Abstract
The Japanese fermented beverage, homemade kefir, contains six lactic acid bacteria: Lactococcus. lactis subsp. Lactis, Lactococcus. lactis subsp. Cremoris, Lactococcus. Lactis subsp. Lactis biovar diacetylactis, Lactobacillus plantarum, Leuconostoc meseuteroides subsp. Cremoris and Lactobacillus casei. In this study, we found that a mixture of the six lactic acid bacteria from kefir increased the cytotoxicity of human natural killer KHYG-1 cells to human chronic myelogenous leukemia K562 cells and colorectal tumor HCT116 cells. Furthermore, levels of mRNA expression and secretion of IFN-γ (interferon gamma) increased in KHYG-1 cells that had been treated with the six lactic acid bacteria mixture from kefir. The results suggest that the six lactic acid bacteria mixture from kefir has strong effects on natural immunity and tumor cell cytotoxicity.
Keywords: kefir, lactic acid bacteria, natural killer cell, cytotoxicity
1. Introduction
The fermented beverage kefir has many health benefits, including its antibacterial, anticarcinogenic, wound healing, antiallergenic, immunomodulation, and gastrointestinal immunity effects [1]. The fermented beverage, homemade kefir, has been available for more than 20 years in Japan. Homemade kefir contains six lactic acid bacteria: Lactococcus. lactis subsp. Lactis [2,3,4,5,6,7,8,9,10,11,12], Lactococcus. lactis subsp. Cremoris [8,9,13], Lactococcus. Lactis subsp. Lactis biovar diacetylactis [4], Lacto bacillus planterun [4,14,15,16], Leuconostoc meseuteroides subsp. Cremoris [7,9], and Lacto bacillus casei [2,5,12,16]. Kefir is able to offer health benefits through modulation of the gastrointestinal immune system, and previous studies showed that kefir increases the levels of some proinflammatory cytokines including TNF-α (tumor necrosis factor alpha), IFN-γ (interferon gamma) and IL-12 (interleukin 12) [1]. Lactic acid bacteria have beneficial effects with respect to diarrhea [17,18], food allergies [19], and inflammatory bowel disease [20,21,22]. Lactic acid bacteria also play an important role in the prevention of colorectal cancer [23,24], and Lacto bacillus casei Shirota increases the activity of natural killer (NK) cells [25,26]. However, the effect of homemade kefir on NK cell activity is not clear. In this study, we found that a six lactic acid bacteria mixture from homemade kefir increases NK cell activity, including cytotoxic activity against human colorectal tumor HCT116 cells. These results suggest that the mixture of six lactic acid bacteria from homemade kefir has important effects on innate immunity and tumor cell cytotoxicity.
2. Materials and Methods
2.1. Materials
Homemade kefir was provided by Nakagaki Consulting Engineer and Co., Ltd. (Osaka, Japan). Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), and human interleukin-2 were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other reagents were of analytical grade.
2.2. Cell Culture
KHYG-1 and K562 cells were provided by JCRB Cell Bank (Osaka, Japan), and HCT116 cells were purchased from American Tissue Culture Collection (ATCC) (Manassas, VA, USA). KHYG-1 cells were cultured in RPMI 1640 medium with 10% fetal bovine serum (Sigma, St. Louis, MO, USA) and 1 μg/mL IL-2. K562 and HCT116 cells were cultured in RPMI 1640 medium and in Dulbecco’s modified Eagle’s medium (DMEM), respectively, with 10% fetal bovine serum.
2.3. Preparation of Lactic Acid Bacteria Mixture
One gram of homemade kefir powder was added to 1 L of de Man, Rogosa and Sharpe (MRS) broth and cultured for 24 h at 22.5 °C. The cultured solution was centrifuged at 3000 rpm for 30 min at 4 °C using a TOMY centrifugation apparatus (TOMY SEIKO, Tokyo, Japan), and the six lactic acid bacteria mixture was obtained from the precipitates. A lactic acid bacteria mixture from killed bacteria was prepared by autoclaving at 120 °C for 15 min. Identification of the lactic acid bacteria cultured in the MRS broth was performed by polymerase chain reaction (PCR) using DNA from the six lactic acid bacteria as templates. After PCR, amplified DNA was separated on 2.0% agarose gels and stained with ethidium bromide. The PCR conditions and primers used are shown in Table 1.
Table 1.
Polymerase chain reaction (PCR) primers and reacting conditions.
| Strain | Primer Sequence | PCR Condition | Ref. | |
|---|---|---|---|---|
| L. lactis subsp. Lactis | sense | 5′-CGTTATGGATTTGATGGATATAAAGC-3′ | 94 °C 9 min, 94 °C 30 s, 50 °C 30 s, 72 °C 60 s × 45 cycles, 72 °C 7 min | [27] |
| antisense | 5′-ACTCTTCTTAAGAACAAGTTTAACAGC-3′ | |||
| L. lactis subsp. Cremoris | sense | 5′-CGTTATGGATTTGATGGATATAAAGC-3′ | 94 °C 9 min, 94 °C 30 s, 50 °C 30 s, 72 °C 60 s × 45 cycles, 72 °C 7 min | |
| antisense | 5′-ACTCTTCTTAAGAACAAGTTTAACAGC-3′ | |||
| L. lactis subsp. lactis biovar diacetylactis | sense | 5′-CTTCGTTATGATTTTACA-3′ | 94 °C 30 s, 94 °C 30 s, 46 °C 60 s, 72 °C 60 s × 30 cycles, 72 °C 2 min | [28,29] |
| antisense | 5′-AATATCAACAATTCCATG-3′ | |||
| Leuconostoc mesenteroides subsp. Cremoris (Genus-specific primers for Leuconostoc) | sense | 5′-AACTTAGTGTCGCATGAC-3′ | 94 °C 5 min, 94 °C 60 s, 60 °C 60 s, 72 °C 60 s × 30 cycles, 72 °C 10 min | [30] |
| antisense | 5′-AGTCGAGTTACAGACTACAA-3′ | |||
| Lactobacillus plantarum | sense | 5′-CCGTTTATGCGGAACACCTA-3′ | 94 °C 3 min, 94 °C 30 s, 54 °C 10 s, 72 °C 30 s × 30 cycles, 72 °C 5 min | [31] |
| antisense | 5′-TCGGGATTACCAAACATCAC-3′ | |||
| Lactobacillus casei | sense | 5-TGCACTGAGATTCGACTTAA-3 | 94 °C 3 min, 94 °C 45 s, 45 °C 45 s, 72 °C 60 s × 30 cycles, 72 °C 5 min | [32] |
2.4. Measurement of NK Cell Activity and Tumor Cytotoxicity
KHYG-1 cells were treated with the six lactic acid bacteria mixture for 24 h at 37 °C in a 5% CO2 incubator. The cells that had been treated with the six lactic acid bacteria mixture were reacted with K562 cells or with HCT116 cells for 4 h at 37 °C in a 5% CO2 incubator. NK cell activity and tumor cytotoxicity were measured using an LDH Cytotoxicity Detection Kit (Takara, Shiga, Japan).
2.5. RT-PCR (Reverse Transcription Polymerase Chain Reaction)
Nucleotide sequences of primers used for RT-PCR were as follows: hIFNγ-F: 5′-GAATGTCCAACGCAAAGCAA-3′, hIFNγ-R: 5′-GCTGCTGGCGACAGTTCAG-3′, hACTB-F: 5′-CTTCCTGGGCATGGAGTC-3′, and hACTB-R: 5′-GGATGTCCACGTCACACTTC-3′. The full RNAs were prepared from cells and subjected to semi-quantitative RT-PCR analyses. PCR conditions were 1 min at 94 °C, 30 s at 94 °C, 30 s at 60 °C, and 35 cycles of 1 min at 72 °C for IFNγ and ACTB. After the reactions, PCR products were separated on 2.0% agarose gels and stained with SAFELOOK™ Pregreen (Wako, Osaka, Japan). Intensities of the bands were quantified using Image J software (https://imagej.nih.gov/ij/). β-actin mRNA was also amplified as a control.
2.6. IFN-γ Measurement
IFN-γ was measured using a Human IFN-γ ELISA (enzyme-linked immunosorbent assay) Development Kit (PeproTech, Rocky Hill, NJ, USA) according to the manufacturer’s instructions.
2.7. Statistical Analysis
Data are expressed as means ± S.E (standard error). Statistical analyses were performed with an analysis of variance (one-way ANOVA) followed by an unpaired Student’s t-test.
3. Results and Discussion
3.1. Induction of NK Activity by the Six Lactic Acid Bacteria from Homemade-Kefir
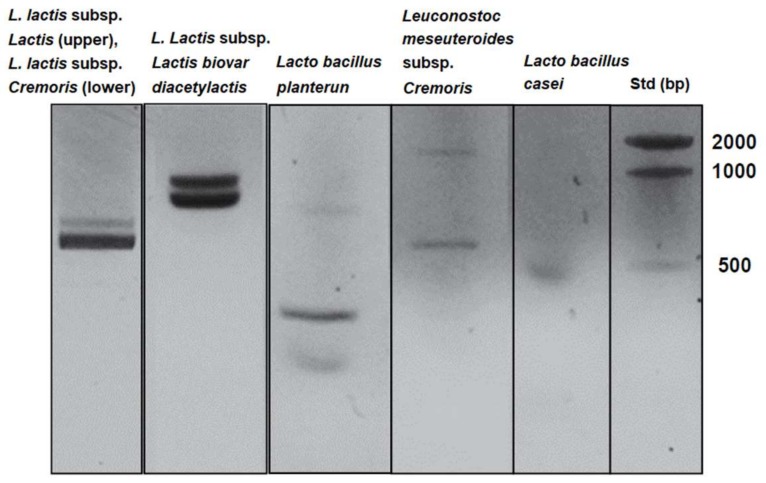
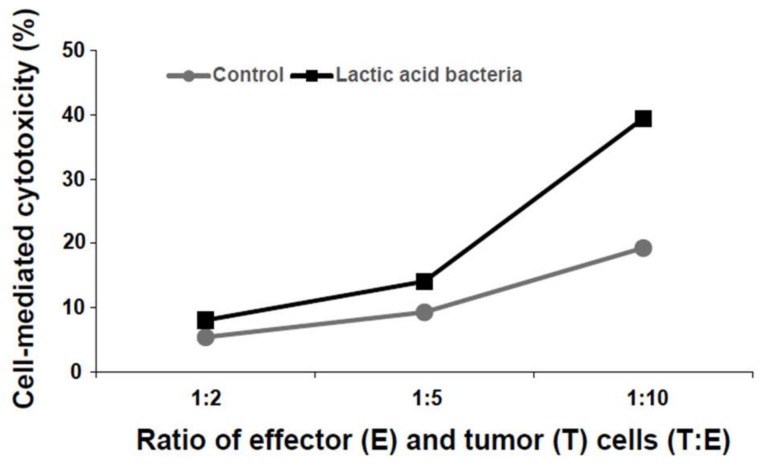
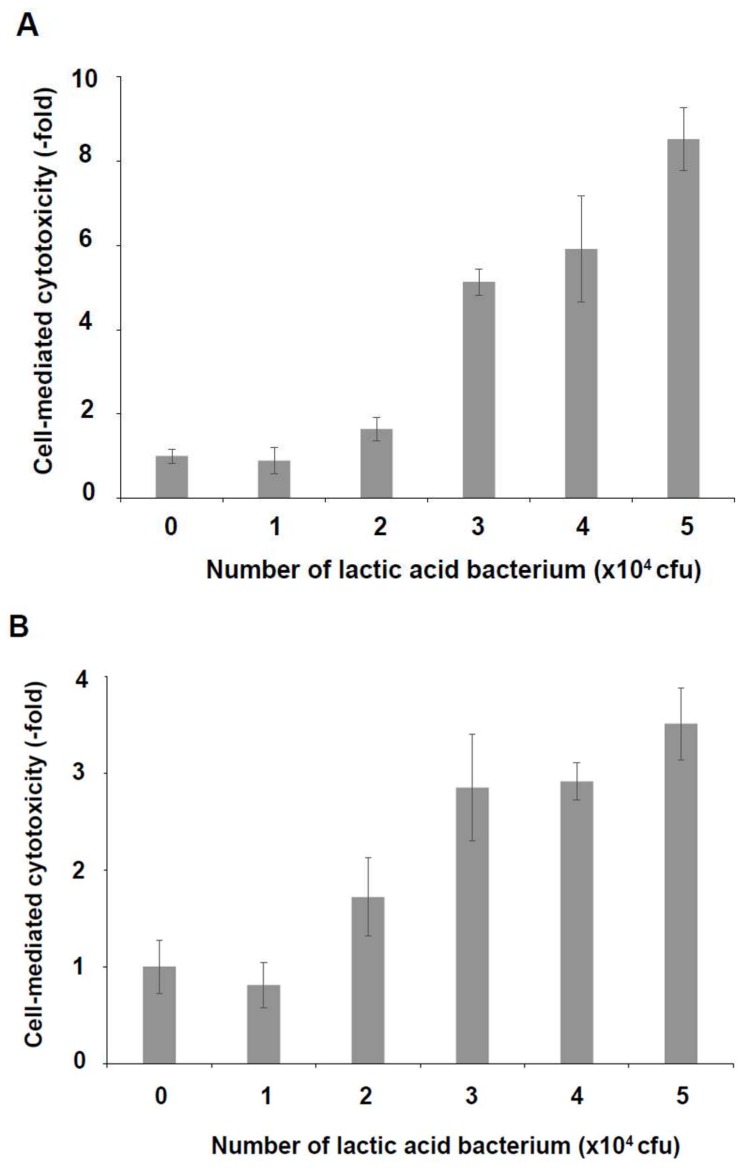
As shown in Figure 1, the six lactic acid bacteria from homemade kefir were detected in MRS broth cultures and identified using PCR with the bacteria’s DNA as templates. The cytotoxicity of KHYG-1 cells that had been treated with the six lactic acid bacteria mixture, against K562 cells, increased in a cell number-dependent manner (Figure 2). As shown in Figure 3A, KHYG-1 cells were activated by the six lactic acid bacteria mixture and increased their cytotoxicity against K562 cells in a dose-dependent manner. Lactic acid bacteria have many effects on biological activities such as apoptosis induction, antioxidant activities, and immune response improvement [33]. Nagao et al. reported that Lactobacillus casei Shirota enhanced NK cell activity [25]. Lacto bacillus plantarum C4 isolated from kefir has also been shown to prevent infection with Yersinia enterocolitice O9 in the intestine in mice. Additionally, a concentrated supernatant from the cultured medium of Lacto bacillus plantarum C4 has antibacterial and anti-tumor activities [34]. Since the six lactic acid bacteria mixture from homemade kefir includes Lacto bacillus plantarum and Lacto bacillus casei, it is thought that these lactic acid bacteria contribute to the induction of NK cell activity.
Figure 1.
Identification of the six lactic acid bacteria cultured in de Man, Rogosa and Sharpe (MRS) broth using polymerase chain reaction (PCR) as described in the Materials and Methods. The amplified DNA after PCR were separated on agarose gels.
Figure 2.
Cell-mediated cytotoxicity of natural killer (NK) cells by a six lactic acid bacteria mixture. KHYG-1 cells were treated with a six lactic acid bacteria mixture for 24 h at 37 °C in a 5% CO2 incubator. The treated cells were reacted with K562 cells for 4 h at 37 °C in a 5% CO2 incubator. Cell-mediated cytotoxicity increased for KHYG-1 cells that had been treated with the six lactic acid bacteria mixture.
Figure 3.
Induction of cell-mediated cytotoxicity by a six lactic acid bacteria mixture. KHYG-1 cells were treated with a six lactic acid bacteria mixture for 24 h at 37 °C in a 5% CO2 incubator. The treated cells were reacted with K562 cells (A) or with HCT116 cells (B) for 4 h at 37 °C in a 5% CO2 incubator. In both cases, cell-mediated cytotoxicity was increased by KHYG-1 cells that had been treated with the six lactic acid bacteria mixture in a dose-dependent manner.
3.2. Induction of Cytotoxicity by the Six Lactic Acid Bacteria Against Human Colorectal Tumor Cells
Cytotoxic activity of KHYG-1 cells against HCT116 cells was increased by the six lactic acid bacteria mixture in a dose-dependent manner (Figure 3B). NK cells have the intrinsic ability to detect and kill cancer cells [35]. The lytic functions of NK cells mediated by granzyme B and perforin have dominated investigations of the role of NK cells in anti-cancer defense [36]. The effector function of NK cells is tightly regulated by activating and inhibiting receptors. Tumor cells can evade the anti-tumor activity of NK cells by modifying the expression of NK cell receptors. In colorectal carcinoma, for instance, the expression of activating receptors—such as CD16 (FCGR3A, Fc fragment of IgG receptor IIIa), NKp46 (NCR1, natural cytotoxicity triggering receptor 1), NKp30 (NCR3, natural cytotoxicity triggering receptor 3), and NKG2D (KLRK1, killer cell lectin like receptor K1)—is decreased. Additionally, the expression of inhibiting receptors, such as KIR3DL1 (killer cell immunoglobulin-like receptor 3DL1) and NKG2A (killer cell lectin like receptor C1), is increased [37]. The six lactic acid bacteria mixture or its secretory compounds may regulate the expression of these receptor genes and inhibit tumor formation by escaping from NK cell-mediated cytotoxicity. Lactic acid bacteria have indeed been demonstrated to have a host of properties for preventing the development of a colorectal tumor, specifically by inhibiting its initiation or progression. In the pathway of the anticancer immune response, lactic acid bacteria also stimulate NK cell activity [33]. Furthermore, a seminal 11-year prospective cohort study conducted in Japan showed an inverse correlation between increase of cancer incidence and the level of NK cell cytotoxicity [38]. These results indicate that the six lactic acid bacteria mixture from homemade kefir reacts with human colorectal tumor HCT116 cells through activation of the immunity of NK cells.
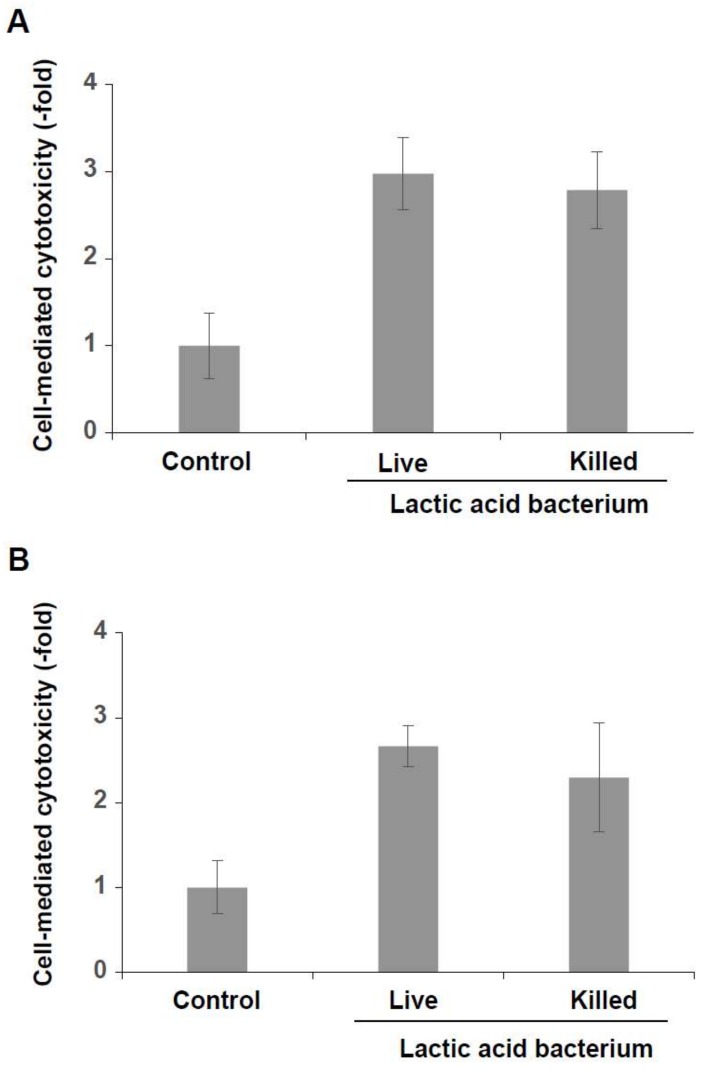
3.3. Induction of Cytotoxicity of KHYG-1 Cells by the Killed Lactic Acid Bacteria Mixture
To examine the distinct effects of live and dead lactic acid bacteria on NK cell activation, KHYG-1 cells were treated with the live and the killed six lactic acid bacteria mixtures and cytotoxicity assays were carried out. As shown in Figure 4A, activation of KHYG-1 cells was increased by treatment with both the live and killed mixtures. Cytotoxicity of KHYG-1 cells against HCT116 cells was also increased by both mixtures (Figure 4B). Previous studies, on the other hand, have shown that 40% to 62% of several strains of L. plantarum survive under the low pH conditions in a simulated intestine [39], and that 81% to 93% of several strains of L. casei can also survive at pH 2.0 for 2 h [40]. The present and previous results, therefore, suggest that both lactic acid bacteria that have been killed by gastric acid and live lactic acid bacteria have effects on NK cell activation in the intestine after the administration of homemade kefir.
Figure 4.
Induction of cell-mediated cytotoxicity of KHYG-1 cells by the live and the killed lactic acid bacteria mixtures. KHYG-1 cells were treated with both mixtures (3 × 104 cfu) for 24 h at 37 °C in a 5% CO2 incubator. The treated cells were reacted with K562 cells (A) or with HCT116 cells (B) for 4 h at 37 °C in a 5% CO2 incubator. In both cases, cell-mediated cytotoxicity was increased in KHYG-1 cells that had been treated with both the live and the killed lactic acid bacteria mixtures.
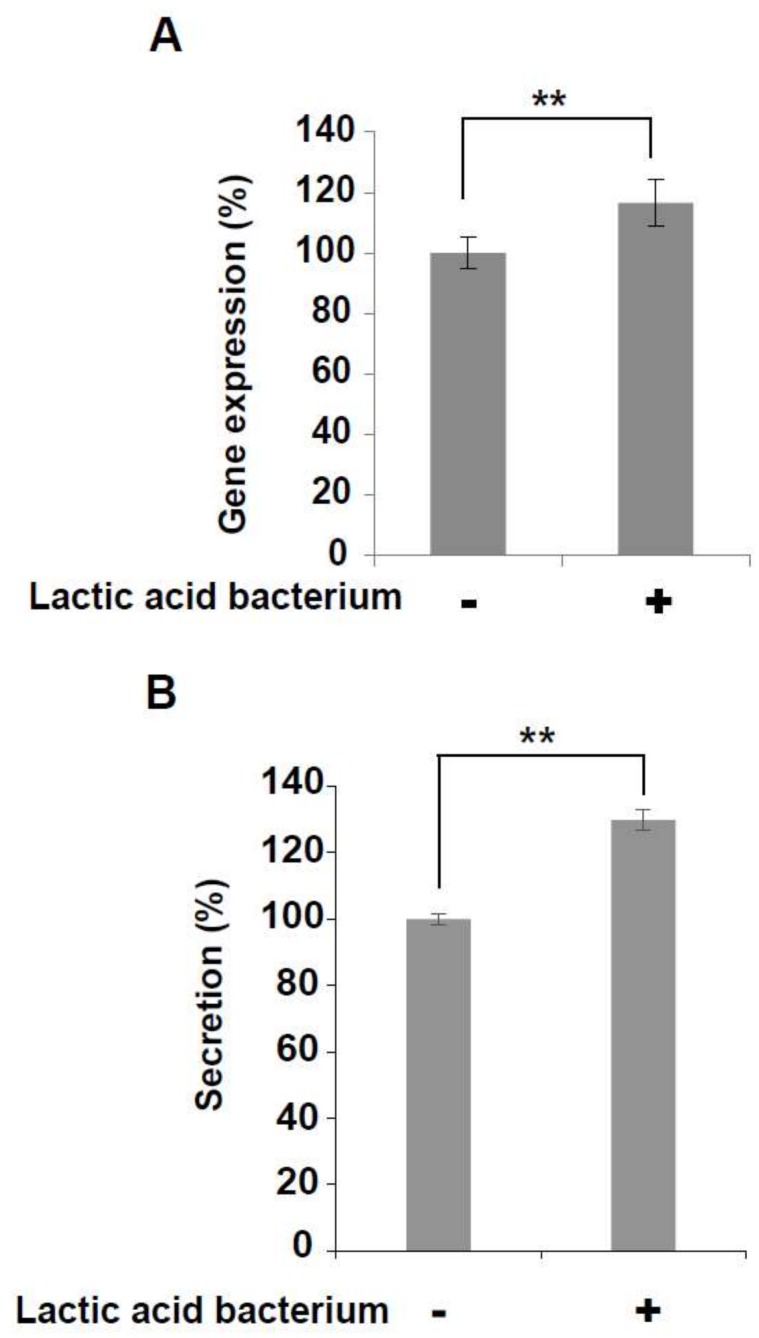
3.4. Increased Expression and Secretion Levels of IFN-γ in KHYG-1 Cells
IFN-γ mRNA and IFN-γ secretion were both increased in KHYG-1 cells that had been treated by the six lactic acid bacteria mixture (Figure 5A,B). NK cells regulate various immune cells through the secretion of IFN-γ and they perform tumor surveillance [41]. Increased IFN-γ expression and secretion induced by the mixture of six lactic acid bacteria from homemade kefir may provide a synergistic effect on anti-tumor activity.
Figure 5.
Increased expression and secretion levels of IFN-γ. (A) The expression level of IFN-γ mRNA was increased in KHYG-1 cells that had been treated with the six lactic acid bacteria mixture (3 × 104 cfu) for 4 h at 37 °C in a 5% CO2 incubator (n = 3, ** p < 0.01). (B) The secretion level of IFN-γ mRNA was increased in the same, treated, KHYG-1 cells (n = 6, ** p < 0.01).
4. Conclusions
This study showed that the cytotoxicity of NK cells and the expression and secretion of IFN-γ in NK cells were increased after treatment of the cells with a six lactic acid bacteria mixture. Further in vitro and in vivo studies using animal models of colorectal cancer are needed to investigate the mechanisms underlying the effects of the six lactic acid bacteria mixture.
Author Contributions
Takuya Yamane, Tatsuji Sakamoto, Takenori Nakagaki and Yoshihisa Nakano conceptualized the study; Takuya Yamane carried out experiments; Takuya Yamane conducted the analyses; and Takuya Yamane wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bourrie B.C., Willing B.P., Cotter P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016;7:647. doi: 10.3389/fmicb.2016.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo L., Lopez E., Lema C. Microflora present in kefir grains of the Galician region (North-West of Spain) J. Dairy Res. 1993;60:263–267. doi: 10.1017/S002202990002759X. [DOI] [PubMed] [Google Scholar]
- 3.Pintado M.E., Da Silva J.A.L., Fernandes P.B., Malcata F.X., Hogg T.A. Microbiological and rheological studies on Portuguese kefir grains. Int. J. Food Sci. Technol. 1996;31:15–26. doi: 10.1111/j.1365-2621.1996.16-316.x. [DOI] [Google Scholar]
- 4.Garrote G.L., Abraham A.G., De Antoni G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001;68:639–652. doi: 10.1017/S0022029901005210. [DOI] [PubMed] [Google Scholar]
- 5.Simova E., Beshkova D., Angelov A., Hristozova T., Frengova G., Spasov Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002;28:1–6. doi: 10.1038/sj/jim/7000186. [DOI] [PubMed] [Google Scholar]
- 6.Witthuhn R.C., Schoeman T., Britz T.J. Isolation and characterization of the microbial population of different South African kefir grains. Int. J. Dairy Technol. 2004;57:33–37. doi: 10.1111/j.1471-0307.2004.00126.x. [DOI] [Google Scholar]
- 7.Witthuhn R., Schoeman T., Britz T. Characterisation of the microbial population at different stages of Kefir production and Kefir grain mass cultivation. Int. Dairy J. 2005;15:383–389. doi: 10.1016/j.idairyj.2004.07.016. [DOI] [Google Scholar]
- 8.Yüksekdağ Z., Beyatli Y., Aslim B. Determination of some characteristics coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic. LWT Food Sci. Technol. 2004;37:663–667. doi: 10.1016/j.lwt.2004.02.004. [DOI] [Google Scholar]
- 9.Mainville I., Robert N., Lee B., Farnworth E.R. Polyphasic characterization of the lactic acid bacteria in kefir. Syst. Appl. Microbiol. 2006;29:59–68. doi: 10.1016/j.syapm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen H.-C., Wang S.-Y., Chen M.-J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008;25:492–501. doi: 10.1016/j.fm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Garofalo C., Osimani A., Milanović V., Aquilanti L., De Filippis F., Stellato G., Di Mauro S., Turchetti B., Buzzini P., Ercolini D., et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015;49:123–133. doi: 10.1016/j.fm.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Zanirati D.F., Abatemarco M., de Cicco Sandes S.H., Nicoli J.R., Nunes Á.C., Neumann E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe. 2015;32:70–76. doi: 10.1016/j.anaerobe.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Korsak N., Taminiau B., Leclercq M., Nezer C., Crevecoeur S., Ferauche C., Detry E., Delcenserie V., Daube G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015;98:3684–3689. doi: 10.3168/jds.2014-9065. [DOI] [PubMed] [Google Scholar]
- 14.Santos A., San Mauro M., Sanchez A., Torres J.M., Marquina D. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 2003;26:434–437. doi: 10.1078/072320203322497464. [DOI] [PubMed] [Google Scholar]
- 15.Da Cruz Pedrozo Miguel M.G., Cardoso P.G., de Assis Lago L., Schwan R.F. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res. Int. 2010;43:1523–1528. doi: 10.1016/j.foodres.2010.04.031. [DOI] [Google Scholar]
- 16.Nalbantoglu U., Cakar A., Dogan H., Abaci N., Ustek D., Sayood K., Can H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014;41:42–51. doi: 10.1016/j.fm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Chouraqui J.P., Van Egroo L.D., Fichot M.C. Acidified milk formula supplemented with Bifidobacterium lactis: Impact on infant diarrhea in residential care settings. J. Pediatr. Gastroenterol. Nutr. 2004;38:288–292. doi: 10.1097/00005176-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Gaón D., García H., Winter L., Rodríguez N., Quintás R., González S.N., Oliver G. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (Buenos Aires) 2003;63:293–298. [PubMed] [Google Scholar]
- 19.Pohjavuori E., Viljanen M., Korpela R., Kuitunen M., Tiittanen M., Vaarala O., Savilahti E. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J. Allergy Clin. Immunol. 2004;114:131–136. doi: 10.1016/j.jaci.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Bourlioux P., Koletzko B., Guarner F., Braesco V. The intestine and its microflora are partners for the protection of the host: Report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am. J. Clin. Nutr. 2003;78:675–683. doi: 10.1093/ajcn/78.4.675. [DOI] [PubMed] [Google Scholar]
- 21.Azcárate-Peril M.A., Sikes M., Bruno-Bárcena J.M. The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G401–G424. doi: 10.1152/ajpgi.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Carmen S., de LeBlanc A.M., Miyoshi A., Rocha C.S., Azevedo V., LeBlanc J.G. Potential application of probiotics in the prevention and treatment of inflammatory bowel diseases. Ulcers. 2011;2011:841651. doi: 10.1155/2011/841651. [DOI] [Google Scholar]
- 23.Rafter J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995;30:497–502. doi: 10.3109/00365529509089779. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama K., Rafter J. The role of lactic acid bacteria in colon cancer prevention: Mechanistic considerations. Antonie Van Leeuwenhoek. 1999;76:391–394. doi: 10.1007/978-94-017-2027-4_25. [DOI] [PubMed] [Google Scholar]
- 25.Nagao F., Nakayama M., Muto T., Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2000;64:2706–2708. doi: 10.1271/bbb.64.2706. [DOI] [PubMed] [Google Scholar]
- 26.Dong H., Rowland I., Tuohy K.M., Thomas L.V., Yaqoob P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin. Exp. Immunol. 2010;161:378–388. doi: 10.1111/j.1365-2249.2010.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura M., Kobayashi M., Okamoto T. Rapid PCR-Based Method Which Can Determine Both Phenotype and Genotype of Lactococcus lactis Subspecies. Appl. Environ. Microbiol. 2002;68:2209–2213. doi: 10.1128/AEM.68.5.2209-2213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beimfohr C., Ludwig W., Schleifer K.H. Rapid Genotypic Differentiation of Lactococcus lactis Subspecies and Biovar. Syst. Appl. Microbiol. 1997;20:216–221. doi: 10.1016/S0723-2020(97)80068-9. [DOI] [Google Scholar]
- 29.Feutry F., Torre P., Arana I., Garcia S., Desmasures N., Casalta E. Lactococcus lactis strains from raw ewe’s milk samples from the PDO Ossau-Iraty cheese area: Levels, genotypic and technological diversity. Dairy Sci. Technol. 2012;92:655–670. doi: 10.1007/s13594-012-0084-3. [DOI] [Google Scholar]
- 30.Kahala M., Maki M., Lehtovaara A., Tapanainen J.M., Katiska R., Juuruskorpi M., Juhola J., Joutsjoki V. Characterization of starter lactic acid bacteria from the Finnish fermented milk product viili. J. Appl. Microbiol. 2008;105:1929–1938. doi: 10.1111/j.1365-2672.2008.03952.x. [DOI] [PubMed] [Google Scholar]
- 31.Torriani S., Felis G.E., Dellaglio F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001;67:3450–3454. doi: 10.1128/AEM.67.8.3450-3454.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward L.J.H., Timmins M.J. Differentiation of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus by polymerase chain reaction. Lett. Appl. Microbiol. 1999;29:90–92. doi: 10.1046/j.1365-2672.1999.00586.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhong L., Zhang X., Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014;20:7878–7886. doi: 10.3748/wjg.v20.i24.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Montijo-Prieto S., Moreno E., Bergillos-Meca T., Lasserrot A., Ruiz-López M.D., Ruiz-Bravo A., Jiménez-Valera M.A. Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. Res. Microbiol. 2015;166:626–632. doi: 10.1016/j.resmic.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson S.E., Keating N., Belz G.T. Natural killer cells and anti-tumor immunity. Mol. Immunol. 2017 doi: 10.1016/j.molimm.2017.12.002. in press. [DOI] [PubMed] [Google Scholar]
- 36.Voskoboinik I., Whisstock J.C., Trapani J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 37.Lee H.H., Kang H., Cho H. Natural killer cells and tumor metastasis. Arch. Pharm. Res. 2017;40:1037–1049. doi: 10.1007/s12272-017-0951-9. [DOI] [PubMed] [Google Scholar]
- 38.Imai K., Matsuyama S., Miyake S., Suga K., Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 39.Tokatlı M., Gülgör G., Bağder Elmacı S., Arslankoz İşleyen N., Özçelik F. In Vitro Properties of Potential Probiotic Indigenous Lactic Acid Bacteria Originating from Traditional Pickles. BioMed Res. Int. 2015;2015:315819. doi: 10.1155/2015/315819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B., Wang Y., Tan Z., Li Z., Jiao Z., Huang Q. Screening of Probiotic Activities of Lactobacilli Strains Isolated from Traditional Tibetan Qula, A Raw Yak Milk Cheese. Asian-Australas. J. Anim. Sci. 2016;29:1490–1499. doi: 10.5713/ajas.15.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda H., Old L.J., Schreiber R.D. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]