Abstract
Background
We hypothesized that pulmonary venous hypertension in heart failure (HF) leads to predominate remodeling of pulmonary veins and that the severity of venous remodeling is associated with the severity of pulmonary hypertension (PH) in HF.
Methods
Patients with HF (n=108; 53 preserved and 55 reduced ejection fraction) with PH (HF-PH; pulmonary artery systolic pressure (PASP) ≥ 40 mmHg) were compared to normal Controls (n=12) and patients with primary pulmonary veno-occlusive disease (PVOD; n=17). In lung specimens from autopsy (Control, HF-PH and 7 PVOD) or surgery (10 PVOD), quantitative histomorphometry was performed in all analyzable arteries (n=4,949), veins (n=7,630) and small indeterminate vessels (IV, n=2,168) to define % medial thickness (%MT) [arteries] and % intimal thickness (%IT) [arteries, veins and IV] relative to external diameter.
Results
The average arterial %MT (Control 6.9; HF-PH 11.0; PVOD 15.0); arterial %IT (Control 4.9; HF-PH 14.9; PVOD 31.1); venous %IT (Control 14.0; HF-PH 24.9; PVOD 43.9) and IV %IT (Control 10.6; HF-PH 25.8; PVOD 50.0) in HF-PH were higher than Controls (p<0.0001 for all) but lower than PVOD (p≤0.005 for all). PASP (mmHg) was lower in HF-PH (median 59 [IQR 50-70]) than PVOD (91 [82-103]). PASP correlated with arterial %MT (r=0.41) and arterial %IT (r=0.35) but more strongly with venous %IT (r=0.49) and IV %IT (r=0.55) (p<0.0001 for all). Associations between PASP and venous or IV %IT remained significant after adjusting for arterial %MT and %IT and did not vary by HF type. In patients with right heart catheterization (30 HF-PH; 14 PVOD) similar associations between the transpulmonary gradient and pulmonary vascular remodeling existed, with numerically stronger associations for venous and IV %IT. While the PASP was slightly higher in HF-PH patients with right ventricular dysfunction, pulmonary vascular remodeling was not more severe. Pulmonary vascular remodeling severity was associated with reductions in the diffusing capacity of the lungs.
Conclusions
In HF, PH is associated with global pulmonary vascular remodeling but the severity of PH correlates most strongly with venous and small IV intimal thickening, similar to the pattern observed in PVOD. These findings expand our understanding of the pathobiology of PH in HF.
Keywords: Heart failure, pulmonary hypertension, right ventricle, heart failure with preserved ejection fraction, pulmonary function, diffusing capacity of the lungs for carbon monoxide
Introduction
Pulmonary hypertension (PH) is a hemodynamic finding which may be due to variable combinations of increased pulmonary blood flow, pulmonary venous hypertension (PVH), pulmonary vasoconstriction or pulmonary vascular (PV) remodeling. Vascular remodeling results in luminal narrowing and increase in resistance to blood flow. The vascular narrowing in PH may be due to hypertrophy or proliferation of smooth muscle in the media of pulmonary arteries or to diverse processes which result in thickening of the intima in pulmonary arteries or veins.
Idiopathic (Group 1.1) pulmonary arterial hypertension (PAH) is known to be due to an isolated pulmonary arteriopathy with sparing of pulmonary veins. The efficacy of PAH therapies was first established in this group.1 Group 1′ PAH (pulmonary veno-occlusive disease, PVOD) is characterized by preferential remodeling of pulmonary venous intima with “secondary” arterial changes.2 In PVOD, there is a risk of pulmonary edema with and limited responsiveness to PAH therapies.3, 4 The nature and severity of venous remodeling in other types of PH and the relationship between venous and arterial remodeling across the spectrum of PH severity is not well defined.1, 5
Heart failure (HF) is a common cause of PH (Group 2 PH)6 and may be associated with preserved (HFpEF) or reduced (HFrEF) ejection fraction (EF). In HF, the severity of PH is associated with adverse outcomes, irrespective of EF.7-10 Pulmonary vascular remodeling may contribute to the severity of PH in HF and support consideration of clinical trials of PAH therapies in HFpEF.11 Previous investigators12-19 have reported pulmonary arterial and venous remodeling in left heart disease (predominately mitral stenosis and congenital lesions), but a systematic study of PV remodeling in typical HFpEF and HFrEF is lacking.
We hypothesized that the chronic PVH in HF may lead to preferential remodeling of pulmonary veins and that the severity of PH and the presence of right ventricular (RV) dysfunction in HF are associated with the severity of venous remodeling, irrespective of EF. Further, as PH associated with left heart disease is reversible, we speculated that the character of the venous intimal remodeling in HF may differ from PVOD, a largely irreversible condition.3 Finally, as the diffusing capacity for carbon monoxide (DLCO) is variably reduced in HF and nearly uniformly reduced in PVOD, we speculated that the severity of PV remodeling may be associated with reductions in DLCO.
Accordingly, we performed comprehensive quantitative histomorphometry with qualitative assessment of intimal character and morphology in pulmonary vessels in autopsy specimens from patients with an antemortem diagnosis of HF (HFpEF and HFrEF) and varying severities of PH. Findings were compared to those in autopsy or surgical specimens from normal Controls and patients with primary PVOD. The severity of PH and the presence of RV dysfunction were defined by antemortem Doppler echocardiography and (when available) right heart catheterization (RHC). The DLCO was measured by antemortem pulmonary function tests (PFT) when available.
Methods
The data, analytic methods, and study materials will not be made available to other investigators.
The study was approved by the Mayo Clinic Institutional Review Board and its Bio-specimens Subcommittee. Only cases with consent for use of their specimens for research were included.
Selection of HF-PH Subjects
Study subjects were identified from autopsy cases from the Mayo Clinic Tissue Registry (MCTR) from 1987–2015. Subjects with a clinical diagnosis of heart failure with a pulmonary artery systolic pressure ≥40 mmHg and meeting the inclusion and exclusion criteria as outlined in the Supplemental Methods and Figure S1 were included. Heart failure patients with other conditions recognized to cause PH were excluded, including those with significant lung disease based on clinical diagnosis, PFT findings or pulmonary histologic findings at autopsy.
Selection of Primary PVOD Cases
Primary PVOD subjects were identified from consecutive cases with histological diagnosis of “PVOD” or “PVOD-like changes” from the MCTR database (1985–2015) and from five PVOD cases (1930–1983) reported in a previously published study20 as outlined in the Supplemental Methods and Figure S2. Patients with conditions recognized to cause “secondary” PVOD were excluded.
Selection of Normal Controls
Twelve normal controls, with similar age and sex as the HF-PH group, without any heart or lung disease or any condition associated with PVOD or other forms of PH reported in medical records or identified at autopsy, were obtained from the MCTR autopsy archives.
Demographics and Clinical and Cardiac Autopsy Characteristics
Clinical information was abstracted from the medical records with echocardiographic, PFT and RHC data extracted from the reports provided by the respective Mayo Clinic clinical laboratories as outlined in the Supplemental Methods.
Histomorphometry
A detailed description of specimen preparation and analysis is provided in the Supplemental Methods. At least two blocks from different lung laterality and lobes were procured from each case (Table S1), stained with hematoxylin and eosin (H&E) and with Verhoeff-van Gieson (VVG) and captured with whole-field digital microscopy (Figure S3). Pulmonary arteries were distinguished from pulmonary veins based on both position and structure. When position and structure of a vessel did not definitively identify it as an artery/arteriole or vein/venule (primarily small [<100 μm] vessels), the vessel was designated as an indeterminate vessel (IV) and analyzed separately. Histomorphometric measurements of vessels utilized rigorous criteria for analytic suitability. All vessels suitable for analysis were measured in each patient (Figure S3). The percent medial thickness (%MT) [arteries] and percent intimal thickness (%IT) [arteries, veins and IV] were calculated relative to external diameter (Figure S4 and Supplemental Methods), as previously described.19, 21
For each vessel measured, intimal morphology was qualitatively categorized as: eccentric, concentric, occlusion, or luminal webs/re-canalization, and the character of intimal remodeling, irrespective of the cellularity, was qualitatively categorized as: dense fibrosis, loose fibrosis, or intimal hyalinosis (Supplemental Methods and Figure S4). Veins with loosely fibrotic intimal remodeling and intimal hyalinosis were combined into one category for analysis.
Statistical Analysis
Data were summarized with frequencies and percentages for categorical variables and with medians and interquartile ranges (IQR) for continuous data. When data were not available in all subjects, the number of subjects with data was reported. Data at the patient-level were compared between groups with chi-square tests or Fisher's exact test for categorical data, and with t-tests or Wilcoxon rank-sum tests for continuous data, as appropriate.
The %MT for all measured arteries and the %IT for all vessels (arteries, veins and IV) were compared between patient groups (control, HF-PH, PVOD) with random-intercept mixed-effects linear regression models to account for the multiple measurements per patient, using a compound symmetry correlation structure. Each model also allowed for heterogeneous variance across the patient groups, after graphically noting a difference in variance between PVOD, HF-PH, and Normal patients at the vessel-level. The normality of the conditional residuals from each model was assessed graphically. The mean and standard error within each group were estimated from these models.
The percentage of arteries, veins, and IVs that had different qualitative remodeling characteristics (eccentric or concentric, dense or loose fibrosis, etc.) was calculated for each patient. The group data were summarized with medians and interquartile ranges of the percent vessels per patient with the qualitative remodeling characteristic, and compared with pairwise Wilcoxon rank-sum tests.
To assess associations with patient-level measurements (PASP, RHC and PFT data), the %MT (arteries) and %IT (arteries, veins or IV) were averaged for each patient, and these patient-level averages were used as predictors in linear regression models, alone and with other adjustment variables. The normality and homoscedasticity assumptions of the residuals for linear regression were assessed graphically. Pearson correlations (r) and the coefficient of determination from linear regression models (R2) were reported. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and figures were generated using R22 or GraphPad Prism (Software, La Jolla CA, USA). P-values less than 0.05 were considered statistically significant, and no adjustment for multiple comparisons was made.
Results
Cohort Demographics and Clinical Characteristics
The HF-PH patients (n=108) were older and more likely to be female than PVOD patients (n=17) but, by design, age and sex were similar in HF-PH and Control (n=12) patients (Table 1). The HF-PH patients were more obese and had or tended to have more cardiovascular risk factors, mild lung disease and renal dysfunction than Control or PVOD patients. A history of smoking was present in a similar percentage of HF-PH, Controls and PVOD patients, but HF-PH patients had or tended to have a higher prevalence of obstructive sleep apnea (OSA) than Controls or PVOD patients.
Table 1. Characteristics of the Study Groups.
| Control (N=12) | HF-PH (N=108) | PVOD (N=17) | P value HF vs PVOD | P Value HF vs Control | |
|---|---|---|---|---|---|
| Age, years | 69 (58, 91) | 74 (62, 85) | 34 (21, 42) | <0.0001 | 0.71 |
| Male | 5 (41.7%) | 51 (47.2%) | 13 (76.5%) | 0.02 | 0.71 |
| BMI (Kg/m2) | 23.3 (19.5, 27.2) | 29.4 (24.7, 37.0) | 22.7 (18.6, 24.9) | <0.0001 | 0.0005 |
| Hypertension | 7 (58.3%) | 94 (87.0%) | 2 (11.8%) | <0.0001 | 0.02 |
| Diabetes | 3 (25.0%) | 51 (47.2%) | 2 (11.8%) | 0.007 | 0.22 |
| Dyslipidemia | 4 (33.3%) | 80 (74.1%) | 4 (23.5%) | 0.0001 | 0.007 |
| Ever Smoker | 4 (33.3%) | 47 (43.5%) | 4 (23.5%) | 0.18 | 0.55 |
| Atrial Fibrillation | 0 (0.0%) | 64 (59.3%) | 0 (0.0%) | <0.0001 | 0.0001 |
| Lung Disease | 0 (0.0%) | 28 (25.9%) | 0 (0.0%) | 0.01 | 0.07 |
| OSA | 2 (17%) | 37 (34%) | 1 (6%) | 0.02 | 0.33 |
| eGFR, ml/min/1.73m2 | <0.0001 | 0.01 | |||
| N | 11 | 108 | 16 | ||
| Median (IQR) | 58 (53, 85) | 48 (29, 60) | 78 (65, 90) | ||
| Echo to death/surgery, days | 101 (19, 869) | 39 (11, 148) | 0.09 | NA | |
| Ejection Fraction, % | 0.04 | NA | |||
| N | 108 | 14 | |||
| Median (IQR) | 48 (25, 63) | 60 (55, 66) | |||
| PASP (echo), mmHg | 59 (50, 70) | 91 (82, 103) | <0.0001 | NA | |
| E/e′ | 0.0008 | NA | |||
| N | 68 | 6 | |||
| Median (IQR) | 21.7 (15.4, 26.9) | 9.5 (7.5, 11.7) | |||
| LA Dilatation | 94/97 (97%) | 2/11 (18%) | <0.0001 | ||
| RV Enlargement | 60/100 (60%) | 16/16 (100%) | 0.001 | NA | |
| RV Dysfunction | 60/98 (61%) | 15/15 (100%) | 0.002 | NA |
Data are n (%), n/n with data (%) or median (Interquartile range).
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HF, heart failure; LA, left atrial; NA, not available or applicable; OSA, obstructive sleep apnea; PASP, pulmonary artery systolic pressure; PVOD, pulmonary veno-occlusive disease; RV, right ventricle
Among patients with echocardiography (HF-PH and PVOD), the median time between the echocardiogram with the highest PASP and death/surgery was 101 days in HF-PH and 39 days in PVOD (Table 1). The HF-PH patients had lower PASP (median 59 [IQR 50-70] mmHg) than PVOD (91 [82-103] mmHg) patients. The HF-PH patients were less likely to have RV enlargement (60%) or dysfunction (61%) than PVOD patients (100% had both). The EF was lower while the E/e′ ratio and prevalence of left atrial enlargement were higher in HF-PH than PVOD.
Among HF-PH patients, those with HFpEF were older and less likely male, more likely obese and had a higher prevalence of hypertension and atrial fibrillation than HFrEF patients (Table S2). The HFpEF patients were less likely to have smoked but had a similar prevalence of lung disease, OSA and renal dysfunction as HFrEF patients. Left ventricular (LV) size was smaller while LV wall thicknesses and mass were greater in HFpEF, but E/e″ ratio and the prevalence of left atrial enlargement and RV enlargement and dysfunction were not significantly different in HFpEF and HFrEF. The PASP was numerically higher in HFpEF but this difference was not statistically significant. At autopsy, heart weights were higher in HFrEF than HFpEF but the percent predicted heart weight (accounting for age, sex and body size23) was similar in the two groups (Table S3). The severity of coronary atherosclerosis, the prevalence of left atrial dilatation, and microscopic evidence of LV and RV hypertrophy and fibrosis were similar in HFpEF and HFrEF.
PV Remodeling in HF-PH
The right upper and left lower lobes were the most frequently sampled lobes in all groups (Table S1). Overall, 14,747 vessels were analyzed. On average, approximately 30 arteries, 50 veins and 7 IV per patient were analyzed in each Control and HF-PH patient, and approximately 50 arteries, 80 veins and 54 IV were analyzed in each PVOD patient. There were more analyzable vessels in PVOD as preparation of surgical and autopsy specimens purposefully maximized small (peripheral) vessels owing to the clinical suspicion of PVOD.
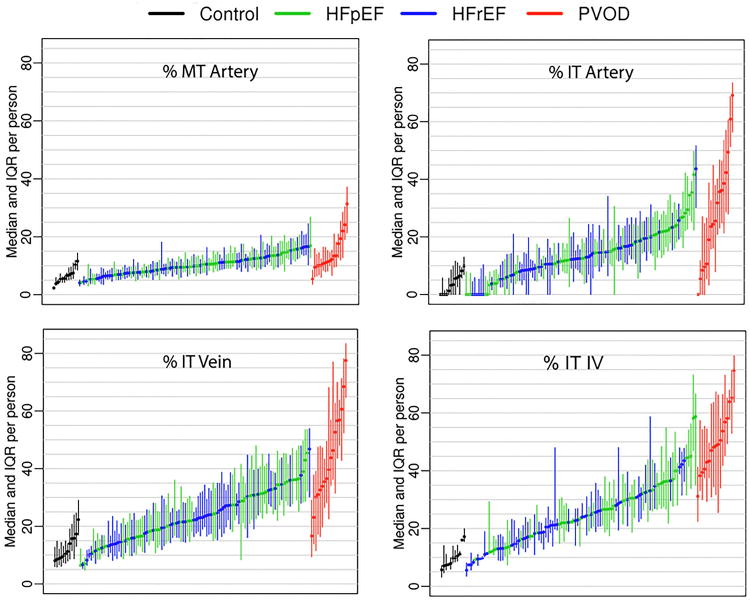
Within the lungs of individual patients, the %MT in arteries and the %IT in arteries, veins and IV varied considerably in HF-PH and PVOD (Figure 1) but were more consistent in Controls. HF-PH patients displayed higher %MT in arteries and greater %IT in arteries, veins and IV as compared with Controls, but % MT in arteries and % IT in arteries, veins and IV were all lower as compared with PVOD (Table 2 and Figures 1 and 2). The percent of veins with arterialization was higher in HF-PH than Controls or PVOD (Table 2). The % MT in arteries and the % IT in arteries and veins tended to be more severe (p=0.07 for all) in HFpEF than HFrEF, and the %IT in IV was significantly higher in HFpEF than HFrEF (Table S3).
Figure 1. Variable pulmonary vascular remodeling in study groups.
For each patient with Heart Failure and Pulmonary Hypertension (with preserved (HFpEF) or reduced (HFrEF) ejection fraction), the median (center dot) and interquartile range (vertical line) for the percent medial thickness (% MT, arteries) and % intimal thickness (%IT, arteries, veins and indeterminate vessels (IV)) in all vessels analyzed in each patient are shown. Similar data are shown in comparator groups (Controls and Pulmonary Veno-occlusive Disease, PVOD).
Table 2. Pulmonary vascular remodeling by study group.
| Normal (N=12) | HF-PH (N=108) | PVOD (N=17) | P Value HF vs PVOD | P Value HF vs Control | |
|---|---|---|---|---|---|
| Arteries - %MT* | 6.9 (0.8) | 11.0 (0.3) | 15.0 (1.4) | 0.005 | <0.0001 |
| Arteries - %IT* | 4.9 (1.0) | 14.9 (0.8) | 31.1 (3.8) | <0.0001 | <0.0001 |
| Veins - % IT* | 14.0 (1.3) | 24.9 (0.8) | 43.9 (3.5) | <0.0001 | <0.0001 |
| IV - %IT* | 10.6 (0.8) | 25.8 (1.0) | 50.0 (2.3) | <0.0001 | <0.0001 |
| % of Veins with arterialization | 5.3 (0.5, 6.1) | 16.4 (9.0, 25.0) | 7.8 (4.4, 12.1) | 0.02 | 0.0002 |
| Intimal character distribution within patients | |||||
| Arteries - % Loose Fibrosis/Hyalinosis | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.87 | 0.46 |
| Veins - % Loose Fibrosis/Hyalinosis | 31 (24, 43) | 25 (11, 49) | 2 (0, 7) | <0.0001 | 0.66 |
| IV - % Loose Fibrosis/Hyalinosis | 0 (0, 25) | 0 (0, 12) | 0 (0, 0) | 0.07 | 0.85 |
| Intimal morphology distribution within patients | |||||
| Arteries | |||||
| % Concentric | 0 (0, 0) | 4 (0, 15) | 20 (11, 40) | <0.0001 | 0.001 |
| % Occluded | 0 (0, 0)† | 0 (0, 0)† | 0 (0, 0) | <0.0001 | 1.0 |
| % Recanalized/webs | 0 (0, 0)† | 0 (0, 0) | 4 (0, 7) | <0.0001 | 0.76 |
| Veins | |||||
| % Concentric | 36 (27, 59) | 53 (39, 66) | 68 (55, 76) | 0.002 | 0.07 |
| % Occluded | 0 (0, 0)† | 0 (0, 0)† | 7 (2, 17) | <0.0001 | 1.0 |
| % Recanalized/webs | 0 (0, 0)† | 0 (0, 0)† | 1.5 (0.4, 7) | <0.0001 | 1.0 |
| Indeterminate vessels | |||||
| % Concentric | 50 (17, 60) | 54 (33, 73) | 66 (59, 84) | 0.05 | 0.20 |
| % Occluded | 0 (0, 0)† | 0 (0, 0)† | 2 (0, 6) | <0.0001 | 1.0 |
| % Recanalized/webs | 0 (0, 0)† | 0 (0, 0) | 6 (1, 9) | <0.0001 | 0.77 |
Data are shown as median (interquartile range), unless otherwise noted.
Means and p values estimated from mixed model to account for repeated measures within patients. Data summarized as mean (standard error of the mean) from these models.
Equal to 0% for all observations (no variability).
Figure 2. Pulmonary arteries, veins and indeterminate vessels in study groups.
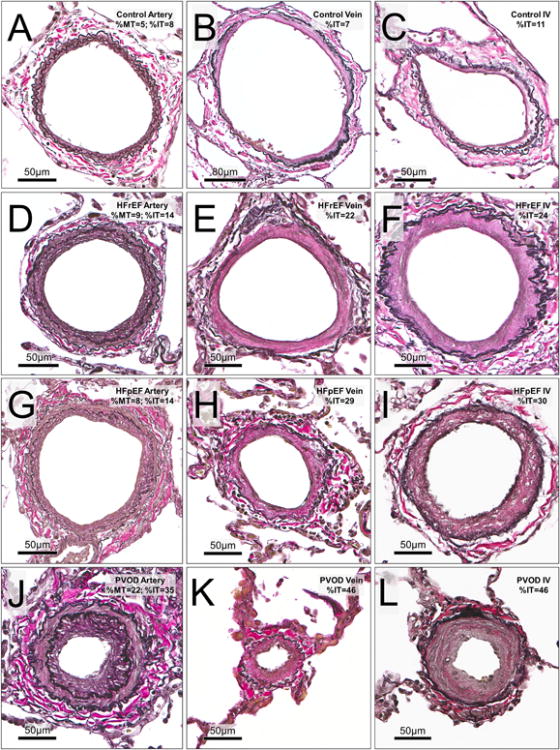
Representative vessels with remodeling approximating the median values for medial and intimal thickening of arteries, veins and indeterminate vessels (IV) in each study group are shown. Rows represent cohort groups (from top; Controls, HFrEF, HFpEF and PVOD). Columns represent vessel type (from left; arteries, veins and indeterminate vessels). In Controls; A (artery:ED152 μm), B (vein: ED 247 μm) and C (IV: ED 121 μm). In HFrEF; D (artery: ED 173 μm), E (vein: ED 181 μm) and F (IV: ED 193 μm). In HFpEF; G (artery: ED 176 μm), H (vein: ED113 μm) and I (IV: ED 175 μm). In PVOD; J (artery: ED 148 μm), K (vein: ED 60 μm) and L (IV; ED135 μm).
Abbreviations: ED, external diameter; HF, Heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; %IT, percent intimal thickness; %MT, percent medial thickness; PH, pulmonary hypertension; PVOD, pulmonary veno-occlusive disease.
The severity of medial thickening (%MT) in arteries increased with greater severity of intimal thickening (%IT) in veins and IV (Figure S5 B and C) but not with the severity of intimal thickening in arteries (Figure S5 A), suggesting that arterial medial hypertrophy developed secondary to venous and IV remodeling. The severity of intimal thickening in arteries increased with the severity of intimal thickening in veins (Figure S5 D); however, the %IT in veins and IV was higher than the %IT in arteries within each group (p<0.0001 for all). As IV may be arterioles or venules, there were strong correlations between the severity of intimal thickening in IV and that of arteries or veins (Figure S5 E and F).
When present, the character of intimal thickening in arteries and IV was rarely that of loose fibrosis/hyalinosis, irrespective of study group (Table 2). In contrast, in veins, the intima thickening in HF-PH could be densely fibrotic or show loose fibrosis or hyalinosis (Figure 3). The frequency of loose fibrosis/hyalinosis did not vary with HF type (Table S3). The venous intima in PVOD was consistently densely fibrotic (Table 2 and Figure 3) and only rarely (68 of 1488 veins) showed loose fibrosis/hyalinosis. The normal intima in Control veins also sometimes displayed a loose fibrosis/hyalinosis character (Table 2 and Figure 3). Of note, in veins from Controls and HF-PH, the severity of intimal thickening (%IT) was greater in veins with loose fibrosis/hyalinosis as compared to veins with dense fibrosis (Figure S6), while in PVOD, the %IT was similar in veins irrespective of intimal character.
Figure 3. Character of pulmonary venous intimal remodeling in study groups.
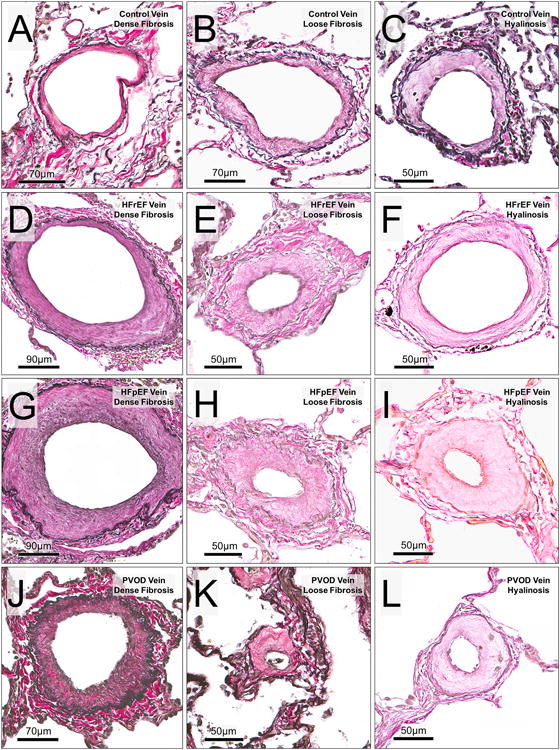
Rows represent cohort groups (from top; controls, HFrEF, HFpEF and PVOD). Columns represent nature of venous intimal remodeling (from left; dense fibrosis, loose fibrosis and intimal hyalinosis). In Controls: A (ED 152 μm), B (ED 183 μm) and C (ED 119 μm). In HFrEF: D (ED 275 μm), E (ED 98μm) and F (ED 162 μm). In HFpEF: G (ED 347 μm), H (ED 118 μm) and I (ED 125 μm). In PVOD: J (ED 206 μm), K (ED 60 μm) and L (ED 93 μm).
The arterial intima was seldom concentric whereas the venous and IV intima were more often concentric, irrespective of study group. Occluded or re-canalized intima was not seen in HF-PH or Controls but was present in a small number of PVOD vessels (Table 2).
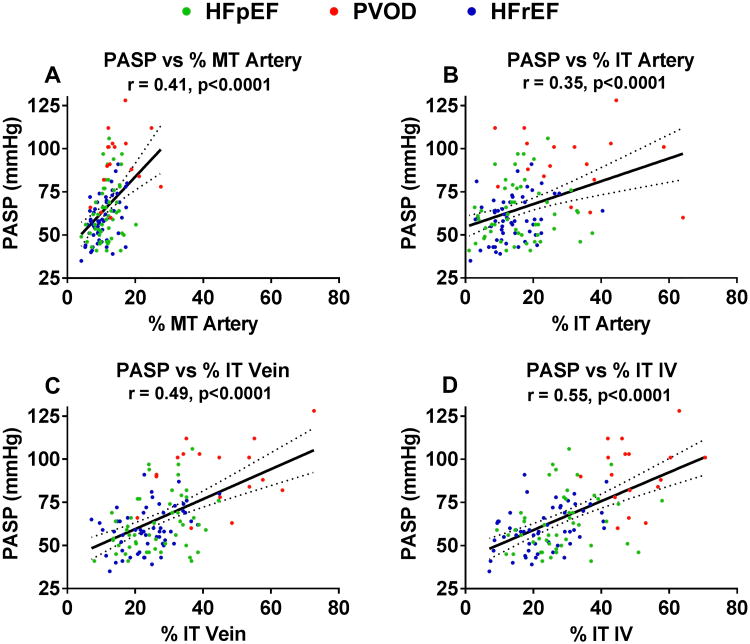
Relationship between PV Remodeling and Pulmonary Hypertension
The severity of medial (Figure 4 A) and intimal (Figure 4B) thickening of arteries and intimal thickening of veins (Figure 4C) and IVs (Figure 4 D) all correlated with the severity of PH (PASP estimated by echocardiography) with the numerically strongest relationships with PASP noted for venous and IV intimal thickening. These relationships persisted after adjustment for lung disease, OSA or renal function (Table S4). Further, the statistically significant relationships between venous or IV intimal thickening and PASP persisted after adjusting for arterial medial and intimal thickening (Table S4). The relationships between PV remodeling and severity of PH did not vary by HF type (Table S5).
Figure 4. Relationship between pulmonary vascular remodeling and severity of pulmonary hypertension.
The relationship between medial (A) and intimal (B) thickening of arteries and intimal thickening of veins (C) and IVs (D) and the severity of pulmonary hypertension as assessed by Doppler echocardiographic estimation of pulmonary artery systolic pressure (PASP) in HF-PH (HFpEF and HFrEF) and pulmonary veno-occlusive disease (PVOD). The solid line represents the estimated PASP via linear regression based on medial/intimal thickening, and the dotted lines represent the 95% confidence interval.
Abbreviations: % MT, percent medial thickening; % IT, percent intimal thickening
Note, one outlier (HFpEF patient with a PASP of 153 mmHg) is not shown on this scale.
Impact of Comorbidities on PV Remodeling
As patients with significant lung disease were excluded from the HF-PH cohort, when present, the severity of lung disease was mild and PASP and PV remodeling were similar in those HF-PH patients with or without mild lung disease (Table S6). Similarly, PASP and PV remodeling were similar in those HF-PH patients with or without OSA (Table S6). While PASP was not significantly different in those HF-PH patients with estimated glomerular filtration rate (eGFR) above versus below the median value in HF-PH, venous and IV intimal remodeling were more severe in patients with worse renal function (Table S6).
Relationship between PV Remodeling and RV function
Among HF-PH patients, PASP was higher in those with (n=60) than without (n=38) RV dysfunction at echocardiography (64 [55-74] versus 52 [46-62] mmHg, p= 0.005). However, the severity of arterial, venous and IV remodeling was not significantly different in those patients with or without RV dysfunction (p>0.23 for all). In 29 patients, the RV dysfunction was characterized as mild and in 30 patients, the RV dysfunction was characterized as moderate or severe. The severity of vascular remodeling in these two groups was not different than those without RV dysfunction (p>0.13 for all).
Findings in Patients with PH Characterized by RHC
A subgroup of HF-PH patients (n=30; 16 with HFrEF and 14 with HFpEF) and nearly all PVOD (n=16) patients had undergone RHC at some point (median of 22 days in HF-PH and 40 days in PVOD) prior to death/surgery. While HF-PH patients undergoing RHC were younger than those who did not, the differences in clinical characteristics between HF-PH and PVOD in the RHC cohorts (Table S7) were similar to that observed in all patients.
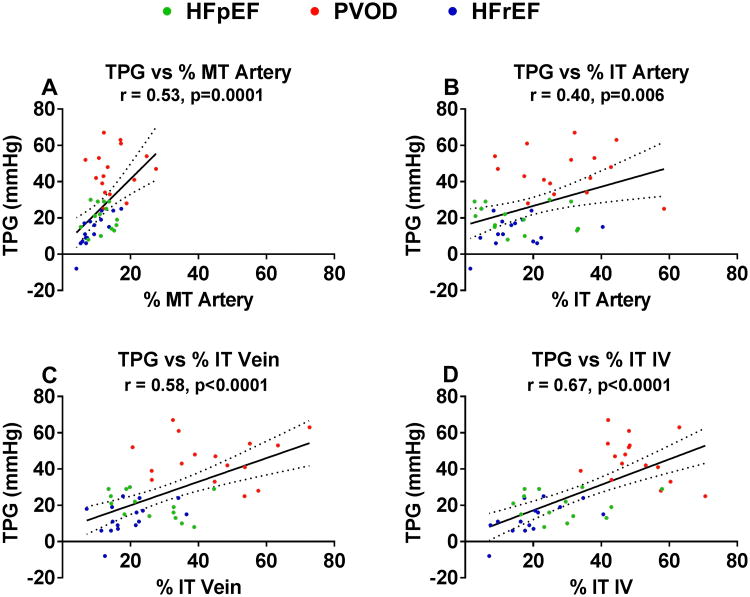
Right atrial and pulmonary artery wedge pressures were higher in HF-PH than PVOD patients (Table S7). The cardiac output tended to be higher in HF-PH than PVOD, but the cardiac index was similar. Pulmonary artery systolic, diastolic and mean pressures were lower in HF-PH than PVOD patients. The trans-pulmonary gradient (TPG) was lower in HF-PH patients than PVOD patients (16 [10-24] versus 45 [37-54] mmHg). Pulmonary vascular resistance (PVR) was also lower in HF-PH than PVOD patients. In the RHC subgroup, the degree of PV remodeling was less severe in HF-PH than in PVOD (Figure 5 and Table S7).
Figure 5. Pulmonary vascular structure in HF-PH and PVOD patients with elevated transpulmonary gradient.
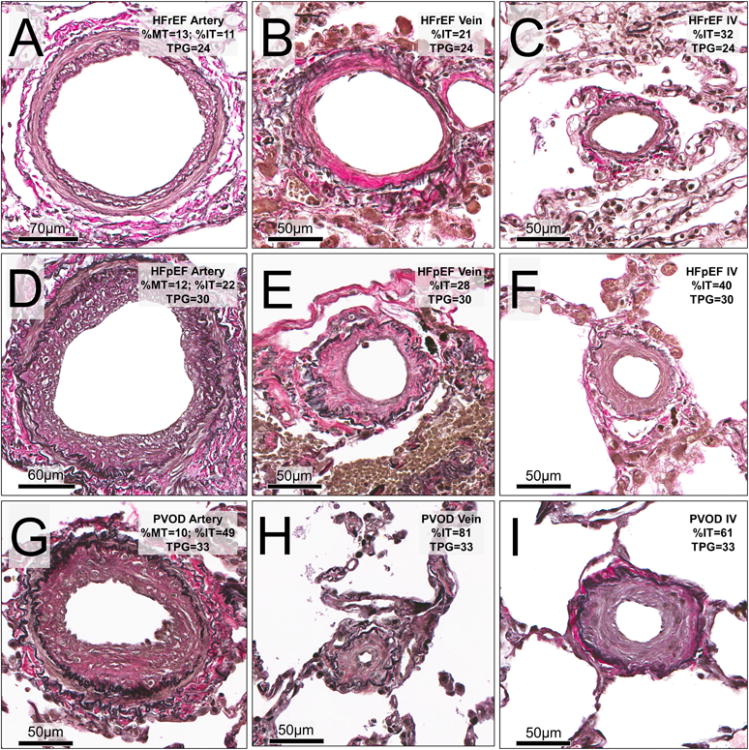
Examples representative of the mean histomorphometry values for all vessels within the patient in three patients with approximately similar degrees of elevation in the transpulmonary gradient (TPG) are shown. Rows indicate cohort group; from top, HFrEF, HFpEF and PVOD). Columns represent vessel type (from left; arteries, veins and indeterminate vessels [IV]). Top row, HFrEF: A (artery, ED 219 μm), B (vein, ED122 μm) and C (IV, ED 60 μm). Middle row, HFpEF: D (artery, ED 220 μm), E (vein, ED 86 μm) and F (IV, ED 69 μm). Bottom row, PVOD: G (artery, ED 151 μm), H (vein, ED 48 μm) and I (IV, ED 86 μm).
Arterial medial and intimal, and venous and IV intimal remodeling correlated with the severity of PH as measured by TPG (Figure 6), with the numerically strongest relationships noted for venous and IV intimal thickening. These associations persisted after adjustment for lung disease, OSA and renal function (Table S8). Similar relationships were present with PVR (Figure S7). The diastolic pressure gradient (DPG) calculated from the reported pulmonary artery wedge and diastolic pressures was lower in HF-PH (6 [2-9] mmHg; n=22) than PVOD (28 [22-40] mmHg; p<0.0001; n=16). The correlations between DPG and PV remodeling were similar to those observed with TPG and PVR (%MT artery: r= 0.36, p=0.03; % IT artery: r= 0.31, p= 0.06: % IT vein, r = 0.56, p=0.0002; % IT IV, r=0.54, p=0.0006).
Figure 6. Relationship between pulmonary vascular remodeling and invasively measured transpulmonary gradient.
The relationship between medial (A) and intimal (B) thickening of arteries and intimal thickening of veins (C) and IVs (D) and the severity of pulmonary hypertension as assessed by the transpulmonary gradient measured at right heart catheterization in HF-PH (HFpEF and HFrEF) and pulmonary veno-occlusive disease (PVOD). The solid line represents the estimated TPG via linear regression based on medial/intimal thickening, and the dotted lines represent the 95% confidence interval.
Abbreviations: % MT, percent medial thickening; % IT, percent intimal thickening
PV Remodeling and Lung Function
A subgroup of HF-PH patients (n=43) and nearly all PVOD (n=14) patients had undergone PFT at some point prior to surgery/death (median, 326 days in HF-PH and 75 days in PVOD). The differences in clinical characteristics between HF-PH and PVOD in the PFT cohort (Table S9) were similar to that observed in all patients. On average, patients with HF displayed a mild mixed obstructive/restrictive pattern on spirometry whereas spirometry was normal in PVOD (Table S9).
Pulmonary arterial vasoconstriction or remodeling can reduce capillary blood volume and thus, DLCO. Increases in pulmonary venous pressure due to left heart disease or remodeling of the small pulmonary veins can lead to interstitial edema and restrictive physiology with reduction in alveolar volume and alveolar-capillary membrane conductance, reducing DLCO. On average, percent predicted total lung capacity (TLC), a surrogate for alveolar volume, was similar in HF-PH and PVOD patients, but the percent predicted DLCO was higher in HF-PH (median 55%) than PVOD (37%; Table S9). In bivariate analysis, DLCO tended to decrease as arterial (p=0.06), venous (p=0.09) and IV (p=0.047) intimal thickening increased (Figure S8). Adjusting for reduction in alveolar percent predicted TLC, reductions in DLCO were associated with increases in intimal thickening in arteries and small IV (Table S10).
Discussion
In this study of PV remodeling in HF with reduced or preserved EF and a spectrum of PH severity, there was significant variability in the extent of arterial, venous and IV remodeling within each HF-PH patient, underscoring the potential for sampling bias if small numbers of vessels are analyzed. Relative to Control patients, HF-PH patients displayed global (arteries, veins and IV) PV remodeling including prominent intimal thickening in veins and IV. This pattern was similar to, but less severe than, that observed in the PVOD patients who, on average, also had more severe PH. The character of venous intimal remodeling was unique in HF-PH as compared to PVOD, with obstruction more often due to loose fibrosis and hyalinosis. In HF-PH and PVOD, the severity of PH was correlated with arterial, venous and IV remodeling, but most strongly/independently with venous and IV intimal thickening. While RV dysfunction was common in HF-PH and associated with higher PASP, the severity of PV remodeling was not greater in patients with versus without RV dysfunction. The DLCO was variably but substantially reduced in HF-PH and markedly reduced in PVOD. Adjusting for differences in lung volumes, both arterial and small IV intimal thickening were associated with reductions in DLCO.
As emphasized in the proceedings from the pathology and pathobiology working group of the Fifth World Symposium on Pulmonary Hypertension5, the histologic alterations of the pulmonary veins are understudied in PH of all etiologies, and there is a need to better understand the relationships between arterial and venous remodeling across the spectrum of mild to severe PH. The importance of pulmonary venous remodeling in PH is underscored by its well-recognized, primary role in causing PH in PVOD2 and its implications for prognosis and therapy. Of all forms of PAH, PVOD has the worst prognosis.3 In PVOD, the presence of extensive venous remodeling can result in increases in the trans-capillary hydrostatic pressure gradient and, consequently, pulmonary edema with increases in pulmonary arterial blood flow in response to PA specific vasodilators.4 While the use of PAH therapies in recognized PVOD is not well studied, they are less effective in PVOD even if tolerated.3, 4
Other types of PAH are believed to be associated with variable combinations of arterial and venous remodeling, but precise documentation of venous remodeling across the spectrum of PAH etiologies and severities is lacking5, in part due to the challenges of pulmonary venous histomorphometry. There is no specific stain to discriminate between small arteries and veins, and histologic techniques to accurately identify veins are laborious and require careful attention to vessel morphology and location.
Previous studies12-19 have documented pulmonary arterial and venous remodeling in left heart disease (primarily mitral stenosis and congenital heart disease), but most were very old and largely qualitative studies without systematic morphometric analysis, assessment of the character of intimal remodeling or rigorous statistical analysis. Most included small numbers of patients, examined small numbers of vessels per patient and did not examine the relationship between arterial and venous remodeling across a range of PH severity or according to HF type and did not relate the relationship between remodeling and pulmonary hemodynamics. Delgado et al measured % MT in a cohort (n=17) of HF patients who died shortly after heart transplant and found higher % MT in those with elevated PVR/TPG.24
More recently, Hunt et al15 performed arterial and venous histomorphometric analysis in 19 controls (failed organ donors; transplant specimens) and 22 HFrEF patients with relatively advanced PH (TPG of 18.6 mmHg, PVR of 4.7 WU), who underwent wedge lung biopsy at the time of left ventricular assist device placement. A median of 6 arteries per patient were analyzed but the number of veins analyzed in each patient was not reported. Average pulmonary arterial and venous medial and intimal thicknesses were all increased in HFrEF, relative to control patients, consistent with our findings. However, the relationships between arterial and venous remodeling, the nature of intimal remodeling, and the relationship between pulmonary hemodynamics and PV remodeling were not described. Importantly, three patients had a repeat lung biopsy after a prolonged period of cardiac unloading and one of the three had dramatic reduction in mean pulmonary arterial pressure associated with marked reductions in arterial and venous medial and intimal thicknesses.
While much emphasis has been placed on pulmonary arterial medial and intimal thickening and its relationship to PH severity in different forms of PAH, here the venous and small IV remodeling appeared more strongly related to PH severity in HF, with pulmonary arterial intimal remodeling having the weakest correlation with PH severity. Thickening of the arterial media correlated well with the severity of venous and IV (but not arterial) intimal thickening. These findings suggest that arterial medial thickening in HF reflects a hypertrophic response to the downstream obstruction provided both by the venous and IV remodeling, as well as the chronic PVH related to their underlying left heart disease.
The venous intimal thickening in HF-PH patients displayed a loosely fibrotic or hyaline type appearance in a subset of veins, co-existing with veins showing densely fibrotic venous intimal thickening. Venous intimal thickening due to loose fibrosis/hyalinosis was rarely seen in PVOD patients. Intimal hyalinosis has not been systematically studied, but has been attributed to deposition of hyaline connective tissue including extracellular matrix; also termed “edematous intima” by some pathologists.17,18,25 In HF-PH patients, a median of 25% of veins showed this appearance but there was significant variability. Indeed, in 25% of HFpEF patients, more than 50% of veins showed this appearance. We speculate that such remodeling may resolve more readily than dense fibrosis and contribute to the reversibility of PH with cardiac unloading in HF. While the severity of PH as assessed by PASP and TPG correlated with the extent of PV remodeling, the relative contributions of remodeling and vasoconstriction to PH severity cannot be ascertained from the current study.
Multiple mechanisms may contribute to PV remodeling in HF.26 The PVH in HF may cause venous endothelial disruption due to edema or mechanical distension of the pulmonary veins and result in activation of growth factors. Inflammation from venous mechanical stress and the pro-inflammatory milieu in HF may also contribute. In situ pulmonary venous thrombosis may play a role.17 The effect of HF related neurohumoral activation on PV remodeling in HF is uncertain although neurohumoral activation is believed to play an important role in contributing to vascular remodeling in other forms of PAH.27
The proceedings from the working group on PH in left heart disease at the Fifth World Symposium on Pulmonary Hypertension advance a paradigm wherein the mechanism of PH in HF progresses from the purely passive effect of PVH, to pulmonary arterial vasoconstriction and finally to PV remodeling with subsequent development of RV dysfunction.11 In the present study, we observed global PV remodeling and particularly, venous and small IV remodeling in HF across a broad spectrum of PH severities. We also found no difference in the severity of pulmonary vascular remodeling among HF patients with vs. without RV dysfunction. These findings suggest that PV remodeling is likely an early and progressive process, and that RV dysfunction may not necessarily be directly related to the severity of pulmonary vascular remodeling. In contrast to most other forms of PAH, HF patients often have intrinsic RV myocardial dysfunction due to ischemic heart disease or cardiomyopathic processes in HFrEF and due to ischemic heart disease, RV pacing, atrial fibrillation or global cardiac microvascular inflammation in HFpEF.9, 28-30 Thus, RV dysfunction may be influenced by, but not as directly linked to the severity of remodeling in HF, as compared to otherwise healthy persons who develop other forms of PAH in the absence of intrinsic myocardial disease.
On average, patients with HF-PH showed mild mixed obstructive/restrictive pulmonary dysfunction, with clinically relevant reductions in the DLCO, whereas patients with PVOD had normal spirometry but more severe reduction in DLCO, consistent with previous studies.2, 31-34 In HF and in PVOD, reductions in DLCO have been attributed to the combined effects of PV remodeling with reduction in pulmonary capillary blood volume and impaired alveolar-capillary conductance due to interstitial edema.2, 32, 34 In a study of alveolar and pulmonary arterial histomorphometry in 20 patients with mitral stenosis, Jordan et al35 concluded that reductions in DLCO correlated more with pulmonary arterial than alveolar structural changes. In our study, adjusting for lung volumes (which correlated strongly with DLCO), intimal thickening in arteries and small IV remained associated with reductions in DLCO, providing a structural basis for the observed impairments in lung gas transfer in HF-PH.
While lung disease, OSA and renal dysfunction are recognized contributors to PH, neither OSA nor the mild lung disease present in some HF-PH patients were associated with differential PV remodeling. Interestingly, worse renal function was associated with more venous and IV intimal remodeling. Whether this is related to more chronic or severe PVH with renal dysfunction or other factors such as inflammation or humoral activation with worsening renal function is not clear.
Our findings have several clinical implications. The current findings may provide insight into the poorer tolerance and lack of response to different PAH therapies in HF-PH that has been noted in some36-39 but not all40-42 studies. While HF patients are known to have “functional PVOD” due to their left heart disease, the pulmonary venous remodeling in HF may also predispose to worsening alveolar edema with pulmonary vasodilators, as in primary PVOD. Whether the anti-proliferative effects of PAH therapies are similar in pulmonary arteries and veins is unclear, but our findings suggest that there is an unmet need for effective treatments that target pulmonary venous remodeling in HF-PH. The frequent presence of loose fibrosis or hyalinosis in pulmonary veins may explain the rapid reversibility of even severe PH in HF with effective decongestion, although this remains highly speculative.
Parenthetically, for clinical investigators and anatomic pathologists studying PVOD, the current study confirms and extends previous studies emphasizing caution in diagnosing PVOD in patients with left heart disease and particularly, the importance of excluding HFpEF as a cause of PVOD-like changes.2
Strengths and Limitations
Strengths of the current study include rigorous case selection, comprehensive histomorphometry of all pulmonary vessels in a relatively large number of patients with HFpEF and HFrEF, pertinent comparison groups, study of a spectrum of PH severity in HF-PH, correlation of remodeling with pulmonary hemodynamics, RV function and lung function, and rigorous analytic techniques. Limitations include the lack of invasive assessment of pulmonary hemodynamics and PFT in all patients, and the lack of these measures immediately before tissue ascertainment. We used semi-quantitative assessment of RV function. However, our previous study in a large HFpEF cohort indicated that semi-quantitative assessment of RV function was strongly related to adverse outcome.9 As non-muscularized arterioles cannot be reliably distinguished from venules without pre-tagging, we are unable to specifically assess non-muscularized arteriolar remodeling. While sections from each lobe were obtained in a random fashion, we cannot rule out biased sampling. This study is largely descriptive, but lays the foundation for future studies investigating the mechanisms of global PV remodeling in HF.
Conclusions
Irrespective of EF, in HF patients with PH, there is remodeling of the pulmonary arteries, veins and small IV. The intimal thickening in veins and IV is prominent in HF and more severe than in arteries, a pattern similar to but less severe than that observed in PVOD patients with more severe PH. The severity of PH correlates most strongly/independently with venous and IV intimal thickening rather than arterial changes. The presence of RV dysfunction was associated with moderately higher PASP but not significantly more severe PV remodeling; suggesting that intrinsic myocardial processes, PVH and pulmonary vasoconstriction contribute to RV dysfunction in HF-PH. Pulmonary vascular remodeling is associated with reductions in DLCO. These findings add to our understanding of the pathobiology of HF related PH.
Supplementary Material
Clinical Perspective.
What is New?
In heart failure (HF) patients with preserved (HFpEF) or reduced (HFrEF) ejection fraction and pulmonary hypertension (HF-PH), there is global pulmonary vascular remodeling with thickening of the media and intima in arteries and thickening of the intima in veins and small pulmonary vessels relative to normal control subjects.
Venous and small vessel intimal thickening was more severe than arterial intimal thickening in HF with a pattern similar to patients with pulmonary veno-occlusive disease (PVOD).
The severity of PH correlated most strongly with venous and small vessel (rather than arterial) remodeling.
What Are the Clinical Implications?
These findings add to our understanding of the pathobiology of HF related PH.
Pulmonary venous remodeling in HF may predispose to worsening alveolar edema with pulmonary vasodilators, as in primary PVOD.
Our findings suggest that there is an unmet need for effective treatments that target pulmonary venous remodeling in HF-PH.
Acknowledgments
None
Sources of Funding: Funding for Dr. Redfield's (P01 HL 76611, U10 HL 110262 and RO1 HL 105418), Dr. Borlaug's (U10 HL 110262, UO1 HL 125205 and RO1 HL128526) and Dr. Frantz's (U01HL125205) time was provided by the National Institutes of Health.
Footnotes
Disclosures: None
References
- 1.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med. 2013;34:639–650. doi: 10.1016/j.ccm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Montani D, Lau EM, Dorfmuller P, Girerd B, Jais X, Savale L, Perros F, Nossent E, Garcia G, Parent F, Fadel E, Soubrier F, Sitbon O, Simonneau G, Humbert M. Pulmonary veno-occlusive disease. Eur Respir J. 2016;47:1518–1534. doi: 10.1183/13993003.00026-2016. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Montani D, O'Callaghan DS, Savale L, Jais X, Yaici A, Maitre S, Dorfmuller P, Sitbon O, Simonneau G, Humbert M. Pulmonary veno-occlusive disease: recent progress and current challenges. Respir Med. 2010;104(1):S23–32. doi: 10.1016/j.rmed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D4–12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98:1805–1811. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Goodale F, Jr, Sanchez G, Friedlich AL, Scannell JG, Myers GS. Correlation of pulmonary arteriolar resistance with pulmonary vascular changes in patients with mitral stenosis before and after valvulotomy. N Engl J Med. 1955;252:979–983. doi: 10.1056/NEJM195506092522303. [DOI] [PubMed] [Google Scholar]
- 13.Heath D, Whitaker W. The pulmonary vessels in mitral stenosis. J Pathol Bacteriol. 1955;70:291–298. doi: 10.1002/path.1700700204. [DOI] [PubMed] [Google Scholar]
- 14.Henry EW. The small pulmonary vessels in mitral stenosis. Br Heart J. 1952;14:406–412. doi: 10.1136/hrt.14.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt JM, Bethea B, Liu X, Gandjeva A, Mammen PP, Stacher E, Gandjeva MR, Parish E, Perez M, Smith L, Graham BB, Kuebler WM, Tuder RM. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am J Physiol Lung Cell Mol Physiol. 2013;305:L725–36. doi: 10.1152/ajplung.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon HD, Kasturi J. Pulmonary vascular changes associated with isolated mitral stenosis in India. Br Heart J. 1975;37:26–36. doi: 10.1136/hrt.37.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagenvoort CA. Morphologic changes in intrapulmonary veins. Hum Pathol. 1970;1:205–213. doi: 10.1016/s0046-8177(70)80034-x. [DOI] [PubMed] [Google Scholar]
- 18.Wagenvoort CA. Pathology of the pulmonary vasculature. Correlation with clinical and haemodynamic data. Schweiz Med Wochenschr. 1985;115:1319–322. [PubMed] [Google Scholar]
- 19.Chazova I, Robbins I, Loyd J, Newman J, Tapson V, Zhdaov V, Meyrick B. Venous and arterial changes in pulmonary veno-occlusive disease, mitral stenosis and fibrosing mediastinitis. Eur Respir J. 2000;15:116–122. doi: 10.1183/09031936.00.15111600. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsson J, Edwards WD. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc. 1985;60:16–25. doi: 10.1016/s0025-6196(12)65277-x. [DOI] [PubMed] [Google Scholar]
- 21.Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- 23.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 24.Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, Sotelo T, Gomez de la Camara A, Cortina J, de la Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7:1011–1016. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017;367:643–649. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulik TJ. Pulmonary hypertension caused by pulmonary venous hypertension. Pulm Circ. 2014;4:581–595. doi: 10.1086/678471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maron BA, Leopold JA. Emerging Concepts in the Molecular Basis of Pulmonary Arterial Hypertension: Part II: Neurohormonal Signaling Contributes to the Pulmonary Vascular and Right Ventricular Pathophenotype of Pulmonary Arterial Hypertension. Circulation. 2015;131:2079–2091. doi: 10.1161/CIRCULATIONAHA.114.006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 31.Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion Capacity and Mortality in Patients With Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:441–449. doi: 10.1016/j.jchf.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Magnussen H, Canepa M, Zambito PE, Brusasco V, Meinertz T, Rosenkranz S. What can we learn from pulmonary function testing in heart failure? Eur J Heart Fail. 2017;19:1222–1229. doi: 10.1002/ejhf.946. [DOI] [PubMed] [Google Scholar]
- 33.Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail. 2015;17:1161–1171. doi: 10.1002/ejhf.417. [DOI] [PubMed] [Google Scholar]
- 34.Olson TP, Johnson BD, Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:490–498. doi: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan SC, Hicken P, Watson DA, Heath D, Whitaker W. Pathology of the lungs in mitral stenosis in relation to respiratory function and pulmonary haemodynamics. Brit Heart J. 1966;28:101–107. doi: 10.1136/hrt.28.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 37.Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D'Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grunig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S. Pre-Capillary, Combined, and Post-Capillary Pulmonary Hypertension: A Pathophysiological Continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 41.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 42.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.