Abstract
Despite advocacy to reduce smoking-related diseases, >1 billion people worldwide continue to smoke. Smoking is immunosuppressive and an important etiological factor in the development of several human disorders including respiratory diseases like chronic obstructive pulmonary disease. However, there is a critical gap in the knowledge of the role of secondhand smoke (SHS) on inflammation and immunity. We therefore studied the influence of SHS on pulmonary inflammation and immune responses to respiratory infection by nontypeable Haemophilus influenzae (NTHI) recurrently found in chronic obstructive pulmonary disease patients. Chronic SHS-exposed mice were chronically infected with NTHI and pulmonary inflammation was evaluated by histology. Immune cell numbers and cytokines were measured by flow cytometry and ELISA, respectively. Chronic SHS exposure impaired NTHI P6 antigen-specific B and T cell responses following chronic NTHI infection as measured by ELISPOT assays, reduced the production of antibodies in serum and bronchoalveolar lavage and enhanced albumin leak into the bronchoalveolar lavage as determined by ELISA. Histopathological examination of lungs revealed lymphocytic accumulation surrounding airways and bronchovasculature following chronic SHS exposure and chronic infection. Chronic SHS exposure enhanced the levels of inflammatory cytokines IL-17A, IL-6, IL-1β and TNF-α in the lungs and impaired the generation of adaptive immunity following either chronic infection or after P6 vaccination. Chronic SHS exposure diminished bacterial clearance from the lungs after acute NTHI challenge, while P6 vaccination improved clearance equivalent to the level seen in air exposed, non-vaccinated mice. Our study provides unequivocal evidence that SHS exposure has long-term detrimental effects on the pulmonary inflammatory microenvironment and immunity to infection and vaccination.
Introduction
Smoking is the leading cause of preventable death worldwide. Mainstream tobacco smoke (MTS) and environmental tobacco smoke exposures are both recognized as major risk factors in the development and pathogenesis of several life-threatening diseases (1–4). It is estimated that tobacco use, either directly or from secondhand smoke exposure (SHS), kills around 6 million people annually (5). The harmful effects of SHS have been recorded since 1928 (6). Epidemiological studies also associate SHS exposure with negative health consequences including respiratory tract infections like chronic obstructive pulmonary disease (COPD), ischemic heart disease, asthma and lung cancer (2), and the economic impact of SHS exposure is estimated at ~$10 billion a year to the US economy (7, 8). These negative health consequences are attributed to the fact that tobacco smoke is a complex, dynamic and reactive mixture of ~5,000 chemicals that are a main source of toxic chemical exposure and chemically-mediated diseases in humans (9). Cigarette smoke (CS) is one of the main forms of tobacco smoking and comprises MTS and sidestream smoke, both varying in chemical composition. SHS is a heterogenous mixture of exhaled MTS and sidestream smoke and has been shown to contain a plethora of different toxic agents. Rodent models of tobacco smoke exposure have clearly shown that chronic daily smoke exposures induce significant pathological changes in mice (10–13). However, tobacco smoke exposures in these studies were chemically similar to ones experienced by primary smokers. Nevertheless, similar kinds of investigations evaluating the effects of SHS at concentrations and for time periods reflective of human exposure are much less studied and poorly evaluated.
Chronic respiratory diseases rank as the third most common cause of death in United States and fifth worldwide causing significant socioeconomic burden (1–5). Human and animal studies have clearly demonstrated that MTS induces inflammation and a number of physiological changes in the airway epithelium leading to pulmonary damage, immune suppression and exacerbation of respiratory infections (14–21). This is highlighted in COPD, in which a subgroup of patients who experience frequent exacerbations account for the majority of health care costs, morbidity and mortality (22). The importance of smoking and infection is not confined to chronic diseases like COPD, as smokers also have increased risk of mortality from influenza and pneumonia (23, 24).
The evidence for the impact of SHS exposure on respiratory infections is less well developed. Environmental tobacco smoke exposure is an important predictor of respiratory health outcome in children and epidemiological studies indicate that adults and children exposed to SHS experience increased risk of respiratory infections (25–28). It is known that approximately 25%–45% COPD patients are non-smokers (29) and many investigators associate SHS exposure to the pathogenesis of respiratory diseases and recognize it as a major cause of COPD in non-smokers (30, 31). However, there are no animal studies that have considered chronic SHS exposure together with chronic infection that allow us to directly investigate mechanism and mitigation strategies. Thus, studies evaluating the impact of SHS exposure on overall lung microenvironment, pulmonary inflammation and development of immunity against common respiratory infections are urgently needed. This is a critical knowledge gap, as chronic infections represent a failure to develop protective immunity that contributes to increased morbidity and mortality.
In the present study, we have used a mouse model to examine the impact of chronic SHS exposure on chronic infection with non-typeable Haemophilus influenzae (NTHI), an important gram negative bacterial pathogen commonly found in COPD exacerbations (32). We evaluated how chronic SHS exposure impacts pulmonary inflammation and the development of adaptive immunity to chronic NTHI infection, and dissected if prophylactic vaccination could help in mitigating SHS-induced defects in adaptive immunity to combat and alleviate future bouts of acute infection. Our results clearly establish that chronic SHS exposure worsens NTHI-mediated pulmonary inflammation and diminishes the generation of adaptive immunity. Moreover, SHS exposure impairs bacterial clearance from the lungs of mice resulting in augmented inflammation and increased lung damage due to defects in the development of adaptive immunity to a candidate vaccine antigen. Our results support the conclusion that chronic exposure to environmental tobacco smoke is an important pulmonary inflammatory insult that translates into worsening lung immunity to respiratory infections and reduces the efficacy of prophylactic vaccination.
Materials and Methods
Mice
Eight week old female C57BL/6J mice (Jackson Laboratory) were used in all experiments. Animals were maintained under specific pathogen-free conditions. Number of animals per group in each experiment was n=10, unless mentioned otherwise in the figure legends. All procedures performed on animals were approved by the Animal Care and Use Committees of both institutions (Roswell Park Cancer Institute and University of Rochester, USA), and complied with all state, federal, and National Institutes of Health regulations.
SHS exposure
Mice were housed in the Inhalation Core Facility at the University of Rochester and were exposed to secondhand smoke 5 hours per day, 5 days per week for 8 weeks. Research cigarettes (3R4F) were combusted using an automated smoking machine (TE-10, Teague Enterprises, Woodland California). Mainstream cigarette smoke was generated using a puff of 35 ml volume and 2 sec duration once per minute (the FTC protocol). Sidestream smoke was collected and mixed with the mainstream smoke at an approximate ratio of 89%/11% (33). The resulting mix of mainstream and sidestream smoke approximates secondhand smoke. The number of cigarettes loaded was adjusted to achieve a target of 90 mg/m3 total particulate matter (TPM); the actual concentration for these experiments was 31±9 mg/m3 (low dose) and 90+28 mg/m3 (high dose) for the studies reported in Fig. 1–5, and 99±3 mg/m3 (high dose) for the study reported in Fig. 6–8 (mean ± standard deviation). Control mice were exposed to filtered air in an identical chamber according to the same schedule. Following the final exposure, mice were transported to Roswell Park Cancer Institute for infection and vaccination experiments.
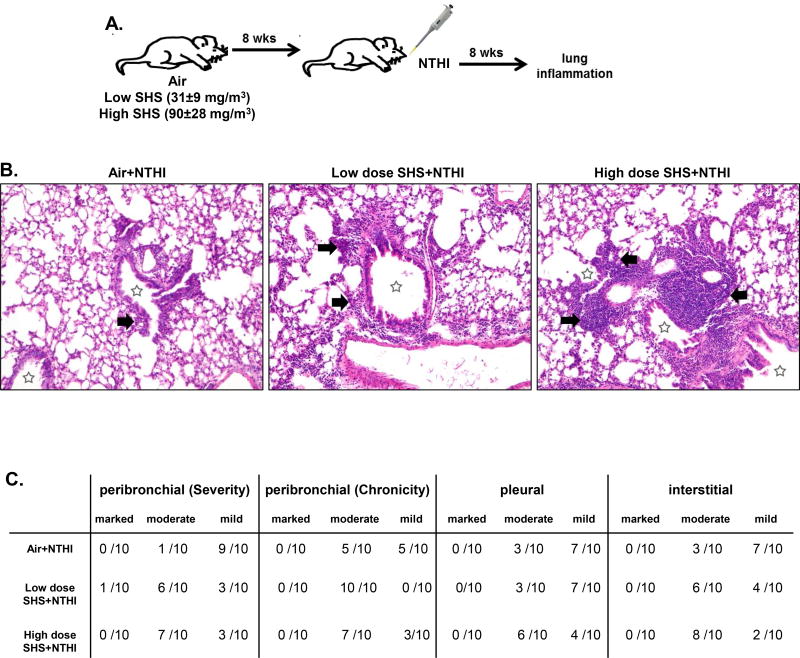
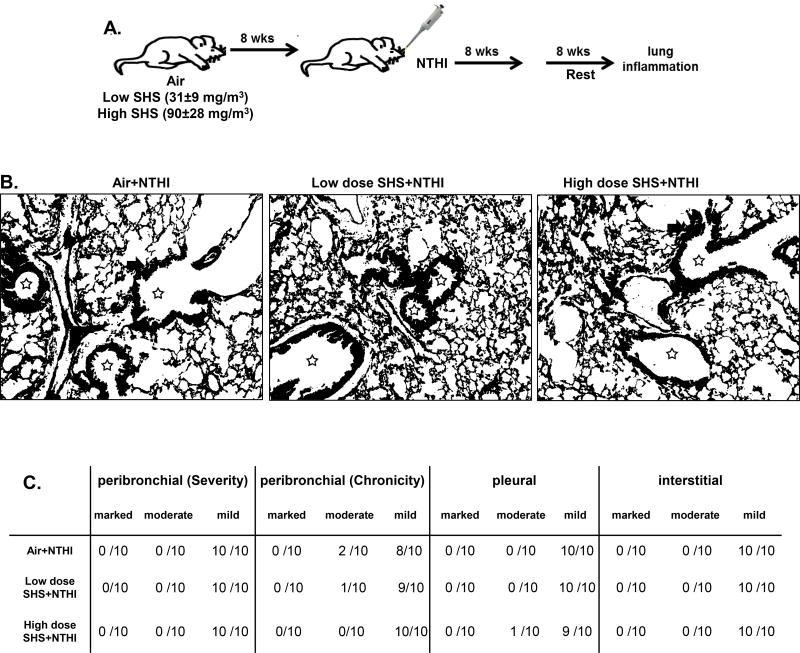
FIGURE 1. Chronic exposure to SHS augments NTHI-mediated chronic pulmonary inflammation.
(A) C57BL/6J female mice were exposed for 8 weeks to SHS or air, followed by 8 weeks of chronic NTHI infection (twice per week). Average smoke exposures were 31±9 (low dose) and 90±28 mg/m3 (high dose) total particulate matter. Animals were euthanized 48 hr after the final infection. (B) H&E-stained lung sections prepared after combined inflammatory insult were evaluated for the severity and chronicity of inflammation. Large areas of lymphocytic inflammation around bronchovascular bundles are depicted by arrows and airway lumen are shown with stars. Original magnification × 100. (C) Consensus scores from two blinded nonconsecutive sessions evaluating respiratory inflammation in peribronchial, pleural, and interstitial regions of the lung were performed by a clinical pathologist (P.N.B.).
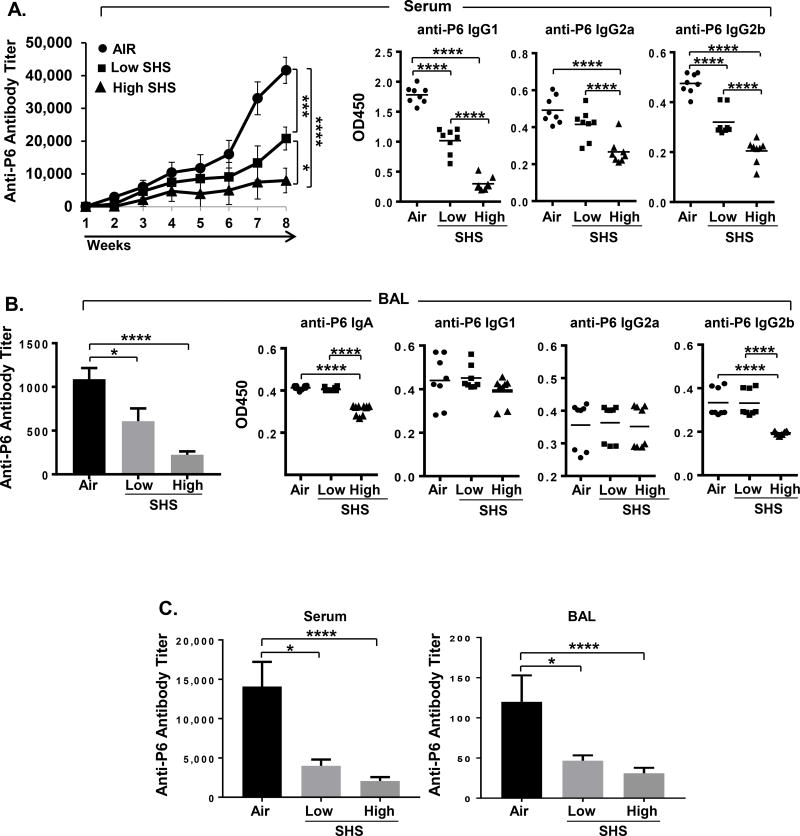
FIGURE 5. SHS exposure diminishes the generation of adaptive immune responses to NTHI-mediated chronic respiratory infection.
(A) Anti-P6 total Ig was measured in weekly serum samples (n=18), and isotype-specific Igs were measured in the final serum sample collected at euthanasia (n=8). (B) Total and isotype-specific anti-P6 Igs were measured in BAL fluid collected at euthanasia from air- or SHS-exposed mice after chronic NTHI infection (n = 8–10). To quantify the antibody subclasses IgG1, IgG2a and IgG2b in serum (A) and IgA, IgG1, IgG2a and IgG2b in the BAL (B) of mice, the OD values at 450 nm were measured using serum dilutions at 1:1600 and BAL dilutions at 1:400. (C) Total anti-P6 Igs were measured in endpoint serum and BAL collected at the time of euthanasia from air- or SHS-exposed mice after 8 wks chronic NTHI infection was followed by another 8 wks of rest period (n = 8–10). Line represents mean. *p≤0.05, ***p≤0.005, ****p≤0.0001 two tailed unpaired t test.
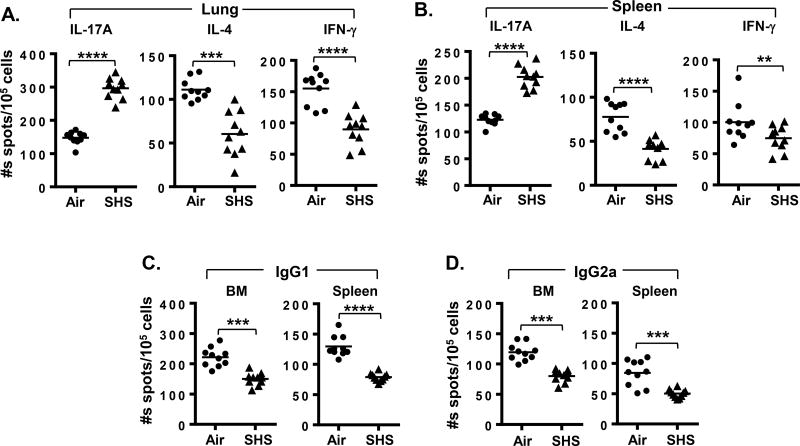
FIGURE 6. SHS induces defect in the generation of adaptive immune responses against NTHI-mediated chronic respiratory infection by impairing the frequencies of antigen-specific cytokine secreting T- and Ab producing B- cells.
Single-cell suspensions of (A) lung lymphocytes and (B) splenocytes were isolated from air- or SHS-exposed mice and incubated with P641–55 peptide-pulsed APCs to measure the frequency of cytokine-secreting T cells by ELISPOT (n = 10). B cell ELISPOTs were performed to quantify the numbers of P6-specific (C) IgG1- and (D) IgG2a-secreting cells from bone marrow (left panels) and spleens (right panels) of air- or SHS-exposed mice (n = 10). Spots were developed with TMB substrate and counted using a CTL ELISPOT reader. Line represents mean. **p≤0.01, ***p≤0.005, ****p≤0.0001 two tailed unpaired t test.
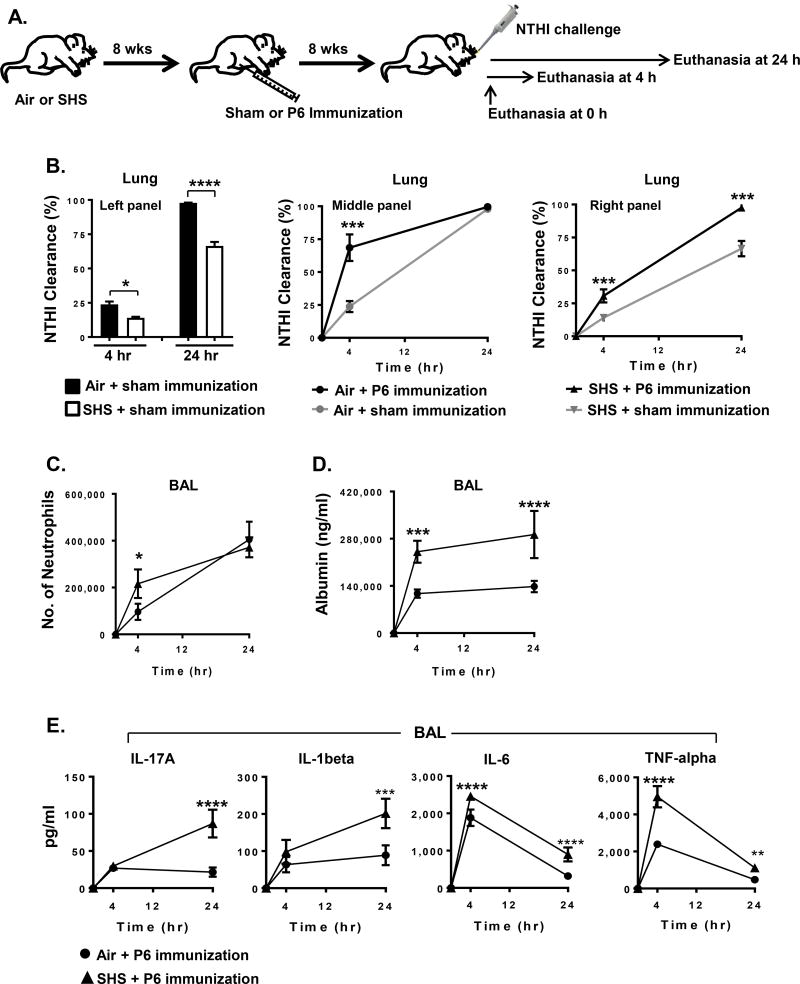
FIGURE 8. Chronic SHS exposure weakens P6 vaccination-induced protection to future bouts of acute respiratory infection by NTHI.
(A) Hallmarks of acute inflammation were measured in air- or SHS-exposed mice 8 weeks after P6 immunization. Parameters associated with acute inflammation were evaluated at baseline (prior to infection) and 4 or 24 hr after acute NTHI challenge. (B) Rate of NTHI clearance in the lungs of mice was measured by bacterial colony-plating assay. (C–D) Accumulation of neutrophils was determined by FACS analysis and the levels of albumin in the BAL were quantified by ELISA. (E) Concentration of proinflammatory cytokines in the BAL was evaluated by ELISA. n=4 mice per group per time point; mean ± SD. Two-way ANOVA *p≤0.05, **p≤0.01, ***p≤0.005, ****p≤0.0001, Bonferroni posttest comparison of air versus SHS.
Pulmonary infections with NTHI
Non-typeable Haemophilus influenzae (NTHI) strain 1479 (clinical isolate from a COPD exacerbation) was used for all experiments. Details for bacterial growth and instillations were exactly as described previously (20). To achieve chronic NTHI-mediated inflammation, mice received 1 × 106 live NTHI bacteria instilled by intratracheal administration twice per week for 8 weeks. A single NTHI instillation (1 × 106 live bacteria) was given to the animals to achieve an acute pulmonary infection and mice were euthanized 4 and 24 hr later and BAL and whole lungs retrieved for evaluating various parameters related to acute inflammation.
P6 immunization
Air and secondhand smoke-exposed mice were immunized either with 40 µg purified P6 antigen in adjuvant or adjuvant alone as described previously (20). Mice were bled retro-orbitally on a weekly schedule and titers of P6-specific Abs were quantified by ELISA.
Lung histology
Lungs were excised and fixed in 10% formaldehyde in PBS overnight, then transferred to ethanol, embedded in paraffin, sectioned, and stained with H&E by the Mouse Tumor Model Resources (MTMR) at Roswell Park Cancer Institute. Images were obtained and lung pathology was evaluated by a pathologist (P.N.B.) as described previously (20). A scoring schema was developed to quantify the extent of inflammation and immune cell infiltration in the lungs of mice exposed to NTHI chronically (34).
Bronchoalveolar lavage
On the day of euthanasia, mice were injected intra-peritoneally with 1 ml warmed 2.5% avertin solution (2,2,2-tribromethanol). Once the animals exhibited no pinch reflex, the thoracic cavity was opened and the trachea exposed for cannulation. Lungs were slowly lavaged by gently injecting 750 µl of ice cold 1% BSA in PBS twice. Cell counts in BAL samples were determined by trypan blue dye method. Cells were stained with cell type-specific antibodies for FACS analysis to measure the frequencies and percentages of various immune subsets in the BAL. Titers of P6-specific Abs, albumin leak and cytokine levels in the cell-free BAL fluid were measured by ELISA.
Isolation of lung lymphocytes, splenocytes and bone marrow cells
Lung lymphocytes from mice were isolated as described (20). Briefly, lungs were minced into small pieces in a 60 mm dish on ice and the lung slurry was mixed with 1 mg/ml Type IA-S collagenase solution containing 50 U/ml DNase I (Sigma) and placed on a rotator for 60 minutes at 37°C. The resulting single-cell suspension was passed through a 40 µm filter to remove large debris and undigested tissue, then underlaid with Ficoll-Paque and centrifuged with brake off. Immune cells at the interface were collected, washed extensively to remove residual Ficoll-Paque and counted. Single cell suspensions of splenocytes were isolated by meshing and passing through a 40 µm filter and collecting cells in a sterile tube on ice. Bone marrow was collected by flushing femur and tibia with sterile PBS on ice. Splenocytes and bone marrow cells were centrifuged at room temperature for 5 minutes at 500 × g, resuspended in complete tissue culture media and counted using trypan blue staining method.
ELISA
Cytokine concentrations in BAL fluid were evaluated using the eBioscience kits following the manufacturer’s instructions. Titers of P6-specific antibodies were measured using ELISA plates coated with 3 µg/ml of purified P6 protein as described previously (20, 35–37). Weekly serum samples and endpoint BAL fluid from individual mice were added to BSA-blocked plates, and bound anti-P6 Igs were detected with HRP-conjugated goat anti-mouse Ig(H+L), IgA, IgG1, IgG2a, IgG2b antibodies (Southern Biotech, Birmingham, AL, USA). Albumin levels in BAL fluid of mice were quantified using a kit from Bethyl Laboratories (Montgomery, TX, USA). Plates were developed with 3,3’,5,5’-tetramethylbenzidine (TMB; for HRP), and absorbance was read at 450 nm.
ELISPOTS
Frequency of P6-specific T cells was evaluated by ELISPOT assay as described (20). Briefly, multiscreen HA plates (Millipore) were coated with 3 µg/ml anti–IL-17, anti–IL-4 or anti–IFN-γ. Lymphocytes were co-cultured with APCs pulsed with 1 µM P641–55 peptide (35). After 48 hr incubation, plates were washed and cytokines detected with biotinylated Abs (anti–IL-17, anti–IL-4 or anti–IFN-γ) followed by addition of streptavidin-HRP (all reagents from ThermoFischer Scientific, GA, USA). Spots were developed with TMB substrate (Vector Labs, CA, USA) and enumerated. Frequency of P6-specific Ab-secreting B cells in bone marrow and spleen was calculated using ELISPOT plates coated with 3 µg/ml native P6. After 48 hr incubation, plates were gently washed and bound anti-P6 Abs were detected with HRP-conjugated secondary Abs to mouse IgG1 and IgG2a (Southern Biotech, Birmingham, AL, USA). Spots were developed with TMB substrate and enumerated after the plates were dried.
Flow cytometry
Frequency of various immune cell types in the BAL and lungs of mice was determined by flow cytometry. Cells were stained with cell type-specific antibodies for FACS analysis to determine the numbers of various immune subsets in BAL and lungs as described earlier (38). Briefly, BAL cells and lung lymphocytes were isolated as described above and 0.5 million cells from each sample were stained with specific antibodies in 100 µL volume of FACS staining buffer (1% BSA in PBS) for 30 minutes at 4°C and subsequently washed in FACS buffer before fixing with cytofix (BD Biosciences, CA, USA). For intracellular staining, cells were first treated with permeabilizing solution (BD Biosciences, CA, USA) and then stained with specific antibodies as described previously (38). Samples were acquired using LSRII-A flow cytometer and data were analyzed using FlowJo software.
Assessment of NTHI clearance
NTHI clearance was performed as described (20). The mice were sacrificed 4 and 24 hr after the final bacterial instillation. Whole lungs were retrieved and gently homogenized on ice in 1 ml PBS. Serial dilutions of lung homogenates were plated onto chocolate agar plates and incubated at 35°C, 5% CO2 for 16 hours (hr). NTHI colonies were enumerated and clearance rates calculated using the initial inoculum of 1 × 106 bacteria.
Statistical analysis
Testing for differences between mean values was determined using either Student t test or two-way ANOVA with posttest comparisons by GraphPad Prism 6 software. The differences between two groups were considered to be significant at P≤0.05.
Results
Chronic SHS exposure augments NTHI-mediated pulmonary inflammation
Initially we wanted to assess how chronic exposure to SHS at low and high doses impacts NTHI-mediated pulmonary inflammation using our previously developed chronic infection mouse model (20, 36, 37). To achieve this, C57BL/6 mice were exposed to SHS as depicted in Fig. 1A for 8 consecutive weeks, with final average exposures of 31±9 (low dose) and 90±28 mg/m3 (high dose) total particulate matter. After completion of exposure to SHS, mice were infected with NTHI twice per week for 8 weeks (Fig. 1A). Histopathological examination of lung sections performed by the pathologist P.N.B. revealed characteristic lymphocytic accumulation surrounding airways and bronchovasculature in all the groups, but the extent of immune cell infiltration was greatly increased in SHS-exposed mice (Fig. 1B).
Pulmonary inflammation was scored using a blinded, semi-quantitative system to determine if any differences existed if mice were exposed to air or SHS prior to NTHI infection (Fig. 1C). The severity of peribronchial inflammation in air-exposed mice was scored primarily as mild (9/10 mice), whereas inflammation in SHS-exposed mice was scored as moderate (6/10) and mild (3/10) at low dose and moderate (7/10) at high dose. The chronicity of bronchovascular inflammation was moderate (5/10) or mild (5/10) in air-exposed mice but almost exclusively moderate in SHS-exposed mice. Furthermore, the extent of pleural inflammation was scored as mild (7/10) in air-exposed mice but it was moderate (3/10) in low dose and (6/10 mice) in high dose SHS-exposed mice. Finally, when we evaluated interstitial inflammation it was found to be mild (7/10) in air exposed mice, while it was moderate (6/10 and 8/10) at low and high SHS exposure. We also investigated the persistence of lung inflammation by resting mice for 8 weeks after the final NTHI instillation. We observed that the extent of inflammation in all groups was now equivalent, suggesting that a rest period helps to reverse the SHS-induced lung inflammation (Fig. 2A–C). These observations conclusively demonstrate that prior exposure to SHS plays a critical role in the escalation of pulmonary inflammation after bacterial infection. Furthermore, in the absence of an ongoing inflammatory stimulus, the pulmonary inflammatory changes subside.
FIGURE 2. Rest period helps to reverse the SHS-induced lung inflammation.
(A) C57BL/6J female mice were exposed for 8 weeks to air, low dose SHS (average smoke exposure 31±9 mg/m3 total particulate matter) or high dose SHS (average smoke exposure 90±28 mg/m3 total particulate matter), followed by 8 weeks of chronic NTHI infection. Animals were euthanized after an 8 week rest period (n = 10). (B) Representative histological sections of H&E stained lung sections from each group showing resolution of inflammation as a consequence of rest period. Original magnification × 100. (C) Consensus scores from two blinded non-consecutive sessions evaluating respiratory inflammation in peribronchial, pleural, and interstitial regions of the lung.
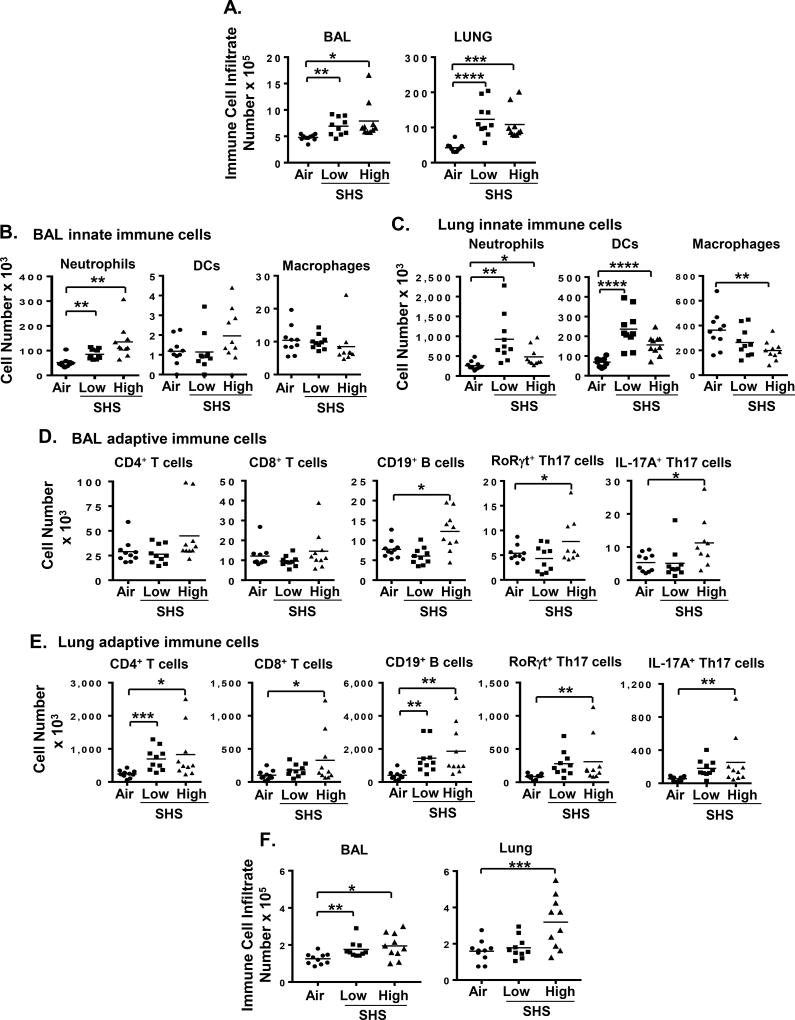
The impact of prior SHS exposure on the composition and extent of accumulation of immune cells in BAL and lungs of the mice in response to chronic NTHI infection was also assessed. We report that SHS exposure at both low and high doses increased total immune cell infiltrate in BAL and lungs of mice compared to air exposed controls (Fig. 3A). After 8 weeks of chronic NTHI infection, we observed significantly higher numbers of neutrophils in BAL and lungs of mice previously exposed to chronic SHS compared with air-exposed animals (Fig. 3B–C). Lung tissue but not BAL contained increased numbers of dendritic cells (DC) and fewer macrophages after SHS exposure (Fig. 3B–C). Furthermore, prior SHS-exposure significantly increased the accumulation of lymphocytes in the BAL (B cells) and lungs (CD4+, CD8+ T cells and B cells) of mice after chronic NTHI infection (Fig. 3D–E). Exposure to the higher concentration of SHS also elevated the numbers of pro-inflammatory RORγ t+ Th17 and IL-17A+ Th17 T cells in the BAL and lungs of mice after chronic NTHI infection compared to air-exposed group, consistent with a pro-inflammatory environment (Fig. 3D–E). Our gating strategy is shown in Supplemental Fig. 1. Mice that were rested for 8 weeks after the final NTHI instillation had substantially fewer inflammatory cells in the BAL than mice evaluated immediately after infection, but we still noted significantly increased numbers of immune cells in mice that had been exposed to SHS prior to NTHI challenge compared to mice exposed to air, indicating that resolution of chronic inflammation was delayed in SHS-exposed mice (Fig. 3F).
FIGURE 3. Chronic SHS exposure modulates airway immune cell composition in response to NTHI-mediated chronic respiratory infection.
(A) Count of total immune cells in BAL (left) and lungs (right) of mice exposed to air or SHS for 8 wks followed by chronic NTHI infection for 8 wks (n = 9–10 per group). Innate (B–C) and adaptive (D–E) immune cell composition in the BAL and lungs of treated mice was determined by flow cytometry using specific markers as described in Materials and Methods. (F) Count of total immune cells in BAL (left) and lungs (right) of mice exposed to air or SHS for 8 wks followed by chronic NTHI infection for 8 wks and then rest for another 8 wks after last NTHI instillation (n = 10 per group) Our gating strategy is shown in Supplemental Figure 1. Line represents mean. *p≤0.05, **p≤0.01, ***p≤0.005, ****p≤0.0001 two tailed unpaired t test.
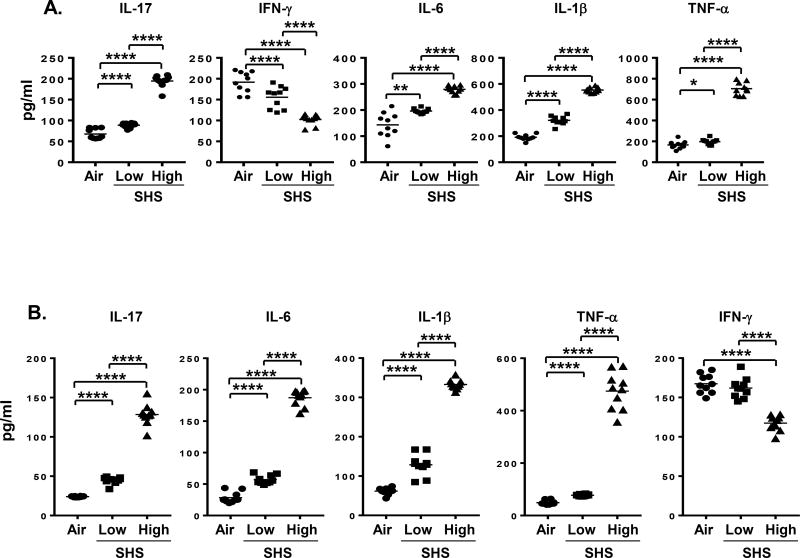
Because the presence of inflammatory cytokines following an infection is considered to be a hallmark of an inflammatory process, we evaluated the levels of several key cytokines previously reported by us to be important in the inflammatory response to chronic pulmonary NTHI infection (20, 36, 37). In mice that were chronically exposed to SHS, levels of IL-17 and IFN-γ cytokines that are connected with Th1/Th17 inflammatory responses were significantly altered in response to chronic NTHI infection. While SHS-exposed mice exhibited increased levels of IL-17 in the BAL, IFN-γ levels were significantly diminished compared to air-exposed mice (Fig. 4A). Moreover, pro-inflammatory IL-6, IL-1β, and TNF-α cytokine levels in the BAL were significantly elevated by prior SHS exposure compared with air controls. Importantly, we observed that a rest period of 8 weeks after the last NTHI infection did not normalize cytokine levels and found that the levels of IL-17A, IL-6, IL-1β and TNF-α were still significantly higher and IFN-γ was still significantly lower in mice that had been exposed to high dose SHS prior to NTHI relative to mice exposed to air prior to NTHI (Fig. 4B). Induction of a heightened pro-inflammatory microenvironment in the lungs of mice against chronic NTHI infection by prior SHS exposure confirms the enhanced pulmonary inflammation that we observed in our histopathological results. Furthermore, a rest period after chronic infection and SHS-exposure cessation only partially improved the pulmonary inflammatory milieu, indicating that SHS exposure has a surprisingly long-term impact.
FIGURE 4. Chronic SHS exposure augments inflammatory cytokine profile in the BAL fluid of mice in a long-term manner in response to NTHI-mediated chronic respiratory infection.
(A) Mice exposed to 8 wks of air or SHS followed by 8 wks of chronic NTHI infection were euthanized and lungs lavaged with 1% BSA solution in PBS, and cytokine levels were determined by ELISA (n = 10 mice per group). (B) A group of mice previously exposed to 8 wks of air or SHS and chronically infected in the lungs with NTHI for 8 wks were rested for another 8 wks period after the last NTHI infection before euthanasia, and BAL was harvested to measure cytokine levels as mentioned above by ELISA (n=10). Line represents mean. *p≤0.05, **p≤0.01, ****p≤0.0001 two tailed unpaired t test.
Suppression of adaptive immune response by prior SHS exposure
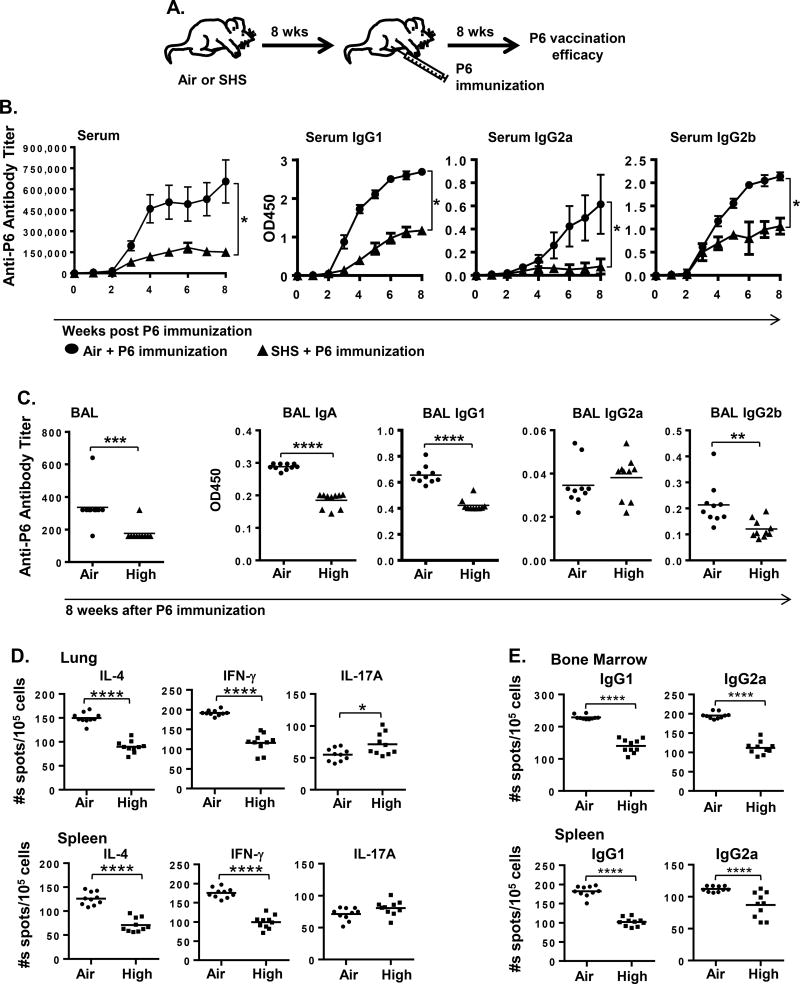
Because our results demonstrated that exposure to SHS augments pulmonary inflammation in response to chronic infection in mice, we next examined how this inflammatory milieu would impact the generation of NTHI-specific adaptive immune responses. To determine this, we measured the levels of anti-P6 antibodies in the BAL and serum by ELISA and the frequencies of Ab-secreting NTHI-specific B cells in the BM and splenocytes by ELISPOT assays. We observed that total anti-P6 Ab levels were drastically reduced in the serum and BAL of SHS-exposed mice compared to air-exposed mice, in a dose-dependent manner (Fig. 5A–B). We also observed that Ab subclasses IgG1, IgG2a and IgG2b in serum and IgA and IgG2b in BAL were significantly diminished in SHS-exposed mice and this was also SHS dose-dependent (Fig. 5A–B). This reduction in the antigen-specific antibody levels between SHS- and air-exposed mice were persistent after a rest period of 8 weeks, demonstrating that SHS exposure has a long lasting detrimental effect on the generation of Ab responses to a pathogen (Fig. 5C).
Because we observed a significant defect in the Ab response to chronic infection by prior SHS exposure, we also assessed its impact on NTHI-specific T cell responses which are essential to anti-bacterial T cell immunity and are critical to the generation of potent Ab responses against the pathogen. To measure the frequencies of cytokine-secreting P6-specific T cells from lung-infiltrating lymphocytes and the spleen, we stimulated T cells with P641–55 immunodominant peptide. For all our subsequent experiments, we used only high dose SHS exposure. Prior SHS-exposure in mice lead to significant increase in the frequencies of P6-specific Th17 cells in lungs and spleens compared with air exposure. Conversely we observed that the frequencies of IL-4 and IFN-γ producing T cells from lungs and spleens of SHS-exposed mice were significantly decreased (Fig. 6A–B); these cytokines are required for Ab class switching to IgG1 and IgG2a respectively. These results indicate that SHS exposure induces a profound defect in anti-bacterial T cell immunity and T helper cell profile that are essential for inducing robust Ab responses to NTHI infection. The profile of Th1 and Th17 P6-specific T cells in the lungs of SHS-exposed mice was consistent with our results of the cytokine profile measured in the BAL (Fig. 4A).
As prior SHS exposure significantly diminished the generation of P6-specific Abs to NTHI infection and suppressed antigen-specific T cell responses in mice (Fig. 5 & Fig. 6A–B), we thus wanted to assess its impact on the frequencies of NTHI-specific IgG1- and IgG2a-secreting B cells from BM and spleen of mice following chronic infection using B-cell ELISPOTs. We noted that prior SHS exposure significantly decreased the frequencies of anti-P6 Ig-secreting B cells for both sub-classes compared with air-exposed mice (Fig. 6C–D). This indicates that while class switching to IgG1and IgG2a occurred however, it was less efficient in mice previously exposed to SHS. Collectively our results clearly establish that chronic SHS exposure induces profound dysfunction in the development of adaptive immune responses by impairing pathogen-specific T and B cell responses.
Prior SHS exposure suppresses efficacy of immunization to combat infections
Immune dysfunction induced by SHS exposure against a chronic infection motivated us to evaluate whether prior SHS exposure has any impact on the immune response to a candidate vaccine antigen. To address this question, mice previously exposed to SHS or air for 8 weeks were immunized with the purified outer membrane lipoprotein P6 antigen (Fig. 7A) and the vaccination efficacy was evaluated by measuring anti-P6 Ab titers. Immunization with P6 antigen induced a robust response in air-exposed mice as previously reported (25, 34), with the development of high titer antibodies in the serum and BAL (Fig. 7B–C). However, our results clearly demonstrated that prior SHS exposure significantly diminished the ability of P6 vaccination to elicit a robust Ab response in mice. SHS exposure slowed down the kinetics of anti-P6 Ab response as well as significantly reduced the absolute serum Ab titers compared with air-exposure (Fig. 7B). The kinetics and the levels of P6-specific IgG1, IgG2a and IgG2b antibody subclasses were also significantly diminished in serum of mice exposed to SHS compared to air exposed controls. Furthermore, SHS exposure significantly reduced end point Ab titers in the BAL and decreased the levels of antigen-specific IgA, IgG1 and IgG2b subclasses compared to control mice (Fig. 7C).
FIGURE 7. Efficacy of immunization is compromised in SHS-exposed mice.
(A) Female C57BL/6J mice after receiving 8 weeks of air or SHS exposure were immunized with 40 µg P6 lipoprotein from NTHI outer membrane as described in Materials and Methods. Vaccination efficacy was determined after immunization by measuring the levels of anti-P6 Ig from (B) weekly serum samples and (C) BAL fluid harvested at euthanasia. OD values at 450 nm were determined to measure the levels of P6-specific IgG1, IgG2a and IgG2b subclasses at 1:1600 dilutions in (B) weekly serum samples, and IgA, IgG1, IgG2a and IgG2b subclasses at 1:400 dilutions in (C) endpoint BAL. Frequencies of (D) cytokine-secreting T cells in the lung (top) and spleen (bottom), and (E) Ab-producing cells in the bone marrow (top) and spleen (bottom) were measured by ELISPOT. Mean ± SD. (n = 10) Line represents mean. *p≤0.05, area under the curve Kruskal–Wallis rank test. *p≤0.05, **p≤0.01, ***p≤0.005, ****p≤0.0001 two tailed unpaired t test.
The impaired serum antibody responses are further reflected in the suppressed generation of P6-specific T and B cells detected by ELISPOTs (Fig. 7D–E). SHS exposure diminished the frequency of P6-specific T cells secreting IL-4 and IFN-γ in lung and spleen (Fig. 7D), implying that SHS impairs Th1 and Th2 responses. Although the frequency of P6-specific T cells secreting IL-17 was elevated in the lungs no change was observed in the spleen (Fig. 7D). Additionally, the frequencies of P6-specific IgG1 and IgG2a Ab-secreting B cells were lower in the bone marrow and spleen of P6-immunized mice that were previously exposed to SHS compared to the air-exposed controls (Fig. 7E).
Because prior SHS exposure impairs the development of a robust immune response to P6 immunization, we next wanted to determine if this level of protection could still be effective against an acute bacterial challenge and facilitate bacterial clearance from the infected lungs. To address this question, we immunized air- or SHS-exposed mice with P6 antigen and challenged them acutely with live NTHI bacteria 8 weeks after immunization. Sham-immunized air- and SHS-exposed mice were utilized as controls to evaluate the effectiveness of P6 vaccination in reducing the bacterial burden 4 and 24 hr after acute NTHI infection in the lungs (Fig. 8A). These results clearly demonstrate that the kinetics of NTHI clearance from the lungs of sham-immunized, SHS-exposed mice was significantly inhibited compared to that observed in sham-immunized, air-exposed mice at both 4 and 24 hr after acute challenge (Fig. 8B, Left panel). The kinetics of NTHI clearance from the lungs was greater in both air- and SHS-exposed mice following P6 immunization compared with their respective sham-immunized controls (Fig. 8B, black curve vs grey curve in middle and right panels), strongly indicating that despite SHS impairing the induction of immune response, P6 vaccination was beneficial in clearing bacteria from the lungs. This protective effect of P6 immunization in mitigating the SHS-exposure induced impairment in bacterial clearance in the lungs of mice was further demonstrated in that the kinetics of bacterial clearance in P6-immunized, SHS-exposed mice was similar to the one observed in sham-immunized, air-exposed mice at both time points (Fig. 8B grey curve in middle panel vs black curve in right panel).
As bacterial clearance in the lungs of P6-vaccinated mice previously exposed to SHS was significantly diminished at an early time point compared with P6-vaccinated air-exposed mice, we sought to assess the infiltration of neutrophils in response to acute NTHI challenge under similar conditions. Surprisingly neutrophil influx was significantly elevated at 4 hr after NTHI challenge in P6-vaccinated mice previously exposed to SHS compared to P6-vaccinated air controls (Fig. 8C). In view of diminished bacterial clearance at this time-point, this observation strongly suggests that the phagocytic function of neutrophils in the lungs of mice exposed to SHS could be severely impaired. A physiological milieu where lungs have decreased bacterial clearance and increased neutrophil influx could result in increased lung epithelial damage. We thus assessed the pulmonary damage induced by quantifying albumin leak into BAL of mice as a surrogate marker of lung damage in response to acute bacterial infection. Albumin leak was markedly elevated in mice previously exposed to SHS and persisted to the 24 hr time-point (Fig. 8D). Finally, we assessed levels of pro-inflammatory cytokines in the BAL. IL-17A, IL-1β, IL-6 and TNF-α were all elevated in mice previously exposed to SHS (Fig. 8E). The kinetics of cytokine induction was different with IL-6 and TNF-α peaking at 4 hr post infection and IL-17A and IL-1β still increasing 24 hr after infection. In all cases SHS-exposed mice exhibited greater levels of inflammatory cytokines.
Discussion
Exposure to environmental SHS is associated with heart diseases, respiratory disorders, cancer, stroke, asthma attacks, middle ear infections and sudden death syndrome in children (4, 39). Under normal physiological milieu, lungs respond to exogenous insults like tobacco smoke via an array of immune cells including resident macrophages, recruited neutrophils and cytokines to safeguard lung homeostasis (40). However, in case of tobacco smoke exposure, persistence of these mediators accounts for exaggerated inflammatory responses as found in smokers with COPD (41). Epidemiological studies have associated passive smoking with increased susceptibility to respiratory infections, especially among vulnerable populations such as hospitality workers, flight attendants, and children whose parents smoke (25–28, 31). Although much work has been done to understand the mechanistic links between tobacco smoke and secondary health effects in smokers, these studies have mainly focused on acute (single) infections following MTS exposure (42–43). Furthermore, we have previously demonstrated that chronic exposure to MTS worsens lung inflammation and diminishes immunity to a chronic NTHI infection in mice (20). However, comparatively less work has been done with SHS, and never in combination with chronic infection. Thus our goal was to specifically interrogate how chronic SHS exposure impacts lung inflammation and modulates the generation of adaptive immunity to a chronic respiratory infection and vaccination using a mouse model. We report here the first study combining chronic SHS exposure with a model of chronic infection, and clearly demonstrate that SHS exposure not only leads to increased lung inflammation, but also impairs innate and adaptive immune responses. We further demonstrate that prior chronic SHS exposure in mice diminishes the efficacy of vaccination with a key antigen that translated into reduced protection to a subsequent acute NTHI infection.
In the present study, mice were exposed to chronic SHS for 8 weeks, followed by chronic NHTI infection. SHS augmented chronic infection-induced pulmonary inflammation, increased infiltration of neutrophils in the lungs and elevated levels of pro-inflammatory cytokines including IL-17A, IL-6 and IL-1β in BAL of mice compared with air exposure, a phenomenon that was observed previously with MTS exposure (20). Enhanced levels of IL-17A could be due to increased expression of IL-22 in T helper type 17 cells as these cells release IL-17 as their effector cytokine under the control of IL-22 and IL-23. Increased IL-22 immunoreactive cells and gene transcripts have been observed in the bronchial mucosa of patients with COPD (44). It is known that worsening of NTHI-induced pulmonary inflammation associates with priming of lung microenvironment to bacterial-exacerbated neutrophilia via IL-1α signaling (45). Another mechanism of pulmonary neutrophil persistence could be SHS-induced impaired cell death processes, as it has been reported that CS induces neutrophil survival by sustaining activation of prosurvival Akt (45). The non-neuronal (or extra-neuronal) cholinergic system represents a regulatory pathway in airway inflammation whose immunomodulatory effects influence inflammation in COPD and asthma (46). It is reported that cholinergic stimulation of neutrophils by nicotine in CS prolongs neutrophil survival in smokers and is implicated in COPD (47). Furthermore, neutrophil persistence in lungs could occur due to impaired bacterial clearance (providing sustained neutrophil trafficking signals) induced by smoke exposure through the inhibition of phagocytic function of macrophages in the airways and sinuses of smokers (20, 48–52). Additionally, studies have shown that tobacco smoke alters the phenotype of alveolar macrophages by decreasing TLR2 expression and inhibiting alveolar macrophage-mediated bacterial phagocytosis (49, 53–54). Tobacco smoke has been shown to suppress proinflammatory cytokine expression and activation of associated signaling pathways in TLR2 and TLR4 agonists-stimulated alveolar macrophages (55). Moreover we have previously shown that TLR2 is critical for the generation of NTHI-specific adaptive immunity in mice (36). We therefore speculate that the reduction in numbers of macrophages by SHS exposure could contribute to impaired bacterial clearance in the lungs of mice, thus enhancing pulmonary inflammation and hindering the induction of a robust adaptive immune response.
To our knowledge, we also report for the first time that prior SHS exposure strongly suppressed the production of systemic as well as mucosal pathogen-specific antibodies against chronic NTHI infection (Fig. 5) and decreased the numbers of antibody-producing B cells in the bone marrow and spleen (Fig. 6C&D). Further, despite increased numbers of total T cells and DCs in lung tissue (Fig. 3C&E), there were decreased numbers of antigen-specific Th1 (IFN-γ producing) and Th2 (IL-4 producing) T cells in both lung and spleen of mice that were exposed to SHS prior to chronic infection (Fig. 6A&B). As T cell responses are critical in inducing a robust antibody response, the SHS-induced impairment in T cell compartment most likely accounts for the suppression of NTHI-specific B cell responses and pathogen-specific antibody production. Our laboratory has previously reported a similar result when mice were exposed to MTS, and observed that MTS induces defects in T cell function and inhibits their ability to produce IL-4, a cytokine primarily required for B cell activation (20). Thus prior chronic SHS exposure by inhibiting the frequency of P6-specific IL-4 and IFN-γ secreting T cells in the lung and spleen could compromise the ability of P6-specific T cells to help NTHI-specific B cells to mount a vigorous antibody response. Although modulation of regulatory T cells (Tregs) by CS remains controversial, some reports show that CS-induced suppression in T cell compartment is attributed to the induction of Tregs. CS is believed to worsen respiratory infections in smokers by augmenting CD4+CD25+ Tregs (56). However, other studies argue that CS in COPD patients could impair immunosuppressive functions of Tregs either by reducing suppressive Tregs or enhancing non-suppressive Treg prevalence, thereby augmenting inflammation and boosting autoimmune component in COPD pathogenesis (57). Our findings clearly establish that SHS impairs pathogen-specific T and B cell responses resulting in diminished generation of antibody responses to chronic infection.
Immune dysfunction triggered by tobacco smoke exposure associates with its cumulative impact in sustaining inflammation and immunosuppression (16, 58). CS affects a wide range of host defense mechanisms ranging from physical, chemical and biological barriers, ultimately leading to pulmonary inflammation and immunosuppression (15, 17, 19, 58–61). In the current study we have demonstrated that chronic SHS exposure modulates Th1, Th2 and Th17 responses to chronic NTHI infection, inhibiting T and B cell functions but augmenting an IL-17 response. IL-17 is shown to be involved in the maintenance of chronic inflammation as observed in asthma and has been detected in the sputum of COPD patients during disease exacerbations. Th-17 cells have also been found in lung biopsies of COPD patients where their role is not completely defined (62). Epidemiologic data showing increased frequencies of inflammatory diseases in smokers linked with Th17 inflammation also suggests that chronic CS exposure may promote Th17 polarized immunity, for example as observed in lung damage during emphysema (58). This is also supported by the observation that chronic CS exposure alters the ratio of Th-17/T-reg cells in mice, leading to increased ROR-γT, IL-17 and IL-6 expression, while simultaneously decreasing Foxp3 and IL-10 expression (63). Moreover, studies have also shown that tissue damage and repair in the central and distal airways of COPD patients might be affected by CS-modulated IL-17A, IL-17F and IL-17 receptor expression (64). In the present study, we provide evidence that chronic SHS exposure also enhances the production of IL-6 and increases the numbers of pulmonary ROR-γT and IL-17+ T cells. Additionally, prior SHS exposure increases the frequency of antigen-specific IL-17-secreting T cells in the lungs and spleen. One of the mechanisms of SHS-induced augmented pulmonary inflammation to chronic infection could be due to multiple aryl hydrocarbon receptor (AhR) ligands in CS activating AhR-dependent transcription which leads to IL-17 signal modulation in the pulmonary microenvironment (65). This is strongly supported by the fact that AhR signaling is critical to IL-17+ cell and ROR-γ T cell differentiation, and also that AhR-deficient mice are incapable of generating Th17 cells (66–67). Another potential mechanism by which SHS could induce pulmonary inflammation might be ROS-dependent signal cascade modulation, leading to inflammatory gene activation and subsequent promotion of chronic immune cell recruitment and inflammation (58). Thus our findings establish that prior SHS exposure modulates T-cell responses by tilting them away from protective responses important for pathogen clearance towards responses that lead to sustenance of chronic inflammation. Our results support the conclusion that SHS exposure has the potential to alter IL-17 signaling axis that could have an overall harmful impact in the mitigation of respiratory infections, thus leading to severe pulmonary damage. These results further demonstrate that SHS impacts pulmonary inflammatory response in a manner similar to mainstream CS in inducing lung damage. We now provide evidence that SHS contributes to increased susceptibility to respiratory infection not only by increasing inflammation but also by impairing and distorting adaptive immune responses.
Smoking cessation is advocated to prevent ongoing adverse health consequences and help rescue smoke-induced damage (68–69). A previous study by our group demonstrated that MTS exposure has long term detrimental impact on lung immunity to respiratory infections (20). In the present study we provide unequivocal evidence that SHS exposure also has long-term detrimental effects on pulmonary inflammatory microenvironment and immune responses to infection (Fig. 3F, 4B & 5C). This highlights the potential of involuntary environmental smoke exposure to negatively impact the generation of protective responses against respiratory infections in a long-term manner. SHS exposure-induced defects in the generation of adequate adaptive immunity against infections or following vaccinations could have serious clinical outcomes as it could lead to poor pathogen clearance resulting in conditions like disease exacerbations (as in non-smoker COPD patients) with resultant increased morbidity and mortality.
To our knowledge, the current study for the first time establishes that chronic SHS exposure worsens bacterial infection-induced pulmonary inflammation and compromises the host’s ability to mount an effective immune response to infection, thus facilitating pulmonary damage that could ultimately increase the susceptibility to further respiratory infections. This has critical implications for people who are either chronically exposed to SHS or are current or former smokers. This inability to mount a strong immune response likely facilitates subsequent bouts of infection by the same pathogen or long-term pathogen colonization. Indeed colonization with new strains of respiratory pathogens is a key finding in COPD patients with frequent acute exacerbations (70–71), and also respiratory infections seen in children exposed to SHS at home are frequently observed (26–27). Furthermore, recurrent infections from newly acquired NTHI strains in COPD patients further aggravate their poor lung function. Thus, it is critical to induce robust and long-lived pathogen-specific immune responses that prevent frequent infections, discourage bacterial colonization and lessen pulmonary inflammation. To address this problem, we investigated whether prophylactic immunization with NTHI candidate vaccine could correct the immune defect in SHS exposed mice. We have previously shown that the NTHI outer membrane P6 protein is a vaccine candidate and protects mice from acute NTHI challenge (20, 35, 72). In the present study we observed that prior chronic SHS exposure strongly diminished the production of both systemic as well as mucosal antigen-specific antibodies after P6 vaccination (Fig. 7B&C). We found that T and B cell responses following P6 immunization were markedly suppressed even 8 weeks after the final SHS exposure, including lung IgA antibodies which are a critical first line of defense against respiratory pathogens (Fig. 7C&D). Moreover, despite vaccination with P6, mice exposed to SHS exhibited greater lung inflammation (neutrophil infiltration), induced augmented levels of inflammatory cytokines in the BAL and greater airway epithelium damage after acute infection, relative to air-exposed and immunized mice (Fig. 8). In our model where chronic SHS exposure reduces pulmonary clearance of NTHI after an acute challenge, P6-immunization increased clearance of bacteria from the lungs comparable to that seen in non-vaccinated, air-exposed mice. These observations corroborate the previous findings from our laboratory and from others that showed CS increases the bacterial burden in the lungs while P6 vaccination after smoke exposure helps to reduce it (20, 73). Other mechanisms that likely also contribute to CS-induced bacterial burden in the lungs include CS-induced impaired phagocytic activity in the lungs as shown by Voss et al (73). They reported that chronic CS exposure in mice impairs the phagocytic activity in granulocytes and monocytes, suggesting that this phenomenon could be strongly associated with bacterial colonization and enhanced burden in the respiratory mucosa. Furthermore, enhanced airway mucus production with poor clearance induced by CS may also augment bacterial burden, and be associated with poor airway health and increased infections in COPD patients (74–75). Increased transcript and protein levels of MUC5B (a mucin gene) are induced in the distal airways of asthmatic patients and in smokers (76). Mice have almost no Muc5AC expression but Muc5B is constitutively produced in airway surface secretory cells, and mice with Muc5B deletion die from lung inflammation (77). However, Muc5AC production in mouse airways is reported to increase after exposure to CS-conditioned media (78). Based on previous findings and our current observations, we conclude that increased bacterial burden in the lungs of mice exposed to SHS could be mediated by impaired innate defenses like defective phagocytic activity and altered mucus production in the lung, as well as SHS-induced impairment in the generation of adaptive immunity including suppressed mucosal IgA production.
From our overall current findings, it appears that vaccination (although partially beneficial) alone is an insufficient strategy to mitigate immune suppression mediated by SHS exposure. Although occupational sources of SHS exposure have ended in most developed countries and lifestyle SHS exposures have been significantly curtailed (39), the persistence of immune dysregulation by SHS exposure has not been determined. It should also be noted that it is not always possible to remove all sources of SHS exposure, such as in private homes. Therefore, more studies are urgently needed on how to mitigate increased susceptibility to respiratory infections due to ongoing and past SHS exposure.
In conclusion, we provide strong new evidence that chronic SHS exposure worsens NTHI-mediated pulmonary inflammation and compromises the host’s ability to combat chronic and acute respiratory infections. Furthermore, SHS potently impairs the host’s ability to induce robust immune responses against a key pathogen-specific vaccine antigen. These results demonstrate that environmental, involuntary SHS exposure has the potential to induce a variety of defects in the host akin to those induced by exposure to MTS and we posit that SHS plays an important role in the pathophysiology of various diseases especially human respiratory disorders.
Supplementary Material
Acknowledgments
This work was supported by FAMRI Clinical Innovators Award to Y.T. and PJS. This work also was supported in part by R01 HL120908 (to PJS and RPP). This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Core facilities: Division of Laboratory Animal Resources (DLAR), Mouse Tumor Model Resource (MTMR) and Flow Cytometry.
Footnotes
Conflict of Interest: None disclosed
References
- 1.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Bookshelf ID: NBK179276. [Google Scholar]
- 5.WHO Facts Sheet- Tobacco. 2016 Available at: http://www.who.int/mediacentre/factsheets/fs339/en/
- 6.Schönherr E. Contribution to the statistical and clinical features of lung tumors. Vol. 27. German: Z Krebsforsch; 1928. pp. 436–450. [Google Scholar]
- 7.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 8.Behan DF, Eriksen MP, Lin Y. Economic effects of environmental tobacco smoke. Schaumburg (IL): Society of Actuaries; 2005. Available at: https://www.soa.org/research-reports/2000-2006/research-economic-effect/ [Google Scholar]
- 9.Talhout R, Schulz T, Florek E, van BJ, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 11.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am. J. Respir. Crit Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 12.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D'Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp. Lung Res. 2005;31:547–562. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- 13.Ma B, Kang MJ, Lee CG, Chapoval S, Liu W, Chen Q, Coyle AJ, Lora JM, Picarella D, Homer RJ, Elias JA. Role of CCR5 in IFN-gamma-induced and cigarette smoke-induced emphysema. J. Clin. Invest. 2005;115:3460–3472. doi: 10.1172/JCI24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am. J. Respir. Crit Care Med. 2004;170:1164–1171. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 15.Thatcher TH, Benson RP, Phipps RP, Sime PJ. High-dose but not low-dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am. J. Physiol Lung Cell Mol. Physiol. 2008;295:L412–L421. doi: 10.1152/ajplung.00392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 17.Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, Schroeder JM, Vogelmeier C. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144–149. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP. Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am. J. Physiol Lung Cell Mol. Physiol. 2011;300:L821–L830. doi: 10.1152/ajplung.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsland BJ, Konigshoff M, Saglani S, Eickelberg O. Immune system dysregulation in chronic lung disease. Eur. Respir. J. 2011;38:500–501. doi: 10.1183/09031936.00103211. [DOI] [PubMed] [Google Scholar]
- 20.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 2014;192:5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John G, Kohse K, Orasche J, Reda A, Schnelle-Kreis J, Zimmermann R, Schmid O, Eickelberg O, Yildirim AO. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin. Sci. (Lond) 2014;126:207–221. doi: 10.1042/CS20130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther. Adv. Chronic. Dis. 2014;5:212–227. doi: 10.1177/2040622314532862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CM, Yang L, Chan KP, Chan WM, Song L, Lai HK, Thach TQ, Ho LM, Chan KH, Lam TH, Peiris JS. Cigarette smoking as a risk factor for influenza-associated mortality: evidence from an elderly cohort. Influenza. Other Respir. Viruses. 2013;7:531–539. doi: 10.1111/j.1750-2659.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bello S, Menendez R, Antoni T, Reyes S, Zalacain R, Capelastegui A, Aspa J, Borderias L, Martin-Villasclaras JJ, Alfageme I, Rodriguez de CF, Rello J, Luis M, Ruiz-Manzano J. Tobacco smoking increases the risk for death from pneumococcal pneumonia. Chest. 2014;146:1029–1037. doi: 10.1378/chest.13-2853. [DOI] [PubMed] [Google Scholar]
- 25.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 26.Colley JR, Holland WW, Corkhill RT. Influence of passive smoking and parental phlegm on pneumonia and bronchitis in early childhood. Lancet. 1974;2:1031–1034. doi: 10.1016/s0140-6736(74)92148-5. [DOI] [PubMed] [Google Scholar]
- 27.Jedrychowski W, Flak E. Maternal smoking during pregnancy and postnatal exposure to environmental tobacco smoke as predisposition factors to acute respiratory infections. Environ. Health Perspect. 1997;105:302–306. doi: 10.1289/ehp.97105302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebbert JO, Croghan IT, Schroeder DR, Murawski J, Hurt RD. Association between respiratory tract diseases and secondhand smoke exposure among never smoking flight attendants: a cross-sectional survey. Environ. Health. 2007;6:28. doi: 10.1186/1476-069X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 30.Goldklang MP, Marks SM, D'Armiento JM. Second hand smoke and COPD: lessons from animal studies. Front Physiol. 2013;4:30. doi: 10.3389/fphys.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim WJ, Song JS, Park DW, Kwak HJ, Moon JY, Kim SH, Sohn JW, Yoon HJ, Shin DH, Park SS, Kim TH. The effects of secondhand smoke on chronic obstructive pulmonary disease in nonsmoking Korean adults. Korean J. Intern. Med. 2014;29:613–619. doi: 10.3904/kjim.2014.29.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King P. Haemophilus influenzae and the lung (Haemophilus and the lung) Clin. Transl. Med. 2012;1:10. doi: 10.1186/2001-1326-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugade AA, Bogner PN, Thanavala Y. Murine model of chronic respiratory inflammation. Adv. Exp. Med. Biol. 2011;780:125–141. doi: 10.1007/978-1-4419-5632-3_11. [DOI] [PubMed] [Google Scholar]
- 35.McMahon M, Murphy TF, Kyd J, Thanavala Y. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine. 2005;23:3590–3596. doi: 10.1016/j.vaccine.2005.01.151. [DOI] [PubMed] [Google Scholar]
- 36.Lugade AA, Bogner PN, Murphy TF, Thanavala Y. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Front Immunol. 2011;2:10. doi: 10.3389/fimmu.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lugade AA, Vethanayagam RR, Nasirikenari M, Bogner PN, Segal BH, Thanavala Y. Nrf2 regulates chronic lung inflammation and B-cell responses to nontypeable Haemophilus influenzae. Am. J. Respir. Cell Mol. Biol. 2011;45:557–565. doi: 10.1165/rcmb.2010-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2014;190:40–50. doi: 10.1164/rccm.201312-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Surgeon General. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention (US), Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (US); 2006. Bookshelf ID: NBK44324. [Google Scholar]
- 40.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorley AJ, Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2007;2:409–428. [PMC free article] [PubMed] [Google Scholar]
- 42.Gaschler GJ, Skrtic M, Zavitz CC, Lindahl M, Onnervik PO, Murphy TF, Sethi S, Stampfli MR. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am. J. Respir. Crit Care Med. 2009;179:666–675. doi: 10.1164/rccm.200808-1306OC. [DOI] [PubMed] [Google Scholar]
- 43.Herr C, Han G, Li D, Tschernig T, Dinh QT, Beisswenger C, Bals R. Combined exposure to bacteria and cigarette smoke resembles characteristic phenotypes of human COPD in a murine disease model. Exp. Toxicol. Pathol. 2015;67:261–269. doi: 10.1016/j.etp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Di SA, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D'Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin. Exp. Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikota JK, Shen P, Morissette MC, Fernandes K, Roos A, Chu DK, Barra NG, Iwakura Y, Kolbeck R, Humbles AA, Stampfli MR. Cigarette smoke primes the pulmonary environment to IL-1alpha/CXCR-2-dependent nontypeable Haemophilus influenzae-exacerbated neutrophilia in mice. J. Immunol. 2014;193:3134–3145. doi: 10.4049/jimmunol.1302412. [DOI] [PubMed] [Google Scholar]
- 46.Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol. Ther. 2007;115:208–222. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Aoshiba K, Nagai A, Yasui S, Konno K. Nicotine prolongs neutrophil survival by suppressing apoptosis. J. Lab Clin. Med. 1996;127:186–194. doi: 10.1016/s0022-2143(96)90077-3. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, Luo HR. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect. Immun. 2010;78:1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green GM, Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N. Engl. J. Med. 1967;276:421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- 51.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 52.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 2004;318:32–37. doi: 10.1016/j.bbrc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J. Infect. Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Cowan MJ, Hasday JD, Vogel SN, Medvedev AE. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J. Immunol. 2007;179:6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- 56.Roos-Engstrand E, Pourazar J, Behndig AF, Bucht A, Blomberg A. Expansion of CD4+CD25+ helper T cells without regulatory function in smoking and COPD. Respir. Res. 2011;12:74. doi: 10.1186/1465-9921-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget. 2017;8:268–284. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sopori M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 60.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42:241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Rijt SH, Keller IE, John G, Kohse K, Yildirim AO, Eickelberg O, Meiners S. Acute cigarette smoke exposure impairs proteasome function in the lung. Am. J. Physiol Lung Cell Mol. Physiol. 2012;303:L814–L823. doi: 10.1152/ajplung.00128.2012. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Chu S, Zhong X, Lao Q, He Z, Liang Y. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int. Immunopharmacol. 2013;15:58–66. doi: 10.1016/j.intimp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Peng W, Weng Y, Ying H, Li H, Xia D, Yu W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int. Immunopharmacol. 2012;14:504–512. doi: 10.1016/j.intimp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Montalbano AM, Riccobono L, Siena L, Chiappara G, Di SC, Anzalone G, Gagliardo R, Ricciardolo FLM, Sorbello V, Pipitone L, Vitulo P, Profita M. Cigarette smoke affects IL-17A, IL-17F and IL-17 receptor expression in the lung tissue: Ex vivo and in vitro studies. Cytokine. 2015;76:391–402. doi: 10.1016/j.cyto.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am. J. Physiol Lung Cell Mol. Physiol. 2005;289:L391–L399. doi: 10.1152/ajplung.00062.2005. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS. One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanieh H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends. Biomed. Res. Int. 2014;2014:520763. doi: 10.1155/2014/520763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu J, Sin DD. Improved patient outcome with smoking cessation: when is it too late? Int. J. Chron. Obstruct. Pulmon. Dis. 2011;6:259–267. doi: 10.2147/COPD.S10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips B, Veljkovic E, Peck MJ, Buettner A, Elamin A, Guedj E, Vuillaume G, Ivanov NV, Martin F, Boue S, Schlage WK, Schneider T, Titz B, Talikka M, Vanscheeuwijck P, Hoeng J, Peitsch MC. A 7-month cigarette smoke inhalation study in C57BL/6 mice demonstrates reduced lung inflammation and emphysema following smoking cessation or aerosol exposure from a prototypic modified risk tobacco product. Food Chem. Toxicol. 2015;80:328–345. doi: 10.1016/j.fct.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proc. Am. Thorac. Soc. 2004;1:109–114. doi: 10.1513/pats.2306029. [DOI] [PubMed] [Google Scholar]
- 71.Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int. J. Chron. Obstruct. Pulmon. Dis. 2011;6:113–123. doi: 10.2147/COPD.S15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abe Y, Murphy TF, Sethi S, Faden HS, Dmochowski J, Harabuchi Y, Thanavala YM. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2002;165:967–971. doi: 10.1164/ajrccm.165.7.2109009. [DOI] [PubMed] [Google Scholar]
- 73.Voss M, Wonnenberg B, Honecker A, Kamyschnikow A, Herr C, Bischoff M, Tschernig T, Bals R, Beisswenger C. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir. Res. 2015;16:41. doi: 10.1186/s12931-015-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baginski TK, Dabbagh K, Satjawatcharaphong C, Swinney DC. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am. J. Respir. Cell Mol. Biol. 2006;35:165–174. doi: 10.1165/rcmb.2005-0259OC. [DOI] [PubMed] [Google Scholar]
- 76.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am. J. Respir. Cell Mol. Biol. 2009;40:277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N. Engl. J. Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp. Lung Res. 2002;28:435–455. doi: 10.1080/01902140290096728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.