Abstract
Objectives
This study was designed to evaluate binaural temporal processing in young and older adults using a binaural masking level difference (BMLD) paradigm. Using behavioral and electrophysiological measures within the same listeners, a series of stimulus manipulations was used to evaluate the relative contribution of binaural temporal fine structure and temporal envelope cues. We evaluated the hypotheses that age-related declines in the BMLD task would be more strongly associated with temporal fine structure than envelope cues and that age-related declines in behavioral measures would be correlated with cortical auditory evoked potential (CAEP) measures.
Design
Thirty adults participated in the study, including ten young normal-hearing, ten older normal-hearing, and ten older hearing-impaired with bilaterally symmetric, mild to moderate sensorineural hearing loss (SNHL). Behavioral and CAEP thresholds were measured for diotic (So) and dichotic (Sπ) tonal signals presented in continuous diotic (No) narrowband noise (50-Hz wide) maskers. Temporal envelope cues were manipulated by using two different narrowband maskers; Gaussian noise (GN) with robust envelope fluctuations and low-noise noise (LNN) with minimal envelope fluctuations. The potential to use temporal fine structure cues was controlled by varying the signal frequency (500 Hz or 4000 Hz), thereby relying on the natural decline in phase-locking with increasing frequency.
Results
Behavioral and CAEP thresholds were similar across groups for diotic conditions, while the masking release in dichotic conditions was larger for younger than older participants. Across all participants, BMLDs were larger for GN than LNN and for 500- than 4000-Hz conditions, where envelope and fine structure cues were most salient, respectively. Specific age-related differences were demonstrated for 500-Hz dichotic conditions in GN and LNN, reflecting reduced binaural temporal fine structure coding. No significant age effects were observed for 4000-Hz dichotic conditions, consistent with similar use of binaural temporal envelope cues across age in these conditions. For all groups, thresholds and derived BMLD values obtained using the behavioral and CAEP methods were strongly correlated, supporting the notion that CAEP measures may be useful as an objective index of age-related changes binaural temporal processing.
Conclusions
These results demonstrate an age-related decline in the processing of binaural temporal fine structure cues with preserved temporal envelope coding that was similar with and without mild-to-moderate peripheral hearing loss. Such age-related changes can be reliably indexed by both behavioral and CAEP measures in young and older adults.
INTRODUCTION
Our ability to perceive sounds of interest in the presence of background competition is aided by the ability of the brain to process subtle differences between sounds arriving at the two ears. An important cue known to improve signal detection in noise is the difference in stimulus phase between the ears (Hirsh, 1948), which can be evaluated using the binaural masking level difference (BMLD) paradigm. In a typical BMLD task, detection thresholds are measured in a baseline diotic condition where tonal signals and noise are presented in-phase at the two ears, (e.g., NoSo) and in a comparison dichotic condition, where either the tones or the noise are out-of-phase at the two ears (e.g., NoSπ; Hirsh, 1948; Pichora-Fuller & Schneider, 1991; Wilson, Moncrieff, Townsend, & Pillion, 2003). The dichotic, antiphasic (NoSπ or NπSo) conditions lead to a measurable improvement in detection threshold relative to the diotic condition, and thus a masking release. Phase values between 0 and π produce intermediate masked thresholds. The BMLD is derived by computing the difference in threshold between the baseline and comparison condition and provides an index of the masking release.
The magnitude of the BMLD is known to be influenced by different characteristics of the signal and masker (e.g., signal frequency, noise bandwidth). For example, a 500-Hz tone presented in an antiphasic condition (NoSπ) with a continuous broadband masker generally produces a large BMLD, on the order of 15 dB (Durlach & Colburn, 1978; Pichora-Fuller & Schneider, 1991). The masking release declines with increasing signal frequency: an effect that has been attributed to the ability of auditory nerve fibers to phase lock to the temporal fine structure of low- versus high-frequency stimuli (Palmer & Russell, 1986). The masking release also increases with decreasing masker bandwidth (Hall and Harvey, 1985). The inverse relationship between BMLD magnitude and masker bandwidth is related to the temporal envelope of the masker. Noise bands are known to have random amplitude fluctuations from moment to moment. The rate of amplitude fluctuation decreases as the noise bandwidth decreases (Rice, 1954), thus producing slow peaks and valleys in the temporal envelope of the masker. Slow amplitude fluctuations are more perceptually salient and are typically perceived as changes in loudness, whereas faster fluctuations are often heard as changes in “roughness” (Fastl & Zwicker, 2007). When a tonal signal is presented in narrowband noise, it results in robust changes in the statistics of the temporal envelope (e.g., Buss, Hall, & Grose, 2003; Eddins & Barber, 1998; Goupell, 2012; Hall, Grose, & Hartmann, 1998). Thus, depending on the characteristics of the signal and masker used in a BMLD paradigm, binaural temporal fine structure and temporal envelope cues may differentially influence the magnitude of the BMLD.
Eddins & Barber (1998) investigated the relative contributions of temporal fine structure and envelope cues to the BMLD by parametrically varying the signal frequency (500 vs. 4000 Hz) to differentially impact the usability of interaural fine-structure cues and masker type (Gaussian noise, GN vs. low-noise noise, LNN) to differentially impact the availability of useful interaural envelope cues. Compared to Gaussian noise, low-noise noise has a relatively flat temporal envelope and can be generated by minimizing the fluctuations in the power of the waveform, as described by Pumplin and Hartman (Hartmann & Pumplin, 1988, 1991; Pumplin, 1985). Alternatively, Kolhrausch et al. (1997) showed that LNN could be generated by extracting the Hilbert envelope of a Gaussian noise, dividing the noise waveform by its envelope, and filtering the stimulus to the desired bandwidth in the frequency domain. Evaluating BMLDs using both GN and LNN, Eddins & Barber (1998) showed that BMLDs were significantly smaller for the 4000-Hz than the 500-Hz stimulus frequencies regardless of masker type, reflecting a reduced ability to use binaural temporal fine structure cues at higher frequencies. At both frequencies, the BMLDs were smaller in LNN versus GN conditions, demonstrating the dependence of the BMLD on available envelope cues (Eddins & Barber, 1998). Similar results using comparable stimulus conditions were reported by Hall et al. (1998). The binaural release from masking has been explained in the context of a model in which signal detection relies on a change in interaural cross-correlation following an initial stage of compression (Bernstein & Trahiotis, 1996; Bernstein, van de Par, & Trahiotis, 1999). That is, when a signal is added out-of-phase (Sπ) to a diotic, homophasic noise (No), it introduces an interaural decorrelation that can be quantified using a normalized cross-correlation metric (Bernstein & Trahiotis, 1996). This model has been used to account for a wide range of binaural detection and discrimination data (Bernstein & Trahiotis, 2009, 2010).
The BMLD also may vary with listener attributes. Specifically, several studies have shown that advancing age results in poorer performance on BMLD tasks, due primarily to poorer thresholds in older adults for dichotic conditions (e.g., NoSπ), leading to smaller BMLDs with increasing age (Grose, Poth, & Peters, 1994; Pichora-Fuller & Schneider, 1991; Strouse, Ashmead, Ohde, & Grantham, 1998). Age effects have been shown for both tonal and speech signals, independent of peripheral hearing loss, and are thought to reflect declines in binaural temporal fine structure coding (Pichora-Fuller & Schneider, 1991; Strouse et al., 1998). Dubno, Ahlstrom, and Horwitz (2008) investigated the effects of age and temporal envelope on BMLDs measured for 500-Hz tones in GN and LNN. Although they reported no significant age effects for a group of young and older listeners with clinically normal pure-tone thresholds, they observed temporal envelope effects such that smaller BMLDs were obtained for LNN versus GN maskers, consistent with previous studies (Eddins & Barber, 1998; Hall et al., 1998). What remains unclear from these investigations is how the relative contributions of both temporal fine-structure and temporal envelope cues to the BMLD change with age.
To examine potential physiological correlates of the BMLD in humans, Fowler & Mikami (1992a, 1992b) used a continuous change paradigm to show that the BMLD could be estimated from auditory cortical responses in young listeners with normal hearing. By varying masker bandwidth in a manner similar to that of Hall and Harvey (1985), they showed that the effect of masker bandwidth, and thus temporal envelope, on electrophysiological estimates of the BMLD was similar to that reported for behavioral measures.
To evaluate binaural temporal fine structure processing, an alternative task that has been used is the detection of interaural phase differences (IPD). In this task, listeners often are asked to detect a change in the interaural phase of amplitude modulated tones over a range of tonal carrier frequencies. Behavioral measures of IPD processing in young, middle-age, and older adults show a gradual decrease in the upper frequency limit of IPD sensitivity such that young listeners can use binaural temporal fine structure cues to detect phase changes up to ~1300 Hz, middle-age listeners to ~1150 Hz, and older listeners to ~950 Hz (Grose & Mamo, 2010; Ross, Fujioka, Tremblay, & Picton, 2007). Comparable age-related declines in the upper frequency limit of IPD sensitivity have also been demonstrated in cortical evoked response measures where younger adults showed sensitivity up to 1225 Hz and older adults up to 760 Hz, consistent with changes in behavioral sensitivity (Papesh, Folmer, & Gallun, 2017; Ross et al., 2007). As such, it might be predicted that age-related declines in binaural temporal fine structure coding also would be observed in other electrophysiological measures such as those used by Fowler & Mikami (1992b) to index the BMLD.
Over the years, a range of auditory evoked response measures have been used to investigate neural correlates of the BMLD along the central auditory pathway, including the auditory brainstem response (ABR), middle latency response (MLR), brainstem frequency-following response (FFR), brainstem and cortical auditory steady-state responses (ASSR- at 500 Hz), and cortical/late auditory evoked responses (CAEPs). While brainstem and midbrain measures have failed to demonstrate reliable and robust BMLDs (Fowler & Mikami, 1996; Ishida & Stapells, 2009), BMLDs have been reported for cortical ASSRs at low (7, 13 Hz) but not high (e.g., 40 or 80) modulation rates carried by 500 Hz (Wong & Stapells, 2004) and for CAEPs (Fowler & Mikami, 1992a, 1992b). Importantly, Fowler and Mikami (1992a, b) showed a strong relationship between CAEP and behavioral BMLDs measured in the same subjects that were larger for 500-Hz tones in narrowband (50-Hz wide) versus broadband noise. One interpretation of those data is that the masker dependency was due to the differential contribution of temporal envelope cues in signal detection. To date, the differential influence of temporal envelope and fine structure coding on the magnitude of the BMLD has not been investigated using electrophysiological responses. Further, it is not certain whether processing of these two prominent temporal cues are equally affected by advancing age and/or elevated pure-tone thresholds. Here, we demonstrate in a parametric investigation that behavioral and electrophysiological BMLDs are highly correlated and that both are reduced in older adults with normal hearing or mild-to-moderate hearing loss, as compared to younger normal-hearing adults. By manipulating the availability of temporal envelope cues, we also show that the age-related changes in the BMLD can be attributed to declines in binaural temporal fine structure coding in the presence of preserved temporal envelope coding.
MATERIALS AND METHODS
Participants
Thirty listeners participated in the study and were grouped according to age and hearing sensitivity; 10 young, normal-hearing adults (YNH) ages 21 to 32 years (mean = 26.3; 8 female) with pure-tone thresholds ≤15 dB HL from 250–4000 Hz, 10 older, normal-hearing adults (ONH) ages 61 to 78 years (mean=69.6; 9 female) with clinically normal mean pure-tone thresholds ≤ 25 dB HL from 250–4000 Hz, and 10 older, hearing-impaired adults (OHI) ages 61 to 81 years (mean = 71.3; 6 female) with symmetric, flat, mild to moderate sensorineural hearing loss (SNHL) with mean pure-tone thresholds of 43 dB HL (right ears) and 44 dB HL (left ears) from 250–4000 Hz. Mean and standard deviation of audiometric results for the three participant groups are shown in Figure 1. The study protocol was approved by the University of South Florida Institutional Review Board. All participants gave written informed consent prior to audiometric and other testing and were paid for their participation.
Figure 1.
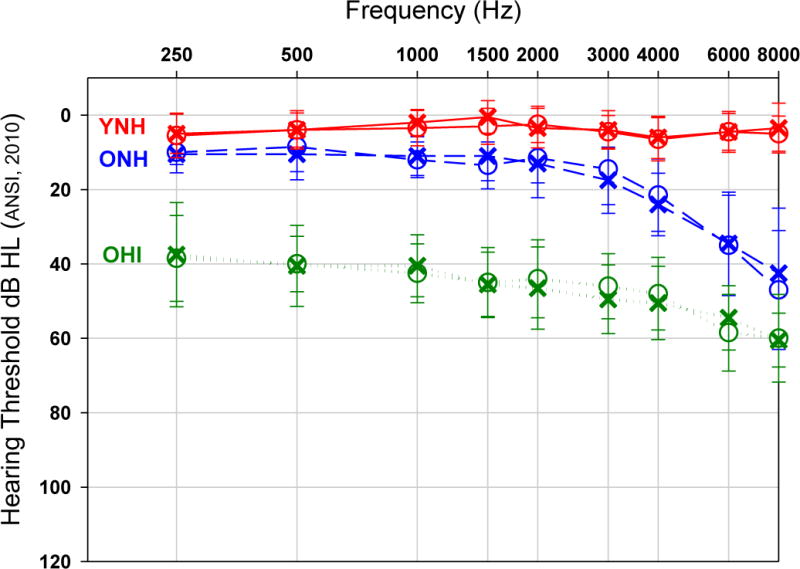
Audiometric thresholds for left (X) and right ears (O) averaged across the participants in each of three groups (YNH, ONH, OHI). Error bars correspond to ± 1SD.
Stimuli
Tonal stimuli consisted of 500- and 4000-Hz tone bursts (duration: 400-ms, 10-ms cos2 rise/fall) generated digitally at a sample rate of 24.4 kHz (Tucker Davis Technologies (TDT) RP2 or RX6). Narrowband (50-Hz wide) noise stimuli were centered at the signal frequency (i.e., 500 Hz or 4000 Hz). Masker stimuli were generated in the frequency domain by filling portions of two buffers with the appropriate magnitudes and phases prior to inverse Fast Fourier Transform (IFFT). For Gaussian noise (GN) maskers, the magnitude buffer included random values from a Rayleigh distribution and the phase buffer included random values from a uniform distribution (0 to 2π). Following the same steps, low-noise noise (LNN) maskers were generated by extracting the Hilbert envelope of a GN, dividing the waveform by its envelope, and filtering the stimulus to the desired BW in the frequency domain (Kohlrausch et al., 1997). Custom MATLAB™ scripts were used to generate a library of masker files (.wav format) 10 minutes in duration that were selected randomly with replacement and played continuously via computer soundcard during behavioral and electrophysiological testing. The tonal signals and maskers were routed through a summer (TDT SM3), headphone buffer (TDT HB6 or HB7) and delivered binaurally via Etymotic ER-2 insert earphones in homophasic (NoSo) and antiphasic (NoSπ) conditions. All stimuli were calibrated at the output of the earphones using a calibrator (B&K 4230), ear simulator (Knowles Electronics DB-100), ½” pressure microphone (B&K 4134), pre-amplifier (B&K 4134), and power conditioner (G.R.A.S. 12AA) routed to a multimeter (Fluke 45).
Procedure
Behavioral thresholds for diotic (So) and dichotic (Sπ) tones were measured in a continuous diotic (No) masker (60 dB spectrum level or 77 dB SPL) using a three interval forced-choice paradigm with a three-down, one-up tracking algorithm estimating 79.4% correct detection (Levitt, 1971). The starting level of the signal was 80 SPL and was the same for all participants. Prior to data collection, the participants were familiarized with the task on at least two, 60-trial blocks. Signal level was adaptively varied in 2-dB step sizes. Threshold was taken as the average level presented on the last even number of reversals, excluding the first three reversals. Mean threshold for a given condition was based on the average of three, 60-trial blocks. When the standard deviation of the threshold estimates was ≥ 2.5 dB, additional runs were administered. In total, there were eight conditions; homophasic (NoSo) and antiphasic (NoSπ) thresholds measured for 500- and 4000-Hz tones in each noise condition (GN and LNN). Stimulus generation, presentation, response collection, and visual feedback were controlled using the TDT SykoFizX software application.
Cortical auditory evoked potential (CAEP; P1-N1-P2) thresholds were measured subsequent to behavioral testing on different days and times. During data collection, the participants were seated in a sound-attenuating booth and listened passively to the stimuli while either watching a closed-captioned video or reading silently. Evoked responses were obtained using a standard clinical montage with three electrodes; the non-inverting electrode on the vertex (Cz), inverting electrode on the right earlobe (A2), and ground on the left earlobe (A1). Electrode impedances were monitored manually throughout data collection and were maintained at ≤ 5 kΩ. Electrodes were connected to a headstage (TDT HS4), routed to a preamplifier (TDT DB4), and then to an A/D converter (TDT RP2). Data were sampled at 25 kHz, recorded over a 500-ms time window post-stimulus onset, band-passed filtered online from 1–30 Hz, amplified × 50,000, and averaged using BioSig software (TDT). Prior to averaging, the software automatically rejected trials as artifacts if response amplitudes exceeded the artifact criterion window (> ±100 μV). No eyeblink monitoring was used.
Tonal stimuli were generated using SigGen software (TDT) and presented at rate of 0.5/sec using BioSig (TDT) software. The masker was controlled with a custom MATLAB™ script and presented continuously via the computer soundcard. A minimum of two replicable evoked responses, based on 100 artifact-free samples, were collected and averaged for each of the eight stimulus conditions. The initial stimulus level was +20 dB re: behavioral thresholds (measured during a separate session) for each condition and was decreased in 4-dB steps until no response was observed. The level was then increased in 2-dB steps until a repeatable response was obtained. Threshold was defined as the lowest level at which the N1 component was present between approximately 90–150 ms in both replications. Figure 2 shows an example of two threshold series measured for a 500-Hz GN masker in the NoSo and NoSπ conditions for one ONH participant, with threshold estimates shown in dashed red lines. BMLDs based on CAEP thresholds were computed in the same manner as those measured for behavioral thresholds (i.e., NoSo – NoSπ). In this example, the BMLD would be 8 dB.
Figure 2.
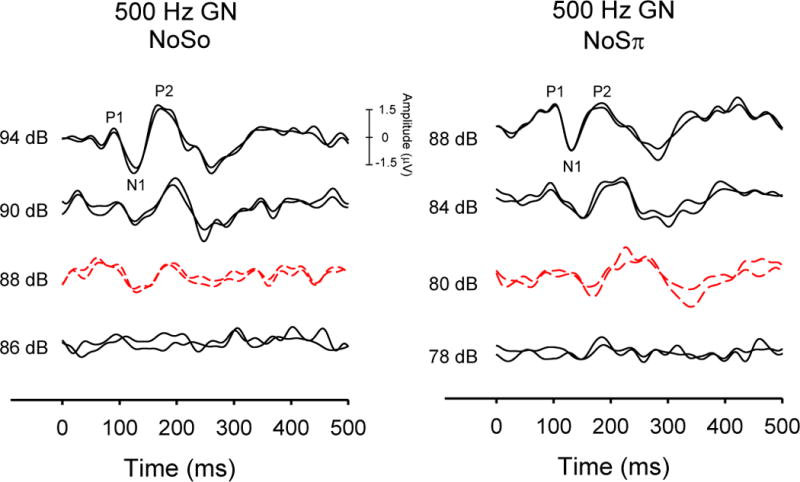
Examples of CAEP waveforms from a representative ONH participant. Responses to 500-Hz tones in Gaussian noise are shown from suprathreshold to threshold levels (shown as dashed red lines) for NoSo (left) and NoSπ (right) conditions. The NoSo threshold is 88 dB SPL and the NoSπ threshold is 80 dB SPL, resulting in a BMLD of 8 dB.
For each listener, peaks in their averaged waveforms were marked manually for N1 and P2 to quantify peak latencies and N1-P2 peak-to-peak amplitude. To account for differences in absolute threshold across participants and conditions, latency and amplitude measures were evaluated at a single level of +20 dB SL relative to each participant’s threshold for each stimulus condition. Two independent, experienced observers judged the thresholds, response latencies, and amplitudes blind to the stimulus condition. The two observers were not both present during the data collection process.
Data Analysis
Preliminary statistical analyses using repeated measures ANOVA indicated that tests for homogeneity of variance, using Levene’s test and p<.05, were not met for some data comparisons (4 of 16 threshold measures and 3 of 8 BMLD measures). As a result, the independent-samples Kruskal-Wallis H test was used to evaluate group effects, incorporating both age and hearing status (YNH, ONH, OHI), for all measures (behavioral and CAEP thresholds, BMLDs, N1-P2 amplitudes, N1 and P2 latency). This is a robust, nonparametric alternative to the ANOVA when the assumptions of normality and equal variance may not be met. With this approach, the data values were replaced by their rank within the full data set being evaluated (e.g., 1–30 in this study), and rank sums were computed for each subject group. The Kruskal-Wallis H statistic, which approximates a chi-square distribution, was computed (after correcting for rank ties) and compared to critical chi-square values to determine significance. When significant differences were observed, post-hoc tests were completed using Dunn’s pairwise comparisons with Bonferroni correction (for multiple comparisons) to quantify mean rank differences across groups. In addition, a Pearson product moment correlation was used to evaluate the relation between behavioral and electrophysiological BMLDs within and across groups. All statistical analyses were completed using IBM SPSS Statistics - Version 22.
RESULTS
Behavioral Thresholds
Figure 3 (left panels) displays the behavioral thresholds for 500-Hz (panel A) and 4000-Hz (panel C) stimuli with the homophasic and antiphasic (NoSo, NoSπ) conditions and noise maskers (GN, LNN) indicated on the abscissa. The boxes span the 25th to 75th percentiles, the horizontal line corresponds to the median, and vertical bars span 10th to 90th percentiles. Statistical results from the Kruskal-Wallis H test and post-hoc analyses for behavioral and CAEP thresholds are reported in Table 1. For behavioral thresholds, Kruskal-Wallis results indicated a significant effect of group only for NoSπ thresholds at 500 Hz in GN maskers (χ2(2) = 12.43, p = 0.002; see Fig 3A, second triad of boxes). Post-hoc pairwise comparisons using mean rank differences demonstrated that thresholds for YNH were significantly lower than both ONH (z = 2.95, p = 0.01) and OHI (z = 3.15, p = 0.005), but the two older groups were not different from each other (z = 0.20, p = 1.0). As seen in Figure 3A and 3C, the reference NoSo thresholds (first triad of boxes) were nearly identical across cohorts. No significant group effects were observed for NoSo or NoSπ thresholds in the 4000-Hz conditions. Although LNN maskers resulted in lower thresholds than GN maskers for all groups, no significant group effects were observed for LNN maskers in either the 500-Hz or 4000-Hz conditions.
Figure 3.
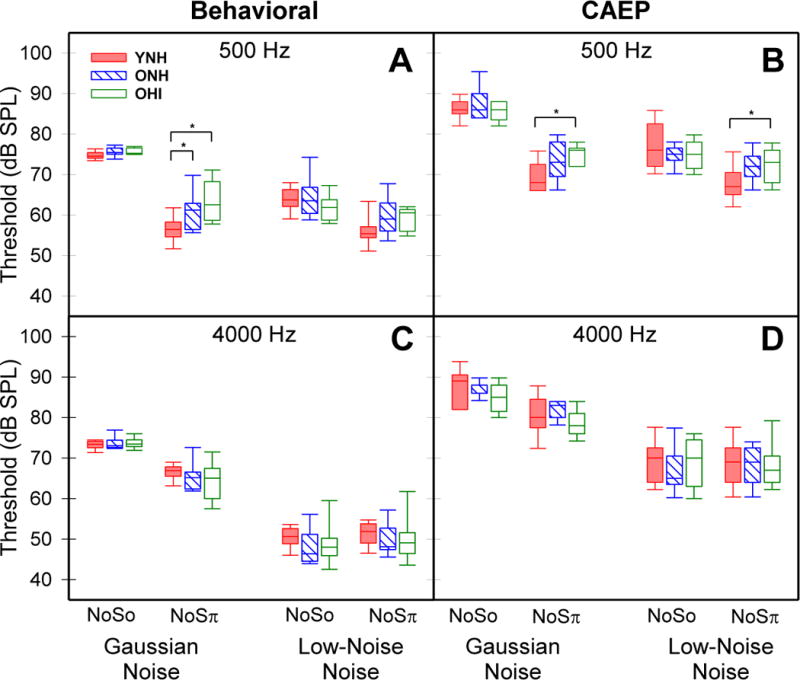
Behavioral and CAEP thresholds for 500-Hz (panels A, B) and 4000-Hz (panels C, D) conditions. Homophasic (NoSo), antiphasic (NoSπ), and noise (GN, LNN) conditions are indicated on the abscissa. Horizontal lines represent the median thresholds. Boxes span the 25th to 75th percentiles and vertical bars span the 10th to 90th percentiles. Groups are differentiated by color and box fill patterns with significant differences between groups (p< 0.05) indicated by bars and asterisks.
TABLE 1.
Results of the Kruskal-Wallis H-test and post-hoc pairwise comparison analyses of behavioral and CAEP threshold measures. Post-hoc analyses were performed only when significant results (p-value adjusted, p < 0.05) were identified for the primary measure.
| Measure | Frequency (Hz) | Masker | Signal Condition | Test Statistic χ2 (df) | Pairwise Comparison | Post-hoc (z-score) | p-value (adjusted) |
|---|---|---|---|---|---|---|---|
| Behavioral Threshold | 500 | GN | NoSo | 6.07 (2) | 0.050 | ||
| NoSπ | 12.43 (2) | 0.001* | |||||
| YNH – ONH | 2.95 | 0.010* | |||||
| YNH – OHI | 3.15 | 0.005* | |||||
| ONH - OHI | 0.20 | 1.000 | |||||
| LNN | NoSo | 2.19 (2) | 0.335 | ||||
| NoSπ | 5.04 (2) | 0.080 | |||||
| 4000 | GN | NoSo | 0.19 (2) | 0.910 | |||
| NoSπ | 2.77 (2) | 0.250 | |||||
| LNN | NoSo | 3.79 (2) | 0.150 | ||||
| NoSπ | 3.47 (2) | 0.177 | |||||
| CAEP Threshold | 500 | GN | NoSo | 0.82 (2) | 0.664 | ||
| NoSπ | 8.44 (2) | 0.015* | |||||
| YNH – ONH | 2.01 | 0.113 | |||||
| YNH – OHI | 2.82 | 0.014* | |||||
| ONH - OHI | 0.81 | 1.000 | |||||
| LNN | NoSo | 0.72 (2) | 0.698 | ||||
| NoSπ | 6.98 (2) | 0.031* | |||||
| YNH – ONH | 2.15 | 0.095 | |||||
| YNH – OHI | 2.41 | 0.048* | |||||
| ONH - OHI | 0.28 | 1.000 | |||||
| 4000 | GN | NoSo | 2.63 (2) | 0.268 | |||
| NoSπ | 4.78 (2) | 0.091 | |||||
| LNN | NoSo | 1.34 (2) | 0.512 | ||||
| NoSπ | 0.23 (2) | 0.891 |
p < 0.05
CAEP Thresholds
Figure 3 (right panels) shows the CAEP thresholds for the 500-Hz (panel B) and 4000-Hz (panel D) conditions. The thresholds were on average 10–15 dB SPL higher than behavioral thresholds, yet they demonstrated a similar pattern across conditions and participant groups. No significant group effect was observed for the NoSo threshold conditions at 500 Hz or for the NoSo or NoSπ conditions at 4000 Hz. There was a significant group effect for NoSπ thresholds at 500 Hz in both GN maskers (χ2(2) = 8.44, p = 0.015) and LNN maskers (χ2(2) = 6.98, p = 0.031; see Fig 3C, second and fourth triad of boxes). Post-hoc pairwise comparisons for GN maskers showed that CAEP thresholds for YNH listeners were significantly different from OHI (z = 2.82, p = 0.014) but not from ONH (z = 2.01, p = 0.133) listeners and the two older groups were not different from each other (z = 0.81, p = 1.0). Likewise, for LNN maskers, NoSπ thresholds for YNH listeners were significantly lower than those for OHI (z = 2.40, p = 0.048) but not for ONH (z = 2.15, p = 0.095), while the two older groups were not different from each other (z = 0.26, p = 1.0). The pattern of CAEP thresholds is consistent with that for behavioral thresholds.
Behavioral BMLDs
The BMLD was derived as the difference between the homophasic (NoSo) and antiphasic (NoSπ) thresholds. Figure 4 (left panels) shows the median, 25th–75th and 10th–90th percentile ranges for behavioral BMLDs computed for the GN and LNN maskers for 500-Hz (panel A) and 4000-Hz (panel C) conditions. Statistical results of the Kruskal-Wallis H test and post-hoc analyses for behavioral and CAEP BMLDs are reported in Table 2. For behavioral BMLDs, the results indicated a significant group difference for 500 Hz for both GN maskers (χ2(2) = 10.75, p = 0.005) and LNN maskers (χ2(2) = 10.56, p = 0.005; Fig 4A). Post-hoc pairwise comparisons for GN maskers showed that BMLDs for YNH listeners were significantly larger than those for ONH (z = −2.80, p = 0.016) and for OHI (z = −2.88, p = 0.012) listeners, while the older groups were not different from each other (z = −0.9, p = 1.0). Although the BMLDs for LNN maskers were smaller than those for GN, pairwise comparisons again showed that BMLDs for YNH listeners were significantly larger than those for ONH (z = −2.54, p = 0.033) and for OHI (z = −3.02, p = 0.007) listeners, but the older groups were not different from each other (z = −0.50, p = 1.0). There were no significant group differences for BMLDs at 4000 Hz for either noise masker.
Figure 4.
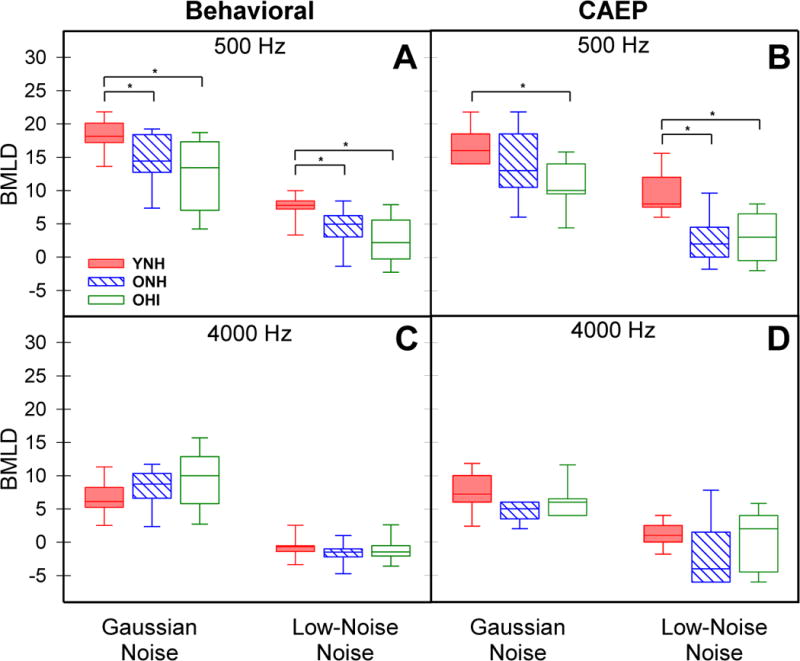
Behavioral and CAEP BMLDs derived from NoSo and NoSπ thresholds for 500-Hz (panels A, B) and 4000-Hz (panels C, D) conditions. Noise (GN, LNN) conditions are indicated on the abscissa. Groups are differentiated by color and box fill patterns with significant differences (p< 0.05) between groups indicated by bars and asterisks.
TABLE 2.
Results of the Kruskal-Wallis H-test and post-hoc pairwise comparison analyses of behavioral and CAEP BMLD measures.
| Measure | Frequency (Hz) | Masker | Test Statistic χ2 (df) | Pairwise Comparison | Post-hoc (z-score) | p-value (adjusted) |
|---|---|---|---|---|---|---|
| Behavioral BMLD | 500 | GN | 10.75 (2) | 0.005* | ||
| YNH – ONH | −2.79 | 0.012* | ||||
| YNH – OHI | −2.88 | 0.016* | ||||
| ONH - OHI | −0.09 | 1.000 | ||||
| LNN | 10.56 (2) | 0.005* | ||||
| YNH – ONH | −2.54 | 0.033* | ||||
| YNH – OHI | −3.02 | 0.007* | ||||
| ONH - OHI | −0.48 | 1.000 | ||||
| 4000 | GN | 3.69 (2) | 0.158 | |||
| LNN | 1.64 (2) | 0.440 | ||||
| CAEP BMLD | 500 | GN | 9.27 (2) | 0.010* | ||
| YNH – ONH | −1.60 | 0.330 | ||||
| YNH – OHI | −3.04 | 0.007* | ||||
| ONH - OHI | −1.45 | 0.445 | ||||
| LNN | 13.78 (2) | 0.001* | ||||
| YNH – ONH | −3.29 | 0.003* | ||||
| YNH – OHI | −3.13 | 0.005* | ||||
| ONH - OHI | 0.17 | 1.000 | ||||
| 4000 | GN | 4.73 (2) | 0.094 | |||
| LNN | 3.18(2) | 0.204 |
p < 0.05
CAEP BMLDs
Figure 4 (right panels) shows the median, 25th–75th and 10th–90th percentile ranges for CAEP BMLDs for 500-Hz (panel B) and 4000-Hz (panel D) stimulus conditions. The pattern of BMLDs estimated from CAEP thresholds was similar to that observed for behavioral BMLDs across both noise and frequency conditions. Results of the Kruskal-Wallis H test indicated a significant group difference in the CAEP BMLD for 500 Hz in both GN maskers (χ2(2) = 9.274, p = 0.010) and LNN maskers (χ2(2) = 13.783, p = 0.001; see Fig 4B). Post-hoc pairwise comparisons for GN maskers showed that BMLDs for YNH listeners were significantly larger than those for OHI (z = −3.04, p=0.007) listeners but not for ONH (z = −1.60, p = 0.33). The two older groups also were not different from each other (z = −1.45, p = 0.445). Similar to the behavioral BMLD results, the LNN maskers led to smaller CAEP BMLDs than GN maskers for all groups, yet group differences were still observed at 500 Hz. Post-hoc comparisons showed that BMLDs for YNH listeners were larger than those for ONH (z = −3.30, p = 0.003) and OHI (z = −3.13, p = 0.005) listeners. The older groups were not different from each other (z = 0.17, p = 1.0). There were no significant group differences for CAEP BMLDs at 4000 Hz for either noise masker.
N1 and P2 Latencies and Amplitudes
Figure 5 illustrates grand average CAEP responses for each group for the four 500-Hz (left) and 4000-Hz (right) stimulus conditions, respectively. The averages were computed from individual responses measured at +20 dB SL relative to their behavioral thresholds for each condition. To account for absolute threshold differences across listeners, group effects for N1 and P2 latencies and N1-P2 peak-to-peak amplitudes were evaluated at +20 dB SL for 500-Hz and 4000-Hz stimuli in both masker (GN, LNN) and phasic (NoSo, NoSπ) conditions. Statistical results from the Kruskal-Wallis H test and post-hoc analyses for both N1 and P2 latencies are reported in Table 3.
Figure 5.
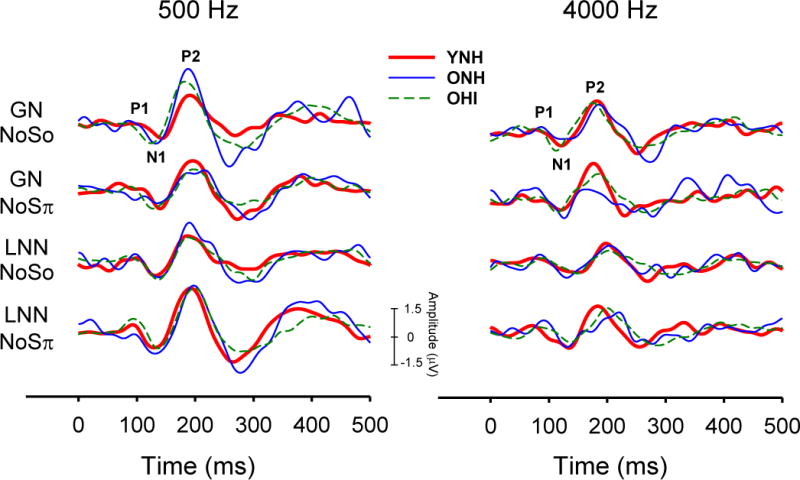
Grand average CAEP responses measured at +20 dB sensation level (re: each individual’s behavioral threshold) are shown for each group and each of four conditions for 500-Hz (left) and 4000-Hz (right) stimuli.
TABLE 3.
Results of the Kruskal-Wallis H-test and post-hoc pairwise comparison analyses of CAEP N1 and P2 latency measures.
| Measure | Frequency (Hz) | Masker | Signal Condition | Test Statistic χ2 (df) | Pairwise Comparison | Post-hoc (z-score) | p-value (adjusted) |
|---|---|---|---|---|---|---|---|
| N1 Latency | 500 | GN | NoSo | 7.247 (2) | 0.027* | ||
| YNH – ONH | −2.41 | 0.047* | |||||
| YNH – OHI | −2.24 | 0.076 | |||||
| ONH - OHI | 0.18 | 1.000 | |||||
| NoSπ | 2.42 (2) | 0.298 | |||||
| LNN | NoSo | 2.95 (2) | 0.229 | ||||
| NoSπ | 1.67 (2) | 0.433 | |||||
| 4000 | GN | NoSo | 9.99 (2) | 0.007* | |||
| YNH – ONH | −2.03 | 0.126 | |||||
| YNH – OHI | −3.11 | 0.006* | |||||
| ONH - OHI | −1.08 | 0.840 | |||||
| NoSπ | 1.37 (2) | 0.503 | |||||
| LNN | NoSo | 7.33 (2) | 0.026* | ||||
| YNH – ONH | −2.34 | 0.058 | |||||
| YNH – OHI | 0.01 | 0.056 | |||||
| ONH - OHI | 2.35 | 1.000 | |||||
| NoSπ | 2.76 (2) | 0.252 | |||||
| P2 Latency | 500 | GN | NoSo | 2.19 (2) | 0.334 | ||
| NoSπ | 4.103 (2) | 0.129 | |||||
| LNN | NoSo | 7.685 (2) | 0.021* | ||||
| YNH – ONH | 2.74 | 0.018* | |||||
| YNH – OHI | 1.72 | 0.259 | |||||
| ONH - OHI | −1.03 | 0.911 | |||||
| NoSπ | 2.15 (2) | 0.341 | |||||
| 4000 | GN | NoSo | 8.55 (2) | 0.014* | |||
| YNH – ONH | 1.54 | 0.372 | |||||
| YNH – OHI | −1.39 | 0.498 | |||||
| ONH - OHI | −2.92 | 0.010* | |||||
| NoSπ | 7.88 (2) | 0.020* | |||||
| YNH – ONH | 2.63 | 0.026* | |||||
| YNH – OHI | 2.13 | 0.098 | |||||
| ONH - OHI | −0.49 | 1.000 | |||||
| LNN | NoSo | 5.67 (2) | 0.059 | ||||
| NoSπ | 6.56 (2) | 0.038* | |||||
| YNH – ONH | 2.52 | 0.036* | |||||
| YNH – OHI | 0.84 | 1.000 | |||||
| ONH - OHI | −1.68 | 0.281 |
p < 0.05
There were significant group effects for N1 latency in two conditions with GN maskers; 500 Hz, NoSo (χ2(2) = 7.243, p = 0.027) and 4000 Hz NoSo (χ2(2) = 9.993, p = 0.007). For the 500-Hz condition, pairwise comparisons showed that YNH listeners had significantly longer N1 latencies than ONH (z = −2.41, p = 0.05) but not OHI listeners (z = −2.24, p = 0.08). In contrast, pairwise comparisons for the 4000-Hz NoSo condition showed YNH had significantly longer N1 latencies than OHI (z = −3.11, p = 0.01) but not ONH listeners (z = −2.03, p = 0.13). The older groups did not differ from one another in any condition (see Figure, Supplemental Digital Content 1, which illustrates N1 latencies across groups and stimulus conditions).
Analyses of group differences for P2 latencies were also completed for measures obtained at +20 dB SL and indicated significant differences in four of the eight test conditions. For 500 Hz, LNN-NoSo (χ2(2) = 7.685, p = 0.021), ONH had longer P2 latencies than YNH (z=2.74, p=0.01) but no other group differences were observed. For 4000 Hz, GN-NoSo (χ2(2) = 8.553, p = 0.014), the only group difference showed that ONH had longer P2 latencies than OHI (z = −2.92, p = 0.01). For 4000 Hz, GN-NoSπ (χ2(2) = 7.81, p = 0.020), ONH had longer P2 latencies than YNH (z = 2.63, p = 0.026) but no other group differences existed. Finally, for 4000 Hz LNN-NoSπ (χ2(2) = 6.56, p = 0.038), ONH had longer P2 latencies than YNH (z = 2.52, p = 0.04). While there were no consistent patterns based on frequency or masker type, the pairwise comparisons for each of the four conditions indicated that P2 latencies were always longer for ONH than either YNH or OHI listeners. All group differences were relatively small in absolute value (see Figure, Supplemental Digital Content 2, which illustrates P2 latencies across groups and stimulus conditions). Statistical results of the Kruskal-Wallis H test for N1-P2 amplitudes, reported in Table 4, revealed no significant group differences across the eight stimulus conditions (see Figure, Supplemental Digital Content 3, which illustrates N1-P2 amplitudes across groups and stimulus conditions, and Table, Supplemental Digital Content 4, which includes latency and amplitude mean data).
TABLE 4.
Results of the Kruskal-Wallis H-test and post-hoc pairwise comparison analyses of CAEP N1-P2 amplitude measures.
| Measure | Frequency (Hz) | Masker | Signal Condition | Test Statistic χ2 (df) | Pairwise Comparison | Post-hoc (z-score) | p-value (adjusted) |
|---|---|---|---|---|---|---|---|
| N1-P2 Amplitude | 500 | GN | NoSo | 6.08 (2) | 0.050 | ||
| NoSπ | 4.34 (2) | 0.114 | |||||
| LNN | NoSo | 2.51 (2) | 0.285 | ||||
| NoSπ | 0.79 (2) | 0.673 | |||||
| 4000 | GN | NoSo | 3.52 (2) | 0.172 | |||
| NoSπ | 0.40 (2) | 0.821 | |||||
| LNN | NoSo | 0.58 (2) | 0.765 | ||||
| NoSπ | 0.68 (2) | 0.711 |
p < 0.05
Behavioral and Electrophysiological Correlations
Figure 6 shows the CAEP BMLDs plotted as a function of behavioral BMLDs for all 30 participants across all frequency, phase, and noise conditions. Pearson product moment correlations were performed for each group to quantify the correlation between behavioral and physiological BMLDs. There was a significant and orderly relationship for all groups with YNH showing the strongest correlation (r = 0.89, p < 0.01), followed by ONH (r = 0.80, p < 0.01) and OHI (r = 0.68, p < 0.01). Using a regression analysis, the slopes of the functions were not significantly different between the YNH and ONH groups (t = −0.405, p=0.686), but the slope for the OHI group was different from YNH (t = 2.310, p=0.024) and ONH (t = 2.239, p=0.028). These results support the contention that BMLDs derived from CAEP thresholds provide a robust neurophysiological index of binaural temporal processing across age and hearing loss.
Figure 6.
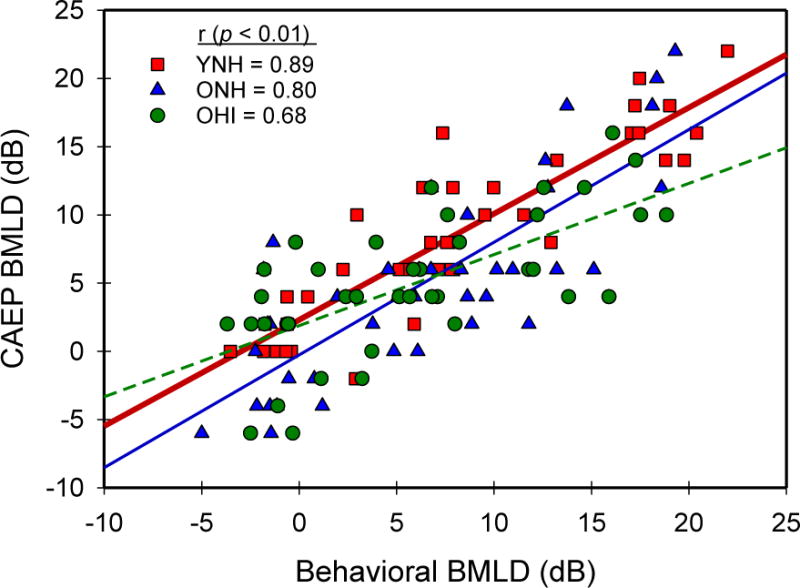
Scatterplot of behavioral and CAEP BMLDs for each participant across all 8 frequency, phase and noise conditions. Groups are differentiated by colored symbols and lines representing linear regression fits of the data. Pearson-product correlation values for each group are indicated in the inset. All correlations were significant (p< 0.01) for each group, and only the OHI group was significantly different (p< 0.01) from the other two groups (YNH, ONH).
DISCUSSION
Using behavioral and electrophysiological methods, the present study investigated masked tone thresholds using the BMLD paradigm and several stimulus manipulations designed to evaluate the potential effects of age and hearing loss on processing of binaural temporal fine structure and binaural temporal envelope cues. The availability of usable temporal fine structure cues was controlled by varying the signal frequency (500 and 4000 Hz) and the availability of usable temporal envelope cues was manipulated by varying the noise type (GN and LNN).
For both behavioral and CAEP measures, there was no effect of group on NoSo thresholds. Thus, masked tone thresholds without binaural cues were not impacted substantially by increasing age or the presence of hearing loss. For the dichotic conditions, comparisons across the various conditions allow a differential evaluation of the contribution of temporal envelope and fine structure cues to the BMLD.
In the 500-Hz GN condition, where robust fine structure and envelope cues should be available, ONH and OHI have smaller behavioral BMLDs than YNH, and OHI have smaller CAEP BMLDs than YNH. This could be due to an age-related reduction in the ability to use either binaural temporal fine structure cues or binaural temporal envelope cues. When comparing the 500-Hz GN and LNN maskers, where the latter effectively minimizes the temporal envelope cues, both behavioral and CAEP BMLDs are reduced by about the same amount for all groups, and the significant group differences between YNH and the two older groups are maintained. These data indicate that all groups rely on binaural temporal envelope cues to the same degree – a result that is consistent with the hypothesis that older listeners are less able to benefit from binaural temporal fine structure cues than younger listeners.
In the 4000-Hz GN condition, where fine structure cues are not usable by the auditory system, the BMLDs are smaller for all groups but the age difference no longer exists. This indicates that the use of binaural temporal envelope cues is similar across all groups and the age differences at 500 Hz were associated with an age-related reduction in the use of binaural temporal fine structure cues. The BMLDs again were reduced (from ~8 dB to 0 dB) in LNN compared to GN maskers by the same amount for all groups. The complete elimination of the BMLD in LNN at 4000 Hz emphasizes the reliance on binaural temporal envelopes cues for BMLDs at high frequencies.
Changes in behavioral thresholds associated with the temporal manipulations used here were consistent with previous psychophysical results employing similar stimulus conditions (e.g., Eddins & Barber, 1998; Hall et al., 1998). Thresholds estimated from CAEP recordings also were consistent with previous CAEP studies investigating the BMLD (e.g., Fowler & Mikami, 1992a, b), even though we did not use identical stimulus frequencies or noise types. Beyond the BMLD, previous psychophysical and electrophysiological studies have demonstrated age-related declines in binaural temporal fine structure coding using alternative stimulus paradigms, such as interaural phase discrimination (Grose & Mamo, 2010; Ross et al., 2007). Here, we extend these results by examining changes in how the auditory system processes both temporal fine structure and temporal envelope cues with advancing age (YNH vs ONH) and hearing loss (ONH vs OHI). There were no group differences for any of the high-frequency conditions, where the BMLD is derived presumably from binaural temporal envelope cues. This indicates that the use of temporal envelope cues in the BMLD paradigm is not markedly compromised by aging. In contrast, reduced behavioral and CAEP BMLDs with age in the low-frequency conditions are consistent with reduced temporal fine structure coding as has been shown with other binaural paradigms (e.g., Grose & Mamo, 2010; Papesh et al., 2017; Ross et al., 2007).
The observed age-effects in our data differ from those reported previously by Dubno et al. (2008). They measured behavioral BMLDs for 500-Hz tones in GN and LNN maskers for a group of younger and older normal-hearing adults and showed no differences between groups. It is likely that the source of these cross-study differences is rooted in methodological differences known to influence the magnitude of masking release such as the choice of noise bandwidth, continuous vs. gated maskers, and LNN stimulus generation techniques. Though it seems less likely, it also is possible that the respective studies sampled subjects from different regions of the same distribution.
More detailed analyses of the CAEP data using the common physiological measures of N1 and P2 latency and N1-P2 amplitude obtained from a single supra-threshold signal level for each participant (+20 dB SL) failed to provide clear differences. The variability across conditions and groups, which was likely impacted by the relatively small sample sizes of 10 per group, rendered both latencies and amplitudes less reliable indices of age-related changes in binaural temporal processing than thresholds and derived BMLDs. Nonetheless, some orderliness in the pattern of results was observed, such as longer latencies for 500- versus 4000-Hz stimuli for many of the conditions.
Although this study was not explicitly designed to assess the clinical utility of using the CAEP to measure the BMLD, the electrophysiological recording methods used here (i.e., electrode montage, threshold search procedure) lend themselves to clinical implementation. Future research may extend the findings reported here by examining binaural temporal fine structure coding over a broader age range to determine when such coding starts to decline and at what rate. It also would be beneficial to include a broader age range of hearing-impaired participants to have a more balanced evaluation of the contributions of age and hearing loss to changes in binaural temporal processing. In addition, it would be useful to evaluate potential improvements in perceptual performance and underlying neural encoding that might occur following different forms of intervention (i.e., assistive technology, amplification, aural rehabilitation, auditory training). Finally, more detailed electrophysiological recordings using an increased number of electrodes and advanced neurophysiological analyses (i.e., source localization) may provide insight into the nature of and the cortical regions associated with the decline in temporal processing with age.
Conclusions
Using a parametric approach to assess changes in central auditory processing of binaural temporal envelope and fine structure cues, this study demonstrated an age-related decline in binaural temporal fine structure coding with preserved binaural temporal envelope coding. We also determined that such changes can be reliably measured and indexed using either behavioral or CAEP threshold measurement techniques. The BMLDs derived from each technique were strongly correlated across all conditions and participant groups, and both derived measures revealed age-related declines in processing binaural temporal fine structure cues.
Supplementary Material
Supplemental Digital Content 1: Figure that illustrates N1 latencies compared across groups and test conditions.pptx.
Supplemental Digital Content 2: Figure that illustrates P2 latencies compared across groups and test conditions.pptx.
Supplemental Digital Content 3: Figure that illustrates N1-P2 peak-to-peak amplitudes compared across groups and test conditions.pptx.
Supplemental Digital Content 4: Table of means and standard deviations of N1 and P2 latencies and N1-P2 peak-to-peak amplitudes computed across groups and test conditions.doc.
Acknowledgments
Both authors contributed to experimental design, data collection and analysis, and manuscript preparation. In addition, the authors thank Makenzie Kline, Bryce Romershausen, and Michelle Arnold for their contributions to data collection and analysis. This research was supported in part by a program project grant from the National Institutes of Health, National Institute of Aging (P01-AG09524).
Footnotes
Portions of this work were presented at the Acoustical Society of America fall meeting, Hong Kong, China, December, 2012, the Association for Research in Otolaryngology Midwinter meeting, February, 2013, and the Aging and Speech Communication conference, October, 2013.
References
- Bernstein LR, Trahiotis C. The normalized correlation: accounting for binaural detection across center frequency. J Acoust Soc Am. 1996;100(6):3774–3784. doi: 10.1121/1.417237. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. How sensitivity to ongoing interaural temporal disparities is affected by manipulations of temporal features of the envelopes of high-frequency stimuli. J Acoust Soc Am. 2009;125(5):3234–3242. doi: 10.1121/1.3101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Accounting quantitatively for sensitivity to envelope-based interaural temporal disparities at high frequencies. J Acoust Soc Am. 2010;128(3):1224–1234. doi: 10.1121/1.3466877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LR, van de Par S, Trahiotis C. The normalized interaural correlation: accounting for NoS pi thresholds obtained with Gaussian and “low-noise” masking noise. J Acoust Soc Am. 1999;106(2):870–876. doi: 10.1121/1.428051. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. The masking level difference for signals placed in masker envelope minima and maxima. J Acoust Soc Am. 2003;114(3):1557–1564. doi: 10.1121/1.1598199. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Ahlstrom JB, Horwitz AR. Binaural advantage for younger and older adults with normal hearing. J Speech Hear Res. 2008;51(2):539–556. doi: 10.1044/1092-4388(2008/039). [DOI] [PubMed] [Google Scholar]
- Durlach NI, Colburn HS. Binaural phenomena. Handbook of perception. 1978;4:365–466. [Google Scholar]
- Eddins DA, Barber LE. The influence of stimulus envelope and fine structure on the binaural masking level difference. J Acoust Soc Am. 1998;103(5 Pt 1):2578–2589. doi: 10.1121/1.423112. [DOI] [PubMed] [Google Scholar]
- Fastl H, Zwicker E. Psychoacoustics : facts and models. 3rd. Berlin; New York: Springer; 2007. [Google Scholar]
- Fowler CG, Mikami CM. Effects of noise bandwidth on the late-potential masking level difference. Electroencephalogr Clin Neurophysiol. 1992a;84(2):157–163. doi: 10.1016/0168-5597(92)90020-c. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Mikami CM. The late auditory evoked potential masking-level difference as a function of noise level. J Speech Hear Res. 1992b;35(1):216–221. doi: 10.1044/jshr.3501.216. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Mikami CM. Phase effects on the middle and late auditory evoked potentials. J Am Acad Audiol. 1996;7(1):23–30. [PubMed] [Google Scholar]
- Goupell MJ. The role of envelope statistics in detecting changes in interaural correlation. J Acoust Soc Am. 2012;132(3):1561–1572. doi: 10.1121/1.4740498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear. 2010;31(6):755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Poth EA, Peters RW. Masking level differences for tones and speech in elderly listeners with relatively normal audiograms. J Speech Hear Res. 1994;37(2):422–428. doi: 10.1044/jshr.3702.422. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH, Hartmann WM. The masking-level difference in low-noise noise. J Acoust Soc Am. 1998;103(5 Pt 1):2573–2577. doi: 10.1121/1.422778. [DOI] [PubMed] [Google Scholar]
- Hall JW, Harvey AD. The binaural masking level difference as a function of frequency, masker level and masking bandwidth in normal-hearing and hearing-impaired listeners. Audiology. 1985;24(1):25–31. doi: 10.3109/00206098509070094. [DOI] [PubMed] [Google Scholar]
- Hartmann WM, Pumplin J. Noise power fluctuations and the masking of sine signals. J Acoust Soc Am. 1988;83(6):2277–2289. doi: 10.1121/1.396358. [DOI] [PubMed] [Google Scholar]
- Hartmann WM, Pumplin J. Periodic signals with minimal power fluctuations. J Acoust Soc Am. 1991;90(4 Pt 1):1986–1999. doi: 10.1121/1.401678. [DOI] [PubMed] [Google Scholar]
- Hirsh IJ. The Influence of Interaural Phase on Interaural Summation and Inhibition. J Acoust Soc Am. 1948;20(4):9. [Google Scholar]
- Ishida IM, Stapells DR. Does the 40-Hz auditory steady-state response show the binaural masking level difference? Ear Hear. 2009;30(6):713–715. doi: 10.1097/AUD.0b013e3181b61cc8. [DOI] [PubMed] [Google Scholar]
- Kohlrausch A, Fassel R, vanderHeijden M, Kortekaas R, vandePar S, Oxenham AJ, Puschel D. Detection of tones in low-noise noise: Further evidence for the role of envelope fluctuations. Acustica. 1997;83(4):659–669. [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(2, Suppl 2):467+. [PubMed] [Google Scholar]
- Palmer AR, Russell IJ. Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear Res. 1986;24(1):1–15. doi: 10.1016/0378-5955(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Papesh MA, Folmer RL, Gallun FJ. Cortical Measures of Binaural Processing Predict Spatial Release from Masking Performance. Front Hum Neurosci. 2017;11:124. doi: 10.3389/fnhum.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA. Masking-level differences in the elderly: a comparison of antiphasic and time-delay dichotic conditions. J Speech Hear Res. 1991;34(6):1410–1422. doi: 10.1044/jshr.3406.1410. [DOI] [PubMed] [Google Scholar]
- Pumplin J. Low-noise noise. J Acoust Soc Am. 1985;78:100–104. [Google Scholar]
- Rice SO. Mathematical analysis of random noise. In: Wax N, editor. Selected papers on noise and stochastic processes. New York: Dover Press; 1954. pp. 133–294. [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27(42):11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104(4):2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Moncrieff DW, Townsend EA, Pillion AL. Development of a 500-Hz masking-level difference protocol for clinic use. J Am Acad Audiol. 2003;14(1):1–8. doi: 10.3766/jaaa.14.1.2. [DOI] [PubMed] [Google Scholar]
- Wong WY, Stapells DR. Brain stem and cortical mechanisms underlying the binaural masking level difference in humans: an auditory steady-state response study. Ear Hear. 2004;25(1):57–67. doi: 10.1097/01.AUD.0000111257.11898.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Figure that illustrates N1 latencies compared across groups and test conditions.pptx.
Supplemental Digital Content 2: Figure that illustrates P2 latencies compared across groups and test conditions.pptx.
Supplemental Digital Content 3: Figure that illustrates N1-P2 peak-to-peak amplitudes compared across groups and test conditions.pptx.
Supplemental Digital Content 4: Table of means and standard deviations of N1 and P2 latencies and N1-P2 peak-to-peak amplitudes computed across groups and test conditions.doc.


