Abstract
There is a strong association between cannabis use and schizophrenia but the underlying cellular links are poorly understood. Neurons derived from human-induced pluripotent stem cells (hiPSCs) offer a platform for investigating both baseline and dynamic changes in human neural cells. Here, we exposed neurons derived from hiPSCs to Δ9-tetrahydrocannabinol (THC), and identified diagnosis-specific differences not detectable in vehicle-controls. RNA transcriptomic analyses revealed that THC administration, either by acute or chronic exposure, dampened the neuronal transcriptional response following potassium chloride (KCl)-induced neuronal depolarization. THC-treated neurons displayed significant synaptic, mitochondrial, and glutamate signaling alterations that may underlie their failure to activate appropriately; this blunted response resembles effects previously observed in schizophrenia hiPSC- derived neurons. Furthermore, we show a significant alteration in THC-related genes associated with autism and intellectual disability, suggesting shared molecular pathways perturbed in neuropsychiatric disorders that are exacerbated by THC.
Introduction
Human-induced pluripotent stem cells (hiPSCs) serve as a tool for the study of developmental processes and disease-relevant models. This has been especially valuable for the study of the human brain where primary tissue for study has been the most difficult to obtain. hiPSCs have provided mechanistic insights into both neurodevelopmental disorders1 and neurodegenerative diseases2,3. Research into psychiatric disorders such as autism4, bipolar disease5, and schizophrenia6 have greatly benefited from the insights afforded by hiPSCs, as these are largely considered human- specific disorders. hiPSC-based models facilitate isogenic investigations into molecular and environmental factors that may exacerbate or ameliorate disease predisposition.
The widespread use of cannabis calls for a concerted effort into increased understanding of both the positive and negative effects of the drug. Brain imaging studies of the primary psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), demonstrated structural and functional changes following regular cannabis use7, while molecular studies uncovered signaling pathways downstream of the two cannabinoid (CB) receptors, CB1, and CB2. Depression of glutamate signaling is a common feature of THC-induced effects via the CB1 receptor in both humans and in animal models8,9.
There is a significant association between cannabis use and schizophrenia in human subjects10–14, however, whether this reflects patient self-medication of prodromal symptoms or an environmental modulation of genetic susceptibility remains an ongoing discussion15,16. We recently reported molecular abnormalities in schizophrenia patient hiPSC-derived neurons in response to neural activity6; here we describe a distinct overlap in hypo-excitability, particularly in the glutamate system, between schizophrenia patient-derived neurons and those treated with THC. THC exposure seems to deregulate glutamate receptors and other genes involved in synaptic function. We observe significant THC-dependent changes in postsynaptic density, ion channel and WNT pathway genes, and epigenetic regulators; and molecular connections to autism and intellectual disability. Although the molecular mechanisms may not be precisely the same, the convergence of glutamatergic hypo-function may partially explain the increased risk for psychiatric disorders amongst those exposed to cannabis.
Materials and methods
Generation of hiPSC neurons and RNA sequencing
Human fibroblasts were obtained from ATCC (CRL-2522) and Coriell (control: GM03440, GM03651, GM04506, AG09319, AG09429; SZ: GM01792, GM02038, GM01835, and GM02497). Limited phenotypic information for each donor is available from the Coriell Cell Repository, and summarized in the methods of Topol et al17. Unfortunately, THC exposure status for each donor is unknown. hiPSCs were reprogrammed using tetracycline-inducible lentiviral vectors and differentiated to neural precursor cells (NPCs) as previously described18. NPCs were differentiated on poly- ornithine/laminin coated plates for 6 weeks. Passage-matched NPCs were used for all experiments. All hiPSC and NPCs used were mycoplasma-free. Forebrain neural progenitor cells were generated from five control and four case hiPSCs as previously reported6,18,19 and neurons were differentiated according to a 6-week maturation protocol. Samples used in RNA sequencing or quantitative RT–PCR can be found in Supplementary Table S1. THC was dissolved in DMSO to 1 mg/ml and prepared as previously described;20 in all experiments, an equivalent volume of DMSO was used as a vehicle control. Acute (1 μM THC for 24 h) and chronic (50 nM THC; five treatments over 7 days) THC exposure (and DMSO-vehicle control) occurred immediately prior to collection. KCl was dissolved in PBS as previously described6; in all experiments, an equivalent volume of PBS was used as a vehicle control. 50 mM KCl treatment occurred for the final three hours prior to collection; consistent with our previous molecular6 and neurotransmitter release21 studies. For RNA-seq experiments, two wells per individual were treated. The RNA Integrity Number (RIN) was determined using an RNA Nano chip (Agilent Technologies) on the Agilent 2100 Bioanalyzer. All samples have high RIN (mean ± SD: 9.54 ± 0.21). 500 ng of total RNA was used as input material for library preparation using the TruSeq Stranded Total RNA Kit (Illumina, USA).
Processing of RNA sequencing data and analyses
RNA sequencing data has been deposited into Sequence Read Archive (SRA; PRNJA419702, “RNA-Seq of iPSC-derived neurons”). Reads were mapped to GRCh38.p5 reference genome using STAR (version 2.5.1a). Known Gencode gene levels (version 24) were quantified by RSEM (version 1.3.0). To facilitate inter-dataset comparisons, we performed ranked (Spearman) and unranked correlation (Pearson) analysis of the controls in both the ±KCl and ±THC datasets, and confirmed that the samples are highly comparable (all control comparisons are ≥ 97%). Differentially expressed genes were identified with edgeR in R after TMM normalization and filtering. p-values and false discovery rate (FDR) were calculated and differentially expressed genes (DEG) were determined as those with an estimated p-value ≤ 0.05 and FDR ≤ 0.01.
Gene sets for enrichment analyses
To further characterize the DEGs we performed enrichment analysis, using a group of gene sets for known molecular pathways and biological processes, including: Gene Ontology (GO) sets of molecular functions (MF), biological processes (BP), and cellular components (CC) (http://www.geneontology.org); the KEGG dataset (http://www.genome.jp/kegg/pathway.html); and the HUGO Gene Nomenclature Committee (HGNC) gene families (http://www.genenames.org). The genes in each gene set were tested for overlap using Fisher’s exact test and FDR correction. Differential expressed genes were (i) separated in upregulated and downregulated genes; (ii) analyzed for full GO overrepresentation according to hypergeometric testing with a significance cutoff FDR = 0.05 in BiNGO (version 3.0.3); (iii) processed with the enrichment map pipeline (https://f1000research.com/posters/5-1235) a p-value cutoff = 0.001, q-value cutoff = 0.05 and Jaccard coefficient cutoff = 0.25 and (iv) visualized in Cytoscape (version 3.5.1).
Quantitative RT–PCR
For qPCR experiments, three wells per individual were treated with either DMSO- vehicle control for 7 days, acute THC exposure (1 μM THC for 24 h) or chronic THC treatment (five treatments with 50 nM THC over 7 days) immediately prior to collection at 6 weeks. Candidate genes were validated for THC-treated and activity-treated alterations using quantitative RT–PCR. cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System (ThermoFisher Scientific, USA). Briefly, 500 ng of total RNA was used and random hexamer primed protocol was followed. Each cDNA sample was amplified in triplicate using SYBR Green PCR Master Mix (ThermoFisher Scientific, USA). Primer pairs used for this analysis are described in Supplementary Table S2.
Generation of gene datasets
As no up to date datasets for associated genes were available for autism, intellectual disability or schizophrenia, we generated our own through extensive literature and database searches. Specific details are available in the Supplemental Information ‘Generation of Gene Databases’.
Results
hiPSC-derived neurons as a model for THC biology
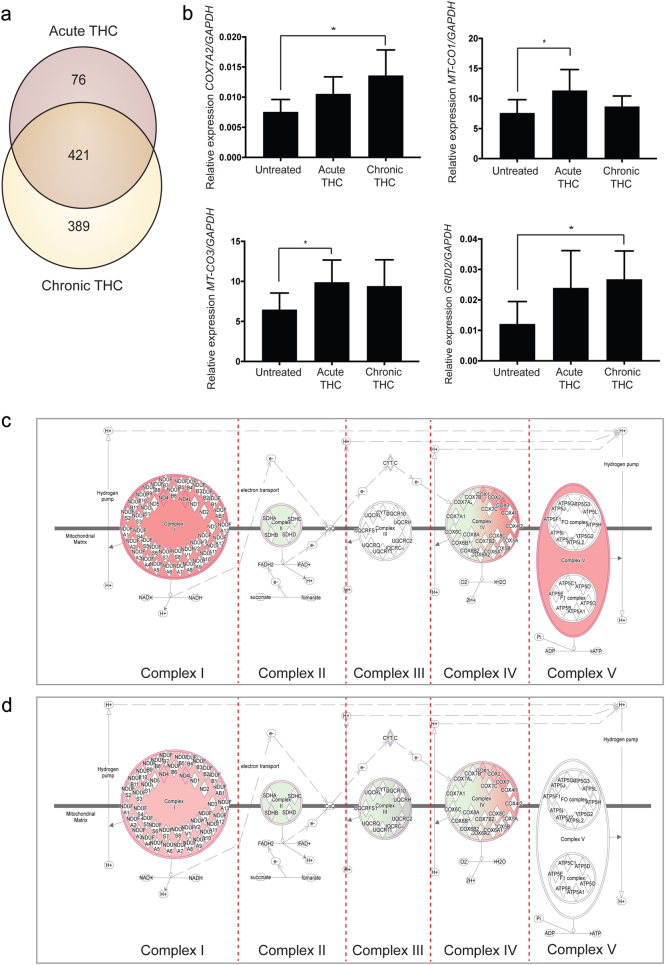
To gain further insight into THC-related molecular mechanisms we utilized hiPSC-derived neurons from four controls as previously reported6. THC (or vehicle control) was added to hiPSC-derived neurons from each individual as acute (1 μM THC for 24 h) or chronic (50 nM THC; five treatments over 7 days) treatments. Acute and chronic THC concentrations were rationally selected from studies of primary mouse neurons22 and experimentally validated in hiPSC neurons23. RNA was extracted and subjected to RNA sequencing (RNA-seq) using the Illumina platform. Our bioinformatic analyses pipeline combined integrated genome/transcriptome alignment using STAR, quantification using RSEM and differential expression using EdgeR. Relative to vehicle treatment, acute THC exposure resulted in 497 genes significantly altered in hiPSC- derived neurons compared to untreated controls, while chronic THC exposure perturbed 810 genes (Fig. 1a; Supplementary Table S3; Supplementary Figure 1). The overlap between acute and chronic exposures was highly significant (421 genes; p- value = 0e + 00, odds ratio = 586.5, Fisher’s exact test). Specific subsets of genes involved in the glutamate receptor pathway and mitochondrial function were altered in response to acute or chronic THC exposure (Supplementary Table S4; Supplementary Figure 2; Fig. 1b–d) and have previously been implicated in THC biology8,24,25. These results provide data to support the use of hiPSC-derived neurons as a model for investigating THC responses in an in vitro human neuronal system.
Fig. 1. THC treatment regulates genes involved in mitochondrial and glutamate pathways.
a RNA sequencing of hiPSC-derived neurons reveals 497 genes (acute) and 810 genes (chronic) are significantly changed following THC exposure, including. b genes involved in mitochondrial (e.g., COX7A2, MT-CO1, and MT-CO3) and glutamate (e.g., GRID2) pathways (Quantitative RT–PCR (qRT–PCR); Ordinary one-way ANOVA with Tukey’s multiple comparisons test: *p < 0.05. n = 5 (see qRT–PCR, Ca–Ce, Supplementary Table S1)). Ingenuity pathway analysis shows that mitochondrial oxidative phosphorylation is strongly altered after both acute c and chronic d THC exposure
RNA sequencing implicates synaptic function, demethylation and ion channel function in THC-treated hiPSC neurons
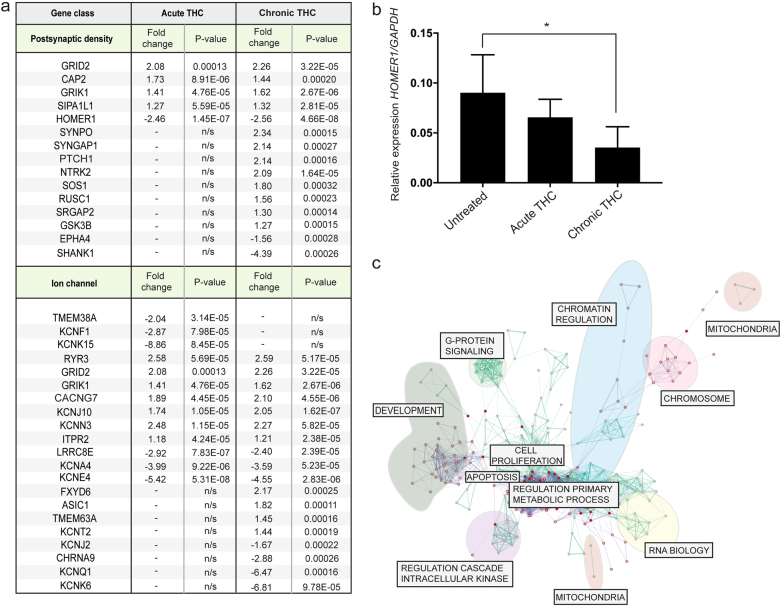
Closer inspection of functional gene clusters associated with THC treatment revealed the potential contribution of genes involved at the postsynaptic density such as HOMER1, GRID2, GRIK1, and SIPA1L1 (Fig. 2a, b). Moreover, chronic THC treatments resulted in the alteration of additional synaptic related genes such as SYNGAP1 and SHANK1. Ion channel genes, especially potassium voltage-gated channel genes (KCNE4, KCNA4, KCNJ10, and KCNN3) are also responsive to both THC treatments with further ion channel genes (KCNJ2, KCNA2, and KCNT2) associated following chronic THC exposure (Fig. 2a). These results strongly implicate synaptic function as a key target of THC-mediated responses. Interestingly, we found epigenetic related transcriptional responses evident in both acute and chronic THC exposures that included the alterations of genes involved in the dynamic methylation/demethylation process (DNMT1, GADD45B, and APOBEC3C); chronic THC exposure resulted in further decreases of histone modification-related proteins such as SETD1A, SETD5, CBX6, KMT2A, KMT2C, and NCOA6 and methyl binding proteins MECP2 and MBD5 (Supplementary Table S5). Network analyses (Enrichment map pipeline in Cytoscape) using the genes altered in response to THC exposure reinforce the involvement of pathways linked to developmental, chromatin regulation and mitochondrial biology (Fig. 2c; Supplementary Table S6).
Fig. 2. Postsynaptic density and ion channel genes are regulated by THC treatment.
a, b Multiple postsynaptic density and ion channel genes are significantly altered in hiPSC-derived neurons following acute or chronic THC exposure, including the postsynaptic gene HOMER1 (Quantitative RT–PCR (qRT–PCR); Ordinary one-way ANOVA with Tukey’s multiple comparisons test: *p < 0.05. n = 5 (see qRT–PCR, Ca–Ce, Supplementary Table S1)). c Network analysis combining all THC-related genes from acute and chronic THC treatment shows broad changes to fundamental cellular functions such as RNA biology, chromatin regulation and development
THC exposure significantly alters genes implicated in autism and intellectual disability
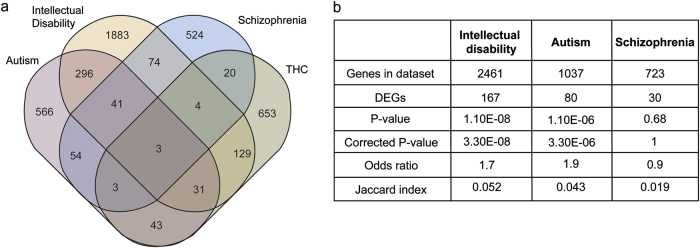
We noticed that many genes implicated in psychiatric disease coincided with genes altered in response to THC treatments. In order to calculate statistical relevance we needed to first update the numbers of genes associated with these disorders and found genes related to autism spectrum disorder (1037 genes), intellectual disability (2461 genes) and schizophrenia (723 genes; see Supplementary Information ‘Generation of Gene Databases’ for details; Supplementary Table S7). Included in our list of significantly altered transcripts following THC exposure is a substantial number of genes linked to autism (80 genes) and intellectual disability (167 genes), with fewer overlapping with schizophrenia (Fig. 3a); autism and intellectual disability associated genes are significant for both p-value and odds ratio using the Fisher’s exact test (Fig. 3b). These data suggest that endogenous THC responsive pathways include many psychiatric disease-associated genes and that changes in these genes, either genetically or epigenetically, may contribute to cannabis-related adverse reactions such as psychosis in some users.
Fig. 3. Genes altered by THC treatment in hiPSC-derived neurons are significantly associated with autism and intellectual disability.
a Venn diagram showing the overlap between THC-related genes and autism, intellectual disability and schizophrenia. b THC-related genes are significantly related to autism and intellectual disability (p-value < 0.05)
Overlapping signaling pathways between THC and schizophrenia
We compared the bioinformatic results from our current THC studies to data obtained from our previous studies of schizophrenia patient hiPSC-derived neurons6,18 to ensure that the quality of the differentiations were comparable across experiments (Supplementary Figure 3). Raw data from all subjects from Roussos et al6. was applied to our bioinformatic pipeline. WNT and mitochondrial pathways (Supplementary Table S8) were significantly altered in both our current THC and previous schizophrenia studies18,26. Genes related to ion channel function were also highly represented in both the THC and schizophrenia gene lists (Supplementary Table S8). Although these pathways were conserved, specific genes related to altered ion channel, WNT or mitochondrial function did not overlap.
Blunted activity-dependent transcriptional response shared between THC and schizophrenia
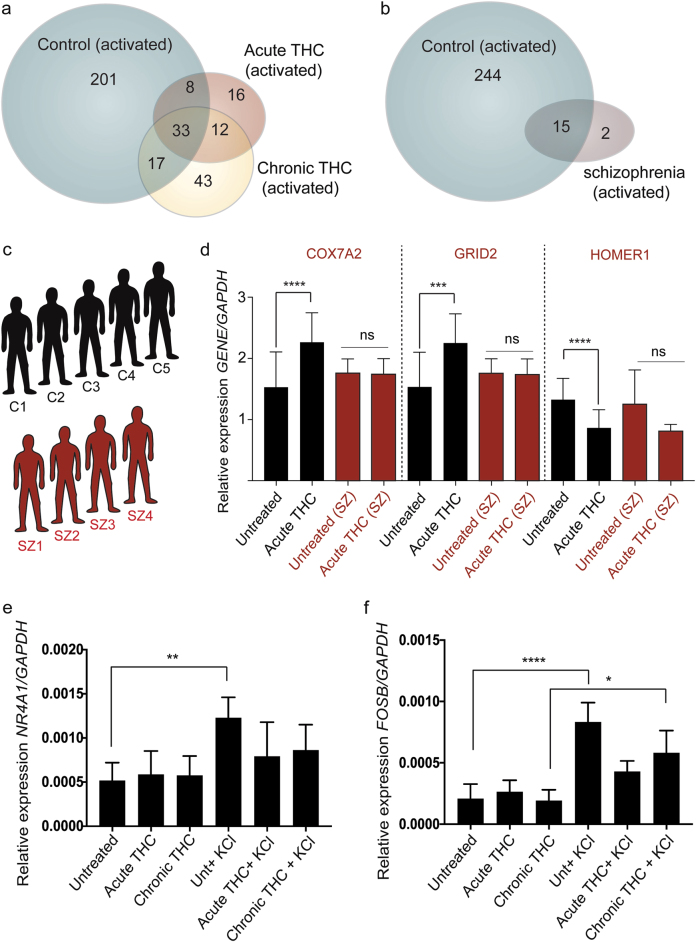
In our previous study6, we demonstrated that schizophrenia-associated hiPSC-derived neurons had a blunted transcriptomic response to KCl relative to controls. We repeated this experimental design on control hiPSC-derived neurons from four individuals, providing either acute (1 μM THC, 24 h), chronic (50 nM THC, 7 days) or vehicle treatment, after which cells were activated using 50 mM KCl (or vehicle) for 3 h as before. We saw a significantly blunted transcriptomic response, more prominent with the acute (75% reduction compared to KCl-activated control neurons; p-value = 1.3e−73, odds ratio = 278.6, Fisher’s exact test) than the chronic exposure (60% reduction compared to KCl-activated control neurons; p-value = 4.4e−83, odds ratio = 181.6, Fisher’s exact test) of THC (Fig. 4a; Supplementary Table S9; Supplementary Figure 4).
Fig. 4. THC treatment results in neuronal hypo-excitability similar to observations using schizophrenia-associated neurons.
a Venn diagram showing impaired transcriptional response following 50 mM KCl treatment for 3 h in THC exposure hiPSC-derived neurons. b A similar decrease in significantly regulated transcripts following 50 mM KCl for 3 h is observed in schizophrenia-associated hiPSC-derived neurons. c A cohort of 5 control (C1–5) and 4 schizophrenia-associated (SZ1-4) cases were used for (d) candidate qRT–PCR analysis investigating COX7A2, GRID2 and HOMER1 following acute THC exposure. e Blunted effect of THC treatment can be seen in immediate early gene transcripts such as NR4A1 and (f) FOSB following KCl-induced activation (Quantitative RT–PCR (qRT–PCR); Ordinary one-way ANOVA with Tukey’s multiple comparisons test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n = 5 controls (see qRT–PCR, Ca–Ce, Supplementary Table S1); n = 4 schizophrenia (see qRT–PCR, S1–S4, Supplementary Table S1))
After re-running the raw schizophrenia-associated data from activity-dependent experiments conducted in Roussos et al6., we again saw a dramatic reduction (~93%; p-value = 4.3e−27, odds ratio = 605.7, Fisher’s exact test) in the schizophrenia-associated transcriptomic response (Fig. 4b; Supplementary Table S10). We tested candidate genes on a cohort of schizophrenia-associated hiPSC-derived neurons (Fig. 4c) and found blunted expression profiles for COX7A2, GRID2 and HOMER1 (Fig. 4d) using quantitative PCR. Quantitative PCR further confirmed this blunting effect of THC exposure; significantly reduced expression of immediate early genes such as NR4A1 and FOSB was observed following KCl treatment (Fig. 4e, f), consistent with what we found previously for these genes in schizophrenia-associated hiPSC-derived neurons6.
Discussion
Our results show that the endogenous response to THC operates through molecular pathways that have been strongly associated with psychiatric disease. This implies that genetic and epigenetic variation present in these specific pathways in individuals might determine the extent of individual susceptibility to adverse response to THC. Genes involved in autism and intellectual disability are prominently involved in THC signaling, while schizophrenia risk may be more linked to similarities when activity- dependent pathways are disrupted.
Understanding human brain subtleties requires a manipulable neuronal model; here we validate hiPSC-derived neuronal networks as a viable system for these types of studies. Others have similarly reported that treatment of hiPSC-derived dopaminergic neurons with THC reproduces effects observed in other mouse and human models27. Mitochondrial pathway dysfunction has been linked to THC exposure24,25,28–30 and schizophrenia26,31, a convergence that is captured in our system (Figs. 1b–d; 2c). Acute and chronic stress have differing effects on mitochondrial genes32 and the different respiratory chain complexes have unique functions during stress responses;33,34 this may explain the parallel differences between acute and chronic THC exposures observed here. Furthermore, links between the glutamatergic and ion channel pathways and THC reported here (Fig. 1b; 2a) are supported by previous studies where both glutamate and ion channel pathways are regulated via cannabinoid receptors8,35. Many of the ion channel proteins identified here are critical for acute synaptic activity36 but are also involved in later stage neural proliferation and differentiation37,38, suggesting that THC exposure may exert short- and/or long-term effects in the developing human brain.
Our experimental system enables relevant comparisons to known disease-causing genes in humans and led us to the significant overlaps in THC-induced genes and those involved in autism and intellectual disability (Fig. 3b). Moreover, by studying activity-related changes instead of baseline differences, we detected similarities between THC-induced hypo-function and schizophrenia (Fig. 4a, b), which would have otherwise been missed in a static system or post-mortem tissue. This is important as we found no overlap of significantly altered ion channel, WNT or mitochondrial genes between THC and schizophrenia datasets (Supplementary Table S8), suggesting that THC- and schizophrenia-related signaling pathways are different with respect to specific genes but convergent in function.
To test whether there was a significant enrichment for psychiatric disease genes in our THC results, we first constructed a list of disease-related genes from the literature, as there were no complete and up to date collections available. We generated comprehensive lists of currently known genes implicated in schizophrenia, autism and intellectual disability, finding significant correlations between THC treatment and autism and intellectual disability (Fig. 3b; Supplementary Table S7). Interestingly, a large recent GWAS uncovered four genes that were significantly associated with lifetime cannabis use39: one, KCNT2, showed significant THC-responsiveness in our hiPSC-derived neurons (Fig. 2a), while two others, NCAM1 and CADM2 (also known as SynCAM2) are important for postsynaptic function40,41, consistent with the enrichment of postsynaptic density genes in THC response (Fig. 2a).
Although we found abrogation of THC-induced changes in schizophrenia-associated hiPSC-derived neurons in candidate genes that were responsive to THC in our study, comprehensive biochemical and functional validation of our THC-induced effects are necessary, across both control and SZ neurons. Moreover, to confirm that these effects are mediated via cannabinoid signaling, future studies should attempt to recapitulate our observed effects using cannabinoid agonists (e.g., anandamide) or block them with selective antagonists (i.e., SR141716A). Consistent with this, we previously reported that THC-induced changes in gene expression in hiPSC neurons were blocked by concurrent 20 nM SR141716A treatment23.
In summary, we found significant associations of THC-related pathways to autism and intellectual disability. Furthermore, we have used a dynamic, human-relevant system to demonstrate a phenotypic link between THC treatment and schizophrenia. We hypothesize that THC exposure, by impacting many of the same synaptic and epigenetic pathways already associated with psychiatric disorders, may serve as an additive risk to existing genetic/epigenetic risk factors.
Electronic supplementary material
Acknowledgements
K.J.B. is a New York Stem Cell Foundation—Robertson Investigator. The Brennand Laboratory is partially supported by a Brain and Behavior Young Investigator Grant, National Institute of Health (NIH) grant R01 MH101454 and the New York Stem Cell Foundation. The Roussos Laboratory is partially supported by the National Institutes of Health (R01AG050986 Roussos and R01MH109677 Roussos), Brain Behavior Research Foundation (20540 Roussos), Alzheimer’s Association (NIRG- 340998 Roussos) and the Veterans Affairs (Merit grant BX002395 Roussos).
Author contributions
G.B., K.J.B. and Y.L.H. conceived of the experiments. K.J.B. generated the hiPSC neurons for RNA-seq while B.G. conducted the RNA-seq analyses guided by M.B., J.H. and P.R. D.C.K. performed RNA-seq experiments, E.A.O. conducted qPCR validation, while I.O. completed the control-SZ THC exposure experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Kristen J. Brennand and Guy Barry.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41398-018-0137-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boland MJ, et al. Molecular analyses of neurogenic defects in a human pluripotent stem cell model of fragile X syndrome. Brain. 2017;140:582–598. doi: 10.1093/brain/aww357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israel MA, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan SD, et al. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetto MC, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry. 2016;22:820–835. doi: 10.1038/mp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madison JM, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol. Psychiatry. 2015;20:703–717. doi: 10.1038/mp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roussos P, Guennewig B, Kaczorowski DC, Barry G, Brennand KJ. Activity-dependent changes in gene expression in schizophrenia human-induced pluripotent stem cell neurons. JAMA Psychiatry. 2016;73:1180–1188. doi: 10.1001/jamapsychiatry.2016.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein A, Livny A, Weizman A. Brain imaging studies on the cognitive, pharmacological and neurobiological effects of cannabis in humans: evidence from studies of adult users. Curr. Pharm. Des. 2016;22:6366–6379. doi: 10.2174/1381612822666160822151323. [DOI] [PubMed] [Google Scholar]
- 8.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci. Biobehav Rev. 2016;64:359–381. doi: 10.1016/j.neubiorev.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossong MG, et al. Effects of delta9-tetrahydrocannabinol on human working memory function. Biol. Psychiatry. 2012;71:693–699. doi: 10.1016/j.biopsych.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol. Psychiatry. 2016;79:526–538. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Volk DW, Lewis DA. The role of endocannabinoid signaling in cortical inhibitory neuron dysfunction in schizophrenia. Biol. Psychiatry. 2016;79:595–603. doi: 10.1016/j.biopsych.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaucher J., et al. Cannabis use and risk of schizophrenia: a Mendelian randomization study. Mol. Psychiatry 2017, https://doi.org/10.1038/mp.2016.252. [DOI] [PMC free article] [PubMed]
- 14.Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol. Psychiatry. 2016;79:549–556. doi: 10.1016/j.biopsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Ksir C, Hart CL. Cannabis and psychosis: a critical overview of the relationship. Curr. Psychiatry Rep. 2016;18:12. doi: 10.1007/s11920-015-0657-y. [DOI] [PubMed] [Google Scholar]
- 16.French L, et al. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry. 2015;72:1002–1011. doi: 10.1001/jamapsychiatry.2015.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topol A, et al. Dysregulation of miRNA-9 in a subset of schizophrenia patient-derived neural progenitor cells. Cell Rep. 2016;15:1024–1036. doi: 10.1016/j.celrep.2016.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennand KJ, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennand K, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol. Psychiatry. 2015;20:361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szutorisz H, Egervari G, Sperry J, Carter JM, Hurd YL. Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol Teratol. 2016;58:107–114. doi: 10.1016/j.ntt.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook V, et al. Human iPSC neurons display activity-dependent neurotransmitter secretion: aberrant catecholamine levels in schizophrenia neurons. Stem Cell Rep. 2014;3:531–538. doi: 10.1016/j.stemcr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortoriello G, et al. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–685. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obiorah MH, IV, Stafford K, Flaherty EK, Brennand KJ. THC treatment alters glutamate receptor gene expression in human stem cell-derived neurons. Mol. Neuropsychiatry. 2017;3:73–84. doi: 10.1159/000477762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh N, Hroudova J, Fisar Z. Cannabinoid-induced changes in the activity of electron transport chain complexes of brain mitochondria. J. Mol. Neurosci. 2015;56:926–931. doi: 10.1007/s12031-015-0545-2. [DOI] [PubMed] [Google Scholar]
- 25.Athanasiou A, et al. Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 2007;364:131–137. doi: 10.1016/j.bbrc.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015;48:10–21. doi: 10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Stanslowsky N, et al. Functional effects of cannabinoids during dopaminergic specification of human neural precursors derived from induced pluripotent stem cells. Addict. Biol. 2016;22:1329–1342. doi: 10.1111/adb.12394. [DOI] [PubMed] [Google Scholar]
- 28.Fisar Z, Singh N, Hroudova J. Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol. Lett. 2014;231:62–71. doi: 10.1016/j.toxlet.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Lipina C, Irving AJ, Hundal HS. Mitochondria: a possible nexus for the regulation of energy homeostasis by the endocannabinoid system? Am. J. Physiol. Endocrinol. Metab. 2014;307:E1–E13. doi: 10.1152/ajpendo.00100.2014. [DOI] [PubMed] [Google Scholar]
- 30.Wolff V, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis-related stroke. Biomed. Res. Int. 2015;2015:323706. doi: 10.1155/2015/323706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncalves VF, Andreazza AC, Kennedy JL. Mitochondrial dysfunction in schizophrenia: an evolutionary perspective. Hum. Genet. 2015;134:13–21. doi: 10.1007/s00439-014-1491-8. [DOI] [PubMed] [Google Scholar]
- 32.Hunter RG, et al. Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc. Natl Acad. Sci. USA. 2016;113:9099–9104. doi: 10.1073/pnas.1602185113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picard M, et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl Acad. Sci. USA. 2015;112:E6614–E6623. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acin-Perez R, Enriquez JA. The function of the respiratory supercomplexes: the plasticity model. Biochim. Biophys. Acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Prog. Chem. Org. Nat. Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voglis G, Tavernarakis N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006;7:1104–1110. doi: 10.1038/sj.embor.7400830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuda T, Adams DJ. Physiological roles of ion channels in adult neural stem cells and their progeny. J. Neurochem. 2010;114:946–959. doi: 10.1111/j.1471-4159.2010.06822.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen D, Yu SP, Wei L. Ion channels in regulation of neuronal regenerative activities. Transl. Stroke Res. 2014;5:156–162. doi: 10.1007/s12975-013-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stringer S, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl. Psychiatry. 2016;6:e769. doi: 10.1038/tp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sytnyk V, Leshchyns’ka I, Schachner M. Neural cell adhesion molecules of the immunoglobulin superfamily regulate synapse formation, maintenance, and function. Trends Neurosci. 2017;40:295–308. doi: 10.1016/j.tins.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Fogel AI, et al. SynCAMs organize synapses through heterophilic adhesion. J. Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.