Abstract
Purpose
To compare the rates of visual field (VF) loss and retinal nerve fiber layer (RNFL) thinning in primary open angle glaucoma (POAG) patients with or without type 2 diabetes mellitus (DM).
Design
Cohort study.
Methods
A total of 197 eyes (55 eyes of 32 POAG patients with DM in POAG/DM group and 142 eyes of 111 age-matched POAG patients without DM in POAG/DM- group) were included from the Diagnostic Innovations in Glaucoma Study (DIGS). Type 2 DM participants were defined by self-report of DM history and use of anti-diabetic medication. The rates of VF loss and RNFL loss were compared in POAG eyes with and without DM using univariate and multivariable mixed effects models.
Results
The median (interquartile range) follow-up was 5.7 years (4.0, 6.4). The mean rate of global RNFL loss in the POAG/DM group was 2-fold slower than in the POAG/DM- group overall (−0.40 µm/year vs. −0.83 µm/year, respectively P = 0.01). Although a slower rate of VF mean deviation and pattern standard deviation loss was found in the POAG/DM group compared to the POAG/DM- group, the difference was not statistically significant.
Conclusions
POAG patients with treated type 2 DM, who had no detectable diabetic retinopathy, had significantly slower rates of RNFL thinning compared to those without diagnosed DM.
Introduction
Primary open angle glaucoma (POAG) is an optic neuropathy characterized by progressive structural changes in the optic nerve head, loss of retinal nerve fiber layer (RNFL), and accompanying damage to the visual field (VF).1, 2 To slow disease progression, identification of risk factors for progression is essential, as it can help determine the strategy of treatment and the frequency of follow-up. However, the risk factors contributing to glaucomatous progression have not been fully characterized.2
Elevation of intraocular pressure (IOP) and impairment of vascular supply to the optic nerve head (ONH) have both been implicated as having a role in the pathophysiology of POAG.3 In this regard, diabetes mellitus (DM), which has been postulated to be related to both pathogenic processes,3, 4 has been suggested as a plausible risk factor for the development of POAG. However, there has been a longstanding debate about the role of DM in POAG. The Ocular Hypertension Treatment Study (OHTS) initially reported that diabetes protects ocular hypertensive patients against the development of glaucoma after 72 months follow-up,5 but this conclusion was not confirmed by analyses utilizing data with a more detailed assessment of diabetic status.6 In contrast, the Los Angeles Latino Eye Study, a cross-sectional study, reported higher prevalence of OAG in participants with type 2 DM.7 Four other large multi-centered randomized clinical trials provided inconsistent results.8 Associations between POAG and DM were reported in the Advanced Glaucoma Intervention Study (AGIS) (6 years follow-up, hazard ratio (HR) 1.87 for DM)9 and the Collaborative Initial Glaucoma Treatment Study (CIGTS) (4 years follow-up, HR 1.59 for DM),10 but not in the Collaborative Normal Tension Glaucoma Study (CNTGS) (5 years follow-up)11 or in the Early Manifest Glaucoma Trial (EMGT) (11 years follow-up).12 Differences in these results can be attributed in part to the potential differences in DM status of the subjects; none of the studies directly evaluated diabetic retinopathy (DR) or other diabetic complications. The stage of POAG may also have possibly affected the findings; more severe disease is associated with more variable VF test results,13 thus reducing the ability to detect progression by VFs. It is notable that most related studies, including the ones above, only focused on the effect of DM on VF progression, and there is a paucity of information about both visual field and RNFL thickness in these patients.
Although VF testing is the most widely used method for assessing functional loss, some patients can show progressive RNFL changes despite the absence of detectable changes on VF.14 Cross-sectional studies have shown that the mean RNFL was thicker in glaucoma eyes of diabetic patients than non-diabetic glaucoma eyes, but this did not reach statistical significance.15 Moreover, diabetes was protective against glaucomatous optic nerve damage when optic disc topographic parameters, including mean rim area and rim volume, were considered.16 Overall, DM is not a well understood risk factor and its association with the progression of POAG is still controversial. Considering the projected increasing global prevalence of DM (more than 420 million people have diabetes globally)17 and the irreversible nature of glaucoma progression, it is important to further clarify the effect of DM on POAG progression.
The purpose of this study was to evaluate the effect of type 2 DM on glaucoma progression, both functionally and structurally, by comparing the rate of VF loss and RNFL thinning in glaucoma eyes with and without type 2 DM.
Methods
This was a cohort study. Participants were recruited from the longitudinal Diagnostic Innovations in Glaucoma Study (DIGS).18 The Institutional Review Boards of the University of California San Diego approved the protocol, and the methodology adhered to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. This study was registered at http://clinicaltrials.gov (no. NCT00221923) on September 14, 2005. Data included in this prospective study were from 2008 - 2016. Written informed consent was obtained from all participants.
Participants
Inclusion criteria for DIGS were open angles with gonioscopy, a best-corrected visual acuity of 20/40 or better, a spherical refraction within ±5.0 diopters (D), and cylinder correction within ±3.0 D. Subjects were excluded if they had a history of intraocular surgery (except for uncomplicated glaucoma and cataract surgery). Subjects with secondary causes of elevated IOP, other intraocular eye disease, or other diseases affecting VF or who were using medications known to affect VF sensitivity also were excluded. Other information such as race, age, systemic disease history, non-ocular medication, blood pressure, heart rate, and central corneal thickness (CCT) was also collected.
For inclusion, POAG patients had reliable (≤33% fixation losses and false negative results and ≤15% false positive results) and repeatable abnormal Standard Automated Perimetry was obtained with the Humphrey 24-2 Swedish Interactive Threshold Algorithm (SITA), and abnormality was based on a Pattern Standard Deviation (PSD) outside the 95% normal limits and Glaucoma Hemifield Test (GHT) results outside normal limits. DM patients were defined by consistent self-reported history and their use of anti-diabetic medication. At each visit, the time of diagnosis and the type of administered drugs was obtained. Type 2 DM was defined if the participant was 30 years or older when diagnosed with DM.7
Patients’ medication history was confirmed with chart review by an investigator masked to the RNFL and VF results. DIGS does not include patients with proliferative DR or diabetic macula edema. Considering evidence showing RNFL thickness changes in early stage DR19 and other retinal diseases, eyes that showed any sign of DR or other retinal diseases (eg, retinal vein occlusion) determined by fundus examination or spectral domain optical coherence tomography (SD-OCT) were also not included. In addition, eyes that had retinal laser treatment were excluded because there would be iatrogenic retinal damage and iatrogenic visual field abnormalities.
POAG eyes were divided into 2 groups: those without diabetes (POAG/DM- group) and those with type 2 DM patients (POAG/DM group). The initial severity of glaucoma was classified based on the severity of their VF damage at the first visit: mild glaucoma was defined as mean deviation (MD) better than -6 decibels (dB), moderate glaucoma was defined as a MD between -6 to -12dB, and severe glaucoma was defined as a MD lower than -12 dB.20–22
Follow-up
Participants were evaluated every 6 months. At the baseline visit and at each annual follow-up visit, subjects underwent complete ophthalmologic examination including slit-lamp biomicroscopy, IOP measurement, dilated stereoscopic fundus examination, and stereophotography of the optic nerve head. VF testing and SD-OCT was completed at baseline and every 6 months during follow-up. The SD-OCT was performed either on the same visit as the VF or within 90 days after the first VF, as well as before the last VF. Diabetes diagnosis and medication were checked each visit. Subjects who no longer reported a history of diabetes or use of anti-diabetic medication were excluded. Also, eyes were excluded during follow-up if they developed DR or other retinal diseases as determined by fundus examination or OCT exam, or if they had received retina laser treatment.
Standard Automated Perimetry
VF (Humphrey Field Analyzer; Carl Zeiss Meditec, Dubin, CA) tests were completed using SITA 24-2 strategies during follow-up. The quality of the VF results was reviewed by the University of California San Diego Visual Field Assessment Center staff. Only reliable tests and VFs without rim and eyelid artifacts, evidence of inattention or fatigue effects, and evidence that the abnormal results of the VF were caused by a disease other than glaucoma were included.
Spectral-Domain Optical Coherence Tomography
The Spectralis SD-OCT (Spectralis HRA+OCT; Heidelberg Engineering Inc., Heidelberg, Germany) was used for ONH and macular imaging (software version 5.4.7.0). The circumpapillary RNFL thickness was measured with parapapillary circle scan. The high resolution RNFL Circle Scan protocol was used; RNFL measurements were calculated in a 10-pixel-wide band along a circle of 12 degrees centered on the ONH. The acquisition rate is 40,000 A- scans per second at an axial resolution of 3.9 mm and a lateral resolution of 6mm. The mean RNFL thickness of temporal (316°−45°), temporal superior (46°–90°), nasal superior (91°–135°), nasal (136°–225°), nasal inferior (226°–270°), temporal inferior (271°–315°), and global area were provided by the software. The software also provides the quality score that ranges from 0dB (poor) to 40dB (excellent). All images were processed and reviewed by the Imaging Data Evaluation and Assessment (IDEA) Center at the University of California, San Diego. Images with non-centered scans, inaccurate segmentation of the RNFL that could not be manually corrected, or quality scores of 15 dB or less were excluded from the analysis.
Statistical Analysis
Descriptive statistics, including mean, standard deviation (SD), and 95% confidence intervals (CI) for normally distributed variables, and median, first quartile, and third quartile for non-normally distributed variables, were computed. Random effects models were used to estimate the effect of type 2 DM on VF loss and the rate of RNFL thickness change (POAG/DM group vs POAG/DM- group). First, a two-way interaction model was used to evaluate whether there was a significant influence of DM on the slope of RNFL and VF loss over time, without considering other explanatory variables.23 Diabetes, time and their interaction were the fixed effects in the model with MD and PSD, RNFL thickness the response with each eye nested within subject as random effects. Akaike information criterion (AIC) was used to measure the relative appropriateness of each model. Secondly, multivariate analyses were performed to correct for potential confounding factors, including age, gender, race, systemic hypertension, mean IOP during follow-up, and initial MD.
Statistical analyses were performed using statistical software R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 14.2 (StataCorp LLC, College Station, TX). Random Coefficient models were fitted using the lme4 package and Wald confidence intervals were calculated for each coefficient. P values less than 0.05 were considered statistically significant.
Results
Study Population and Baseline Factors
Of the 143 glaucoma patients (197 eyes), 32 (22%) subjects (55 eyes) had type 2 DM. The two groups of subjects were age-matched. Baseline demographics and ophthalmic characteristics of the POAG/DM group and the POAG/DM- group are summarized in Table 1. The median (interquartile range, IQR) duration of diabetes of these DM patients was 13 years (8, 19) and all reported using anti-diabetic medicines of insulin and/or metformin. Some participants with DM were also on other anti-diabetic pills (e.g. glipizide). At baseline, there was no significant difference between the POAG/DM-and POAG/DM groups in terms of gender, IOP, mean ocular perfusion pressure (MOPP, 2/3 mean arterial pressure - IOP), VF MD, VF PSD, global RNFL thickness, axial length, and CCT. The groups differed by race, and self-reported history of system hypertension; the POAG/DM- group had a higher proportion of Caucasian and lower incidence of hypertension. Although the prevalence of self-reported hypertension in the POAG/DM group is higher, the blood pressure was within normal limits and not different from the POAG/DM- group. According to initial VF MD, 78.2% and 80.2% of the eyes in POAG/DM group and POAG/DM- group, respectively, had mild glaucoma. Prior and during the follow-up period, 27.3% and 35.9% of the eyes in POAG/DM group and POAG/DM- group, respectively, underwent glaucoma surgery (P=0.24). The median (IQR) number of visits for VF testing was 11 (8, 16), and the median number of visits with good quality RNFL thickness measurements was similar at 10 (7, 15). The median (IQR) follow-up was 5.7 years (4.0, 6.4) based on SD-OCT visit. There were no significant differences in the number of VF visits, RNFL thickness measurements or years of follow-up between the POAG/DM and POAG/DM- groups. As all of these patients had VF testing before SD-OCT was available, we also completed an analysis using the full follow-up time available with VF testing (median (IQR) follow-up of 10.6 years (5.0, 14.8) and number of visits 19 (11, 25)). The baseline mean VF MDs of this analysis was similar in the 2 groups, −4.3 dB (95% CI −5.7, −3.0) in POAG/DM group and −4.3 dB (95% CI −5.2, −3.5) in POAG/DM- group (P=0.99).
Table 1.
Baseline Demographics and Ophthalmic Characteristics of POAG/DM and POAG/DM- Group
| POAG/DM | POAG/DM- | P value | |
|---|---|---|---|
| By Subject (No.) | 32 | 111 | |
| Age (yrs) | 74.9±1.9 | 73.8±1.0 | 0.60 |
| Gender (M/F) | 20/12 | 60/51 | 0.40 |
| Race, no. (%) | <0.001* | ||
| Caucasian | 11 (34.4%) | 84 (75.68%) | |
| African American | 17 (53.1%) | 13 (11.71 %) | |
| Other | 4 (12.5%) | 14 (12.61%) | |
| Diabetes history (yrs) | 13 (8, 19) | N/A | |
| Anti-diabetes medicine, no. (%) | |||
| Insulin | 5 (15.6%) | N/A | |
| Metformin | 23 (71.9%) | N/A | |
| Both | 4 (12.5%) | N/A | |
| Hypertension, no. (%) | 28 (87.5%) | 54 (48.7%) | <.0001* |
| Diastolic BP (mmHg) | 80.5±2.1 | 80.0±1.2 | 0.85 |
| Systolic BP (mmHg) | 131.7±3.5 | 127.5±1.9 | 0.28 |
| Mean arterial pressure (mmHg) | 97.6±2.4 | 95.8±1.3 | 0.52 |
| Heart rate (/min.) | 69.8±2.0 | 66.3±1.1 | 0.13 |
| By Eye (No.) | 55 | 142 | |
| Follow-up (yrs) | 6.2 (4.0, 6.6) | 5.6 (3.8, 6.4) | 0.59 |
| Visits of visual fields | 12.0 (8.0, 16.0) | 11.0 (8.0, 15.0) | 0.71 |
| Visits of RNFL circle scans | 11.0 (7.8, 15.0) | 10.5 (7.0, 15.0) | 0.83 |
| MOPP (mmHg) | 49.9 (46.5, 53.3) | 48.8 (47.0, 50.7) | 0.60 |
| Axial Length (mm) | 23.8 (23.3, 24.3) | 24.2 (23.9, 24.4) | 0.19 |
| CCT (µm) | 550.3 (537.0, 563.6) | 538.8 (531.5, 546.2) | 0.14 |
| Initial IOP (mmHg) | 18.5 (16.5, 16.8) | 18.0 (16.9, 19.1) | 0.67 |
| Mean IOP during follow-up (mmHg) | 15.7 (14.5, 16.8) | 15.2 (14.6, 15.9) | 0.53 |
| Initial MD (dB) | −4.5 (−5.3, −3.6) | −4.7 (−6.0, −3.4) | 0.80 |
| MD > −6 dB | 43 (78.2%) | 114 (80.2%) | |
| MD −6~−12 | 6 (10.9%) | 19 (13.4%) | |
| MD < −12 dB | 6 (10.9%) | 9 (6.3%) | |
| Initial PSD (dB) | 5.2 (2.1, 8.3) | 6.4 (1.3, 4.5) | 0.09 |
| Initial global RNFLT (µm) | 72.9 (70.2, 75.7) | 78.8 (74.7, 83.0) | 0.06 |
| Glaucoma surgery#, no. (%) | 15 (27.3%) | 51 (35.9%) | 0.24 |
| Cataract surgery#, no. (%) | 22 (40%) | 58 (40.9%) | 0.91 |
For normally distributed variables by subject, results are shown in mean ± standard deviation; for non-normally distributed variables, results are shown in mean (interquartile range). Normally distributed variables by eye are shown in mean (95% confident interval).
, statistically significant;
, prior and during follow-up.
Abbreviations: MOPP=mean ocular perfusion pressure; CCT=central corneal thickness; IOP=intraocular pressure; MD=mean deviation; PSD=pattern standard deviation; BP=blood pressure; RNFLT= retinal nerve fiber layer thickness; M=male; F=female; yrs=years; POAG/DM= primary open angle glaucoma eyes with type 2 DM patients; POAG/DM-= primary open angle glaucoma eyes without diabetes.
Visual Field and Retinal Nerve Fiber Layer Thickness Change
Table 2 summarizes mean univariate rates of change of MD, PSD, global and sectoral RNFL thickness in POAG/DM- and POAG/DM eyes, and shows the association of type 2 DM with the VF and RNFL thickness change based on univariate analysis. The POAG/DM had a slower rate of MD change (−0.21dB/year vs. −0.38dB/year, 0.17 dB/year difference) and slower rate of PSD change (0.12dB/year vs. 0.23dB/year, 0.11 dB/year difference), but neither MD slope (p=0.07) or PSD slope (p=0.12) was statistically different between the two groups. However, the mean rate of global RNFL loss in the POAG/DM group was significantly slower (−0.40 µm/year) compared to the POAG/DM-group (−0.83 µm/year). Moreover, in the temporal superior region, the mean rates of RNFL loss in the POAG/DM- group were approximately four times faster compared to the POAG/DM group (−1.38 µm/year vs. −0.33 µm/year in temporal superior region, P <0.01). The model of global RNFL comparison had the lowest AIC indicating the most appropriate model. The wide distribution of the rate of MD change, and global RNFL loss in eyes of POAG/DM- and POAG/DM group is shown in Figure 1. Differences between POAG/DM- and POAG/DM eyes in the rates of RNFL loss in other regions (except for temporal superior region) did not reach statistical significance. In addition, since the mean baseline RNFL thickness was higher in the nondiabetic group compared to diabetic group (78.8 µm vs. 72.9 µm), RNFL loss rates based on the percent change (%/year) of RNFL thickness also was assessed. Consistent with the µm/year analysis, the mean percent/year rate of global RNFL thinning in the POAG/DM group was significantly slower (−0.49 %/year) compared to the POAG/DM- group (−1.13%/year) (P=0.005). In the temporal superior region, the mean percent change rates of RNFL thickness in the POAG/DM- group and the POAG/DM group were −1.48 %/year vs. −0.29 %/year (P <0.001). The model of global RNFL percent change comparison had the lowest AIC. Given that eyes that had undergone glaucoma surgery would likely have a slower rate of RNFL loss, the rate of RNFL loss in the POAG/DM- and POAG/DM groups stratified by glaucoma surgery status also was compared. For both the eyes that had undergone glaucoma surgery and the eyes that had not undergone glaucoma surgery, the rate of RNFL thinning was slower in the POAG/DM group compared to the POAG/DM- group (−0.54 µm/year vs. −1.06 µm/year, P= 0.01, and 0.04 and −0.54 P=0.09 respectively, Table 3).
Table 2.
Rates and Difference of Visual Field Loss and Retinal Nerve Fiber Layer Thinning in the POAG/DM and POAG/DM- Patients
| POAG/DM (n=55 eyes) |
POAG/DM- (n=142 eyes) |
Difference (univariate analysis) |
|||
|---|---|---|---|---|---|
|
|
|||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P | AIC | |
| Visual Field Slope Value (dB/year) | |||||
| MD | −0.21 (−0.36, −0.05) | −0.38 (−0.48, −0.29) | 0.17 (−0.01, 0.36) | 0.07 | 8720 |
| PSD | 0.12 (−0.00, 0.24) | 0.23 (0.16, 0.30) | −0.11 (−0.25, 0.03) | 0.12 | 7462 |
| Retinal Nerve Fiber Layer Thickness Slope value (µm/year) | |||||
| Global | −0.40 (−0.67, −0.12) | −0.83 (−1.00, −0.66) | 0.44 (0.11, 0.76) | 0.01* | 10384 |
| Temporal | −0.50 (−0.78, −0.22) | −0.77 (−0.95, −0.59) | 0.27 (−0.07, 0.61) | 0.12 | 10902 |
| TS | −0.33 (−0.88, 0.21) | −1.38 (−1.71, −1.06) | 1.05 (0.42, 1.68) | 0.002* | 13077 |
| TI | −1.14 (−1.63, −0.64) | −1.34 (−1.68, −1.01) | 0.21 (−0.39,0.81) | 0.49 | 13829 |
| Nasal | −0.23 (−0.51, 0.05) | −0.39 (−0.57, −0.20) | 0.16 (−0.18, 0.49) | 0.36 | 11391 |
| NS | −0.20 (−0.66, 0.25) | −0.56 (−0.84, −0.28) | 0.36 (−0.18, 0.89) | 0.19 | 12804 |
| NI | −0.55 (−1.01, −0.09) | −0.93 (−1.23, −0.64) | 0.38 (−0.16, 0.93) | 0.17 | 13117 |
, statistically significant.
Abbreviations: POAG/DM= primary open angle glaucoma eyes with type 2 DM patients; POAG/DM-= primary open angle glaucoma eyes without diabetes; CI= confidence interval; MD=mean deviation; PSD=pattern standard deviation; AIC= Akaike information criterion; TS= temporal superior; TI= temporal inferior; NS= nasal superior; NI= nasal inferior.
Figure 1.
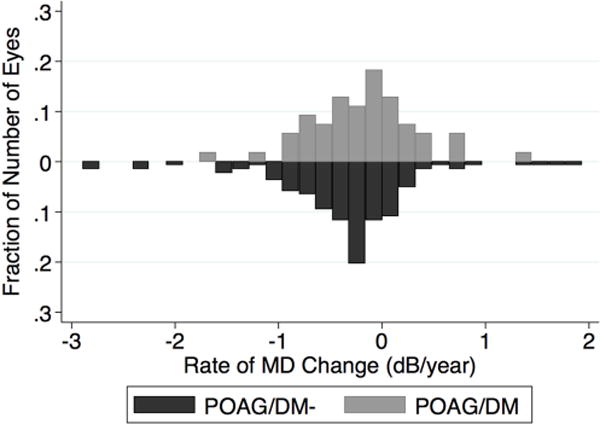
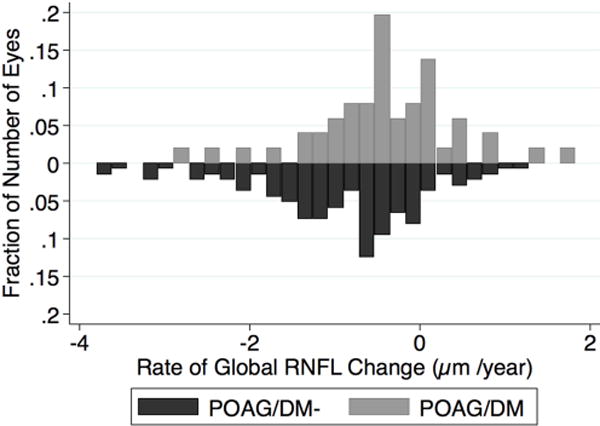
Bar graph showing the distributions of the rates of mean deviation loss and global retinal nerve fiber layer thinning in eyes of POAG/DM and POAG//DM- patients. MD=mean deviation; RNFL= retinal nerve fiber layer.
Table 3.
Rates and Difference of Global Retinal Nerve Fiber Layer Thinning in the POAG/DM and POAG/DM- Eyes with or without glaucoma surgery
| POAG/DM | POAG/DM- | Difference | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. (eyes) | Mean (95% CI) (µm/year) | No. (eyes) | Mean (95% CI) (µm/year) | Mean (95% CI) (µm/year) | P | |
| Glaucoma surgery | 15 | −0.04 (−0.55, 0.45) | 51 | −0.54 (−0.80, −0.27) | 0.48 (−0.08, 1.05) | 0.09 |
| Non-glaucoma surgery | 40 | −0.54 (−0.86, −0.22) | 91 | −1.06 (−1.26, −0.85) | 0.52 (0.14, 0.90) | 0.01* |
, statistically significant.
Abbreviations: POAG/DM= primary open angle glaucoma eyes with type 2 DM patients; POAG/DM- = primary open angle glaucoma eyes without diabetes; CI= confidence interval.
Table 4 presents the fixed effects from the fitted multivariate mixed effects model, showing the effects of type 2 DM and other potentially important factors on changes in RNFL thickness (global and temporal superior regions) over time. Aside from DM, other factors included in the analysis included age, gender, race, mean IOP during follow-up, initial MD and hypertension. After adjustment of these factors, the differences in the rate of RNFL loss in global and temporal superior regions were still statistically significant between the POAG/DM- and POAG/DM groups. POAG/DM patients had significantly slower rates of RNFL thickness change compared with POAG/DM- patients (0.46 µm/year and 1.19 µm/year slower thinning in global and temporal superior RNFL thickness respectively; P=0.011 and P=0.001, respectively). In addition to type 2 DM, mean IOP during follow-up was also associated with rates of global and temporal superior RNFL loss (P=0.001 and P=0.007 respectively). Other factors included in the analysis, although associated with RNFL thickness measurements in some cases, were not significantly associated with the rates of RNFL loss.
Table 4.
Effects of Type 2 Diabetes Mellitus and Other Factors on Retinal Nerve Fiber Layer Thickness (multivariate analysis)
| Global RNFLT (µm) | Temporal Superior RNFLT (µm) | |||
|---|---|---|---|---|
|
|
||||
| Coefficient (95%CI) | P value | Coefficient (95%CI) | P value | |
| Intercept | 83.85 (76.67, 91.03) | <0.001* | 111.48 (99.15, 123.80) | <0.001* |
| Diabetes | −0.42 (−5.92, 5.07) | 0.880 | −2.27 (−11.61, 7.08) | 0.635 |
| Time (years) | −0.88 (−1.34, −0.41) | <0.001* | −2.10 (−2.99, −1.22) | <0.001* |
| Age (years) | −0.04 (−0.25, 0.18) | 0.741 | 0.24 (−0.13, 0.61) | 0.209 |
| Gender (reference, male) | −4.46 (−8.63, −0.28) | 0.038* | −6.37 (−13.54, 0.8) | 0.084 |
| VF MD (dB) | 1.72 (1.35, 2.09) | <0.001* | 2.69 (2.01, 3.37) | <0.001* |
| Mean IOP during follow-up (mmHg) | 0.38 (−0.19, 0.96) | 0.195 | 0.05 (−0.99, 1.09) | 0.921 |
| Caucasian (reference, AA) | −10.86 (−17.00, −4.72) | 0.001* | −15.23 (−25.76, −4.7) | 0.005* |
| Other race (reference, AA) | −8.04 (−16.20, 0.11) | 0.055 | −8.31 (−22.28, 5.65) | 0.246 |
| Hypertension | 0.29 (−4.75, 5.32) | 0.911 | −2.13 (−10.82, 6.56) | 0.631 |
| Diabetes × Time | 0.46 (0.11, 0.81) | 0.011* | 1.19 (0.52, 1.85) | 0.001* |
| Age × Time | 0.01 (−0.01, 0.02) | 0.337 | 0.01 (−0.02, 0.03) | 0.713 |
| Gender × Time | 0.01 (−0.27, 0.30) | 0.926 | −0.13 (−0.67, 0.42) | 0.645 |
| VF MD ×Time | −0.01 (−0.05, 0.02) | 0.381 | −0.04 (−0.1, 0.02) | 0.236 |
| Mean IOP during follow-up × Time | −0.07 (−0.11, −0.03) | 0.001* | −0.11 (−0.19, −0.03) | 0.007* |
| Caucasian (reference, AA) ×Time | 0.01 (−0.38, 0.41) | 0.948 | 0.61 (−0.14, 1.37) | 0.116 |
| Other race (reference, AA) × Time | −0.05 (−0.59, 0.49) | 0.844 | −0.08 (−1.11, 0.94) | 0.872 |
| Hypertension × Time | 0.08 (−0.26, 0.42) | 0.651 | 0.64 (−0.01, 1.3) | 0.056 |
, statistically significant in univariate analysis.
Abbreviations: RNFLT= retinal nerve fiber layer thickness; CI= confidence interval; MD=mean deviation; AA=African American. Multivariate analysis adjusted for age, gender, ethnicity, hypertension, mean IOP during follow-up, and initial MD.
In the analysis of the rate of VF loss with longer follow-up (median follow-up: 10.6 years) on the same population, the mean MD slopes were −0.06 dB/year in the POAG/DM group and −0.19 dB/year in the POAG/DM- group. The POAG/DM group also showed a slower change in PSD (mean PSD slope 0.03 dB/year in the POAG/DM group vs. 0.13 dB/year in the POAG/DM- group), but the differences in the rate of MD and rate of PSD change were not statistically different between the two groups (P values were 0.14 and 0.21 respectively).
Discussion
Although the eyes of glaucoma patients without type 2 DM were not different than the eyes of those with type 2 DM with respect to visual field progression, they did show a significantly faster rate of RNFL thinning. In other words, the RNFL of these type 2 DM patients without observable DR had a slower rate of thinning than those without type 2 DM, and the presence of diabetes or its treatment may be protective against RNFL loss in the former group.
In the current study, eyes in the POAG/DM group had a slower rate of RNFL thinning in both the global area and the temporal superior region than the eyes in the POAG/DM- group, regardless of whether the unit of analysis was µm/year or %/year. In addition, eyes of the POAG/DM group had slower RNFL thinning than those of the POAG/DM- group, regardless of whether they had undergone glaucoma surgery. The mean global RNFL slope of the POAG/DM- group (−0.83 µm/year) is similar to a recently reported 5 years follow-up RNFL slope of POAG patients with VF progression (−0.93 µm/year), while the mean global RNFL slope of the POAG/DM group (−0.40 µm/year) is similar to that of the VF non-progressors (−0.46 µm/year). 24, 25 One should keep in mind that the ability to detect glaucoma progression by VF versus SD-OCT is significantly influenced by the stage of disease. Eyes with less severe disease at baseline have a higher chance of being detected as progressing by SD-OCT compared with VF.14 In our study, most of the eyes in both groups had mild glaucoma with MD > −6dB (POAG/DM group, 78.2%; POAG/DM- group, 80.2%) at baseline.
One reason that DM has been considered to be associated with POAG is the end-organ effect of uncontrolled glucose levels. For every 10 mg/dL increase in fasting serum glucose, IOP increases by 0.09 mmHg in men and 0.11 mmHg in women.26 This highlights the importance of considering the treatment of DM when addressing the effect of DM on glaucoma. With comprehensive treatment, type 2 DM patients who can avoid hyperglycemia may have lower IOP than those with hyperglycemia. On the other hand, the main proposed mechanism of association between DM and POAG (the so-called neurodegeneration theory27) is based on the idea that chronic hyperglycemia4, 28 can directly harm the retina.27 This theory is supported by a previous study that showed that compared to normal eyes, RNFL thickness was significantly lower in the superior areas in diabetic eyes without DR.29 But, more recent studies reported that there was no significant difference in RNFL thickness between nondiabetic controls and diabetic patients with no or mild DR.30 Both RNFL thickness and the number of macular ganglion cells were not correlated with the duration of type 2 DM and retinopathy, but correlated significantly with the severity of diabetic polyneuropathy (DPN).31 However, the previous evidence showing ganglion cell damage in diabetes was collected from type 1 diabetes patients32 and streptozotocin induced diabetic animal models, a widely studied animal model of type 1 diabetes.28 These results suggest that the type and overall condition of DM should be considered when evaluating the effect of DM on POAG.
To characterize the type 2 DM patients in the current study, only those with high awareness of their diabetes as determined by their persistent self-report of diagnosis time and medication were included in the study. And, DM patients with DR were excluded from the study. Moreover, it is notable that most of the DM patients (71.9%) were only using metformin during the entire follow-up. Metformin is the first line medicine for type 2 DM, and often is combined with other drugs when hemoglobin A1c is above 7%.17 Therefore, the participants in the current POAG/DM group can be regarded as a population of well-controlled type 2 DM patients that have not developed DR.
In the current study, eyes in POAG/DM group showed slower RNFL thinning than those in the POAG/DM- group. The potential mechanisms of this protective effect against glaucomatous damage might be indirectly associated with the underlying type 2 DM. Vascular endothelial growth factor (VEGF) blockade significantly increased neuronal cell death in an ocular hypertensive glaucoma model.33 But VEGF overexpresses in the diabetic retina,4 which may be a protective strategy of the retina to rescue and protect retinal neurons.34
The type of anti-diabetic medication, particularly metformin, also may account for the differences observed between the groups. In the current study, all patients in the POAG/DM group had been using metformin and/or insulin. As mitochondrial dysfunction and retinal ganglion cell loss35 are both key events of glaucoma pathophysiology, their protection36,37 by metformin might have had a salutary effect against glaucoma progression, A retrospective cohort study showed metformin use was associated with reduction in the risk of developing OAG.38 In the current study, the global and sectoral RNFL thinning rates between metformin and non-metformin users in POAG/DM patients were compared to determine if metformin has a protective effect. However, no significant difference was found (all P vales >0.1). As most subjects in the POAG/DM group were on metformin (84.4%, solo or combined), this analysis is limited by the small sample size of the non-metformin users (5 subjects/7 eyes). Indeed, as the first line anti-diabetes medicine, metformin is prescribed to the majority of the type 2 diabetes patients. Another explanation of these results is related to a hypothesis by Faiq et al that glaucoma is a brain specific diabetes.39 Based on this theory, insulin and other antidiabetic pills could be a potential remedy for glaucoma.39 Further studies are needed to understand whether anti-diabetic medicines are protective against glaucomatous damage.
A limitation of the current study relates to the self-report used to classify the diabetic and control groups as this may have led to information bias. A similar limitation initially arose with analysis of the OHTS data. The initial OHTS report showed that a history of DM at baseline appeared to be protective against developing POAG. But after a more complete assessment of the diabetic status of OHTS, the initial protective effect of self-reported DM on the development of glaucoma utilizing 3 different definitions of diabetes was not confirmed.5, 6 Specifically, a history of DM at baseline or follow-up was considered as “high sensitivity and low specificity”. In contrast, current use of anti-diabetic medication was deemed as “low sensitivity and high specificity”, and patients reporting dietary treatment of diabetes were considered “moderate sensitivity and specificity”.6 As an alternative to self-report, diabetes-related blood tests also have been used in some studies. However, this too may introduce bias as they may not be appropriate for confirming a DM diagnosis; the results could be normal after appropriate treatment. However, by limiting inclusion to patients on DM medications, the inclusion of non-DM patients in the study as a diabetic is minimized. To optimize the reliability of self-reported DM in the current study, diabetic patients were defined as consistently self-reporting a history of type 2 DM, using anti-diabetic medication, and also specifying diagnosis time and the medicine type at every visit during follow-up.
Another limitation of this study is that hemoglobin A1c values were not available. Hemoglobin A1c, a measure of glycemic control, reflects only the three-month average plasma glucose concentration. Many studies, including the current one, have a much longer follow-up period, so that glycemic control for a large part of the follow-up time would still not be known unless hemoglobin A1c was measured serially. Moreover, there is accumulating evidence suggesting that lower glucose levels alone may not predictably reduce the risk of complications, including retinopathy, and that hemoglobin A1c may not be a sufficient outcome measure in clinical trials.40 Although hemoglobin A1c values were not available, the data that were collected is strongly suggestive that the participants in the current POAG/DM group were well-controlled type 2 DM patients that have not developed DR.
Some studies have suggested an ethnic difference in the association of diabetes with POAG.3 Race was also included in our model, but no significant racial difference was found in the rates of VF and RNFL loss. However, there was a small sample size for each racial group in our study. A larger sample size is needed to clarify this issue.
Another consideration and potential limitation relates to the difficulty in separating the effect of the treatment from the effect of the disease. Moreover, in patients with progression there often is additional treatment that may obfuscate the delineation of risk factors. Diabetic patients may have retinal edema that precedes microaneurysms or other signs of DR, and that might account for characteristic OCT findings. To best avoid this issue, all DIGS participants underwent extensive ophthalmological examinations, and eyes with evidence of diabetic macula edema or any sign of DR were excluded. It should be noted that the patients in the POAG/DM group did not have DR even with a median diabetes history of 13 years. DR is related to long diabetes duration and poor glycemic and blood pressure control.41 Therefore, still another limitation of our study is that there might be selection bias with only inclusion of mild or well controlled type 2 DM patients without DR. And, we cannot extrapolate directly the results of type 2 DM patients in this study to all types of diabetes or to diabetics with DR. In addition, it is possible that undiagnosed type 2 DM patients might have been included in the POAG/DM- group, resulting in a misclassification bias which reduces the likelihood of finding a difference between the groups. If there was misclassification, however, it is likely our results of significant differences in the rate RNFL loss may underestimate the difference between patients with and without DM.
In conclusion, although there was no difference in visual field progression between eyes of POAG patients without and with type 2 DM without detectable diabetic retinopathy, there was a significantly slower rate of RNFL thickness loss in POAG patient eyes with treated type 2 DM than those without DM. Our results should be confirmed in a larger longitudinal study.
Supplementary Material
Acknowledgments
a. Funding/Support:
National Institutes of Health/National Eye Institute Grants EY011008 (L.M.Z.), EY14267 (L.M.Z.), and EY019869 (L.M.Z.), Core Grant P30EY022589, an unrestricted grant from Research to Prevent Blindness (New York, NY), and grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen.
b. Financial Disclosures:
Huiyuan Hou: none; Takuhei Shoji: Financial support-Alcon; Linda Zangwill: Research support-National Eye Institute, Carl Zeiss Meditec, Heidelberg Engineering, Topcon, and Nidek; Sasan Moghimi: none; Luke J. Saunders: none; Kyle Hasenstab: none; Elham Ghahari: none; Patricia Isabel C. Manalastas: none; Tadamichi Akagi: none; Mark Christopher: none; Rafaella C. Penteado: none; Robert N. Weinreb: Research support-Carl Zeiss Meditec, Genentech, Heidelberg Engineering, Konan, National Eye Institute, Optos, Optovue, Tomey and Topcon; Consultant- Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Eyenovia, Novartis, Unity, Valeant.
c. Other Acknowledgment: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Tham YC, Cheng CY. Associations between chronic systemic diseases and primary open angle glaucoma: an epidemiological perspective. Clin Exp Ophthalmol. 2017;45:24–32. doi: 10.1111/ceo.12763. [DOI] [PubMed] [Google Scholar]
- 4.Song BJ, Aiello LP, Pasquale LR. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr Diab Rep. 2016;16:124. doi: 10.1007/s11892-016-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MO, Beiser JA, Kass MA, Ocular Hypertension Treatment Study G Is a history of diabetes mellitus protective against developing primary open-angle glaucoma? Arch Ophthalmol. 2008;126:280–1. doi: 10.1001/archophthalmol.2007.35. [DOI] [PubMed] [Google Scholar]
- 7.Chopra V, Varma R, Francis BA, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–232 e1. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(Suppl 1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol. 2002;134:499–512. doi: 10.1016/s0002-9394(02)01659-8. [DOI] [PubMed] [Google Scholar]
- 10.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 11.Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study G Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106:2144–53. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner SK, Mansberger SL. Effect of Restricting Perimetry Testing Algorithms to Reliable Sensitivities on Test-Retest Variability. Invest Ophthalmol Vis Sci. 2016;57:5631–5636. doi: 10.1167/iovs.16-20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci. 2016 Oct;57:421–8. doi: 10.1167/iovs.15-18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akkaya S, Can E, Ozturk F. Comparison of the corneal biomechanical properties, optic nerve head topographic parameters, and retinal nerve fiber layer thickness measurements in diabetic and non-diabetic primary open-angle glaucoma. Int Ophthalmol. 2016;36:727–36. doi: 10.1007/s10792-016-0191-x. [DOI] [PubMed] [Google Scholar]
- 16.Akkaya S, Can E, Ozturk F. Comparison of Optic Nerve Head Topographic Parameters in Patients With Primary Open-Angle Glaucoma With and Without Diabetes Mellitus. J Glaucoma. 2016;25:49–53. doi: 10.1097/IJG.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 17.Reusch JE, Manson JE. Management of Type 2 Diabetes in 2017: Getting to Goal. JAMA. 2017;317:1015–1016. doi: 10.1001/jama.2017.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson KB, Frydkjaer-Olsen U, Grauslund J. Vascular Changes and Neurodegeneration in the Early Stages of Diabetic Retinopathy: Which Comes First? Ophthalmic Res. 2016;56:1–9. doi: 10.1159/000444498. [DOI] [PubMed] [Google Scholar]
- 20.Hodapp E, Parish IIRK, Anderson DR. Clinical Decisions in Glaucoma. St Louis, MO: Mosby; 1993. pp. 52–61. [Google Scholar]
- 21.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology. 2016;123:2309–2317. doi: 10.1016/j.ophtha.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammel N, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol. 2017;178:38–50. doi: 10.1016/j.ajo.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Weinreb RN. The Relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–33. e1–3. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin JW, Sung KR, Lee GC, Durbin MK, Cheng D. Ganglion Cell-Inner Plexiform Layer Change Detected by Optical Coherence Tomography Indicates Progression in Advanced Glaucoma. Ophthalmology. 2017 doi: 10.1016/j.ophtha.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–8. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen E, Kramer M, Shochat T, Goldberg E, Krause I. Relationship Between Serum Glucose Levels and Intraocular Pressure, a Population-based Cross-sectional Study. J Glaucoma. 2017;26:652–656. doi: 10.1097/IJG.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 27.Costa L, Cunha JP, Amado D, Pinto LA, Ferreira J. Diabetes Mellitus as a Risk Factor in Glaucoma’s Physiopathology and Surgical Survival Time: A Literature Review. J Curr Glaucoma Pract. 2015;9:81–5. doi: 10.5005/jp-journals-10008-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom. 2011;94:4–23. doi: 10.1111/j.1444-0938.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–85. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan S, Dehghani C, Pritchard N, et al. Corneal and Retinal Neuronal Degeneration in Early Stages of Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:6365–6373. doi: 10.1167/iovs.17-22736. [DOI] [PubMed] [Google Scholar]
- 31.Salvi L, Plateroti P, Balducci S, et al. Abnormalities of retinal ganglion cell complex at optical coherence tomography in patients with type 2 diabetes: a sign of diabetic polyneuropathy, not retinopathy. J Diabetes Complications. 2016;30:469–76. doi: 10.1016/j.jdiacomp.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–5. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013;182:1379–90. doi: 10.1016/j.ajpath.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez C, Dal Monte M, Simo R, Casini G. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J Diabetes Res. 2016;2016:9508541. doi: 10.1155/2016/9508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamel K, Farrell M, O’Brien C. Mitochondrial dysfunction in ocular disease: Focus on glaucoma. Mitochondrion. 2017;35:44–53. doi: 10.1016/j.mito.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Kim YS, Kim M, Choi MY, et al. Metformin protects against retinal cell death in diabetic mice. Biochem Biophys Res Commun. 2017;492:397–403. doi: 10.1016/j.bbrc.2017.08.087. [DOI] [PubMed] [Google Scholar]
- 37.Abdelgadir E, Ali R, Rashid F, Bashier A. Effect of Metformin on Different Non-Diabetes Related Conditions, a Special Focus on Malignant Conditions: Review of Literature. J Clin Med Res. 2017;9:388–395. doi: 10.14740/jocmr2922e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin HC, Stein JD, Nan B, et al. Association of Geroprotective Effects of Metformin and Risk of Open-Angle Glaucoma in Persons With Diabetes Mellitus. JAMA Ophthalmol. 2015;133:915–23. doi: 10.1001/jamaophthalmol.2015.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faiq MA, Dada T. Diabetes Type 4: A Paradigm Shift in the Understanding of Glaucoma, the Brain Specific Diabetes and the Candidature of Insulin as a Therapeutic Agent. Curr Mol Med. 2017 doi: 10.2174/1566524017666170206153415. [DOI] [PubMed] [Google Scholar]
- 40.Lipska KJ, Krumholz HM. Is Hemoglobin A1c the Right Outcome for Studies of Diabetes? JAMA. 2017;317:1017–1018. doi: 10.1001/jama.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G. Optical Coherence Tomography Angiography of Diabetic Retinopathy. Dev Ophthalmol. 2016;56:107–12. doi: 10.1159/000442801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


