Abstract
The pattern of peltate glandular trichome initiation and ontogeny on expanding peppermint (Mentha × piperita) leaves was defined by surveying the populations of peltate glands in each of seven developmental stages within sampling areas of leaf apical, mid-, and basal zones for both abaxial and adaxial surfaces. It was shown that new peltate glands continue to form until leaf expansion ceases and that regions of active gland initiation are unevenly distributed. The distribution of gland initiation reflects the basipetal pattern of leaf maturation, with relatively immature regions at the leaf base continuing to produce oil glands long after gland production has stopped at the leaf apex. The proportion of glands in the secretory stage as a function of leaf development and the direct observations of living glands over a period of 33 h indicate that a period of only 20 to 30 h of secretory activity is required for filling of the gland storage compartment with essential oil. These findings are discussed in relation to earlier literature describing age-related changes in glandular essential oil content.
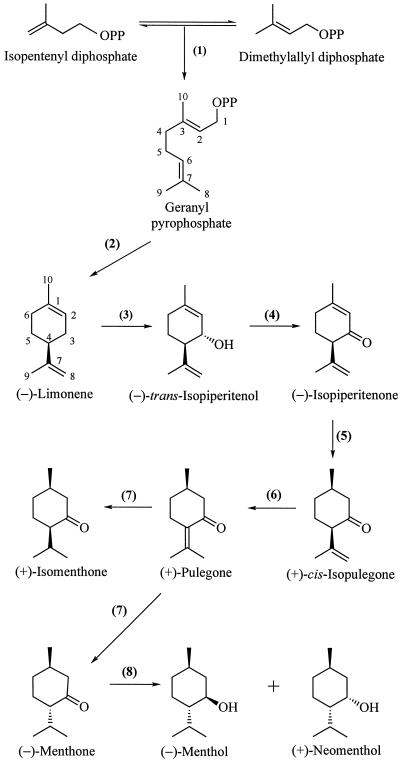
Monoterpenes, the C10 members of the terpenoid (isoprenoid) family of natural products, have been implicated as constitutive defenses against a variety of herbivores and pathogens (Langenheim, 1994). Although widely distributed among many plant families (Charlwood and Charlwood, 1991), the monoterpenes are best known as the principal constituents of the essential oils and resins of the common herbs and spices, to which they often impart the characteristic odors and flavors (Guenther, 1972). The most important of the commercial essential oil-producing species is peppermint (Mentha × piperita), a perennial herb of the family Lamiaceae that produces high levels of p-menthane monoterpenes, including menthone and menthol (Lawrence, 1981). During leaf development, the total content of monoterpenes increases with age and the composition of the monoterpenes undergoes significant change (Burbott and Loomis, 1969; Croteau and Martinkus, 1979; Maffei et al., 1989; Brun et al., 1991). The pathway of monoterpene biosynthesis in peppermint (Fig. 1) has been well established (Croteau and Gershenzon, 1994; Wise and Croteau, 1999), and recent evidence has indicated that the principal determinant of monoterpene production in this species is the rate of biosynthesis (Gershenzon et al., 2000) as defined by the developmentally regulated levels of the responsible biosynthetic enzymes and their corresponding messages (McConkey et al., 2000). This evidence, which demonstrates the role of production rate and eliminates significant involvement of monoterpene catabolism or volatilization in influencing yield (Gershenzon et al., 2000), has focused interest on the cell differentiation processes associated with monoterpene accumulation that provide structural context for biochemical and regulatory studies.
Figure 1.
The principal pathway for monoterpene biosynthesis in peppermint. The responsible enzymes are: geranyl diphosphate synthase (1), (4S)-(−)-limonene synthase (2), cytochrome P450 (−)-limonene-3-hydroxylase (3), (−)-trans-isopiperitenol dehydrogenase (4), (−)-isopiperitenone reductase (5), (+)-cis-isopulegone isomerase (6), (+)-pulegone reductase (7), and (−)-menthone reductase (8).
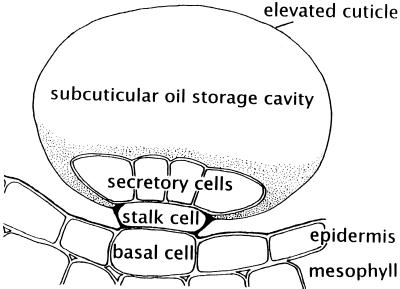
The site of monoterpene biosynthesis in peppermint has been specifically localized to the secretory cells of the glandular trichomes (Gershenzon et al., 1989; McCaskill et al., 1992) located upon the aerial surfaces (Amelunxen, 1965; Fahn, 1979). Three types of trichomes occur on peppermint leaves: non-glandular, multicellular, simple hairs; small, capitate glandular trichomes, with a single secretory head cell; and peltate glandular trichomes, with an eight-celled apical disc of secretory cells (Fahn, 1979; Maffei et al., 1989; Brun et al., 1991). The peltate glands are surmounted by a large sub-cuticular storage space that is formed by separation of the cuticle from the apical walls of the disc cells and that fills with the largely monoterpenoid-containing essential oil (Fig. 2). In general, capitate glandular trichomes of the Lamiaceae have only limited storage capacity, and there is some evidence to suggest that their secretion consists mainly of a complex mixture of carbohydrates, lipids, and proteins (Werker et al., 1985; Ascensão and Pais, 1998). However, the capitate glandular trichomes of peppermint have been shown to contain small amounts of the monoterpenes characteristic of the essential oil (Amelunxen et al., 1969). Whatever the exact nature of the capitate gland secretory products, it is clear that the bulk of the monoterpenes of peppermint essential oil is produced by and stored in the peltate glandular trichomes.
Figure 2.
Schematic diagram of a peppermint leaf peltate glandular trichome illustrating the placement of these epidermal structures and the relationship of the disc of secretory cells to the stalk and basal cells and to the sub-cuticular storage space.
The rate of initiation and the developmental progress of peltate glands in the Lamiaceae vary considerably between different regions of a leaf and depend to a large extent on the maturity of tissues within the different regions (Werker et al., 1993). In addition, the age of the glands strongly influences the composition of the oil that they contain (Maffei et al., 1986, 1989; Voirin and Bayet, 1996; Rohloff, 1999). To better understand how the pattern of gland initiation and development affects the biosynthetic capacity of mint leaves, a survey was conducted to record the numbers and developmental stages of glands in different regions of expanding peppermint leaves. Although previous studies have provided estimates of overall peltate gland densities for peppermint leaves (Maffei et al., 1989; Colson et al., 1993) and for leaves of other members of the Lamiaceae (Maffei et al., 1986; Werker et al., 1993; Bosabalidis and Skoula, 1998), no data are available for populations of the different gland development stages as a function of leaf growth. Here we provide estimates of population numbers for glands at seven ontogenic stages during leaf development and use these data to deduce the rate of essential oil filling of the gland sub-cuticular storage space. The estimate, based on population considerations, was confirmed to be very rapid by direct observations of gland filling. By comparing these glandular developmental data with data on biosynthetic enzyme activities and corresponding immunoblot and mRNA-blot analyses (McConkey et al., 2000) and data relating to total monoterpene accumulation (Gershenzon et al., 2000), it is possible to correlate gland developmental stages with rates of monoterpene production and target those developmental stages of greatest significance for ultrastructural investigation (Turner et al., 2000).
RESULTS
Estimate of the Plastochron
The rate of leaf initiation was determined for 4- to 7-week-old non-flowering shoots, a period of vigorous vegetative growth. Leaf initiation remained constant over this period, with an average production of approximately three new leaf pairs per week, or one leaf pair every 2.3 d. There was little variation in the plastochron from week to week, ranging from 2.2 to 2.4 d per leaf. Estimates of leaf age, based on leaf position and a 2.3-d plastochron, suggest that, on average, it takes approximately 16 d for an expanding leaf blade to reach 15 mm (about one-half of the mature length) and 22 d to complete leaf expansion with a leaf blade length of 35 mm.
Gland Initiation and Development
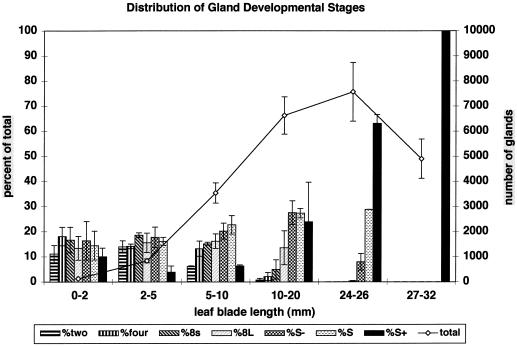
Total gland numbers increased from about 100 for small, 2-mm-long leaf primordia to about 7,500 for 25-mm-long peppermint leaves (Fig. 3). Since leaf expansion results from vacuolar expansion of cells in maturing tissues where gland initiation has ceased, average gland surface density decreases from a maximum of about 100 glands/mm2 for 2- to 10-mm-long leaves to about 6.5 glands/mm2 for fully expanded, mature leaves.
Figure 3.
Distribution of peltate gland developmental stages among leaves of various ages. Bars indicate the percentage of the total gland number represented by each developmental stage (left axis). The line graph indicates the total number of peltate glands (right axis) for each leaf size class. Peltate gland stages: 2, two-celled apical disc; 4, four-celled; 8s, eight-celled, small; 8L, eight-celled, large; S−, early secretory stage; S, middle secretory stage; S+, post-secretory stage. Error bars represent ses.
The proportions of presecretory glands (stages 2, 4, 8s, and 8L), secretory stage glands (S− and S), and post-secretory glands (S+) remained roughly the same during the early phases of leaf expansion (Fig. 3). After leaves reached about one-half of their mature size, there was an evident decline in the overall proportion of presecretory glands (Fig. 3), at which point gland initiation became largely limited to the basal zones of the leaves (Fig. 4). Gland initiation stopped entirely with the completion of leaf expansion.
Figure 4.
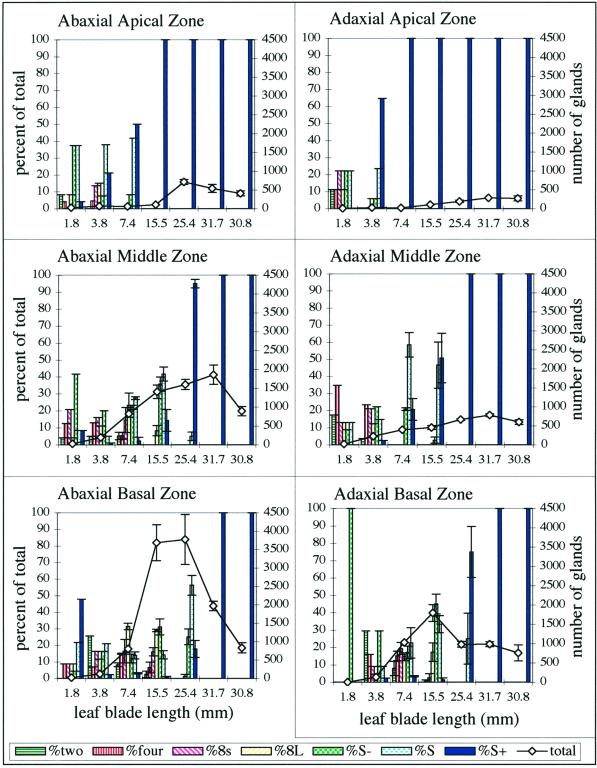
Distribution of peltate gland developmental stages among six leaf zones for young leaves of a single peppermint shoot. Bars indicate the percentage of the total peltate gland number represented by each gland developmental stage (left axes). The line graphs indicate the total number of peltate glands (right axes) for each leaf size class. Peltate gland stages: 2, two-celled apical disc; 4, four-celled; 8s, eight-celled, small; 8L, eight-celled, large; S−, early secretory stage; S, middle secretory stage; S+, post-secretory stage. Error bars represent ses. Calculations for bar graphs are based on sampling areas of six contiguous grid squares. Error bars are not applicable for leaves <7.4 mm because these were too small to accommodate more than one sampling area.
Gland distribution and the regions of active gland initiation were not uniformly distributed over the leaf surface, with distinct differences between the abaxial and adaxial epidermis and between the apical and basal regions of the leaf (Fig. 4). There are approximately twice as many glands produced on the abaxial surface than the adaxial surface, and more glands are produced within the basal and middle regions of the leaf surface than in the apical region (Fig. 4).
The skewed distribution of glands at early developmental stages suggests that different regions of expanding leaves mature at different times (Fig. 4). Within any one zone (apical, middle, or basal), initiation of new glands ceased on the adaxial epidermis about 1 plastochron (1 leaf age) before the abaxial epidermis. Gland initiation stopped in the apical zone about 3 plastochrons before the same epidermis of the basal zone. At the two extremes (i.e. the adaxial apical zone and the abaxial basal zone), cessation of gland initiation differed in time by about 4 plastochrons (more than 9 d).
Observations of Gland Filling
Late-presecretory glands (8L stage) were observed to fill completely with essential oil within the 33-h observation period. It was estimated from the observation intervals that this secretory phase (i.e. from the 8L stage to the S+ stage) lasts from 20 h to no more than 30 h, representing a very short period in the overall time frame of leaf development. During this same period, the observations indicated that small peltate glands (stage 2 or 4) increased in size to the late-presecretory stage (8L), indicating that the presecretory phase of gland development is also very rapid, lasting approximately 30 h.
DISCUSSION
Our observations with peppermint confirm earlier findings for Lamiaceae species (Croteau et al., 1981; Maffei et al., 1986, 1989; Colson et al., 1993; Werker et al., 1993) that continuous initiation of new peltate glands results in a continuous increase in gland number throughout the period of leaf expansion. Gland formation stops with completion of leaf growth. We also found that gland densities and regions of gland formation are not uniformly distributed on the leaf surface, with prolonged gland initiation and the largest numbers of glands occurring in abaxial basal and abaxial middle zones. The early termination of gland initiation in the apical zone probably reflects regional maturation of the epidermis because cessation of gland formation progresses in a basipetal pattern, similar to the pattern of maturation in typical Eudicot leaves (Esau, 1977; Werker et al., 1993), and because the apical region of expanding mint leaves attains the dark green color and relatively rigid texture of mature leaves much sooner than the basal regions do. Hülskamp et al. (1994) described a similar pattern of maturation for the non-glandular trichomes of Arabidopsis. Similar distributions of peltate glands, with the greatest abundance on the abaxial leaf surface, have been reported for a variety of Lamiaceae spp. (Antunes and Sevinate-Pinto, 1991; Bourett et al., 1994; Ascensão et al., 1995, 1998; Gavalas et al., 1998), although this pattern does not hold for all species. Bosabalidis and Skoula (1998) mapped the distribution of mature peltate glands on leaves of Origanum × intercedens and found that peltate gland density was highest on the adaxial (upper) epidermis and that, on each surface, the glands were more or less evenly distributed with similar densities in apical and basal regions.
Our results also show that peltate glands mature very quickly. The sequence of leaf size classes shown in Figure 3 corresponds to a progression of leaf ages. For most of the sequence, the combined number of middle-secretory (S) and post-secretory (S+) glands approximates the total number of glands (of all stages) found in the next younger leaf class. This distribution indicates that once initiated, glands progress through their development to the secretory phase without delay and that the duration of their development is close to 1 plastochron, or approximately 55 h. The number of glands at any developmental stage should be proportional to the relative duration of that developmental stage. During early leaf development, each of the gland developmental stages, prior to the S+ stage, have roughly equivalent proportions (Fig. 3), indicating that each stage lasts about 9.2 h. By this estimate, filling of the sub-cuticular oil storage space (S− through S stage) should take approximately 18.3 h. Direct observations of gland filling confirm that glands progress from the 8L stage to the S+ stage in 20 to 30 h, and that the entire process of peltate gland development requires approximately 60 h from initiation to filling.
It was recently shown that the large increase in monoterpene content of 12- to 20-d-old peppermint leaves coincides with the peak period of monoterpene biosynthesis as determined by 14CO2 incorporation (Gershenzon et al., 2000). Monoterpene biosynthesis and accumulation in younger leaves are negligible, while in leaves older than 20 d, the rate of synthesis declines precipitously and monoterpene accumulation ceases. A more refined analysis, using oil glands isolated from leaves of different ages and in vitro assay of the eight sequential enzymes responsible for the biosynthesis of the principal monoterpene (−)-menthol (Fig. 1), indicated that most of these activities were highest in 10- to 20-mm-long leaves (approximately 14–17 d old) (McConkey et al., 2000). This time course, with a peak at around 15 d, was paralleled by developmental immunoblot analysis of limonene synthase, which catalyzes the committed step of the pathway (Kjonaas and Croteau, 1983), and by RNA-blot analyses of the genes encoding enzymes of the early pathway steps. High monoterpene production rates extend from younger leaves (5–10 mm, 12 d) to older leaves (25–30 mm, 20 d), but biosynthetic activity drops to negligible levels when leaf expansion is complete and all glands are in the post-secretory stage (S+). This observation indicates that the S+ glands are not biosynthetically productive. All stages of gland development are present on 15-mm leaves when biosynthetic activity is maximal; however, the proportions of presecretory-stage structures (2–8L stages) are very low when leaves reach a length of approximately 25 mm and oil production rates are still high. These observations confirm that glands in presecretory stages lack monoterpene biosynthetic capability and that maximum production rates per leaf correlate with the number of glands present in secretory (S) phase.
Although the present study is the first to compare the proportions of different gland developmental stages in different regions of developing peppermint leaves, a number of recent investigations on Mentha spp. have included surveys of gland density, examinations of the essential oil from young and mature regions of leaves, and compositional characterization of the oil obtained from individual glandular trichomes. The bulk of this work was directed to rationalizing the yield increase and profound monoterpene compositional changes that accompany development (Burbott and Loomis, 1969; Croteau and Martinkus, 1979; Brun et al., 1991; Court et al., 1993). Limonene and menthone are the major monoterpenes present in the youngest leaves and the proportion of limonene declines rapidly with development, whereas menthone increases in prominence with increasing oil yield and declines only at later stages as menthol becomes the dominant monoterpene constituent (Fig. 1) (Burbott and Loomis, 1969; Croteau and Martinkus, 1979; Brun et al., 1991). Consistent with the compositional change, (−)-menthone reductase, catalyzing the conversion of (−)-menthone to (−)-menthol as the last step of the pathway (Kjonaas et al., 1982), exhibits a delayed time course of development compared to all of the earlier enzymes of monoterpene biosynthesis in peppermint, with a peak of activity centered at approximately 21 d (instead of 15 d for preceding enzymatic steps) (McConkey et al., 2000).
Maffei et al. (1986, 1989), using scanning electron microscopy to estimate gland numbers and densities on developing leaves of Mentha virdis and peppermint, found that immature leaves contained fewer glandular trichomes than older leaves, indicating sustained gland production during leaf growth. The total number of glands produced and their oil content depended on the physiological state of the plant (Maffei et al., 1986). In the case of peppermint, considerable variation in monoterpene content between individual trichomes was observed, although the trend toward production of menthol and related isomers did correlate with leaf size and age (Maffei et al., 1989). The variation in oil content was taken as evidence that glands of different ages occur in close proximity. We have shown that new glands are continually produced during leaf growth and that newly initiated glands do occur together with mature glands in growing regions, such that neighboring glands within the same leaf zone are often of different ages. This developmental arrangement could lead to a heterogeneous pattern in oil content. A similar pattern of gland initiation was found in Ocimum by Werker et al. (1993). These investigators reported localized regions of apparently meristematic activity within Ocimum leaves, especially in basal zones at which new glands continue to be produced during leaf expansion. They also described differences in composition of oil from mature and young zones of Ocimum leaves, as well as compositional differences in the oil from leaves of different ages.
Related studies by Colson et al. (1993) evaluated the numbers of peltate glands on peppermint leaves produced over a growing season, and revealed that, during vegetative growth, the total number of peltate glands steadily increases (with a decline just prior to flowering), such that the first leaves produced in a season could have only 2,000 glands each, whereas leaves of similar size, but 10 nodes younger, could have 17,000 glands per leaf. These results explain the lower number of glands (relative to younger leaves) observed for the most mature leaves in the present study (Fig. 3) and are consistent with earlier data relating to leaf oil yield as a function of plant development (Burbott and Loomis, 1967, 1969).
Brun et al. (1991) and Voirin and Bayet (1996), in extending the classical studies of Burbott and Loomis (1969), examined variations in essential oil content within regions of individual leaves and between leaves of various ages on shoots of peppermint using an analytical technique that permitted the sampling of very small leaf areas. These studies revealed a relatively high menthone content in young leaves and a high menthol content in older leaves with an increase in menthyl acetate content during leaf senescence. Significant differences in the menthone and menthol content of glands were shown to correlate with gland position along the leaf axis of expanding leaves. While differing in detail from some of the positional effects observed by Maffei et al. (1989), these studies indicated that glands from the leaf apex of expanding leaves had a high menthol content, whereas those from near the leaf base contained mostly menthone. Voirin and Bayet (1996) also noted that regions of high menthol and low menthone content shifted toward the leaf base during leaf growth. This shift was correlated with changes in chlorophyll content that appear to mark leaf maturation, supporting the proposal that the changes in oil composition from menthone to menthol are related to leaf and gland maturation. This suggestion is entirely consistent with the present data that show gland initiation stops at the leaf apex long before it stops at the basal zone and that there appears to be an axial gradient in the average gland maturation state.
None of the above studies directly addresses the question of whether the conversion of menthone to menthol by menthone reductase occurs in all glands upon reaching maturity or whether this capability is largely a property of a subpopulation of glands that are initiated relatively late in development and are dedicated to menthol production (the overall monoterpene compositional dynamics might be rationalized either way). Several recent observations bear on this question. The compositional change is substantial in that the bulk of the menthone produced over the course of leaf development is converted to menthol (McConkey et al., 2000). Over 90% of this metabolic transformation occurs in nearly full-sized leaves (25–30 mm, 20 d), upon which over 90% of the glands are in the secretory (approximately 30% S) or post-secretory (60% S+) phase. The kinetics of the appearance of methone reductase in developing leaves demonstrate that the peak of this activity is at 21 d and that reductase capacity persists after preceding biosynthetic activities (peaking at 15 d) have largely disappeared (McConkey et al., 2000). Thus the kinetic evaluation, coupled with the population distribution of glands involved in this dynamic process, suggest that mature glands (S+ stage) do participate substantially in the reduction of previously synthesized (−)-menthone to (−)-menthol. This conclusion implies that, following gene expression to initiate (and complete) monoterpene biosynthesis in secretory stage glands (McConkey et al., 2000), a later period of transcriptional and translational activity occurs in these mature structures to provide menthone reductase, and perhaps other late-stage enzymes, for the metabolism of accumulated (−)-menthone. The molecular basis of these events, and the physical alterations in these glandular structures that accompany monoterpene production and metabolism, intracellular trafficking, and secretion (export) of oil to the sub-cuticular storage space, are only poorly understood. This recent work on Mentha sp. and related species of the Lamiaceae family has refocused attention on the ultrastructure of oil gland development, which is the subject of the following paper (Turner et al., 2000).
MATERIALS AND METHODS
Plant Material and Plastochron Estimate
Peppermint (Mentha × piperita L. cv Black Mitcham) plants were propagated from rhizomes and grown in a controlled environment as described in detail elsewhere (Gershenzon et al., 2000). To estimate the rate of leaf initiation and the ages of leaves of various sizes, the total number of leaf nodes on each shoot for a minimum of five vegetative shoots were counted every week over a 3-week period. The shoot apices were dissected with the aid of a stereo dissection microscope in order to include the smallest leaf primordia. The shoots were obtained from a flat of mature peppermint plants that were cut to the soil surface about 4 weeks before the first samples were taken so that all shoots would be non-flowering and approximately the same age.
Peltate Gland Counts
Leaves from 4-week-old shoots were fixed overnight in 10% (v/v) formalin, 5% (v/v) glacial acetic acid, 50% (v/v) ethanol, and 35% (v/v) water (Berlyn and Miksche, 1976), rinsed in 50% (v/v) ethanol, and then stained overnight in a saturated solution of Sudan Red 7B (Sigma, St. Louis) in 50% (v/v) ethanol (Brundrett et al., 1991). After rinsing in 50% (v/v) ethanol, specimens were stored in 50% (v/v) glycerol until their use in the gland distribution study.
Electron microscopy specimen grids (size-75 mesh) were placed on stained leaf whole mounts to frame samplingareas for estimating gland distribution. Each grid was 3 mm in diameter and contained 35 grid squares. Each grid square enclosed an area of about 0.07 mm2. Typically, 12 grid squares were sampled (two sets of six contiguous grid squares) representing a sampling area of approximately 0.84 mm2. Whole mounts were observed with a compound light microscope and all glands within the sampling areas were scored for developmental stage.
One leaf from each node of a shoot was sampled. Leaves were divided into three sampling zones (apical, middle, and basal) representing equal distances along the length of the blade. Both abaxial and adaxial leaf surfaces were sampled. For leaves with blades larger than 9 mm in length, a minimum of two grids were placed in each zone. For large mature leaves, 16 grids were typically sampled, representing about 2% of the leaf surface area. Larger percentages were sampled for younger leaves, with approximately 10% of the total leaf surface area sampled for a leaf with a 15-mm-long blade (about one-half of mature length), and approximately 20% of the surface area sampled for a leaf with a 7-mm-long leaf blade. Complete counts of all glands were made for leaves smaller than 2 mm in length.
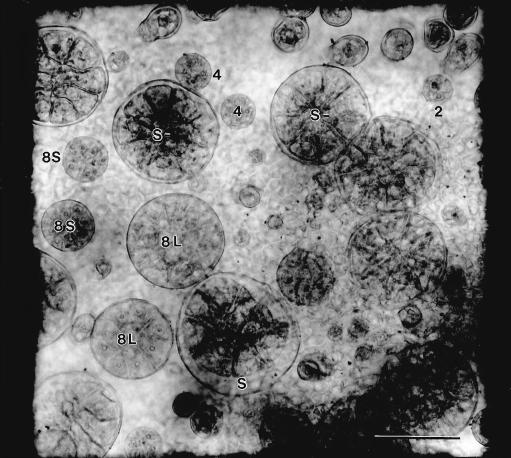
Seven developmental stages were chosen that reflect differences in the number of glandular disc cells and the amount of cuticular separation accompanying formation of the sub-cuticular storage space during oil secretion (Fig. 5). The seven stages were as follows: 2, with two glandular cap cells; 4, with four glandular cap cells; 8s, a relatively small gland with eight cap cells; 8L, a large, nearly full-sized gland with eight cap cells but with little or no cuticular expansion; S−, a full-sized gland with eight cap cells and beginning separation of a sub-cuticular space; S, similar to S− but with a larger and obviously filling sub-cuticular space; and S+, a post-secretory gland with a fully expanded sub-cuticular space that was filled with essential oil.
Figure 5.
Whole mount of a young (7.5 mm) peppermint leaf showing peltate glands of various developmental stages within a 0.07 mm2 counting square placed within the abaxial, basal zone. Gland stages are: 2, two-celled apical disc; 4, four-celled; 8s, eight-celled, small; 8L, eight-celled, large; S−, early secretory stage; S, middle secretory stage. Bar = 50 μm.
A Pulnix TM-7 CCD camera (Pulnix America, Sunnyvale, CA) mounted on a compound microscope (model BH-2, Olympus, Tokyo), with a Quadra 950 (Apple Computer, Cupertino, CA) and NIH Image (version 160, developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image), was used to capture digitized images of specimens for measurements of leaf blade lengths, blade areas, and sampling areas, and to produce printed images of sampling areas on which the locations and developmental stages of individual glands were mapped. Sampling areas within grids were chosen at low magnifications (with 4× and 10× objective lenses) where glands were not easily seen, whereas counting of glands and developmental classification were done at high magnification (40× objective lens).
Direct Observations of Gland Filling
The rate of gland filling with essential oil was estimated by using a Wild stereo dissection microscope (Leica Microsystems, Wetzlar, Germany) to observe and photograph individual glands in a small areole in the abaxial, basal zone of a growing leaf of a potted peppermint plant at 4- to 7-h intervals over a 33-h period. The plant was returned to the growth chamber after each photographic session. The observed region was marked with a small spot of ink on a raised vein near the areole. Glands were photographed at a magnification of 50× with Kodak Technical pan film (Eastman-Kodak, Rochester, NY), and enlarged prints were made with Ilford Multigrade IV photographic paper (Ilford Imaging, Paramus, NJ). Individual glands were identified by position and scored for developmental stage in each of the micrographs to follow the developmental progress of individual glands.
ACKNOWLEDGMENTS
We thank Thom Koehler for raising the plants, Vincent Franceschi for helpful discussions, and Joyce Tamura for typing the manuscript.
Footnotes
This work was supported in part by the U.S. Department of Energy, Division of Energy Biosciences; by the Mint Industry Research Council; and by the Agricultural Research Center, Washington State University (project no. 0268).
LITERATURE CITED
- Amelunxen F. Elektronenmikroskopische Untersuchungen an den Drüsenschuppen von Mentha piperita L. Plant Med. 1965;13:457–473. [Google Scholar]
- Amelunxen F, Wahlig T, Arbeiter H. Über den Nachweis des ätherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Z Pflanzenphysiol. 1969;61:68–72. [Google Scholar]
- Antunes T, Sevinate-Pinto I. Glandular trichomes of Teucrium scorodonia L. morphology and histochemistry. Flora. 1991;185:65–70. [Google Scholar]
- Ascensão L, Figueiredo AC, Barroso JG, Pedro LG, Schripsema J, Deans SG, Scheffer JJC. Plectranthus madagascariensis: morphology of the glandular trichomes, essential oil composition, and its biological activity. Int J Plant Sci. 1998;159:31–38. [Google Scholar]
- Ascensão L, Marques N, Pais MS. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae) Ann Bot. 1995;75:619–626. [Google Scholar]
- Ascensão L, Pais MS. The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Ann Bot. 1998;81:263–271. [Google Scholar]
- Berlyn GP, Miksche JP. Botanical Microtechnique and Cytochemistry. Ames: Iowa State University Press; 1976. [Google Scholar]
- Bosabalidis AM, Skoula M. A comparative study of the glandular trichomes on the upper and lower leaf surfaces of Origanum × intercedens Rech. J Essential Oil Res. 1998;10:277–286. [Google Scholar]
- Bourett TM, Howard RJ, O'Keefe DP, Hallahan DL. Gland development on leaf surfaces of Nepeta racemosa. Int J Plant Sci. 1994;155:623–632. [Google Scholar]
- Brun N, Colson M, Perrin A, Voirin B. Chemical and morphological studies of the effects of aging on monoterpene composition in Mentha × piperita leaves. Can J Bot. 1991;69:2271–2278. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. Efficient lipid staining in plant material with sudan red 7b or florol yellow 088 in polyethylene glycol-glycerol. Biotech Histochem. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Burbott AJ, Loomis WD. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967;42:20–28. doi: 10.1104/pp.42.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbott AJ, Loomis WD. Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol. 1969;44:173–179. doi: 10.1104/pp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood BV, Charlwood KA. Monoterpenoids. In: Charlwood BV, Banthorpe DV, editors. Terpenoids. London: Academic Press; 1991. pp. 43–98. [Google Scholar]
- Colson M, Pupier R, Perrin A. Étude biomathématique du nombre de glandes peltées des feuilles de Mentha × piperita. Can J Bot. 1993;71:1202–1211. [Google Scholar]
- Court WA, Roy RC, Pocs R. Effect of harvest date on the yield and quality of the essential oil of peppermint. Can J Plant Sci. 1993;73:815–824. [Google Scholar]
- Croteau R, Felton M, Karp F, Kjonaas R. Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis) Plant Physiol. 1981;67:820–824. doi: 10.1104/pp.67.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Gershenzon J. Genetic control of monoterpene biosynthesis in mints (Mentha:Lamiaceae) Recent Adv Phytochem. 1994;28:193–229. [Google Scholar]
- Croteau R, Martinkus C. Metabolism of monoterpenes: demonstration of (+)-neomenthyl-β-d-glucoside as a major metabolite of (−)-menthone in peppermint (Mentha piperita) Plant Physiol. 1979;64:169–175. doi: 10.1104/pp.64.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants. Ed 2. New York: John Wiley and Sons; 1977. [Google Scholar]
- Fahn A. Secretory Tissues in Plants. New York: Academic Press; 1979. pp. 162–164. [Google Scholar]
- Gavalas N, Bosabalidis AM, Kokkini S. Comparative study of leaf anatomy and essential oils of the hybrid Mentha × villoso-nervata and its parental species M. longifolia and M. spicata. Isr J Plant Sci. 1998;46:27–33. [Google Scholar]
- Gershenzon J, Maffei M, Croteau R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata) Plant Physiol. 1989;89:1351–1357. doi: 10.1104/pp.89.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, McConkey M, Croteau R. Regulation of monoterpene accumulation in leaves of peppermint (Mentha × piperita L) Plant Physiol. 2000;122:205–213. doi: 10.1104/pp.122.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther E. The Essential Oils. I-VI (reprinted) Huntington, NY: Robert E. Krieger Publishing Company; 1972. [Google Scholar]
- Hülskamp M, Miséra S, Jürgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Kjonaas R, Croteau R. Demonstration that limonene is the first cyclic intermediate in the biosynthesis of oxygenated p-menthane monoterpenes in Mentha piperita and other Mentha species. Arch Biochem Biophys. 1983;220:79–89. doi: 10.1016/0003-9861(83)90389-2. [DOI] [PubMed] [Google Scholar]
- Kjonaas R, Martinkus-Taylor C, Croteau R. Metabolism of monoterpenes: conversion of l-menthone to l-menthol and d-neomenthol by stereospecific dehydrogenases from peppermint (Mentha piperita) leaves. Plant Physiol. 1982;69:1013–1017. doi: 10.1104/pp.69.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenheim JH. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- Lawrence BM. Monoterpene interrelationships in the Mentha genus: a biosynthetic discussion. In: Mookherjee BD, Mussinan CJ, editors. Essential Oils. Wheaton, IL: Allured Publishing Company; 1981. pp. 1–81. [Google Scholar]
- Maffei M, Chialva F, Sacco T. Glandular trichomes and essential oils in developing peppermint leaves: I. Variation of peltate trichome number and terpene distribution within leaves. New Phytol. 1989;111:707–716. doi: 10.1111/j.1469-8137.1989.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Maffei M, Gallino M, Sacco T. Glandular trichomes and essential oils of developing leaves in Mentha viridis lavanduliodora. Planta Med. 1986;52:187–193. [Google Scholar]
- McCaskill D, Gershenzon J, Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- McConkey M, Gershenzon J, Croteau R. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint (Mentha × piperita L.) Plant Physiol. 2000;122:215–223. doi: 10.1104/pp.122.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff J. Monoterpene composition of essential oil from peppermint (Mentha × piperita L.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J Agric Food Chem. 1999;47:3782–3786. doi: 10.1021/jf981310s. [DOI] [PubMed] [Google Scholar]
- Voirin B, Bayet C. Developmental changes in the monoterpene composition of Mentha × piperita leaves from individual peltate trichomes. Phytochemistry. 1996;43:573–580. [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae) Ann Bot. 1993;71:43–50. [Google Scholar]
- Werker E, Ravid U, Putievsky E. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Isr J Bot. 1985;34:31–45. [Google Scholar]
- Wise ML, Croteau R. Monoterpene biosynthesis. In: Cane DE, editor. Comprehensive Natural Products Chemistry. 2, Isoprenoids Including Carotenoids and Steroids. Oxford: Elsevier Science; 1999. pp. 97–153. [Google Scholar]