Abstract
Purpose
The decision to prescribe packed red blood cell (PRBC) transfusions in patients with chemotherapy-induced anemia (CIA) includes assessment of clinical features such as the patient’s cancer type and treatment regimen, severity of anemia symptoms, and presence of comorbidities. We examined contemporary transfusion practices in patients with nonmyeloid cancer and CIA.
Methods
Key inclusion criteria were age ≥ 18 years with nonmyeloid cancer, receiving first/second-line myelosuppressive chemotherapy, baseline hemoglobin (Hb) ≤ 10.0 g/dL, and planned to receive ≥ 1 PRBC transfusions. Exclusion criteria were receipt of erythropoiesis-stimulating agents within 8 weeks of screening and/or chronic renal insufficiency. Data were collected from patients’ medical records, laboratory values, and physician/provider questionnaires. Proportion of patients for each clinical consideration leading to a decision to prescribe a PRBC transfusion and 95% exact binomial confidence intervals were determined.
Results
The study enrolled 154 patients at 18 sites in USA; 147 (95.5%) received a PRBC transfusion. Fatigue was the most common symptom affecting the decision to prescribe a PRBC transfusion (101 [69.2%] patients). Of the three reasons selected as primary considerations for prescribing a PRBC transfusion, anemia symptoms (106 [72.1%] patients) was the most frequently reported, followed by Hb value (37 [25.2%] patients) and medical history (4 [2.7%] patients).
Conclusions
In this study, the primary consideration for prescribing a PRBC transfusion was anemia symptoms in 72.1% of patients, with only 25.2% of patients prescribed a transfusion based exclusively on Hb value. Results indicate that clinical judgment and patient symptoms, not just Hb value, were used in decisions to prescribe PRBC transfusions.
Electronic supplementary material
The online version of this article (10.1007/s00520-017-4035-7) contains supplementary material, which is available to authorized users.
Keywords: Chemotherapy-induced anemia, Comorbidities, Fatigue, Hemoglobin, Packed red blood cell
Introduction
Chemotherapy-induced anemia (CIA) often develops in patients with cancer who are treated with myelosuppressive chemotherapy [1–3] and can be associated with diminished physical functioning, poor prognosis, and decreased quality of life [4, 5]. Transfusion with packed red blood cells (PRBCs) is a key supportive measure in the care of patients with CIA [3], offering the benefit of rapid correction of anemia in these patients.
In general, the uses and indications for transfusion therapy have changed over the last few years with a shift toward a more restrictive transfusion policy [6, 7]. In clinical practice, any attendant comorbidities or treatment that a patient is receiving should also be considered in the decision on whether or not to prescribe a blood transfusion, especially for cancer patients receiving chemotherapy [2, 3, 8–10]. Additionally, the benefit of a blood transfusion has to be balanced with known risks that include the potential for transfusion-related reactions such as transfusion-associated circulatory overload, transfusion-related acute lung injury, allergic reaction, and febrile nonhemolytic transfusion reaction [7, 11–13] and the potential for transmission of blood-borne pathogens [14]. Other factors to consider include time constraints associated with administration of transfusions [15], the inconvenience to both patients and healthcare professionals [16], and the associated costs [17, 18].
Current clinical guidelines from the National Comprehensive Cancer Network (NCCN) [3] support a restrictive transfusion policy for cancer-related anemia that limits the use of transfusions to achieve a hemoglobin (Hb) concentration of ≥ 7 g/dL but acknowledge that transfusion may be reasonable when patients exhibit anemia symptoms or when patients have comorbidities such as cardiac disease, chronic pulmonary disease, or cerebrovascular disease. While a large body of data with respect to patients with CIA has been generated from studies evaluating support with erythropoiesis-stimulating agents (ESAs) [16, 19–25], there remains a lack of data regarding clinical factors that form the basis for making decisions on when to transfuse patients with CIA [3, 8]. As such, a study evaluating the clinical factors used in making decisions for prescribing transfusions for patients with CIA can inform practitioners on current practice patterns.
This prospective, observational study was designed to evaluate physician-assessed clinical considerations for prescribing PRBC transfusion therapy in patients with a baseline Hb concentration of ≤ 10.0 g/dL who were to receive chemotherapy without support of ESAs.
Methods
Study sites, physicians, and patients
This was a multicenter, prospective, observational study conducted in the USA. Study sites were selected through a site evaluation process from a pool of suburban, urban, and academic oncology centers assembled from sites in the sponsor’s clinical trial management system and sites recommended by the medical market research vendor Adelphi Real World. Potential study sites were selected based on the following criteria: site follows PRBC transfusion guidelines (e.g., NCCN guidelines), number of potentially eligible patients receiving treatment at the site, investigator interest and/or willingness to enroll patients, ESA use, access to facilities for PRBC transfusions (i.e., proximity of site to transfusion center), and ability to integrate the study protocol into existing clinic and transfusion workflow. Selected study sites that did not enroll patients within 3 months of site initiation could be closed and replaced at the sponsor’s discretion.
Physicians were selected based on the following criteria: primary specialty must be oncology or hematology oncology, must be board certified or board eligible, must spend at least 50% of time in direct patient care, at least 50% of patient population treated must be ≥ 18 years of age, must treat at least 30 patients with metastatic cancer per month, and a minimum of 40% of the patients with metastatic cancer treated must be receiving chemotherapy.
Patients were screened for study eligibility when a PRBC transfusion was ordered. Eligible patients were ≥ 18 years of age, had nonmyeloid cancer, were receiving first- or second-line myelosuppressive chemotherapy, had baseline Hb ≤ 10.0 g/dL, and were planned to receive 1 or more PRBC transfusions. Key exclusion criteria were receipt of an ESA within 8 weeks prior to screening and/or chronic renal insufficiency. Patients who met the eligibility criteria were enrolled into the study. No study drug was administered as part of this study.
The study protocol was approved by an independent ethics committee or institutional review board at each center. All subjects provided written informed consent.
Datasources and study procedures
For each study site, data were collected on site location (urban, suburban, or rural), site type (academic or nonacademic), location of transfusion center at site (yes or no), and ESA usage (yes or no). Medical records of patients were evaluated to obtain information on sex, age, cancer type, cancer stage at initial diagnosis, date of initial cancer diagnosis, date and regimen of myelosuppressive chemotherapy received, comorbidities, and signs and symptoms of anemia. The most recent laboratory reports were evaluated to obtain the last Hb concentration before a PRBC transfusion. Questionnaires were completed by physicians prior to the transfusion to document considerations for prescribing a PRBC transfusion (see Online Resource 1 for study survey/questionnaire). Physicians selected and ranked the “most important (primary) consideration,” “second-most important consideration,” or “least important consideration” from the list of three considerations: (1) anemia symptoms, (2) Hb value, or (3) medical history (including comorbidities). Physicians also recorded the signs and symptoms of anemia affecting the decision to prescribe a PRBC transfusion.
Study outcomes and data assessment
Data on site location, site type, whether or not the transfusion center was located on the site, and ESA usage were summarized. The proportions of patients with specific signs and symptoms of anemia were determined. Signs and symptoms of anemia affecting the decision to prescribe a PRBC transfusion were also evaluated. The overall and stratified proportions of patients for each primary clinical consideration leading to a PRBC transfusion were determined and 95% exact binomial confidence intervals were estimated. Baseline covariate strata included sex (male vs female), age (< 65 years vs ≥ 65 years), primary tumor type, chemotherapy type (platinum vs non-platinum), chemotherapy line (first vs second line), and site type (academic vs nonacademic).
Results
Study period and patient enrollment
The study recruitment period was from 30 September 2014 to 31 October 2015, with the last visit of the last patient in October 2015. Database lock was on 7 January 2016. A total of 154 patients were enrolled at 18 sites in the USA, out of the 25 recruited sites (Online Resource 2). Of the 18 sites that enrolled patients, most (10 [55.6%] sites) were in urban areas, with a few in suburban (4 [22.2%] sites) and rural areas (4 [22.2%] sites). Most sites (16 [88.9%]) were nonacademic and most (12 [66.7%]) reported routine use of ESAs. Ten (55.6%) sites did not have on-site transfusion centers.
PRBC transfusion, units of blood received, and last Hb value prior to PRBC transfusion
Of the 154 patients enrolled, 147 (95.5%) received a PRBC transfusion and 7 (4.5%) did not (Table 1). Most patients (120 [77.9%]) received 2 units of blood and 27 (17.5%) patients received 1 unit of blood; information on units of blood received was missing for 7 (4.5%) patients (Table 1). Mean (range) last Hb value before a PRBC transfusion was 8.1 (4.9–9.9) g/dL. For one of the patients, Hb value was measured after a PRBC transfusion; therefore, data for this patient were excluded from the analysis. Of the 146 patients included in the analysis, 140 (95.9%) patients received a transfusion at Hb ≥ 7.0 g/dL and 70 (47.9%) patients received a transfusion at Hb ≥ 8.0 g/dL.
Table 1.
PRBC transfusion, units of blood received, and last Hb value prior to PRBC transfusion
| Total patients enrolled in the study N = 154 | |
|---|---|
| PRBC transfusion, n (%) | |
| Received a PRBC transfusion | 147 (95.5) |
| Did not receive a PRBC transfusion | 7 (4.5) |
| Units of blood received, n (%) | |
| 1 | 27 (17.5) |
| 2 | 120 (77.9) |
| Missing | 7 (4.5) |
| Last Hb value prior to PRBC transfusion | |
| n | 146a |
| Mean (range), g/dL | 8.1 (4.9–9.9) |
| Hb category, n (%) | |
| < 7.0 g/dL | 6 (4.1) |
| ≥ 7.0 g/dL | 140 (95.9) |
| < 8.0 g/dL | 76 (52.1) |
| ≥ 8.0 g/dL | 70 (47.9) |
Hb hemoglobin, PRBC packed red blood cell
aOne patient was excluded from the analysis as the Hb value was measured after a PRBC transfusion
Patient demographics
Baseline demographics and clinical characteristics for the 154 total patients enrolled in the study and the 147 patients who received a PRBC transfusion are shown in Table 2. Of the patients who received a PRBC transfusion, most (100 [68.0%]) were female and over half (81 [55.1%]) were ≥ 65 years of age. The most common tumor type was gynecological cancer (38 [25.9%] patients), followed by non-small cell lung cancer (32 [21.8%] patients) and small cell lung cancer (24 [16.3%] patients). Most of the patients were receiving platinum-containing chemotherapy (107 [72.8%]) and most were receiving first-line chemotherapy (95 [64.6%]). Signs and symptoms of anemia were reported by a high proportion of the patients (133 [90.5%]). Most patients (106 [72.1%]) did not present with comorbidities relevant to the anemia. In the 41 (27.9%) patients who did, the most common comorbidity was chronic pulmonary disease (22 [15.0%] patients). Only 9 (6.1%) patients had underlying cardiovascular disease.
Table 2.
Baseline demographic and clinical characteristics
| Characteristic | Total patients enrolled in the study N = 154 | Patients who received a PRBC transfusion N = 147 |
|---|---|---|
| Sex, n (%) | ||
| Female | 107 (69.5) | 100 (68.0) |
| Male | 47 (30.5) | 47 (32.0) |
| Age, mean (SD), years | 65.3 (9.8) | 65.3 (9.9) |
| < 65 years, n (%) | 70 (45.5) | 66 (44.9) |
| ≥ 65 years, n (%) | 84 (54.5) | 81 (55.1) |
| Primary tumor type, n (%) | ||
| Gynecological cancer | 43 (27.9) | 38 (25.9) |
| Non-small cell lung cancer | 32 (20.8) | 32 (21.8) |
| Small cell lung cancer | 25 (16.2) | 24 (16.3) |
| Gastrointestinal cancer | 19 (12.3) | 18 (12.2) |
| Breast cancer | 14 (9.1) | 14 (9.5) |
| Urogenital cancer | 7 (4.5) | 7 (4.8) |
| Hematological malignancies | 7 (4.5) | 7 (4.8) |
| Othera | 7 (4.5) | 7 (4.8) |
| Chemotherapy type, n (%) | ||
| Platinum | 113 (73.4) | 107 (72.8) |
| Non-platinum | 41 (26.6) | 40 (27.2) |
| Chemotherapy line, n (%) | ||
| First | 100 (64.9) | 95 (64.6) |
| Second | 54 (35.1) | 52 (35.4) |
| Signs and symptoms of anemia, n (%) | ||
| Yes | 137 (89.0) | 133 (90.5) |
| No | 17 (11.0) | 14 (9.5) |
| Comorbidities relevant to the anemia, n (%) | ||
| Yes | – | 41 (27.9) |
| Chronic pulmonary disease | – | 22 (15.0) |
| Congestive heart failure or coronary heart disease | – | 9 (6.1) |
| Cerebral vascular disease | – | 2 (1.4) |
| Otherb | – | 17 (11.6) |
| No | – | 106 (72.1) |
PRBC packed red blood cell, SD standard deviation
aIncludes bone/sarcoma, brain, head and neck, skin, thyroid cancers, or missing
bNot specified
Signs and symptoms of anemia
The proportions of patients with specific signs and symptoms of anemia are shown in Online Resource 3. In patients who received a PRBC transfusion, fatigue was the most common sign and symptom of anemia (127 [86.4%] patients), followed by dyspnea on exertion (58 [39.5%] patients) and pallor (40 [27.2%] patients) (Online Resource 3a). Similarly, fatigue was the most common sign and symptom of anemia affecting the physician’s decision to prescribe a PRBC transfusion (101 [69.2%] patients), followed by dyspnea on exertion (49 [33.6%] patients) and pallor (33 [22.6%] patients) (Online Resource 3b).
Primary clinical considerations for prescribing a PRBC transfusion
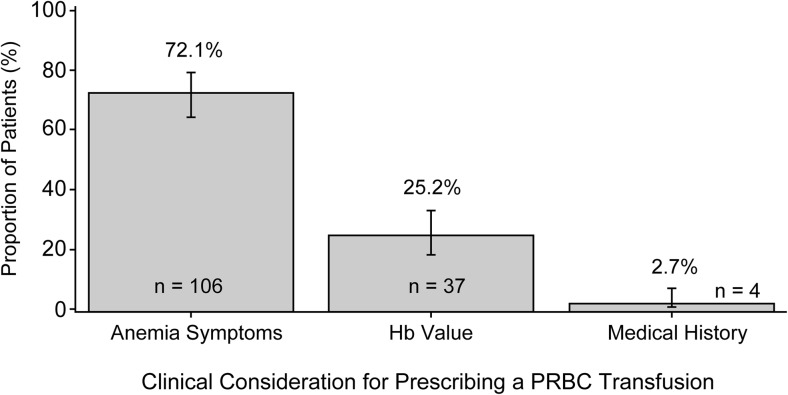
Physician-assessed clinical considerations for prescribing a PRBC transfusion are shown in Fig. 1 and Table 3. The most frequently reported primary clinical consideration for prescribing a PRBC transfusion was anemia symptoms in 106 (72.1%) patients, followed by Hb value in 37 (25.2%) patients, and medical history in 4 (2.7%) patients. The mean Hb concentration at which a decision to give a PRBC transfusion was made ranged from 8.1 to 8.5 g/dL, whether transfusion was primarily given because of anemia symptoms (8.1 g/dL, range: 6.2–9.9 g/dL), Hb value (8.1 g/dL, range: 4.9–9.9 g/dL), or medical history (8.5 g/dL, range: 7.8–9.8 g/dL) (Table 3).
Fig. 1.
Physician-assessed primary clinical considerations for prescribing PRBC transfusions. The proportions of patients for each primary clinical consideration leading to a PRBC transfusion were determined. Error bars are 95% exact binomial confidence intervals. Hb hemoglobin, PRBC packed red blood cell
Table 3.
Primary clinical considerations for prescribing a PRBC transfusion
| Patients who received a PRBC transfusion N = 147 | ||
|---|---|---|
| n (%) or mean (range), g/dL | 95% CI (% or g/dL) | |
| Anemia symptoms | 106 (72.1) | 64.1, 79.2 |
| Last Hb value prior to a PRBC transfusion | 8.1 (6.2–9.9) | 7.9, 8.3 |
| Hb value | 37 (25.2) | 18.4, 33.0 |
| Last Hb value prior to a PRBC transfusion | 8.1 (4.9–9.9) | 7.8, 8.5 |
| Medical history | 4 (2.7) | 0.7, 6.8 |
| Last Hb value prior to a PRBC transfusion | 8.5 (7.8–9.8) | 7.0, 9.9 |
Physicians selected the “most important (primary) consideration,” “second-most important consideration,” or “least important consideration” from the list of three considerations of anemia symptoms, Hb value, or medical history (including comorbidities)
CI confidence interval, Hb hemoglobin, PRBC packed red blood cell
Primary clinical considerations for prescribing a PRBC transfusion stratified by baseline covariates
Online Resources 4 to 7 show primary clinical considerations for prescribing a PRBC transfusion stratified by baseline covariates of sex (male vs female), age (< 65 years vs ≥ 65 years), primary tumor type, chemotherapy type (platinum vs non-platinum), chemotherapy line (first vs second), and site type (academic vs nonacademic). When stratified by age, approximately twice the number of patients ≥ 65 years of age received a PRBC transfusion based on Hb value: 25 (30.9%) patients who were ≥ 65 years old vs 12 (18.2%) patients who were < 65 years old (Online Resource 4). Primary tumor type, chemotherapy type, or chemotherapy line did not influence the decision to transfuse above and beyond anemia symptoms (Online Resources 5 to 7).
Discussion
Anemia is common in patients with cancer and often occurs before patients receive treatment for the cancer [3, 26, 27]. Anemia is also one of the most frequent adverse effects of chemotherapy as shown by studies in different regions [27–31]. An analysis of data for cancer patients receiving chemotherapy obtained from pooled placebo arms of six randomized controlled trials (RCTs) of darbepoetin alfa and from an aggregated US community oncology EMR database showed that 58% of patients in the RCTs and 46% EMR episodes had a Hb decline from < 10 to < 9 g/dL at week 9 [28]. In a multicenter observational study of patients receiving chemotherapy for nonmyeloid malignancies in Spain [29], almost half the patients (48%) had a Hb level < 12 g/dL. A multicenter survey of Japanese patients receiving chemotherapy [30] reported an average Hb level prior to treatment of 9.5 g/dL. A study of chemotherapy-associated anemia in Western Denmark reported a median Hb level before transfusion of 9.0 g/dL [31]. In data reported by the European Cancer Anemia Study, mean Hb level in cancer patients before initiation of either iron supplementation, transfusion, or use of an ESA was 9.7 g/dL [27].
Since the seminal paper by Herbert et al. evaluating transfusion requirements in critical care units in Canada [32], numerous studies and clinical guidelines have supported the use of a more restrictive transfusion policy with a target Hb concentration of 7 to 8 g/dL in patients who are not actively bleeding. These studies encompass a wide range of patient groups including patients treated in critical care units, coronary care units, and orthopedic and cardiac surgery patients, as well as patients in the setting of sickle cell disease, end stage renal disease, gastrointestinal bleeding, and sepsis [6, 7, 33–36].
Transfusion requirements in cancer patients receiving myelosuppressive chemotherapy have not been prospectively studied [37, 38]. Data on transfusion practices generated in Europe show a wide variation in transfusion practices as it relates to cancer patients with anemia who are receiving chemotherapy [38–41]. These data may not be directly applicable to the US population in view of variations in treatment guidelines, approved supportive agents such as ESAs, and differences in reimbursement policies. In addition, since the US Food and Drug Administration placed restrictions on ESA use in 2007, a number of studies have shown a change in patterns of transfusion practice with a documented increase in transfusion frequency seen in most studies [9, 40, 42–44].
In our prospective, multicenter, observational study, we sought to understand current transfusion practice patterns and the factors that contribute to a decision to transfuse patients with anemia who were receiving myelosuppressive chemotherapy for nonmyeloid malignancies, in an era of restricted ESA use. The most common reason to prescribe a transfusion in our patient population was anemia symptoms in 72.1% of patients, with fatigue being the predominant symptom in 69.2% of patients. Absolute Hb value was only a secondary consideration, being cited as the reason to prescribe a transfusion in 25.2% of patients (Fig. 1 and Table 3). Even though comorbidities were reported in 27.9% of patients, physicians considered them relevant to prescribing a transfusion only 2.7% of the time.
While patients in our study were treated according to current clinical guidelines for transfusion of cancer-related anemia [3], 47.9% received a transfusion at a Hb level ≥ 8.0 g/dL (Table 1). This pattern was consistent across multiple covariates including sex, cancer type, and chemotherapy type (platinum versus non-platinum) (Online Resources 4 to 7). There was a trend to prioritize Hb value over anemia symptoms in patients ≥ 65 years of age (Online Resource 4).
Anemia symptoms appear to have been an important clinical consideration in the decision to transfuse in our study. In general, fatigue has not been a major indication for transfusion outside of the cancer setting [45, 46]. There may be a number of factors that distinguish patients with cancer from other non-cancer patient groups. Cancer-related fatigue is common and typically multifactorial. As noted above, many patients with cancer may already be anemic prior to starting treatment for their cancer. The physical and psychological effects of the cancer itself, treatment effects (which may or may not include myelosuppression), the effect of repetitive cycles of chemotherapy, and the recovery interval between treatments impact on the decision making for management of patients with cancer.
Although we did not attempt to quantify the degree of fatigue in our patient group, fatigue was the most frequently recorded clinical consideration for prescribing a PRBC transfusion over the absolute Hb value or medical history/comorbidities. Additional factors that may have influenced the decision for PRBC transfusion at a higher Hb value as observed in our study may relate to the fact that ESA use was excluded in this study, and thus treating physicians may have anticipated a further fall in Hb levels due to planned chemotherapy treatment.
The major strength of our study is the prospective design with predefined measures and endpoints that enabled the evaluation of PRBC transfusion patterns in cancer patients with anemia who were receiving chemotherapy in a real-world setting. However, the findings from this study should be considered in the context of a number of limitations. The assessments of iron stores or iron treatments were not recorded. Also, any attendant complications associated with transfusion therapy were not recorded. Our study relied on questionnaires completed by the physicians; patients did not fill out comparable questionnaires. Patient-reported symptoms were abstracted from the subject’s EMR or reported to the oncology nurse and then recorded. Physicians were not asked to comment on how they might have integrated ESAs in their transfusion algorithm or whether their decision to transfuse was correlated with the number of chemotherapy cycles anticipated. Anemia signs and symptoms were only assessed on a 10-point prespecified list without using a commonly accepted, validated instrument and without severity grading. Likewise, validated instruments were not used to assess quality of life measures for patients in our study.
In conclusion, in this prospective, multicenter, observational study of transfusion practices in patients with CIA, the primary clinical consideration for prescribing a PRBC transfusion was anemia symptoms in 72.1% of patients. The absolute Hb value was the second clinical consideration for the decision to transfuse in 25.2% of patients. Overall, 47.9% of patients received a transfusion at a Hb level ≥ 8 g/dL. The decision to transfuse was independent of sex, medical comorbidities, cancer type, or chemotherapy type (platinum or non-platinum), with a trend to rely more on Hb value when transfusing patients ≥ 65 years of age. Clinical judgment based on individual patient signs and symptoms of anemia, in particular fatigue, and not only the absolute Hb value, were used in the decision to prescribe a PRBC transfusion.
Electronic supplementary material
(PDF 499 kb)
Acknowledgments
This study was supported by Amgen Inc. Amgen Inc. was involved in the design, data collection, analysis, and data interpretation. Sharon Hunter, a consultant for Amgen Inc., provided statistical support for many of the analyses. Martha Mutomba (on behalf of Amgen Inc.) and Susanna Mac from Amgen Inc. provided writing assistance.
Compliance with ethical standards
Conflict of interest
James Granfortuna, Kaye Shoffner, and Stephen E. DePasquale have no financial relationships or conflicts of interest to disclose. Sejal Badre was an employee of Amgen Inc. at the time of the study and owns/may have owned stock in Amgen Inc. Chet Bohac was an employee of Amgen Inc. at the time of the study and owns/may have owned stock in Amgen Inc.; he is currently an employee of and owns stock in Immune Design. Cisio De Oliveira Brandao is an employee of and owns stock in Amgen Inc.
Research involving human participants and/or animals
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm. 2007;64(3 Suppl 2):S5–13. doi: 10.2146/ajhp060601. [DOI] [PubMed] [Google Scholar]
- 2.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN® clinical practice guidelines in oncology. Cancer- and chemotherapy-induced anemia version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/anemia.pdf Accessed 8 December 2017
- 4.Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2–7. doi: 10.1159/000088282. [DOI] [PubMed] [Google Scholar]
- 5.Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LV, van der Wall E, Richel DJ, Nooij MA, Voest EE, Hupperets P, Mulder NH, van der Graaf WT, TenVergert EM, van Tinteren H, de Vries EG. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med. 2017;377(13):1261–1272. doi: 10.1056/NEJMra1612789. [DOI] [PubMed] [Google Scholar]
- 8.Prescott LS, Taylor JS, Lopez-Olivo MA, Munsell MF, VonVille HM, Lairson DR, Bodurka DC. How low should we go: a systematic review and meta-analysis of the impact of restrictive red blood cell transfusion strategies in oncology. Cancer Treat Rev. 2016;46:1–8. doi: 10.1016/j.ctrv.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Xu H, Cannavale K, Sattayapiwat O, Rodriguez R, Page JH, Chao C. Trends in anemia treatment among patients with five non-myeloid malignancies treated with chemotherapy in a large integrated health care delivery system in California, 2000-2013. Support Care Cancer. 2016;24(7):2989–2998. doi: 10.1007/s00520-016-3078-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, Bayliss S, Moss P, Stanworth S, Hyde C. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess. 2007;11(1–202):iii–iiv. doi: 10.3310/hta11130. [DOI] [PubMed] [Google Scholar]
- 11.Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, Henderson KM. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2002;2:CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrijvers D. Management of anemia in cancer patients: transfusions. Oncologist. 2011;16(Suppl 3):12–18. doi: 10.1634/theoncologist.2011-S3-12. [DOI] [PubMed] [Google Scholar]
- 14.Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med. 2006;355(13):1303–1305. doi: 10.1056/NEJMp068178. [DOI] [PubMed] [Google Scholar]
- 15.Corey-Lisle PK, Desrosiers MP, Collins H, De La Orden M, Payne KA, Levache CB, Dumont P. Transfusions and patient burden in chemotherapy-induced anaemia in France. Ther Adv Med Oncol. 2014;6(4):146–153. doi: 10.1177/1758834014534515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaspy JA, Jadeja JS, Justice G, Kessler J, Richards D, Schwartzberg L, Tchekmedyian NS, Armstrong S, O'Byrne J, Rossi G, Colowick AB. Darbepoetin alfa given every 1 or 2 weeks alleviates anaemia associated with cancer chemotherapy. Br J Cancer. 2002;87(3):268–276. doi: 10.1038/sj.bjc.6600465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berndt E, Crown W, Kallich J, Long S, Song X, Lyman GH. The impact of anaemia and its treatment on employee disability and medical costs. PharmacoEconomics. 2005;23(2):183–192. doi: 10.2165/00019053-200523020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lyman GH, Berndt ER, Kallich JD, Erder MH, Crown WH, Long SR, Lee H, Song X, Finkelstein SN. The economic burden of anemia in cancer patients receiving chemotherapy. Value Health. 2005;8(2):149–156. doi: 10.1111/j.1524-4733.2005.03089.x. [DOI] [PubMed] [Google Scholar]
- 19.Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19(11):2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 20.Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E, Taylor K, Belch A, Altes A, Martinelli G, Watson D, Matcham J, Rossi G, Littlewood TJ, Darbepoetin Alfa 20000161 Study Group Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 21.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 22.Vansteenkiste J, Hedenus M, Gascon P, Bokemeyer C, Ludwig H, Vermorken J, Hamilton L, Bridges K, Pujol B. Darbepoetin alfa for treating chemotherapy-induced anemia in patients with a baseline hemoglobin level <10 g/dL versus ≥10 g/dL: an exploratory analysis from a randomized, double-blind, active-controlled trial. BMC Cancer. 2009;9(1):311. doi: 10.1186/1471-2407-9-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, Siena S, Gateley J, Tomita D, Colowick AB, Musil J, Aranesp 980297 Study Group Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 24.Pirker R, Ramlau RA, Schuette W, Zatloukal P, Ferreira I, Lillie T, Vansteenkiste JF. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol. 2008;26(14):2342–2349. doi: 10.1200/JCO.2007.15.0748. [DOI] [PubMed] [Google Scholar]
- 25.Pirker R, Hedenus M, Vansteenkiste J, Hernandez E, Belton L, Terwey JH. Effectiveness of darbepoetin alfa for chemotherapy-induced anemia when initiated at hemoglobin ≤10 g/dL. Clin Ther. 2016;38(122–135):e126. doi: 10.1016/j.clinthera.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M, Nortier J, Olmi P, Schneider M, Schrijvers D. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40(15):2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Pirker R, Pirolli M, Quigley J, Hulnick S, Legg J, Collins H, Vansteenkiste J. Hemoglobin decline in cancer patients receiving chemotherapy without an erythropoiesis-stimulating agent. Support Care Cancer. 2013;21(4):987–992. doi: 10.1007/s00520-012-1617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steegmann JL, Sanchez Torres JM, Colomer R, Vaz A, Lopez J, Jalon I, Provencio M, Gonzalez-Martin A, Perez M. Prevalence and management of anaemia in patients with non-myeloid cancer undergoing systemic therapy: a Spanish survey. Clin Transl Oncol. 2013;15(6):477–483. doi: 10.1007/s12094-012-0953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka A, Yoshino I, Makino S, Katsumata N, Takahashi K, Kuwano H, Maehara Y, Nishiyama M. Questionnaire-based survey on chemotherapy-induced anemia. Int J Clin Oncol. 2014;19(3):411–420. doi: 10.1007/s10147-014-0677-3. [DOI] [PubMed] [Google Scholar]
- 31.Yong M, Riis AH, Fryzek JP, Moller BK, Johnsen SP. Predictors and patterns of red blood cell transfusion use among newly diagnosed cancer patients with chemotherapy-associated anemia in Western Denmark (1998-2003) Clin Epidemiol. 2011;3:91–99. doi: 10.2147/CLEP.S17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hébert Paul C., Wells George, Blajchman Morris A., Marshall John, Martin Claudio, Pagliarello Giuseppe, Tweeddale Martin, Schweitzer Irwin, Yetisir Elizabeth. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. New England Journal of Medicine. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 33.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J, Investigators FOCUS. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 35.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettilä V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Müller RG, Møller MH, Steensen M, Tjäder I, Kilsand K, Odeberg-Wernerman S, Sjøbø B, Bundgaard H, Thyø MA, Lodahl D, Maerkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A, TRISS Trial Group, Scandinavian Critical Care Trials Group Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 36.Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165(964–971):e961. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkins T, Surowiecka MK, McCullough J. Transfusion indications for patients with cancer. Cancer Control. 2015;22(1):38–46. doi: 10.1177/107327481502200106. [DOI] [PubMed] [Google Scholar]
- 38.Lai-Tiong F, Brami C, Dubroeucq O, Scotte F, Cure H, Jovenin N. Management of anemia and iron deficiency in a cancer center in France. Support Care Cancer. 2016;24(3):1091–1096. doi: 10.1007/s00520-015-2877-4. [DOI] [PubMed] [Google Scholar]
- 39.Aapro M, Abraham I, Bokemeyer C, Ludwig H, Macdonald K, Soubeyran P, Turner M. The background and methodology of the anaemia cancer treatment (A.C.T.) study: a global retrospective study of practice patterns and outcomes in the management of anaemia in cancer patients and their congruence with evidence-based guidelines. Support Care Cancer. 2008;16(2):193–200. doi: 10.1007/s00520-007-0311-2. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig H, Aapro M, Bokemeyer C, Macdonald K, Soubeyran P, Turner M, Albrecht T, Abraham I. Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: findings from the anaemia cancer treatment (ACT) study. Eur J Cancer. 2009;45(9):1603–1615. doi: 10.1016/j.ejca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig H, Aapro M, Bokemeyer C, Glaspy J, Hedenus M, Littlewood TJ, Osterborg A, Rzychon B, Mitchell D, Beguin Y. A European patient record study on diagnosis and treatment of chemotherapy-induced anaemia. Support Care Cancer. 2014;22(8):2197–2206. doi: 10.1007/s00520-014-2189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollingsworth K, Romney MC, Crawford A, McAna J. Risk evaluation mitigation strategy: impact of application of the Food and Drug Adminstration’s strategy on use of erythropoiesis-stimulating agents and transfusion in patients with chemotherapy-induced anaemia. J Clin Pharm Ther. 2015;40(3):299–303. doi: 10.1111/jcpt.12269. [DOI] [PubMed] [Google Scholar]
- 43.Mouysset J-L, Freier B, van den Bosch J, Levache CB, Bols A, Tessen HW, Belton L, Bohac C, Terwey JH, Tonini G. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Care Manag Res. 2016;8:1–10. doi: 10.2147/CMAR.S88110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry DH, Langer CJ, McKenzie RS, Piech CT, Senbetta M, Schulman KL, Stepanski EJ. Hematologic outcomes and blood utilization in cancer patients with chemotherapy-induced anemia (CIA) pre- and post-national coverage determination (NCD): results from a multicenter chart review. Support Care Cancer. 2012;20(9):2089–2096. doi: 10.1007/s00520-011-1318-2. [DOI] [PubMed] [Google Scholar]
- 45.Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carson JL, Strair R. Transfusion strategies in hematologic and nonhematologic disease. Hematology Am Soc Hematol Educ Program. 2014;2014(1):548–552. doi: 10.1182/asheducation-2014.1.548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 499 kb)