Abstract
Objective
To examine the potential effects of lifestyle factors on male reproductive health. Evidence of a global decline in human sperm quality over recent decades has been accumulating. Environmental, occupational, and modifiable lifestyle factors may contribute to this decline. This review focuses on key lifestyle factors that are associated with male infertility such as smoking cigarettes, alcohol intake, use of illicit drugs, obesity, psychological stress, advanced paternal age, dietary practices, and coffee consumption. Other factors such as testicular heat stress, intense cycling training, lack of sleep and exposure to electromagnetic radiation from mobile phone use are briefly discussed.
Materials and method
A comprehensive literature search was performed to identify and synthesise all relevant information, mainly from within the last decade, on the major lifestyle factors associated with male infertility and semen quality. Database searches were limited to reports published in English only. A manual search of bibliographies of the reports retrieved was conducted to identify additional relevant articles.
Results
In all, 1012 articles were identified from the database search and after reviewing the titles and abstract of the reports, 104 articles met the inclusion criteria. Of these, 30 reports were excluded as the full-text could not be retrieved and the abstract did not have relevant data. The remaining 74 reports were reviewed for data on association between a particular lifestyle factor and male infertility and were included in the present review.
Conclusion
The major lifestyle factors discussed in the present review are amongst the multiple potential risk factors that could impair male fertility. However, their negative impact may well be mostly overcome by behaviour modification and better lifestyle choices. Greater awareness and recognition of the possible impact of these lifestyle factors are important amongst couples seeking conception.
Abbreviations: AAS, anabolic–androgenic steroids; APA, advanced paternal age; ASIH, anabolic steroid-induced hypogonadism; ART, assisted reproductive technology; BMI, body mass index; Chk1, checkpoint kinase 1; ECS, endogenous cannabinoid system; GnIH, gonadotropin-inhibitory hormone; HADS, Hospital Anxiety and Depression Score; HPA, hypothalamus–pituitary–adrenal; HPG, hypothalamus–pituitary–gonadal; IVF, in vitro fertilisation; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; MMP, mitochondrial membrane potential; ROS, reactive oxygen species; SOD, superoxide dismutase
Keywords: Male infertility, Lifestyle, Risk factors, Semen quality, Sperm DNA fragmentation
Introduction
There has been increasing evidence on the global decline in human sperm quality over the past few decades [1], [2], [3], [4]. In the most recent report, Levine’s group performed a systematic review and meta-regression analysis of the current trends in sperm counts. The comprehensive study involved 42 935 men with samples spanning over ∼40 years. They reported a significant decline of 50–60% in sperm counts amongst men from North America, Europe, Australia and New Zealand [5]. This latest finding has sparked even greater concern over the reasons behind the apparent decline in the sperm count of Western men.
As male fertility can be influenced by a variety of factors, one possible explanation for the declining trend would be that there are environmental and/or occupational factors along with lifestyle practices that contribute to the deterioration of semen quality [6], [7], [8], [9].
The present article reviews the available evidence examining the potential effects of lifestyle factors on male reproductive health. It focuses on the following lifestyle factors that are associated with male infertility: smoking cigarettes, alcohol intake, use of illicit drugs, obesity, psychological stress, advanced paternal age, dietary practices, and coffee consumption. Other factors such as testicular heat stress, intense cycling training, lack of sleep, and exposure to electromagnetic radiation from mobile phone use are also briefly touched upon.
Materials and methods
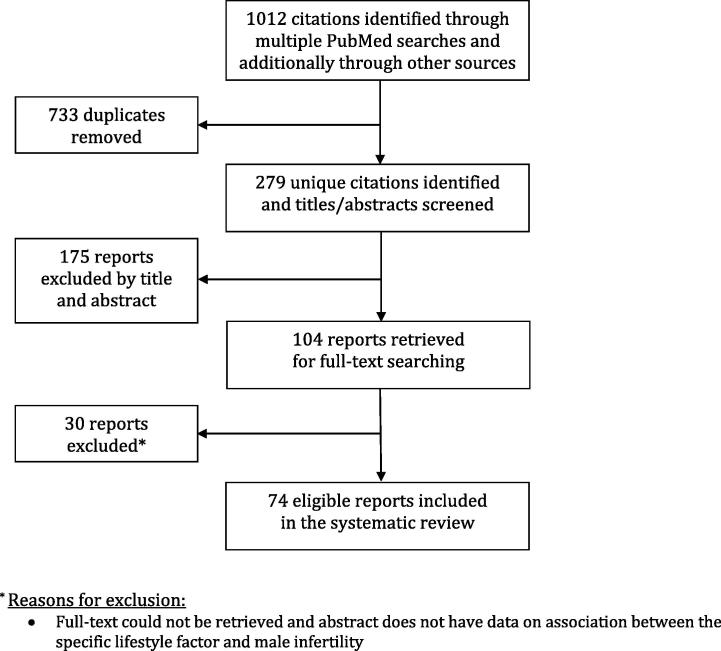
A systematic review of literature was conducted using PubMed over the last 10 years (from December 2008 to November 2017) for all published reports on the major lifestyle factors associated with male infertility (Fig. 1). The inclusion criterion was English language studies reporting on the lifestyle factors associated with male infertility such as ‘smoking’, ‘alcohol’, ‘marijuana’, ‘cocaine’, ‘anabolic steroids’, ‘diet’, ‘obesity’, ‘BMI’, ‘psychological stress, ‘advanced paternal age’, and ‘caffeine’. The keyword search terms for each of these factors were used in combination with the following search terms: ‘male infertility’, ‘male fertility’, ‘sperm quality’, ‘semen parameters’, ‘DNA fragmentation’, ‘paternal’, ‘maternal’, ‘ART’, and ‘IVF’. Pertinent studies that were published prior to the 10-year timeframe were included at the discretion of the author, as were some studies on testicular heat stress, intense cycling training, lack of sleep, and exposure to electromagnetic radiation from mobile phone use. Reference lists of the reports were searched for further relevant citations, which were subject to the inclusion criteria. All non-English language studies and those without a published abstract were not included.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart outlining the process of database search for study identification, screening, eligibility, and inclusion.
Results
In all, 1012 articles were identified from the database search and after reviewing the titles and abstract of the reports, 104 articles met the inclusion criteria. Of these, 30 reports were excluded as the full-text could not be retrieved and the abstract did not have data on the association between the lifestyle factor of interest and male infertility. The remaining 74 reports were reviewed for data on the association between a particular lifestyle factor and male infertility. Available evidence pertaining to the potential adverse effects of these lifestyle factors on male fertility vary in strength. Certain factors, such as cigarette smoking and alcohol intake are likely to exert an additive effect, whilst other factors may pose a threat when exposed along with other environmental and occupational factors.
Smoking
Cigarette smoke contains >7000 chemicals, including highly carcinogenic tobacco-specific nitrosamines, [e.g. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosonornicotine], polycyclic aromatic hydrocarbons (e.g. benzo[a]pyrene), and volatile organic compounds (e.g. benzene) [10]. Cigarette smokers have increased exposure to hazardous substances such as tar, nicotine (which is highly addictive), carbon monoxide, and heavy metals (e.g. cadmium and lead) [11].
Cigarette smoking is a known potential risk factor for decreased male fertility. Smoking is associated with leucocytospermia, a major endogenous source of reactive oxygen species (ROS). Moreover, tobacco smoke contains ROS at levels that can overwhelm the endogenous antioxidant defences. Increased seminal levels of ROS in smokers expose spermatozoa to oxidative stress, consequently impairing sperm function and ultimately compromising male fertility (reviewed in [12]). However, the mechanisms underlying the effects of smoking on sperm quality have not been fully clarified.
A large meta-analysis involving males from 26 countries/regions concluded that smoking causes a decline in sperm quality in both fertile and infertile men [13]. Sperm concentration in male smokers was reported to be typically 13–17% lower than that of non-smokers [14]. Moreover, cigarette smoking has been negatively associated with sperm count, motility, and morphology. The decline in semen quality was found to be more marked in heavy (>20 cigarettes/day) and moderate (10–20 cigarettes/day) smokers compared to mild smokers (1–10 cigarettes/day). The effect size was higher in infertile males than in the general population [15].
Besides its association with impaired male fertility, tobacco smoking is also responsible for increases in DNA damage, aneuploidies, and mutations in sperm [16]. One study suggested that the deterioration of semen quality amongst male smokers was correlated with increased DNA fragmentation rates and decreased expression of checkpoint kinase 1 (Chk1). Without the activation of Chk1 in response to DNA damage, there would be a decline in sperm repair leading to increased sperm apoptosis, which could lower semen quality [17].
Thus, the general effect of cigarette smoking on male fertility may result from the combined roles of elevated oxidative stress, DNA damage, and cell apoptosis, which could explain not only the reduction in semen quality, but also impaired spermatogenesis, sperm maturation, and sperm function reported to be present in smokers compared to non-smokers. Contributing factors leading to these effects in male smokers include the presence of nicotine and its metabolite, cotinine, benzo(a)pyrene, as well as cadmium levels [11]. For example, a meta-analysis of 13 317 men found that smoking was associated with higher mean testosterone levels, which could be attributed to the inhibition of testosterone breakdown by cotinine [18].
Pre-conception paternal smoking presents an increased risk of several morbidities in the offspring, which could perhaps be mediated via epigenetic modifications transmitted through spermatozoa. Male smokers showed a tendency towards increased alterations in methylation patterns genome-wide in their sperm DNA compared with never-smokers [19]. Maternal smoking during pregnancy and lactation could potentially cause harmful effects on male offspring fertility. Maternal cigarette smoke exposure during the gestation and weaning period was shown to cause diminished germ cell population, germ cell DNA damage, and defective sperm in the male offspring [20].
With paternal smoking being a significant risk factor for in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) failure, paternal smoking could perhaps contribute to decreased assisted reproductive technology (ART) success rates as much as maternal smoking (a risk factor only for IVF failure) does or more [21]. Moreover, male smoking could even influence the clinical pregnancy rate per intrauterine insemination (IUI) cycle [22]. Amongst former smokers, every additional year following the male partner’s smoking cessation reduced the risk of ART failure by 4%, particularly between clinical pregnancy and live birth [23].
Despite there being no concrete potential relationship between smoking and male infertility as of yet, available evidence on cigarette smoking and male fertility support the recommendation of smoking cessation and minimising exposure to tobacco smoke amongst couples who are trying to conceive.
Alcohol
A meta-analysis involving 29 914 men reported a significant association between alcohol intake and lower semen volume, but not sperm parameters [13]. However, a recent meta-analysis involving 16 395 men reported that alcohol intake has a detrimental effect on semen volume and sperm morphology [24]. Direct exposure of spermatozoa to alcohol (at concentrations corresponding to that of serum after moderate and heavy drinking) was found to be harmful to sperm motility and morphology in a dose-dependent manner [25].
The actions of alcohol on the male reproductive system seem to occur at all levels of the hypothalamus–pituitary–gonadal (HPG) axis. Alcohol appears to interfere with the production of GnRH, FSH, LH, and testosterone, as well as impair the functions of Leydig and Sertoli cells. As a result, the production, morphological development and maturation of spermatozoa could be impaired [26]. Spermatogenesis appears to gradually decline with increasing levels of alcohol intake [27]. Partial or complete spermatogenic arrest and Sertoli cell-only syndrome were more commonly present amongst heavy drinkers compared to non-drinkers [28].
Chronic alcohol intake was found to have a detrimental effect on both semen quality and the levels of male reproductive hormones [29]. Conversely, a study comprising 8344 healthy male volunteers found that moderate alcohol intake was associated with higher testosterone levels but not with semen quality [30]. Chronic ethanol administration has been shown to decrease testicular steroidogenic and antioxidant enzyme activities resulting in increased oxidative stress [31], which could disrupt testosterone synthesis and compromise fertility.
A study on the male partners of couples facing primary infertility found that teratozoospermia was present in 63% and 72% of males who drank alcohol moderately (40–80 g/day) and heavily (>80 g/day), respectively. None of the heavy alcohol drinkers were normozoospermic and most were oligozoospermic (64%), which is suggestive of progressive testicular damage in relation to increasing daily alcohol intake [32]. Similarly, another study found alcohol consumption rates to be significantly higher in men with severe oligozoospermia and with non-obstructive azoospermia compared to fertile controls [33].
Although the effects of alcohol on male reproductive function are dependent on the intake amount, a threshold amount of alcohol beyond which the risk of male infertility increases has not yet been determined. Moreover, it must be kept in mind that whilst alcohol intake and cigarette smoking alone did not affect sperm parameters, both habits together appear to exert an additive effect that could adversely alter sperm parameters [34].
Recreational drugs
Marijuana, cocaine, anabolic–androgenic steroids (AAS), opiates (narcotics), and methamphetamines are examples of illicit drugs that exert a negative impact on male fertility. The adverse effects of these drugs could impair the HPG axis, testicular architecture, and sperm function [35].
Cannabis or commonly referred to as marijuana, is the most abused illicit drug globally and has predominantly male users. Regular marijuana smoking (more than once weekly within the last 3 months) was found to lower sperm concentration and total sperm count amongst young men, and this effect was further exacerbated when marijuana was used in combination with other recreational drugs [36]. Deregulation of the endogenous cannabinoid system (ECS) was shown to significantly impair spermatogenesis resulting in lower total sperm count and motility [37]. Competition between the phytocannabinoids in marijuana and endocannabinoids for binding to the cannabinoid and vanilloid receptors leads to disruption of the homoeostasis of the ECS, which could consequently impair male fertility [38]. Sperm from infertile men have a marked modulation of endocannabinoid metabolism and reduction in endocannabinoid biosynthesis compared to sperm from fertile men [39].
Cocaine is a highly addictive, strong stimulant drug. Male rats given high doses of cocaine chronically before mating had lower pregnancy rates and offspring birth weights [40]. Both acute and chronic exposure to cocaine disrupted spermatogenesis and damaged the testicular ultrastructure [40], [41]. These changes could have been brought about by cocaine-induced apoptosis [42]. Long-term (≥5 years) cocaine users were associated with lower sperm concentration and motility, and a higher fraction of sperm with abnormal morphology [43]. Compared to other infertile men, infertile cocaine users are more likely to have other concurrent risk factors of male infertility such as smoking, (other) substance abuse, as well as a prior history of sexually transmitted infections [44].
Testosterone and its derivatives comprise a family of hormones called AAS. AAS are used primarily by males to enhance their athletic performance and/or personal appearance [45]. The use of AAS has expanded beyond that of professional athletes and the prevalence of anabolic steroid-induced hypogonadism (ASIH) amongst young men and teenagers is on the rise [46]. A retrospective study found that ASIH was the most common cause of profound hypogonadism (≤50 ng/dL testosterone) amongst men who sought treatment for hypogonadism [47]. Increased levels of exogenous testosterone, resulting from AAS use, exert a negative feedback on the HPG axis causing reversible suppression of spermatogenesis, testicular atrophy, and infertility. This may result in transient azoospermia with a recovery period of possibly even up to 2 years. Additionally, ASIH induced by AAS abuse can also result in loss of libido and erectile dysfunction [48]. Consequences from AAS use and subsequently treatment of the resulting hypogonadotrophic hypogonadism depend on the dose, duration, and type of AAS used [49]. Some studies have pointed towards a more permanent repercussion of steroid use on male fertility and as such, AAS use should be highly discouraged [50].
Obesity
Overweight and obesity are associated with excessive fat accumulation, which can be evaluated using the body mass index (BMI). Overweight (BMI 25–<30 kg/m2) and obese (BMI ≥30 kg/m2) males are associated with a decrease in sperm quality and a greater risk of infertility. A systematic review of 30 studies comprising 115 158 males found that paternal obesity was associated with lowered male reproductive potential. Men who were obese had a higher percentage of sperm with DNA fragmentation, abnormal morphology, and low mitochondrial membrane potential (MMP), and were more likely to be infertile [51]. Sperm with high DNA fragmentation and low MMP are associated with high levels of ROS [52].
A meta-analysis involving 13 077 men reported that obese men were more likely to be oligozoospermic or azoospermic compared to men within a normal weight range [53]. A population-based study found that as BMI and waist circumference increased, the prevalence of low ejaculate volume, sperm concentration, and total sperm count were also greater in overweight and obese men of unknown fertility. However, they did not find an association between body size and sperm motility, morphology or DNA damage [54]. A smaller study comprising 23.3% obese men showed that males who were overweight and obese had no increased relative risk of abnormal semen parameters, although their testosterone and sex hormone-binding globulin levels decreased with increasing BMI [55]. Additionally, inhibin B levels are decreased, whilst oestradiol levels are increased in overweight or obese men [56].
The presence of excess white adipose tissue in obese individuals causes increased conversion of testosterone to oestrogen, and affects the HPG axis leading to a reduction in gonadotrophin release. These effects result in secondary hypogonadism and impaired spermatogenesis [57]. Increased production of leptin by the white adipose tissue decreases testosterone production. Adipokines stimulate the production of ROS by leucocytes. Insulin resistance and dyslipidaemia can induce systemic inflammation, leading to oxidative stress [58]. Increased scrotal adiposity leads to testicular heat stress and causes oxidative stress. Increased scrotal temperature along with lack of activity impairs spermatogenesis. Increased oxidative stress impairs sperm motility, DNA integrity, and sperm–oocyte interaction [59].
Obese men who attempt ART have reduced rates of live birth per ART cycle [51]. Increased paternal BMI is associated with decreased blastocyst development, pregnancy, and live-birth rates, but not early embryo development [60].
One pilot study investigated if maternal obesity could influence the semen quality of her male offspring. Increasing maternal BMI had a negative relationship with the offspring’s inhibin B levels. Although the study found a suggestive trend of impaired fertility status in the offspring of overweight mothers, it was not statistically significant [61].
The severity of the consequences of obesity on the hormonal profile, sperm parameters, and DNA damage, as well as pregnancy outcomes may be varied due to the presence of other co-morbidities. Weight loss and lowering of BMI have helped improve sperm quality in some, but not all men [62].
Psychological stress
Stress, in its many forms, may be detrimental to male reproductive potential. The classical stress response activates the sympathetic nervous system and involves the hypothalamus–pituitary–adrenal (HPA) axis [63]. Both the HPA axis and gonadotrophin-inhibitory hormone (GnIH) exert an inhibitory effect on the HPG axis and testicular Leydig cells. The resulting inhibition of the HPG axis reduces testosterone levels. This leads to changes in Sertoli cells and the blood–testis barrier, which ultimately causes spermatogenesis to be suppressed. Impairment of testosterone secretion forms the main basis underlying the detrimental effects of psychological stress on spermatogenesis [64]. Raised corticosterone levels in stressed rats were found to suppress both testosterone and inhibin levels [65].
One study evaluated the effects of psychological stress on reproductive hormones and sperm quality in the male partner of infertile couples. The level of psychological stress was assessed using the Hospital Anxiety and Depression Score (HADS) questionnaire and 27% of the study population was found to have significant psychological stress. Men who were significantly stressed (HADS ≥8) had lower levels of testosterone, and higher levels of FSH and LH than men with normal HADS (HADS <8). However, GnRH levels were unchanged. Testosterone was negatively correlated, whilst FSH and LH were positively correlated with their HADS. Men with HADS ≥8 had a lower sperm count, motility, and normal morphology compared to those with normal HADS. Serum testosterone levels were positively correlated, whilst LH and FSH were negatively correlated with sperm count and motility [66].
Similarly, in an animal model study, acute restraint stress was shown to suppress sperm motility from 30 min of restraint onwards. Plasma levels of ACTH and corticosterone were elevated, whilst FSH, LH, and testosterone were decreased. It appears that the increased HPA axis activity had inhibited HPG activity [67].
A meta-analysis of 57 cross-sectional studies involving 29 914 participants reported that psychological stress could lower sperm concentration and progressive motility, and increase the fraction of sperm with abnormal morphology [13].
One study investigated the association between psychological stress in the form of occupational, life stress, family functioning, and semen quality. They found that occupational stress was negatively associated with semen quality, with a positive association between stress and percentage of sperm with DNA damage. Satisfaction with family functioning was negatively associated with the percentage of motile sperm cells. However, life stress did not correlate with semen quality [68]. Another study evaluated the associations between work-related stress, stressful life events, and perceived stress on semen quality. Of these, perceived stress and stressful life events were negatively associated with semen quality, particularly sperm motility and percentage normal morphology. However, work-related stress was not associated with semen parameters [69].
In another study amongst healthy volunteers, the effect of examination stress was investigated on seminal antioxidant content and sperm quality. During the stressful period just before examination, seminal glutathione and free sulphydryl content, as well as sperm motility was reduced, whilst the percentage of sperm with abnormal morphology was higher compared to a non-stress period 3 months after examination [70]. Seminal antioxidant enzymes, superoxide dismutase (SOD) and catalase were also measured. During the stressful pre-examination period, stress scores and SOD activity was increased, whilst sperm concentration and motility were decreased compared to the post-examination non-stress period. However, catalase activity remained unchanged [71].
Psychological stress is associated with reduced paternity and abnormal semen parameters, and thus could be a causative factor in affecting male infertility.
Advanced paternal age (APA)
Advanced maternal age is defined as the age of 35 years, beyond which there is significantly increased risks of adverse reproductive outcome for women [72]. However, APA has not been as well-defined, with studies commonly defining it to be between 35 and 50 years of age or categorising it into age ranges of 5 years [73]. A meta-analysis of 90 studies involving 93 839 participants reported an age-associated decline in semen volume, sperm total, and progressive motility, normal sperm morphology along with an increase in DNA fragmentation. However, despite its decline over time, sperm concentration did not decline with increasing male age [74].
Analysis of semen parameters of healthy men over a wide age range (22–80 years) showed that semen volume and sperm motility declined gradually and continuously with age without a specific age threshold [75]. However, a retrospective study involving 5081 men (aged 16.5–72.3 years) suggested that the decline in sperm parameters and its corresponding age threshold were as follows: total sperm count and total motile sperm - after 34 years of age; sperm concentration and fraction of sperm with normal morphology - after 40 years of age; sperm motility and progressive parameters of motile sperm - after 43 years of age; ejaculate volume - after 45 years of age; ratio of Y:X-bearing sperm in ejaculates - after 55 years of age. Thus, the authors proposed that independent of the woman’s age, the likelihood of pregnancy declines after intercourse with men aged >34 years [76].
The exact mechanisms underlying the age-associated decline in male fertility have not been determined [77]. These age‐dependent changes in semen quality could probably be attributed to normal physiological changes in the reproductive tract that occur with ageing, decreased capacity for cellular and tissue repair of damage induced by exposure to toxicants or diseases, and increased chances with age of having reproductive damage resulting from exogenous exposures such as smoking or infections [75]. The fact that both normal physiological processes and environmental factors could be held responsible for the effects of ageing on the male reproductive system adds to its complexity [78].
As men grow older, testicular function and metabolism deteriorates as the testis undergoes age-related morphological changes such as decrease in the number of germ cells, Leydig and Sertoli cells, as well as structural changes, including the narrowing of seminiferous tubules (reviewed in [78]). Concentrations of free and total testosterone steadily decline with increasing male age, leading to primary hypogonadism. Regulation of the HPG axis is also altered as men age. Accumulation of ROS in male germ cells throughout the course of ageing leads to oxidative stress and damage to sperm DNA. Apoptosis is also increased in the ageing testes (reviewed in [78]). These age-related changes inevitably lead to deterioration of sperm quality and quantity.
APA-induced increase in sperm DNA fragmentation adversely affects the success rates of ART outcomes, as well as early embryo development [79]. A study of donor ovum cycles indicated a 26% lower odds of live birth with each 5-year increase in paternal age [80]. In couples undergoing IVF, implantation and pregnancy rates decreased with increasing paternal age when the maternal age was between 30–34 years [81]. However, paternal age did not seem to affect ART outcomes when ICSI and good quality oocytes were used [82]. APA negatively influenced the number of high-quality embryos but did not affect pregnancy outcomes in couples undergoing ICSI cycles [83].
Independent of maternal age, APA is associated with increased rates of spontaneous abortion and lower pregnancy rates amongst couples attempting to conceive either naturally or using IUI [77]. The association between APA and foetal loss suggests that DNA mutations originating from the ageing male are detrimental to the offspring’s health [72]. Paternal ageing causes genetic and epigenetic changes in spermatozoa, which could proceed through fertilisation into the offspring, causing a variety of diseases in the resulting offspring [78].
Therefore, couples must be counselled with equal emphasis on the contribution of APA and advanced maternal age as being potential risk factors of negative pregnancy outcomes and impaired offspring health.
Diet
Diet and nutrition plays an important role in semen quality. A recent exhaustive systematic review of observational studies concluded that intake of a healthy, balanced diet could improve semen quality and fecundity rates amongst males [84]. For example, the Mediterranean diet, which is enriched with omega-3 fatty acids, antioxidants, and vitamins, and low in saturated and trans-fatty acids, were found to be inversely associated with low semen quality parameters [84]. Thus, greater compliance to the Mediterranean diet may aid in improving semen quality [85].
Another study defined a typical ‘Western’-style diet as one that was high in red and processed meat, refined grains, and high-energy drinks, whilst a more ‘Prudent’ diet comprised mainly of white meat, fruit, vegetables, and whole grains. The healthier ‘Prudent’ diet was positively associated with sperm progressive motility, but not sperm concentration and morphology [86]. In fact, a healthy dietary intake was reported to improve at minimum one measure of semen quality [87]. To begin with, the practice of substituting processed red meats with fish may have a positive impact on sperm counts and morphology [88].
Vegetables and fruits, fish and poultry, cereals and low-fat dairy products were amongst the foods positively associated with sperm quality. However, diets consisting of processed meat, full-fat dairy products, alcohol, coffee, and sugar-sweetened beverages were associated with poor semen quality and lower fecundity rates [84].
Caffeine
Most studies have not found an association between moderate caffeine intake and male fertility. A large meta-analysis with 29 914 participants found no significant effects of coffee consumption on semen quality [13]. Another study involving 2554 young Danish men also found no association between moderate caffeine (≤800 mg/day) or cola (≤1 L/day) intake and reduction in semen quality [89].
However, Belloc et al. [90] found that nearly 76% of caffeine consumers (3.0 ± 1.8 cups of coffee/day) had a slight increase in semen volume, whereas fertile vasectomy patients who drank ≥6 cups of coffee/day presented with higher sperm motility [91]. A recent systematic review involving 19 967 men found that in most of the studies, semen parameters were affected by cola-containing beverages and caffeine-containing soft drinks, but not by caffeine intake from mainly coffee, tea, and cocoa drinks [92].
Caffeine intake may impair male reproductive function possibly through sperm DNA damage. The Ricci et al. [92] meta-analysis suggests that caffeine intake could be associated with double-strand DNA breaks and sperm aneuploidy, but not DNA adducts. Independent of age, healthy non-smoking men whose daily coffee intake amounted to >308 mg (∼2.9 cups) have shown increased double-strand sperm DNA damage [93]. By contrast, the Belloc et al. [90] study found that caffeine intake was associated with a lower risk of elevated DNA fragmentation (odds ratio 0.92, 95% CI 0.92–0.99).
Some studies have even reported that coffee consumption in males was associated with prolonged time to pregnancy [92]. Amongst couples undergoing IVF, male caffeine consumption was not significantly associated with live-birth, fertilisation and implantation rates [94]. Male caffeine intake was associated with a lower probability of achieving a clinical pregnancy and live birth per ART cycle, particularly in men consuming ≥272 mg caffeine/day [95]. Male consumption of coffee had a negative relationship with ICSI fertilisation rate, but did not seem to affect implantation, pregnancy, and miscarriage rates [96]. Amongst couples with successful IVF/gamete intra-fallopian transfer (GIFT) outcomes, male caffeine consumption had no effect on fertilisation, pregnancy or live-birth rates. However, the odds of multiple gestations increased by 3.0 (95% CI 1.2–7.4) for men who consumed an additional 100 mg caffeine daily during the week of the initial clinic visit [97].
The potential developmental toxicity of caffeine in the reproductive function of the male progeny has also been examined. Men exposed in utero to increasing maternal coffee consumption displayed a tendency toward decreasing semen volume and testosterone levels. Whilst current caffeine consumption in adult males was not associated with semen quality, males with high caffeine intake had increased serum testosterone levels compared to those with low intake [98]. Maternal coffee consumption during gestation and lactation impaired gonadal development and seemed to exert permanent detrimental effects on the reproductive potential of male offspring rats [99].
In summary, based on the current available data, there is no firm potential relationship between caffeine intake and male infertility.
Other lifestyle risk factors
Genital heat stress resulting from scrotal hyperthermia is a substantial risk factor for male infertility. Prolonged hours of sitting or exposure to radiant heat, varicocele, and cryptorchidism can all lead to testicular heat stress [100]. Elevated scrotal temperatures lead to spermatogenic arrest, germ cell apoptosis, oxidative stress, and sperm DNA damage [59]. Cycling as a sport is associated with increased generation of testicular heat. Intense cycling training for 16 weeks in young healthy male road cyclists was found to induce an increase in seminal ROS and malondialdehyde levels, along with a decrease in enzymatic antioxidants and total antioxidant capacity. These changes were maintained even after 4 weeks of recovery [101]. Furthermore, seminal interleukin levels were raised in these non-professional cyclists and sperm parameters were suppressed despite the 4 weeks of recovery [102].
Sleep disturbances may possibly have adverse effects on male fertility, as semen volume was lower in patients with difficulty in initiating sleep, including those who smoked or were overweight [103]. Sleep loss was found to affect sperm function in an animal study [104]. Constant use of electronic devices also contributes to poor sleep hygiene. Exposure to radiofrequency electromagnetic waves radiation emitted by mobile phone use could potentially exert harmful effects on the testis [105]. One meta-analysis found that mobile phone exposure is associated with reduced sperm motility and viability [106], whilst another study found this adverse effect on sperm motility and viability occurs only in vitro [107].
Conclusion
There are a wide variety of risk factors that could potentially influence sperm quality. These include lifestyle factors such as cigarette smoking, alcohol intake, use of illicit drugs, obesity, psychological stress, APA, diet, and caffeine intake. The adverse effects of these factors could even become intensified from one generation to the next, and then passed on to the resulting offspring. However, their negative effects can be overcome to a large extent by behaviour modification and better lifestyle choices. In this manner, the harmful impact of these factors on the male reproductive potential could also be alleviated and thus result in a more favourable outcome.
The evidence supporting the adverse effect of each risk factor on male fertility, as discussed in the present review, are of varying strength. Almost all the studies focus on the specific effects of one or at most two risk factors that were under evaluation. However, in reality, exposure to these risk factors does not occur individually but rather simultaneously, with each one being at a varying duration and severity of exposure. It could then be said that without insight into the broader picture of these complex exposures, we may already be underestimating the consequences of each exposure.
The additive effects from the risk factors of male infertility such as smoking and alcohol intake on sperm parameters have been recognised. Moreover, the other groups of risk factors of male fertility, such as environmental and occupational factors, may also pose a simultaneous underlying threat to male fertility. Exposure to the confounding factor(s) should also be taken into consideration when planning the study design.
Perhaps by maintaining an overall positive lifestyle, the burden of the multiple factors that could influence sperm quality and male fecundity, may begin to slowly improve. In that respect, awareness and recognition of the possible impact of risk factors present in daily life is crucial amongst couples seeking conception. As the influence of several of these factors governing male infertility may be reversible, therefore the couple may benefit from early counselling and clinical intervention.
Conflict of interest
None.
Etiology
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Carlsen E., Giwercman A., Keiding N., Skakkebaek N.E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jørgensen N., Andersen A.G., Eustache F., Irvine D.S., Suominen J., Petersen J.H. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- 3.Aitken J.R. Falling sperm counts twenty years on: where are we now? Asian J Androl. 2013;15:204–207. doi: 10.1038/aja.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sengupta P., Borges E., Jr, Dutta S., Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Hum Exp Toxicol. 2017 doi: 10.1177/0960327117703690. [DOI] [PubMed] [Google Scholar]
- 5.Levine H., Jørgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurewicz J., Radwan M., Sobala W., Radwan P., Bochenek M., Hanke W. Effects of occupational exposure - is there a link between exposure based on an occupational questionnaire and semen quality? Syst Biol Reprod Med. 2014;60:227–233. doi: 10.3109/19396368.2014.907837. [DOI] [PubMed] [Google Scholar]
- 7.Knez J. Endocrine-disrupting chemicals and male reproductive health. Reprod Biomed Online. 2013;26:440–448. doi: 10.1016/j.rbmo.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Bonde J.P. Occupational causes of male infertility. Curr Opin Endocrinol Diabetes Obes. 2013;20:234–239. doi: 10.1097/MED.0b013e32835f3d4b. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R., Biedenharn K.R., Fedor J.M., Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (US) National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (US) Publications and Reports of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta (GA): 2010. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. [PubMed] [Google Scholar]
- 11.Dai J.B., Wang Z.X., Qiao Z.D. The hazardous effects of tobacco smoking on male fertility. Asian J Androl. 2015;17:954–960. doi: 10.4103/1008-682X.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlev A., Agarwal A., Gunes S.O., Shetty A., du Plessis S.S. Smoking and male infertility: an evidence-based review. World J Mens Health. 2015;33:143–160. doi: 10.5534/wjmh.2015.33.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Lin H., Li Y., Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Vine M.F., Margolin B.H., Morrison H.I., Hulka B.S. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61:35–43. [PubMed] [Google Scholar]
- 15.Sharma R., Harlev A., Agarwal A., Esteves S.C. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Beal M.A., Yauk C.L., Marchetti F. From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat Res. 2017;773:26–50. doi: 10.1016/j.mrrev.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Cui X., Jing X., Wu X., Wang Z., Li Q. Potential effect of smoking on semen quality through DNA damage and the downregulation of Chk1 in sperm. Mol Med Rep. 2016;14:753–761. doi: 10.3892/mmr.2016.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Leung J.Y., Lin S.L., Schooling C.M. Cigarette smoking and testosterone in men and women: A systematic review and meta-analysis of observational studies. Prev Med. 2016;85:1–10. doi: 10.1016/j.ypmed.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins T.G., James E.R., Alonso D.F., Hoidal J.R., Murphy P.J., Hotaling J.M. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology. 2017;5:1089–1099. doi: 10.1111/andr.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobinoff A.P., Sutherland J.M., Beckett E.L., Stanger S.J., Johnson R., Jarnicki A.G. Damaging legacy: maternal cigarette smoking has long-term consequences for male offspring fertility. Hum Reprod. 2014;29:2719–2735. doi: 10.1093/humrep/deu235. [DOI] [PubMed] [Google Scholar]
- 21.Kovac J.R., Khanna A., Lipshultz L.I. The effects of cigarette smoking on male fertility. Postgrad Med. 2015;127:338–341. doi: 10.1080/00325481.2015.1015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thijssen A., Creemers A., Van der Elst W., Creemers E., Vandormael E., Dhont N. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: a prospective cohort study. Reprod Biomed Online. 2017;34:463–472. doi: 10.1016/j.rbmo.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Vanegas J.C., Chavarro J.E., Williams P.L., Ford J.B., Toth T.L., Hauser R. Discrete survival model analysis of a couple's smoking pattern and outcomes of assisted reproduction. Fertil Res Pract. 2017;3:5. doi: 10.1186/s40738-017-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci E., Al Beitawi S., Cipriani S., Candiani M., Chiaffarino F., Vigano P. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34:38–47. doi: 10.1016/j.rbmo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly G.P., McClure N., Kennedy M.S., Lewis S.E. Direct effect of alcohol on the motility and morphology of human spermatozoa. Andrologia. 1999;31:43–47. [PubMed] [Google Scholar]
- 26.Emanuele M.A., Emanuele N.V. Alcohol's effects on male reproduction. Alcohol Health Res World. 1998;22:195–201. [PMC free article] [PubMed] [Google Scholar]
- 27.Pajarinen J., Karhunen P.J., Savolainen V., Lalu K., Penttila A., Laippala P. Moderate alcohol consumption and disorders of human spermatogenesis. Alcohol Clin Exp Res. 1996;20:332–337. doi: 10.1111/j.1530-0277.1996.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 28.Pajarinen J.T., Karhunen P.J. Spermatogenic arrest and 'Sertoli cell-only' syndrome–common alcohol-induced disorders of the human testis. Int J Androl. 1994;17:292–299. doi: 10.1111/j.1365-2605.1994.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 29.Muthusami K.R., Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril. 2005;84:919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Jensen T.K., Swan S., Jorgensen N., Toppari J., Redmon B., Punab M. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum Reprod. 2014;29:1801–1809. doi: 10.1093/humrep/deu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maneesh M., Jayalekshmi H., Dutta S., Chakrabarti A., Vasudevan D.M. Effect of chronic ethanol administration on testicular antioxidant system and steroidogenic enzyme activity in rats. Indian J Exp Biol. 2005;43:445–449. [PubMed] [Google Scholar]
- 32.Gaur D.S., Talekar M.S., Pathak V.P. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z.B., Jiang Y.T., Yun X., Yang X., Wang R.X., Dai R.L. Male infertility in Northeast China: a cytogenetic study of 135 patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet. 2012;29:83–87. doi: 10.1007/s10815-011-9670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martini A.C., Molina R.I., Estofan D., Senestrari D., Fiol de Cuneo M., Ruiz R.D. Effects of alcohol and cigarette consumption on human seminal quality. Fertil Steril. 2004;82:374–377. doi: 10.1016/j.fertnstert.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Fronczak C.M., Kim E.D., Barqawi A.B. The insults of illicit drug use on male fertility. J Androl. 2012;33:515–528. doi: 10.2164/jandrol.110.011874. [DOI] [PubMed] [Google Scholar]
- 36.Gundersen T.D., Jorgensen N., Andersson A.M., Bang A.K., Nordkap L., Skakkebaek N.E. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol. 2015;182:473–481. doi: 10.1093/aje/kwv135. [DOI] [PubMed] [Google Scholar]
- 37.Lewis S.E., Paro R., Borriello L., Simon L., Robinson L., Dincer Z. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. Int J Androl. 2012;35:731–740. doi: 10.1111/j.1365-2605.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 38.du Plessis S.S., Agarwal A., Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. 2015;32:1575–1588. doi: 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis S.E., Rapino C., Di Tommaso M., Pucci M., Battista N., Paro R. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS One. 2012;7:e47704. doi: 10.1371/journal.pone.0047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George V.K., Li H., Teloken C., Grignon D.J., Lawrence W.D., Dhabuwala C.B. Effects of long-term cocaine exposure on spermatogenesis and fertility in peripubertal male rats. J Urol. 1996;155:327–331. [PubMed] [Google Scholar]
- 41.Rodriguez M.C., Sanchez-Yague J., Paniagua R. Effects of cocaine on testicular structure in the rat. Reprod Toxicol. 1992;6:51–55. doi: 10.1016/0890-6238(92)90020-t. [DOI] [PubMed] [Google Scholar]
- 42.Li H., Jiang Y., Rajpurkar A., Dunbar J.C., Dhabuwala C.B. Cocaine induced apoptosis in rat testes. J Urol. 1999;162:213–216. doi: 10.1097/00005392-199907000-00070. [DOI] [PubMed] [Google Scholar]
- 43.Bracken M.B., Eskenazi B., Sachse K., McSharry J.E., Hellenbrand K., Leo-Summers L. Association of cocaine use with sperm concentration, motility, and morphology. Fertil Steril. 1990;53:315–322. doi: 10.1016/s0015-0282(16)53288-9. [DOI] [PubMed] [Google Scholar]
- 44.Samplaski M.K., Bachir B.G., Lo K.C., Grober E.D., Lau S., Jarvi K.A. Cocaine use in the infertile male population: a marker for conditions resulting in subfertility. Curr Urol. 2015;8:38–42. doi: 10.1159/000365687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanayama G., Hudson J.I., Pope H.G., Jr. Illicit anabolic-androgenic steroid use. Horm Behav. 2010;58:111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karavolos S., Reynolds M., Panagiotopoulou N., McEleny K., Scally M., Quinton R. Male central hypogonadism secondary to exogenous androgens: a review of the drugs and protocols highlighted by the online community of users for prevention and/or mitigation of adverse effects. Clin Endocrinol. 2015;82:624–632. doi: 10.1111/cen.12641. [DOI] [PubMed] [Google Scholar]
- 47.Coward R.M., Rajanahally S., Kovac J.R., Smith R.P., Pastuszak A.W., Lipshultz L.I. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200–2205. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Nieschlag E., Vorona E. Mechanisms in endocrinology: Medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions. Eur J Endocrinol. 2015;173:R47–58. doi: 10.1530/EJE-15-0080. [DOI] [PubMed] [Google Scholar]
- 49.Rahnema C.D., Lipshultz L.I., Crosnoe L.E., Kovac J.R., Kim E.D. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271–1279. doi: 10.1016/j.fertnstert.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 50.de Souza G.L., Hallak J. Anabolic steroids and male infertility: a comprehensive review. BJU Int. 2011;108:1860–1865. doi: 10.1111/j.1464-410X.2011.10131.x. [DOI] [PubMed] [Google Scholar]
- 51.Campbell J.M., Lane M., Owens J.A., Bakos H.W. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online. 2015;31:593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Lobascio A.M., De Felici M., Anibaldi M., Greco P., Minasi M.G., Greco E. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology. 2015;3:265–270. doi: 10.1111/andr.302. [DOI] [PubMed] [Google Scholar]
- 53.Sermondade N., Faure C., Fezeu L., Shayeb A.G., Bonde J.P., Jensen T.K. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenberg M.L., Kim S., Chen Z., Sundaram R., Schisterman E.F., Buck Louis G.M. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29:193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macdonald A.A., Stewart A.W., Farquhar C.M. Body mass index in relation to semen quality and reproductive hormones in New Zealand men: a cross-sectional study in fertility clinics. Hum Reprod. 2013;28:3178–3187. doi: 10.1093/humrep/det379. [DOI] [PubMed] [Google Scholar]
- 56.Chavarro J.E., Toth T.L., Wright D.L., Meeker J.D., Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer N.O., Bakos H.W., Fullston T., Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012;2:253–263. doi: 10.4161/spmg.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González A., Esquivel-Chirino C. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durairajanayagam D., Agarwal A., Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online. 2015;30:14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Bakos H.W., Henshaw R.C., Mitchell M., Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95:1700–1704. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 61.Ramlau-Hansen C.H., Nohr E.A., Thulstrup A.M., Bonde J.P., Storgaard L., Olsen J. Is maternal obesity related to semen quality in the male offspring? A pilot study. Hum Reprod. 2007;22:2758–2762. doi: 10.1093/humrep/dem219. [DOI] [PubMed] [Google Scholar]
- 62.Kahn B.E., Brannigan R.E. Obesity and male infertility. Curr Opin Urol. 2017;27:441–445. doi: 10.1097/MOU.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 63.McGrady A.V. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;13:1–7. doi: 10.3109/01485018408987495. [DOI] [PubMed] [Google Scholar]
- 64.Nargund V.H. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12:373–382. doi: 10.1038/nrurol.2015.112. [DOI] [PubMed] [Google Scholar]
- 65.Tohei A., Tomabechi T., Mamada M., Akai M., Watanabe G., Taya K. Effects of repeated ether stress on the hypothalamic-pituitary-testes axis in adult rats with special reference to inhibin secretion. J Vet Med Sci. 1997;59:329–334. doi: 10.1292/jvms.59.329. [DOI] [PubMed] [Google Scholar]
- 66.Bhongade M.B., Prasad S., Jiloha R.C., Ray P.C., Mohapatra S., Koner B.C. Effect of psychological stress on fertility hormones and seminal quality in male partners of infertile couples. Andrologia. 2015;47:336–342. doi: 10.1111/and.12268. [DOI] [PubMed] [Google Scholar]
- 67.Ren L., Li X., Weng Q., Trisomboon H., Yamamoto T., Pan L. Effects of acute restraint stress on sperm motility and secretion of pituitary, adrenocortical and gonadal hormones in adult male rats. J Vet Med Sci. 2010;72:1501–1506. doi: 10.1292/jvms.10-0113. [DOI] [PubMed] [Google Scholar]
- 68.Jurewicz J., Radwan M., Merecz-Kot D., Sobala W., Ligocka D., Radwan P. Occupational, life stress and family functioning: does it affect semen quality? Ann Hum Biol. 2014;41:220–228. doi: 10.3109/03014460.2013.849755. [DOI] [PubMed] [Google Scholar]
- 69.Janevic T., Kahn L.G., Landsbergis P., Cirillo P.M., Cohn B.A., Liu X. Effects of work and life stress on semen quality. Fertil Steril. 2014;102:530–538. doi: 10.1016/j.fertnstert.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eskiocak S., Gozen A.S., Yapar S.B., Tavas F., Kilic A.S., Eskiocak M. Glutathione and free sulphydryl content of seminal plasma in healthy medical students during and after exam stress. Hum Reprod. 2005;20:2595–2600. doi: 10.1093/humrep/dei062. [DOI] [PubMed] [Google Scholar]
- 71.Eskiocak S., Gozen A.S., Kilic A.S., Molla S. Association between mental stress & some antioxidant enzymes of seminal plasma. Indian J Med Res. 2005;122:491–496. [PubMed] [Google Scholar]
- 72.Ramasamy R., Chiba K., Butler P., Lamb D.J. Male biological clock: a critical analysis of advanced paternal age. Fertil Steril. 2015;103:1402–1406. doi: 10.1016/j.fertnstert.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu C., Lipshultz L.I., Kovac J.R. The role of advanced paternal age in modern reproductive medicine. Asian J Androl. 2016;18:425. doi: 10.4103/1008-682X.179251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson S.L., Dunleavy J., Gemmell N.J., Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Eskenazi B., Wyrobek A.J., Sloter E., Kidd S.A., Moore L., Young S. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 76.Stone B.A., Alex A., Werlin L.B., Marrs R.P. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–958. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 77.Belloc S., Hazout A., Zini A., Merviel P., Cabry R., Chahine H. How to overcome male infertility after 40: Influence of paternal age on fertility. Maturitas. 2014;78:22–29. doi: 10.1016/j.maturitas.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Gunes S., Hekim G.N., Arslan M.A., Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33:441–454. doi: 10.1007/s10815-016-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma R., Agarwal A., Rohra V.K., Assidi M., Abu-Elmagd M., Turki R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35. doi: 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robertshaw I., Khoury J., Abdallah M.E., Warikoo P., Hofmann G.E. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21:590–593. doi: 10.1177/1933719113506497. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y., Kang X., Zheng H., Liu H., Liu J. Effect of paternal age on reproductive outcomes of in vitro fertilization. PLoS One. 2015;10:e0135734. doi: 10.1371/journal.pone.0135734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Begueria R., Garcia D., Obradors A., Poisot F., Vassena R., Vernaeve V. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29:2114–2122. doi: 10.1093/humrep/deu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Y., Kang X., Zheng H., Liu H., Huang Q., Liu J. Effect of paternal age on reproductive outcomes of intracytoplasmic sperm injection. PLoS One. 2016;11:e0149867. doi: 10.1371/journal.pone.0149867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salas-Huetos A., Bullo M., Salas-Salvado J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 85.Karayiannis D., Kontogianni M.D., Mendorou C., Douka L., Mastrominas M., Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod. 2017;32:215–222. doi: 10.1093/humrep/dew288. [DOI] [PubMed] [Google Scholar]
- 86.Gaskins A.J., Colaci D.S., Mendiola J., Swan S.H., Chavarro J.E. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giahi L., Mohammadmoradi S., Javidan A., Sadeghi M.R. Nutritional modifications in male infertility: a systematic review covering 2 decades. Nutr Rev. 2016;74:118–130. doi: 10.1093/nutrit/nuv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Afeiche M.C., Gaskins A.J., Williams P.L., Toth T.L., Wright D.L., Tanrikut C. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144:1091–1098. doi: 10.3945/jn.113.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen T.K., Swan S.H., Skakkebaek N.E., Rasmussen S., Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883–891. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- 90.Belloc S., Cohen-Bacrie M., Dalleac A., Amar E., Hazout A., de Mouzon J. Caffeine intake and sperm parameters. Analysis of a cohort of 4474 consecutive semen samples. Fertil Steril. 2013;100(Suppl.):S212. [Google Scholar]
- 91.Sobreiro B.P., Lucon A.M., Pasqualotto F.F., Hallak J., Athayde K.S., Arap S. Semen analysis in fertile patients undergoing vasectomy: reference values and variations according to age, length of sexual abstinence, seasonality, smoking habits and caffeine intake. Sao Paulo Med J. 2005;123:161–166. doi: 10.1590/S1516-31802005000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ricci E., Vigano P., Cipriani S., Somigliana E., Chiaffarino F., Bulfoni A. Coffee and caffeine intake and male infertility: a systematic review. Nutr J. 2017;16:37. doi: 10.1186/s12937-017-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmid T.E., Eskenazi B., Baumgartner A., Marchetti F., Young S., Weldon R. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 94.Choi J.H., Ryan L.M., Cramer D.W., Hornstein M.D., Missmer S.A. Effects of caffeine consumption by women and men on the outcome of in vitro fertilization. J Caffeine Res. 2011;1:29–34. doi: 10.1089/jcr.2011.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karmon A.E., Toth T.L., Chiu Y.H., Gaskins A.J., Tanrikut C., Wright D.L. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology. 2017;5:354–361. doi: 10.1111/andr.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braga D.P., Halpern G., Figueira Rde C., Setti A.S., Iaconelli A., Jr, Borges E., Jr. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97:53–59. doi: 10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 97.Klonoff-Cohen H., Bleha J., Lam-Kruglick P. A prospective study of the effects of female and male caffeine consumption on the reproductive endpoints of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2002;17:1746–1754. doi: 10.1093/humrep/17.7.1746. [DOI] [PubMed] [Google Scholar]
- 98.Ramlau-Hansen C.H., Thulstrup A.M., Bonde J.P., Olsen J., Bech B.H. Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod. 2008;23:2799–2805. doi: 10.1093/humrep/den331. [DOI] [PubMed] [Google Scholar]
- 99.Dorostghoal M., Erfani Majd N., Nooraei P. Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin Exp Reprod Med. 2012;39:144–152. doi: 10.5653/cerm.2012.39.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Durairajanayagam D., Sharma R., Du Plessis S.S., Agarwal A. Testicular heat stress and sperm quality. In: Du Plessis S.S., editor. Male infertility: a complete guide to lifestyle and environmental factors. Springer Science + Business Media; New York: 2014. [Google Scholar]
- 101.Maleki B.H., Tartibian B., Vaamonde D. The effects of 16 weeks of intensive cycling training on seminal oxidants and antioxidants in male road cyclists. Clin J Sport Med. 2014;24:302–307. doi: 10.1097/JSM.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 102.Hajizadeh Maleki B., Tartibian B. Long-term low-to-intensive cycling training: impact on semen parameters and seminal cytokines. Clin J Sport Med. 2015;25:535–540. doi: 10.1097/JSM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 103.Vigano P., Chiaffarino F., Bonzi V., Salonia A., Ricci E., Papaleo E. Sleep disturbances and semen quality in an Italian cross sectional study. Basic Clin Androl. 2017;27:16. doi: 10.1186/s12610-017-0060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alvarenga T.A., Hirotsu C., Mazaro-Costa R., Tufik S., Andersen M.L. Impairment of male reproductive function after sleep deprivation. Fertil Steril. 2015;103:1355–1362. doi: 10.1016/j.fertnstert.2015.02.002. e1. [DOI] [PubMed] [Google Scholar]
- 105.Agarwal A., Durairajanayagam D. Are men talking their reproductive health away? Asian J Androl. 2015;17:433–434. doi: 10.4103/1008-682X.140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adams J.A., Galloway T.S., Mondal D., Esteves S.C., Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int. 2014;70:106–112. doi: 10.1016/j.envint.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 107.Liu K., Li Y., Zhang G., Liu J., Cao J., Ao L. Association between mobile phone use and semen quality: a systemic review and meta-analysis. Andrology. 2014;2:491–501. doi: 10.1111/j.2047-2927.2014.00205.x. [DOI] [PubMed] [Google Scholar]