Abstract
Elevated levels of hypoxia inducible factor-1 (HIF1) are linked to tumor metastasis, angiogenesis, poor patient prognosis and response to chemotherapy. HIF1α is a master regulator of the hypoxic response, including in cancer cells, through transcriptional activation of several target genes. Previously, we identified compound CJ-3k with high anti-HIF1α activity based on the structure of a well-known HIF1α inhibitor, YC-1. In this study, the CJ-3k scaffold was systematically modified to explore the structure–activity relationships. Fifty-three new CJ-3k analogs were synthesized and screened for their anti-HIF-1α activity in a luciferase-transfected human breast cancer cell line (MDA-MB-231). Some of these new analogs have a significantly greater activity than that of CJ-3k and hold potential for development as new therapeutic agents for the treatment of cancer.
Keywords: Hypoxia, benzimidazoles, HIF-1α inhibitors, breast cancer
Mammalian cells usually require adequate levels of oxygen to execute aerobic metabolism and energy production to support their biological functions. Solid tumors are characterized by an aggressive rate of proliferation and the formation of large solid masses which obstruct the vascularity surrounding them. Consequently, the regular normal blood supply into tumor cells is impaired, resulting in a low oxygen level, hypoxia (1, 2). Under this unique microenvironment of hypoxia, intra-tumoral cells start to activate the transcriptional factor, hypoxia inducible factor-1 (HIF1) that mediates the transcription of several target genes. These genes encode different carriers, hormones and enzymes assisting hypoxic tumor cells to carry-out their essential biological processes (glycolysis, angiogenesis, erythropoiesis, and cell migration) despite the deprivation of oxygen (1, 3–8).
HIF1 is a heterodimer transcription factor belonging to the basic-helix-loop-helix protein superfamily (9). It is composed of two subunits: the HIF1α subunit, which is the oxygen-sensitive subunit, and the HIF1β subunit, which is constitutively expressed (9). Under normoxia, HIF1α is continually degraded via hydroxylation, ubiquitination and proteasomal degradation due to its short half-life (5 minutes) (10). Under hypoxic conditions, HIF1α is stabilized and dimerized with HIF1β. The HIF1 heterodimer is subsequently translocated to the nucleus where it binds with co-factors p300/cAMP-response element-binding protein, forming the active transcription complex. This assembled complex is able to interact with hypoxia-response elements (HRE) to initiate their transcription (7, 11).
HIF1α is highly expressed in various types of solid tumor and high levels of HIF1α have been strongly associated with tumor angiogenesis, tumor initiation, progression, invasion, metastasis, and drug resistance (12, 13). In the past two decades, several HIF1α inhibitors have been developed which interrupt the HIF1 pathway at different levels, however, currently there is no Food and Drug Administration-approved drug that selectively targets HIF1α in hypoxic tumor cells (14).
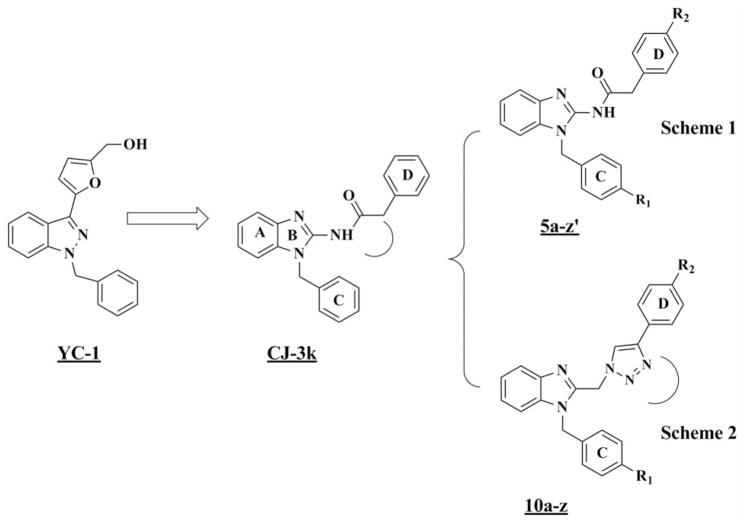
Previously, we identified compound CJ-3k (15) to be a novel HIF1α inhibitor based on the structure of a well-known HIF1α inhibitor, 3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) (16–20).
Here we systematically modified the structure of CJ-3k in order to elucidate the structure–activity relationships (SAR) for this scaffold. We designed and synthesized 53 new CJ-3k analogs and tested their ability to reduce HIF1α transcriptional activity using the triple-negative breast cancer cell line MDA-MB-231 as our cancer model.
Materials and Methods
Reagents and materials
Unless otherwise mentioned, all reagents and starting materials were obtained from commercial sources; Sigma-Aldrich (St. Louis, MO, USA), Alfa Aesar (Ward Hill, MA, USA), and Fischer Scientific (Pittsburg, PA, USA) and used as received (analytical reagents) without further purification. High performance liquid chromatography or reagent-grade solvents were purchased from either Sigma-Aldrich or Fisher Scientific and used without further purification. Reaction progress was monitored by thin-layer chromatography on aluminum-backed Uniplates (Analtech, Newark, DE, USA), and spots were visualized using UV light (254 and 365 nm). Column chromatography was performed with silica gel (particle size). Flash chromatography was carried out using Merck silica gel (230–400 mesh). Electrospray ionization (ESI) spectrometry was determined on a Bruker Esquire LC Ion Trap spectrometer (Billerica, MA, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance II spectrometer (400 MHz) (Bruker, Billerica, MA, USA). The chemical shifts are defined as δ values (parts per million) relative to tetramethylsilane (TMS) internal standard. Significant 1H-NMR data are reported in the following order: multiplicity (s, singlet; d, doublet; t, triplet; m, multiplet), number of protons, type of proton. High resolution mass spectrometry (HRMS) spectra were conducted on a Waters Xevo G2 Quadrupole Time-of-Flight LC/MS instrument (Milford, MA, USA). All reported yields are for purified products.
Design and synthesis of compounds 5a to 5z′ and 10a to 10z
We took two distinct approaches in order to design and synthesize new CJ-3k analogs as shown in Figure 1: Scheme 1: we modified the CJ-3k scaffold by adding different substituent groups to rings C and D to explore the effects on their biological activities (compounds 5a to 5z′); Scheme 2: we used bioisostereic replacement of the amide linker by a triazole ring to explore the conformational flexibility for this scaffold (compounds 10a to 10z).
Figure 1.
Design of novel hypoxia-inducible factor1α inhibitors based on the scaffold of YC-1 and CJ-3k.
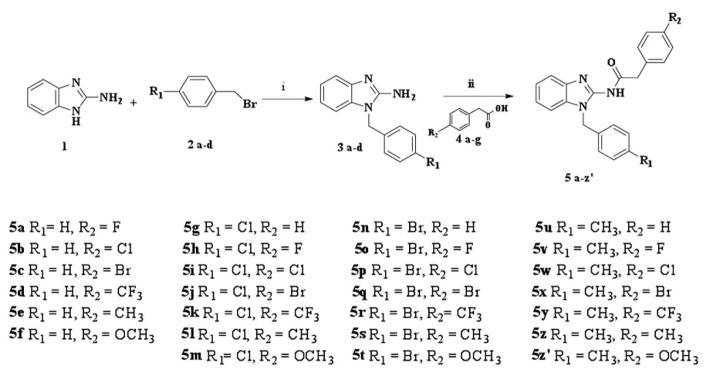
Figure 2.
Scheme 1: Synthetic route for compounds 5a–z′. Reagents and conditions: (i) potassium hydroxide, ethanol, room temperature, 18 h (ii) N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, hydroxybenzotriazole, dimethyl formamide, room temperature, 24 h.
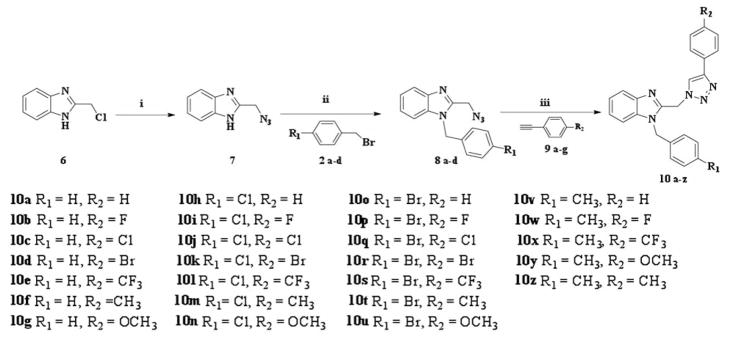
Figure 3.
Scheme 2: Synthetic route for compounds 10a–z. Reagents and conditions: (i) sodium azide, dimethyl sulphoxide, room temperature, 5 h (ii) potassium hydroxide, ethanol, room temperature, 18 h (iii) copper sulphate pentahydrate, sodium ascorbate, dichloromethane/water/tertiary butanol, room temperature, 24 h.
Scheme 1
Benzylation of compound 1 was carried out at room temperature by stirring with the corresponding benzyl bromide derivatives (2a–2d) in ethanol and potassium hydroxide flakes for 18 h (21–24). Substitution occurred exclusively at the N-1 position, yielding 50–70% yields of the pure intermediates 3a–3d after column purification. The final compounds 5a to 5z′ were synthesized by the amidation of the appropriate phenyl acetic acid derivatives 4a–4g with the intermediate amines 3a–3d using the traditional coupling reagents N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI.HCl) and 4-hydroxy-1H-benzotriazole (HOBt) in dimethyl formamide (DMF) (25, 26). Condensation proceeded successfully within 24 h yielding compounds 5a–z′ which were subsequently purified via crystallization (Figure 2).
General procedure for the preparation of compounds 3a–3d
2-Aminobenzimidazole (1) (6 mmol), potassium hydroxide flakes (9 mmol) and the appropriate benzyl bromide derivative (2a–2d) (6.3 mmol) were stirred in 15 ml ethanol at room temperature for 18 h. The solution was then concentrated under vacuum and washed with brine solution (2×20 ml). The formed product was extracted three times with ethyl acetate. The organic layer was separated, dried with magnesium sulfate and concentrated under vacuum to remove the ethyl acetate. The crude product was purified by flash column chromatography [0%–6–8% methanol/dichloromethane (DCM) eluent] to yield compounds 3a–3d as white solid.
General procedure for the synthesis of N-(1-phenyl-1H-benzo[d]imidazol-2-yl)-2-(4-substituted phenyl) acetamide (compounds 5a–5z′, Scheme 1)
A mixture of the appropriate 4-substituted phenyl acetic acid 4 (1 mmol), and HOBt (0.20 g, 1.5 mmol) were dissolved in DMF. The reaction mixture was cooled to 0°C, EDCI.HCl (0.29 g, 1.5 mmol) was added and the solution was stirred for 1 h. Then the appropriate amine 3 (1.05 mmol) and triethanol amine (TEA) (0.2 ml, 0.15 g, 1.5 mmol) were added to the solution. The resulting reaction mixture was stirred overnight at room temperature (RT), and then the solvent was removed under reduced pressure. The solid was washed with water, filtered, and dried. The products were purified by crystallization from methanol.
Scheme 2
The 2-azidomethyl benzimidazole (7) was prepared via nucleophilic substitution of 2-chloromethyl benzimidazole (6) using sodium azide solution in dimethyl sulphoxide (DMSO) (27). Benzylation of 7 at N-1 position was performed as described previously (21–24) giving the target compounds 8a–8d. Then click chemistry was employed to afford the final compounds 10a–10z. This was accomplished via the reaction of the intermediates 8a–8d with the selected phenylacetylenes (9a–9g) in the presence of copper (II)-ascorbate active catalyst in an aqueous-based solvent system (Figure 3). Compounds 10a–10z were separated and further purified using flash chromatography column (28, 29).
2-(Azidomethyl)-1H-benzimidazole (7)
To a 0.5 M solution of NaN3 in DMSO (0.468 g, 7.2 mmol) was added 2-chloromethyl benzimidazole (6) (0.99 g, 6 mmol), and the resulting mixture was stirred at room temperature for 5 h. After completion, the reaction mixture was extracted with diethyl ether. The combined organic extracts were washed with brine and water, and dried over sodium sulfate. After the organic solvent was removed under reduced pressure, the residue was purified by flash chromatography to provide the azido compound 7.
General procedure for the preparation of azido compounds 8a–8d
2-(Azidomethyl)-1H-benzoimidazole (7) (1 mmol), potassium hydroxide flakes (1.5 mmol) and the appropriate benzyl bromide derivative (2a–2d) (1.05 mmol) were stirred in ethanol at r.t. for 18–20 h. The solution was then concentrated under vacuum and washed with brine solution. The formed product was extracted three times with ethyl acetate. The organic extracts were combined, separated, dried with sodium sulphate and concentrated under vacuum to remove the ethyl acetate. The crude product was purified by flash chromatography (50% ethyl acetate/hexane eluent), to yield compounds 8a–8d as yellow solid.
General procedure for the preparation of 1-(4-substituted benzyl)-2-((4-(4-substitutedphenyl)-1H-1, 2,3-triazol-1-yl) methyl)-1H-benzo[d]imidazoles (Compounds 10a–10z, Scheme 2)
In a 1:1:1 mixture of t-butyl alcohol/water/DCM (10 ml), the appropriate terminal alkyne (1.0 equiv.) was dissolved. The appropriate azido compound (1.2 equiv.) was added, followed by the addition of CuSO4·5H2O (15%) and sodium ascorbate (45%). The reaction mixture was stirred at room temperature for 24 h. The solution was washed with sat. aqueous NH4Cl solution, then extracted from the aqueous layer with DCM for three times. The organic extracts were combined, separated, dried over with sodium sulphate and concentrated under vacuum to remove the DCM. The crude product was purified by flash chromatography (ethyl acetate/hexane eluent) to yield compounds 10a–10z.
Cell lines
The parental human breast cancer cell line MDA-MB-231 was originally obtained from the American Type Culture Collection (Manassas, VA, USA) and authenticated prior to use in these experiments by DDC Medical (www.ddmedical.com). The metastatic breast cancer cell line MDA-MB-231-HRE-Luc was generated and validated in our laboratory.
Establishment of the aggressive human breast cancer cell line MDA-MB-231-HRE-Luc
The parental human breast cancer cell line MDA-MB-231 was plated in 6-well plate at seeding density of 350,000 cells in 2 ml of growth medium without antibiotics, allowed to grow and reach 90–95% confluence at the time of transfection. The cells were the stably transfected with pGL4.42 vector (Promega, Madison, WI, USA) using the lipid-based transfection reagent Lipofectamine 2000 (Invitrogen-Life Technologies, Inc., Grand Island, NY, USA). Optimal conditions for MDA-MB-231 transfection were 500 μl of a 2.5:1 mixture (reagent: DNA). After 24 h of transfection, the medium was freshly replaced, and cells were allowed to recover for an additional 24 h before the addition of the selection antibiotic marker Hygromycin B. Forty-eight hours following the transfection, Hygromycin B was added to the transfected cells at 1 mg/ml.
Validation of the human breast cancer cell line MDA-MB-231-HRE-Luc
The parental cells (MDA-MB-231) were plated in two 96-well formats in Dulbecco’s modified Eagle’s medium (DMEM) at densities of 5,000, 10,000, and 20,000 cells/well, while the transfected MDA-MB-231-HRE-Luc cells were plated in another two 96-well plates in DMEM supplemented with 1 mg/ml Hygromycin B at the same densities. Cells were allowed to grow and form a monolayer overnight. On the next day, the media (DMEM and DMEM/Hygromycin B) were freshly replaced and cells were incubated for 6 h in normoxia (21% O2, 5% CO2, and 74% N2) at 37°C. Following this, some of the MDA-MB-231 parental cells as well as the transfected cells (MDA-MB-231-HRE-Luc) were incubated for 18 h in hypoxia. Hypoxia was induced in two different ways separately [hypoxia chamber: (1% O2, 5% CO2, and 94% N2) and hypoxia mimic: 300 μM desferroxamine (DFO)]. The other portions of both cell lines were further incubated for another 18 h under normoxia. After these 24 h of incubation, the cells were washed with 100 μl of cold Dulbecco’s phosphate-buffered saline (DPBS) buffer (CORNING, Cellgro Manassas, VA, USA), lysed by adding 20 μl of 1× passive lysis buffer (PLB). Plates were rocked for 15 minutes at 25°C to ensure complete coverage of the cells with the lysis buffer. Luciferase assay reagent was mixed with 20 μl of cell lysate and luminescence intensity was measured by Synergy MxMonochromator-Based Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Assays were performed in triplicates.
Cell culture
MDA-MB-231-HRE-Luc cells were routinely maintained in high-glucose DMEM (CORNING, Cellgro) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA), and 1 mg/ml Hygromycin B (Invitrogen-Life Technologies, Inc.). Cells were incubated at 37°C in a humidified incubator with 5% CO2, 21% O2 in air (normoxia). Hypoxia was induced by flushing the incubator chamber with a mixture of 1% O2, 5% CO2 and 94% N2. Cell growth and morphology were monitored by phase-contrast microscopy.
HIF-dependent luciferase assay
MDA-MB-231-HRE-Luc cells were plated in 96-well plates (BD, Falcon, Franklin Lakes, NJ, USA) at a seeding density of 15,000 cells/well. Cells were allowed to adhere and form a monolayer before adding the test compounds (~70% confluence). After attachment, cells were treated individually with 100 μl of fresh media containing CJ-3k, Chetomin (Cayman Chemicals Ann Arbor, MI, USA) or the test compounds (5f, 5h, 5i, 5p, 5r, and 5q) at different concentrations (100, 30, 10, 3, 1, 0.3, and 0.1 μM). Control samples were treated with cell culture media only. Cells were incubated under normoxia (21% O2, 5% CO2, and 74% N2) at 37°C for 24 h, followed by culture under hypoxia (1% O2, 5% CO2, and 94% N2) for another 24 h. The cells were washed with 100 μl of cold DPBS buffer, then cell lysates were isolated by adding 20 μl of 1× PLB supplemented with 1× of halt protease and phosphatase single-use cocktail (Thermo Scientific) in order to ensure the stability of proteins. Plates were rocked for 30 minutes at 25°C to ensure complete coverage of the cells with the lysis buffer. Subsequently 100 μl of Luciferase assay reagent (Promega) was added to the 20 μl of cell lysate through an automated injector. Luminescence intensity was measured by Synergy MxMonochromator-Based Multi-Mode Microplate Reader (BioTek).
Results
Chemistry
1-Benzyl-1H-benzimidazol-2-amine (3a)
Yield: 70.0%. 1H NMR (400 MHz, DMSO): δ 5.34 (s, 2H, CH2), 6.50 (s, 2H, NH2), 6.80 (t, 1H, Ar-H), 6.89 (t, 1H, Ar-H), 6.95 (dd, 1H, Ar-H), 7.25–7.35 (m, 6H, Ar-Hs). ESI-MS: m/z 223.9 [M+H]+.
1-(4-Chlorobenzyl)-1H-benzimidazol-2-amine (3b)
Yield: 42.3%. 1H NMR (400 MHz, CDCl3): δ 4.57 (s, 2H, NH2), 5.26 (s, 2H, CH2), 6.83 (dt J=7.6 and 1.1Hz, 1H, Ar-H), 6.92 (dt J=7.6 and 1.1Hz, 1H, Ar-H), 7.06 (1H, d J=7.6Hz, Ar-H), 7.14 (1H, d J=7.7Hz, Ar-H), 7.23 (bd J=8.5Hz, 2H, Ar-Hs), 7.40 (bd J=8.5Hz, 2H, Ar-Hs). ESI-MS: m/z 258.0 [M+H]+.
1-(4-Bromobenzyl)-1H-benzimidazol-2-amine (3c)
Yield: 50.0%. 1H NMR (400 MHz, CDCl3): δ 5.14 (s, 2H, CH2), 7.05–7.54 (m, 8H, Ar-Hs). ESI-MS: m/z 302.0 [M+H]+.
1-(4-Methylbenzyl)-1H-benzoimidazol-2-amine (3d)
Yield: 50.0%. 1H NMR (400 MHz, CDCl3): δ 2.34 (s, 3H, CH3), 5.14 (s, 2H, CH2), 6.95–7.55 (m, 8H, Ar-Hs). ESI-MS: m/z 238.0 [M+H]+.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(4-fluorophenyl) acetamide (5a)
Yield=82.3%. 1H NMR (400 MHz, CDCl3): δ 3.79 (s, 2H, CH2-CO); 5.32 (s, 2H, benzylic CH2); 6.98–7.41 (m, 13H, Ar-Hs); ESI-MS: m/z 360.4 [M+H]+. HRMS (ESI) m/z for C22H19FN3O [M+H]+, calcd 360.1512, found 360.1507.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(4-chlorophenyl) acetamide (5b)
Yield=55.3%. 1H NMR (400 MHz, CDCl3): δ 3.78 (s, 2H, CH2-CO); 5.31 (s, 2H, benzylic CH2); 7.14–7.38 (m, 13H, Ar-Hs); ESI-MS: m/z 374.1 [M−H]+. HRMS (ESI) m/z for C22H19ClN3O [M+H]+, calcd 376.1217, found 376.1205.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(4-bromophenyl) acetamide (5c)
Yield=52.3%. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 2H, CH2-CO); 5.30 (s, 2H, benzylic CH2); 7.18–7.48 (m, 13H, Ar-Hs); ESI-MS: m/z 419.9 [M+H]+. HRMS (ESI) m/z for C22H19BrN3O [M+H]+, calcd 420.0712, found 420.0699.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide (5d)
Yield=82.3%. 1H NMR (400 MHz, CDCl3): 3.87 (s, 2H, CH2-CO); 5.31 (s, 2H, benzylic CH2); 7.09–7.66 (m, 13H, Ar-Hs); ESI-MS: m/z 410.3 [M+H]+. HRMS (ESI) m/z for C23H19F3N3O [M+H]+, calcd 410.1480 found 410.1470.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(p-tolyl) acetamide (5e)
Yield=72.0%. 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H, CH3); 3.79 (s, 2H, CH2-CO); 5.32 (s, 2H, benzylic CH2); 7.12–7.27 (m, 6H, Ar-Hs); 7.30–7.35 (m, 7H, Ar-Hs); ESI-MS: m/z 356.4 [M+H]+. HRMS (ESI) m/z for C23H22N3O [M+H]+, calcd 356.1763, found 356.1757.
N-(1-Benzyl-1H-benzo[d]imidazol-2-yl)-2-(4-methoxyphenyl) acetamide (5f)
Yield=75.0%. 1H NMR (400 MHz, CDCl3): δ 3.67 (s, 2H, CH2-CO); 3.72 (s, 3H, OCH3); 5.22 (s, 2H, benzylic CH2); 6.78–7.28 (m, 13H, Ar-Hs); ESI-MS: m/z 394.2 [M+Na]+. HRMS (ESI) m/z for C23H22N3O2 [M+H]+, calcd 372.1712, found 372.1706.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-phenylacetamide (5g)
Yield=23.8%. 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 2H, CH2-CO), 5.26 (s, 2H, benzylic CH2), 7.07–7.26 (m, 8H, Ar-Hs); 7.34–7.37 (m, 3H, Ar-Hs); 7.40–7.44 (m, 2H, Ar-Hs); ESI-MS: m/z 374.0 [M−H]+. HRMS (ESI) m/z for C22H19ClN3O [M+H]+, calcd 376.1217, found 376.1212.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-fluorophenyl) acetamide (5h)
Yield=92.9%. 1H NMR (400 MHz, CDCl3): δ 3.69 (s, 2H, CH2-CO), 5.17 (s, 2H, benzylic CH2), 6.89–6.95 (m, 2H, Ar-Hs); 7.01–7.19 (m, 7H, Ar-Hs); 7.21 (s, 1H, Ar-H); 7.23–7.30 (m, 2H, Ar-Hs); ESI-MS: m/z 394.2 [M+H]+. HRMS (ESI) m/z for C22H18ClFN3O [M+H]+, calcd 394.1122, found 394.1112.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-chlorophenyl) acetamide (5i)
Yield=97.1%. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 2H, CH2-CO), 5.23 (s, 2H, benzylic CH2), 7.10–7.32 (m, 12H, Ar-Hs); ESI-MS: m/z 408.0 [M−H]+. HRMS (ESI) m/z for C22H18Cl2N3O [M+H]+, calcd 410.0827, found 410.0818.
2-(4-Bromophenyl)-N-(1-(4-chlorobenzyl)-1H-benzo[d]imidazol-2-yl) acetamide (5j)
Yield=50.1%. 1H NMR (400 MHz, CDCl3): δ 3.79 (s, 2H, CH2-CO), 5.23 (s, 2H, benzylic CH2), 7.15–7.47 (m, 12H, Ar-Hs); ESI-MS: m/z 478.0 [M+Na]+. HRMS (ESI) m/z for C22H18BrClN3O [M+H]+, calcd 454.0322, found 454.0314.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide (5k)
Yield=84.0%. 1H NMR (400 MHz, CDCl3): δ 3.87 (s, 2H, CH2-CO), 5.26 (s, 2H, benzylic CH2), 7.01–7.64 (m, 12H, Ar-Hs); ESI-MS: m/z 444.1 [M+H]+. HRMS (ESI) m/z for C23H18ClF3N3O [M+H]+, calcd 444.109, found 444.1081.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(p-tolyl) acetamide (5l)
Yield=74.0%. 1H NMR (400 MHz, CDCl3): δ 2.26 (s, 3H, CH3), 3.68 (s, 2H, CH2-CO), 5.17 (s, 2H, benzylic CH2), 7.03–7.21 (m, 12H, Ar-Hs); ESI-MS: m/z 390.2 [M+H]+. HRMS (ESI) m/z for C23H21ClN3O [M+H]+, calcd 390.1373, found 390.1367.
N-(1-(4-Chlorobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-methoxyphenyl) acetamide (5m)
Yield=78.0%. 1H NMR (400 MHz, CDCl3): δ 3.66 (s, 2H, CH2-CO), 3.72 (s, 3H, OCH3), 5.17 (s, 2H, benzylic CH2), 6.71–6.84 (m, 2H, Ar-Hs); 7.00–7.18 (m, 8H, Ar-Hs); 7.02–7.26 (m, 2H, Ar-Hs); ESI-MS: m/z 406.2 [M+H]+. HRMS (ESI) m/z for C23H21ClN3O2 [M+H]+, calcd 406.1322, found 406.1312.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-phenylacetamide (5n)
Yield=84.9%. 1H NMR (400 MHz, CDCl3): δ 3.76 (s, 2H, CH2-CO), 5.23 (s, 2H, benzylic CH2), 7.05–7.28 (m, 9H, Ar-Hs); 7.40–7.49 (m, 4H, Ar-Hs); ESI-MS: m/z 419.8 [M−H]+. HRMS (ESI) m/z for C22H19BrN3O [M+H]+, calcd 420.0712, found 420.0704.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-fluorophenyl) acetamide (5o)
Yield=85.7%. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 2H, CH2-CO), 5.19 (s, 2H, benzylic CH2), 6.89–7.36 (m, 12H, Ar-Hs); ESI-MS: m/z 437.9 [M−H]+. HRMS (ESI) m/z for C22H18BrFN3O [M+H]+, calcd 438.0617, found 438.0607.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-chlorophenyl) acetamide (5p)
Yield=69.1%. 1H NMR (400 MHz, CDCl3): δ 3.68 (s, 2H, CH2-CO); 5.14 (s, 2H, benzylic CH2); 7.05–7.12 (m, 6H, Ar-Hs); 7.22–7.34 (m, 6H, Ar-Hs); ESI-MS: m/z 456.1 [M+H]+. HRMS (ESI) m/z for C22H18BrClN3O [M+H]+, calcd 454.0322, found 454.0311.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-bromophenyl) acetamide (5q)
Yield=96.1%. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 2H, CH2-CO), 5.26 (s, 2H, benzylic CH2), 7.12–7.47 (m, 12H, Ar-Hs); ESI-MS: m/z 499.9 [M+H]+. HRMS (ESI) m/z for C22H18Br2N3O [M+H]+, calcd 497.9817, found 497.9801.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide (5r)
Yield=71.6%. 1H NMR (400 MHz, CDCl3): δ 3.78 (s, 2H, CH2-CO); 5.25 (s, 2H, benzylic CH2); 7.12–7.20 (m, 5H, Ar-Hs); 7.24–7.48 (m, 7H, Ar-Hs); ESI-MS: m/z 488.0 [M+H]+. HRMS (ESI) m/z for C23H18BrF3N3O [M+H]+, calcd 488.0585 found 488.0595.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(p-tolyl) acetamide (5s)
Yield=87.9%. 1H NMR (400 MHz, CDCl3): δ 2.26 (s, 3H, CH3); 3.68 (s, 2H, CH2-CO); 5.15 (s, 2H, benzylic CH2); 6.70–7.35 (m, 12H, Ar-Hs); ESI-MS: m/z 436.2 [M+H]+. HRMS (ESI) m/z for C23H21BrN3O2 [M+H]+, calcd 434.0868, found 434.0858.
N-(1-(4-Bromobenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-methoxyphenyl) acetamide (5t)
Yield=89.1%. 1H NMR (400 MHz, CDCl3): δ 3.66 (s, 2H, CH2-CO); 3.72 (s, 3H, OCH3); 5.15 (s, 2H, benzylic CH2); 6.73–6.83 (m, 2H, Ar-Hs); 7.00–7.18 (m, 6H, Ar-Hs); 7.20–7.26 (m, 2H, Ar-Hs); 7.31–7.36 (m, 2H, Ar-Hs);. ESI-MS: m/z 474.0 [M+Na]+. HRMS (ESI) m/z for C23H21BrN3O2 [M+H]+, calcd 450.0817, found 450.0804.
N-(1-(4-Methylbenzyl)-1H-benzo[d]imidazol-2-yl)-2-phenylacetamide (5u)
Yield=60.4%. 1H NMR (400 MHz, CDCl3): δ 2.23 (s, 3H, CH3); 3.76 (s, 2H, CH2-CO); 5.21 (s, 2H, benzylic CH2); 7.02 (d, 2H, Ar-Hs); 7.09–7.19 (m, 7H, Ar-Hs); 7.25 (t, 2H, Ar-Hs); 7.33–7.39 (m, 2H, Ar-Hs); ESI-MS: m/z 378.2 [M+Na]+. HRMS (ESI) m/z for C23H22N3O [M+H]+, calcd 356.1763, found 356.1757.
2-(4-Fluorophenyl)-N-(1-(4-methylbenzyl)-1H-benzo[d] imidazol-2-yl) acetamide (5v)
Yield=91.0%. 1H NMR (400 MHz, CDCl3): δ 2.31 (s, 3H, CH3); 3.76 (s, 2H, CH2-CO); 5.25 (s, 2H, benzylic CH2); 6.97–7.03 (m, 2H, Ar-Hs); 7.07–7.12 (m, 2H, Ar-Hs); 7.12–7.25 (m, 6H, Ar-Hs); 7.23–7.39 (m, 2H, Ar-Hs); ESI-MS: m/z 374.3 [M+H]+. HRMS (ESI) m/z for C23H21FN3O [M+H]+, calcd 374.1669 found 374.1660.
2-(4-Chlorophenyl)-N-(1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl) acetamide (5w)
Yield=51.2%. 1H NMR (400 MHz, CDCl3): δ 2.25 (s, 3H, CH3); 3.70 (s, 2H, CH2-CO); 5.17 (s, 2H, benzylic CH2); 6.94–7.40 (m, 12H, Ar-Hs); ESI-MS: m/z 412.2 [M+Na]+. HRMS (ESI) m/z for C23H21ClN3O [M+H]+, calcd 390.1373 found 390.1363.
2-(4-Bromophenyl)-N-(1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl) acetamide (5x)
Yield=60.0%. 1H NMR (400 MHz, CDCl3): δ 2.34 (s, 3H, CH3); 3.79 (s, 2H, CH2-CO); 5.29 (s, 2H, benzylic CH2); 7.10–7.15 (m, 2H, Ar-Hs); 7.15–7.28 (m, 6H, Ar-Hs); 7.30–7.33 (m, 2H, Ar-Hs); 7.43–7.48 (m, 2H, Ar-Hs); ESI-MS: m/z 456.0 [M+Na]+. HRMS (ESI) m/z for C23H21BrN3O [M+H]+, calcd 434.0868, found 434.0857.
N-(1-(4-Methylbenzyl)-1H-benzo[d]imidazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide (5y)
Yield=92.1%. 1H NMR (400 MHz, CDCl3): δ 2.30–2.32 (s, 3H, CH3); 3.85 (s, 2H, CH2-CO); 5.24 (s, 2H, benzylic CH2); 7.08–7.58 (m, 12H, Ar-Hs); ESI-MS: m/z 424.2 [M+H]+. HRMS (ESI) m/z for C24H21F3N3O [M+H]+, calcd 424.1637 found 424.1628.
N-(1-(4-thylbenzyl)-1H-benzo[d]imidazol-2-yl)-2-(p-tolyl) acetamide (5z)
Yield=72.1%. 1H NMR (400 MHz, CDCl3): δ 2.33 (s, 3H, CH3); 2.35 (s, 3H, CH3); 3.79 (s, 2H, CH2-CO); 5.27 (s, 2H, benzylic CH2); 7.08–7.26 (m, 10H, Ar-Hs); 7.31–7.34 (m, 2H, Ar-Hs); ESI-MS: m/z 392.3 [M+Na]+. HRMS (ESI) m/z for C24H24N3O [M+H]+, calcd 370.1919, found 370.191.
2-(4-Methoxyphenyl)-N-(1-(4-methylbenzyl)-1H-benzo[d]imidazol-2-yl) acetamide (5z′)
Yield=98.5%. 1H NMR (400 MHz, CDCl3): δ 2.31 (s, 3H, CH3); 3.74 (s, 2H, CH2-CO); 3.79 (s 3H, OCH3); 5.32 (s, 2H, benzylic CH2); 6.84–6.89 (m, 2H, Ar-Hs); 7.17–7.12 (m, 2H, Ar-Hs); 7.12–7.25 (m, 6H, Ar-Hs); 7.30–7.53 (m, 2H, Ar-Hs); ESI-MS: m/z 386.3 [M+H]+. HRMS (ESI) m/z for C24H24N3O2 [M+H]+, calcd 386.1869, found 386.1863.
2-(Azidomethyl)-1H-benzimidazole (7)
Yield=71.5%. 1H NMR (400 MHz, CDCl3): δ 4.81 (s, 2H, CH2-N3); 6.99–7.32 (dd, 2H, Ar-Hs); 7.64 (dd, 2H, Ar-Hs). ESI-MS: m/z 173.7 [M+H]+.
2-(Azidomethyl)-1-benzyl-1H-benzimidazole (8a)
Yield: 50.0%. 1H NMR (400 MHz, CDCl3): δ 4.5 (s, 2H, CH2-N3); 5.3 (s, 2H, benzylic CH2); 7.0 (m, 2H, Ar-Hs); 7.25 (m, 6H, Ar-Hs); 7.75 (m, 1H, Ar-H). ESI-MS: m/z 286.1 [M+Na]+.
2-(Azidomethyl)-1-(4-chlorobenzyl)-1H-benzimidazole (8b)
Yield: 45.2%. 1H NMR (400 MHz, CDCl3): δ 4.5 (s, 2H, CH2-N3); 5.3 (s, 2H, benzylic CH2); 6.95 (d, 2H, Ar-Hs); 7.25 (m, 5H, Ar-Hs); 7.75 (m, 1H, Ar-H). ESI-MS: m/z 298.1 [M+H]+.
2-(Azidomethyl)-1-(4-bromobenzyl)-1H-benzimidazole (8c)
Yield: 49.5%.1H NMR (400 MHz, CDCl3): δ 4.5 (s, 2H, CH2-N3); 5.3 (s, 2H, benzylic CH2); 6.9 (td, 2H, Ar-Hs); 7.25 (m, 5H, Ar-Hs); 7.75 (m, 1H, Ar-H). ESI-MS: m/z 366.1 [M+Na]+.
2-(Azidomethyl)-1-(4-methylbenzyl)-1H-benzimidazole (8d)
Yield: 60.3%. 1H NMR (400 MHz, CDCl3): δ 2.2 (s, 3H, CH3); 4.5 (s, 2H, CH2-N3); 5.3 (s, 2H, benzylic CH2); 6.9 (d, 2H, Ar-Hs); 7.1 (d, 2H, Ar-Hs); 7.25 (m, 3H, Ar-Hs); 7.75 (m, 1H, Ar-H). ESI-MS: m/z 300.1 [M+Na]+.
1-Benzyl-2-((4-phenyl-1H-1,2,3-triazol-1-yl) methyl)-1H-benzo[d]imidazole (10a)
Yield=72.5%. 1H NMR (400 MHz, CDCl3): δ 5.45 (s, 2H, triazole-CH2); 5.76 (s, 2H, benzylic CH2); 6.96 (d, 2H, Ar-Hs); 7.10–7.30 (m, 9H, Ar-Hs); 7.68–7.85 (m, 4H, Ar-Hs); ESI-MS: m/z 388.2 [M+Na]+. HRMS (ESI) m/z for C23H20N5 [M+H]+, calcd 366.1719, found 366.1720.
1-Benzyl-2-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10b)
Yield=71.2%. 1H NMR (400 MHz, CDCl3): δ 5.50 (s, 2H, triazole-CH2); 5.81 (s, 2H, benzylic CH2); 7.00–7.04 (m, 2H, Ar-Hs); 7.06–7.10 (m, 2H, Ar-Hs); 7.23–7.26 (m, 2H, Ar-Hs); 7.27–7.29 (m, 1H, Ar-Hs); 7.32–7.36 (m, 3H, Ar-Hs); 7.68–7.73 (m, 2H, Ar-Hs); 7.82–7.87 (m, 2H, Ar-Hs); ESI-MS: m/z 406.3 [M+Na]+. HRMS (ESI) m/z for C23H19FN5 [M+H]+, calcd 384.1624, found 384.1623.
1-Benzyl-2-((4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10c)
Yield=43.7%. 1H NMR (400 MHz, CDCl3): δ 5.50 (s, 2H, triazole-CH2); 5.82 (s, 2H, benzylic CH2); 7.0–7.02 (m, 2H, Ar-Hs); 7.22–7.25 (m, 3H, Ar-Hs); 7.33–7.37 (m, 5H, Ar-Hs); 7.63–7.69 (m, 2H, Ar-Hs); 7.81–7.88 (m, 2H, Ar-Hs); ESI-MS: m/z 422.1 [M+Na]+. HRMS (ESI) m/z for C23H19ClN5 [M+H]+, calcd 400.1329, found 400.1331.
1-Benzyl-2-((4-(4-bromophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10d)
Yield=45.0%. 1H NMR (400 MHz, CDCl3): δ 5.50 (s, 2H, triazole-CH2); 5.81 (s, 2H, benzylic CH2); 6.99–7.01 (m, 2H, Ar-Hs); 7.24–7.25 (m, 3H, Ar-Hs); 7.33–7.34 (m, 2H, Ar-Hs); 7.50–7.52 (m, 2H, Ar-Hs); 7.60–7.61 (m, 2H, Ar-Hs); 7.83–7.88 (m, 2H, Ar-Hs); ESI-MS: m/z 468.0 [M+Na]+. HRMS (ESI) m/z for C23H19BrN5 [M+H]+, calcd 444.0824, found 444.0820.
1-Benzyl-2-((4-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10e)
Yield=40.3%. 1H NMR (400 MHz, CDCl3): δ 5.51 (s, 2H, triazole-CH2); 5.84 (s, 2H, benzylic CH2); 7.0 (d, 2H, Ar-Hs); 7.25 (d, 3H, Ar-Hs); 7.33 (s, 3H, Ar-Hs); 7.64 (d, 2H, Ar-Hs); 7.84 (d, 3H, Ar-Hs); 7.96 (s, 1H, Ar-Hs); ESI-MS: m/z 456.1 [M+H]+. HRMS (ESI) m/z for C24H19F3N5 [M+H]+, calcd 434.1593, found 434.1591.
1-Benzyl-2-((4-(p-tolyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10f)
Yield=71.8%. 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H, CH3); 5.5 (s, 2H, triazole-CH2); 5.81 (s, 2H, benzylic CH2); 6.94–6.98 (m, 2H, Ar-Hs); 7.15–7.35 (m, 8H, Ar-Hs); 7.58 (d, 2H, Ar-Hs); 7.85 (s, 2H, Ar-Hs); ESI-MS: m/z 380.0 [M+H]+. HRMS (ESI) m/z for C24H22N5 [M+H]+, calcd 380.1875, found 380.1871.
1-Benzyl-2-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10g)
Yield=37.7%. 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H, OCH3); 5.50 (s, 2H, triazole-CH2); 5.80 (s, 2H, benzylic CH2); 7–7.9 (m, 14H, Ar-Hs); ESI-MS: m/z 418.2 [M+Na]+. HRMS (ESI) m/z for C24H22N5O [M+H]+, calcd 396.1824, found 396.1822.
1-(4-Chlorobenzyl)-2-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10h)
Yield=39.1%. 1H NMR (400 MHz, CDCl3): δ 5.47 (s, 2H, triazole-CH2); 5.82 (s, 2H, benzylic CH2); 6.91 (d, 2H, Ar-Hs); 7.2 (d, 2H, Ar-Hs); 7.33–7.39 (m, 6H, Ar-Hs); 7.72 (dd, 2H, Ar-Hs); 7.8 (m, 2H, Ar-Hs); ESI-MS: m/z 400.2 [M+H]+. HRMS (ESI) m/z for C23H19ClN5 [M+H]+, calcd 400.1329, found 400.1331.
1-(4-Chlorobenzyl)-2-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10i)
Yield=33.6%. 1H NMR (400 MHz, CDCl3): δ 5.40 (s, 2H, triazole-CH2); 5.75 (s, 2H, benzylic CH2); 6.83 (d, 2H, Ar-Hs); 7.01 (d, 2H, Ar-Hs); 7.13 (d, 2H, Ar-Hs); 7.22–7.29 (m, 3H, Ar-Hs); 7.59–7.63 (m, 2H, Ar-Hs); 7.74–7.78 (m, 2H, Ar-Hs); ESI-MS: m/z 440.2 [M+Na]+. HRMS (ESI) m/z for C23H18ClFN5 [M+H]+, calcd 418.1235, found 418.1231.
1-(4-Chlorobenzyl)-2-((4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10j)
Yield=40.0%. 1H NMR (400 MHz, CDCl3): δ 5.39 (s, 2H, triazole-CH2); 5.75 (s, 2H, benzylic CH2); 6.82 (d, 2H, Ar-Hs); 7.13 (d, 2H, Ar-Hs); 7.21–7.30 (d, 5H, Ar-Hs); 7.56–7.58 (m, 2H, Ar-Hs); 7.76–7.79 (m, 2H, Ar-Hs); ESI-MS: m/z 456.1 [M+Na]+. HRMS (ESI) m/z for C23H18Cl2N5 [M+H]+, calcd 434.0939, found 434.0935.
2-((4-(4-Bromophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1-(4-chlorobenzyl)-1H-benzo[d]imidazole (10k)
Yield=33.6%. 1H NMR (400 MHz, CDCl3): δ 5.40 (s, 2H, triazole-CH2); 5.75 (s, 2H, benzylic CH2); 6.83 (d, 2H, Ar-Hs); 7.13 (d, 2H, Ar-Hs); 7.20–7.30 (m, 3H, Ar-Hs); 7.43–7.53 (m, 4H, Ar-Hs); 7.787.80 (m, 2H, Ar-Hs); ESI-MS: m/z 502.0 [M+Na]+. HRMS (ESI) m/z for C23H18BrClN5 [M+H]+, calcd 478.0434, found 478.0438.
1-(4-Chlorobenzyl)-2-((4-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10l)
Yield=29.3%. 1H NMR (400 MHz, CDCl3): δ 5.41 (s, 2H, triazole-CH2); 5.78 (s, 2H, benzylic CH2); 6.83 (d, 2H, Ar-Hs); 7.13 (d, 2H, Ar-Hs); 7.27–7.29 (m, 3H, Ar-Hs); 7.58 (d, 2H, Ar-Hs); 7.77–7.80 (m, 3H, Ar-Hs); 7.87 (s, 1H, Ar-H); ESI-MS: m/z 490.0 [M+Na]+. HRMS (ESI) m/z for C24H18ClF3N5 [M+H]+, calcd 468.1203, found 468.1208.
1-(4-Chlorobenzyl)-2-((4-(p-tolyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10m)
Yield=33.8%. 1H NMR (400 MHz, CDCl3): δ 2.28 (s, 3H, CH3); 5.40 (s, 2H, triazole-CH2); 5.74 (s, 2H, benzylic CH2); 6.85 (d, 2H, Ar-Hs); 7.11–7.16 (m, 4H, Ar-Hs); 7.25 (d, 3H, Ar-Hs); 7.54 (d, 2H, Ar-Hs); 7.76–7.80 (m, 2H, Ar-Hs); ESI-MS: m/z 436.2 [M+H]+. HRMS (ESI) m/z for C24H21ClN5 [M+H]+, calcd 414.1485, found 414.1482.
1-(4-Chlorobenzyl)-2-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10n)
Yield=34.9%. 1H NMR (400 MHz, CDCl3): δ 3.85 (s, 3H, OCH3); 5.49 (s, 2H, triazole-CH2); 5.83 (s, 2H, benzylic CH2); 6.93–6.96 (m, 4H, Ar-Hs); 7.23–7.27 (m, 2H, Ar-Hs); 7.31–7.36 (m, 3H, Ar-Hs); 7.66–7.68 (m, 2H, Ar-Hs); 7.81 (s, 1H, Ar-H); 7.86–7.88 (m, 1H, Ar-H); ESI-MS: m/z 452.1 [M+Na]+. HRMS (ESI) m/z for C24H21ClN5O [M+H]+, calcd 430.1435, found 430.1444.
1-(4-Bromobenzyl)-2-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10o)
Yield=55.6%. 1H NMR (400 MHz, CDCl3): δ 5.5 (s, 2H, triazole-CH2); 5.88 (s, 2H, benzylic CH2); 6.91 (d, 2H, Ar-Hs); 7.32–7.45 (m, 8H, Ar-Hs); 7.73–7.77 (m, 2H, Ar-Hs); 7.85–7.89 (m, 1H, Ar-H); 7.94 (s, 1H, Ar-H); ESI-MS: m/z 468.0 [M+Na]+. HRMS (ESI) m/z for C23H19BrN5 [M+H]+, calcd 444.0824, found 444.0815.
1-(4-Bromobenzyl)-2-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10p)
Yield=57.0%. 1H NMR (400 MHz, CDCl3): δ 5.40 (s, 2H, triazole-CH2); 5.77 (s, 2H, benzylic CH2); 6.77 (d, 2H, Ar-Hs); 7.01 (t, 2H, Ar-Hs); 7.18–7.35 (m, 6H, Ar-Hs); 7.59–7.63 (m, 2H, Ar-Hs); 7.78 (s, 1H, Ar-H); ESI-MS: m/z 374 [M+1]+. ESI-MS: m/z 486.0 [M+Na]+. HRMS (ESI) m/z for C23H18BrFN5 [M+H]+, calcd 462.0730, found 462.0721.
1-(4-Bromobenzyl)-2-((4-(4-chlorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10q)
Yield=52.3%. 1H NMR (400 MHz, CDCl3): δ 5.45 (s, 2H, triazole-CH2); 5.82 (s, 2H, benzylic CH2); 6.83 (d, 2H, Ar-Hs); 7.29–7.37 (m, 7H, Ar-Hs); 7.64–7.68 (m, 2H, Ar-Hs); 7.82–7.88 (m, 2H, Ar-Hs); ESI-MS: m/z 480.1 [M+H]+. HRMS (ESI) m/z for C23H18BrClN5 [M+H]+, calcd 478.0434, found 478.0431.
1-(4-Bromobenzyl)-2-((4-(4-bromophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10r)
Yield=58.0%. 1H NMR (400 MHz, CDCl3): δ 5.45 (s, 2H, triazole-CH2); 5.82 (s, 2H, benzylic CH2); 6.85 (d, 2H, Ar-Hs); 7.36–7.45 (m, 5H, Ar-Hs); 7.52–7.58 (m, 4H, Ar-Hs); 7.80–7.90 (m, 2H, Ar-Hs); ESI-MS: m/z 546.0 [M+Na]+. HRMS (ESI) m/z for C23H18Br2N5 [M+H]+, calcd 521.9929, found 521.9927.
1-(4-Bromobenzyl)-2-((4-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10s)
Yield=54.2%. 1H NMR (400 MHz, CDCl3): δ 5.48 (s, 2H, triazole-CH2); 5.85 (s, 2H, benzylic CH2); 6.85 (d, 2H, Ar-Hs); 7.28–7.40 (m, 5H, Ar-Hs); 7.65 (d, 2H, Ar-Hs); 7.82–7.88 (m, 3H, Ar-Hs); 7.93 (s, 1H, Ar-H); ESI-MS: m/z 536.0 [M+Na]+. HRMS (ESI) m/z for C24H18BrF3N5 [M+H]+, calcd 512.0698, found 512.0695.
1-(4-Bromobenzyl)-2-((4-(p-tolyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10t)
Yield=62.7%. 1H NMR (400 MHz, CDCl3): δ 2.28 (s, 3H, CH3); 5.41 (s, 2H, triazole-CH2); 5.77 (s, 2H, benzylic CH2); 6.79 (d, 2H, Ar-Hs); 7.12 (d, 2H, Ar-Hs); 7.18–7.38 (m, 5H, Ar-Hs); 7.54 (d, 2H, Ar-Hs); 7.77–7.85 (m, 2H, Ar-Hs); ESI-MS: m/z 480.1 [M+Na]+. HRMS (ESI) m/z for C24H21BrN5 [M+H]+, calcd 458.0980, found 458.0979.
1-(4-Bromobenzyl)-2-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10u)
Yield=57.0%. 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H, OCH3); 5.45 (s, 2H, triazole-CH2); 5.80 (s, 2H, benzylic CH2); 6.86 (d, 2H, Ar-Hs); 6.93 (d, 2H, Ar-Hs); 7.28–7.38 (m, 5H, Ar-Hs); 7.63–7.70 (m, 2H, Ar-Hs); 7.78 (s, 1H, Ar-H); 7.82–7.83 (m, 2H, Ar-Hs); ESI-MS: m/z 498.0 [M+Na]+. HRMS (ESI) m/z for C24H21BrN5O [M+H]+, calcd 474.0929, found 474.0931.
1-(4-Methylbenzyl)-2-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10v)
Yield=59.4%. 1H NMR (400 MHz, CDCl3): δ 2.15 (s, 3H, CH3); 5.39 (s, 2H, triazole-CH2); 5.76 (s, 2H, benzylic CH2); 6.82 (d, 2H, Ar-Hs); 6.97 (d, 2H, Ar-Hs); 7.27–7.37 (m, 6H, Ar-Hs); 7.65 (d, 2H, Ar-Hs); 7.78 (s, 2H, Ar-Hs); ESI-MS: m/z 380.3 [M+H]+. HRMS (ESI) m/z for C24H22N5 [M+H]+, calcd 380.1875, found 380.1877.
2-((4-(4-Fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1-(4-methylbenzyl)-1H-benzo[d]imidazole (10w)
Yield=41.7%. 1H NMR (400 MHz, CDCl3): δ 2.22 (s, 3H, CH3); 5.44 (s, 2H, triazole-CH2); 5.81 (s, 2H, benzylic CH2); 6.88 (d, 2H, Ar-Hs); 7.03–7.09 (m, 4H, Ar-Hs); 7.34–7.35 (m, 3H, Ar-Hs); 7.67–7.70 (m, 2H, Ar-Hs); 7.78 (s, 1H, Ar-H); 7.82–7.85 (m, 1H, Ar-H); ESI-MS: m/z 420.2 [M+Na]+. HRMS (ESI) m/z for C24H21FN5 [M+H]+, calcd 398.1781, found 398.1784. 1-(4-Methylbenzyl)-2-((4-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10x): Yield=22.3%. 1H NMR (400 MHz, CDCl3): δ 2.20 (s, 3H, CH3); 5.46 (s, 2H, triazole-CH2); 5.86 (s, 2H, benzylic CH2); 6.88 (d, 2H, Ar-Hs); 7.02 (d, 2H, Ar-Hs); 7.32–7.36 (m, 3H, Ar-Hs); 7.52 (d, 2H, Ar-Hs); 7.64 (d, 3H, Ar-Hs); 7.83 (m, 1H, Ar-Hs); ESI-MS: m/z 448.2 [M+H]+. HRMS (ESI) m/z for C25H21F3N5 [M+H]+, calcd 448.1749, found 448.1747.
2-((4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-1-(4-methylbenzyl)-1H-benzo[d]imidazole (10y)
Yield=35.1%. 1H NMR (400 MHz, CDCl3): δ 2.23 (s, 3H, CH3); 3.82 (s, 3H, OCH3); 5.44 (s, 2H, triazole-CH2); 5.80 (s, 2H, benzylic CH2); 6.88–6.92 (m, 4H, Ar-Hs); 7.03–7.05 (d, 2H, Ar-Hs); 7.32–7.36 (m, 3H, Ar-Hs); 7.63–7.66 (m, 2H, Ar-Hs); 7.74 (s, 1H, Ar-H); 7.82–7.85 (m, 1H, Ar-H); ESI-MS: m/z 432.2 [M+Na]+. HRMS (ESI) m/z for C25H24N5O [M+H]+, calcd. 410.1981, found 410.1979.
1-(4-Methylbenzyl)-2-((4-(p-tolyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (10z)
Yield=40.7%. 1H NMR (400 MHz, CDCl3): δ 2.23 (s, 3H, CH3); 2.35 (s, 3H, CH3); 5.44 (s, 2H, triazole-CH2); 5.81 (s, 2H, benzylic CH2); 6.83 (d, 2H, Ar-Hs); 6.98 (d, 2H, Ar-Hs); 7.12 (d, 2H, Ar-Hs); 7.27–7.33 (m, 3H, Ar-Hs); 7.52–7.58 (m, 2H, Ar-Hs); 7.77–7.85 (m, 2H, Ar-Hs); ESI-MS: m/z 394.4 [M+H]+. HRMS (ESI) m/z for C25H24N5 [M+H]+, calcd. 394.2023, found 394.2029.
Establishment of the stable luciferase-transfected MDA-MB-231-HRE-Luc cell line from the parental MDA-MB-231 breast cancer cell line
The parental human breast cancer cell line (MDA-MB-231) was stably transfected with pGL4.42 vector containing four copies of an HRE that drives transcription of the luciferase reporter gene luc2P and a gene for hygromycin B resistance for selection of stably transfected mammalian cells. MDA-MB-231-HRE-Luc cells were validated by measuring and comparing its luciferase activity with that of the parental cells (MDA-MB-231) under normoxia and different conditions of hypoxia (hypoxia mimic DFO)/hypoxia incubator 1% O2) and at different cell densities (5000, 10,000, and 20,000). Results indicated that MDA-MB-231-HRE-Luc cells contain a stably integrated HIF-inducible luciferase reporter gene producing recognizable luciferase induction under hypoxia conditions in comparison to the parental cells.
Identification of compounds 5f, 5h, 5i, 5p, 5q, and 5r as HIF1α Inhibitors through HIF-luciferase assay on the MDA-MB-231-HRE-Luc cells
All the newly synthesized compounds (5a–z′ and 10a–z) along with CJ-3k were first screened for their effect on HIF1α transcriptional activity at a concentration of 10 μM under hypoxia in the human breast cancer cell line (MDA-MB-231-HRE-Luc) using a high-throughput luciferase assay. As inhibitors of HIF1α transcriptional activity, YC-1 and Chetomin (30) were used as positive controls at 10 μM and 200 nM, respectively. This preliminary screening revealed that some compounds (5f, 5h, 5i, 5p, 5q, and 5r) had a potent inhibitory effect on HIF1α expression in comparison to the untreated control (>50% inhibition). Yet none of the triazole series of compounds 10a–z suppressed HIF1α expression by more than 50%.
The most potent inhibitors of HIF1α: 5r, 5h, and 5q
In order to quantitatively compare the anti-HIF activities of the potent molecules (5f, 5h, 5i, 5p, 5q, and 5r), IC50 values were further determined by measuring luciferase activity at different concentrations (100, 30, 10, 3, 1, 0.3, and 0.1 μM) using the luciferase assay. The three compounds 5r, 5h, and 5q were identified to have the best potency, with IC50 values in the range 1.53–1.78 μM (Table I).
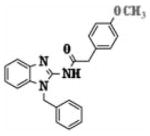
Table I.
Half maximal inhibitory concentration (IC50) values for selected compounds using the luciferase reporter assay in MDA-MB-231-HRE-Luc cells. Assays were carried out in triplicates.
| Compound | Structure | Menu IC50 ± SEM (μM) |
|---|---|---|
| 5f |
|
8.12 ± 3.86 |
| 5h |
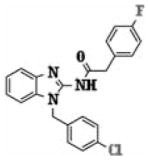
|
1.58 ± 0.75 |
| 5i |
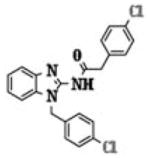
|
2.77 ± 1.39 |
| 5p |
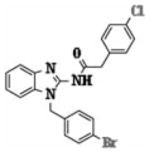
|
3.58 ± 1.38 |
| 5q |
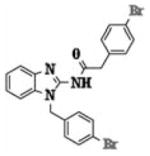
|
1.78 ± 0.75 |
| 5r |
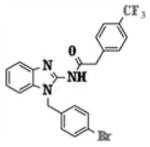
|
1.53 ± 0.78 |
| CJ-3k |
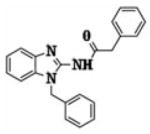
|
12.45 ± 10.83 |
| Chetomin |
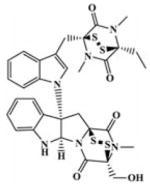
|
0.04 ± 0.01 |
Discussion
As a master regulator for multiple survival pathways in solid tumors under hypoxia, HIF1α has wildly considered as a prominent cancer drug target. However, targeting this transcriptional factor has proven to be challenging and currently there are no approved specific HIF1α inhibitors. YC-1 is a well-known small molecule HIF1α inhibitor, but it has strong inhibition of platelet aggregation which could lead to severe internal bleeding side effects (31, 32). Based on our previously discovered scaffold that is represented by the structure of CJ-3k, we performed systematic structure-activity relationship studies for this scaffold to further optimize the structures in this report. We also developed a sensitive high-throughput luciferase assay by stably transfected the human breast cancer cell line (MDA-MB-231-HRE-Luc) and used this assay to evaluate the HIF1α inhibitory activity for all fifty-three new analogs. We identified several new potent analogs (5f, 5h, 5i, 5p, 5q, and 5r) in this lead optimization study. The IC50 values for these compounds suggested increased potency for HIF1α inhibition when compared to their unsubstituted congener CJ-3k, with 5r, 5h, and 5q as the most potent analogs. Results from this study suggested that the addition of an lipophilic electron-withdrawing groups (e.g. F, Cl, Br and CF3) on rings C and D of the parent molecule (CJ-3k) can increase the HIF1α inhibitory activity, while the incorporation of a triazole moiety to replace the amide bond is detrimental to this activity. While this scaffold was originally derived based on the structure of YC-1, compounds belonging to this scaffold have significantly lower potency in platelet aggregation inhibition and thus are expected to have lower liability for potential internal bleeding side effects. Further optimization of this scaffold will likely to produce a unique HIF1α inhibitor as a potential therapeutic agent for breast cancer and other solid tumors.
Acknowledgments
This work was partially supported by the NIH grant 1R01CA148706 to WL. The content is solely the responsibility of the Authors and does not necessarily represent the official views of the National Institutes of Health. The Authors thank Drs Tiffany Seagroves and Danielle Brooks (Department of Pathology) for their generous help and guidance in establishing the MDA-MB-231 Luc-HRE cell line. They would also like to thank Dr. Bob Moore for the access to the microplate reader.
Footnotes
Conflicts of Interest
The Authors declare that there are no conflicts of interest.
References
- 1.Semenza GL. HIF1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 2.Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 3.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. HIF1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. HIF1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting HIF1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Bardos JI, Ashcroft M. Negative and positive regulation of HIF1: a complex network. Biochim Biophys Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001;31:847–855. doi: 10.1016/s0891-5849(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 11.Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94:5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319–331. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- 13.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, Pollack IF, Park DM. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Wang J, Schwab LP, Park KT, Seagroves TN, Jennings LK, Miller DD, Li W. Novel Benzimidazole Analogs as Potent Hypoxia Inducible Factor (HIF1α) Inhibitors: Synthesis, Biological Evaluation, Profiling Drug-like Properties, and Biotransformation. Anticancer Res. 2014;34:3891–904. [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu HK, Juan SH, Ho PY, Liang YC, Lin CH, Teng CM, Lee WS. YC-1 inhibits proliferation of human vascular endothelial cells through a cyclic GMP-independent pathway. Biochem Pharmacol. 2003;66:263–271. doi: 10.1016/s0006-2952(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 17.Pan SL, Guh JH, Peng CY, Wang SW, Chang YL, Cheng FC, Chang JH, Kuo SC, Lee FY, Teng CM. YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] inhibits endothelial cell functions induced by angiogenic factors in vitro and angiogenesis in vivo models. J Pharmacol Exp Ther. 2005;314:35–42. doi: 10.1124/jpet.105.085126. [DOI] [PubMed] [Google Scholar]
- 18.Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- 19.Moon SY, Chang HW, Roh JL, Kim GC, Choi SH, Lee SW, Cho KJ, Nam SY, Kim SY. Using YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral Oncol. 2009;45:915–919. doi: 10.1016/j.oraloncology.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang SX, Pei ZZ, Wu XH, Li JK, Yang YJ. Effect of YC-1 on HIF1 alpha and VEGF expression in human hepatocarcinoma cell lines. Chinese journal of hepatology. 2009;17:308–309. [PubMed] [Google Scholar]
- 21.Guida X, Jianhua H, Xiaomin L. Synthesis and QSAR studies of novel 1-substituted-2-aminobenzimidazoles derivatives. Eur J Med Chem. 2006;41:1080–1083. doi: 10.1016/j.ejmech.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Spinks D, Ong HB, Mpamhanga CP, Shanks EJ, Robinson DA, Collie IT, Read KD, Frearson JA, Wyatt PG, Brenk R, Fairlamb AH, Gilbert IH. Design, synthesis and biological evaluation of novel inhibitors of Trypanosoma brucei pteridine reductase 1. ChemMedChem. 2011;6:302–308. doi: 10.1002/cmdc.201000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterz K, Mollmann L, Jacobs A, Baumert D, Wiese M. Activators of P-glycoprotein: Structure-activity relationships and investigation of their mode of action. ChemMedChem. 2009;4:1897–1911. doi: 10.1002/cmdc.200900283. [DOI] [PubMed] [Google Scholar]
- 24.Lee DY, Singh N, Kim MJ, Jang DO. Chromogenic and fluorescent recognition of iodide with a benzimidazole-based tripodal receptor. Org Lett. 2011;13(12):3024–3027. doi: 10.1021/ol2008846. [DOI] [PubMed] [Google Scholar]
- 25.Xue F, Luo X, Ye C, Ye W, Wang Y. Inhibitory properties of 2-substituent-1H-benzimidazole-4-carboxamide derivatives against enteroviruses. Bioorg Med Chem. 2011;19:2641–2649. doi: 10.1016/j.bmc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Jlalia I, Lensen N, Chaume G, Dzhambazova E, Astasidi L, Hadjiolova R, Bocheva A, Brigaud T. Synthesis of an MIF-1 analogue containing enantiopure (S)-alpha-trifluoromethyl-proline and biological evaluation on nociception. Eur J Med Chem. 2013;62:122–129. doi: 10.1016/j.ejmech.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 27.Hou J, Li Z, Fang Q, Feng C, Zhang H, Guo W, Wang H, Gu G, Tian Y, Liu P, Liu R, Lin J, Shi YK, Yin Z, Shen J, Wang PG. Discovery and extensive in vitro evaluations of NK-HDAC-1: a chiral histone deacetylase inhibitor as a promising lead. J Med Chem. 2012;55:3066–3075. doi: 10.1021/jm201496g. [DOI] [PubMed] [Google Scholar]
- 28.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 30.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Teng CM, Wu CC, Ko FN, Lee FY, Kuo SC. YC-1, a nitric oxide-independent activator of soluble guanylate cyclase, inhibits platelet-rich thrombosis in mice. Eur J of Pharmacol. 1997;320:161–166. doi: 10.1016/s0014-2999(96)00911-9. [DOI] [PubMed] [Google Scholar]
- 32.Mullershausen F, Russwurm M, Friebe A, Koesling D. Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41–2272. Circulation. 2004;109:1711–1713. doi: 10.1161/01.CIR.0000126286.47618.BD. [DOI] [PubMed] [Google Scholar]