Abstract
Dry eye disease (DED), age-related macular degeneration (AMD), and Uveitis are ocular diseases that significantly affect the quality of life of millions of people each year. In these diseases, the action of chemokines, pro-inflammatory cytokines, and immune cells drives a local inflammatory response that results in ocular tissue damage. Multiple therapeutic strategies have been developed to either address the symptoms or abate the underlying cause of these diseases. Herein, we will review the challenges to deliver drugs to the relevant location in the eye for each of these diseases as well as current and innovative therapeutic approaches that attempt to restore homeostasis within the ocular microenvironment.
Keywords: dry eye disease, age-related macular degeneration, uveitis, drug delivery, modern therapeutic approaches
1. Introduction
With the global ophthalmic drug delivery market estimated to grow at two-and-a –half times the overall rate of the pharmaceutical industry, many commercial opportunities exist for the development of new ophthalmic drugs.[1] Ideal candidates for improved drug delivery treatments are those ocular diseases that drastically affect patients’ quality of life including dry eye disease (DED), age-related macular degeneration (AMD), and uveitis.[2–4] These three common ocular diseases affect different regions of the eye and have immuno-mechanistic characteristics in their disease pathogenesis. For instance, DED affects the ocular surface and is thought to be primarily due to inflammation mediated by T cell infiltration.[5,6] Although, the disease pathogenesis of uveitis is also thought to be mediated via T cells, inflammation occurs in the uveal tract of the eye. On the other hand, AMD primarily afflicts the macula tissue of the eye, and is thought to be caused by the complement immune system (innate immunity), chronic oxidative stress, and neovascularization.[7,8] Though, all these diseases affect different regions of the eye and possess different pathology, one common underlying link associated with these ocular diseases is the involvement of inflammation.[7,9,10] When properly regulated, inflammation is both healthy and essential for the elimination of pathogens and healing. However, excessive, unregulated inflammation can lead to chronic diseases where immune-mediated damage to the ocular tissues elicits an inflammatory response that causes further damage.[11–13] In order to either treat the damage caused by unregulated inflammation or halt the inflammatory cycle, current and new therapies have been developed.[7,14,15] Moreover, modern therapeutic approaches are interdisciplinary in nature, utilizing a combination of synthetic materials, cells, biologics and small molecule based treatments in order to address the underlying inflammatory imbalance. Ultimately, these modern therapeutic approaches can even be inspired by the body’s own method of restoring homeostasis. Specifically, some of the methods of administration for these modern therapeutic approaches include: topical administration, injections, contact lenses, and implants.[16,17] However, there are several limitations associated with these methods of drug administration, such as anatomical barriers, poor bioavailability, and patient compliance issues. For this reason, new treatment strategies intend to address one or more of these barriers. In this review, we discuss the challenges of ocular drug delivery, and the currently used (and also new, investigative) treatments aimed at targeting the pathological factors of dry eye disease, age-related macular degeneration, and uveitis.
2. Routes of Ocular Administration
2.1. Anterior segment
2.1.1. Topical
A key challenge of ocular drug delivery systems for the treatment of diseases affecting the anterior segment of the eye is to obtain therapeutic levels of drug in the ocular tissues, while minimizing systemic side effects.[18] Indeed, even the currently approved therapies for pathologies of the anterior portion of the eye (ex: DED and anterior uveitis), are plagued by short resident time on the ocular surface and poor bioavailability.[19]
Currently, the standard of care for the treatment of diseases affecting the ocular surface and the anterior segment is the topical administration of ophthalmic medications such as eye drops, suspensions, gels, or ointments (Figure 2).[18] Although topically administered drugs are generally well accepted and tolerated methods of delivering medication by patients,[19,20] a major limitation is patient compliance, especially for individuals affected by chronic pathologies such as uveitis, and DED. In fact, these pathologies require the self-administration of topical medication several times a day, which can severely decrease patient compliance.[21] Moreover, this frequent dosing may cause either systemic or local side effects due to the high amounts of total drug administered. Another limitation of topical formulations is their low bioavailability at the site of action.[22] In particular, it is reported that approximately only 5–10% of the administered drug reaches the target tissue, while the remaining 90–95% is eliminated.[23] This elimination occurs through natural, precorneal mechanisms of protection from foreign substance such as drainage through the nasolacrimal duct, blinking, tear film, tear turn over, and induced lacrimation (Figure 1).[24–26] In particular, after the administration of an ophthalmic medication, the drug is first diluted in the lacrimal fluid, which reduces the effective concentration of the applied drug. Moreover, the precorneal tear drainage washes away topical medication within the first 15–30 seconds after application, reducing the amount of time the drug remains in contact with the ocular surface, and absorption.[27] Furthermore, another factor reducing the effectiveness of topical eye drops is the anatomic volume of the cul-de-sac, which is approximately 7–10 μL, while the dosing volume of instillation is approximately 20–50 μL.[25] This difference leads to either the spill of the excess volume on the cheek or to a rapid elimination through the nasolacrimal duct.[25] Despite these limitations, topical administration of ophthalmic drugs is still the most widely prescribed route of administration as it offers numerous advantages including noninvasiveness, ease of administration, and low absorption into systemic circulation.[18] Examples of topical ophthalmic drugs are those used for pathologies affecting the surface of the eye, such as DED, in which artificial tears and lubricants are topically administered to relieve symptoms.[28] However, the development of new methods to enhance drug bioavailability and reduce the frequency of drug administration would greatly improve patient compliance and overall effectiveness of treatment. Recently, A few examples of alternative approaches are discussed in the following sections.
Figure 2.
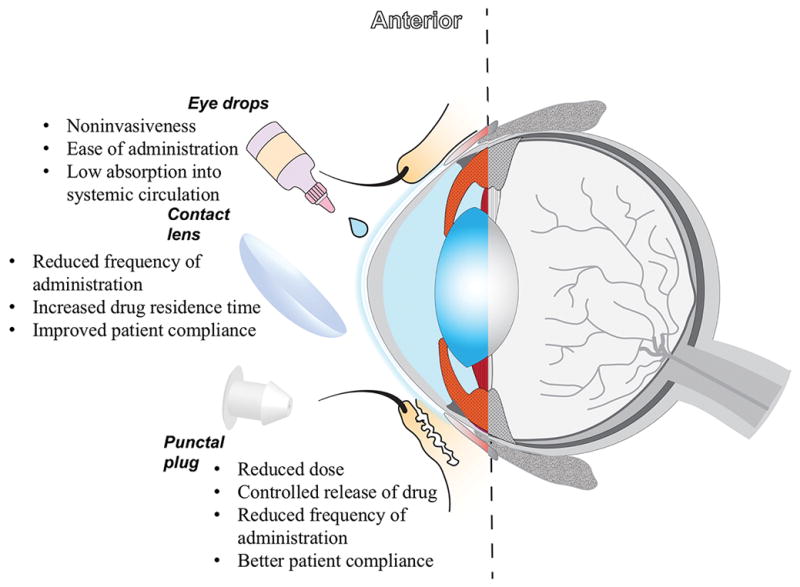
Representative Image of the Anterior Segment of the eye and some examples of different routes of administration.
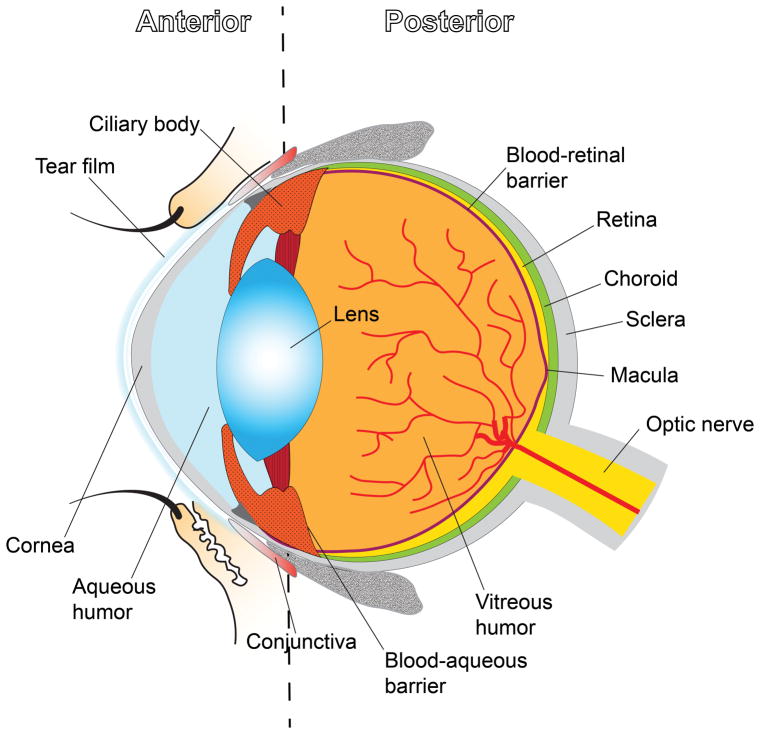
Figure 1.
Schematic illustration of the overall structure of the eye.
2.1.2. Contact lenses
Therapeutic contact lenses (Figure 2) have been widely studied for controlled and sustained drug delivery in order to overcome the limitations associated with topical eye drops.[29] Since contact lenses can be worn for a longer length of time, their use for the release of an ophthalmic medication helps to improve patient compliance by reducing the frequency of administration.[30] Furthermore, in comparison to eye drop formulations, contact lenses allow an increased residence time associated with greater than 50% more bioavailability at the site of action.[30] Consequently, the administered dosage to obtain therapeutic levels at the desired site can be reduced, limiting systemic absorption and its associated side effects.[30] Thanks to these advantages, drug loaded contact lenses are under investigation as a possible drug delivery system for pathologies affecting the surface of the eye such as DED. In particular, contact lenses for the release of cyclosporine have been studied in order to provide increased ocular contact time thus enhancing the drug bioavailability, in addition to a controlled and sustained drug release profile.[31]
The simplest way to obtain drug-loaded contact lenses is by absorption of the drug (soaking the lens into a drug solution), which will be then released on the ocular surface.[30] The ability to load the drug into the contact lens strongly depends on the water content, thickness, concentration of drug solution, molecular weight of the drug, and soaking time.[30] Over the years, this technique has been used for loading contact lenses with different ophthalmic medications such as timolol, brimonidine, pirfinedone, cyclosporine, and dexamethasone.[31–35] Despite the simplicity of fabricating a soaked contact lens, it can take a few hours to absorb the drug, and the amount of drug that can be incorporated in the lens matrix is low, especially for hydrophobic drugs.[36] Moreover, when the drug is incorporated into the lens matrix by soaking, it can quickly diffuse out of the lens, with release times typically limited to a few hours.[36] Therefore, contact lenses could be a promising device to achieve sustained delivery of ophthalmic medications. However, their commercialization is still limited because of the need to address some issues that negatively impact lens properties such as transparency, ion and oxygen permeability, water content, and mechanical properties, each of which is coupled to the properties of the drug and the amount of drug that is loaded.[30] For this reason, alteration of any of these critical properties of contact lenses could result in affected visual ability in patients, presenting significant design challenges for long-term delivery with large amounts of loaded drug.
2.1.3. Punctal plugs
Punctal plugs (Figure 2) are a non-invasive therapeutic method and generally well accepted by both patients and physicians, and were originally used for treating DED by blocking tear drainage, thus improving tear film quantity and residual contact time.[37] Recently, punctal plugs have been proposed for the controlled release of topically administered medications to the ocular surface.[38,39] For this purpose, punctal plugs are generally coated on all sides (except the head portion) with a material that is impermeable to the tear fluids and the drug. Release is controlled through diffusion of drug following contact of the head of the plug with tear fluid. Common issues associated with the use of punctal plugs are eye irritation, excessive tearing, ocular discomfort, and spontaneous loss of the plug from the punctum.[19,40,41] However, drug eluting punctal plugs could offer a new approach for the treatment of chronic pathologies, thanks to several potential advantages over topical administration such as dose reduction, controlled release of drugs, reduction in the frequency of administration and potentially better patient compliance with the therapy.[41]
2.2. Posterior segment
2.2.1. Topical and systemic administration
Treating the less accessible posterior segment of the eye is more challenging for topical delivery than addressing anterior diseases, due to the longer diffusional distance, that the drug has to overcome before reaching the posterior tissues, characterized by additional physical and diffusional barriers.[42,43] In particular, topical administration is inefficient in delivering medications to the posterior segment because of the rapid drainage through the nasolacrimal ducts,[44] as discussed in section 2.1.1. To reach the posterior segment of the eye, a topically administered drug must penetrate through the cornea (Figure 1), which represents a barrier from external agents that naturally serves to hinder the transport of either exogenous substances from the pre-corneal pockets.[45,46] The cornea allows for only the passage of small, moderately lipophilic molecules, while drug solutions made of macromolecules can often penetrate through the cornea only at very low rates, making it difficult to achieve therapeutic efficacy.[45] An additional challenge for topically administered drugs to reach the intraocular environment is represented by the blood-aqueous barrier (Figure 1), consisting of endothelial cells in the uvea and of the non-pigmented layer of the ciliary body epithelium. Specifically, the blood-aqueous barrier forms tight junctions that regulate the exchange of solutes between the anterior and posterior segments, thus impeding nonspecific drug penetration into the inner ocular tissues.[47,48]
Another possible approach for locating drug molecules to the back of the eye consists in systemic administration (intravenous or oral), however the delivery is limited by blood dilution of the drug, presence of inner and outer blood-retinal barriers (Figure 1), and in case of oral route, gastrointestinal barriers.[49] The presence of these anatomical barriers requires a high drug concentration circulating in the plasma to achieve therapeutic levels in the eye, and such high doses may result in systemic side effects.[49,50] Consequently, treating disorders that affect the posterior segment of the eye would greatly benefit from specific localized targeting that could be achieved (for instance) by the more invasive intravitreal injections and implants.
2.2.2. Intravitreal injections
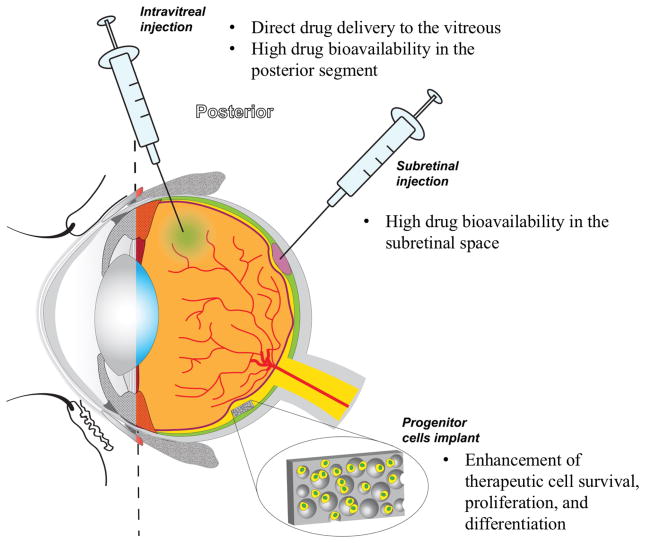
Intravitreal injection (Figure 3) is a route of administration that intends to target the posterior segment of the eye. This approach consists in a direct delivery of the drug to the vitreous, thereby avoiding passage through the ocular barriers and (in turn) leading to a high availability of the ophthalmic medication in the posterior segment tissues.[51] Intravitreal injections are currently used for the administration of anti-VEGF drugs for the treatment of AMD and macular edema.[52,53]
Figure 3.
Illustration of the Posterior Segment of the eye and a few examples of some methods of therapeutic administration.
Despite the advantage of delivering medication locally, intravitreal injections are considered an invasive procedure with consequent potential complications, such as raised intraocular pressure (IOP), transient blurry vision, retinal detachment, and cataracts.[54] Moreover, several injections are often needed to ensure optimal therapeutic drug levels at the site of action due to the short half-life of most ophthalmic drugs, thus increasing the risks of side effects and decreasing overall patient compliance.[17,55,56] Therefore, alternative methods to deliver ophthalmic formulations to the posterior segment that require less frequent dosing could be extremely beneficial for patients, with the advantage of avoiding the aforementioned complications related to repeated injections, and reducing the risk of rapid clearance.
2.2.3. Intravitreal implants
Intravitreal implants can be used as controlled/sustained drug delivery systems that can overcome several limitations of topically, systemically, and intravitreal administered medications.[57] If designed appropriately, implants have the potential to promote the sustained delivery of relatively steady therapeutic levels of drug to the site of action over long periods of time with only one implantation procedure. Moreover, a significantly lower amount of drug is required (due to reduction in clearance and protection of the unreleased dose), thereby reducing the associated potential risks of systemic administration and intravitreal injections.[57]
Intravitreal implants are classified as either non-biodegradable or biodegradable polymeric devices and are each capable to release drug molecules from a few months to several years depending upon the design.[21] Typically, non-biodegradable implants can be utilized to achieve a slower rate of release over a longer period of time than biodegradable implants, however, they require surgical removal once the loaded drug is exhausted.[57] A non-biodegradable implant containing fluocinolone acetonide (Retisert, Bausch & Lomb, Rochester, NY, United States) was the first to be approved by the FDA for the treatment of severe, non-infectious uveitis.[58] Vitrasert® (Bausch & Lomb, Rochester, NY, United States) is another example of a non-biodegradable implant. Specifically, Vitrasert® is the first implantable ganciclovir delivery system approved for the treatment of cytomegalovirus retinitis. Clinically used in the United States since 1996, Vitrasert® releases the drug over a period of eight months.[59] Overall, non-biodegradable implants have been demonstrated to be a valid alternative to intravitreal injections to obtain prolonged release of the therapeutic in the posterior segment with only one implantation procedure. However, despite the safety and efficacy demonstrated by non-biodegradable implants, surgical removal can lead to ocular complications.[57] Hence, biodegradable implants that ultimately do not need to be removed (and refilled and re-implanted or otherwise replaced when the drug is exhausted) would be a highly desirable alternative. Biodegradable implants are generally composed of biocompatible polymers that either degrade into non-toxic byproducts, or solubilize in vivo and can be eliminated safely by the human body, thus avoiding permanent chronic foreign-body reaction.[60] One of the most commonly utilized biodegradable polymers for controlled release formulations is poly lactic-co-glycolic acid (PLGA), which is FDA approved for a number of applications.[60–62] PLGA degrades into acidic byproducts such as lactic acid and glycolic acid, and although adverse reactions are generally mild to non-detectable, the context will dictate the importance of these effects.[63,64] Notably, the biocompatibility of PLGA has been investigated in ocular tissues and has shown to possess greater tolerability than when placed in non-ocular tissues, explaining why it is still one of the most widely utilized biodegradable polymers for controlled release today.[60]
One example of a biodegradable implant is represented by the bioerodible Ozurdex (Allergan Inc., Irvine, CA, United States), approved by the FDA for the treatment of uveitis and macular edema.[65] It consists in a PLGA matrix that releases dexamethasone for up to 4 four months.[65] Recently, the use of Ozurdex has been investigated as additional therapy in patients affected by AMD and refractive to ranibizumab.[66]The results of the study suggest the effectiveness of the dexamethasone-based implant in stabilizing vision, thus encouraging further investigation of the use of Oxurdex as a possible treatment for AMD.[66] Despite the advantage of requiring only one procedure to be implanted, biodegradable implants (like non-degradable implants) can still move from the original site of injection/implantation in the intraocular environment. Also, if not designed properly, a sudden increase of drug release may occur.[65] However, recent studies have shown how these matrices degrade, which can be correlated to initial conditions such as the polymer molecular weight distribution, polymer type, copolymer ratio, size, shape, and type of drug.[67–69] More so, these properties can be tuned to not only eliminate burst effect, but also to provide a customized release profile for practically any drug.[67–69] Overall, both non-biodegradable and biodegradable implants represent potential advantages and disadvantages, and represent a potential solution to the many limitations associated with traditional methods of administration of ophthalmic drugs.
2.3. Engineered drug delivery systems: microparticles and nanoparticles
New biodegradable polymeric carriers with convenient size/shape, such as microparticles with size in the range of 1–1000 μm and nanoparticles with size of less than 1 μm, represent a promising tool for ocular drug delivery.[70–74] In particular, micro- and nanoparticles enable the achievement of sustained intraocular therapeutic drug concentrations without requiring the surgical implantation of a drug delivery device (as they can be injected through a needle and syringe), offering a release of drug that can last for weeks or even months.[57,70,75] Particulates are most often administered intravitreally as a less invasive procedure compared to surgical implantation.[57] Moreover, these particular drug delivery systems can be engineered to target certain cells type, reducing the risks of systemic side effects.[57] Micro- and nanoparticles can be classified as “micro- and nanospheres”, and “ micro- and nanocapsules”.[76] In particular, in micro- and nanospheres, the drug and polymer are typically combined, and the drug is dispersed throughout the polymeric matrix.[76] In such a matrix system, the release of the active molecules is controlled by diffusion through the polymer matrix with simultaneous polymer degradation, which will non-linearly increase the diffusivity over time.[77] On the other hand, in micro-and nanocapsules, the drug particles or droplets are entrapped in a polymeric membrane.[76] Active molecules can be encapsulated in micro and nanocapsules via an emulsion-diffusion procedure (for example) while solvent evaporation techniques are used to fabricate drug-loaded micro and nanospheres (for example).[78,79]
Micro and nanoparticles can be formulated from a variety of polymeric materials. However the most commonly used synthetic polymers consist in aliphatic polyesters such as polycaprolactone (PCL) polylactic acid (PLA), polyglycolic acid (PGA), and PLGA, due to the advantages that characterized such polymers, as stated in the previous section.[80–82] As discussed in the prior section, the desired drug release profile can be engineered through varying the molecular weight of the polymer and copolymer formulation (as well as other formulation variables), allowing the tuning of the duration of release that can range from weeks to months.[83] One example of PLGA microspheres that are capable of providing one-month of release of an ophthalmic medication following subconjunctival injection has been recently developed.[84] Specifically, an in vitro study suggests that sustained release of the drug can be achieved with an amount of medication that is well above the lower limit of absorption for the entire period of the study.[84] Moreover, microspheres that were subconjunctivally injected in New Zealand white rabbits led to no observable foreign body response or infection over the course of one month.[84] Additionally, PLGA-based release systems have been studied as a promising candidate for the treatment of DED and uveitis, and they have been demonstrated a valid candidate for sustained release of therapeutics after a single administration through injection into ocular tissues.[85,86] In addition, a unique gelling, eye drop-like formulation has been recently reported that is able to comfortably retain the therapeutic drug in the lower fornix (topically) for a period of one month, while simultaneously releasing glaucoma medication over the period of time (without any injection into ocular tissues).[87] Although micro- and nanoparticles seem to possess significant potential as ocular drug delivery systems, limitations include encapsulation efficiency of drug (especially in smaller, nanoparticle formulations with high surface area), stability of the molecules during particle fabrication, control of particle size and drug release rate, and large-scale manufacturing of sterile preparations.[83]
3. Ocular Diseases
3.1. Dry eye Disease
3.1.1. Background of the Pathology/A Few Examples of Current Treatments of DED
Dry eye disease affects the tears and ocular surface, afflicting more than 10 million individuals in the United States alone.[88–92] Epidemiological studies suggest that aging and female sex are two of the most common risks factors for DED.[5] Several other risk factors for this particular ocular condition include autoimmune diseases (rheumatoid arthritis, Sjögren’s Syndrome), thyroid disease, hormonal changes, and refractive laser surgery.[2] Typically, patients with one or more of these risks factors will also experience symptoms such as ocular irritation, dryness, tear hyperosmolarity, and foreign body sensation.[93,94] In severe cases, DED can lead to the risk of developing infections and corneal ulcerations resulting in blindness.[6] Moreover, these symptoms can have a significant effect on the patients’ quality of life by affecting their visual ability to complete daily tasks (ex: reading or driving), which may lead to psychological side effects such as anxiety and depression.[94] Given the surprisingly serious nature of these side effects, a variety of methods has been explored in an attempt to mitigate these symptoms.
One common therapeutic strategy to help minimize the symptoms of dry eye is tear plugs (as described in section 2.1.3), [95] which preserve the health of the ocular surface by conserving tears.[95] Plugs (Figure 2) are classified by the location of insertion, which can include either the puncta or canaliculi (nasolacrimal drainage ducts) and plugs can be either permanently or temporarily inserted.[96] A factor that contributes to the intended duration of usage is the composition of the tear plug, which could be made of degradable collagen, gelatin, as well as non-degradable materials such as silicone, Teflon, and hydromethylacrylate.[95] Even though tear plugs are considered safe and have shown to be effective for maintaining ocular lubrication, some individuals experience complications associated with plug retention rates and infection.[95] It also has been demonstrated that closing the puncta exposes the ocular surface to high levels of pro-inflammatory cytokines in the tears, which can lead to exacerbated symptoms of DED.[96]
A common alternative to help lubricate the ocular surface for individuals with dry eye symptoms is the use of artificial tears[97]. As administered in eye-drop format, artificial tears can help to reduce the friction between the ocular surface and eyelids, providing relief for some (but not all) patients.[94] However, preservatives that are included in the formulation can result in hyperosmolarity of the tear film, leading to ocular surface inflammation.[94] One type of preservative known as benzalkonium chloride (BAK) has been speculated to cause hyperosmolarity of the tears, induce ocular irritation, lower cell viability, and induce oxidative stress on conjunctival epithelial cells in long-term treated dry eye patients.[98] Due to these potential side effects, new formulations have been developed that contain electrolyte-based artificial tear substitutes with a buffering component to help decrease the hyperosmolarity of the tears and aid to preserve the ocular surface.[99]
Ultimately, although artificial tears and punctal plugs have proven to lessen various symptoms of DED in some patients (such as ocular irritation and discomfort), they are not designed to address the underlying cause of the condition.[5] More recently, the inflammatory response has been identified to play a prominent role in the development and propagation of DED.[12,14,100–102] Specifically, inflammation leads to hyperosmolarity of the tear film and, ultimately, tissue destruction.[94] One of the primary mediators of ocular inflammation and tissue destruction are pathogenic effector T lymphocytes.[6] Generally, these lymphocytes are associated with chronic inflammation.[103] Adoptive transfer of pathogenic CD4+ T lymphocytes from mice that have induced DED into a nude mice develops DED in cell recipients.[104] Also, ocular inflammation is associated with increased expression of CCR5, which, in turn, results in the recruitment and infiltration of pathogenic effector T cells to the ocular tissue.[6,104–106] Building upon this evidence, current and new investigative therapeutic approaches have been developed to reduce ocular inflammation in order to restore the ocular microenvironment in DED (Table 1).[107–109]
Table 1.
Summary of Treatments for DED
| Treatment | Type of Study | Results | Ref. |
|---|---|---|---|
| Lipids | Murine | Topical administration of omega-3 fatty acids reduced corneal fluorescein staining and altered pro-inflammatory cytokine milieu in the ocular tissue. | [111] |
| LipiFlow | Clinical | Approved in 20011 by the FDA, LipiFlow is a medical device that uses vectored thermal pulsation to stimulate the release of meibum. | [113] [114] [115] [116] [117] |
| Corticosteroids | Murine | This class of steroid hormones can suppress molecular stress responses through reducing inflammation and resolving signs of DED. | [121] [122] |
| Doxycycline | Murine | PLGA-based microspheres loaded with doxycycline were able to modulate the effects (ex: corneal fluorescein staining) of DED. | [108] |
| Cyclosporine A (CsA) | Clinical | Restasis©; Allergan Inc, Irvine, California is a cyclosporine A ophthalmic emulsion used to treat patients with chronic DED. | [109] [124] |
| Contact Lenses | Rabbit | In order to overcome the low bioavailability of topically administered drugs to the ocular surface, contact lens (ex: silicone based and hyaluronic acid-laden ring implants) have been utilized to enhance drug residence time. | [28] [136] |
| CCR2 | Murine | Biological immune antagonists have shown to decrease mRNA expression levels of cytokines and reduce the infiltration of antigen-presenting cells to the ocular surface. | [125] |
| Lifitegrast | Murine and Clinical | An FDA approved integrin antagonist of LFA-1 demonstrated the ability to reduce ocular surface inflammation in a desiccating stress murine model and significantly improved ocular irritation in clinical trials. | [107] [139] [141] |
| Regulatory T cells | Murine | The ex vivo expansion of Tregs into a mouse with DED was able to resolve signs of inflammation. | [154] |
| Synthetic Approaches to Recruit Tregs | Murine | PLGA-based microspheres loaded with a chemokine, CCL22, was able to resolve signs of DED and shift the ratio of Tregs to effector T cells in the lacrimal gland tissue. | [85] |
3.1.2. Anti-Inflammatory Based Treatments for Dry eye Disease
Lipids and LipiFlow
One therapeutic strategy for DED is the administration of fatty acids such as omega-3s, which are known to reduce inflammation through the downstream effects on the NF-κB pathway.[110] Topical administration of omega-3 was explored in attempt to mitigate DED symptoms such as corneal fluorescein staining,[111] as an increase in corneal staining is an indicator of corneal disease severity.[112] Specifically, the fluorescein dye stains dead squamous epithelial cells and can diffuse into areas where cellular tight junctions have been compromised.[112] The results of the sample scoring suggest that the fluorescein staining was decreased in animals treated with fatty acids.[111] In addition to a reduction of corneal fluorescein staining, mRNA levels of pro-inflammatory cytokines in the cornea and conjunctiva (e.g. IL-1 and TNF-α) were lower in treated animals, suggesting that omega-3 fatty acids can alter the pro-inflammatory milieu and lessen the signs of dry eye.[91,93,102,113]
Other types of lipid-based treatment approaches have also been developed to mitigate the symptoms associated with the disease including a device known as LipiFlow (Figure 4).[114] This particular medical device uses a 12-minute vectored thermal pulsation (VTP) treatment that applies heat to the eyelid while also applying pressure to the outer eyelids to enable the release of meibum (oil like substance found in the tears).[113,115] A clinical trial revealed that LipiFlow was able to improve symptoms of ocular irritation, and subsequently in 2011, the FDA approved LipiFlow as a medical device.[116,117] Although the treatment is an effective therapy for some patients, it is still not widely available due to its high cost.[117] Hence, additional numerous topical cost-effective pharmaceutical agents are being screened as a potential therapy for DED.[118]
Figure 4.
Representation of the LipiFlow Disposable. Black arrows show the Eye Cup and Lid Warmer. Reproduced with permission.[114] Copyright 2012, Lippincott Williams & Wilkins.
Corticosteroids
Corticosteroids (glucocorticosteriods) are a class of steroid hormones widely exploited for a range of inflammatory and immune-based diseases.[119] A few inflammatory conditions treated with the administration of corticosteroids include: asthma, chronic obstructive pulmonary disease (COPD), uveitis, and age-related macular degeneration.[58,119,120] Corticosteroids have multiple methods of action to abate inflammation.[119] Classically, one prominent method of action is through the glucocorticoid receptor mediated pathways, which act to inhibit the synthesis of multiple inflammatory proteins thereby suppressing pro-inflammatory genes and lymphocyte activation.[119] Since inflammation and lymphocyte activation are recognized in diseases such as dry eye, others have examined whether glucocorticosteriods can resolve DED symptoms.[121,122] Several murine studies have suggested that the administration of corticosteroids can suppress molecular stress responses through lowering the levels of pro-inflammatory cytokines, and improving clinical signs of disease such as corneal fluorescein staining.[121,122] However, even though corticosteroids have exhibited to be efficacious for DED in short-term studies, there are many potential deleterious side effects associated with their long-term usage including cataracts, high blood pressure, increased risk of infection, and corticosteroid-induced glaucoma resulting from an increase of intraocular pressure (IOP).[123] Thus, in order to circumvent the potential long-term side effects associated with corticosteroid usage, other types of treatments have been examined as a therapy for patients with symptoms of dry eye.[124–126]
Doxycycline
Doxycycline is antibiotic classified as a tetracycline derivative used for a variety of conditions ranging from rosacea to cancer.[108,127] Mechanistically, doxycycline acts as a matrix metalloproteinase (MMP-proteolytic enzymes) inhibitor and [128] can suppress the expression of pro-inflammatory cytokines.[129] In DED, it has been observed that the upregulation of several MMPs can result in the breakdown of tight junction protein degradation and an increase of epithelial desquamation to the ocular surface.[108] Due to the effects of MMPs in DED, doxycycline was subconjunctivally administered in order to modulate the effects of these proteolytic enzymes.[108] Specifically, doxycycline-loaded polymer microspheres (made from PLGA), that controllably release the doxycycline over time, abated the effects of desiccating stress induced DED in a murine model.[108] Ultimately, this investigation suggests that doxycycline PLGA-based microspheres resolved corneal barrier disruption in mice as compared to the unloaded (no drug) microspheres.[108]
Cyclosporine A
Cyclosporine A (CsA) is an immunosuppressive agent utilized for several inflammatory conditions such as organ transplantation, rheumatoid arthritis, and uveitis.[130–133] CsA inhibits calcineurin, (a serine/threonine phosphatase), decreasing the expression of specific genes that are involved in T-cell activation and the production of interleukins (IL-2), which acts as a lymphocyte mitogen.[134] A recent clinical trial evaluated the use of topical CsA ophthalmic emulsion 0.05%, for the treatment of DED (Restasis©; Allergan Inc, Irvine, California).[109] One-hundred and fifty-eight subjects ranging in severity from mild, moderate and chronic DED were monitored for a period of 3–16 months, and by the end of the study, the administration of CsA appeared to be responsible for significant reduction in clinical symptoms of DED.[109] In addition, several dosages of the CsA ophthalmic emulsion were explored such as (0.05%, 0.1%, 0.2%, and 0.4%), with the most beneficial doses of CsA being 0.05% and 0.1%.[124] Notably, however, it can take several months for CsA to have a therapeutic effect in some patients.[135] Therefore, new treatments continue to be developed with the goal of achieving a more rapid onset of action and sustained delivery while simultaneously addressing the underlying inflammation mediating DED.[125,135]
Contact Lenses
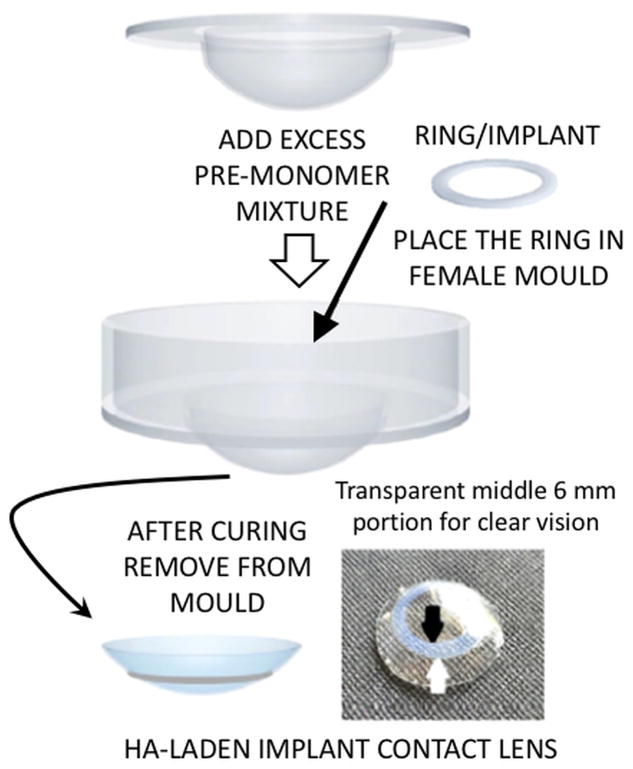
As an approach to overcome the low bioavailability of topically administered cyclosporine A, a silicone-based contact lens was investigated.[31] Specifically, the incorporation of vitamin E and cyclosporine A into a silicone-based contact lens appeared to enhance the release duration of the drug to more than 1-month with only utilizing 10% of vitamin E added into the lens.[31] However, the incorporation of vitamin E into the contact lens induced a minor alteration in the refractive index of the contact lens.[31] In an attempt to evade this issue, others have attempted to achieve sustained ophthalmic drug delivery without altering the optical properties of the contact lens with a new hyaluronic acid-laden ring-implant contact lens (Figure 5). The combination of the ring/implant (separation of drug to the outer rim of the lens leaving the central portion over the pupil unloaded) enabled the sustained delivery of the drug while maintaining ideal optical properties over the pupil for vision. [136] This delivery system showed hyaluronic acid (HA) was released in the therapeutic range for up to nine days, and the ocular healing was considerably faster in the rabbits treated with HA implanted contact lenses as compared to the untreated group.[136] The extended release of hyaluronic acid was accomplished through optimizing the amount of cross linker and the thickness of the implant.
Figure 5.
Image of a hyaluronic acid-laden implant contact lens fabricated to enable the sustained delivery of hyaluronic acid while maintaining ideal optical properties over the pupil for accurate vision. Reproduced with permission.[26] Copyright 2017, Elsevier.
3.1.3. Biological/Small Molecule Antagonist Therapies
CCR2
Immune antagonists/agonists (ex: chemokine, interleukin, and ICAM-1) are a biologically-oriented approach to halt effector T lymphocytes that can generate destructive inflammation.[107,125,137] One specific type of immune antagonist that has been analyzed as a potential treatment for DED is the chemokine receptor, CCR2 antagonist.[125] Topical administration of CCR2 antagonist can reduce mRNA expression levels of interleukins, IL-1α, IL-1β, and TNF-α in the cornea and conjunctiva, thereby affecting the pro-inflammatory microenvironment in the ocular tissue.[125] Furthermore, the CCR2 antagonist decreased the number of CD11b+ monocytes (type of antigen-presenting cell on the ocular surface) in the conjunctiva and cornea, which is important because antigen-presenting cells located in the cornea can significantly affect corneal disease pathogenesis.[88,125] Importantly, the lower levels of pro-inflammatory cytokine expression and cellular infiltrates in the ocular tissue contributed to a reduction of disease severity.[125] Despite these promising results, the administration of immunological antagonists may require additional investigation given the associated, serious side effects.[138] For example, treatment with anti-TNF-α therapy increases the patients’ chances of developing infections, congestive heart failure, and their overall rate of mortality.[138] Given this evidence, studies are needed to determine the side effects of administering a topical antagonist to chemokine receptors in order to determine whether this type of treatment has severe side effects similar to anti-TNF-α therapy.
Lifitegrast
Lifitegrast is an integrin antagonist (small molecule-”tetrahydroisoquinoline”) therapy that acts to block the binding of two cell surface proteins known as lymphocyte function-associated antigen (LFA-1) and intercellular adhesion molecule 1 (ICAM-1).[107] This interaction is essential to a number of T-cell interactions such as T-cell activation by antigen-presenting cells and strong adhesion to the endothelial cells during extravasation.[107,139] Due to the role of LFA-1 in T-cell function, an antagonist of LFA-1 was investigated for the treatment of DED.[139] In a desiccating stress murine model, a reduction of ocular surface inflammation was observed.[140] Furthermore, the drug was assessed in a clinical trial of 588 masked, randomized subjects who either were given a placebo (control) or received topically administered Lifitegrast (5.0%) (Twice a day) for a period of 84 days.[141] The subjects were evaluated at days 14, 42, and 84, and the primary measurement of efficacy was to observe a mean change from baseline inferior corneal staining score (ICSS). [141] The data revealed that Lifitegrast markedly reduced corneal fluorescein, and improved symptoms of ocular discomfort when compared to the placebo control group.[141] Lifitegrast ophthalmic solution is currently approved by the FDA and is commercially marketed as Xiidra© (Shire Pharmaceuticals, Lexington, MA, USA).[139]
3.1.4. Cell-Based Therapy
Regulatory T cells
As an alternative to blocking or suppressing T-cell mediated inflammation, it may be possible to take advantage of a natural mechanism the body uses to regulate inflammation.[142] In the healthy steady state, our bodies regulate inflammation through directing the migration of lymphocytes to areas of inflammation in order to resolve tissue damage and ultimately promote immune regulation.[143] Within the classification of lymphocytes is a subset population of immunosuppressive lymphocytes known as regulatory T cells (Tregs), which are utilized by the body to control pathogenic effector T cells, regulating the destructive inflammation that can lead to tissue damage.[144–148] Disruption in the function, development or number of Tregs can lead to autoimmune and inflammatory diseases.[149,150] Moreover, it is now understood that an immunological balance of effector T cells and Tregs between the two populations is critical to maintain a healthy microenvironment.[149] Overall, Tregs are naturally tuned to regulate the proliferation of pathogenic effector T cells, and maintain immunological homeostasis in the ocular tissue.[151]
Accordingly, Treg-based cell therapies have been explored (the ex vivo differentiation/expansion and re-implantation of live cells) for the treatment of diseases such as DED.[152,153] It also has been suggested that regulatory T cells (Tregs) could be harvested from peripheral blood, expanded ex vivo and injected back into the patient in order to boost circulating Treg numbers thereby reducing/resolving the destructive inflammation.[152] Such would represent a biologically oriented “drug” that is multi-modal, dynamic, and responsive in the local environment and capable of communicating to the immunological milieu. Siemasko et al. demonstrated that the ex vivo expansion of Tregs injected into a mouse with DED were able to suppress ocular surface inflammation.[154] Although adoptive transfer of Treg represents tremendous promise (with potential to be more effective than any “drug” while eliminating severe side effects), there are still several issues with the clinical translation of ex vivo expanded Tregs.[152] For instance, expanding sufficient numbers of Tregs can be challenging, and current good manufacturing practices and FDA criteria need to be maintained during ex vivo culture to ensure that contamination does not occur.[152] Likewise, the plasticity of Tregs causes regulatory concerns, given that some Tregs may differentiate into effector T cells in situ.[152] Also, differentiation into effector T cells in situ can lead to an increase of abnormally high levels of IL-2, which can result in vascular leakage syndrome, a life threating condition.[152,155] Collectively, there are still many hurdles to ensure safety and efficacy before being implemented as a clinical therapy.[152]
3.1.5. Synthetic approaches to recruitment of endogenous Tregs
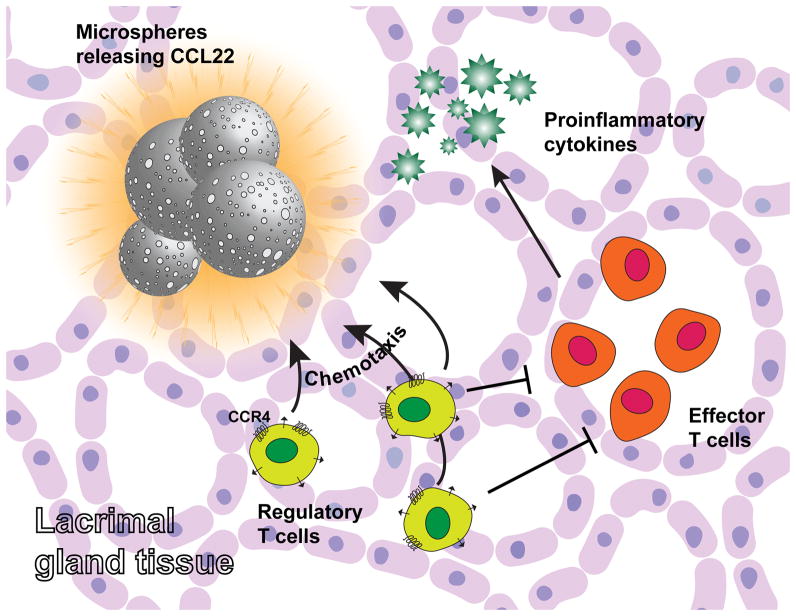
Recent studies have suggested that it may be possible to recruit the body’s own repertoire of Tregs (5–15%), without the need for ex vivo cell therapy.[156] This approach employs controlled release technology based on biodegradable polymers (PLGA mentioned briefly earlier in this article), which has been utilized in a number of FDA approved drug delivery applications[62,157]. These controlled release formulations have been shown to sustain a biological gradient of the chemokine, CCL22, effectively recruiting regulatory T cells (which preferentially express the CCR4 ligand for this chemokine) to the site of implantation of the controlled release system.[143,158] Local delivery (or delivery from a point source to establish a gradient) appears to be important as bolus administration of the chemokine was proven to be ineffective.[158,159] This endogenous Treg-recruiting treatment also demonstrated to effectively attract Tregs in a model of periodontitis, resolving inflammation and dramatically reducing symptoms.[143,158] Interestingly, there are similarities between the pathology of periodontitis and DED, as both diseases are characterized by a pro-inflammatory environment the can lead to local tissue destruction.[5,158] It was also recently hypothesized that such formulations could recruit Treg to the lacrimal gland and prevent inflammation associated with DED. These endogenous Treg-recruiting formulations were indeed shown to be capable of shifting the ratio of Tregs and CD4+ IFN-γ+ cells in the lacrimal gland (Figure 6).[85] In addition, the local administration of Treg-recruiting microspheres prevented the symptoms of DED such as aqueous tear production, goblet cell density and corneal fluorescein staining.[85] Ultimately, this evidence suggests that recruitment of endogenous Treg can prevent the signs and underlying inflammation associated with dry eye.
Figure 6.
Synthesis strategy to recruit Tregs and shift T effectors and Treg balance for the prevention of dry eye disease.
3.2. Age-Related Macular Degeneration
3.2.1. Pathology of AMD
Age-Related Macular Degeneration (AMD) is the leading cause of blindness in the elderly population with an average estimated Medicare cost of 724 million dollars in the United States alone.[160,161] The disease affects the central areas of the macula region of the retina, composed of light sensing cells that enable central vision.[162] When the central area of the macula is impacted, retinal pigment cells begin to slowly degenerate leading to blurry central vision and metamorphopsia (a type central visual distortion).[162] Although, most vision loss occurs in the advanced stages of the disease, the early onset can be characterized by the presence of drusens (hard/soft yellow deposits formed from acellular debris under the retina)and/or retinal pigmentary abnormalities (Figure 7).[8,120] As the disease progresses this can lead to a chronic inflammatory response, resulting in the formation of retinal atrophy (also known as “geographic atrophy”), and/or the secretion of angiogenic cytokines (ex: vascular endothelial growth factor-VEGF). Ultimately, these pathological features have been classified into two distinct, advanced clinical classification stages.[120]
Figure 7.
Characteristic features associated with the pathology of Age-Related Macular Degeneration. (A) Intermediate state of AMD with drusen (B) Loss of retinal pigment epithelial cells and choroidal vessels. (C) Neovascular AMD with retinal hemorrhage. Reproduced with permission.[160] Copyright 2009, Elsevier, The Lancet.
The two advanced stages of the disease are characterized as either dry/non-neovascular AMD or wet/neovascular AMD (Figure 7).[120] Dry/non-neovascular AMD causes slow degradation of vision due to the loss of photoreceptors and development of geographic atrophy.[120] On the other hand, wet/neovascular AMD is characterized by choroidal neovascularization, leading to sub retinal fluid, retinal pigment epithelium detachment, and formation of fibrotic scars (Figure 7).[8,161] Typically, these clinical signs can be diagnosed during examination using fluorescent angiography (fluorescein highlights leaky vessels), which is a useful diagnostic tool to identify choroidal neovascularization, and optical coherence tomography (OCT) to detect thinning of the macula tissue.[120] Upon diagnosis, preventative therapies such as PreserVision (a vitamin and mineral supplement), may be prescribed to abate the risk of advanced stage AMD and the associated vision loss. [163]However, this therapy may not be useful for all patients. For instance, the use of supplements such as beta-carotene can increase the risk of lung cancer in smokers.[120] In addition, high doses of vitamin E can increase the risk of heart failure in patients with diabetes and heart disease. [120]
Due to the potential side effects, studies have examined the pathogenesis of the disease in order to develop new effective therapies.[164–166] New studies of AMD progression suggest that disease is associated with higher levels of biomarkers that are indicative of inflammation.[7] It is currently thought that the activation of the innate immune system, upregulation of complement factors, and the secretion of chemokines and cytokines lead to ocular tissue damage in AMD.[7,11] Although the full pathogenesis has not been elucidated, current (and experimental) treatments have attempted to address the local inflammation in order to decrease the progression of vision loss (Table 2).[11,162,167]
Table 2.
Summary of Treatments for Age-Related Macular Degeneration
| Treatment | Type of Study | Results | Ref. |
|---|---|---|---|
| Rapamycin | Preclinical-Rat | The oral administration of rapamycin was able to lessen abnormalities of the retinal tissue observed in ocular histological sections. | [168] |
| Doxycycline | Murine | Lower expression levels of M-2 type macrophages markers such as Arg1 and reduced neovascularization were detected with the administration of doxycycline. | [170] |
| HIF-Antagonist | Murine | The hypoxia-inducible factor (HIF-1) antagonist has shown to reduce levels of pro-angiogenic factors in choroidal neovascularization and may serve as a treatment for wet AMD. | [172] |
| Anti-VEGF | Clinical | Ranibizumab (Lucentis) and Bevacizumab (Avastin) are both VEGF-A monoclonal antibodies, which have demonstrated clinical efficacy as a therapy for wet AMD. Although, this treatment may lead to hemorrhage and cataract formation. | [162] [175] |
| Gene Therapy | Murine | Preclinical and phase I human trails demonstrated that an adenoviral vector expressing pigment epithelium-derived factor (PEDF) lessened choroidal neovascularization. | [178] [179] |
| Complement Inhibition | In Phase II clinical trials, Lampalizumab (a humanized monoclonal antibody fragment) has shown to inhibit a component of the complement immune system thereby reducing geographic atrophy observed in AMD. | [180] [181] |
|
| IL-18 | Murine/Primate | Administration of IL-18 reduced choroidal neovascularization in non-human primates. | [182] |
| Human Embryonic Stem Cells (hESCs) | Rodent/Clinical | Transplanted hESCs in the subretinal space of rodents was able to maintain visual function. In addition, to assess safety of transplanted hESC-derived RPE in humans, a clinical trial was performed. The subjects did not have any adverse effects from the stem cells. | [184] [185] |
| Induced Pluripotent Stem Cells (iPSCs) | Human | An iPSC trial completed in Japan demonstrated that the stem-cells were able to prevent the loss of vision in a woman with AMD. Although, the genetic mutations were observed in the cells of the other trial subject and thus the trial was halted. | [187] |
| Retinal Progenitor Cells (RPCs) | Murine | A scaffold composed of poly (lactic) acid and poly (lactic-co-glycolic) acid seeded with RPCs was able to enhance survival of RPCs. Additionally, a polycaprolactone scaffold was utilized to seed stem cells. | [187] [189] [190] |
3.2.2. Anti-Inflammatory Therapy Based Treatments for Age-Related Macular Degeneration
Immunosuppressive Agent: Rapamycin
Rapamycin (Sirolimus) is an immunosuppressive treatment utilized for a several conditions, such as organ transplantation and ocular inflammatory diseases.[11,109,130] Rapamycin inhibits a downstream target known as mTOR (mammalian target of rapamycin) that is needed for upregulation of IL-2 production, which sustains T cell activation and proliferation.[130] The mTOR pathway has also been linked to effects on cellular aging; therefore, mTOR inhibitors, such as rapamycin, prevent the conversion of quiescence to senescence, which has revealed to slow down aging in mice.[168] Slowing down the aging process with rapamycin may also be relevant to the progression of age-related diseases such as AMD.[168] Kolosova et al. demonstrated rapamycin could affect retinopathy in senescence-accelerated AMD rat model by reducing histological abnormalities of the ocular retinal tissue.[168] Overall, pre-clinical evidence suggest rapamycin did not cause any adverse side-effects when administered orally and may have a potential advantage due to its low renal toxicity.[130]
Doxycycline
Doxycycline (as described in section 3.1.2.3) has also exhibited anti-inflammatory and anti-angiogenic properties, making it a potential candidate for the treatment of AMD.[169] He et al. hypothesized that inhibiting the polarization of a subset of pro-angiogenic immune cells, M2 type macrophages, with doxycycline could lead to lower expression levels of pro-angiogenic cytokines and thereby diminish neovascularization.[170] To test this hypothesis, mice were injected intraperitoneally with doxycycline one day prior to exposing them to laser photocoagulation (to cause choroidal neovascularization injury) and, thereafter, doxycycline was injected daily until the conclusion of the study.[170] With the administration of doxycycline, there was a significant reduction in the expression of the M2-type macrophage markers such as Arg1 and subsequent neovascularization.[170] Furthermore, doxycycline can inhibit choroidal neovascularization in other experimental pre-clinical models.[169] Even though, pre-clinical studies demonstrate doxycycline had a significant effect on neovascularization, there are other types of anti-angiogenic treatments that do not require daily systemic administration. [52,171]
3.2.3. Anti-Angiogenic Treatments for AMD
Sustained Delivery of a HIF-Antagonist
Pro-angiogenic factors can cause disease progression of AMD, and specific promoters for genes encoding these pro-angiogenic factors have been identified.[172] These promoters possess a hypoxia response element, and they are activated by the hypoxia-inducible factor-1 (HIF-1).[173] Consequently, a possible strategy to block pro-angiogenic factors is to develop inhibitors of HIF-1, since it is involved in the upregulation of many pro-angiogenic factors.[172] In particular, doxorubicin (DXR) has been demonstrated to be a potent inhibitors of HIF-1-mediated gene transcription by blocking the binding of HIF-1 on DNA.[172] For instance, it has been demonstrated that DXR released from polymeric particles was able to significantly reduce the levels of different pro-angiogenic factors (VEGF-A, PDGF-BB, and SDF-1) in an established pre-clinical model of choroidal neovascularization.[172] Accordingly, these results demonstrate the ability of DXR to suppress HIF-1, representing a promising approach that may be effectively applied as a treatment for AMD.
Anti-VEGF Therapy
The pro-angiogenic vascular endothelial growth factor A (VEGF-A) plays a role in disease propagation.[52] To directly hinder the effects of VEGF-A, new anti-VEGF treatments have been developed, such as Ranibizumab (Lucentis) (a recombinant monoclonal antibody), which promises significant improvement in visual acuity and reduced angiographic lesions after a two-year clinical follow-up of a multicenter clinical trial.[52,174] Ophthalmologists originally began treating neovascular AMD off-label with bevacizumab (Avastin), another VEGF-A monoclonal antibody originally developed as a treatment for advanced colon or rectal cancer, and costs less than Ranibizumab.[175] Although bevacizumab was being used off-label, there was an absence of clinical-trial data supporting its use for AMD. Therefore, the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) compared the efficacy and safety of bevacizumab to ranibizumab. The results indicated both drugs possessed similar efficacy concerning visual acuity.[175] Despite the clinical efficacy of anti-VEGF therapies for AMD, these medications can increase the risk of thromboembolic events, and intravitreal injections have been associated with several risks including cataract formation, bacterial endophthalmitis, hemorrhage, and retinal detachment.[162] Moreover, many patients required frequent injections (sometimes every six weeks) for a prolonged period of time to prevent vision loss.[167] In order to avoid these side effects, gene therapies for AMD have been explored as ways to enable effective suppression of the VEGF pathway.[167,176]
Gene Therapy for AMD
A different therapeutic strategy that could resolve the issue of the short half-life of protein-based treatments may be the use of viral vectors to deliver sustained transgene expression of anti-angiogenic factors.[177,178] Specifically, approaches using an adenoviral vector expressing pigment epithelium-derived factor (PEDF) to counteract the effects of VEGF have been evaluated in pre-clinical (ex: primate) and phase I human trials.[177,179] Evidence from these investigations reported lessened choroidal neovascularization and no significant adverse events or dose-limiting toxicities were observed.[178] In spite of this evidence, there are still concerns surrounding the possible side effects of gene therapy. In particular, viral vectors can induce T-cell responses against the expressed transgene products, and recent evidence has also demonstrated that the usage of viral vectors can result in mutagenesis, ultimately leading to cancer.[176] Overall, more investigation is warranted for gene- based therapies.
3.2.4. Complement Inhibition
An underlying factor that is linked to the development of AMD is activation (or deregulation) of the complement system.[7,180] Activation of complement pathways leads to a membrane attack complex (MAC), which can result in cell lysis, the release of chemokines and increase of capillary permeability.[180] A member of the chymotrypsin family of serine proteases known as complement factor D (CFD) is an enzyme involved in regulating the alternative complement pathway.[164] Moreover, some of the factors that influence the alternative complement pathway include genetic variations associated with CFD gene single nucleotide polymorphisms (SNP) and AMD.[164] Due to the association between AMD and genes encoding aspects of the complement system, new AMD therapies have been investigated to block components of the complement system. Specifically, Lampalizumab (Anti-Factor D), a humanized monoclonal antibody fragment administered intravitreously acts to inhibit CFD involved in the amplification of the alternative pathway.[164] In a Phase II study, there was a reduction of disease progression in patients treated with anti-factor D.[181] As Phase III clinical trials have begun, evaluations will be required to determine whether the immunogenicity of these types of antibody-based therapeutics can cause any undesirable immunological responses potentially impacting drug efficacy.
3.2.5. IL-18 Therapy
Drusens contribute to the activation of an inflammatory response through NLRP3 inflammasomes.[166] When stimulated by a damage signal, NLRP3 forms an inflammasome, which leads to the activation and secretion of IL-1β and IL-18.[166] Interestingly, studies on IL-1receptor knockout mice demonstrated that IL-1 did not have a significant effect on the progression of AMD (choroidal neovascularization). While on the other hand, injecting IL-18–neutralizing antibodies resulted in a significant increase of choroidal neovascularization development. This suggests that IL-18 might prevent the formation of vascularization.[166] Building upon this evidence, tolerability and efficacy of IL-18 was explored in a mouse and non-human primate model of AMD.[182] Notably, the (Figure 8), suggesting that IL-18 could prevent the choroidal neovascularization in AMD.[182] Ultimately, the administration of IL-18 reduced the pathology associated with AMD in both murine and non-human primate models, suggesting that this new type of immune-therapy may be able to prevent AMD progression.[182]
Figure 8.
(A) Representative images of fundus fluorescein angiography show a reduction of fluorescein stained lesions in the treatment (IL-18) group (B) The amount of fluorescein lesions were significantly decreased in the IL-18 group suggesting that the immunotherapy, IL-18, can prevent choroidal neovascularization. Modifications/Reproduced with permission.[180] Copyright 2015, Investigative Ophthalmology and Visual Science.
3.2.6. Cellular-Based Therapies
Human Embryonic Stem Cells (hESCs)
New stem cell-based treatments are being investigated to regenerate the retinal pigment epithelial cells that are destroyed in AMD.[183] For example, the use of human embryonic stem cell-derived retinal pigment epithelial cells (hESC-derived RPE) preserved visual function and ensured the health of the photoreceptors in a rodent model.[183] Moreover, the administration of hESCs did not result in the formation of a teratoma (tumor) in the sub-retinal area of transplantation, and ultimately, the long-term data suggested that hESCs did not result in adverse pathological reactions. [183]
In addition to a long-term pre-clinical rodent test, two prospective phase I/II clinical studies were designed to examine the medium- and long-term safety of human embryonic stem cells (hESCs) transplanted into patients.[184] Primary endpoints of safety were assessed concerning the sub-retinal transplantation of hESC-derived RPE in AMD subjects that received three different cell doses and were followed for 22 months.[184] The evidence collected in this trial indicated that patients did not suffer from any adverse rejection, nor from any systemic effect from the transplanted cells.[184] However, even though no serious adverse effects were observed, there are still concerns associated with the use of embryonic stem cells, because they have been known to form teratomas in some pre-clinical models.[185] Furthermore, use of hESC-derived RPE cells is ethically and politically controversial since the stem cells originate from human embryos.[185]
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) derived from retinal pigment epithelia cells were proposed as an alternative to hESCs as they bypass some of the associated ethical concerns. Although iPSCs have progressed from pre-clinical to clinical trials,[186,187] there are still concerns about their potential immune rejection.[186] The promise of iPSC therapy and potential concerns were both highlighted by a recent clinical study carried out in Japan.[186] In this trial, iPSCs were transplanted into a woman with AMD, and resulted in improved prevention of vision loss.[186] However, the stem-cell trial was halted after genetic mutations that can potentially carry the risk of cancer, were discovered in the cells of the second trial participant.[186] Overall, this clinical trial demonstrated that additional investigation is required to examine the potential immunogenicity, possibility of genetic mutations leading to cancer, and likely requirement of immunosuppressive drugs before iPSCs therapy is implemented as a safe clinical treatment.
Retinal Progenitor Cells (RPCs)
Retinal progenitor cells possess the ability to differentiate into unique types of retinal cells such as photoreceptors, and may be utilized as a cellular-based therapy for the treatment of AMD.[188] However, delivering living cells into an unorganized and inflamed ocular microenvironment could affect cell survival. For this reason, new tissue-engineering approaches (such as scaffolds) can potentially provide a unique micro-environmental to enable cells to differentiate and organize into functional layers to repair damaged tissue.[189] For instance, porous, biodegradable scaffolds composed of a combination of poly (L-lactic acid) and PLGA were fabricated, and subsequently RPCs were seeded on the scaffold and cultured (Figure 9).[188] An in vivo study was performed on rats using the polymer scaffolds seeded with RPCs, which demonstrated that the implantation of the seeded scaffold enabled enhanced survival of the RPCs.[188] In addition, another study explored a 3-D thin-film, polycaprolactone-based scaffold seeded with retinal progenitor cells to treat AMD.[190] The cells were able to stay in close contact with one another, the porosity allowed for diffusion of nutrients, and provided an environment for the cells to adhere. [190] Overall, three-dimensional polymer-based scaffolds are a new, promising approach to provide an environment that enhances therapeutic cell survival, proliferation, and differentiation.
Figure 9.
SEM micrographs of PLGA-based scaffolds fabricated using a phase-inversion technique. (A) Representative image of the water-exposed side. (B) Representative image of the glass side. (C) Representative image of the cross section. Reproduced with permission.[188] Copyright 2004, Elsevier.
3.3. Uveitis
3.3.1. Background of the Pathology
Uveitis is a term used to refer to various inflammatory conditions of the eye, and is often associated with irreversible ocular damage, visual impairment or blindness, and with consequent reduction in the quality of life.[191] Uveitis is estimate to causes 10% of visual loss in the United States each year, and up to 25% of cases in the developing countries.[192,193] Approximately 70–90% of patients aged between 20–60 years, which represents the age range where individuals are most productive from an economical point of view, are most affected by uveitis. In particular, when vision is lower than 20/40, the ability of a person to accomplish tasks in her/his productive years is impaired.[194] This leads to a significant encumbrance to the US economy, with cost estimated to be around 242.6 million dollars each year.[15]
Uveitis typically starts in the uveal tract (ciliary body, iris and choroids), but it can also affect other structures including vitreous humor, retina, vessels and optic nerve.[195] The disease can be of either infectious or non-infectious nature.[196] Specifically, infectious uveitis is the most common form, representing approximately 15–20% of all cases in the United States.[197] It is initiated through an immune response directed against exogenous pathogens such as viruses, fungi, parasites, and bacteria.[198] Infectious uveitis can affect different parts of the eye, leading to either anterior or posterior uveitis.[197] However the most devastating cases are those causing posterior involvement such as acute retinal necrosis due to herpes viruses or toxoplasmosis retinochoroiditis.[197]
Conversely, non-infectious uveitis is often autoimmune-oriented, and is associated with systemic pathologies (for example sarcoidosis, Vogt-Koyanagi-Harada syndrome, Behçet’s disease), or local conditions such as punctate inner chorioretinopathy, birdshot chorioretinopathy, multifocal choroiditis, and serpiginous chorioretinopathy.[199] Non-infectious uveitis is the result of an abnormal response of the immune system to retinal soluble antigens (S-Ag) or interphotoreceptor retinoid-binding protein (IRBP). Such response leads to a non-infectious inflammation of the eye, which is mediated by T-cells and propagated by pro-inflammatory cytokines.[13,200,201] In particular, during natural development, T-cells migrate from the bone marrow to the thymus, where they differentiate and “learn” how to recognize self-antigens that make up our own tissues. However, thymic education is not always effective, and inadequate elimination from the thymus of effector T-cell precursors that are able to recognize antigens may lead to circulating, non-tolerized T-cells in healthy individuals.[202] Moreover, when non-tolerized T-cells become activated when exposed to retinal or cross-reactive antigens these cells can differentiate into pathogenic effector T-cells, which can ultimately migrate to the eye. Consequently, this can result in a cascade of inflammatory events initiated by the recognition of ocular antigen by these T-cells, ultimately resulting in the breakdown of the blood-retinal barrier and the recruitment of leukocytes from circulation, which leads to the ocular inflammation observed in uveitis.[199,202,203]
In order to better elucidate the pathophysiology of non-infectious uveitis and develop new therapies, preclinical models of experimental autoimmune uveitis (EAU) have been investigated.[4,204,205] The most common EAU models utilize mice and rats by actively immunizing them with retinal antigens (S-Ag or IRBP), which are recognized by lymphocytes of uveitis patients.[206] Some of the characteristics of EAU in animals are retinal vasculitis, photoreceptor damage, retinal and/or choroidal inflammation, and loss of vision function, thus reproducing the main clinical-pathological features of human uveitis.[206] Different stages of the EAU model are shown in Figure 10. In particular, mice immunized using IRPB are characterized by a decrease in retinal inflammation severity over time, while chronic inflammation persists for more than 120 days post immunization (Figure 10I).[207] Moreover, optic disk images have confirmed inflammation characterized by retinal edema and vasculitis (Figure 10B) with presence of active and old lesions in the chronic stage of EAU (Figure 10E and G).[207] Overall, EAU models have been revealed as a valid tool toward a better understanding of uveitis, thus helping the development of current and new therapeutic strategies for managing the associated inflammation (Table 3).
Figure 10.
Images showing retinal inflammation characterizing EAU in mice at different time periods after immunization using IRPB. (A) Non-immunized mouse retina. (B) Mouse fundus (25 days post immunization) characterized by severe optic disk inflammation and vasculitis (white arrows). (C) Mouse fundus (60 days post immunization) characterized by retinal atrophy, vascular sheathing (white arrows), and small retinal infiltrates. (D) Mouse fundus (80 days post immunization) characterized by inferior vitreous infiltrates (asterisks) and vascular sheathing. (E) Mouse fundus (80 post immunization) characterized by multiple infiltrates. The blue arrow indicates an area of gliosis or scar. (F) Mouse fundus (90 days post immunization) characterized by vascular sheathing (white arrow) and multiple retinal infiltrates (white arrowheads). (G) Mouse fundus (120 days post immunization) characterized by large scars. (H) Mouse fundus (120 days post immunization) characterized by pigment deposition. (I) The retinal inflammation in the images was quantified with a clinical score and grouped according to the time period after immunization. Reproduced with permission.[207] Copyright 2012, American Society for Investigative Pathology.
Table 3.
Treatment approaches for uveitis
| Treatment | Type of study | Results | Ref. |
|---|---|---|---|
| Corticosteroids | Human | Topical corticosteroids are effective in controlling inflammation in anterior uveitis. Periocular or intraocular injections or oral administration are required for treating posterior uveitis. | [191] [195] |
| Methotrexate | Human | This folic acid analog has demonstrated to be effective in controlling inflammation. | [211] |
| Mycophenolate mofetil | Human | This prodrug of mycophenolic acid has shown to control intraocular inflammation, and improve or stabilize visual acuity. Mycophenolate mofetil is well tolerated by patients with low recurrence of uveitis. | [217] [219] |
| Cyclosporine | Human | Cyclosporine is effective in controlling inflammation with sustained effects even after the reduction of corticosteroids. In a retrospective cohort study, cyclosporine has been demonstrated to control inflammation at six months and one year. | [131] [221] [222] |
| Tacrolimus | Human | Tacrolimus is an antibiotic impairing T-cell activity and cytokine production. The drug has demonstrated to possess a more favorable safety profile than cyclosporine. | [226] [227] [228] [229] |
| Anti-TNF-α | Human | Infliximab (Remicade, Janssen biotech Inc., Titusville, NJ) is a monoclonal antibody antagonist of TNF-α. It has been shown to effectively suppress occurrence of uveitis attacks. | [233] [237] |
| Anti-IL-2 - Daclizumab | Human | A phase I/II single armed interventional study has provided preliminary evidence that regular infusions of daclizumab can be administered for years as an alternative to standard immunosuppressive drugs. | [248] |
| Anti-IL-17A | Human | Secukinumab (Novartis Pharma AG, Basel, Switzerland) is a monoclonal antibody antagonist of IL-17A. Phase III studies have shown secukinumab has beneficial effects for patients with non-infectious uveitis. | [254] |
| Anti-CD28 - Abatacept | Human | Abatacept (Bristol-Myers Squibb, New York, NY, United States) is a treatment for JIA-related uveitis. Several studies have supported its efficacy in controlling JIA-uveitis in children and young adults. | [256] [257] [258] |
| Retisert | Human | Retisert (Baush and lomb, Rochester, NY, United States) is an FDA approved non-biodegradable implant containing fluocinolone acetonide. A double blind, prospective case series has demonstrated an improvement or stabilization of visual acuity, with no recurrence of ocular inflammation. | [262] [263] |
| Cyclosporine-releasing microparticles | Animal model - Rabbits | Cyclosporine-loaded microparticles have shown sustained concentration of cyclosporine in choroid-retina and iris-ciliary body for at least 65 days after intravitreous injection in a rabbit model. | [87] |
| Gene therapy Ad-IL-10 | Animal model - Rats | Systemic administration of Ad-IL-10 in rats has shown to reduce leukocyte infiltration and subsequently decrease inflammation in the anterior chamber. | [269] |
3.3.2. Anti-Inflammatory Based Treatments for Uveitis
Corticosteroids
Corticosteroids are the primary anti-inflammatory therapy utilized for the treatment of non-infectious uveitis.[15,208] Corticosteroids have different methods of action to manage the inflammation, as discussed in section 3.1.2.2. [209] As therapeutic strategy for uveitis, corticosteroids can be administered systemically, such as oral prednisone or intravenous methylprednisolone sodium succinate, or topically in the form of injections.[15] The choice of the most appropriate route of administration of corticosteroid strongly depends on the site and activity of uveitis. In particular, topical administration of corticosteroids is effective in treating anterior uveitis, but the drug does not typically penetrate adequately to the posterior segment.[191] For this reason, topical corticosteroids may not be an ideal effective treatment for posterior uveitis, which often requires periocular or intraocular procedures[191] or oral administration of corticosteroids.[195] Accordingly, long-term administration can lead to many side effects including hypertension, diabetes, cataract, and glaucoma.[210] To reduce corticosteroid dose and associated side effects, immunosuppressive agents such as methotrexate, mycophenolate mofetil, cyclosporine, or tacrolimus are administered as steroid-sparing agents.[210]
Methotrexate
Methotrexate is an analog of folic acid that irreversibly binds and inactivates dihydrofolate reductase, resulting in the inhibition of rapidly dividing cells such as lymphocytes.[15,210] Methotrexate was first introduced in 1948 as an antineoplastic agent, and subsequently found to have anti-inflammatory effects.[211] The FDA approved the use of methotrexate as a treatment of rheumatoid arthritis in 1988, becoming the standard antirheumatic drug.[211,212] Moreover, methotrexate is a commonly used immunosuppressive agent for the treatment of ocular inflammation, and it can be administered orally, parenterally, or by intraocular injection.[15,212] In particular, in uveitis patients methotrexate has demonstrated to be effective for controlling inflammation and for achieving corticosteroid-sparing.[211] Even if several months may be required for therapeutic success, methotrexate is generally well tolerated by most patients, and it seems to have little risks of serious side effects.[212]
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is a pharmacologically inactive drug (prodrug) that, after administration, is metabolized to its active form, the mycophenolic acid.[213] MMF suppresses the immune system by inhibiting inosine-5-monophosphate dehydrogenase, thus selectively halting
T and B lymphocyte replication.[214] It is currently used as a treatment for organ transplant rejection and for several autoimmune diseases.[15] The efficacy of MMF therapy has been demonstrated in the treatment of posterior segment intraocular inflammation even when cyclosporine and tacrolimus were not effective. Moreover, MMF inhibits the development of EAU,[215] and its use in the treatment of uveitis is well documented.[216–218] In particular, MMF is effective both in combination with steroids or another immunomodulatory treatments, and also as monotherapy.[15] MMF is generally well tolerated by patients, with a low recurrence of the pathology after discontinuation of the therapy, as demonstrated in a retrospective study of 60 uveitis patients.[217] In addition, MMF can be used as a safe and well tolerated immunosuppressant for the treatment of uveitis in children, with the possibility to decrease the dose of systemic steroids required to control inflammation.[219]
Cyclosporine
Cyclosporine is often topically used for the treatment of immune-mediated ocular pathologies involving activation of T-cells, as mentioned in section 3.2.2.4.[220] As a treatment for patients with uveitis, cyclosporine is effective in controlling inflammation, and its effects are sustained even after the reduction of corticosteroid dosage.[131,221] For example, a retrospective cohort study on 373 patients demonstrated clinically acceptable control over inflammation at 6 months and 1 year for 33.4% and 51.9% of patients, respectively.[222] Despite the efficacy in managing the inflammation, cyclosporine can lead to severe nephrotoxicity,[223,224] and in addition, some patients can be refractory to treatment.[225]
Tacrolimus
Tacrolimus is an antibiotic that also impairs T-cell activity and cytokine production via inhibition of the calcineurin enzyme.[226] Tacrolimus was initially approved as a systemic immunosuppressant for liver transplantation, and currently has a broad range of usage.[226] For instance, tacrolimus is a treatment of choice in uveitis patients refractory to cyclosporine either because of lack of therapeutic effect or undesirable side effects.[225] Additionally, even though tacrolimus and cyclosporine can have similar efficacy for posterior and intermediate uveitis, tacrolimus therapy has exhibited a more favorable safety profile.[227] In the treatment of uveitis, tacrolimus has been demonstrated to be effective over time.[228] Studies have also shown that corticosteroids can be withdrawn in patients treated with tacrolimus.[229]
3.3.3. Biologic therapeutic approaches
Anti-TNF-α
Anti-TNF-α was identified as a potential candidate for the treatment of patients affected by uveitis, who either did not show an improvement in disease symptoms or did not tolerate traditional immunomodulatory therapies.[209,230] TNF-α is a pro-inflammatory cytokine, which has been implicated in a number of immune-mediated pathologies, including intraocular tissue damage associated with uveitis.[231] Specifically, TNF-α recruits leukocyte to the eye in the initial phase of uveitis and favors the adhesion of leukocytes to the vascular endothelium. TNF-α is also a crucial factor in the dendritic cell maturation, macrophages activation, activation of effector function of infiltrating T cells, as well as in the apoptosis of resident ocular cells.[231] Moreover, it has been indicated that intraocular levels and expression of TNF-α are high during the course of EAU, and systemic neutralization of TNF-α has a suppressive effect on the severity and incidence of EAU.[231,232] Since TNF-α plays an integral role in the propagation of EAU, the use of biological therapies to block the action of TNF-α has been investigated. One example of such is Infliximab (Remicade, Janssen Biotech Inc., Titusville, NJ), a monoclonal antibody acting against TNF-α.[233] Its efficacy has been extensively studied as a treatment for many different diseases, including spondiloarthritis [234], ulcerative colitis [235], and sarcoidosis, [236] and its use has been also explored for the treatment of uveitis. Intravenous administration of Infliximab results in a half-life of 9.5 days, however the drug is usually given every 4–8 weeks in the maintenance phase of treatment, since the biological effects extend beyond its serum half-life.[231]
Several studies have reported the efficacy of Infliximab for the treatment of non-infectious uveitis. For example, the effects of infliximab on the occurrence of uveitis attacks and on visual prognosis were investigated in patients affected by uveitis resulting from Behçet’s disease. Moreover, patients involved in the trial did not have any therapeutic effect with the combination therapies of azathioprine, corticosteroids, and cyclosporine.[237] The results from this trial suggest that Infliximab can effectively suppress the occurrence of uveitis attacks. Moreover, Infliximab has a corticosteroid-sparing effect, and positive consequences for the visual prognosis were observed.[237] However, the beneficial effects of visual acuity are not necessarily preserved over time.[237] Moreover, in another study, the efficacy of low-dose (<10 mg/kg), moderate-dose (≥10–15 mg/kg), and high-dose (≥15–20 mg/kg) of Infliximab for the treatment of uveitis was compared.[238] Although the administration of infliximab had beneficial effects in treating uveitis, an increase in dose up to four times above the approved dosage was often necessary to control the disease. In addition, the study highlighted that doses <10 mg/kg administered every four weeks may not be sufficient.[238] Overall, the high dose administration of Infliximab has not caused concern of serious side effects, suggesting it is a relatively safe treatment approach for uveitis.
Anti-IL-2
IL-2 is a cytokine regulating lymphocyte homeostasis and function. Studies in EAU models suggest that IL-2 is one of the predominant cytokines produced in the early phases of the disease.[239] One example of anti-IL-2 therapy is represented by daclizumab, a humanized blocking monoclonal antibody acting against an epitope of the alpha subunit of the IL-2 receptor (CD25), which is located on activated T-cells and other immune cells.[240] Daclizumab has been used in patients experiencing acute reaction episodes following organ transplantation, including heart,[241,242] pancreas,[243] liver,[244] and lung.[245] The use of daclizumab for the treatment of intraocular inflammation, including uveitis,[246] has been investigated in a multicenter, noncomparative, interventional case series. In this study, daclizumab was subcutaneously administered to investigate the possibility of whether the drug could safely reduce the need of standard systemic corticosteroids or other immunosuppressive treatments in patients with non-infectious uveitis.[247] Induction treatment with subcutaneous daclizumab at 2 mg/kg followed by 1 mg/kg maintenance treatments every other week was determined to be safe. In addition, the administration of intravenous daclizumab for the treatment of non-infectious uveitis was explored in a long-term, phase I/II single armed interventional study.[248] This study provided preliminary evidence that regularly administered infusions of daclizumab can be given as an alternative to standard immunosuppressive therapies for years to treat severe uveitis.
Anti-IL-17A
T-helper 17 (Th17) cells are a sub-set of pro-inflammatory T helper cells and one of the main pathogenic effectors in autoimmune uveitis. Specifically, Th17 cells produce the pro-inflammatory cytokine IL-17A and other effector cytokines, including IL-17F and IL-22.[249] The upregulation of IL-17A in patients with uveitis has led to experimental treatments specific to this target.[250] For instance, secukinumab (Novartis Pharma AG, Basel, Switzerland) is a selective, high-affinity and fully-human monoclonal antibody that binds to human IL-17A. This binding is thought to inhibit the expression of other pro-inflammatory cytokines and effector proteins, thus preventing the downstream activation of neutrophil granulocytes, macrophages, epithelial cells, and fibroblasts.[251] Secukinumab blocks inflammation in patients affected by psoriasis, rheumatoid arthritis, and uveitis.[252] Intravenous dosing of secukinumab has shown greater efficacy than subcutaneous dosing in patients with non-infectious uveitis, suggesting that patients may not receive a sufficient amount of drug with subcutaneous administration.[253] Moreover, three multicenter, randomized, double-masked, phase III studies in the United States have examined the efficacy and safety of different doses of Secukinumab in patients with non-infectious uveitis.[254] Although the study suggested that secukinumab administration resulted in a beneficial effect and allowed for reduction of the use of concomitant immunosuppressive medication, the authors did not discover any dissimilarities in uveitis recurrence between placebo groups and secukinumab treatment groups.[254] On these bases, further research may be needed to assess the efficacy of secukinumab in managing non-infectious uveitis in patients who are refractory to routine immunosuppressive treatments.
Anti-CD28 - Abatacept
Abatacept (Bristol-Myers Squibb, New York, NY, United States) is a fusion protein composed of the Fc region of the immunoglobulin IgG1 and the extracellular domain of cytotoxic T-lymphocyte antigen 4.[255] T-cell activation involves both the T-cell receptor and a co-stimulatory signal provided through the binding of CD28 on the T-cell to the B7 protein on an antigen-presenting cell such as a dendritic cell. Specifically, abatacept acts by binding to the B7 protein, thus preventing co-stimulatory signaling, and ultimately leading to impedance of T cell activation. Abatacept is currently an approved treatment for juvenile idiopathic arthritis (JIA) - related uveitis and rheumatoid arthritis, and can be administered either as an intravenous infusion or subcutaneous injection.[256,257] Several studies support the efficacy of abatacept in controlling or improving JIA-uveitis in children and young adults. Particularly, a study carried out on seven patients affected by JIA-related uveitis and refractory or intolerant to immunosuppressive demonstrated an improvement in all patients, although only one patient had complete remission over a follow-up period of 7–11 months.[258] In addition, the therapy was well tolerated in six of the seven patients. Another small study performed on two patients showed that the use of abatacept may result in complete remission of uveitis after several months of treatment.[259] However, both studies involved a small sample size (seven and two respectively), and, for this reason, a larger series of studies and a longer term follow-up may be required to confirm the efficacy and cost effectiveness of this therapy.
3.3.4. Engineering Approaches to Treat Uveitis
Fluocinolone acetonide implants - Retisert
Retisert (Bausch & Lomb, Rochester, NY, United States), is a non-biodegradable implant containing a synthetic corticosteroid, fluocinolone acetonide, and designed for a long-term, local release of the therapeutic agent (Figure 11). The pharmacokinetics of fluocinolone acetonide was initially tested in vivo[260,261] in 24 rabbits receiving implants of either 0.5 mg or 2 mg.[260] After the first month a constant release of the active principle was observed for a period of 12 months, with minimal systemic absorption as demonstrated by urine and plasma concentration below the detection limits.[260] Similar results were obtained in another study where the release rate was constant over the one-year testing period.[261] The effectiveness of the fluocinolone acetonide implant (releasing approximately 2 μg/day) was investigated in a pilot study composed of five patients with severe posterior uveitis.[262] After a ten month follow-up, visual acuity was either improved or stabilized, and inflammation was under control in all patients treating with the implant.[262] Successively, in a double-blind study patients were randomized to receive fluocinolone acetonide intravitreal implants of 0.59 mg (0.6 μg/day) or 2.1 mg (2 μg/day) for 58 months.[263] The outcome of the results of the study showed an improvement in mean visual acuity with no recurrences of ocular inflammation during the first two years after implantation.[263] In 2005, Retisert was approved by the FDA for the treatment of chronic, non-infectious, posterior uveitis.[264,265] Retisert is an ocular the implant composed of a silicone elastomer containing 0.59 mg fluocinolone acetonide, which is surgically implanted in the posterior segment. Over the first month following implantation, the device delivers the medication at a rate of 0.6 μg/day, followed by a continuous delivery of 0.3–0.4 μg/day for 30 months. The drug delivery rate depends on different factors such as surface area, permeability of polymers, drug solubility, and rate of drug clearance.
Figure 11.
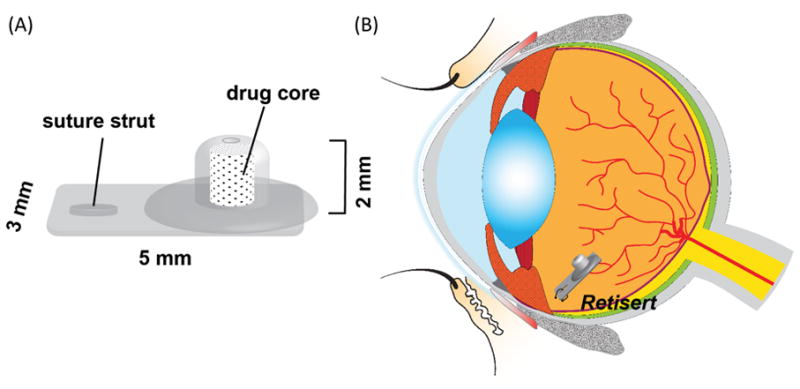
(A) Schematic and (B) site of implantation of Retisert.
Cyclosporine-releasing microparticles
As discussed previously, cyclosporine has been proven to be effective in controlling inflammation in patients with uveitis. However, there are limitations (low bioavailability and systemic side effects) associated with the commonly used formulations of cyclosporine (topically, systemically administered).[266,267] To overcome these limitations, microparticles containing cyclosporine have been under investigation as an alternative system for delivering the drug for a prolonged period of time, thus achieving a prolonged drug action with reduced side effects (Figure 12A).[86,266] In particular, it has been demonstrated that PLGA-based microparticles containing cyclosporine allow for a sustained concentration of the drug in the iris-ciliary body and choroid-retina of healthy rabbits for at least 65 days after injection (Figure 12B).[86] Moreover, the mean residence time of the drug loaded in the microparticles was ten times higher than cyclosporine solution.[86] Similar results have been obtained using cyclosporine-loaded lipid microspheres, capable of a prolonged release of the medication with cyclosporine concentration much higher than in the traditional ocular emulsion.[268] These results suggest that patients affected by uveitis could potentially benefit from the use of sustained-release drug formulations, representing a way to localized and deliver the drug more efficiently than topical or systemic administration.
Figure 12.
Scanning electron microscopy images (SEM) of PLGA microparticles loaded with cyclosporine (A) SEM image of microparticles before the in vitro release assay (B) SEM image after two weeks of in vitro release (C) SEM image taken after two months of in vitro release (10 μm scale bar). (D) Diagram showing the concentration of cyclosporine released overtime in different ocular tissues and blood subsequently after the cyclosporine microparticles are intravitreally injected for the treatment of uveitis. (×) Iris-ciliary body; (□) cornea; (△) conjunctiva; (○) aqueous humor; (◇) blood; (□) vitreous body; (●) choroid-retina; (◆) sclera; (▲) lens. Reproduced with permission.[86]Copyright 2006, Association for Research in Vision and Ophthalmology.
3.3.5. Gene therapy for Uveitis
Although gene therapy of retinal degeneration has been under investigation in diseases such as AMD, it is also a new approach for uveitis, and only few studies on animal models have been attempted.[209] For example, an adenoviral-mediated transfer of the interleukin (IL)-10 gene for the inhibition of autoimmune anterior uveitis was investigated using a rat model.[269] Specifically, the adenoviral construct expressing IL-10 (Ad-IL-10) or carrying no cytokine transgene was systemically administered to the rats. The results from the study suggested that rats receiving one or two divided administrations of Ad-IL-10 had a reduction of leukocyte infiltration in the anterior chamber of the eye. In another experimental study, a lentiviral vector was developed for the delivery of genes encoding murine IL-1Ra (mIL-1Ra) and murine IL-10 to the anterior chamber, in order to determine whether it could affect the inflammatory response.[270] A significant reduction in the severity of experimental uveitis was demonstrated, suggesting that the utilization of lentiviral-mediated expression of immunomodulatory could be promising as a potential, future treatment for the anterior chamber of the eye.
4. Conclusion
Ophthalmic drug delivery has undergone substantial transformation, with treatment strategies now being created that specifically address the underlying disease mechanisms. Prior to their application to ophthalmic pathologies, antibiotics (ex: doxycycline) and immunosuppressant agents (ex: rapamycin) were employed for a variety of conditions ranging from rosacea to organ transplantation. These drugs have now been repurposed for additional types of diseases that involve inflammation, which include DED, AMD and Uveitis. Newer approaches include targeted biologics, controlled drug delivery systems that modulate the immunological homeostasis, and gene therapies. A key element of each of these new methods is local delivery to the ocular tissue to halt the subsequent effects of inflammation and ultimately shift the ocular microenvironment towards a homeostatic milieu. Although, these modern drug delivery systems may benefit patients that do not respond well to current conventional therapies, the existing regulatory guidelines make it extremely difficult to facilitate clinical translational of these complex therapeutic modalities. While the regulations concerning traditional pharmaceuticals are well established and require straightforward approaches that measure purity and bioactivity, new modern drug delivery therapies (ex: gene therapies) are far more complex from a regulatory perspective, requiring evaluation of multiple factors with unpredictable downstream effects. Yet new technology is consistently being developed that is more specific, leading to better safety and efficacy. For example, a new targeted gene-editing technology is CRISPR-Cas9 (gene-based technology), which could be a powerful future treatment of ocular diseases since it has demonstrated promise in a preclinical wet AMD model.[271] Furthermore, as little as picograms-to-nanograms of active agent are required in new local delivery systems that could induce the body’s own cells to treat diseases such as DED.[85] In addition, modern ophthalmic therapeutic approaches are becoming more interdisciplinary, combining biologicals/small molecules/cells with engineered polymeric materials in order to create drug delivery systems that even mimic the body’s natural functions. As the understanding of these disease mechanisms has evolved, the body’s natural process of restoring homeostasis may serve as an important inspiration for the development of safer, targeted ocular drug delivery therapies.
Acknowledgments
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000145, the Wallace H. Coulter Foundation (Translational Research Award to SRL), the Camille and Henry Dreyfus Foundation (Camille Dreyfus Teacher Scholar Award to SRL), NIH-R01EY024039, P30 EY008098, and the Ri.MED Foundation (to RG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Michelle L. Ratay, Department of Bioengineering, University of Pittsburgh, 427 Benedum Hall 3700 O’Hara Street Pittsburgh, Pa 15261.
Elena Bellotti, Department of Chemical Engineering, University of Pittsburgh, 427 Benedum Hall 3700 O’Hara Street Pittsburgh, Pa 15261.
Riccardo Gottardi, Department of Chemical Engineering, Department of Orthopedic Surgery, Ri.MED Foundation, 427 Benedum Hall 3700 O’Hara Street Pittsburgh, Pa 15261.
Steven R. Little, Department of Chemical Engineering, Department of Bioengineering, Department of Ophthalmology, Department of Immunology, Department of Pharmaceutical Sciences, The McGowan Institute for Regenerative Medicine, 940 Benedum Hall 3700 O’Hara Street Pittsburgh Pa 15261.
References
- 1.Gower NJD, Barry RJ, Edmunds MR, Titcomb LC, Denniston AK. BMC Ophthalmol. 2016;16:11. doi: 10.1186/s12886-016-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman NJ. Curr Opin Ophthalmol. 2010;21:310. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JR, Stempel AJ, Bharadwaj A, Appukuttan B. Clin Transl Immunol. 2016;5:e63. doi: 10.1038/cti.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessen M, Akpek EK. J Ophthalmic Vis Res. 2014;9:240. [PMC free article] [PubMed] [Google Scholar]
- 6.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Invest Ophthalmol Vis Sci. 2009;50:3802. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Cell Mol Life Sci. 2016;73:1765. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman HR, Chan C, FLF, Chew EY. Lancet. 2009;372:1835. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemp GN, Foulks Michael A. Ocul Surf. 2007:75. Dry Eye Wo. [Google Scholar]
- 10.Kulkarni P. J Ocul Pharmacol Ther. 2001;17:181. doi: 10.1089/10807680151125537. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wang VM, Chan C-C. Eye. 2011;25:127. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. Adv Ther. 2000;17:84. doi: 10.1007/BF02854841. [DOI] [PubMed] [Google Scholar]
- 13.Barry RJ, Nguyen QD, Wlee R, Imurray P, Denniston AK. Clin Ophthalmol. 2014;8:1891. doi: 10.2147/OPTH.S47778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Yi, et al. Eye Contact Lens. 2014;40:248. doi: 10.1097/ICL.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson T, Nussenblatt RB, Sen HN. Expert Opin Emerg Drugs. 2011;16:309. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fangueiro JF, Veiga F, Silva AM, Souto EB. Curr Pharm Des. 2016;22:1135. doi: 10.2174/1381612822666151216145900. [DOI] [PubMed] [Google Scholar]
- 17.Urtti A. Adv Drug Deliv Rev. 2006;58:1131. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. J Ocul Pharmacol Ther. 2013;29:106. doi: 10.1089/jop.2012.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molokhia SA, Thomas SC, Garff KJ, Mandell KJ, Wirostko BM. J Ocul Pharmacol Ther. 2013;29:92. doi: 10.1089/jop.2012.0241. [DOI] [PubMed] [Google Scholar]
- 20.Baranowski P, Karolewicz B, Gajda M, Pluta J. Sci World J. 2014;2014 doi: 10.1155/2014/861904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuno N, Fujii S. Polymers (Basel) 2011;3:193. [Google Scholar]
- 22.Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. J Ocul Pharmacol Ther. 2013;29:106. doi: 10.1089/jop.2012.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng CC, Kim J, Chauhan A. Biomaterials. 2010;31:4032. doi: 10.1016/j.biomaterials.2010.01.113. [DOI] [PubMed] [Google Scholar]
- 24.Gaudana R, Ananthula HK, Parenky A, Mitra AK. 2010;12:348. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jünemann AGM, Chorągiewicz T, Ozimek M, Grieb P, Rejdak R. Ophthalmol J. 2016;1:29. [Google Scholar]
- 26.Xu Q, Kambhampati S, Kannan R. Middle East Afr J Ophthalmol. 2013;20:26. doi: 10.4103/0974-9233.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicine Nanotechnology, Biol Med. 2009;5:394. doi: 10.1016/j.nano.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Guzman-Aranguez A, Fonseca B, Carracedo G, Martin-Gil A, Martinez-Aguila A, Pintor J. Eye Contact Lens Sci Clin Pract. 2015;42:1. doi: 10.1097/ICL.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Conway A, Chauhan A. Biomaterials. 2008;29:2259. doi: 10.1016/j.biomaterials.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Maulvi FA, Soni TG, Shah DO. Drug Deliv. 2016;7544:1. doi: 10.3109/10717544.2016.1138342. [DOI] [PubMed] [Google Scholar]
- 31.Peng CC, Chauhan A. J Control Release. 2011;154:267. doi: 10.1016/j.jconrel.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Jung HJ, Abou-Jaoude M, Carbia BE, Plummer C, Chauhan A. J Control Release. 2013;165:82. doi: 10.1016/j.jconrel.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Schultz CL, Poling TR, Mint JO. Clin Exp Optom. 2009;92:343. doi: 10.1111/j.1444-0938.2009.00370.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Yang Y, Lei M, Ye C, Zhao C, Xu J, Wu K, Yu M. Drug Deliv. 2016;23:3538. doi: 10.1080/10717544.2016.1204570. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Peng CC, Chauhan A. J Control Release. 2010;148:110. doi: 10.1016/j.jconrel.2010.07.119. [DOI] [PubMed] [Google Scholar]
- 36.Gulsen D, Anuj C. IOVS. 2004;45:2342. doi: 10.1167/iovs.03-0959. [DOI] [PubMed] [Google Scholar]
- 37.Kompella UB, Kadam RS, Lee VH. Ther Deliv. 2010;1:435. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta C, Chauhan A. J Control Release. 2011;150:70. doi: 10.1016/j.jconrel.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Yellepeddi VK, Sheshala R, McMillan H, Gujral C, Jones D, Raghu Raj Singh T. Drug Discov Today. 2015;20:884. doi: 10.1016/j.drudis.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Gooch N, Molokhia SA, Condie R, Burr RM, Archer B, Ambati BK, Wirostko B. Pharmaceutics. 2012;4:197. doi: 10.3390/pharmaceutics4010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kompella UB, Kadam RS, Lee VHL. Ther Deliv. 2010;1:435. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boddu SH, Gupta H, Patel S. Recent Pat Drug Deliv Formul. 2014;8:27. doi: 10.2174/1872211308666140130093301. [DOI] [PubMed] [Google Scholar]
- 43.Schopf LR, Popov AM, Enlow EM, Bourassa JL, Ong WZ, Nowak P, Chen H. Transl Vis Sci Technol. 2015;4:11. doi: 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison PW, Khutoryanskiy VV. Ther Deliv. 2014;5:1297. doi: 10.4155/tde.14.75. [DOI] [PubMed] [Google Scholar]
- 45.Cholkar K, Patel A, Vadlapudi AD, Mitra AK. Recent Pat Nanomed. 2014;2:82. doi: 10.2174/1877912311202020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YC, Chiang B, Wu X, Prausnitz MR. J Control Release. 2014;190:172. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha-Vaz J, Marques FB, Fernandes R, Alves C, Velpandian T. 2016:37. [Google Scholar]
- 48.Lee J, Pelis RM. Drug Metab Dispos. 2016;44:1675. doi: 10.1124/dmd.116.069369. [DOI] [PubMed] [Google Scholar]
- 49.Kompella UB, Amrite AC, Pacha Ravi R, Durazo SA. Prog Retin Eye Res. 2013;36:172. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro A. Retin Today. 2016:1. [Google Scholar]
- 51.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM, Wong WT. Invest Ophthalmol Vis Sci. 2010;51:5403. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY MARINA Study Group. N Engl J Med. 2006;355:1419. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 53.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Nat Rev Drug Discov. 2006;5:123. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Hou H, Nan K, Sailor MJ, Freeman WR, Cheng L. Exp Eye Res. 2014;129:74. doi: 10.1016/j.exer.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Zhang X, Li J, Wang Y, Chen Q, Hou C, Garrett Q. Investig Ophthalmol Vis Sci. 2013;54:6287. doi: 10.1167/iovs.13-12081. [DOI] [PubMed] [Google Scholar]
- 56.Shah SS, Denham LV, Elison JR, Bhattacharjee PS, Clement C, Huq T, Hill JM. Expert rev Ophthalmol. 2010;5:75. doi: 10.1586/eop.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.del Amo EM, Urtti A. Drug Discov Today. 2008;13:135. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Hunter RS, Lobo A-M. Clin Ophthalmol. 2011;5:1613. doi: 10.2147/OPTH.S17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyer DS, Posalski J. Am J Ophthalmol. 1999;127:349. doi: 10.1016/s0002-9394(98)00176-7. [DOI] [PubMed] [Google Scholar]
- 60.Lee SS, Hughes P, Ross AD, Robinson MR. Pharm Res. 2010;27:2043. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 61.Bertram JP, Jay SM, Hynes SR, Robinson R, Criscione JM, Lavik EB. Acta Biomater. 2009;5:2860. doi: 10.1016/j.actbio.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya AP, Lewis JS, Keselowsky BG. Biomaterials. 2013;34:3422. doi: 10.1016/j.biomaterials.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumari A, Yadav SK, Yadav SC. Colloids Surfaces B Biointerfaces. 2010;75:1. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Makadia HK, Siegel SJ. Polymers (Basel) 2011;3:1377. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee DJ. J Funct Biomater. 2015;6:650. doi: 10.3390/jfb6030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calvo P, Ferreras A, Al Adel F, Wang Y, Brent MH. Br J Ophthalmol. 2014:1. doi: 10.1136/bjophthalmol-2014-305684. [DOI] [PubMed] [Google Scholar]
- 67.Rothstein SN, Federspiel WJ, Little SR. J Mater Chem. 2008;18:1873. [Google Scholar]
- 68.Rothstein SN, Federspiel WJ, Little SR. Biomaterials. 2009;30:1657. doi: 10.1016/j.biomaterials.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothstein SN, Little SR. J Mater Chem. 2011;21:29. [Google Scholar]
- 70.Moshfeghi AA, Peyman GA. Adv Drug Deliv Rev. 2005;57:2047. doi: 10.1016/j.addr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Ramazani F, Hiemstra C, Steendam R, Kazazi-Hyseni F, Van Nostrum CF, Storm G, Kiessling F, Lammers T, Hennink WE, Kok RJ. Eur J Pharm Biopharm. 2015;95:368. doi: 10.1016/j.ejpb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Elsaid N, Jackson TL, Elsaid Z, Alqathama A, Somavarapu S. Mol Pharm. 2016;13:2923. doi: 10.1021/acs.molpharmaceut.6b00335. [DOI] [PubMed] [Google Scholar]
- 73.Kamaleddin MA. Nanomedicine Nanotechnology, Biol Med. 2017:0. [Google Scholar]
- 74.Salama HA, Ghorab M, Mahmoud AA, Abdel Hady M. AAPS PharmSciTech. 2017 doi: 10.1208/s12249-017-0710-8. [DOI] [PubMed] [Google Scholar]
- 75.Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang JC, Tang L, Heflin T, Alwadani S, Eberhart CG, Stark WJ, Hanes J. J Control Release. 2015;201:32. doi: 10.1016/j.jconrel.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Short BG. Toxicol Pathol. 2008;36:49. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 77.Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Prog Retin Eye Res. 2004;23:253. doi: 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Moinard-Chécot D, Chevalier Y, Briançon S, Beney L, Fessi H. J Colloid Interface Sci. 2008;317:458. doi: 10.1016/j.jcis.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 79.Wischke C, Schwendeman SP. Int J Pharm. 2008;364:298. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 80.Andrés-Guerrero V, Zong M, Ramsay E, Rojas B, Sarkhel S, Gallego B, De Hoz R, Ramirez AI, Salazar JJ, Trivino A, Ramirez JM, Del Amo EM, Cameron N, De-Las-Heras B, Urtti A, Mihov G, Dias A, Herrero-Vanrell R. J Control Release. 2015;211:105. doi: 10.1016/j.jconrel.2015.05.279. [DOI] [PubMed] [Google Scholar]
- 81.Peters T, Kim S-W, Castro V, Stingl K, Strasser T, Bolz S, Schraermeyer U, Mihov G, Zong M, Andres-Guerrero V, Herrero Vanrell R, Dias AA, Cameron NR, Zrenner E. Biomaterials. 2017;124:157. doi: 10.1016/j.biomaterials.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Chen YS, Green CR, Wang K, Danesh-Meyer HV, Rupenthal ID. Eur J Pharm Biopharm. 2014;95:378. doi: 10.1016/j.ejpb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 83.Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug Discov Today. 2011;16:270. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Fedorchak MV, Conner IP, Medina CA, Wingard JB, Schuman JS, Little SR. Exp Eye Res. 2014;125:210. doi: 10.1016/j.exer.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ratay ML, Glowacki AJ, Balmert SC, Acharya AP, Polat J, Andrews LP, Fedorchak MV, Schuman JS, Vignali DAA, Little SR. J Control Release. 2017 doi: 10.1016/j.jconrel.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y, Liu Y, Liu Y, Wang J, Zhang X, Lu W, Ma Z, Zhu X, Zhang Q. Investig Ophthalmol Vis Sci. 2006;47:3983. doi: 10.1167/iovs.05-1373. [DOI] [PubMed] [Google Scholar]
- 87.Fedorchak MV, Conner IP, Cugini A, Schuman JS, Little SR. Sci Rep. 2017 doi: 10.1038/s41598-017-09379-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stevenson W, Chauhan SK, Dana R. Arch Ophthamology. 2012;130:90. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolando M, Zierhut M. Surv Ophthalmol. 2001;45(Suppl 2):S203. doi: 10.1016/s0039-6257(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 90.Brewitt H, Sistani F. Surv Ophthalmol. 2001;45:199. doi: 10.1016/s0039-6257(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 91.Gayton JL. Clin Ophthalmol. 2009;3:405. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu J, Asche CV, Fairchild CJ. Cornea. 2011;30:379. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 93.Lemp MA. Am J Ophthalmol. 2008;146:350. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 94.Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M. Ocul Surf. 2013;11:246. doi: 10.1016/j.jtos.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Tai M-C, Cosar CB, Cohen EJ, Rapuano CJ, Laibson PR. Cornea. 2002;21:135. doi: 10.1097/00003226-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Jehangir N, Bever G, Mahmood SMJ, Moshirfar M. J Ophthalmol. 2016;2016 doi: 10.1155/2016/9312340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tong L, Beuerman R, Simonyi S, Hollander DA, Stern ME. Ocul Surf. 2016;14:233. doi: 10.1016/j.jtos.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Clouzeau C, Godefroy D, Riancho L, Rostène W, Baudouin C, Brignole-Baudouin F. Mol Vis. 2012;18:851. [PMC free article] [PubMed] [Google Scholar]
- 99.Pflugfelder SC, Solomon A, Stern ME, Ph D. Cornea. 2000;19:644. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 100.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. Exp Eye Res. 2004;78:409. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mol Ther. 2014:1. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Mucosal Immunol. 2010;3:425. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stern ME, Schaumburg CS, Pflugfelder SC. Int Rev Immunol. 2013;32:19. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, Siemasko K. J Immunol. 2006;176:3950. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 105.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. J Immunol. 2009;182:1247. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Mucosal Immunol. 2014;7:38. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Ocul Surf. 2016;14 doi: 10.1016/j.jtos.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Chang E, Mcclellan AJ, Farley WJ, Li D, Stephen C. J Clin Exp Ophthamology. 2013;2:1. [Google Scholar]
- 109.Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, Savage HE. Arch Ophthalmol. 2008;126:1046. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 110.Lim A, Wenk MR, Tong L. Trends Mol Med. 2015;21:1. doi: 10.1016/j.molmed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg Da, Dana MR. Arch Ophthalmol. 2008;126:219. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 112.Bron AJ, Argueso P, Irkec M, Bright FV. Prog Retin Eye Res. 2015;44:36. doi: 10.1016/j.preteyeres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 113.Thode AR, Latkany RA. Drugs. 2015;75:1177. doi: 10.1007/s40265-015-0432-8. [DOI] [PubMed] [Google Scholar]
- 114.Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, Holland EJ, Lemp Ma, McDonald JE, Silbert DI, Blackie Ca, Stevens Ca, Bedi R. Cornea. 2012;31:396. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 115.Blackie CA, Coleman CA, Holland EJ. Clin Ophthalmol. 2016;10:1385. doi: 10.2147/OPTH.S109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Y, Veerappan A, Yeo S, Rooney DM, Acharya RU, Tan JH, Tong L. Eye Contact Lens Sci Clin Pract. 2016;0:1. doi: 10.1097/ICL.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kabat AG, Sowka JW. Rev Optom. 2010:109. [Google Scholar]
- 118.Perry DE, Henry D, Donnenfeld Curr Opin Ophthamology. 2004;15:299. doi: 10.1097/00055735-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 119.Barnes PJ. Eur J Pharmacol. 2006;533:2. doi: 10.1016/j.ejphar.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 120.Jager RD, Mieler WF, Miller JW. N Engl J Med. 2008;358:2606. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Z, Yang WZ, Zhu ZZ, Hu QQ, Chen YF, He H, Chen YX, Liu ZG. Investig Ophthalmol Vis Sci. 2014;55:2963. doi: 10.1167/iovs.13-13577. [DOI] [PubMed] [Google Scholar]
- 122.De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Exp Eye Res. 2006;83:526. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 123.Kersey JP, Broadway DC. Eye. 2006;20:407. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 124.Stevenson D, Tauber J, Reis BL. Ophthamology. 2000;107:967. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 125.Goyal S, Chauhan SK, Zhang Q, Dana R. Arch Ophthalmol. 2009;127:882. doi: 10.1001/archophthalmol.2009.125. [DOI] [PubMed] [Google Scholar]
- 126.Kang H, Cha KH, Cho W, Park J, Park HJ, Sun BK, Hyun SM, Hwang SJ. Int J Nanomedicine. 2016;11:2921. doi: 10.2147/IJN.S107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duivenvoorden WCM, Seidlitz E, Hirte HW, Tozer RG, Singh G. Cancer Res. 2002;62:1588. [PubMed] [Google Scholar]
- 128.Stechmiller J, Cowan L, Schultz G. Biol Res Nurs. 2010;11:336. doi: 10.1177/1099800409346333. [DOI] [PubMed] [Google Scholar]
- 129.Li D-Q, Chen Z, Song XJ, Luo L, Pflugfelder SC. Invest Ophthalmol Vis Sci. 2004;45:4302. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 130.Fisher JD, Acharya AP, Little SR. Clin Immunol. 2015;160:24. doi: 10.1016/j.clim.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee SH, Chung H, Yu HG. Korean J Ophthalmol. 2012;26:21. doi: 10.3341/kjo.2012.26.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moore CP, McHugh JB, Thorne JG, Phillips TE. Investig Ophthalmol Vis Sci. 2001;42:653. [PubMed] [Google Scholar]
- 133.Kitahara K, Kawai S. Curr Opin Rheumatol. 2007;19:238. doi: 10.1097/BOR.0b013e328099af80. [DOI] [PubMed] [Google Scholar]
- 134.Roberts CW, Carniglia PE, Brazzo BG. Cornea. 2007;26:805. doi: 10.1097/ICO.0b013e318074e460. [DOI] [PubMed] [Google Scholar]
- 135.Okanobo A, Chauhan SK, Dastjerdi MH, Kodati S, Dana R. Am J Ophthalmol. 2012;154:63. doi: 10.1016/j.ajo.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maulvi FA, Shaikh AA, Lakdawala DH, Desai AR, Pandya MM, Singhania SS, Vaidya RJ, Ranch KM, Vyas BA, Shah DO. Acta Biomater. 2017:1. doi: 10.1016/j.actbio.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 137.Dinarello CA, van der Meer JWM. Semin Immunol. 2013;25:469. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Antoni C, Braun J. Clin Exp Rheumatol. 2002 [PubMed] [Google Scholar]
- 139.Pflugfelder SC, Stern M, Zhang S, Shojaei A. J Ocul Pharmacol Ther. 2016;33:5. doi: 10.1089/jop.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krauss AH, Corrales RM, Pelegrino FSA, Tukler-Henriksson J, Pflugfelder SC, de Paiva CS. Investig Ophthalmol Vis Sci. 2015;56:5888. doi: 10.1167/iovs.15-17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sheppard JD, Torkildsen GL, Lonsdale JD, D’Ambrosio FA, McLaurin EB, Eiferman RA, Kennedy KS, Semba CP. Ophthalmology. 2014;121:475. doi: 10.1016/j.ophtha.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 142.Tang Q, Bluestone Ja. Nat Immunol. 2008;9:239. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jhunjhunwala S, Raimondi G, Glowacki AJ, Hall SJ, Maskarinec D, Thorne SH, Thomson AW, Little SR. Adv Mater. 2012;24:4735. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vignali DAA, Collison LW, Workman CJ. Nat Rev Immunol. 2008;8 doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Christodoulou MI, Moutsopoulos NM, Moutsopoulos HM. Am J Pathol. 2008;173:1389. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yadav M, Stephan S, Bluestone JA. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miyara M, Sakaguchi S. Trends Mol Med. 2007;13:108. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 148.Stein-Streilein J, Taylor AW. J Leukoc Biol. 2007;81:593. doi: 10.1189/jlb.0606383. [DOI] [PubMed] [Google Scholar]
- 149.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Cell. 2008;133:775. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 150.Stewart JM, Keselowsky BG. Adv Drug Deliv Rev. 2017:1. doi: 10.1016/j.addr.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yagci A, Gurdal C. Int Ophthalmol. 2014;34:1291. doi: 10.1007/s10792-014-9969-x. [DOI] [PubMed] [Google Scholar]
- 152.Riley JL, June CH, Blazar BR. Immunity. 2009;30:656. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Glowacki AJ, Gottardi R, Yoshizawa S, Cavalla F, Garlet GP, Sfeir C, Little SR. Ann Biomed Eng. 2015;43:593. doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siemasko KF, Gao J, Calder VL, Hanna R, Calonge M, Pflugfelder SC, Niederkorn JY, Stern ME. Invest Ophthalmol Vis Sci. 2008:5434. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 155.Baluna R, Vitetta ES. Immunopharmacology. 1997;37:117. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 156.Nelson BH. J Immunol. 2004;172:3983. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 157.Anderson JM, Shive MS. Adv Drug Deliv Rev. 2012;64:72. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 158.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR. Proc Natl Acad Sci U S A. 2013;110:18525. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y, Irvine DJ. Biomaterials. 2011;32:4903. doi: 10.1016/j.biomaterials.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Day S, Acquah K, Lee PP, Mruthyunjaya P, Sloan FA. Am J Ophthalmol. 2011;152:1014. doi: 10.1016/j.ajo.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gottlieb JL. JAMA Ophthamology. 2002;288:2233. doi: 10.1001/jama.288.18.2233. [DOI] [PubMed] [Google Scholar]
- 162.Singer M. F1000Prime Rep. 2014;6:29. doi: 10.12703/P6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.A-REDS Group. Arch Ophthalmol. 2001;119:1417. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stanton CM, Yates JRW, den Hollander AI, Seddon JM, Swaroop A, Stambolian D, Fauser S, Hoyng C, Yu Y, Atsuhiro K, Branham K, Othman M, Chen W, Kortvely E, Chalmers K, Hayward C, Moore AT, Dhillon B, Ueffing M, Wright AF. Invest Ophthalmol Vis Sci. 2011;52:8828. doi: 10.1167/iovs.11-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roychoudhury J, Herndon JM, Yin J, Apte RS, Ferguson TA. Investig Ophthalmol Vis Sci. 2010;51:3560. doi: 10.1167/iovs.09-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang A, Humphries MM, Ed C. Nat Med. 201AD;18:791. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Campochiaro PA, Lauer AK, Sohn EH, Mir TA, Naylor S, Anderton MC, Kelleher M, Harrop R, Ellis S, Mitrophanous KA. Hum Gene Ther. 2016;28:99. doi: 10.1089/hum.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Am J Pathol. 2012;181:472. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 169.Cox CA, Amaral J, Salloum R, Guedez L, Reid TW, Jaworski C, John-aryankalayil M, Freedman KA, Campos MM, Martinez A, Becerra SP, Carper DA. Ophthalmology. 2010;117:1782. doi: 10.1016/j.ophtha.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He L, Marneros AG. J Biol Chem. 2014;289:8019. doi: 10.1074/jbc.M113.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Am Acad Ophthalmol. 2006;113 doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 172.Iwase T, Fu J, Yoshida T, Muramatsu D, Miki A, Hashida N, Lu L, Oveson B, Lima E, Silva R, Seidel C, Yang M, Connelly S, Shen J, Han B, Wu M, Semenza GL, Campochiaro PA, Hanes J. J Control Release. 2013;172 doi: 10.1016/j.jconrel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Circ Res. 2003:93. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 174.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. N Engl J Med. 2006;355:1419. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 175.TCR Group. N Engl J Med. 2011;364:1897. [Google Scholar]
- 176.Thomas CE, Ehrhardt A, Kay Ma. Nat Rev Genet. 2003;4:346. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 177.Cashman SM, Bowman L, Christofferson J, Kumar-Singh R. Investig Ophthalmol Vis Sci. 2006;47:3496. doi: 10.1167/iovs.05-1610. [DOI] [PubMed] [Google Scholar]
- 178.Binley K, Widdowson PS, Kelleher M, de Belin J, Loader J, Ferrige G, Carlucci M, Esapa M, Chipchase D, Angell-Manning D, Ellis S, Mitrophanous K, Miskin J, Bantseev V, Nork TM, Miller P, Naylor S. Hum Gene Ther. 2012;23:980. doi: 10.1089/hum.2012.008. [DOI] [PubMed] [Google Scholar]
- 179.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, Dibartolomeo R, Wei LL. Hum Gene Ther. 2006;17:167. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 180.Peter F, Ziipfel Nadine Lauer CS. Adv Exp Med Biol. 2010;703:9. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- 181.Gemenetzi M, Lotery AJ. Eye. 2016;30:1. doi: 10.1038/eye.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Doyle SL, Lopez FJ, Celkova L, Brennan K, Mulfaul K, Ozaki E, Kenna PF, Kurali E, Hudson N, Doggett T, Ferguson TA, Humphries P, Adamson P, Campbell M. Investig Ophthalmol Vis Sci. 2015;56:5424. doi: 10.1167/iovs.15-17264. [DOI] [PubMed] [Google Scholar]
- 183.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Stem Cells. 2009;27:2126. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 184.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman J, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R, Del Priore LV. Lancet. 2014;6736:1. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 185.De Wert G, Mummery C. Hum Reprod. 2003;18:672. doi: 10.1093/humrep/deg143. [DOI] [PubMed] [Google Scholar]
- 186.Trounson A, DeWitt ND. Nat Rev Mol Cell Biol. 2016;17:194. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 187.Fields M, Cai H, Gong J, Del Priore L. Cells. 2016;5:44. doi: 10.3390/cells5040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Biomaterials. 2005;26:3187. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 189.Hynes SR, Lavik EB. Graefe’s Arch Clin Exp Ophthalmol. 2010;248:763. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 190.Sodha S, Wall K, Redenti S, Klassen H, Young MJ, Tao SL. J Biomater Sci Polym Ed. 2011;22:443. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 191.de Smet MD, Taylor SRJ, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, Zierhut M, Barisani-Asenbauer T, LeHoang P, Lightman S. Prog Retin Eye Res. 2011;30:452. doi: 10.1016/j.preteyeres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 192.Nguyen QD, Merrill PT, Clark WL, Banker AS, Fardeau C, Franco P, LeHoang P, Ohno S, Rathinam SR, Thurau S, Abraham A, Wilson L, Yang Y, Shams N. Ophthalmology. 2016;123:2413. doi: 10.1016/j.ophtha.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 193.Kim JS, Knickelbein JE, Nussenblatt RB, Sen N. Int Ophthalmol Clin. 2015;55:79. doi: 10.1097/IIO.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, Uchida A. JAMA Ophthalmol. 2013;131:1405. doi: 10.1001/jamaophthalmol.2013.4237. [DOI] [PubMed] [Google Scholar]
- 195.Mérida S, Palacios E, Navea A, Bosch-Morell F. Int J Mol Sci. 2015;16:18778. doi: 10.3390/ijms160818778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Papotto PH, Marengo EB, Sardinha LR, Goldberg AC, Rizzo LV. Autoimmun Rev. 2014;13:909. doi: 10.1016/j.autrev.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lin P. Curr Ophthalmolmology Rep. 2015;3:170. [Google Scholar]
- 198.Takase H, Futagami Y, Yoshida T, Kamoi K, Sugita S, Imai Y, Mochizuki M. Investig Ophthalmol Vis Sci. 2006;47:1557. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 199.Caspi RR. J Clin Invest. 2010;120:3073. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Agarwal RK, Caspi RR. Methods Mol Med. 2004;102:395. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 201.Becker MD, Adamus G, Davey MP, Rosenbaum JT. Ocul Immunol Inflamm. 2000;8 doi: 10.1076/0927-3948(200006)8. [DOI] [PubMed] [Google Scholar]
- 202.Sen ES, Dick AD, Ramanan AV. Nat Rev Rheumatol. 2015 doi: 10.1038/nrrheum.2015.20. [DOI] [PubMed] [Google Scholar]
- 203.Caspi RR. Investig Ophthalmol Vis Sci. 2011;52:1873. doi: 10.1167/iovs.10-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Palareti G, Legnani C, Cosmi B, Antonucci E, Erba N, Poli D, Testa S, Tosetto A. Int J Lab Hematol. 2016;38:42. [Google Scholar]
- 205.Sakoda Y, Nagai T, Murata S, Mizuno Y, Kurosawa H, Shoda H, Morishige N, Yanai R, Sonoda K-H, Tamada K. J Immunol. 2016;196:2947. doi: 10.4049/jimmunol.1501742. [DOI] [PubMed] [Google Scholar]
- 206.Chen J, Qian H, Horai R, Chan CC, Falick Y, Caspi RR. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen M, Copland DA, Zhao J, Liu J, Forrester JV, Dick AD, Xu H. Am J Pathol. 2012;180:235. doi: 10.1016/j.ajpath.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 208.Servat JJ, Mears KA, Black EH, Huang JJ. Expert Opin Biol Ther. 2012;12:311. doi: 10.1517/14712598.2012.658366. [DOI] [PubMed] [Google Scholar]
- 209.Srivastava A, Rajappa M, Kaur J. Clin Chim Acta. 2010;411:1165. doi: 10.1016/j.cca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 210.You C, Sahawneh HF, Ma L, Kubaisi B, Schmidt A, Foster CS. Clin Ophthalmol. 2017;11:257. doi: 10.2147/OPTH.S121734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Samson CM, Waheed N, Baltatzis S, Foster CS. Ophthamology. 2001;6420:1134. doi: 10.1016/s0161-6420(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 212.Gangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Kempen JH. Ophthalmology. 2009;116:2188. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Allison AC. Lupus. 2005;14:s2. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 214.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Ophthalmology. 2005;112:1472. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 215.Chanaud NP, Vistica BP, Eugui E, Nussenblatt RB, Allison AC, Gery I. Exp Eye Res. 1995;61:429. doi: 10.1016/s0014-4835(05)80138-1. [DOI] [PubMed] [Google Scholar]
- 216.Siepmann K, Huber M, Stübiger N, Deuter C, Zierhut M. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:788. doi: 10.1007/s00417-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 217.Doycheva D, Zierhut M, Blumenstock G, Stuebiger N, Deuter C. Graefe’s Arch Clin Exp Ophthalmol. 2011;249:1235. doi: 10.1007/s00417-011-1731-8. [DOI] [PubMed] [Google Scholar]
- 218.Sobrin L, Christen W, Foster CS. Ophthalmology. 2008;115:1416. doi: 10.1016/j.ophtha.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 219.Al-Khatib S, Suhler E, Djalilian A, Sen H, Nussenblatt R, Buggage R. Invest Ophthalmol Vis Sci. 2002;43:4278. [Google Scholar]
- 220.Il Gum S, Kim Y-H, Jung J-C, Kim IG, Lee JS, Lee KW, Park YJ. Biochem Biophys Res Commun. 2016;482:1148. doi: 10.1016/j.bbrc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 221.Vitale AT, Rodriguez A, Foster CS, Acs F. Ophthalmology. 1996;103:365. doi: 10.1016/s0161-6420(96)30683-0. [DOI] [PubMed] [Google Scholar]
- 222.Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. Ophthalmology. 2010;117:576. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Isnard Bagnis C. J Am Soc Nephrol. 2002;13:2962. doi: 10.1097/01.asn.0000034945.61533.26. [DOI] [PubMed] [Google Scholar]
- 224.Myers BD, Sibley R, Newton L, Tomlanovich SJ, Boshkos C, Stinson E, Luetscher Ja, Whitney DJ, Krasny D, Coplon NS. Kidney Int. 1988;33:590. doi: 10.1038/ki.1988.38. [DOI] [PubMed] [Google Scholar]
- 225.Sloper CM, Powell RJ, Dua HS. Ophthalmology. 1999;106:723. doi: 10.1016/S0161-6420(99)90156-2. [DOI] [PubMed] [Google Scholar]
- 226.Abud TB, Amparo F, Saboo US, Di Zazzo A, Dohlman TH, Ciolino JB, Hamrah P, Dana R. Ophthalmology. 2016;123:1449. doi: 10.1016/j.ophtha.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Murphy CC. Arch Ophthalmol. 2005;123:634. doi: 10.1001/archopht.123.5.634. [DOI] [PubMed] [Google Scholar]
- 228.Hogan AC, McAvoy CE, Dick AD, Lee RWJ. Ophthalmology. 2007;114 doi: 10.1016/j.ophtha.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 229.Lee RWJ, Greenwood R, Taylor H, Amer R, Biester S, Heissigerova J, Forrester JV, Dick AD. Ophthalmology. 2012;119:1223. doi: 10.1016/j.ophtha.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 230.Hale S, Lightman S. Cytokine. 2006;33:231. doi: 10.1016/j.cyto.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 231.Cordero-Coma M, Sobrin L. Surv Ophthalmol. 2015;60:575. doi: 10.1016/j.survophthal.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 232.Busch M, Bauer D, Hennig M, Wasmuth S, Thanos S. IOVS. 2013;54:39. doi: 10.1167/iovs.12-10138. [DOI] [PubMed] [Google Scholar]
- 233.Kruh JN, Yang P, Suelves AM, Foster CS. Ophthalmology. 2014;121:358. doi: 10.1016/j.ophtha.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 234.Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Golder W, Kellner H, Schneider M, So H. Argtritis Rheum. 2003;48:2224. doi: 10.1002/art.11104. [DOI] [PubMed] [Google Scholar]
- 235.Reinisch W, Van Assche G, Befrits R, Connell W, Haens GD, Ghosh S, Michetti P, Ochsenkühn T, Panaccione R, Schreiber S, Silverberg MS, Sorrentino D, Van Der Woude CJ, Vermeire S, Panes J. J Crohn’s Colitis. 2012;6:248. doi: 10.1016/j.crohns.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 236.Hostettler KE, Tamm M, Brutsche H. Respiration. 2012;83:218. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 237.Tugal-Tutkun I, Mudun A, Urgancioglu M, Kamali S, Kasapoglu E, Inanc M, Gu A. Arthritis Rheum. 2005;52:2478. doi: 10.1002/art.21231. [DOI] [PubMed] [Google Scholar]
- 238.Sukumaran S, Marzan K, Shaham B, Reiff A. ISRB Rheumatol. 2012 doi: 10.5402/2012/765380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gasparin F, Takahashi BS, Scolari MR, Gasparin F, Pedral LS, Damico FM. Arq Bras Oftalmol. 2012;75:143. doi: 10.1590/s0004-27492012000200016. [DOI] [PubMed] [Google Scholar]
- 240.Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J. N Engl J Med. 1998;338:161. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 241.Vlad G, Ho EK, Vasilescu ER, Fan J, Liu Z, Cai JW, Jin Z, Burke E, Deng M, Cadeiras M, Cortesini R, Itescu S, Marboe C, Mancini D, Suciu-foca N. Transpl Immunol. 2007;18:13. doi: 10.1016/j.trim.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 242.Mullen JC, Kuurstra EJ, Oreopoulos A, Bentley MJ, Wang S. Transplant Res. 2014;3:1. doi: 10.1186/2047-1440-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rasaiah SB, Light JA, Sasaki TM, Currier CB, Light A, Sasaki TM. Clin Transplant. 2000;14:409. doi: 10.1034/j.1399-0012.2000.14040902.x. [DOI] [PubMed] [Google Scholar]
- 244.Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, Peltekian KM, Lilly LB, Scudamore CH, Bain VG, Wall WJ, Roy A. Liver Transplant. 2005;11:1064. doi: 10.1002/lt.20490. [DOI] [PubMed] [Google Scholar]
- 245.Mullen JC, Oreopoulos A, Lien DC, Bentley MJ, Modry DL, Stewart K, Winton TL, Jackson K. J Hear Lung Transplant. 2003;26:504. doi: 10.1016/j.healun.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 246.Papaliodis GN, Chu D, Foster CS. Ophthalmology. 2003;110:786. doi: 10.1016/S0161-6420(02)01932-2. [DOI] [PubMed] [Google Scholar]
- 247.Nussenblatt RB, Peterson JS, Foster CS. Ophthalmology. 2005;112:764. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 248.Nussenblatt RB, Thompson DJS, Li Z, Peterson JS, Robinson RR, Shames RS, Nagarajan S, Tang MT, Mailman M, Velez G, Roy C, Levy-clarke GA, Suhler EB, Djalilian A, Sen HN, Al-khatib S, Ursea R, Srivastava S, Bamji A, Mellow S, Sran P, Waldmann TA, Buggage RR. J Autoimmun. 2003;21:283. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 249.Patel DD, Lee DM, Kolbinger F, Antoni C. Ann Rheum Dis. 2013;72(Suppl 2):ii116. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 250.Maya JR, Sadiq MA, Zapata LJ, Hanout M, Sarwar S, Rajagopalan N, Guinn KE, Sepah YJ, Nguyen QD. J Ophthalmol. 2014 doi: 10.1155/2014/310329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Strober B, Gottlieb AB, Sherif B, Mollon P, Gilloteau I, McLeod L, Fox T, Mordin M, Gnanasakthy A, Papavassilis C, Lebwohl MG. J Am Acad Dermatol. 2017 doi: 10.1016/j.jaad.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 252.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster SC, Kim RY, Samson MC, Falk NS, Chu DS, Callanan Q, Nguyen QD, Rose K, Haider A, Di Padova F. Am J Ophthalmol. 2010;2 doi: 10.1016/0002-9394(82)90197-0. [DOI] [PubMed] [Google Scholar]
- 253.Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, Group AS. OPHTHA. 2015;122:939. doi: 10.1016/j.ophtha.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 254.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, Androudi S. Ophthalmology. 2013;120:777. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 255.Lin P, Suhler EB, Rosenbaum JT. Ophthalmology. 2014;121:365. doi: 10.1016/j.ophtha.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schwartzman S. Best Pract Res Clin Rheumatol. 2016;30:304. doi: 10.1016/j.berh.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 257.Pasadhika S, Rosenbaum JT. Biol Target Ther. 2014;20:67. doi: 10.2147/BTT.S41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, Cimino L, Zannin ME. Arthritis Care Res. 2010;62:821. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- 259.Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Graefe’s Arch Clin Exp Ophthalmol. 2011;249:297. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]
- 260.Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Invest Ophthalmol. 2000;41:3569. [PubMed] [Google Scholar]
- 261.Driot J-Y, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. J Ocul Pharmacol Ther. 2004;20:269. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- 262.Jaffe GJ, Ben-nun J, Guo H, Dunn JP, Ashton P. Ophthalmology. 2000;107:2024. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 263.Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Ophthalmology. 2005;112:1192. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 264.Mohammad DA, Sweet BV, Elner SG. Ann Pharmacother. 2007;41:449. doi: 10.1345/aph.1H540. [DOI] [PubMed] [Google Scholar]
- 265.Hsu J. Curr Opin Ophthalmol. 2007;18:235. doi: 10.1097/ICU.0b013e3281108000. [DOI] [PubMed] [Google Scholar]
- 266.He Y, Wang J-C, Liu Y-L, Ma Z-Z, Zhu X-A, Zhang Q. J Ocul Pharmacol Ther. 2006;22:121. doi: 10.1089/jop.2006.22.121. [DOI] [PubMed] [Google Scholar]
- 267.Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Eur J Pharm Biopharm. 2003;56:307. doi: 10.1016/s0939-6411(03)00138-3. [DOI] [PubMed] [Google Scholar]
- 268.Wolska E, Sznitowska M. Int J Pharm. 2013;441:449. doi: 10.1016/j.ijpharm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 269.Fang I-M, Lin C-P, Yang C-H, Chiang B-L, Yang C-M, Chau L-Y, Chen M-S. J Ocul Pharmacol Ther. 2005;21:420. doi: 10.1089/jop.2005.21.420. [DOI] [PubMed] [Google Scholar]
- 270.Trittibach P, Barker SE, Broderick CA, Natkunarajah M, Duran Y, Robbie SJ, Bainbridge JW, Smith AJ, Sarra GM, Dick AD, Ali RR. Gene Ther. 2008;15:1478. doi: 10.1038/gt.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim K, Park SW, Kim JH, Lee SH, Kim D, Koo T, Kim KE, Kim JH, Kim JS. Genome Res. 2017;27:419. doi: 10.1101/gr.219089.116. [DOI] [PMC free article] [PubMed] [Google Scholar]