Abstract
Chromatin structure regulates both genome expression and dynamics in eukaryotes, where large heterochromatic regions are epigenetically silenced through the methylation of histone H3K9, histone deacetylation, and the assembly of repressive complexes. Previous genetic screens with the fission yeast Schizosaccharomyces pombe have led to the identification of key enzymatic activities and structural constituents of heterochromatin. We report here on additional factors discovered by screening a library of deletion mutants for silencing defects at the edge of a heterochromatic domain bound by its natural boundary—the IR-R+ element—or by ectopic boundaries. We found that several components of the DNA replication progression complex (RPC), including Mrc1/Claspin, Mcl1/Ctf4, Swi1/Timeless, Swi3/Tipin, and the FACT subunit Pob3, are essential for robust heterochromatic silencing, as are the ubiquitin ligase components Pof3 and Def1, which have been implicated in the removal of stalled DNA and RNA polymerases from chromatin. Moreover, the search identified the cohesin release factor Wpl1 and the forkhead protein Fkh2, both likely to function through genome organization, the Ssz1 chaperone, the Fkbp39 proline cis-trans isomerase, which acts on histone H3P30 and P38 in Saccharomyces cerevisiae, and the chromatin remodeler Fft3. In addition to their effects in the mating-type region, to varying extents, these factors take part in heterochromatic silencing in pericentromeric regions and telomeres, revealing for many a general effect in heterochromatin. This list of factors provides precious new clues with which to study the spatiotemporal organization and dynamics of heterochromatic regions in connection with DNA replication.
Keywords: chromatin remodeling, cohesins, DNA replication, fission yeast, gene silencing, genetic screen, heterochromatin, mating-type region, nuclear organization, proline isomerase
The various chromatin states found in eukaryotes fall into two histologically distinct types: euchromatin, which sustains gene expression, and heterochromatin, with repressive functions. While numerous forms of euchromatin can be distinguished based on histone modifications, heterochromatin is more homogenously characterized by the methylation of histone H3 at lysine 9 (H3K9me). H3K9me silences repeats and transposable elements that together constitute more than half of the human genome (Gregory 2005), and recent investigations of embryonic stem cells and cell reprogramming have further shown the importance of H3K9me in maintaining cell identity in differentiated cells through the formation of facultative heterochromatin (Becker et al. 2016). Beyond the control of gene expression, chromatin fulfills structural roles and participates in DNA recombination, and in the repair, replication, and condensation of chromosomes. How these various functions are coordinated to accommodate each other at the chromatin level is an important aspect of eukaryotic genome biology.
Much knowledge of chromatin structure and function stems from studies of the fission yeast Schizosaccharomyces pombe. Genetic screens in this organism produced mutants of the H3K9 methyltransferase Clr4 (Ekwall and Ruusala 1994; Thon et al. 1994), which together with its metazoan homolog SUVAR39 was the first identified histone methyltransferase (Rea et al. 2000). Clr4 and SUVAR39 establish large heterochromatic domains. The genetic screens that identified the clr4 locus relied on derepression of the silent mating-type loci and nearby reporters. Not only do clr4 mutants express these normally silent genes due to loss of H3K9me heterochromatin, but several aspects of mitotic and meiotic recombination are also altered in the mutants (Thon et al. 1994; Ellermeier et al. 2010). Thus, the system provides an example where chromatin structure coordinately controls gene expression and recombination through H3K9me. In addition to Clr4, genetic screens have produced mutants in: the HP1-like protein Swi6 (Gutz and Schmidt 1985; Lorentz et al. 1994); the Clr7 (a.k.a. Raf2, Cmc2, and Dos2), Clr8 (a.k.a. Raf1, Cmc1, and Dos1), and Rik1 subunits of the Clr4-associated complex CLRC (Li et al. 2005; Thon et al. 2005); the Clr1, Clr2, and Clr3 subunits of the SHREC histone deacetylase complex (Thon and Klar 1992; Ekwall and Ruusala 1994; Thon et al. 1994; Sugiyama et al. 2007; Job et al. 2016); Clr5 (Hansen et al. 2011); the Clr6 histone deacetylase (Grewal et al. 1998); and the Ubc4 E2 ubiquitin ligase (Irvine et al. 2009). This mutant assortment mirrors mutants isolated in Drosophila (Elgin and Reuter 2013) and mouse (Blewitt et al. 2005), and emphasizes the evolutionarily conserved properties of heterochromatin, such as H3K9me and histone hypoacetylation.
Major players in S. pombe heterochromatin formation were identified by the screens mentioned above, although not all players. This is partly because some requirements for heterochromatin formation are location-specific. At centromeres, heterochromatin is strongly driven by RNA interference (RNAi), whereas this is less the case in the mating-type region (Ekwall et al. 1999; Volpe et al. 2002). The formation of telomeric heterochromatin depends on RNAi and on the Shelterin protein Taz1 (Kanoh et al. 2005; Hansen et al. 2006). Small heterochromatic islands dispersed in the genome depend on Red1 or Shelterin components (Hiriart et al. 2012; Zofall et al. 2016). In addition, some factors possibly eluded original screens due to their relatively small contribution to heterochromatic gene silencing, this would be the case for the Swi6 paralog Chp2 (Thon and Verhein-Hansen 2000), the impaired growth of the mutants, as for the Pcu4 cullin (Thon et al. 2005), or to functional redundancy, as for Atf1/Pcr1 redundant with RNAi to establish heterochromatin in the mating-type region (Jia et al. 2004). Also, the screens were not saturated, as some loci appeared as mutational hotspots while others were underrepresented. Other loci affecting heterochromatin formation or function were subsequently identified in candidate approaches (references in Table 1). Recently, a screen of the Bioneer deletion library recapitulated some of these findings for pericentromeric silencing by isolating factors required for the silencing of an ade6+ reporter placed in a dg repeat of centromere 1 (Bayne et al. 2014).
Table 1. List of silencing factors identified in this study, with references to prior identification in genetic screens or candidate and biochemical approaches.
| Gene ID | Product | Description | Reference |
|---|---|---|---|
| Histone deacetylase complexes | |||
| SPBC2D10.17 | Clr1 | SHREC complex cryptic loci regulator 1 subunit | Thon and Klar (1992), Sugiyama et al. (2007) |
| SPAC1B3.17 | Clr2 | SHREC complex cryptic loci regulator 2 subunit | Ekwall and Ruusala (1994), Thon et al. (1994), Sugiyama et al. (2007) |
| SPBC800.03 | Clr3 | SHREC complex cryptic loci regulator 3 subunit (histone deacetylase) | Ekwall and Ruusala (1994), Thon et al. (1994), Sugiyama et al. (2007) |
| SPBP35G2.10 | Mit1 | SHREC complex ATP-dependent DNA helicase subunit Mit1 | Sugiyama et al. (2007) |
| SPAC16C9.05 | Cph1 | Clr6 histone deacetylase-associated PHD protein-1 | Nicolas et al. (2007) |
| Histone H3K9 methyltransferase complex CLRC and Dos2 complexes | |||
| SPBC428.08c | Clr4 | Cryptic loci regulator 4 (histone H3K9 methyltransferase) | Ekwall and Ruusala (1994), Thon et al. (1994), Hong et al. (2005), Horn et al. (2005) |
| SPCC970.07c | Raf2/Dos2/Cmc2/Clr7 | Rik1-associated factor 2/delocalization of Swi6 factor 2/cullin-associated methyltransferase complex protein 2/cryptic loci regulator 7 | Hong et al. (2005), Horn et al. (2005), Li et al. (2005), Thon et al. (2005) |
| SPCC613.12c | Raf1/Dos1/Cmc1/Clr8 | Rik1-associated factor 1/delocalization of Swi6 factor 2/cullin-associated methyltransferase complex protein 1/cryptic loci regulator 8 | Hong et al. (2005), Horn et al. 2005), Li et al. (2005), Thon et al. (2005) |
| SPCC11E10.08 | Rik1 | Silencing protein Rik1 | Ekwall and Ruusala (1994), Hong et al. (2005), Horn et al. (2005), Thon et al. (2005) |
| SPAC1071.02 | Mms19 | Dos2 silencing complex subunit | Li et al. (2011) |
| HP1 family and associated factors | |||
| SPAC664.01c | Swi6 | Chromodomain protein Swi6 | Gutz and Schmidt (1985) |
| SPAC1851.03 | Ckb1 | CK2 family regulatory subunit | Shimada et al. (2009), Braun et al. (2011) |
| CRL4 | |||
| SPAC17H9.19c | Cdt2 | WD repeat protein Cdt2 | Braun et al. (2011), Bayne et al. (2014) |
| SPAC17H9.10c | Ddb1 | Damaged DNA binding protein 1 | Braun et al. (2011), Bayne et al. (2014) |
| CSN | |||
| SPBC215.03c | Csn1 | COP9 signalosome complex subunit Csn1 | Bayne et al. (2014) |
| RPC | |||
| SPAC694.06c | Mrc1 | Mediator of replication checkpoint 1 | |
| SPAPB1E7.02c | Mcl1 | DNA polymerase α accessory factor | Mamnun et al. (2006), Natsume et al. (2008) |
| SPBC216.06c | Swi1 | Replication fork protection complex subunit Swi1 | |
| SPBC30D10.04 | Swi3 | Replication fork protection complex subunit Swi3 | |
| SPBC609.05 | Pob3 | FACT complex subunit | Lejeune et al. (2007) |
| Ubiquitin–SUMO-related | |||
| SPCC338.16 | Pof3 | F-box protein | Katayama et al. (2002), Mamnun et al. (2006) |
| SPBC354.10 | Def1 | RNAPII degradation factor (predicted) | |
| SPAC1687.05 | Pli1 | SUMO E3 ligase | Xhemalce et al. (2004) |
| Genome organization/replication timing | |||
| SPBC16G5.15c | Fkh2 | Forkhead transcription factor Fkh2 | |
| SPBC428.17c | Wpl1 | Cohesin loading/unloading factor (WAPL) Wpl1 | |
| SPAC6F6.17 | Rif1 | Telomere length regulator protein Rif1 | Toteva et al. (2017) |
| SPBC1198.11C | Reb1 | RNA polymerase I transcription termination factor /replication block | Jakociunas et al. (2013) |
| Chromatin remodelers/chaperones | |||
| SPAC25A8.01c | Fft3 | Fission yeast fun 30 related protein Fft3 ATP-dependent helicase, chromatin remodeler | Stralfors et al. (2011) |
| SPAC31G5.19 | Abo1 | ATPase with bromodomain protein | Gal et al. (2016) |
| SPBC1347.02 | Fkpb39 | FKBP-type peptidyl-prolyl cis-trans isomerase (predicted) | |
| SPAC57A7.12 | Ssz1 | Heat shock protein | |
| Histone acetyltransferase | |||
| SPAC1952.05 | Gcn5 | SAGA complex histone acetyltransferase catalytic subunit Gcn5 | |
| RNA-associated proteins | |||
| SPAC4F10.14c | Btf3 | Nascent polypeptide-associated complex β subunit/basic transcription factor Btf3 | |
| SPCC4G3.15c | Not2 | CCR4-NOT complex NOT box subunit Not2 | Cotobal et al. (2015) |
| SPAC29B12.06c | Rcd1 | CCR4-NOT complex RNA-binding protein subunit Rcd1 | Cotobal et al. (2015) |
| Miscellaneous | |||
| SPBC428.07 | Meu6 | Meiotic chromosome segregation protein Meu6 (adjacent to clr4+ gene) | |
ID, identifier; PHD, Plant homeodomain; CLRC, Clr4 complex; FACT, facilitates chromatin transcription; RNAPII, RNA polymerase II.
Comprehensive models for heterochromatin formation and maintenance have been derived from the phenotypes of S. pombe mutants, with noncoding RNAs, RNAi, and DNA-binding proteins participating in the establishment of heterochromatic domains whose subsequent maintenance invokes epigenetic components. Domains in the 20–100 kb range are formed at centromeres, telomeres, and the mating-type region. Smaller heterochromatin patches are formed at internal chromosomal locations, some of which participate in the repression of meiotic genes during vegetative growth.
The new screens reported here build on our recent identification of DNA elements capable of functionally replacing the natural chromatin boundary, IR-R+, at the edge of the mating-type region (Jakociunas et al. 2013; Toteva et al. 2017). IR-R+ deletion leads to reduced heterochromatic silencing (Thon et al. 2002) that can be restored by artificial boundaries. Thus, five genomic elements, whose boundary activity requires the regulators of replication origin firing Taz1 and Rif1, can substitute for IR-R+ (STAR1-5, for Sensitive to Taz1 and Rif1), as well as another uncharacterized element originating from a unique chromosomal location, BTH1 (Boundary To Heterochromatin 1) (Toteva et al. 2017). At their endogenous chromosomal locations, STAR elements are adjacent to late replication origins. They are physically bound by Taz1, which, together with Rif1, delays origin firing (Hayano et al. 2012; Tazumi et al. 2012). The effects of Taz1, Rif1, and STAR elements on replication and chromatin structure appear to be mechanistically connected, perhaps through protein phosphatase 1 (Toteva et al. 2017). rDNA repeats can also form ectopic boundaries, in this case highlighting the essential role of the replication-blocking protein Reb1 in boundary formation (Jakociunas et al. 2013). Additional phenotypes suggest coordinated control of DNA replication and boundary positioning. In particular, the heterochromatic part of the mating-type region replicates early in S phase in contrast to the flanking euchromatin. Deletion of the IR-R+ boundary perturbs the replication pattern at the edge of the domain in addition to perturbing heterochromatin (Toteva et al. 2017). Our new screens confirm and extend these previous observations by showing that multiple proteins associated with the replisome participate in heterochromatic gene silencing. This is in addition to several other factors with hitherto undocumented roles in heterochromatin, whose identification further increases our understanding of the interplay between genome organization and gene expression.
Materials and Methods
Strains, genetic manipulations, media, and oligonucleotides
The S. pombe strains used in this study are listed in Supplemental Material, Table S3 in File S1. Oligonucleotide sequences are provided in Table S4 in File S1. Media and yeast handling were conducted according to Ekwall and Thon (2017). NBA medium (Yeast Nitrogen Base) is identical to Saccharomyces cerevisiae SC medium.
Screens of Bioneer deletion library
An S. pombe deletion library of haploid strains (Bioneer, version 3) was screened in an arrayed format with the help of a ROTOR HDA robot (Singer Instruments), as described in Toteva et al. (2017) and based on Baryshnikova et al. (2010). Compared to Toteva et al. (2017), where the B6 and B7 strains were used as query strains, the following additional strains were mated here to the library: B14, B15, B16, B17, B18, and B19. In all cases, recombinant progeny of the crosses were selected for G418 resistance, conferred by the ORF deletions, and uracil prototrophy, conferred by the mating-type region of the query strains. (EcoRV)::ade6+ derepression was tested by growth in the absence of adenine, first in two rounds of high-throughput screening and subsequently by spot tests of a ten-fold dilution series for potential hits. Isolated colonies for the best-looking hits were obtained by streaking Bioneer library strains. The purified strains were then checked by PCR to confirm the expected ORF deletions, using Taq polymerase and primer Cp-N10 or Cp-C3, which anneal within the kanR cassette (Kim et al. 2010), in combination with loci-specific primers. Cp-N10 was used with Cp5 loci-specific primers and Cp-C3 with Cp3 loci-specific primers. Once confirmed, the purified strains were crossed again with the query strains to test the resulting Ade phenotype in ade6-DN/N progeny. ade6-DN/N was identified by PCR on genomic DNA with primers GTO-232 and GTO-233.
Construction of mcl1, wpl1, and dpb4 deletion strains
To retest the phenotypes of mcl1Δ::kanR (SPAPB1E7.02cΔ), wpl1Δ::kanR (SPBC428.17cΔ), and dpb4Δ::kanR (SPBC3D6.09Δ), deletion cassettes were amplified from the corresponding Bioneer deletion library strains, respectively: V3-P19-76, V3-32P-77, and V3-P02-69, with the oligonucleotide pairs GTO-1332 and GTO-1333, GTO-1365 and GTO-1366, and GTO-1363 and GTO-1364, and Phusion HF DNA polymerase (Thermo Fisher Scientific). The mcl1Δ::kanR amplicon was cloned into the EcoRV site of plasmid pJET1.2 (Thermo Fisher Scientific), generating plasmid pPA7. This plasmid was digested with BglII to release the mcl1Δ::kanR insert for S. pombe transformation, while the purified amplicons for wpl1Δ::kanR and dpb4Δ::kanR were used directly. Each deletion was introduced into the S. pombe strains PG3950, PC152, PT600, and PG3947, and transformants were selected on YES plates with G418. Gene replacements were confirmed by PCR with primers Cp-N10 or Cp-C3 (Kim et al. 2010) employed as above with locus-specific primers: Cp5-mcl1D, Cp5-wpl1D, and Cp5-dpb4D were used with Cp-N10, and Cp3-mcl1D, Cp3-wpl1D, and Cp3-dpb4D were used with Cp-C3. The PG3950, PC152, PT600, and PG3947 transformants with mcl1Δ::kanR were named PA71, PA72, PA73, and PA74; those with dpb4Δ::kanR were named PA77, PA78, PA79, PA80, and PA81; and those with wpl1Δ::kanR were named PA82, PA83, PA84, and PA85. During the construction of these strains, transformants were replica-plated onto medium with limited adenine (YE) and medium lacking adenine (AA-ade) at several steps, including following the original G418 selection of transformants, during the colony purification of multiple candidates to gauge effects on (EcoRV)::ade6+ expression, and to ensure that the phenotypes of the strains saved in the collection were representative.
RNA preparation and RT-QPCR
(EcoRV)::ade6+ RNA levels were monitored as described previously (Hansen et al. 2005; Jakociunas et al. 2013). Briefly, for each strain to be examined, three isolated colonies were first patched on YES and subsequently propagated overnight in 50 ml EMM2 cultures supplemented with adenine, leucine, and uracil, to an OD600 of 0.4. Cell pellets were flash frozen in liquid nitrogen and stored at −80°. Following RNA extraction, 2 µg RNA were treated with RQ1 DNase (Promega), diluted with DEPC-treated H2O to a final volume of 200 µl, and stored at −20°. QPCRs were set up with 7.5 µl DNase-treated RNA samples in 25 µl reactions using a QuantiFast Multiplex RT-PCR kit (QIAGEN) and two primers at 1 µM each. Three technical replicates were set up for each reaction. The reactions were performed as suggested by the manufacturer: 10 min at 50°, followed by 5 min at 95° and 39 cycles of 10 sec at 95°, and 30 sec at 60°, with data capture at each cycle, in a Bio-Rad CFX96 Thermal Cycler. Melting curves were produced on the final products and no RT controls were included. Reactions were set up with the primer pairs GTO-218 and GTO-219 (ade6+), and TJO-55 and TJO-58 (act1+). Data were analyzed using Bio-Rad CFX Manager and Microsoft Excel, normalizing ade6+ expression to act1+ in all samples. Mean normalized expression (MNE) values were calculated according to the following equation: MNE = (Eact1)CTact1, mean/(Eade6) CTade6, mean, where Eact1 is the efficiency of act1+ DNA amplification determined on 10-fold serial dilutions of genomic DNA, CTact1,mean is the mean cycle threshold for act1+ RNA amplification, Eade6 is the efficiency of amplification of the target of interest (ade6+) determined on 10-fold serial dilutions of genomic DNA, and CTade6,mean is the mean cycle threshold for ade6+ RNA amplification.
Data availability
Strains are available upon request. All Supplemental Tables and Figures are regrouped in File S1. Table S1 in File S1 presents a list of Bioneer deletion strains selected for the second and third mutant screens and Table S2 in File S1 gives a list of potential false positives. Figures S1–S8 in File S1 show the phenotypes of mutants examined in the second screen, while Figures S9–S11 in File S1 show the phenotypes of mutants examined in the third screen. Table S3 in File S1 contains a list of strains and their genotypes, while Table S4 in File S1 contains oligonucleotide sequences.
Results and Discussion
Screen for factors required for heterochromatin integrity close to domain boundaries
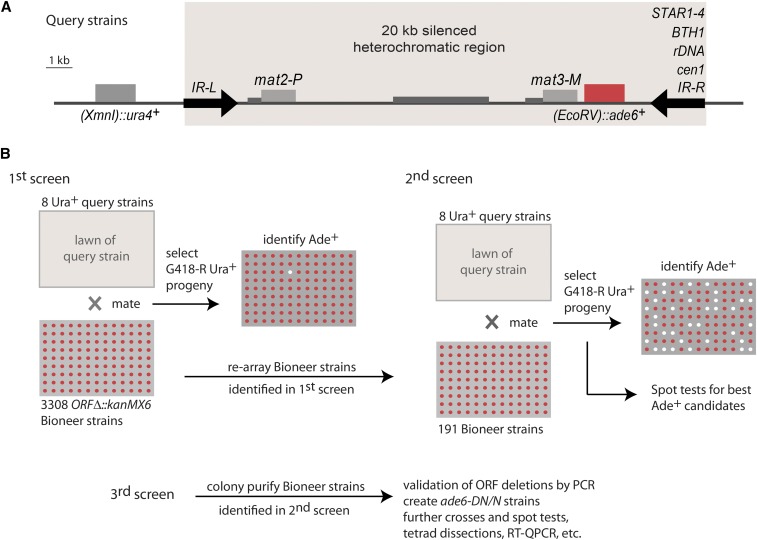
An arrayed library of 3308 S. pombe ORF deletion strains (Kim et al. 2010) was used to determine the requirements for heterochromatic gene silencing at the edge of the mating-type region in eight strains: a strain with the wild-type IR-R+ boundary and strains with, respectively: STAR1-4, BTH1, an rDNA repeat, or the edge of cen1 in the place of IR-R+ (Figure 1A). The eight query strains contained the (EcoRV)::ade6+ reporter gene close to the edge of the heterochromatic domain to provide a readout for gene silencing (Thon et al. 1999), and a euchromatic ura4+ gene tightly linked to the mating-type region to allow for its selection ((XmnI)::ura4+). The query strains were crossed with the deletion library in parallel large-scale experiments. Progeny combining the mating-type region of interest with each ORF deletion were selected according to the scheme in Figure 1B and tested for (EcoRV)::ade6+ expression. Candidate factors whose deletions appeared to permit the expression of (EcoRV)::ade6+ were identified. At this stage, even deletions with a dubious phenotype were chosen for further examination alongside deletions with a potentially strong effect. The corresponding 196 strains from the Bioneer library were rearrayed on a single plate (191 strains) or processed individually (five strains) and retested by genetic crosses against all query strains, leading to a shorter list of 48 factors potentially required for (EcoRV)::ade6+ repression (Figures S1–S8 and Table S1 in File S1). Factors with extensively documented effects on heterochromatin formation were not tested further, and we focused instead on factors whose effects on heterochromatin had not been documented before, or not tested in the mating-type region. Recombinant Ura+ G418-resistant progeny were colony-purified and spotted on indicator and selective plates to better evaluate (EcoRV)::ade6+ expression, leading to a short list of 33 candidate genes with a heterochromatic silencing defect in at least one of the queried mating-type regions. For these candidates, single colonies were isolated from the Bioneer collection, the ORF deletions were verified by PCR using locus-specific primers, and we obtained recombinant progeny combining the mating-type regions of interest with each ORF deletion in an ade6-DN/N background. The ade6-DN/N background is well suited to the monitoring of ade6+ expression by colony color on a limiting concentration of adenine or by RT-QPCR with primers that specifically recognize ade6+. In addition to Bioneer strains, three deletion strains that were not present in the library were added to the analysis (swi1Δ::kanR, swi3Δ::kanR, and clr8Δ::kanR).
Figure 1.
Experimental design. (A) Mating-type region of query strains, showing the (EcoRV)::ade6+ reporter used to monitor heterochromatic silencing close to the wild-type boundary element IR-R+ or ectopic boundaries. Silencing of (EcoRV)::ade6+ results in red colony formation on the medium used, while expression results in white colonies. The euchromatic (XmnI):ura4+ gene was used to select the mating-type region in crosses with the Bioneer collection. (B) ORF deletions allowing (EcoRV)::ade6+ expression were identified in three rounds of screening as depicted. Candidates were retested as described in the text. ORF, open reading frame; RT-QPCR, reverse transcription-quantitative polymerase chain reaction.
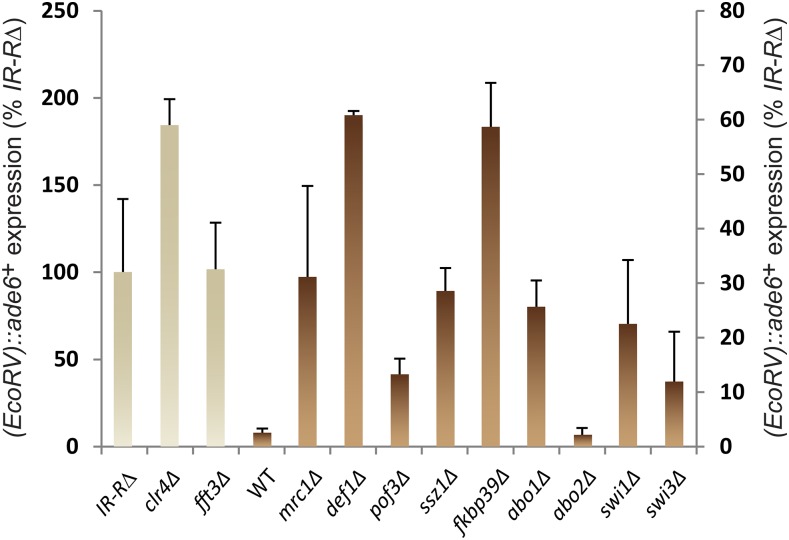
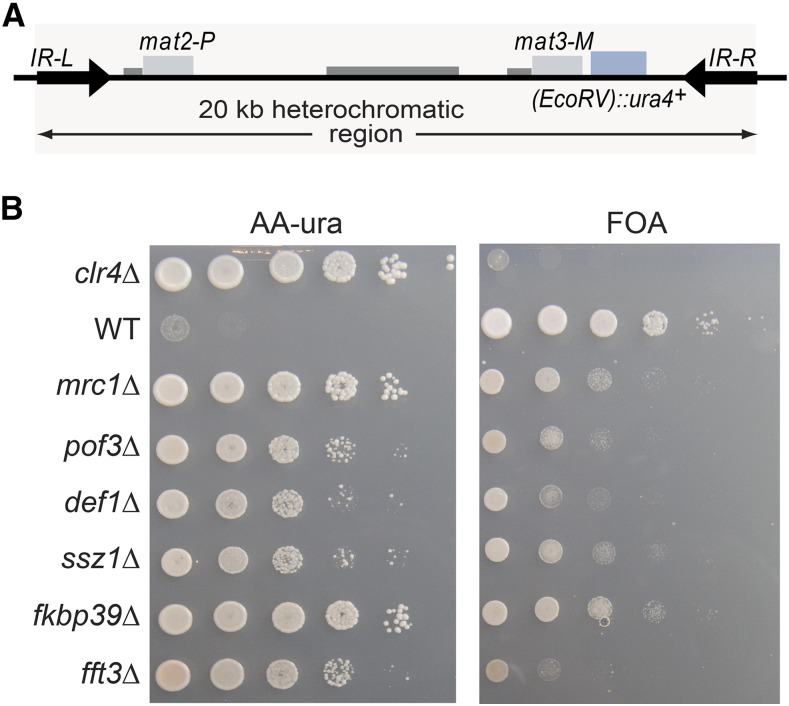
Comprehensive spot-test analyses assessing silencing by factors that made it through the entire screen are shown in Figures S9–S11 in File S1. A subset of factors are presented in Figure 2 and Figure 3 for their effects on all query strains, and in Figure 4 and Figure 5 for their effects with the wild-type boundary IR-R+. Derepression of (EcoRV)::ade6+ occurs in IR-RΔ and clr4Δ cells, resulting in white or light pink colonies on medium with limiting adenine and improved growth in the absence of adenine. While not as pronounced as for clr4Δ, the phenotypes of the other mutations tested were distinctive and were confirmed by quantification of the (EcoRV)::ade6+ transcript (Figure 5). Increases ranging from 5- to 40-fold over wild-type were measured and normalized to (EcoRV)::ade6+ expression in the IR-RΔ strain in Figure 5 (abo2Δ is shown for comparison with the deletion of its paralog abo1Δ). A different reporter, (EcoRV)::ura4+, was affected in the same way. In particular, mrc1Δ, pof3Δ, def1Δ, ssz1Δ, fkbp39Δ, and fft3Δ cells grew better in the absence of uracil than in the presence of fluoroorotic acid (FOA), unlike the parental wild-type strain for which (EcoRV)::ura4+ repression leads to the opposite growth pattern (Figure 6).
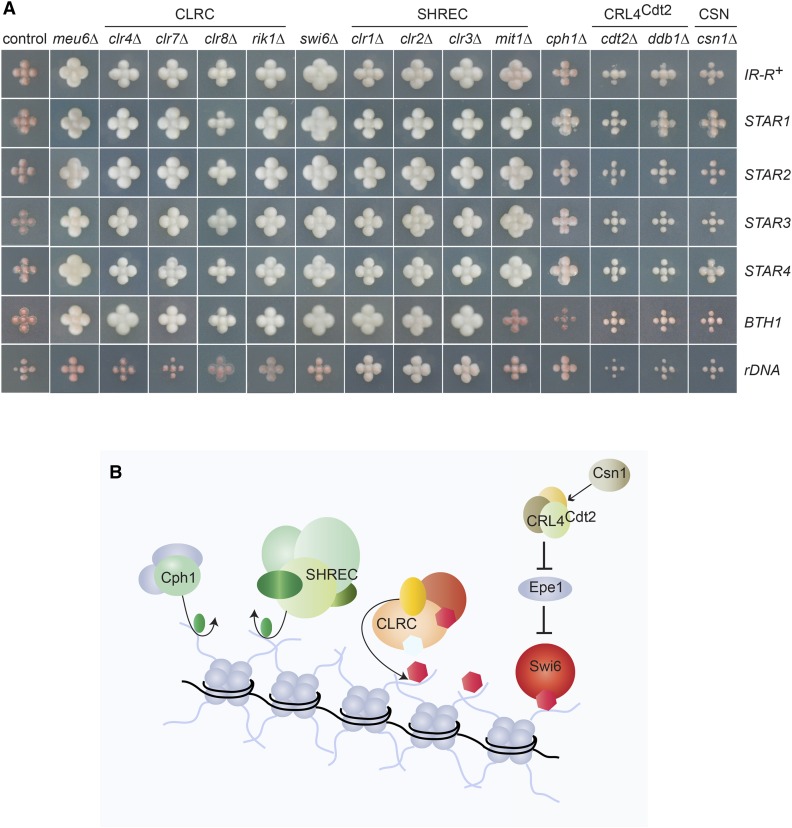
Figure 2.
Factors identified in the screens include histone modifiers and readers, and their regulators. (A) Bulk progeny of crosses combining the indicated boundary elements and ORF deletions, replicated onto medium lacking adenine [NBA (yeast nitrogen base) supplemented with leucine] to assay (EcoRV)::ade6+ expression. Light colors reflect (EcoRV)::ade6+ expression. Control: an ORF deletion with no effect on (EcoRV)::ade6+ expression (V3-P25-45, SPAC15E1.07c). (B) Schematic representation of factors and complexes shown in (A). HDACs remove acetyl groups (in green); CLRC methylates H3K9 (in red); CRL4Cdt2, positively regulated by Csn1, destabilizes the antisilencing factor Epe1. HDACs, histone deacetylases; ORF, open reading frame.
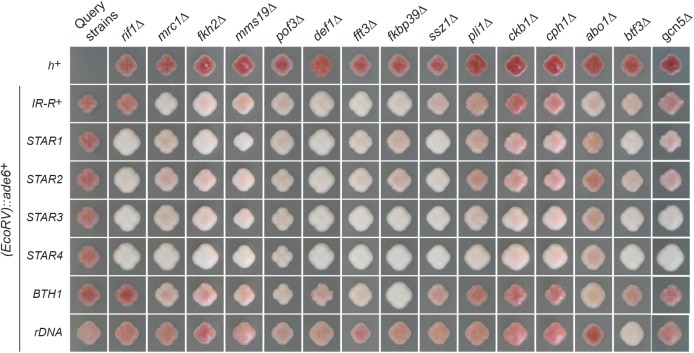
Figure 3.
Overview of other factors identified in the screen. Purified ade6-DN/N strains combining the indicated boundary elements and open reading frame deletions were propagated on medium lacking adenine [NBA (yeast nitrogen base) supplemented with leucine] to assay (EcoRV)::ade6+ expression. Spot tests for these strains are presented in Figures S9–S11 in File S1.
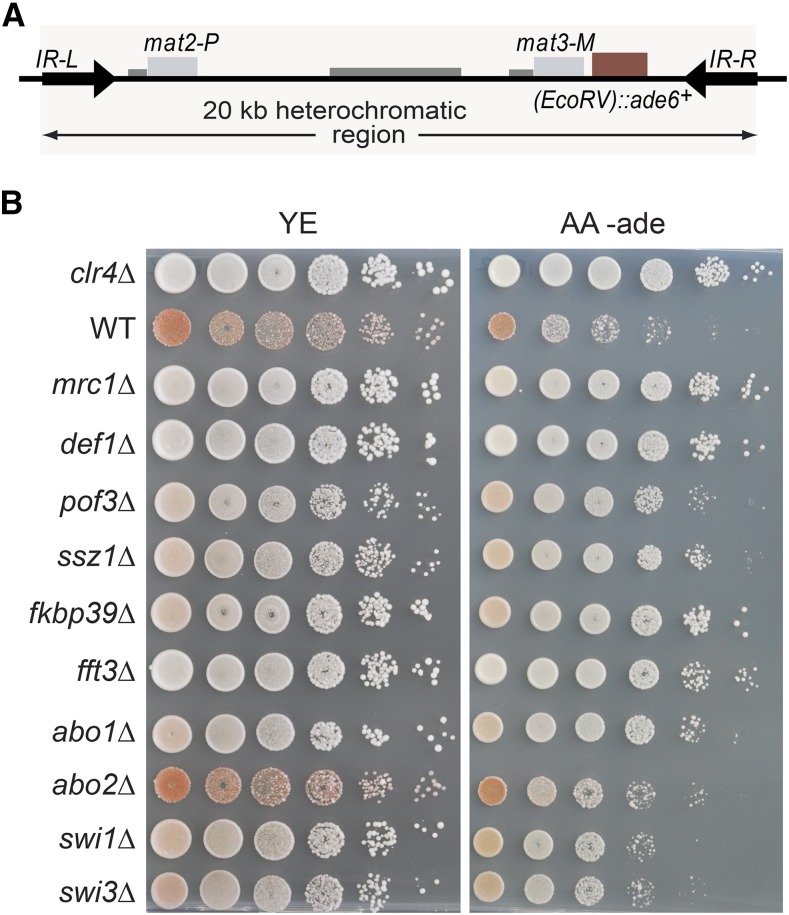
Figure 4.
Effects of selected factors on (EcoRV)::ade6+ in the presence of the WT boundary IR-R+. (A) Mating-type region in this experiment. (B) Growth assays with 10-fold serial dilutions of indicated deletion strains. Strains LJ99, PG3950, LJ102, LJ100, LJ220, LJ203, LJ104, LJ103, LJ199, LJ105, PM20, and PM24 were used. AA -ade, medium lacking adenine; WT, wild-type; YE, medium with limited adenine.
Figure 5.
Effects of selected factors on (EcoRV)::ade6+ transcript in the presence of the WT boundary IR-R+, assayed by RT-QPCR. The strains are the same as in Figure 4. The means of three biological replicates are displayed. (EcoRV)::ade6+ RNA level was normalized to act1+ in each strain and is expressed as % expression in IR-RΔ cells. The y-axis labeling on the left applies to the three strains on that side that have the highest (EcoRV)::ade6+ expression. The labeling on the right applies to the other strains. Errors bars represent the SE of the mean. RT-QPCR, reverse transcription-quantitative polymerase chain reaction; WT, wild-type.
Figure 6.
Effects of selected factors on (EcoRV)::ura4+ in the presence of the WT boundary IR-R+. (A) Mating-type region in this experiment. (B) Growth assays with 10-fold serial dilutions of indicated deletion strains. Strains used for spot tests: LJ177, PG1899, LJ176, LJ178, LJ179, and LJ175. AA-ura, medium lacking uracil; FOA, fluoroorotic acid; WT, wild-type.
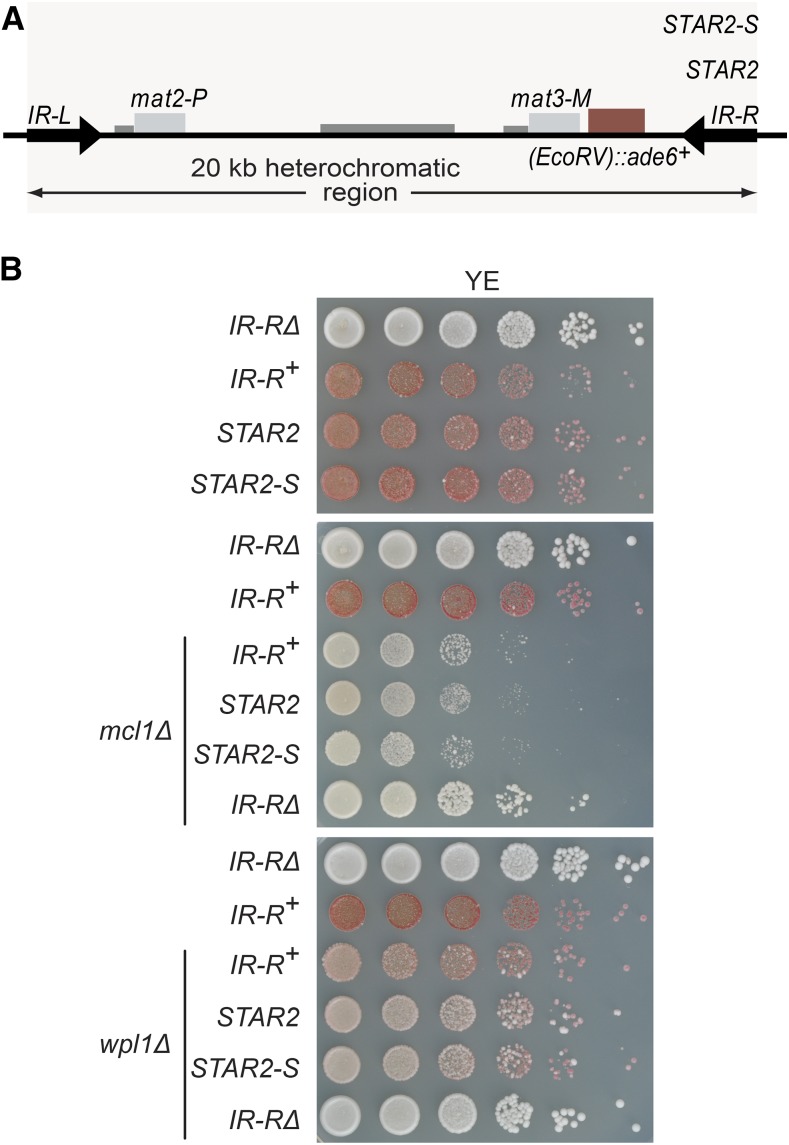
A few Bioneer strains could not be reliably retested by crossing. Instead, for three of them (mcl1Δ and wpl1Δ, scored as positive in first and second screens, and dpb4Δ, scored as false positive in first screen), the kanMX6 cassette that replaces each deleted ORF (Kim et al. 2010) was amplified by PCR, together with 500 bp–1 kb flanking DNA from genomic DNA of the Bioneer deletion strain, and used to transform tester strains to confirm effects suggested by the genetic screens. Little effect was seen in dpb4Δ cells, suggesting that the originally observed phenotype was artifactual, while derepression by mcl1Δ and wpl1Δ was confirmed (Figure 7).
Figure 7.
Effects of Mcl1 and Wpl1 on heterochromatic silencing. (A) The mating-type regions used in this experiment featured two ectopic boundaries, STAR2 and the 85 bp STAR2-S element (Toteva et al. 2017), in addition to wild-type IR-R+. (B) mcl1Δ and wpl1Δ mutants with the indicated boundaries were spotted on low adenine to compare (EcoRV)::ade6+ expression with the wild-type background. mcl1Δ confers a pronounced growth defect; however, the formed (EcoRV)::ade6+ mcl1Δ colonies are Ade+. wpl1Δ induces strong variegation. The strains were wild-type: PG3947, PG3950, PC152, and PT600; mcl1Δ: PA71, PA72, PA73, and PA74; and wpl1Δ: PA82, PA83, PA84, and PA85. YE, medium with limited adenine.
The silencing factors that were retested throughout the whole scheme are listed in Table 1, together with references to prior literature for those that had been described previously. In addition to known components of heterochromatin, the screens identified factors that had eluded all previous screens or that had not been examined for effects in the mating-type region. A few were element-specific, such as Rif1 acting with the STAR elements (Toteva et al. 2017), while others were required for (EcoRV)::ade6+ silencing in all or nearly all query strains.
Requirement for histone deacetylases and methyltransferases
The sole proteins required for (EcoRV)::ade6+ silencing in all configurations of the mating-type region tested were Clr1, Clr2, and Clr3; that is, the deacetylase module of the SHREC complex (Figure 2). Most boundary elements also relied on the chromatin remodeler module of SHREC, Mit1, with the exception of BTH1. Some independence of the deacetylase and remodeler modules of SHREC is predicted by a structure–function analysis of the complex (Job et al. 2016). The histone H3K9 methyltransferase Clr4, the Clr7, Clr8, and Rik1 subunits of the CLRC complex, and the chromodomain protein Swi6 were also broadly identified, in all screens except for the full-length rDNA repeat (Figure 2A). The rDNA repeat is exceptional in that it silences (EcoRV)::ade6+ tightly through antisense transcription and histone deacetylation, even in the absence of Clr4 (Jakociunas et al. 2013). The Clr6 histone deacetylase-associated PHD protein-1 Cph1 was also identified in several screens, albeit with relatively weak phenotypes (Figure 2). Moreover, deletion of the meu6+ gene adjacent to clr4+ resulted in the same strong derepression as clr4Δ (Figure 2), possibly due to impaired clr4 expression. Altogether, this first set of mutants emphasizes the central roles of deacetylation and methylation in heterochromatic gene silencing, and validates the screening strategy.
Requirement for Ddb1-Cdt2 and Csn1
Nearly all screens also identified the Ddb1, Cdt2, and Csn1 proteins (Figure 2 and Figure S9 in File S1 for ddb1Δ). Ddb1 and Cdt2 are part of the Cullin 4 ubiquitin ligase CRL4Cdt2 that targets specific proteins for degradation by the proteasome. In Cullin complexes, an adaptor protein, Cdt2 in this case, interacts with cognate substrates to display them to the ubiquitin ligase activity. Three CRL4Cdt2 substrates are known in S. pombe: the putative histone H3K9 demethylase Epe1 (Braun et al. 2011), the licensing factor Cdt1 that is required for the firing of replication origins (Ralph et al. 2006); and the inhibitor of ribonucleotide reductase Spd1 (Liu et al. 2003). According to previous work, stabilization of Epe1 in the cdt2Δ and ddb1Δ mutants and its accumulation in heterochromatin would suffice to derepress (EcoRV)::ade6+ (Braun et al. 2011). Deletion of the COP9 signalosome (CSN) subunit Csn1 also resulted in reduced silencing (Figure 2). Reduced silencing in the csn1Δ mutant might result from CRL4Cdt2 deregulation (Liu et al. 2003), or from the destabilization of other cullin ligases such as SCFPof3, whose F-box component Pof3 is proteolyzed in CSN mutants (Schmidt et al. 2009). Indicative of a general requirement in heterochromatin, Csn1 was also identified in a recent genetic screen for heterochromatic silencing defects at centromeres (Bayne et al. 2014).
Requirement for Def1, Pof3, and Pli1
Further links to ubiquitylation were revealed with the identification of the proposed homolog of S. cerevisiae Def1 and the F-box protein Pof3 (Figure 3, Figure 4, Figure 5, Figure 6, and Figures S9 and S10 in File S1). To our knowledge, this is the first evidence for an effect of Def1 in heterochromatin. In S. cerevisiae, Def1 mediates the ubiquitylation and degradation of the Rpb1 subunit of RNA polymerase II (RNAPII) during transcriptional stress (Wilson et al. 2013), and of Pol3, the catalytic subunit of the replicative DNA polymerase δ, in postreplicative repair (Daraba et al. 2014). In S. pombe, Def1 might control polymerase usage in heterochromatin, remove RNAPII from heterochromatin to promote transcriptional silencing, or prevent collisions between DNA and RNA polymerases, a proposed cause of heterochromatin instability (Zaratiegui et al. 2011). The functions of Pof3, homologous to Dia2 in S. cerevisiae, are understood in much greater detail than those of Def1. In S. pombe, chromosomes are lost in the absence of Pof3, telomeres are shortened, telomere and centromere silencing is alleviated, and the DNA damage checkpoint is continuously activated (Katayama et al. 2002; Mamnun et al. 2006). Pof3 associates with the replisome. Its targets, inferred from the increased stability in pof3Δ cells, include the mediator of replication checkpoint Mrc1, Pol ε, and subunits of the CMG helicase, each of which could be relevant to the observed effects (see below; Roseaulin et al. 2013a,b; Maculins et al. 2015). Other targets of Pof3 potentially relevant to heterochromatic silencing would be Ams2, a regulator of histone gene transcription (Takayama et al. 2010), or other DNA-binding proteins that might be removed by Pof3 as Pof3 travels with the replication fork.
The screens also detected a small contribution of the SUMO E3 ligase Pli1 to (EcoRV)::ade6+ silencing (Figure 3), compatible with previously observed limited derepression of centromeric reporters in pli1Δ cells (Xhemalce et al. 2004).
Requirement for the RPC
Mrc1/Claspin, Mcl1 (Ctf4 in S. cerevisiae), Swi1 (Timeless in animals or Tof1 in S. cerevisiae), Swi3 (Tipin or Csm3), and the histone chaperone FACT are part of the RPC essential to normal replication fork progression (Gambus et al. 2006; Foltman et al. 2013; Yeeles et al. 2017). Here, Mrc1, Mcl1, Swi1, Swi3, and the Pob3 subunit of FACT were all necessary for tight (EcoRV)::ade6+ silencing (Figure 4, Figure 7, and Figure S9 in File S1). Mrc1, Mcl1, Swi1, and Swi3 also ensure replication fork protection at replication blocks (Noguchi et al. 2004; Shimmoto et al. 2009) and telomeric repeats (Gadaleta et al. 2016). Structurally, Mcl1/Ctf4 forms a trimer that connects the CMG helicase to the lagging-strand primase Polα, and various accessory factors to the replisome (Williams and McIntosh 2002, 2005; Tsutsui et al. 2005; Simon et al. 2014; Samora et al. 2016; Villa et al. 2016). Pof3/Dia2 in particular, also identified in our screen, is associated with the replisome in an Mcl1- and Mrc1-dependent manner (Mamnun et al. 2006; Morohashi et al. 2009). Hence, the silencing defect in RPC mutants might be due to a failed association of Pof3 with the fork in these mutants. Since Pof3 is responsible for the short half-life of Mrc1 in wild-type S. pombe cells, and mediates the degradation of DNA polymerases and MCM helicases in the absence of the RPC (Roseaulin et al. 2013a,b), the silencing defect in pof3Δ cells might also result from a misregulated RPC.
Further requirements for DNA replication/genome organization factors
Other proteins implicated in the control of DNA replication or genome organization identified in the screens were the forkhead protein Fkh2, whose S. cerevisiae homologs Fkh1 and Fkh2 organize the long-range clustering and activation of early-firing origins (Knott et al. 2012) and long-range chromosomal interactions occurring during mating-type switching (Li et al. 2012), and the cohesin release factor Wings apart-like Wpl1 (Figure 3, Figure 7, and Figure S9 in File S1). In the absence of Wpl1, (EcoRV)::ade6+ showed a strongly variegated phenotype (Figure 7). In mammals, ΔWAPL cells display altered chromatin loop structure, reduced long-range chromosomal interactions in cis, and decreased nuclear compartmentalization that correlates with gene derepression in normally silent compartments (Haarhuis et al. 2017). These effects might result from the aberrant distribution or accumulation of cohesion, and might be evolutionarily conserved since cohesins participate in S. pombe genome architecture (Mizuguchi et al. 2014) and a rad21 mutation causes heterochromatin loss in subtelomeric regions (Dheur et al. 2011). Furthermore, components of the RPC (including Swi1, Swi3, Mrc1, and Mcl1) are required for sister chromatid cohesion (e.g., Williams and McIntosh 2002; Xu et al. 2004; Lengronne et al. 2006; Ansbach et al. 2008), hence the effects of the RPC, and Wpl1 might overlap mechanistically.
The screens also confirmed the importance of the iron–sulfur cluster assembly protein Mms19 to heterochromatin (Figure 3). Mms19 copurifies with the silencing factor Clr7/Dos2 (Li et al. 2011).
Requirement for chromatin remodelers and chaperones
In addition to the SHREC subunit Mit1 and the FACT subunit Pob3, the SMARCAD1 family ATP-dependent DNA helicase Fft3 and the ATPase with bromodomain Abo1 were also broadly required for silencing by the various boundary elements used in the screens (Figure 3, Figure 4, Figure 5, Figure 6, and Figure S10 in File S1). In previous studies, Fft3 was found to affect heterochromatic boundaries at centromeres and telomeres (Stralfors et al. 2011), and functions of Abo1 in heterochromatin were recently documented as well (Gal et al. 2016). In our study, deletion of Abo1 conferred relatively modest, albeit distinguishable, phenotypes (Figure 3, Figure 4, Figure 5, Figure 6, and Figure S10 in File S1). The Abo1 paralogue Abo2 was not isolated in the initial screens, but was examined for comparison with Abo1 and displayed more minor effects, if any.
Newly identified factors whose deletion caused a pronounced derepression of (EcoRV)::ade6+ included the predicted peptidyl-prolyl cis-trans isomerase Fkbp39 and the Ssz1 chaperone, an HSP70 family protein (Figure 3, Figure 4, and Figure 5 for effects on (EcoRV)::ade6+ with IR-R+; Figure S10 in File S1 for all elements; and Figure 6 for effects on (EcoRV)::ura4+). Both Fkbp39 and Ssz1 are predominantly nuclear in S. pombe (Matsuyama et al. 2006), which is compatible with them having effects on chromatin. The Fkbp39 homolog Fpr4 in S. cerevisiae catalyzes P30 and P38 isomerization in histone H3 (Nelson et al. 2006); in this light, our findings indicate a potential importance for this isomerization in heterochromatin.
Requirement for other factors and modifying enzymes
Finally, Btf3, the Not2 and Rcd1 subunits of the CCR4-Not complex previously implicated in heterochromatic silencing in fission yeast (Cotobal et al. 2015; Bronner et al. 2017), and the histone acetyltransferase Gcn5 were also required, to various degrees, for silencing (Figure 3 and Figure S11 in File S1). Given the strong requirement for histone deacetylation in heterochromatin, a requirement for an opposing activity is intriguing, yet silent chromatin is not altogether deacetylated; Gcn5 might function in concert with Btf3 or Not2, or transient acetylation might facilitate heterochromatin maintenance through effects at the replication fork (Espinosa et al. 2010; Kurat et al. 2017).
Effects at telomeres and centromeres
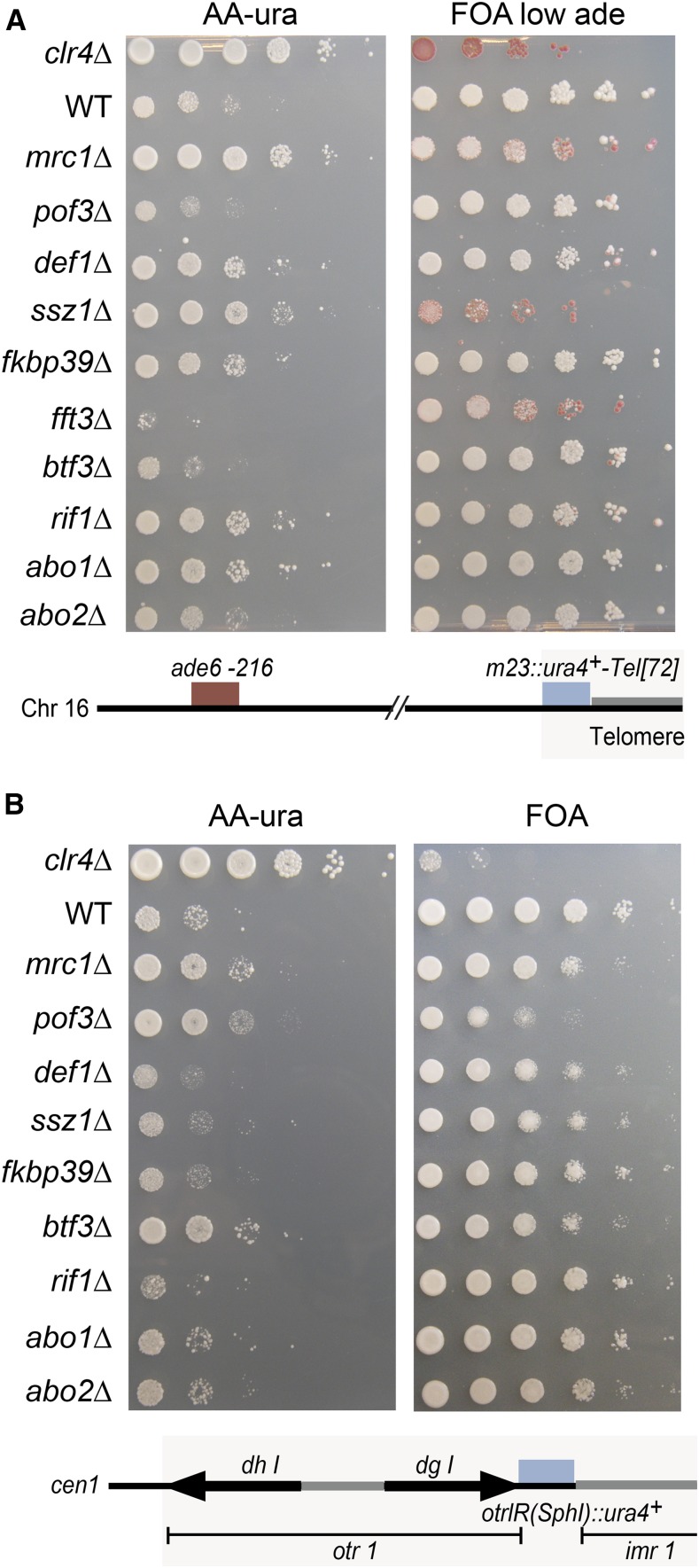
The effects of selected ORF deletions on telomeric silencing was assayed with the m23::ura4+-Tel[72] reporter close to the end of a truncated minichromosome Ch16 ((Nimmo et al. 1994); Figure 8A). In this assay, cells containing the minichromosome are Ade+ due to intragenic complementation between the ade6-210 allele on chromosome 3 and ade6-216 on the minichromosome. Several deletions (mrc1Δ, def1Δ, ssz1Δ, fkbp39Δ, rif1Δ, and rif1Δ) increased growth on medium lacking uracil, indicative of m23::ura4+-Tel[72] derepression. For some strains (mrc1Δ, ssz1Δ, and fft3Δ in Figure 8A), FOA strongly selected for cells that had lost their minichromosome, resulting in red colonies on FOA with limiting adenine. This is expected for mutants in which ura4+ cannot be repressed effectively; only cells that have lost the mini-chromosome are phenotypically Ura−.
Figure 8.
Effects of selected factors on telomeric and centromeric silencing. Serial dilutions (10-fold) of cell suspensions were spotted. (A) Effects on the m23::ura4+-Tel[72] telomeric reporter gene. Derepression of the reporter improves plating efficiency on AA-ura and decreases white colony formation on FOA medium with low adenine concentration (where only cells with a repressed reporter can form white colonies). Strains used: LJ171, FY520, LJ163, LJ172, LJ173, LJ162, LJ174, LJ165, LJ170, LJ168, LJ161, and LJ166. (B) Effects on centromeric otr1(SphI)::ura4+ reporter gene. Strains used: LJ185, FY648, LJ180, LJ186, LJ190, LJ192, LJ187, LJ183, LJ181, LJ191, and LJ189. AA-ura, medium lacking uracil; ade, adenine; Chr, chromosome; FOA, fluoroorotic acid; WT, wild-type.
The otr1R(SphI)::ura4+ insertion in centromere 1 (Allshire et al. 1995) was used to test effects on pericentromeric silencing. These were less pronounced than for the edge of the mating-type region or subtelomere (Figure 8B), perhaps due to the location of the otr1R(SphI)::ura4+ reporter, embedded in RNAi-responsive heterochromatin.
Interplays between replication and heterochromatin
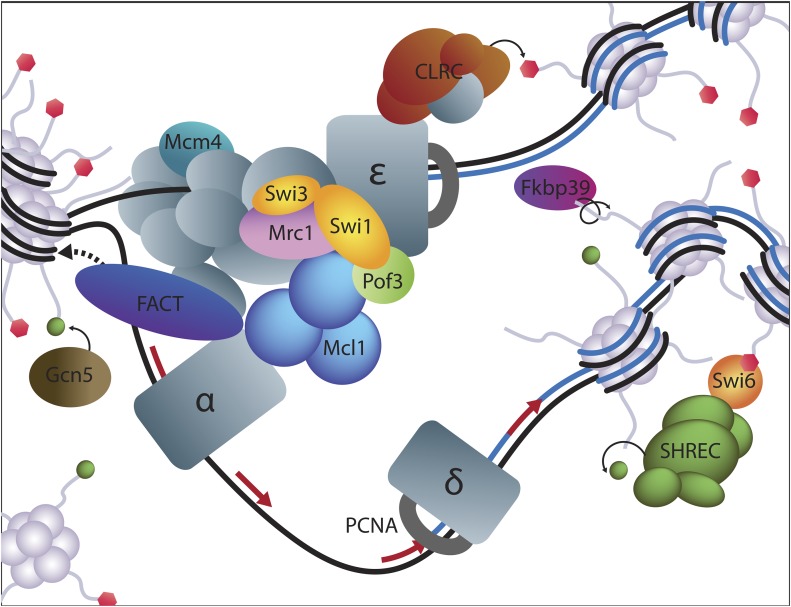
Physical and functional links between DNA replication and heterochromatin formation have been discovered in numerous organisms, and are proposed to participate in the epigenetic maintenance of heterochromatin during cell division. In S. pombe, The Clr7/Dos2 subunit of the H3K9 methyltransferase complex CLRC binds Polε, and Polε mutants with reduced affinity for Dos2/Clr7 exhibit heterochromatin defects (Li et al. 2011). Mutations in the primase Polα (Nakayama et al. 2001; Natsume et al. 2008) or in the Mcm4 helicase (Toteva et al. 2017) also cause heterochromatin defects, as well as mutations in nonessential replication factors (Natsume et al. 2008). Our present results emphasize the tight links between replication and heterochromatin integrity by identifying multiple RPC components and associated chromatin modifiers (Figure 9).
Figure 9.
Silencing factors identified in this screen have roles in DNA replication. The drawing schematizes a replication fork. Factors identified in the screen are in color, while other subunits of the represented complexes are in gray.
A first mechanism through which RPC components might facilitate the maintenance of heterochromatin is through the stabilization of ongoing replication forks. It has been observed that replisome components are, under some circumstances, degraded upon replication stress (Roseaulin et al. 2013a,b; Daraba et al. 2014); in particular, both Polε and replicative helicases become unstable in S. pombe in the absence of the RPC component Swi1 (Roseaulin et al. 2013a,b). The breakdown of replisomes at replication forks that encounter an impediment in heterochromatin might lead to the dissociation of CLRC from the fork, or to a fork restart using different DNA polymerases that do not interact with CLRC, thereby weakening heterochromatin (Zaratiegui et al. 2011; Lee et al. 2013; Lee and Russell 2013). Similarly, control of homologous recombination, both during and outside S phase, might favor heterochromatin maintenance by preventing the use of polymerases other than Polε (Zaratiegui et al. 2011; Miyabe et al. 2015). A requirement for the stabilization of replication forks in heterochromatic regions would be compounded by the fact that heterochromatin appears to be difficult to replicate and prone to fork stalling (Zaratiegui et al. 2011; Lee et al. 2013; Lee and Russell 2013).
A second mechanism through which RPC components might reinforce heterochromatin is through the control of replication origin firing. Mrc1/Claspin has dual roles in replication fork progression and in the DNA replication timing program. Other factors isolated in our screen also affect replication origin usage. Thus Rif1, necessary for boundary activity and heterochromatin protection by the STAR boundaries (Toteva et al. 2017), controls the replication timing program across species (Hayano et al. 2012; Yamazaki et al. 2012; Peace et al. 2014). In S. pombe, both Mrc1 and Rif1 mutations alter DNA replication genome-wide and bypass the requirement for the DDK kinase Hsk1 (Matsumoto et al. 2011; Hayano et al. 2012). Consistent with coordinated control of origin firing and heterochromatin maintenance, a mutation in the N-terminus of the Mcm4 helicase, which removes a part of the protein that is predicted to confer DDK control on origin firing, weakens heterochromatic silencing close to IR-R+ or artificial boundaries (Toteva et al. 2017). In addition, the forkhead protein Fkh2 has well-documented roles in replication origin usage in S. cerevisiae (Knott et al. 2012). Altogether, these observations indicate that the separation of replication domains, possibly facilitated by boundary elements, is of importance to heterochromatin integrity, for instance by controlling the type of replisomes that operate in particular domains.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300341/-/DC1.
Acknowledgments
We thank members of the laboratory for discussions, Janne Verhein-Hansen for preparing media and solutions, and the University of Copenhagen and the Carlsberg Foundation for financial support.
Footnotes
Communicating editor: C. Hoffman
Literature Cited
- Allshire R. C., Nimmo E. R., Ekwall K., Javerzat J. P., Cranston G., 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9: 218–233. [DOI] [PubMed] [Google Scholar]
- Ansbach A. B., Noguchi C., Klansek I. W., Heidlebaugh M., Nakamura T. M., et al. , 2008. RFCCtf18 and the Swi1-Swi3 complex function in separate and redundant pathways required for the stabilization of replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe. Mol. Biol. Cell 19: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikova A., Costanzo M., Dixon S., Vizeacoumar F. J., Myers C. L., et al. , 2010. Synthetic genetic array (SGA) analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Methods Enzymol. 470: 145–179. [DOI] [PubMed] [Google Scholar]
- Bayne E. H., Bijos D. A., White S. A., de Lima Alves F., Rappsilber J., et al. , 2014. A systematic genetic screen identifies new factors influencing centromeric heterochromatin integrity in fission yeast. Genome Biol. 15: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. S., Nicetto D., Zaret K. S., 2016. H3K9me3-dependent heterochromatin: barrier to cell fate changes. Trends Genet. 32: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewitt M. E., Vickaryous N. K., Hemley S. J., Ashe A., Bruxner T. J., et al. , 2005. An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc. Natl. Acad. Sci. USA 102: 7629–7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Garcia J. F., Rowley M., Rougemaille M., Shankar S., et al. , 2011. The Cul4-Ddb1(Cdt)(2) ubiquitin ligase inhibits invasion of a boundary-associated antisilencing factor into heterochromatin. Cell 144: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner C., Salvi L., Zocco M., Ugolini I., Halic M., 2017. Accumulation of RNA on chromatin disrupts heterochromatic silencing. Genome Res. 27: 1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotobal C., Rodriguez-Lopez M., Duncan C., Hasan A., Yamashita A., et al. , 2015. Role of Ccr4-Not complex in heterochromatin formation at meiotic genes and subtelomeres in fission yeast. Epigenet. Chromatin 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraba A., Gali V. K., Halmai M., Haracska L., Unk I., 2014. Def1 promotes the degradation of Pol3 for polymerase exchange to occur during DNA-damage–induced mutagenesis in Saccharomyces cerevisiae. PLoS Biol. 12: e1001771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheur S., Saupe S. J., Genier S., Vazquez S., Javerzat J. P., 2011. Role for cohesin in the formation of a heterochromatic domain at fission yeast subtelomeres. Mol. Cell. Biol. 31: 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K., Ruusala T., 1994. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics 136: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K., Thon G., 2017. Genetic analysis of Schizosaccharomyces pombe, pp. 31–62 in Fission Yeast: A Laboratory Manual, edited by Hagan I., Carr A. M., Grallert A., Nurse P. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Cranston G., Allshire R. C., 1999. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics 153: 1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C., Reuter G., 2013. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 5: a017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C., Higuchi E. C., Phadnis N., Holm L., Geelhood J. L., et al. , 2010. RNAi and heterochromatin repress centromeric meiotic recombination. Proc. Natl. Acad. Sci. USA 107: 8701–8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa M. C., Rehman M. A., Chisamore-Robert P., Jeffery D., Yankulov K., 2010. GCN5 is a positive regulator of origins of DNA replication in Saccharomyces cerevisiae. PLoS One 5: e8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltman M., Evrin C., De Piccoli G., Jones R. C., Edmondson R. D., et al. , 2013. Eukaryotic replisome components cooperate to process histones during chromosome replication. Cell Reports 3: 892–904. [DOI] [PubMed] [Google Scholar]
- Gadaleta M. C., Das M. M., Tanizawa H., Chang Y. T., Noma K., et al. , 2016. Swi1Timeless prevents repeat instability at fission yeast telomeres. PLoS Genet. 12: e1005943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal C., Murton H. E., Subramanian L., Whale A. J., Moore K. M., et al. , 2016. Abo1, a conserved bromodomain AAA-ATPase, maintains global nucleosome occupancy and organisation. EMBO Rep. 17: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus A., Jones R. C., Sanchez-Diaz A., Kanemaki M., van Deursen F., et al. , 2006. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 8: 358–366. [DOI] [PubMed] [Google Scholar]
- Gregory T. R., 2005. Synergy between sequence and size in large-scale genomics. Nat. Rev. Genet. 6: 699–708. [DOI] [PubMed] [Google Scholar]
- Grewal S. I., Bonaduce M. J., Klar A. J., 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H., Schmidt H., 1985. Switching genes in Schizosaccharomyces pombe. Curr. Genet. 9: 325–331. [DOI] [PubMed] [Google Scholar]
- Haarhuis J. H. I., van der Weide R. H., Blomen V. A., Yáñez-Cuna J. O., Amendola M., et al. , 2017. The cohesin release factor WAPL restricts chromatin loop extension. Cell 169: 693–707.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. R., Burns G., Mata J., Volpe T. A., Martienssen R. A., et al. , 2005. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol. Cell. Biol. 25: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. R., Ibarra P. T., Thon G., 2006. Evolutionary-conserved telomere-linked helicase genes of fission yeast are repressed by silencing factors, RNAi components and the telomere-binding protein Taz1. Nucleic Acids Res. 34: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. R., Hazan I., Shanker S., Watt S., Verhein-Hansen J., et al. , 2011. H3K9me-independent gene silencing in fission yeast heterochromatin by Clr5 and histone deacetylases. PLoS Genet. 7: e1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano M., Kanoh Y., Matsumoto S., Renard-Guillet C., Shirahige K., et al. , 2012. Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes Dev. 26: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiriart E., Vavasseur A., Touat-Todeschini L., Yamashita A., Gilquin B., et al. , 2012. Mmi1 RNA surveillance machinery directs RNAi complex RITS to specific meiotic genes in fission yeast. EMBO J. 31: 2296–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E. J., Villen J., Gerace E. L., Gygi S. P., Moazed D., 2005. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3–K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol. 2: 106–111. [DOI] [PubMed] [Google Scholar]
- Horn P. J., Bastie J. N., Peterson C. L., 2005. A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev. 19: 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine D. V., Goto D. B., Vaughn M. W., Nakaseko Y., McCombie W. R., et al. , 2009. Mapping epigenetic mutations in fission yeast using whole-genome next-generation sequencing. Genome Res. 19: 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakociunas T., Domange Jordo M., Ait Mebarek M., Bunner C. M., Verhein-Hansen J., et al. , 2013. Subnuclear relocalization and silencing of a chromosomal region by an ectopic ribosomal DNA repeat. Proc. Natl. Acad. Sci. USA 110: E4465–E4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Noma K., Grewal S. I., 2004. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304: 1971–1976. [DOI] [PubMed] [Google Scholar]
- Job G., Brugger C., Xu T., Lowe B. R., Pfister Y., et al. , 2016. SHREC silences heterochromatin via distinct remodeling and deacetylation modules. Mol. Cell 62: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh J., Sadaie M., Urano T., Ishikawa F., 2005. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr. Biol. 15: 1808–1819. [DOI] [PubMed] [Google Scholar]
- Katayama S., Kitamura K., Lehmann A., Nikaido O., Toda T., 2002. Fission yeast F-box protein Pof3 is required for genome integrity and telomere function. Mol. Biol. Cell 13: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. U., Hayles J., Kim D., Wood V., Park H. O., et al. , 2010. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott S. R., Peace J. M., Ostrow A. Z., Gan Y., Rex A. E., et al. , 2012. Forkhead transcription factors establish origin timing and long-range clustering in S. cerevisiae. Cell 148: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurat C. F., Yeeles J. T., Patel H., Early A., Diffley J. F., 2017. Chromatin controls DNA replication origin selection, lagging-strand synthesis, and replication fork rates. Mol. Cell 65: 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Russell P., 2013. Brc1 links replication stress response and centromere function. Cell Cycle 12: 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Rozenzhak S., Russell P., 2013. gammaH2A-binding protein Brc1 affects centromere function in fission yeast. Mol. Cell. Biol. 33: 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune E., Bortfeld M., White S. A., Pidoux A. L., Ekwall K., et al. , 2007. The chromatin-remodeling factor FACT contributes to centromeric heterochromatin independently of RNAi. Curr. Biol. 17: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne A., McIntyre J., Katou Y., Kanoh Y., Hopfner K. P., et al. , 2006. Establishment of sister chromatid cohesion at the S. cerevisiae replication fork. Mol. Cell 23: 787–799. [DOI] [PubMed] [Google Scholar]
- Li F., Goto D. B., Zaratiegui M., Tang X., Martienssen R., et al. , 2005. Two novel proteins, dos1 and dos2, interact with rik1 to regulate heterochromatic RNA interference and histone modification. Curr. Biol. 15: 1448–1457. [DOI] [PubMed] [Google Scholar]
- Li F., Martienssen R., Cande W. Z., 2011. Coordination of DNA replication and histone modification by the Rik1-Dos2 complex. Nature 475: 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Coic E., Lee K., Lee C. S., Kim J. A., et al. , 2012. Regulation of budding yeast mating-type switching donor preference by the FHA domain of Fkh1. PLoS Genet. 8: e1002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Powell K. A., Mundt K., Wu L., Carr A. M., et al. , 2003. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 17: 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz A., Ostermann K., Fleck O., Schmidt H., 1994. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene 143: 139–143. [DOI] [PubMed] [Google Scholar]
- Maculins T., Nkosi P. J., Nishikawa H., Labib K., 2015. Tethering of SCF(Dia2) to the replisome promotes efficient ubiquitylation and disassembly of the CMG helicase. Curr. Biol. 25: 2254–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamnun Y. M., Katayama S., Toda T., 2006. Fission yeast Mcl1 interacts with SCF(Pof3) and is required for centromere formation. Biochem. Biophys. Res. Commun. 350: 125–130. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Hayano M., Kanoh Y., Masai H., 2011. Multiple pathways can bypass the essential role of fission yeast Hsk1 kinase in DNA replication initiation. J. Cell Biol. 195: 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A., Arai R., Yashiroda Y., Shirai A., Kamata A., et al. , 2006. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 24: 841–847. [DOI] [PubMed] [Google Scholar]
- Miyabe I., Mizuno K., Keszthelyi A., Daigaku Y., Skouteri M., et al. , 2015. Polymerase delta replicates both strands after homologous recombination-dependent fork restart. Nat. Struct. Mol. Biol. 22: 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T., Fudenberg G., Mehta S., Belton J. M., Taneja N., et al. , 2014. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature 516: 432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi H., Maculins T., Labib K., 2009. The amino-terminal TPR domain of Dia2 tethers SCF(Dia2) to the replisome progression complex. Curr. Biol. 19: 1943–1949. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Allshire R. C., Klar A. J., Grewal S. I., 2001. A role for DNA polymerase alpha in epigenetic control of transcriptional silencing in fission yeast. EMBO J. 20: 2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume T., Tsutsui Y., Sutani T., Dunleavy E. M., Pidoux A. L., et al. , 2008. A DNA polymerase alpha accessory protein, Mcl1, is required for propagation of centromere structures in fission yeast. PLoS One 3: e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. J., Santos-Rosa H., Kouzarides T., 2006. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 126: 905–916. [DOI] [PubMed] [Google Scholar]
- Nicolas E., Yamada T., Cam H. P., Fitzgerald P. C., Kobayashi R., et al. , 2007. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat. Struct. Mol. Biol. 14: 372–380. [DOI] [PubMed] [Google Scholar]
- Nimmo E. R., Cranston G., Allshire R. C., 1994. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 13: 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E., Noguchi C., McDonald W. H., Yates J. R., III, Russell P., 2004. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 24: 8342–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace J. M., Ter-Zakarian A., Aparicio O. M., 2014. Rif1 regulates initiation timing of late replication origins throughout the S. cerevisiae genome. PLoS One 9: e98501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph E., Boye E., Kearsey S. E., 2006. DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1. EMBO Rep. 7: 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S., Eisenhaber F., O’Carroll D., Strahl B. D., Sun Z. W., et al. , 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599. [DOI] [PubMed] [Google Scholar]
- Roseaulin L. C., Noguchi C., Martinez E., Ziegler M. A., Toda T., et al. , 2013a Coordinated degradation of replisome components ensures genome stability upon replication stress in the absence of the replication fork protection complex. PLoS Genet. 9: e1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseaulin L. C., Noguchi C., Noguchi E., 2013b Proteasome-dependent degradation of replisome components regulates faithful DNA replication. Cell Cycle 12: 2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samora C. P., Saksouk J., Goswami P., Wade B. O., Singleton M. R., et al. , 2016. Ctf4 links DNA replication with sister chromatid cohesion establishment by recruiting the Chl1 helicase to the replisome. Mol. Cell 63: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. W., McQuary P. R., Wee S., Hofmann K., Wolf D. A., 2009. F-box-directed CRL complex assembly and regulation by the CSN and CAND1. Mol. Cell 35: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A., Dohke K., Sadaie M., Shinmyozu K., Nakayama J., et al. , 2009. Phosphorylation of Swi6/HP1 regulates transcriptional gene silencing at heterochromatin. Genes Dev. 23: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmoto M., Matsumoto S., Odagiri Y., Noguchi E., Russell P., et al. , 2009. Interactions between Swi1-Swi3, Mrc1 and S phase kinase, Hsk1 may regulate cellular responses to stalled replication forks in fission yeast. Genes Cells 14: 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. C., Zhou J. C., Perera R. L., van Deursen F., Evrin C., et al. , 2014. A Ctf4 trimer couples the CMG helicase to DNA polymerase alpha in the eukaryotic replisome. Nature 510: 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stralfors A., Walfridsson J., Bhuiyan H., Ekwall K., 2011. The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet. 7: e1001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Cam H. P., Sugiyama R., Noma K., Zofall M., et al. , 2007. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128: 491–504. [DOI] [PubMed] [Google Scholar]
- Takayama Y., Mamnun Y. M., Trickey M., Dhut S., Masuda F., et al. , 2010. Hsk1- and SCF(Pof3)-dependent proteolysis of S. pombe Ams2 ensures histone homeostasis and centromere function. Dev. Cell 18: 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazumi A., Fukuura M., Nakato R., Kishimoto A., Takenaka T., et al. , 2012. Telomere-binding protein Taz1 controls global replication timing through its localization near late replication origins in fission yeast. Genes Dev. 26: 2050–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Klar A. J., 1992. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics 131: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Verhein-Hansen J., 2000. Four chromo-domain proteins of Schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 155: 551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Cohen A., Klar A. J., 1994. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Bjerling K. P., Nielsen I. S., 1999. Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151: 945–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Bjerling P., Bunner C. M., Verhein-Hansen J., 2002. Expression-state boundaries in the mating-type region of fission yeast. Genetics 161: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Hansen K. R., Altes S. P., Sidhu D., Singh G., et al. , 2005. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics 171: 1583–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toteva T., Mason B., Kanoh Y., Brogger P., Green D., et al. , 2017. Establishment of expression-state boundaries by Rif1 and Taz1 in fission yeast. Proc. Natl. Acad. Sci. USA 114: 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y., Morishita T., Natsume T., Yamashita K., Iwasaki H., et al. , 2005. Genetic and physical interactions between Schizosaccharomyces pombe Mcl1 and Rad2, Dna2 and DNA polymerase alpha: evidence for a multifunctional role of Mcl1 in DNA replication and repair. Curr. Genet. 48: 34–43. [DOI] [PubMed] [Google Scholar]
- Villa F., Simon A. C., Ortiz Bazan M. A., Kilkenny M. L., Wirthensohn D., et al. , 2016. Ctf4 is a hub in the eukaryotic replisome that links multiple CIP-Box proteins to the CMG helicase. Mol. Cell 63: 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe T. A., Kidner C., Hall I. M., Teng G., Grewal S. I., et al. , 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297: 1833–1837. [DOI] [PubMed] [Google Scholar]
- Williams D. R., McIntosh J. R., 2002. mcl1+, the Schizosaccharomyces pombe homologue of CTF4, is important for chromosome replication, cohesion, and segregation. Eukaryot. Cell 1: 758–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., McIntosh J. R., 2005. Mcl1p is a polymerase alpha replication accessory factor important for S-phase DNA damage survival. Eukaryot. Cell 4: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. D., Harreman M., Taschner M., Reid J., Walker J., et al. , 2013. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 154: 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhemalce B., Seeler J. S., Thon G., Dejean A., Arcangioli B., 2004. Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 23: 3844–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Boone C., Klein H. L., 2004. Mrc1 is required for sister chromatid cohesion to aid in recombination repair of spontaneous damage. Mol. Cell. Biol. 24: 7082–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Ishii A., Kanoh Y., Oda M., Nishito Y., et al. , 2012. Rif1 regulates the replication timing domains on the human genome. EMBO J. 31: 3667–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeeles J. T., Janska A., Early A., Diffley J. F., 2017. How the eukaryotic replisome achieves rapid and efficient DNA replication. Mol. Cell 65: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaratiegui M., Castel S. E., Irvine D. V., Kloc A., Ren J., et al. , 2011. RNAi promotes heterochromatic silencing through replication-coupled release of RNA Pol II. Nature 479: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofall M., Smith D. R., Mizuguchi T., Dhakshnamoorthy J., Grewal S. I., 2016. Taz1-Shelterin promotes facultative heterochromatin assembly at chromosome-internal sites containing late replication origins. Mol. Cell 62: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. All Supplemental Tables and Figures are regrouped in File S1. Table S1 in File S1 presents a list of Bioneer deletion strains selected for the second and third mutant screens and Table S2 in File S1 gives a list of potential false positives. Figures S1–S8 in File S1 show the phenotypes of mutants examined in the second screen, while Figures S9–S11 in File S1 show the phenotypes of mutants examined in the third screen. Table S3 in File S1 contains a list of strains and their genotypes, while Table S4 in File S1 contains oligonucleotide sequences.