Summary
Generating an unlimited source of human insulin-producing cells is a prerequisite to advance β cell replacement therapy for diabetes. Here, we describe a 3D culture system that supports the expansion of adult human pancreatic tissue and the generation of a cell subpopulation with progenitor characteristics. These cells display high aldehyde dehydrogenase activity (ALDHhi), express pancreatic progenitors markers (PDX1, PTF1A, CPA1, and MYC), and can form new organoids in contrast to ALDHlo cells. Interestingly, gene expression profiling revealed that ALDHhi cells are closer to human fetal pancreatic tissue compared with adult pancreatic tissue. Endocrine lineage markers were detected upon in vitro differentiation. Engrafted organoids differentiated toward insulin-positive (INS+) cells, and circulating human C-peptide was detected upon glucose challenge 1 month after transplantation. Engrafted ALDHhi cells formed INS+ cells. We conclude that adult human pancreatic tissue has potential for expansion into 3D structures harboring progenitor cells with endocrine differentiation potential.
Keywords: pancreas, organoid, human, ALDH, endocrine differentiation, beta cells, insulin, progenitor, fetal, diabetes
Highlights
-
•
A 3D culture system can support the expansion of adult human pancreatic tissue
-
•
An ALDHhi cell subpopulation is identified in these organoids
-
•
ALDHhi cells, and not ALDHlo cells, are capable of forming new organoids
-
•
ALDHhi cells show endocrine differentiation potential
In the context of β cell replacement therapy for diabetes, de Koning and colleagues describe a 3D culture platform that supports ex vivo expansion of human pancreatic tissue as organoids. These organoids harbor a subpopulation of ALDHhi cells that display proliferative capacity and can differentiate to an endocrine fate.
Introduction
β Cell replacement therapy is an attractive therapy to achieve normoglycemia in patients with diabetes mellitus due to severe β cell failure (Nijhoff et al., 2016, Ricordi et al., 1989). The shortage of organ donors severely limits the number of patients that are eligible for current β cell replacement therapy, i.e., pancreas or islet transplantation. Mature human β cells cannot be expanded in vitro without complex dedifferentiation and redifferentiation processes (Russ et al., 2008, Gershengorn et al., 2004). Thus, there is an unmet clinical need to generate insulin-producing cells from alternative cell sources to make this therapy more widely available.
Several types of cells have been studied as possible sources of insulin-producing cells, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (iPSCs). While the phenotype of these cells has long been characterized by immature maturation (Hrvatin et al., 2014), recently more glucose-responsive cells have been generated from human pluripotent stem cells in vitro (Pagliuca et al., 2014, Rezania et al., 2014), but safety remains a major concern for any regenerative strategy using hESCs or iPSCs (Lund et al., 2012, Mummery, 2011). An attractive alternative could be the use of putative progenitor cells from adult human pancreas that give rise to the endocrine lineage. Histological studies of human pancreas indicate that neogenesis of insulin-producing cells is associated with the ductal tree in obesity and pregnancy (Butler et al., 2003, Butler et al., 2010). Other studies have also shown that some insulin-producing cells can be generated from cultured human pancreatic ductal tissue (Bonner-Weir et al., 2000, Yatoh et al., 2007, Lee et al., 2010, Klein et al., 2015). We recently showed that in silico analysis of single-cell transcriptome profiles of human adult pancreatic cells using a StemID algorithm predicts a distinct subpopulation of ductal cells with multipotential differentiation potential (Grun et al., 2016). In mice, the existence of postnatal endocrine progenitors within the pancreatic ductal population has become controversial, with lineage-tracing experiments showing contradictory results. Although several studies were able to detect endocrine cells derived from the ductal lineage postnatally or after injury (Inada et al., 2008, Xu et al., 2008, Criscimanna et al., 2011, Al-Hasani et al., 2013), others did not find this (Solar et al., 2009, Kopp et al., 2011, Furuyama et al., 2011).
At present, expansion of human pancreatic cells in a standard, 2D culture system is hampered by the transition of both islet (Russ et al., 2009, Gershengorn et al., 2004) and duct cells (Gao et al., 2003, Seeberger et al., 2006, Todorov et al., 2006) to a mesenchymal cell-like phenotype during passaging. This approach does not provide the natural 3D environment of tissues, and thus important information of cell orientation and polarity for proliferation, growth, and differentiation are lost. In fact, proper alignment and polarization of progenitor cells is known to be required for successful differentiation of fetal pancreatic progenitor cells (Kesavan et al., 2009, Cortijo et al., 2012), and 3D culture of fetal murine pancreatic progenitors can be used to unravel and mimic niches important in pancreas development (Greggio et al., 2013). Thus, it is tempting to hypothesize that 3D culture of adult human pancreatic tissue may provide a microenvironment that enhances expansion and differentiation of pancreatic progenitors.
A Matrigel-based 3D culture system was developed in our institute that yields organoids from stem cells in different organs, with the capacity for long-term expansion and generation of functional differentiated organ-specific cells (Sato et al., 2011, Huch et al., 2013a, Huch et al., 2013b). Single isolated adult mouse pancreatic progenitor cells can be expanded by forming colonies or organoids in a Matrigel-based system (Greggio et al., 2013, Huch et al., 2013a, Jin et al., 2013). We observed that these progenitor cells are derived from the ductal tree, express the stem cell marker leucine-rich repeat containing G protein-coupled receptor 5 (Lgr5) in culture and are able to differentiate toward the endocrine lineage (Huch et al., 2013a).
Results
Human Pancreatic Tissue Expands as Budding Organoids
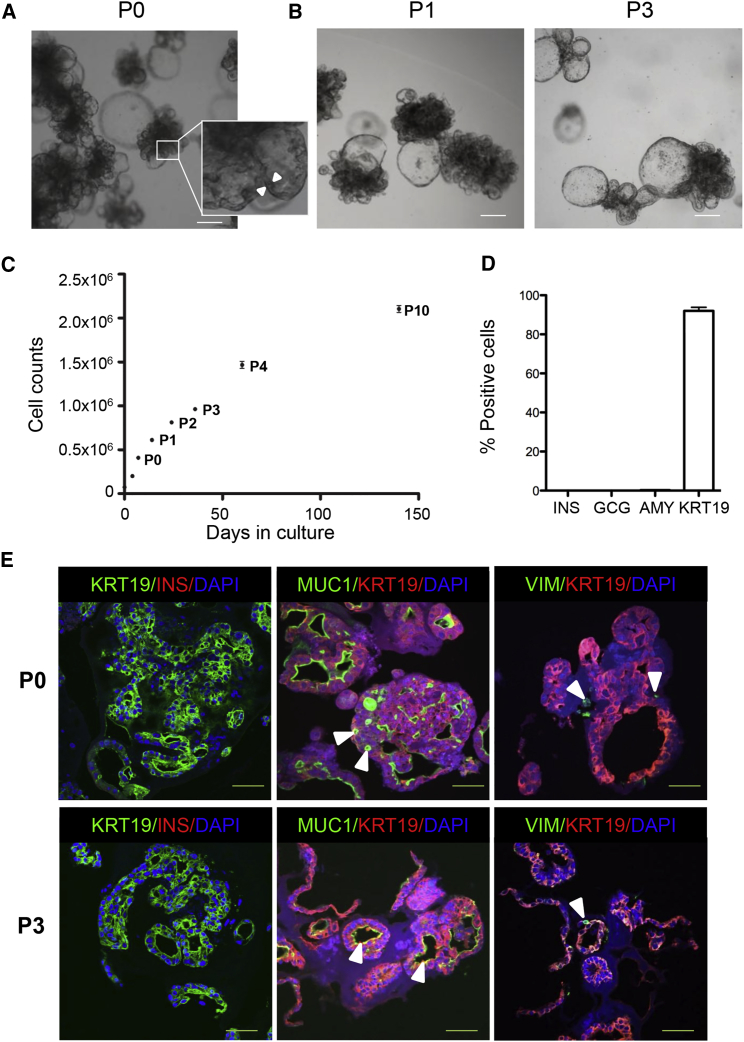
Islet-depleted pancreatic tissue after collagenase digestion was obtained from 35 non-diabetic organ donors (age 53.6 ± 12.1 years and BMI 24.7 ± 4.0 kg/m2) and one organ donor with a history of type 1 diabetes (age 48 years, BMI 21 kg/m2). After mechanical dissociation the small clumps of tissue were embedded in Matrigel and supplied with an epidermal growth factor/Noggin/R-spondin-based expansion medium. More than 90% of small pancreatic cell clusters formed budding structures within 3 days (Movie S1), and expanded with a cauliflower-like appearance by day 7 (Figure 1A). Some larger cyst-like structures were present in the organoids (Figure 1A). Organoids could be passaged without macroscopic changes in phenotype (Figure 1B), and maintained in culture for at least 10 passages (Figure 1C). The calculated rate for cell doubling was ∼67 hr during passage 0 (P0) (n = 3). Growth rate slightly diminished upon passaging (Figure 1C). The proportion of small budding structures and cyst-like structures varied among donors (Figure S1A). Organoids could also be generated from pancreatic tissue from an organ donor with a history of type 1 diabetes (Figure S1B).
Figure 1.
Characterization of Human Pancreatic Organoids during Expansion in a 3D Culture System
(A) Bright-field image of pancreatic organoids (P0), expanded for 7 days in a 3D Matrigel-based culture system, reveals extensive growth with multiple budding structures. Some larger buds have a cyst-like appearance. The insert shows a close-up image of the cell lining (between arrowheads) in a budding structure. Scale bar, 100 μm.
(B) Passaged organoids (P1 and P3 are shown) expanded in similar conditions as P0 (A), also forming budding structures in a cauliflower-like configuration. Scale bar, 100 μm.
(C) Growth curve of pancreatic organoids cultured for 140 days. Cell numbers were counted on days 0, 4, and 7 (P0), and at the end of subsequent passages. Cells in five wells per time point were counted (mean ± SEM). The expansion curve is representative of n = 3 donors.
(D) Quantification of number of cells positive for INS, GCG, AMY, or KRT19 by immunohistochemistry in organoids on day 7 of expansion culture (n = 8 donors; at least 10 organoids/donor were counted; mean ± SEM). DAPI was used as nuclear counterstain.
(E) Non-passaged organoids (P0, upper panels) have similar features compared with passaged organoids (P3, lower panels). Left panels: the majority of the cells are KRT19+ (green), with no INS+ cells (red) detected during the expansion phase. Middle panels: MUC1 (green) staining at the apical cell border indicates polarization of the duct cells. Small lumena are visible within the organoids (arrowheads). Right panels: few vimentin-positive (VIM+) cells (green) are present in the organoids (arrowheads). n = 5 donors; >15 organoids/donor were stained. DAPI was used as nuclear counterstain. Scale bars, 50 μm. See also Figure S1.
Next, we analyzed the cellular composition of organoids in the expansion phase. At day 7 (P0), 92.3% ± 5.4% of organoid cells were positive for the epithelial marker keratin 19 (KRT19), indicating a ductal phenotype (Figures 1D and 1E). Also at passage 3 (P3), the vast majority of the cells still had a ductal phenotype (87.2% ± 8.4% of organoid cells were KRT19+). No insulin-positive (INS+), glucagon-positive (GCG+), or amylase-positive (AMY+) cells were detected in the organoids at day 7 of the expansion phase (P0) (Figures 1D and 1E). The organoids were organized as KRT19+ cells lining cystic or smaller elongated luminal spaces (Figure 1E). In both early and late passages, cells appeared polarized with positive mucin-1 (MUC1) staining at the luminal side of the KRT19+ epithelial lining (Figure 1E). Although human pancreatic cells in 2D culture systems acquire a mesenchymal cell-like phenotype (Gershengorn et al., 2004, Gao et al., 2003, Seeberger et al., 2006, Todorov et al., 2006, Ouziel-Yahalom et al., 2006), few vimentin-positive cells (<2%) were observed in our organoid cultures at P0 or P3 (Figure 1E). Thus, small adult human pancreatic cell clusters can be expanded and passaged in 3D culture, generating polarized ductal budding structures.
Human Pancreatic Organoids Display Pancreatic Progenitors Clustered toward the Tips of the Budding Structures
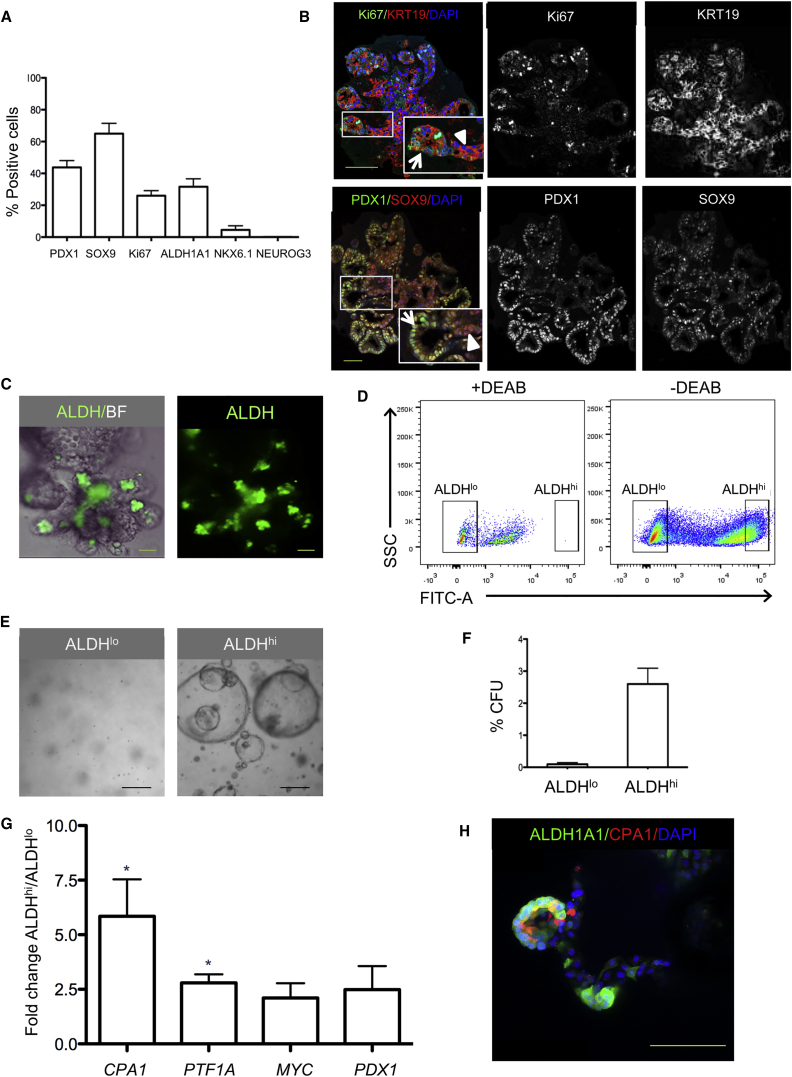
Since extensive growth by budding was observed, we determined the proliferative capacity of the budding structures. Quantification showed that 27.3% ± 8.7% of the cells within an organoid were Ki67+ at day 7 of expansion (Figure 2A). Only a few Ki67+ cells were negative for the duct marker KRT19 (1.3% ± 0.8% of Ki67+ cells). Ki67+ cells were mainly observed in tips of buds and were not frequently observed in trunk regions (Figure 2B). Also, budding structures with wide tips and narrow trunks were present in the organoids (Figures 2B and 2C). Labeling with the nucleoside analog 5-ethynyl-2′-deoxyuridine that incorporates into newly synthesized DNA further confirmed the location of the proliferative cells toward the tip region (data not shown).
Figure 2.
Tips of Budding Structures of Pancreatic Organoids Contain Cells with Progenitor Cell Characteristics
(A) Quantification of cells positive for pancreatic progenitor and proliferation markers in organoids on day 7 of expansion culture. Organoids derived from four to six donors were analyzed (at least ten organoids/donor). Data are expressed as mean ± SEM.
(B) Confocal image of pancreatic organoids on day 7 of expansion. Top: organoids were stained for the proliferation marker Ki67 (green) and ductal marker KRT19 (red). Organoid budding structures show a narrow trunk region (arrowhead) and a wider tip region (arrow). Bottom: organoids were stained for SOX9 (red) and PDX1 (green). SOX9+ cells were present both in the buds and in the trunk region of the organoids (arrowhead). PDX1+ cells (green), which frequently co-expressed SOX9 (yellow), were mainly located in the budding structures with strong staining for PDX1 often observed in cells at the outermost tip regions (arrow). Both overlay and individual channels are depicted. DAPI was used as nuclear counterstain. Scale bars, 100 μm.
(C) Organoids in expansion phase for 7 days were labeled with the Aldefluor fluorescent reagent system, marking progenitor cells characterized by high ALDH activity. Most ALDHhi cells (green) were located in the tips of organoid buds. Scale bar, 50 μm.
(D) FACS analysis of dispersed organoid cells (after 7 days in expansion culture) labeled with Aldefluor with and without the ALDH inhibitor DEAB. The FACS plot shows how ALDHhi cells (i.e., cells that express high ALDH activity) and ALDHlo cells (i.e., cells that express low ALDH activity) are selected. Representative plot from n = 10 donors.
(E) Bright-field images of sorted and expanded single ALDHlo cells (left panel) and ALDHhi cells (right panel) in Matrigel for 14 days. Scale bar, 100 μm.
(F) Proportion of sorted ALDHlo and ALDHhi cells with colony-forming (organoid-forming) potential. Data represent mean ± SEM (n = 3 donors).
(G) Gene expression of CPA1, PTF1A, MYC, and PDX1 in sorted ALDHlo and ALDHhi cells derived from organoids expanded for 7 days. The graph shows the gene expression ratio in ALDHhi to ALDHlo cells for the different markers. Mean ± SEM (n = 3 donors) ∗p < 0.05.
(H) Whole-mount immunostaining for ALDH1A1 and CPA1 of organoids expanded for 7 days. Confocal images show ALDH1A1+ cells (green) and CPA1+ cells (red) in the tip of the budding structures. Some cells co-express the two markers. Scale bar, 50 μm.
CFU, colony-forming unit. See also Figures S2 and S3.
Based on the configuration of the budding structures, we hypothesized that the tips of the budding structures would be enriched for pancreatic progenitor cells, as has been reported for mouse fetal pancreatic development (Zhou et al., 2008, Shih et al., 2013). Immunostaining of the organoids for the pancreatic progenitor markers pancreatic and duodenal homeobox 1 (PDX1) and SRY (sex-determining region Y) box 9 (SOX9) (Figure 2B) showed high PDX1 expression in the budding structures of the organoids, particularly in tip regions, while SOX9 was more homogeneously distributed (Figure 2B). SOX9 and PDX1 gene expression increased during expansion (Figure S2A). Furthermore, gene expression of LGR5 increased during this time (Figure S2B), and a subset of cells in budding structures clearly expressed LGR5 mRNA, as assessed by smFISH (Figure S2C). No neurogenin-3-positive (NEUROG3+) cells were observed in organoids in the expansion phase (Figure 2A). Next, we exposed the organoids to a fluorescent reagent (Aldefluor) that identifies progenitor cells based on their increased aldehyde dehydrogenase activity (ALDH) activity. Pancreatic progenitors with high expression of ALDH1 isoforms have recently been identified in both developing and adult mouse pancreas (Ioannou et al., 2013, Rovira et al., 2010). Cells concentrated in the tips of the budding structures showed high ALDH activity (Figure 2C). The Aldefluor reagent is optimized to detect enzyme activity of ALDH1 isoforms, and we found that ALDH1A1 immunostaining co-localized with both KRT19+ and KRT19− cells in the tips of the organoid buds, indicating the presence of heterogeneous populations of ALDH1A1+ cells (data not shown). High ALDH activity arose during organoid culture as no ALDHhi cells were found in the d0 islet-depleted tissue (n = 3; data not shown). LGR5 gene expression was higher in sorted ALDHhi compared with ALDHlo cells (Figure S2D).
Single ALDHhi Cells Show Progenitor Characteristics
To determine whether ALDHhi cells have characteristics of progenitor cells, organoids were dispersed into single cells, labeled with Aldefluor, and sorted by fluorescence-activated cell sorting (FACS) (ALDHlo cells 34.0% ± 7.4% and ALDHhi cells 25.4% ± 6.0%, n = 5) (Figure 2D). A subpopulation of ALDHhi cells derived from organoids was able to generate small cyst-like colonies in 3D culture (2.2% ± 0.8% of ALDHhi cells), in marked contrast to ALDHlo cells that formed no colonies (Figures 2E, 2F, and S3A). When organoids derived from single-sorted ALDHhi cells were immunostained for ALDH1A1, both ALDH1A1+ and ALDH1A1− cells were observed, indicating the generation of a heterogeneous cell population from a single ALDHhi cell (Figure S3B). When these labeled organoids were sorted once more, yet again only ALDHhi cells showed colony-forming potential in 3D culture (data not shown). Interestingly, complex budding structures were not observed when single ALDHhi cells were expanded, which could indicate the requirement of supporting cells for self-assembly into a ductal-tree-like configuration (Figure S3A).
Next, we characterized the ALDHhi cell population for the presence of markers previously described for multipotent progenitor cells in mouse pancreatic organogenesis (Zhou et al., 2008). Gene expression levels of the markers carboxypeptidase A1 (CPA1) and pancreas-specific transcription factor, 1a (PTF1A) were significantly upregulated in ALDHhi cells compared with the ALDHlo fraction (Figure 2G). Immunoreactivity for CPA1 was found in ALDH1A1+ cells in the tips of organoid budding regions, but some ALDH1A1+ cells were CPA1− (Figure 2H). Importantly, these ALDH1A1+ cells were negative for the acinar cell marker amylase (Figure S3C). Many ALDH1A1+ cells in the tips of budding regions buds also co-stained for PDX1 (Figure S3D). Genes that are known to be upregulated in mouse centroacinar (CAC) cells, such as HES1, SOX9, SCA1, MET, NES, and HEY1 (Rovira et al., 2010, Kopinke and Murtaugh, 2010), were analyzed in sorted ALDHhi and ALDHlo cells. No upregulation of these markers was present in ALDHhi compared with ALDHlo cells (Figure S3E). HES1+ cells were present in expanded organoids after 7 days, but their distribution was not well defined (Figure S3F). Thus, a subpopulation of ALDHhi cells within human pancreatic organoids has colony formation capacity and expresses pancreatic progenitor markers.
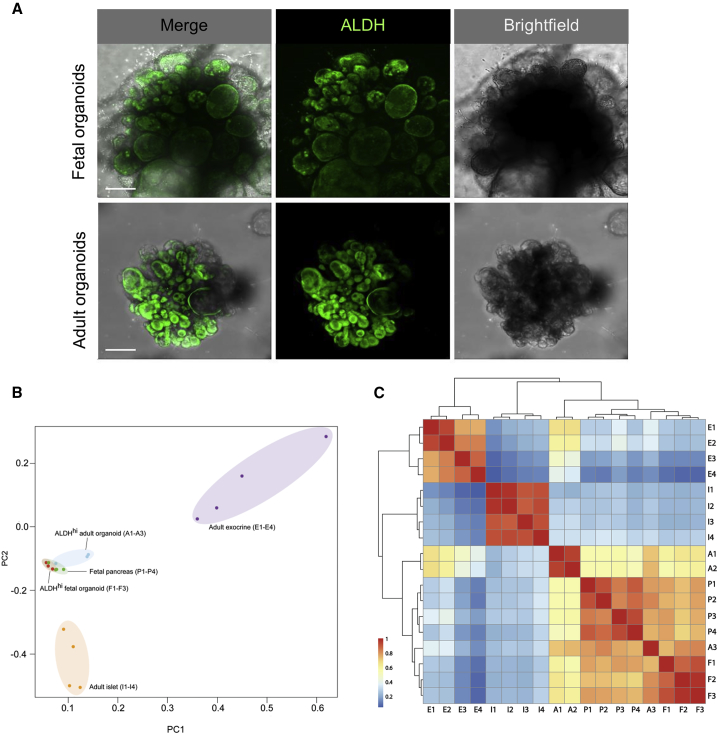
Transcriptional Profiling Shows Clustering of ALDHhi Cells from Adult and Fetal Pancreatic Organoids
Since the self-assembly of adult human pancreatic tissue into organoids in our 3D culture system resembles budding structures previously described in pancreatic development, we compared characteristics of organoids derived from adult pancreatic tissue (adult pancreatic organoids) with human fetal pancreatic tissue and organoids derived from this fetal pancreatic tissue (fetal pancreatic organoids). We observed that fetal pancreatic organoids displayed a similar morphology to adult pancreatic organoids under the same culture conditions (Figure 3A). ALDHhi cells were predominantly located in budding structures (Figure 3A). This observation strengthened our hypothesis that ALDHhi cells that are concentrated in tip regions have pancreatic progenitor characteristics. We set out to determine the level of similarity of these putative ALDHhi progenitors isolated from adult pancreatic organoids with ALDHhi cells derived from fetal pancreatic organoids. Global gene expression analysis revealed that ALDHhi cells from adult pancreatic organoids are transcriptionally closer to ALDHhi cells isolated from fetal pancreatic organoids than to the adult exocrine tissue they originated from (Figures 3B and 3C). In contrast, ALDHhi cells from fetal pancreatic organoids retained their more primitive identity, clustering closely to fetal pancreatic tissue (Figure 3B). Furthermore, expression levels of the multipotent progenitor markers CPA1, PTF1A, PDX1, and MYC in ALDHhi cells from adult pancreatic organoids were comparable with those in ALDHhi cells from organoids derived from fetal pancreas (no significant difference, p < 0.05; data not shown).
Figure 3.
Transcriptional Profiling Shows Clustering of ALDHhi Cells from Adult and Fetal Pancreatic Organoids
(A) Aldefluor labeling of adult and fetal human pancreatic organoids expanded for 7 days. Maximum projection (confocal imaging). Scale bar, 100 μm.
(B) Principal-component analysis for gene expression profiles of the following samples: A1–A3: ALDHhi cells sorted from organoids after 7 days expansion derived from human adult pancreatic tissue (age 50.0 ± 18.7 years, BMI 22.0 ± 4.0 kg/m2). F1–F3: ALDHhi cells sorted from organoids after 7 days expansion derived from human fetal pancreatic tissue (gestational age: F1 9 weeks, F2 20 weeks, and F3 22 weeks). P1–P4: primary fetal pancreatic tissue (gestational age: P1 9 weeks, P2 18 weeks, P3 14 weeks, and P4 10 weeks). I1–I4: adult human islets (age 34.5 ± 17.3 years, BMI 23.8 ± 4.5 kg/m2). E1–E4: adult exocrine (islet-depleted) pancreatic tissue (age 63.5 ± 5.7 years, BMI 25.5 ± 3.7 kg/m2).
(C) Correlation cluster analysis for the gene expression profiles of the samples described in (B).
Pancreatic Organoids Can Differentiate toward an Endocrine Fate In Vitro
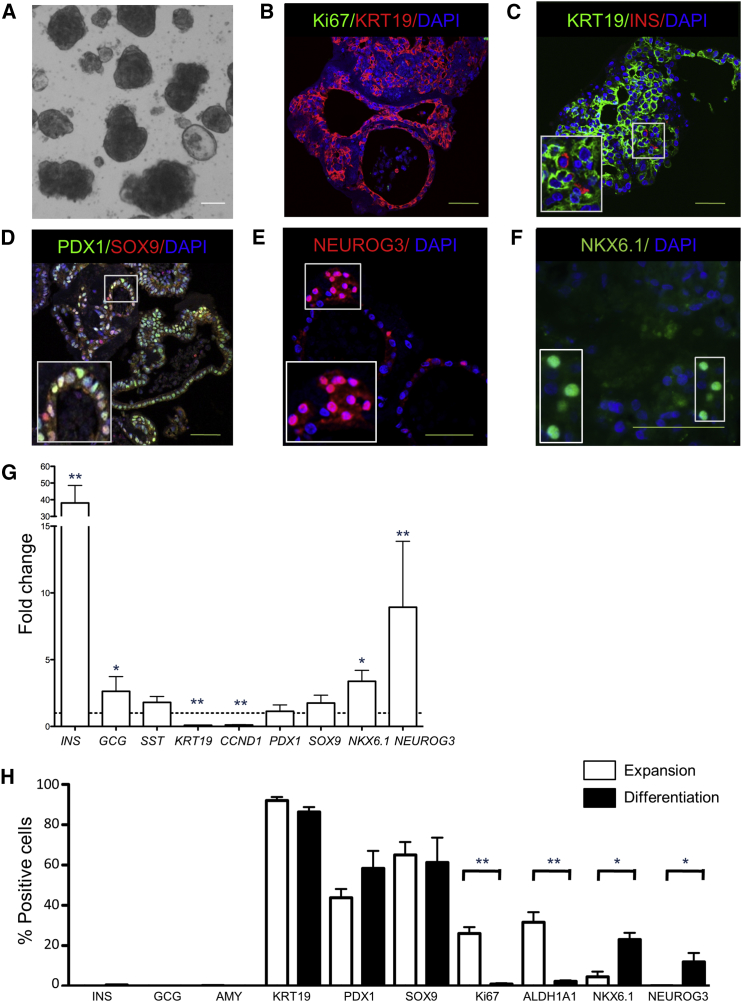
Since pancreatic organoids expanded from adult human exocrine tissue express progenitor markers during expansion, their differentiation potential was studied using culture conditions reported to differentiate human duct cells (Yatoh et al., 2007, Gao et al., 2005). When P0 organoids were transferred to low attachment plates containing differentiation medium, their budding structure appeared to collapse and a rounded shape was seen after 7 days (Figure 4A). By then, growth had almost completely stopped as indicated by the near absence of Ki67+ cells (0.9% ± 0.4% of total cells; Figures 4B and 4H) and reduced Cyclin D1 (CCND1) gene expression (Figure 4G). Furthermore, the proportion of ALDH1A1+ cells decreased considerably from 27.0% ± 14.5% (P0 expansion day 7) to 1.4% ± 1.3% of total cells (differentiation day 7) (Figure 4H).
Figure 4.
Endocrine Cell Markers in Pancreatic Organoids after 7 Days of In Vitro Differentiation
(A) Bright-field image of human pancreatic organoids initially expanded for 7 days followed by differentiation culture for 7 days. DAPI was used as nuclear counterstain. Scale bar, 100 μm.
(B) Confocal image of pancreatic organoids immunostained for Ki67 (green) and KRT19 (red). The majority of cells were KRT19+. No cells were Ki67+. DAPI was used as nuclear counterstain. Scale bar, 50 μm.
(C) Confocal image of pancreatic organoids immunostained for KRT19 (green) and INS (red). Few INS+ (0.52%) cells were present. DAPI was used as nuclear counterstain. Scale bar, 50 μm.
(D) Confocal image of pancreatic progenitor markers PDX1 (green) and SOX9 (red). DAPI was used as nuclear counterstain. Scale bar, 50 μm.
(E) Confocal image of pancreatic progenitor marker NEUROG3 (red). DAPI was used as nuclear counterstain. Scale bar, 50 μm.
(F) Confocal image of pancreatic progenitor marker NKX6.1 (green). DAPI was used as nuclear counterstain. Scale bar, 50 μm.
(G) Gene expression of several markers in organoids on day 7 of differentiation compared with organoids on day 7 of expansion. Gene expression in expansion organoids were set to 1 (dotted line). Mean ± SEM, n = 8 donors. ∗p < 0.05 and ∗∗p < 0.01.
(H) Percentage of cells immunostained for different markers on day 7 of expansion (white bars) compared with day 7 of differentiation (black bars) using confocal images. More than 10 organoids per donor were assessed. Mean ± SEM, n = 4–6 donors. ∗p < 0.05 and ∗∗p < 0.01.
See also Figure S4.
A marked increase in insulin gene expression after 7 days of differentiation was observed when compared with the end of the expansion phase (Figure 4G). Despite the considerable increase in insulin gene expression with differentiation, few INS+ cells were observed by immunostaining (0.52% ± 0.22%; Figures 4C and 4H). GCG gene expression was only slightly increased between expansion and differentiation (Figure 4G), and no GCG+ cells were present in the differentiation phase (Figure 4H). To exclude the possibility that insulin was taken up from the medium, we confirmed that INS+ cells were also positive for human C-peptide (Figure S4A). No AMY+ cells were observed and the majority of cells remained KRT19+ during differentiation (83.4% ± 14.5% of cells; Figure 4H), even though gene expression of KRT19 decreased (Figure 4G).
Gene expression of the endocrine progenitor marker NEUROG3 and β cell marker NKX 6 homeobox 1 (NKX6.1) were significantly upregulated during the differentiation phase (Figure 4G). NEUROG3+ cells could also be identified by immunostaining in the “collapsed” organoids (Figures 4E and 4H). In addition, more cells expressed NKX6.1 in the differentiation phase compared with the expansion phase (Figures 4F and 4H). Conversely, no change was found in gene expression of the pancreatic progenitor markers PDX1 and SOX9 upon differentiation (Figure 4G) or the number of PDX1+ and SOX9+ cells (Figures 4D and 4H). Thus, upon in vitro differentiation, pancreatic progenitors can be directed along the endocrine lineage.
Human Pancreatic Tissue Can Be Cryopreserved without Losing Expansion or Differentiation Capacities
With therapeutic purposes in mind, we explored the possibility of cryopreserving pancreatic tissue before organoid expansion. Freshly retrieved tissue (day 0) was compared with cryopreserved tissue (day 0) from the same donors (n = 4). Growth of organoids with budding structures was observed from both groups (Figure S4B), but the organoids grown from cryopreserved starting material had more cystic appearance than organoids grown from freshly isolated material. When subjected to in vitro differentiation, no significant differences in gene expression were found (Figure S4C). Thus, organoids can be grown from cryopreserved primary human adult pancreatic tissue.
Human Pancreatic Organoids Generate Insulin-Producing Cells In Vivo
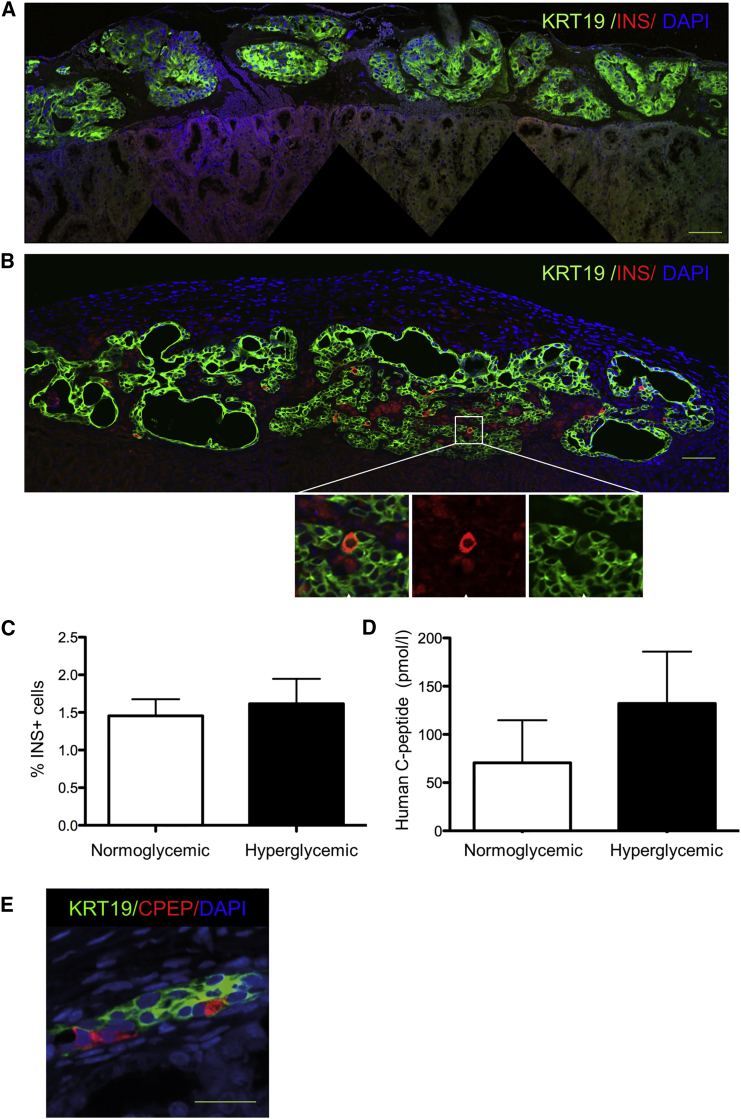
While the in vitro differentiation experiments indicated differentiation toward an endocrine lineage based on gene expression analysis, only a few INS+ cells were observed. It is well known that human ESC differentiate into insulin-producing cells after implantation into mice (Kroon et al., 2008). Therefore, we tested the capacity of pancreatic organoids to further differentiate in vivo after transplantation under the kidney capsule of immunodeficient mice. One day after transplantation the majority of grafted cells were KRT19+ and no INS+ cells were observed (Figure 5A). However, pancreatic organoids from the same donor 1 month after transplantation showed INS+ cells within the ductal lining (Figure 5B). Organoids derived from each donor (n = 8) were able to generate 1.5% ± 0.2% INS+ cells within the ductal lining (n = 8; Figure 5B). No difference in the proportion of INS+ cells was observed in the hyperglycemic animals (Figure 5C). Production of insulin was confirmed by immunostaining of C-peptide in INS+ cells (CPEP) (Figure S5A). Furthermore, insulin was co-expressed with several functional endocrine markers (PDX1, IAPP, NKX6.1, and SYP) but not with GCG (Figures S5B–S5E). The differentiation phase in vitro was necessary for the appearance of INS+ cells as transplantation of organoids 1 week after expansion yielded either no grafts or only large cystic structures with no hormone-positive cells (data not shown).
Figure 5.
Human Pancreatic Organoids Generate Insulin-Producing Cells In Vivo
Grafts composed of in vitro differentiated organoids derived from islet-depleted exocrine pancreatic tissue were transplanted under the mouse kidney capsule after 7 days of expansion and 7 days of differentiation.
(A) Day 1 after transplantation. Immunostaining for KRT19 (green) and INS (red). No INS+ cells are present. DAPI (blue) was used as nuclear counterstain. Scale bar, 50 μm.
(B) Day 30 after transplantation. Transplanted organoids formed a ductal network (KRT19, green) and many INS+ cells (red) are present within the ductal lining (arrows indicate INS+ cells). DAPI (blue) was used as nuclear counterstain. Scale bar, 50 μm.
(C) Percentage of INS+ cells in the ductal lining 1 month after transplantation. For each donor, grafts were transplanted in both normoglycemic and hyperglycemic mice, and two mice were transplanted per donor. No significant difference in the number of INS+ cells between the two groups of mice was found. Mean ± SEM, n = 8 donors.
(D) Human C-peptide concentration after a glucose challenge in mice 1 month after organoid transplantation. Grafts were transplanted in either normoglycemic mice or hyperglycemic mice, and two mice were transplanted per donor. No significant difference in human C-peptide concentration between the two groups of mice was observed. Mean ± SEM, n = 8 donors.
(E) Confocal image of a graft of sorted ALDHhi cells isolated from organoids after expansion for 7 days. The ALDHhi cells were differentiated for 7 days in vitro before transplantation under the kidney capsule of normoglycemic mice (n = 3 donors, 1 mouse per donor). The graft was retrieved after 50 days and contained both KRT19+ and CPEP+ cells. Cells co-expressing KRT19 and C-peptide indicate a transition stage from a duct (-like) phenotype to a β (-like) cell phenotype. DAPI (blue) was used as nuclear counterstain. Scale bar, 50 μm.
See also Figure S5.
Human C-peptide could be readily measured in normoglycemic and hyperglycemic mice after a glucose challenge indicating that human insulin was released into the circulation upon a common β cell stimulus (Figure 5D). Basal human C-peptide was also present in normoglycemic mice before the glucose challenge (89.2 ± 59.9 pmol/L, n = 8). Blood glucose of grafted mice did not decrease significantly in either hyperglycemic mice (26.5 ± 3.9 mmol/L at day 0 versus 24.3 ± 3.8 mmol/L at day 30, n = 8) or in the normoglycemic group (8.6 ± 1.3 mmol/L at day 0 versus 6.7 ± 2.4 mmol/L at day 30, n = 8). Thus, graft function was detectable but not sufficient to restore normoglycemia within 1 month after organoid transplantation in hyperglycemic mice.
Sorted ALDHhi cells derived from organoids expanded for 7 days were also differentiated for 1 week in vitro and subsequently transplanted under the kidney capsule of normoglycemic immunodeficient mice. After 50 days, retrieved grafts contained both KRT19+ and C-peptide+ cells (Figure 5E).
Discussion
Our data indicate that adult human pancreatic tissue can be expanded as 3D organoids and long-term expansion can be achieved by passaging these pancreatic organoids. Cryopreservation of the pancreatic tissue is possible without losing these characteristics. A subpopulation of cells from the organoids have progenitor characteristics and give rise to endocrine cells after transplantation.
Progress to expand human adult pancreatic tissue and thus exploration of its capacity for endocrine cell differentiation has been hampered by epithelial-to-mesenchymal transition in 2D culture systems. The 3D culture system we have developed here provides an environment that allows substantial growth of human pancreatic cells starting from minced tissue. Recently we showed that adult mouse pancreatic organoids can also be expanded using similar culture conditions (Huch et al., 2013a). We and others described Matrigel-based culture methods for expansion of dispersed exocrine cells from adult mouse (Huch et al., 2013a, Jin et al., 2013) and human (Boj et al., 2015) pancreas and for expansion of genetically modified human pancreatic ductal cells (Lee et al., 2013). The striking aspect of our expansion protocol is the self-organization of the tissue into organoids with complex budding structures. These organoids display a remarkable KRT19+ epithelial tree-like structure with apical-basal polarity. This configuration has been shown for mouse embryonic pancreas tissue in vivo and in vitro (Greggio et al., 2013, Zhou et al., 2008), and is present during human pancreatic organogenesis (Jennings et al., 2013). Similar culture systems enabled the expansion of human intestine and stomach in complex cell configurations, also called organoids, that appear to recapitulate organogenesis and tissue regeneration (Sato et al., 2011, Barker et al., 2010).
During embryogenesis, the tip regions of the expanding ductal trees in the developing pancreas harbor progenitor cells (Zhou et al., 2008, Jennings et al., 2013, Solar et al., 2009); and, in adult human tissue, we predicted, from single-cell transcriptome data, that the progenitor population in human adult pancreatic cells resides among the ductal population (Grun et al., 2016). The morphology of the pancreatic organoids (derived from adult pancreatic tissue) in our study allowed us to search for putative progenitors in the budding structures. ALDHhi cells had differentially high expression of the progenitor markers PDX1, CPA1, MYC, and PTF1A (Zhou et al., 2008). In addition, we established organoid culture from human fetal pancreatic tissue. These fetal pancreatic organoids also contained ALDHhi cells in budding structures. Interestingly, transcriptional profiling revealed that ALDHhi cells from adult pancreatic organoids were closer to ALDHhi cells from fetal pancreatic organoids than to adult exocrine tissue (i.e., the islet-depleted tissue before expansion). This indicates a reprogramming of a subset of adult pancreatic cells to a progenitor-like stage.
Organoids expanded from human pancreas and transplanted in immunodeficient mice gave rise to insulin-producing cells after 1 month. The location of the insulin-producing cells in the grafts, i.e., in close proximity of or within the ductal lining, and the increased number of insulin-positive cells after engraftment compared with the initial cell population, support the concept that these cells were newly generated. However, while human C-peptide could be detected in the circulation the number of β cells was not sufficient to restore normoglycemia in diabetic mice 1 month after transplantation. In a recent study by Lee et al. (2013), the authors also showed that a subpopulation of expanded ductal cells could differentiate to insulin-secreting cells using an alternative approach of adenovirus-mediated expression of pro-endocrine factors and co-transplantation with mouse embryonic fibroblasts. In summary, although culture conditions need to be optimized to increase graft efficacy, our study provides a proof-of-concept that this 3D culture system can be used to expand primary human ductal cells that can differentiate to an endocrine fate without the need for genetic modification.
The origin of the ALDHhi cells in our system is unclear. An ALDHhi progenitor cell population has been identified in a subset of adult ductal/CAC cells (Rovira et al., 2010) and in the developing pancreas (Ioannou et al., 2013) of mice, and these cells had the ability to self-renew and to differentiate into both endocrine and exocrine cells.
Notably, several studies have recently attributed a functional role to ALDH1 isoforms during pancreas development in humans (Li et al., 2014), mice (Ostrom et al., 2008), and zebrafish (Matsuda et al., 2013). Our data indicate that the specific culture conditions we developed support this progenitor state. Whether these ALDHhi cells have a CAC origin or not warrants further investigation. At present this question is challenging as there is a lack of markers specific for human adult CACs. In mature murine pancreas, HES1 expression marks terminal ductal or CACs, whereas it identifies MPCs in early embryonic pancreas (until e11.5), and exocrine-restricted progenitors from e13.5 until birth (Kopinke and Murtaugh, 2010). Here, we found that HES1 was slightly upregulated at the mRNA level in ALDHhi cells, but HES1 protein expression was not restricted to the tips of the budding structures where the majority of ALDHhi cells reside. Finally, the ALDHhi population is heterogeneous, with only a small proportion of cells able to expand in vitro. Therefore, additional cell surface markers will be necessary to efficiently enrich and characterize the progenitor subpopulation from this heterogeneous population of ALDHhi cells.
It has been unclear whether an endocrine/multipotent progenitor population exists in the adult human pancreas. Indeed, classical definitions of what constitutes an adult stem cell population are under debate (Clevers, 2015). However, we provide a proof-of-concept for the existence of a ALDHhi population of human pancreatic cells (most likely KRT19+ cells) that appears under specific culture conditions, and that has the capacity to differentiate into an endocrine cell phenotype. Aldefluor has been used to detect ALDHhi stem/progenitor cells in multiple tissues (Balber, 2011). Here, we show that this cell population expresses the pancreatic progenitor markers CPA1, PDX1, MYC, and PTF1A. Furthermore, the gene expression profile of adult pancreatic ALDHhi population presents a high degree of similarity with fetal pancreatic ALDHhi cells.
Finally, since these cells are derived from the adult human pancreas and, thus, have already committed to a pancreatic fate, differentiation to the β cell lineage may be easier to achieve than differentiation of other adult stem/progenitor cells. In current allogeneic and autologous islet transplantation contaminating non-islet cells from the human pancreas are always co-transplanted with islets into human recipients without adverse effects (Ichii et al., 2008). Therefore, expanded and differentiated adult human pancreatic cells are likely to be relatively safe compared with embryonic or induced pluripotent cell lines for future β cell replacement therapy programs.
Experimental Procedures
Human Primary Tissue
Adult Pancreatic Tissue
Islet-depleted pancreatic tissue and human islets that could not be used for clinical transplantation were used in the studies according to national laws and if research content was available.
Fetal Pancreatic Tissue
Fetal pancreatic tissue from elective abortions was used after written (parental) informed consent was provided. Collection and use of human fetal tissue for research was approved by the Medical Ethics committee of the LUMC.
Generation of Human and Fetal Pancreatic Organoids
Human adult islet-depleted pancreatic tissue was obtained after human islet isolation procedures. For culture of adult pancreatic organoids, small pieces of exocrine tissue were plated and expanded in Matrigel (BD Biosciences). Human fetal pancreatic tissue was obtained from fetuses with a gestational age of 9, 20, and 22 weeks. For culture of fetal pancreatic organoids, small pieces of human fetal pancreatic tissue were plated and expanded in Matrigel similar to adult pancreatic organoids. A progenitor population within the organoids was identified using the Aldefluor fluorescent reagent system (STEMCELL), which is based on detection of high ALDH activity in progenitor cells. ALDHhi and ALDHlo cell populations were sorted and plated in Matrigel before analysis. Human and fetal pancreatic organoids were analyzed by immunohistochemistry, RNA sequencing, qPCR, and smFISH, For a detailed description see Supplemental Experimental Procedures.
In Vitro and In Vivo Differentiation of Human Pancreatic Organoids
After expansion, adult pancreatic organoids were retrieved from the Matrigel and analyzed, cryopreserved in liquid nitrogen, or transferred to a differentiation culture medium (3 mL), characterized by absence of growth factors, in six-well hydrophobic plates (Corning). Organoids were cultured for 7–18 days before analysis or transplantation under the kidney capsule in normoglycemic or hyperglycemic immunodeficient mice. Human C-peptide was measured and grafts were analyzed up to 1 month after transplantation. For a detailed description see Supplemental Experimental Procedures.
Author Contributions
C.J.M.L. and N.W.G. performed the key experiments and co-wrote the manuscript with E.J.P.d.K., H.C., and F.C. J.B. performed the experiments with fetal pancreatic organoids. F.R. and L.v.G. provided technical assistance. M.H., S.F.B., T.S., H.C., R.G.J.V., and H.H. provided expertise on organoids culture systems and culture of human cells. S.B.-W. provided expertise on human pancreatic duct cells. T.J.R. provided the infrastructure for human pancreatic tissue isolation. L.K. and A.v.O. gave support for the RNA sequencing experiments. S.M.C.d.S.L. and M.S.R. provided human fetal pancreatic tissue. M.A.E. provided human adult pancreatic tissue.
Acknowledgments
This work was financially supported by the DON foundation, the Dutch Diabetes Research Foundation, the Tjanka Foundation, and the Diabetes Cell Therapy Initiative. We thank Dr. Vrolijk for help with analyses of immunostaining (ImageJ-based data analyses), Yves Heremans (H.H. lab) and Annemieke Tons (Leiden University Medical Center, Leiden) for helping with the NEUROG3 staining, the Hubrecht imaging center for microscope assistance, and Stefan van der Elst (Geijsen lab, Hubrecht Institute, Utrecht) for help with FACS and analysis.
Published: March 13, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, two tables, and one movie and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.02.005.
Accession Numbers
The accession number for the RNA sequencing data reported in this paper is GEO: GSE108854.
Supplemental Information
References
- Al-Hasani K., Pfeifer A., Courtney M., Ben-Othman N., Gjernes E., Vieira A., Druelle N., Avolio F., Ravassard P., Leuckx G. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell. 2013;26:86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Balber A.E. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 2011;29:570–575. doi: 10.1002/stem.613. [DOI] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Boj S.F., Hwang C.I., Baker L.A., Chio I.I., Engle D.D., Corbo V., Jager M., Ponz-Sarvise M., Tiriac H., Spector M.S. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S., Taneja M., Weir G.C., Tatarkiewicz K., Song K.H., Sharma A., O'Neil J.J. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Butler A.E., Cao-Minh L., Galasso R., Rizza R.A., Corradin A., Cobelli C., Butler P.C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. STEM CELLS. What is an adult stem cell? Science. 2015;350:1319–1320. doi: 10.1126/science.aad7016. [DOI] [PubMed] [Google Scholar]
- Cortijo C., Gouzi M., Tissir F., Grapin-Botton A. Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep. 2012;2:1593–1606. doi: 10.1016/j.celrep.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimanna A., Speicher J.A., Houshmand G., Shiota C., Prasadan K., Ji B., Logsdon C.D., Gittes G.K., Esni F. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology. 2011;141:1451–1462. doi: 10.1053/j.gastro.2011.07.003. 1462.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K., Kawaguchi Y., Akiyama H., Horiguchi M., Kodama S., Kuhara T., Hosokawa S., Elbahrawy A., Soeda T., Koizumi M. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Gao R., Ustinov J., Pulkkinen M.A., Lundin K., Korsgren O., Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Gao R., Ustinov J., Korsgren O., Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48:2296–2304. doi: 10.1007/s00125-005-1935-8. [DOI] [PubMed] [Google Scholar]
- Gershengorn M.C., Hardikar A.A., Wei C., Geras-Raaka E., Marcus-Samuels B., Raaka B.M. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- Greggio C., De F.F., Figueiredo-Larsen M., Gobaa S., Ranga A., Semb H., Lutolf M., Grapin-Botton A. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun D., Muraro M.J., Boisset J.C., Wiebrands K., Lyubimova A., Dharmadhikari G., van den Born M., van E.J., Jansen E., Clevers H. De novo prediction of stem cell identity using single-cell transcriptome data. Cell Stem Cell. 2016;19:266–277. doi: 10.1016/j.stem.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S., O'Donnell C.W., Deng F., Millman J.R., Pagliuca F.W., DiIorio P., Rezania A., Gifford D.K., Melton D.A. Differentiated human stem cells resemble fetal, not adult, beta cells. Proc. Natl. Acad. Sci. USA. 2014;111:3038–3043. doi: 10.1073/pnas.1400709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Bonfanti P., Boj S.F., Sato T., Loomans C.J., van de Wetering M., Sojoodi M., Li V.S., Schuijers J., Gracanin A. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii H., Miki A., Yamamoto T., Molano R.D., Barker S., Mita A., Rodriguez-Diaz R., Klein D., Pastori R., Alejandro R. Characterization of pancreatic ductal cells in human islet preparations. Lab. Invest. 2008;88:1167–1177. doi: 10.1038/labinvest.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou M., Serafimidis I., Arnes L., Sussel L., Singh S., Vasiliou V., Gavalas A. ALDH1B1 is a potential stem/progenitor marker for multiple pancreas progenitor pools. Dev. Biol. 2013;374:153–163. doi: 10.1016/j.ydbio.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R.E., Berry A.A., Kirkwood-Wilson R., Roberts N.A., Hearn T., Salisbury R.J., Blaylock J., Piper H.K., Hanley N.A. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514–3522. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Feng T., Shih H.P., Zerda R., Luo A., Hsu J., Mahdavi A., Sander M., Tirrell D.A., Riggs A.D. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc. Natl. Acad. Sci. USA. 2013;110:3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan G., Sand F.W., Greiner T.U., Johansson J.K., Kobberup S., Wu X., Brakebusch C., Semb H. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Klein D., Alvarez-Cubela S., Lanzoni G., Vargas N., Prabakar K.R., Boulina M., Ricordi C., Inverardi L., Pastori R.L., Dominguez-Bendala J. BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes. 2015;64:4123–4134. doi: 10.2337/db15-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D., Murtaugh L.C. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC. Dev. Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J.L., Dubois C.L., Schaffer A.E., Hao E., Shih H.P., Seymour P.A., Ma J., Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Jo J., Hardikar A.A., Periwal V., Rane S.G. Cdk4 regulates recruitment of quiescent beta-cells and ductal epithelial progenitors to reconstitute beta-cell mass. PLoS One. 2010;5:e8653. doi: 10.1371/journal.pone.0008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Sugiyama T., Liu Y., Wang J., Gu X., Lei J., Markmann J.F., Miyazaki S., Miyazaki J., Szot G.L. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Feng Z.C., Yeung F.S., Wong M.R., Oakie A., Fellows G.F., Goodyer C.G., Hess D.A., Wang R. Aldehyde dehydrogenase 1 activity in the developing human pancreas modulates retinoic acid signalling in mediating islet differentiation and survival. Diabetologia. 2014;57:754–764. doi: 10.1007/s00125-013-3147-y. [DOI] [PubMed] [Google Scholar]
- Lund R.J., Narva E., Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 2012;13:732–744. doi: 10.1038/nrg3271. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Parsons M.J., Leach S.D. Aldh1-expressing endocrine progenitor cells regulate secondary islet formation in larval zebrafish pancreas. PLoS One. 2013;8:e74350. doi: 10.1371/journal.pone.0074350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery C. Induced pluripotent stem cells – a cautionary note. N. Engl. J. Med. 2011;364:2160–2162. doi: 10.1056/NEJMcibr1103052. [DOI] [PubMed] [Google Scholar]
- Nijhoff M.F., Engelse M.A., Dubbeld J., Braat A.E., Ringers J., Roelen D.L., van Erkel A.R., Spijker H.S., Bouwsma H., van der Boog P.J. Glycemic stability through islet-after-kidney transplantation using an Alemtuzumab-based induction regimen and long-term triple-maintenance immunosuppression. Am. J. Transplant. 2016;16:246–253. doi: 10.1111/ajt.13425. [DOI] [PubMed] [Google Scholar]
- Ostrom M., Loffler K.A., Edfalk S., Selander L., Dahl U., Ricordi C., Jeon J., Correa-Medina M., Diez J., Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouziel-Yahalom L., Zalzman M., Anker-Kitai L., Knoller S., Bar Y., Glandt M., Herold K., Efrat S. Expansion and redifferentiation of adult human pancreatic islet cells. Biochem. Biophys. Res. Commun. 2006;341:291–298. doi: 10.1016/j.bbrc.2005.12.187. [DOI] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van D.A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Lacy P.E., Scharp D.W. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- Rovira M., Scott S.G., Liss A.S., Jensen J., Thayer S.P., Leach S.D. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. USA. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ H.A., Bar Y., Ravassard P., Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- Russ H.A., Ravassard P., Kerr-Conte J., Pattou F., Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G., van Es J.H., van den Brink S., Van Houdt W.J., Pronk A., Van G.J., Siersema P.D. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Seeberger K.L., Dufour J.M., Shapiro A.M., Lakey J.R., Rajotte R.V., Korbutt G.S. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab. Invest. 2006;86:141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- Shih H.P., Wang A., Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu. Rev. Cell Dev. Biol. 2013;29:81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- Solar M., Cardalda C., Houbracken I., Martin M., Maestro M.A., De M.N., Xu X., Grau V., Heimberg H., Bouwens L. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Todorov I., Omori K., Pascual M., Rawson J., Nair I., Valiente L., Vuong T., Matsuda T., Orr C., Ferreri K. Generation of human islets through expansion and differentiation of non-islet pancreatic cells discarded (pancreatic discard) after islet isolation. Pancreas. 2006;32:130–138. doi: 10.1097/01.mpa.0000202945.78331.93. [DOI] [PubMed] [Google Scholar]
- Xu X., D'Hoker J., Stange G., Bonne S., De L.N., Xiao X., Van de C.M., Mellitzer G., Ling Z., Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yatoh S., Dodge R., Akashi T., Omer A., Sharma A., Weir G.C., Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.