Abstract
There has recently been an increasing interest in model-based evaluation and comparison of different treatment options in radiation oncology studies. This is partly driven by the considerable technical advancements in radiation therapy of the last decade, leaving radiation oncologists with a multitude of options to consider. In lieu of randomized trials comparing all of these different treatment options for varying indications, which is unfeasible, treatment evaluations based on normal-tissue complication probability (NTCP) models offer a practical alternative.
The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) effort culminating in a number of reports published in 2010 provided a basis for many of the since implemented dose-response models and dose-volume constraints, and was a key component for model-based treatment evaluations. Given that seven years have past since the QUANTEC publications and that patient-reported outcomes has emerged as an important consideration in recent years, an updated summary of the published radiation dose-response literature, that includes a focus on patient-reported quality of life outcomes, is warranted.
Here, we provide a systematic review of quantitative dose-response models published after January 1st 2010 for endpoints relevant to radiation therapy for head and neck cancer, as these patients are typically at risk for a variety of treatment-induced normal tissue complications.
Keywords: Dose-response models, radiation therapy, head and neck, patient-reported outcomes
Introduction
Patients treated with radiation therapy (RT) for head and neck cancer are at risk for a variety of normal tissue complications and as technological advancements have made intensity-modulated radiation therapy (IMRT) and multi-modality imaging readily available, risk-adaptive treatment strategies are being increasingly utilized.1–3 These improvements, and the increase in the number of HPV p16 positive tumors in recent years, have led to loco-regional control at the level of close to 80% for patients receiving definitive RT.2,4,5 This means that organ at risk (OAR) determination as well as identifying important normal tissue complication probability (NTCP) dose-effect relationships and thresholds are key to facilitate further reduction of adverse effects and improvements of quality of life, since these are now considered critical factors in head and neck RT.6,7
The laudable effort by the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) group reported in 2010 provided a thorough review of the published clinical evidence for normal tissue dose-effect relationships.8 Especially relevant to head and neck RT were the reports on salivary glands, esophagus, brainstem, hearing loss, larynx and pharynx.9–13
Given the rising interest in patient-reported outcomes as key components in normal tissue toxicity evaluation and treatment tailoring14,15, as well as the emergence of new evidence since the QUANTEC reports, there is a need for an updated review of NTCP dose-response models for head and neck RT. This is especially relevant as model-based comparisons involving new RT treatment modalities such as proton therapy are becoming increasingly common and should always be based on the latest and most reliable evidence.16–18
To this end we performed a systematic review of studies presenting quantitative NTCP dose-response models for endpoints relevant to head and neck RT that were published after the reports from the QUANTEC group.
Methods and Materials
Search strategies and inclusion criteria
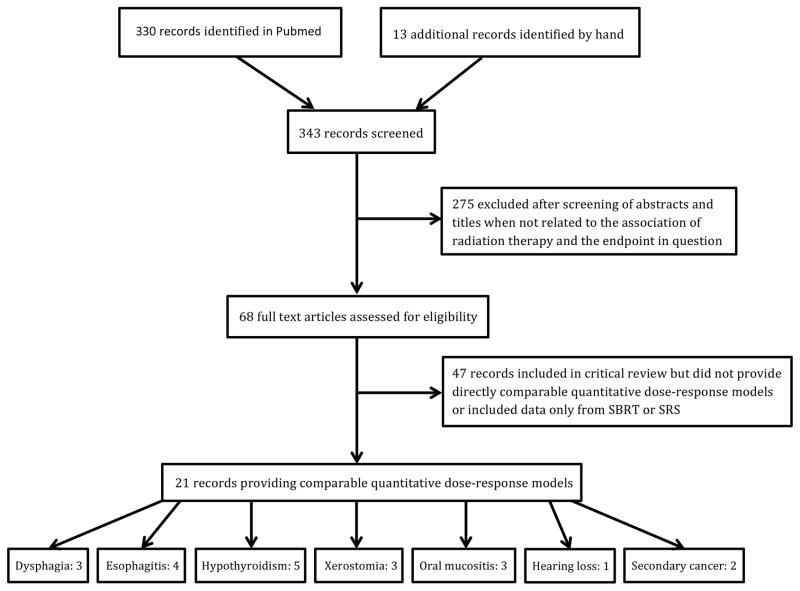
Potentially relevant records published after the QUANTEC reports, which became available in early 2010, were identified through a Pubmed search using various combinations of “Radiation therapy” or “Radiation-induced” and “Dose-volume” or “Dose-response” for each of the following endpoints; dysphagia, esophagitis, laryngeal edema, xerostomia, hypothyroidism, brainstem injury, optic neuropathy, oral mucositis, hearing loss, fatigue and secondary cancer. Records were filtered by publication date (between 01/01/2010 and 12/31/2016), language (English) and age (Adults ≥ 19 years), focusing on linear accelerator based fractionated RT for this review. Details of the selection process for records with quantitative dose-response models are provided in Figure 1. The specific search terms applied for each endpoint can be found as supplementary material.
Figure 1.
Flow chart illustrating the selection process for identifying studies with quantitative dose-response models included for detailed review.
All of the included records were reviewed with respect to dose-volume parameters and NTCP models for the corresponding OARs, including 95% confidence intervals, as well as for any clinical risk factors affecting the dose-response models, and whether the endpoints were scored by the physician or self-reported by the patients.
Mathematical expressions for relevant dose-response models
The following section provides the mathematical relationship and variable explanation for the different quantitative dose-response models encountered in this review.
Logistic regression
| (1) |
β0 is a constant and βi represents the regression coefficient for the i-th covariate xi.
Logit model
| (2) |
D50 is the dose leading to a 50% complication rate, D is the dose to the organ and k represents the slope of the dose-response curve.
Lyman equivalent model using generalized equivalent uniform dose (gEUD)
| (3) |
D50 is the dose leading to a 50% complication rate, mrepresents the slope of the dose-response curve, vi is the i-th volume fraction of the organ, Di is the dose to the i-th volume fraction and n describes the volume effect of the organ dose-response. For a mean dose model n =1. Note, that in this expression for gEUD the usual parameter a is replaced by a =1 n, i.e. a is identified with the inverse of the volume effect parameter.
Logistic model
| (4) |
D50 is the dose leading to a 50% complication rate, D is the dose to the organ and γ50 is the relative change in complication rate per unit change in dose at the 50% level.
Log-logistic model using gEUD
| (5) |
Plateau excess absolute risk (EAR) model based on organ-equivalent dose (OED)
| (6) |
Critically evaluating various dose-response models for a given endpoint
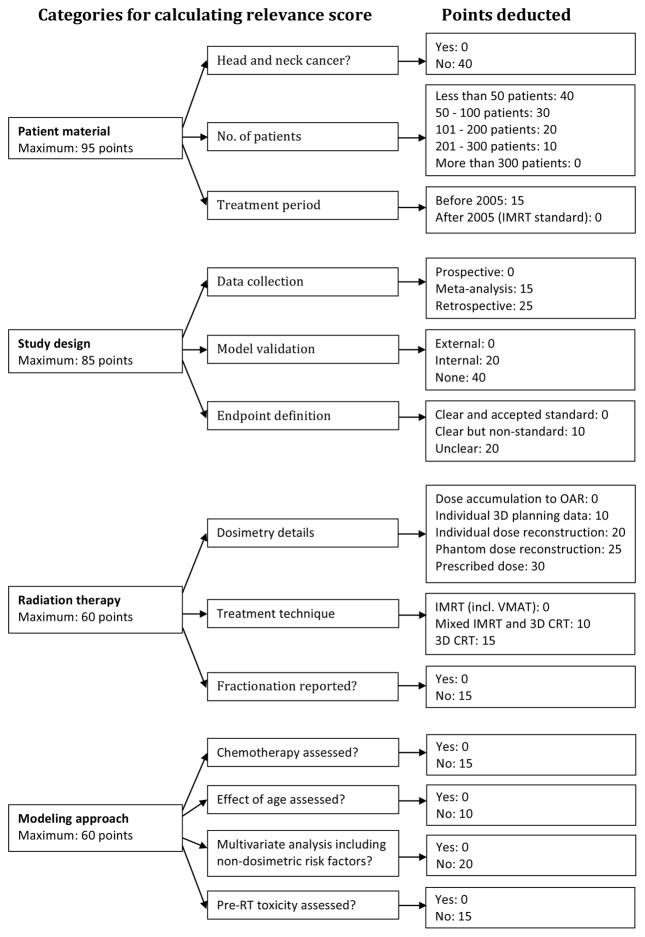
It is not straightforward to compare dose-response models from different studies due to varying choice of mathematical model, adjustment for multiple factors and variation in patient material and treatment. Therefore, we adopted the previously published concept of computing a relevance score for each report with a quantitative dose-response model, as a measure of how relevant the model is for estimating NTCP for the patient population in question.17 The flow chart in Figure 2 depicts our adaptation of how to calculate the relevance score for head and neck cancer patients undergoing RT or chemo-RT in the modern IMRT era. The categories and scores were derived based on clinical and analytical judgment, as well as by adhering to the critical points brought to light in the QUANTEC reports related to head and neck patients.
Figure 2.
Schema illustrating the relevance score computation and the weights assigned to the various categories. The highest relevance score a report can be assigned is 300 and the lowest score is zero. The number of points deducted in each category represents the weight assigned to deviation from the ideal scenario.
Although the relevance score addresses the overall relevance of various models for the patient population in question, it does not provide much granularity in regards to the appropriateness of the applied statistical methodology. Therefore, we further included a checklist depicting whether key items from the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) consensus statement on model development and validation were addressed in the reviewed studies.19
To determine the consistency between various dose-response models we calculated the corresponding NTCP for each model based on the dose distribution from a randomly selected head and neck patient who received comprehensive nodal irradiation at our institution with static field IMRT. We further added a 10% variation in the relevant OAR dose metric to each calculation to illustrate whether models were consistent across some variation in dose.
Results
We identified a total of 59 eligible full-text records that after further review resulted in 21 records with quantitative dose-response models for either dysphagia, esophagitis, hypothyroidism, xerostomia, oral mucositis, hearing loss or secondary cancers, as depicted in Figure 1. We did not find any post-QUANTEC studies presenting data on brainstem injury after standard fraction RT, and hence refer to the QUANTEC report for the most recent data.11
The following sections provide a detailed overview based on the records reviewed for each of the endpoints included in this review. For endpoints covered in the QUANTEC reports, comparisons are made between the suggested QUANTEC dose-volume constraints and the results presented by the papers included in this review.
The identified quantitative dose-response models are presented in Table 1 for patient-reported quality of life (QoL) endpoints, in Table 2 for endpoints scored by physical examination or laboratory tests and in Table 3 for secondary cancer endpoints. It is worth mentioning that model parameters cannot be directly compared between studies since parameters in multivariable models depend on the other covariates.
Table 1.
Dose-response models for head and neck normal tissue toxicity endpoints scored by patient self-reporting using quality of life questionnaires. Relevance scores presented as total and (patient material, study design, radiation therapy, modeling approach).
| Endpoint definition | Patient material | Time point | Significant non-dosimetric risk factors | Model and parameters (95% CIs) | Reference |
|---|---|---|---|---|---|
| Dysphagia | |||||
| Moderate to severe liquid swallowing based on EORTC QLQ-H&N35* | 354 head and neck cancer patients treated with chemo-RT 1.5 – 2 Gy/fx |
6 months post RT | Radiation technique: (IMRT vs. 3DCRT) | Logistic regression: βSG larynx mean dose = 0.074 (0.030, 0.11) βRadiation technique = −1.21 (−2.12, −0.27) Constant = −5.98 AUC = 0.75 (0.68–0.83) |
Christianen et al. 201220 Relevance score: 215 (80, 35, 40, 60) |
|
|
|
||||
| Moderate to severe soft food swallowing based on EORTC QLQ-H&N35 | Age: (>65 vs. 18–65 y) Tumor site: (Oro/nasopharynx vs. Other) Radiation technique: (IMRT vs. 3DCRT) |
Logistic regression: βMiddle PCM mean dose = 0.061 (0.030, 0.095) βAge = 1.20 (0.41, 2.00) βTumor site = 1.12 (0.31, 1.93) βRadiation technique = −0.91 (−1.77, −0.073) Constant = −5.83 AUC = 0.79 (0.72–0.86) |
|||
|
|
|
||||
| Moderate to severe soft food swallowing based on EORTC QLQ-H&N35 | Age: (>65 vs. 18–65 y) | Logistic regression: βSuperior PCM mean dose = 0.049 (0.030, 0.068) βSG larynx = 0.048 (0.010, 0.086) βAge = 0.80 (0.020, 1.57) Constant = −6.89 AUC = 0.77 (0.70–0.84) |
|||
|
|
|
||||
| Moderate to severe choking when swallowing based on EORTC QLQH& N35 | - | Logistic regression: βEsophagus inlet muscle V60 = 0.020 (0.010, 0.030) βSG larynx mean dose = 0.066 (0, 0.31) Constant = −7.07 AUC = 0.77 (0.67–0.86) |
|||
|
| |||||
| Xerostomia | |||||
| Xerostomia based on EORTC QLQH& N35 criteria | 178 head and neck cancer patients treated with IMRT or chemo-IMRT 2 Gy/fx |
6 months post RT | Baseline xerostomia: (Yes vs. No) | Logistic regression: βContralat. parotid mean dose = 0.047 (0.020, 0.077) βBaseline xerostomia = 0.72 (0.0, 1.44) Constant = −1.44 AUC = 0.68, R2 = 0.13 |
Beetz et al. 201230 Relevance score: 240 (75, 55, 50, 60) |
|
|
|
||||
| Sticky saliva based on EORTC QLQH& N35 criteria | - | Logistic regression: βContralat. submand. mean dose = 0.075 (0.030, 0.12) βSublingual glands mean dose = −0.06 (−0.10, −0.02) βSoft palate mean dose = 0.026 (0.0, 0.049) Constant = −3.24 AUC = 0.70, R2 = 0.17 |
|||
AUC – Area under the receiver operating characteristics curve
R2 – Pseudo R2 values measuring goodness-of-fit
European Organisation for Research and Treatment of Cancer head and neck cancer module quality of life questionnaire 35
Table 2.
Dose-response models for head and neck normal tissue toxicity endpoints scored by the treating physician based on clinical examination or laboratory tests. Relevance scores presented as total and (patient material, study design, radiation therapy, modeling approach).
| Endpoint definition | Patient material | Time point | Significant non-dosimetric risk factors | Model and parameters (95% CIs) | Reference |
|---|---|---|---|---|---|
| Dysphagia | |||||
| Grade 2–4 swallowing dysfunction based on RTOG/EORTC SWALM6* | 354 head and neck cancer patients treated with chemo-RT 1.5 – 2 Gy/fx |
6 months post RT | - | Logistic regression: βSuperior PCM mean dose = 0.057 (0.039, 0.077) βSG larynx mean dose = 0.037 (0.010, 0.058) Constant = −6.09 AUC = 0.80 (0.75–0.85) |
Christianen et al. 201220 Relevance score: 215 (80, 35, 40, 60) |
|
| |||||
|
Physician-scored Grade ≥3 pharyngeal dysphagia based on CTCAE† v3.0 |
253 head and neck cancer patients treated with RT or chemo-RT 1.8 – 2.4 Gy/fx |
Within 8 weeks post RT | Concurrent chemo: (Yes vs. No) Gender‡ |
Logistic regression: βInferior PCM mean dose = 0.03 (0.01, 0.53) βConcurrent chemo = 0.81 (0.09, 1.73) βGender = 0.27 Constant = −2.39 AUC = 0.62 (0.55–0.69) |
Otter et al. 201524 Relevance score: 215 (85, 45, 40, 45) |
|
| |||||
|
Physician-scored Grade ≥3 dysphagia based on CTCAE v3.0 |
148 head and neck cancer patients treated with induction chemotherapy followed by concurrent chemo-IMRT 2.2 – 2.4 Gy/fx |
Within 8 weeks post RT | - | Logit model: D50, total PCM = 44.5 (36, 53) ktotal PCM = 2.6 (0.8, 4.5) R2 = 0.65 |
Bhide et al. 201235 Relevance score: 170 (75, 45, 50, 0) |
|
| |||||
| Esophagitis | |||||
| RTOG grade ≥2 acute esophagitis | 374 NSCLC patients treated with pretreatment or concurrent chemotherapy and 3DCRT 1.8 – 2.5 Gy/fx |
Within 3–4 weeks from start of RT | Concurrent chemo: (Yes vs. No) | Logistic regression: βEsophagus mean dose = 0.069 (0.058, 0.080) βConcurrent chemo = 1.50 (1.19, 1.81) Constant = −3.13 AUC = 0.83 |
Huang et al. 201226 Relevance score: 160 (40, 40, 35, 45) |
|
| |||||
| Acute esophageal toxicity grade ≥2 based on CTC v3.0 | 139 NSCLC patients treated with concurrent chemotherapy and IMRT 2.75 Gy/fx |
Within 3 months from start of RT | - | Logistic regression: βEsophagus V50 = 0.027 (0.005, 0.049) Constant = −0.52 Performance measures N/A |
Kwint et al. 201227 Relevance score: 145 (35, 45, 50, 15) |
|
| |||||
| Acute esophageal toxicity grade ≥2 based on RTOG grading | 149 NSCLC patients treated with chemo- IMRT 2 Gy/fx |
During RT and within a few weeks post RT | Concurrent chemo: (Yes vs. No) Gender: (Female vs. Male) T stage: (cT3/4 vs. cT1/2) |
Logistic regression: βEsophagus mean dose = 0.12 (0.06, 0.17) βConcurrent chemo = 2.65 (1.55, 3.74) βGender = 1.20 (0.31, 2.10) βT stage = 0.99 (0.11, 1.87) Constant = −6.42 AUC = 0.82, R2 = 0.41 |
Wijsman et al. 201528 Relevance score: 170 (35, 40, 50, 45) |
|
| |||||
| Acute esophageal toxicity grade ≥2 based on CTCAE v4.0 | 125 patients with central lung tumors treated with SBRT 6 – 20 Gy/fx |
During RT or within 4 months post RT | - | Logistic regression (BED10 doses): βEsophagus D5cc = 0.106 (0.047, 0.165) βEsophagus Dmax = 0.044 (0.019, 0.070) Constant = −3.90†† Performance measures N/A |
Wu et al. 201429 Relevance score: 105 (35, 20, 50, 0) |
|
| |||||
| Hypothyroidism | |||||
| Clinical or sub-clinical hypothyroidism based on CTCAE v4.02 | 65 head and neck cancer patients treated with RT or chemo-RT, excluding nasopharynx if pituitary gland dose >40 Gy 1.8 – 2 Gy/fx |
Within 12 months post RT | - | Lyman EUD mean dose model: D50, Thyroid mean dose = 60 (56, 73) mThyroid mean dose = 0.27 (0.20, 0.39) Performance measures N/A |
Bakhshandeh et al. 201343 Relevance score: 130 (50, 45, 35, 0) |
|
| |||||
| Clinical or sub-clinical hypothyroidism as elevated TSH§ with or without reduced T4 levels | 105 head and neck cancer patients treated with chemo-RT, excluding nasopharynx 1.5 – 2 Gy/fx |
2 years post RT | Thyroid volume (cm3) | Logistic regression: βThyroid mean dose = 0.062 (0.029, 0.096) βThyroid volume = −0.19 (−0.30, −0.08) Constant = 0.011 AUC = 0.85 (0.78–0.92) |
Boomsma et al. 201244 Relevance score: 215 (75, 55, 40, 45) |
|
| |||||
| Clinical or sub-clinical hypothyroidism as elevated TSH and/or reduced free T3 or T4 levels | 53 patients with Hodgkin’s Lymphoma treated with sequential chemo-RT 1.5 – 1.8 Gy/fx |
Median follow-up 32 months post RT | Thyroid volume (cm3) Gender: (Male vs. female) |
Logistic regression: βThyroid V30 (cm3) = 0.26 (0.08, 0.44) βThyroid volume = −0.27 (−0.49, −0.05) βGender = −2.21 (−3.88, −0.54) Constant = 1.94 AUC = 0.87 (0.75–0.95) AUCExternal = 0.91 (0.76–0.98) |
Cella et al. 201249 Relevance score: 155 (10, 50, 35, 60) Externally validated |
|
| |||||
| Biochemical hypothyroidism as elevated TSH levels | 198 head and neck cancer patients, excluding nasopharynx, treated with RT or chemo-RT 2 – 2.1 Gy/fx |
Within 25 months post RT | Thyroid volume (cm3) | Logistic regression: βThyroid mean dose = 0.18 (0.10, 0.37) βThyroid volume = −0.30 (−0.62, −0.15) Constant = −4.92 Calibration plot Pearson’s r = 0.97 |
Ronjom et al. 201545 Relevance score: 260 (75, 75, 50, 60) Externally validated |
|
| |||||
| Clinical or sub-clinical hypothyroidism based mainly on elevated TSH | Meta-analysis of 4 studies for dose-response data for RT | Between 1 and 11 years post RT | - | Logistic model: D50, Thyroid mean dose = 45 (28, 62) γ50, Thyroid mean dose = 1.40 (0.50, 2.20) Performance measures N/A |
Vogelius et al. 201150 Relevance score: 110 (40, 30, 15, 25) |
|
| |||||
| Xerostomia | |||||
| Xerostomia based on post-treatment salivary excretion function ratio <45% on scintigraphy (per parotid gland) | 31 head and neck cancer patients treated with IMRT or chemo-IMRT 1.8 – 2 Gy/fx |
1 or 2 years post RT | # | Lyman EUD mean dose model (1 year): D50, Parotid mean dose = 43.6 (CI N/A) mParotid mean dose = 0.18 (CI N/A) Performance measures N/A |
Chen et al. 201331 Relevance score: 185 (55, 35, 50, 45) |
|
| |||||
| Lyman EUD mean dose model (2 years): D50, Parotid mean dose = 44.5 (CI N/A) mParotid mean dose = 0.30 (CI N/A) Performance measures N/A | |||||
|
| |||||
| Grade 4 xerostomia based on LENTSOMA ¶ criteria for salivary flow measurements | 66 head and neck cancer patients treated with RT or chemo-RT 1.7 – 2.7 Gy/fx |
3 months post RT | - | Logistic model: D50, Spared parotid mean dose = 22.2 (16.4, 28.4) γ50, Spared parotid mean dose = 0.83 (0.44, 1.36) |
Moiseenko et al. 201232 Relevance score: 150 (65, 45, 40, 0) Validated QUANTEC criteria |
|
| |||||
| 12 months post RT | - | Logistic model: D50, Spared parotid mean dose = 32.4 (26.3, 39.9) γ50, Spared parotid mean dose = 0.97 (0.58, 1.53) NPV for QUANTEC criteria = 94% |
|||
|
| |||||
| Oral mucositis | |||||
| Grade ≥3 oral mucositis based on CTCAE v2 | 351 head and neck cancer patients from 6 different trials treated with RT or chemo-RT 2 – 2.4 Gy/fx |
Within 8 weeks post RT | Unknown primary: (Yes vs. No) Parotid primary: (Yes vs. No) |
Penalized logistic regression (per fx): βV180cGy = 0.212*(V180cGy-53.6)/27.5 βV220cGy = 0.194*(V220cGy-10.5)/11.1 βUnknown primary = −0.025*((Yes=1, No=0) −0.044)/0.205 βParotid primary = −0.303*((Yes=1, No=0) −0.209)/0.406 AUC = 0.72 ± 0.09, Brier score = 0.23 ± 0.02 |
Dean et al. 201636 Relevance score: 245 (95, 65, 40, 45) |
|
| |||||
| Grade ≥3 oral mucositis based on CTCAE v3.0 | 253 head and neck cancer patients treated with RT or chemo-RT 1.8 – 2.4 Gy/fx |
Within 8 weeks post RT | - | Logistic regression: βOral cavity mean dose = 0.030 (0.0, 0.058) AUC = 0.62 (0.55–0.69) |
Otter et al. 201524 Relevance score: 215 (85, 45, 40, 45) |
|
| |||||
| Grade ≥3 oral mucositis based on CTCAE v3.0 | 164 oropharyngeal cancer patients treated with IMRT or chemo-IMRT 1.3 – 2.2 Gy/fx |
During RT | Concurrent chemo: (Yes vs. No) | Logistic regression: βOral cavity D21cc/week = 0.016 (0.009, 0.023) βConcurrent chemo = 1.42 (0.51, 2.32) Constant = −5.4 AUCD21cc/week cutoff = 0.77 (0.69–0.85) |
Sanguineti et al. 201237 Relevance score: 200 (60, 45, 50, 45) |
|
| |||||
| Grade ≥3 oral mucositis based on CTCAE v3.0 | 144 head and neck cancer patients treated with induction chemotherapy followed by concurrent chemo-IMRT 2.2 – 2.4 Gy/fx |
Within 8 weeks post RT | - | Logit model: D50, oral cavity = 51 (40, 61) koral cavity = 1.0 (0.6, 1.5) R2 = 0.80 |
Bhide et al. 201235 Relevance score: 170 (75, 45, 50, 0) |
|
| |||||
| Hearing loss | |||||
| Grade 1–2 hearing loss based on CTCAE v4.03 | 140 patients treated with skull base RT with combined proton and photon RT 1.8 – 2 Gy/fx |
Within 2 years post RT | - | Log-logistic gEUD model (Cochlea): D50, Cochlea = 56.0 (53.6, 58.5) γ50, Cochlea = 2.8 (1.9, 4.2) a, Cochlea = 1.2 (0.1, 3.6) AUC = 0.81 ± 0.04 |
De Marzi et al. 201551 Relevance score: 75 (20, 20, 35, 0) |
|
| |||||
| Log-logistic gEUD model (Inner ear): D50, Inner ear = 53.6 (51.8, 55.4) γ50, Inner ear = 2.8 (2.1, 3.7) a, Inner ear = 0.1 (0.1, 0.6) AUC = 0.86 ± 0.03 | |||||
AUC – Area under the receiver operating characteristics curve
R2 – Pseudo R2 values measuring goodness-of-fit
Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer Late Radiation Morbidity Scoring Criteria assessed at 6 months
Common Terminology Criteria for Adverse Events
No ref info for gender in logistic regression model
Constant value estimated from Fig. 2 in Wu et al. (29)
Thyroid-stimulating hormone
Certain non-dosimetric risk factors affecting quality of life but not part of dose-response model
Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic criteria
Table 3.
Dose-response models for secondary cancers in the head and neck region based on cohort studies of long-term cancer survivors. Relevance scores presented as total and (patient material, study design, radiation therapy, modeling approach).
| Secondary cancer | |||||
|---|---|---|---|---|---|
| Endpoint definition | Patient material | Time point | Significant non-dosimetric risk factors | Model and parameters (95% CIs) | Reference |
| Treatment-related esophageal cancer in a cohort of breast cancer survivors | Nested case-control study of 289,748 breast cancer survivors with 252 cases and 488 matched controls | Between 5 and 37 years post breast cancer treatment | Smoking: (Current vs. Nonsmoker) Hormonal therapy in last 5 years: (Yes vs. No) |
Linear model: EOR/Gy Esophagus mean dose = 0.09 (0.04, 0.16) ORSmoking = 2.4 (1.1, 5.7) ORHormonal therapy = 0.4 (0.2, 0.8) Performance measures N/A |
Morton et al. 201265 Relevance score: 110 (40, 20, 5, 45) |
|
| |||||
| Radiation-induced secondary cancer of the mouth and pharynx | Cohort study based on atomic bomb survivors receiving high doses and Hodgkin’s lymphoma patients treated with RT | Time factors included in model | Age at exposure: (Continuous) Attained age: (Continuous) |
EAR plateau OED model: αMouth and pharynx = 0.045 βEAR = 0.73 (0.30, 1.60) γage at exposure = −0.024 γattained age = 2.38 Performance measures N/A |
Schneider et al. 201167 Relevance score: 85 (40, 10, 5, 30) |
Graphical illustrations of all listed dose-response models are provided as supplementary material along with a “.txt” file with Matlab code to generate a data file containing all model parameters presented in Tables 1 – 3. Furthermore, the calculated NTCP estimates comparing the various different models are provided as supplementary material.
Dysphagia
Several reports identified the pharyngeal constrictor muscles (PCMs) as a critical OAR for treatment-induced dysphagia20–22 along with the supraglottic larynx20,23, whereas some separated the constrictor muscles into the superior, middle and inferior parts. Table 1 shows that age, tumor site and radiation technique can be important predictors for patient-reported dysphagia, whilst Otter et al. identified concurrent chemotherapy as an important predictor for grade ≥3 physician-scored dysphagia.24 The timeline for dysphagia scoring ranged from within 8 weeks to 6 months following treatment, which should be taken into account when comparing various models since acute dysphagia may not necessarily have the same dose-response characteristics as late dysphagia.
Mean doses in the range of 50 to 60 Gy to the PCMs were found to be indicative of an increased risk of dysphagia in several studies21,22, whereas a mean dose higher than 40 or 50 Gy to the larynx was associated with increased risk.22,25 The QUANTEC report on larynx and pharynx recommends keeping the dose to the larynx and pharyngeal constrictors to below 60 Gy when possible, and to limit the volume receiving more than 50 Gy.12 These recommendations are well in line with what was found in the updated reports included in this review, even with the considerable variability in endpoint definitions among the studies included in the QUANTEC review.
Although the reports included here were all studies of head and neck cancer patients treated with reasonably homogeneous radiation therapy regimens, some variability in the results obtained from the different studies is expected due to the use of different measurement tools and dysphagia definitions. It also appears that patient-reported outcomes are becoming an increasingly common tool for measuring dysphagia in a QoL-setting20,22,23, and as such it would be pertinent to consider how subjectively scored dysphagia compares to for example Common Terminology Criteria for Adverse Events (CTCAE) grading.
Esophagitis
We identified four studies with quantitative dose-response models for esophagitis, defined as grade ≥2 acute esophageal toxicity, presented in Table 2. These models are based on data from cohorts of non-small cell lung cancer (NSCLC) patients treated with radiation and chemotherapy26–28 as well as one study of central lung tumors treated with SBRT that was included for comparison with standard fractionation studies.29 Two studies identified concurrent chemotherapy as an independent predictor of acute esophagitis with an OR of 4.5 (95% CI: 3.3, 6.1) in Huang et al.26 and 14.1 (95% CI: 4.7, 42.2) in Wijsman et al.28, whereas all patients in Kwint et al.27 received concurrent chemotherapy so this was not assessable as an independent risk factor.
Esophageal toxicity was scored within a few weeks or months from the start of radiation therapy and esophageal mean dose or V50 were identified as the independent dosimetric predictors for the studies employing standard fractionation. In the study of SBRT the dose to the hottest 5 cm3 of the esophagus (D5cc) and the maximum dose, both converted to BED10, were significant predictors. The recommendations from the reports utilizing standard fractionation are to limit the esophageal mean dose or the V50, without specifying any specific cutoff points for dose constraints. The QUANTEC report on acute esophagitis summarized that the volume of esophagus receiving >40–50 Gy was indicative of increased esophagitis risk, but could also not determine any specific dose limits13, essentially concluding that the mean esophageal dose should be limited to the extent that is reasonable in terms of still adequately treating the tumor target.
Since all dose-response models for esophagitis identified in this review are based on patients treated for lung cancer, the limitations of translating the models to treatment of head and neck cancer should be considered. For the models based on standard fractionation the dose per fraction would be similar to that in head and neck RT, although more likely including the inferior esophagus for lung cancer patients and the superior part for head and neck cancer patients. The potential difference in chemotherapy regimens should also be considered, with concurrent regimens consisting of cisplatin27 or etoposide and cisplatin.28 There is also a likely interplay between acute esophageal toxicity and later appearing dysphagia after head and neck cancer treatment that may not be portrayed in studies of lung cancer patients.
Xerostomia
For xerostomia the parotid glands are usually considered the critical OARs for stimulated salivary flow and the QUANTEC recommendations that are now widely implemented clinically for head and neck IMRT suggest limiting the mean dose to both parotid glands to <25 Gy, or at least one parotid gland to <20 Gy.10 This was based on the endpoint definition of <25% of baseline salivary flow, while some of the updated reports included in this review used alternative definitions such as patient-reported QoL for dry mouth or sticky saliva30, and scintigraphy of salivary excretion function.31 While most of the dose-response models in the included reports were based on parotid gland dose, the model for patient-reported sticky saliva found dose to the contralateral submandibular gland, sublingual glands and soft palate to be significant predictors.30 The protective effect of increasing mean dose to the sublingual glands in relation to the risk of sticky saliva could be biologically plausible as mentioned by the authors, since the sublingual glands are mainly responsible for mucous saliva secretion.
The study by Moiseenko and colleagues tested the validity of the QUANTEC xerostomia recommendations on an independent prospectively acquired dataset, and found that the suggested constraints performed well with a negative predictive value of 94%.32 A subsequent study by Beetz and colleagues, aimed at testing the validity of the QUANTEC constraints in an independent dataset of patient-reported xerostomia, showed that whether or not the QUANTEC criteria were met was a significant risk factor at 6, 12, 18 and 24 months post treatment.33 Some level of Xerostomia at baseline prior to RT was also found to be an important risk factor and should be considered when this information is available.30,33
Although the mean dose to the parotid glands, or at least the contralateral gland, has been shown to be strongly associated with xerostomia recent evidence suggests that there are stem cell regions within the parotid glands that are critical to the maintenance of the functionality of the gland, and hence may warrant targeted partial-gland sparing.34
Oral mucositis
Several reports with quantitative dose-response models for acute oral mucositis were identified, all based on head and neck cancer patient cohorts, with some variation in the anatomical OAR definition. The volume encompassing the oral cavity and in some cases parts of the pharynx was most commonly used24,35–37, although some studies used a mucosal surface OAR definition.38–40 In a comparative analysis Dean et al. concluded that models based on an oral cavity definition and mucosal surface definition performed similarly for estimating acute mucositis and they recommend using the simpler oral cavity OAR contour.39 The mucositis endpoint was homogeneously defined as CTCAE grade ≥3 acute oral mucositis, occurring during RT or up to 8 weeks post treatment.
The mean dose to the oral cavity was found to be an independent predictor of oral mucositis24,35, as well as the volume of oral cavity receiving high doses per fraction36 and the dose to the hottest 21 cm3.37 One report identified concurrent chemotherapy as an independent predictor along with oral cavity dose37, whereas for the model presented by Bhide et al. all patients were treated with concurrent chemo-RT.35
Strigari et al. performed a meta-analysis of previously published studies to compare the ability of various NTCP models to identify tolerable vs. intolerable treatment schedules in relation to acute oral mucositis.41 By comparing varying schedules of total dose in 2-Gy fractions (EQD2) and overall treatment time they showed how well the different models could distinguish tolerable from intolerable treatments, with a general trend that treatment times longer than 38 days were considered tolerable. While most studies focused on the risk of developing oral mucositis, one study of 66 oropharyngeal cancer patients investigated whether the duration of grade 3 acute mucositis was related to the dose received by the oral mucosa, but failed to find a significant association.42
Although there was no QUANTEC report focused directly on oral mucositis, it has been recognized that dysphagia occurring at a later onset can be a consequence of preceding acute mucositis, and NTCP models for oral mucositis may be relevant to consider in relation to the risk of late dysphagia as well.12
Hypothyroidism
Hypothyroidism as determined through elevated TSH, or reduced T3 and T4 levels, typically appearing 1–2 years after treatment remains a fairly common normal tissue complication after head and neck RT. The mean thyroid dose43–45 has been shown as important independent predictors of radiation-induced hypothyroidism (RIHT). Also, several studies have identified the thyroid volume receiving more than 30 to 35 Gy (V30-V35) as an important dose-volume constraint associated with the risk of RIHT.46–48
Importantly, the thyroid volume prior to treatment was found to be an important risk factor as well, with increasing pre-treatment thyroid volume showing a decreased risk of RIHT.44,45,49 This further supports the consideration of the thyroid as a parallel structure organ, suggesting that limiting the irradiated volume would be key to reducing the complication risk.
As RIHT is an endocrine complication it is not sufficient to consider only the thyroid gland as an OAR, since irradiation of the pituitary gland is also associated with an increased risk of RIHT. Thus, several of the studies included in this analysis excluded patients with nasopharyngeal cancer or limited the inclusion to patients with doses to the pituitary gland <40 Gy, as this is considered an important cutoff point.43–45
A meta-analysis of 33 published studies performed by Vogelius et al. in 2011 identified female gender, partial or hemi-thyroidectomy, Caucasian descent and lymphangiography as significant risk factors for hypothyroidism, whereas age and chemotherapy were not.50 They also found a significant dose-response effect but highlighted the considerable uncertainty in the NTCP model parameters when comparing the results from different studies.
There was no QUANTEC report that covered RIHT.
Hearing loss
Several post-QUANTEC studies of radiation-induced hearing loss were identified but only one that reported quantitative dose-response models.51 Some studies reported on hearing loss for patients treated with Gamma Knife radiosurgery and are not discussed further in this review, but referred to here for the interested reader.52,53
De Marzi et al. reported dose-response models separately based on considering the inner ear, cochlea or internal auditory canal as the critical OAR for 140 patients treated to the skull base with a mixture of photon and proton RT.51 We only include the models based on inner ear and cochlea in Table 2 since these performed considerably better than the model based on the internal auditory canal, with areas under the receiver operating characteristics curve of 0.86 and 0.81 compared to 0.72, respectively. The mean cochlear dose for patients with grade 1–2 hearing loss was 54.6 ± 16 GyRBE (with a relative biological effectiveness (RBE) of 1.1 used for protons) and 36.8 ± 14 GyRBE for those without.
Another study of 17 patients treated with post-parotidectomy RT showed no evidence of ipsilateral or contralateral hearing loss during 2 years of follow-up, with all patients receiving a mean cochlear dose <45 Gy.54 Furthermore, Champ et al. presented audiometric evaluation data from 154 patients treated for acoustic neuromas with RT in 1.8 Gy fractions.55 These authors found that separating patients into groups receiving either ≤40 Gy or >40 Gy to be a significant predictor of hearing impairment.
Although there was no clear threshold dose determined in the QUANTEC report on radiation-induced hearing loss it recommends that the mean cochlear dose be kept ≤45 Gy, or ≤35 Gy if possible9, which seems to agree well with the results from the studies included in this updated review.
Optic neuropathy/vision impairment
We identified only a limited number of studies reporting on radiation-induced optic neuropathy (RION) or visual impairment after standard fractionation RT and neither presented a quantitative dose-response model. In a study by Farzin et al. in 2016 only two out of 213 patients treated for meningioma had visual problems attributed to RT, both with maximum doses to the optic nerve and chiasm close or just above 54 Gy.56 Another study found that IMRT resulted in lower doses to the optic nerves and chiasm, although not significantly affecting the risk of RION in patients with meningioma and pituitary adenoma.57
In a small study of 10 meningioma patients higher mean eye dose appeared to be related to deteriorated vision, although with such limited patient numbers this should be considered anecdotal.58
The QUANTEC report on optic nerves and chiasm concluded that RION was rare if maximum doses to these structures were <55 Gy with standard fractionation, with a marked increase in risk >60 Gy.59 The limited number of reports on RION post QUANTEC could reasonably be explained as a result of the widespread implementation of these recommendations and few patients receiving doses to the optic nerves and chiasm above the recommended 55 Gy constraint.
Fatigue
Fatigue has long been a known serious consequence of RT, especially in combination with concurrent chemotherapy. However, addressing this complication as dose-dependent phenomenon and attempting to identify the responsible OARs has only recently been undertaken and as such this was not addressed specifically in the QUANTEC reports. The definition typically involves the feeling of fatigue not relieved by rest, or how much fatigue impacts ones QoL on a daily basis, and can be scored using the CTCAE scale or using validated patient questionnaire instruments.60,61
In a retrospective analysis of the data from the PARSPORT trial, investigators found a significant association between mean dose to the brainstem, cerebellum and posterior fossa and risk of grade ≥2 fatigue, translating into an increased risk for patients receiving IMRT.62 In a multivariate analysis of patients from the same trial, the mean dose to the cerebellum, basal ganglia and pituitary gland were significantly associated with increased risk of fatigue when adjusting for multiple clinical factors, albeit in a limited sample of only 40 patients.63 Although this study found no association between chemotherapy and fatigue, a prospective study based on patient-reported questionnaires found significantly worse fatigue scores in patients receiving concurrent chemotherapy, compared to RT alone.61
Before quantitative dose-response models can be derived, the challenge remains to identify the pertinent OAR for radiation-induced fatigue and validation studies of the potential central nervous system OARs identified in the aforementioned studies should be undertaken, as well as further exploratory analyses and small-animal investigations.64
Secondary cancer
Studies of secondary cancer following RT require large cohorts with long follow-up, mainly due to the fact that they are rare events often associated with very long latency. We did, however, identify a few reports analyzing the risk of radiation-induced secondary cancers in the head and neck area, with dose-response models detailed in Table 3.
The study by Morton et al. found an excess odds ratio per Gy of 0.09 (95% CI: 0.04, 0.16) for secondary esophageal cancer in a cohort of breast cancer survivors, along with a protective effect of hormonal therapy and a multiplicative effect of smoking and radiation.65 Several studies have identified the importance of secondary thyroid cancer in childhood cancer patients treated with RT, but this does not appear to translate to adult patients.66
The study by Schneider et al. combined cohort data from atomic bomb survivors and long-term survivors of Hodgkin’s lymphoma treated with RT to estimate dose-response parameters for several different anatomical sites, including mouth and pharynx and salivary glands.67 Importantly, any models estimating the risk of secondary cancer induction are subject to considerable uncertainty not only because of sparse data but also due to the inherent assumption that the limited radiation exposure information from long-term follow-up studies can be translated to modern RT treatment settings.
Comparing various NTCP models for multiple endpoints
When rating different RT options for head and neck cancer patients a variety of endpoints need to be considered, as demonstrated by the multitude of normal tissue complications included in this review. While hard endpoints such as brainstem necrosis or spinal cord myelopathy should always be prioritized it is less clear whether dysphagia, xerostomia, mucositis or esophagitis should be considered more important for treatment comparisons. In addition several NTCP models may exist for the same endpoint and while previous efforts have attempted to compare models based on the quality of input data and appropriateness in relation to the patients being studied17, this remains a pertinent issue. Here, we applied the computation of a relevance score to compare various NTCP models with regards to their relevance in estimating the risk of radiation-induced toxicities after head and neck RT. The checklist of items from the TRIPOD consensus statement highlights the variation in statistical methodology and reporting in the various modeling studies and can be found as supplementary table S1.
For comparing proton therapy to photon therapy options Blanchard et al. decided to test various NTCP models derived from photon treatments on a patient cohort treated with proton therapy.16 They found that the photon-derived models performed well for estimating the risk of dysphagia, xerostomia and hypothyroidism, but less well for acute mucositis. When comparing NTCP estimates between photon and proton therapy another validation of the results would be concordance of results between different models evaluating the same endpoints, whereas discordant results would suggest that one treatment option might not be clearly preferable.
When it comes to simultaneously evaluating multiple different endpoints one approach could be to estimate the relative impact on quality of life from the various endpoints for example as quality-adjusted life years.68 This would potentially allow for a single measure common scale assessment of all different endpoints, and would go along the lines of implementing patient-reported outcomes as key components in radiation oncology decision making.69,70
Alternative strategies to overcome NTCP modeling limitations
As noted in the QUANTEC reports and many of the papers included in this review, classical NTCP modeling is subject to several limitations such as dealing with highly correlated dosimetric parameters, or the lack of spatial information in dose-volume histograms.
To overcome the limitation of correlated data, a functional data analysis approach was implemented by Dean et al. to model the risk of grade ≥3 dysphagia and oral mucositis.71 This approach models dose-volume data as a continuous function, with components determined using unsupervised (principal components analysis), or supervised (partial least squares regression) analysis, as well as including clinical covariates in the model. Reducing the dimensionality of the dose data in this manner should provide more robust estimates of dose-response parameters and improve generalizability of the model, but does add more complexity to the interpretation of the model parameters.
Another intriguing alternative approach is presented by Buettner et al. in which they demonstrate a novel morphological model that includes regional dose variations throughout the parotid gland to predict patient-reported xerostomia.72 Their results show that including the spatial information of the dose distribution revealed areas of the parotid gland with apparently increased radiation sensitivity, and performed significantly better than a model based on mean parotid dose. These results are in agreement with the recent evidence of stem cell regions within the parotid gland that may be chiefly responsible for the salivary function and post-RT recovery.34 In contrast, the study by Dean et al. showed similar performance between models based on dose-volume data and those incorporating spatial dose information to predict grade ≥3 oral mucositis.36
Recent efforts have also been focused on moving away from OAR-based dose-response modeling, in favor of voxel-based analyses correlating risk of toxicity with three-dimensional dose maps. Monti et al. performed such an analysis for acute grade ≥3 dysphagia and found significantly higher doses in voxels corresponding to the anatomical location of the cricopharyngeus muscle and cervical esophagus.73
Conclusions
Given the variety of available dose-response models published since the QUANTEC reports and the increased awareness of the importance of model validation, dealing with correlated dosimetric data, spatial variation in radiation sensitivity and the importance of patient-reported outcomes, data-driven decision making is becoming a reality in modern day radiation oncology. The NTCP estimates provided in the supplementary material illustrate that models for hypothyroidism, oral mucositis, and xerostomia (depending on time point and definition) are generally consistent, whereas the estimates for dysphagia and esophagitis vary considerably between models. When models disagree the corresponding relevance score should provide an indication as to which models are more reliable in the setting of modern day head and neck RT.
Despite this, it remains vital to encourage data sharing to allow sufficiently powered validation studies, and to implement prospective model testing in trials comparing different treatment approaches, so that the models’ ability to truly distinguish between optimal and sub-optimal treatment options can be evaluated.
Supplementary Material
Acknowledgments
Dr. Brodin acknowledges support from the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071 and UL1TR001073.
Footnotes
Conflicts of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Garg MK, Glanzman J, Kalnicki S. The evolving role of positron emission tomography-computed tomography in organ-preserving treatment of head and neck cancer. Seminars in nuclear medicine. 2012;42(5):320–7. doi: 10.1053/j.semnuclmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Gregoire V, Langendijk JA, Nuyts S. Advances in Radiotherapy for Head and Neck Cancer. J Clin Oncol. 2015;33(29):3277–84. doi: 10.1200/JCO.2015.61.2994. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y, Tome WA. Risk-adaptive optimization: selective boosting of high-risk tumor subvolumes. Int J Radiat Oncol Biol Phys. 2006;66(5):1528–42. doi: 10.1016/j.ijrobp.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal DI, Harari PM, Giralt J, Bell D, Raben D, Liu J, et al. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol. 2016;34(12):1300–8. doi: 10.1200/JCO.2015.62.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto DE, Kessler ML, Piert M, Eisbruch A. Correlation between pretreatment FDG-PET biological target volume and anatomical location of failure after radiation therapy for head and neck cancers. Radiother Oncol. 2008;89(1):13–8. doi: 10.1016/j.radonc.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer F, Fortin A, Gelinas M, Nabid A, Brochet F, Tetu B, et al. Health-related quality of life as a survival predictor for patients with localized head and neck cancer treated with radiation therapy. J Clin Oncol. 2009;27(18):2970–6. doi: 10.1200/JCO.2008.20.0295. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Hu C, Eisbruch A. Organ-sparing radiation therapy for head and neck cancer. Nature reviews Clinical oncology. 2011;8(11):639–48. doi: 10.1038/nrclinonc.2011.106. [DOI] [PubMed] [Google Scholar]
- 8.Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S3–9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S50–7. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S36–41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rancati T, Schwarz M, Allen AM, Feng F, Popovtzer A, Mittal B, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S64–9. doi: 10.1016/j.ijrobp.2009.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S86–93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA oncology. 2015;1(8):1051–9. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt H, Merkel D, Koehler M, Flechtner HH, Sigle J, Klinge B, et al. PRO-ONKO-selection of patient-reported outcome assessments for the clinical use in cancer patients-a mixed-method multicenter cross-sectional exploratory study. Support Care Cancer. 2015 doi: 10.1007/s00520-015-3055-4. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard P, Wong AJ, Gunn GB, Garden AS, Mohamed AS, Rosenthal DI, et al. Toward a model-based patient selection strategy for proton therapy: External validation of photon-derived normal tissue complication probability models in a head and neck proton therapy cohort. Radiother Oncol. 2016;121(3):381–6. doi: 10.1016/j.radonc.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodin NP, Maraldo MV, Aznar MC, Vogelius IR, Petersen PM, Bentzen SM, et al. Interactive decision-support tool for risk-based radiation therapy plan comparison for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2014;88(2):433–45. doi: 10.1016/j.ijrobp.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107(3):267–73. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Annals of internal medicine. 2015;162(10):735–6. doi: 10.7326/L15-5093-2. [DOI] [PubMed] [Google Scholar]
- 20.Christianen ME, Schilstra C, Beetz I, Muijs CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107–14. doi: 10.1016/j.radonc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Mazzola R, Ricchetti F, Fiorentino A, Fersino S, Giaj Levra N, Naccarato S, et al. Dose-volume-related dysphagia after constrictor muscles definition in head and neck cancer intensity-modulated radiation treatment. Br J Radiol. 2014;87(1044):20140543. doi: 10.1259/bjr.20140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen HR, Jensen K, Aksglaede K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiother Oncol. 2013;107(3):288–94. doi: 10.1016/j.radonc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Vainshtein JM, Moon DH, Feng FY, Chepeha DB, Eisbruch A, Stenmark MH. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91(5):925–33. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otter S, Schick U, Gulliford S, Lal P, Franceschini D, Newbold K, et al. Evaluation of the Risk of Grade 3 Oral and Pharyngeal Dysphagia Using Atlas-Based Method and Multivariate Analyses of Individual Patient Dose Distributions. Int J Radiat Oncol Biol Phys. 2015;93(3):507–15. doi: 10.1016/j.ijrobp.2015.07.2263. [DOI] [PubMed] [Google Scholar]
- 25.Anderson NJ, Wada M, Schneider-Kolsky M, Rolfo M, Joon DL, Khoo V. Dose-volume response in acute dysphagia toxicity: Validating QUANTEC recommendations into clinical practice for head and neck radiotherapy. Acta Oncol. 2014;53(10):1305–11. doi: 10.3109/0284186X.2014.933874. [DOI] [PubMed] [Google Scholar]
- 26.Huang EX, Bradley JD, El Naqa I, Hope AJ, Lindsay PE, Bosch WR, et al. Modeling the risk of radiation-induced acute esophagitis for combined Washington University and RTOG trial 93–11 lung cancer patients. Int J Radiat Oncol Biol Phys. 2012;82(5):1674–9. doi: 10.1016/j.ijrobp.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwint M, Uyterlinde W, Nijkamp J, Chen C, de Bois J, Sonke JJ, et al. Acute esophagus toxicity in lung cancer patients after intensity modulated radiation therapy and concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;84(2):e223–8. doi: 10.1016/j.ijrobp.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Wijsman R, Dankers F, Troost EG, Hoffmann AL, van der Heijden EH, de Geus-Oei LF, et al. Multivariable normal-tissue complication modeling of acute esophageal toxicity in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy. Radiother Oncol. 2015;117(1):49–54. doi: 10.1016/j.radonc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Wu AJ, Williams E, Modh A, Foster A, Yorke E, Rimner A, et al. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiother Oncol. 2014;112(2):267–71. doi: 10.1016/j.radonc.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105(1):101–6. doi: 10.1016/j.radonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Chen WC, Lai CH, Lee TF, Hung CH, Liu KC, Tsai MF, et al. Scintigraphic assessment of salivary function after intensity-modulated radiotherapy for head and neck cancer: correlations with parotid dose and quality of life. Oral Oncol. 2013;49(1):42–8. doi: 10.1016/j.oraloncology.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Moiseenko V, Wu J, Hovan A, Saleh Z, Apte A, Deasy JO, et al. Treatment planning constraints to avoid xerostomia in head-and-neck radiotherapy: an independent test of QUANTEC criteria using a prospectively collected dataset. Int J Radiat Oncol Biol Phys. 2012;82(3):1108–14. doi: 10.1016/j.ijrobp.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beetz I, Steenbakkers RJ, Chouvalova O, Leemans CR, Doornaert P, van der Laan BF, et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014;53(5):597–604. doi: 10.3109/0284186X.2013.831186. [DOI] [PubMed] [Google Scholar]
- 34.van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Science translational medicine. 2015;7(305):305ra147. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhide SA, Gulliford S, Schick U, Miah A, Zaidi S, Newbold K, et al. Dose-response analysis of acute oral mucositis and pharyngeal dysphagia in patients receiving induction chemotherapy followed by concomitant chemo-IMRT for head and neck cancer. Radiother Oncol. 2012;103(1):88–91. doi: 10.1016/j.radonc.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Dean JA, Wong KH, Welsh LC, Jones AB, Schick U, Newbold KL, et al. Normal tissue complication probability (NTCP) modelling using spatial dose metrics and machine learning methods for severe acute oral mucositis resulting from head and neck radiotherapy. Radiother Oncol. 2016;120(1):21–7. doi: 10.1016/j.radonc.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanguineti G, Sormani MP, Marur S, Gunn GB, Rao N, Cianchetti M, et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):235–42. doi: 10.1016/j.ijrobp.2011.06.2000. [DOI] [PubMed] [Google Scholar]
- 38.Dean JA, Welsh LC, McQuaid D, Wong KH, Aleksic A, Dunne E, et al. Assessment of fully-automated atlas-based segmentation of novel oral mucosal surface organ-at-risk. Radiother Oncol. 2016;119(1):166–71. doi: 10.1016/j.radonc.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean JA, Welsh LC, Wong KH, Aleksic A, Dunne E, Islam MR, et al. Normal Tissue Complication Probability (NTCP) Modelling of Severe Acute Mucositis using a Novel Oral Mucosal Surface Organ at Risk. Clinical oncology. 2017;29(4):263–73. doi: 10.1016/j.clon.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musha A, Shimada H, Shirai K, Saitoh J, Yokoo S, Chikamatsu K, et al. Prediction of Acute Radiation Mucositis using an Oral Mucosal Dose Surface Model in Carbon Ion Radiotherapy for Head and Neck Tumors. PLoS One. 2015;10(10):e0141734. doi: 10.1371/journal.pone.0141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strigari L, Pedicini P, D’Andrea M, Pinnaro P, Marucci L, Giordano C, et al. A new model for predicting acute mucosal toxicity in head-and-neck cancer patients undergoing radiotherapy with altered schedules. Int J Radiat Oncol Biol Phys. 2012;83(5):e697–702. doi: 10.1016/j.ijrobp.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Yahya S, Benghiat H, Nightingale P, Tiffany M, Sanghera P, Hartley A. Does Dose to an Oral Mucosa Organ at Risk Predict the Duration of Grade 3 Mucositis after Intensity-modulated Radiotherapy for Oropharyngeal Cancer? Clinical oncology. 2016;28(12):e216–e9. doi: 10.1016/j.clon.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Bakhshandeh M, Hashemi B, Mahdavi SR, Nikoofar A, Vasheghani M, Kazemnejad A. Normal tissue complication probability modeling of radiation-induced hypothyroidism after head-and-neck radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(2):514–21. doi: 10.1016/j.ijrobp.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Boomsma MJ, Bijl HP, Christianen ME, Beetz I, Chouvalova O, Steenbakkers RJ, et al. A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys. 2012;84(3):e351–6. doi: 10.1016/j.ijrobp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Ronjom MF, Brink C, Bentzen SM, Hegedus L, Overgaard J, Petersen JB, et al. External validation of a normal tissue complication probability model for radiation-induced hypothyroidism in an independent cohort. Acta Oncol. 2015;54(9):1301–9. doi: 10.3109/0284186X.2015.1064160. [DOI] [PubMed] [Google Scholar]
- 46.Akgun Z, Atasoy BM, Ozen Z, Yavuz D, Gulluoglu B, Sengoz M, et al. V30 as a predictor for radiation-induced hypothyroidism: a dosimetric analysis in patients who received radiotherapy to the neck. Radiat Oncol. 2014;9:104. doi: 10.1186/1748-717X-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujiwara M, Kamikonya N, Odawara S, Suzuki H, Niwa Y, Takada Y, et al. The threshold of hypothyroidism after radiation therapy for head and neck cancer: a retrospective analysis of 116 cases. J Radiat Res. 2015;56(3):577–82. doi: 10.1093/jrr/rrv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MY, Yu T, Wu HG. Dose-volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer. Japanese journal of clinical oncology. 2014;44(4):331–7. doi: 10.1093/jjco/hyt235. [DOI] [PubMed] [Google Scholar]
- 49.Cella L, Liuzzi R, Conson M, D’Avino V, Salvatore M, Pacelli R. Development of multivariate NTCP models for radiation-induced hypothyroidism: a comparative analysis. Radiat Oncol. 2012;7:224. doi: 10.1186/1748-717X-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogelius IR, Bentzen SM, Maraldo MV, Petersen PM, Specht L. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer. 2011;117(23):5250–60. doi: 10.1002/cncr.26186. [DOI] [PubMed] [Google Scholar]
- 51.De Marzi L, Feuvret L, Boule T, Habrand JL, Martin F, Calugaru V, et al. Use of gEUD for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br J Radiol. 2015;88(1048):20140413. doi: 10.1259/bjr.20140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown M, Ruckenstein M, Bigelow D, Judy K, Wilson V, Alonso-Basanta M, et al. Predictors of hearing loss after gamma knife radiosurgery for vestibular schwannomas: age, cochlear dose, and tumor coverage. Neurosurgery. 2011;69(3):605–13. doi: 10.1227/NEU.0b013e31821a42f3. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 53.Hayden Gephart MG, Hansasuta A, Balise RR, Choi C, Sakamoto GT, Venteicher AS, et al. Cochlea radiation dose correlates with hearing loss after stereotactic radiosurgery of vestibular schwannoma. World Neurosurg. 2013;80(3–4):359–63. doi: 10.1016/j.wneu.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jereczek-Fossa BA, Rondi E, Zarowski A, D’Onofrio A, Alterio D, Ciocca M, et al. Prospective study on the dose distribution to the acoustic structures during postoperative 3D conformal radiotherapy for parotid tumors: dosimetric and audiometric aspects. Strahlenther Onkol. 2011;187(6):350–6. doi: 10.1007/s00066-011-2170-5. [DOI] [PubMed] [Google Scholar]
- 55.Champ CE, Shen X, Shi W, Mayekar SU, Chapman K, Werner-Wasik M, et al. Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery. 2013;73(3):489–96. doi: 10.1227/NEU.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 56.Farzin M, Molls M, Kampfer S, Astner S, Schneider R, Roth K, et al. Optic toxicity in radiation treatment of meningioma: a retrospective study in 213 patients. J Neurooncol. 2016;127(3):597–606. doi: 10.1007/s11060-016-2071-7. [DOI] [PubMed] [Google Scholar]
- 57.Astradsson A, Wiencke AK, Munck af Rosenschold P, Engelholm SA, Ohlhues L, Roed H, et al. Visual outcome after fractionated stereotactic radiation therapy of benign anterior skull base tumors. J Neurooncol. 2014;118(1):101–8. doi: 10.1007/s11060-014-1399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abouaf L, Girard N, Lefort T, D’Hombres A, Tilikete C, Vighetto A, et al. Standard-fractionated radiotherapy for optic nerve sheath meningioma: visual outcome is predicted by mean eye dose. Int J Radiat Oncol Biol Phys. 2012;82(3):1268–77. doi: 10.1016/j.ijrobp.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28–35. doi: 10.1016/j.ijrobp.2009.07.1753. [DOI] [PubMed] [Google Scholar]
- 60.Aynehchi BB, Obourn C, Sundaram K, Bentsianov BL, Rosenfeld RM. Validation of the Modified Brief Fatigue Inventory in head and neck cancer patients. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;148(1):69–74. doi: 10.1177/0194599812460985. [DOI] [PubMed] [Google Scholar]
- 61.Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer. 2014;120(13):1975–84. doi: 10.1002/cncr.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gulliford SL, Miah AB, Brennan S, McQuaid D, Clark CH, Partridge M, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: an analysis from the PARSPORT Phase III trial. Radiother Oncol. 2012;104(2):205–12. doi: 10.1016/j.radonc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Powell C, Schick U, Morden JP, Gulliford SL, Miah AB, Bhide S, et al. Fatigue during chemoradiotherapy for nasopharyngeal cancer and its relationship to radiation dose distribution in the brain. Radiother Oncol. 2014;110(3):416–21. doi: 10.1016/j.radonc.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 64.Renner M, Feng R, Springer D, Chen MK, Ntamack A, Espina A, et al. A murine model of peripheral irradiation-induced fatigue. Behavioural brain research. 2016;307:218–26. doi: 10.1016/j.bbr.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morton LM, Gilbert ES, Hall P, Andersson M, Joensuu H, Vaalavirta L, et al. Risk of treatment-related esophageal cancer among breast cancer survivors. Ann Oncol. 2012;23(12):3081–91. doi: 10.1093/annonc/mds144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. 1995. Radiat Res. 2012;178(2):AV43–60. doi: 10.1667/rrav05.1. [DOI] [PubMed] [Google Scholar]
- 67.Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Model. 2011;8:27. doi: 10.1186/1742-4682-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health policy and planning. 2006;21(5):402–8. doi: 10.1093/heapol/czl018. [DOI] [PubMed] [Google Scholar]
- 69.Niska JR, Halyard MY, Tan AD, Atherton PJ, Patel SH, Sloan JA. Electronic patient-reported outcomes and toxicities during radiotherapy for head-and-neck cancer. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2017 doi: 10.1007/s11136-017-1528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shuman AG, Larkin K, Thomas D, Palmer FL, Fins JJ, Baxi SS, et al. Patient Reflections on Decision Making for Laryngeal Cancer Treatment. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017;156(2):299–304. doi: 10.1177/0194599816683377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dean JA, Wong KH, Gay H, Welsh LC, Jones AB, Schick U, et al. Functional Data Analysis Applied to Modeling of Severe Acute Mucositis and Dysphagia Resulting From Head and Neck Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;96(4):820–31. doi: 10.1016/j.ijrobp.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buettner F, Miah AB, Gulliford SL, Hall E, Harrington KJ, Webb S, et al. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol. 2012;103(1):82–7. doi: 10.1016/j.radonc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Monti S, Palma G, D’Avino V, Gerardi M, Marvaso G, Ciardo D, et al. Voxel-based analysis unveils regional dose differences associated with radiation-induced morbidity in head and neck cancer patients. Scientific reports. 2017;7(1):7220. doi: 10.1038/s41598-017-07586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.