Abstract
Wild relatives provide an important source of useful traits in wheat breeding. Wheat and wild relative hybrids have been widely used in breeding programs to introduce such traits into wheat. However, successful introgression is limited by the low frequency of homoeologous crossover (CO) between wheat and wild relative chromosomes. Hybrids between wheat carrying a 70 Mb deletion on chromosome 5B (ph1b) and wild relatives, have been exploited to increase the level of homoeologous CO, allowing chromosome exchange between their chromosomes. In ph1b-rye hybrids, CO number increases from a mean of 1 CO to 7 COs per cell. CO number can be further increased up to a mean of 12 COs per cell in these ph1b hybrids by treating the plants with Hoagland solution. More recently, it was shown that the major meiotic crossover gene ZIP4 on chromosome 5B (TaZIP4-B2) within the 70 Mb deletion, was responsible for the restriction of homoeologous COs in wheat-wild relative hybrids, confirming the ph1b phenotype as a complete Tazip4-B2 deletion mutant (Tazip4-B2 ph1b). In this study, we have identified the particular Hoagland solution constituent responsible for the increased chiasma frequency in Tazip4-B2 ph1b mutant-rye hybrids and extended the analysis to Tazip4-B2 TILLING and CRISPR mutant-Ae variabilis hybrids. Chiasma frequency at meiotic metaphase I, in the absence of each Hoagland solution macronutrient (NH4 H2PO4, KNO3, Ca (NO3)2·4H2O or Mg SO4·7H2O) was analyzed. A significant decrease in homoeologous CO frequency was observed when the Mg2+ ion was absent. A significant increase of homoeologous CO frequency was observed in all analyzed hybrids, when plants were irrigated with a 1 mM Mg2+ solution. These observations suggest a role for magnesium supplementation in improving the success of genetic material introgression from wild relatives into wheat.
Keywords: wheat, wild relatives, magnesium, Ph1 locus, TaZIP4-B2, homoeologous crossover, CRISPR/Cas9 system
Introduction
Despite possessing related ancestral genomes (genome AABBDD), bread wheat behaves as a diploid during meiosis. Deletion of chromosome 5B in tetraploid and hexaploid wheat results in a level of incorrect chromosome pairing and exchange, visualized as a low level of multivalents at metaphase I, and homoeologous crossovers (COs) between related chromosomes in wheat-wild relative hybrids (Riley and Chapman, 1958; Sears and Okamoto, 1958). From these observations, it was proposed that chromosome 5B carries a locus termed Pairing homoeologous 1 (Ph1), which evolved on wheat's polyploidisation and restricted chromosome pairing and COs to true homologs (Riley and Chapman, 1958). A hexaploid wheat cv. Chinese Spring (CS) line carrying a 70 Mb deletion on the long arm of chromosome 5B (ph1b) has been exploited over the last 40 years to allow exchange between wild relative and wheat chromosomes. Recently, it was shown that on wheat's polyploidisation, a segment of 3B carrying the major crossover gene ZIP4 and a block of heterochromatin, duplicated and inserted between two CDK2-like genes within a cluster of CDK2-like and methyl-transferase genes (Griffiths et al., 2006; Al-Kaff et al., 2008; Martín et al., 2014, 2017). Using exploitation of TILLING mutants, it was shown that the duplicated ZIP4 gene (TaZIP4-B2) within this cluster, both promotes homologous CO and restricts homoeologous CO (Rey et al., 2017). Therefore, TaZIP4-B2 within the 70 Mb ph1b deletion region is responsible for the effect on homoeologous CO in wheat-wild relative hybrids, and as such the ph1b line can be described as a complete-deletion (or complete loss-of-function) mutant of Tazip4-B2 (Tazip4-B2 ph1b mutant). In terms of the effect on chromosome synapsis/pairing, cell biological studies reveal that the ph1b deletion in wheat has little effect, with most synapsis occurring during clustering of the telomeres as a bouquet. Furthermore, in wheat-wild relative hybrids, which only possess homoeologues, the ph1b deletion also has little effect on the level of synapsis, except that most pairing occurs after dispersal of the telomere bouquet. In wheat itself, a few chromosomes also undergo delayed pairing until after dispersal of the bouquet, with the subsequent incorrect pairing leading to the low level of multivalents observed at metaphase I (Martín et al., 2014, 2017).
For the last 40 years, the wheat CS ph1b deletion line has been exploited in crosses with wild relatives to allow exchange between chromosomes at meiosis. As indicated previously, in these hybrids, the extent of chromosome synapsis is similar whether the line carries the ph1b deletion or not. Moreover, on the synapsed chromosomes, similar numbers of MLH1 sites (normally a marker for CO), are observed (Martín et al., 2014). However significant site CO frequency is only observed in those hybrids carrying the ph1b deletion. However even in this case, the frequency of resulting COs still does not reflect the number of available MLH1 sites (Martín et al., 2014). This implies that there is potential for increased processing of MLH1 sites into COs. Fortuitously, it has been observed that a nutrient solution (Hoagland's solution) added to the soil when Tazip4-B2 ph1b mutant-rye hybrids are growing resulted in increased CO frequency, although it was not known which nutrient component was responsible for the effect (Martín et al., 2017).
Mineral elements are essential nutrients for plants to complete their life cycle. They are classified into macro and micronutrients, which are required in relatively large and small amounts, respectively (Hoagland and Arnon, 1950). The importance of each of these macronutrients has been reported in numerous physiological processes, such as plant growth, cell division, and metabolism (Huber, 1980; Maathuis, 2009). However, limited studies have been performed as to their effect on meiosis. Early studies have previously reported that alterations of external factors, such as temperature, or nutrient composition, can produce profound effects on chiasma frequency (Grant, 1952; Wilson, 1959; Law, 1963; Bennett and Rees, 1970; Fedak, 1973).
The main objective of the present study was to determine whether a specific macronutrient present in the Hoagland solution was responsible for the observed increased homoeologous CO frequency in Tazip4-B2 ph1b mutant-rye hybrids described in Martín et al. (2017). We also analyzed whether this macronutrient increased homoeologous CO frequency in each of the Tazip4-B2 ph1b (complete deletion), TILLING (point mutation) and CRISPR (partial deletion) mutant-Ae. variabilis hybrids.
Materials and methods
Plant material
Plant material used in this study included: Triticum aestivum (2n = 6x = 42; AABBDD) cv. Chinese Spring Tazip4-B2-ph1b mutant line (Sears, 1977); Triticum aestivum cv. Chinese Spring-rye hybrids—crosses between the Tazip4-B2-ph1b mutant line hexaploid wheat and rye [Secale cereal cv. Petkus (2n = 2x = 14; RR)]; Triticum aestivum cv. Chinese Spring-Aegilops variabilis hybrids—crosses between Tazip4-B2-ph1b mutant and Ae. variabilis Eig. (2n = 4x = 28; UUSvSv); Triticum aestivum cv. Cadenza-Ae. variabilis hybrids—crosses between Cad1691 and Cad0348, Tazip4-B2 TILLING mutants and Ae. variabilis (Krasileva et al., 2017; Rey et al., 2017); and Triticum aestivum cv. Fielder-Ae. variabilis hybrids—crosses between Tazip4-B2 CRISPR mutant and Ae. variabilis (see Production of TaZIP4-B2 knock-out using RNA-guided Cas9, section Materials and Methods).
Nutrient solution treatments
The total number of plants used in this work is described in Table S1. All seedlings were vernalized for 3 weeks at 7°C under a photoperiod of 16 h light/8 h dark, and then transferred to a controlled environmental room until meiosis (approximately 2 months later for all genotypes used in this study). The growth conditions were 16 h/8 h, light/dark photoperiod at 20°C day and 15°C night, with 70% humidity. At least 2 weeks before meiosis, irrigation of plants with a Hoagland solution (100 mL per plant) was commenced following the method previously described in Martín et al. (2017). Briefly, plants were irrigated once a week with a Hoagland solution (100 mL) from the stem elongation stage of the vegetative stage (stage 7–8, Feeke's scale). The composition of the Hoagland solution was: (macronutrients) KNO3 (12 mM), Ca (NO3)2·4H2O (4 mM), NH4 H2PO4 (2 mM), Mg SO4·7H2O (1 mM); and (micronutrients) NaFe-EDTA (60 mM), KCl (50 μM), H3BO3 (25 μM), Mn SO4·H2O (2 μM), Zn SO4 (4 μM), Cu SO4·5H2O (0.5 μM), H2MoO4 (0.5 μM). Four treatments were carried out to analyze the effect of the absence of NH4 H2PO4, KNO3, Ca (NO3)2·4H2O or Mg SO4·7H2O from the Hoagland solution on homoeologous CO frequency in Tazip4-B2-ph1b mutant-rye hybrids. For each treatment, a different Hoagland solution was prepared in the absence of each macronutrient (NH4 H2PO4, KNO3, Ca (NO3)2·4H2O or Mg SO4·7H2O). Moreover, the effect of the presence of only Mg SO4·7H2O (Mg SO4·7H2O is designed as Mg2+ in the manuscript) in only water rather than in Hoagland solution on CO frequency was also analyzed in Tazip4-B2-ph1b mutant-rye hybrids in comparison to the effects of Hoagland solution. Also, two different concentrations of Mg2+ (1 and 2 mM of Mg2+ in water) were used to assess the homoeologous CO frequency in Tazip4-B2-ph1b mutant-rye hybrids. The treatment with either Mg2+ in water alone or Hoagland solution in Tazip4-B2 ph1b mutant-Ae. variabilis, and Tazip4-B2 TILLING and CRISPR mutant-Ae. variabilis hybrids was also assessed.
Assessment of the addition of Mg2+ in water alone on homoeologous CO frequency was also made on non-irrigated plants, by injecting into Tazip4-B2 ph1b mutant-rye hybrids tillers a solution containing 1 mM Mg2+ in water (0.5 mL per spike) just above every spike at the stage 9 on the Feekes's scale (three spikes with water alone and three spikes with Mg2+ in water). All spikes were analyzed 24–48 h after the injection.
Feulgen-stained analysis
After either irrigating with Hoagland or Mg2+ solution, or injecting the Mg2+ solution, tillers were harvested when the flag leaf was completely emerged, and only anthers at meiotic metaphase I were collected and fixed in 100% ethanol/acetic acid 3:1 (v/v). The anthers used in this study were taken from spikelets in the lower half of the spike. From each spikelet, the 2 largest florets (on opposing sides of the floret) were used. From each dissected floret, one of the three synchronized anthers was squashed in 45% acetic acid/distilled water (v/v) and the meiocytes assessed for being at meiotic metaphase I by observation under a phase contrast microscope [LEICA DM2000 microscope (LeicaMicrosystems, http://www.leica-microsystems.com/)]. The two remaining anthers were left then fixed in 100% ethanol/acetic acid 3:1 (v/v) for cytological analysis of meiocytes. The anthers were incubated in ethanol/acetic acid at 4°C for at least 24 h. Cytological analysis of meiocytes at metaphase I was performed using Feulgen reagent as previously described in Sharma and Sharma (2014). Metaphase I meiocytes were observed under a phase contrast microscope equipped with a Leica DFC450 camera and controlled by LAS v4.4 system software (Leica Biosystems, Wetzlar, Germany). The digital images were used to determine the meiotic configurations of the meiocytes by counting the number of univalents, rod (1 chiasma) and ring (2 chiasmata) bivalents and multivalents (trivalents (1–2 chiasmata), tetravalents (3 chiasmata) and pentavalents (4 chiasmata)). Two different methods depending on the number of chiasma (single or double chiasmata) were used to calculate chiasma frequency per meiocyte (see Figure S1 for examples of the scored structures). Images were processed using Adobe Photoshop CS5 (Adobe Systems Incorporated, US) extended version 12.0 × 64.
Production of TaZIP4-B2 CRISPR mutants using RNA-guided Cas9
Three single guide RNAs (sgRNA) were designed manually to specifically target TaZIP4-B2. These guides were in the limited regions where there was sufficient variation between ZIP4 on 5BL and homoeologous group 3 chromosomes (Figure 3). The specific guides were: Guide 4: 5′GATGAGCGACGCATCCTGCT3′, Guide 11: 5′GATGCGTCGCTCATCCTCCG3′ and Guide 12: 5′GAAGAAGGATGCGGCCTTGA3′ (Figure 3). Two constructs were assembled using standard Golden Gate assembly (Werner et al., 2012) with each construct containing the Hygromycin resistance gene under the control of a rice Actin1 promoter, Cas9 under the control of the rice ubiquitin promoter and two of the sgRNAs each under the control of a wheat U6 promoter (Figure 3). Construct 1 contained guides 4 and 12 and construct 2 contained guides 11 and 12. To produce each gRNA, a PCR reaction was performed using Phusion High-Fidelity Polymerase (Thermo Scientific M0530S) with a forward primer containing the gRNA sequence, and a standard reverse primer 5′TGTGGTCTCAAGCGTAATGCCAACTTTGTAC3′ using the plasmid pICSL70001::U6p::gRNA (Addgene plasmid 46966) as template. Each gRNA was cloned individually into the level 1 vectors pICH47751 (gRNA4 & 11) and pICH47761 (gRNA12). Level 1 construct pICH47802-RActpro::Hpt::NosT (selection maker), pICH47742-ZmUbipro::Cas9::NosT and the gRNAs were then assembled in the binary Level 2 vector pGoldenGreenGate (pGGG) a Golden Gate compatible vector based on pGreen (Hellens et al., 2000) (Figure 3).
The two constructs were introduced to T. aestivum cv. Fielder by Agrobacterium-mediated inoculation of immature embryos. 450 immature embryos were inoculated with Agrobacterium strain AGL1 containing each construct. Briefly, after 3 days co-cultivation with Agrobacterium, immature embryos were selected on 15 mg/l hygromycin during callus induction for 2 weeks and 30 mg/l hygromycin for 3 weeks in the dark at 24°C on Murashige and Skoog medium (MS; Murashige and Skoog, 1962) 30 g/l Maltose, 1.0 g/l Casein hydrolysate, 350 mg/l Myo-inositol, 690 mg/l Proline, 1.0 mg/lThiamine HCl (Harwood et al., 2009) supplemented with 2 mg/l Picloram, 0.5 mg/l 2,4-Dichlorophenoxyacetic acid (2,4-D). Regeneration was under low light (140 μmol.m-2.s-1) conditions on MS medium with 0.5 mg/l Zeatin and 2.5 mg/l CuSO45H2O.
Primary transgenic plants (T0) were analyzed by PCR across the region of interest. The sequences for the forward and reverse primers used for the screening in T0 were 5′GCCGCCATGACGATCTCCGAG3′ and 5′GGACGCGAGGGACGCGAG3′, respectively (Rey et al., 2017), followed by direct sequencing. The PCR was performed using RedTaq ReadyMix PCR Reaction Mix (Sigma, St. Louis, MO, USA; R2523) according to the manufacturer's instructions. PCR conditions were: 3 min 95C, 35 cycles of 15 s at 95C, 15 s at 58C and 30 s at 72C. T0 plants with edits in TaZIP4-B2 were progressed to the T1 generation and 24 T1 seedlings from each original T0 plant were analyzed in the same way for the presence of edits.
Statistical analyses
Statistical analyses were performed using STATISTIX 10.0 software (Analytical Software, Tallahassee, FL, USA). Analysis of variance (ANOVA) in Tazip4-B2 ph1b mutant-rye hybrids, Tazip4-B2 TILLING mutant-Ae. variabilis hybrids and Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids was based on a completely randomized design. Several transformations were carried out: tangent (ring bivalents), arcsine (trivalents), and logarithm (double CO) transformations in the analysis of the effect of absence of each macronutrients in homoeologous CO frequency in Tazip4-B2 ph1b mutant-rye hybrids; exponential (ring bivalents) transformation in Tazip4-B2 ph1b mutant-Ae. variabilis hybrids; exponential (rod bivalents, rings bivalents and trivalents) transformation in Tazip4-B2 TILLING mutant (Cad1691)-Ae. variabilis hybrids; and square root (ring bivalents) and exponential (trivalents) transformations in Tazip4-B2 TILLING mutant (Cad0348)-Ae. variabilis hybrids. Means were separated using the Least Significant Difference (LSD) test with a probability level of 0.05. Both Tazip4-B2 CRISPR mutant lines and Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids were analyzed by the Kruskal–Wallis test (non-parametric one-way analysis of variance). Means were separated using the Dunn's test with a probability level of 0.05.
Results
Magnesium increases homoeologous COs in Tazip4-B2 ph1b mutant-rye hybrids
The Tazip4-B2 ph1b mutant-rye hybrids were obtained by crosses between the hexaploid wheat cv. Chinese Spring Tazip4-B2 ph1b mutant and rye. These hybrids were used to analyse which macronutrient (NH4 H2PO4, KNO3, Ca (NO3)2·4H2O or Mg SO4·7H2O) present within in the Hoagland solution detailed in Martín et al. (2017) could be responsible for the increased CO number observed in the Tazip4-B2 ph1b mutant-rye hybrids. To assess the effect of the absence of each macronutrient in homoeologous CO frequency in meiotic metaphase I, we irrigated several Tazip4-B2 ph1b mutant-rye hybrids with: (1) Hoagland solution; (2) water alone; (3) Hoagland solution minus KNO3; (4) Hoagland solution minus Ca (NO3)2·4H2O; (5) Hoagland solution minus NH4 H2PO4; (6) Hoagland solution minus MgSO4·7H2O (MgSO4·7H2O is designed as Mg2+ in the manuscript) (Table 1). The absence of each Hoagland solution macronutrient caused a slight increase in homoeologous CO frequency, except for the treatment lacking Mg2+, where a significant decrease in homoeologous CO frequency per meiocyte was observed at meiotic metaphase I in these hybrids (Table 1). No significant differences in CO frequency at metaphase I were observed between hybrids treated with water alone and those treated with the Hoagland solution minus Mg2+ [a mean of 7.91 chiasmata for hybrids treated with water alone and 8.09 chiasmata for hybrids treated with Hoagland solution minus Mg2+ (Table 1)].
Table 1.
Effect of the absence of each macronutrient in the Hoagland solution on the homoeologous CO frequency of T. aestivum cv. Chinese Spring Tazip4-B2 ph1b mutant-rye hybrids.
| No. of cell examined | Univalents | Rod bivalents | Ring bivalents | Trivalents | Chiasma frequency | ||
|---|---|---|---|---|---|---|---|
| Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | ||
| Single chiasma | Double chiasmata | ||||||
| Hoagland solution | 109 | 12.36 ± 0.21c (7–18) |
4.39 ± 0.14a (2–8) |
2.57 ± 0.10abc (0–5) |
0.58 ± 0.07a (0–3) |
10.74 ± 0.16a (7–14) |
12.03 ± 0.22a (7–18) |
| Water alone | 108 | 15.93 ± 0.16a (12–20) |
4.03 ± 0.13ab (1–7) |
1.68 ± 0.09bc (0–4) |
0.22 ± 0.04bc (0–1) |
7.91 ± 0.13d (5–10) |
8.15 ± 0.15d (5–12) |
| Hoagland solution - NH4 H2PO4 | 92 | 13.61 ± 0.22b (8–17) |
3.83 ± 0.14b (2–8) |
2.78 ± 0.13a (0–6) |
0.39 ± 0.06ab (0–2) |
10.25 ± 0.18b (7–14) |
11.03 ± 0.23b (7–17) |
| Hoagland solution - KNO3 | 93 | 13.86 ± 0.22b (6–18) |
4.34 ± 0.16a (2–8) |
2.35 ± 0.12abc (0–5) |
0.25 ± 0.05c (0–2) |
9.57 ± 0.18c (7–16) |
9.96 ± 0.19c (7–16) |
| Hoagland solution - Ca(NO3)2·4H2O | 102 | 13.84 ± 0.20b (9–18) |
4.14 ± 0.13ab (1–7) |
2.50 ± 0.09c (0–4) |
0.29 ± 0.05bc (0–2) |
9.79 ± 0.15c (7–14) |
10.12 ± 0.17c (7–15) |
| Hoagland solution - Mg SO4·7H2O | 90 | 15.63 ± 0.24a (10–20) |
4.12 ± 0.16ab (1–8) |
1.68 ± 0.13ab (0–4) |
0.26 ± 0.06bc (0–2) |
8.09 ± 0.20d (5–12) |
8.62 ± 0.22d (5–13) |
| P-value | 0.0000 | 0.0538 | 0.0592 | 0.0516 | 0.0000 | 0.0000 | |
Frequencies of univalents, bivalents, trivalents and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 ph1b mutant-rye hybrids. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test. Different letters indicate significant differences between treatments.
Additionally, we scored all meiocytes for the occurrence of double chiasmata in the metaphase I chromosomal configurations (examples highlighted by arrows in Figure S1). When double chiasmata were considered in the chiasma frequency, a mean of 8.15 chiasmata and 8.62 chiasmata was observed respectively in Tazip4-B2 ph1b mutant-rye hybrids treated with water alone, and those treated with the Hoagland solution minus Mg2+ (Table 1). As expected, no significant differences were observed between the two treatments when double chiasmata were considered in these Tazip4-B2 ph1b hybrids.
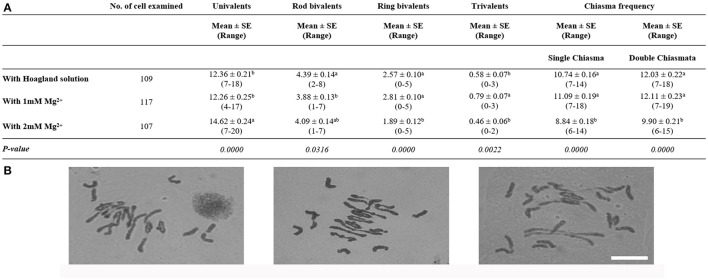
Once the absence of Mg2+ was demonstrated to decrease homoeologous CO frequency in Tazip4-B2 ph1b mutant-rye hybrids, the effect of irrigating with only Mg2+ present at a final concentration of 1 mM in water rather than in the Hoagland solution, was also analyzed on homoeologous COs in Tazip4-B2 ph1b mutant-rye hybrids (Figure 1). Treatment with a solution containing only Mg2+ also increased homoeologous COs at metaphase I per meiocyte in these hybrids, showing no significant difference in comparison to the Hoagland solution treatment. A mean of 11.09 chiasmata was observed after treatment with 1 mM Mg2+ in water, and 10.74 chiasmata after treatment with the Hoagland solution (Figure 1), when a single chiasma was considered. A similar situation was seen when double chiasmata were considered: no significant differences were observed in homoeologous COs per meiocyte in Tazip4-B2 ph1b mutant-rye hybrids after treatment with either 1 mM Mg2+ or Hoagland solutions (Figure 1).
Figure 1.
Effect of either 1 mM or 2 mM Mg2+ on homoeologous CO frequency of T. aestivum cv. Chinese Spring Tazip4-B2 ph1b mutant-rye hybrids. (A) Frequencies of univalents, bivalents, trivalents, and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 ph1b mutant-rye hybrids treated with either Hoagland solution, 1 mM Mg2+ or 2 mM Mg2+solution. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test. Different letters indicate significant differences between treatments. (B) Representative meiotic configurations of Tazip4-B2 ph1b mutant-rye hybrids. From left to right: treatment with Hoagland solution, 1 mM Mg2+or 2 mM Mg2+ solution. Bar: 20 μm.
The concentration of Mg2+ was subsequently increased to a final concentration of 2 mM to assess whether the number of homoeologous COs could be increased further (Figure 1). Surprisingly, numbers of COs were reduced under these conditions (mean 11.09 for 1 mM Mg2+ and 8.84 for 2 mM Mg2+ treatments respectively, when single chiasma were considered, and 12.11 and 9.90, respectively, when double chiasmata were considered).
In addition to irrigating the plants with either Hoagland or Mg2+ solutions, we analyzed the effect of treatment with 1 mM Mg2+ in water following injection into the tillers of Tazip4-B2 ph1b mutant-rye hybrids. Injections were made just above each spike. Once again, homoeologous CO frequency was significantly increased in hybrids treated with 1 mM Mg2+ when the solution was injected into the tiller (Table 2). A mean of 8.98 chiasmata in hybrids treated with water alone and 10.60 chiasmata in hybrids treated with 1 mM Mg2+ was observed in the hybrids considering a single chiasma (Table 2) and a mean of 9.67 chiasmata and 11.30 chiasmata considering double chiasmata (Table 2).
Table 2.
Effect of injecting 1 mM Mg2+ solution into the tillers of Tazip4-B2 ph1b mutant-rye hybrids.
| No. of cell examined | Univalents | Rod bivalents | Ring bivalents | Trivalents | Chiasma frequency | ||
|---|---|---|---|---|---|---|---|
| Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | ||
| Single chiasma | Double chiasmata | ||||||
| Water alone | 87 | 15.02 ± 0.26a (3–20) |
3.43 ± 0.16b (0–7) |
2.12 ± 0.10 (0–4) |
0.63 ± 0.08 (0–3) |
8.98 ± 0.18b (6–16) |
9.67 ± 0.21b (6–17) |
| With 1 mM Mg2+ | 79 | 12.32 ± 0.26b (5–18) |
4.32 ± 0.17a (1–8) |
2.37 ± 0.12 (0–5) |
0.77 ± 0.08 (0–3) |
10.60 ± 0.19a (7–16) |
11.30 ± 0.22a (8–18) |
| P-value | 0.0000 | 0.0001 | 0.1079 | 0.2312 | 0.0000 | 0.0000 | |
Frequencies of univalents, bivalents, trivalents, and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 ph1b mutant-rye hybrids treated with water alone and with 1 mM Mg2+ solution. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test. Different letters indicate significant differences between treatments.
Magnesium increases homoeologous COs in Tazip4-B2 ph1b mutant-Ae. variabilis hybrids
The addition of Mg2+ is thus identified as responsible for the increase in homoeologous CO at meiotic metaphase I in Tazip4-B2 ph1b mutant-rye hybrids. We then assessed the effect of 1 mM Mg2+ on T. aestivum cv. Chinese spring Tazip4-B2 ph1b mutant-Ae. variabilis hybrids. Firstly, we scored the number of univalents, bivalents and multivalents, and total chiasma frequency in this hybrid, to compare the level of chiasma frequency to that previously reported by Kousaka and Endo (2012) in T. aestivum cv. Chinese spring-Ae. variabilis hybrids in the absence of chromosome 5B. We observed a similar chiasma frequency in our hybrid (mean 14.15 chiasmata per meiocyte), to that previously reported in T. aestivum cv. Chinese spring-Ae. variabilis hybrids in the absence of chromosome 5B (mean of 14.09 chiasmata per meiocyte), confirming a similar level of meiotic metaphase I configuration in these hybrids.
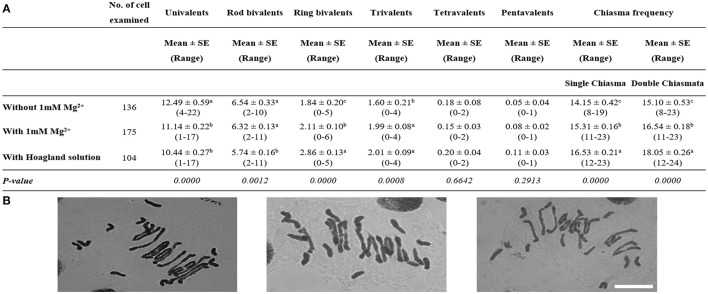
We then analyzed the effect of treatment with water alone and with either 1 mM Mg2+ solution or complete Hoagland solution on the Tazip4-B2 ph1b mutant-Ae. variabilis hybrids. The total number of COs was significantly higher after treatment with 1 mM Mg2+ than after treatment with water alone (without Mg2+ control), both in the case of single chiasma and double chiasmata, showing a mean of 15.31 and 14.15 chiasmata in the case of single chiasma, and a mean of 16.54 and 15.10 chiasmata in the case of double chiasmata, respectively (Figure 2). The number of univalents was significantly decreased and the number of trivalents was significantly increased when the plants were treated with 1 mM Mg2+ solution in comparison to when Mg2+ was absent (a mean of 11.14 and 1.99, respectively, after treatment with 1 mM Mg2+ and a mean of 12.49 and 1.60, respectively, after treatment with water alone were observed in Figure 2). With regard to the Hoagland solution treatment, significant differences were observed between hybrids treated with water alone and hybrids treated with Hoagland solution (Figure 2). Hoagland solution treatment showed the highest chiasma frequency, followed by 1 mM Mg2+ and water alone [means of 16.53, 15.31, and 14.15 chiasmata were observed, respectively, when a single chiasma was considered, and means of 18.05, 16.54, and 15.10 chiasmata were observed, respectively, when double chiasmata were considered (Figure 2)].
Figure 2.
Effect of either 1 mM Mg2+ or Hoagland solution on homoeologous CO frequency of T. aestivum cv. Chinese Spring Tazip4-B2 ph1b mutant-Ae. variabilis hybrids. (A) Frequencies of univalents, bivalents, trivalents, tetravalents, pentavalents and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 ph1b mutant-Ae. variabilis hybrids treated with either 1 mM Mg2+ or Hoagland solution. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test. Different letters indicate significant differences between treatments. (B) Representative meiotic configurations of Tazip4-B2 ph1b mutant-Ae. variabilis hybrids. From left to right: water alone, 1 mM Mg2+ treatment and Hoagland solution treatment. Bar: 20 μm.
Magnesium increases homoeologous COs in wheat Tazip4-B2 TILLING mutant-Ae. variabilis mutant hybrids
Recently we reported that Tazip4-B2 TILLING mutants crossed with Ae. variabilis exhibited homoeologous COs at meiotic metaphase I (Rey et al., 2017). We therefore decided to analyse whether the level of homoeologous COs induced by Tazip4-B2 TILLING mutants was also affected by treatment with 1 mM Mg2+ solution. To assess the effect of 1 mM Mg2+ on homoeologous CO frequency at metaphase I, we added 100 mL per plant of a solution of either 1 mM Mg2+ in water or Hoagland solution once a week to the soil in which these hybrids were growing. In this experiment, we analyzed both Tazip4-B2 TILLING mutant lines (Cad1691 and Cad0348) (Rey et al., 2017), crossed with Ae. variabilis. Both TILLING mutant hybrids showed a significant increase in chiasma frequency after treatment with 1 mM Mg2+, compared to chiasma frequency obtained in both the hybrids treated with water alone. The Tazip4-B2 TILLING mutant (Cad1691)-Ae. variabilis and the Tazip4-B2 TILLING mutant (Cad0348)-Ae. variabilis hybrids showed means of 13.41 and 13.66 single chiasma frequency, respectively, after treatment with 1 mM Mg2+ and means of 12.21 and 12.23 single chiasma frequency, respectively, in water alone (Table 3; Figure S2). Significant differences were also observed when double chiasmata were scored in both mutant lines (Table 3; Figure S2). Numbers of univalents and trivalents were also affected by treatment with 1 mM Mg2+ in both mutant lines as in the Tazip4-B2 ph1b mutant-Ae. variabilis hybrids described in the previous section. Numbers of univalents were significantly decreased both in Tazip4-B2 TILLING mutant (Cad1691)-Ae. variabilis and Tazip4-B2 TILLING mutant (Cad0348)-Ae. variabilis hybrids treated with 1 mM Mg2+ [means of 12.74 and 12.11 univalents respectively with Mg2+ and means of 14.74 and 14.63 univalents respectively with water alone (Table 3)]. Numbers of trivalents were significantly increased both in wheat (Cad1691)-Ae. variabilis and wheat (Cad0348)-Ae. variabilis hybrids, after treatment with 1 mM Mg2+ [means of 1.63 and 1.93 trivalents respectively with Mg2+, and means of 1.05 and 1.27 trivalents respectively with water alone (Table 3)].
Table 3.
Effect of either 1 mM Mg2+ or Hoagland solution on the homoeologous CO frequency of T. aestivum cv. Cadenza (Cad1691-Tazip4-B2)-Ae. variabilis and T. aestivum cv. Cadenza (Cad0348-Tazip4-B2)-Ae. variabilis hybrids.
| No. of cell examined | Univalents | Rod bivalents | Ring bivalents | Trivalents | Tetravalents | Pentavalents | Chiasma frequency | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | |||
| Single chiasma | Double chiasmata | |||||||||
| Wateralone* | Cad1691 × Ae. variabilis hybrids | 106 | 14.74 ± 0.29a (7–26) |
6.75 ± 0.17 (3–11) |
1.26 ± 0.08 (0–4) |
1.05 ± 0.08b (0–4) |
0.22 ± 0.04 (0–2) |
0.03 ± 0.02 (0–1) |
12.21 ± 0.19b (8–18) |
12.74 ± 0.21b (8–20) |
| Cad0348 × Ae. variabilis hybrids | 102 | 14.63 ± 0.28a (6–21) |
6.64 ± 0.18 (3–10) |
1.12 ± 0.10 (0–4) |
1.27 ± 0.11b (0–4) |
0.21 ± 0.04 (0–1) |
0.05 ± 0.02 (0–1) |
12.23 ± 0.20b (7–19) |
12.68 ± 0.23b (7–21) |
|
| With 1 mM Mg2+ | Cad1691 × Ae. variabilis hybrids | 95 | 12.74 ± 0.33b (5–19) |
7.06 ± 0.20 (2–11) |
1.18 ± 0.11 (0–3) |
1.63 ± 0.13a (0–5) |
0.17 ± 0.30 (0–1) |
0.04 ± 0.02 (0–1) |
13.41 ± 0.22a (8–18) |
13.75 ± 0.23a (8–19) |
| Cad0348 × Ae. variabilis hybrids | 110 | 12.11 ± 0.32b (3–20) |
6.93 ± 0.18 (1–12) |
1.51 ± 0.11 (0–4) |
1.93 ± 0.11a (0–5) |
0.24 ± 0.04 (0–2) |
0.07 ± 0.02 (0–1) |
14.21 ± 0.23a (9–21) |
14.90 ± 0.25a (10–22) |
|
| With Hoagland solution | Cad1691 × Ae. variabilis hybrids | 118 | 11.94 ± 0.33b (2–20) |
6.76 ± 0.23 (0–12) |
1.17 ± 0.10 (0–4) |
1.50 ± 0.11a (0–5) |
0.26 ± 0.05 (0–2) |
0.04 ± 0.02 (0–1) |
13.66 ± 0.21a (9–20) |
14.14 ± 0.22a (9–21) |
| Cad0348 × Ae. variabilis hybrids | 142 | 12.48 ± 0.25b (2–20) |
6.70 ± 0.17 (1–12) |
1.37 ± 0.09 (0–4) |
1.76 ± 0.10a (0–5) |
0.23 ± 0.04 (0–2) |
0.04 ± 0.02 (0–1) |
13.84 ± 0.19a (9–21) |
14.30 ± 0.21a (9–22) |
|
| P-value | Cad1691 × Ae. variabilis hybrids | 0.0000 | 0.1930 | 0.6630 | 0.0026 | 0.3278 | 0.9376 | 0.0000 | 0.0000 | |
| Cad0348 × Ae. variabilis hybrids | 0.0000 | 0.3220 | 0.5967 | 0.0003 | 0.9720 | 0.3650 | 0.0000 | 0.0000 | ||
Frequencies of univalents, bivalents, trivalents, tetravalents, pentavalents, and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 TILLING mutant-Ae. variabilis hybrids treated with either 1 mM Mg2+ or Hoagland solution. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test.
This data published in Rey et al. (2017). Different letters indicate significant differences between treatments.
Finally, we assessed the effect of treating with Hoagland solution and with water alone, finding significant differences in homoeologous COs between the two treatments, both in Tazip4-B2 TILLING mutant (Cad1691)-Ae. variabilis and Tazip4-B2 TILLING mutant (Cad0348)-Ae. variabilis hybrids. Numbers of univalents and trivalents were also affected to the same extent (Table 3). In the Tazip4-B2 TILLING mutant (Cad1691)-Ae. variabilis hybrid, means of 14.74 univalents and 1.05 trivalents were observed in hybrids treated with water alone, and means of 11.94 univalents and 1.50 trivalents observed in hybrids treated with Hoagland solution (Table 3). In the Tazip4-B2 TILLING mutant (Cad0348)-Ae. variabilis hybrid, means of 14.63 univalents and 1.27 trivalents were observed in hybrids with water alone and means of 12.48 univalents and 1.76 trivalents in hybrids treated with Hoagland solution (Table 3).
Phenotypic analysis of Tazip4-B2 mutants generated by CRISPR/Cas9 system
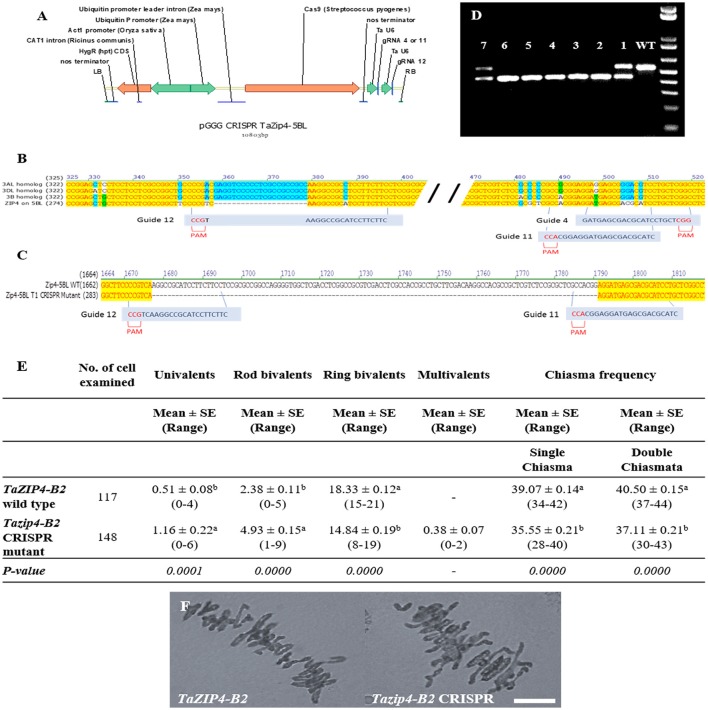
Firstly, eighty-one primary transgenic plants (T0) were analyzed by PCR followed by direct sequencing. Four plants were identified with edits in the target region. One plant had a perfect 115 bp deletion between guides G11 and G12. Twenty-four T1 plants from this line were screened and 5 homozygous edited plants with the 115 bp deletion were recovered. These plants were used to score the number of univalents, bivalents, and multivalents, and total chiasma frequency in the Tazip4-B2 mutant CRISPR lines (Figure 3). Wild-type Fielder lines were used as control plants (Figure 3). The Tazip4-B2 CRISPR mutant lines exhibited a significant reduction in ring bivalents, from a mean of 18.33 to 14.84 in the wild-type Fielder and CRISPR mutant lines respectively (Figure 3). A significant increase in the number of univalents and rod bivalents was also observed, from means of 0.51 univalents and 2.38 rod bivalents in the wild-type Fielder line, to means of 1.16 univalents and 4.93 rod bivalents in the CRISPR mutant lines (Figure 3). This indicates a significant reduction in homologous COs in these Tazip4-B2 mutant lines (Figure 3). Chiasma frequency decreased from a mean of 39.07 single chiasma and 40.50 double chiasmata in the wild-type Fielder line, to a mean of 35.55 single chiasma and 37.11 double chiasmata in the Tazip4-B2 CRISPR mutant (Figure 3).
Figure 3.
Development and phenotypic analysis of Tazip4-B2 CRISPR mutants generated using RNA-guided Cas9. (A) Schematic of the structure of the pGGG CRISPR TaZip4-B2 vector used in this study. (B) Alignment of all copies of the ZIP4 gene in wheat showing sequences and positions of the three sgRNAs designed to specifically target TaZIP4-B2 with their corresponding protospacer-adjacent motif (PAM). (C) Alignment of TaZIP4-B2 wild type and Tazip4-B2 CRISPR mutant sequences sowing the localization of the large deletion (115 bp) in TaZIP4-B2. (D) Genotypic assays for the identification of homozygous edited lines (lines: #2, #3, #4, #5, and #6) and heterozygous lines (lines: #1 and #7). Wild-type Fielder (WT) was used as a control line. (E) Frequencies of univalents, bivalents and multivalents, and total chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in wild-type Fielder and Tazip4-B2 CRISPR mutant. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to Dunn's test. Different letters indicate significant differences between treatments. (F) Representative meiotic metaphase I configurations of wild-type Fielder and Tazip4-B2 CRISPR Fielder mutants. Left: wheat cv. Fielder and right: Tazip4-B2 CRISPR mutant. Bar: 20 μm.
Magnesium also increases homoeologous COs in wheat Tazip4-B2 CRISPR mutant-Ae. variabilis mutant hybrids
For this study, a wild-type Fielder and a Tazip4-B2 CRISPR Fielder mutant line were crossed with Ae. variabilis to assess the level of homoeologous COs in the resulting hybrids (Table S2). Frequency of univalents, bivalents and multivalents, and total chiasma frequency were scored at meiotic metaphase I (Table S2). Tazip4-B2 CRISPR mutant hybrids exhibited a significant increase in single chiasma frequency, from a mean of 3.15 in the wild-type Fielder-Ae. variabilis hybrid to 16.66 in the Tazip4-B2 CRISPR-Ae. variabilis hybrid (Table S2). Double chiasma frequency was also increased in the Tazip4-B2 CRISPR mutant hybrids (Table S2). There was also a similar increase in the chiasma frequency to that reported previously in Tazip4-B2 TILLING-Ae. variabilis hybrids (Rey et al., 2017).
Having observed the effect of treatment with Mg2+ on homoeologous CO frequency in the Tazip4-B2 TILLING mutant hybrids, we also analyzed the effect of this ion on Tazip4-B2 CRISPR mutants-Ae. variabilis hybrids. We added 100 mL of a solution of 1 mM Mg2+ in water or Hoagland solution once a week to the soil in which the hybrids were growing. As expected, the addition of nutrients to these mutant hybrids caused a significant increase in chiasma frequency (Table 4; Figure S2). Tazip4-B2 CRISPR-Ae. variabilis hybrids treated with water alone exhibited means of 16.66 single chiasma frequency and 18.10 double chiasma frequency. Addition of 1 mM Mg2+ caused a significant increase in chiasma frequency of these mutant hybrids (means of 17.67 and 18.75 single and double chiasma frequency respectively) (Table 4). Also, the addition of Hoagland solution increased the homoeologous COs in these Tazip4-B2 CRISPR hybrids. Means of 18.34 and 19.82 single and double chiasma frequency respectively, were observed in those plants treated with Hoagland solution (Table 4).
Table 4.
Effect of either 1 mM Mg2+or Hoagland solution on the homoeologous CO frequency of wheat Tazip4-B2 CRISPR-Ae. variabilis mutant hybrids.
| No. of cell examined | Univalents | Rod bivalents | Ring bivalents | Trivalents | Tetravalents | Pentavalents | Hexavalents | Chiasma frequency | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | Mean ± SE (Range) | ||
| Single chiasma | Double chiasmata | |||||||||
| Water alone | 124 | 9.64 ± 0.27a (3–17) |
5.64 ± 0.17b (2–10) |
1.94 ± 0.11b (0–6) |
2.37 ± 0.11 (0–6) |
0.52 ± 0.06 (0–3) |
0.20 ± 0.04a (0–2) |
0.00 ± 0.00 (0–0) |
16.66 ± 0.21c (11–22) |
18.10 ± 0.23c (12–24) |
| With 1 mM Mg2+ | 135 | 7.92 ± 0.25b (0–14) |
6.19 ± 0.15a (3–11) |
2.02 ± 0.09b (0–6) |
2.68 ± 0.09 (0–3) |
0.50 ± 0.06 (0–2) |
0.10 ± 0.01b (0–1) |
0.01 ± 0.01 (0–1) |
17.67 ± 0.17b (14–23) |
18.75 ± 0.20b (14–24) |
| With Hoagland solution | 125 | 7.90 ± 0.28b (0–15) |
5.56 ± 0.17b (1–11) |
2.82 ± 0.11a (0–6) |
2.53 ± 0.11 (0–5) |
0.59 ± 0.07 (0–3) |
0.08 ± 0.03b (0–2) |
0.00 ± 0.00 (0–0) |
18.34 ± 0.21a (14–24) |
19.82 ± 0.23a (15–25) |
| P-value | 0.0000 | 0.0121 | 0.0000 | 0.1021 | 0.5929 | 0.0259 | 0.1575 | 0.0000 | 0.0000 | |
Frequencies of univalents, bivalents, trivalents, tetravalents, pentavalents, and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids treated with either 1 mM Mg2+ or Hoagland solution. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to LSD test. Different letters indicate significant differences between treatments.
Discussion
Introgression of genetic material from relative species into bread wheat has been used in plant breeding for over 50 years, although classical plant breeding methods to introgress wild relative segments into wheat are both inefficient and time consuming (Ko et al., 2002). Recent availability of SNP based arrays, combined with classical cytogenetic approaches, significantly enhanced our ability to exploit wild relatives (King et al., 2017a,b), using lines carrying a deletion of either the whole of chromosome 5B, or a smaller 70 Mb segment (ph1b) (Riley and Chapman, 1958; Sears and Okamoto, 1958; Sears, 1977), to increase the level of homoeologous crossovers between wild relatives and wheat chromosomes. Recombination between wild relative chromosomes and wheat chromosomes is, however, still limited. Thus, there is a need to find abiotic or biotic treatments such as temperature, nutritional availability, DNA-damaging agents, among others (Lambing et al., 2017) to enhance recombination. Martín et al. (2017) recently reported an alternative tool to increase CO number in Tazip4-B2 ph1b mutant-rye hybrids, using the addition of a Hoagland solution to the soil in which the plants are grown. Martín et al. (2017) also showed that the presence of the Hoagland solution did not affect the homoeologous CO number in wild-type wheat-rye hybrids.
Here, we report the successful identification of the particular Hoagland solution constituent responsible for the observed increase in homoeologous CO frequency. After analyzing Tazip4-B2 ph1b mutant-rye hybrids in the absence of each separate Hoagland solution macronutrient, we observed a significant reduction in homoeologous CO frequency when the Mg2+ ion was absent. This suggests that the Mg2+ ion is mainly responsible for the effect of Hoagland solution on homoeologous COs described previously by Martín et al. (2017). These observations were obtained after cytogenetic analysis of meiotic configurations at meiotic metaphase I. The analysis involved scoring single and double chiasmata in the chromosomal structures (Figure S1). Single chiasma counting has commonly been used in many studies to measure chiasma frequency in wheat (Dhaliwal et al., 1977; Sears, 1977; Roberts et al., 1999). However, other studies have suggested that double chiasmata may occur in these chromosomal configurations (Gennaro et al., 2012; Dreissig et al., 2017). Double chiasmata were considered in the present study, as a high number of MLH1 sites were previously reported in Tazip4-B2 ph1b mutant-rye hybrids in Martín et al. (2014). In our studies, up to 19 chiasmata were scored in Tazip4-B2 ph1b mutant-rye hybrids, which is similar to the number of MLH1 sites observed previously (Martín et al., 2014).
The effect of treatment with a solution of 1mM Mg2+ in water, was analyzed to confirm whether that the Mg2+ ion was responsible for the increase in homoeologous COs observed in these hybrids. The effect of treatment with this solution was assessed either by irrigation of, or injection into Tazip4-B2 ph1b mutant-rye hybrids. Surprisingly, both methods of application increased homoeologous CO frequency in the Tazip4-B2 ph1b mutant-rye hybrids. Thus, the results from the injection method of application suggested that the 1 mM Mg2+ concentration was directly responsible for the increased homoeologous CO effect seen in the Tazip4-B2 ph1b mutant-rye hybrids, rather than through indirect effects on the plant growth or development. However, homoeologous CO frequency was decreased when the Mg2+ concentration was increased further (Figure 1). This reduction in COs was associated with a significant increase in the number of univalents, and decrease in the number of ring bivalents and trivalents.
A recent study revealed that TaZIP4-B2 within the 5B region defined by the 70 Mb ph1b deletion, was responsible for the suppression of homoeologous COs in hybrids (Rey et al., 2017). Tazip4-B2 TILLING mutants (one with a missense mutation and another with a nonsense mutation), when crossed with Ae. variabilis, exhibit similar levels of homoeologous CO that observed in ph1b-Ae. variabilis hybrids (Rey et al., 2017). It was therefore important to assess the effect of 1 mM Mg2+ solution on these Tazip4-B2 TILLING mutant-Ae. variabilis hybrids to confirm that the effect was associated with Tazip4-B2, and that the Mg2+ effect could also be observed in a different hybrid. Moreover, we also applied the CRISPR/Cas9 genome editing system in hexaploid wheat cv. Fielder to the mutant TaZIP4-B2 to compare its mutant phenotype with those observed in TILLING mutant lines, and their Ae. variabilis hybrids. Tazip4-B2 CRISPR mutants showed a significant decrease in homologous COs compared to control plants (TaZIP4-B2 wild type wheat), similar to that already reported for Tazip4-B2 TILLING mutants (Rey et al., 2017). Also, as expected, a significant increase was observed in Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids, similar to that observed in both ph1b- Ae. variabilis and Tazip4-B2 TILLING mutant-Ae. variabilis hybrids. Furthermore, the addition of 1 mM Mg2+ to all these hybrids increased the frequency of homoeologous CO. This confirms that the Mg2+ effect is associated with Tazip4-B2, and occurs in different hybrids. The only difference observed with the Tazip4-B2 CRISPR and TILLING mutants was the occurrence of multivalents in the CRISPR mutants compared to the TILLING mutants (Figure 3 and Rey et al., 2017). This suggests that TaZIP4-B2 not only promotes homologous COs and restricts homoeologous COs, but also contributes to the efficiency of homologous pairing. We hypothesize that the CRISPR deletion disrupts more of the TaZIP4-B2 function than the TILLING mutants. Interestingly, in rice, ZIP4 mutants have previously been reported to show a delay in completing homologous synapsis (Shen et al., 2012), however, in that diploid species, this does not lead to homoeologous COs because only homologs are present. However, in the ph1b mutant, delayed pairing of some homologs is observed until after the telomere bouquet, allowing some subsequent homoeologous pairing to take place. This delayed pairing of homologs in the ph1b mutant is consistent with a ZIP4 mutant phenotype.
Magnesium is one of the most important nutrients, mainly involved in the general promotion of plant growth and development. In terms of CO function, Mg2+ may affect multiple proteins in the class I interference crossover pathway either in a positive or negative manner. For example, recent studies have suggested that Mg2+is required for the endonuclease activity of the MLH1-MLH3 heterodimer (Rogacheva et al., 2014). The MLH1-MLH3 heterodimer shows a strong preference for HJs in the absence of Mg2+ (Ranjha et al., 2014). Whatever the target, our present study reveals that homoeologous COs can be increased by the 1 mM Mg2+ treatment of Tazip4-B2 (ph1b, TILLING or CRISPR derived) mutant-wild relative hybrids. Thus, this treatment can be used as a tool to enhance the introgression of wild relative traits into wheat.
Author contributions
M-DR, AM, PS, and GM: conceived and designed the study; MS, SH, and WH: participated in the development of the Tazip4-B2 mutant in bread wheat cv. Fielder by CRISPR/Cas9 system; M-DR: analyzed the research results and wrote the first draft; PS and GM: modified the paper. All authors have read and approved the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ali Pendle (John Innes Centre, UK) for her valuable comments in the writing of the manuscript. This work was supported by the UK Biotechnology and Biological Research Council (BBSRC), through a grant part of the Designing Future Wheat (DFW) Institute Strategic Programme (BB/P016855/1), three grants (Grant BB/J004588/1; Grant BB/M009599/1; Grant BB/J007188/1); and by a Marie Curie Fellowship Grant (H2020-MSCA-IF-2015-703117).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00509/full#supplementary-material
Chromosomal configurations with single chiasma or double chiasmata highlighted with arrows. These structures marked by an arrow were counted as either single or double chiasmata in all analyzed meiocytes. Both datasets are shown in all analyzed genotypes. Bar: 20 μm.
Representative meiotic configurations of Triticum aestivum cv. Cadenza (Cad1691-Tazip4-B2 mutant)-Ae. variabilis (A) and Triticum aestivum cv. Cadenza (Cad0348-Tazip4-B2 mutant)-Ae. variabilis (B) and wheat Tazip4-B2 CRISPR mutant-Ae. variabilis mutant (C) hybrids. From left to right: water alone, treated with either 1 mM Mg2+ or Hoagland solution. Bar: 20 μm.
Genotypes and number of plants used for analyzing the effect of a nutrient solution in homoeologous CO frequency in wheat and its relative species.
Frequencies of univalents, bivalents, multivalents and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in wheat Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to Dunn's test. Different letters indicate significant differences between treatments.
References
- Al-Kaff N., Knight E., Bertin I., Foote T., Hart N., Griffiths S., et al. (2008). Detailed dissection of the chromosomal region containing the Ph1 locus in wheat Triticum aestivum: with deletion mutants and expression profiling. Ann. Bot. 101, 863–872. 10.1093/aob/mcm252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. D., Rees H. (1970). Induced variation in chiasma frequency in rye in response to phosphate treatments. Genet. Res. 16, 325–331. 10.1017/S0016672300002585 [DOI] [Google Scholar]
- Dhaliwal H. S., Gill B. S., Waines J. G. (1977). Analysis of induced homoeologous pairing in a ph mutant wheat x rye hybrid. J. Hered. 68, 207–209. 10.1093/oxfordjournals.jhered.a108815 [DOI] [Google Scholar]
- Dreissig S., Fuchs J., Himmelbach A., Mascher M., Houben A. (2017). Sequencing of single pollen nuclei reveals meiotic recombination events at megabase resolution and circumvents segregation distortion caused by postmeiotic processes. Front. Plant Sci. 8:1620. 10.3389/fpls.2017.01620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedak G. (1973). Increased chiasma frequency in desynaptic barley in response to phosphate treatments. Can. J. Genet. Cytol. 15, 647–649. 10.1139/g73-076 [DOI] [PubMed] [Google Scholar]
- Gennaro A., Forte P., Panichi D., Lafiandra D., Pagnotta M. A., D'Egidio M. G., et al. (2012). Stacking small segments of the 1D chromosome of bread wheat containing major gluten quality genes into durum wheat: transfer strategy and breeding prospects. Mol. Breed. 30, 149–167. 10.1007/s11032-011-9606-6 [DOI] [Google Scholar]
- Grant V. (1952). Cytogenetics of the hybrid Gilia millefoliata x achilleaefolia. 1. Variations in meiosis and polyploidy rate as affected by nutritional and genetics conditions. Chromosoma 5, 372–390. 10.1007/BF01271494 [DOI] [PubMed] [Google Scholar]
- Griffiths S., Sharp R., Foote T. N., Bertin I., Wanous M., Reader S., et al. (2006). Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439, 749–752. 10.1038/nature04434 [DOI] [PubMed] [Google Scholar]
- Harwood W. A., Bartlett J. G., Alves S. C., Perry M. A., Smedley M., Leyland N., et al. (2009). Barley transformation using Agrobacterium-mediated techniques, in Methods in Molecular Biology, Transgenic Wheat, Barley and Oats, Vol. 478, eds Jones H. D., Shewry P. R. (Human press; ), 137–147. [DOI] [PubMed] [Google Scholar]
- Hellens R. P., Edwards E. A., Leyland N. R., Bean S., Mullineaux P. M. (2000). pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832. 10.1023/A:1006496308160 [DOI] [PubMed] [Google Scholar]
- Hoagland D. R., Arnon D. I. (1950). The water-culture method of growing plants without soil. Calif. Agr. Expt. Sta. Circ. 347, 1–39. [Google Scholar]
- Huber D. M. (1980). The role of mineral nutrition in defense. Plant Disease 5, 381–405. [Google Scholar]
- King J., Grewal S., Yang C. Y., Hubbart Edwards S., Scholefield D., Ashling S., et al. (2017a). Introgression of Aegilops speltoides segments in Triticum aestivum and the effect of the gametocidal genes. Ann. Bot. 121, 229–240. 10.1093/aob/mcx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Grewal S., Yang C. Y., Hubbart S., Scholefield D., Ashling S., et al. (2017b). A step change in the transfer of interspecific variation into wheat from Amblyopyrum muticum. Plant Biotechnol. J. 15, 217–226. 10.1111/pbi.12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J. M., Seo B. B., Suh D. Y., Do G. S., Park D. S., Kwack Y. H. (2002). Production of a new wheat line possessing the 1BL.1RS wheat-rye translocation derived from Korean rye cultivar Paldanghomil. Theor. Appl. Genet. 104, 171–176. 10.1007/s00122-001-0783-2 [DOI] [PubMed] [Google Scholar]
- Kousaka R., Endo T. R. (2012). Effect of a rye B chromosome and its segments on homoeologous pairing in hybrids between common wheat and Aegilops variabilis. Genes Genet. Syst. 87, 1–7. 10.1266/ggs.87.1 [DOI] [PubMed] [Google Scholar]
- Krasileva K. V., Vasquez-Gross H. A., Howell T., Bailey P., Paraiso F., Clissold L., et al. (2017). Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. U.S.A. 114, E913–E921. 10.1073/pnas.1619268114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambing C., Franklin F. C. H., Wang C. J. R. (2017). Understanding and manipulating meiotic recombination in plants. Plant Physiol. 173, 1530–1542. 10.1104/pp.16.01530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. N. (1963). An effect of potassium on chiasma frequency and recombination. Genetica 33, 313–329. 10.1007/BF01725768 [DOI] [Google Scholar]
- Maathuis F. J. (2009). Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258. 10.1016/j.pbi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Martín A. C., Rey M. D., Shaw P., Moore G. (2017). Dual effect of the wheat Ph1 locus on chromosome synapsis and crossover. Chromosoma 126, 669–680. 10.1007/s00412-017-0630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A. C., Shaw P., Phillips D., Reader S., Moore G. (2014). Licensing MLH1 sites for crossover during meiosis. Nat. Commun. 5, 1–5. 10.1038/ncomms5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Ranjha L., Anand R., Cejka P. (2014). The Saccharomyces cerevisiae Mlh1-Mlh3 heterodimer is an endonuclease that preferentially binds to Holliday junctions. J. Biol. Chem. 289, 5674–5686. 10.1074/jbc.M113.533810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M. D., Martín A. C., Higgins J., Swarbreck D., Uauy C., Shaw P., et al. (2017). Exploiting the ZIP4 homologue within the wheat Ph1locus has identified two lines exhibiting homoeologous crossover in wheat-wild relative hybrids. Mol. Breed. 37:95. 10.1007/s11032-017-0700-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R., Chapman V. (1958). Genetic control of the cytological diploid behaviour of hexaploid wheat. Nature 182, 713–715. 10.1038/182713a0 [DOI] [Google Scholar]
- Roberts M. A., Reader S. M., Dalgliesh C., Miller T. E., Foote T. N., Fish L. J., et al. (1999). Induction and characterisation of the Ph1 wheat mutants. Genetics 153, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogacheva M. V., Manhart C. M., Chen C., Guarne A., Surtees J., Alani E. (2014). Mlh1-Mlh3, a meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J. Biol. Chem. 289, 5664–5673. 10.1074/jbc.M113.534644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears E. R. (1977). Induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 19, 585–593. 10.1139/g77-063 [DOI] [Google Scholar]
- Sears E. R., Okamoto M. (1958). Intergenomic chromosome relationships in hexaploid wheat. Proc Xth Internat Congr Genet, Montreal, 2, 258–259. [Google Scholar]
- Sharma A. K., Sharma A. (2014). Chromosome Techniques: Theory and Practice. Michigan, IN: Butterworth-Heinemann. [Google Scholar]
- Shen Y., Tang D., Wang K., Wang M., Huang J., Luo W., et al. (2012). ZIP4 in homologous chromosome synapsis and crossover formation in rice meiosis. J. Cell Biol. 125, 2581–2591. 10.1242/jcs.090993 [DOI] [PubMed] [Google Scholar]
- Werner S., Engler C., Weber E., Gruetzner R., Marillonnet S. (2012). Fast track assembly of multigene constructs using golden gate cloning and the MoClo system. Bioeng. Bugs 3, 38–43. 10.4161/bbug.3.1.18223 [DOI] [PubMed] [Google Scholar]
- Wilson J. Y. (1959). Chiasma frequency in relation to temperature. Genetica 29, 290–303. 10.1007/BF01535715 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chromosomal configurations with single chiasma or double chiasmata highlighted with arrows. These structures marked by an arrow were counted as either single or double chiasmata in all analyzed meiocytes. Both datasets are shown in all analyzed genotypes. Bar: 20 μm.
Representative meiotic configurations of Triticum aestivum cv. Cadenza (Cad1691-Tazip4-B2 mutant)-Ae. variabilis (A) and Triticum aestivum cv. Cadenza (Cad0348-Tazip4-B2 mutant)-Ae. variabilis (B) and wheat Tazip4-B2 CRISPR mutant-Ae. variabilis mutant (C) hybrids. From left to right: water alone, treated with either 1 mM Mg2+ or Hoagland solution. Bar: 20 μm.
Genotypes and number of plants used for analyzing the effect of a nutrient solution in homoeologous CO frequency in wheat and its relative species.
Frequencies of univalents, bivalents, multivalents and chiasma frequency (single and double chiasmata) were scored at meiotic metaphase I in wheat Tazip4-B2 CRISPR mutant-Ae. variabilis hybrids. Values in parenthesis indicate range of variation between cells. P < 0.05 indicates significant differences according to Dunn's test. Different letters indicate significant differences between treatments.