Abstract
Although cerebrospinal fluid (CSF) shunt placement is the most common procedure performed by pediatric neurosurgeons, shunts remain among the most failure-prone life-sustaining medical devices implanted in modern medical practice. This article provides an overview of the mechanisms of CSF shunt failure for the 3 most commonly employed definitive CSF shunts in the practice of pediatric neurosurgery: ventriculoperitoneal, ventriculopleural, and ventriculoatrial. The text has been partitioned into the broad modes of shunt failure: obstruction, infection, mechanical shunt failure, overdrainage, and distal catheter site-specific failures. Clinical management strategies for the various modes of shunt failure are discussed as are research efforts directed towards reducing shunt complication rates. As it is unlikely that CSF shunting will become an obsolete procedure in the foreseeable future, it is incumbent on the pediatric neurosurgery community to maintain focused efforts to improve our understanding of and management strategies for shunt failure and shunt-related morbidity.
Keywords: Catheter obstruction, Hydrocephalus, Shunt failure, Ventriculoatrial shunt, Ventriculoperitoneal shunt, Ventriculopleural shunt
Introduction
Cerebrospinal fluid (CSF) shunts remain among the most failure-prone life-sustaining medical devices implanted in modern medical practice, with failure rates of 30–40% at 1 year and approximately 50% at 2 years in pediatric patients [1–7] . Over the last decade, the creation of the Hydrocephalus Clinical Research Network (HCRN), a consortium of 14 North American Pediatric Hospitals, has allowed for more organized efforts in the clinical study and prevention of shunt failure. Unfortunately, aside from the recognition that the institution of standardized operating room protocols can successfully reduce shunt infection rates [8] , 21st century clinical literature has been essentially devoid of any surgeon-modifiable factors that significantly and consistently reduce the essentially stagnant overall shunt failure rate, with advancements in endoscopic technology and frameless stereotactic image guidance as well as the introduction of novel shunt hardware failing to confer improved shunt longevity. Endoscopic shunt placement, while theoretically very attractive as it allows the surgeon to visually confirm entry into the ventricular system, does not significantly improve shunt placement accuracy [9] and may actually increase failure rates in pediatric patients [3]. Frameless stereotactic image-guided ventricular catheter placement fares a bit better as it does appear to improve rates of radiographically optimal shunt placement; however, this has not definitively translated into fewer shunt failures [10]. Moreover, with respect to shunt failure rates, equipoise persists among modern shunt hardware in clinical use. Although the advent of flow-regulated valves [11, 12], anti-siphon devices [13], adjustable differential-pressure valves, and gravitational valves [14] has provided neurosurgeons with numerous options for managing patients with CSF overdrainage, no modern commercialized shunt valve has been definitively shown to reduce overall shunt failure rates [2, 3, 15–18]. Prospective comparisons between flow-controlled and pressure-dependent valves [19] as well as between programmable and nonprogrammable valves [4, 20] have demonstrated no significant differences in shunt revision rates. Although 1 retrospective study has suggested that programmable valves are associated with higher failure rates than nonprogrammable valves, it remains unclear if this effect is simply a reflection of surgeon preference in favor of programmable valve implantation in more complex/ severe cases [21]. Similarly, since the abandonment of distal slit valve catheters, which were associated with higher rates of distal catheter obstruction [1, 22], no commonly employed ventricular or distal catheters have demonstrated superiority with respect to noninfectious shunt failure rates. Studies have been mixed regarding the efficacy of antibiotic-impregnated and silver-coated catheters with respect to successfully reducing shunt infection rates [23, 24], although overall it seems that these antimicrobial catheter modifications may modestly reduced early postoperative infections [25]. While some studies have noted reduced shunt failure and infection rates when shunt placement is performed by a high-volume surgeon [26] , others have failed to identify a correlation between surgeon experience and shunt complications [3, 27].
It is worth noting that patient-specific factors associated with higher shunt failure rates have been identified, although, as these factors are immutable when approaching the care of an individual patient, they will not be the focus of this article. Riva-Cambrin et al. [3] recently published HCRN data representing the largest prospective series of pediatric patients with CSF shunts that solidifies the finding that younger patients (particularly infants less than 6 months of age) tend to experience higher rates of shunt failure and additionally introduced complex chronic cardiac comorbidities as a novel independent predictor for poor shunt survival. Notably, hydrocephalus etiology was not found to be associated with shunt survival in this series. While this was not the first study that did not identify hydrocephalus etiology as a major determinant of shunt failure rates [28, 29] , it should be noted that others have identified myelomeningocele [30], intraventricular hemorrhage, tumor, and post-meningitic hydrocephalus as etiologies associated with higher rates of shunt failure [31]. These findings were in part reproduced by Lazareff et al. [32], who found the latter 3 etiologies to be overrepresented in patients requiring 4 or more shunt revisions. Additional patient-specific variables identified in the literature as shunt failure risk factors include prematurity [33], an increased number of prior shunt revisions or short time intervals between revisions [29, 31–33], and the presence of baseline ventriculomegaly or slit-like ventricles when the patient’s shunt is functioning normally [30, 34].
This article will seek to provide an overview of the mechanisms of CSF shunt failure for the 3 most commonly employed definitive CSF shunts in the practice of pediatric neurosurgery: ventriculoperitoneal (VP), ventriculopleural (VPL), and ventriculoatrial (VA) shunts. Throughout the text, “shunt failure” will be defined as any instance in which a patient with an indwelling CSF shunt requires an operative intervention for shunt exploration/ replacement or management of persistent/worsening hydrocephalus symptomatology. The text has been partitioned into the broad modes of shunt failure: obstruction, infection, mechanical shunt failure, overdrainage, and distal catheter site-specific failures. This review will not cover lumboperitoneal shunts, El Shafei’s retrograde ventriculojugular and ventriculosinus shunts [35–37], or rarely employed distal catheter sites such as the gall-bladder (ventriculocholecystic) [38] and urinary bladder (ventriculovesical) [39].
Obstruction
Complete obstruction of CSF flow at any point along the length of a CSF shunt from ventricular catheter to valve to distal catheter results in a clinical presentation consistent with acutely elevated intracranial pressure (ICP). Infants will generally present with difficulty feeding, nausea/vomiting, and irritability. Physical examination will disclose a bulging fontanel. Older children and adults usually present with headache, cognitive difficulties, nausea/vomiting, and drowsiness/somnolence. Fundoscopic examination will disclose papilledema. Additionally, it is important to note that shunted myelomeningocele patients may present with symptomology more commonly associated with tethered cord, syringomyelia, and Chiari malformation-related hindbrain dysfunction, including weakness/regression in motor skills, difficulty ambulating, bowel/bladder dysfunction, worsening scoliosis, and lower cranial nerve palsies.
Shunt obstruction will not result in any abnormalities on the plain radiographic shunt series as the shunt hardware remains intact, but is generally heralded by imaging evidence of increasing ventricular size by cross-sectional cranial imaging. However, it is important to recognize that up to 15% of shunted pediatric patients will have such profound alterations in brain compliance that their ventricles will not enlarge in the face of shunt failure and increased ICP [40]. Additionally, when comparing a patient’s ventricular size to baseline studies, interpretation should take into account that the decline in ventricular size following initial shunt placement does not reach a plateau until approximately 14 months, regardless of whether a standard differential-pressure, a siphon-reducing differential-pressure, or flow-limiting valve was employed [30].
Ventricular Catheter
Ventricular catheter obstruction with cells or tissue accounts for over 50% of shunt failures in the pediatric population [2, 31, 34, 40], although the literature has been mixed with respect to the cell types implicated in the pathophysiology of catheter obstruction [41]. Clinicians and scientists alike have observed an array of cells and tissues bound to CSF shunt catheter material, including choroid plexus, astrocytes, macrophages/microglia/foreign body giant cells/granulomatous reactions, eosinophils, lymphocytes, monocytes, brain parenchyma, ependyma, connective tissue and fibrin networks, leptomeninges, necrotic debris, hemorrhage, calcification, neoplastic cells, foreign bodies, and embolic material [42]. Choroid plexus, in particular, is frequently cited as the primary tissue type responsible for noninfectious shunt failures within the general neurosurgical literature and textbooks, but the body of literature specifically focused on ventricular catheter-cell interactions has progressively downplayed the importance of choroid plexus in ventricular catheter obstructions over the last half-century. In 1969, Hakim [43] reported choroid plexus to be the obstructive material in 80% of “15 or more” catheters examined, thus establishing choroid plexus as the primary culprit behind shunt obstructions. A little over a decade later, Sekhar et al. [44] performed a histologic examination of luminal obstructions in 91 explanted ventricular catheters using standard clinical pathology techniques and found the obstructive material contained choroid plexus in only 38.5% of obstructions; choroid plexus was less common than “glial tissue” (39.6%), “connective tissue” (53.8%), or “chronic inflammatory responses” (49.5%). In their discussion, Sekhar et al. [44] reasoned that their findings were consistent with the knowledge that astrocytes are highly proliferative, whereas choroid plexus and ependymal cells have limited proliferative capacity in nonpathologic conditions. Del Bigio’s [45] 1998 review of ventricular catheter obstruction pathophysiology made a strong case for choroid plexus being secondary to both astroglial proliferation and chronic inflammatory/granulomatous reactions, citing not only the limited “reactivity” of choroid plexus but also work from his own laboratory demonstrating the growth of vascularized astroglial pedicles from the ventricular walls of rats and rabbits subjected to ventricular puncture [46–48]. More recently in 2013, Blegvad et al. [49] published similar results, showing that over 50% of analyzed ventricular catheters contained intraluminal vascularized glial tissue, inflammatory macrophages/giant cells, and occasional eosinophils. In 2014, Sarkiss et al. [50] published findings on obstructive luminal material in 85 explanted ventricular catheters using immunohistochemistry and light microscopy and demonstrated that choroid plexus luminal obstructions were a relatively rare finding (7% overall). They classified each luminal obstruction as being primarily inflammatory (“presence of inflammatory cells like activated macrophages, activated microglia, and lymphocytes”), reactive (“presence of reactive astrocytes, Rosenthal fibers, dense fibroconnective tissue, and/ or macrophages along with foreign body giant cell or multinucleated giant cells”), or choroid plexus. The authors found primarily “inflammatory” tissue in 31% (26/85) and primarily “reactive” tissue in 59% (50/85) of catheter samples. While representative imaging is not presented and it is unclear how mixed cellular responses were classified, this study nevertheless furthers the progressive 50-year erosion of the notion that choroid plexus is the primary suspect in shunt obstructions.
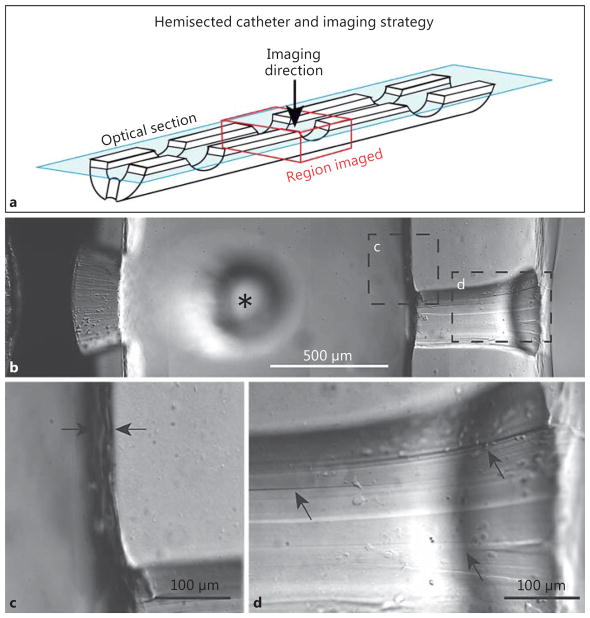
Work by our group, utilizing comprehensive multi-channel, 3-dimensional confocal microscopy imaging of explanted ventricular catheters from shunt-dependent Seattle Children’s Hospital patients further shifts the focus away from choroid plexus and narrows in on astrocytes and microglia, which are by far the most common cell types bound directly to catheter surfaces [40]. Based on this work, we have created an astrocyte/microglia centric model for ventricular catheter occlusion (Fig. 1). Our working model for noninfectious ventricular catheter occlusion can be conceptualized as a 5-stage process [40, 41]. First, within microseconds of catheter implantation, extracellular, CSF, and serum proteins are adsorbed on the poly(dimethylsiloxane) (silicone; PDMS) catheter surfaces, providing a chemically permissible substrate for cells to attach and migrate (Fig. 1: “stage 1”) [51] . Protein adsorption is dynamic and can make the catheter surface more hydrophilic, and it can provide ligands by which cells and tissues can bind; however, adsorption alone is not sufficient to cause occlusion [52, 53] . Secondary to the tissue/microvascular injury caused by catheter placement and the ongoing presence of a foreign body, microglia and astrocytes become activated and coalesce around the catheter shank within the brain parenchyma, with the microglia being most intimately associated with the catheter surface (Fig. 1: “stage 2”). The parenchymal source for obstructing cells is supported not only by our imaging of explanted ventricular catheters but also by findings from explanted neuronal recording electrode arrays [54–56] and animal studies that have demonstrated the generation of parenchyma-based reactive astroglial pedicles protruding into the cerebral ventricles following ventricular puncture [48]. We suspect reactive microglia and astrocytes fan out broadly across the catheter surface (attempting to clear this rather large foreign body) but ultimately become concentrated at the CSF intake hole edges, where PDMS surface irregularities allow for robust cell attachment ( Fig. 2) [57, 58]. Preferential attachment and subsequent occlusion appears to first occur at the most distal catheter CSF intake holes, which, in spite of greater CSF flow and shear stress at these sites, appear be at risk given their proximity to the parenchyma, the primary source of infiltrating cells [59, 60]. Generally, we have observed that microglia are the first cells to attach to the CSF intake holes in great numbers, based on the observation that failed catheters with a microglia-dominant cellular response have generally been implanted for shorter durations (mean: 24.7 days) than those demonstrating an astrocyte-dominant cellular response (mean: 1,183 days; p = 0.027) (Fig. 1: “stage 3”). Astrocytes may be seen at the CSF intake holes within the 1st week of implantation; however, they are generally not seen in significant numbers in catheters implanted for less than 2 months (Fig. 1 : “stage 4”). It seems likely that the delayed arrival of astrocytes is simply a reflection of slower migration rates, as microglia are known to be exceptionally mobile in vivo following tissue injury [61, 62]. However, other factors, including co-stimulatory cytokine signaling with microglia [63], may be critical for promoting astrocyte migration in this context. Lastly, over weeks to months, the astrocytes begin to outnumber microglia at the catheter CSF intake holes. Moreover, astrocytes bound to the catheter surface serve as a substrate for the binding of less reactive/proliferative cell types (choroid plexus, ependymal cells) which would otherwise not readily become affixed to a bare PDMS catheter surface (Fig. 1 : “stage 5”). Although not depicted in Figure 1, our model for shunt obstruction cannot exclude the possibility that reactive “free-floating” cells within the CSF may contribute to shunt obstruction. With the knowledge that ependymal dysfunction and sloughing is common in the hydrocephalus population [16], it is entirely possible that reactive cells are shed directly into the ventricular system and play a role in the pathophysiology of ventricular catheter obstruction. Also, notably absent from Figure 1 is the array of less common cell types reported in prior studies, including lymphocytes, multinucleated giant cells (peripheral macrophages), and fibroblasts [44, 50, 64]. Recognizing that catheter placement results in microvascular injury and inherent localized breakdown of the blood-brain barrier, a variety of peripheral immune cells are likely to be present on and around all implanted ventricular catheters, but their role appears to be rather limited (at least by way of cell numbers) in the vast majority of noninfectious ventricular catheter obstructions. However, with bacterial shunt infections, a dramatic increase in peripheral immune cells (pleocytosis), particularly neutrophils, can be seen on CSF cytology. Also note that CSF eosinophilia has been well documented in patients with allergic reactions to the ventricular catheter material [65].
Fig. 1.
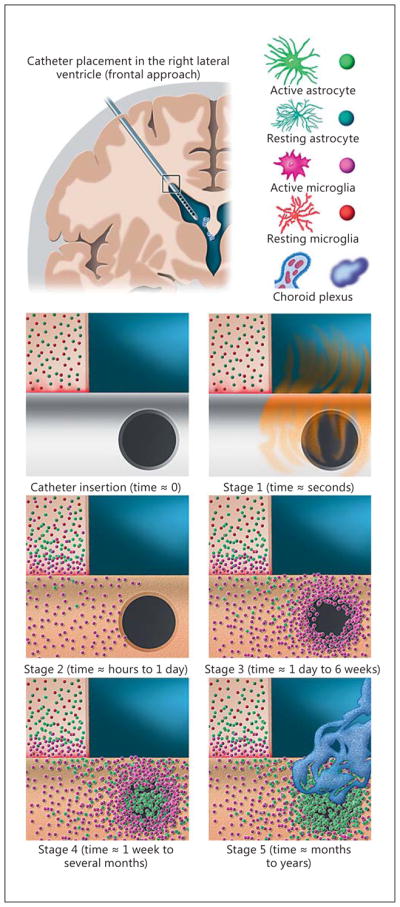
Model of the parenchymal response to ventricular catheter placement and the most common form of noninfectious ventricular catheter obstruction. In the upper left, a coronal brain section containing a right frontal ventricular catheter is shown for reference. The box at the catheter entry point into the right lateral ventricle corresponds to the zoomed-in view of the subpanels below. In the upper right, a key to the cell types depicted in the model is provided. The subpanels correspond to the 5 stages described of the astrocyte/microglia centric model for ventricular catheter occlusion described in the text. The 1st subpanel, “catheter insertion” depicts the theoretical moment of catheter insertion prior to protein adsorption. In reality, as protein adsorption (“stage 1” sub-panel) occurs within microseconds of catheter placement, this process would actually be nearly complete by the time the surgeon has fully inserted the catheter. “Stage 2” depicts the initial tissue response to the implanted catheter, with microglia and astrocytes becoming activated and coalescing around the catheter shank within the brain parenchyma. The highly motile microglia serve as the leading front in this response and become most intimately associated with the portion of the catheter surface within the brain parenchyma. These microglia are the first to appear on the catheter surface in great numbers and, as they migrate along the catheter surface in a Brownian fashion, they adhere most readily to the irregular edges of the CSF intake holes and begin to accumulate at these sites in “stage 3.” After at least 1 week, if not more, in vivo astrocytes begin to appear in greater numbers, and, as depicted in “stage 4” can be seen forming cellular bridges spanning across the CSF intake holes. Over months to years the astrocytes begin to outnumber (or outcompete) the microglia. As depicted in “stage 5,” these astrocytes serve as a robust substrate for the secondary attachment of other cell types including choroid plexus (depicted) and sloughed ependymal cells (not depicted).
Fig. 2.
a–d Differential interference contrast images of transparent ventricular catheter illustrating imperfections on the surfaces of the CSF flow holes and the unevenness of the luminal surface. The images presented are of single optical sections illustrating approximately 2.5 μm in depth and were collected using a 3-dimensional, multispectral, spinning-disk confocal microscope (Olympus IX81 inverted microscope with motorized x-y-z stage, broad-spectrum light source, and charge-coupled device camera). Prior to imaging, the ventricular catheter was cut longitudinally, allowing a clear view of the irregular luminal and CSF intake hole surfaces.
Although our model suggests that choroid plexus attachment is not the primary inciting event in the pathophysiology of shunt obstruction [40], it remains critical that the practicing neurosurgeon remain aware of the possibility of choroid plexus attachment when extracting long-term indwelling cathethers. Removal of these catheters can result in avulsion of a bound (secondary or otherwise), well-vascularized, pedicle of choroid plexus, resulting in an intraventricular hemorrhage. With this in mind, we advocate liberal use of the Bugbee wire to cauterize any attached vascularized choroid plexus pedicles when encountering even the slightest resistance when attempting to remove the catheter [66].
It is should be noted that a subset of patients will experience repeated ventricular catheter obstructions which tend to occur more rapidly with each subsequent obstruction [32] . This could be a reflection of a proinflammatory state which is only exacerbated by the trauma incurred with each ventricular catheter revision and has led some to advocate that ventricular catheter tracts associated with recurrent failures should, if anatomically possible, be abandoned in favor of virgin tracts (contralateral side, anterior vs. posterior approach). Repeated, short-interval, ventricular catheter obstruction should also raise suspicion for an allergic response, a diagnosis strongly supported by CSF eosinophilia in the context of sterile cultures [67, 68]. Although the PDMS catheter material is the most commonly implicated allergen [65, 69], some have even posited that the ethylene oxide used to sterilize many commercialized shunts may incite an immune response [70] . In cases of suspected shunt hardware allergy, exchanging the PDMS catheter for a polyurethane or “extracted” PDMS catheter, which has been through a series of solvents to extract any unpolymerized silicone oil and polymerization catalysts, may alleviate the problem [65].
The literature remains mixed with respect to the optimal trajectory for ventricular catheter implantation, with some authors favoring the frontal approach [7, 71, 72] and others the posterior parietal/occipital approach [73]. Given conflicting reports, a randomized controlled trial of anterior versus posterior entry sites for ventricular catheter placement is currently enrolling patients across 9 HCRN centers in the hope of providing clarity to this issue [74] . Although the optimal ventricular catheter placement trajectory remains to be determined, multiple studies have identified a correlation between ventricular catheter obstruction rates and ventricular anatomy [30, 34] . Sainte-Rose et al. [34] reviewed data from 1,719 hydrocephalic children and found obstruction rates to be lowest in those patients with “normal” ventricular size (27.1%), with increased rates of obstruction seen in both patients with enlarged ventricles at post-shunt baseline (36.1%) and slit ventricles (44.3%). Similar findings were reported by Tuli et al. [30], noting that once ventricular anatomy had reached a post-shunt baseline approximately 14 months following initial shunt placement, each increased Evan’s ratio unit was associated with more than a 2-fold increased risk of shunt failure. However, while baseline post-shunting ventriculomegaly was a risk factor for failure, patients with slit ventricles (defined as the ventricular catheter tip being completely surrounded by parenchyma without intervening CSF by imaging) experienced a nearly 5-fold higher failure rate compared to those patients with ventricular catheter tips completely surrounded by CSF.
Although numerous groups have attempted to improve ventricular catheter design through both structural [75] and chemical [76] modifications, no ventricular catheter has demonstrated superiority with respect to noninfectious failure rates. Future research should continue to explore novel ways to improve the interaction of shunts with the brain, potentially through inhibition of the inflammatory cascade or utilization of catheter materials more resistant to cellular attachment/migration. A discussion of the efficacy of antibiotic-impregnated, silver-coated, and hydrogel-coated ventricular catheters with respect to shunt infection rates can be found in the “Infection” section (below).
Valve
Obstruction or mechanical malfunction of modern shunt valves is considerably less common than ventricular catheter obstruction, accounting for only 4–6% of shunt failures [33]. Given the closed nature of a CSF shunt system it would stand to reason that the cell types responsible for ventricular catheter obstruction are similarly responsible for occlusion/obstruction of the valve, although definitive studies confirming this hypothesis are lacking, in part due to the complexity of imaging cellular material within valves. Of those that have been imaged, similar cells have been found in valves as compared to ventricular catheters, with a nearly uniform response regardless of intraoperative confirmation of valve obstruction [49]. Given the well-established presence of cells within the ventricular catheter [40, 44, 50] it is conceivable that reactive cells may progressively occlude the valve via migration with the flow of CSF through the catheter lumen. It is also possible that cell masses break free from points of attachment at ventricular catheter CSF intake holes and travel up the catheter lumen with anterograde CSF flow as an embolic event, with the potential to obstruct/occlude the shunt system when passing through a point of stricture within the valve. The authors favor the later mechanism as the cause for acute shunt obstructions that, upon intraoperative interrogation, are entirely limited to the valve with otherwise excellent flow through the ventricular and distal catheters. Valve obstructions occurring within hours to days of shunt placement are likely to be related to direct embolization of clotted blood products generated at the time of ventricular catheter insertion. With this in mind, some have advocated for allowing CSF egress from a newly implanted ventricular catheter to allow for clearance of blood products and cellular debris prior to connecting the catheter to the valve may reduce early valve failures; however, this practice has not been systematically studied [1]. Very rarely, membrane-controlled antisiphon devices (which may be implanted in conjunction with a valve lacking an antisiphon device or incorporated into a valve) can become functionally obstructed by external compression of the membrane by formation of a collagenous tissue capsule within the subcutaneous space, and, in such cases, resection of the capsule restricting the antisiphon device membrane can restore the patency/functionality of the shunt system [77].
Distal Catheter
Distal catheter obstruction tends to occur in a delayed fashion [78] with one group finding that the odds of shunt failure being related to distal catheter obstruction increases 1.45-fold per year following shunt placement [33]. While placement of distal catheters with inadequate length to allow for growth of the pediatric patient may be partially responsible for the time-dependent nature of distal catheter malfunctions in the historical pediatric literature, this fails to fully explain the phenomenon [33]. When distal catheter malfunction is identified intraoperatively at the time of shunt revision, the continued appropriateness of the patient’s current distal catheter site should be carefully considered. As will be discussed below in the Distal Catheter Site-Specific Modes of Shunt Failure section, functional obstruction of a distal peritoneal catheter should raise suspicion for an intra-abdominal pseudocyst or extensive intra-abdominal adhesions, which may preclude safe placement of a new distal catheter into the peritoneum.
Since the recognition that slit valve distal catheters are associated with higher rates of distal catheter failure in the late 1990s, which naturally prompted a rapid decline in their use, the literature has been devoid of any surgeon-modifiable factors that might reduce distal catheter failure rates [22]. Importantly, the method of distal catheter placement, including open peritoneal placement, trocar insertion, and laparoscopic placement, does not significantly impact rates of distal catheter obstruction [3]. Not dissimilar to ventricular catheters, an allergic inflammatory response to distal catheter components may, in rare cases, contribute to distal shunt obstruction [79].
Infection
The overall reported rate of shunt infection in the literature ranges from 3 to 15% [4, 8, 16, 28, 29, 33, 80]; however, the proportion of shunt failures related to infection falls off rapidly after first several months following implantation, with 90% of infections occurring within the first 6 months [81] . McGirt et al. [33] found that while infection was responsible for 45% of shunt failures within the 1st month of implantation, by 2 years after implantation, infection constituted only 6% of failures. The majority of infections are caused by skin flora seeded onto the shunt hardware at the time of surgery, with coagulase-negative Staphylococcus isolated in approximately 60% of cases and Staphylococcus aureus in just under one-fifth [82]. Shunt infection risk factors include younger patient age, history of prior neurosurgical procedures/shunt revisions, and the presence of the gastrostomy tube [80, 83].
Reinfection following a primary shunt infection is a challenge that plagues hydrocephalus treatment, occurring in 26% of pediatric patients [84] . Interestingly, patient factors, treatment, and diagnostic factors matter less than evidence of difficulty clearing the primary shunt infection, with intermittent clearance and reemergence of the pathologic organism on serial CSF cultures during the treatment course serving as a negative prognostic indicator [84]. New research aims at understanding the role of established inflammatory schemes, including the influx of activated M2 macrophages, neutrophils, and chemokines CXCL1, CCL2, and IL-17 in the presence of bacterial biofilm-infected shunt hardware [85, 86]. Mechanisms to inhibit biofilm formation on both external ventricular drains and CSF shunts are being explored [87].
Unlike the problem of noninfectious shunt obstruction, efforts directed towards reducing shunt infection rates, including the study of operating room procedures and the production of ventricular catheters with antimicrobial properties, have been marginally more gratifying over the last 20 years. In 2011, it was shown that implementation of a standardized 11-step operating room protocol for shunt procedures successfully reduced shunt infection rates from 8.8 to 5.7% across 4 centers within the HCRN [8]. Included in this standardized HCRN protocol were measures such as posting a sign on the door to limit operating room foot traffic, standardize systemic administration of pre- and postoperative systemic antibiotics, and administration of intrathecal vancomycin and gentamicin just prior to skin closure.
Both antibiotic-impregnated (rifampin with either clindamycin or minocycline) and silver-coated catheters (combination of metallic silver and an insoluble silver salt), designed with an eye towards reducing shunt infection complications, have become widely adopted in clinical practice in North America although studies have been mixed with respect to their efficacy [23, 25, 88]. Although a recent meta-analysis from Konstantelias et al. [25] concluded that antibiotic-impregnated and silver-coated catheters appear to reduce the rates of early postoperative infections, it was noted that the infections that do occur with these modified catheters tend to be associated with more virulent organisms, including methicillin-resistant S. aureus and gram-negative bacilli, and certainly this observation warrants further study. On the heels of this meta-analysis, the HCRN published results from a prospective shunt implantation protocol which included the standardized use of antibiotic-impregnated catheters and demonstrated no significant difference in infection rates across 8 centers (6.0%, 95% CI: 5.1–7.2% compared with the historical control of 5.7%, 95% CI: 4.6–7.0%) [23]. However, it is to be noted that concurrent with the addition of antibiotic-impregnated catheter use to the HCRN protocol, standardized administration of intrathecal antibiotics at the time of ventricular catheter implantation was removed from the protocol [23]. While some have raised concerns about the potential for antibiotic-induced neurotoxicity [89], prior work demonstrating the potential benefit of prophylactic intrathecal gentamicin and vancomycin is enticing [90] , and it remains an open question as to whether the combined use of antibiotic-impregnated catheters and intraoperative intrathecal antibiotics could further reduce infection rates. It also bears mentioning that in addition to more widely adopted antimicrobial catheters, one commercialized ventricular catheter with a polyvinylpyrrolidone hydrogel coating showed promise in an early in vitro study which found reduced bacterial attachment to the hydrogel surface [13]. Unfortunately, clinical studies have failed to demonstrate reduced infection rates with the use of polyvinylpyrrolidone-coated catheters [14] , and 1 study even found statistically significant increases in infection rates [15].
Lastly, although peritoneal pseudocyts are generally associated with an indolent low-grade VP shunt infection, this generally late infectious complication, which often comes to clinical attention secondary to abdominal/ gastrointestinal complaints, will be covered below in the Distal Catheter Site-Specific Modes of Shunt Failure section.
Mechanical Shunt Failure
Fracture
Fracture of the shunt tubing is typically a late complication and occurs almost exclusively along the distal catheter between the valve and the peritoneum. Ideally, the catheter will remain flexible and free to slide within the fibrous subcutaneous tract. With time, however, the material may become calcified or tethered by scarring, both of which increase the risk of fracture [1]. Calcification reduces the flexibility of the material, predisposing it to crack formation and breakage in the neck, where it is most mobile [91, 92]. Calcifications or scarring along the tract can also tether the catheter to the adjacent tissues and create tension as the child grows, ultimately leading to fracture of the tubing anywhere along its course [1]. Early shunt tubing fractures can also occur, usually due to lacerations at the time of initial placement; care must be taken during surgery to ensure that the proximal and distal catheters remain undamaged.
Fracture of the shunt tubing accounts for 3–21% of all shunt failures [91, 92], although our experience is more in line with the lower end of this range. It has been suggested that the use of smaller diameter catheters is associated with a higher risk of breakage, perhaps due to higher mechanical stress generated by the reduced cross section [91]. Additional risk factors have yet to be identified.
Once a fracture occurs, CSF flow may stop immediately or it may continue temporarily through the fibrous subcutaneous tract between the broken fragments. As a consequence, patients can present with fulminant hydrocephalus or mild, more insidious symptoms [93]. Rarely, there may be intermittent complaints influenced by patient position and neck rotation, which can open and occlude the tract [1]. Some children may also present with pain along the catheter, and physical examination may reveal palpable scarring or calcifications along its course. The evaluation of suspected shunt failure should always include plain X-rays of the shunt system, which is the most useful modality for identifying broken shunt tubing. In many cases, however, the break is discovered incidentally during surveillance examinations.
While it is tempting to assume that the patient is shunt independent when these breaks are discovered in the absence of symptoms, we caution against this; delayed deterioration of asymptomatic children with shunt fractures has been reported in the literature [94] , and it has been seen in our practice. We, therefore, advocate surgical replacement in all cases of shunt catheter fracture. Children with acute hydrocephalus require emergent revision, whereas those who are asymptomatic can undergo revision in an urgent, semi-elective time frame. However, this practice is not uniform across the country. At some centers, children with asymptomatic shunt fractures are observed; at others, the shunt is explored and, if it is found to be nonfunctional, it is explanted, liberating the patient from the shunt.
If shunt revision is pursued, the broken catheter fragments are removed if possible. However, it is not uncommon for segments of the distal catheter to be adherent to the tract and challenging to remove; these pieces are typically left in place. It is also possible for the distal end of the catheter to migrate completely into the peritoneum. If this portion can be easily identified and removed at the time of surgery, we do so; however, these fragments are often impossible to remove via standard shunt insertion incisions, and thus they are frequently left behind. More aggressive attempts at retrieval are made in cases of intra-abdominal infection or otherwise unexplained abdominal pain [1].
Disconnection
The majority of CSF shunts are constructed from separate components, and disconnection between these can cause shunt failure (Fig. 3b). This type of failure typically occurs relatively early after placement, and is often due to errors at the time of surgery. Catheters that are not fully hubbed on connectors may loosen, and poorly tied knots may inadequately secure points of connection. All multi-component shunt systems are potentially at risk for disconnection. In recent years, only 1 shunt system, a snap shunt system utilizing polyvinylpyrrolidone-coated catheters, has been recalled given an excessively high rate of ventricular catheter migration, likely because the lubricious hydrogel catheter surface increased its propensity for disconnection from the shunt valve at the snap connection point [95].
Fig. 3.
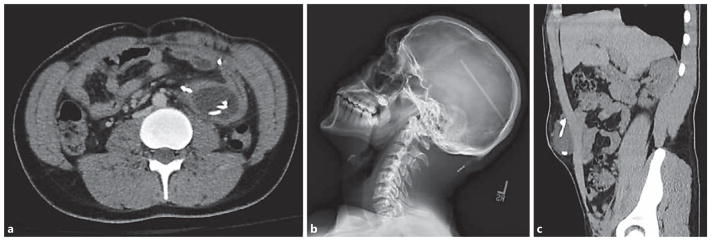
Selected imaging illustrating distal catheter complications. a This chronically shunted teenage male presented with a 4-day history of abdominal pain and a 1-day history of headache associated with nausea and emesis. In this axial CT scan, the distal VP shunt catheter tubing is noted to be tightly coiled within a fluid collection contained within the intraperitoneal space, consistent with an abdominal pseudocyst. As is typical for abdominal pseudocysts, cultured samples of this fluid collection demonstrated the presence of Propionibacterium acnes. b This patient presented with new-onset headache and by radiographic shunt series was noted to have disconnection of his distal catheter at the site of a straight connector within the shunt system. c This patient present with localized, superficial abdominal swelling in the context of progressively worsening headache approximately 1 week after a distal catheter shunt revision. This coronal CT scan demonstrates distal VP shunt catheter tubing tightly coiled within a fluid collection contained within the preperitoneal space, most likely a reflection of suboptimal catheter placement at the time of the recent shunt revision surgery.
Disconnections in the system impede CSF flow, causing symptoms of hydrocephalus. As with shunt tubing fractures, more mild, insidious symptoms can also be seen, and the child may on occasion be asymptomatic. On physical examination, ballotable fluid pockets may be identified at the site of a disconnection; these collections of CSF can be visualized with cross-sectional imaging, such as CT or MRI.
A disconnected shunt requires surgical repair. Often, the disconnected fragments can be reconnected without replacement, but occasionally replacement of 1 or more components is required. The system should be evaluated for excessive tension on catheters, which can cause disconnection, and the surgeon must also ensure that the securing knots are tied to the appropriate tightness. In our practice, the shunt system is assembled on a sterile table away from the incision; this approach permits meticulous attention to shunt assembly and allows the surgeon to test proper shunt function before placement. Unitized shunt systems do not require assembly and theoretically reduce the risk of disconnection, but they pose additional challenges when shunt revision is required, and they can limit the valve choice.
Migration
Migration of the proximal or distal catheter can cause shunt failure after successful initial placement. As the child grows, the catheter tips can withdraw from their original locations, blocking drainage of CSF. This phenomenon can occur with both the proximal catheter, which withdraws from the ventricle, and the distal catheter, which withdraws from the cardiac atrium, pleural space, or peritoneum. While some cases of ventricular catheter migration may be due to withdrawal of the tip from the ventricle as the head grows, this is relatively rare. In the majority of children, the tip of a catheter that is placed to the appropriate depth during the original surgery will remain in the ventricle through development and into adulthood. More commonly, the distal elements of the shunt, such as the valve, can become tethered; as the child grows, traction on the distal components gradually pulls the intraventricular catheter out of the ventricles and into the brain parenchyma, occluding the catheter inlets and blocking CSF flow. Shunt systems featuring right angle connectors or Rickham reservoirs tend to resist proximal catheter migration [1] . The diagnosis of a migrated ventricular catheter is typically made radiographically when cross-sectional imaging studies demonstrate withdrawal of the catheter tip from the ventricle. Treatment requires surgical replacement of the ventricular catheter, with attention paid to any obvious causes of withdrawal. To prevent migration, we typically suture the proximal catheter or the valve to the pericranium to anchor the system close to the burr hole.
Distal catheter migration also occurs with growth of the patient, particularly when the terminus is placed at the cavoatrial junction (VA shunt) of a young child. As he or she grows, the tip will withdraw into the venous system, and it may ultimately retract into the soft tissues of the neck. When this occurs, CSF drainage comes to a halt and shunt failure ensues. This phenomenon is less common in VP and VPL shunts; because these distal catheters need not be cut to precise lengths, additional tubing length can be inserted into the target cavity at the time of placement, providing room for growth. Given this consideration, VP shunt failures caused by preperitoneal positioning of the distal catheter are most commonly associated with misplacement of the distal catheter at the time of implantation rather than reflecting a true migration-related complication (Fig. 3c). In spite of the comparatively low risk of migration complications in VP and VPL shunts, we advocate surveillance imaging as the child grows to monitor for withdrawal of the distal catheter. When migration occurs, or appears imminent on imaging, replacement with new distal tubing is required.
Overdrainage
Overdrainage complications occur when a functioning shunt is draining more CSF than is optimal for a given patient. If there is rapid decompression of a very large ventricular system, the resulting drop in pressure over the extra-axial convexities will promote the development of extra-axial fluid collections and/or subdural hematomas. More chronic overdrainage is thought to be the underlying cause of the slit ventricle syndrome (SVS) [96] and is primarily thought to be related to a distal catheter siphoning effect. The siphoning of CSF is caused by the gravitational force acting on the fluid column within the distal catheter tubing, which terminates in a location dependent to the cranium, an effect generally amplified by upright posture. Moreover, in the case of VA and VPL shunts, the suction created by the intermittently negative ambient pressure at the distal catheter terminus can worsen the siphon effect [97]. Based on clinical, endoscopic, and imaging observations in chronically shunted patients, researchers at the University of Wisconsin hypothesize that repetitive proximal shunt obstructions and compliance problems result from a chronic overdrainage syndrome that may remain asymptomatic for years [98]. Great effort has been put into the development of improved shunt valves/antisiphon devices that reduce both acute and chronic overdrainage complications related to the siphoning effect of the distal catheter. However, in spite of varied engineering approaches (membrane controlled, flow regulated, gravity-assisted), no modern device has eliminated the problem or even definitively demonstrated superiority over its peers [2, 3, 30, 99]. However, the use of lumboperitoneal shunts in patients with SVS has recently shown promise at reducing shunt failure rates in this challenging cohort of patients [100].
Extra-Axial Fluid Collections
Extra-axial fluid collections tend to occur as early complications following shunting of an older child with pronounced preoperative ventriculomegaly and are seen following approximately 3% of all new shunt placements [16]. When encountered, there are several reasonable management strategies that can be selectively employed depending on the child’s clinical condition and the shunt hardware they have in place.
If the child is in extremis from mass effect referable to the extra-axial collection then prompt evacuation via bur hole or craniotomy is warranted, with possible placement of a subdural drain. If necessary, chronic drainage of slowly resolving or recurrent extra-axial fluid collection can be achieved by splicing a subdural drain into the shunt system distal to the valve [1] . Splicing distal to the valve promotes a transmantle pressure gradient favoring ventricular expansion; the ventricles are maintained at higher pressure determined by the resistance of the valve, whereas the subdural space is free to drain with minimal resistance (or even negative pressure secondary to the siphoning effect). If the patient is only mildly symptomatic and has an adjustable differential pressure shunt valve in place, then the pressure on the valve may simply be dialed up to reduce CSF outflow, often resulting in progressive reduction in the size of the extra-axial collection. Lastly, if the patient does not have an adjustable valve in place then a shunt revision surgery may be considered so that an antisiphon device can be added to the system and/or a fixed valve can be replaced with an adjustable valve. Some have argued that, in spite of their higher price tag, adjustable valves are cost effective because they allow for avoidance of a subset of overdrainage-related shunt revisions; however, it should be noted that a rather high rate of severe overdrainage symptoms (21%) was noted in this cost analysis [101].
Slit Ventricle Syndrome
SVS, also known as “normal volume hydrocephalus” [102] or “noncompliant ventricle syndrome” [103] in the historical literature, is frequently discussed but lacks a consistent definition [97, 104]. SVS has been used to describe a broad cohort of chronically shunted patients with small ventricles, both with and without symptoms, which makes comparison of incidence rates across studies challenging [17] . Studies with relatively short-term follow-up have reported SVS rates of 1% or less [2, 105]; however, when patients shunted since early childhood are followed for more than a decade, the incidence of SVS appears to be closer to 10% [106].
Patients undergoing initial shunt placement at a younger age or with hydrocephalus related to infection, trauma, or aqueductal stenosis are at greater risk for SVS [107]. Although most studies have failed to demonstrate any significant differences in SVS rates between shunt valves employed [16, 30], one study did find a two-thirds reduction in the rate of SVS with the use of a flow-regulated valve as compared to differential pressure valves with and without the inclusion of an antisiphon device [107] . Part of the difficulty in establishing superiority of a valve or antisiphon device with respect to rates of SVS is simply the long time course over which this condition tends to develop and become symptomatic. Tuli at al. [30] demonstrated that ventricular anatomy decreases exponentially following initial shunt placement only to ultimately plateau at approximately 14 months regardless of whether the child was implanted with a standard differential pressure valve, a differential pressure valve with an incorporated antisiphon device, or a flow-limiting valve. Even if ventricular anatomy reaches a plateau by 14 months, the intractable, generally postural, headaches, and recurrent shunt failures associated with SVS may not develop until many years after stability of ventricular size, with a mean interval of 6.5 years between initial shunting and symptomatic SVS in one study [108]. With this in mind, it is not surprising that one study demonstrating reduced SVS rates with the use of an antisiphon device relied entirely on historical controls, severely limiting the strength of the findings [109]. Nevertheless, given the management challenge that a symptomatic SVS patient poses, many surgeons have opted to routinely place antisiphon devices (or valves incorporating these devices) into every shunt system they implant, primary or revision, based on physiologic principles and a recognition that there appears, at the very least, to be no harm associated with the use of these devices [109, 110]. While the literature has not coalesced around the superiority of a single antisiphon valve/device design, a practical consideration that bares mentioning is that, while gravity-assisted antisiphon device/valve mechanisms are becoming increasingly adopted in the management of adult hydrocephalus, this particular antisiphon mechanism is generally not suitable to the pediatric population as the weight of the ball-in-cone mechanism must correspond appropriately to the effective height of the distal catheter fluid column, which remains a moving target in growing children [109, 110].
As alluded to earlier, the management of a patient with symptomatic SVS can be quite challenging. It seems self-evident that SVS patients’ diminutive ventricular anatomy, which can at times be hard to cannulate even with stereotactic guidance, makes ventricular catheter revisions, which SVS patients need at higher rates [73], more complex. Moreover, the very poor brain compliance of SVS patients makes them both highly sensitive to perturbations in shunt function and more prone to becoming more rapidly comatose in the context of acute shunt failure [111] . The combination of poor brain compliance and small ventricles also raises the practical consideration of minimizing intraoperative CSF egress from newly placed ventricular catheters when performing shunt revision surgery on an SVS patient.
For those SVS patients with debilitating symptoms in spite of a patent ventricular CSF shunt, a number of management strategies have been described, although consensus is lacking regarding the appropriate escalation scheme for these interventions, so clinical judgment is generally required on a case-by-case basis. For some patients with mild-to-moderate SVS-related headaches, conservative pharmacologic and lifestyle modification strategies may be effective. It has been reported that more than a third of mild-to-moderately symptomatic SVS patients will benefit from scheduled periods of supine rest during the day or antimigraine therapies [112–114]. While it remains unclear if the efficacy of migraine medications represents a mistake in diagnosis, it has been proposed that, in the context of the poorly compliant brain of an SVS patient, these drugs may be reducing or stabilizing cerebral blood flow, effectively decreasing ambient pressure and pulsatility within the intracranial environment [96].
SVS patients with more severe symptoms or those who fail to respond to conservative management strategies generally require more invasive interventions. Generally, we begin by considering whether or not the patient’s current ventricular CSF shunt system has been fully optimized from the perspective of valve selection. Certainly, an antisiphon device or siphon-limiting valve should be incorporated into the shunt system of all symptomatic SVS patients. Additionally, if there is uncertainty as to what baseline ICP will minimize headache symptoms in a particular SVS patient, then a period of invasive ICP monitoring with an external ventriculostomy drain or parenchymal ICP monitor may help to resolve this ambiguity and guide valve selection [96]. Moreover, conversion from a fixed valve system to an adjustable valve system is advised in these cases so that noninvasive adjustments can be made in the clinic.
For those SVS patients with persistent symptoms following thoughtful optimization of their ventricular shunt system, there are several described management strategies that may be trialed, including lumboperitoneal shunt placement [100, 115], endoscopic third ventriculostomy (ETV) [116] , and subtemporal decompression/cranial vault expansion [103], but an agreed-upon treatment algorithm for this patient population is still lacking. Recently, there has been a growing body of data to support the use of lumboperitoneal shunts in the management of pediatric patients with SVS [100]. Although iatrogenic hindbrain herniation remains a feared complication associated with the use of lumboperitoneal shunts, with a series demonstrating radiographic hindbrain herniation in over 70% of young children (average age 3.3) who had undergone placement of a lumboperitoneal shunt as a primary hydrocephalus treatment [99], the rates of hind-brain herniation with this treatment strategy appear to be dramatically lower in older children with SVS who have previously been treated with ventricular shunt systems [100, 115]. Moreover, the risk of hindbrain herniation may be limited by incorporating a valve designed to limit overdrainage complications into the lumboperitoneal shunt system [115]. When managing patients with radiographic SVS and frequent ventricular catheter failures, some authors have advocated conversion to a lumboperitoneal shunt system, with continued use of a ventricular shunt system only in the face of a trapped ventricle [100], while others have simply implanted a lumboperitoneal shunt system in addition to an already functional ventricular shunt system [117] . The precise mechanism by which lumboperitoneal shunts reduce SVS symptoms and ventricular shunt failures is not entirely clear; however, it may be that simply the provision of a second, extra-axial, site for CSF egress decreases flow through the ventricular catheter thereby decreasing the driving force for ventricular collapse. It is also possible that the 2nd shunt outside of the ventricular system is beneficial in so far as it provides an avenue for CSF egress at times when the ventricular catheter may be functionally obstructed by collapse of the ventricular walls effectively covering the catheter’s CSF intake holes.
ETV has also proved useful in a subset of SVS patients [116] , presumably by dampening arterial pressure waves within the ventricular system by creating a new path for direct egress of CSF from the ventricles to the subarachnoid space. However, from a practical perspective, safe performance of an ETV is nearly impossible when dealing with true slit ventricle anatomy given the limited working room within the patient’s collapsed ventricles. As such, prior to attempting an ETV in an SVS patient, their ventricular system must be slowly dilated (by progressively raising an external ventriculostomy drain) under close in-patient supervision and continuous ICP monitoring.
Lastly, the most invasive intervention for SVS patients is performance of a subtemporal decompressive craniectomy with or without cranial vault expansion [103]. Given the associated morbidity of such an extensive neurosurgical procedure, we view this as an option of last resort. That being said, it does appear that successful temporal decompression cannot only improve SVS symptomatology in a subset of patients but may also decrease subsequent shunt failure rates, with Buxton and Punt [103] finding a 68% reduction in shunt failure rates in the 3 years following performance of a decompressive procedure in this population.
Distal Catheter Site-Specific Modes of Shunt Failure
Peritoneal Shunts
Given the ease with which it can be accessed surgically and the large surface area it offers for CSF absorption, the peritoneal cavity is the preferred location for distal catheter placement and is used for all initial shunt placements unless a contraindication exists. Thus far, no association has been established between shunt or distal catheter failure rates and the surgical technique employed for peritoneal catheter implantation. Although there is equipoise regarding peritoneal catheter placement via open minilaparotomy, trocar placement, and laparoscopic placement in the pediatric hydrocephalus population at large, the authors stress that when the possibility of intraperitoneal adhesions exists consultation with general surgery colleagues for laparoscopic shunt peritoneal catheter placement should be considered. This technique allows for direct visual assessment and lysis of intraperitoneal adhesions, confirmation that the catheter has not been placed into a walled-off adhesion loculation, and direct visual confirmation of CSF egress from the distal catheter tip prior to closure [118] . For patients with no history of abdominal surgery or trauma selected for minimally invasive trocar placement of the peritoneal catheter, the authors strongly advocate placement a urinary catheter at the outset of the case so as to ensure that the patient’s bladder is fully collapsed prior to puncturing through the abdominal musculature, thereby minimizing the risk of bladder perforation.
When a shunt system’s distal catheter is placed in the peritoneal space, it is important to recognize that intra-abdominal pressure can be a major determinant of the effective resistance to CSF flow, particularly when a low-pressure/high-flow system is required by a patient prone to bouts of constipation. In such patients, constipation can result in significantly elevated intra-abdominal pressures that decrease CSF outflow to the point of causing symptoms consistent with shunt failure [119–121]. When we encounter VP shunt patients with known histories of constipation who present with mild-to-moderate headache/nausea but an otherwise reassuring neurologic examination and no evidence of papilledema on fundoscopic examination, admission for an aggressive bowel regimen (while performing ongoing neurologic examinations) is often sufficient to address the issue. Certainly, if the constipation episodes continue to recur in spite of instituting a more aggressive prophylactic bowel regimen in consultation with a gastroenterologist, then repositioning the distal catheter to a different site should be considered, either electively or at the next instance of frank shunt malfunction.
Peritoneal pseudocysts are a unique manifestation of indolent low-grade VP shunt infections that often present with symptoms consistent with functional shunt obstruction, often in association with abdominal pain/distention and gastrointestinal distress (Fig. 3a). Between 30 and 100% of pseudocyst complications are found to be associated with culture-positive shunt infection with either Propionibacterium acnes or Staphylococcus epidermidis [122–124] . The pseudocyst itself is the result of chronic inflammation-related thickening of a peritoneal serous membrane. Generally, when a patient experiences shunt failure secondary to formation of a peritoneal pseudocyst, an external ventriculostomy drain system is placed and subsequent replacement of the distal catheter into the peritoneal cavity is avoided [96] . After obtaining CSF and pseudocyst aspirate cultures in the operating room, empiric antibiotics should be initiated. The culture results will dictate the timing of shunt internalization, with internalization of the shunt proceeding after approximately one week if cultures remain sterile (allowing adequate time for the slow-growing P. acnes organism to appear in culture) or longer if cultures are positive. While there is no standardized protocol for shunt implantation following confirmed infection, our institutional practice is to confirm CSF sterilization with repeated cultures and determine the appropriating timing of shunt internalization jointly with our infectious disease colleagues based on the virulence of the cultured organism.
While rare, recurrent distal catheter failures might also be an indication that the patient has an allergy to a component of the PDMS shunt tubing, particularly when these failures occur with exuberant intra-abdominal adhesions and repeatedly clean culture results. Under these circumstances, replacement with a polyurethane-based or “extracted” PDMS shunt hardware should be performed [65, 79].
Rarely, shunt tubing can erode into a hollow viscus in a delayed fashion following the original shunt insertion [96] . When peritoneal catheter tubing erodes through the bowel or bladder walls the patient may present with tubing migrating out of the anus or urethra, generally without concurrent symptoms of peritonitis. Unlike an acute episode of peritonitis, the site of entry into the hollow viscus usually heals without intervention once the tubing is removed [125, 126]. It has been proposed that the use of more stiff distal shunt catheters may predispose to this complication [125]. Even more rarely, shunt tubing may migrate into an intravascular location. There is one case report of a distal VP catheter migrating intravascularly, ultimately terminating within the pulmonary artery, requiring staged removal employing the use of an endovascular snare [127].
Pleural Shunts
Patients with VPL shunts will sometimes develop profound respiratory distress/failure from CSF accumulation within the pleural cavity [128–131]. Reported rates of symptomatic pleural effusions requiring shunt revision in children range from 20 to 62%, with agreement in the field that young patients are at higher risk for developing pleural effusions [132, 133]. It is most commonly believed that this is simply a reflection of the smaller pleural surface area and, therefore, reduced CSF-absorptive capacity in young children. Many neurosurgeons avoid VPL shunt placement in children below a certain age with various proposed age thresholds, ranging from 3 to 7 years of age, reported [133] . The risk of pleural effusions and VPL shunt failures overall continues to decline through late childhood and teenage years, as evidenced by a series of 131 VPL shunt patients with a mean age of 14 ± 5 years which demonstrated a symptomatic pleural effusion rate of 13.7% [134] . Of the 112 patients in this series with a minimum follow-up period of 1 year, 46% of patients 11 years of age or older experienced shunt failure, compared with 70% of patients under the age of 11 (p < 0.05) [134]. The observation of lower failure rates in older patients is certainly consistent with the dramatically lower rates of complications quoted in the adult literature, with the largest series of adult patients with VPL shunts finding a 4.5% shunt revision rate for symptomatic pleural effusions [135].
For the rare patient with contraindications to other commonly employed distal catheter sites as well as recurrent symptomatic unilateral pleural effusions from VPL shunting, the use of a “bipleural” distal catheter system can be considered in order to better distribute CSF into the entire pleural cavity so as to maximize its absorptive capacity [136] . Additionally, when a patient with a VPL shunt has respiratory compromise requiring mechanical ventilation, use of acetazolamide might be considered to decrease the burden of CSF volume being shunted to the pleural space [137].
Atrial Shunts
VA shunts are the only widely used CSF shunt with an intravascular distal catheter position and, not surprisingly, are associated with several unique modes of shunt failure. Access to the vascular system is generally achieved via the jugular vein using the Seldinger technique, although subclavian and facial vein approaches can also be employed [1] . Intraoperative fluoroscopy or transesophageal echocardiography should be used to confirm that the distal catheter tip is placed within the proximal atrium or distal superior vena cava, ideally at the cavoatrial junction [138, 139]. Given this narrow zone for optimal distal catheter tip positioning, adjusting the distal catheter length in synchrony with the growth of pediatric patients is the most frequent indication for VA shunt revision surgery in children, account for 66% of revisions in a series [140] . Should the catheter tip be situated too deeply within the right atrium or ventricle, it can cause arrhythmias secondary to direct irritation of the sinoatrial and/or atrioventricular nodes, with premature ventricular contractions being the most commonly observed arrhythmia. Moreover, deep positioning of the catheter terminus within the right atrium increases the risk of atrial thrombus formation [139] . Much in the same way that deep catheter placement can cause arrhythmias, these can also be seen as an early sign of right atrial thrombus formation [141]. Formation of a thrombus at the distal catheter terminus is not only a unique cause of VA shunt distal catheter obstruction, but, in rare cases, this thrombus can dislodge and travel to the pulmonary artery, resulting in life-threatening cor pulmonale [142]. Given the potential for blood products to cause distal VA catheter obstructions, we advocate flushing the distal catheter with preservative-free saline prior to connecting it to the valve/ proximal catheter at the time of implantation. Interestingly, it has been postulated that the lower observed rates of distal VA catheter thrombus formation in elderly normal pressure hydrocephalus patients may be related to the frequent administration of anticoagulants for comorbid conditions in this population [138], although the authors are not aware of any studies trialing the use of prophylactic anticoagulation medications in pediatric VA shunt patients.
The intravascular location of VA shunt distal catheters is of particular significance in the context of infection, as 0.7–2.3% of VA shunt infections are associated with the development of shunt nephritis, one of the most serious VA shunt complications [1, 143, 144]. Shunt nephritis was first reported by Black et al. [145] in 1965 and has been well documented in numerous reports since that time [143, 144] . Shunt nephritis generally manifests as an insidious progressive secondary renal disease associated with proteinuria; however, depending on the severity of the condition, patients may present with the full-blown nephrotic syndrome, hematuria, fever, anemia, hepatosplenomegaly, nonthrombocytopenic purpura, and hypertension [1, 143]. Pathological analysis of kidney biopsies in these patients has revealed the presence of a proliferative glomerulonephritis, associated with sub-endothelial deposits of C3, C4, and IgM [143]. S. epidermidis is the causal organism in more than 80% of cases [146], and not surprisingly S. epidermidis antigens have been identified in association with circulating cyroglobulins in cases of shunt nephritis [147]. As this complication is caused by infection with skin flora, it tends to occur within several months of shunt placement [143], but cases of shunt nephritis occurring more than a decade after shunt placement have also been reported [148]. When encountered, shunt nephritis should be immediately treated by removing of the VA shunt system (with placement of an external ventriculostomy drain for temporary CSF diversion) and initiation of broad antibiotic coverage while blood/CSF culture results are pending. Shunt nephritis can be deadly if not promptly identified and treated [149], although with appropriate care more than half of patients will go on to make a full recovery without evidence of permanent renal damage [146].
Conclusions
It is the hope of the authors that this review serves as an up-to-date summary of the current shunt failure literature with an eye towards providing an overview of the panoply of shunt-related complications encountered in clinical practice. Although this article frequently notes the failure of shunt and operating room technology to make a significant impact on persistently high shunt failure rates, we wish to close by noting those interventions that can be recommended for routine clinical practice given available data and generally low risk. (The authors wish to note that recommendations provided do not attempt to factor in health care cost considerations.)
Administration of systemic antibiotics with excellent skin flora coverage prior to shunt surgery skin incision and in the 24-h period after surgery.
Limit operating room foot traffic and standardize surgical antisepsis.
Protocolization of shunt operations to maximize operative efficiency and safety while minimizing unnecessary handling of shunt hardware.
Universal use of stereotactic guidance for ventricular catheter placement (as improved catheter placement accuracy without introduction of additional procedural risk clearly favors this intervention even it has not yet been demonstrated to improve failure rates).
Use of either antimicrobial shunt catheters or prophylactic intrathecal antibiotics (or even both, as it has yet to be thoroughly studied whether the combination could further drive down failure rates).
Maintaining attention to patient physiology-specific valve selection, with the authors favoring incorporation of a siphon-limiting device in virtually all primary pediatric shunt placements given the limited risk associated with these devices.
Aggressively instituting workups for frequently failing shunts rather than repeatedly performing identical revision surgeries. Specifically, we recommend the judicious use of invasive ICP monitoring to better correlate symptomatology with objective pressure measurements so that shunt hardware can be intelligently selected. In addition, we advocate checking for CSF eosinophilia so that shunt hardware allergies can be promptly identified and addressed with hypoallergenic shunt hardware. The clinician should also consider the possibility of an indolent shunt infection, another cause of recurrent failures.
Consideration of alternative distal site selection in cases of recurrent distal catheter failure.
Develop familiarity with the described treatment strategies for managing symptomatic SVS patients.
As is highlighted throughout the article, there are many facets of the shunt failure problem that deserve our continued attention and research efforts. As ventricular catheter obstructions remain the most common cause of pediatric shunt failures, improvement in our understanding of this phenomenon will hopefully lead to the development of catheters designed to resist cellular attachment and/or evade the brain’s innate immune response. Moreover, continued efforts to deepen our understanding of the nuances of CSF physiology will hopefully result in continued improvements in shunt valve design, because, although the modern neurosurgeon has a number of siphon reduction devices and a variety of adjustable and fixed shunt valves at their disposal, which can be very useful in managing acute overdrainage syndromes and some low-pressure headaches when employed appropriately, these devices have yet to definitively demonstrate reductions in SVS development [30]. Moreover, when broadly considering quality of life and nonoperative shunt-related morbidities in shunt-dependent pediatric patients as they progress into young adulthood, it is evident that a much broader cohort of patients, beyond those with radiographic SVS, suffer from chronic symptomatology that intuitively seems referable to the inability shunt systems to effectively restore CSF physiology. A patient-reported survey, which was completed by nearly 2,000 hydrocephalic young adults who were diagnosed and treated in childhood, found that more than 50% of patients diagnosed before 18 months of age and more than 40% of patients diagnosed during their teen years suffer from chronic baseline headaches into adulthood. Nearly three quarters of those diagnosed before the age of 18 months reported a history of depression and 45% had sought treatment for this diagnosis, further underscoring the lasting impact that childhood hydrocephalus and its associated treatment failures have on the overall well-being and quality of life of these patients [1, 150]. While one might hope that we have yet to fully realize the long-term benefits of the wide adoption of shunt valves with built-in siphon-limiting devices, the lack of a clear effect within the first several years of shunt placement suggests that any potential benefit beyond this time frame will be modest at best and should serve to inspire continued efforts in intelligent shunt valve design. With the knowledge that CSF shunts are unlikely to ever be capable of fully recapitulating CSF physiology, we eagerly anticipate the development of early pharmacologic and procedural interventions that might reduce the population of shunt-dependent children in the coming decades. However, as it is highly unlikely that CSF shunting will become an obsolete procedure in the foreseeable future, it is incumbent on the pediatric neurosurgery community of clinicians and researchers to maintain focused efforts to improve understanding and management strategies for shunt failure and shunt-related morbidity.
Acknowledgments
We would like to acknowledge Kate Sweeney, University of Washington medical illustrator, for the Figure 1 artwork as well as the Seattle Children’s Research Institute for providing financial support for the creation of this figure.
Footnotes
Disclosure Statement
Dr. Browd has ownership in Aqueduct Neurosciences Inc., Aqueduct Critical Care Inc., and Navisonics Inc.
References
- 1.Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: part I: obstruction and mechanical failure. Pediatr Neurol. 2006;34:83–92. doi: 10.1016/j.pediatrneurol.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, Piatt J, Haines S, Schiff S, Cochrane D, Steinbok P, MacNeil N. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230–236. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 3.Riva-Cambrin J, Kestle JR, Holubkov R, Butler J, Kulkarni AV, Drake J, Whitehead WE, Wellons JC, Shannon CN, Tamber MS, Limbrick DD, Rozzelle C, Browd SR, Simon TD, Network HCR. Risk factors for shunt malfunction in pediatric hydrocephalus: a multi-center prospective cohort study. J Neurosurg Pediatr. 2016;17:382–390. doi: 10.3171/2015.6.PEDS14670. [DOI] [PubMed] [Google Scholar]
- 4.Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery. 1999;45:1399–1408. doi: 10.1097/00006123-199912000-00026. discussion 1408–1311. [DOI] [PubMed] [Google Scholar]
- 5.Scott RM, Madsen JR. Shunt technology: contemporary concepts and prospects. Clin Neurosurg. 2003;50:256–267. [PubMed] [Google Scholar]
- 6.Liptak GS, McDonald JV. Ventriculoperitoneal shunts in children: factors affecting shunt survival. Pediatr Neurosci. 1985;12:289–293. doi: 10.1159/000120268. [DOI] [PubMed] [Google Scholar]
- 7.Buster BE, Bonney PA, Cheema AA, Glenn CA, Conner AK, Safavi-Abbasi S, Andrews MB, Gross NL, Mapstone TB. Proximal ventricular shunt malfunctions in children: factors associated with failure. J Clin Neurosci. 2016;24:94–98. doi: 10.1016/j.jocn.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Kestle JR, Riva-Cambrin J, Wellons JC, 3rd, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM, Luerssen TG, Simon TD, Holubkov R Hydrocephalus Clinical Research Network. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr. 2011;8:22–29. doi: 10.3171/2011.4.PEDS10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehead WE, Riva-Cambrin J, Wellons JC, Kulkarni AV, Holubkov R, Illner A, Oakes WJ, Luerssen TG, Walker ML, Drake JM, Kestle JR, Network HCR. No significant improvement in the rate of accurate ventricular catheter location using ultrasound-guided CSF shunt insertion: a prospective, controlled study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2013;12:565–574. doi: 10.3171/2013.9.PEDS1346. [DOI] [PubMed] [Google Scholar]
- 10.Levitt MR, O’Neill BR, Ishak GE, Khanna PC, Temkin NR, Ellenbogen RG, Ojemann JG, Browd SR. Image-guided cerebrospinal fluid shunting in children: catheter accuracy and shunt survival. J Neurosurg Pediatr. 2012;10:112–117. doi: 10.3171/2012.3.PEDS122. [DOI] [PubMed] [Google Scholar]
- 11.Hanlo PW, Cinalli G, Vandertop WP, Faber JA, Bøgeskov L, Børgesen SE, Boschert J, Chumas P, Eder H, Pople IK, Serlo W, Vitzthum E. Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. J Neurosurg. 2003;99:52–57. doi: 10.3171/jns.2003.99.1.0052. [DOI] [PubMed] [Google Scholar]
- 12.Decq P, Barat JL, Duplessis E, Leguerinel C, Gendrault P, Keravel Y. Shunt failure in adult hydrocephalus: flow-controlled shunt versus differential pressure shunts – a cooperative study in 289 patients. Surg Neurol. 1995;43:333–339. doi: 10.1016/0090-3019(95)80058-o. [DOI] [PubMed] [Google Scholar]
- 13.Portnoy HD, Schulte RR, Fox JL, Croissant PD, Tripp L. Anti-siphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J Neurosurg. 1973;38:729–738. doi: 10.3171/jns.1973.38.6.0729. [DOI] [PubMed] [Google Scholar]
- 14.Tschan CA, Antes S, Huthmann A, Vulcu S, Oertel J, Wagner W. Overcoming CSF over-drainage with the adjustable gravitational valve proSA. Acta Neurochir (Wien) 2014;156:767–776. doi: 10.1007/s00701-013-1934-3. discussion 776. [DOI] [PubMed] [Google Scholar]
- 15.Gebert AF, Schulz M, Schwarz K, Thomale UW. Long-term survival rates of gravity-assisted, adjustable differential pressure valves in infants with hydrocephalus. J Neurosurg Pediatr. 2016;17:544–551. doi: 10.3171/2015.10.PEDS15328. [DOI] [PubMed] [Google Scholar]
- 16.Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J, Haines S, Schiff SJ, Cochrane DD, Steinbok P, MacNeil N. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294–303. doi: 10.1097/00006123-199808000-00068. discussion 303–295. [DOI] [PubMed] [Google Scholar]
- 17.Drake JM, Kestle JR, Tuli S. CSF shunts 50 years on – past, present and future. Childs Nerv Syst. 2000;16:800–804. doi: 10.1007/s003810000351. [DOI] [PubMed] [Google Scholar]
- 18.Baird LC, Mazzola CA, Auguste KI, Klimo P, Flannery AM Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 5. Effect of valve type on cerebrospinal fluid shunt efficacy. J Neurosurg Pediatr. 2014;14(suppl 1):35–43. doi: 10.3171/2014.7.PEDS14325. [DOI] [PubMed] [Google Scholar]
- 19.Jain H, Sgouros S, Walsh AR, Hockley AD. The treatment of infantile hydrocephalus: “differential-pressure” or “flow-control” valves. A pilot study. Childs Nerv Syst. 2000;16:242–246. doi: 10.1007/s003810050505. [DOI] [PubMed] [Google Scholar]
- 20.Carmel PW, Albright AL, Adelson PD, Canady A, Black P, Boydston W, Kneirim D, Kaufman B, Walker M, Luciano M, Pollack IF, Manwaring K, Heilbrun MP, Abbott IR, Rekate H. Incidence and management of sub-dural hematoma/hygroma with variable- and fixed-pressure differential valves: a randomized, controlled study of programmable compared with conventional valves. Neurosurg Focus. 1999;7:e7. doi: 10.3171/foc.1999.7.4.2. [DOI] [PubMed] [Google Scholar]
- 21.Hatlen TJ, Shurtleff DB, Loeser JD, Ojemann JG, Avellino AM, Ellenbogen RG. Nonprogrammable and programmable cerebrospinal fluid shunt valves: a 5-year study. J Neurosurg Pediatr. 2012;9:462–467. doi: 10.3171/2012.1.PEDS10482. [DOI] [PubMed] [Google Scholar]
- 22.Cozzens JW, Chandler JP. Increased risk of distal ventriculoperitoneal shunt obstruction associated with slit valves or distal slits in the peritoneal catheter. J Neurosurg. 1997;87:682–686. doi: 10.3171/jns.1997.87.5.0682. [DOI] [PubMed] [Google Scholar]
- 23.Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD, Luerssen TG, Jerry Oakes W, Riva-Cambrin J, Rozzelle C, Simon TD, Walker ML, Wellons JC, Browd SR, Drake JM, Shannon CN, Tamber MS, Whitehead WE Hydrocephalus Clinical Research Network. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2016;17:391–396. doi: 10.3171/2015.8.PEDS15253. [DOI] [PubMed] [Google Scholar]
- 24.Klimo P, Thompson CJ, Baird LC, Flannery AM Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 7. Antibiotic-impregnated shunt systems versus conventional shunts in children: a systematic review and meta-analysis. J Neurosurg Pediatr. 2014;14(suppl 1):53–59. doi: 10.3171/2014.7.PEDS14327. [DOI] [PubMed] [Google Scholar]
- 25.Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 2015;122:1096–1112. doi: 10.3171/2014.12.JNS14908. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38:295–301. doi: 10.1159/000070413. [DOI] [PubMed] [Google Scholar]
- 27.Pang D, Grabb PA. Accurate placement of coronal ventricular catheter using stereotactic coordinate-guided free-hand passage. Technical note J Neurosurg. 1994;80:750–755. doi: 10.3171/jns.1994.80.4.0750. [DOI] [PubMed] [Google Scholar]
- 28.Griebel R, Khan M, Tan L. CSF shunt complications: an analysis of contributory factors. Childs Nerv Syst. 1985;1:77–80. doi: 10.1007/BF00706686. [DOI] [PubMed] [Google Scholar]
- 29.Piatt JH, Carlson CV. A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg. 1993;19:233–241. doi: 10.1159/000120738. discussion 242. [DOI] [PubMed] [Google Scholar]
- 30.Tuli S, O’Hayon B, Drake J, Clarke M, Kestle J. Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery. 1999;45:1329–1333. doi: 10.1097/00006123-199912000-00012. discussion 1333–1325. [DOI] [PubMed] [Google Scholar]
- 31.Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg. 2000;92:31–38. doi: 10.3171/jns.2000.92.1.0031. [DOI] [PubMed] [Google Scholar]
- 32.Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C. Multiple shunt failures: an analysis of relevant factors. Childs Nerv Syst. 1998;14:271–275. doi: 10.1007/s003810050223. [DOI] [PubMed] [Google Scholar]
- 33.McGirt MJ, Leveque JC, Wellons JC, Villavicencio AT, Hopkins JS, Fuchs HE, George TM. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–255. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 34.Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ, Humphreys RP, Hendrick EB. Mechanical complications in shunts. Pediatr Neurosurg. 1991;17:2–9. doi: 10.1159/000120557. [DOI] [PubMed] [Google Scholar]
- 35.El Shafei IL, El Shafei HI. The retrograde ventriculo-sinus shunt (El Shafei RVS shunt). Rationale, evolution, surgical technique and long-term results. Pediatr Neurosurg. 2005;41:305–317. doi: 10.1159/000088733. [DOI] [PubMed] [Google Scholar]
- 36.Fernandes de Oliveira M, Teixeira MJ, Reis RC, Petitto CE, Gomes Pinto FC. Failed ventriculoperitoneal shunt: is retrograde ventriculosinus shunt a reliable option? World Neurosurg. 2016;92:445–453. doi: 10.1016/j.wneu.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 37.El-Shafei IL, El-Shafei HI. The retrograde ventriculovenous shunts: the El-Shafei retrograde ventriculojugular and ventriculosinus shunts. Pediatr Neurosurg. 2010;46:160–171. doi: 10.1159/000316639. [DOI] [PubMed] [Google Scholar]
- 38.Demetriades AK, Haq IZ, Jarosz J, McCormick D, Bassi S. The ventriculocholecystic shunt: two case reports and a review of the literature. Br J Neurosurg. 2013;27:505–508. doi: 10.3109/02688697.2013.771135. [DOI] [PubMed] [Google Scholar]
- 39.West CG. Ventriculovesical shunt. Technical note. J Neurosurg. 1980;53:858–860. doi: 10.3171/jns.1980.53.6.0858. [DOI] [PubMed] [Google Scholar]
- 40.Hanak BW, Ross EF, Harris CA, Browd SR, Shain W. Toward a better understanding of the cellular basis for cerebrospinal fluid shunt obstruction: report on the construction of a bank of explanted hydrocephalus devices. J Neurosurg Pediatr. 2016:1–11. doi: 10.3171/2016.2.PEDS15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris CA, McAllister JP. What we should know about the cellular and tissue response causing catheter obstruction in the treatment of hydrocephalus. Neurosurgery. 2012;70:1589–1601. doi: 10.1227/NEU.0b013e318244695f. discussion 1601–1582. [DOI] [PubMed] [Google Scholar]
- 42.Harris C, McAllister JP. Obstruction of cerebrospinal fluid drainage systems. In: Kombogiorgas D, editor. The Cerebrospinal Fluid Shunts. Hauppauge: Nova Science Publishers; 2016. pp. 273–292. [Google Scholar]
- 43.Hakim S. Observations on the physiopathology of the CSF pulse and prevention of ventricular catheter obstruction in valve shunts. Dev Med Child Neurol Suppl. 1969;20:42–48. doi: 10.1111/j.1469-8749.1969.tb09243.x. [DOI] [PubMed] [Google Scholar]
- 44.Sekhar LN, Moossy J, Guthkelch AN. Malfunctioning ventriculoperitoneal shunts. Clinical and pathological features. J Neurosurg. 1982;56:411–416. doi: 10.3171/jns.1982.56.3.0411. [DOI] [PubMed] [Google Scholar]
- 45.Del Bigio MR. Biological reactions to cerebrospinal fluid shunt devices: a review of the cellular pathology. Neurosurgery. 1998;42:319–325. doi: 10.1097/00006123-199802000-00064. discussion 325–316. [DOI] [PubMed] [Google Scholar]
- 46.Bruni JE, Del Bigio MR. Reaction of periventricular tissue in the rat fourth ventricle to chronically placed shunt tubing implants. Neurosurgery. 1986;19:337–345. doi: 10.1227/00006123-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Del Bigio MR, Bruni JE. Reaction of rabbit lateral periventricular tissue to shunt tubing implants. J Neurosurg. 1986;64:932–940. doi: 10.3171/jns.1986.64.6.0932. [DOI] [PubMed] [Google Scholar]
- 48.Del Bigio MR, Fedoroff S. Short-term response of brain tissue to cerebrospinal fluid shunts in vivo and in vitro. J Biomed Mater Res. 1992;26:979–987. doi: 10.1002/jbm.820260802. [DOI] [PubMed] [Google Scholar]
- 49.Blegvad C, Skjolding A, Broholm H, Laursen H, Juhler M. Pathophysiology of shunt dysfunction in shunt treated hydrocephalus. Acta Neurochir (Wien) 2013;155:1763–1772. doi: 10.1007/s00701-013-1729-6. [DOI] [PubMed] [Google Scholar]
- 50.Sarkiss CA, Sarkar R, Yong W, Lazareff JA. Time dependent pattern of cellular characteristics causing ventriculoperitoneal shunt failure in children. Clin Neurol Neurosurg. 2014;127:30–32. doi: 10.1016/j.clineuro.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Harris C, McAllister JI. What we should know about the cellular and tissue response causing catheter obstruction in the treatment of hydrocephalus. Neurosurgery. 2012;70:1589–1602. doi: 10.1227/NEU.0b013e318244695f. [DOI] [PubMed] [Google Scholar]
- 52.Harris C, Resau J, Hudson E, West R, Moon C, Black A, McAllister JI., 2nd Reduction of protein adsorption and macrophage and astrocyte adhesion on ventricular catheters by polyethylene glycol and N-acetyl-L-cysteine. J Biomed Mater Res A. 2011;98:425–433. doi: 10.1002/jbm.a.33130. [DOI] [PubMed] [Google Scholar]
- 53.Weisenberg S, TerMaath S, Seaver C, Killeffer J. Ventricular catheter development: past, present, and future. J Neurosurg. 2016 doi: 10.3171/2015.12.JNS151181. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 55.Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- 56.Turner AM, Dowell N, Turner SW, Kam L, Isaacson M, Turner JN, Craighead HG, Shain W. Attachment of astroglial cells to microfabricated pillar arrays of different geometries. J Biomed Mater Res. 2000;51:430–441. doi: 10.1002/1097-4636(20000905)51:3<430::aid-jbm18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 57.Czernicki Z, Strzałkowski R, Walasek N, Gajkowska B. What can be found inside shunt catheters. Acta Neurochir Suppl. 2010;106:81–85. doi: 10.1007/978-3-211-98811-4_13. [DOI] [PubMed] [Google Scholar]
- 58.Guevara JA, La Torre J, Denoya C, Zúccaro G. Microscopic studies in shunts for hydrocephalus. Childs Brain. 1981;8:284–293. doi: 10.1159/000119991. [DOI] [PubMed] [Google Scholar]
- 59.Thomale U, Hosch H, Koch A, Schulz M, Stoltenburg G, Haberl E, Sprung C. Perforation holes in ventricular catheters – is less more? Childs Nerv Syst. 2010;26:781–789. doi: 10.1007/s00381-009-1055-8. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Morris M, Olivero W, Boop F, Sanford R. Computational and experimental study of proximal flow in ventricular catheters. Technical note. J Neurosurg. 2003;99:426–431. doi: 10.3171/jns.2003.99.2.0426. [DOI] [PubMed] [Google Scholar]
- 61.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 62.Kim JV, Dustin ML. Innate response to focal necrotic injury inside the blood-brain barrier. J Immunol. 2006;177:5269–5277. doi: 10.4049/jimmunol.177.8.5269. [DOI] [PubMed] [Google Scholar]
- 63.Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi Y, Ohkura A, Hirohata M, Tokutomi T, Shigemori M. Ultrastructure of obstructive tissue in malfunctioning ventricular catheters without infection. Neurol Med Chir (Tokyo) 1998;38:399–404. doi: 10.2176/nmc.38.399. discussion 403–394. [DOI] [PubMed] [Google Scholar]
- 65.Ellis MJ, Kazina CJ, Del Bigio MR, McDonald PJ. Treatment of recurrent ventriculoperitoneal shunt failure associated with persistent cerebrospinal fluid eosinophilia and latex allergy by use of an “extracted” shunt. J Neurosurg Pediatr. 2008;1:237–239. doi: 10.3171/PED/2008/1/3/237. [DOI] [PubMed] [Google Scholar]
- 66.Chambi I, Hendrick EB. A technique for removal of an adherent ventricular catheter. Pediatr Neurosci. 1988;14:216–217. doi: 10.1159/000120392. [DOI] [PubMed] [Google Scholar]
- 67.Traynelis VC, Powell RG, Koss W, Schochet SS, Kaufman HH. Cerebrospinal fluid eosinophilia and sterile shunt malfunction. Neurosurgery. 1988;23:645–649. doi: 10.1227/00006123-198811000-00019. [DOI] [PubMed] [Google Scholar]
- 68.Tung H, Raffel C, McComb JG. Ventricular cerebrospinal fluid eosinophilia in children with ventriculoperitoneal shunts. J Neurosurg. 1991;75:541–544. doi: 10.3171/jns.1991.75.4.0541. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka T, Ikeuchi S, Yoshino K, Isoshima A, Abe T. A case of cerebrospinal fluid eosinophilia associated with shunt malfunction. Pediatr Neurosurg. 1999;30:6–10. doi: 10.1159/000028752. [DOI] [PubMed] [Google Scholar]
- 70.Pittman T, Williams D, Rathore M, Knutsen AP, Mueller KR. The role of ethylene oxide allergy in sterile shunt malfunctions. Br J Neurosurg. 1994;8:41–45. doi: 10.3109/02688699409002391. [DOI] [PubMed] [Google Scholar]
- 71.Becker DP, Nulsen FE. Control of hydrocephalus by valve-regulated venous shunt: avoidance of complications in prolonged shunt maintenance. J Neurosurg. 1968;28:215–226. doi: 10.3171/jns.1968.28.3.0215. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman HJ, Smith MS. The use of shunting devices for cerebrospinal fluid in Canada. Can J Neurol Sci. 1986;13:81–87. doi: 10.1017/s0317167100035952. [DOI] [PubMed] [Google Scholar]
- 73.Sainte-Rose C. Shunt obstruction: a preventable complication? Pediatr Neurosurg. 1993;19:156–164. doi: 10.1159/000120722. [DOI] [PubMed] [Google Scholar]
- 74.Whitehead W. A randomized controlled trial of anterior versus posterior entry site for CSF shunt insertion. 2014 doi: 10.3171/2021.9.PEDS21391. http://hcrn.org/research/entry-site/ [DOI] [PubMed]
- 75.Harris CA, McAllister JP. Does drainage hole size influence adhesion on ventricular catheters? Childs Nerv Syst. 2011;27:1221–1232. doi: 10.1007/s00381-011-1430-0. [DOI] [PubMed] [Google Scholar]
- 76.Harris CA, Resau JH, Hudson EA, West RA, Moon C, Black AD, McAllister JP. Effects of surface wettability, flow, and protein concentration on macrophage and astrocyte adhesion in an in vitro model of central nervous system catheter obstruction. J Biomed Mater Res A. 2011;97:433–440. doi: 10.1002/jbm.a.33078. [DOI] [PubMed] [Google Scholar]
- 77.Drake JM, da Silva MC, Rutka JT. Functional obstruction of an antisiphon device by raised tissue capsule pressure. Neurosurgery. 1993;32:137–139. doi: 10.1227/00006123-199301000-00023. [DOI] [PubMed] [Google Scholar]
- 78.Piatt JH. Cerebrospinal fluid shunt failure: late is different from early. Pediatr Neurosurg. 1995;23:133–139. doi: 10.1159/000120950. [DOI] [PubMed] [Google Scholar]
- 79.Hussain NS, Wang PP, James C, Carson BS, Avellino AM. Distal ventriculoperitoneal shunt failure caused by silicone allergy. Case report. J Neurosurg. 2005;102:536–539. doi: 10.3171/jns.2005.102.3.0536. [DOI] [PubMed] [Google Scholar]
- 80.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 81.Choux M, Genitori L, Lang D, Lena G. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992;77:875–880. doi: 10.3171/jns.1992.77.6.0875. [DOI] [PubMed] [Google Scholar]
- 82.Campbell J. Shunt infections. In: Albright A, Pollack IF, Adelson PD, editors. Principles and Practice of Pediatric Neurosurgery. New York: Thieme; 2008. pp. 1141–1147. [Google Scholar]
- 83.Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR, Kulkarni AV, Langley M, Limbrick DD, Mayer-Hamblett N, Tamber M, Wellons JC, Whitehead WE, Riva-Cambrin J, Network HCR. Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. J Pediatr. 2014;164:1462.e2–1468.e2. doi: 10.1016/j.jpeds.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon T, Mayer-Hamblett N, Whitlock K, Langley M, Kestle J, Riva-Cambrin J, Rosenfeld M, Thorell E. Few patient, treatment, and diagnostic or microbiological factors, except complications and intermittent negative cerebrospinal fluid (CSF) cultures during first CSF shunt infection, are associated with reinfection. J Pediatric Infect Dis Soc. 2014;3:15–22. doi: 10.1093/jpids/pit050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snowden J, Beaver M, Smeltzer M, Kielian T. Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system. Infect Immun. 2012;80:3206–3214. doi: 10.1128/IAI.00645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanke M, Kielian T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front Cell Infect Microbiol. 2012;2:62. doi: 10.3389/fcimb.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stoodley P, Braxton E, Jr, Nistico L, Hall-Stoodley L, Johnson S, Quigley M, Post J, Ehrlich G, Kathu S. Direct demonstration of Staphylococcus biofilm in an external ventricular drain in a patient with a history of recurrent ventriculoperitoneal shunt failure. Pediatr Neurosurg. 2010;46:127–132. doi: 10.1159/000319396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;7:38. doi: 10.1186/1471-2334-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamber MS, Klimo P, Mazzola CA, Flannery AM Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 8. Management of cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2014;14(suppl 1):60–71. doi: 10.3171/2014.7.PEDS14328. [DOI] [PubMed] [Google Scholar]
- 90.Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105:242–247. doi: 10.3171/jns.2006.105.2.242. [DOI] [PubMed] [Google Scholar]
- 91.Cuka GM, Hellbusch LC. Fractures of the peritoneal catheter of cerebrospinal fluid shunts. Pediatr Neurosurg. 1995;22:101–103. doi: 10.1159/000120884. [DOI] [PubMed] [Google Scholar]
- 92.Langmoen IA, Lundar T, Vatne K, Hovind KH. Occurrence and management of fractured peripheral catheters in CSF shunts. Childs Nerv Syst. 1992;8:222–225. doi: 10.1007/BF00262852. [DOI] [PubMed] [Google Scholar]
- 93.Aldrich EF, Harmann P. Disconnection as a cause of ventriculoperitoneal shunt malfunction in multicomponent shunt systems. Pediatr Neurosurg. 1990;16:309–311. doi: 10.1159/000120549. discussion 312. [DOI] [PubMed] [Google Scholar]
- 94.James HE, Schut L. Pitfalls in the diagnosis of arrested hydrocephalus. Acta Neurochir (Wien) 1978;43:13–17. doi: 10.1007/BF01809223. [DOI] [PubMed] [Google Scholar]
- 95.Chen HH, Riva-Cambrin J, Brockmeyer DL, Walker ML, Kestle JR. Shunt failure due to intracranial migration of BioGlide ventricular catheters. J Neurosurg Pediatr. 2011;7:408–412. doi: 10.3171/2011.1.PEDS10389. [DOI] [PubMed] [Google Scholar]
- 96.Browd SR, Gottfried ON, Ragel BT, Kestle JR. Failure of cerebrospinal fluid shunts: part II: overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006;34:171–176. doi: 10.1016/j.pediatrneurol.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Anderson R, Garton HJL, Kestle JRW. Treatment of hydrocephalus with shunts. In: Albright A, Pollack IF, Adelson PD, editors. Principles and Practice of Pediatric Neurosurgery. New York: Thieme; 2008. pp. 109–130. [Google Scholar]
- 98.Kraemer M, Iskandar BJ. Overdrainage-Related Ventricular Tissue Is a Significant Cause of Proximal Shunt Obstruction. Chicago: American Association of Neurological Surgeons; 2016. [Google Scholar]
- 99.Chumas PD, Armstrong DC, Drake JM, Kulkarni AV, Hoffman HJ, Humphreys RP, Rutka JT, Hendrick EB. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J Neurosurg. 1993;78:568–573. doi: 10.3171/jns.1993.78.4.0568. [DOI] [PubMed] [Google Scholar]
- 100.Sood S, Barrett RJ, Powell T, Ham SD. The role of lumbar shunts in the management of slit ventricles: does the slit-ventricle syndrome exist? J Neurosurg. 2005;103:119–123. doi: 10.3171/ped.2005.103.2.0119. [DOI] [PubMed] [Google Scholar]
- 101.Arnell K, Eriksson E, Olsen L. The programmable adult Codman Hakim valve is useful even in very small children with hydrocephalus. A 7-year retrospective study with special focus on cost/benefit analysis. Eur J Pediatr Surg. 2006;16:1–7. doi: 10.1055/s-2006-923904. [DOI] [PubMed] [Google Scholar]
- 102.Engel M, Carmel PW, Chutorian AM. Increased intraventricular pressure without ventriculomegaly in children with shunts: “normal volume” hydrocephalus. Neurosurgery. 1979;5:549–552. doi: 10.1227/00006123-197911000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Buxton N, Punt J. Subtemporal decompression: the treatment of noncompliant ventricle syndrome. Neurosurgery. 1999;44:513–518. doi: 10.1097/00006123-199903000-00045. discussion 518–519. [DOI] [PubMed] [Google Scholar]
- 104.Rekate HL. The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg. 2004;40:259–263. doi: 10.1159/000083737. [DOI] [PubMed] [Google Scholar]
- 105.Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of non-tumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst. 1994;10:321–327. doi: 10.1007/BF00335171. [DOI] [PubMed] [Google Scholar]
- 106.Sgouros S, Malluci C, Walsh AR, Hockley AD. Long-term complications of hydrocephalus. Pediatr Neurosurg. 1995;23:127–132. doi: 10.1159/000120949. [DOI] [PubMed] [Google Scholar]
- 107.Kan P, Walker ML, Drake JM, Kestle JR. Predicting slitlike ventricles in children on the basis of baseline characteristics at the time of shunt insertion. J Neurosurg. 2007;106:347–349. doi: 10.3171/ped.2007.106.5.347. [DOI] [PubMed] [Google Scholar]
- 108.Major O, Fedorcsák I, Sipos L, Hantos P, Kónya E, Dobronyi I, Paraicz E. Slit-ventricle syndrome in shunt operated children. Acta Neurochir (Wien) 1994;127:69–72. doi: 10.1007/BF01808550. [DOI] [PubMed] [Google Scholar]
- 109.Gruber RW, Roehrig B. Prevention of ventricular catheter obstruction and slit ventricle syndrome by the prophylactic use of the Integra antisiphon device in shunt therapy for pediatric hypertensive hydrocephalus: a 25-year follow-up study. J Neurosurg Pediatr. 2010;5:4–16. doi: 10.3171/2008.7.17690. [DOI] [PubMed] [Google Scholar]
- 110.Rekate HL. Antisiphon device. J Neurosurg Pediatr. 2010;5:1–2. doi: 10.3171/2009.8.00249. discussion 2–3. [DOI] [PubMed] [Google Scholar]
- 111.Epstein F, Lapras C, Wisoff JH. “Slit-ventricle syndrome”: etiology and treatment. Pediatr Neurosci. 1988;14:5–10. [PubMed] [Google Scholar]
- 112.Walker ML, Fried A, Petronio J. Diagnosis and treatment of the slit ventricle syndrome. Neurosurg Clin N Am. 1993;4:707–714. [PubMed] [Google Scholar]
- 113.Nowak TP, James HE. Migraine headaches in hydrocephalic children: a diagnostic dilemma. Childs Nerv Syst. 1989;5:310–314. doi: 10.1007/BF00274520. [DOI] [PubMed] [Google Scholar]
- 114.Obana WG, Raskin NH, Cogen PH, Szymanski JA, Edwards MS. Antimigraine treatment for slit ventricle syndrome. Neurosurgery. 1990;27:760–763. doi: 10.1097/00006123-199011000-00014. discussion 763. [DOI] [PubMed] [Google Scholar]
- 115.Rekate HL, Wallace D. Lumboperitoneal shunts in children. Pediatr Neurosurg. 2003;38:41–46. doi: 10.1159/000067562. [DOI] [PubMed] [Google Scholar]
- 116.Reddy K, Fewer HD, West M, Hill NC. Slit ventricle syndrome with aqueduct stenosis: third ventriculostomy as definitive treatment. Neurosurgery. 1988;23:756–759. doi: 10.1227/00006123-198812000-00013. [DOI] [PubMed] [Google Scholar]
- 117.Le H, Yamini B, Frim DM. Lumboperitoneal shunting as a treatment for slit ventricle syndrome. Pediatr Neurosurg. 2002;36:178–182. doi: 10.1159/000056054. [DOI] [PubMed] [Google Scholar]
- 118.Jea A, Al-Otibi M, Bonnard A, Drake JM. Laparoscopy-assisted ventriculoperitoneal shunt surgery in children: a series of 11 cases. J Neurosurg. 2007;106:421–425. doi: 10.3171/ped.2007.106.6.421. [DOI] [PubMed] [Google Scholar]
- 119.Martínez-Lage JF, Martos-Tello JM, Rosde-San Pedro J, Almagro MJ. Severe constipation: an under-appreciated cause of VP shunt malfunction: a case-based update. Childs Nerv Syst. 2008;24:431–435. doi: 10.1007/s00381-007-0514-3. [DOI] [PubMed] [Google Scholar]
- 120.Muzumdar D, Ventureyra EC. Transient ventriculoperitoneal shunt malfunction after chronic constipation: case report and review of literature. Childs Nerv Syst. 2007;23:455–458. doi: 10.1007/s00381-006-0232-2. [DOI] [PubMed] [Google Scholar]
- 121.Powers CJ, George T, Fuchs HE. Constipation as a reversible cause of ventriculoperitoneal shunt failure. Report of two cases. J Neurosurg. 2006;105:227–230. doi: 10.3171/ped.2006.105.3.227. [DOI] [PubMed] [Google Scholar]
- 122.Gaskill SJ, Marlin AE. Pseudocysts of the abdomen associated with ventriculoperitoneal shunts: a report of twelve cases and a review of the literature. Pediatr Neurosci. 1989;15:23–26. doi: 10.1159/000120436. discussion 26–27. [DOI] [PubMed] [Google Scholar]
- 123.Burchianti M, Cantini R. Peritoneal cerebro-spinal fluid pseudocysts: a complication of ventriculoperitoneal shunts. Childs Nerv Syst. 1988;4:286–290. doi: 10.1007/BF00271925. [DOI] [PubMed] [Google Scholar]
- 124.Hahn YS, Engelhard H, McLone DG. Abdominal CSF pseudocyst. Clinical features and surgical management. Pediatr Neurosci. 1985;12:75–79. [PubMed] [Google Scholar]
- 125.Adeloye A. Protrusion of ventriculo peritoneal shunt through the anus: report of two cases. East Afr Med J. 1997;74:337–339. [PubMed] [Google Scholar]
- 126.Ghritlaharey RK, Budhwani KS, Shrivastava DK, Gupta G, Kushwaha AS, Chanchlani R, Nanda M. Trans-anal protrusion of ventriculo-peritoneal shunt catheter with silent bowel perforation: report of ten cases in children. Pediatr Surg Int. 2007;23:575–580. doi: 10.1007/s00383-007-1916-8. [DOI] [PubMed] [Google Scholar]
- 127.Morell RC, Bell WO, Hertz GE, D’Souza V. Migration of a ventriculoperitoneal shunt into the pulmonary artery. J Neurosurg Anesthesiol. 1994;6:132–134. doi: 10.1097/00008506-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 128.Alam S, Manjunath NM. Severe respiratory failure following ventriculopleural shunt. Indian J Crit Care Med. 2015;19:690–692. doi: 10.4103/0972-5229.169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Küpeli E, Yilmaz C, Akçay S. Pleural effusion following ventriculopleural shunt: case reports and review of the literature. Ann Thorac Med. 2010;5:166–170. doi: 10.4103/1817-1737.65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beach C, Manthey DE. Tension hydrothorax due to ventriculopleural shunting. J Emerg Med. 1998;16:33–36. doi: 10.1016/s0736-4679(97)00238-2. [DOI] [PubMed] [Google Scholar]
- 131.Irani F, Elkambergy H, Okoli K, Abou DS. Recurrent symptomatic pleural effusion due to a ventriculopleural shunt. Respir Care. 2009;54:1112–1114. [PubMed] [Google Scholar]
- 132.Hoffman HJ, Hendrick EB, Humphreys RP. Experience with ventriculo-pleural shunts. Childs Brain. 1983;10:404–413. doi: 10.1159/000120142. [DOI] [PubMed] [Google Scholar]
- 133.Jones RF, Currie BG, Kwok BC. Ventriculo-pleural shunts for hydrocephalus: a useful alternative. Neurosurgery. 1988;23:753–755. doi: 10.1227/00006123-198812000-00012. [DOI] [PubMed] [Google Scholar]
- 134.Melamed EF, Christian E, Krieger MD, Berry C, Yashar P, McComb JG. 200 age as a novel risk factor for revision of ventriculo-pleural shunt in pediatric patients. Neurosurgery. 2016;63(suppl 1):178–179. [Google Scholar]
- 135.Megison DP, Benzel EC. Ventriculo-pleural shunting for adult hydrocephalus. Br J Neurosurg. 1988;2:503–505. doi: 10.3109/02688698809029605. [DOI] [PubMed] [Google Scholar]
- 136.Ratliff M, Unterberg A, Bächli H. Ventriculo-bipleural shunt as last resort in a 4-year-old child in whom a VP and VA shunt failed. J Neurosurg Pediatr. 2016;17:285–288. doi: 10.3171/2015.7.PEDS15218. [DOI] [PubMed] [Google Scholar]
- 137.Carrion E, Hertzog JH, Medlock MD, Hauser GJ, Dalton HJ. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child. 2001;84:68–71. doi: 10.1136/adc.84.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McGovern RA, Kelly KM, Chan AK, Morrissey NJ, McKhann GM. Should ventriculoatrial shunting be the procedure of choice for normal-pressure hydrocephalus? J Neurosurg. 2014;120:1458–1464. doi: 10.3171/2014.1.JNS131808. [DOI] [PubMed] [Google Scholar]
- 139.McGrail KM, Muzzi DA, Losasso TJ, Meyer FB. Ventriculoatrial shunt distal catheter placement using transesophageal echocardiography: technical note. Neurosurgery. 1992;30:747–749. [PubMed] [Google Scholar]
- 140.Vernet O, Campiche R, de Tribolet N. Long-term results after ventriculoatrial shunting in children. Childs Nerv Syst. 1995;11:176–179. doi: 10.1007/BF00306265. [DOI] [PubMed] [Google Scholar]
- 141.Natarajan A, Mazhar S. Right heart complications of ventriculoatrial shunt. Eur Heart J. 2011;32:2134. doi: 10.1093/eurheartj/ehr164. [DOI] [PubMed] [Google Scholar]
- 142.Ladouceur D, Giroux M. Echocardiographic detection of intracardiac thrombi complicating ventriculoatrial shunt. Report of two cases. Pediatr Neurosurg. 1994;20:68–72. doi: 10.1159/000120767. [DOI] [PubMed] [Google Scholar]
- 143.Searle M, Lee HA. Ventriculoatrial shunt nephritis. Postgrad Med J. 1982;58:566–569. doi: 10.1136/pgmj.58.683.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noe HN, Roy S. Shunt nephritis. J Urol. 1981;125:731–733. doi: 10.1016/s0022-5347(17)55183-6. [DOI] [PubMed] [Google Scholar]
- 145.Black JA, Challacombe DN, Ockenden BG. Nephrotic syndrome associated with bacteraemia after shunt operations for hydrocephalus. Lancet. 1965;2:921–924. doi: 10.1016/s0140-6736(65)92901-6. [DOI] [PubMed] [Google Scholar]
- 146.Narchi H, Taylor R, Azmy AF, Murphy AV, Beattie TJ. Shunt nephritis. J Pediatr Surg. 1988;23:839–841. doi: 10.1016/s0022-3468(88)80235-5. [DOI] [PubMed] [Google Scholar]
- 147.Dobrin RS, Day NK, Quie PG, Moore HL, Vernier HL, Michael AF, Fish AJ. The role of complement, immunoglobulin and bacterial antigen in coagulase-negative staphylococcal shunt nephritis. Am J Med. 1975;59:660–673. doi: 10.1016/0002-9343(75)90227-2. [DOI] [PubMed] [Google Scholar]
- 148.Burström G, Andresen M, Bartek J, Fytagoridis A. Subacute bacterial endocarditis and subsequent shunt nephritis from ventriculoatrial shunting 14 years after shunt implantation. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204655. bcr2014204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McKenzie SA, Hayden K. Two cases of “shunt nephritis”. Pediatrics. 1974;54:806–808. [PubMed] [Google Scholar]
- 150.Gupta N, Park J, Solomon C, Kranz DA, Wrensch M, Wu YW. Long-term outcomes in patients with treated childhood hydrocephalus. J Neurosurg. 2007;106:334–339. doi: 10.3171/ped.2007.106.5.334. [DOI] [PubMed] [Google Scholar]