Abstract
Background and Purpose
Brain arteriovenous malformation (bAVM) is an important risk factor for intracranial hemorrhage. Current treatments for bAVM are all associated with considerable risks. There is no safe method to prevent bAVM hemorrhage. Thalidomide reduces nose bleeding in patients with hereditary hemorrhagic telangiectasis (HHT), an inherited disorder characterized by vascular malformations. In this study, we tested whether thalidomide and its less toxic analogue, lenalidomide, reduce bAVM hemorrhage using a mouse model.
Methods
BAVMs were induced through induction of brain focal activin-like kinase 1 (Alk1, an AVM causative gene) gene deletion and angiogenesis in adult Alk1-floxed mice. Thalidomide was injected intraperitoneally twice per week for six weeks, starting either 2 weeks or 8 weeks after AVM induction. Lenalidomide was injected intraperitoneally daily starting 8 weeks after AVM induction for six weeks. Brain samples were collected at the end of the treatments for morphology, mRNA and protein analyses. The influence of Alk1 down regulation on platelet-derived growth factor (PDGFB) expression was also studied on cultured human brain microvascular endothelial cells (HBMECs). The effect of PDGFB in mural cell recruitment in bAVM was explored by injection of a PDGFB overexpressing lentiviral vector to the mouse brain.
Results
Thalidomide or lenalidomide treatment reduced the number of dysplastic vessels and hemorrhage, and increased mural cell (vascular smooth muscle cells and pericytes) coverage in the bAVM lesion. Thalidomide reduced the burden of CD68+ cells, and the expression of inflammatory cytokines in the bAVM lesions. PDGFB expression was reduced in ALK1-knockdown HBMECs and in mouse bAVM lesion. Thalidomide increased Pdgfb expression in bAVM lesion. Overexpression of PDGFB mimicked the effect of thalidomide.
Conclusions
Thalidomide and lenalidomide improve mural cell-coverage of bAVM vessels and reduce bAVM hemorrhage, which is likely through upregulation of Pdgfb expression.
Keywords: thalidomide, lenalidomide, brain arteriovenous malformation, hemorrhage, mural cell coverage
Subject Terms: angiogenesis, cerebrovascular malformations, intracranial hemorrhage, animal models of human diseases
Introduction
Brain AVM is an important risk factor for intracranial hemorrhage.1 Many patients are not treated due to high risks associated with currently available interventions.2 There is no specific medical treatment available for bAVM patients.
The causative gene for sporadic bAVM is still unknown. About 5% of the bAVM patients have a genetic disorder called hereditary hemorrhagic telangiectasia (HHT). The two main subtypes of HHT (1 & 2) are caused by mutations in the endoglin (ENG) gene or the activing receptor-like kinase 1 (ALK1 or ACVLR1) gene1. The familial forms of the more common sporadic disorders have been used to study the disease mechanisms of sporadic cerebrovascular diseases3. We took this concept to consider HHT as a familial form of the more common sporadic bAVM, as HHT bAVM possesses a phenotype that is similar to sporadic bAVM. Knowledge of the inherited disease can shed light on sporadic bAVM pathogenesis.
We have established several adult onset bAVM mouse models through conditional knockout of either Eng or Alk1 gene and focal brain angiogenic stimulation 4–7. These bAVM models have several key phenotypes resembling those of human bAVMs, such as dilated tortuous vessels, direct arterial-venous shunts 4–7, hemorrhage, macrophage infiltration and reduced mural cell-coverage 8, 9.
In the Alk1-deficient bAVM mouse model, many AVM vessels do not have vascular smooth muscle cells (vSMCs) and have fewer pericytes than normal brain vessels, which are associated with vascular leakage and hemorrhage4, 5 Platelet-derived growth factor B (PDGFB) and platelet-derived growth factor receptor-β (PDGFR-β) signaling plays an important role in promoting pericyte and vSMC recruitment to endothelial tubes during vascular maturation. We found Pdgfrβ expression in the bAVM is reduced5, suggesting that Pdgfb/pdgfrβ signaling might be impaired in bAVM and may be a potential therapeutic target.
Thalidomide belongs to a class termed immunomodulatory drugs (IMID). As a result of thalidomide’s well-known adverse effects, e.g., peripheral neuropathy and drowsiness,10, less toxic second generation of IMIDs, such as lenalidomide, have been identified11. Thalidomide inhibits gastrointestinal bleeding and stabilizes telangiectasia vessels in HHT patients, increases Pdgfb expression and improves mural cell recruitment in the retina of Eng+/− mice12. However, it si not clear if these drugs can stabilize bAVM vessels.
In this study, we tested whether thalidomide and lenalidomide can reduce bAVM hemorrhage using an Alk1 bAVM model and found that both agents increased vascular pericyte- and vSMC-coverage, reduced bAVM hemorrhage. In addition, overexpression of PDGFB in the bAVM showed a similar effect to thalidomide, suggesting that PDGFB is the critical factor responsible for the beneficial effects of thalidomide.
Materials and Methods
All data and supporting materials are available with the article and its online supplementary.
Animals
A total of 148 8 to 10-week old Alk12f/2f mice13 in C57BL background with loxP sites flanking exons 4–6 and 4 wild type C57BL mice (the Jackson Laboratory, Bar Harbor, ME) were used. Equal numbers of male and female mice were included. Experimental procedures for using laboratory animals were approved by the Institution of Animal Care and Use Committee of the University of California, San Francisco.
Injection of viral vectors into mouse brain
Mice were anesthetized through inhalation of 4% isoflurane, and placed in a stereotactic frame with a holder (David Kopf Instruments, Tujunga, CA). A burr hole was drilled in the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. A total of 2 μl viral vectors were stereotactically injected into the basal ganglia 4 3 mm beneath the brain surface. Mice were randomly assigned to each treatment groups.
For mice with thalidomide treatment starting 2 weeks after model induction, 2X109 genome copies (gc) of AAV-VEGF (an adeno-associated viral vector expressing human vascular endothelial growth factor) and 1X107 plaque forming units (pfu) of Ad-Cre (an adenoviral vector expressing Cre recombinase) were injected. Since the level of VEGF has been shown to be correlate with bAVM hemorrhage14, in order to induce significant hemorrhage in established bAVM, we used a higher AAV1-VEGF dose (5X109 gc) to induce bAVM in the mice that have thalidomide and lenalidomide treatment started 8 weeks after model induction. For overexpression of human PDGFB, 2X109 gc of AAV1-VEGF, 1X107 pfu of Ad-Cre or Ad-GFP (control) and 1.5X109 gc of Lenti-PDGFB or Lenti-GFP (control) were used. Ad-Cre and Ad-GFP were purchased from Vector Biolabs (Cat #1700 and Cat #1060, respectively, Malvern, PA) and AAV-VEGF was made by our laboratory using method described previously 15. Lenti-PDGFB and Lenti-GFP were purchased from GeneCopoeia (Cat #LP-EGFP-Lv105 and LP-A0380-Lv105-0200-S, Rockville, MD).
Thalidomide (75mg/kg of body weight, Sigma-Aldrich, St Louis, MO), or dimethyl sulfoxide (DMSO, vehicle) was administered intra-peritoneally twice a week for six weeks starting 2- or 8-weeks after model induction (Figure S1A&B). Lenalidomide (50mg/kg of body weight, Hangzhou ICH Biopham Co., Ltd., Hangzhou, China) or DMSO was administered intraperitoneally daily for six weeks starting 8-weeks after model induction (Figure S1C). Body weight was measured weekly or bi-weekly as indicated.
Dissection of basal ganglia microvessels
After the mice were anesthetized with isoflurane, the brain samples were collected and immersed immediately in Hank’s buffer supplemented with 15 mM HEPES. Brain tissue around vector injection site was dissected and homogenized using a Dounce’s grinder in Hank’s buffer supplemented with 15 mM HEPES, 0.5% (w/v) BSA, 10 mM Glucose, 20 mM sodium bicarbonate and 1 mM sodium pyruvate (pH 7.4). Homogenates were centrifuged in 15% Dextran at 6,000g for 20 min at 4°C. Supernatant was removed. Pellets (micro-vessels) were resuspended in RNAzol (Molecular Research Center, Cincinnati, OH) for RNA extraction. Due to the limited amount of RNA that can be extracted from the microvessels collected from the viral injection region of individual mouse, samples from 4–9 similar treated animals were pooled.
Statistics
For quantification of vessels density, dysplastic vessels, pericytes and vSMC coverage and Prussian blue positive areas, section numbers were scrambled. The quantification was done by researchers who were blinded to the treatment groups. Data are presented as mean ± standard deviation (SD). Due to the skewed nature of the Prussian blue positive area, the data observations were log-transformed prior to the analysis. All data were analyzed using one-way ANOVA followed by Sidak’s multiple comparisons or Student’s t test to compare the means of two groups. A p value < 0.05 was considered to be significant. Sample sizes were indicated in Figures.
Additional methodologies are described in the online-only Data Supplement.
Results
Thalidomide treatment inhibits bAVM development
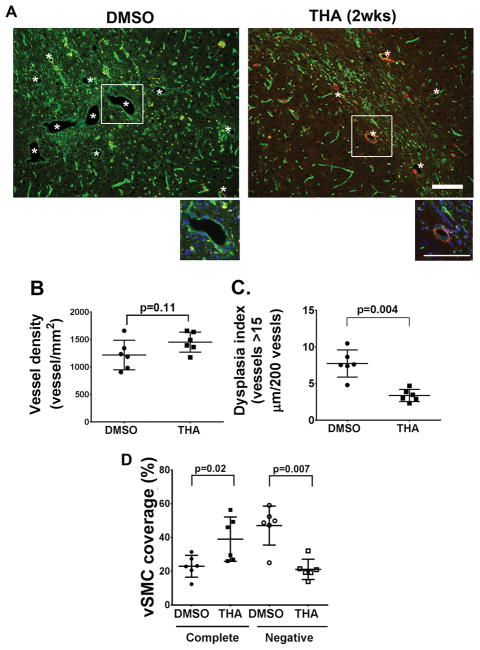
The AVMs are formed 8-weeks after induction of Alk1 deletion and angiogenesis 4. To test whether thalidomide inhibits bAVM formation, thalidomide treatment was started 2-weeks after AVM induction at the onset of AAV-VEGF induced brain angiogenesis16, and continued for 6 weeks. The bAVM phenotypes were analyzed following the completion of the treatment (Figure IA in the online-only Data Supplement). The thalidomide and DMSO groups have similar vascular densities (p = 0.11). However, the thalidomide group had fewer dysplasia vessels (3.38±0.83/200 vessels) than that of DMSO group (7.74±1.85, p=0.004, Figure 1). In addition, the thalidomide group has more vessels completely covered by vSMCs (39.1±13.1%) than DMSO group (23±6.5%, p=0.02) and fewer vSMC-negative vessels (11.6±6%) than DMSO groups (47.1±11.6%, p=0.007, Figure 1A & D). These data suggest that thalidomide reduces the formation of dysplastic vessels and may promote vSMC-recruitment.
Figure 1. Thalidomide inhibits bAVM development.
A. Representative images of brain sections. Vessels were stained by lectin (green). Vascular smooth muscles were stained with an antibody against α-smooth muscle actin (red). *: indicates dysplastic vessels. The enlarged images of white rectangle areas are shown below the pictures. Scale bars: 100 μm. B. Quantification of vessel density. P=0.11, by t-test analysis. C. Dysplasia index (numbers of vessels that are larger than 15 μm in diameter per 200 vessels). P=0.004, by t-test analysis. D. Quantification of vSMC coverage. The data are the percentage of dysplastic vessels that were covered (Complete) or not covered (Negative) by vSMCs. THA (2wks): mice received thalidomide treatment starting 2 weeks after model induction. P values were determined by one-way ANOVA followed by Sidak’s multiple comparisons. N=6 for all analyses.
Thalidomide and lenalidomide increase mural cell-coverage and reduce hemorrhage in established bAVMs
To test if thalidomide and lenalidomide reduce hemorrhage of established bAVM, we treated mice 8-weeks after the model induction when bAVMs have fully developed (Figure IB & IC in the online-only Data Supplement) 4. Since abnormally high level of VEGF has been shown to be associated with bAVM hemorrhage14, to increase the severity of bAVM hemorrhage, we used a higher dose of AAV-VEGF (5X109 gc) to induce the bAVM phenotype.
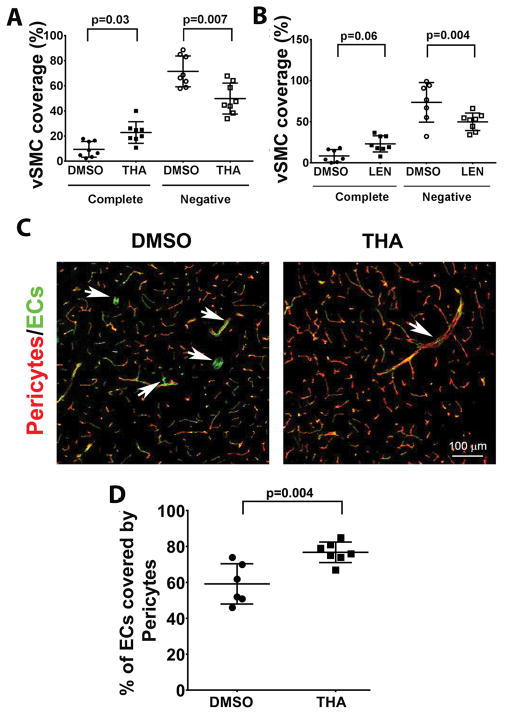
Thalidomide or lenalidomide did not alter vessel density (p=0.61), and the number of abnormal vessels (p=0.1, Figure II in the online-only Data Supplement) in the established bAVMs. However, the treatments improved vSMC-coverage in the bAVMs. Compared to DMSO groups, the thalidomide group has more vessels that are completely covered by vSMCs (DMSO vs. thalidomide: 9.5±6.3% vs. 22.9±8.6%, p=0.03) and the lenalidomide group showed a trend of increase of vSMCs coverage (DMSO vs. lenalidomide: 8.4±7.7 vs. 22.1±9.8, p=0.06). Thalidomide and lenalidomide treated groups also have fewer vSMC-negative vessels than DMSO groups (DMSO vs. thalidomide: 71.6±12.3% vs. 49.9±12.3%, p=0.007; DMSO vs. lenalidomide: 73.6±24.1% vs. 49.9±10.7%, p=0.004, Figure 2A & B, and Figure III in the online-only Data Supplement).
Figure 2. Thalidomide and lenalidomide increase mural cell-coverage in established bAVMs.
A & B. Quantification of vSMC (α-smooth muscle actin positive cells)-coverage. The data are the percentage of dysplastic vessels that were covered (complete) or not covered (Negative) vSMCs. N=8 for thalidomide (THA) treated group and its DMSO control. N=8 for lenalidomide (LEN) treated group and N=7 for its DMSO control. P values were determined by one-way ANOVA followed by Sidak’s multiple comparisons. C. Representative confocal images of brain sections stained with antibodies specific to endothelial cells (CD31, green) and pericytes (CD13, red). Arrows indicate pericyte negative vessel segments. Scale bars: 100 μm. D. Quantification of the percentage of endothelial cells (ECs) covered by pericytes. N=7 for thalidomide (THA) treated group and N=6 for its DMSO control. P=0.004, which was determined by one-way ANOVA followed by Sidak’s multiple comparisons. 8wks: treatments starting 8 weeks after model induction.
We have also analyzed pericyte-coverage. In the hemisphere contralateral to the bAVM lesion, the pericyte-coverage was 77±5%, which is similar to that reported for normal brain 17. In bAVMs, pericyte-coverage was reduced (59±4.6%, DMSO group, p=0.004). Thalidomide treatment restored pericyte-coverage (77±2.2%, p=0.004, Figure 2C & D).
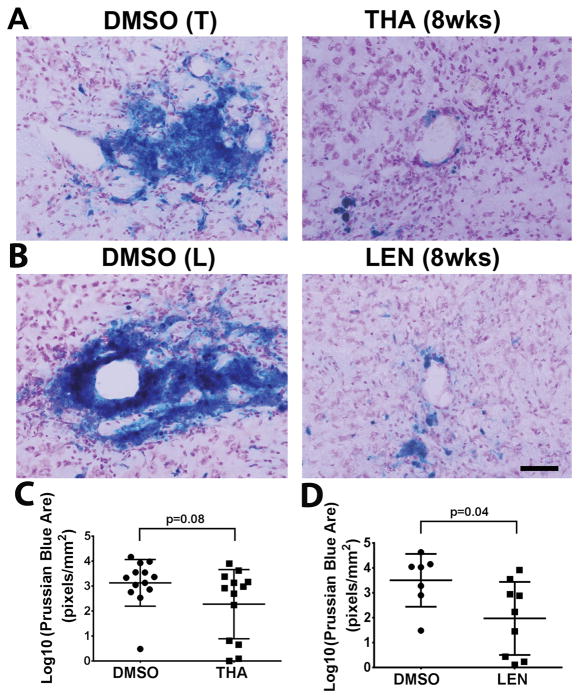
BAVM hemorrhage was measured using Prussian blue staining of iron deposition. Compared to DMSO controls (3.13±0.93 pixel/mm2 of Prussian blue positive area), thalidomide group showed a trend of reduction of Prussian-blue positive area (2.28±1.38 pixel/mm2, p=0.08). Lenalidomide treated group had smaller Prussian-blue positive area (1.98±1.47 pixel/mm2) than its DMSO control group (3.5±1.06 vs, p=0.04, Figure 3). These data suggest that thalidomide and lenalidomide treatments improve mural cell coverage and reduce hemorrhage in established bAVMs.
Figure 3. Thalidomide and lenalidomide reduces hemorrhage in the established bAVM.
A & B. Representative images of Prussian blue stained sections. Scale bar: 100 μm. C & D. Quantification of Prussian blue-positive area. Log 10: the data were 10 log conversed. THA (8wks): mice received thalidomide treatment starting 8 weeks after model induction. LEN (8wks): mice received lenalidomide treatment starting 8 weeks after model induction. DMSO (T): control for thalidomide treated-group; DMSO (L): control for lenalidomide treated-group. N=13 for thalidomide group and its DMSO control. N=9 for lenalidomide group and N=7 for its DMSO control. P values were determined by t-test analysis.
Thalidomide restored Pdgfb and Pdgfrβ Expression in bAVM vasculature
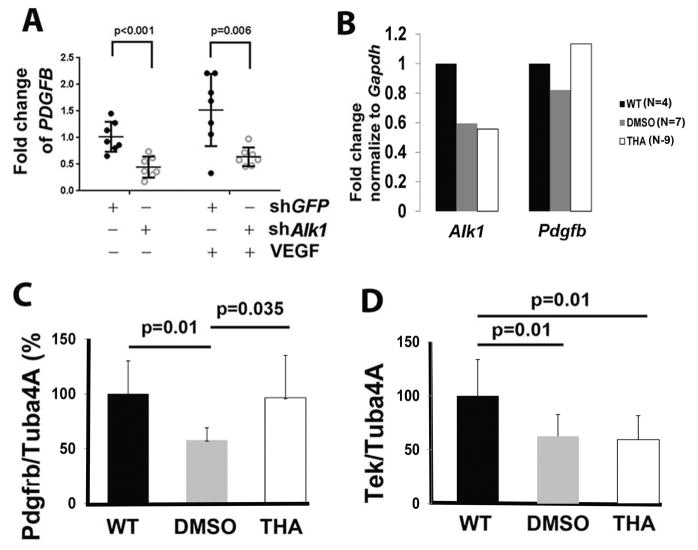
To explore mechanisms that might mediate the reduction of mural cell-coverage in bAVM vessels, we performed in vitro studies first using human brain microvascular endothelial cells (HBMECs). When ALK1 expression was knocked down to 30% of its normal level by lenti-shALK1 (a lentiviral vector expressing human ALK1 shRNA, Figure IV and Table II in the online-only Data Supplement), PDGFB expression was reduced to 44% in the absence of VEGF (p=0.001) and to 64% in the presence of VEGF (p=0.006, Figure 4A and Table III in the online-only Data Supplement). We then analyzed gene expression in the bAVM vessels. Compared to the microvessels isolated from wild type mouse brain, the microvessels isolated from the bAVMs showed a ~50% reduction of Alk1 expression in both DMSO- and thalidomide-treated mice. This was associated with an 18% reduction of Pdgfb expression in DMSO-treated mice. Thalidomide treatment restored Pdgfb expression (Figure 4B). These data suggest that a reduction of PDGFB expression in ALK1 mutated endothelial cells could be responsible for the loss of mural cell-coverage in the bAVM vessels, which may be restored with thalidomide treatment.
Figure 4. Thalidomide treatment upregulated Pdgfb and Pdgfrβ expression.
A. PDGFB expression was reduced in ALK1 knockdown HBMECs. The Y axis is fold changes compared to the mean of Lenti-GFP (shGFP) treated controls without VEGF treatment. N=7. P values were determined by one-way ANOVA analysis followed by Sidak’s multiple comparisons. B. Quantification of Alk1 and Pdgfb expression in bAVM microvessels. In order to obtained enough RNA for the analysis. microvessels from mice in same group were pooled: 4 wild type (WT) mice, 7 DMSO treated mice and 9 thalidomide (THA) treated mice. C & D. Quantification of western blot results. Tuba4a was used as an internal control for normalization of protein load. Data was shown by percentage (%) normalized to the WT group. N=6. P values were determined by one-way ANOVA followed by Sidak’s multiple comparisons.
We next quantified the expression Pdfgrβ and Tek, which are two important factors in mural cells recruitment, in both bAVM vessels and the surrounding brain tissue by western blot analysis (Figure V in the online-only Data Supplement). Compared to normal mice, DMSO-treated bAVM mice express 56% lower Pdfgrβ (p=0.01) and 38% lower Tek (p=0.01). Thalidomide treatment increased Pdfgrβ expression (p=0.035 vs. DMSO group, Figure 4C), but did not alter Tek expression in bAVM vessels and their surrounding tissue (Figure 4D). Therefore, it is likely that thalidomide improves mural cell-coverage of bAVM vessels through an upregulation of Pdgfb/Pdgfrβ signaling pathway.
Thalidomide reduced inflammation in bAVM lesion
Studies on the genetics and cytokine expressions suggest that inflammation may contribute to AVM progression and rupture9. To test if thalidomide reduces bAVM inflammation, the expression of inflammatory cytokines, Cxcr4, Il1b and Tnfα were analyzed by qRT-PCR. We found that levels of Cxcr4, Il1b and Tnfα were higher in bAVM microvessels than that in the WT micovessels. Thalidomide reduced the levels of these cytokines in bAVM microvessels (Figure VIA in the online-only Data Supplement). Thalidomide has also reduced CD68+ burden in bAVM. Compared to DMSO-treated mice (513±270 cells/mm2), thalidomide-treated mice have fewer CD68+ cells in bAVM (210±188 cells/mm2, p=0.02). The number of CD68+ cells is positively correlated with the number of vSMC negative vessels (r=0.71, p<0.0001), and Prussian blue positive area (r=0.46, p=0.012, (Figure VII in the online-only Data Supplement).
Overexpression of PDGFB mimicked thalidomide effect
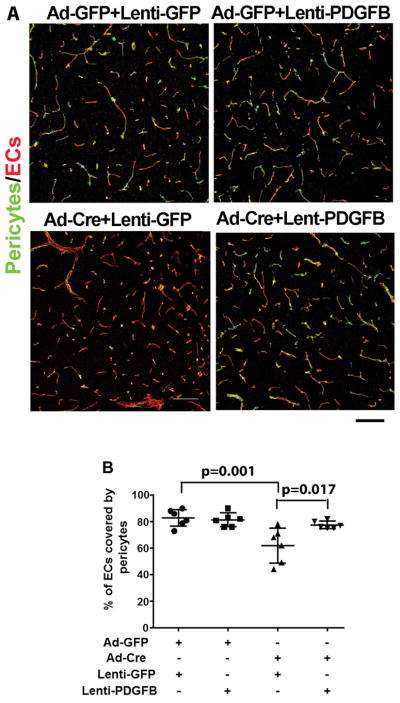
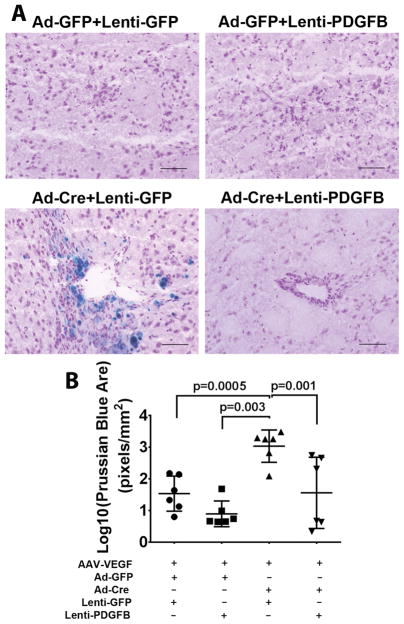
To test whether upregulation of Pdgfb mimics thalidomide’s therapeutic effect, we overexpressed human PDGFB in mouse bAVM lesion through lentiviral vector-mediated gene transfer, because protein sequences of human and mouse PDGFB are 96% homology (Figure VIII in the online-only Data Supplement). Lenti-PDGFB or Lenti-GFP (control) was injected to the brain at the time of bAVM induction. PDGFB expression was detected at the injection site 8-weeks after viral injection and GFP expression was detected 3 and 7 days after injection (Figure IX in the online-only Data Supplement). Overexpression of PDGFB did not alter the vessel densities (Figure IX in the online-only Data Supplement), but reduced the number of dysplastic vessels (Dysplasia Index) in bAVM lesion (Lenti-PDGFB vs. Lenti-GFP: 5.25±1.75 vs. 8.35±3.03, p=0.04, Figure X in the online-only Data Supplement); and increased pericyte-coverage of bAVM vessels (Lenti-PDGFB vs. Lenti-GFP: 78±2.9% vs. 62±13.1%, p=0.017. Figure 5A & B). The Prussian blue positive area in mice co-injected Lenti-PDGFB with Ad-Cre and AAV-VEGF (1.56±1.23 pixels/mm2) was smaller than that of mice received co-injection of Lenti-GFP with Ad-Cre and AAV-VEGF (3.03±0.51pixel/mm2, p=0.001), and was similar to that of the two control groups that injected with lenti-GFP, Ad-GFP and AAV-VEGF or Lenti-PDGFB, Ad-GFP and AAV-VEGF (Ps>0.05, Figure 6A & B). Therefore, overexpression of PDGFB mimicked thalidomide’s effect, which suggests that upregulation of Pdgfb expression contributes to thalidomide’s beneficial effect.
Figure 5. Overexpression of PDGFB increases pericyte covered bAVM vessels.
A. Representative confocal images of brain sections stained with antibodies specific to CD31 (an endothelial cell-marker, red) and CD13 (a pericyte-marker, green). Scale bars: 100 μm. B. Quantification of the percentage of endothelial cells (ECs) covered by pericypes. All mice were Alk12f/2f mice and were treated with AAV-VEGF to induce brain angiogenesis. Ad-GFP+Lenti-GFP: Controls for normal angiogenesis; Ad-GFP+Lenti-PDGFB: Overexpressing PDGFB in angiogenic brain; Ad-Cre+Lenti-GFP: Untreated bAVM; Ad-Cre+Lenti-PDGFB; Overexpression of PDGFB in bAVM. N=6. P values were determined by one-way ANOVA followed by Sidak’s multiple comparisons.
Figure 6. Overexpression of PDGFB reduced hemorrhage in the bAVM lesions.
A. Representative images of Prussian blue stained sections. Scale bar: 100 μm. B. Quantification of Prussian blue positive area. The data are 10 log conversed (Log 10). All mice are treated with AAV-VEGF to induce brain angiogenesis. Ad-GFP+Lenti-GFP: Controls for normal angiogenesis; Ad-GFP+Lenti-PDGFB: Overexpressing PDGFB in angiogenic brain; Ad-Cre+Lenti-GFP: Untreated bAVM; Ad-Cre+Lenti-PDGFB; Overexpression of PDGFB in bAVM. N=6. P values were determined by one-way ANOVA followed by Sidak’s multiple comparisons.
Thalidomide and lenalidomide did not alter mouse body weight
No obvious abnormal behavior was observed in the thalidomide- or lenalidomide- treated mice. The body weights are similar among all groups throughout the treatment period (Figure XII in the online-only Data Supplement). Mortality was reported in (Table IV in the online-only Data Supplement).
Discussion
In this study, using an Alk1− deficient bAVM mouse model4, we showed that thalidomide inhibits bAVM formation, and improves mural cell-coverage and reduces hemorrhage in established bAVMs. Pdgfb and Pdgfrβ expression are reduced in the Alk1-deficient bAVM. Thalidomide restores their expression.
PDGFB/PDGFRβ signaling is indispensable for mural cell recruitment during angiogenesis 18. In the brain, disruption of PDGFB or its receptor PDGFRβ leads to a reduction of vascular mural cells, causing various vascular abnormalities, which microaneurysm, and chronic microhemorrhage17, 19. The role of PDGFB/PDGFRβ signaling in human bAVM pathogenesis is largely unknown. Increased PDGFB expression has been detected in a subset of surgical resected human sporadic bAVMs14, 20. However, studies including ours demonstrated that vessels in bAVMs and their surroundings have fewer mural cell-coverage than normal cerebrovasculature21. We showed in this study that lentivirus mediated overexpression of PDGFB mimicked the effects of thalidomide, which suggest that upregulation of Pdgfb/Pdgfrβ pathway could be a underlying mechanism of thalidomide effect. However, more studies will be needed to confirm the role of PDGFB in bAVM pathogenesis.
In prior reports, thalidomide improves mural cell recruitment in the retina of Eng+/− mice 12. Thalidomide stabilizes small capillaries in the telangiectasia, reduces nose bleeds and the requirement of blood transfusions in HHT patients12. We showed previously that bAVMs in Alk1 deficient model have fewer mural cells, which is associated with a reduction of Pdgfrβ expression5. We showed in the present study that both thalidomide and lenalidomide reduce hemorrhage and improves mural cell coverage in bAVMs, which is most likely through upregulation of PDGFB/PDGFRβ signaling.
Anti-angiogenesis has been shown to be effective in treating bAVM 22, 23. Although previous studies suggested that thalidomide has anti-angiogenic function, we found neither thalidomide nor lenalidomide altered vessel densities in bAVMs, suggesting that the beneficial effects shown in this study are not through an anti-angiogenesis.
Interestingly, studies discovered CRBN (cereblon), a substrate receptor of the CUL4–RBX1–DDB1 ubiquitin ligase complex (CRL4), is a direct and primary target of thalidomide and lenalidomide in in vitro cell models and in zebrafish embryos24, 25. The association between CRBN and PDGFB is unclear. Thalidomide has been suggested to indirectly reduce the expression of certain cytokines, such as, IL-6, IL-1β and TNF-α 26. We showed that thalidomide reduced Cxcr4, Il1b and Tnfα in mouse bAVM microvessels. In addition, elevation of PDGFB in human bAVM may reflect a potential compensatory mechanism in response to the reduction of mural cells. Further characterization of endothelial-to-mural cell signaling and quantitative studies of mural cell recruitment and turnover are needed to better delineate the role of mural cells in human bAVM pathogenesis.
Since both thalidomide and lenalidomide are currently used in clinical practice, the doses and side effects of these drugs in human have mostly been defined. In this study, our goal is to test their therapeutic efficacy in bAVM. The doses we used were selected based on previous studies. For thalidomide, both 75 mg/kg and 150 mg/kg (i.p.) had probably near maximal therapeutic effects12; however, 150 mg/kg retarded mouse growth. For lenalidomide, based on available rodent studies27, we have empirically chosen a lower dose compared to thalidomide and administrated the drug daily based on its pharmacokinetics28.
Thalidomide has well-known adverse effects that limit its applicability in treating bAVM patients10. We showed that lenalidomide, one of the newer derivatives of thalidomide, has similar effects to that of thalidomide. Therefore, the newer and safer derivatives of thalidomide could be better options for treating bAVM patients.
Our study had several limitations. (1) Ad-Cre that expressing Cre in all cell-types has been used to induce focal Alk1 deletion during model induction. However, our previous studies showed that this model has many phenotypes that resembles human bAVM4, 5. (2) Different viral vectors have been used in experimental mice, which may lead to more severe inflammation than those occurred in human bAVMs. However, we found thalidomide treatment reduces inflammation in this model, suggesting that thanlidomde could reduce inflammation in human bAVMs.
Summary/Conclusions
In summary, our study is the first to show that thalidomide and lenalidomide inhibit bAVM development and improve vascular integrity of existing bAVMs. Our data suggest that thalidomide and its safer derivatives should be further explored as therapeutic options to reduce bAVM hemorrhage in patients.
This study was supported by grants to H. Su from the NIH (R01 NS027713, R01 HL122774 and R21 NS083788), and from the Michael Ryan Zodda Foundation and a grant from University of California San Francisco Research Evaluation and Allocation Committee.
Supplementary Material
Acknowledgments
We thank Dr. S. Paul Oh at Barrow Neurological Institute, Phoenix, AZ for providing us the Alk1 floxed mice.
Footnotes
Conflict of Interests:
The authors have declared that no conflict of interest exists.
Author Contributions
W.Z., W.C., D.Z., L.W., C.B., D.S., S.W., L.Z., M.C., E.W., Z.L., Z.L., M.Z., F.S., and S.S. performed the experiments and collected samples. W.Z., W.C., L.W., C.B., S.W., M.C., E.W., Z.L., and Z.L., performed data analyses. W.Z. and H.S. designed the study and wrote the manuscript.
References
- 1.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: A response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (aruba): A multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders RD, Avidan MS. Postoperative cognitive trajectories in adults: The role of inflammatory processes. Anesthesiology. 2013;118:484–486. doi: 10.1097/ALN.0b013e3182838b67. [DOI] [PubMed] [Google Scholar]
- 4.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33:305–310. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, et al. De novo cerebrovascular malformation in the adult mouse after endothelial alk1 deletion and angiogenic stimulation. Stroke. 2014;45:900–902. doi: 10.1161/STROKEAHA.113.003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9:e88511. doi: 10.1371/journal.pone.0088511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012;43:1240–1246. doi: 10.1161/STROKEAHA.111.647263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62:1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 11.Quach H, Kalff A, Spencer A. Lenalidomide in multiple myeloma: Current status and future potential. Am J Hematol. 2012;87:1089–1095. doi: 10.1002/ajh.23234. [DOI] [PubMed] [Google Scholar]
- 12.Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 13.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. Alk5- and tgfbr2-independent role of alk1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (hht2) Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56:1058–1065. discussion 1058–1065. [PubMed] [Google Scholar]
- 15.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, et al. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, et al. Endothelium-specific ablation of pdgfb leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 19.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim O, Bicer A, Ozkan A, Kurtkaya O, Cirakoglu B, Kilic T. Expression of platelet-derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J Clin Neurosci. 2010;17:1557–1562. doi: 10.1016/j.jocn.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Tu J, Stoodley MA, Morgan MK, Storer KP. Ultrastructure of perinidal capillaries in cerebral arteriovenous malformations. Neurosurgery. 2006;58:961–970. doi: 10.1227/01.NEU.0000210248.39504.B5. discussion 961–970. [DOI] [PubMed] [Google Scholar]
- 22.Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, et al. Bevacizumab attenuates vegf-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43:1925–1930. doi: 10.1161/STROKEAHA.111.647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Shen F, Mao L, Zhan L, Kang S, Sun Z, et al. Soluble flt1 gene therapy alleviates brain arteriovenous malformation severity. Stroke. 2017;48:1420–1423. doi: 10.1161/STROKEAHA.116.015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang J, Liu X, Bolanos L, Barker B, Rigolino C, Cortelezzi A, et al. A calcium- and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nat Med. 2016;22:727–734. doi: 10.1038/nm.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichner R, Heider M, Fernandez-Saiz V, van Bebber F, Garz AK, Lemeer S, et al. Immunomodulatory drugs disrupt the cereblon-cd147-mct1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016;22:735–743. doi: 10.1038/nm.4128. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio A, Adriani G, Catalano A, Carocci A, Rao L, Lentini G, et al. A mini-review on thalidomide: Chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr Med Chem. 2017;24:2736–2744. doi: 10.2174/0929867324666170601074646. [DOI] [PubMed] [Google Scholar]
- 27.Henry JY, Lu L, Adams M, Meyer B, Bartlett JB, Dalgleish AG, et al. Lenalidomide enhances the anti-prostate cancer activity of docetaxel in vitro and in vivo. Prostate. 2012;72:856–867. doi: 10.1002/pros.21488. [DOI] [PubMed] [Google Scholar]
- 28.Pather K, Helsby NA, Palmer BD, Ching L-M. Development and validation of a high performance liquid chromatography assay for the determination of a fluorinated analogue of thalidomide, n-(2,6-dioxopiperidin-3-yl)-3,4,5,6-tetrafluorophthalamic acid, and lenalidomide. Journal of Liquid Chromatography & Related Technologies. 2011;34:83–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.