Abstract
Clinically, many nail disorders accompany bone deformities, but whether the two defects are causally related is under debate. To investigate the potential interactions between the two tissue types, we analyzed epithelial-specific β-catenin deficient mice, in which nail differentiation is abrogated. These mice showed regression of not only the nail plate but also of the underlying digit bone. Characterization of these bone defects revealed active bone resorption, which is suppressed by Wnt activation in osteoblast and osteoclast precursors. Furthermore, we found that Wntless (Wls) expression, essential for Wnt ligand secretion, was lacking in the β-catenin deficient nail epithelium and that genetic deletion of Wls in the nail epithelium led to the lack of Wnt activation in osteoblast and osteoclast precursors and subsequently led to defective regression of the underlying digit bone. Together, these data show epithelial Wnt ligands can ultimately regulate Wnt signaling in osteoblasts and osteoclast precursors, known to regulate bone homeostasis. These results reveal a critical role for the nail epithelium on the digit bone during homeostatic regeneration and show that Wnt/β-catenin signaling is critical for this interaction.
Introduction
Mammalian fingertip is mainly composed of the nail plate, nail epithelium and underlying terminal phalanx, which is the most distal bone of the limb. The nail plate is a keratinized structure that grows continuously owing to maturation and keratinization of the nail matrix, the proximal part of the nail epithelium, which is lined with the thin dermis that is attached to the terminal phalanx (Baran et al., 2003).
Nail disorders often accompany bone deformities, and such cases have been reported during the diagnosis and treatments of a wide range of skin/nail diseases including psoriatic arthritis(Resnick et al., 1977), psoriasis(Serarslan et al., 2007), hypertrophic pulmonary osteoarthropath(Baran et al., 2003), hyperparathyroidism(Baran et al., 2003) and congenital onychodysplasia of the index fingers (COIF. Iso-Kikuchi syndrome)(Iso, 1969). Despite their divergent pathogenesis, as well as the distinct cellular and molecular characteristics of each nail disorder, the associated bone defects are characterized by their similar malformation and regression of the terminal phalange, the most distal bone of the digits, where the nail disorder occurs. Given the clinical diversity of nail disorders associated with bone defects, it was hypothesized that the nail organ may play a fundamental role in homeostasis of the underlying bone (Blaydon et al., 2006). However, this theory has not been tested experimentally, and whether the nail organ influences the homeostasis of the underlying bone remains unknown.
In response to Wnt ligands, cytoplasmic β-catenin accumulates, enabling it to translocate to the nucleus where it interacts with members of the LEF/TCF family of DNA binding proteins to form a transcriptional complex, resulting in transcription of target genes to exert diverse biological effects (Nusse, 1999).
In the osteo-lineage, Wnt signaling promotes differentiation from mesenchymal stem cells to preosteoblast, precursor of osteoblasts that produce bone, while it inhibits maturation of preosteoblast to osteoblasts that breakdown the bone (Baron et al., 2006). Loss of β-catenin in mesencymal progenitors and inactivation of Lrp5, one of the Wnt coreceptor, prevents osteoblast differentiation resulting in low bone mass (Gong et al., 2001; Kato et al., 2002). Moreover, Wnt activation in differentiated osteoblasts promotes the ability of osteoblast to inhibit osteoclast differentiation and their activity (Albers et al., 2013; Glass et al., 2005; Spencer et al., 2006). Disruption of the well-tuned balance of the production of bone matrix by osteoblasts, and the resorption by osteoclast causes disorders such as osteoporosis (Baron and Kneissel, 2013).
In the nail epithelium, Wnt signaling is essential for nail stem cell differentiation and loss of Wnt signaling in the nail epithelium eventually results in the complete loss of the nail plate (Takeo et al., 2013). Lgr6, an agonist of the Wnt pathway, is expressed in stem cells in the nail matrix that gives rise to the nail plate. Conventional loss of Lgr6 results in larger digit bone after normal development. However, whether the changes observed in the bone are due to the changes in nail epithelium is unknown, because Lrg6 is also expressed in a subset of osteolineage cells in mouse digits (Lehoczky and Tabin, 2015).
In this study, we demonstrate the critical role of nail epithelial cells in homeostasis of the terminal phalanx through epithelium-specific modulation of Wnt signaling components and proposes a mechanism of how defects in nail differentiation causes deformities of the terminal phalanx under homeostatic conditions.
Results
Epithelial-specific deletion of β-catenin leads to the degeneration of the terminal phalanx
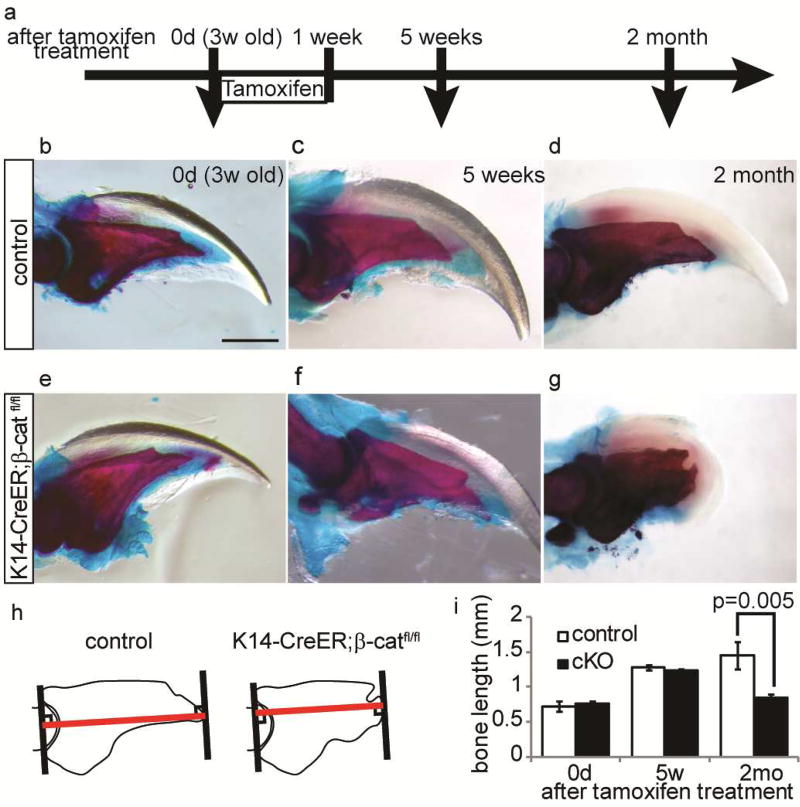
To address if defects in nail formation can affect the homeostasis of the underlying digit bone we utilized epithelial-specific β-catenin deficient mice (K14-creER: β-cateninfl/fl) in which nail differentiation is known to be abrogated (Takeo et al., 2013). These mice lack β-catenin expression, following tamoxifen (TAM) treatment, specifically in Keratin 14-expressing epithelial cells including nail matrix cells. Since K14 is not expressed by cells in the osteo-lineage, these mice are useful for determining whether genetically induced deficiency within the nail epithelium influences homeostasis of the bone. By 2 months after TAM treatment, the digit tip of β-catenin conditional knockout (β-catenin cKO) mice lacked an apparent nail plate as previously reported(Takeo et al., 2013), due to the requirement of Wnt/β-catenin signaling for differentiation of nail epithelial cells (Fig. 1a – g). Visualization of the bone by glycerol treatment revealed that the distal part of the terminal phalanx was regressed in all β-catenin cKO mice (Fig. 1h and i). For the morphometric analysis of the terminal phalanx, we stained the digits with alizarin red/alucian blue and found that the terminal phalanx of β-catenin cKO mice showed a significantly shorter and atypical knob shape with a pitted uneven surface (Fig. 1g and h). This is in contrast to the normal terminal phalanx in control mice which was triangular in shape with a sharp-pointed tip (Fig. 1d). These results show that changes in epithelial cells, and not cells of the bone lineage, can influence the maintenance of the underlying bone.
Figure 1. Removal of β-catenin in epithelial cells leads to digit bone defect.
(a) Experimental scheme. Three-week-old old β-catenin cKO mice and littermates were treated with tamoxifen for 7 d, and analyzed at the indicated time points. (b – g) Whole mount alizarin red/alcian blue analysis of control (b - d) and β-catenin cKO digit (e – g) at 0d (b, e), 5 weeks (c, f) and 2 month (d, g) after tamoxifen treatment. Failure of nail growth and digit bone deformation was observed in cKO mice at 2 month after tamoxifen treatment. (h) Schematic illustration of bone length measurement. Length of red line was measured. (i) Quantification analyses of bone length at indicated time point. Data are presented as the mean ± SD (n=16). Scale bar, 500 µm.
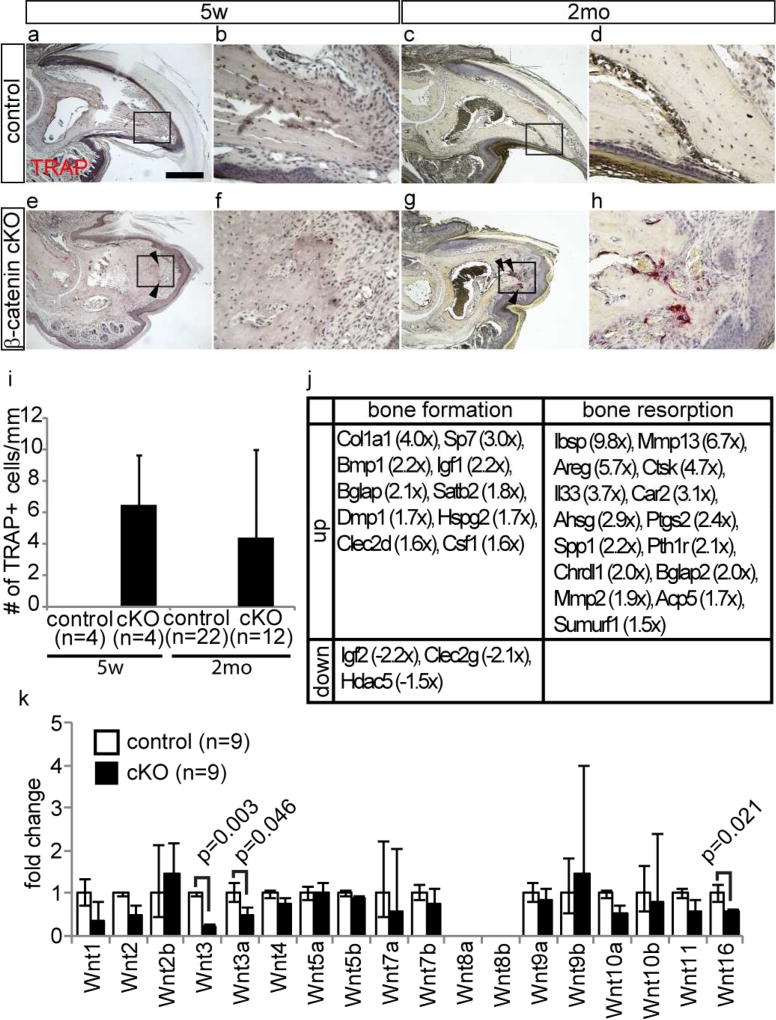
To characterize the bone regression phenotype of these mice, we examined the activity of tartrate-resistant acid phosphatase (TRAP), a specific marker of osteoclastogenesis(Minkin, 1982). The terminal phalanx in β-catenin cKO mice showed strong TRAP signal at 5 weeks and 2 months after tamoxifen treatment, demonstrating the presence of active osteoclasts, which are known to be responsible for bone resorption (Fig. 2e–i). In contrast, the control terminal phalanx lacked TRAP activity (Fig. 2a–d and i). TRAP signals in β-catenin cKO mice were observed on the surface of the distal tip of the terminal phalanx, where bone regression was observed. On the other hand, the proximal part of the terminal phalanx did not show TRAP activity and the morphology of this area was unaltered in the cKO mouse digits (Fig. 2a and e). Although some autoimmune diseases, such as rheumatoid arthritis are characterized by secretion of T cell derived cytokines that induce bone resorption (Kong et al., 1999; Sato et al., 2006; Takayanagi et al., 2000), immunostaining did not indicate any increase in the infiltration of CD3+ mature T cells, and only a few T cells were present, similar to what is observed in human digit(Ito et al., 2005) (Fig. S1). Moreover, expression of interferon was not upregulated in the microarray data of β-catenin cKO mouse digits (data not shown).
Figure 2. Depletion of epithelial β-catenin results in the activation of bone resorption and down-regulation of Wnt ligand expression.
(a–h) TRAP staining on control (a–d) and β-catenin cKO (e–h) digit at 5 weeks (a, b, e, and f) and 2 month (c, d, g, and h) after tamoxifen treatment. (b, d, f, and h) are magnified field of boxed area in (a, c, e, and g), respectively left panels. (i) Quantification analysis of the number of TRAP positive cells on the surface of terminal phalanx at indicated time points. (j) List of differentially expressed genes associated with bone formation and resorption between control and β-catenin cKO digit at 2 month after tamoxifen treatment. (k) Relative Wnt ligand expression level between control and β-catenin cKO mice at 2 month after tamoxifen treatment. Arrowheads indicate TRAP positive cells. Data are presented as the mean ± SD. Scale bar, 500 µm.
Wnt ligand expression is down regulated in β-catenin deficient nail epithelium
Microarray analysis (Table S1. GEO accession numbers; GSM1875156, GSM1875157, GSM1875158, GSM1875159, GSM1875160, GSM1875161) and subsequent overrepresentation analysis of Gene Ontology (GO) terms using cDNA prepared from digit tips of control and β-catenin cKO mice revealed that the expression levels of several genes known to promote bone resorption, including integrin binding sialoprotein (Ibsp, 9.5x) (Malaval et al., 2008), matrix metallopeptidase 13 (Mmp13, 6.7x) (Kosaki et al., 2007; Liu et al., 2012), amphiregulin (Areg, 5.7x) (Lu et al., 2009) and cathepsin K (Ctsk, 4.2x) (Wilson et al., 2009), were markedly up-regulated in β-catenin cKO digits (Fig. 2j). In combination with qPCR analysis using whole mount digit, we also found that several canonical Wnt ligands are down-regulated in cKO mouse digit (Fig. 2k). Further qPCR analysis of isolated nail epithelium from the control digits identified that Wnt ligands are expressed by nail epithelial cells, including Wnt3a, which is known to be capable of activating Wnt signaling in osteoblasts (Spencer et al., 2006) (Fig. S2). This is striking given that canonical Wnt signaling inhibits osteoclast differentiation and promotes osteoblast-mediated inhibition of osteoclastogenesis (Albers et al., 2013; Glass et al., 2005; Spencer et al., 2006).
Importantly, immunohistochemical analysis for Wls protein, known to be essential for Wnt ligand secretion (Banziger et al., 2006; Bartscherer et al., 2006), revealed that osteoblasts and osteoclasts were localized on the dorso-distal surface of the terminal phalanx, immediately beneath the nail matrix cells with Wls expression (Fig. 3a–d and Fig. S2). In contrast, the nail epithelium of β-catenin cKO mouse digits did not express Wls at 5 weeks and 2 months after TAM treatment (Fig. S2). This is reasonable given that nail epithelial stem cells in β-catenin cKO mice lack the ability to give rise to distal matrix cells that contain Wnt-secreting cells with expression of Wls (Takeo et al., 2013).
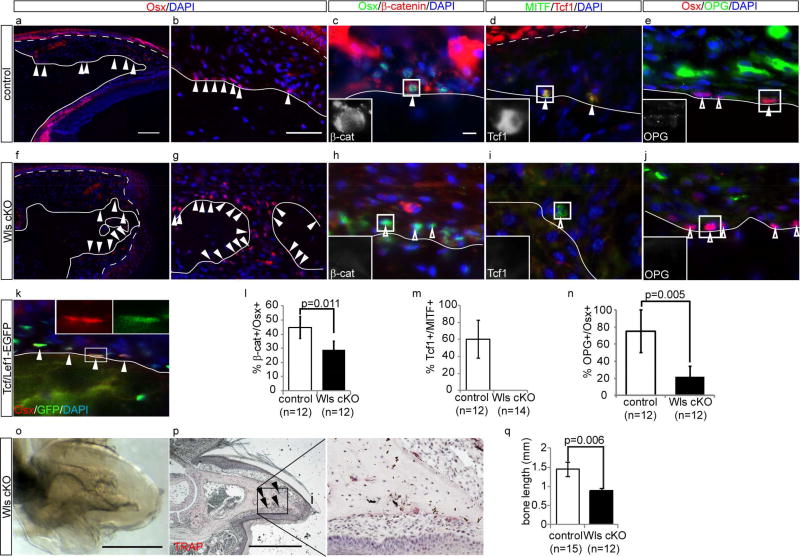
Figure 3. Loss of Wls in epithelium leads to suppression of Wnt signaling in osteoblast and osteoclast precursors and activation of bone resorption.
(a–j) Immunohistochemistory for Osx ( a, b, f and g), Osx and β-catenin (c and h), MITF and Tcf1 (d and i), and Osx and OPG (e and j) on control (a – e) and Wls cKO (f – j) at 2 month after tamoxifen treatment. Insets in (c, d, e, h, i, and j) indicate single channel image of β-catenin (c and h), Tcf1 (d and i), and OPG (e and j) of boxed cell, respectively. (k) Immunohistochemistry of Osx on digit of Tcf/Lef1-GFP reporter mice. (l–n) Quantification analysis of the percentage of nuclear β-catenin positive cells in Osx positive cells (l), the percentage of nuclear Tcf1 positive cells in MITF positive cells (m), and the percentage of OPG positive cells in Osx positive cells (n) at 2 month after tamoxifen treatment. (o and p) Whole-mount transparent specimen (o) and TRAP staining (p) of Wls cKO digit at 2 month after tamoxifen treatment. Left panel of (p) is magnified image of boxed area. (q) Quantification analysis of the bone length of Wls cKO digit at 2 month after tamoxifen treatment. Lines indicate border between terminal phalanx and dermis. Dashed lines indicate border between epithelium and dermis in (a–j). Solid arrowheads indicate Oxs+ cells (a, b, f, g), double positive cells (c – e, k), and TRAP positive cells (p). Open arrowheads indicate single positive cells for Osx (e, h, j) and MITF (i), Data are presented as the mean ± SD. Scale bars, 500 µm in ; (o, p); 100 µm in (a); 50 µm in (b) ; 10 µm in (c).
Wnt ligands secreted from nail epithelium are required for Wnt activation in underlying osteoblasts and osteoclast precursors
To test if Wnt ligands from epithelial cells are essential for Wnt activation in underlying osteoblasts and osteoclast precursors, we generated K14-creER;Wntless mice (Wls cKO) in which Wls is depleted in epithelial cells following TAM treatment (Fig. S2). We then detected Wnt signaling activity in osteoblast/osteoclast precursors, which were identified with the following markers, Osterix (Osx)(Nakashima et al., 2002) and Microphthalmia-associated transcription factor (MITF)(Kawaguchi and Noda, 2000), respectively. In control mice, Osx+ osteoblasts are concentrated in the dorsal side of the terminal phalanx and these osteoblasts display active Wnt signaling, which was evidenced by nuclear signal of β-catenin immunostaining (Fig. 3a – c and l). Additionally, we detected GFP signal in these cells using TCF/LEF –GFP mice (Ferrer-Vaquer et al., 2010) in which GFP is expressed under a control of Wnt responsive element and a heat shock protein 1B minimal promoter (Fig. 3k). MITF+ osteoclast precursors expressed Tcf1 (van de Wetering et al., 1997), a nuclear mediator of Wnt/β-catenin signaling, suggesting that the Wnt pathway is activated in osteoclast precursors (Fig. 3d and m). By 2 months after deletion of Wls gene from nail epithelium, the terminal phalanx of these mice showed regression and TRAP activity, similar to β-catenin cKO mice (Fig. 3o – q). Furthermore, Wls cKO mice showed mislocalized osteoblasts, suppression of Wnt signaling in osteoblasts and osteoclast precursors (Fig. 3f – i, l and m). Remarkably, Osx+ osteoblasts of Wls cKO mice exhibited reduced expression of osteoprotegerin (Opg), which is known to inhibit osteoclastgenesis as a result of Wnt activation in osteoblasts (Glass et al., 2005)(Fig. 3e, j and n). These results show that osteoblasts and osteoclast precursors on the surface of the terminal phalanx directly or indirectly require Wnt ligand(s) produced by the nail epithelium to ultimately activate Wnt signaling, which is necessary for preventing bone regression.
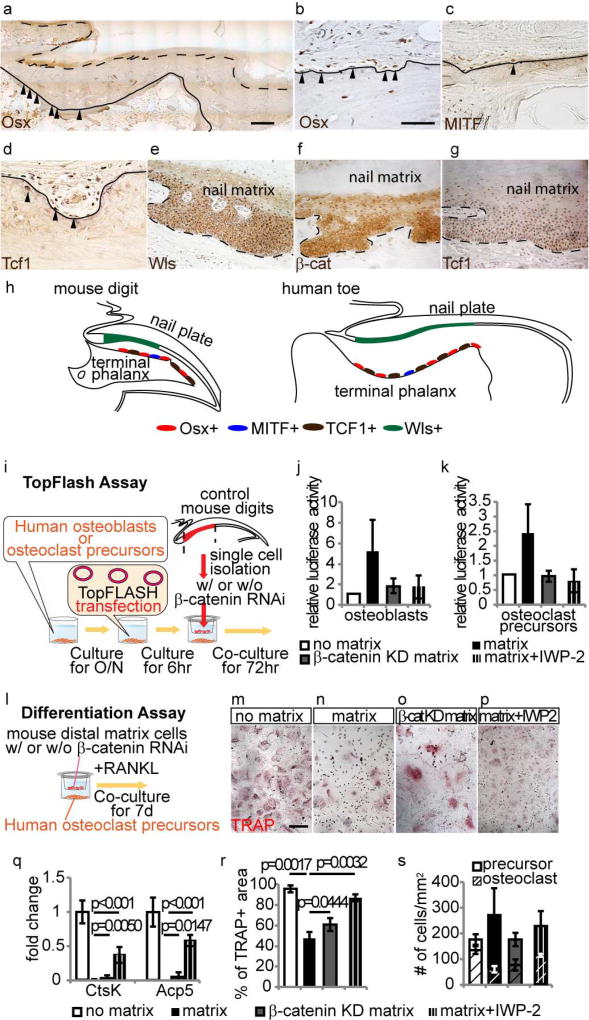
Given the structure of the digit is essentially conserved in mice and humans(Fleckman et al., 2013), we sought to test if a similar principle can be applied to understand the homeostasis of the distal phalanx in humans. To this, we first examined whether a similar population of osteoblasts and osteoclast precursors exist in the corresponding area of the human terminal phalanx. We found that Osx+, MITF+ and Tcf1+ cells were located on the dorsal surface of the terminal phalanx, while Wls expressing cells was observed only in nail matrix cells overlying the terminal phalanx, in which the expression of nuclear β-catenin and Tcf1 were detectable, similar what is observed in mice (Fig. 4a–h). We thus tested the ability of Wnts from nail matrix cells to activate Wnt signaling in human osteoblasts and osteoclast precursors. We co-cultured human osteoblasts and osteoclast precursors with nail matrix cells isolated from mouse digits using transwell inserts, and three days after co-culture, they upregulated the TopFlash signal, indicating activation of Wnt signal(Korinek et al., 1997) (Fig. 4i–k). Furthermore, osteoclast precursors co-cultured with nail matrix showed less differentiation into mature osteoclasts that are characterized by TRAP activity and the large multinucleated morphology at 7 days after induction of differentiation by RANK ligand treatment (Fig. 4l–n). Similar to the changes observed with TRAP staining, the presence of nail matrix cells significantly inhibited the expression of markers for mature osteoclasts including Cathepsin K (CtsK) and Acid Phosphatase 5 (Acp5) (Fig. 4q). This up-regulation of Wnt activation and inhibition of osteoclast differentiation were neutralized by knocking down β-catenin in nail matrix cells or addition of IWP2 inhibitor, an inhibitor of Wnt ligand secretion(Chen et al., 2009) (Fig. 4j, k and o–r). In all treatments, the total number of osteoclast precursors and mature osteoclast were similar, suggesting that these treatments did not affect cell viability (Fig. 4s). These results suggest that maintenance of the human distal phalange may also be governed, at least in part, by the overlying nail epithelial cells through their capacity to extrinsically promote Wnt signaling via secretion of Wnt ligands in the bone microenvironment.
Figure 4. Human osteoblast and osteoclast precursors have the ability to respond to nail epithelium.
(a–g) Immunohistochemistory for Osx (a anb b), MITF (c), Tcf1 (d and g), Wls (e) and β-catenin (f) on terminal phalanx and nail epithelium of human toe. (h) Schematic illustration of the distribution pattern of Osx+, MITF+, Tcf1+ and Wls+ cells in mouse digit and human toe. (i) Experimental scheme of co-culture experiment and TopFlash assay on human osteoblasts and osteoclast precursors. (j and k) Representative result of TopFlash assay of human osteoblast and human osteoclast precursors. (l) Experimental scheme of differentiation assay of human osteoclast precursors. (m–p) Differentiation analysis of human osteoclast precursors cultured with no nail matrix (m), nail matrix (n), nail matrix with β-catenin knockdown (o), and nail matrix and IWP-2 inhibitor (p). (q) qPCR analysis of osteoclast differentiation markers using cells at 3 days after induction of osteoclast differentiation.(r and s) Quantification analysis of the area that covered by mature osteoclast (r) and number of osteoclast precursors and mature osteoclasts (s). Lines indicate border between terminal phalanx and dermis. Dashed lines indicate border between epithelium and dermis. Arrowheads indicate cells that are positive for indicated marker. Data are presented as the mean ± SD (n=6). Scale bars, 1mm in (a); 50 µm in (b); 200 µm in (h).
Extrinsic regulation of bone homeostasis by epithelial cells is uniquely seen in the distal terminal phalange
Above results demonstrate a mechanism for the extrinsic regulation of bone homeostasis by epithelial cells. This further raised a question of whether this mechanism is also in play during homeostasis of the long bone. To address this, we examined Wls expression in the long bone (i. e. middle phalanx). In contrast to the terminal phalanx, Wls-expressing cells were observed in the bone trabeculae, which is where osteoblasts reside in the long bone of control mice (Fig. S3). This suggests that the osteoblasts have the ability of to activate Wnt signaling in a cell autonomous manner unlike those in the terminal phalange. Accordingly, deletion of β-catenin or Wls genes in epithelial cells did not lead to the up-regulation of TRAP activity in these bones (Fig. S3). These results show that regulation of Wnt signaling in osteoblasts and osteoclast precursors by epithelial cells is one of the mechanisms that is unique to the maintenance of the distal terminal phalange (Fig. 5).
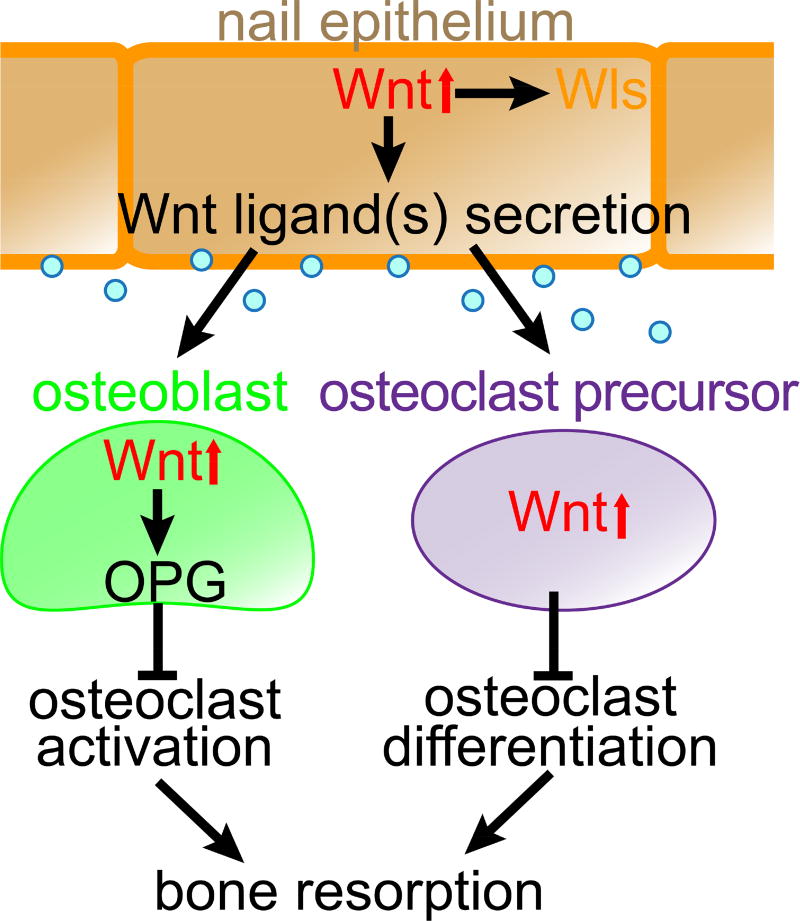
Figure 5. Nail epithelium maintains the underlying bone by secreting Wnt ligands that extrinsically promote Wnt signaling in bone microenvironment.
In the presence of Wnt ligands derived from the nail epithelium, osteoblasts that form the bone and precursors of osteoclasts that break down the bone both display Wnt pathway activation. Wnt activation in osteoblasts results in osteoprotegerin (Opg) expression, which is known to inhibit osteoclast activation and differentiation. Wnt activation in osteoclast precursors, on the other hand, inhibits their differentiation into mature osteoclasts, together ensuring the bone maintenance.
Discussion
In several skin diseases, including scleroderma and psoriasis, acro-osteolysis of the terminal phalanx is associated with a nail dystrophy phenotype (Sakthiswary et al., 2011), however, it was generally not known how these two conditions were related. In this study, we revealed that the nail epithelium is essential for digit bone maintenance (Fig. 1 and 5). In essence, we deleted genes specifically in nail epithelial cells and demonstrated that changes in the nail epithelium can modulate the function of osteoblasts/osteoclasts that maintains the underlying digit bone. The significance of these findings is that many clinical studies report the association between disorders of the nail and digit bone (e.g. psoriasis, hyperparathyroidism, and Iso-Kikuchi syndrome), yet which cells need to be targeted for therapeutic interventions has been unclear. Intriguingly, a recent report shows that Wnt signaling is down-regulated in epidermal cells of psoriasis patients and it is known that some of psoriasis patients develop malformation of terminal phalanx (Gudjonsson et al., 2010). Our study suggests that the important roles for nail epithelium presented in this study need to be taken into consideration in treating such diseases instead of solely focusing on the osteo-lineage.
We showed that deletion of β-catenin specifically in nail epithelial cells results in the down regulation of Wnt ligand secretion from the nail epithelium and depletion of Wnt ligand from nail epithelium leads to the down-regulation of Wnt activation in osteoblast and osteoclast precursors, which may cause bone resorption (Fig. 2 and 3). The precise mechanism of how loss of β-catenin leads to down-regulation of Wnt ligand secretion from nail epithelium remains to be addressed. Previous studies showed that several Wnt ligands are downstream targets of Wnt/β-catenin signaling in skin keratinocytes (Deschene et al., 2014; Zhang et al., 2009). Thus, it is possible that a similar mechanism that promotes Wnt ligand expression in a positive-feedback mechanism is present in the nail epithelium. Another remaining question is if and how other cell types such as mesenchymal cells in the nail organ respond to the loss of Wnts in epithelial cells and whether they indirectly contribute to the bone resportion in the terminal phalanx. Our results demonstrating that Wnt ligand secretion in epithelial cells is ultimately required for digit bone maintenance may serve as an important initial step in dissecting the dynamic interactions between distinct tissues during homeostasis of this organ.
In development, the terminal phalanx forms late in gestation by endochondral ossification of mesenchymal condensates. Postnatally, elongation of the distal end of the terminal phalanx occurs by direct ossification on the surface of the terminal phalanx, whereas proximal elongation results from endochondral growth plate (Han et al., 2008; Nakamura et al., 1989). In humans, mutations in Wnt related genes such as Rspo4, PORCN, Wnt7a and Wnt10a are associated with several diseases that developed severe disorders in limb development including the nail and terminal phalanx, such as anonychia, focal dermal hypoplasia, and odonto-onycho-dermal dysplasia (Adaimy et al., 2007; Blaydon et al., 2006; Grzeschik et al., 2007; Kantaputra et al., 2010; Wang et al., 2007). These observations suggest that a similar dependency of terminal phalanx on nail epithelium may exist during embryonic development, although the mechanism of ossification of terminal phalanx is different between embryonic development and postnatal homeostasis. Nonetheless, not all mutation in Wnt related genes result in the deformation of the both nail and terminal phalanx. In such case, mutation in Fzd6, which is expressed in the keratogenous zone in humans, results in nail abnormalities but not in digit bone malformation (Frojmark et al., 2011; Naz et al., 2012). Therefore, further investigation, such as Wnt ligand secretion, in these mutant mice may help elucidate the relationship between the formation of nail and terminal phalanx during embryogenesis.
Injuries of the terminal phalange are common and represent more than 50% of amputation related injuries(Doraiswamy and Baig, 2000). These observations highlight the vulnerability of this area and raise the possibility that animals and humans may have evolved mechanisms to form the nail, a specialized epithelium to protect the digit tip from injury. In response to nail loss, osteoclastogenesis ensues and the organism spontaneously loses the distal tip of the terminal phalange, the most fragile and finest part of the digit bone, avoiding further bone fractures. Overall, this phenomenon may represent a strategy in which the organ protects itself from injuries. Our study provides insight into the evolutionary dynamics of bone maintenance and has implications in designing strategies for the prevention and treatment of bone resorption by focusing on the epithelium.
Materials and Methods
Mice and sample collections
All mice were obtained from Jackson Laboratories, and maintained in the Animal Facility at NYU Langone Medical Center. All animal protocols were approved by IACUC at NYU School of Medicine. Cre recombination in K14-CreER;β-cateninfl/fl and K14-CreER;Wntlessfl/fl mice was induced by TAM injection as published(Rabbani et al., 2011). For nail sample collections, we sacrificed mice by CO2 narcosis, and harvested the middle 3 digits of the hind limbs.
Alucian blue/Alizarin red staining
Whole mount Alucian blue/Alizarin red staining was performed as described in previous paper with minor modification (McLeod, 1980). Briefly, digits were fixed in 4% PFA/PBS at 4°C for overnight, removed skin and muscle, and stained with 0.015% Alucian blue 8GX/20% Glacial acetic acid/80% EtOH at RT for overnight. After wash in 95% EtOH twice, digits were stained with 0.0075% Alizarin red S/1% KOH at RT for overnight. After washing in 1% KOH in H2O, digits were incubated serially in 20% glycerol/1% KOH for 3–6 h at RT, 50% glycerol/1% KOH for 4 – 16 h at RT and 100% glycerol O/N at RT.
Gene expression profiling by microarray
Three-week-old K14-CreER; β-cateninfl/fl mice and their littermate were treated with tamoxifen for 7 days to induce Cre recombination in K14 positive epithelial cells including nail epithelium. At 2 month after tamoxifen treatment, six digits each from 3 different mice were harvested and processed to total RNA isolation using TRIzol (Life Technologies, CA). To obtain a sufficient amount of RNA for oligonucleotide gene chip hybridization, we used the Ovation RNA Amplification System V2 (Nugen, CA) for mRNA amplification. The amplified mRNA was labeled and hybridized to the ExonExprChip (Affymetrix, CA). Data was analyzed with GeneSpring X software, and genes that were differentially regulated at least 1.5 fold were selected for further analysis. GEO accession numbers; GSM1875156, GSM1875157, GSM1875158, GSM1875159, GSM1875160, GSM1875161.
TRAP staining
Nails were fixed in 10% Zinc buffered formalin at 4°C for 48 hours and washed in PBS twice. After decalcification in 22.5% formic acid/10% sodium citrate buffer at RT for 2 hr, nails were dehydrated through ethanol and xylene, embedded in paraffin, and cut into 6 µm sections. Following rehydration, paraffin-sectioned tissues were incubated in Naphtol-AS-BI-phosphate (Sigma-Aldrich, PA)/Fast Red Violet LB (pH 5.2, Sigma-Aldrich. PA) with 50 µ M sodium tartrate for 4 h at 37 °C. Slides were counterstained with Mayer's Hematoxylin.
Immunohistochemistry
Mouse digit samples were fixed in zinc-buffered formalin at 4°C for 48 h and processed for paraffin tissue sections following decalcification in 22.5% formic acid/10% sodium citrate buffer at RT for 2 hr or 10% EDTA (pH 7.0) at RT for 24 hr . Following rehydration, antigen retrieval was performed by microwaving sections for 6 min on high-wattage setting in 1x TE buffer (pH. 8.0). Sections were blocked in 10% fetal bovine serum (FBS)/PBS at RT for 1 hr , then incubated with primary antibodies against Osx (1:100, abcam, MA, ab22552) , Tcf1 (1:50, Cell signaling, MA, 2203S), β-catenin (1:500, Sigma-Aldrich, PA,C7207 ), Wls (1:100, abcam, MA, ab82897), MITF (1:50, Sigma-Aldrich, PA, M6065-100UG), OPG (1:100, abcam, MA, ab79064) and CD3 (1;100, Ventana Medical Systems, AZ, 790–4341) at 4 °C overnight, followed by incubation with fluorescein conjugated (Life Technologies, CA, 1:200), or biotinylated secondary antibodies (Vector Laboratories, CA, 1;100) at RT for 2 hr. For biotinylated secondary antibodies, a third amplification step with streptavidin-conjugated TRITC (1:200, Vector Laboratories, CA) or Horseradish peroxidase (HRP, 1:500, Vector Laboratories, CA) were performed. A diaminobenzidine (DAB) substrate solution (Sigma-Aldrich, PA) was used for developing signals for HRP. All antibodies were diluted in 0.1% Triton-X 100/PBS. For immunohistochemistry on Tcf/Lef1-GFP reporter mice, digit samples were embedded in OCT-compound (Sakura Finetek, CA) following fixation by zinc-buffered formalin and wash in PBS as described above and cut into 10µm section. Sections were incubated in 1x PBS for 5 min. prior to blocking step in 10% FBS. For immunostaining on human digit toe, we obtained de-identified human great toe from human cadaver, and was processed using the same protocol as above. The Institutional Review Board of NYU approved the procedure as an exempt protocol.
Human osteoblasts/osteoclast precursors and mouse nail epithelial cell co-culture with TopFlash Assay
Human osteoblasts and osteoclast precursors were purchased from PromoCell GmbH (Germany, C-12760) and Lonza (MD, 711.98) and cultured according to the manufacturer’s instruction. Osteoblast at passage 3 or 4 and osteoclast precursors were plated in the well of 24-well culture plate at the density of 25,000 cells/well in osteoblast growth media (PromoCell GmbH, Germany, C-27001) and cultured for overnight at 37 °C, 5% CO2. Osteoblasts and osteoclast precursors were transfected with 500ng of TopFLASH or FopFLASH vector (Millipore, MA, 17-285) and 50 ng pRL-TK vector (Promega, WI, E2241), using Lipofectamine 2000 (Invitrogen, CA, 52758). Six hours after lipofection, culture media was replaced, and co-cultured with distal matrix that are transfected with 50nM siRNA against for β-catenin (Invitrogen, CA, MSS202664) or control siRNA (Invitrogen, 462001), using Lipofectamine RNAiMAX (Invitrogen, CA, 56532). Distal matrix cells were dissected and isolated as previously described in previous paper (Takeo et al., 2013). After 72 hr of co-culture with DMSO or 5µM IWP-2(Sigma-Aldrich, PA) in DMSO, distal matrix were removed and luciferase activity measured using Dual-Glo Luciferase activity assay system (Promega, WI, E2920) according to manufacturer’s instructions.
Differentiation assay of human osteoclast precursors
Induction of human osteoclast precursors (Lonza, MD, 711.98) was performed according to the manufacturer’s instruction. Briefly, osteoclast precursors were plated at the density of 25,000 cells/well in 24 well plate and differentiation was induced by adding RANK ligand. At 7th day in culture, differentiation status was examined by TRAP staining.
Beads implantation
We performed bead implantation experiment using previously described method with the following modifications (Takeo et al., 2013; Yu et al., 2010). Affi-Gel Blue Gel beads (Bio-Rad) were washed with PBS then soaked with recombinant mouse Wnt3a (R&D Systems, MN, 1324-WN-002/CF) at a concentration of 40 µg/ml or PBS as a control for 2 hours at room temperature. Beads implantation was performed at 6 month after initial tamoxifen treatment in K14-CreER;Wls cKO mice.
Statistical analysis
Student’s t-test was used to calculate p-values on Microsoft Excel, with two-tailed tests and unequal variance.
Supplementary Material
Acknowledgments
We thank the Genome Technology Center, and The Flow Cytometry and Cell Sorting Center at NYU, and the Center for Functional Genomics at University at Albany for performing microarray. The Genome Technology Center is partially supported by the NYU Cancer Institute Cancer Center Support Grant 5P30CA0016087-32, and NIH/NCI grant P30 CA016087-30. The Flow Cytometry and Cell Sorting Center is partially supported by the NYU Cancer Institute Cancer Center Support Grant 5P30CA0016087-33. M.I. is grateful for the support from NIH/NIAMS grant 1R01AR059768-01A1, 1R01AR066022-01A1, the Arnold and Mabel Beckman Foundation, Ellison medical foundation and funding from Department of Dermatology and Cell Biology, and the Helen and Martin Kimmel Center for Stem Cell Biology at NYU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing or financial interests.
References
- Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81:821–8. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, et al. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200:537–49. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–22. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Baran R, Dawber PR, Haneke E, Tosti A, Bristow I. A Text Atlas of Nail Disorders; Technique in Investigation and Diagnosis. 3. 2003. pp. 1–8. [Google Scholar]
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–27. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–33. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006;38:1245–7. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, et al. -Catenin Activation Regulates Tissue Growth Non-Cell Autonomously in the Hair Stem Cell Niche. Science. 2014;343:1353–6. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy NV, Baig H. Isolated finger injuries in children--incidence and aetiology. Injury. 2000;31:571–3. doi: 10.1016/s0020-1383(00)00052-8. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckman P, Jaeger K, Silva KA, Sundberg JP. Comparative anatomy of mouse and human nail units. Anat Rec (Hoboken) 2013;296:521–32. doi: 10.1002/ar.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojmark AS, Schuster J, Sobol M, Entesarian M, Kilander MB, Gabrikova D, et al. Mutations in Frizzled 6 cause isolated autosomal-recessive nail dysplasia. Am J Hum Genet. 2011;88:852–60. doi: 10.1016/j.ajhg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833–5. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. The Journal of investigative dermatology. 2010;130:1849–59. doi: 10.1038/jid.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Yang X, Lee J, Allan CH, Muneoka K. Development and regeneration of the neonatal digit tip in mice. Dev Biol. 2008;315:125–35. doi: 10.1016/j.ydbio.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso R. Congenital nail defects of the index finger and reconstructive surgery. Seikeigeka Orthopedic surgery. 1969;20:1383–4. [PubMed] [Google Scholar]
- Ito T, Ito N, Saathoff M, Stampachiacchiere B, Bettermann A, Bulfone-Paus S, et al. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol. 2005;125:1139–48. doi: 10.1111/j.0022-202X.2005.23927.x. [DOI] [PubMed] [Google Scholar]
- Kantaputra PN, Mundlos S, Sripathomsawat W. A novel homozygous Arg222Trp missense mutation in WNT7A in two sisters with severe Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Med Genet A. 2010;152A:2832–7. doi: 10.1002/ajmg.a.33673. [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi N, Noda M. Mitf is expressed in osteoclast progenitors in vitro. Exp Cell Res. 2000;260:284–91. doi: 10.1006/excr.2000.5020. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354:846–51. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- Lehoczky JA, Tabin CJ. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci U S A. 2015;112:13249–54. doi: 10.1073/pnas.1518874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Song F, Sun J, Yu H, Liu SS. Suture compression induced bone resorption with intensified MMP-1 and 13 expressions. Bone. 2012;51:695–703. doi: 10.1016/j.bone.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–94. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205:1145–53. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–90. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Hori M, Imamura T, Horii E, Miura T. Method for measurement and evaluation of carpal bone angles. J Hand Surg Am. 1989;14:412–6. doi: 10.1016/0363-5023(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Naz G, Pasternack SM, Perrin C, Mattheisen M, Refke M, Khan S, et al. FZD6 encoding the Wnt receptor frizzled 6 is mutated in autosomal-recessive nail dysplasia. Br J Dermatol. 2012;166:1088–94. doi: 10.1111/j.1365-2133.2011.10800.x. [DOI] [PubMed] [Google Scholar]
- Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15:1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–55. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick D, Feingold ML, Curd J, Niwayama G, Goergen TG. Calcaneal abnormalities in articular disorders. Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and Reiter syndrome. Radiology. 1977;125:355–66. doi: 10.1148/125.2.355. [DOI] [PubMed] [Google Scholar]
- Sakthiswary R, Naicker AS, Htwe O, Shahrir MS, Sazliyana SS. Severe psoriatic acroosteolysis in the absence of psoriatic arthropathy. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.09.2011.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serarslan G, Guler H, Karazincir S. The relationship between nail- and distal phalangeal bone involvement severity in patients with psoriasis. Clin Rheumatol. 2007;26:1245–7. doi: 10.1007/s10067-006-0476-y. [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–96. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499:228–32. doi: 10.1038/nature12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–99. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–8. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Peters C, Saftig P, Bromme D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J Biol Chem. 2009;284:2584–92. doi: 10.1074/jbc.M805280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee EC, Lee J, Muneoka K. BMP signaling induces digit regeneration in neonatal mice. Development. 2010;137:551–9. doi: 10.1242/dev.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. Journal of Molecular and Cellular Cardiology. 2009;46:370–7. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.