Abstract
Recognition of high-grade endometrial stromal sarcoma is important because of its aggressive clinical behavior. Morphologic features of YWHAE-NUTM2 high-grade endometrial stromal sarcoma may overlap with other uterine sarcoma types. BCOR immunoexpression was studied in these tumors and their morphologic mimics to assess its diagnostic utility. BCOR immunohistochemical staining was performed on archival tissue from 28 high-grade endometrial stromal sarcomas with classic morphology (20 YWHAE-NUTM2, 5 ZC3H7B-BCOR, 3 BCOR-ZC3H7B), 3 high-grade endometrial stromal sarcomas with unusual morphology and unknown gene rearrangement status, 66 low-grade endometrial stromal sarcomas, 21 endometrial stromal nodules, 38 uterine leiomyosarcomas, and 19 uterine leiomyomas. Intensity of nuclear staining and percentage of positive tumor cells were recorded. Strong diffuse nuclear BCOR staining (defined as >95% of tumor cells) was seen in the round cell component of all 20 (100%) classic YWHAE-NUTM2 high-grade endometrial stromal sarcomas and the 3 unusual high-grade endometrial stromal sarcomas which prompted FISH studies confirming YWHAE rearrangement in 2 tumors. Genomic PCR confirmed the presence of BCOR exon 16 internal tandem duplication in the third case. Diffuse BCOR staining was strong in 3 and weak in 1 BCOR-rearranged high-grade endometrial stromal sarcoma while absent in the remaining 4 BCOR-rearranged tumors. BCOR staining was weakly positive in <5% of tumor cells in 4 of 66 (6%) low-grade endometrial stromal sarcoma and 1 of 18 (6%) endometrial stromal nodules and weakly to moderately positive in <5% to 40% of tumor cells in 6 of 31 (19%) leiomyosarcomas. No BCOR staining was seen in the remaining low-grade endometrial stromal sarcomas, endometrial stromal nodules, leiomyosarcomas, or any of the leiomyomas. BCOR immunohistochemical staining is a highly sensitive marker for YWHAE-NUTM2 high-grade endometrial stromal sarcoma with both classic and unusual morphology and identifies a subset of high-grade endometrial stromal sarcoma with BCOR alterations, including BCOR rearrangement and internal tandem duplication.
INTRODUCTION
Endometrial stromal tumors are rare, but comprise the second most common type of uterine mesenchymal neoplasia after smooth muscle tumors, exhibiting a wide variety of morphologic patterns, genetic aberrations, and clinical outcomes. These neoplasms are currently classified as endometrial stromal nodule, low-grade endometrial stromal sarcoma, high-grade endometrial stromal sarcoma, and undifferentiated uterine sarcoma by the World Health Organization (1). Endometrial stromal nodules and low-grade endometrial stromal sarcomas are both characterized by small, bland, ovoid cells resembling proliferative-phase endometrial stroma and harbor rearrangement of genes often involved in transcriptional regulation with JAZF1-SUZ12 fusion being most common (2–4). In contrast, undifferentiated uterine sarcoma consists of highly pleomorphic cells bearing little resemblance to endometrial stroma, and most have complex karyotypes with numerous numerical and structural aberrations (5). High-grade endometrial stromal sarcoma in the current World Health Organization classification is limited to tumors characterized by high-grade round cell morphology sometimes associated with a low-grade fibromyxoid component and harboring t(10;17)(q22;p13) resulting in YWHAE-NUTM2 fusion (1, 6, 7). However, another recently described endometrial stromal sarcoma sharing morphologic overlap with myxoid leiomyosarcoma was found to harbor t(X;22)(p11.4;q13.2) resulting in ZC3H7B-BCOR fusion and has been proposed as another morphologic variant of high-grade endometrial stromal sarcoma due to its aggressive clinical behavior (8). Other types of morphologically high-grade endometrial stromal sarcoma lacking YWHAE, JAZF1, PHF1 and CCND1 rearrangements have also been described (9). Recognition of high-grade endometrial stromal sarcoma as a distinct entity is important due to its prognosis being intermediate between low-grade endometrial stromal sarcoma and undifferentiated uterine sarcoma and different from leiomyosarcoma (7, 10).
Immunohistochemistry is often helpful in aiding the diagnosis of high-grade endometrial stromal sarcoma harboring YWHAE-NUTM2 fusion, especially when molecular assays such as fluorescence in situ hybridization (FISH), reverse transcription or genomic polymerase chain reaction, and RNA sequencing are not readily available. CD10, estrogen receptor (ER), and progesterone receptor (PR) are usually negative in the high-grade round cell component, while positive in the low-grade fibromyxoid component, if present, similar to the immunohistochemical profile seen in typical low-grade endometrial stromal sarcoma (6). Homogeneous moderate to strong cyclin D1 staining in ≥70% of tumor cells is also characteristic of the round cell component of YWHAE-NUTM2 high-grade endometrial stromal sarcoma (11). However, when present, cyclin D1 expression is variable in the low-grade fibromyxoid component of YWHAE-NUTM2 high-grade endometrial stromal sarcoma and can be strong and diffuse in undifferentiated uterine sarcoma and rarely in uterine leiomyosarcomas (11). In our practice, we have also encountered examples of YWHAE-NUTM2 high-grade endometrial stromal sarcoma in which cyclin D1 expression was weak and/or seen in <70% of tumor cells in the round cell component, but the gene fusion was ultimately confirmed by FISH.
Recent studies have shown BCOR mRNA upregulation in most small blue round cell tumors of the soft tissues (12) and clear cell sarcomas of the kidney (13) harboring BCOR genetic abnormalities or YWHAE rearrangements. BCOR expression by immunoblotting and immunohistochemistry has also been reported in clear cell sarcoma of the kidney (14) (15). BCOR immunostaining has now emerged as a robust marker of EWSR1-negative small blue round cell tumors of the soft tissues harboring YWHAE-NUTM2, BCOR-CCNB3, and BCOR-MAML3 fusions, as well as BCOR internal tandem duplications (ITD) (16). Its utility in the evaluation of uterine sarcomas, including YWHAE-NUTM2 high-grade endometrial stromal sarcoma, has not yet been investigated. In this study, we assessed the sensitivity and specificity of BCOR expression in YWHAE-NUTM2 high-grade endometrial stromal sarcoma.
Materials and Methods
Case Selection
A total of 20 high-grade endometrial stromal sarcomas with YWHAE-NUTM2 fusion and classic histology, 8 BCOR-rearranged high-grade endometrial stromal sarcomas, and 3 high-grade endometrial stromal sarcomas with unusual morphology and unknown gene rearrangement status were collected from four institutions. Eight high-grade endometrial stromal sarcomas with FISH confirmation of YWHAE rearrangement were identified by searching the Memorial Sloan Kettering Cancer Center (New York, NY, USA) clinical database for the terms, “high-grade endometrial stromal sarcoma,” “undifferentiated endometrial sarcoma,” and “undifferentiated uterine sarcoma.” YWHAE rearrangement was identified by FISH as part of the clinical work up in 5 of the cases and a previous study in the remainder (unpublished data). An additional 7 and 6 YWHAE-NUTM2 high-grade endometrial stromal sarcomas confirmed by FISH were obtained from Vancouver General Hospital (Vancouver, Canada) and King Edward Memorial Hospital (Perth, Australia), respectively, and were previously reported (6, 17, 18). Six high-grade endometrial stromal sarcomas with BCOR rearrangement originated from Memorial Sloan Kettering Cancer Center, including 3 that were previously reported (8), and 2 from Vancouver General Hospital. All 8 BCOR-rearranged high-grade endometrial stromal sarcomas were confirmed by FISH (2 cases) or next-generation sequencing platforms using Archer FusionPlex (5 cases), a targeted RNA sequencing assay that detects gene fusions and oncogenic isoforms in selected protein-coding exons of 35 genes, or MSK-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) (1 case) (19). ZC3H7B-BCOR fusion was present in 5 tumors, while its reciprocal fusion by detected in 3 (Table 1). Three high-grade endometrial stromal sarcomas with unusual morphology and unknown rearrangement status were contributed by Memorial Sloan Kettering Cancer Center, Vancouver General Hospital, and Massachusetts General Hospital (Boston, MA, USA). A representative hematoxylin and eosin (H&E) slide of each tumor was reviewed for independent diagnostic confirmation by at least 2 gynecologic pathologists (S. C., C. H. L., C. J. R. S., E. O., L. N. H.). Four- or 5-μm unstained slides of formalin-fixed, paraffin-embedded tissue sections from each of 28 high-grade endometrial stromal sarcomas were obtained.
Table 1.
Fusion transcripts of BCOR-rearranged HGESS detected by Archer FusionPlex and correlation with BCOR expression.
| Fusion | BCOR expression (intensity, % cells) |
|---|---|
|
BCOR-ZC3H7B In frame, BCOR exon 6 and ZC3H7B exon 11 |
Negative |
|
ZC3H7B-BCOR* In frame, ZC3H7B exon 6 and BCOR exon 14 |
Positive (strong, >95%) |
|
BCOR-ZC3H7B In frame, BCOR exon 7 and ZC3H7B exon 11 |
Positive (strong, >95%) |
|
ZC3H7B-BCOR In frame, ZC3H7B exon 10 and BCOR exon 7 |
Positive (strong, >95%) |
|
ZC3H7B-BCOR In frame, ZCH37B exon 10 and BCOR exon 7 |
Positive (weak, >95%) |
The reciprocal fusion was also detected.
To investigate the specificity of BCOR expression, staining in additional uterine mesenchymal tumors was also assessed. This cohort included triplicate 0.6 mm diameter core tissue microarrays of 38 previously reported uterine leiomyosarcomas and19 previously reported uterine leiomyomas from Memorial Sloan Kettering Cancer Center (20–22); duplicate and triplicate 0.6 mm diameter core tissue microarrays and whole tissue sections of 66 previously published LGESS from Massachusetts General Hospital, Memorial Sloan Kettering Cancer Center, and King Edward Memorial Hospital, respectively, respectively (2, 17, 20–22); and duplicate and triplicate 0.6 mm diameter core tissue microarrays of 21 endometrial stromal nodules from Massachusetts General Hospital and Memorial Sloan Kettering Cancer Center (2, 20–22). A subset of low-grade endometrial stromal sarcomas and endometrial stromal nodules were obtained from the consultation files of one of the authors (E.O.) and the late Dr. Robert E. Scully, while all other tumors were obtained from the surgical pathology files of the participating institutions. Gene rearrangement status by FISH was known and previously reported for 34 low-grade endometrial stromal sarcomas and 8 endometrial stromal nodules, including 24, 3, and 2 tumors with JAZF1-SUZ12, JAZF1-PHF1, and EPC-PHF1 fusions, respectively, and 9 and 4 tumors with JAZF1 and PHF1 rearrangement with no known fusion partner, respectively (2, 17). All low-grade endometrial stromal sarcomas and endometrial stromal nodules were previously screened for YWHAE rearrangement by FISH (7, 17). Four- to 5-μm unstained slides of the tissue microarrays and whole tumor tissue sections of 17 low-grade endometrial stromal sarcomas were obtained.
Immunohistochemistry
Immunohistochemical staining for BCOR was performed using a commercially available monoclonal antibody, clone C-10 (sc-514576; Santa Cruz, Dallas, TX) at 1:150 dilution (1.7 μg/mL). Staining was performed on the Leica Bond-3 autostaining system (Leica, Buffalo Grove, IL), using heat-based antigen retrieval, a high pH buffer solution (Leica, ER2, 30 min.), 30 min. primary incubation time, and a polymer detection system (Refine, Leica) as previously reported (16). A carrier-based multitissue block comprising of normal skin, colon, lung, testis, spleen, placenta, pancreas, liver, and kidney served as negative controls (23). Results were evaluated by a gynecologic pathologist (S.C.). Intensity (strong, moderate, weak, and negative) and estimated percentage of positive tumor cells (nuclear staining only) were evaluated. BCOR immunohistochemistry was repeated on 5-μm whole tumor tissue sections for cases that showed any nuclear staining on the tissue microarray.
Fluorescence in situ hybridization
Break-apart FISH for YWHAE, BCOR, and BCORL1 rearrangement was performed on 5-μm whole tissue sections of tumors with no known gene rearrangement status demonstrating any nuclear BCOR expression by immunohistochemistry as previously described (8, 12). Custom probes were made by bacterial artificial chromosomes (BAC) clones flanking the YWHAE (RP11-105D11, RP11-1142D6, RP11-170J13, RP11-806J5), BCOR (RP11-21D3, RP11-1105N2, RP11-37K20, RP11-973F20), and BCORL1 (RP11-671B10, RP11-246J10, RP11-460L15, RP11-383B16) genes and obtained from BAC/PAC Resources (Children’s Hospital Oakland Research Institute, Oakland, CA). BAC clones were labeled with nick translation and validated on normal metaphase chromosomes. Briefly, 5-μm whole tissue sections from FFPE tissue blocks were mounted on charged slides. Slides were deparaffinized, pretreated, and hybridized with denatured probes overnight, followed by post-hybridization washes and counterstaining with DAPI. Slides were examined on a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) using Isis 5 software (Metasystems). Two hundred tumor nuclei were counted, and cases with >20% of nuclei with break-apart signals were considered positive.
PCR for BCOR exon 16 internal tandem duplication (ITD)
FFPE tumor and adjacent normal myometrial tissue were macrodissected in tumors with positive BCOR expression and no evidence of YWHAE, BCOR, and BCORL1 rearrangement by FISH. Briefly, tumor and normal DNA were extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen). Primer sequences targeted exon 16 of BCOR (Fwd-1: 5′-GTCCTCCCGCATATTTCGC-3′ or Fwd-2: 5′-GACCTGGAAGCCTTCAACCC-3′ and Rev: 5′-CAAGCTGGACCCACCATGTAC-3′). PCR was performed using Advantage 2 PCR kit at an annealing temperature of 65.2°C (Fwd-1) or 66.5°C (Fwd-2) for 38 cycles, and PCR products were analyzed by agarose gel electrophoresis. Amplicons larger than wild type were subjected to Sanger sequencing to confirm the presence of internal tandem duplication.
RESULTS
Strong and diffuse BCOR expression in the round cell component of all YWHAE-NUTM2 high-grade endometrial stromal sarcomas
Strong nuclear BCOR staining in >95% of tumor cells was seen in the round cell component of all 20 (100%) YWHAE-NUTM2 high-grade endometrial stromal sarcomas (Figure 1). Six of these tumors also showed a low-grade fibrous or fibromyxoid component in which BCOR staining was variable in intensity and extent, ranging from weak (2 cases) to strong (1 case) and expression in <5% to 75% (mean, 30%) of tumor cells (Figure 1).
Figure 1.
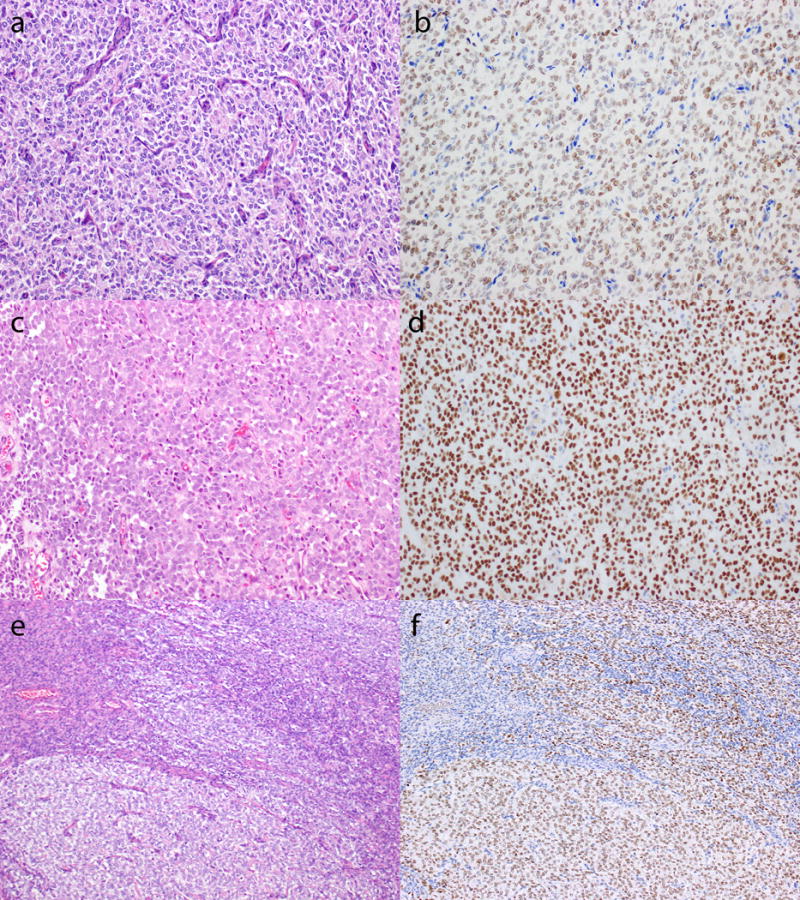
Distinct BCOR expression in the low-grade spindled cell and round cell components of YWHAE-NUTM2 high-grade endometrial stromal sarcoma. Representative H&E (a, c) and immunohistochemical stains (b, d) demonstrating diffuse nuclear BCOR staining that may be moderate (b) or strong (d) in intensity. The low-grade component (e, top left) shows focal, weak BCOR immunostaining (f, top left) compared to strong and diffuse BCOR expression (f, bottom right) in the round cell component (e, bottom right).
BCOR expression identifies high-grade endometrial stromal sarcomas with unusual morphology, including one harboring BCOR internal tandem duplication
BCOR immunohistochemistry was helpful in the assessment of 3 high-grade endometrial stromal sarcomas demonstrating unusual morphologic features. One tumor was a pulmonary metastasis from a patient with known YWHAE-NUTM2 fusion previously confirmed in the primary uterine tumor. While the primary tumor showed typical morphologic features of YWHAE-NUTM2 high-grade endometrial stromal sarcoma, the pulmonary metastasis was less cellular and mitotically active and consisted of spindled to stellate cells with slightly enlarged ovoid nuclei with prominent nucleoli, embedded in abundant myxoid stroma (Figure 2a and 2b). Strong and diffuse nuclear BCOR expression was seen throughout the metastatic tumor (Figure 2c).
Figure 2.
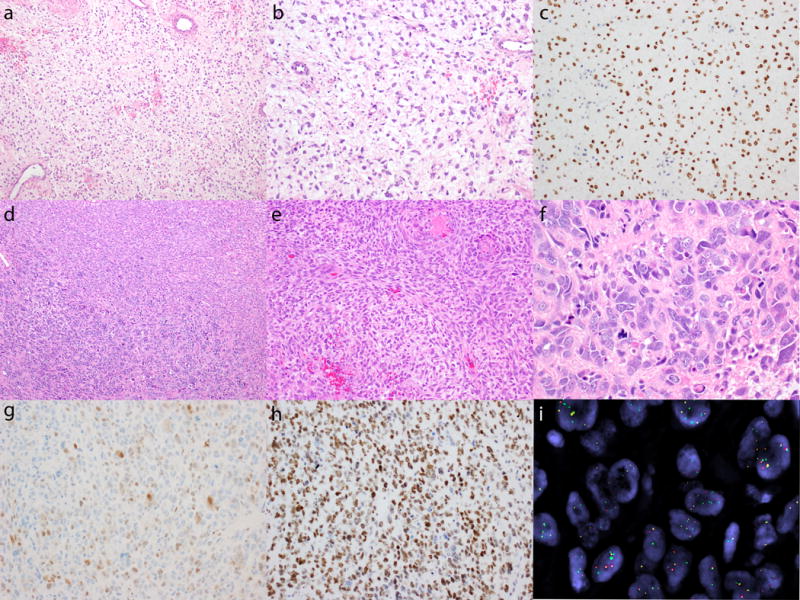
BCOR expression in 2 cases of YWHAE-NUTM2 high-grade endometrial stromal sarcoma with unusual histology. A pulmonary metastasis composed of intermediate-grade fibromyxoid spindled cells with low mitotic index (a, b) exhibits strong and diffuse BCOR immunoexpression (c). Pleomorphic undifferentiated sarcoma (d, bottom left, f) composed of cells with marked nuclear pleomorphism, brisk mitotic activity, and atypical mitoses in a background of hyalinized stroma adjacent to high-grade endometrial stromal sarcoma (d, top right, e) demonstrating spindled cells with ovoid nuclei and prominent nucleoli vaguely whorling around delicate arterioles. The pleomorphic sarcoma component shows only focal weak to moderate cyclin D1 staining (g), but diffuse and strong BCOR expression (h). Separation of green 5′ and red 3′ signals and single green 5′ signals (i) confirm YWHAE rearrangement by break-apart fluorescence in situ hybridization.
The second high-grade endometrial stromal sarcoma was a primary uterine tumor that predominately consisted of highly atypical cells with marked nuclear pleomorphism and prominent nucleoli arranged in sheets and nests surrounded by hyalinized to myxoid stroma (pleomorphic undifferentiated uterine sarcoma) (Figure 2d and 2f). The mitotic index was 56/10 high power fields, including numerous atypical mitoses. Within the dominant pleomorphic component, there was a distinct focus of smaller, more uniform spindled cells with ovoid nuclei associated with delicate vasculature and a mitotic index of 10/10 high power fields (Figure 2d and 2e). While the pleomorphic sarcoma component demonstrated only weak and focal cyclin D1 positivity (Figure 2g), BCOR expression was diffuse and strong (Figure 2h), prompting FISH studies which confirmed the presence of a YWHAE rearrangement (Figure 2i).
The third tumor was a uterine sarcoma in a 25 year old patient that demonstrated some features similar to and other features differing from YWHAE-NUTM2 high-grade endometrial stromal sarcoma. The tumor had a tongue-like pattern of myometrial invasion typical of stromal sarcomas (Figure 3a) and had a low-grade fibromyxoid component with small spindled cells (Figure 3b) and a high-grade round cell component with larger, round nuclei (Figure 3c). However, focal myxoid stroma within the round cell component (Figure 3d) was noted, and some spindled areas demonstrated larger cells with striking nuclear atypia (Figure 3e). The immunophenotype showed strong and diffuse cyclin D1 staining, only focal CD10 positivity, and absent ER and PR expression. BCOR expression was strong and diffuse throughout the tumor, including the round and spindled cell components (Figure 3f). This pattern of BCOR expression prompted FISH analysis for BCOR, BCORL1, and YWHAE rearrangement, but none were detected. However, an in-frame BCOR internal tandem duplication was identified by genomic PCR and targeted DNA sequencing in which the duplicated sequence from BCOR exon 16 spanned 86 bp with a 4-bp insertion between duplicated sequences (Figure 4). BCOR internal tandem duplication was absent in the adjacent normal myometrium.
Figure 3.
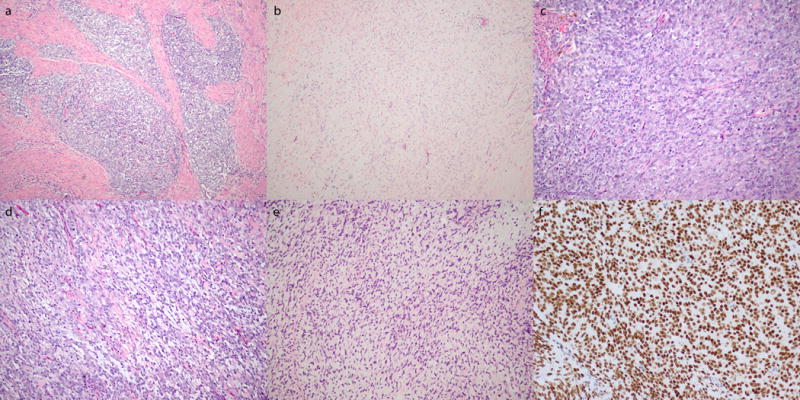
Morphologic features and BCOR staining pattern of high-grade endometrial stromal sarcoma harboring BCOR internal tandem duplication. a) Tongue-like myometrial invasion. Low-grade fibromyxoid (b) and high-grade round cell (c) components similar to features seen in YWHAE-NUTM2 high-grade endometrial stromal sarcoma. d) Foci of myxoid stroma within the round cell component. e) More extensive nuclear atypia within the fibromyxoid component. f) Diffuse and strong BCOR expression.
Figure 4.

BCOR exon 16 internal tandem duplication in high-grade endometrial stromal sarcoma. a) Schematic demonstrating wild type BCOR (top) and BCOR internal tandem duplication (bottom) sequences. b) Duplicated region (highlighted in beige) overlapping with the BCOR wild type sequence with a 4-bp insertion (highlighted in blue) in between duplicated regions.
A subset of BCOR-rearranged high-grade endometrial stromal sarcomas demonstrates BCOR immunoreactivity
We evaluated BCOR expression in whole tissue sections of 5 ZC3H7B-BCOR and 3 BCOR-ZC3H7B high-grade endometrial stromal sarcomas, and found nuclear staining in >95% of tumor cells that was strong in 3 cases (2 ZC3H7B-BCOR and 1 BCOR-ZC3H7B) (Figure 5a and 5b) and weak in another (ZC3H7B-BCOR) (Figure 5c and 5d) (Table 1). BCOR staining was absent in the remaining 4 tumors. Among the 3 ZC3H7B-BCOR high-grade endometrial stromal sarcomas with fusion transcriptome data, the reciprocal fusion was detected in only one tumor that showed strong and diffuse BCOR expression.
Figure 5.
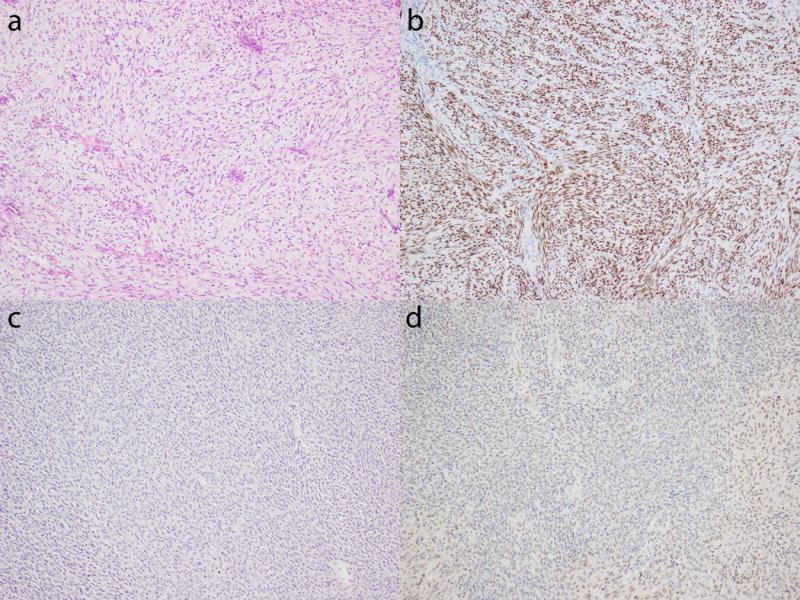
BCOR expression in ZC3H7B-BCOR high-grade endometrial stromal sarcoma. a and b) Myxoid spindled cell sarcoma demonstrating strong and diffuse BCOR staining. c and d) Spindled cell sarcoma with less obvious myxoid features showing only weak BCOR expression.
BCOR expression is not seen in most other uterine mesenchymal tumors
BCOR immunohistochemical staining was evaluated in 31 of 38 (81.6%) leiomyosarcomas, 18 of 19 (95%) leiomyomas, all 66 (100%) low-grade endometrial stromal sarcomas, and 18 of 21 (86%) endometrial stromal nodules. Staining was not evaluable in the remaining tumors in which tissue cores were no longer present on the tissue microarray slides (Figure 6). Weak BCOR staining in <5% of tumor cells was seen in 4 (6%) low-grade endometrial stromal sarcomas and 1 of 18 (6%) endometrial stromal nodules. Gene rearrangement status was known in 2 of the low-grade endometrial stromal sarcomas exhibiting focal, weak BCOR expression with JAZF1-SUZ12 fusion in one and JAZF1 rearrangement with no known partner in the other. Weak to moderate BCOR staining was seen in <5% to 40% (mean, 22%) of tumor cells in 6 of 31 (19%) leiomyosarcomas. Among 4 leiomyosarcomas for which whole tissue sections were available, weak to moderate BCOR expression was seen in <5% of cells in 3 tumors and 50% of cells in one tumor. Genomic PCR was performed on the leiomyosarcomas demonstrating more extensive BCOR staining, and BCOR exon 16 internal tandem duplication was not detected. BCOR staining was not seen in any of the leiomyomas.
Figure 6.
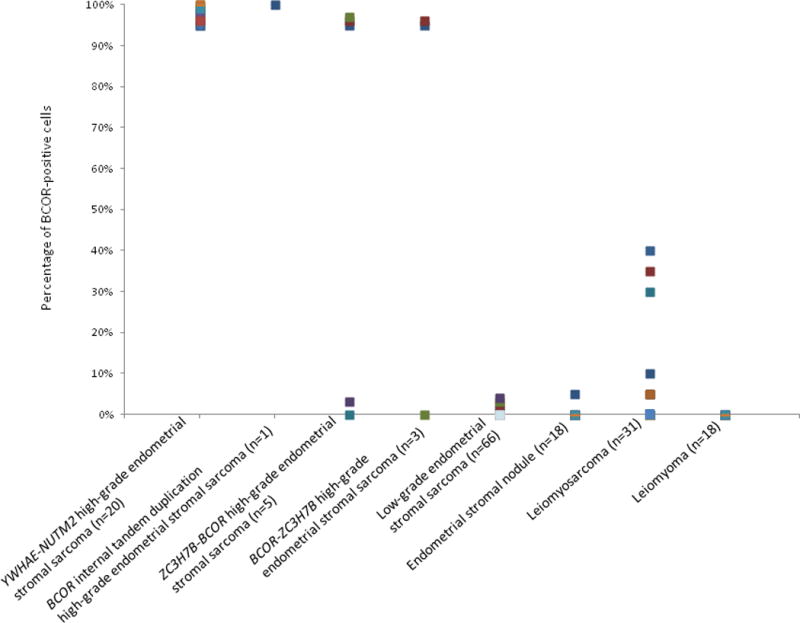
Percentage of BCOR-positive tumor cells in high-grade endometrial stromal sarcoma harboring YWHAE-NUTM2, ZC3H7B-BCOR, and BCOR-ZC3H7B fusions and BCOR internal tandem duplication, low-grade endometrial stromal sarcoma, endometrial stromal nodule, leiomyosarcoma, and leiomyoma.
DISCUSSION
Identification of YWHAE-NUTM2 high-grade endometrial stromal sarcomas is important due to its different prognosis and management from low-grade endometrial stromal sarcomas and undifferentiated uterine sarcomas, and novel commercially available biomarkers such as BCOR may help in distinguishing high-grade endometrial stromal sarcomas from these and other types of uterine sarcomas potentially in the differential diagnosis. Herein, we showed that BCOR nuclear immunoexpression was strong and diffuse in the round cell component of all 20 YWHAE-NUTM2 high-grade endometrial stromal sarcomas of classic histology studied. A similar staining pattern was seen in 3 high-grade endometrial stromal sarcomas of unusual morphology which prompted FISH confirmation of YWHAE rearrangement in 2 and identification of a novel somatic BCOR in-frame internal tandem duplication by genomic PCR and targeted DNA sequencing of BCOR exon 16 in the third tumor. Diffuse BCOR expression was also seen in 50% (4 of 8) of BCOR-rearranged high-grade endometrial stromal sarcomas, including tumors harboring ZC3H7B-BCOR and its reciprocal fusion. Staining was largely negative in other uterine mesenchymal tumors, with only limited, weak to moderate reactivity seen in 6% (4 of 66) of low-grade endometrial stromal sarcomas, 6% (1 of 18) of endometrial stromal nodules and 19% (6 of 31) of leiomyosarcomas.
Since the recent histologic description of YWHAE-NUTM2 high-grade endometrial stromal sarcomas (6), cyclin D1 along with CD10, ER, and PR have so far served as the most helpful immunohistochemical markers in the diagnosis of these tumors (11). Strong and diffuse cyclin D1 staining coupled with absent CD10, ER, and PR expression is highly characteristic of the round cell component of YWHAE-NUTM2 high-grade endometrial stromal sarcomas, while variable cyclin D1 and positive CD10, ER, and PR staining is seen in the low-grade component if present (11). Based on our study, BCOR appears to be at least equally robust in identifying the round cell component of these tumors, while the staining varies in the low grade component, similar to what has been reported with cyclin D1 (11). However, our findings also suggest that the utility of BCOR immunohistochemistry may surpass that of cyclin D1 particularly in YWHAE-NUTM2 tumors demonstrating unusual morphology or in the minority of classic YWHAE-NUTM2 high-grade endometrial stromal sarcomas that fail to demonstrate diffuse cyclin D1 expression. While we did not perform a direct comparison of cyclin D1 and BCOR in our study, at least one of our YWHAE-NUTM2 high-grade endometrial stromal sarcomas with classic histology had only focal, weak cyclin D1 staining, while BCOR was strongly positive. BCOR expression was also strong and diffuse in a metastatic paucicellular YWHAE-NUTM2 high-grade endometrial stromal sarcomas with a myxoid background as well as a pleomorphic undifferentiated sarcoma showing only focal cyclin D1 expression of variable intensity, but demonstrating YWHAE rearrangement by FISH. The latter tumor is particularly noteworthy given that de-differentiation or transformation into undifferentiated sarcoma may occur in YWHAE-NUTM2 high-grade endometrial stromal sarcomas similar to what has rarely been reported in low-grade endometrial stromal sarcomas (24, 25). Therefore, BCOR immunohistochemistry may be informative regarding the histogenesis of some pleomorphic undifferentiated uterine sarcomas.
In addition to identifying the round cell component of YWHAE-NUTM2 high-grade endometrial stromal sarcomas, BCOR expression led to the discovery of a novel in-frame BCOR internal tandem duplication in one of our high-grade endometrial stromal sarcomas sharing both morphologic and immunophenotypic features with YWHAE-NUTM2 high-grade endometrial stromal sarcomas, but lacking YWHAE, BCOR, and BCORL1 rearrangement. Highly recurrent BCOR internal tandem duplications have recently been discovered in clear cell sarcoma of the kidney and small blue round cell tumors of the soft tissues in infants (12, 14, 15, 26, 27). The discovery of BCOR internal tandem duplication in one of our high-grade endometrial stromal sarcomas now expands the spectrum of cancers in which BCOR internal tandem duplication appears to be a genetic driver of tumorigenesis. While BCOR internal tandem duplication is present in the vast majority of clear cell sarcomas of the kidney (14, 15, 27), its prevalence among uterine sarcomas is currently unknown. However, with the application of BCOR immunohistochemistry in the routine evaluation of uterine sarcomas, particularly those that are morphologically reminiscent of YWHAE-NUTM2 high-grade endometrial stromal sarcomas or undifferentiated uterine sarcomas of uniform type lacking YWHAE or other gene rearrangements more common among endometrial stromal sarcomas, additional examples of high-grade endometrial stromal sarcomas with this unusual genetic abnormality may be identified. BCOR internal tandem duplication and YWHAE-NUTM2 fusion appear mutually exclusive in clear cell sarcoma of the kidney (26, 27). Future studies confirming this finding in high-grade endometrial stromal sarcomas should be undertaken.
Our findings demonstrate that BCOR expression is present in 50% of BCOR-rearranged high-grade endometrial stromal sarcomas and ZC3H7B-BCOR fusion was recently described in an uncommon group of endometrial stromal sarcomas (7, 28). In a previous study, we described the unique histologic features of this tumor which significantly overlap with myxoid leiomyosarcoma. While our cohort was small, the clinical data suggested that ZC3H7B-BCOR endometrial stromal sarcoma was clinically aggressive compared to low-grade endometrial stromal sarcoma which typically follows an indolent course (8). Compared to the 3 ZC3H7B-BCOR high-grade endometrial stromal sarcomas that were previously published (8), the 5 additional BCOR-rearranged high-grade endometrial stromal sarcomas in our current study displayed similar morphologic and immunohistochemical features, including extensive myxoid change, focal fascicular architecture, brisk mitotic activity of at least 10 mitoses/10 high power fields, diffuse CD10 expression, variable ER and PR staining, and either negative or only focal desmin or SMA positivity. While BCOR expression was absent in 2 previously reported ZC3H7B-BCOR high-grade endometrial stromal sarcomas (16), we have shown here for the first time that diffuse BCOR staining may be seen in other high-grade endometrial stromal sarcomas harboring ZC3H7B-BCOR or its reciprocal fusion. The BCOR antibody clone C-10 detects the epitope encoded by exons 1, 2, and 3 and part of exon 4 of BCOR. Based on our findings, BCOR immunoexpression does not appear to correlate with BCOR breakpoints in the fusion transcripts. Among the 3 ZC3H7B-BCOR high-grade endometrial stromal sarcomas with fusion transcriptome data, the reciprocal fusion was observed only in one case which demonstrated strong BCOR expression. Given the identification of ZC3H7B-BCOR and BCOR-ZC3H7B fusions among our cohort of high-grade endometrial stromal sarcomas and lack of correlation between BCOR expression pattern and either gene fusion, it remains uncertain whether ZC3H7B or BCOR constitutes the genetic driver of these tumors.
Our results also suggest that BCOR expression is a reliable marker that can distinguish high-grade endometrial stromal sarcomas with YWHAE-NUTM2, ZC3H7B-BCOR, BCOR-ZC3H7B, and BCOR internal tandem duplication from other uterine mesenchymal tumors. In contrast to strong and diffuse BCOR staining in high-grade endometrial stromal sarcomas harboring YWHAE-NUTM2 fusion or BCOR internal tandem duplication and a subset of BCOR-rearranged high-grade endometrial stromal sarcomas, BCOR immunoreactivity of only weak to moderate intensity was present in <5 to 30% of tumor cells among <6% of low-grade endometrial stromal sarcomas and endometrial stromal nodules and approximately 20% of leiomyosarcomas. The presence of focal BCOR staining in a subset of leiomyosarcomas may be a potential caveat since this tumor type is in the differential diagnosis of high-grade endometrial stromal sarcomas, particularly those harboring BCOR rearrangements. BCOR expression was not found in the remaining tumors or any of the leiomyomas tested.
The mechanism by which BCOR expression is upregulated in tumors harboring BCOR genetic aberrations and YWHAE-NUTM2 gene fusions is unclear. BCOR is encoded by the BCL-6 corepressor (BCOR) gene located on Xp11.4 and interacts with PCGF1 within a variant polycomb repressive complex (PRC1) that suppresses gene expression by histone modification (29, 30). Ubiquitous expression of BCOR mRNA is seen in a variety of normal human tissues (29), and among tumors, high expression of BCOR transcripts and protein have been found in most round cell sarcomas of the bone and soft tissues and clear cell sarcomas of the kidney with BCOR genetic aberrations or YWHAE-NUTM2 fusion (12, 14, 15). In tumors with BCOR internal tandem duplication, the duplicated BCOR region resides within a PUFD domain that facilitates binding with PCGF1, another member of the variant PRC1, and may disrupt the structure and/or function of the PRC1 in epigenetic modification (14, 15). Further studies are needed to investigate the impact of BCOR internal tandem duplication, BCOR and YWHAE rearrangements on PRC1 function and the mechanism of high-grade endometrial stromal sarcoma oncogenesis.
In summary, BCOR expression is highly sensitive in identifying high-grade endometrial stromal sarcomas harboring YWHAE-NUTM2 fusion and BCOR internal tandem duplication as well as a subset of BCOR-rearranged high-grade endometrial stromal sarcomas. Diffuse and strong expression should prompt FISH, RT-PCR, or RNA sequencing confirmation of YWHAE and BCOR rearrangement or targeted DNA sequencing for BCOR internal tandem duplication if those rearrangements are not detected. Identification of BCOR internal tandem duplication and similar BCOR immunophenotype in high-grade endometrial stromal sarcomas adds to the growing body of histologic, immunophenotypic, and genetic evidence uniting these tumors with clear cell sarcoma of the kidney and soft tissue round cell sarcomas.
Footnotes
DISLOSURE/DUALITY OF INTEREST
The authors have no conflicts of interest to disclose.
References
- 1.Oliva E, Carcangiu ML, Carinelli SG, et al. Mesenchymal tumors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. 4th. Lyon, France: International Agency for Research on Cancer; 2014. pp. 135–47. [Google Scholar]
- 2.Chiang S, Ali R, Melnyk N, et al. Frequency of known gene rearrangements in endometrial stromal tumors. Am J Surg Pathol. 2011;35:1364–72. doi: 10.1097/PAS.0b013e3182262743. [DOI] [PubMed] [Google Scholar]
- 3.Chiang S, Oliva E. Recent developments in uterine mesenchymal neoplasms. Histopathology. 2013;62:124–37. doi: 10.1111/his.12048. [DOI] [PubMed] [Google Scholar]
- 4.Chiang S, Oliva E. Cytogenetic and molecular aberrations in endometrial stromal tumors. Hum Pathol. 2011;42:609–17. doi: 10.1016/j.humpath.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Halbwedl I, Ullmann R, Kremser ML, et al. Chromosomal alterations in low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma as detected by comparative genomic hybridization. Gynecol Oncol. 2005;97:582–7. doi: 10.1016/j.ygyno.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–53. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Ou WB, Marino-Enriquez A, et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci U S A. 2012;109:929–34. doi: 10.1073/pnas.1115528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang LN, Aneja A, Conlon N, et al. Novel high-grade endometrial stromal sarcoma: a morphologic mimicker of myxoid leiomyosarcoma. Am J Surg Pathol. 2017;41:12–24. doi: 10.1097/PAS.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciallis AP, Bedroske PP, Schoolmeester JK, et al. High-grade endometrial stromal sarcomas: a clinicopathologic study of a group of tumors with heterogenous morphologic and genetic features. Am J Surg Pathol. 2014;38:1161–72. doi: 10.1097/PAS.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 10.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820–30. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Ali RH, Rouzbahman M, et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with YWHAE-FAM22 rearrangement. Am J Surg Pathol. 2012;36:1562–70. doi: 10.1097/PAS.0b013e31825fa931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao YC, Sung YS, Zhang L, et al. Recurrent BCOR internal tandem duplication and ywhae-nutm2b fusions in soft tissue undifferentiated round cell sarcoma of infancy: overlapping genetic features with clear cell sarcoma of kidney. Am J Surg Pathol. 2016;40:1009–20. doi: 10.1097/PAS.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astolfi A, Melchionda F, Perotti D, et al. Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget. 2015;6:40934–9. doi: 10.18632/oncotarget.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno-Yokohata H, Okita H, Nakasato K, et al. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet. 2015;47:861–3. doi: 10.1038/ng.3338. [DOI] [PubMed] [Google Scholar]
- 15.Roy A, Kumar V, Zorman B, et al. Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun. 2015;6:8891. doi: 10.1038/ncomms9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao YC, Sung YS, Zhang L, et al. BCOR overexpression is a highly sensitive marker in round cell sarcomas with BCOR genetic abnormalities. Am J Surg Pathol. 2016;40:1670–8. doi: 10.1097/PAS.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart CJ, Leung YC, Murch A, Peverall J. Evaluation of fluorescence in-situ hybridization in monomorphic endometrial stromal neoplasms and their histological mimics: a review of 49 cases. Histopathology. 2014;65:473–82. doi: 10.1111/his.12406. [DOI] [PubMed] [Google Scholar]
- 18.Isphording A, Ali RH, Irving J, et al. YWHAE-FAM22 endometrial stromal sarcoma: diagnosis by reverse transcription-polymerase chain reaction in formalin-fixed, paraffin-embedded tumor. Hum Pathol. 2013;44:837–43. doi: 10.1016/j.humpath.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes C, Karamurzin Y, Frizzell N, et al. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod Pathol. 2014;27:1020–7. doi: 10.1038/modpathol.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson SE, Nonaka D, Chuai S, et al. p53, epidermal growth factor, and platelet-derived growth factor in uterine leiomyosarcoma and leiomyomas. Int J Gynecol Cancer. 2006;16:849–53. doi: 10.1111/j.1525-1438.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 22.Leitao MM, Soslow RA, Nonaka D, et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer. 2004;101:1455–62. doi: 10.1002/cncr.20521. [DOI] [PubMed] [Google Scholar]
- 23.Frosina D, Jungbluth AA. A novel technique for the generation of multitissue blocks using a carrier. Appl Immunohistochem Mol Morphol. 2016;24:668–72. doi: 10.1097/PAI.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurihara S, Oda Y, Ohishi Y, et al. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol. 2008;32:1228–38. doi: 10.1097/PAS.0b013e31816a3b42. [DOI] [PubMed] [Google Scholar]
- 25.Koontz JI, Soreng AL, Nucci M, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci U S A. 2001;98:6348–53. doi: 10.1073/pnas.101132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson J, Valind A, Gisselsson D. BCOR internal tandem duplication and YWHAE-NUTM2B/E fusion are mutually exclusive events in clear cell sarcoma of the kidney. Genes Chromosomes Cancer. 2016;55:120–3. doi: 10.1002/gcc.22316. [DOI] [PubMed] [Google Scholar]
- 27.Kenny C, Bausenwein S, Lazaro A, et al. Mutually exclusive BCOR internal tandem duplications and YWHAE-NUTM2 fusions in clear cell sarcoma of kidney: not the full story. J Pathol. 2016;238:617–20. doi: 10.1002/path.4693. [DOI] [PubMed] [Google Scholar]
- 28.Micci F, Gorunova L, Agostini A, et al. Cytogenetic and molecular profile of endometrial stromal sarcoma. Genes Chromosomes Cancer. 2016;55:834–46. doi: 10.1002/gcc.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huynh KD, Fischle W, Verdin E, Bardwell VJ. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14:1810–23. [PMC free article] [PubMed] [Google Scholar]
- 30.Gearhart MD, Corcoran CM, Wamstad JA, Bardwell VJ. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol Cell Biol. 2006;26:6880–9. doi: 10.1128/MCB.00630-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


