Abstract
In response to the outbreak of Zika virus (ZIKV) infection in the Western Hemisphere and the recognition of a causal association with fetal malformations, the Global Virus Network (GVN) assembled an international taskforce of virologists to promote basic research, recommend public health measures and encourage the rapid development of vaccines, antiviral therapies and new diagnostic tests. In this article, taskforce members and other experts review what has been learned about ZIKV-induced disease in humans, its modes of transmission and the cause and nature of associated congenital manifestations. After describing the make-up of the taskforce, we summarize the emergence of ZIKV in the Americas, Africa and Asia, its spread by mosquitoes, and current control measures. We then review the spectrum of primary ZIKV-induced disease in adults and children, sites of persistent infection and sexual transmission, then examine what has been learned about maternal-fetal transmission and the congenital Zika syndrome, including knowledge obtained from studies in laboratory animals. Subsequent sections focus on vaccine development, antiviral therapeutics and new diagnostic tests. After reviewing current understanding of the mechanisms of emergence of Zika virus, we consider the likely future of the pandemic.
Keywords: Zika virus, Arbovirus, Congenital manifestations, Maternal-fetal transmission, Antiviral therapy, Vaccines
1. Introduction
Following on the heels of the Ebola epidemic in West Africa, the Zika virus (ZIKV) outbreak in the Western Hemisphere has led to the rapid mobilization of scientific resources to study the disease and initiate the development of specific countermeasures. As part of this response, the Global Virus Network (GVN) formed a task force of investigators from its worldwide Centers of Excellence to develop a coordinated program of research and to serve as a resource for scientists, physicians and public health officials dealing with the outbreak.
In this article, members of the GVN Zika task force and other experts review what has been learned about the disease in humans since an association with severe congenital manifestations was identified in 2015 (Weaver et al., 2016). The first section describes the make-up of the task force and its research program. The following sections review the emergence of the virus in the Americas, Africa and Asia, its transmission, and current control measures. We then review the spectrum of primary Zika virus (ZIKV) -induced disease in adults and children, sites of persistent infection and modes of sexual transmission, then examine what has been learned about maternal-fetal transmission and congenital Zika syndrome (CZS), including knowledge obtained from studies in laboratory animals. Subsequent sections focus on vaccine development, antiviral therapeutics and new diagnostic tests. The concluding section of the paper reviews what has been learned about the mechanisms of emergence of Zika virus and considers the probable future course of the epidemic.
2. The Global Virus Network Zika taskforce (Natalia Mercer, Edward McSweegan)
The mission of the GVN is to strengthen medical research and the public health response to emerging viruses and persisting viral threats (Mann, 2011). Since its inception, the network has sought to carry out its mission through collaborative research projects, international meetings and training courses, professional publications and commentaries, and the engagement of expert medical virologists to advise on outbreak responses and research priorities. The GVN currently consists of 38 Centers and 6 Affiliates in 24 countries, focusing on all aspects of medical virology. For further information, readers are referred to the report of the most recent annual meeting and the website at http://gvn.org.
In early 2016, the GVN joined with 30 other global health or-ganizations to pledge support for the rapid and open sharing of research data on ZIKV. That pledge arose from an earlier World Health Organization consensus statement, in which international journal editors acknowledged that “timely and transparent pre-publication sharing of data and results during public health emergencies must become the global norm” (WHO, 2015) The GVN’s Zika task force was organized in February 2016 and widely announced at a meeting of American, European and Brazilian virologists (“Bridging the Sciences: Zika Virus,” Atlanta, Georgia, May 1–3, 2016). The expert members of the Zika taskforce are listed in Table 1.
Table 1.
Members of the Global Virus Network Task Force on Zika Virus.
| Member | Institute | |
|---|---|---|
| 1 | Scott Weaver, Chairman | Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX |
| 2 | Xavier Abad | IRTA-CReSA, Centre de Recerca en Sanitat Animal, Barcelona, Spain |
| 3 | Sazaly AbuBakar | University of Malaya, Kuala Lumpur, Malaysia |
| 4 | Nuria Busquets | IRTA-CReSA, Centre de Recerca en Sanitat Animal, Barcelona, Spain |
| 5 | Michael Diamond | Washington University School of Medicine, Seattle, WA |
| 6 | Susan J. Fisher | University of California, San Francisco, CA |
| 7 | Robert Gallo | Institute of Human Virology, Univesity of Maryland School of Medicine, Baltimore, MD |
| 8 | Antoine Gessain | Institut Pasteur, Laboratory Oncogenic Virus Epidemiology and Pathophysiology, Paris |
| 9 | Diane Griffin | Johns Hopkins Bloomberg School of Public Health, Baltimore, MD |
| 10 | Andrew Haddow | U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD |
| 11 | Giuseppe Ippolito | National Institute for Infectious Diseases, Rome, Italy |
| 12 | Esper G. Kallas | University of Sao Paulo, Brazil |
| 13 | Albert Ko | Yale University School of Public Health, CT |
| 14 | Alain Kohl | MRC-University of Glasgow, Centre for Virus Research, Scotland |
| 15 | Marc Lecuit | Institut Pasteur, Biology of Infection Unit, Paris, France |
| 16 | Julius Lutwama | Uganda Virus Research Institute, Entebbe, Uganda |
| 17 | John Mackenzie | Curtin University, Perth, Australia |
| 18 | Gene Morse | University at Buffalo, Buffalo, NY |
| 19 | Kenneth Olson | Colorado State University, Ft. Collins, CO |
| 20 | Jorge Osorio | University of Wisconsin and University of Antioquia Medical School, Medellin, Colombia |
| 21 | Janusz T. Paweska | National Institute for Communicable Diseases, Johannesburg, South Africa |
| 22 | Giovanni Rezza | Istituto Superiore di Sanità, Rome, Italy |
| 23 | Amadou Sall | Institut Pasteur de Dakar, Senegal |
| 24 | Raymond Schinazi | Emory University School of Medicine, Atlanta, GA |
| 25 | Cameron Simmons | University of Melbourne, Australia |
| 26 | Ed Tramont | National Institutes of Health, Bethesda, MD |
| 27 | Nikos Vasilakis | Institute for Human Infections and Immunity, University of Texas Medical Branch, TX |
| 28 | David Watkins | University of Miami, Miami, FL |
| 29 | Stephen Whitehead | National Institutes of Health, Bethesda, MD |
In a survey of research needs, Center directors and task force members identified a lack of serum samples from definitively diagnosed patients as a major impediment to developing sensitive and specific diagnostic assays. Subsequently, the GVN obtained funds from the Allergan Foundation to support the establishment of a repository of high quality immune sera collected from a variety of convalescent patients with a definitive ZIKV diagnosis (http://gvn.org/donation-allergan-foundation). In addition to aiding diagnostics development, these samples will be useful for the evaluation and comparison of immune responses to natural infection and future candidate vaccines.
Consultation among task force members has also led to a number of proposed research initiatives intended to help answer some of the many public health questions presented by the emergence and pandemic spread of ZIKV. They include:
identifying opportunities to expand epidemiological studies;
developing rapid diagnostics able to distinguish among regional arboviruses;
developing and testing vaccines in susceptible populations;
screening for existing and novel drugs to improve therapeutic options;
performing basic research to identify mechanisms of viral infectivity, persistence, and the pathogenesis of congenital defects and neurological complications.
These initiatives are discussed in the following sections.
3. Emergence and spread of ZIKV infection
3.1. The Americas (Esper Kallas)
After occurring for decades throughout Africa and Asia, ZIKV became a major topic of intense discussion after a ravaging epidemic of infection was identified in Brazil. This resulted in several cases of Guillain Barre syndrome (GBS) (Paploski et al., 2016; do Rosario et al., 2016) and an unexpected epidemic of newborns with microcephaly and other neurological defects (Oliveira Melo et al., 2016; Schuler-Faccini et al., 2016; Mlakar et al., 2016; Calvet et al., 2016). More than one year later (Brito and Cordeiro, 2016), intensive investigation revealed that a Polynesian ZIKV strain was probably introduced into Northeast Brazil coinciding with the 2013 Confederations Cup, a preparatory tournament for the football World Cup in Brazil. Everything seems to have started around the Tahiti vs. Spain match, in Recife, Pernambuco State capital (Faria et al., 2016).
ZIKV spread to neighboring States and regions (Brito et al., 2016), reaching most of the Brazilian Northeast, the North, the Midwest, and parts of the Southeast (Cardoso et al., 2015). It did not take long to reach other countries in South (WHO, 2015; Pacheco et al., 2016) and Central America (Lozier et al., 2016), accompanied by an epidemic of GBS cases (Dos Santos et al., 2016) and microcephaly (Cuevas et al., 2016). Patients infected with ZIKV began to be identified in the U.S., initially imported from South and Central American countries (McCarthy, 2016; Hamer et al., 2017). Autochthonous cases of Zika were later detected in 2016 in the continental United States in Florida (Likos et al., 2016) and later Texas [https://emergency.cdc.gov/han/han00399.asp]. As the virus continues to spread, it is still unclear how many cases of GBS, CZS, and other complications of ZIKV infection will be seen.
Several underlying conditions were in place to facilitate such a fast spread of ZIKV in the Americas. The first is the extensive presence of an efficient vector, Aedes aegypti, in vast swaths of the Americas, from Argentina to the U.S., with widely variable and sometimes quite inefficient mosquito control programs (Ali et al., 2017). Second, the absence of previous ZIKV epidemics in the Americas, resulting in a massive susceptible population. The third is the virus’s ability to induce viremia shortly after the infectious mosquito bite (Dudley et al., 2016), rendering each infected person an efficient amplification host for several days. Fourth, the mobility of people among states and countries in very short periods of time enhanced rapid spread among areas where the vector is present, with the potential of seeding new foci of transmission (Hamer et al., 2017; Ali et al., 2017).
Sexual transmission of ZIKV, reported since 2011 (Foy et al., 2011), is also believed to have contributed to virus spread in Americas. As the virus can be detected in different body fluids (Paz-Bailey et al., 2017), including semen from vasectomized men (Froeschl et al., 2017), sexual transmission may be responsible for an unknown proportion of cases within epidemic and endemic areas, as well as cases in non-endemic regions who acquired the virus from their sexual partners returning from areas of ZIKV transmission (Moreira et al., 2017a). The contribution to the epidemic in regions with active mosquito-borne transmission is difficult to estimate because mosquito transmission of Ae. aegypti-borne viruses such as DENV often occurs nearly simultaneously within a household.
What is next? Areas with a large naïve population and abundant Ae. aegypti are expected to experience epidemic patterns of ZIKV transmission. However, as we accumulate areas with mounting herd immunity, ZIKV tends to spread in smaller outbreaks in the remaining susceptible groups. Although the susceptible populations in the Americas may be diminishing as future amplifiers of ZIKV, it is anticipated that further transmission may still occur.
3.2. Africa (Andrew Haddow)
Since 2015, the vast majority of ZIKV research has focused on those strains circulating outside of Africa; however, research in Africa has remained neglected and virus characterization and pathogenesis studies involving African strains have unfortunately been discounted by many – albeit inappropriately – as irrelevant. There is much to be gained through a thorough understanding of the ecology, epidemiology and pathogenesis of those ancestral ZIKV strains circulating in Africa. Such data will further our understanding of those ZIKV strains responsible for the large outbreaks reported throughout the tropics, which are known to cause severe clinical manifestations following infection in a subset of patients.
To date, the only continent where both members of the Spondweni flavivirus serogroup, ZIKV and Spondweni virus (SPONV), are known to circulate is Africa (Haddow and Woodall, 2016; Haddow et al., 2016). While ZIKV strains constitute two phylogenetic lineages, the ancestral African lineage and the Asian lineage, these lineages represent a single virus serotype (Haddow et al., 2012, 2016; Dowd et al., 2016a; Marchette et al., 1969; Aliota et al., 2016a; Faye et al., 2014). Symptomatic cases of ZIKV and SPONV infection both present as acute febrile illnesses, making clinical diagnosis in Africa challenging (Haddow and Woodall, 2016). Additionally, serologic cross-reactivity has resulted in the misidentification of virus isolates and has traditionally confounded serosurveys where non-specific diagnostic assays were utilized (Haddow and Woodall, 2016; Haddow et al., 1964; Simpson, 1964; Draper, 1965).
Sustained arbovirus surveillance efforts led to the original isolation of ZIKV from a sentinel rhesus macaque exposed in the Zika Forest, Uganda in 1947 (strain MR 766); a second isolate was made from a pool of Ae. africanus mosquitoes collected the following year (strain E1/48) (Dick et al., 1952). The first human infection was reported in Uganda in 1962, probably resulting from a mosquito bite in the Zika Forest (Simpson, 1964). Due to the historic misidentification of the Chuku strain of SPONV as a ZIKV strain (Haddow et al., 1964; Simpson, 1964; Draper, 1965; Macnamara, 1954), some early case reports of ZIKV infection actually represented SPONV infection. Furthermore, early experimental vector competence and virus characterization studies utilized SPONV rather than ZIKV (Haddow and Woodall, 2016; Macnamara, 1954; Bearcroft, 1956, 1957). Due to their close relationship, further studies of cross-protection in mammalian hosts, as well as the potential for superinfection exclusion in competent mosquito vectors, are needed.
Our present knowledge regarding the geographic distribution of ZIKV in Africa primarily comes from surveillance efforts of a few laboratories East and West Africa in the second half of the 20th Century (Haddow et al., 2012). These studies indicate that ZIKV circulates in various niches throughout sub-Saharan Africa, and long-term enzootic circulation was recently demonstrated by serosurveys in several countries with previously reported ZIKV circulation (Gambia, Nigeria, Senegal and Tanzania) (Buechler et al., 2017; Herrera et al., 2017). However, the majority of surveillance has focused only on specific locales, resulting in an underestimation of the geographic distribution of ZIKV, as well as amplification hosts and mosquito vectors. Furthermore, shifts in the predominant vector species may have occurred during recent years, masking potential enzootic transmission cycles.
Field studies in East and West Africa, as well as experimental infections, indicate that ZIKV is primarily maintained in enzootic cycles involving sylvatic mosquitoes and nonhuman primates (NHPs) (Haddow et al., 1964, 2016; Dick et al., 1952). Although evidence of present or prior ZIKV infection has been reported in several African NHP species, including the genera Cercocebus, Cercopithecus, Chlorocebus, Colobus and Erythrocebus (Haddow et al., 2012; Buechler et al., 2017; Musso and Gubler, 2016), the primary NHP species involved in the ZIKV enzootic transmission cycle remain unknown. Serological evidence of past infection has also been reported in water buffalo, elephants, goats, hippos, impala, kongoni, lions, sheep, wildebeest and zebra (Haddow et al., 2012; Hayes, 2009). Yet, without viremia data, their role as amplification/reservoir hosts remains unresolved. While the ability of African rodents and birds serve as ZIKV amplification hosts remains unclear, their role may be limited based on previous field and experimental work involving SPONV (Haddow et al., 2016).
Commonly incriminated mosquito vectors include: Ae. africanus, Ae. furcifer, Ae. opok, Ae. vittatus and Ae. luteocephalus; while potential amplification hosts likely involve multiple NHP species. Transovarial transmission in mosquitoes (Diallo et al., 2014) and sexual transmission among NHPs (Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017 Aug. https://na01.safelinks.protection.outlook.com/?url=https%3A%2F%2Fdoi.org%2F10.3201%2Feid2308170036&data=02%7C01%7Csweaver%40utmb.edu%7C190b765df0df48128df608d4b7f3f0a3%7C7bef256d85db4526a72d31aea2546852%7C0%7C0%7C636335705719675148&sdata=8JDEPmF77mCiUFZeugFxTWqwj%2B5bsnX8KCoJ5J6iFV8%3D&reserved=0) represent secondary ZIKV maintenance mechanisms. While Aedes spp. mosquitoes likely serve as the primary sylvatic mosquito vectors, future research should investigate the competence of additional mosquito genera, such as Mansonia spp., from which both ZIKV and SPONV have been isolated (Haddow et al., 2016). Studies investigating variation in vector competence among geographically distinct mosquito populations are also needed.
Human infections with either African or Asian lineage ZIKV strains result in similar clinical presentations (Heang et al., 2012; Plourde and Bloch, 2016). Severe manifestations, such as GBS and CZS, have only been reported following infection with Asian lineage strains (Musso and Gubler, 2016; Plourde and Bloch, 2016; Petersen et al., 2016a), although recent cases of CZS in Guinea-Bissau await confirmation of maternal ZIKV infection (Gulland, 2016). The lack of detected congenital defects in Africa is not well understood; it may be due to phenotypic variation between African and Asian strains, underreporting, misdiagnosis and/or immune protection resulting from ZIKV or related African flavivirus infection prior to puberty (Haddow and Woodall, 2016). Although there have been multiple reports of sexual ZIKV transmission involving Asian lineage strains (Moreira et al., 2017b), the first sexually transmitted case was reported in the female sexual partner of a ZIKV-infected male who infected in Senegal (Foy et al., 2011). Clinical and epidemiological studies are needed to determine if severe manifestations result from ZIKV infection with African lineage strains, as well as the role of sexual transmission in virus maintenance, transmission and spread.
In 2015, an Asian lineage ZIKV strain(s) caused an outbreak of human disease in the Cape Verde Islands, and this strain may have been introduced from the Americas, potentially leading to competition with African phenotypes. However, recent in vivo and in vitro experimental work using a limited number of ZIKV strains demonstrated that the African lineage exhibits increased fitness/virulence in vertebrates as well as mosquito vectors compared to Asian lineage strains (Bowen et al., 2017; Weger-Lucarelli et al., 2016; Lazear et al., 2016; Roundy et al., 2017; Azar et al., 2017). Characterization of additional African strains in mosquito vectors, reservoir hosts and models for human infection, including early African isolates not available in reference collections are needed to fully explore the evolution of ZIKV, as well as to identify mutations potentially associated with differing phenotypes.
The lack of reported ZIKV epidemics in Africa must be taken with a grain of salt, as the majority of infections are asymptomatic and ZIKV co-circulates with a multitude of other pathogens that cause acute febrile illness, making diagnosis challenging. Seventy years after its discovery, the detection of ZIKV infections in Africa remains hindered by a lack of affordable and specific diagnostic assays, as well as support for longitudinal surveillance needed to better understand epidemiology, ecology and mechanisms of ZIKV maintenance and emergence. Improved understanding of the evolution of ZIKV and its pathogenicity as well as the emergence of epidemic cycles will depend on improved surveillance and epidemiologic studies in its ancestral Africa.
3.3. Asia (Lisa F. P. Ng)
The 2015–2016 ZIKV epidemic in Latin America that led to cases of devastating neuropathology and congenital neurological manifestation prompted a surge in awareness and monitoring of the virus in Asia (Fauci and Morens, 2016). However, it is known that ZIKV has been present in Asia for decades, and many countries have reported occasional or sporadic outbreaks of ZIKV infection (Musso and Gubler, 2016). In Southeast Asia, ZIKV was first isolated in Malaysian mosquitoes in the 1950s (Smithburn, 1954), and human infections were reported from Indonesia as early as in 1977 (Olson et al., 1981). Reports of local transmission in Cambodia (Heang et al., 2012), the Philippines (Alera et al., 2015), Malaysia and Thailand have also been documented (Buathong et al., 2015; Wikan et al., 2016). Furthermore, indirect serological data of ZIKV infection using non-acute blood samples from Thailand, Malaysia, Indonesia and Vietnam suggest that ZIKV is endemic and widespread in this region (Haddow et al., 2012). However, natural ZIKV reservoirs, such as potentially NHPs, remain elusive and to date, no other arthropod species has been reported to harbor ZIKV other than Aedes spp. mosquitoes. Further surveillance will be crucial to understanding the pathogenicity of Asian ZIKV strains as well as their maintenance, transmission and spread in these countries.
More recently, Singapore reported its first case of local ZIKV transmission on 27 August 2016 and phylogenetic analysis revealed that the virus strains form an earlier phylogenetic branch distinct from the 2015 outbreaks in Latin American (Maurer-Stroh et al., 2016). This observation suggests that there are still multiple strains of ZIKV in circulation with wide antigenic diversity and immunity. The presence of different ZIKV strains poses great challenges not only in the development of specific detection reagents, but also in the development of vaccines and therapeutics such as monoclonal antibodies.
Finally, phylogenetic studies indicate that Southeast Asia is the likely source of introduced ZIKV epidemics in Yap Island, 2007 (Haddow et al., 2012) and independently into French Polynesia beginning in 2013, followed by spread to the Americas (Faria et al., 2016; Musso and Gubler, 2016). However, due to the limited sampling in Asia, the exact source location in Southeast Asia remains unknown. Additional surveillance to identify genetic diversity in the Asian lineage may ultimately pinpoint the source of the Oceania/American outbreaks as well as any phenotypic variation critical to spread and pathogenicity seen in these recent outbreaks.
4. Mosquito transmission and control measures (Nikos Vasilakis)
ZIKV transmission has been documented in two ecologically and evolutionarily distinct cycles: an ancestral, enzootic, sylvatic cycle, where the virus circulates between arboreal Aedes spp. mosquitoes and NHPs; and a human or urban cycle, between humans and peridomestic/domestic Aedes spp. mosquitoes (Fig. 1). Enzootic transmission has been documented in Africa (Dick et al., 1952) and there is indirect evidence that ZIKV may be circulating in the forests of Southeast Asia (Wolfe et al., 2001). The major vectors in the African sylvatic cycle are Ae. africanus, Ae. luteocephalus, Ae. taylori and Ae. furcifer (Haddow et al., 1964; Dick et al., 1952; Diallo et al., 2014; Berthet et al., 2014; Fagbami, 1979; Cornet et al., 1979a), as well as several other arboreal Aedes species (Diallo et al., 2014; Berthet et al., 2014; Akoua-Koffi et al., 2001). Non-Aedine mosquitoes such as, Anopheles coustani and Mansonia uniformis, which inhabit various rural habitats, have also been potentially implicated in enzootic transmission. The isolation of ZIKV from Ae. vittatus sampled in an agricultural village within the ‘zone of emergence’ supports its putative role as a bridge vector into the human transmission cycle (Diallo et al., 2014) (Fig. 1). ZIKV transmission in the urban cycle mainly involves the anthropophilic Ae. aegypti mosquito (Marchette et al., 1969; Weger-Lucarelli et al., 2016; Roundy et al., 2017; Olson et al., 1981; Guerbois et al., 2016; Li et al., 2012; Boorman and Porterfield, 1956; Cornet et al., 1979b; Hall-Mendelin et al., 2016; Richard et al., 2016; Chouin-Carneiro et al., 2016), and to lesser degree the peridomestic Ae. albopictus (Azar et al., 2017; Grard et al., 2014; Wong et al., 2013), Ae. hensilli (Ledermann et al., 2014) and Ae. polynesiensis (Richard et al., 2016; Musso et al., 2014a) as vectors in niche ecotypes. Aedes albopictus is a highly invasive species, which has significantly expanded its global distribution in tropical as well as temperate settings, thus positioning it to become a significant ZIKV vector if conditions permit. However, its behavior is not as conducive to interhuman transmission as that of Ae. aegypti. So, taking into consideration its similar vector competence (Azar et al., 2017), Ae. albopictus is expected to play a secondary role in regions inhabited by similar populations.
Fig. 1.
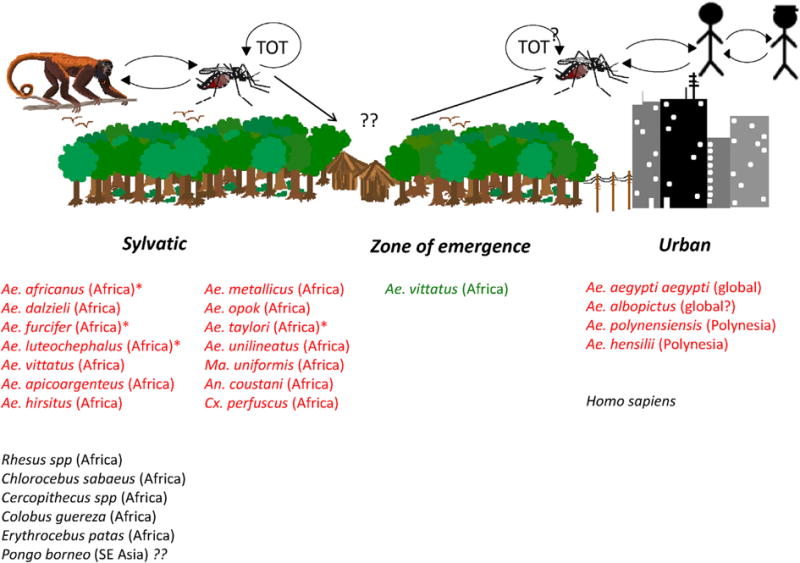
Sylvatic and urban cycling of Zika virus and its mosquito vectors.
To explain its spectacular global spread, it was suggested that ZIKV underwent adaptive evolution for more efficient urban transmission by Ae. aegypti mosquitoes, or for higher viremia in humans, which could enhance fetal infection. To date, most experimental studies (Weger-Lucarelli et al., 2016; Roundy et al., 2017; Azar et al., 2017) have failed to support these hypotheses, and cases of microcephaly possibly associated with maternal ZIKV infection in Thailand, Vietnam and Guinea Bissau (Gulland, 2016) suggest that Asian and African ZIKV strains may be capable of producing CZS. However, a recent study reported that NS1 antigen levels affect ZIKV oral infectivity of Ae. aegypti (Liu et al., 2017). In this study, virus strains from the Americas were more infectious than the FSS13025 2010 Cambodian strain, and an NS1-A188V substitution that evolved prior to spread into the Western Hemisphere enhanced both NS1 production and infection of this urban vector. Because other studies involving ZIKV strains that differ in this NS1 residue have not reported this infectivity difference, additional experiments with low-generation mosquito populations from other locations are needed to determine if this NS1 substitution may explain the explosive transmission and spread in the Americas.
The intensity of ZIKV transmission in the Americas is undoubtedly influenced by other factors, certainly including the stochastic introduction into regions with hundreds-of-millions of immunologically naïve humans. In mid-2016 an unsubstantiated report in the popular press from Brazil suggested that Cx. quinquefasciatus mosquitoes may be competent vectors of ZIKV transmission, followed by peer-reviewed report from China (Guo et al., 2016). However, several experimental studies (Fernandes et al., 2016; Boccolini et al., 2016; Amraoui et al., 2016; Huang et al., 2016a; Aliota et al., 2016b) demonstrated that American populations of this species as well as of the closely related Culex pipiens are refractory to ZIKV infection and incapable of transmission. A possible explanation for these contradictory findings is that factors such as the mosquito virome and/or microbiome, or genetic differences in geographic mosquito populations, affects vector competence. Laboratory vector competence is only meaningful if a mosquito species repeatedly feeds on humans, and widely divergent results have been obtained by studies of Cx. quinquefasciatus feeding patterns (Molaei et al., 2007; de Carvalho et al., 2014; Azmi et al., 2015; Guo et al., 2014).
The absence of licensed vaccines and therapeutics offer limited options, at least in the short term, to control the explosive global spread of ZIKV. The only currently viable and effective methods include reduction of contact between the vector and susceptible humans, and the elimination and/or reduction of vector populations. Aedes aegypti populations can theoretically be reduced using cost-effective approaches such as: community engagement and personal responsibility for eliminating or treating larval habitats; application of adulticide aerosols within homes or other places where people are exposed to biting vectors; release of genetically modified mosquitoes that express a dominant lethal gene resulting in the death of all offspring from matings with wild females, thus eliminating the risk for persistence of the transgene in nature; release of Ae. aegypti harboring endosymbiotic Wolbachia bacteria, which interfere with ZIKV replication and transmission; and the use of use of inexpensive and relatively maintenance-free lethal traps [reviewed in (Weaver et al., 2016; Ritchie and Devine, 2016; Walker and Sinkins, 2016; Olson and Franz, 2016; Vasilakis and Weaver, 2016)]. All these novel approaches will face logistical, technical and financial challenges to be implemented and in some cases sustained on a scale to protect large urban populations at risk.
Lastly a major determinant of ZIKV stability in the Americas will be its ability to establish an enzootic, NHP-hosted transmission cycle in the Americas. A recent modeling study (Althouse et al., 2016) demonstrated a high probability of enzootic establishment across a wide range of biologically plausible parameters, such as host and vector population sizes, host birthrates, and the ZIKV force of infection. Several arboreal New World mosquitoes involved in the enzootic transmission of YFV, including Haemagogus alboma-culatus, Hg. spegazzini, Hg. janthinomys, Sabethes chloropterus, Sa. albipivus, Sa. glaucodaemon, Sa. soperi, and Sa. cyaneus, Psorophora ferox and Ae. serratus [reviewed in (Hanley et al., 2013)] could serve as enzootic ZIKV vectors. Their ZIKV vector competence as well as the host competence of New World monkeys and other small mammals that have begun to be tested (Ragan et al., 2017), should be evaluated experimentally. Importantly, establishment of a ZIKV sylvatic transmission cycle in the Americas would render future eradication efforts practically impossible, and also might inhibit our ability to control the ongoing outbreak of CZS.
5. Features of primary human infection
5.1. Benign illness in adults and children (Scott Weaver)
Evidence that Zika virus typically causes an inapparent or benign illness dates back to the the first carefully documented case of human ZIKV infection by Simpson in Uganda, possibly a laboratory infection [earlier reports from West Africa were actually infections with the closely related Spondweni virus (Haddow and Woodall, 2016)]. The illness included a slight headache on day one with no other signs or symptoms. On day 2 a “diffuse pink maculopapular rash, which covered the face, neck, trunk and upper arms” appeared, gradually spreading to involve all four limbs, the palms of the hands and the soles of the feet. A low-grade fever (99.4 F) also appeared along with slight malaise and back pain. By day 3, the patient returned to normal aside from a persistent rash on the trunk and limbs, disappearing by day 5.
During the first well-characterized (albeit mainly retrospectively) 2007 outbreak in Yap, many children and adults (an estimated 68-to 77% of persons 3 years of age or older were infected). Common signs and symptoms included rash, fever, arthralgia, and conjunctivitis, with myalgia, headache, retro- orbital pain, edema, and vomiting less common, but no severe manifestations, hospitalizations, or deaths associated with ZIKV infection (Duffy et al., 2009). Only 18% of persons infected were estimated to have had clinical illness (95% CI: 10–27%). As ZIKV has spread to other parts of Oceania and to the Western Hemisphere, and as outbreaks have been detected in Asian locations such as Singapore, this typical clinical syndrome has remained, although estimated apparent:inapparent ratios have ranged slightly higher, probably due to increased awareness of the virus among patients and health care workers. However, rash, fever, arthralgia and conjunctivitis have remained common signs and symptoms in most outbreaks (Salehuddin et al., 2017).
5.2. Persistent infection and sexual transmission (Geraldine O. Schott-Lerner, Shelton S. Bradrick and Mariano A. Garcia-Blanco)
5.2.1. Features of persistent infection
Dramatic findings during the past 2 years if the outbreak in the Americas have demonstrated the persistence of ZIKV in several tissues following human infection. Several studies have investigated the presence and persistence of ZIKV in the male and female genitourinary (GU) tract by testing sperm, urine, and vaginal secretions from infected patients over extended periods of time. Remarkably, ZIKV RNA can persist at high levels in sperm months after resolution of signs and symptoms (Musso et al., 2015; Mansuy et al., 2016a, 2016b; Nicastri et al., 2016). In female patients, vRNA was detected in vaginal secretions for up to ~2 weeks after onset symptoms (Paz-Bailey et al., 2017; Murray et al., 2017). The sexual transmissibility of ZIKV and its persistence in reproductive tract tissues and secretions are features that were not commonly observed in other flaviviral infections in humans.
Animal studies have recapitulated some of these clinical observations concerning GU persistence and tropism of ZIKV (Table 2). Two studies examined the effects of ZIKV infection on murine male reproductive tissues and demonstrated persistence of virus in testis and epididymis as well as histopathologic tissue lesions and inflammation (Govero et al., 2016; Ma et al., 2016). Although the translation to humans of these studies is unclear, they nonetheless raise the question of long-term consequences of ZIKV in the human male reproductive system. Studies in pregnant wild-type (WT) female mice showed that vaginal exposure to ZIKV results in local infection, and growth restriction, and brain infection of developing fetuses (Yockey et al., 2016). Intraperitoneally or intravenously inoculated WT mice normally do not develop viremia, so these results highlight a strong tropism of ZIKV for the GU tract. A third study showed persistent shedding of ZIKV RNA in semen from infected immunodeficient male mice and sexual transmission to uninfected females (Duggal et al., 2017). One study in rhesus and cynomolgus macaques showed that ZIKV persisted in seminal fluids and male and female reproductive tissues, and another indicated longer persistence of vRNA in maternal blood of infected pregnant rhesus macaques (Dudley et al., 2016; Osuna et al., 2016).
Table 2.
Animal models for ZIKV infection of the genitourinary tract and sexual transmission.
| Reference, title | Model | Summary |
|---|---|---|
| Govero et al. (2016) Zika virus infection damages the testes in mice. | WT male C57BL/6 mice treated with IFNα and IFNβ receptor 1-blocking monoclonal antibody | ZIKV persisted in testis and epididymis of male mice, preferentially infecting spermatogonia, primary spermatocytes and Sertoli cells. This was associated with tissue injury and seminiferous tubule destruction, and linked to lowered testosterone and oligospermia. |
| Ma et al. (2016) Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. | Ifnar1−/− and WT C57BL/6 male mice | ZIKV induced inflammation in the testis and epididymis of male mice infected intraperitoneally and tissue damage persisting up to 60 days post-infection in mice. |
| Yockey et al. (2016) Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. | Ifnar1−/− and WT C57BL/6 female mice | Intravaginal exposure to ZIKV in WT pregnant C57BL/6 female mice led to persistence of viral RNA and infectious particles in the vagina and fetal growth restriction and brain infection. |
| Duggal et al. (2017) Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. | Ifnar1−/− mice | Infected male AG129 mice shed ZIKV in semen and infected female mice via sexual transmission; infectious virus was detected in semen from vasectomized and non-vasectomized males up to 58 days after infection, and 50% of females became infected post-mating. Fetal infection was detected in resulting pregnancies. |
| Osuna et al. (2016) Zika viral dynamics and shedding in rhesus and cynomolgus macaques. | Rhesus and cynomolgus macaques | Rhesus and cynomolgus macaques are susceptible to infection by ZIKV. Viral RNA was detected in saliva and seminal fluid 3 weeks after resolution of viremia in blood plasma. |
| Dudley et al. (2016) A rhesus macaque model of Asian-lineage Zika virus infection. | Rhesus macaques | Pregnant animals maintained persistent plasma viremia for more than 10 days post-infection, not the case for non-pregnant animals. |
5.2.2. Evidence of sexual and other forms of non-mosquito-borne transmission
Foy et al. described the case of two patients returning from Senegal in 2008 that were diagnosed with ZIKV infection after serological testing. Although their clinical signs and symptoms were consistent with flavivirus infection, one developed prostatitis and hematospermia. His wife subsequently developed a febrile illness and serological tests (viral neutralization assays) were consistent with ZIKV infection. It was established that she had never been to Asia or Africa nor had travelled outside the U.S. in over a year, suggesting that the virus was sexually transmitted (Foy et al., 2011). In 2016, several reports confirmed sexual transmission of ZIKV from males to females and both male-to-male, and female-to-male transmission has also been documented, albeit less frequently (Hills et al., 2016; Venturi et al., 2016; D’Ortenzio et al., 2016; Deckard et al., 2016; Davidson et al., 2016).
Other than sexual transmission, the most likely non-mosquito transmission route is via contaminated blood products (Musso et al., 2014b) and this route has been documented since the inception of the ZIKV epidemic in the Americas (Cunha et al., 2016; Barjas-Castro et al., 2016; Motta et al., 2016). Two studies looking at the presence of ZIKV RNA in donated blood in the United States (U.S.) concluded that few samples tested positive (1 in 93,000 and 1 in ~25,000) and screening of donors’ travel history should prevent contaminated blood and infection of recipients; hence blood transfusion is not believed to be a major mode of transmission in the U.S. (Galel et al., 2017; Williamson et al., 2017). Specific guidelines to handle blood products are being reviewed and updated (Petersen et al., 2016b; Vasquez et al., 2016). Upcoming discussions as to how to screen blood donations are a matter of urgent importance, while novel techniques of high-throughput screening are under development (Katz and Rossmann, 2017).
5.2.3. Models of sexual transmission
Animal models have been valuable to study mechanisms of both sexual and vertical transmission of ZIKV, including both mice (see VI.D.2) and NHPs (see VI.D.3). Although it is clear that ZIKV tropism is critical to its transmission and biology, few published studies have examined mechanisms of GU tract infection and more research is needed to understand why this virus is so successful in those tissues compared to other closely related flaviviruses. Furthermore, the connections between sexual transmission and vertical transmission (i.e. can the sexual route of infection increase the chance of fetal infection?) remain unexplored.
6. Mechanisms and consequences of maternal-fetal transmission
6.1. Spectrum of congenital defects (Caroline Marrs, George Saade)
ZIKV infection during pregnancy can cause both pregnancy loss and congenital malformations, including microcephaly and a range of other central nervous system and ocular malformations (Rasmussen et al., 2016) (Table 3). Counseling pregnant and reproductive-aged women has been difficult due to complex diagnostic algorithms and evolving data on the risk of both sexual and vertical transmission (D’Ortenzio et al., 2016).
Table 3.
Pregnancy and neonatal outcomes reported in mothers with ZIKV infection.
| Outcome | Reference |
|---|---|
| Early pregnancy loss | Meaney-Delman et al. (2016b) |
| Stillbirth | Martines et al. (2016); Sarno et al. (2016); Brasil et al. (2016) |
| Microcephaly | Mlakar et al. (2016); Reynolds et al. (2017) |
| Ocular abnormalities | de Paula Freitas et al. (2016); Ventura et al. (2016) |
| Hearing loss | Leal et al. (2016) |
| Central nervous system lesions, including calcifications | Reynolds et al. (2017); Brasil et al. (2016) |
| Growth restriction | Brasil et al. (2016) |
The screening approach in pregnancy is based on patient history of potential exposure to ZIKV through residence, travel or sexual contact, maternal ZIKV antibody testing and/or detection of viral RNA with PCR testing of maternal blood and urine, prenatal fetal ultrasound, as well as amniocentesis in some cases. Diagnosis can be difficult due to antibody cross-reactivity between ZIKV and other flaviviruses, the limited window of time that some antibodies and/or viral particles persist in the bloodstream, and the asymptomatic nature of most ZIKV infections. Laboratory testing and prenatal ultrasound screening algorithms have been published by the Centers for Disease Control and Prevention (CDC) and updated with the evolving scientific knowledge (Rabe et al., 2016; Oduyebo et al., 2016).
It is important to note that there is prolonged persistence of viral RNA in human blood and urine in pregnancy. ZIKV RNA has been detected in the serum of non-pregnant persons up to 11–13 days after symptom onset, while ZIKV RNA has been detected in serum of pregnant women up to 10 weeks after symptom onset (Lanciotti et al., 2008; Driggers et al., 2016; Meaney-Delman et al., 2016a). However, the presence of RNA does not necessarily indicate infectious Zika virus, and therefore there is still confusion about how long a woman and her fetus are at risk of maternal-fetal transmission. There are very limited data about the risk of maternal-child transmission via breastmilk. Case reports and small case series have not found evidence of transmission in spite of presence of viral particles and even infective particles in breastmilk (Besnard et al., 2014; Cavalcanti et al., 2017). The long-term effect of neonatal infection is unknown. Given the numerous benefits of breastfeeding, the World Health Organization (WHO) and the CDC have recommended that infants born to mothers with suspected or confirmed ZIKV infection, or who are at risk of exposure, should be fed according to normal infant feeding guidelines (WHO, 2017; CDC).
There is substantial evidence for a causal relationship between maternal ZIKV infection and fetal/neonatal microcephaly and other brain insults (Rasmussen et al., 2016). Table 3 lists the other birth defects that have been reported in pregnant women with suspected or confirmed ZIKV infection. Most defects are of the central nervous system, and there is evidence that ZIKV has a predilection for neural cells (Dick, 1952; Bell et al., 1971; Tetro, 2016; Souza et al., 2016). Microcephaly is not typically apparent until the late second or early third trimester, when a woman is often past the legal window for termination of pregnancy should she desire it.
Since the initial outbreak in Brazil, there has been an effort to standardize the prenatal definition of microcephaly, which previously had not been defined consistently in the literature. This issue is further compounded by the limitation in fetal measurements: fetal head size can only be estimated prenatally, and this estimate has a wide overlap between normal and abnormal. The Society for Maternal Fetal Medicine (SMFM) published a statement regarding ultrasound diagnosis of microcephaly in the setting of the ZIKV outbreak (SMFM, 2016). In summary, the Society recommended defining isolated microcephaly as a fetal head circumference of ≥3 standard deviations below the mean for gestational age, while pathologic microcephaly is considered certain when the fetal head circumference is smaller than 5 standard deviations below the mean for gestational age. This is similar to the WHO’s recommendations (WHO, 2016a).
Because the measurements are related to average fetal dimensions for gestational age, it is crucial to ensure accurate pregnancy dating and to use an appropriate reference growth curve. In the U.S., a recent large multicenter cohort study reported nomograms for various fetal biometry measurements, including head circumference (HC) (the NICHD National Fetal Growth Study), stratified by race/ethnicity (Buck Louis et al., 2015). The drawback in this setting is that it does not report cutoffs lower than the third percentile [which is roughly equivalent to the 2nd standard deviation (SD)]. The International Fetal and Newborn Growth Consortium (INTERGROWTH-21st) collected data on large populations of healthy pregnant women across the globe to describe normal fetal growth and has published the nomograms (Villar et al., 2013). Using these more modern growth references should help better identify truly pathologic microcephaly.
In addition to CNS malformations, there is evidence that ZIKV infection can lead to early pregnancy loss and later fetal demise (Meaney-Delman et al., 2016b; Martines et al., 2016; Sarno et al., 2016). While the primary concern has been microcephaly, some have raised the concern for long-term, more subtle effects of prenatal infection (Oliveira Melo et al., 2016).
The CDC, in collaboration with local and state health departments, established the U.S. Zika Pregnancy Registry to monitor outcomes of pregnant women with laboratory evidence of possible ZIKV infection and their infants (Reynolds et al., 2017). ZIKV-associated birth defects were reported in 5% of infants with laboratory evidence of possible ZIKV infection, and in 10% of infants with confirmed infection. The rate was 15% in women with confirmed infection in the first trimester, suggesting early infection leads to worse outcomes. Brazil and other South American countries are likewise collecting registries in an attempt to better characterize incidence, risk of transmission, and outcomes. As new data emerge, national and international guidance for testing, surveillance, and management of maternal ZIKV infection will evolve.
6.2. Epidemiology of congenital Zika syndrome (Guilherme Ribeiro, Uriel Kitron)
Between July and September of 2015, physicians in Northeastern Brazil performing prenatal ultrasound began to notice an increase in the frequency of fetuses with congenital brain abnormalities. In October 2015, following the continued rise in the number of fetuses and newborns presenting with microcephaly, particularly in the State of Pernambuco, the Brazilian Health Ministry issued a declaration of a national public health emergency, which was followed by a global declaration by the WHO in February 2016. At that time, there was no direct scientific evidence of a causal relationship between ZIKV infection during pregnancy and congenital brain defects in fetuses or newborns, but such an association was highly suspected because epidemics of ZIKV infection preceded the rise in congenital malformations in northeastern Brazil. In addition, one month after the Brazilian public health emergency declaration, researchers from French Polynesia, where a large outbreak of ZIKV infection occurred between October 2013 and March 2014, also reported retrospectively an unusual increase in the number of fetuses and newborns with brain abnormalities approximately one year following the beginning of the ZIKV outbreak (Besnard et al., 2016).
Evidence for a causal association between ZIKV and congenital abnormalities has accumulated based on clinical, epidemiological, and experimental studies. Several case reports have shown the presence of ZIKV, viral RNA (vRNA), and/or IgM antibodies against ZIKV in blood, amniotic fluid and other tissues of newborns and stillbirths presenting with congenital brain defects (Oliveira Melo et al., 2016; Mlakar et al., 2016; Sarno et al., 2016). In addition, strong spatial and temporal correlations between ZIKV epidemics and GBS in adults, as well as microcephaly in newborns, were observed in Salvador, Brazil (Paploski et al., 2016). There, a lag time of 30–33 weeks between peaks in the number of exanthematous cases suspected of ZIKV infection and the number of suspected cases of microcephaly was demonstrated, suggesting a greater risk of congenital malformations when women are infected by ZIKV during the first trimester of pregnancy. A population-level study performed on French Polynesian data also showed that ZIKV infections during the first trimester of pregnancy were associated with a higher risk of microcephaly (Cauchemez et al., 2016).
Definitive epidemiological evidence of a link between ZIKV infection during pregnancy and CZS was derived from two studies. In a cohort of pregnant women undergoing fetal ultrasonography in Rio de Janeiro, congenital abnormalities were detected in 12 (29%) of fetuses from 42 ZIKV-positive women, but in none (0%) of 16 ZIKV-negative women (Brasil et al., 2016). Another study conducted in Recife enrolled 32 microcephaly cases, as well as 64 control neonates without microcephaly, and found that 13 (41%) of the cases and none of the controls had laboratory evidence for ZIKV infection (OR 55.5; 95% CI: 8·6–∞) (de Araujo et al., 2016). Overall, analysis of data from several sites indicated that the risk of microcephaly is mainly in the first trimester of pregnancy (Johansson et al., 2016). In vitro and experimental animal studies also support the ability of ZIKV to cause abnormalities during brain development, and models for CZS are detailed above (Section 3.2). The evidence for causality of ZIKV infection and birth defects has been extensively reviewed by Rasmussen et al. (2016) and Broutet et al. (2016).
Since the first cases of microcephaly were detected in northeastern Brazil, 24 countries and territories in the Americas have reported CZS and by March 10, 2017 the Pan American Health Or-ganization (PAHO) recorded 2767 confirmed cases associated with ZIKV infection (PAHO, 2017). Brazil accounts for the vast majority of congenital disease with 2386 (86%) of all confirmed cases, followed by Colombia (128 cases). The U.S. has reported 52 cases of CZS (PAHO, 2017). However, the actual numbers are likely greater given the difficulty in performing laboratory confirmation of ZIKV infection by serology during pregnancy, and in newborns suspected of congenital malformations (see Section 9).
While microcephaly has been the most obvious congenital complication associated with ZIKV, there is growing evidence for additional manifestations that may not be as pronounced at birth or that may only manifest later in infancy. However, during the initial months after recognition of this emerging congenital disorder, identification and reporting of CZS cases were based mainly on head circumference measurements, targeting only microcephaly. Now that it is clear that a small head size (based on sex and gestational age) at birth may not accompany all cases of CZS, pediatricians should be aware of potential congenital deficits among infants and children presenting with delayed neuromotor and cognitive development, as well as visual and hearing impairments.
Several epidemiological questions regarding CZS remain unanswered, and research priorities need to be revisited. Ongoing cohort studies of ZIKV-infected pregnant women and their fetuses and newborns will help define and quantify the risks for vertical transmission, miscarriage, abortion, and CZS among newborns. In addition, these studies may help to identify modulating factors that increase or reduce the risk of congenital defects [i.e. the route of ZIKV acquisition by the mother – mosquito-borne vs. sexual - and the preexistence of antibodies against other flaviviruses following natural infections, such as dengue virus (DENV), or via immunization, e.g., yellow fever (YF) vaccination]. The follow-up of children with CZS will also help elucidate the types and degrees of cognitive, motor, visual, hearing, and other neurological impairments, as well as survival rates.
Finally, until all pregnant women are provided with accurate laboratory diagnosis to detect both symptomatic and asymptomatic ZIKV infections, novel screening criteria that can reliably identify in utero and peripartum congenital Zika cases must be developed and evaluated. Such criteria may have to incorporate the history of an illness clinically compatible with ZIKV infection during pregnancy, and results from periodic ultrasonography performed during gestation, in addition to the head circumference measures already in use.
6.3. Pathologic manifestations (Diane E. Griffin)
Pathologic changes associated with ZIKV infection have been evaluated both in clinical specimens from humans and in tissues from experimentally infected immunocompetent mice and nonhuman primates (NHP). Organs susceptible to infection that have received the most attention are the placenta, brains and eyes after congenital infection.
Placenta
In humans, histologic examination of placentas from women with a history of ZIKV infection showed viral antigen in Hofbauer cells and histiocytes accompanied by villous inflammation, edema, trophoblastic epithelial lesions and calcifications (Noronha et al., 2016). Placentas from infants carried to term were small and showed chronic villitis, chorionitis, deciduitis and stromal fibrosis (Chimelli et al., 2017). In monkeys, ZIKV was most abundant in the chorionic villous tissue (Adams Waldorf et al., 2016). In immunocompetent mice infected by intra-uterine inoculation there was infection of trophoblasts and endothelial cells accompanied by a loss of definition between placental layers, reduction of the labyrinth and hemorrhage suggesting compromise of the trophoblast-endothelial layer (Vermillion et al., 2017).
Brain and spinal cord
In the few infected human fetuses that have been examined pathologically, gross abnormalities included microcephaly with cortical thinning, agyria, hydrocephalus and calcifications in the cortex and subcortical white matter (Mlakar et al., 2016; Strafela et al., 2017). Histopathology showed mono-nuclear inflammation, microglial nodules and hyperplasia, astrogliosis and neuronophagia in the affected regions (Mlakar et al., 2016; Noronha et al., 2016). Similar macroscopic and microscopic abnormalities, along with abrupt slowing of white matter expansion, were observed in the brain of the fetus of a pigtail macaque infected subcutaneously at the equivalent of 28 weeks gestation (Adams Waldorf et al., 2016).
Neuropathologic examination of infants carried to term that were stillborn or died shortly after birth has shown frequent presence of arthrogryposis, microophthalmia and small brains with multiple abnormalities including thickened leptomeninges, agyria, ventriculomegaly, parenchymal thinning and calcifications. Histopathology of these cases showed abnormal neuronal migration with polymicrogyria, meningeal glioneuronal heterotopia, and motor neuron loss and cerebellar cortical dysplasia. Viral antigen was most often detected in the meninges and inflammation was not prominent (Chimelli et al., 2017). Pups born to Swiss Jim Lambert (SJL), but not C57BL/6, mice intravenously infected with large amounts [4 × 1010–1012 plaque-forming units (pfu)] of ZIKV at 10–13 days gestation displayed intrauterine growth restriction and abnormal brains with cortical thinning and evidence of neuronal cell death (Cugola et al., 2016). After intrauterine infection, placentally transferred ZIKV infects endothelial cells, micro-glial cells and neural progenitor cells with evidence of microglial cell activation and cortical thinning (Vermillion et al., 2017). Direct intracerebral infection of embryonic day 13.5 mice leads to infection of neural progenitor cells in the subventricular zone, decreased proliferation of radial glial cells and cortical thinning (Wu et al., 2016; Li et al., 2016a). Intracerebral infection of 1 or 3-week-old C57BL/6 mice leads to widespread infection and microglial cell (Iba1+) and astrocyte (GFAP+) activation. Apoptotic neuronal cell death is more abundant in younger animals. Particularly vulnerable neuronal populations were in the hippocampus, layers II and V of the cerebral cortex and cortical spinal tract (Huang et al., 2016b).
Eye
Eye lesions in congenitally infected infants with and without microcephaly include malformation, optic neuritis and atrophy, chorioretinal scarring and atrophy, macular pigment stippling and lens subluxation (de Paula Freitas et al., 2016; Ventura et al., 2016; Culjat et al., 2016). Ocular malformations are also observed in congenitally infected mice (Cugola et al., 2016), and in human adults after infection (Parke et al., 2016).
6.4. Laboratory models
6.4.1. In vitro studies (Marc Lecuit)
As with many other flaviviruses, ZIKV can replicate in a wide variety of human and non-human cultured cells (Zhu et al., 2016). Yet, ZIKV infection of humans displays unique clinical features among flaviviruses (Miner and Diamond, 2017). They correlate with its specific in vivo tissue and cell tropisms, which are still being characterized and deciphered. Cardinal features of ZIKV, of critical clinical importance are that (i) it is transmitted by a mosquito bite but can also be transmitted sexually, (ii) it is able to actively cross the placental barrier and replicate in the placenta, and (iii) it can disseminate to the fetus and its developing brain, where it leads to severe neurodevelopmental defects, in particular in the developing cortex, resulting in microcephaly. Studies of cell and tissue samples from infected humans, as well as experimentally infected NHP and mouse lines, have shown that ZIKV infects a wide variety of tissues and cells, including the skin (human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells) (Hamel et al., 2015), the testis (Leydig cells, sertoli cells, spermatogonia) (Govero et al., 2016; Ma et al., 2016), vaginal epithelium and uterine fibroblasts (Yockey et al., 2016; Chen et al., 2016), placenta (trophoblasts, endothelial cells, Hofbauer cells) (Noronha et al., 2016; El Costa et al., 2016; Simoni et al., 2017; Quicke et al., 2016), and the brain (cortical progenitors, mature neurons and astrocytes) (Gabriel et al., 2017; Li et al., 2017; Qian et al., 2016; Tang et al., 2016; Xu et al., 2016; Brault et al., 2016). It may also infect the eye (Ganglion cells, bipolar neurons, the optic nerve, cornea) and be found in body fluids including tears, saliva, semen, cervical mucus and urine (Miner et al., 2016a; Barzon et al., 2016; Zambrano et al., 2017).
In vivo and in vitro studies have shown that ZIKV cell tropism may reflect the expression pattern of virus co-receptors, such as AXL, a member of TAM receptor family, either directly, or as a signaling molecule that may modulate the type I-interferon receptor (IFNAR) pathway (Hamel et al., 2015; Nowakowski et al., 2016; Meertens et al., 2017; Pagani et al., 2017; Tabata et al., 2016; Richard et al., 2017). TIM1 has also been reported to act as a co-receptor (Hamel et al., 2015; Tabata et al., 2016). Yet, because neither of these putative receptors is required for productive ZIKV infection, their significance with regard to ZIKV cell and tissue tropism remains to be fully determined (Wells et al., 2016). As one would expect for an RNA virus, type I and III interferon and interferon-stimulated genes are also key restriction factors that modulate cell permissiveness to ZIKV (Lazear et al., 2016; Bayer et al., 2016).
The blood phase of ZIKV infection has not been studied in detail so far, and it remains to be determined if ZIKV infects in vivo polymorphonuclear cells, lymphocytes and/or monocytes, and whether this may have an impact on ZIKV’s ability to cross the placental and blood-brain barriers.
Crossing of the placental barrier
Crossing of the placental barrier: whereas it has been established that ZIKV’s ability to cross the placental barrier is a key property that leads to CZS, the precise mechanism of crossing of the placental barrier remains only partially understood. Ongoing cohort studies will determine precisely the impact of pregnancy term on transmission efficiency. Studies with in vitro cultured cells and human placental explants have shown that mature syncytio-trophoblasts are not permissive to ZIKV, in contrast to extravillous cytotrophoblasts (EVT) (Tabata et al., 2016; Sheridan et al., 2017). Yet, how ZIKV reaches EVT and whether EVT infection is the only factor that determines the ability to traverse the placental barrier remains to be determined. The non-permissiveness of syncytio-trophoblasts to ZIKV reflects, at least in part, its capacity to produce type III IFN, in contrast to EVT (Bayer et al., 2016). Although ZIKV has not been shown to replicate in syncytiotrophoblasts, it may transcytose these cells, although no experimental data have yet been published to support this hypothesis. Once in the placental tissue, ZIKV replicates in Hofbauer cells, the resident macrophages of the placenta; this has been observed in clinical samples of human placentas, in human placental explants infected experimentally, and in cultured Hofbauer cells. ZIKV replication in Hofbauer cells (Simoni et al., 2017; Quicke et al., 2016; Tabata et al., 2016), as well as infection of endothelial cells of placental villus capillaries may constitute key amplification steps and lead to prolonged viral release in the fetal circulation, from where ZIKV can disseminate to the brain (Noronha et al., 2016; Vermillion et al., 2017; Tabata et al., 2016; Richard et al., 2017).
Infection of the fetal brain and neuropathology
Most if not all viruses associated with fetal systemic infection also invade the brain. The precise mechanism by which ZIKV infects the brain remains unknown. The developing fetal blood-brain barrier is not as tight as in adults, and systemic infection and associated innate immune responses may also compromise the blood-brain barrier. Whether ZIKV actively infects endothelial cells critical to the blood-brain barrier or whether it transcytoses these cells remains unknown. Once in the brain parenchyma, ZIKV infects cortical progenitors, and mature neurons as well as astrocytes (Gabriel et al., 2017; Li et al., 2017; Qian et al., 2016; Tang et al., 2016; Xu et al., 2016; Zhang et al., 2016; Retallack et al., 2016). In contrast to other flaviviruses such as West Nile and dengue virus-4 (DENV-4), ZIKV is able to infect cortical progenitors, and this is likely a critical feature that accounts for ZIKV-associated microcephaly (Brault et al., 2016). The basis for ZIKV tropism for cortical progenitors and the mechanism by which it induces microcephaly remains unknown. The high level of AXL expression in these cells has been proposed as a factor accounting for their permissiveness to ZIKV (Nowakowski et al., 2016).
6.4.2. Rodent models (Shannan Rossi)
In December of 2015, the total knowledge of ZIKV animal models was found in a handful of manuscripts written decades earlier. The first attempt to create a small animal model to understand ZIKV infection and pathogenesis used outbred white mice, rabbits, and guinea pigs (Dick et al., 1952). It was immediately clear that, without adaptation, ZIKV did not produce any discernible disease in white mice (Dick, 1952). To overcome this limitation, genetically modified mice with type-I interferon response deficits were used. By March 2016, the first report of lethal neurologic disease in mice lacking either type-I (IFNAR1) or types-I and –II interferon receptors was published (Rossi et al., 2016) (Table 4). These initial findings were quickly corroborated and extended by other investigators (Lazear et al., 2016; Dowall et al., 2016; Aliota et al., 2016c); reviewed in (Morrison and Diamond, 2017).
Table 4.
Mouse models of Zika virus infection.
| Reference | Mouse/age | Routea | Virus strain (dose)b | Pathologic findings |
|---|---|---|---|---|
| Adult Mouse Models | ||||
| Rossi et al. (2016) | A129/various AG129/various |
IP, ID | FSS13025 (1 × 105 pfu) |
A129 (age-dependent) and AG129 (age-independent) mortality, weight loss and viremia. High titers in organs like brain and testis. |
| Lazear et al. (2016) | Various KO strains/4–6-weeks | SQ | H/PF/2013 (1 × 102–3 pfu) |
Immunocompromised mice required for mortality, high titers in organs like brain, spinal cord and testis |
| Dowall et al. (2016) | A129, 129/5–6-weeks | SQ | MP1751 [1962 mosquito isolate] (1 × 106 pfu) |
Immunocompromised mice required for mortality, vRNA detected in ovaries of 129 and A129 |
| Aliota et al. (2016d) | AG129/8-weeks | SQ | H/PF/2013 (various) |
Muscle pathology observed |
| Miner et al. (2016a) | AG129/4-weeks Ifnar1−/−/4–8week |
IP, SQ | H/PF/2013, Paraiba 2015 |
Establishes a model for evaluating treatments for ZIKV infections in the eye |
| Yockey et al. (2016) | C57BL/6/7e22 week Ifnar1−/−/4–5 week |
IVAG IVAG |
FSS13025 (various) |
Establishes vaginal tract as a highly susceptible site of ZIKV replication |
| Govero et al. (2016) | C57BL/6/7 week | SQ | DakAr41519 H/PF/2013 |
Establishes consequences of ZIKV infection in the male reproductive tract of mice |
| Reference | Mouse | Route (dose)b | Virus | Gestational Age | Findings |
|---|---|---|---|---|---|
| Fetal Mouse Models | |||||
| Miner et al. (2016b) | A129xwt wt treated α-IFN | SQ/1 × 103 | H/PF/2013 | E6.5 | Fetal demise, IGR. ZIKV vRNA found in placenta, infected trophoblasts |
| Cugola et al. (2016) | C57Bl/6 SJL |
IV/1 × 1010 | Brasil 2016 | E10–13 | IGR, Overall brain destruction, reduced number cortical neurons. Apoptotic neural progenitor cells (NPC) |
| Li et al. (2016a) | ICR | IC/6.5 × 105 | SZ01 | E13.5 | NPC cell-cycle arrest and defects in differentiation, induces immune response in brain and apoptosis of post-mitotic neurons |
| Wu et al. (2016) | C57Bl/6 | IC, IP/3 × 105 | SZ01 | E13.5 | Infected radial glia cells of the fetuses and NPC, reduced cavity of lateral ventricles |
| Sapparapu et al. (2016) | C57Bl/6 Ifnar1−/− |
SQ/1 × 103 | H/PF/2013 | E5.5–7.5 | Monoclonal antibody treatment markedly reduced tissue pathology, placental and fetal infection, and mortality in mice. |
IP – intraperitoneal; ID – intradermal; SQ – subcutaneous; IVAG – intravaginal; IV – intravenous.
pfu – plaque forming units.
Once adult mouse models were established, it became critical to develop models that exhibited severe disease in developing fetuses and neonates to better understand CZS. Building on the success of the initial immunodeficient mouse models, females [either WT treated with anti-IFNAR1 antibody (Ab), or IFNAR1-deficient] were mated to immunocompetent males and infected with ZIKV during pregnancy. The resulting pups were either resorbed or showed an intrauterine growth-restricted (IUGR) phenotype, depending on the gestational age of the pups at the time of infection (Miner et al., 2016b). ZIKV was detected in the heads and bodies of pups as well as in the placenta (Miner et al., 2016b; Sapparapu et al., 2016). In another murine model, the SJL mouse, ZIKV also infects fetuses, leading to intrauterine growth restriction and microcephaly (Cugola et al., 2016).
However, a limitation of these IFN-deficient murine models is the lack of a normal innate response, even though the pups in most cases are phenotypically WT. Therefore, immunocompetent (WT) pregnant mice have also been used, infected either intravaginally, intrauterinely, intravenously, or intraperitoneally, resulting in pups born with IUGR, brain and eye abnormalities, as well as viral loads in the brain, spleen and placenta (Yockey et al., 2016; Vermillion et al., 2017; Cugola et al., 2016; Wu et al., 2016). Direct intracerebral infection of embryonic ICR mouse brains results in cell-cycle arrest, apoptosis, and inhibition of neural stem cell differentiation (Li et al., 2016b). Direct inoculation into the uterine wall on embryonic day 10 but not 14 leads to increased infection of placental and fetal tissues fetal death (Vermillion et al., 2017). Intraperitoneal infection of pregnant C57BL/6 mice results in infection of radial glia cells in the dorsal ventricular zone of fetuses, the primary neural progenitors of the cortex, reducing this population and leading to a reduced cavity of lateral ventricles and decreased cortical surface area (Wu et al., 2016).
More recently, the mouse model has also allowed for the study of sexual ZIKV transmission, as seen in humans (Section 5.2.2). ZIKV replicates in the male mouse reproductive tract (Govero et al., 2016; Rossi et al., 2016; Ma et al., 2017), is present in the seminal fluid, and can be efficiently transmitted to naïve female mice during intercourse (Duggal et al., 2017). Currently, the contribution of human sexual transmission to congenital ZIKV infection is poorly understood, but could result in an altered pathogenesis compared to mosquito-transmitted infection. Given that intravaginal infection of WT mice can result in a localized ZIKV infection in the uterus (Yockey et al., 2016), these models may provide a critical to understanding potential differences.
Murine models are currently being fine-tuned to better mimic human infection. Nevertheless, these models already provide an important approach to screen therapeutics and vaccines that can reduce or block transmission to the fetus. However, caution must be used when interpreting the results because the anatomy and gestational timing in mice is very different than that of humans.
6.4.3. Nonhuman primate models (Matthew T. Aliota, Thaddeus G. Golos, Thomas C. Friedrich, David H. O’Connor and Jorge E. Osorio)
Zika virus (ZIKV) likely originated and still is maintained in a sylvatic transmission cycle between NHPs and arboreal mosquitoes in tropical Africa and possibly Asia, where ZIKV antibodies have been detected in several monkey species (Buechler et al., 2017); for a review see (Musso and Gubler, 2016). Indeed, ZIKV was first isolated from a sentinel rhesus macaque (Macaca mulatta) in 1947 in Uganda (Dick et al., 1952), but until recently, data regarding ZIKV pathogenesis in NHP were limited. This has prompted the development of NHP models that now serve as useful platforms to study ZIKV pathogenesis, candidate therapies, and vaccines. Macaque monkeys [e.g., rhesus, cynomolgus (Macaca fascicularis), and pigtail (Macaca nemestrina)] have been the species of choice for ZIKV NHP studies to date (Table 5). Macaques are widely used in both infectious disease and obstetric research because their close relationship with humans provides similarities in immunobiology, fetal development, and disease outcomes, among others. Macaques also support ZIKV replication without viral adaptation. Because of the animals’ size, macaque studies allow longitudinal and invasive tissue and fluid sampling to understand the kinetics of virus replication and antiviral immunity in an immunocompetent host. Macaques thus provide a system for rigorous preclinical evaluations of interventions. This model also comes with some limitations including cost, reduced power because of small group sizes, and the limited number of centers with the expertise and size to conduct macaque studies.
Table 5.
Nonhuman primate models of ZIKV infection and disease.
| Species | Virus inoculation route(s), strain(s)a | Pathology(ies) or fetal outcomes | Site(s) of virus detection | Reference |
|---|---|---|---|---|
| Rhesus macaque (Macaca mulatta) | 104–106 PFU sc, ZIKV strain H/PF/2013, ZIKV strain MR766 | No clinical signs | Plasma, saliva, urine, CSF, vaginal secretions | Dudley et al. (2016); Aliota et al. (2016a) |
| Pigtail macaque (Macaca nemestrina) | 107 PFU50 sc (given 5 times), ZIKV strain FSS13025 | Fetal brain lesions, mild deciduitis in the dam | Fetal brain, eyes, testes; maternal eyes, kidneys, and chorionic villi of the placenta. | Adams Waldorf et al. (2016) |
| Cynomologus macaque (Macaca fascicularis), rhesus macaque | 105 TCID50 units sc, ZIKV strain PRVABC59; 106 PFU sc Thai ZIKV isolate | No clinical signs | Plasma, urine, saliva, CSF, semen, vaginal secretions; lymphoid, neurologic, and reproductive tissues | Osuna et al. (2016) |
| Rhesus macaque | 105 PFU IV, 2015 Brazilian ZIKV isolate | No clinical signs, no major histopathological changes | Plasma, whole blood, urine, saliva, CSF; lymphoid, cardiopulmonary, gastrointestinal, integument, and genitourinary tissues | Coffey et al. (2017) |
| Rhesus macaque | 104–106 FFU sc (as 10,100 μl injections), ZIKV strain PRVABC59 | Rash, fever, lymphadenopathy | Plasma, urine, Brain; lymphoid, neurologic, joint, and reproductive tissues | Hirsch et al. (2017) |
| Rhesus macaque | 104 PFU sc, ZIKV strain H/PF/2013 | No clinical signs in the dam, prolonged viremia in the dam,; decreased fetal head growth velocity, neutrophilic infiltration at the maternal-fetal interface and in fetal tissues, ocular pathology in the fetus | Maternal plasma, urine, saliva, spleen, lymph node, and decidua of the dam; amniotic fluid, fetal optic nerve, lymph node, pericardium, lung, placenta, bone marrow, liver, reproductive tract | Nguyen et al. (2017) |
| Rhesus macaque | 103–106 PFU sc, ZIKV strain PRVABC59, ZIKV strain Brazil/ZKV/2015 | No clinical signs | Plasma, whole blood CSF; lymphoid and colorectal tissue | Aid et al. (2017) |
| Cynomologus macaque | 1 × 104 and 5 × 105 PFU sc, ZIKV strain PRVAC59, ZIKV strain FSS13025, ZIKV strain IBH30656 | No clinical signs | Plasma, urine, saliva, and testes | Koide et al. (2016) |
| Rhesus macaque | 105 PFU sc, ZIKV strain GZ01/2016 | Fever | Plasma, urine, saliva, lacrimal fluid, CSF; Brain, lymphoid, and digestive tract tissues | Li et al. (2016c) |
PFU e plaque-forming units; sc – subcutaneous’ TCID50 – tissue culture infectious dose 50%.
Recent studies in macaques established that Asian/American-lineage ZIKV infection of NHPs recapitulates key features of human infection in both pregnant and non-pregnant animals (Dudley et al., 2016; Osuna et al., 2016; Coffey et al., 2017), including mild weight loss, rash, and elevated liver enzymes at early times post-infection (Hirsch et al., 2017). In some experiments, ZIKV infection also resulted in elevated body temperature for up to 10 days post-infection (Li et al., 2016c). Viremia peaked 2 to 6 days after infection and typically became undetectable by day 10 in ZIKV-inoculated rhesus macaques (Dudley et al., 2016; Osuna et al., 2016). ZIKV RNA also has been detected in urine, saliva, and the cerebrospinal fluid of some animals after clearance from the blood (Dudley et al., 2016; Hirsch et al., 2017), and sporadically in seminal fluid and vaginal secretions (Dudley et al., 2016; Osuna et al., 2016). Although vRNA is typically cleared from blood within approximately 10 days, in some studies vRNA also was detected in tissues including secondary lymphoid organs, the male reproductive tract, the intestines, brain and spinal cord several weeks after acute infection (Coffey et al., 2017; Hirsch et al., 2017; Li et al., 2016c). Infected animals developed humoral and cell-mediated immune responses that protected against challenge with homologous and heterologous ZIKV strains (Aliota et al., 2016a; Osuna et al., 2016), indicating that this model will be useful for preclinical evaluation of vaccine candidates as well as the protective efficacy of passive immunization against ZIKV (Abbink et al., 2016; Dowd et al., 2016b).
The structure of the placenta and the organization of the maternal-fetal interface is remarkably diverse among mammals (Wildman et al., 2006). The hemochorial villous structure of the macaque placenta, and the villous and extravillous pathways of trophoblast differentiation represent the closest available experimental model to human placentation (Carter and Pijnenborg, 2011; Enders et al., 2001; Enders, 2007). In particular, extravillous placental trophoblasts migrating from the placenta to remodel endometrial spiral arteries are noted in both macaque and human pregnancy (Enders, 2007; Bondarenko et al., 2007). Extravillous trophoblasts have been proposed as a target of ZIKV and a conduit of ZIKV access to the fetal compartment (Tabata et al., 2016). There are unique populations of immune cells in the decidua (Golos et al., 2010) and within the placenta itself, including placental macrophages (Hofbauer cells) (Bulmer and Johnson, 1984). Both cell populations are permissive for ZIKV infection (Quicke et al., 2016; Tang et al., 2016; Weisblum et al., 2017; Jurado et al., 2016), which reinforce the strength and relevance of the NHP model. The long gestation period (165–180 days) of macaques also provides a realistic model for human fetal development. Thus, macaques provide a close representation of the human morphological, developmental, and immune environment at the maternal-fetal interface, giving them a unique role in modeling the impact of pathogens on pregnancy and both fetal and maternal well-being.
In pregnant rhesus macaques, ZIKV viremia can persist for at least 71 days (Dudley et al., 2016), and is associated with decreased fetal head growth velocity and consistent vertical transmission (Nguyen et al., 2017). Strikingly, significant ocular pathology was noted in fetuses of dams infected during the first trimester (Nguyen et al., 2017). Persistent viremia and retinal and optic nerve pathology and visual dysfunction have been noted in pregnant women (Driggers et al., 2016; Meaney-Delman et al., 2016a; van der Eijk et al., 2016) and neonates (Costello et al., 2016), respectively. Similarly, infection of a single pregnant pigtail macaque resulted in in utero transmission with reduced growth of the fetal brain, white matter deficiency and gliosis, and axonal damage (Adams Waldorf et al., 2016). ZIKV RNA was detected in the chorionic villous tissue of the placenta as well as the fetal brain and liver, suggesting trans-placental transmission followed by ZIKV invasion and injury to the fetal brain. Although the dam showed no clinical signs of infection, ZIKV RNA was detected in the maternal brain, eyes, spleen, and liver (Adams Waldorf et al., 2016). These studies indicate that macaques can serve as a powerful model to investigate ZIKV pathogenesis in the developing fetus and can be used to elucidate the subtleties of CZS, in which microcephaly is the most severe of a range of possible sequelae.
Both field- and laboratory-based studies in other NHP species, particularly in New World monkeys, should be a research priority; the conditions exist for ZIKV to establish an enzootic monkey-mosquito cycle in the Americas, as occurred for YFV hundreds of years ago following its importation from Africa during the slave trade. The public health implications of sylvatic ZIKV would be complex, but establishment of an enzootic cycle would make elimination from the Americas next to impossible, and might increase human exposure in rural areas. Such studies will be vital for understanding potential reservoir hosts and the transmission dynamics in the Americas.
7. Current status of vaccine development (Chao Shan and Pei-Yong Shi)
In response to the recent ZIKV epidemics, vaccine candidates have been developed at an unprecedented pace. Four distinct approaches have been taken: subunit vaccines, inactivated vaccines, chimeric flavivirus vaccines, and live-attenuated vaccines. Among these candidates, subunit and inactivated vaccines have shown efficacy in both mice and NHPs, and several of these candidates have already advanced to phase 1-II clinical evaluation in humans (Barouch et al., 2017). Chimeric virus and live-attenuated vaccines have shown murine efficacy (Xie et al., 2017; Shan, 2017), but their efficacies in NHPs remains to be reported. These promising preclinical results are not surprising because similar approaches have been successful for development for other flavivirus vaccines. Clinically approved vaccines are currently available for four flavi-viruses, including (i) a live-attenuated vaccine for YFV (YFV 17D); (ii) an inactivated vaccine for tick-borne encephalitis virus; (iii) inactivated and live-attenuated (JEV SA 14-14-2) vaccines for Japanese encephalitis; chimeric lavivirus (YFV 17D backbone expressing JEV prM-E genes) vaccines for JEV; and (iv) chimeric flavivirus (YFV 17D backbone expressing DENV1-4 prM-E genes) vaccines for DENV (Shan et al., 2016a).
Subunit vaccines have been developed expressing ZIKV prM-E or M-E proteins using one of the three vectors, including plasmid DNA (Dowd et al., 2016b; Larocca et al., 2016), modified mRNA (Pardi et al., 2017; Richner et al., 2017), or adenovirus serotype 52 (Abbink et al., 2016). For the modified mRNA approach, RNA was in vitro-transcribed using 1-methylpseudouridine (instead of natural uridine to minimize the indiscriminate activation of innate immunity) and encapsulated within lipid nanoparticles for in vivo delivery. To minimize the potential adverse effect of cross-reactive Ab-mediated enhancement among ZIKV and other flaviviruses, the prM-E coding sequence was modified to eliminate the flavivirus-conserved fusion-loop epitope in the E protein; immunization of animals with this mRNA vaccine diminished the production of antibodies capable of enhancing DENV infection in cells and mice (Richner et al., 2017). For the plasmid DNA approach, two doses were needed to protect NHPs from ZIKV challenge. In contrast, a single dose of mRNA or adenovirus serotype 52-vectored vaccine conferred protection, among which a high dose of 50 μg of the mRNA vaccine elicited sterilizing immunity.
A purified inactivated vaccine using ZIKV strain PBVABC59 required two doses to confer protection in NHPs (Abbink et al., 2016). This inactivated vaccine is currently under joint development by the Walter Reed Army Institute of Research and Sanofi Pasteur file://localhost/(https/:www.statnews.com:pharmalot:2016:12:22:zika-vaccines-army-sanofi:). To increase the manufacture yield of this vaccine, Yang el al. engineered the ZIKV PBVABC59 strain with three Vero cell-adaptive mutations that could increase titers by 25–300-fold when cultured on Vero cells, which are an approved vaccine substrate (Yang et al., 2017). This technology has the potential to significantly reduce the cost of this promising vaccine.
A chimeric flavivirus vaccine uses available flavivirus vaccine backbones [e.g., YFV 17D or 3′untranslated genome region (UTR) deletion DENV vaccine] to express ZIKV prM-E genes. To support the feasibility of this approach, Xie et al. recently reported a chimeric DENV-2 containing the ZIKV prM-E genes that fully protected against WT ZIKV challenge in the A129 mouse model; reciprocally, a chimeric ZIKV containing the DENV-2 prM-E genes completely protected WT DENV-2 challenge (Xie et al., 2017). Along the same lines, Stephen Whitehead and colleagues are currently developing such chimeric vaccines using the 3′UTR deletion DENV vaccine backbones (personal communications). Together with the NIAID dengue vaccine currently in phase III clinical trials, the addition of the chimeric ZIKV vaccine may provide a dual dengue-and-Zika vaccine, which could be useful for populations living in regions endemic for both DENV and ZIKV.
A live-attenuated ZIKV vaccine has the advantages of single-dose immunization, a rapid and robust immune response, and potential long-lived protection. Using an infectious cDNA clone of Cambodian strain FSS13025 of ZIKV closely related to all American strains (Shan et al., 2016b), Shan et al. engineered a live-attenuated vaccine candidate with a 10-nucleotide deletion in the 3′UTR. This vaccine candidate elicited a sterilizing immunity and robust T cell response in mice after immunization with a single dose of 10 infectious particles. Besides potent efficacy, this live-attenuated vaccine candidate also exhibited reduced neurovirulence and other indications of a high safety profile (Shan, 2017). Although the correlation between protection against viremia and neutralizing Ab titers has been well established (Dowd et al., 2016b; Larocca et al., 2016), it is unknown whether sterilizing immunity and robust T cell response are required to avert trans-placental transmission of ZIKV during pregnancy. Answering this question will be critical for the development of a vaccine to protect against CZS. Two other questions critical to Zika vaccine development are: (i) whether antibody enhancement between the closely related DENV and ZIKV flaviviruses might result in the potential for vaccination to influence disease related to the other, and; (ii) how to eliminate the potential risk of vaccine-triggered GBS.
8. Current status of antiviral therapeutics (Raymond F. Schinazi, Christina Gavegnano, Bryan Cox, and Leda Bassit)
8.1. The need for safe, potent Zika virus antiviral agents
Recent estimates suggest that over 1.5 million individuals were infected with ZIKV during the American continental outbreak of 2014–2015 (WHO, 2015). As described above, ZIKV infection can result in neurodegenerative disorders, GBS, fetal abnormalities, and fetal microcephaly following infection of pregnant women (Faria et al., 2016; Driggers et al., 2016; Tang et al., 2016; Dang et al., 2016; Garcez et al., 2016; Muller and Miller, 2016). There are currently >3000 pregnant American women with laboratory signs of ZIKV infection (https://www.cdc.gov/zika/geo/pregwomen-uscases.html). To date, there are no FDA-approved drugs to treat ZIKV infection, and the spread of this virus and ongoing pandemic necessitates expeditious identification of novel antiviral agents.
8.2. Drug characteristics to treat or prevent ZIKV infection
Due to its distinct pathogenesis, ZIKV presents unique challenges in developing and identifying antiviral agents that are safe, potent, and specific to prevent and treat infection in pregnant women. An ideal antiviral drug will meet unique pharmacologic criteria as a preventative or treatment option (Fig. 2). First and foremost, the drug must be pregnancy category A/B, as these agents do not impair the growing fetus or compromise the maternal-fetal interface. The drug must potently inhibit ZIKV replication devoid of toxicity across all relevant and permissive cell types including fibroblast, epithelial, dendritic, liver, neuronal, and placental Hofbauer and trophoblast cells (Noronha et al., 2016; Hamel et al., 2015; Simoni et al., 2017; Quicke et al., 2016; Tang et al., 2016). An ideal drug would be orally bioavailable for maintenance dosing with an expansive distribution profile. In addition, one can envision a slow release, nanoformulated drug that could be administered once by injection to release active antiviral agent over the gestation period. The agent should accumulate in vivo at concentrations sufficient to eliminate viral replication, thereby mitigating development of resistant virus mutants. The innate antiviral type I interferon-response should ideally remain intact during therapy since these paracrine and autocrine cascades, and subsequent cellular activation, impact viral transmission across the placenta to the unborn fetus (Quicke et al., 2016; Bayer et al., 2016; Daffis et al., 2009; Grant et al., 2016; Munoz-Jordan and Fredericksen, 2010; Gavegnano et al., 2017). At the time of publication, several promising candidates have been identified that meet some of these criteria (Fig. 3) (Xu et al., 2016; Cheng et al., 2016; Cox et al., 2015; Goebel et al., 2016; Kincaid, 2016; Zmurko et al., 2016; Barrows et al., 2016).
Fig. 2.
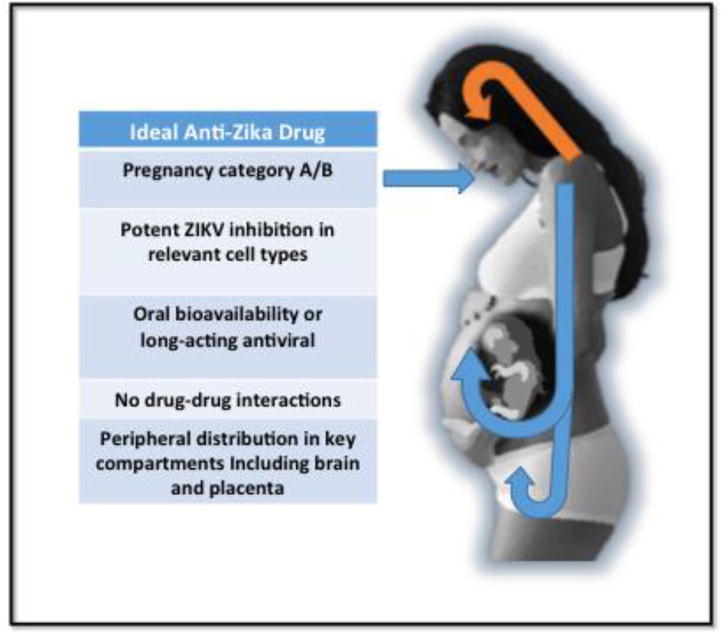
Characteristics of ideal anti-Zika drugs (see text).
Fig. 3.
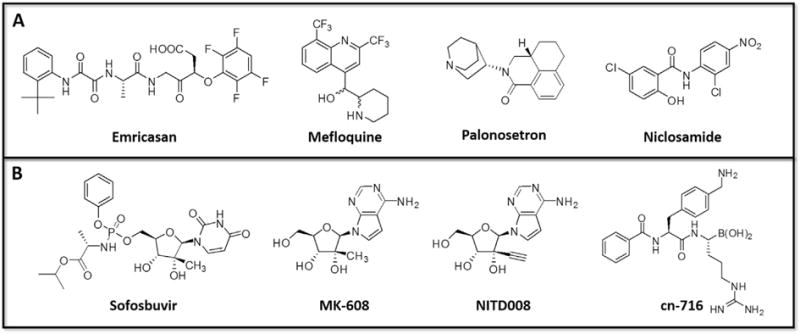
Chemical structures of reported ZIKV inhibitors. A) FDA-approved drugs or B) Novel antiviral agents.
8.3. Landscape of ZIKV inhibitors - repurposing efforts and limitations of identified agents
Drug repurposing offers the opportunity to expedite drug discovery. Barrow et al., reported 24 FDA-approved agents that inhibit ZIKV in Huh-7 hepatocytes and immortalized human stem cells (Barrows et al., 2016). Some of these are pregnancy category B drugs, like daptomycin, mefloquine, and palonosetron, but these compounds suffer from limited anti-ZIKV activity and fail to inhibit the virus in critical cell types. Xu et al. screened >6000 compounds, including >2000 FDA-approved agents, using caspase-3 activity followed by ZIKV protein expression and cell viability as a readout for antiviral potency (Xu et al., 2016). The screen identified niclosamide (a pregnancy category B drug) as a weak ZIKV inhibitor and emricasan as a potent inhibitor. Emricasan is a phase 2 inhibitor of caspase 3 under investigation for fatty acid liver disease. Although promising, it is unclear if long-term, steady-state plasma concentrations efficiently eliminate ZIKV. Additionally, it is uncertain if a capase-3 inhibitor with anti-inflammatory properties impacts development of the unborn fetus and ability to mount innate and adaptive immunity in vivo.
Novel ZIKV inhibitors that act as traditional, direct-acting antiviral agents have also been reported. Nucleoside analogs, like sofosbuvir and 7-deaza-2′-C-methyladenosine (MK-608), potently inhibit ZIKV replication in cellular assays and are efficacious in animal models (Zmurko et al., 2016). The recent crystal structure of the ZIKV polymerase, the target of nucleoside antiviral agents, will have great impact for the discovery of novel antiviral agents (Andre et al., 2007). Peptidomimetic agents like CN-716 inhibit the ZIKV protease in vitro, but only weakly inhibit viral replication due to poor cellular penetration [summarized in (Makhluf et al., 2016)]. Due to potential safety concerns, these compounds may not translate as therapeutic options for pregnant women, but could apply to other infected individuals.
8.4. Towards a ZIKV cure
Drug repurposing and discovery efforts have generated promising leads that block ZIKV replication. Elucidating treatment options for ZIKV requires understanding pharmacology, drug activity across relevant cell types, adequate drug distribution, safety, and intact immunity. Information obtained to date provides an excellent foundation for informed drug discovery efforts that address each of these factors in vitro and ex vivo. The ultimate result holds the promise of safe, specific, and potent antiviral agents that prevent transmission as pre- exposure prophylaxis or eradication of ZIKV infection.
9. Current status of diagnostic testing (Diogo M. Magnani, Michael Ricciardi, Esper G. Kallas, David I. Watkins)
While it is relatively straightforward to detect ZIKV nucleic acids during the acute phase in blood, urine, saliva, and semen, it has proven more difficult to design rapid and effective diagnostics for prior ZIKV exposure (Chua et al., 2017). This is reflected by the U.S. Food and Drug Administration (FDA) website where Emergency Use Authorization (EUA) has been obtained for 11 RNA-based assays and only two tests for IgM (FDA, 2017). Additionally, the Euroimmun kit detects IgM and IgG antibodies against the NS1 protein of ZIKV and has been approved by the Brazilian equivalent of the FDA, Agência Nacional de Vigilância Sanitária (ANVISA) (Huzly et al., 2016; Steinhagen et al., 2016). In patients who have received a flaviviral vaccine (DENV, YFV, or JEV) and/or have been infected with any flaviviruses in the past, some of these IgM assays may be difficult to interpret due to the cross-reactivity (Rabe et al., 2016; Huzly et al., 2016; Fields et al., 2007; Barba-Spaeth et al., 2016; Stettler et al., 2016; Speer and Pierson, 2016). Thus, a positive IgM test needs to be confirmed with a laborious plaque reduction neutralization test (PRNT), which itself may not even have sufficient specificity for persons who have been infected with multiple flaviviruses. To replace the labor- intensive PRNT assay, a reporter virus system was recently developed to allow high-throughput and rapid quantification of neutralizing antibody titers for ZIKV and DENV (Shan et al., 2017). IgM antibodies persist for 2–12 weeks in serum, and sera from individuals previously infected for more than 12 weeks would also need attempted confirmation with a virus neutralization-based method (Rabe et al., 2016). Additionally, since it is thought that the majority of ZIKV infections are asymptomatic (Musso and Gubler, 2016), RNA- and IgM-based assays are only used for symptomatic infections and may therefore underestimate the actual number of infected individuals.
Detection of prior ZIKV infection, especially in dengue endemic areas, is therefore exceedingly difficult after 12 weeks post-infection and there are no FDA Emergency Use Authorized tests for detection of Zika-specific IgG after the acute phase. Indeed, Eurimmun offers the only kit for detection of Zika-specific IgG. The high levels of Ab cross-reactivity among flaviviruses pose a formidable challenge for specific infection diagnosis in the chronic phase. A multiplex microsphere immunoassay using DENV and ZIKV E, NS1, and NS5 proteins was shown to improve diagnostic accuracy (Wong et al., 2017). More recently a nanotechnology platform has been developed to detect IgG avidity against the NS1 protein of the ZIKV and this has been submitted to the FDA for EUA (Zhang et al., 2017).
A new assay for the detection of ZIKV-specific IgG responses during the chronic phase was recently developed. This assay appears to be specific even in DENV-exposed individuals. The Z-Quick Test (Fig. 4) has been validated in more than 280 individuals from several cohorts, including 107 blinded samples from dengue-endemic regions. This test can detect previous ZIKV exposure at two weeks or later post onset of symptoms or three weeks or later after asymptomatic infection. A human IgG mAb P1F12 was isolated from a ZIKV-infected individual (who developed GBS) in Brazil. Remarkably, despite being entirely germline-encoded, P1F12 exhibits high affinity and specificity for ZIKV and does not cross-react with any of the four DENV serotypes (Magnani et al., 2017). It appears that all ZIKV-infected individuals mount Ab responses against the epitope recognized by P1F12. This epitope is recognized by Abs in individuals previously infected by ZIKV, thereby preventing the binding of P1F12 to intact ZIKV. By contrast, Abs in the plasma from individuals previously infected by any of the DENV serotypes, do not prevent binding of P1F12. P1F12 may, therefore have potential as a diagnostic.
Fig. 4.
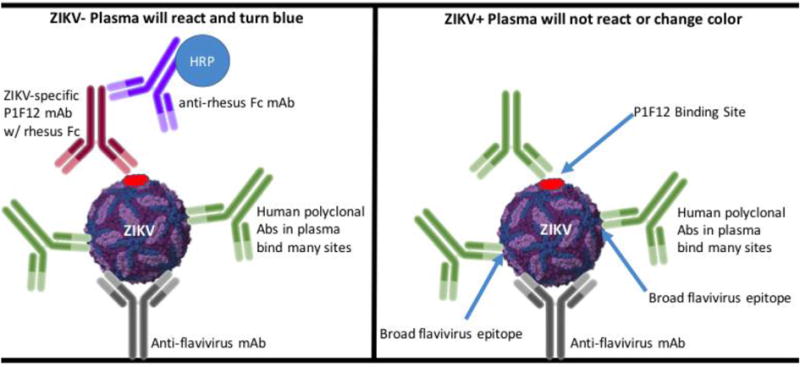
Z-Quick Test overview (Magnani et al., 2017). Ninety-six-well ELISA plates are coated with an anti-flavivirus mAb overnight. The next day, plates are washed with PBS-T and block with 5% non-fat milk for 1 h at 37 °C. The plates are then washed, ZIKV is added to each well, and the plates are incubated at room temperature for 1 h. Plates are washed again, patient plasma is added to wells, and the plates are incubated for 1 h at 37 °C. The plates are then washed, the P1F12 ZIKV-specific antibody is added, and incubated for 1 h at 37 °C. During this step, if the patient was exposed to ZIKV, the antibodies in the patient’s plasma should block the binding of the P1F12 mAb. Next, the plates are washed, a HRP detection antibody is added, and the plates are incubated at 37 °C for 1 h. Lastly, the wells are washed, TMB is used to develop the HRP, and the wells are read using a spectrophotometer.
More tests to detect ZIKV-specific antibodies are urgently needed, as extensive epidemiological studies should be carried out in stored and previously collected cohort samples. More importantly, we need to provide tools for the decision-making process and counseling of pregnant women in areas at risk for ZIKV epidemics.
10. Current understanding of Zika emergence mechanisms (Scott C. Weaver, Andrew Haddow)
The reasons for the sudden and dramatic Zika epidemic in the Americas remain poorly understood. Undoubtedly increases in air travel with ever-increasing urbanization of the neotropics and reinfestation of rapidly expanding cities, combined with completely naïve populations provided ideal conditions for efficient ZIKV transmission and spread. Prolonged sexual transmission, unique among arboviruses, may also be contributing to transmission.
The epidemic in the Americas may also have been facilitated by changes in ZIKV virulence mediated by increased viremia or placental tropism, although there is no direct evidence for this; in fact, some African ZIKV strains are more virulent in murine models than strains from the Americas (Rossi et al., 2016). Urban vector-adaptive evolution is suggested by recent studies of an NS1 substitution that enhances Ae. aegypti infection (Liu et al., 2017), but other studies with ZIKV strains differing in this substitution have not identified comparable differences (Weger-Lucarelli et al., 2016; Roundy et al., 2017). Additional studies with wild or low-generation, colonized mosquito populations are needed to further evaluate this NS1 change.
The competing hypothesis for phenotypic changes in ZIKV, which accompanied its introduction into the Americas, enhancing transmission or pathogenesis, is simply a stochastic introduction into a region primed for explosive transmission and spread. If cases of microcephaly linked to ZIKV infection reported in Thailand and Vietnam (WHO, 2016b) are confirmed and are representative of Asian strain virulence, and endemic circulation maintains herd immunity sufficient to prevent major epidemics, no adaptive evolution would be needed to explain the 2015–2017 epidemic in the Americas. Better surveillance in Asia and Africa, facilitated by improved serodiagnostics, should eventually lead to answers to these questions.
11. The future of the pandemic (Scott C. Weaver, Andrew Haddow)
The very limited information on the levels of circulation and geographic range of ZIKV in the Old World, and the lack of seroprevalence data from most of the Americas due to the serodiagnostic limitations described above in section 9, place major limitations on our ability to predict the future of the ongoing ZIKV epidemic and to assess current and future endemic transmission, including potential spillover from enzootic circulation as discussed above. A combination of spatial analyses and modeling indicated that over 1 million pregnant women and nearly 100 million persons living in the Americas could be infected during the current epidemic, suggesting tens of thousands of cases of CZS (Perkins et al., 2017). However, the trajectory of the American outbreak before herd immunity slows transmission, and the level of residual endemic exposure during the coming years is difficult to predict because basic parameters needed to accurately model transmission (e.g. minimum extrinsic incubation period in Ae. aegypti, length and magnitude of infectious viremia in humans, typical vector longevity) are lacking. These include vector transmission efficiency (or the R0 of typical infections) because the typical pattern of human infectious viremia remains poorly characterized (most studies only measure RNA genome copies and not infectious titers, and recently identified cell-associated ZIKV that persists in blood for many weeks (Mansuy et al., 2017) may or may not be infectious for mosquito vectors) and the contribution of sexual and other forms of direct transmission are difficult to estimate as discussed above. Although transmission efficiency may differ based on viremia levels, vector competence and sexual transmission, the endemicity of the 4 DENV serotypes in the Americas for many decades suggests that ZIKV will continue to circulate in the human transmission cycle for the foreseeable future.
The recent arrival of CHIKV in the Americas, with epidemic circulation beginning about 18 months before that of ZIKV was detected, may provide additional clues regarding the future of ZIKV. Although exact comparisons of transmission efficiency are difficult due to limited data like those mentioned above, CHIKV and ZIKV are believed to have identical Ae. aegypti-borne human transmission cycles in the Americas. After its detection in the Caribbean in late 2013, epidemic CHIKV circulation peaked there the following summer, followed by a dramatic decline in reported cases in 2015. Combined with data on a 2015 reduction in imported cases in the U.S., which may serve as sentinels for levels of circulation in the Caribbean and Latin America, these results suggest that the ZIKV epidemic may typically peak regionally within one year. Although some regions of South America have not yet experienced major epidemics and thus may not yet have seen their peak of transmission, the ZIKV epidemic may be subsiding in some regions of the Americas.
Some models suggest that the cessation of American ZIKV epidemics could be followed by relatively low incidence of infection and disease for several decades (Ferguson et al., 2016). However, the experience in India with CHIKV, where a major 2006–2007 epidemic after decades of absence was followed in 2016 by another wave of infections, suggests that herd immunity during the initial Indian CHIKV epidemic did not reach levels sufficient to inhibit transmission for these long time periods. Until levels of ZIKV herd immunity can be accurately measured serologically using improved assays less subject to flavivirus cross-reactions, it will remain difficult to predict future levels of endemic circulation.
The risk for a major ZIKV epidemic in Asia, even if American strains are found to be more transmissible or virulent for congenital infections, may be limited by long-term endemic exposure there, as discussed above. If endemic circulation in Asia and also probably Africa is maintained at levels that provide relatively stable herd immunity, outbreaks on the scale seen in completely naïve populations of the Americas may not be possible, and many women may become immune prior to pregnancy. Levels of CHIKV herd immunity measured in the Philippines, which are estimated to range from approximately 20-50% over time (Salje et al., 2016), suggest such a scenario for ZIKV. Although estimates of ZIKV infection incidence in Cambodia during 2007 were only about 1/8 those of DENV (Duong et al., 2017), this equates to about ½ that of each DENV serotype, still indicating extensive exposure considering the high levels of DENV hyperendemicity there. However, only with better surveillance facilitated by improved serodiagnostics will the data needed to understand ZIKV epidemiology and predict future trends become available.
12. Concluding remarks (Scott Weaver)
The ZIKV epidemic in Oceania and the Americas and the discovery since 2013 of severe outcomes of infection including GBS and CZS have triggered remarkable advances in understanding the transmission, spread and adverse outcomes of infection. They have also driven unprecedented, rapid progress in animal model development, as well as in vaccine and therapeutic discovery with several Phase I-II clinical trials already completed. However, critical questions regarding the cause of the ongoing outbreak of CZS remain, especially:
Did the emergence occur due to changes pathogenicity or transmissibility in the ZIKV strain that spread into French Polynesia followed by the Americas, or did the outbreak occur following a stochastic series of introductions into naïve populations followed by air travel and mosquito-borne amplification?
Why were rates of CZS apparently higher in northeastern Brazil than in other regions of Latin American and the Caribbean?
Does immunity from prior flavivirus exposure affect ZIKV pathogenesis and the risk for fetal infection in pregnant women?
Will vaccines and therapeutics to prevent and control ZIKV infection become widely available considering the scientific, financial and logistic challenges?
Can ZIKV and other emerging, urban arboviruses such as DENV, CHIKV and YFV be controlled through improvements in traditional vector control programs, new approaches using genetically modified mosquitoes, Wolbachia bacteria, lethal trapping, combinations of these methods with vaccines?
Novel strategies are needed to contain ZIKV spread in the Americas and around the world. Better tools to understand virus spread, such as more reliable, specific, inexpensive, and high-throughput, point-of-care serology assays, and the development of highly efficacious vaccines can enhance nearly all control methods under development. Hopefully, support for ZIKV research in all of these areas will be better sustained than in the past so that we will be better prepared to anticipate and respond to reemerging arboviruses such as CHIKV, DENV and YFV, as well as similar arboviruses yet to emerge.
Acknowledgments
Work from the authors’ laboratories was funded by in part by U.S. National Institutes of Health grants: R01NS087539-S1 (to DEG), R21AI129607 (to RFS), and R24AI120942, R01AI121452 (to SCW) R01 AI107157-01A1 (to TGG), R01AI116382-01A1S1 (to DHO), U01AI115577 (to NV) and P51 OD011106 (to the WNPRC).
References
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353(6304):1129–1132. doi: 10.1126/science.aah6157. http://dx.doi.org/10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22(11):1256–1259. doi: 10.1038/nm.4193. http://dx.doi.org/10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell. 2017;169(4):610–620. doi: 10.1016/j.cell.2017.04.008. http://dx.doi.org/10.1016/j.cell.2017.04.008e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoua-Koffi C, Diarrassouba S, Benie VB, Ngbichi JM, Bozoua T, Bosson A, et al. Investigation surrounding a fatal case of yellow fever in Cote d’Ivoire in 1999. Bull Soc Pathol Exot. 2001;94(3):227–230. [PubMed] [Google Scholar]
- Alera MT, Hermann L, Tac-An IA, Klungthong C, Rutvisuttinunt W, Manasatienkij W, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis. 2015;21(4):722–724. doi: 10.3201/eid2104.141707. http://dx.doi.org/10.3201/eid2104.141707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Gugliemini O, Harber S, Harrison A, Houle L, Ivory J, et al. Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLoS Neglected Trop Dis. 2017;11(2):e0005135. doi: 10.1371/journal.pntd.0005135. http://dx.doi.org/10.1371/journal.pntd.0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, et al. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS Neglected Trop Dis. 2016a;10(12):e0005168. doi: 10.1371/journal.pntd.0005168. http://dx.doi.org/10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg Infect Dis. 2016b;22(10):1857–1859. doi: 10.3201/eid2210.161082. http://dx.doi.org/10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Correction: characterization of lethal Zika virus infection in AG129 mice. PLoS Neglected Trop Dis. 2016c;10(5):e0004750. doi: 10.1371/journal.pntd.0004682. http://dx.doi.org/10.1371/journal.pntd.0004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of lethal Zika virus infection in AG129 mice. PLoS Neglected Trop Dis. 2016d;10(4):e0004682. doi: 10.1371/journal.pntd.0004682. http://dx.doi.org/10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse BM, Vasilakis N, Sall AA, Diallo M, Weaver SC, Hanley KA. Potential for Zika virus to establish a sylvatic transmission cycle in the Americas. PLoS Negl Trop Dis. 2016;10(12):e0005055. doi: 10.1371/journal.pntd.0005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amraoui F, Atyame-Nten C, Vega-Rua A, Lourenco-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016;21(35) doi: 10.2807/1560-7917.ES.2016.21.35.30333. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre S, Godoy Gustavo MA, Lima Ketllyn IZ, Oliveira Naiara U, Torres Fernando V, Maluf Rafael VC, Guido Glaucius, Oliva Crystal structure of Zika virus NS5 RNA-dependent RNA polymerase. Nat Commun. 2017 Mar;8:14764. doi: 10.1038/ncomms14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo TV, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo AP, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16(12):1356–1363. doi: 10.1016/S1473-3099(16)30318-8. http://dx.doi.org/10.1016/S1473-3099(16)30318-8. [DOI] [PubMed] [Google Scholar]
- Azar Sasha R, Stark Pamela M, Debboun Mustapha, Vela Jeremy, Roundy Christopher M, Rossi Shannan L, Nava Martin R, Hanley Kathryn A, Ribeiro Guilherme S, Kitron Uriel, Yun Ruimei, Huang Jing H, Fernandez-Salas Ildefonso, Leal Grace, Vasilakis Nikos, Weaver Scott C, Vitek Christopher J, Paploski Igor AD. Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. Am J Trop Med Hyg. 2017 doi: 10.4269/ajtmh.16-0969. Online 08 May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi SA, Das S, Chatterjee S. Seasonal prevalence and blood meal analysis of filarial vector Culex quinquefasciatus in coastal areas of Digha, West Bengal, India. J Vector Borne Dis. 2015;52(3):252–256. [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016 doi: 10.1038/nature18938. http://dx.doi.org/10.1038/nature18938. [DOI] [PubMed]
- Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016;56(7):1684–1688. doi: 10.1111/trf.13681. http://dx.doi.org/10.1111/trf.13681. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Thomas SJ, Michael NL. Prospects for a Zika virus vaccine. Immunity. 2017;46(2):176–182. doi: 10.1016/j.immuni.2017.02.005. http://dx.doi.org/10.1016/j.immuni.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20(2):259–270. doi: 10.1016/j.chom.2016.07.004. http://dx.doi.org/10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin E, Lavezzo E, et al. Isolation of infectious Zika virus from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy, January 2016. Euro Surveill. 2016;21(10):30159. doi: 10.2807/1560-7917.ES.2016.21.10.30159. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.10.30159. [DOI] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19(5):705–712. doi: 10.1016/j.chom.2016.03.008. http://dx.doi.org/10.1016/j.chom.2016.03.008. Epub 2016/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50(5):442–448. [PubMed] [Google Scholar]
- Bearcroft WGC. The histopathology of the liver of yellow fever-infected rhesus monkeys. J Pathol. 1957;74(2):295–303. [Google Scholar]
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35(2):183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- Berthet N, Nakoune E, Kamgang B, Selekon B, Descorps-Declere S, Gessain A, et al. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14(12):862–865. doi: 10.1089/vbz.2014.1607. http://dx.doi.org/10.1089/vbz.2014.1607. [DOI] [PubMed] [Google Scholar]
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13) [PubMed] [Google Scholar]
- Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastere S, Bost-Bezeaud F, Marcelis L, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill. 2016;21(13) doi: 10.2807/1560-7917.ES.2016.21.13.30181. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.13.30181. [DOI] [PubMed] [Google Scholar]
- Boccolini D, Toma L, Di Luca M, Severini F, Romi R, Remoli ME, et al. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro Surveill. 2016;21(35) doi: 10.2807/1560-7917.ES.2016.21.35.30328. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.35.30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko GI, Burleigh DW, Durning M, Breburda EE, Grendell RL, Golos TG. Passive immunization against the MHC class I molecule Mamu-AG disrupts rhesus placental development and endometrial responses. J Immunol. 2007;179(12):8042–8050. doi: 10.4049/jimmunol.179.12.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50(3):238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- Bowen JR, Quicke KM, Maddur MS, O’Neal JT, McDonald CE, Fedorova NB, et al. Zika virus antagonizes type I interferon responses during infection of human dendritic cells. PLoS Pathog. 2017;13(2):e1006164. doi: 10.1371/journal.ppat.1006164. http://dx.doi.org/10.1371/journal.ppat.1006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. doi: 10.1056/NEJMoa1602412. http://dx.doi.org/10.1056/NEJ-Moa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault JB, Khou C, Basset J, Coquand L, Fraisier V, Frenkiel MP, et al. Comparative analysis between flaviviruses reveals specific neural stem cell tropism for zika virus in the mouse developing neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. http://dx.doi.org/10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito CA, Cordeiro MT. One year after the Zika virus outbreak in Brazil: from hypotheses to evidence. Rev Soc Bras Med Trop. 2016;49(5):537–543. doi: 10.1590/0037-8682-0328-2016. http://dx.doi.org/10.1590/0037-8682-0328-2016. [DOI] [PubMed] [Google Scholar]
- Brito CA, Brito CC, Oliveira AC, Rocha M, Atanasio C, Asfora C, et al. Zika in Pernambuco: rewriting the first outbreak. Rev Soc Bras Med Trop. 2016;49(5):553–558. doi: 10.1590/0037-8682-0245-2016. http://dx.doi.org/10.1590/0037-8682-0245-2016. [DOI] [PubMed] [Google Scholar]
- Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, et al. Zika virus as a cause of neurologic disorders. N Engl J Med. 2016;374(16):1506–1509. doi: 10.1056/NEJMp1602708. http://dx.doi.org/10.1056/NEJMp1602708. [DOI] [PubMed] [Google Scholar]
- Buathong R, Hermann L, Thaisomboonsuk B, Rutvisuttinunt W, Klungthong C, Chinnawirotpisan P, et al. Detection of zika virus infection in Thailand, 2012-2014. Am J Trop Med Hyg. 2015;93(2):380–383. doi: 10.4269/ajtmh.15-0022. http://dx.doi.org/10.4269/ajtmh.15-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213(4):449. doi: 10.1016/j.ajog.2015.08.032. http://dx.doi.org/10.1016/j.ajog.2015.08.032 e1-e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler CR, Bailey AL, Weiler AM, Barry GL, Breitbach ME, Stewart LM, et al. Seroprevalence of zika virus in wild African green monkeys and baboons. mSphere. 2017;2(2) doi: 10.1128/mSphere.00392-16. http://dx.doi.org/10.1128/mSphere.00392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Johnson PM. Macrophage populations in the human placenta and amniochorion. Clin Exp Immunol. 1984;57(2):393–403. [PMC free article] [PubMed] [Google Scholar]
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653–660. doi: 10.1016/S1473-3099(16)00095-5. http://dx.doi.org/10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- Cardoso CW, Paploski IA, Kikuti M, Rodrigues MS, Silva MM, Campos GS, et al. Outbreak of exanthematous illness associated with zika, Chi-kungunya, and dengue viruses, salvador, Brazil. Emerg Infect Dis. 2015;21(12):2274–2276. doi: 10.3201/eid2112.151167. http://dx.doi.org/10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):249–257. doi: 10.1016/j.bpobgyn.2010.10.010. http://dx.doi.org/10.1016/j.bpobgyn.2010.10.010. [DOI] [PubMed] [Google Scholar]
- de Carvalho GC, Malafronte Rdos S, Miti Izumisawa C, Souza Teixeira R, Natal L, Marrelli MT. Blood meal sources of mosquitoes captured in municipal parks in Sao Paulo, Brazil. J Vector Ecol. 2014;39(1):146–152. doi: 10.1111/j.1948-7134.2014.12081.x. http://dx.doi.org/10.1111/j.1948-7134.2014.12081.x. [DOI] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387(10033):2125–2132. doi: 10.1016/S0140-6736(16)00651-6. http://dx.doi.org/10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti MG, Cabral-Castro MJ, Goncalves JL, Santana LS, Pimenta ES, Peralta JM. Zika virus shedding in human milk during lactation: an unlikely source of infection? Int J Infect Dis. 2017;57:70–72. doi: 10.1016/j.ijid.2017.01.042. http://dx.doi.org/10.1016/j.ijid.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Zika Virus CDC. Transmission and Risks: CDC; [cited 2017 May 16 2017] Available from: https://www.cdc.gov/zika/transmission/index.html.
- Chen JC, Wang Z, Huang H, Weitz SH, Wang A, Qiu X, et al. Infection of human uterine fibroblasts by Zika virus in vitro: implications for viral transmission in women. Int J Infect Dis. 2016;51:139–140. doi: 10.1016/j.ijid.2016.07.015. http://dx.doi.org/10.1016/j.ijid.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Cheng F, Murray JL, Rubin DH. Drug repurposing: new treatments for zika virus infection? Trends Mol Med. 2016;22(11):919–921. doi: 10.1016/j.molmed.2016.09.006. http://dx.doi.org/10.1016/j.molmed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Chimelli L, Melo AS, Avvad-Portari E, Wiley CA, Camacho AH, Lopes VS, et al. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1699-5. http://dx.doi.org/10.1007/s00401-017-1699-5. [DOI] [PubMed]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to zika virus. PLoS Neglected Trop Dis. 2016;10(3):e0004543. doi: 10.1371/journal.pntd.0004543. http://dx.doi.org/10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua A, Prat I, Nuebling CM, Wood D, Moussy F. Update on zika diagnostic tests and WHO’s related activities. PLoS Neglected Trop Dis. 2017;11(2):e0005269. doi: 10.1371/journal.pntd.0005269. http://dx.doi.org/10.1371/journal.pntd.0005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey LL, Pesavento PA, Keesler RI, Singapuri A, Watanabe J, Watanabe R, et al. Zika virus tissue and blood compartmentalization in acute infection of rhesus macaques. PloS One. 2017;12(1):e0171148. doi: 10.1371/journal.pone.0171148. http://dx.doi.org/10.1371/journal.pone.0171148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet M, Robin Y, Chateau R, Heme G, Adam C. Isolementd’arbovirus au Senegal oriental a partir de moustiques (1972–1977) et note surl’epide-miologie des virus transmis par les Aedes, en particulier du virus amaril. ORSTOM, Entomol Med Parasitol. 1979a;17:149–163. [Google Scholar]
- Cornet M, Robin Y, Adam C, Valade M, Calvo M. Comparison between experimental transmission of yellow fever and zika viruses in Aedes aegypti. Cah ORSTOM ser Ent med Parasitol. 1979b;17:47–53. [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, et al. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6:35296. doi: 10.1038/srep35296. http://dx.doi.org/10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A, Dua T, Duran P, Gulmezoglu M, Oladapo OT, Perea W, et al. Defining the syndrome associated with congenital Zika virus infection. Bull. World Health Organ. 2016;94(6):406–A. doi: 10.2471/BLT.16.176990. http://dx.doi.org/10.2471/BLT.16.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BD, Stanton RA, Schinazi RF. Predicting Zika virus structural biology: challenges and opportunities for intervention. Antivir Chem Chemother. 2015;24(3–4):118–126. doi: 10.1177/2040206616653873. http://dx.doi.org/10.1177/2040206616653873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas EL, Tong VT, Rozo N, Valencia D, Pacheco O, Gilboa SM, et al. Preliminary report of microcephaly potentially associated with zika virus infection during pregnancy - Colombia, January-November 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1409–1413. doi: 10.15585/mmwr.mm6549e1. http://dx.doi.org/10.15585/mmwr.mm6549e1. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–271. doi: 10.1038/nature18296. http://dx.doi.org/10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjat M, Darling SE, Nerurkar VR, Ching N, Kumar M, Min SK, et al. Clinical and imaging findings in an infant with zika embryopathy. Clin Infect Dis. 2016;63(6):805–811. doi: 10.1093/cid/ciw324. http://dx.doi.org/10.1093/cid/ciw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MS, Esposito DL, Rocco IM, Maeda AY, Vasami FG, Nogueira JS, et al. First complete genome sequence of zika virus (flaviviridae, flavivirus) from an autochthonous transmission in Brazil. Genome Announc. 2016;4(2) doi: 10.1128/genomeA.00032-16. http://dx.doi.org/10.1128/genomeA.00032-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of zika virus. N Engl J Med. 2016;374(22):2195–2198. doi: 10.1056/NEJMc1604449. http://dx.doi.org/10.1056/NEJMc1604449. Epub 2016/04/13. [DOI] [PubMed] [Google Scholar]
- Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 2009;5(10):e1000607. doi: 10.1371/journal.ppat.1000607. http://dx.doi.org/10.1371/journal.ppat.1000607. Epub 2009/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19(2):258–265. doi: 10.1016/j.stem.2016.04.014. http://dx.doi.org/10.1016/j.stem.2016.04.014. Epub 2016/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of zika virus - New York city, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(28):716–717. doi: 10.15585/mmwr.mm6528e2. http://dx.doi.org/10.15585/mmwr.mm6528e2. Epub 2016/07/22. [DOI] [PubMed] [Google Scholar]
- Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-Male sexual transmission of zika Virus–Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65(14):372–374. doi: 10.15585/mmwr.mm6514a3. http://dx.doi.org/10.15585/mmwr.mm6514a3. Epub 2016/04/15. [DOI] [PubMed] [Google Scholar]
- Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PloS One. 2014;9(10):e109442. doi: 10.1371/journal.pone.0109442. http://dx.doi.org/10.1371/journal.pone.0109442. Epub 2014/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. Epub 1952/09/01. [DOI] [PubMed] [Google Scholar]
- Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for zika virus infection. PLoS Neglected Trop Dis. 2016;10(5):e0004658. doi: 10.1371/journal.pntd.0004658. http://dx.doi.org/10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, et al. Broadly neutralizing activity of zika virus-immune sera identifies a single viral serotype. Cell Rep. 2016a;16(6):1485–1491. doi: 10.1016/j.celrep.2016.07.049. http://dx.doi.org/10.1016/j.cel-rep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016b;354(6309):237–240. doi: 10.1126/science.aai9137. http://dx.doi.org/10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper CC. Infection with the Chuku strain of Spondweni virus. West Afr Med J. 1965;14:16–19. Epub 1965/02/01. [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142–2151. doi: 10.1056/NEJMoa1601824. http://dx.doi.org/10.1056/NEJMoa1601824. Epub 2016/03/31. [DOI] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. http://dx.doi.org/10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. http://dx.doi.org/10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, et al. Frequent zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep. 2017;18(7):1751–1760. doi: 10.1016/j.celrep.2017.01.056. http://dx.doi.org/10.1016/j.celrep.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V, Ong S, Leang R, Huy R, Ly S, Mounier U, et al. Low circulation of zika virus, Cambodia, 2007-2016. Emerg Infect Dis. 2017;23(2):296–299. doi: 10.3201/eid2302.161432. http://dx.doi.org/10.3201/eid2302.161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mogling R, van Kampen JJ, et al. Miscarriage associated with zika virus infection. N Engl J Med. 2016;375(10):1002–1004. doi: 10.1056/NEJMc1605898. http://dx.doi.org/10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- Enders AC. Implantation in the macaque: expansion of the implantation site during the first week of implantation. Placenta. 2007;28(8–9):794–802. doi: 10.1016/j.placenta.2006.11.001. http://dx.doi.org/10.1016/j.placenta.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Enders AC, Blankenship TN, Fazleabas AT, Jones CJ. Structure of anchoring villi and the trophoblastic shell in the human, baboon and macaque placenta. Placenta. 2001;22(4):284–303. doi: 10.1053/plac.2001.0626. http://dx.doi.org/10.1053/plac.2001.0626. [DOI] [PubMed] [Google Scholar]
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepide-miological investigations in Oyo State. J Hyg (Lond) 1979;83(2):213–219. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria NR, Azevedo Rdo S, Kraemer MU, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352(6283):345–349. doi: 10.1126/science.aaf5036. http://dx.doi.org/10.1126/science.aaf5036. Epub 2016/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Morens DM. Zika virus in the Americas–yet another arbovirus threat. N Engl J Med. 2016;374(7):601–604. doi: 10.1056/NEJMp1600297. http://dx.doi.org/10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Neglected Trop Dis. 2014;8(1):e2636. doi: 10.1371/journal.pntd.0002636. http://dx.doi.org/10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Zika Virus Emergency Use Authorization. 2017 Mar 10; Available from: https://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm-zika.
- Ferguson NM, Cucunuba ZM, Dorigatti I, Nedjati-Gilani GL, Donnelly CA, Basanez MG, et al. EPIDEMIOLOGY. Countering the Zika epidemic in Latin America. Science. 2016;353(6297):353–354. doi: 10.1126/science.aag0219. http://dx.doi.org/10.1126/science.aag0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RM, Barbosa da Silva KA, Castro MG, et al. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS Neglected Trop Dis. 2016;10(9):e0004993. doi: 10.1371/journal.pntd.0004993. http://dx.doi.org/10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM. Fields virology. fifth. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. http://dx.doi.org/10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeschl G, Huber K, von Sonnenburg F, Nothdurft HD, Bretzel G, Hoelscher M, et al. Long-term kinetics of Zika virus RNA and antibodies in body fluids of a vasectomized traveller returning from Martinique: a case report. BMC Infect Dis. 2017;17(1):55. doi: 10.1186/s12879-016-2123-9. http://dx.doi.org/10.1186/s12879-016-2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, et al. Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell. 2017;20(3):397–406. doi: 10.1016/j.stem.2016.12.005. http://dx.doi.org/10.1016/j.stem.2016.12.005 e5. [DOI] [PubMed] [Google Scholar]
- Galel SA, Williamson PC, Busch MP, Stanek D, Bakkour S, Stone M, et al. First Zika-positive donations in the continental United States. Transfusion. 2017 doi: 10.1111/trf.14029. http://dx.doi.org/10.1111/trf.14029. Epub 2017/02/05. [DOI] [PubMed]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–818. doi: 10.1126/science.aaf6116. http://dx.doi.org/10.1126/science.aaf6116. Epub 2016/04/12. [DOI] [PubMed] [Google Scholar]
- Gavegnano C, Bassit LC, Cox BD, Hsiao HM, Johnson EL, Suthar M, Chakraborty R, Schinazi RF. Jak Inhibitors Modulate Production of Replication-Competent Zika Virus in Human Hofbauer, Trophoblasts and Neuroblastoma cells. Pathogens and Immunity. 2017;2:199–203. doi: 10.20411/pai.v2i2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S, Snyder B, Sellati T, Saeed M, Ptak R, Murray M, et al. A sensitive virus yield assay for evaluation of Antivirals against Zika Virus. J Virol Methods. 2016;238:13–20. doi: 10.1016/j.jviromet.2016.09.015. http://dx.doi.org/10.1016/j.jviromet.2016.09.015. Epub 2016/10/23. [DOI] [PubMed] [Google Scholar]
- Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental Major Histocompatibility Complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol. 2010;54(2–3):431–443. doi: 10.1387/ijdb.082797tg. http://dx.doi.org/10.1387/ijdb.082797tg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540(7633):438–442. doi: 10.1038/nature20556. http://dx.doi.org/10.1038/nature20556. Epub 2016/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19(6):882–890. doi: 10.1016/j.chom.2016.05.009. http://dx.doi.org/10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Neglected Trop Dis. 2014;8(2):e2681. doi: 10.1371/journal.pntd.0002681. http://dx.doi.org/10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, et al. Outbreak of zika virus infection, Chiapas state, Mexico, 2015 and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis. 2016 doi: 10.1093/infdis/jiw302. http://dx.doi.org/10.1093/infdis/jiw302. [DOI] [PMC free article] [PubMed]
- Gulland A. Continued spread of Zika raises many research questions, WHO says. BMJ. 2016;354:i4812. doi: 10.1136/bmj.i4812. http://dx.doi.org/10.1136/bmj.i4812. [DOI] [PubMed] [Google Scholar]
- Guo XX, Li CX, Wang G, Zheng Z, Dong YD, Zhang YM, et al. Host feeding patterns of mosquitoes in a rural malaria-endemic region in hainan island, China. J Am Mosq Control Assoc. 2014;30(4):309–311. doi: 10.2987/14-6439R.1. http://dx.doi.org/10.2987/14-6439R.1. [DOI] [PubMed] [Google Scholar]
- Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5(9):e102. doi: 10.1038/emi.2016.102. http://dx.doi.org/10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Woodall JP. Distinguishing between zika and Spondweni viruses. Bull World Health Organ. 2016;94(10):711–711A. doi: 10.2471/BLT.16.181503. http://dx.doi.org/10.2471/BLT.16.181503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve isolations of zika virus from Aedes (stegomyia) africanus (theobald) taken in and above a Uganda forest. Bull World Health Organ. 1964;31:57–69. Epub 1964/01/01. [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Neglected Trop Dis. 2012;6(2):e1477. doi: 10.1371/journal.pntd.0001477. http://dx.doi.org/10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Nasar F, Guzman H, Ponlawat A, Jarman RG, Tesh RB, et al. Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni virus. PLoS Neglected Trop Dis. 2016;10(10):e0005083. doi: 10.1371/journal.pntd.0005083. http://dx.doi.org/10.1371/journal.pntd.0005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Mendelin S, Pyke AT, Moore PR, Mackay IM, McMahon JL, Ritchie SA, et al. Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of zika virus in Australia. PLoS Neglected Trop Dis. 2016;10(9):e0004959. doi: 10.1371/journal.pntd.0004959. http://dx.doi.org/10.1371/journal.pntd.0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of zika virus infection in human skin cells. J Virol. 2015;89(17):8880–8896. doi: 10.1128/JVI.00354-15. http://dx.doi.org/10.1128/JVI.00354-15. Epub 2015/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer DH, Barbre KA, Chen LH, Grobusch MP, Schlagenhauf P, Goorhuis A, et al. Travel-associated zika virus disease acquired in the Americas through February 2016: a GeoSentinel analysis. Ann Intern Med. 2017;166(2):99–108. doi: 10.7326/M16-1842. http://dx.doi.org/10.7326/M16-1842. [DOI] [PubMed] [Google Scholar]
- Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2013 doi: 10.1016/j.meegid.2013.03.008. http://dx.doi.org/10.1016/j.meegid.2013.03.008. Epub 2013/03/26. [DOI] [PMC free article] [PubMed]
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. http://dx.doi.org/10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heang V, Yasuda CY, Sovann L, Haddow AD, Travassos da Rosa AP, Tesh RB, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis. 2012;18(2):349–351. doi: 10.3201/eid1802.111224. http://dx.doi.org/10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera BB, Chang CA, Hamel DJ, Mboup S, Ndiaye D, Imade G, et al. Continued transmission of zika virus in humans in West Africa, 1992–2016. J Infect Dis. 2017 doi: 10.1093/infdis/jix182. http://dx.doi.org/10.1093/infdis/jix182. [DOI] [PMC free article] [PubMed]
- Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of zika virus through sexual contact with travelers to areas of ongoing transmission - continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(8):215–216. doi: 10.15585/mmwr.mm6508e2. http://dx.doi.org/10.15585/mmwr.mm6508e2. Epub 2016/03/04. [DOI] [PubMed] [Google Scholar]
- Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13(3):e1006219. doi: 10.1371/journal.ppat.1006219. http://dx.doi.org/10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Ayers VB, Lyons AC, Unlu I, Alto BW, Cohnstaedt LW, et al. Culex species mosquitoes and zika virus. Vector Borne Zoonotic Dis. 2016a;16(10):673–676. doi: 10.1089/vbz.2016.2058. http://dx.doi.org/10.1089/vbz.2016.2058. [DOI] [PubMed] [Google Scholar]
- Huang WC, Abraham R, Shim BS, Choe H, Page DT. Zika virus infection during the period of maximal brain growth causes microcephaly and cortico-spinal neuron apoptosis in wild type mice. Sci Rep. 2016b;6:34793. doi: 10.1038/srep34793. http://dx.doi.org/10.1038/srep34793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flavi-viruses. Euro Surveill. 2016;21(16) doi: 10.2807/1560-7917.ES.2016.21.16.30203. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.16.30203. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375(1):1–4. doi: 10.1056/NEJMp1605367. http://dx.doi.org/10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1(13) doi: 10.1172/jci.insight.88461. http://dx.doi.org/10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Rossmann SN. Zika and the blood supply: a work in progress. Arch Pathol Lab Med. 2017;141(1):85–92. doi: 10.5858/arpa.2016-0430-RA. http://dx.doi.org/10.5858/arpa.2016-0430-RA. [DOI] [PubMed] [Google Scholar]
- Kincaid EA. Second look: efforts to repurpose old drugs against Zika cast a wide net. Nat Med. 2016;22(8):824–825. doi: 10.1038/nm0816-824. http://dx.doi.org/10.1038/nm0816-824. [DOI] [PubMed] [Google Scholar]
- Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, et al. Development of a zika virus infection model in cynomolgus macaques. Front Microbiol. 2016;7:2028. doi: 10.3389/fmicb.2016.02028. http://dx.doi.org/10.3389/fmicb.2016.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. http://dx.doi.org/10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474–478. doi: 10.1038/nature18952. http://dx.doi.org/10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A mouse model of zika virus pathogenesis. Cell Host Microbe. 2016;19(5):720–730. doi: 10.1016/j.chom.2016.03.010. http://dx.doi.org/10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MC, Muniz LF, Ferreira TS, Santos CM, Almeida LC, Van Der Linden V, et al. Hearing loss in infants with microcephaly and evidence of congenital zika virus infection - Brazil, november 2015-may 2016. MMWR Morb Mortal Wkly Rep. 2016;65(34):917–919. doi: 10.15585/mmwr.mm6534e3. http://dx.doi.org/10.15585/mmwr.mm6534e3. [DOI] [PubMed] [Google Scholar]
- Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Neglected Trop Dis. 2014;8(10):e3188. doi: 10.1371/journal.pntd.0003188. http://dx.doi.org/10.1371/journal.pntd.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (stegomyia) aegypti (linnaeus) to zika virus. PLoS Neglected Trop Dis. 2012;6(8):e1792. doi: 10.1371/journal.pntd.0001792. http://dx.doi.org/10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016a;19(1):120–126. doi: 10.1016/j.stem.2016.04.017. http://dx.doi.org/10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016b;19(5):672. doi: 10.1016/j.stem.2016.10.017. http://dx.doi.org/10.1016/j.stem.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, et al. Char-acterization of a 2016 clinical isolate of zika virus in non-human primates. EBioMedicine. 2016c;12:170–177. doi: 10.1016/j.ebiom.2016.09.022. http://dx.doi.org/10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell. 2017;20(3):385–396. doi: 10.1016/j.stem.2016.11.017. http://dx.doi.org/10.1016/j.stem.2016.11.017 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. Local mosquito-borne transmission of zika virus - miami-dade and broward Counties, Florida, June-august 2016. MMWR Morb Mortal Wkly Rep. 2016;65(38):1032–1038. doi: 10.15585/mmwr.mm6538e1. http://dx.doi.org/10.15585/mmwr.mm6538e1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature. 2017 doi: 10.1038/nature22365. http://dx.doi.org/10.1038/nature22365. [DOI] [PMC free article] [PubMed]
- Lozier M, Adams L, Febo MF, Torres-Aponte J, Bello-Pagan M, Ryff KR, et al. Incidence of zika virus disease by age and sex - Puerto Rico, november 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(44):1219–1223. doi: 10.15585/mmwr.mm6544a4. http://dx.doi.org/10.15585/mmwr.mm6544a4. [DOI] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167(6):1511–1524. doi: 10.1016/j.cell.2016.11.016. http://dx.doi.org/10.1016/j.cell.2016.11.016 e10. [DOI] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2017;168(3):542. doi: 10.1016/j.cell.2017.01.009. http://dx.doi.org/10.1016/j.cell.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Magnani DM, Silveira CGT, Rosen BC, Ricciardi M, Pedreño-Lopez N, Martin J, Gutman MJ, et al. A human inferred germline antibody binds to an immunodominant epitope and neutralizes Zika virus. PLoS Neglected Trop Dis. 2017 doi: 10.1371/journal.pntd.0005655. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhluf H, Kim K, Shresta S. Novel strategies for discovering inhibitors of Dengue and Zika fever. Expert Opin Drug Discov. 2016;11(10):921–923. doi: 10.1080/17460441.2016.1212013. http://dx.doi.org/10.1080/17460441.2016.1212013. [DOI] [PubMed] [Google Scholar]
- Mann A. New organization pledges scientific expertise for viral outbreaks. Nat Med. 2011;17(4):394. doi: 10.1038/nm0411-394a. http://dx.doi.org/10.1038/nm0411-394a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy JM, Pasquier C, Daudin M, Chapuy-Regaud S, Moinard N, Chevreau C, et al. Zika virus in semen of a patient returning from a non-epidemic area. Lancet Infect Dis. 2016a;16(8):894–895. doi: 10.1016/S1473-3099(16)30153-0. http://dx.doi.org/10.1016/S1473-3099(16)30153-0. [DOI] [PubMed] [Google Scholar]
- Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405. doi: 10.1016/S1473-3099(16)00138-9. http://dx.doi.org/10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- Mansuy JM, Mengelle C, Pasquier C, Chapuy-Regaud S, Delobel P, Martin-Blondel G, Izopet J. Zika virus infection and prolonged viremia in whole-blood specimens. Emerg Infect Dis. 2017;23:863–865. doi: 10.3201/eid2305.161631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. Epub 1969/05/01. [DOI] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, et al. Notes from the field: evidence of zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal Losses–Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):159–160. doi: 10.15585/mmwr.mm6506e1. http://dx.doi.org/10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Mak TM, Ng YK, Phuah SP, Huber RG, Marzinek JK, et al. South-east Asian Zika virus strain linked to cluster of cases in Singapore, August 2016. Euro Surveill. 2016;21(38) doi: 10.2807/1560-7917.ES.2016.21.38.30347. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.38.30347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. First US case of Zika virus infection is identified in Texas. BMJ. 2016;352:i212. doi: 10.1136/bmj.i212. http://dx.doi.org/10.1136/bmj.i212. [DOI] [PubMed] [Google Scholar]
- Meaney-Delman D, Oduyebo T, Polen KN, White JL, Bingham AM, Slavinski SA, et al. Prolonged detection of zika virus RNA in pregnant women. Obstet Gynecol. 2016a;128(4):724–730. doi: 10.1097/AOG.0000000000001625. http://dx.doi.org/10.1097/AOG.0000000000001625. [DOI] [PubMed] [Google Scholar]
- Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Hennenfent AK, et al. Zika virus infection among U.S. Pregnant trav-elers - August 2015-February 2016. MMWR Morb Mortal Wkly Rep. 2016b;65(8):211–214. doi: 10.15585/mmwr.mm6508e1. http://dx.doi.org/10.15585/mmwr.mm6508e1. [DOI] [PubMed] [Google Scholar]
- Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 2017;18(2):324–333. doi: 10.1016/j.celrep.2016.12.045. http://dx.doi.org/10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Diamond MS. Zika virus pathogenesis and tissue tropism. Cell Host Microbe. 2017;21(2):134–142. doi: 10.1016/j.chom.2017.01.004. http://dx.doi.org/10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 2016a;16(12):3208–3218. doi: 10.1016/j.celrep.2016.08.079. http://dx.doi.org/10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016b;165(5):1081–1091. doi: 10.1016/j.cell.2016.05.008. http://dx.doi.org/10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651. http://dx.doi.org/10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77(1):73–81. [PubMed] [Google Scholar]
- Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017a doi: 10.1016/j.cmi.2016.12.027. http://dx.doi.org/10.1016/j.cmi.2016.12.027. [DOI] [PubMed]
- Moreira J, Peixoto TM, Siqueira Md, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017b doi: 10.1016/j.cmi.2016.12.027. http://dx.doi.org/10.1016/j.cmi.2016.12.027. [DOI] [PubMed]
- Morrison TE, Diamond MS. Animal models of zika virus infection, pathogenesis, and immunity. J Virol. 2017;91(8) doi: 10.1128/JVI.00009-17. http://dx.doi.org/10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta IJ, Spencer BR, Cordeiro da Silva SG, Arruda MB, Dobbin JA, Gonzaga YB, et al. Evidence for transmission of zika virus by platelet transfusion. N Engl J Med. 2016;375(11):1101–1103. doi: 10.1056/NEJMc1607262. http://dx.doi.org/10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Miller ES. Preliminary results from the US zika pregnancy Registry: untangling risks for congenital anomalies. Jama. 2016 doi: 10.1001/jama.2016.18632. http://dx.doi.org/10.1001/jama.2016.18632. Epub 2016/12/14. [DOI] [PubMed]
- Munoz-Jordan JL, Fredericksen BL. How flaviviruses activate and suppress the interferon response. Viruses. 2010;2(2):676–691. doi: 10.3390/v2020676. http://dx.doi.org/10.3390/v2020676. Epub 2010/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23(1):99–101. doi: 10.3201/eid2301.161394. http://dx.doi.org/10.3201/eid2301.161394. Epub 2017/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016;29(3):487–524. doi: 10.1128/CMR.00072-15. http://dx.doi.org/10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014a;20(10):O595–O596. doi: 10.1111/1469-0691.12707. http://dx.doi.org/10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014b;19(14) doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. http://dx.doi.org/10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano 3rd S, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13(5):e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21(32) doi: 10.2807/1560-7917.ES.2016.21.32.30314. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.32.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Memorias do Inst Oswaldo Cruz. 2016;111(5):287–293. doi: 10.1590/0074-02760160085. http://dx.doi.org/10.1590/0074-02760160085. Epub 2016/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18(5):591–596. doi: 10.1016/j.stem.2016.03.012. http://dx.doi.org/10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduyebo T, Petersen EE, Rasmussen SA, Mead PS, Meaney-Delman D, Renquist CM, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible zika virus exposure - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127. doi: 10.15585/mmwr.mm6505e2. http://dx.doi.org/10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. http://dx.doi.org/10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- Olson JG, Ksiazek TG, Suhandiman Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389–393. doi: 10.1016/0035-9203(81)90100-0. Epub 1981/01/01. [DOI] [PubMed] [Google Scholar]
- Olson KE, Franz AWE. Geneticaly modified vectors for control of arbovi-ruses. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control Caister. Academic Press; Norfolk, UK: 2016. pp. 315–336. [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22(12):1448–1455. doi: 10.1038/nm.4206. http://dx.doi.org/10.1038/nm, 4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco O, Beltran M, Nelson CA, Valencia D, Tolosa N, Farr SL, et al. Zika virus disease in Colombia - preliminary report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1604037. http://dx.doi.org/10.1056/NEJMoa1604037. [DOI] [PubMed]
- Pagani I, Ghezzi S, Ulisse A, Rubio A, Turrini F, Garavaglia E, et al. Human endometrial stromal cells are highly permissive to productive infection by zika virus. Sci Rep. 2017;7:44286. doi: 10.1038/srep44286. http://dx.doi.org/10.1038/srep44286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO. Regional Zika Epidemiological Update (Americas) - 10 March 2017 [cited 2017 MArch 13] 2017 Available from: http://www.paho.org/hq/index.php?option=com_content&id=11599&Itemid=41691.
- Paploski IA, Prates AP, Cardoso CW, Kikuti M, Silva MM, Waller LA, et al. Time lags between exanthematous illness attributed to zika virus, Guillain-barre syndrome, and microcephaly, salvador, Brazil. Emerg Infect Dis. 2016;22(8):1438–1444. doi: 10.3201/eid2208.160496. http://dx.doi.org/10.3201/eid2208.160496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017 doi: 10.1038/nature21428. http://dx.doi.org/10.1038/nature21428. [DOI] [PMC free article] [PubMed]
- Parke DW, 3rd, Almeida DR, Albini TA, Ventura CV, Berrocal AM, Mittra RA. Serologically confirmed zika-related unilateral acute maculopathy in an adult. Ophthalmology. 2016;123(11):2432–2433. doi: 10.1016/j.ophtha.2016.06.039. http://dx.doi.org/10.1016/j.ophtha.2016.06.039. [DOI] [PubMed] [Google Scholar]
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, et al. JAMA Ophthalmol. Brazil: 2016. Ocular Findings in Infants with Microcephaly Associated with Presumed Zika Virus Congenital Infection in Salvador. http://dx.doi.org/10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of zika virus in body fluids - preliminary report. N Engl J Med. 2017 doi: 10.1056/NEJMoa1613108. http://dx.doi.org/10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed]
- Perkins TA, Siraj AS, Ruktanonchai CW, Kraemer MU, Tatem AJ. Erratum: model-based projections of Zika virus infections in childbearing women in the Americas. Nat Microbiol. 2017;2:17051. doi: 10.1038/nmicrobiol.2017.51. http://dx.doi.org/10.1038/nmicrobiol.2017.51. [DOI] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016a doi: 10.1056/NEJMc1606769. http://dx.doi.org/10.1056/NEJMra1602113. [DOI] [PubMed]
- Petersen EE, Polen KN, Meaney-Delman D, Ellington SR, Oduyebo T, Cohn A, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible zika virus Exposure–United States, 2016. MMWR Morb Mortal Wkly Rep. 2016b;65(12):315–322. doi: 10.15585/mmwr.mm6512e2. http://dx.doi.org/10.15585/mmwr.mm6512e2. [DOI] [PubMed] [Google Scholar]
- Plourde AR, Bloch EV. A literature review of zika virus. Emerg Infect Dis. 2016;22(7) doi: 10.3201/eid2207.151990. http://dx.doi.org/10.3201/eid2207.151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. doi: 10.1016/j.cell.2016.04.032. http://dx.doi.org/10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, et al. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20(1):83–90. doi: 10.1016/j.chom.2016.05.015. http://dx.doi.org/10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, et al. Interim guidance for interpretation of zika virus antibody test results. MMWR Morb Mortal Wkly Rep. 2016;65(21):543–546. doi: 10.15585/mmwr.mm6521e1. http://dx.doi.org/10.15585/mmwr.mm6521e1. [DOI] [PubMed] [Google Scholar]
- Ragan IK, Blizzard EL, Gordy P, Bowen RA. Investigating the potential role of North american animals as hosts for zika virus. Vector Borne Zoonotic Dis. 2017;17(3):161–164. doi: 10.1089/vbz.2016.2099. http://dx.doi.org/10.1089/vbz.2016.2099. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338. http://dx.doi.org/10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408–14413. doi: 10.1073/pnas.1618029113. http://dx.doi.org/10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. Vital signs: update on zika virus-associated birth defects and evaluation of all U.S. Infants with congenital zika virus exposure - U.S. Zika pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(13):366–373. doi: 10.15585/mmwr.mm6613e1. http://dx.doi.org/10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French polynesian Aedes aegypti and Aedes polynesiensis for zika virus. PLoS Neglected Trop Dis. 2016;10(9):e0005024. doi: 10.1371/journal.pntd.0005024. http://dx.doi.org/10.1371/journal.pntd.0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AS, Shim BS, Kwon YC, Zhang R, Otsuka Y, Schmitt K, et al. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc Natl Acad Sci U S A. 2017;114(8):2024–2029. doi: 10.1073/pnas.1620558114. http://dx.doi.org/10.1073/pnas.1620558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017 doi: 10.1016/j.cell.2017.03.016. http://dx.doi.org/10.1016/j.cell.2017.02.017. [DOI] [PubMed]
- Ritchie SA, Devine G. Conventional vector control: evidence it controls arboviruses. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control Caister. Academic Press; Norfolk, UK: 2016. pp. 281–290. [Google Scholar]
- do Rosario MS, de Jesus PA, Vasilakis N, Farias DS, Novaes MA, Rodrigues SG, et al. Guillain-barre syndrome after zika virus infection in Brazil. Am J Trop Med Hyg. 2016;95(5):1157–1160. doi: 10.4269/ajtmh.16-0306. http://dx.doi.org/10.4269/ajtmh.16-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a novel murine model to study zika virus. Am J Trop Med Hyg. 2016;94(6):1362–1369. doi: 10.4269/ajtmh.16-0111. http://dx.doi.org/10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, Fernandez-Salas I, Vitek CJ, Paploski IA, Kitron U, Ribeiro GS, Hanley KA, Weaver SC, Vasilakis N. Variation in Aedes aegypti mosquito competence for zika virus transmission. Emerg Infect Dis. 2017;23(4):625–632. doi: 10.3201/eid2304.161484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehuddin AR, Haslan H, Mamikutty N, Zaidun NH, Azmi MF, Senin MM, et al. Zika virus infection and its emerging trends in Southeast Asia. Asian Pac J Trop Med. 2017;10(3):211–219. doi: 10.1016/j.apjtm.2017.03.002. http://dx.doi.org/10.1016/j.apjtm.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Salje H, Cauchemez S, Alera MT, Rodriguez-Barraquer I, Thaisomboonsuk B, Srikiatkhachorn A, et al. Reconstruction of 60 Years of Chikungunya epidemiology in the Philippines demonstrates episodic and focal transmission. J Infect Dis. 2016;213(4):604–610. doi: 10.1093/infdis/jiv470. http://dx.doi.org/10.1093/infdis/jiv470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, et al. Zika virus and the Guillain-barre syndrome - case series from seven countries. N Engl J Med. 2016;375(16):1598–1601. doi: 10.1056/NEJMc1609015. http://dx.doi.org/10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, Bombardi RG, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540(7633):443–447. doi: 10.1038/nature20564. http://dx.doi.org/10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydra-nencephaly and fetal demise. PLoS Neglected Trop Dis. 2016;10(2):e0004517. doi: 10.1371/journal.pntd.0004517. http://dx.doi.org/10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible association between zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. http://dx.doi.org/10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, Weaver SC, Vasconcelos PFC, Rossi SL, Shi PY. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017;23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xie X, Barrett AD, Garcia-Blanco MA, Tesh RB, Vasconcelos PF, Vasilakis N, Weaver SC, Shi PY. Zika virus: diagnosis, therapeutics, and vaccine. ACS Infect Dis. 2016a;2(3):170–172. doi: 10.1021/acsinfecdis.6b00030. [DOI] [PubMed] [Google Scholar]
- Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, et al. An infectious cDNA clone of zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe. 2016b;19(6):891–900. doi: 10.1016/j.chom.2016.05.004. http://dx.doi.org/10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xie X, Ren P, Loeffelholz MJ, Yang Y, Furuya A, et al. A rapid zika diagnostic assay to measure neutralizing antibodies in patients. EBioMedicine. 2017;17:157–162. doi: 10.1016/j.ebiom.2017.03.006. http://dx.doi.org/10.1016/j.ebiom.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017;114(9):E1587. doi: 10.1073/pnas.1616097114. http://dx.doi.org/10.1073/pnas.1616097114. E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S. Zika virus infection of Hofbauer cells. Am J Reprod Immunol. 2017;77(2) doi: 10.1111/aji.12613. http://dx.doi.org/10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335–338. [PubMed] [Google Scholar]
- SMFM. SMFM statement: ultrasound screening for fetal microcephaly following Zika virus exposure. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.02.043. [Internet] [DOI] [PubMed] [Google Scholar]
- Smithburn KC. Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am J Hyg. 1954;59(2):157–163. doi: 10.1093/oxfordjournals.aje.a119630. [DOI] [PubMed] [Google Scholar]
- Souza BS, Sampaio GL, Pereira CS, Campos GS, Sardi SI, Freitas LA, et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep. 2016;6:39775. doi: 10.1038/srep39775. http://dx.doi.org/10.1038/srep39775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer SD, Pierson TC. VIROLOGY. Diagnostics for Zika virus on the horizon. Science. 2016;353(6301):750–751. doi: 10.1126/science.aah6187. http://dx.doi.org/10.1126/science.aah6187. [DOI] [PubMed] [Google Scholar]
- Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill. 2016;21(50) doi: 10.2807/1560-7917.ES.2016.21.50.30426. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 doi: 10.1126/science.aaf8505. http://dx.doi.org/10.1126/science.aaf8505. [DOI] [PubMed]
- Strafela P, Vizjak A, Mraz J, Mlakar J, Pizem J, Tul N, et al. Zika virus-associated micrencephaly: a thorough description of neuropathologic findings in the fetal central nervous system. Arch Pathol Lab Med. 2017;141(1):73–81. doi: 10.5858/arpa.2016-0341-SA. http://dx.doi.org/10.5858/arpa.2016-0341-SA. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20(2):155–166. doi: 10.1016/j.chom.2016.07.002. http://dx.doi.org/10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587–590. doi: 10.1016/j.stem.2016.02.016. http://dx.doi.org/10.1016/j.stem.2016.02.016. Epub 2016/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetro JA. Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect. 2016;18(3):167–168. doi: 10.1016/j.micinf.2015.12.010. http://dx.doi.org/10.1016/j.micinf.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Vasilakis N, Weaver SC. Flavivirus transmission focusing on Zika. Curr Opin Virol. 2016;22:30–35. doi: 10.1016/j.coviro.2016.11.007. http://dx.doi.org/10.1016/j.coviro.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez AM, Sapiano MR, Basavaraju SV, Kuehnert MJ, Rivera-Garcia B. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted zika virus Infection–Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(14):375–378. doi: 10.15585/mmwr.mm6514e1. http://dx.doi.org/10.15585/mmwr.mm6514e1. [DOI] [PubMed] [Google Scholar]
- Ventura CV, Maia M, Dias N, Ventura LO, Belfort R., Jr Zika: neurological and ocular findings in infant without microcephaly. Lancet. 2016;387(10037):2502. doi: 10.1016/S0140-6736(16)30776-0. http://dx.doi.org/10.1016/S0140-6736(16)30776-0. [DOI] [PubMed] [Google Scholar]
- Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21(8):30148. doi: 10.2807/1560-7917.ES.2016.21.8.30148. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- Vermillion MS, Lei J, Shabi Y, Baxter VK, Crilly NP, McLane M, et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun. 2017;8:14575. doi: 10.1038/ncomms14575. http://dx.doi.org/10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Altman DG, Purwar M, Noble JA, Knight HE, Ruyan P, et al. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG. 2013;120(s2):9–26. doi: 10.1111/1471-0528.12047. [DOI] [PubMed] [Google Scholar]
- Walker T, Sinkins SP. Biological control of arbovirus vectors. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control Caister. Academic Press; Norfolk, UK: 2016. pp. 291–302. [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: history, emergence, biology, and prospects for control. Antivir Res. 2016 doi: 10.1016/j.antiviral.2016.03.010. http://dx.doi.org/10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed]
- Weger-Lucarelli J, Ruckert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, et al. Vector competence of american mosquitoes for three strains of zika virus. PLoS Neglected Trop Dis. 2016;10(10):e0005101. doi: 10.1371/journal.pntd.0005101. http://dx.doi.org/10.1371/journal.pntd.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, et al. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J Virol. 2017;91(4) doi: 10.1128/JVI.01905-16. http://dx.doi.org/10.1128/JVI.01905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell. 2016;19(6):703–708. doi: 10.1016/j.stem.2016.11.011. http://dx.doi.org/10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- WHO. Zika virus outbreaks in the Americas. Wkly Epidemiol Rec. 2015;90:609–610. [PubMed] [Google Scholar]
- WHO. Interim Guidance Update: Pregnancy Management in the Context of Zika Virus Infection. Geneva: WHO; 2016a. [cited 2017 May 16, 2017]. [DOI] [PubMed] [Google Scholar]
- WHO. Zika Situation Report, 6 October 2016. Geneva: WHO; 2016b. [cited 2017 May 21, 2017] Available from: http://www.who.int/emergencies/zika-virus/situation-report/6-october-2016/en/. [Google Scholar]
- WHO. Guideline: Infant Feeding in Areas of Zika Virus Transmission. Geneva: WHO; 2017. [cited 2017 May 16, 2017]. Available from: http://www.who.int/nutrition/publications/guidelines/infantfeeding_zikavirus_transmission/en/. [Google Scholar]
- Wikan N, Suputtamongkol Y, Yoksan S, Smith DR, Auewarakul P. Immunological evidence of Zika virus transmission in Thailand. Asian Pac J Trop Med. 2016;9(2):141–144. doi: 10.1016/j.apjtm.2016.01.017. http://dx.doi.org/10.1016/j.apjtm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci U S A. 2006;103(9):3203–3208. doi: 10.1073/pnas.0511344103. http://dx.doi.org/10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PC, Linnen JM, Kessler DA, Shaz BH, Kamel H, Vassallo RR, et al. First cases of Zika virus-infected US blood donors outside states with areas of active transmission. Transfusion. 2017 doi: 10.1111/trf.14041. http://dx.doi.org/10.1111/trf.14041. Epub 2017/02/23. [DOI] [PubMed]
- Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64(5–6):310–316. doi: 10.4269/ajtmh.2001.64.310. Epub 2001/07/21. [DOI] [PubMed] [Google Scholar]
- Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albo-pictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Neglected Trop Dis. 2013;7(8):e2348. doi: 10.1371/journal.pntd.0002348. http://dx.doi.org/10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SJ, Furuya A, Zou J, Xie X, Dupuis AP, 2nd, Kramer LD, et al. A multiplex microsphere immunoassay for zika virus diagnosis. EBioMedicine. 2017;16:136–140. doi: 10.1016/j.ebiom.2017.01.008. http://dx.doi.org/10.1016/j.ebiom.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26(6):645–654. doi: 10.1038/cr.2016.58. http://dx.doi.org/10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Yang Y, Muruato AE, Zou J, Shan C, Nunes BT, et al. Understanding zika virus stability and developing a chimeric vaccine through functional analysis. MBio. 2017;8(1) doi: 10.1128/mBio.02134-16. http://dx.doi.org/10.1128/mBio.02134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101–1107. doi: 10.1038/nm.4184. http://dx.doi.org/10.1038/nm. 4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shan C, Zou J, Muruato AE, Bruno DN, de Almeida Medeiros Daniele B, et al. A cDNA clone-launched platform for high-yield production of inactivated zika vaccine. EBioMedicine. 2017;17:145–156. doi: 10.1016/j.ebiom.2017.02.003. http://dx.doi.org/10.1016/j.ebiom.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, et al. Vaginal exposure to zika virus during pregnancy leads to fetal brain infection. Cell. 2016;166(5):1247–1256. doi: 10.1016/j.cell.2016.08.004. http://dx.doi.org/10.1016/j.cell.2016.08.004 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano H, Waggoner J, Leon K, Pinsky B, Vera K, Schettino M, et al. High incidence of Zika virus infection detected in plasma and cervical cytology specimens from pregnant women in Guayaquil, Ecuador. Am J Reprod Immunol. 2017;77(2) doi: 10.1111/aji.12630. http://dx.doi.org/10.1111/aji.12630. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hammack C, Ogden SC, Cheng Y, Lee EM, Wen Z, et al. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016;44(18):8610–8620. doi: 10.1093/nar/gkw765. http://dx.doi.org/10.1093/nar/gkw765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Pinsky BA, Ananta JS, Zhao S, Arulkumar S, Wan H, et al. Diagnosis of Zika virus infection on a nanotechnology platform. Nat Med. 2017 doi: 10.1038/nm.4302. http://dx.doi.org/10.1038/nm.4302. [DOI] [PubMed]
- Zhu Z, Chan JF, Tee KM, Choi GK, Lau SK, Woo PC, et al. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect. 2016;5:e22. doi: 10.1038/emi.2016.48. http://dx.doi.org/10.1038/emi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J. The viral polymerase inhibitor 7-deaza-2′-C-methyladenosine is a potent inhibitor of in vitro zika virus replication and delays disease progression in a robust mouse infection model. PLoS Neglected Trop Dis. 2016;10(5):e0004695. doi: 10.1371/journal.pntd.0004695. http://dx.doi.org/10.1371/journal.pntd.0004695. Epub 2016/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]


