephens T. 2009. Asthma disparities in urban environments. J Allergy Clin Immunol 123(6):1199–1206, PMID: 19501229, 10.1016/j.jaci.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Bundy KW, Gent JF, Beckett W, Bracken MB, Belanger K, Triche E, et al. 2009. Household airborne penicillium associated with peak expiratory flow variability in asthmatic children. Ann Allergy Asthma Immunol 103(1):26–30, PMID: 19663123, 10.1016/S1081-1206(10)60139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. 2011. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med 165(8):741–748, PMID: 21810636, 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Hargreave FE. 1990. Airway hyperresponsiveness. Relevance of random population data to clinical usefulness. Am Rev Respir Dis 142(3):497–500, PMID: 2202243, 10.1164/ajrccm/142.3.497. [DOI] [PubMed] [Google Scholar]
- Douwes J, Zuidhof A, Doekes G, van der Zee SC, Wouters I, Boezen MH, et al. 2000. (1–>3)-beta-D-glucan and endotoxin in house dust and peak flow variability in children. Am J Respir Crit Care Med 162(4 Pt 1):1348–1354, PMID: 11029343, 10.1164/ajrccm.162.4.9909118. [DOI] [PubMed] [Google Scholar]
- Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. 2005. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol 95(6):518–524, PMID: 16400889, 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- Frey U, Bielicki JA. 2017. Fluctuation metrics as novel endpoints for clinical trials in asthma. Am J Respir Crit Care Med 195(8):967–968, PMID: 28409680, 10.1164/rccm.201611-2286ED. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Liu N, Kaur A, Lakshminarayanan M, Zhou Y, Nelsen LM, et al. 2012. Airway obstruction lability helps distinguish levels of disease activity in asthma. Respir Med 106(4):500–507, PMID: 22301379, 10.1016/j.rmed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Hetzel MR, Clark TJ. 1980. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax 35(10):732–738, PMID: 7466721, 10.1136/thx.35.10.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden-Chapman P, Pierse N, Nicholls S, Gillespie-Bennett J, Viggers H, Cunningham M, et al. 2008. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ 337:a1411, PMID: 18812366, 10.1136/bmj.a1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad PU, Setton EM, Allen RW, Keller PC, Brauer M. 2009. Modeling residential fine particulate matter infiltration for exposure assessment. J Expos Sci Environ Epidemiol 19(6):570–579, PMID: 18716606, 10.1038/jes.2008.45. [DOI] [PubMed] [Google Scholar]
- Johnston FH, Hanigan IC, Henderson SB, Morgan GG. 2013. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: retrospective analysis of daily mortality, 1994-2007. BMJ 346:e8446, PMID: 23299843, 10.1136/bmj.e8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. 1996. Measuring quality of life in children with asthma. Qual Life Res 5(1):35–46, PMID: 8901365, 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- Kaminsky DA, Wang LL, Bates JH, Thamrin C, Shade DM, Dixon AE, et al. 2017. Fluctuation analysis of peak expiratory flow and its association with treatment failure in asthma. Am J Respir Crit Care Med 195(8):993–999, PMID: 27814453, 10.1164/rccm.201601-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps AW, Roorda RJ, Brand PL. 2001. Peak flow diaries in childhood asthma are unreliable. Thorax 56(3):180–182, PMID: 11182008, 10.1136/thorax.56.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Takaro TK, Song L, Weaver M. 2005. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health 95(4):652–659, PMID: 15798126, 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MD, Krzyzanowski M, Quackenboss JJ, O'Rourke MK. 1997. Diurnal variation of PEF and its use in epidemiological studies. Eur Respir J Suppl 24:49S–56S, PMID: 9098711. [PubMed] [Google Scholar]
- Levy JI, Brugge D, Peters JL, Clougherty JE, Saddler SS. 2006. A community-based participatory research study of multifaceted in-home environmental interventions for pediatric asthmatics in public housing. Soc Sci Med 63(8):2191–2203, PMID: 16781807, 10.1016/j.socscimed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Magzamen S, Mortimer KM, Davis A, Tager IB. 2005. School-based asthma surveillance: a comparison of student and parental report. Pediatr Allergy Immunol 16(8):669–678, PMID: 16343089, 10.1111/j.1399-3038.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- McGowan JA, Hider RN, Chacko E, Town GI. 2002. Particulate air pollution and hospital admissions in Christchurch, New Zealand. Aust N Z J Public Health 26(1):23–29, PMID: 11895020, 10.1111/j.1467-842X.2002.tb00266.x. [DOI] [PubMed] [Google Scholar]
- McNamara M, Noonan C, Ward T. 2011. Correction factor for continuous monitoring of wood smoke fine particulate matter. Aerosol Air Qual Res 11(3):315–322, PMID: 25364330, 10.4209/aaqr.2010.08.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R 3rd, et al. 2004. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 351(11):1068–1080, PMID: 15356304, 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- Mortimer KM, Redline S, Kattan M, Wright EC, Kercsmar CM. 2001. Are peak flow and symptom measures good predictors of asthma hospitalizations and unscheduled visits? Pediatr Pulmonol 31(3):190–197, PMID: 11276131, 10.1002/ppul.1028. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, et al. 2007. Woodsmoke health effects: a review. Inhal Toxicol 19(1):67–106, PMID: 17127644, 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. 2007. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, MD:National Heart, Lung, and Blood Institute. [Google Scholar]
- Noonan CW, Navidi W, Sheppard L, Palmer CP, Bergauff M, Hooper K, et al. 2012. Residential indoor PM2.5 in wood stove homes: follow-up of the Libby changeout program. Indoor Air 22(6):492–500, PMID: 22607315, 10.1111/j.1600-0668.2012.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan CW, Ward TJ. 2012. Asthma randomized trial of indoor wood smoke (ARTIS): rationale and methods. Contemp Clin Trials 33(5):1080–1087, PMID: 22735495, 10.1016/j.cct.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan CW, Ward TJ, Semmens EO. 2015. Estimating the number of vulnerable people in the United States exposed to residential wood smoke. Environ Health Perspect 123(2):A30, PMID: 25642637, 10.1289/ehp.1409136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segala C, Fauroux B, Just J, Pascual L, Grimfeld A, Neukirch F. 1998. Short-term effect of winter air pollution on respiratory health of asthmatic children in Paris. Eur Respir J 11(3):677–685, PMID: 9596121. [PubMed] [Google Scholar]
- Self TH, George CM, Wallace JL, Patterson SJ, Finch CK. 2014. Incorrect use of peak flow meters: are you observing your patients? J Asthma 51(6):566–572, PMID: 24720711, 10.3109/02770903.2014.914218. [DOI] [PubMed] [Google Scholar]
- Semmens EO, Noonan CW, Allen RW, Weiler EC, Ward TJ. 2015. Indoor particulate matter in rural, wood stove heated homes. Environ Res 138:93–100, PMID: 25701812, 10.1016/j.envres.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigsgaard T, Forsberg B, Annesi-Maesano I, Blomberg A, Bølling A, Boman C, et al. 2015. Health impacts of anthropogenic biomass burning in the developed world. Eur Respir J 46(6):1577–1588, PMID: 26405285, 10.1183/13993003.01865-2014. [DOI] [PubMed] [Google Scholar]
- Simons FE. 1997. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. N Engl J Med 337(23):1659–1665, PMID: 9385125, 10.1056/NEJM199712043372304. [DOI] [PubMed] [Google Scholar]
- Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. 2014. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health 35:185–206, PMID: 24641558, 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- Sweet LL, Polivka BJ, Chaudry RV, Bouton P. 2014. The impact of an urban home-based intervention program on asthma outcomes in children. Public Health Nurs 31(3):243–252, PMID: 24720657, 10.1111/phn.12071. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Energy. 2009. Residential Energy Consumption Survey, Table HC6.7 Space Heating in U.S. Homes, by Census Region. Washington, DC. [Google Scholar]
- van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. 1992. Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. The Dutch Chronic Non-specific Lung Disease Study Group. Am Rev Respir Dis 146(3):547–554, PMID: 1355640, 10.1164/ajrccm/146.3.547. [DOI] [PubMed] [Google Scholar]
- Waalkens HJ, Gerritsen J, Koëter GH, Krouwels FH, van Aalderen WM, Knol K. 1991. Budesonide and terbutaline or terbutaline alone in children with mild asthma: effects on bronchial hyperresponsiveness and diurnal variation in peak flow. Thorax 46(7):499–503, PMID: 1877037, 10.1136/thx.46.7.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T, Lange T. 2010. The impact of wood smoke on ambient PM2.5 in northern Rocky Mountain valley communities. Environ Pollut 158(3):723–729, PMID: 19897293, 10.1016/j.envpol.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Ward T, Boulafentis J, Simpson J, Hester C, Moliga T, Warden K, et al. 2011. Lessons learned from a woodstove changeout on the Nez Perce Reservation. Sci Total Environ 409(4):664–670, PMID: 21144555, 10.1016/j.scitotenv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Ward TJ, Rinehart L, Lange T. 2006. The 2003/2004 Libby, Montana PM2.5 Source Apportionment Research Study. Aerosol Sci Tech 40(3):166–177, 10.1080/02786820500494536. [DOI] [Google Scholar]
- Ward TJ, Semmens EO, Weiler E, Harrar S, Noonan CW. 2017. Efficacy of interventions targeting household air pollution from residential wood stoves. J Expos Sci Environ Epidemiol 27(1):64–71, PMID: 26555475, 10.1038/jes.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Y, Choung JT, Yu J, Kim d. K, Koh YY. 2008. Acute effects of Asian dust events on respiratory symptoms and peak expiratory flow in children with mild asthma. J Korean Med Sci 23(1):66–71, PMID: 18303201, 10.3346/jkms.2008.23.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoos HL, Kitzman H, McMullen A, Henderson C, Sidora K. 2002. Symptom monitoring in childhood asthma: a randomized clinical trial comparing peak expiratory flow rate with symptom monitoring. Ann Allergy Asthma Immunol 88(3):283–291, PMID: 11926622, 10.1016/S1081-1206(10)62010-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Graphical representation showing study locations according to years of participation (Winter No. 1, 2008-2009; Winter No. 2, 2009-2010; Winter No. 3, 2010-2011; Winter No. 4, 2011-2012; and Winter No. 5, 2012-2013), and the corresponding sample sizes for the three arms. The study locations are as follows: Hamilton, MT [F (n equals 4), W (n equals 5), and P (n equals 6)]; Missoula, MT; Nez Perce, ID [F (n equals 9), W (n equals 10), and P (n equals 10)]; Butte, MT; Fairbanks, AK [F (n equals 5), W (n equals 4), and P (n equals 5)]; and Western MT [F (n equals 25) and P (n equals 24)].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/bf7f/5915210/d52b38286130/EHP849_f2.jpg)