Abstract
Antibodies have exquisite specificity to differentiate foreign antigens that mimic “self”, but it remains unclear how such specificity is acquired. We generated B-cells displaying an antibody that cross-reacts with two related protein antigens expressed on self versus foreign cells. B-cell anergy was imposed by self antigen but reversed upon challenge with high-density foreign antigen, leading to germinal center recruitment and antibody gene hypermutation. Single-cell analysis revealed rapid selection for mutations that decrease self affinity and slower selection for epistatic mutations that specifically increase foreign affinity. Crystal structures revealed the mutations exploited subtle topological differences to achieve 5,000-fold preferential binding to foreign targets over self epitopes. Strikingly, resolution of antigenic mimicry drove the optimal affinity maturation trajectory, highlighting the value of retaining self-reactive clones as substrates for protective antibody responses.
Antibodies often distinguish nearly identical foreign and self antigens, such as the glycolipids on the cell wall of Campylobacter jejuni and those on human nerve cells, with less than 0.1% of infected people producing cross-reactive antibodies that result in paralysis and Guillain–Barré Syndrome (1). Apparent limits to antibody self-foreign discrimination are exploited by Human Immunodeficiency Virus (HIV), Lymphocytic choriomeningitis virus and Lassa fever viruses. They establish persistent infections and evade antibodies by mimicking self and cloaking their foreign envelope proteins with self-glycans (2–5). Although self-reactivity can be removed from antibodies by V(D)J recombination (6) or by V-region hypermutation (7–9), the cellular basis and mutational pathways for resolving foreign–self mimicry after infection or immunization remain undefined.
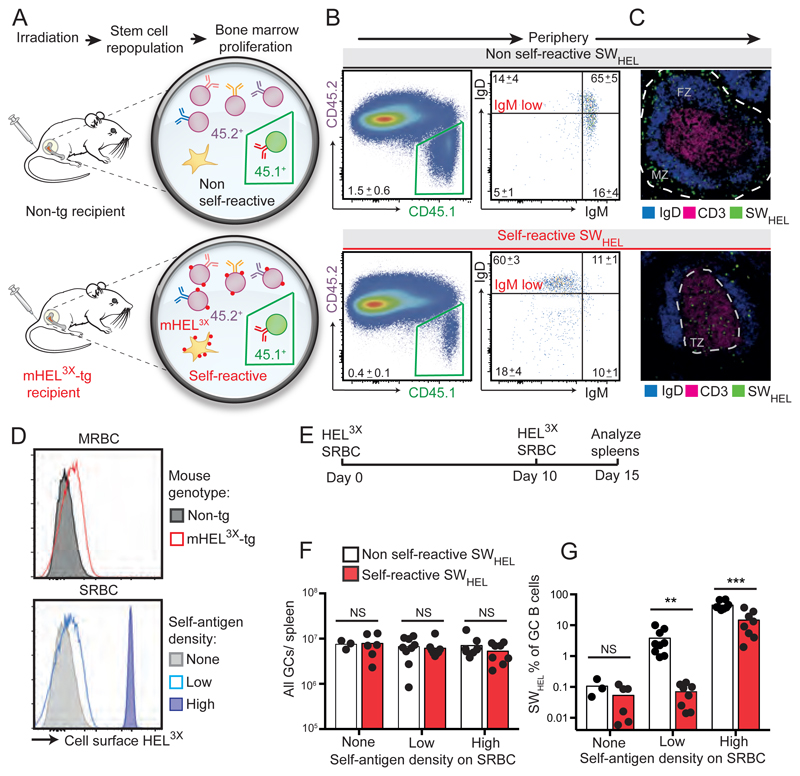
We engineered bone marrow chimeric mice (Figure 1A, B, fig. S1-S4) in which the majority of developing B-cells reaching the spleen from the bone marrow are polyclonal and CD45.2+. However 1% of transitional B-cells and 0.1% of mature follicular B-cells were CD45.1+ SWHEL cells, which carry on their surface HyHEL10 antibody with a defined structure and low affinity for a self protein (hen egg lysozyme with three substitutions (10–13), HEL3X; 1/KD = 1.2 × 107 M-1) and for a structurally similar foreign protein (duck egg lysozyme, DEL; 1/KD = 2.5 × 107 M-1). In one group of chimeric mice, the self protein was displayed on all cells as an integral membrane protein, mHEL3X, encoded by a transgene with a ubiquitin promoter (14). When SWHEL B-cells were self-reactive, they reached the spleen as short-lived anergic cells with decreased surface IgM but normal surface IgD (Figure 1B, fig. S1-S4), located primarily in the T-cell zone (Figure 1C), as in other anergic models (15–17). The frequency of anergic SWHEL cells was lower than the circulating frequency of anergic IgD+ IgMlo VH4-34+ B-cells that recognize ubiquitous cell surface antigens and mutate away from self reactivity in humans(8).
Figure 1. Recruitment of anergic cells into GC requires higher foreign antigen density.
(A) Construction of parallel groups of hematopoietic chimeras and analysis of their spleen by: (B) flow cytometry of all B cells (left) or CD45.1+ SWHEL B cells (right) (n=14 per group; mean ±(SEM); or (C) immunohistology, showing localisation of SWHEL B-cells (green), other B-cells (blue) and T-cells (magenta). (D) Relative abundance of self HEL3X on mouse red blood cells (MRBCs) from mHEL3X-transgenic or non-tg mice and on foreign sheep red blood cells (SRBCs) conjugated with 0 (none), 0.1µg/mL (low) or 10µg/mL (high) HEL3X. (E) Timing of chimera immunizations. (F) Total GC cells per spleen. (G) Percentage of SWHEL cells among GC B-cells. NS P>0.05, ** P<0.01, *** P<0.001 by Student’s t-test. Data points represent one chimera (two experiments, 16-26 chimeras in each).
We first tested if self-reactive SWHEL B-cells could respond to a foreign antigen that perfectly mimicked self. Sheep red blood cells (SRBCs) were covalently coupled with self antigen at surface densities either the same as on the endogenous mouse red blood cells (MRBCs) or 30 fold higher (Figure 1D). Despite equal T-cell help for germinal center (GC) responses by the diverse repertoire of other B-cells (Figure 1F), self-reactive SWHEL B-cells only entered GCs when SRBCs carried high antigen density (Figure 1G). SRBCs with low antigen density could nevertheless induce GC responses from SWHEL B-cells that were not self-reactive. These results are consistent with previous evidence that helper T-cells only cooperate with anergic B-cells when B-cell receptor cross-linking by foreign antigen is greater than that induced by self antigen (18).
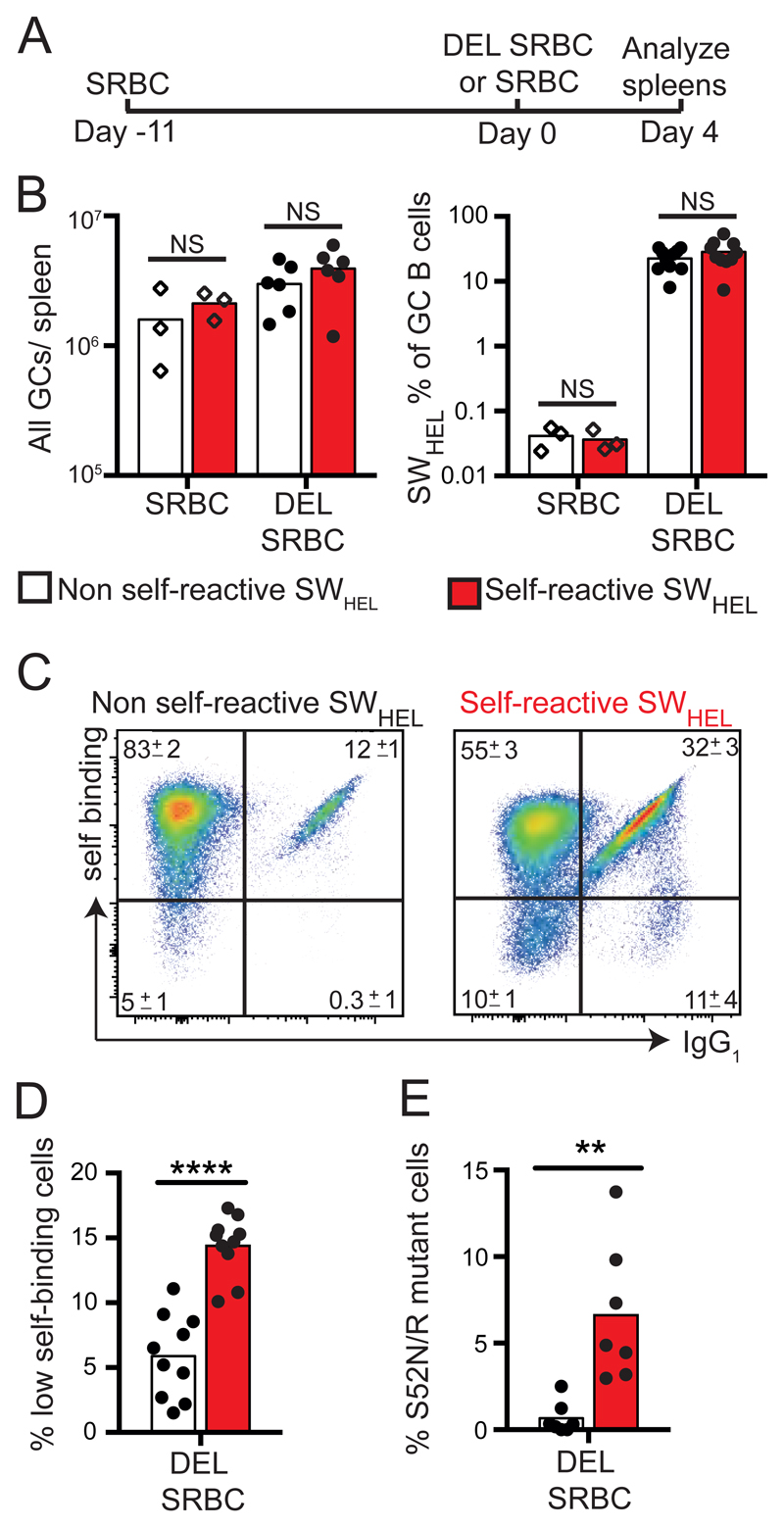
Next we tested the response of self-reactive SWHEL B-cells to DEL, which differs from self antigen at four residues that contact the HyHEL10 H-chain (fig S5, S6A). GC reactions were initiated with unconjugated SRBCs and, 11 days later, SWHEL B-cells recruited into the reaction synchronously by boosting with DEL coupled at high density to SRBCs (Figure 2A). Four days after immunization with DEL-SRBCs, SWHEL B-cells comprised ~20% of all GC B-cells and were present in comparable total numbers regardless of self-reactivity (Figure 2B, fig. S5 and S6B, C). When the SWHEL GC B-cells were self-reactive, they had lower surface IgG1 per cell (Figure 2C and fig. S6D), likely caused by engagement with self antigen on neighboring cells. At this early timepoint, the frequencies and numbers of IgG1− and IgG1+ SWHEL B-cells with low binding to self antigen were increased when the cells were self-reactive (Figure 2C, D and fig. S6C). These low binding cells had increased frequencies of missense mutations (fig. S6E, F), with 55% having acquired S52R or S52N mutations in complementarity determining region 2 (CDR2) (Figure 2E). Both mutations drastically decreased affinity for both self and foreign protein (fig. S7 and table S1).
Figure 2. Binding to similar foreign and self antigens triggers rapid mutation away from self.
(A) Timing of chimera immunizations. (B) Total GC cells per spleen and percent SWHEL cells among GC cells in chimeras receiving DEL-SRBC or unconjugated SRBC. (C) Analysis of SWHEL GC B-cells, showing percentage that bind 0.14µM HEL3X or express cell surface IgG1 (mean ±(SEM), and (D) percentage non-binding cells. (E) Percentage of sorted and single-cell-sequenced SWHEL GC cells with S52N or S52R mutations. NS p>0.05 ** P<0.01 **** P<0.0001; Student’s t-test. Data points represent one mouse. Data are from at least two independent experiments each involving 3-4 mice in DEL-SRBC groups.
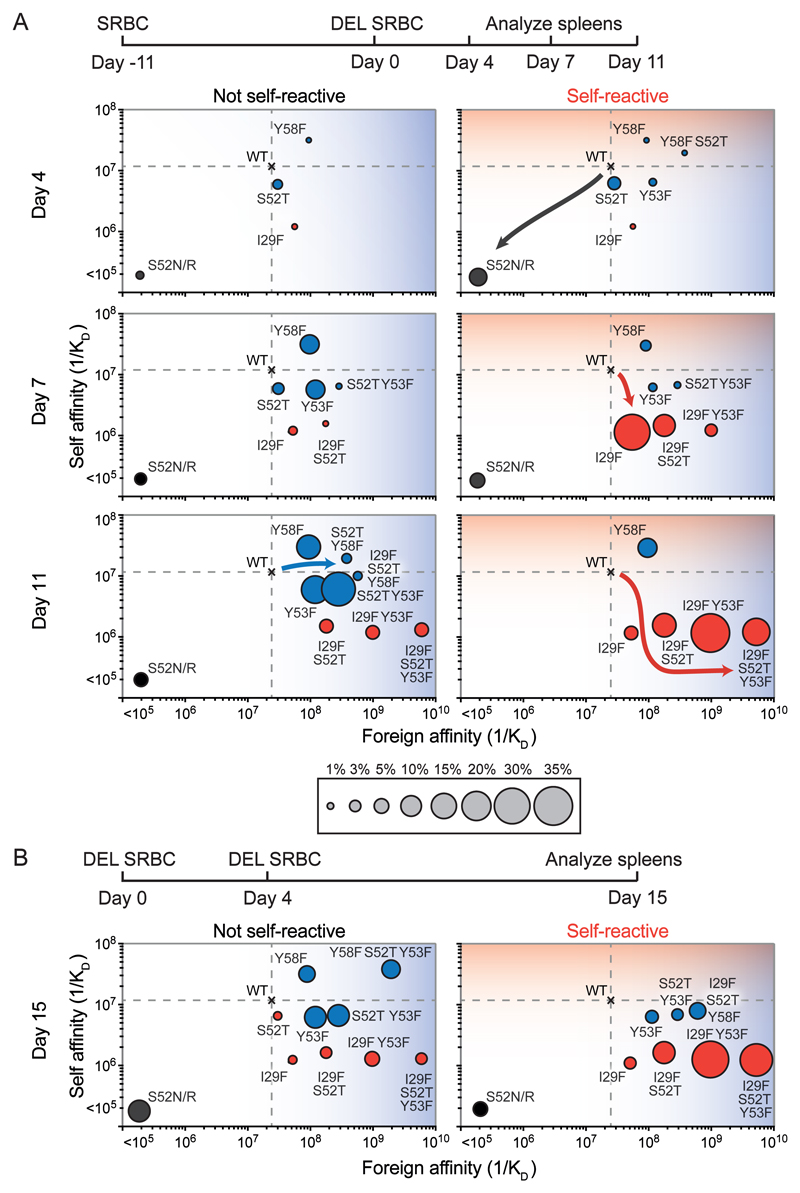
To determine if rapid selection for mutant GC B-cells with decreased affinity for self was followed by affinity maturation towards foreign, we analyzed antibody mutations 4, 7, and 11 days after SWHEL B-cells were challenged with DEL-SRBC (Figure 3A and fig. S8). On day 4, S52R or S52N mutations were again significantly increased when SWHEL B-cells were self-reactive (11.55% vs 3.55%, P = 0.0093). However, their frequency decreased on days 7 and 11. Instead, an I29F mutation in CDR1 became prevalent on day 7, occurring as a single substitution in 31% of SWHEL B-cells when they were self-reactive compared with only 1.7% when they were not. I29F had the unique property of distinguishing foreign from self, causing a 10-fold decrease in self affinity and a 2.6-fold increase in foreign affinity (Figure 3A, fig. S7 and table S1).
Figure 3. Self-reactive cells follow distinct mutational trajectories in order to lose self-binding capacity, leading to optimal affinity against foreign antigen.
Chimera immunization timepoints were to: (A) to synchronize recruitment of SWHEL B-cells into established GCs; or (B) to recruit SWHEL B-cells into GCs from the outset. SWHEL GC B-cells were single-cell sequenced. Dashed lines show affinity of unmutated (WT) antibody for self and foreign proteins. Circles show the affinity of recurring mutant antibodies for self and foreign proteins, with area denoting the percentage of SWHEL B-cells with the indicated mutation. Red circles indicate mutations more frequent in self-reactive SWHEL B-cells and blue circles represent mutations more frequent in the non-self-reactive SWHEL B-cells. Data are from one experiment, representative of two, each involving 2-3 mice per group at each timepoint.
The I29F mutation became paired with S52T or Y53F mutations in CDR2, starting as a small subset of self-reactive cells on day 7 but becoming the most prevalent as pairs or a trio by day 11. S52T or Y53F were rarely found individually but combined with the I29F foundation mutation increased foreign-self discrimination, retaining 1 × 106 M−1 affinity for self but progressively increasing foreign affinity to 6 × 109 M−1. Strong epistatic (non-additive) effects were observed. For example, the I29F S52T YF3F trio increased the apparent ΔΔG for binding foreign antigen by −3.3 kcal/mol, compared with −1.6 kcal/mol expected for additive effects of the individual mutations (table S1). This trio of mutations became even more prevalent when self-reactive SWHEL B-cells were recruited at the outset of the GC reaction and analyzed 15 days later (Figure 3B and fig. S9). Thus, an antibody that was initially unable to distinguish foreign from self had evolved 5000-fold differential binding to foreign over self, by first mutating away from binding self and then towards binding foreign. SWHEL-derived cells that had lost self-binding but retained foreign were also frequent among the IgG1+ memory B-cell compartment (fig. S10). Foreign-specific IgG1 serum titers were increased in mice with initially self-reactive SWHEL B-cells (fig. S11).
A different, less optimal evolutionary trajectory prevailed when SWHEL B-cells were not self-reactive, dominated by acquisition of a CDR2 mutation, Y58F, either alone, paired, or in trio with S52T and Y53F (Figure 3). Y58F alone or with S52T and Y53F increased self-affinity four-fold, explaining why this trajectory was not taken by self-reactive SWHEL cells. The Y58F-S52T-Y53F trio increased foreign affinity to 2 × 109 M−1, which was three-fold lower than the I29F-S52T-Y53F trio selected through the self-reactive trajectory.
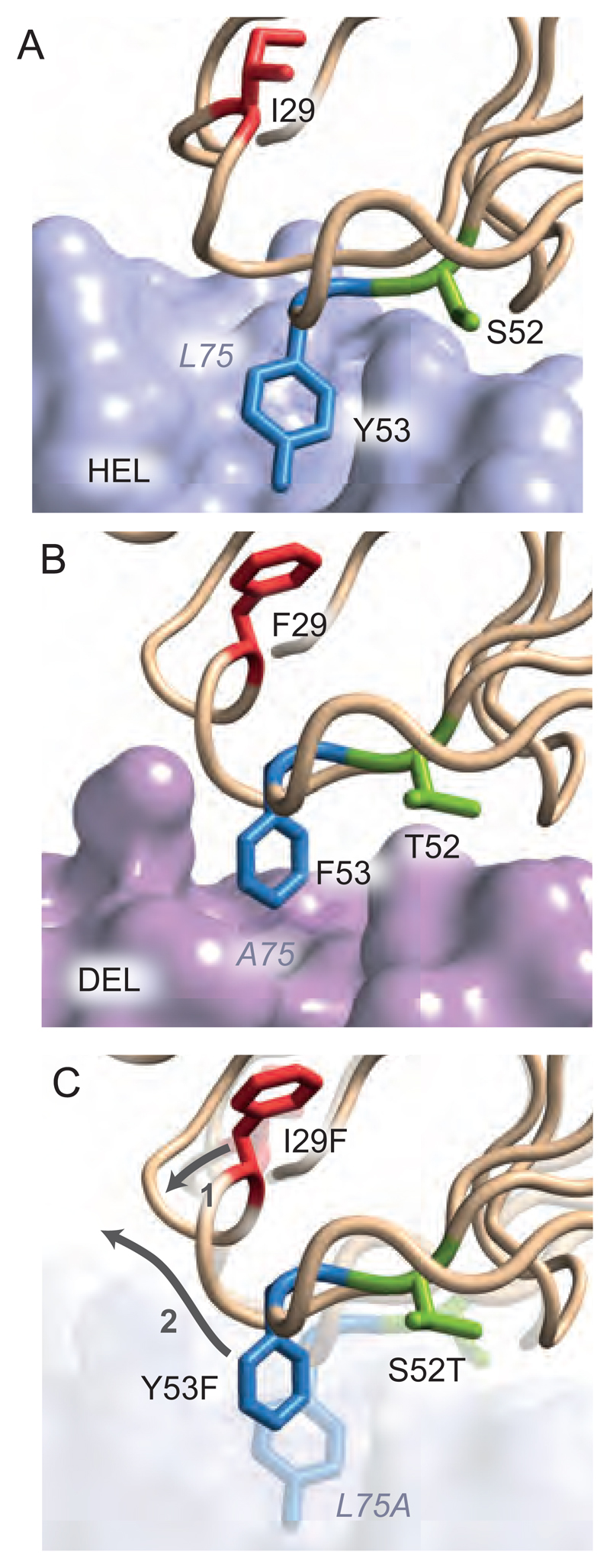
To understand how these three mutations conferred a 5000-fold differential binding to foreign over self, we used X-ray crystallography to analyze the structure of HyHEL10I29F, S52T, Y53F complexed with DEL (Figure 4, table S2, movie S1) compared to HyHEL10WT complexed with HEL (19). I29F resulted in a structural rearrangement of the CDR1 loop to accommodate the larger phenylalanine side chain. Displacement of this loop (1) opened up additional structural adjustments of CDR2 (2), and in particular repositioned Y53F to interact with a hydrophobic pocket formed on the surface of DEL by the short Alanine 75 side chain compared to the much longer Leucine in HEL. The CDR2 backbone adjustments also allowed replacement of the smaller S52 side chain with threonine. Thus, our structural analyses were in agreement with the observed mutational trajectory, whereby the I29F foundation mutation introduces structural rearrangements into CDR1 and CDR2; these enable secondary mutations at positions 52 and 53, which selectively increase foreign affinity in an epistatic manner. Binding studies confirmed I29F confers 50-fold lower binding to self versus foreign antigen by exploiting the L75A foreign pocket coupled with the adjacent E73K charge reversal (table S1). This was further confirmed by solving the structure of HyHEL10I29F complexed with DEL (fig. S12).
Figure 4. Structural basis of mutation away from self.
X-ray crystallographic structures of (A) unmutated HyHEL10 in complex with HEL and (B) HyHEL10I29F,S52T,Y53F triple mutant antibody in complex with DEL. (C) Overlay of both structures showing the structural rearrangement of the CDR1 loop caused by the I29F mutation (1) and the complementary structural adjustments of positions 52 and 53 in the CDR2 loop to exploit the Leu75Ala pocket in the foreign antigen (2).
We next identified anergic B-cells in the mHEL3Xtg mice within a polyclonal repertoire that possessed micromolar affinity for the same self antigen and tested if they too could resolve antigenic mimicry. HEL3X-binding B-cells comprised 2.7% of IgD+ IgMlo anergic B-cells and 0.5% of all spleen B-cells (fig. S13A). These were sorted and added at 0.5% frequency to unselected CD45.1+ B-cells, and the polyclonal mixture injected with T-cells into mHEL3Xtg Rag1−/− mice immunized with DEL-SRBC. In the recipients, 96% of the GC response was derived from the unselected CD45.1+ B-cells, presumably mostly recognizing SRBC antigens. By contrast, 61% of the DEL-binding GC response was derived from the polyclonal HEL3X–binding anergic CD45.2+ B-cells (fig. S13B). Only 9.7% of these cells still bound self antigen, whereas 53% bound foreign DEL selectively (fig. S13C). Thus, in a normal repertoire, cells with micromolar affinity for self-HEL3X are dominant contributors to the GC response against the self-mimic DEL and rapidly lose binding to self.
The findings here extend evidence for autoantibody redemption in human antibodies (7–9) by showing mutation away from self precedes mutation towards foreign to create unique epistatic trajectories. Self-reactivity, rather than being a barrier to immunization, directed cells down an alternative trajectory, which produced a higher final affinity against the foreign immunogen. The higher threshold to activate anergic cells and recruit them to GC reactions is nevertheless an important constraint: for instance, low density of Env molecules on HIV virions may fail to activate anergic B-cells with moderate cross-reactive affinity for self glycans attached to foreign and self polypeptides, precluding mutation trajectories away from self-reactivity.
Antibody mutation away from self in GC reactions defers the need to acquire stringent self-tolerance until after an infection. This is complementary to the concept of purging self-reactive antibodies from the pre-immune repertoire before they can be tested for binding foreign antigen (1, 6, 20–22) as well as to Jerne’s hypothesis of mutation away from self in the bone marrow/bursa (23). Both concepts create a “holes in the repertoire” problem if applied too stringently (24, 25). Crucially, autoantibody redemption minimizes the potential for microbes to evolve antigens that are “almost self”, which could otherwise only be recognized by pre-immune antibodies that had been deleted or edited in the bone marrow. Mutation away from self in response to one foreign antigen may allow progeny B-cells to respond to an unrelated foreign antigen later. For example, intestinal microbes may induce polyspecific B-cells to mutate away from self, providing a self-tolerant repertoire that would not be available in individuals treated with antibiotics or raised in a more hygienic environment. The evolution of an antibody along a limited set of mutation trajectories, driven by two selection pressures for higher affinity to one ligand and lower affinity for another, provides an example of deterministic molecular evolution. We conclude that our findings provide insights into the GC reaction and the evolution of specificity in antibody-antigen interactions.
Supplementary Material
One Sentence Summary.
Antibodies cross-reacting with self and foreign proteins evolve on consistent trajectories to shed self binding, which enhances foreign binding
Acknowledgments
We thank the Garvan Institute Australian BioResources, Garvan Molecular Genetics, and Flow Cytometry facilities for expert animal husbandry, genotyping, and cell sorting.
Funding: This work was supported by Program Grants 1016953 & 1113904, Project Grant 1108800, and Fellowships 585490 & 1081858 from the NHMRC, Discovery Grants 160104915 & 140103465 from the ARC and by the Bill and Patricia Ritchie Family Foundation.
Footnotes
Author contributions: DLB performed and analyzed mouse experiments; PS performed and analyzed binding affinity experiments; DBL performed and analyzed crystallography experiments; JJ and KB generated antibodies and antigens; TDC JHR and JRH developed mHEL3X transgenic mice; RB devised and developed the mHEL3X x SWHEL system; CCG supervised B-cell biology aims; DC supervised structural and biophysical aims; DLB, PS, DBL, RB, DC, CCG designed and interpreted experiments; DLB, DBL, PS, KP, RB, DC, CCG prepared figures; BTP generated movies; DLB drafted & RB, DC, CCG revised the manuscript.
Competing interests: Authors have no competing interests.
Data and materials availability: Coordinates and structure factors have been deposited in the Protein Data Bank, with accession codes 5VJO and 5VJQ.
References
- 1.Wakerley BR, et al. Expert Rev Clin Immu. 2013;9:627. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, et al. Science. 2005;308:1906. [Google Scholar]
- 3.Pinschewer DD, et al. J Clin Invest. 2004;114:988. doi: 10.1172/JCI22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyatt R, et al. Nature. 1998;393:705. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 5.Gristick HB, et al. Nat Struct Mol Biol. 2016;23:906. doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemazee D, et al. J Exp Med. 2000;191:1813. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J, et al. Nature. 2016;529:105. doi: 10.1038/nature16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed JH, et al. J Exp Med. 2016;213:1255. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabouri Z, et al. Proc Natl Acad Sci. 2014:1. [Google Scholar]
- 10.Phan TG, et al. J Exp Med. 2003;197:845. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan TG, et al. JEM. 2006;203:2419. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padlan EA, et al. P Natl Acad Sci USA. 1989;86:5938. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paus D, et al. JEM. 2006;203:1081. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan TD, et al. Immunity. 2012;37:893. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cyster JG, et al. Nature. 1994;371:389. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 16.Fulcher DA, et al. J Exp Med. 1994;179:125. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodnow CC, et al. Nature. 1988;334 [Google Scholar]
- 18.Cooke MP, et al. J Exp Med. 1994;179:425. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acchione M, et al. Molecular Immunology. 2009;47:457. doi: 10.1016/j.molimm.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnet FM. The clonal selection theory of acquired immunity. Vanderbilt University Press; Nashville: 1959. [Google Scholar]
- 21.Lederberg J. Science. 1959;129:1649. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 22.Wardemann H, et al. Science. 2003;301:1374. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 23.Jerne NK. Eur J Immunol. 1971;1:1. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 24.Nemazee D. Immunol Today. 1996;17:25. doi: 10.1016/0167-5699(96)80565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perelson AS, et al. J Theor Biol. 1979;81:645. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.