Abstract
We have previously shown that in Nicotiana sylvestris cytoplasmic male-sterile (CMS) mutants where the mtDNA lacks the nad7 gene coding for a subunit of respiratory Complex I (NADH:ubiquinone oxidoreductase, EC 1.6.5.3), glycine (Gly) oxidation was lower than in the wild type and insensitive to rotenone, suggesting Complex I dysfunction. In contrast, the oxidation rate of exogenous NADH and the capacity of the cyanide-resistant respiration (AOX) were enhanced. Here we report that, in contrast to Gly, the rate of malate oxidation was not affected, but proceeded totally in a rotenone-insensitive pathway, strongly suggesting that survival of CMS plants depends on the activation of internal and external alternative NAD(P) H dehydrogenases and that Gly decarboxylase activity depends on Complex I functioning. A similar defect in Complex I activity and Gly oxidation was found in the NMS1 nuclear mutant, defective in the processing of the nad4 transcript, but alternative NAD(P) H dehydrogenases were less activated. In CMS and NMS1, the fraction of the AOX pathway was increased, as compared to wild type, associated with higher amounts of aox transcripts, AOX protein, and plant resistance to cyanide. Non-phosphorylating respiratory enzymes maintained normal in vivo respiration levels in both mutants, but photosynthesis was decreased, in correlation with lower leaf conductance, emphasizing mitochondrial control on photosynthesis.
In most eukaryotes with the exception of some lower fungi such as Saccharomyces cerevisiae, Complex I (NADH:ubiquinone oxidoreductase, EC 1.6.5.3) is the more complex element of electron input in the mitochondrial respiratory chain, catalyzing electron transfer from matrix-derived NAD(P) H to ubiquinone via flavin mononucleotide and a number of iron-sulfur clusters (Weiss et al., 1991; Walker, 1992). This reaction is coupled with proton extrusion from the matrix to the intermembrane space, generating an electrochemical gradient necessary for ATP synthesis. In plants as in other organisms, Complex I is a multimeric enzyme of more than 35 polypeptides (Leterme and Boutry, 1993). The minimal Complex I found in bacteria consists of 14 subunits (Friedrich, 1998), all of which are conserved in eukaryotic mitochondrial Complex I: animals (Chomyn et al., 1985, 1986), plants (Rasmusson et al., 1998), and fungi (Videira, 1998). In mammals, seven subunits (known as NAD1 to NAD6 and NAD4L) are mitochondrial-encoded (Walker, 1992), whereas the remainder are encoded by the nuclear genome, translated in the cytosol, and imported into the organelle. In Neurospora, Complex I is formed of two subcomplexes: a peripheral arm, facing the matrix that consists of 13 nuclear-encoded subunits, including polypeptides of 30, 38, and 49 kD; and a hydrophobic intramembrane arm that comprises all mitochondrial-encoded NAD subunits and the remaining nuclear-encoded subunits. In higher plants the 30- and 49-kD subunits, named NAD7 and NAD9 respectively, are mitochondrial-encoded (Gäbler et al., 1994; Lamattina et al., 1993).
Complex I defects should be lethal for all aerobic cells since the near totality of their energy requirement is ensured by mitochondrial respiration. However, plant and fungal mitochondria contain two sets of alternative respiratory pathways that bypass proton-pumping complexes located in the internal mitochondrial membrane (Rasmusson et al., 1998) and have no equivalent in mammalian mitochondria. These pathways are: (a) At least four additional NAD(P) H:ubiquinone oxidoreductases (alternative dehydrogenases) bypassing Complex I. Two of them, facing the intermembrane space, oxidize cytosolic NADH or NADPH, respectively; two others, facing the matrix, oxidize matrix-derived NADH or NADPH, respectively. All are considered insensitive to rotenone, a Complex I-specific inhibitor (Douce et al., 1973; Roberts et al., 1995; Melo et al., 1996; Møller, 1997), and (b) downstream of the ubiquinone pool, the alternative oxidase (AOX) bypasses Complex III (cytochrome bc1-containing complex) and Complex IV (cytochrome c oxidase) and is cyanide-insensitive (Moore and Siedow, 1991; Siedow and Umbach, 1995). The enzyme is a homodimer of 35-kD monomers that may be covalently linked by a disulfide bond. It is active when the latter is reduced and is further stimulated by pyruvate (Millar et al., 1993, Umbach et al., 1994). AOX is influenced by developmental and environmental factors and may be regulated at the level of gene expression or enzyme activity (Umbach and Siedow, 1993; Vanlerberghe and McIntosh, 1997).
The role of these alternative pathways is not clearly elucidated and their contribution to respiration could be dependent on Complex I activity. Use of respiratory mutants may help to understand their physiological significance and regulation.
Many nuclear and mitochondrial respiratory mutants have been characterized in fungi and algae, but in higher plants such mutants have so far been reported in only two species. In Zea mays, the NCS2 mutant carries a deletion in the nad4 mitochondrial gene (Marienfeld and Newton, 1994). NCS2 plants, which are maintained at the heteroplasmic state (a mixture of normal and deleted mt genomes), show impaired development of the sporophyte with striped leaves. The stripes consist of alternate yellowish pale-green and normal green sectors harboring respectively mutated and wild-type mitochondria. In Nicotiana sylvestris, the near homoplasmic cytoplasmic male sterile (CMS) I and CMSII mutants have large deletions in their mtDNA (Chétrit et al., 1992) comprising the nad7 gene sequence (Pla et al., 1995) and the upstream region of the nad1 first exon (Lelandais et al., 1998; Gutierres et al., 1999). In addition to the lack of NAD7 and NAD1, their Complex I is similarly defective for NAD9 and the nuclear-encoded 38-kD subunit (Gutierres et al., 1997). Respiration measurements on mitochondria isolated from either CMSI or CMSII (further collectively referred to as CMS) leaf tissues showed that Gly oxidation was lower than in wild type and insensitive to rotenone, suggesting Complex I dysfunction. On the other hand, the oxidation rate of exogenous NADH and the capacity of the cyanide-resistant respiration were enhanced in CMS.
In this paper we show that in contrast to Gly, the rate of malate oxidation was not affected in CMS, but is totally insensitive to rotenone, suggesting enhancement of rotenone-insensitive internal NAD(P) H dehydrogenase activity. Furthermore, we compare the respiratory behavior of CMS with that of the nuclear NMS1 Complex I mutant affected in the processing of the Complex I nad4 gene (Brangeon et al., 2000). As CMS, NMS1 plants possess a defective Complex I and present severe developmental defects, but their phenotypic abnormalities, including male sterility, are more pronounced (De Paepe et al., 1990). For all genotypes, respiratory measurements on isolated mitochondria were completed by in planta gas exchange experiments and analysis of aox gene expression.
RESULTS
Respiration of Purified Leaf Mitochondria
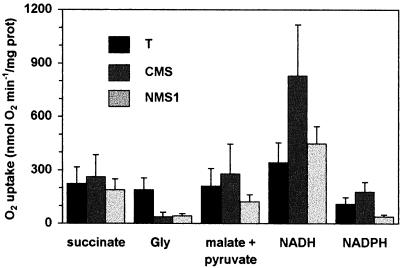
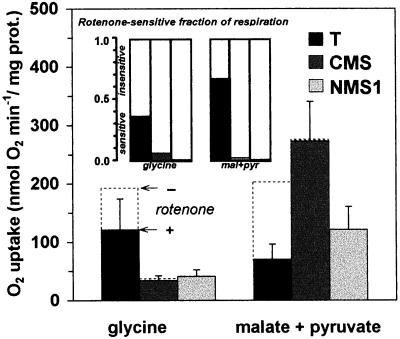
Oxygen uptake by purified wild-type and mutant mitochondria was compared using various respiratory substrates, after either ADP addition (state 3) or in presence of carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; Fig. 1). Succinate oxidation, which does not pass through Complex I, was the same in mutants and in wild type, indicating that the redox chain downstream of (and including) the ubiquinone pool was not affected. In contrast to Gly oxidation, which was dramatically reduced in NMS1 as previously found in CMS (Gutierres et al., 1997), malate oxidation was reduced in NMS1 only; pyruvate was usually added to malate to minimize the progressive back pressure of the oxaloacetate formed, but the initial rate of malate oxidation with or without pyruvate was similar (results not shown). Other tricarboxylic acid cycle derived substrates, such as α-oxoglutarate or isocitrate, gave similar results as malate, with however much lower overall rates (not shown). In order to determine whether Complex I was functionally altered, respiration was measured in presence of rotenone, a specific Complex I inhibitor (Fig. 2). Using either Gly or malate, the rotenone-inhibited fraction mediated by Complex I was indeed negligible in both mutants (Fig. 2, inset). Thus, malate oxidation, where the rotenone-inhibited fraction constitutes about 70% of the total activity in wild type, was near completely mediated in mutants by a rotenone-insensitive matrix-facing NAD(P) H-dehydrogenase activity. Nevertheless, the activity of this pathway was lower in NMS1 than in CMS.
Figure 1.
Respiration of purified leaf mitochondria. Substrate concentrations were 10 mm except NAD(P) H was 1 mm. Oxidation rates were followed in presence of 150 μm ADP (state 3) or 2 μm FCCP for NAD(P) H (uncoupling). Means of at least five independent experiments on each genotype (except for NADPH with two—three experiments) with a mitochondrial suspension containing approximately 80 μg protein mL−1 in the oxygen-electrode cuvette. These protein amounts correspond to approximately 35 g of leaf fresh mass in T, 25 g in CMS, and 12 g in NMS1 plants at the rosette stage.
Figure 2.
Absolute rates of oxygen uptake in presence of rotenone, a specific Complex I inhibitor. Respiration before (dashed columns) and after (gray columns) addition of rotenone, 50 μm final. Inset, Rotenone-sensitive (gray columns) and insensitive (white columns) fractions of respiration. For conditions and numerical values of uninhibited rate, see Figure 1.
Oxidation of exogenously supplied NADH, which was the best respiratory substrate in all genotypes, was not significantly changed in NMS1 as compared with wild-type témoin (T), whereas it was more than doubled in CMS, confirming previous results (Gutierres et al., 1997). This suggests that activity of NADH-dehydrogenase facing the intermembrane-space was stimulated in CMS only. Similarly, NADPH oxidation was increased in CMS but not in NMS1.
As expected, the lack of the major coupling site represented by Complex I was associated with a reduced “respiratory control ratio” (rate in phosphorylating state 3/rate in resting state 4) in both mutants (Table I). The values of P/O ratio using malate as a substrate are decreased in both CMS and NMS1, and are similar to those obtained with succinate and NADH.
Table I.
Comparison of respiratory rate in the presence of added ADP to the rate following its expenditure (RCR) and the ADP transformed per oxygen consumed (P/O) ratios between wild type (T) and mutants (CMS and NMS1)
| Substrates | RCR
|
P/O
|
||||
|---|---|---|---|---|---|---|
| T | CMS | NMS1 | T | CMS | NMS1 | |
| Succinate | 2.4 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 |
| Gly | 2.6 ± 0.8 | 1.0 ± 0.4 | 1.0 ± 0.2 | 2.3 ± 0.5 | – | – |
| Malate | 3.3 ± 0.7 | 1.4 ± 0.3 | 1.2 ± 0.2 | 2.2 ± 0.2 | 1.6 ± 0.4 | 1.2 ± 0.4 |
| NADH | 2.7 ± 0.7 | 2.1 ± 0.5 | 2.0 ± 0.3 | 1.5 ± 0.2 | 1.4 ± 0.4 | 1.2 ± 0.1 |
| NADPH | 2.7 ± 0.1 | 2.0 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.1 |
Means ± se of at least four independent experiments.
Cyanide-Resistant Respiration
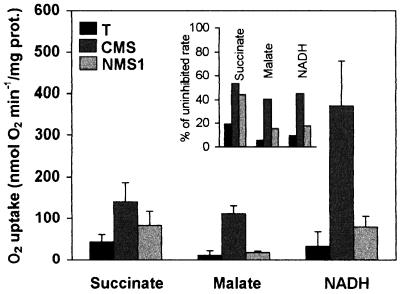
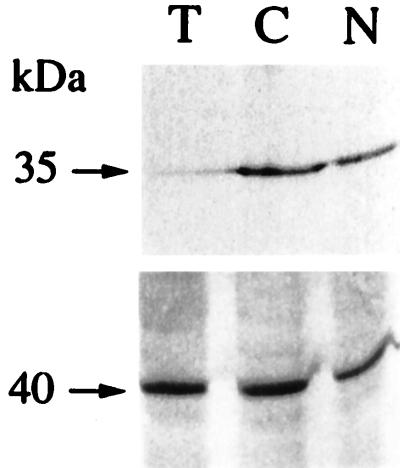
The AOX capacity (respiration in presence of KCN minus the residual respiration, i.e. oxygen uptake in presence of n-propyl gallate and KCN), was measured in conditions of maximum activity of the enzyme, i.e. after thiol reduction by dithiothreitol (DTT) and activation by pyruvate (Millar et al., 1993; Umbach et al., 1994). As expected, AOX rates were higher than in previous experiments performed without these effectors (Gutierres et al., 1997). Moreover, significant rates of residual respiration were also observed, averaging 60 nmol O2 min−1/mg protein (not shown). Such high rates were reported by Purvis (1997) in similar experimental conditions, but remained unexplained. Whatever the mechanism involved, values of residual respiration were similar in all genotypes. In contrast, the AOX capacity was higher in CMS than in wild-type T, whatever the respiratory substrate used, succinate, malate or NADH (Fig. 3). These data confirmed previous results obtained in basic conditions of AOX activity (Gutierres et al., 1997). In the NMS1 mutant, enhancement of the AOX capacity was less apparent than in CMS; in particular, use of malate did not reveal any differences as compared with T. However, in both mutants, the fraction of cyanide-resistant respiration as regards to total respiration was higher than in the wild-type. In addition, the AOX protein was detected in higher amounts in NMS1 than in T mitochondria (Fig. 4), as previously reported for CMS (Gutierres et al., 1997).
Figure 3.
Capacity of AOX. n-Propyl gallate or salicylhydroxamic acid inhibited steady-rate respiration in the presence of 1 mm freshly prepared KCN, 5 mm DTT (AOX reducer), and 5 mm pyruvate (AOX stimulator). From left to right, substrates, 10 mm succinate, 10 mm malate in state 3, and 1 mm NADH uncoupled by 2 μm FCCP. Means of three to five experiments. For general conditions, see Figure 1. Inset, Fraction of AOX capacity (percentagess of uninhibited rates).
Figure 4.
Western immunodetection of AOX on wild-type (T), CMSII (C), and NMS1 (N) leaf mt proteins. Top, 35-kD signal obtained using the Sauromatom guttatum anti-AOX antibody; bottom, 40-kD signal obtained using the potato antiformate dehydrogenase (FDH) antibody as control; 10 μg of mt proteins per lane.
In Vivo AOX Assessment
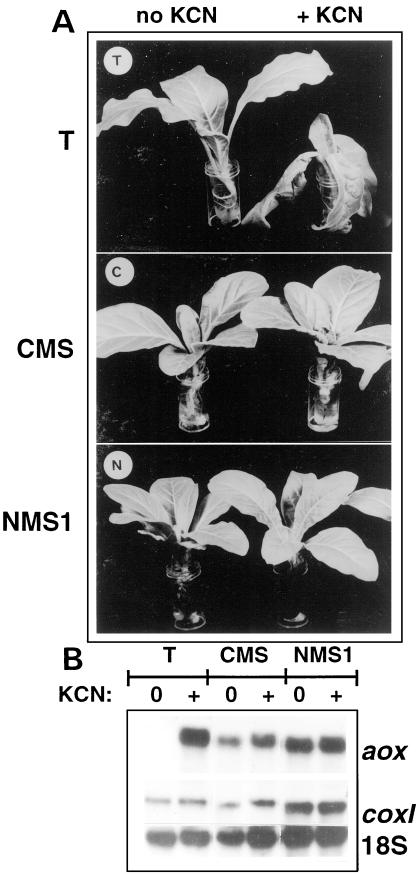
The in vitro measured AOX capacity does not necessary reflect the in vivo activity of the enzyme (Millar et al., 1995) and in order to determine to what extent this pathway could be operating in vivo, we proceeded by incubating plantlets in the presence of 5 mm KCN; plantlets incubated in water were used as control. After 18–24 h of incubation in the KCN solution (according to the experiments), wild-type T leaves were wrinkled, whereas CMS and NMS1 leaves did not show any visible alterations (Fig. 5A). After 2 d of incubation, CMS plants were only slightly affected (not shown).
Figure 5.
In planta assessment of cyanide resistance and AOX expression. A, T, CMS, and NMS1 plantlets of similar developmental stage were maintained for 24 h in water with or without 5 mm KCN under greenhouse conditions. Due to the lower growth rates of mutant plants as regards to T plants (De Paepe et al., 1990; Gutierres et al., 1997), T plantlets were about 6 weeks old, CMS plantlets were 8 weeks old, and NMS1 plantlets were 12 weeks old. B, Corresponding northern analysis; AOX (aox), subunit 1 of cytochrome oxidase (coxI), and 18S mitochondrial rRNA as control.
The effect of KCN on aox gene expression was analyzed by northern experiments (Fig. 5B). In wild-type, steady-state levels of aox transcripts, about 1.7 kb in size, were dramatically increased in KCN-treated plants as compared with control plants, in which they were only detectable after long time exposure (not shown). In contrast, aox transcripts were present in large amounts in watered CMS and NMS1 plantlets, and their levels did not significantly increase in the presence of KCN. Whatever the genotype, transcript levels of the mitochondrial coxI gene coding for a subunit of cytochrome oxidase were not affected by the KCN treatment.
In Vivo Respiration and Photosynthesis
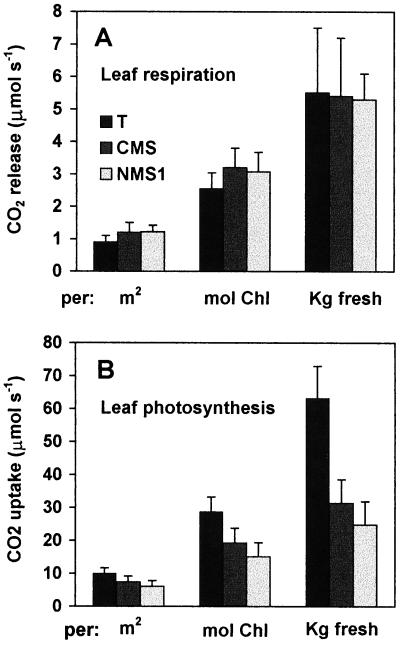
Global respiration of intact leaves attached to the plant was measured by gas exchange at current atmospheric CO2 concentration (350 μmol mol−1 air). Plants were pre-conditioned for about 18 h in total darkness. The steady-state rate of CO2 release in the dark (respiration) was similar for both mutants and wild-type, irrespective of quantification of biological material, unit area (m2), mole of chlorophyll, or fresh mass (Fig. 6A). Similar results were obtained using plants previously exposed to 1 h of saturating illumination at 668 nm (= 1,000 μmol photons m−2 s−1).
Figure 6.
Steady-state rates of in vivo leaf respiration and net photosynthesis. Before experiments, plants were preconditioned in complete darkness for 18 h. A, Means plus se of respiration, either directly, after pre-conditioning, or after a subsequent 1-h illumination (these two conditions gave same values); means of six (T and NMS1) to 10 (CMS) independent experiments. B, Means plus se of net photosynthesis at 300 μmol photons s−1 m−2 light incident; data are expressed per unit leaf area, chlorophyll content, or mass unit of leaf fresh matter (same results with dry matter). Means of six (T and NMS1) to 10 (CMS) independent experiments.
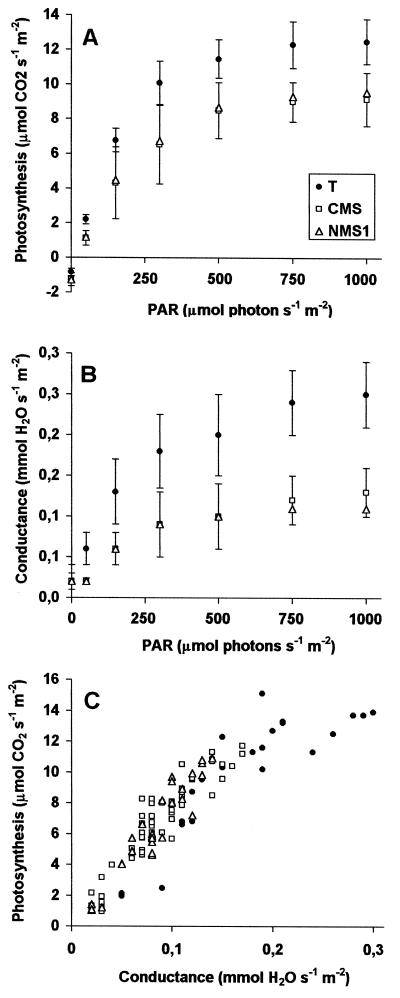
The net photosynthesis was then measured by the same method under various light intensities. Quite unexpectedly, it was similarly reduced for CMS and NMS1 relative to wild-type T (Fig. 6B). The differences were more apparent if fresh matter was used for reference: whereas the respiration of CMS, NMS1 and T was then equal, photosynthesis was 50% reduced. The light intensity curves (Fig. 7A) showed that net photosynthesis was lowered in mutants at different light intensities, correlated with a decrease in leaf conductance (Fig. 7B). This correlation between photosynthesis and leaf conductance, which governs diffusion of external CO2 to cells and thence to chloroplasts, was the same for all genotypes and over a broad light range (Fig. 7C).
Figure 7.
Light-intensity curves of net photosynthesis (A), leaf conductance to water (B), and the correlation between the two (C). Means plus sd are given in A and B. For clarity of presentation, the sd of mutants were pooled together. For C, individual values are shown, from low to high illumination. Same conditions as for Figure 6.
DISCUSSION
Previous respiration studies (Gutierres et al., 1997) performed on isolated mitochondria using Gly as a substrate had shown a collapse of both rotenone-sensitive and rotenone-insensitive respiration in N. sylvestris CMS mutants in which the mitochondrial genome is deleted for the nad7 gene encoding the NAD7 Complex I subunit (Li et al., 1988; Chétrit et al., 1992; Pla et al., 1995). In contrast, rates of NADH oxidation and of cyanide-resistant respiration (AOX) were enhanced. In this paper we compare the respiratory behavior of the CMS mutant with that of the nuclear NMS1 Complex I mutant, of which the mtDNA is not affected, but which is defective in the splicing of the Complex I nad4 gene (Brangeon et al., 2000). Although NMS1 plants are similarly affected in Complex I functioning as CMS plants, the activity of alternative NAD(P) H dehydrogenases, both external and internal, as well as the absolute AOX capacity, measured in conditions of maximum activity of the enzyme, are lower in NMS1 than in CMS. High in planta operation of alternative respiratory pathways in both mutants is suggested on the basis of maintenance of the whole plant respiration, over-expression of the aox gene and resistance to KCN.
Absence of Complex I Activity Is Not Lethal for Higher Plants
The present work is the first report in higher plants on a nuclear mutant lacking a functional Complex I. Nuclear Complex I mutants have already been described in fungi, for example the Neurospora mutants devoid of the 49 kD subunit, homologous to NAD7 (Guénebaut et al., 1997, and refs. therein), or of the 30 kD subunit, homologous to NAD9 (Duarte et al., 1995), and the nuo51 mutant of Aspergillus niger, disrupted in the 51 kD subunit, the proposed NADH binding site. In higher plants, the previously described Complex I mutants, i.e. the maize NCS2 mutants (Marienfeld and Newton, 1994; Karpova and Newton, 1999) and the N. sylvestris CMS mutants (Pla et al., 1995; Gutierres et al., 1997; Lelandais et al., 1998) carry a deletion in their mtDNA. The viability of NCS2 plants was proposed to be ensured by their heteroplasmy, yet N. sylvestris CMS are near-homoplasmic for the deletion, making it unlikely that the remaining low substoichiometric amounts of normal mtDNA (Lelandais et al., 1998) could ensure plant survival. In the NMS1 homozygous (ms1/ms1) mutant, Complex I activity is totally absent, confirming that Complex I dysfunction is not lethal for plants, even though it is associated with severe developmental defects.
In Plants As in Fungi, Alternative NAD(P)H Dehydrogenases Are Likely to Sustain Plant Development in the Absence of Complex I Activity
In fungi, survival of Complex I mutants is thought to be due to the activation of rotenone-insensitive NAD(P) H dehydrogenases. Although it has first been reported that the so-called “small Complex I” assembled in N. crassa chloramphenicol-treated cells would carry a rotenone-insensitive respiratory activity (Tuschen et al., 1990), further studies revealed that this activity was in fact carried out by a matrix-faced NADH dehydrogenase (Nehls et al., 1992). Similarly, the Aspergillus niger nuo51 mutant was shown to have a 2-fold increase in matrix-faced NADH dehydrogenase activity (Prömper et al., 1993). In the N. sylvestris CMS mutants, both external, intermembrane-space-facing NAD(P) H dehydrogenases (Gutierres et al., 1997) and internal, matrix-facing NAD(P) H dehydrogenases (this paper) were stimulated. Activation of these two rotenone-insensitive pathways would prevent the blockage of plant metabolism: the former, in association with AOX, would permit glycolysis turnover and thus the lowering of upstream carbon metabolism pressure by continuous regeneration of NAD(P), and the latter would maintain Krebs cycle functioning.
The concept that alternative NAD(P) H dehydrogenases sustain plant development is also supported by the fact that they are more active in CMS than in NMS1 plants, the latter also having the more severe phenotypic alterations (De Paepe et al., 1990). Whatever the exact relationship between phenotype and NAD(P) H dehydrogenase activities, differences between mutants should be of genetic origin. Indeed, whereas CMS mtDNA is deleted for the nad7 sequence (Pla et al., 1995; Lelandais et al., 1998), in NMS1 the mitochondrial genome looks normal (De Paepe et al., 1990) and their nuclear recessive mutation affects the processing of the nad4 gene (Brangeon et al., 2000). It is interesting that no enhanced external NADH dehydrogenase activity was found in the NAD4-deficient maize NCS2 mutant (Marienfeld and Newton, 1994; Karpova and Newton, 1999). In mammalian mitochondria, NAD4 was proposed to play a role in the assembly of the Complex I membrane arm (Hofhaus and Attardi, 1993) and to participate in a domain allowing the association of Complex I with matricial dehydrogenating enzymes (Majander et al., 1991).
As it has been reported that NAD(P) H activities are calcium dependent (Møller et al., 1981), it is likely that experiments presented here, using classical respiration media without calcium, did not allow maximum activities of these enzymes, and that higher activities would be obtained in presence of calcium. This point should be investigated in the future.
Gly Oxidation Is Dependent on Complex I Activity
In contrast to the rotenone-insensitive oxidation of tricarboxylic acid cycle cycle substrates higher in CMS than in NMS1, that of Gly was collapsed in NMS1, as it has previously been shown for CMS (Gutierres et al., 1997), suggesting that Gly decarboxylase (GDC) activity is dependent on Complex I functioning. This could be explained by an increase in the NADH/NAD+ ratio that would retro-inhibit GDC. Indeed, this enzyme binds NADH (Ki = 15 μm) and NAD+ (Km = 75 μm) in a competitive manner (Neuburger et al., 1986) and its activity would be rapidly blocked at NADH concentrations too low to be efficiently oxidized by alternative matrix-facing NAD(P) H dehydrogenases (Km = 80 μm: Møller, 1986). In an alternate manner, the Complex I defect could directly affect GDC structure. In plants as in other organisms, Complex I has been shown to carry an acyl-carrier protein (Runswick et al., 1991; Sackmann et al., 1991; Shintani and Ohlrogge 1994) proposed to play a role in lipid biosynthesis, more precisely lipoic acid (Wada et al., 1997), a cofactor of the GDC complex. However, in this case activities of other lipoic acid-containing enzymes, such as oxoglutarate dehydrogenase and pyruvate dehydrogenase, would also be affected. Whatever the mechanism involved, our results attest to the subordination of Gly oxidation to Complex I integrity. Further determination of GDC activity in Complex I mutants would be of great interest for elucidating Complex I and GDC interactions.
In Vitro and in Vivo Operation of Alternative Respiratory Pathways: Retrograde Regulation from Mitochondria to Nucleus
In contrast to previous results with CMS (Gutierres et al., 1997), absolute rates of cyanide-resistant respiration were not clearly above wild-type values in NMS1, especially using malate as a substrate. However, when proportional rates relative to total respiration were considered, they were enhanced in both mutants. Moreover, as do CMS plants, NMS1 plants have more aox steady-state transcripts and AOX proteins than T plants, and are more resistant to cyanide, suggesting a high engagement of the AOX route in planta. Such increased levels of aox transcripts were previously found in antimycin-treated tobacco cell suspensions (Vanlerberghe and McIntosh, 1994), but the N. sylvestris CMS and NMS1 mutant systems are the only cases where a breakdown of the electron transport chain upstream of the ubiquinone pool results in higher aox transcripts and AOX activity. As AOX has been recently demonstrated to act as a detoxification enzyme (Wagner, 1995; Popov et al., 1997; Maxwell et al., 1999) controlling the levels of reactive oxygen species, it can be hypothesized that, as found in mammalian mitochondria (Pitkanen and Robinson, 1996), the collapse of Complex I activity would result in an increased generation of reactive oxygen species, which would in turn induce aox gene transcription. Up-regulation of nuclear gene expression in response to metabolic status has been described in yeast mitochondria where it was called retrograde regulation (Liao and Butow, 1993).
Mitochondrial Control on Photosynthesis
The normal rate of leaf respiration in mutants (Fig. 6A) suggests the high efficiency of rotenone-insensitive NAD(P) H dehydrogenases in vivo and thus of glycolysis and Krebs cycle turnover. As confirmed by the unaltered rate of succinate oxidation, electron transport is efficiently assured downstream of the ubiquinone pool, keeping these dehydrogenases coupled to the two other sites of proton translocation contributing to ATP production (Rayner and Wiskich, 1983). However, plant respiration in CMS and NMS1 should not be energetically as efficient as in the wild type, considering the lack of the first coupling site and the stimulation of AOX. This is in good agreement with the lower respiratory control and P/O ratios in both mutants (Table I). ATP deprivation could affect ion uptake and proton efflux through the plasma membrane of guard cells, and thus stomatal pore opening. As light-induced CO2 uptake is strictly correlated to leaf gas conductance, a lower CO2 diffusion would explain the decreased photosynthetic activity (Figs. 6B and 7C). It has already been suggested that the energy required by stomatal movements could be mainly provided by mitochondria, as guard cells have a high respiratory activity (Parvathi and Raghavendra, 1995). Such indirect mitochondrial control of CO2 uptake does not exclude a more direct interaction between photosynthesis and respiration by exchange of reducing power and various metabolites. Gly represents the key metabolic substrate of photosynthesizing C3 plants and its oxidative decarboxylation interconnects photosynthesis, photorespiration, and mitochondrial electron transport. Thus it is an important point of redox state regulation—of bioenergetics in general—in photosynthesizing plant cells (Douce and Neuburger, 1989). The observed collapse of Gly oxidation could then be another cause for the decrease of photosynthesis in such mutants. Although the exact mechanisms accounting for these respiration/photosynthesis interactions are not elucidated, it is interesting to note that a much more dramatic photosynthetic impairment is presented by all the maize NCS mitochondrial mutants, including the Complex I NCS2 mutant (Roussel et al., 1991).
CONCLUSION
Learning from Similarities and Differences between Nuclear and Mitochondrial Mutants
Complex I is a major site for mitochondrial energy production, thus its dysfunction is likely to result in severe developmental defects, even in the presence of alternative NAD(P) H dehydrogenases activities. In wild-type N. sylvestris, as in other plant species, nothing is known about the real in vivo partitioning of activities between Complex I and the rotenone-insensitive NAD(P) H dehydrogenases, due to the lack of specific inhibitors and appropriate noninvasive experimental tools that have been developed for the AOX, i.e. 16O/18O isotopic discrimination (Robinson et al., 1995). Their activation could constitute a physiological strategy under metabolic or environmental constraints susceptible to affect Complex I functioning. Our results demonstrate that alternative NAD(P) H dehydrogenases, in association with Complex II, are able to ensure plant survival and development in the absence of Complex I functioning. It is interesting to note that these activities are lower in NMS1 than in CMS plants, in line with the more severe phenotype of the former (De Paepe et al., 1990). However, total leaf respiration is similar in both mutants, suggesting that NMS1 plants compensate for the lower engagement of alternative pathways by other mechanisms, such as a higher number of mitochondria per cell or leaf area. In accordance with this, the yield of leaf mitochondrial proteins is 2-fold higher in NMS1 than in CMS plants (see the Fig. 1 legend). The less efficient respiration, the photosynthetic decrease and the collapse of Gly oxidation would altogether contribute to the defective mutant phenotype. It remains to be determined to what extent the alternative respiratory pathways demonstrated in leaves are also active in non-photosynthetic tissues such as male gametophytes.
MATERIALS AND METHODS
Plant Material
The Nicotiana sylvestris wild parental type (T) is a fertile botanical line of the Institut des Tabacs (Bergerac, France). Cytoplasmic (CMSI and CMSII) or nuclear (NMS1) mutants were derived from protoplast cultures (Li et al., 1988). CMS plants were maintained by backcrossing with wild-type T as the male and NMS1 by production of a few seeds under high light (De Paepe et al., 1990). The plants were grown on vermiculite in a greenhouse under 16 h of fluorescent light at a day/night temperature of 24°C/17°C.
Purification of Leaf Mitochondria
All operations were carried out at 4°C. About 50 to 100 g of freshly harvested leaves were homogenized in 600 mL of a medium (pH 7.5) containing 0.6 m mannitol, 40 mm MOPS [3-(N-morpholino)-propanesulfonic acid], 0.6% (w/v) insoluble polyvinylpyrrolidone, 3 mm EGTA, 4 mm Cys, and 0.5% (w/v) bovine serum albumin (BSA). The juice was filtered through a 30-μm mesh and centrifuged at 900g for 7 min. The supernatant was centrifuged at 10,000g for 15 min and the pellet was resuspended in a medium (pH 7.2) containing 0.6 m mannitol, 10 mm MOPS, and 0.1% (w/v) BSA. Washed mitochondria were obtained after a second cycle of centrifugations and then purified on a self-forming 32% (v/v) Percoll gradient (Pharmacia, Uppsala). Protein amount was determined according to Smith et al. (1985) using BSA as standard (bicinchominic acid method, Pierce, Rockford, IL). We consistently obtained higher amounts of mitochondrial proteins from mutants than from wild-type T: about 2. 5, 3. 3, and 6. 5 μg g−1 leaf fresh mass for T, CMS, and NMS1, respectively.
Respiration Measurements of Purified Leaf Mitochondria
Oxygen uptake was measured at 25°C with a Clark electrode (Rank Brothers, UK). The chamber contained 2 mL of the following medium (pH 7.2): 0.6 m mannitol, 30 mm KCl, 5 mm MgCl2, 0.2 mm ATP, and 10 mm potassium phosphate. First, mitochondria (50–150 μg of protein mL−1 final) were added and, after equilibration, one of the following substrates was injected: 10 mm Gly, 10 mm succinate, 10 mm malate plus 10 mm pyruvate, or 1 mm NADH or NADPH. The next sequence of addition consisted of: 80 to 150 μm ADP, to reach maximum respiration rate by phosphorylation coupling (state 3), or by uncoupling using 2 μm FCCP; 25 to 50 μm rotenone, to inhibit Complex I; 0.2 mm freshly prepared KCN, to inhibit cytochrome oxidase; and finally 50 μm n-propyl gallate or 1 mm salicylhydroxamic acid to inhibit AOX. To study the maximum AOX capacity, no inhibitors such as rotenone were present, and the following additions were successively made after the steady-state control rate was attained: 0.1 to 3 mm KCN; 5 mm DTT, and 5 mm pyruvate as AOX thiol-reducer and activator, respectively; and 50 to 100 μm n-propyl gallate as AOX inhibitor. All concentrations used were chosen to get maximum effect, low volume injections were made to avoid dilution and solvent artifacts (checked in controls), and at least 2 min were allowed after each addition to reach steady state. Respiratory data were compared using Student tests (5% level). Error bars refer to standard deviations.
Western Immunodetections
SDS-PAGE of leaf mitochondrial proteins (10 μg per lane) and immunodetection of protein, using mice monoclonal Sauromatum guttatum anti-AOX antibody (dilution 1:50) and rabbit Solanum tuberosum anti-formate dehydrogenase antibody (dilution 1:1,000), were as described in Gutierres et al. (1997).
Gas Exchange Measurements
Measurement of CO2 release (respiration) or uptake (photosynthesis) was carried out on the second fully expanded leaf (no. 4 from the apex of non-flowering plants) using an open, portable, infrared gas-exchange system (LI-COR 6400 Inc., Lincoln, NE). A middle part (excluding the central midrib) of the leaf, still attached to the plant, was clamped in the chamber such that an area of 7 cm2 was exposed to gas flow and illumination. The following environmental parameters were continuously monitored (values in parentheses). Incident red light, from light-emitting diodes at 668 nm with 32 nm one-half-bandwidth (0–2,000 μmol photons s−1 m−2); leaf and air temperature (25°C); barometric pressure (100.4 kPa); and air concentrations of water vapor (65% humidity) and of CO2 (350 μmol mol−1 air). Plants were preconditioned in total darkness for about 18 h, and respiration was measured either before or after 1 h of illumination, the two values being essentially equal at steady state. Photosynthesis was recorded on the same sample and leaf conductance was directly computed from water vapor data.
Northern Analysis of KCN-Treated Plants
Plantlets were harvested with their intact roots, washed three times, and placed in distilled water for 1 h before addition of KCN (5 mm final). They were kept for 1 d in greenhouse conditions. Small leaf pieces (100 mg) were harvested in liquid nitrogen and stored at −80°C before extraction of total RNAs by the Trizol-chloroform procedure (Gibco-BRL, UK). Ten micrograms of total RNA resuspended in loading buffer (pH 7) containing 50% (w/v) glycerol, 0.2% (w/v) bromphenol blue, 20 mm MOPS, 5 mm sodium acetate, and 0.5 mm EDTA was electrophoresed in 5%/12% (v/v) formaldehyde/agarose gels containing 0.5 μg mL−1 ethidium bromide, blotted onto nylon-based membranes (Appligene, France), and hybridized with 32P-labeled homologous probes for aox (1-kb PCR fragment, oligonucleotide O1: 5′GATCTGACGAAACACCAC3′, O2: 5′CAACAATACGATGAGCC3′, designed according to the N. tabacum aox cDNA sequence (Vanlerberghe and McIntosh, 1994), and coxI, carried out by a 7.2-kb SacI fragment from an N. sylvestris cosmid mtDNA library [Vitart et al., 1992]).
ACKNOWLEDGMENTS
The authors are grateful to Drs. Gabriel Cornic and Bernard Genty (Biodiversité, Ecologie et Sytématique Végétales, Université Paris-Sud, Orsay, France) for access to the LI-COR apparatus and for helpful discussions. They also thank Drs. M. Hodges and S. Brown for critical reading of the manuscript.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique.
LITERATURE CITED
- Brangeon J, Sabar M, Gutierres S, Combettes B, Bove J, Gendy C, Chétrit P, Colas des Francs-Small C, Pla M, Vedel F, De Paepe R. Defective splicing of the first nad4 intron is associated with lack of several Complex I subunits in the Nicotiana sylvestris NMS1 nuclear mutant. Plant J. 2000;21:269–280. doi: 10.1046/j.1365-313x.2000.00679.x. [DOI] [PubMed] [Google Scholar]
- Chétrit P, Rios R, De Paepe R, Vitart V, Gutierres S, Vedel F. Cytoplasmic male sterility is associated with large deletions in the mitochondrial DNA of two Nicotiana sylvestris protoclones. Curr Genet. 1992;21:131–137. doi: 10.1007/BF00318472. [DOI] [PubMed] [Google Scholar]
- Chomyn A, Cleeter MWJ, Ragan CI, Rilley M, Doolittle RF, Attardi G. URF6, last unidentified frame of human mtDNA codes for an NADH dehydrogenase subunit. Science. 1986;234:614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A, Mariottini P, Cleeter MWJ, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF, Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985;314:592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- De Paepe R, Chétrit P, Vitart V, Ambard-Bretteville F, Prat D, Vedel F. Several nuclear genes control both male sterility and mitochondrial protein synthesis in Nicotiana sylvestris protoclones. Mol Gen Genet. 1990;222:206–210. doi: 10.1007/BF00633819. [DOI] [PubMed] [Google Scholar]
- Douce R, Manella CA, Bonner WD., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973;292:105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M. The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:371–414. [Google Scholar]
- Duarte M, Sousa R, Videira A. Inactivation of genes encoding subunits of the peripheral and membrane arms of Neurospora mitochondrial Complex I and effects on enzyme assembly. Genetics. 1995;139:1211–1221. doi: 10.1093/genetics/139.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T. The NADH: ubiquinone oxidoreductase (Complex I) from Escherichia coli. Biochim Biophys Acta. 1998;1364:134–146. doi: 10.1016/s0005-2728(98)00024-3. [DOI] [PubMed] [Google Scholar]
- Gäbler L, Herz U, Liddell A, Leaver CJ, Schröder W, Brennicke A, Grohmann L. The 42.5 kDa subunit of the NADH: ubiquinone oxidase (Complex I) in higher plants is encoded by the mitochondrial nad7 gene. Mol Gen Genet. 1994;244:33–40. doi: 10.1007/BF00280184. [DOI] [PubMed] [Google Scholar]
- Guénebaut V, Vicentelli R, Mills D, Weiss H, Leonard KR. Three-dimensional structure of NADH-dehydrogenase from Neurospora crassa by electron microscopy and conical tilt reconstruction. J Mol Biol. 1997;265:409–418. doi: 10.1006/jmbi.1996.0753. [DOI] [PubMed] [Google Scholar]
- Gutierres S, Combettes B, De Paepe R, Mirande M, Lelandais C, Vedel F, Chétrit P. In the Nicotiana sylvestris CMSII mutant, a recombination-mediated change 5′ to the first exon of the mitochondrial nad1 gene is associated to lack of the NADH-ubiquinone oxidoreductase (complex I) NAD1 subunit. Eur J Biochem. 1999;261:361–370. doi: 10.1046/j.1432-1327.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Gutierres S, Sabar M, Lelandais C, Chétrit P, Diolez P, Degand H, Boutry M, Vedel F, de Kouchkovsky Y, De Paepe R. Lack of mitochondrial and nuclear-encoded subunits of Complex I and alteration of the respiratory chain in Nicotiana sylvestris mitochondrial deletion mutants. Proc Natl Acad Sci USA. 1997;94:3436–3441. doi: 10.1073/pnas.94.7.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofhaus G, Attardi G. Lack of assembly of mitochondrial DNA-encoded subunits of respiratory NADH dehydrogenase and loss of enzyme activity in a human cell mutant lacking the mitochondrial ND4 gene product. EMBO J. 1993;12:3043–3048. doi: 10.1002/j.1460-2075.1993.tb05973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova OV, Newton KJ. A partially assembled complex I in NAD4-deficient mitochondria of maize. Plant J. 1999;17:511–521. [Google Scholar]
- Lamattina L, Gonzalez D, Gualberto J, Grienenberger JM. Higher plant mitochondria encode a homologue of the nuclear-encoded 30-kDa subunit of bovine mitochondrial Complex I. Eur J Biochem. 1993;217:831–838. doi: 10.1111/j.1432-1033.1993.tb18311.x. [DOI] [PubMed] [Google Scholar]
- Lelandais C, Albert B, Gutierres S, De Paepe R, Godelle B, Vedel F, Chétrit P. Organization and expression of the mitochondrial genome in the Nicotiana sylvestris CMSII mutant. Genetics. 1998;150:873–882. doi: 10.1093/genetics/150.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterme S, Boutry M. Purification and preliminary characterization of mitochondrial Complex I (NADH:ubiquinone reductase) from broad bean (Vicia faba L.) Plant Physiol. 1993;102:435–443. doi: 10.1104/pp.102.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Chétrit P, Mathieu C, Vedel F, De Paepe R, Rémy R, Ambard-Bretteville F. Regeneration of cytoplasmic male sterile protoclones of Nicotiana sylvestris with mitochondrial variations. Curr Genet. 1988;13:261–266. [Google Scholar]
- Liao X, Butow RA. RTG1 and RTG2: two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell. 1993;72:61–71. doi: 10.1016/0092-8674(93)90050-z. [DOI] [PubMed] [Google Scholar]
- Majander A, Huoponen K, Savontaus MI, Nikoskelainen E, Wikström M. Electron transfer properties of NADH:ubiquinone reductase in the ND1/3460 and the ND4/11778 mutations of the Leber hereditary optic neuroretinopathy (LHON) FEBS Lett. 1991;292:289–292. doi: 10.1016/0014-5793(91)80886-8. [DOI] [PubMed] [Google Scholar]
- Marienfeld JR, Newton KJ. The maize NCS2 abnormal growth has a chimeric nad4-nad7 mitochondrial gene and is associated with reduced Complex I function. Genetics. 1994;138:855–863. doi: 10.1093/genetics/138.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AMP, Roberts TH, Møller IM. Evidence of the presence of two rotenone-insensitive NAD(P) H dehydrogenases on the inner surface of the inner membrane of potato tuber mitochondria. Biochim Biophys Acta. 1996;1276:133–139. [Google Scholar]
- Millar AH, Atkin OK, Lambers H, Wiskich JT, Day DA. A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol Plant. 1995;95:523–532. [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day D. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Møller IM. Membrane-bound NAD(P) H dehydrogenases in plant mitochondria. Physiol Plant. 1986;67:517–520. [Google Scholar]
- Møller IM. The oxidation of cytosolic NAD(P) H by external NAD(P) H dehydrogenases in respiratory chain of plant mitochondria. Physiol Plant. 1997;100:85–90. [Google Scholar]
- Møller IM, Johnston SP, Palmer JM. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981;194:487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Siedow JN. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta. 1991;1059:121–140. doi: 10.1016/s0005-2728(05)80197-5. [DOI] [PubMed] [Google Scholar]
- Nehls U, Friedrich T, Schmiede A, Ohnishi T, Weiss H. Characterization of assembly intermediates of NADH:ubiquinone oxidoreductase (Complex I) accumulated in Neurospora mitochondria by gene disruption. J Mol Biol. 1992;227:1032–1042. doi: 10.1016/0022-2836(92)90519-p. [DOI] [PubMed] [Google Scholar]
- Neuburger M, Bourguignon J, Douce R. Isolation of a large complex from matrix of pea leaf mitochondria involved in the rapid transformation of glycine into serine. FEBS Lett. 1986;207:18–22. [Google Scholar]
- Parvathi K, Raghavendra AS. Bioenergetic processes in guard cells related to stomatal function. Physiol Plant. 1995;93:146–154. [Google Scholar]
- Pitkanen S, Robinson BH. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Investig. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla M, Mathieu C, De Paepe R, Chétrit P, Vedel F. Deletion of the two exons of the mitochondrial nad7 gene results in lack of the NAD7 polypeptide in a Nicotiana sylvestris CMS mutant. Mol Gen Genet. 1995;248:79–88. doi: 10.1007/BF02456616. [DOI] [PubMed] [Google Scholar]
- Popov VN, Simonian RA, Skulachev VP, Starkov AA. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Letters. 1997;415:87–90. doi: 10.1016/s0014-5793(97)01099-5. [DOI] [PubMed] [Google Scholar]
- Prömper C, Schneider R, Weiss H. The role of the proton-pumping and alternative respiratory chain NADH:ubiquinone oxidoreductase in overflow catabolism of Aspergillus niger. Eur J Biochem. 1993;216:223–230. doi: 10.1111/j.1432-1033.1993.tb18136.x. [DOI] [PubMed] [Google Scholar]
- Purvis AC. Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol Plant. 1997;100:165–170. [Google Scholar]
- Rasmusson AG, Heiser V, Zabaleta E, Brennicke A, Grohmann L. Physiological, biochemical and molecular aspects of mitochondrial Complex I in plants. Biochim Biophys Acta. 1998;1364:1401–111. doi: 10.1016/s0005-2728(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Rayner JR, Wiskich JT. Development of NADH oxidation by red beet mitochondria on slicing and aging of the tissues. Aust J Plant Physiol. 1983;10:55–63. [Google Scholar]
- Roberts TH, Fredlund KM, Møller IM. Direct evidence for the presence of two external NAD(P) H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Lett. 1995;373:307–309. doi: 10.1016/0014-5793(95)01059-n. [DOI] [PubMed] [Google Scholar]
- Robinson SA, Ribas-Carbos M, Yakir D, Giles L, Reuveni Y, Berry JA. Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using isotope discrimination. Aust J Plant Physiol. 1995;22:487–496. [Google Scholar]
- Roussel DL, Thompson DL, Pallardy SG, Miles D, Newton KJ. Chloroplast structure and function is altered in the NCS2 maize mitochondrial mutant. Plant Physiol. 1991;96:232–238. doi: 10.1104/pp.96.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick JM, Fearnley IM, Skehel JM, Walker JE. Presence of an acyl carrier protein in NADH:ubiquinone oxidoreductase from bovine heart mitochondria. FEBS Lett. 1991;286:121–124. doi: 10.1016/0014-5793(91)80955-3. [DOI] [PubMed] [Google Scholar]
- Sackmann U, Zensen R, Rohlen D, Jahnke U, Weiss H. The acyl-carrier protein in Neurospora crassa mitochondria is a subunit of NADH:ubiquinone reductase (Complex I) Eur J Biochem. 1991;200:463–469. doi: 10.1111/j.1432-1033.1991.tb16205.x. [DOI] [PubMed] [Google Scholar]
- Shintani DK, Ohlrogge JB. The characterization of a mitochondrial acyl carrier protein isoform isolated from Arabidopsis thaliana. Plant Physiol. 1994;104:1221–1229. doi: 10.1104/pp.104.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN, Umbach AL. Plant mitochondrial electron transfer and molecular biology. Plant Cell. 1995;7:821–831. doi: 10.1105/tpc.7.7.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;156:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tuschen G, Sackmann U, Nehls U, Haiker H, Buse G, Weiss H. Assembly of NADH:ubiquinone reductase (Complex I) in Neurospora mitochondria: independent pathways of nuclear-encoded and mitochondrially encoded subunits. J Mol Biol. 1990;213:845–857. doi: 10.1016/S0022-2836(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Covalent and non-covalent dimers of cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Wiskich JT, Siedow JN. Regulation of alternative oxidase kinetics by pyruvate and intermolecular disulphide bond redox status in soybean seedling mitochondria. FEBS Lett. 1994;348:181–184. doi: 10.1016/0014-5793(94)00600-8. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Videira A. Complex I from the fungus Neurospora crassa. Biochim Biophys Acta. 1998;1364:89–100. doi: 10.1016/s0005-2728(98)00020-6. [DOI] [PubMed] [Google Scholar]
- Vitart V, De Paepe R, Mathieu C, Chétrit C, Vedel F. Amplification of substoichiometric recombinant mitochondrial DNA sequences in a nuclear male sterile mutant regenerated from protoplast culture in Nicotiana sylvestris. Mol Gen Genet. 1992;233:193–200. doi: 10.1007/BF00587579. [DOI] [PubMed] [Google Scholar]
- Wada H, Shintani D, Ohlrogge J. Why do mitochondria synthesize fatty acids: evidence for involvement in lipoic acid production. Proc Natl Acad Sci USA. 1997;94:1591–1596. doi: 10.1073/pnas.94.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM. A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Letters. 1995;368:339–342. doi: 10.1016/0014-5793(95)00688-6. [DOI] [PubMed] [Google Scholar]
- Walker JE. The NADH:ubiquinone oxidoreductase (Complex I) of respiratory chain. Q Rev Biophys. 1992;25:253–324. doi: 10.1017/s003358350000425x. [DOI] [PubMed] [Google Scholar]
- Weiss H, Friedrich T, Hofhaus G, Preis D. The respiratory chain NADH dehydrogenase (Complex I) of mitochondria. Eur J Biochem. 1991;197:563–576. doi: 10.1111/j.1432-1033.1991.tb15945.x. [DOI] [PubMed] [Google Scholar]