Abstract
The Mongolian gerbil is an efficient, robust, and cost-effective rodent model that recapitulates many features of H. pylori-induced gastric inflammation and carcinogenesis in humans, allowing for targeted investigation of the bacterial determinants and environmental factors and, to a lesser degree, host constituents that govern H. pylori-mediated disease. This chapter discusses means through which the Mongolian gerbil model has been used to define mechanisms of H. pylori-inflammation and cancer as well as the current materials and methods for utilizing this model of microbially induced disease.
Keywords: Helicobacter pylori, Mongolian gerbil, Gastric inflammation, Gastric cancer
1 Introduction
1.1 Helicobacter pylori and Gastric Cancer
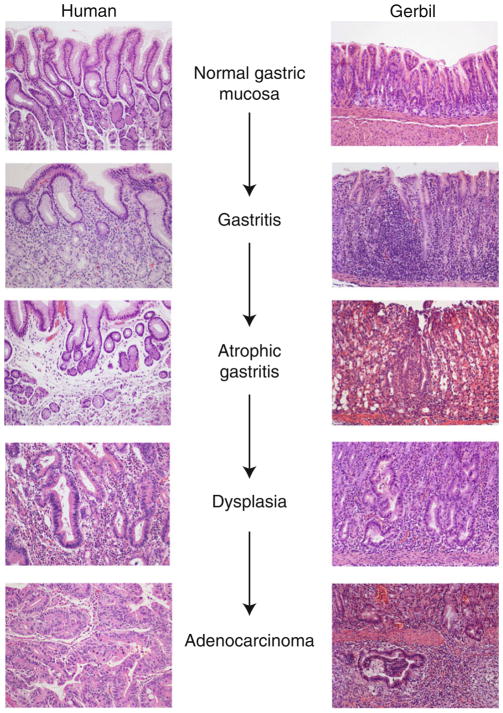
Gastric adenocarcinoma is the third leading cause of cancer-related death worldwide, accounting for over 700,000 deaths each year [1]. There are two main histologically distinct forms of gastric adenocarcinoma, diffuse- and intestinal-type. Diffuse-type cancer is characterized by non-cohesive neoplastic cells that infiltrate the stroma and is not associated with histological precancerous lesions, while intestinal-type cancer progresses through a series of well-defined pathological steps from normal gastric mucosa to chronic superficial gastritis, atrophic gastritis, intestinal metaplasia, and finally dysplasia and adenocarcinoma [2] (Fig. 1).
Fig. 1.
Chronological steps in the progression to gastric cancer in humans and gerbils. Representative hematoxylin and eosin (H&E)-stained human (left ) or gerbil (right ) gastric tissue sections showing the various stages in the progression and transformation from normal gastric mucosa to gastric adenocarcinoma, including gastritis, atrophic gastritis, dysplasia, and adenocarcinoma at 20× magnification
Chronic gastric inflammation induced by Helicobacter pylori is the strongest known risk factor for the development of gastric pre-malignant lesions and cancer, and H. pylori eradication significantly reduces the intensity of premalignant lesions and the subsequent incidence of gastric adenocarcinoma [3–5]. Despite more than half of the world’s population being colonized with H. pylori, only a fraction of individuals ever develop gastric dysplasia or adenocarcinoma. The discrepancy in overall H. pylori infection rates versus disease outcomes is governed by specific relationships among host inflammatory responses, strain-specific bacterial virulence determinants, and environmental factors, which ultimately influence interactions between H. pylori and its human host.
1.2 Mongolian Gerbils
The Mongolian gerbil (Meriones unguiculatus) is a small rodent member of the Cricetidae family (Table 1). The gerbil has been increasingly used in research focused on H. pylori pathogenesis as it represents an efficient and cost-effective rodent model that recapitulates many features of H. pylori-induced gastric inflammation and carcinogenesis in humans (Fig. 1). The first published description of this model in 1991 reported that H. pylori colonized gerbils and induced mild gastritis following a 2-month infection [6]. Numerous other studies demonstrated that H. pylori-infected gerbils developed gastric ulcers, duodenal ulcers, and intestinal metaplasia following experimental challenge [7–11]. Subsequent studies showed that gerbils develop gastric carcinoma following co-administration of H. pylori and chemical carcinogens such as N-methyl-N-nitrosourea (MNU) or N-methyl-N-nitro-N--nitrosoguanidine (MNNG) [12–15]. In 1998, two seminal studies demonstrated that Mongolian gerbils developed gastric adenocarcinoma following long-term H. pylori infection in the absence of additional chemical carcinogens [16, 17], which was subsequently confirmed by other groups [18, 19]. Carcinomas that developed in H. pylori-infected gerbils typically occurred in the distal stomach and the pyloric region and contained well-differentiated intestinal-type epithelium, reflecting many features of intestinal-type gastric adenocarcinoma in humans. Consistent with reports in humans, H. pylori eradication in the gerbil model significantly reduces the severity of gastritis, premalignant lesions, and incidence of gastric adenocarcinoma [8, 20–25].
Table 1.
Characteristics of the Mongolian gerbil model
| Characteristic | Description |
|---|---|
| Family | Cricetidae |
| Genus species | Meriones unguiculatus |
| Coat | Agouti |
| Weight | 70–100 g as adult |
| Activity | Nocturnal, burrowing |
| Water | ~4 mL/day |
| Food | ~8 g/day |
| Breeding | Monogamous, pair 10–12 weeks of age |
| Disposition | Friendly, not prone to fighting or biting |
Similar to the human disease, the colonization of the gerbil gastric mucosa by H. pylori elicits a mixed inflammatory infiltrate in the lamina propria consisting of neutrophils and mononuclear leukocytes. Severe inflammation may be accompanied by pit abscesses, formation of lymphoid follicles, and epithelial hyperplasia. In the Mongolian gerbil, inflammation is usually more pronounced in the transitional mucosa between the corpus and the antrum and spreads proximal and distally as the disease progresses. Over time, there is loss of parietal and chief cells that usually starts in distal corpus and progresses towards the proximal mucosa. The loss of these specialized cells in the oxyntic mucosa is usually accompanied by hyperplasia of mucous neck cells (sometimes called mucous metaplasia) and the base of fundic glands may show features of spasmolytic polypeptide expressing metaplasia (also known as pseudopyloric metaplasia). In more advanced stages of H. pylori infection, epithelial dysplastic changes may be observed, which usually start in the deep portion of the glands in the transitional mucosa. These changes are characterized by epithelial nuclear crowding and by irregularity, budding, and branching of the glands. Dysplastic glandular epithelium tends to spread laterally, parallel to the muscularis mucosa. The penetration of dysplastic glands through the muscularis mucosa into the submucosa is characteristic of invasive adenocarcinoma. However, one caveat of using the Mongolian gerbil model of gastric cancer is that there has been some difficulty in distinguishing herniation of non-neoplastic mucosa from invasive carcinoma, particularly within the setting of inflammation. To address this issue, guidelines have been established for pathological interpretation of these lesions [26]. Although these guidelines were originally formulated for evaluation of intestinal tumors, they have been applied to gastric lesions by numerous investigators [27–29], and in many studies of H. pylori-induced cancer in gerbils, several features have been used to distinguish invasive carcinoma from mucosal herniation (Table 2).
Table 2.
Features that distinguish invasive carcinoma from herniated mucosa
| Feature | Description |
|---|---|
| 1 | Invasive cells differ from overlying mucosa with atypia exceeding low-grade dysplasia |
| 2 | Desmoplasia, unassociated with a predominant inflammatory infiltrate |
| 3 | Irregular, sharp, or angulated glands in the invasive component |
| 4 | Invading glands spread laterally deep to the surface mucosa |
| 5 | Loss of epithelial cells from invading glands |
| 6 | Greater than 2 invading glands in submucosa |
| 7 | Absence of basement membrane around invading glands |
1.3 Host Constituents
Although limited due to their outbred nature, Mongolian gerbils have been used to study the role of a subset of host constituents on the development of gastric cancer. IL-1β is a Th1-type cytokine that inhibits acid secretion and is increased within gastric mucosa of H. pylori-infected individuals [30]. In humans, polymorphisms in the IL-1β gene cluster are associated with increased IL-1β production and a significantly increased risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma, but only among H. pylori-infected individuals [31–33]. These relationships were investigated in gerbils infected with H. pylori by quantifying changes in gastric acid secretion that were mediated by IL-1β. Compared to uninfected animals, gerbils infected with H. pylori exhibited elevated levels of IL-1β expression within the gastric mucosa, which was accompanied by decreased gastric acidity. Treatment of H. pylori-infected gerbils with an IL-1β antagonist abolished loss of acid secretion, implicating IL-1β in the development of achlorhydria within an H. pylori-infected gerbil stomach [34].
Other groups have also developed novel reagents specific for analyses in gerbils. Numerous primers targeting chemokines and cytokines have been developed based on species-specific gerbil cDNA to assess H. pylori-induced inflammatory responses in gerbils [35–41]. In addition to chemokines and cytokines, H. pylori has been shown to alter expression of other inflammatory mediators in gerbils including iNOS and COX2 [37, 42]. The role of NF-κB activation has also been assessed in gerbils within the context of H. pylori-induced inflammation. One group developed a gastric cell line derived from a gerbil gastric cancer specimen and demonstrated that H. pylori can activate NF-κB signaling in an in vitro, species-specific model [43], while another group demonstrated that activation of NF-κB was essential for H. pylori-induced inflammation in gerbils [44]. H. pylori infection of gerbils has also been shown to increase serum levels of gastrin, which can promote cell growth, and increased gastrin levels were directly related to heightened gastric epithelial cell proliferation [45, 46]. Furthermore, a recent study demonstrated that a gastrin antagonist prevents H. pylori-induced gastritis in gerbils [47]. Overall, despite the fact that the gerbil model is not genetically tractable, many host responses have been successfully investigated and targeted in this system.
1.4 Bacterial Determinants
In contrast to constraints regarding the study of host factors, the gerbil model is particularly robust for studying cancer-associated microbial determinants. The cag pathogenicity island (cag PAI), one of the most well-studied H. pylori virulence determinants, encodes a bacterial type IV secretion system (T4SS) that allows for the delivery of the bacterial effector protein, CagA, into host gastric epithelial cells [48, 49]. Transgenic mice that overexpress CagA develop gastric epithelial cell hyperproliferation and gastric adenocarcinoma [50], implicating this molecule as a bacterial oncoprotein. One limitation of using murine models of H. pylori infection is that cag+ strains frequently lose function of the cag island following chronic infection [51, 52]. In contrast, cag+ strains of H. pylori efficiently colonize gerbils and maintain a functional cag T4SS secretion system [45], which allows for examination of the role of this key virulence determinant in the context of H. pylori-induced inflammation and cancer. Compared to gerbils infected with cag- H. pylori, gerbils challenged with cag+ strains develop significantly more severe gastritis [18, 53–56], indicating the importance of the cag island in H. pylori-mediated inflamma-tion. Rieder et al. investigated alterations not only in the intensity but also in the topography of inflammation in gerbils infected with wild-type H. pylori compared to isogenic cagA- mutants. Loss of cagA resulted in an inflammatory response primarily restricted to the gastric antrum, and which did not significantly involve the acid-secreting corpus [57]. Cumulatively, these results indicate that a functional cag T4SS is required for H. pylori-induced corpus-predominant gastritis, a precursor in the progression to intestinal-type gastric adenocarcinoma, which can be easily evaluated in the Mongolian gerbil model.
As previously demonstrated by Watanabe et al., gerbils develop gastric cancer in response to H. pylori [16]; however, the prolonged time-course required for transformation in these early studies precluded large-scale analyses that comprehensively evaluate mediators that are critical to gastric carcinogenesis. Since serial passage of H. pylori in rodents can increase colonization efficiency, Franco et al. investigated whether in vivo adaptation of a human H. pylori strain (B128) would enhance its carcinogenic potential [58]. A single gerbil was infected with a human H. pylori isolate B128 and then sacrificed 3 weeks post-challenge. A single colony output derivative (H. pylori strain 7.13) was isolated and used to infect an independent population of gerbils. Although the levels of inflammation induced by both the parental strain B128 and output strain 7.13 were similar, gastric dysplasia and adenocarcinoma only developed in gerbils infected with the output strain 7.13 [58]. These findings indicated that in vivo adaptation of H. pylori strains can increase the virulence potential of H. pylori strains in the gerbil model.
1.5 Environmental Factors
Host constituents and/or H. pylori virulence determinants are not solely responsible for the development of gastric cancer in humans or animal models, as environmental factors, such as diet, are also important risk factors for disease [59]. However, epidemiologic studies of diet in humans are subject to many limitations, including a reliance on patient reporting and difficulty in ascertaining diets that were consumed decades prior to the development of gastric cancer. Moreover, it is difficult to determine whether dietary parameters are causally linked to the development of gastric cancer or merely represent markers for other factors that are important in gastric cancer pathogenesis. To further investigate potential relationships between diet and gastric cancer risk, several studies have examined the role of dietary factors in the gerbil model of H. pylori-induced gastric carcinogenesis.
Increased salt consumption has been shown to increase the risk for gastric cancer in humans [60], and the effects of high-salt diets on H. pylori infection and gastric cancer have been investigated using the gerbil model. One study reported that H. pylori infection and a high-salt diet could independently induce gastric atrophy and intestinal metaplasia in Mongolian gerbils [61]. Other studies have demonstrated a synergistic effect of H. pylori infection and high-salt diets on gastric carcinogenesis in the presence of a chemical carcinogen, MNU [62–64]. To further investigate the direct effect of a high-salt diet on H. pylori-induced carcinogenesis in gerbils, Gaddy et al. maintained Mongolian gerbils on high-salt or normal-salt diets and then challenged gerbils with H. pylori. Compared to infected gerbils maintained on normal-salt diets, the incidence of gastric adenocarcinoma was significantly increased among H. pylori-infected animals maintained on a high-salt diet [65].
Another dietary factor that increases the risk for gastric cancer is iron deficiency [66]. To address the effect of dietary iron depletion on H. pylori-induced gastric carcinogenesis, Noto et al. maintained gerbils on iron-replete or iron-depleted diets and then challenged gerbils with H. pylori [67]. H. pylori induced more severe gastritis and increased the incidence and frequency of gastric dysplasia and gastric adenocarcinoma among gerbils maintained on iron-depleted diets compared to gerbils maintained on iron-replete diets [67], phenotypes that were abrogated in animals infected with an cagA- isogenic mutant strain. H. pylori cagA- strains also exhibited a significant decrease in colonization density [68] and altered colony morphology [69], but only among gerbils maintained on iron-depleted diets. Cumulatively, these data demonstrate that dietary factors, including high salt and low iron, significantly increase the severity and frequency of H. pylori-induced premalignant and malignant lesions in the gerbil model of gastric cancer.
2 Materials
2.1 Helicobacter pylori Culture
Personal protective equipment (PPE).
Helicobacter pylori strains (see Note 1).
Sterile cotton-tipped applicators.
Trypticase soy agar plates with 5 % sheep blood.
Baffled-bottom flasks.
Sterile Brucella broth with 10 % fetal bovine serum and vancomycin (10 μg/mL final concentration).
Spectrophotometer.
37 °C incubator with 5 % CO2 with platform shaker.
Glass slides.
Gram stain reagents.
Oxidase test.
Catalase test.
Microscope.
2.2 Gerbil Challenge with H. pylori
50 mL conical tubes.
Tabletop centrifuge.
1 mL syringes with 18 G feeding needles.
Sterile Brucella broth (control).
H. pylori resuspended in Brucella broth (2 × 109 CFU/mL).
Biosafety cabinet.
Male Mongolian gerbils (see Note 2).
2.3 Gerbil Dissection
Dissecting tray with pins.
70 % ethanol.
1 mL syringes with 27 G needles.
Sterile blunt forceps.
Sterile blunt scissors.
Sterile sharp forceps.
Sterile sharp scissors.
Sterile scalpel.
Sterile surgical blades.
100 % ethanol for tool sterilization.
Sterile petri dishes.
Sterile 1× phosphate-buffered saline (PBS).
Pencil or solvent-resistant marker for labeling histology cassettes.
Histology cassettes.
Sponges for histology cassettes.
10 % formalin for histology.
Microcentrifuge tubes.
Freezer tubes.
Dry ice.
2.4 Sample Preparation After Dissection
Microcentrifuge.
Digital scale.
Sterile tissue homogenizer.
Tryptic soy agar with 5 % sheep blood.
Antifungal/antibiotics (amphotericin, bacitracin, nalidixic acid, vancomycin; Table 3).
GasPak™ Campy Container sachets.
100 % ethanol for histology.
Equipment for paraffin embedding and sectioning.
Hematoxylin & eosin (H&E).
Microscope.
Table 3.
Antifungal/antibiotic concentrations for isolation of H. pylori by quantitative culture
| Antifungal/antibiotic | Stock concentration (mg/mL) | Final concentration (μg/mL) |
|---|---|---|
| Amphotericin | 20 | 2 |
| Bacitracin | 30 | 30 |
| Nalidixic acid | 10 | 10 |
| Vancomycin | 20 | 20 |
3 Methods
3.1 Helicobacter pylori Culture
Recover minimally passaged H. pylori strains from −80 °C freezer stocks onto trypticase soy agar plates with 5 % sheep blood. Incubate at 37 °C with 5 % CO2 for 2–4 days (see Note 3).
Expand H. pylori strains onto 1–2 trypticase soy agar plates with 5 % sheep blood and incubate at 37 °C with 5 % CO2 for 24–48 h.
Continue expanding H. pylori strains onto trypticase soy agar plates with 5 % sheep blood and incubate at 37 °C with 5 % CO2 for 24 h until desired amount is obtained (see Note 4).
Collect H. pylori from the 24-h plates using sterile cotton-tipped applicators. Using sterile technique, inoculate Brucella broth culture (see Note 5) with a starting OD600>0.15 (see Note 6). Incubate on a platform shaker at 37 °C with 5 % CO2, ~150 rpm for 16–18 h.
The next day, harvest H. pylori broth cultures. Remove 1 mL of H. pylori broth culture to measure OD600 and a small sample to perform Gram stain, oxidase, and catalase tests. H. pylori should be in the log phase of growth and typically OD600>1.0 (range=1.0–2.0) (see Note 6).
Transfer broth culture into 50 mL conicals and subject to centrifugation at 4000 rpm for 10 min.
Discard supernatants and resuspend bacterial pellets to a concentration of 2 × 109 CFU/mL. To do so, multiply the final OD600 measurement to determine CFU/mL (1 OD600=5.5×108 CFU/mL). Multiply total CFU/mL by the total culture volume to determine total CFU. Next, divide total CFU by the desired concentration of bacteria needed per gerbil (2×109 CFU/mL). This calculation will provide the volume required to resuspend the bacterial pellet so that there are 2×109 CFU/mL. The following example calculation would result in enough inoculum to infect 41 gerbils.
Example calculation:
3.2 Gerbil Challenge with H. pylori
Order male Mongolian gerbils (36–49 days old or 41–50 g) (see Note 2). Adapt gerbils to new environment in the animal housing facility for at least 1 week prior to challenge.
Fast the gerbils for 8–12 h prior to first challenge (see Note 7).
Orally gavage gerbils with 0.5 mL of Brucella broth as a control or 0.5 mL of H. pylori (1×109 CFU) using sterile feeding needles (one feeding needle per strain of H. pylori). For oral gavage, hold the gerbil by the ears and support and straighten the body. Guide the feeding needle along the roof of the mouth to the back of the throat and pass it through the esophagus into the stomach. Dispense 0.5 mL of bacteria (1 × 109 CFU). After challenging, fast the gerbils for an additional 2–4 h (see Note 8). Reintroduce food and wait 24–48 h before the second challenge (see Note 9).
Fast the gerbils for 8–12 h prior to second challenge.
Orally gavage gerbils with 0.5 mL of Brucella broth as a control or 0.5 mL of H. pylori (1×109 CFU) using sterile feeding needles. After challenging, fast the gerbils for an additional 2–4 h and then reintroduce food.
Monitor gerbils throughout the course of infection to address any health concerns, such as changes in body weight (see Note 10).
3.3 Preparation for Gerbil Dissection
Prepare and autoclave tryptic soy agar. Prepare enough plates to have approximately 3–4 per gerbil (see Note 11).
Once media has adequately cooled to ~56 °C, add 5 % sheep blood and antifungal/antibiotics (Table 3 and see Note 12).
Pour plates and allow them to solidify overnight. Store plates at 4 °C for short-term storage.
Prepare collection tubes and histology cassettes for gerbil dissection and tissue collection (Table 4).
Table 4.
Collection tubes and histology cassettes required for gerbil dissection and tissue collection
| Collection tube/cassette | Purpose |
|---|---|
| Tube 1 | Microcentrifuge tube for blood collection |
| Tube 2a | Microcentrifuge tube with 1 mL of sterile 1× PBS with antifungal/antibiotics for gastric tissue collection for quantitative culturea |
| Tube 3 | Freezer tube for gastric tissue collection for long-term storage at −80 °C |
| Cassette | Histology cassette labeled with pencil or solvent-resistant marker for gastric tissue collection for histology |
| Tube 4 | Microcentrifuge tube with 900 μL sterile 1× PBS with antifungal/antibiotics for 1:10 serial dilution |
| Tube 5 | Microcentrifuge tube with 900 μL sterile 1× PBS with antifungal/antibiotics for 1:100 serial dilution |
| Tube 6 | Microcentrifuge tube with 900 μL sterile 1× PBS with antifungal/antibiotics for 1:1000 serial dilution |
Weigh tube 2 prior to collection of tissue to ultimately determine CFU per gram of gastric tissue.
3.4 Gerbil Dissection
Euthanize gerbils according to the practices and procedures designated by the Institutional Animal Care and Use Committee (IACUC) and individual animal housing facility. Following euthanasia, perform cervical dislocation.
Secure gerbil in the supine position on the dissecting tray. Drench the anterior chest and abdomen in 70 % ethanol.
Using a 27-G syringe, collect blood by cardiac puncture and place in first collection tube, Tube 1 (Table 4 and see Note 13).
Using sterile blunt forceps and scissors, open the top layer of skin to expose the peritoneum.
Using sharp forceps and scissors, open the peritoneum to expose the stomach.
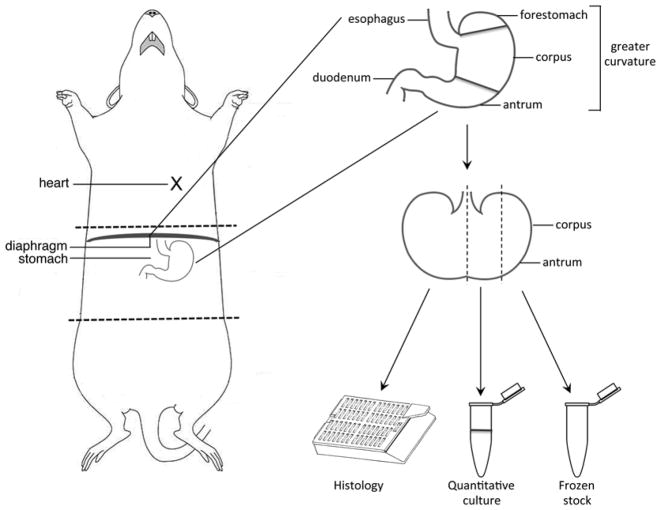
Excise the stomach, including a portion of the duodenum. Discard the forestomach lined with squamous epithelium and place the stomach in a sterile petri dish (Fig. 2).
Cut the stomach along the greater curvature so that it lies flat (Fig. 2) and remove any remaining food.
Cut the stomach, extending from the squamocolumnar junction through the proximal duodenum, in half longitudinally and place one-half into a histology cassette (Fig. 2) and submerge in 10 % formalin (see Note 14).
Cut the remaining half of the stomach in half, creating two one-quarter sections (Fig. 2). Place one-quarter of the stomach into the second collection tube with sterile 1× PBS and antifungal/antibiotics (Tube 2) for quantitative culture. Place the other one-quarter of the stomach into the third collection tube (Tube 3) and place on dry ice.
Fig. 2.
Gerbil dissection and isolation of gastric tissue. Perform cardiac puncture for blood collection. Open the peritoneum to expose the stomach. Excise the stomach, including a portion of the duodenum. Cut the stomach along the greater curvature so that it lies flat. Cut the stomach, extending from the squamocolumnar junction through the proximal duodenum, in half longitudinally and place one-half into a histology cassette. Cut the remaining half of the stomach in half, creating two one-quarter sections. Place one-quarter of the stomach into a collection tube for quantitative culture. Place the other one-quarter of the stomach into a collection tube for long-term storage
3.5 Blood Samples and Frozen Gastric Tissue Samples
For blood samples, subject samples to centrifugation for 5 min at 5000 rpm. Transfer serum/supernatant to a new microcentrifuge tube and repeat centrifugation for 5 min at 5000 rpm or 2400×g. Transfer serum/supernatant to a fresh microcentrifuge tube and store at −20 °C until needed (see Note 15).
For gastric tissue samples temporarily stored on dry ice, transfer and store gastric tissue samples at −80 °C for long-term storage until needed (see Note 16).
3.6 Gastric Tissue Samples for Quantitative Culture
-
1
Weigh the microcentrifuge tube containing gastric tissue to determine the weight of each gastric tissue section. Subtract the initial tube weight to determine weight of each piece of tissue to ultimately determine CFU per gram of gastric tissue by quantitative culture.
-
2
Transfer each gastric tissue sample into a sterile tissue homogenizer containing 1 mL sterile 1× PBS + antifungal/antibiotics.
-
3
Homogenize the gastric tissue and plate 100 μl of homogenate onto tryptic soy agar plates containing 5 % sheep blood and antifungal/antibiotics.
-
4
Perform serial dilutions and plate 100 μL of each serial dilution out to a 1:1000 dilution (Tubes 4, 5, and 6).
-
8
Incubate plates at 37 °C with 5 % CO2 for 5–7 days in GasPak™ Campy Container sachets and then count colonies to determine CFU per gram of gastric tissue.
-
9
Isolate and expand H. pylori single colony isolates. Perform Gram stain, oxidase, and catalase tests on single colonies and then freeze for future analyses of in vivo-derived strains (see Note 17).
3.7 Gastric Tissue Samples for Histology
Incubate histology cassettes in 10 % formalin overnight and then transfer to 100 % ethanol.
Paraffin embed and section gastric tissue.
Stain gastric tissue sections with H&E and have a pathologist assess acute and chronic inflammation, and determine disease diagnosis based on the most advanced lesions present within the gastric tissue sections.
Footnotes
Some strains of Helicobacter pylori are commercially available through ATCC.
Due to the higher incidence of disease among males, male gerbils are typically used for assessing H. pylori-induced inflammation and gastric disease. Mongolian gerbils are classified as a USDA animal and are subject to different restrictions than murine models. Mongolian gerbils are commercially available from Charles River Laboratories.
Wait until single colonies are clearly visible on plate. Passing plates too early will lead to insufficient growth and not enough inoculum for broth cultures.
At least 2–3 plates per H. pylori strain are required for a 50 mL broth culture. Some laboratory strains of H. pylori grow very well, while clinical strains frequently do not, so adjust the number of plates depending on the growth rates of individual H. pylori strains.
Choose the appropriate volume of broth culture depending on the number of gerbils in the experiment. A 50 mL volume should be enough for approximately 20 animals, depending on the final OD600. See example calculation (Subheading 3.1, step 7).
Optical density readings can differ between spectrophotometer instruments. For reference: 1 OD600=5.5×108 CFU. If the starting OD600 is too low, H. pylori will not grow. Determine the best starting OD600 based on individual spectrophotometers and CFU. Some laboratory strains of H. pylori grow very well, while other clinical strains do not, so adjust the starting OD600 based on spectrophotometer readings as well as growth rates of individual H. pylori strains.
Fasting gerbil prior to infection allows for more efficient H. pylori colonization.
Oral gavage is usually performed when the gerbils are awake and not sedated; however, the gerbils can be sedated for this procedure, but it is a much more time-intensive process.
The amount of time between challenges is due to the requirement for fasting. Fasting the gerbils for extended periods of time can have adverse effects on the health; therefore, adequate time is needed for re-feeding between infections.
Typically a 12-week infection with a gerbil-adapted H. pylori strain, such as 7.13, should be adequate to observe gastric dysplasia and adenocarcinoma. Longer infection times may be necessary for less well-adapted H. pylori strains.
One liter should yield approximately 30–40 plates.
If media is not adequately cooled prior to the addition of blood or antifungal/antibiotics, blood will lyse and antifungal/antibiotics will be inactivated.
Regarding cardiac puncture, collect blood with a single attempt to avoid lysis of red blood cells. However, if unsuccessful, more attempts can be made or blood can be collected immediately after the animal has been dissected, exposing the heart. Remove the needle following blood collection for dispensing into the microcentrifuge tube. This will prevent further red blood cell lysis.
For histology, harvest both the gastric antrum and corpus in each section because indices of inflammation are scored in both the antrum and corpus. The strips of tissue for paraffin embedding should be no greater than 3 mm wide.
The serum should be clear to yellow in color. Serum that is pink or red indicates lysis of red blood cells in the sample.
Frozen samples can be used for a variety of purposes, including but not limited to flow cytometry, quantitative PCR, and Western blotting.
If no colonies are isolated from serial dilutions, collectively sweep each plate onto a new tryptic soy agar plate with 5 % sheep blood and antifungal/antibiotics to determine if H. pylori can be isolated. This is not quantitative, but will provide information on whether the animal was successfully colonized. PCR can also be performed to determine H. pylori colonization.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, et al. Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Correa P, Chen VW. Gastric cancer. Cancer Surv. 1994;20:55–76. [PubMed] [Google Scholar]
- 3.Wong BC, Lam SK, Wong WM, Chen JS, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Re: long term follow up of patients treated for Helicobacter pylori infection. Gut. 2007;56:436. doi: 10.1136/gut.2006.108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokota K, Kurebayashi Y, Takayama Y, Hayashi S, et al. Colonization of Helicobacter pylori in the gastric mucosa of Mongolian gerbils. Microbiol Immunol. 1991;35:475–480. doi: 10.1111/j.1348-0421.1991.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in Mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara S, Ikeda F, Yokota Y, Karita M. Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J Med Microbiol. 1997;46:391–397. doi: 10.1099/00222615-46-5-391. [DOI] [PubMed] [Google Scholar]
- 9.Honda S, Fujioka T, Tokieda M, Gotoh T, Nishizono A, Nasu M. Gastric ulcer, atrophic gastritis, and intestinal metaplasia caused by Helicobacter pylori infection in Mongolian gerbils. Scand J Gastroenterol. 1998;33:454–460. doi: 10.1080/00365529850171990. [DOI] [PubMed] [Google Scholar]
- 10.Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkusa T, Okayasu I, Miwa H, Ohtaka K, Endo S, Sato N. Helicobacter pylori infection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. Gut. 2003;52:797–803. doi: 10.1136/gut.52.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatematsu M, Yamamoto M, Shimizu N, Yoshikawa A, et al. Induction of glandular stomach cancers in Helicobacter pylori-sensitive Mongolian gerbils treated with N-methyl-N-nitrosourea and N-methyl-N′-nitro-N-nitrosoguanidine in drinking water. Jpn J Cancer Res. 1998;89:97–104. doi: 10.1111/j.1349-7006.1998.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama A, Maruta F, Ikeno T, Ishida K, et al. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 14.Shimizu N, Inada K, Nakanishi H, Tsukamoto T, et al. Helicobacter pylori infection enhances glandular stomach carcinogenesis in Mongolian gerbils treated with chemical carcinogens. Carcinogenesis. 1999;20:669–676. doi: 10.1093/carcin/20.4.669. [DOI] [PubMed] [Google Scholar]
- 15.Tokieda M, Honda S, Fujioka T, Nasu M. Effect of Helicobacter pylori infection ontheN-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in Mongolian gerbils. Carcinogenesis. 1999;20:1261–1266. doi: 10.1093/carcin/20.7.1261. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 18.Ogura K, Maeda S, Nakao M, Watanabe T, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Q, Chen XY, Shi Y, Xiao SD. Development of gastric adenocarcinoma in Mongolian gerbils after long-term infection with Helicobacter pylori. J Gastroenterol Hepatol. 2004;19:1192–1198. doi: 10.1111/j.1440-1746.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu N, Ikehara Y, Inada K, Nakanishi H, et al. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. Cancer Res. 2000;60:1512–1514. [PubMed] [Google Scholar]
- 21.Keto Y, Ebata M, Okabe S. Gastric mucosal changes induced by long term infection with Helicobacter pylori in Mongolian gerbils: effects of bacteria eradication. J Physiol. 2001;95:429–436. doi: 10.1016/s0928-4257(01)00059-6. [DOI] [PubMed] [Google Scholar]
- 22.Nozaki K, Shimizu N, Tsukamoto T, Inada K, et al. Reversibility of heterotopic proliferative glands in glandular stomach of Helicobacter pylori-infected Mongolian gerbils on eradication. Jpn J Cancer Res. 2002;93:374–381. doi: 10.1111/j.1349-7006.2002.tb01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirayama F, Takagi S, Yokoyama Y, Yamamoto K, Iwao E, Haga K. Long-term effects of Helicobacter pylori eradication in Mongolian gerbils. J Gastroenterol. 2002;37:779–784. doi: 10.1007/s005350200130. [DOI] [PubMed] [Google Scholar]
- 24.Brzozowski T, Konturek PC, Kwiecien S, Konturek SJ, et al. Triple eradication therapy counteracts functional impairment associated with Helicobacter pylori infection in Mongolian gerbils. J Physiol Pharmacol. 2003;54:33–51. [PubMed] [Google Scholar]
- 25.Nozaki K, Shimizu N, Ikehara Y, Inoue M, et al. Effect of early eradication on Helicobacter pylori-related gastric carcinogenesis in Mongolian gerbils. Cancer Sci. 2003;94:235–239. doi: 10.1111/j.1349-7006.2003.tb01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boivin GP, Washington K, Yang K, Ward JM, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 27.Houghton J, Stoicov C, Nomura S, Rogers AB, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 28.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 29.Hagiwara T, Mukaisho K, Nakayama T, Sugihara H, Hattori T. Long-term proton pump inhibitor administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in Mongolian gerbils infected with Helicobacter pylori. Gut. 2011;60:624–630. doi: 10.1136/gut.2010.207662. [DOI] [PubMed] [Google Scholar]
- 30.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 31.El-Omar EM, Carrington M, Chow WH, McColl KE, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 32.Figueiredo C, Machado JC, Pharoah P, Seruca R, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 33.Santos JC, Ladeira MS, Pedrazzoli J, Jr, Ribeiro ML. Relationship of IL-1 and TNF-alpha polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res. 2012;45:811–817. doi: 10.1590/S0100-879X2012007500099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–773. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto N, Sakagami T, Fukuda Y, Koizuka H, et al. Influence of Helicobacter pylori infection on development of stress-induced gastric mucosal injury. J Gastroenterol. 2000;35:332–340. doi: 10.1007/s005350050357. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara S, Shibata H, Takahashi M, Ishikawa F, Yokokura T, Sugimura T, Wakabayashi K. Cloning of Mongolian gerbil cDNAs encoding inflammatory proteins, and their expression in glandular stomach during H. pylori infection. Cancer Sci. 2004;95:798–802. doi: 10.1111/j.1349-7006.2004.tb02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaoka Y, Yamauchi K, Ota H, Sugiyama A, et al. Natural history of gastric mucosal cytokine expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect Immun. 2005;73:2205–2212. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda T, Tsukamoto T, Takasu S, Shi L, Hirano N, Ban H, Kumagai T, Tatematsu M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer. 2009;125:1786–1795. doi: 10.1002/ijc.24586. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 2009;100:2152–2159. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Helicobacter pylori outer membrane proteins on gastric mucosal interleukin 6 and 11 expression in Mongolian gerbils. J Gastroenterol Hepatol. 2011;26:1677–1684. doi: 10.1111/j.1440-1746.2011.06817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai T, Fukui H, Franceschi F, Penland R, et al. Cyclooxygenase expression during Helicobacter pylori infection in Mongolian gerbils. Dig Dis Sci. 2003;48:2139–2146. doi: 10.1023/b:ddas.0000004517.83166.26. [DOI] [PubMed] [Google Scholar]
- 43.Nozaki K, Tanaka H, Ikehara Y, Cao X, et al. Helicobacter pylori-dependent NF-kappa B activation in newly established Mongolian gerbil gastric cancer cell lines. Cancer Sci. 2005;96:170–175. doi: 10.1111/j.1349-7006.2005.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanai A, Maeda S, Shibata W, Hikiba Y, et al. Activation of IkappaB kinase and NF-kappaB is essential for Helicobacter pylori-induced chronic gastritis in Mongolian gerbils. Infect Immun. 2008;76:781–787. doi: 10.1128/IAI.01046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peek RM, Wirth HP, Moss SF, Yang M, et al. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48–59. doi: 10.1016/s0016-5085(00)70413-6. [DOI] [PubMed] [Google Scholar]
- 46.Konturek PC, Brzozowski T, Konturek SJ, Kwiecień S, et al. Functional and morphological aspects of Helicobacter pylori-induced gastric cancer in Mongolian gerbils. Eur J Gastroenterol Hepatol. 2003;15:745–754. doi: 10.1097/01.meg.0000059155.68845.9d. [DOI] [PubMed] [Google Scholar]
- 47.Sordal O, Waldum H, Nordrum IS, Boyce M, Bergh K, Munkvold B, Qvigstad G. The gastrin receptor antagonist netazepide (YF476) prevents oxyntic mucosal inflammation induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2013;18:397–405. doi: 10.1111/hel.12066. [DOI] [PubMed] [Google Scholar]
- 48.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 49.Censini S, Lange C, Xiang Z, Crabtree JE, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohnishi N, Yuasa H, Tanaka S, Sawa H, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sozzi M, Crosatti M, Kim SK, Romero J, Blaser MJ. Heterogeneity of Helicobacter pylori cag genotypes in experimentally infected mice. FEMS Microbiol Lett. 2001;203:109–114. doi: 10.1111/j.1574-6968.2001.tb10828.x. [DOI] [PubMed] [Google Scholar]
- 52.Philpott DJ, Belaid D, Troubadour P, Thiberge JM, Tankovic J, Labigne A, Ferrero RL. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell Microbiol. 2002;4:285–296. doi: 10.1046/j.1462-5822.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 53.Akanuma M, Maeda S, Ogura K, Mitsuno Y, et al. The evaluation of putative virulence factors of Helicobacter pylori for gastro-duodenal disease by use of a short-term Mongolian gerbil infection model. J Infect Dis. 2002;185:341–347. doi: 10.1086/338772. [DOI] [PubMed] [Google Scholar]
- 54.Saito H, Yamaoka Y, Ishizone S, Maruta F, et al. Roles of virD4 and cagG genes in the cag pathogenicity island of Helicobacter pylori using a Mongolian gerbil model. Gut. 2005;54:584–590. doi: 10.1136/gut.2004.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohnita K, Isomoto H, Honda S, Wada A, et al. Helicobacter pylori strain-specific modulation of gastric inflammation in Mongolian gerbils. World J Gastroenterol. 2005;11:1549–1553. doi: 10.3748/wjg.v11.i10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibata W, Hirata Y, Maeda S, Ogura K, et al. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol. 2006;210:306–314. doi: 10.1002/path.2040. [DOI] [PubMed] [Google Scholar]
- 57.Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce pre-cancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–1242. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 58.Franco AT, Israel DA, Washington MK, Krishna U, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cover TL, Peek RM., Jr Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes. 2013;4(6):482–493. doi: 10.4161/gmic.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 61.Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig Dis Sci. 2003;48(3):475–485. doi: 10.1023/a:1022524313355. [DOI] [PubMed] [Google Scholar]
- 62.Nozaki K, Shimizu N, Inada K, Tsukamoto T, et al. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083–1089. doi: 10.1111/j.1349-7006.2002.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato S, Tsukamoto T, Mizoshita T, Tanaka H, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–1566. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 64.Gamboa-Dominguez A, Ubbelohde T, Saqui-Salces M, Romano-Mazzoti L, et al. Salt and stress synergize H. pylori-induced gastric lesions, cell proliferation, and p21 expression in Mongolian gerbils. Dig Dis Sci. 2007;52:1517–1526. doi: 10.1007/s10620-006-9524-3. [DOI] [PubMed] [Google Scholar]
- 65.Gaddy JA, Radin JN, Loh JT, Zhang F, et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pra D, Rech Franke SI, Pegas Henriques JA, Fenech M. A possible link between iron deficiency and gastrointestinal carcinogenesis. Nutr Cancer. 2009;61:415–426. doi: 10.1080/01635580902803701. [DOI] [PubMed] [Google Scholar]
- 67.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan S, Noto JM, Romero-Gallo J, Peek RM, Jr, Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7(5):e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noto JM, Lee JY, Gaddy JA, Cover TL, Amieva MR, Peek RM., Jr Regulation of Helicobacter pylori virulence within the context of iron deficiency. J Infect Dis. 2015;211:1790–1794. doi: 10.1093/infdis/jiu805. [DOI] [PMC free article] [PubMed] [Google Scholar]