Abstract
Degradation of fatty acids having cis-double bonds on even-numbered carbons requires the presence of auxiliary enzymes in addition to the enzymes of the core β-oxidation cycle. Two alternative pathways have been described to degrade these fatty acids. One pathway involves the participation of the enzymes 2,4-dienoyl-coenzyme A (CoA) reductase and Δ3-Δ2-enoyl-CoA isomerase, whereas the second involves the epimerization of R-3-hydroxyacyl-CoA via a 3-hydroxyacyl-CoA epimerase or the action of two stereo-specific enoyl-CoA hydratases. Although degradation of these fatty acids in bacteria and mammalian peroxisomes was shown to involve mainly the reductase-isomerase pathway, previous analysis of the relative activity of the enoyl-CoA hydratase II (also called R-3-hydroxyacyl-CoA hydro-lyase) and 2,4-dienoyl-CoA reductase in plants indicated that degradation occurred mainly through the epimerase pathway. We have examined the implication of both pathways in transgenic Arabidopsis expressing the polyhydroxyalkanoate synthase from Pseudomonas aeruginosa in peroxisomes and producing polyhydroxyalkanoate from the 3-hydroxyacyl-CoA intermediates of the β-oxidation cycle. Analysis of the polyhydroxyalkanoate synthesized in plants grown in media containing cis-10-heptadecenoic or cis-10-pentadecenoic acids revealed a significant contribution of both the reductase-isomerase and epimerase pathways to the degradation of these fatty acids.
Degradation of fatty acids is mediated by the β-oxidation cycle (Kunau et al., 1995). In mammalian cells both mitochondria and peroxisomes possess the enzymes of the β-oxidation cycle, whereas most fungi, including Candida tropicalis and Saccharomyces cerevisiae, have only a peroxisomal β-oxidation pathway. Although it is well established that peroxisomes are also the main site of fatty acid degradation in plants (Gerhardt, 1993; Kindl, 1993), there are studies suggesting the presence of some of the β-oxidation enzymes in mitochondria (Dieuaide et al., 1993; Gerhardt et al., 1995; Bode et al., 1999).
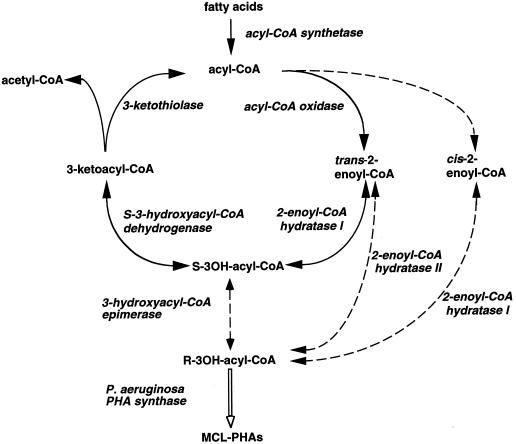
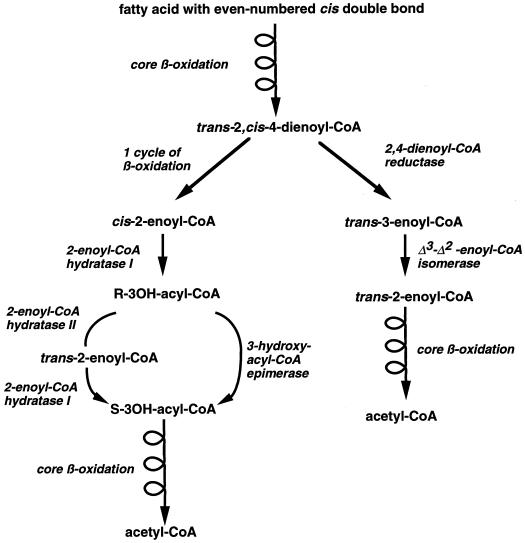
Degradation of saturated fatty acids by the core β-oxidation pathway requires the presence of four enzyme activities (Fig. 1). The first enzyme is either in the mitochondria an acyl-coenzyme A (CoA) dehydrogenase or in the peroxisomes an acyl-CoA oxidase, both enzymes converting acyl-CoAs to trans-2-enoyl-CoAs. The enzyme 2-enoyl-CoA hydratase I then hydrates the trans-2-enoyl-CoA to the S-isomer of 3-hydroxyacyl-CoA, which is subsequently converted to 3-ketoacyl-CoA by the S-3-hydroxyacyl-CoA dehydrogenase. The last enzyme, 3-ketothiolase, completes the cycle by cleaving 3-ketoacyl-CoA to generate acetyl-CoA and acyl-CoA. The core β-oxidation enzymes are not capable of completely degrading unsaturated fatty acids with cis double bonds at an even-numbered carbon since hydration of cis-2-enoyl-CoA by the 2-enoyl-CoA hydratase I generates the R-isomer of 3-hydroxyacyl-CoA, which is not a substrate for the S-3-hydroxyacyl-CoA dehydrogenase. Additional enzymes have thus been described, which contribute to β-oxidation to avoid this metabolic block created by unsaturated bonds (Hiltunen et al., 1996). Two pathways have been proposed for the degradation of fatty acids having cis-double bonds at even-numbered carbons (Fig. 2; Schulz and Kunau, 1987). In one pathway, the unsaturated fatty acid is degraded by the core β-oxidation cycle to trans-2,cis-4-enoyl-CoA, which is subsequently reduced to trans-3-enoyl-CoA by the 2,4-dienoyl-CoA reductase. The trans-3-enoyl-CoA is then converted to trans-2-enoyl-CoA by the enzyme Δ3-Δ2-enoyl-CoA isomerase before returning to the core β-oxidation cycle (Fig. 2). In an alternative pathway, the unsaturated fatty acid is degraded by the core β-oxidation enzymes to R-3-hydroxyacyl-CoA, which is then epimerized to S-3-hydroxyacyl-CoA before rejoining the β-oxidation cycle. Epimerization can be achieved either directly by a 3-hydroxyacyl-CoA epimerase or indirectly by the combined action of a 2-enoyl-CoA hydratase II (also called R-3-hydroxyacyl- CoA hydro-lyase), converting R-3-hydroxyacyl-CoA to 2-trans-enoyl-CoA, and the 2-enoyl-CoA hydratase I, converting 2-trans-enoyl-CoA to S-3-hydroxyacyl-CoA (Fig. 2; Engeland and Kindl, 1991; Gerhardt 1992; Kindl, 1993).
Figure 1.
Core β-oxidation cycle and synthesis of medium chain length-polyhydroxyalkanoate (MCL-PHA) in transgenic plants. Enzymatic reactions involved in the core β-oxidation cycle are indicated by solid lines. Reactions involved in the synthesis of R-3-hydroxyacyl-CoA are shown by dashed lines and the synthesis of MCL-PHAs through the expression of the P. aeruginosa PHA synthase is shown by a white arrow.
Figure 2.
β-Oxidation of fatty acids with cis-double bonds on even-numbered carbons. The main branch on the right is referred as the reductase-isomerase pathway, whereas the main branch on the left is referred as the epimerase pathway. In the epimerase pathway, conversion of S-3-hydroxyacyl-CoA to R-3-hydroxyacyl-CoA can be mediated either via a 3-hydroxyacyl-CoA epimerase or the combined action of the 2-enoyl-CoA hydratase I and 2-enoyl-CoA hydratase II.
Only the reductase-isomerase pathway occurs in mammalian mitochondria since this organelle does not have detectable epimerase activity (Chu and Schulz, 1985). In contrast, in peroxisomes of mammals and plants, as well as in bacteria, the epimerase and the reductase-isomerase pathways appear operative (Chu and Schulz, 1985; Yang et al., 1986; Behrends et al., 1988). Two main approaches have been used to determine the relative importance of the two alternative pathways for the degradation of fatty acids having cis-double bonds at even-numbered carbons. Kinetic analysis of the degradation of trans-2,cis-4-decadienoyl-CoA in rat liver peroxisomes and Escherichia coli have shown that even-numbered unsaturated fatty acids are overwhelmingly degraded by the reductase-isomerase pathway in these organisms (Yang et al., 1986). This conclusion was reinforced by genetic studies showing that inactivation of the gene encoding the 2,4-dienoyl-CoA reductase in E. coli makes the bacterium unable to grow on petroselenic acid (C18:1Δ6cis) whereas growth is normal on acetate or oleic acid (C18:1Δ9cis; You et al., 1989). In contrast, comparisons of enzyme activities present in the cotyledons or isolated peroxisomes of cucumber seedlings indicated that the pathway via 2,4-dienoyl-CoA reductase was much less effective than the epimerase pathway in plants (Behrends et al., 1988; Engeland and Kindl, 1991).
Medium chain length-polyhydroxyalkanoates (MCL-PHAs) are high-Mr polyesters synthesized in a variety of pseudomonads through the polymerization of 3-hydroxyacyl-CoAs generated by the degradation of alkanoic acids by the β-oxidation cycle (Poirier et al., 1995; Steinbüchel and Füchtenbusch, 1998). These polyesters have properties of biodegradable plastics and elastomers and their synthesis in plants is seen as an attractive alternative to bacterial fermentation for their large-scale production at low cost (Poirier et al., 1992, 1995; Poirier, 1999). Synthesis of MCL-PHAs in plants has been achieved by targeting the bacterial PHA synthase from Pseudomonas aeruginosa in the peroxisomes (Mittendorf et al., 1998). In these transgenic plants PHA is synthesized from saturated and unsaturated 3-hydroxyacyl-CoA intermediates generated by the β-oxidation of fatty acids (Fig. 1). Since PHA is made only from the R-isomer of 3-hydroxyacids, it was hypothesized that either the S-3-hydroxyacyl-CoA intermediates generated by the β-oxidation cycle are converted to the R-isomer by a 3-hydroxyacyl-CoA epimerase or that distinct 2-enoyl-CoA hydratases convert 2-trans-enoyl-CoA and 2-cis-enoyl-CoA to R-3-hydroxyacyl-CoAs (Mittendorf et al., 1998; Fig. 1). It has been further shown that the monomer composition of MCL-PHAs produced in transgenic plants grown in liquid cultures can be directly influenced by the addition of fatty acids to the media, demonstrating that external free or esterified fatty acids can enter the β-oxidation cycle and that intermediates are included into MCL-PHAs (Mittendorf et al., 1999). The monomer composition of MCL-PHA thus reflects both the nature and quantity of the fatty acids degraded by the peroxisomal β-oxidation cycle (Mittendorf et al., 1999; Poirier et al., 1999). In the present study we have examined the in vivo contribution of the two alternative pathways for the degradation of fatty acids with double bonds on even-numbered carbons by analyzing the monomer composition of MCL-PHA synthesized in plants fed with cis-10-heptadecenoic and cis-10-pentadecenoic acids.
RESULTS
Synthesis of PHA in Plants Fed with 10-Heptadecenoic Acid
Transgenic plants producing MCL-PHA in their peroxisomes were grown in liquid media containing the detergent Tween-80 and various free fatty acids. Since the amount of odd-chain monomers found in plant MCL-PHAs synthesized from the endogenous pool of fatty acids is relatively low, we have added to the growth media free odd-chain unsaturated fatty acids to clearly distinguish the odd-chain 3-hydroxy acid monomers derived from the external free fatty acids from the even-chain monomers derived from either the endogenous fatty acids or the fatty acids conjugated to Tween-80 (70 mol% oleic acid, with the balance being even-chain fatty acids).
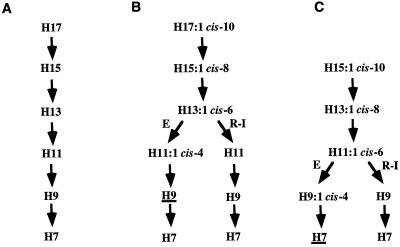
The 3-hydroxyacyl-CoAs generated by the degradation of heptadecanoic acid and cis-10-heptadecenoic acid are shown in Figure 3, A and B. Only the S-isomer of 3-hydroxyacyl-CoAs is directly generated during the degradation of heptadecanoic acid by the core β-oxidation enzymes (Fig. 1). Whereas the degradation of cis-10-heptadecenoic acid by the reductase-isomerase pathway also generates directly only the S-isomer of 3-hydroxyacyl-CoAs, degradation via the epimerase pathway generates R-3-hydroxynonanoyl-CoA (H9; all 3-hydroxyacids are designated by the prefix H; Fig. 3B). The epimerase pathway will also generate the unique CoA intermediate H11:1Δ4, whereas the reductase-isomerase pathway will generate the CoA intermediate H11. Thus degradation of cis-10-heptadecenoic acid via the epimerase or reductase-isomerase pathway could be distinguished by the relative abundance of either H11 and H11:1Δ4 monomers, as well as the amount of H9:0 monomer, in MCL-PHA.
Figure 3.
Spectrum of 3-hydroxyacyl-CoAs generated by the degradation of heptadecanoic acid (A), cis-10-heptadecenoic acid (B), and cis-10-pentadecenoic acid (C). The PHA synthase from P. aeruginosa can only incorporate into MCL-PHA 3-hydroxyacyl-CoAs ranging from six to 16 carbons. The 3-hydroxyacyl-CoAs with less then six carbons have therefore not been indicated. For the degradation of cis-10-heptadecenoic acid and cis-10-pentadecenoic acid, the branched points of the epimerase pathway (E) and the reductase-isomerase pathway (R–I) are indicated. All 3-hydroxyacyl-CoAs generated by the β-oxidation of the saturated and unsaturated fatty acids are in the S configuration, with the exception of R-3-hydroxynonanoyl-CoA and R-3-hydroxyheptanoyl-CoA (underlined in B and C) generated by the degradation of cis-10-heptadecenoic acid and cis-10-pentadecenoic acid, respectively, via the epimerase pathway.
The monomer composition of MCL-PHA purified from plants grown in media supplemented with only Tween-80, or with Tween-80 and free fatty acids, is shown in Table I. As expected, the major changes in the PHA monomer composition created by the addition of heptadecanoic acid to Tween-80 is an increase in the proportion of all odd-chain monomers, ranging from a 50-fold increase of H15 to a 3-fold increase of H7 (Fig. 3A; Table I). When plants are fed with cis-10-heptadecenoic acid and Tween-80, two novel monomers appear in the PHA, namely H15:1 and H13:1. It is striking that the H11:1 monomer predicted to be generated by the epimerase pathway is undetectable in the PHA (Fig. 3B; Table I). Furthermore, the amount of H11 monomer present in the PHA of plants fed with cis-10-heptadecenoic acid is comparable with plants fed with heptadecanoic acid, whereas the amount of H13 and H15 monomers remains very low and is comparable with plants grown in the absence of odd-chain fatty acids. These results are expected if the degradation of cis-10-heptadecenoic acid is mainly mediated by the reductase-isomerase pathway. However, PHA isolated from cultures fed with cis-10-heptadecenoic acid also show a significant increase in proportion of the H9 monomer. Whereas the ratio of H7:H9:H11 monomers in plants fed with Tween-80 and heptadecanoic acid is 1:1.1:0.5, the ratio in plants fed with Tween-80 and cis-10-heptadecenoic acid is 1: 2.4:0.5. Such an increase in H9 can be rationalized by the degradation of cis-10-heptadecenoic acid via the epimerase, which generates the R-isomer of 3-hydroxynonanoyl-CoA, which can be used directly by the PHA synthase for MCL-PHA synthesis. In this context, the absence of the monomer H11:1Δ4 could be explained by the inability of the PHA synthase of P. aeruginosa to use 3-hydroxyacyl-CoA substrates having a double bond at the fourth carbon and adjacent to the hydroxyl group which contributes to the formation of the ester bond in PHA (see “Discussion”).
Table I.
PHA synthesis in transgenic plants fed with odd-chain fatty acids
| Mediumb | PHA Monomer Compositiona
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H6 | H7 | H8:1 | H8 | H9:1 | H9 | H10:1 | H10 | H11:1 | H11 | H12:1 | H12:2 | H12 | H13:1 | H13 | H14:2 | H14:3 | H14:1 | H14 | H15:1 | H15 | H16:2 | H16:3 | H16:1 | H16 | |
| mol % | |||||||||||||||||||||||||
| T-80 | 4.3 | 2.3 | 8.5 | 35 | nd | 2.0 | 1.4 | 11 | nd | 0.5 | 3.3 | 1.6 | 8.3 | nd | 0.1 | 4.4 | 4.0 | 3.9 | 2.5 | nd | 0.04 | 1.9 | 4.0 | 0.3 | 0.8 |
| T-80 + C17 | 4.3 | 6.1 | 4.3 | 32 | nd | 6.9 | 1.1 | 12 | nd | 3.3 | 2.1 | 0.8 | 8.2 | nd | 3.5 | 2.2 | 2.1 | 2.5 | 2.2 | nd | 2.4 | 1.1 | 2.5 | 0.2 | 0.6 |
| T-80 + C17:1c | 4.0 | 5.4 | 8.4 | 25 | nd | 13.2 | 1.5 | 8.2 | nd | 2.0 | 2.5 | 0.6 | 5.8 | 4.4 | 0.2 | 2.9 | 2.7 | 2.7 | 2.1 | 1.6 | 0.07 | 1.7 | 3.9 | 0.2 | 1.1 |
| T-80 + C17:1t | 3.6 | 8.3 | 6.9 | 22 | nd | 9.8 | 1.3 | 7.6 | nd | 5.8 | 2.1 | 1.2 | 5.6 | 8.2 | 0.2 | 2.5 | 2.5 | 2.8 | 2.1 | 1.4 | 0.06 | 1.4 | 3.5 | 0.2 | 1.0 |
| T-80 + C15:1c | 3.2 | 13.4 | 6.3 | 21 | nd | 4.2 | 1.2 | 5.9 | 2.5 | 0.3 | 2.0 | 0.8 | 4.7 | 8.6 | 0.1 | 2.5 | 2.3 | 2.9 | 1.9 | 9.9 | 0.04 | 1.3 | 3.7 | 0.2 | 0.9 |
| T-80 + C15:1t | 3.0 | 8.0 | 5.1 | 23 | nd | 9.1 | 1.3 | 6.2 | 5.5 | 0.3 | 1.6 | 0.7 | 4.9 | 8.7 | 0.1 | 1.9 | 2.1 | 2.7 | 1.4 | 9.2 | 0.03 | 1.5 | 2.7 | 0.2 | 0.6 |
3-Hydroxyacid monomers are denoted by the prefix H. Data are the mean of three to four measurements. se are not indicated for simplification of the table, but are less than 15% of the mean values for all data except for the H14:2 monomer from the T-80 treatment which is 4.4 ± 1.0. nd, Not detected.
T-80, Tween-80; C17, heptadecanoic acid; C17:1c, cis-10-heptadecenoic acid; C17:1t, trans-10-heptadecenoic acid; C15:1c, cis-10-pentadecenoic acid; C15:1t, trans-10-pentadecenoic acid.
Fatty acids having a trans-double bond at the even-numbered carbon can be degraded completely by the core β-oxidation enzymes since only trans-2 enoyl-CoA intermediates would be generated. However, the reductase-isomerase pathway could still act on these fatty acids since the 2,4-dienoyl-CoA reductase can also convert trans-2,trans-4-dienoyl-CoA to trans-3-enoyl-CoA (Dommes and Kunau, 1984; Behrends et al., 1988). Thus the degradation of trans-10-heptadecenoic acid via the reductase-isomerase pathway is expected to generate a similar range of 3-hydroxyacid monomers into PHA as the degradation of cis-10-heptadecenoic acid, including the distinctive H11 monomer. In a similar manner, degradation of trans-10-heptadecenoic acid via the core β-oxidation cycle is expected to generate a range of 3-hydroxyacid monomers into PHA comparable to the degradation of cis-10-heptadecenoic acid via the epimerase pathway, with the notable exception that the 3-hydroxynonanoyl-CoA generated by the degradation of trans-10-heptadecenoic is in the S-configuration, whereas it is in the R-configuration for cis-10-heptadecenoic acid. Results shown in Table I show that feeding plants with Tween-80 and trans-10-heptadecenoic acid leads to the appearance in PHA of the odd-chain unsaturated monomers H15:1 and H13:1, as well as an increase in H11, whereas the H13 and H15 monomers remain low at levels similar to plants fed only with Tween-80. These results indicate that degradation of trans-10-heptadecenoic acid is also mediated by the reductase-isomerase pathway. No H11:1 monomer is detected in PHA isolated from plants fed with trans-10-heptadecenoic acid. However, in contrast to experiments conducted with cis-10-heptadecenoic acid, feeding with the trans isomer does not lead to a significant elevation of the proportion of H9 compared with H7 and H11, the ratio H7:H9:H11 being 1:1.2:0.7. Although these data do not directly indicate whether some fraction of the trans-10-heptadecenoic acid is also degraded by the pathway comprising only the core β-oxidation enzymes, the specific elevation of H9 monomer in PHA isolated from plants fed with the cis isomer and not the trans isomer of 10-heptadecenoic acid supports the hypothesis that a significant proportion of the cis unsaturated fatty acids are degraded via the epimerase pathway.
Synthesis of PHA in Plants Fed with 10-Pentadecenoic Acid
To confirm the degradation of unsaturated fatty acids via both the epimerase and reductase-isomerase pathway in plants, the PHA monomers generated by the degradation of pentadecenoic acid was examined. As shown in Figure 3C, degradation of cis-10-pentadecenoic acid via the reductase-isomerase pathway would generate the H9 intermediate, whereas degradation via the epimerase would generate the H9:1Δ4 intermediate. Furthermore, degradation via the epimerase pathway would also lead to the direct synthesis of the R-isomer 3-hydroxyheptanoyl-CoA, whereas all other 3-hydroxyacyl-CoAs would be generated as S-isomers. PHA from plants fed with either trans-10-pentadecenoic acid or cis-10-pentadecenoic acid contain the odd-chain unsaturated monomers H15:1, H13:1, and H11:1 (Table I). There is also a 2- to 4.5-fold increase in the H9 monomer compared with plants fed only Tween-80 whereas H11, H13, and H15 are as low as in the Tween-80 control plants. These data are again consistent with the degradation of both cis- and trans-10-pentadecenoic acid via the reductase-isomerase pathway. Despite the absence of the monomer H9:1Δ4 in PHA isolated from plants fed with either trans- or cis-10-pentadecenoic acid, the degradation of a portion of the cis unsaturated fatty acid via the epimerase pathway is indicated by the increased in the proportion of the H7 monomer, as shown by the H7:H9 ratio of 1:0.3 for plants fed with cis-10-pentadecenoic acid compared with 1:1.1 for plants fed with either trans-10-pentadecenoic acid or pentadecanoic acid.
Synthesis of PHA in Pseudomonas putida Grown on Heptadecenoic Acid and Pentadecenoic Acid
Studies using purified β-oxidation enzymes from E. coli and Pseudomonas fragii have shown that the intermediate trans-2,cis-4-decadienoyl-CoA is efficiently degraded only via the reductase-isomerase pathway, whereas degradation via the epimerase pathway represents at best only a minor pathway (Yang et al., 1986; Imamura et al., 1990). We have therefore compared the monomer composition of PHA synthesized in P. putida grown on the same fatty acids with that used in the plant feeding experiments (Table II).
Table II.
PHA synthesis in P. putida KT2442 fed with odd-chain fatty acids
| Medium | PHA Monomer Compositiona
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H6 | H7 | H8 | H9:1 | H9 | H10 | H11:1 | H11 | H12 | H13:1 | H13 | H14:1 | H14 | H15:1 | H15 | |
| mol% | |||||||||||||||
| T-80 | 9.2 | 0.9 | 32 | nd | 0.8 | 30 | nd | 0.3 | 12 | nd | 0.03 | 13 | 0.7 | nd | nd |
| T-80 + C17 | 2.8 | 20 | 13 | nd | 31 | 11 | nd | 10 | 3.7 | nd | 4.3 | 2.7 | 0.2 | nd | 1.3 |
| T-80 + C17:1c | 4.0 | 18 | 18 | nd | 25 | 14 | nd | 6.7 | 4.8 | 5.3 | 0.02 | 3.7 | 0.2 | 0.7 | nd |
| T-80 + C17:1t | 1.3 | 25 | 6.1 | nd | 38 | 3.7 | nd | 12 | 1.0 | 11 | 0.02 | 0.7 | 0.06 | 0.8 | nd |
| T-80 + C15:1c | 4.0 | 17 | 17 | nd | 23 | 14 | 7.3 | 0.3 | 5.2 | 5.7 | 0.02 | 4.1 | 0.2 | 1.6 | nd |
| T-80 + C15:1t | 3.3 | 18 | 13 | nd | 26 | 12 | 11 | 0.5 | 4.3 | 5.7 | 0.03 | 3.4 | 0.2 | 1.5 | nd |
Data are the mean of three measurements. se are not indicated for simplification of the table, but are less than 14% and 20% of the mean values for odd-chain and even-chain monomers, respectively. nd, Not detected.
The range of PHA monomers found in P. putida grown on Tween-80 with or without fatty acid is considerably simpler than for plants. This is explained by the fact that in bacteria almost all PHA monomers are derived from the degradation of exogenous fatty acids, whereas in plant PHA a significant proportion of monomers are derived from the degradation of endogenous fatty acids of the reserve triacylglycerides. Growth of bacteria in Tween-80 alone leads to the synthesis of PHA incorporating mostly the degradation intermediates of oleic acid comprised between six and 14 carbons, namely H14:1, H12, H10, H8, and H6 (Table II). Odd-chain monomers represent only a small fraction of the PHA monomers (≈2 mol%) and are probably derived from the α-oxidation of some medium-chain fatty acids. When P. putida is fed with Tween-80 and heptadecanoic acid, there is a major increase in all odd-chain monomers, but clearly some even-chain monomers are still derived from the oleic acid esterified to Tween-80.
The monomer composition of PHA synthesized from both cis- and trans-unsaturated fatty acids shows that the reductase-isomerase is involved in their degradation. This is seen by the increase in the H11 monomer in PHA from bacteria fed with either cis- or trans-10-heptadecenoic acid and the increase in H9 monomer in PHA from bacteria fed with either cis-or trans-10-pentadecenoic acid (Table II). It is also clear that as in the plant feeding experiments, bacterial PHA made from the degradation of both isomers of either heptadecenoic acid or pentadecenoic acid do not contain detectable levels of the H11:1 or H9:1 monomers, respectively. However, in contrast with plant PHA, there is no significant increase in the H9 monomer in bacteria fed with cis-10-heptadecenoic acid compared with trans-10-heptadecenoic acid or heptadecanoic acid, the ratios between the monomers H7:H9:H11 remaining relatively stable at 1:1.4 to 1.5:0.4 to 0.5. In a similar manner, there is no increase in the H7 monomer in bacteria fed with cis-10-pentadecenoic acid compared with trans-10-pentadecenoic acid or pentadecanoic acid. Together, these data indicate that the epimerase pathway contributes substantially less to the degradation of fatty acids with cis-unsaturated bonds at even-numbered carbons in P. putida compared with plants.
DISCUSSION
Complete degradation of fatty acids having cis double bonds at even-numbered carbons requires the participation of auxiliary enzymes acting in concert with the four enzyme activities of the core β-oxidation cycle. This is because hydration of the cis-2-enoyl-CoA intermediate, instead of the usual trans-2-enoyl-CoA, by the 2-enoyl-CoA hydratase I generates the R-isomer of 3-hydroxyacyl-CoA, which cannot be converted to 3-ketoacyl-CoA by the S-3-hydroxyacyl-CoA dehydrogenase of the core β-oxidation cycle. According to the original pathway proposed by Stoffel and Caesar (1965), a 3-hydroxyacyl-CoA epimerase is involved in converting R-3-hydroxyacyl-CoA to the corresponding S-isomer, which can then re-enter the β-oxidation cycle.
Epimerization of R-3-hydroxyacyl-CoA to the S-isomer has been shown to involve several mechanisms. In mammalian peroxisomes, epimerization of 3-hydroxyacyl-CoA was shown to occur by the combined action of two stereospecific hydratases: the classic 2-enoyl-CoA hydratase I of the core β-oxidation complex, converting trans-2-enoyl-CoA to S-3-hydroxyacyl-CoA, and a novel 2-enoyl-CoA hydratase II converting trans-2-enoyl-CoA to R-3-hydroxyacyl-CoA (Hiltunen et al., 1989). Since these two reactions are reversible, epimerization of R-3-hydroxyacyl-CoA would be mediated by the reverse reaction of the 2-enoyl-CoA hydratase II with the forward reaction of the 2-enoyl-CoA hydratase I (Fig. 2). In E. coli and plants, epimerase activity is found associated with the multifunctional protein (MFP), a polypeptide possessing 2-enoyl-CoA hydratase I, S-3-hydroxyacyl-CoA dehydrogenase, Δ3-Δ2-enoyl-CoA isomerase, and 3-hydroxyacyl-CoA epimerase activities (Preisig-Müller et al., 1994; Yang et al., 1995). Mutagenesis experiments with the E. coli MFP indicated that a single amino acid was involved in the dehydration of both R- and S-3-hydroxyacyl-CoA to trans-2-enoyl-CoA, thereby indicating that epimerization occurs also via a dehydration-hydration reaction (Yang et al., 1995). In contrast, mutagenesis experiments with the plant MFP has shown that epimerization is independent of the hydratase activity and does not require the removal of water, implying that the plant enzyme is a true epimerase (Preisig-Müller et al., 1994). In addition, plants also appear to have monofunctional 2-enoyl-CoA hydratase II enzymes (also named R-3-hydroxyacyl-CoA hydro-lyase), thus indicating that epimerization could also occur in plants by the combined action of two stereospecific hydratases like in mammalian peroxisomes (Engeland and Kindl, 1991).
Although 3-hydroxyacyl-CoA epimerase activity has been detected in peroxisomes of mammals and plants, as well as in bacteria, no significant epimerase activity has been found associated with mammalian mitochondria, although this organelle can degrade fatty acids having cis-double bonds at even numbered carbons (Chu and Schulz, 1985). Kunau and Dommes (1978) have described the enzyme 2,4-dienoyl-CoA reductase, which in combination with the Δ3-Δ2-enyol-CoA isomerase, could sequentially convert the intermediate trans-2,cis-4-dienoyl-CoA to trans-3-enyol-CoA and trans-2-enoyl-CoA, and thus avoid a block in the β-oxidation cycle without the need of an epimerase (Fig. 2). 2,4-Dienoyl-CoA reductase and Δ3-Δ2-enyol-CoA isomerase are found in mammalian mitochondria, peroxisomes of mammals, plants, and fungi, as well as in bacteria (Hiltunen et al., 1996). Thus in mammalian mitochondria, only the reductase-isomerase pathway seems to be operative for the degradation of fatty acids with cis-double bonds on even-numbered carbons, whereas both reductase-isomerase and epimerase pathways could contribute to the degradation of these fatty acids in peroxisomes and bacteria.
Estimation of the degradation of the intermediate trans-2,cis-4-dienoyl-CoA through the epimerase and reductase-isomerase pathways indicated that for E. coli and rat liver peroxisomes, only approximately 2% to 3% of the intermediate was metabolized through the epimerase pathway (Yang et al., 1986). The preponderance of the reductase-isomerase pathway in bacteria was also shown by the poor degradation of petroselenic (C18:1Δ6cis) in an E. coli mutant deficient in the enzyme 2,4-dienoyl-CoA reductase (You et al., 1989), as well as the monomer composition of MCL-PHA synthesized in P. putida fed with linoleic acid (de Waard et al., 1993). Furthermore, the reconstituted β-oxidation complex from P. fragi could not completely degrade linoleic acid (C18:2Δ9cis, 12cis) unless 2,4-dienoyl-CoA reductase was added (Imamura et al., 1990). In contrast to these experiments in bacteria and mammalian peroxisomes, Engeland and Kindl (1991) have shown that in purified glyoxysomes or crude extracts of cotyledons of cucumber seedlings, the ratio of 2-enoyl-CoA hydratase II to 2,4-dienoyl-CoA reductase activity was 100:1. The activity of the 2,4-dienoyl-CoA reductase was also low compared with all other enzymes participating in β-oxidation and was thus regarded as rate-limiting (Engeland and Kindl, 1991). Together, these data suggested that in contrast to mammalian peroxisomes and bacteria, plant peroxisomes degrade fatty acids with cis-double bonds on even-numbered carbons mainly by the epimerase pathway and that the reductase-isomerase pathway participates little, if any, to their degradation. It is, however, unclear whether measurements of in vitro enzyme activities adequately reflect the flux through pathways in a cellular environment where numerous substrates must compete for similar enzymes and where micro-environment or substrate channeling may occur.
Transgenic plants expressing the PHA synthase from the bacterium P. aeruginosa in peroxisomes synthesize MCL-PHA derived from the polymerization of a broad range of 3-hydroxyacyl-CoA intermediates of β-oxidation (Mittendorf et al., 1998). It has been previously shown that the monomer composition of the MCL-PHA in plants can be directly influenced by the nature of the fatty acids being degraded by β-oxidation. For example, plants grown in media containing odd-chain or branched-chain fatty acids produce PHA containing an increased proportion of odd-chain and branched-chain 3-hydroxyacid monomers, respectively (Mittendorf et al., 1999). Thus the composition of plant PHA can be used as a tool to analyze how fatty acids are degraded in plants. In this study we have used these transgenic plants to analyze how fatty acids having a double bond at an even-numbered carbon are degraded.
Degradation via the reductase-isomerase pathway of cis- and trans-10-heptadecenoic acid, as well as cis- and trans-10-pentadecenoic acid, in plants and bacteria is clearly shown by the abundance of the H11 and H9 monomers in PHAs, respectively. Direct evidence for degradation of unsaturated fatty acids via the epimerase pathway would have been provided by the presence of the H11:1Δ4 monomer in PHA derived from cis-10-heptadecenoic acid and of the H9:1Δ4 monomer in PHA derived from cis-10-pentadecenoic acid. However, these monomers are not detectable in the respective PHA samples either in plants or bacteria. Nevertheless, indirect evidence for the presence of the epimerase pathway in plants is provided by the increase in the proportion of the H9 monomer in PHA derived from cis-10-heptadecenoic acid relative to PHA derived from trans-10-heptadecenoic acid, as well as the increase in the proportion of the H7 monomer in PHA derived from cis-10-pentadecenoic acid relative to PHA derived from trans-10-pentadecenoic acid. It is reasoned that these monomers are increased because their respective 3-hydroxyacyl-CoA are generated directly as the R-isomer by the epimerase pathway, and thus can be used by the PHA synthase without the need of epimerization. The reason for the absence of the H11:1Δ4 monomer in PHA derived from cis-10-heptadecenoic acid and of the H9:1Δ4 monomer in PHA derived from cis-10-pentadecenoic acid despite the activity of the epimerase pathway is likely due to the inefficiency of the PHA synthase from P. aeruginosa to use 3-hydroxyacyl-CoAs having a double bond on the fourth carbon and near the hydroxyl group participating in the formation of the ester bond. The absence of the monomer H10:2Δ4cis, 7cis in PHA derived from linolenic acid (C18:3Δ9cis, 12cis, 15cis) has been noted previously and reinforces this hypothesis (Mittendorf et al., 1998, 1999). It is also possible that the absence of the H11:1Δ4 and H9:1Δ4 monomers in PHAs may be due to the inability of the plant enzymes to epimerize S-3-hydroxyacyl-CoAs having a double bond on the fourth carbon.
Analysis of the PHA monomer composition from plants grown in the various unsaturated fatty acids does not allow for the precise calculation of the relative flux of fatty acids degraded via the reductase-isomerase and the epimerase pathways. This is in part because the PHA synthase must compete with several other enzymes for the 3-hydroxyacyl-CoAs, including 2-enoyl-CoA hydratase I and S-3-hydroxyacyl-CoA dehydrogenase, and that affinities of all these enzymes for the various saturated and unsaturated 3-hydroxyacyl-CoAs are not known. Nevertheless, the shifts in PHA monomer composition between the various fatty acids are sufficiently large to conclude that the flux of fatty acids toward the reductase-isomerase pathway is quite substantial. For example, levels of the H11 monomer in PHA are in the same range whether the plants were grown in heptadecanoic acid, or cis- or trans-heptadecenoic acid, whereas the monomer H13 is 10 times lower in plants grown in cis- or trans-heptadecenoic acid compared with plants grown in heptadecanoic acid. Such monomer composition would not be expected if the reductase-isomerase was a minor pathway.
A precise determination of the flux toward the epimerase pathway is made difficult because we do not know the in vivo kinetics of the conversion between the R- and S-isomers of 3-hydroxyacyl-CoAs, an important factor considering that the PHA synthase accepts only the R-isomers. Comparison of the PHA monomer composition in plants and bacteria nevertheless clearly indicates that the proportion of unsaturated fatty acids degraded via the epimerase pathway is larger in plants compared with bacteria.
The apparent discrepancy between the present study and the results of Engeland and Kindl (1991), which suggest a minor involvement of the reductase-isomerase pathway, could be explained by the different methodologies used. For example, it may be possible that the 2-enoyl-CoA hydratase II activity detected in cucumber cotyledons may be more involved in sterol metabolism and degradation of branched-chain fatty acids then in the degradation of straight-chain fatty acid, as reported for a similar enzyme in mammals (Dieuaide-Noubhani et al., 1997; Qin et al., 1997). The high ratio between 2-enoyl-CoA hydratase II activity and 2,4-dienoyl-CoA reductase activity detected in cucumber cotyledons (Engeland and Kindl, 1991) may thus have provided a distorted view of the relative importance of the epimerase pathway versus the reductase-isomerase pathway in the degradation of straight-chain unsaturated fatty acids. Because PHA is synthesized in living cells and in an intact subcellular environment, it provides a valuable alternative view of the metabolic pathways participating in fatty acid degradation.
MATERIALS AND METHODS
Bacterial Strain and Plant Material
Pseudomonas putida KT2442 (Bagdasarian et al., 1981) was grown in shake flasks at 28°C. For synthesis of MCL-PHAs, P. putida was first grown overnight in Luria broth, cells were washed in water, and resuspended at a dilution of 1:20 in one-half-strength E2 medium (Lageveen et al., 1988) containing a final concentration of 0.1% (w/v) free fatty acids, 0.4% (v/v) ethanol, and 1.5% (w/v) Tween-80. Cells were harvested after 18 h and washed consecutively with water, 50% (v/v) ethanol, and methanol before being analyzed for PHA. The fatty acids used were heptadecanoic acid, cis-10-pentadecenoic acid, trans-10-pentadecenoic acid, cis-10-heptadecenoic acid, and trans-10-heptadecenoic acid (Nu-Check-Prep, Elysian, MN).
Transgenic Arabidopsis line 3.3 expressing the P. aeruginosa PhaC1 synthase in the peroxisomes has been previously described (Mittendorf et al., 1998). Axenic plants were grown under constant agitation (100 rpm) for 7 d in liquid media containing one-half-strength Murashige and Skoog salts and 1% (w/v) Suc, and then grown for an additional 7 d in the same media supplemented with a final concentration of 0.02% (w/v) free fatty acids, 0.08% (v/v) ethanol, and 1% (w/v) Tween-80. The plant material was harvested, rinsed with water, lyophilized, and frozen until used for PHA extraction.
PHA Extraction and Analysis
Extraction of PHA from plant material and analysis by gas-chromatography and mass spectrometry (GC-MS) was done essentially as previously described (Mittendorf et al., 1998). Dried frozen plant material was ground in a mortar and extracted with methanol in a Soxhlet apparatus for 24 h followed by PHA extraction with chloroform for 24 h. The PHA-containing chloroform was concentrated using a Rotovapor and filtered over glass wool to remove residual solid particles. PHA was precipitated by the addition of 10 volumes of cold methanol and subsequently purified by two cycles of chloroform solubilization and methanol precipitation. PHA dissolved in chloroform was transesterified by acid methanolysis (Huijberts et al., 1992). In some experiments trimethylsilyl derivatization of methyl esters of 3-hydroxyacids was accomplished by the addition of 50 μL of N-methyl-N-(trimethylsilyl)-2,2,2-trifluoroacetamide to 1 mL of the sample and the mixture heated at 80°C for 10 min. The sample was subsequently dried under a nitrogen flux to evaporate the unreacted N-methyl-N-(trimethylsilyl)-2,2,2-trifluoroacetamide and resuspended in chloroform. The esterified PHA monomers were analyzed by GC-MS using a gas chromatograph (5890, HP-5MS column, Hewlett-Packard, Palo Alto, CA) coupled to a 5972 mass spectrometer (Hewlett-Packard). Identification of monomers present in plant PHA was facilitated by the use of commercial standards and purified bacterial PHAs for which the monomer composition was determined by GC-MS as well as heteronuclear NMR (obtained from G. Eggink, ATO-DLO, Wageningen, The Netherlands).
ACKNOWLEDGMENTS
The authors wish to thank Christiane Nawrath and Hans Weber for critical reading of the manuscript as well as Stephanie Stolz and Giovanni Ventre for their excellent technical assistance.
Footnotes
This work was supported in part by a grant from the Herbette Foundation and the État de Vaud. L.A. was a recipient of a fellowship from the Georgine Claraz Foundation.
LITERATURE CITED
- Bagdasarian M, Lurz R, Ruchert B, Franklin FCH, Bagdasarian MM, Frey J, Timmis KN. Specific purpose plasmid cloning vectors: II. Broad host range, high copy number, RFS1010-derived vectors for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Behrends W, Thieringer R, Engeland K, Kunau W-H, Kindl H. The glyoxysomal β-oxidation system in cucumber seedlings: identification of enzymes required for the degradation of unsaturated fatty acids. Arch Biochem Biophys. 1988;263:170–177. doi: 10.1016/0003-9861(88)90625-x. [DOI] [PubMed] [Google Scholar]
- Bode K, Hooks MA, Couée I. Identification, separation, and characterization of acyl-coenzyme A dehydrogenase involved in mitochondrial β-oxidation in higher plants. Plant Physiol. 1999;119:1305–1314. doi: 10.1104/pp.119.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Schulz H. 3-Hydroxyacyl-CoA epimerase is a peroxisomal enzyme and therefore not involved in mitochondrial fatty acid oxidation. FEBS Lett. 1985;185:129–134. doi: 10.1016/0014-5793(85)80755-9. [DOI] [PubMed] [Google Scholar]
- de Waard P, van der Wal H, Huijberts GNM, Eggink G. Heteronuclear NMR analysis of unsaturated fatty acids in poly(3-hydroxyalkanoates): study of β-oxidation in Pseudomonas putida. J Biol Chem. 1993;268:315–319. [PubMed] [Google Scholar]
- Dieuaide M, Couée I, Pradet A, Raymond P. Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for mitochondrial fatty acid β-oxidation and acyl-CoA dehydrogenase activity in a higher plant. Biochem J. 1993;296:199–207. doi: 10.1042/bj2960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Novikov D, Vandekerckhove J, van Veldhoven PP, Mannaerts GP. Identification and characterization of the 2-enoyl-CoA hydratase involved in peroxisomal β-oxidation in rat liver. Biochem J. 1997;321:253–259. doi: 10.1042/bj3210253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommes V, Kunau W-H. 2,4-Dienoyl coenzyme A reductase from bovine liver and Escherichia coli: comparison of properties. J Biol Chem. 1984;259:1781–1788. [PubMed] [Google Scholar]
- Engeland K, Kindl H. Evidence for a peroxisomal fatty acid β-oxidation involving d-3-hydroxyacyl-CoAs: characterization of two forms of hydro-lyase that convert d-(-)-3-hydroxyacyl-CoA. Eur J Biochem. 1991;200:171–178. doi: 10.1111/j.1432-1033.1991.tb21064.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. Fatty acid degradation in plants. Prog Lipid Res. 1992;31:417–446. doi: 10.1016/0163-7827(92)90004-3. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. Catabolism of fatty acids (α- and β-oxidation) In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Gerhardt B, Fischer K, Maier U. Effect of palmitoylcarnitine on mitochondrial activities. Planta. 1995;196:720–726. [Google Scholar]
- Hiltunen JK, Filppula SA, Koivuranta KT, Siivari K, Qin Y-M, Häyrinen H-M. Peroxisomal β-oxidation and polyunsaturated fatty acids. Ann N Y Acad Sci. 1996;804:116–128. doi: 10.1111/j.1749-6632.1996.tb18612.x. [DOI] [PubMed] [Google Scholar]
- Hiltunen JK, Palosaari PM, Kunau W-H. Epimerization of 3-hydroxyacyl-CoA esters in rat liver: involvement of two 2-enoyl-CoA hydratase. J Biol Chem. 1989;264:13536–13540. [PubMed] [Google Scholar]
- Huijberts GNM, Eggink G, de Waard P, Huisman GW, Witholt B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol. 1992;58:536–544. doi: 10.1128/aem.58.2.536-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Ueda S, Mizugaki M, Kawaguchi A. Purification of the multienzyme complex for fatty acid oxidation from Pseudomonas fragi and reconstitution of the fatty acid oxidation system. J Biochem. 1990;107:184–189. doi: 10.1093/oxfordjournals.jbchem.a123023. [DOI] [PubMed] [Google Scholar]
- Kindl H. Fatty acid degradation in plant peroxisomes: function and biosynthesis of the enzymes involved. Biochimie. 1993;75:225–230. doi: 10.1016/0300-9084(93)90080-c. [DOI] [PubMed] [Google Scholar]
- Kunau W-H, Dommes P. Degradation of unsaturated fatty acids: identification of intermediates in the degradation of cis-4-decenoly-CoA by extracts of beef-liver mitochondria. Eur J Biochem. 1978;91:533–544. doi: 10.1111/j.1432-1033.1978.tb12707.x. [DOI] [PubMed] [Google Scholar]
- Kunau W-H, Dommes V, Schulz H. β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol. 1988;54:2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf V, Bongcam V, Allenbach L, Coullerez G, Martini N, Poirier Y. Polyhydroxyalkanoate synthesis in transgenic plants as a new tool to study carbon flow through β-oxidation. Plant J. 1999;20:45–55. doi: 10.1046/j.1365-313x.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Mittendorf V, Robertson EJ, Leech RM, Krüger N, Steinbüchel A, Poirier Y. Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid β-oxidation. Proc Natl Acad Sci USA. 1998;95:13397–13402. doi: 10.1073/pnas.95.23.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y. Production of new polymeric compounds in plants. Curr Opin Biotechnol. 1999;10:181–185. doi: 10.1016/s0958-1669(99)80032-9. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Dennis DE, Klomparens K, Somerville C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992;256:520–523. doi: 10.1126/science.256.5056.520. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio/Technology. 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Ventre G, Caldelari D. Increased flow of fatty acids towards β-oxidation in developing seeds of Arabidopsis deficient in diacylglycerol acyltransferase activity or synthesizing medium-chain-length fatty acids. Plant Physiol. 1999;121:1359–1366. doi: 10.1104/pp.121.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig-Müller R, Gühnemann-Schäfer K, Kindl H. Domains of the tetrafunctional protein acting in glyoxysomal fatty acid β-oxidation: demonstration of epimerase and isomerase activities on a peptide lacking hydratase activity. J Biol Chem. 1994;269:20475–20481. [PubMed] [Google Scholar]
- Qin Y-M, Haapalainen AM, Conry D, Cuebas DA, Hiltunen JK, Novikov DK. Recombinant 2-enoyl-CoA hydratase derived from rat peroxisomal multifunctional enzyme 2: role of the hydratase reaction in bile acid synthesis. Biochem J. 1997;328:377–382. doi: 10.1042/bj3280377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Kunau W-H. Beta-oxidation of unsaturated fatty acids: a revised pathway. Trends Biochem Sci. 1987;12:403–406. [Google Scholar]
- Steinbüchel A, Füchtenbusch B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998;16:419–427. doi: 10.1016/s0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Caesar H. Der Stoffelwechsel der ungesättigten Fettssaüren: V. Zur β-Oxidation der Mono- und Polyenfettsäuren. Der Mechanismus der enzymatischen Reaktionen an Δ2cis-enoyl-CoA Verbindungen. Hoppe Seyler's Z Physiol Chem. 1965;341:76–83. [PubMed] [Google Scholar]
- Yang S-Y, Cuebas D, Schulz H. 3-Hydroxyacyl-CoA epimerase of rat liver peroxisomes and Escherichia coli function as auxiliary enzymes in the β-oxidation of polyunsaturated fatty acids. J Biol Chem. 1986;261:12238–12243. [PubMed] [Google Scholar]
- Yang S-Y, He X-Y, Schulz H. Glutamate 139 of the large α-subunit is the catalytic base in the dehydration of both d- and l-3-hydroxyacyl-coenzyme A but not in the isomerization of Δ3-Δ2-enoyl-coenzyme A catalyzed by the multienzyme complex of fatty acid oxidation from Escherichia coli. Biochemistry. 1995;34:6441–6447. doi: 10.1021/bi00019a025. [DOI] [PubMed] [Google Scholar]
- You S-Y, Cosloy S, Schulz H. Evidence for the essential function of 2,4-dienoyl-coenzyme A reductase in the β-oxidation of unsaturated fatty acids in vivo: isolation and characterization of an Escherichia coli mutant with a defective 2,4-dienoyl-coenzyme A reductase. J Biol Chem. 1989;264:16489–16495. [PubMed] [Google Scholar]