ABSTRACT
Dermatophytosis, the commonest superficial fungal infection, has gained recent attention due to its change of epidemiology and treatment failures. Despite the availability of several agents effective against dermatophytes, the incidences of chronic infection, reinfection, and treatment failures are on the rise. Trichophyton rubrum and Trichophyton interdigitale are the two species most frequently identified among clinical isolates in India. Consecutive patients (n = 195) with suspected dermatophytosis during the second half of 2014 were included in this study. Patients were categorized into relapse and new cases according to standard definitions. Antifungal susceptibility testing of the isolated Trichophyton species (n = 127) was carried out with 12 antifungal agents: fluconazole, voriconazole, itraconazole, ketoconazole, sertaconazole, clotrimazole, terbinafine, naftifine, amorolfine, ciclopirox olamine, griseofulvin, and luliconazole. The squalene epoxidase gene was evaluated for mutation (if any) in 15 T. interdigitale and 5 T. rubrum isolates exhibiting high MICs for terbinafine. A T1189C mutation was observed in four T. interdigitale and two T. rubrum isolates. This transition leads to the change of phenylalanine to leucine in the 397th position of the squalene epoxidase enzyme. In homology modeling the mutant residue was smaller than the wild type and positioned in the dominant site of squalene epoxidase during drug interaction, which may lead to a failure to block the ergosterol biosynthesis pathway by the antifungal drug.
KEYWORDS: dermatophytes, allylamines, antifungal resistance, mutational studies
INTRODUCTION
In recent years, dermatophytosis, the commonest superficial fungal infection in dermatology practice, has gained attention due to its change of epidemiology and treatment failure. Increased numbers of cases are noted in diabetics and in aging and immunocompromised populations (1). Trichophyton rubrum and Trichophyton mentagrophyte complex (Trichophyton interdigitale) are the most commonly isolated species but may vary in different geographical locations (2). Despite the availability of many effective antidermatophytic agents in practice, the prevalence of dermatophytosis remains unaltered. The rise in the incidence of chronic and recurrent infections and reinfection in susceptible populations and treatment failure are implicated in this situation (3). Of course, poor compliance with therapy, steroid use, self-medication, and possible antifungal resistance are other factors leading to the present menace. In recent years, several cases with unusual, atypical, and chronic/relapse/recalcitrant presentations have been encountered in India (3). The in vitro antifungal susceptibility testing for dermatophytes, its in vivo correlation, and the mechanism of antifungal resistance have not been studied in detail. No breakpoint is yet determined to guide antifungal therapy. Relapse is usually encountered while managing tinea unguium (4) and to some extent attributed to terbinafine resistance (5–7). Mukherjee et al. (5) showed a good correlation between clinical resistance to terbinafine and the high MIC of this drug for T. rubrum isolates. They characterized those isolates and identified a missense mutation in the squalene epoxidase (SE) gene leading to L393F and F397L substitutions (6, 8). However, even with low MICs for SE inhibitors (allylamines), onychomycosis cases not responding to this drug have been reported (4, 9). Those reports indicate poor understanding of allylamine susceptibility testing and mechanism of resistance. The present study was conducted enrolling patients with both fresh and relapsed/recurrent cases of dermatophytosis to identify allylamine resistance in Trichophyton species and to evaluate the possible molecular mechanism of resistance. A homology model was studied to assess the impact of the identified mutation on the general structure of the squalene epoxidase protein.
RESULTS
Demography and clinical details.
A total of 195 consecutive patients clinically suspected of dermatophytosis and further confirmed by demonstrating septate thin hyphae on direct microscopy of skin scrapings were included in the study. The majority, 73.8% (144), of the patients were male, with a median age of 33.5 years (interquartile range [IQR], 24 to 47 years; mean ± standard deviation [SD], 36.33 ± 16.6); 7 (3.6%) and 4 (2%) were children and neonates, respectively. Occupations of the patients included homemaker (49 [25.1%]), office worker (42 [21.5%]), student (39 [20%]), field worker (29 [14.9%]), agriculturist (23 [11.8%]), and others (13 [6.7%]). In 60 (30.8%) patients, lesions were distributed in more than one site, whereas 135 (69.2%) patients had a solitary lesion. Among the patients with single-site involvement, the majority were diagnosed as having tinea corporis (59 [30.2%]), followed by tinea cruris (55 [28.2%]), tinea pedis (14 [7.1%]), tinea faciei (5 [2.5%]), and tinea capitis (2 [1%]). Tinea corporis and tinea cruris (18 [9.2%]) were the most commonly coexistent forms of disease in the 60 patients with multiple-site involvement. Spreading lesions were noted in 159 patients; the lesions were erythematous and pigmented in 132 patients and 65 patients, respectively. The majority (117 [60%]) of the patients were classified as having recurrent dermatophytosis. A history of contact with infected humans and animals and with soil was noted for 57 (29.2%), 28 (14.3%), and 26 (13.3%) patients, respectively.
Patients sought medical attention at various durations of illness. Seventy-six patients (38.9%) sought medical attention within 2 to 6 months after onset of lesions; 26 (13.3%) patients within a period of 1 month, 33 (16.9%) patients within 7 to 12 months, 23 (11.8%) patients within 1 to 2 years, and 22 (11.3%) patients after 2 years. Fifteen (7.7%) patients could not specify the duration of the disease. The majority (123 [63.1%]) of the patients treated themselves before consulting a physician. A definite history of previous antifungal exposure (topical or systemic) could be ascertained for 63 (32.3%) patients. Prior antifungal therapy for current infection was significantly higher in recurrent dermatophytosis cases than in fresh cases (67 versus 16 cases; P < 0.0001). Diabetes mellitus as a comorbidity was noted for 12 (6.1%) patients, whereas 17 (8.7%) patients had other comorbidities, like high blood pressure, history of kidney transplant, pancreatitis, trauma, tuberculosis, and hepatitis C virus (HCV) infection.
Microbiological investigation.
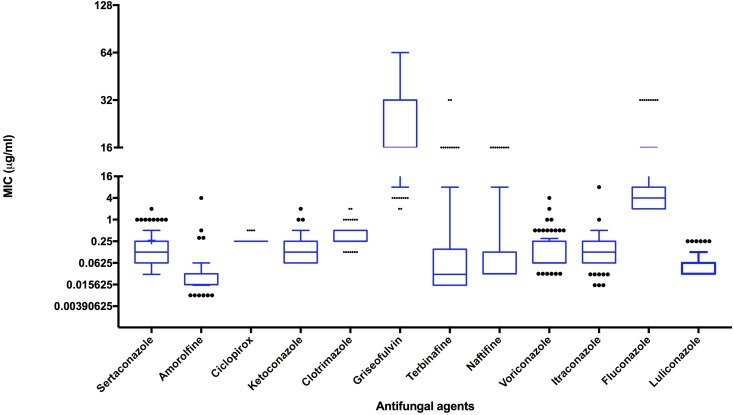
Dermatophytes were isolated from 133 (68.2%) of 195 patients. T. interdigitale was the predominant isolate (88 [66.1%]), followed by T. rubrum (35 [26.3%]), T. tonsurans (4 [3%]), Microsporum gypseum (4 [3%]), and Microsporum canis (2 [1.5%]). Antifungal susceptibility testing was performed for 127 Trichophyton isolates with 12 antifungal agents. The antifungal susceptibility profile of those isolates is depicted in Table 1 and Fig. 1. The MICs and other salient features of azole- and/or allylamine-resistant isolates are provided in Table 2.
TABLE 1.
Drug susceptibility profile of Trichophyton species
| Antifungal | Type of value | Value for organism (μg/ml) |
||
|---|---|---|---|---|
| T. interdigitale | T. rubrum | T. tonsurans | ||
| Fluconazole | Range | 2–32 | 2–32 | 2–8 |
| GMa | 5.03 | 4.08 | 4 | |
| MIC50 | 4 | 4 | 4 | |
| MIC90 | 16 | 8 | 4 | |
| Ketoconazole | Range | 0.0625–2 | 0.0625–1 | 0.125–0.5 |
| GM | 0.17 | 0.13 | 0.21 | |
| MIC50 | 0.125 | 0.125 | 0.125 | |
| MIC90 | 0.5 | 0.5 | 0.25 | |
| Sertaconazole | Range | 0.03–1 | 0.03–2 | 0.125–0.5 |
| GM | 0.13 | 0.15 | 0.25 | |
| MIC50 | 0.125 | 0.125 | 0.25 | |
| MIC90 | 0.5 | 1 | 0.25 | |
| Clotrimazole | Range | 0.125–2 | 0.125–2 | 0.25–0.5 |
| GM | 0.36 | 0.35 | 0.35 | |
| MIC50 | 0.25 | 0.25 | 0.25 | |
| MIC90 | 0.5 | 0.5 | 0.5 | |
| Voriconazole | Range | 0.0312–2 | 0.0312–4 | 0.0625–0.125 |
| GM | 0.12 | 0.08 | 0.07 | |
| MIC50 | 0.125 | 0.0625 | 0.0625 | |
| MIC90 | 0.5 | 0.25 | 0.0625 | |
| Itraconazole | Range | 0.15–8 | 0.015–1 | 0.0625–0.25 |
| GM | 0.13 | 0.09 | 0.14 | |
| MIC50 | 0.125 | 0.0625 | 0.125 | |
| MIC90 | 0.5 | 0.25 | 0.25 | |
| Terbinafine | Range | 0.015–32 | 0.015–16 | 0.015–2 |
| GM | 0.06 | 0.05 | 0.14 | |
| MIC50 | 0.03 | 0.015 | 0.5 | |
| MIC90 | 4 | 2 | 2 | |
| Naftifine | Range | 0.0312–16 | 0.0312–16 | 0.0312–4 |
| GM | 0.1 | 0.007 | 0.14 | |
| MIC50 | 0.0312 | 0.0312 | 0.0312 | |
| MIC90 | 8 | 1 | 0.125 | |
| Amorolfine | Range | 0.007–4 | 0.007–0.0625 | 0.156–0.03 |
| GM | 0.02 | 0.02 | 0.01 | |
| MIC50 | 0.0156 | 0.0312 | 0.0156 | |
| MIC90 | 0.0625 | 0.0625 | 0.0156 | |
| Ciclopirox olamine | Range | 0.25–0.5 | 0.25 | 0.25–0.5 |
| GM | 0.25 | 0.25 | 0.3 | |
| MIC50 | 0.25 | 0.25 | 0.25 | |
| MIC90 | 0.25 | 0.25 | 0.25 | |
| Griseofulvin | Range | 2–128 | 2–128 | 32 |
| GM | 26.31 | 27.31 | 0.07 | |
| MIC50 | 32 | 32 | 32 | |
| MIC90 | 64 | 128 | 32 | |
| Luliconazole | Range | 0.0312–0.25 | 0.0312–0.25 | 0.0625–0.0312 |
| GM | 0.05139 | 0.0509 | 0.0441 | |
| MIC50 | 0.0312 | 0.0312 | 0.0312 | |
| MIC90 | 0.125 | 0.125 | 0.0625 | |
GM, geometric mean.
FIG 1.
Box plot of 12 antifungals tested.
TABLE 2.
Salient features of dermatophytosis due to azole- and/or allylamine-resistant Trichophyton speciesa
| Patient ID | Organism | NCCPF no./GenBank accession no. | Antifungal exposure | Relapse | MIC (μg/ml) |
Mutation | ||
|---|---|---|---|---|---|---|---|---|
| Terbinafine | Naftifine | Fluconazole | ||||||
| 12 | T. interdigitale | 800032/MG587085 | Clotrimazole + miconazole | Yes | 2 | 4 | 2 | No |
| 29 | T. interdigitale | 800033/MG587086 | No | No | 16 | >16 | 16 | No |
| 68 | T. interdigitale | 800022/KX906451 | No | Yes | 16 | >16 | 8 | F397L |
| 89 | T. interdigitale | 800023/KX906452 | No | No | 16 | >16 | 32 | F397L |
| 93 | T. interdigitale | 800024/KX906453 | No | No | 32 | >16 | 2 | No |
| 98 | T. interdigitale | 800040/KX906463 | Terbinafine | Yes | 4 | 8 | 2 | F397L |
| 106 | T. interdigitale | 800030/KX906456 | Herbal remedies | No | 2 | 1 | 4 | No |
| 125 | T. interdigitale | 800025/KX906454 | No | Yes | 16 | >16 | 32 | No |
| 139 | T. interdigitale | 800026/KX906455 | No | No | 16 | 8 | 4 | F397L |
| 143 | T. interdigitale | 800027/MG587087 | Herbal remedies | No | 4 | >16 | 8 | No |
| 145 | T. interdigitale | 800028/MG587088 | Fluconazole + luliconazole | Yes | 4 | 8 | 8 | No |
| 157 | T. interdigitale | 800050/MG587089 | Terbinafine | Yes | 16 | 8 | 8 | No |
| 173 | T. interdigitale | 800029/MG587090 | Not known | No | 16 | 1 | 4 | No |
| 174 | T. interdigitale | 800031/MG587091 | Not known | Yes | 8 | 16 | 16 | No |
| 198 | T. interdigitale | 800051/MG587092 | No | No | 32 | 16 | 32 | No |
| 46 | T. rubrum | 900038/KX906447 | Fluconazole | Yes | 2 | 4 | 2 | No |
| 118 | T. rubrum | 900039/KX906448 | Clotrimazole + terbinafine | Yes | 16 | 0.0625 | 8 | No |
| 126 | T. rubrum | 900042/MG587093 | No | No | 2 | 1 | 4 | No |
| 137 | T. rubrum | 900040/KX906449 | Fluconazole | Yes | 16 | 8 | 4 | F397L |
| 156 | T. rubrum | 900041/KX906473 | Luliconazole | No | 8 | >16 | 4 | F397L |
ID, identifier; NCCPF, National Culture Collection of Pathogenic Fungi.
Fifteen (17%) T. interdigitale isolates exhibited high terbinafine MICs, 2, 4, 8, 16, and 32 μg/ml for 2, 3, 1, 7, and 2 isolates, respectively, whereas 5 isolates (14.3%) of T. rubrum exhibited high terbinafine MICs, 2, 8, and 16 μg/ml for 2, 1, and 2 isolates, respectively. Of these 20 Trichophyton isolates with high terbinafine MICs, 10 isolates were from recurrent cases (Table 2). The majority of the isolates with high terbinafine MICs also showed high MICs for naftifine, except 1 T. rubrum isolate showing a high MIC for terbinafine only (this patient had prior exposure to terbinafine). Griseofulvin was the most inactive drug (in vitro), with a modal MIC of 32 μg/ml. Among azoles, fluconazole had poor in vitro activity, with MICs of ≥8 μg/ml for 45 (35.4%) isolates. Cumulative frequencies of the isolates exhibiting high MICs are depicted in Fig. 2. Among 15 patients who had history of azole treatment before visiting our hospital, 13 patients were categorized as having recurrent cases, and fluconazole MICs of the isolates ranged between 2 and 8 μg/ml. The MIC90s of voriconazole, itraconazole, sertaconazole, clotrimazole and ketoconazole were 0.5 μg/ml. Amorolfine, ciclopirox olamine, and luliconazole had low MIC90s, 0.06, 0.25, and 0.125 μg/ml, respectively.
FIG 2.
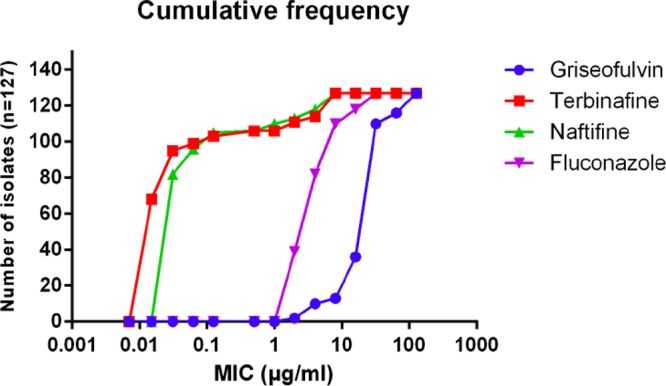
Cumulative frequency of the four antifungal drugs with highest MICs.
The DNA sequences of the squalene epoxidase genes of the terbinafine isolates with high MICs were compared with those from terbinafine-sensitive isolates. A T1189C transition of the open reading frame of the SE gene was noted in two of the five T. rubrum isolates with high terbinafine MICs. SE gene sequences of four randomly selected terbinafine-sensitive isolates did not show this mutation. Similarly, 4 of the 15 T. interdigitale isolates with high MICs for terbinafine showed the same T1189C mutation, but none of the 9 terbinafine-sensitive isolates showed it (see Fig. S1 in the supplemental material). Further analysis showed that this missense mutation leads to a change of phenylalanine at the 397th position to leucine (Phe397Leu). We selected one wild-type isolate and one non-wild-type (Phe397Leu) isolate and subjected them to homology modeling to assess the impact of this substitution on the general structure of the squalene epoxidase protein. Homology modeling revealed that the mutant residue is smaller than the wild-type residue and the mutation was in the domain of the binding site of the molecule, which may lead to the failure of drug-enzyme interaction (Fig. 3).
FIG 3.
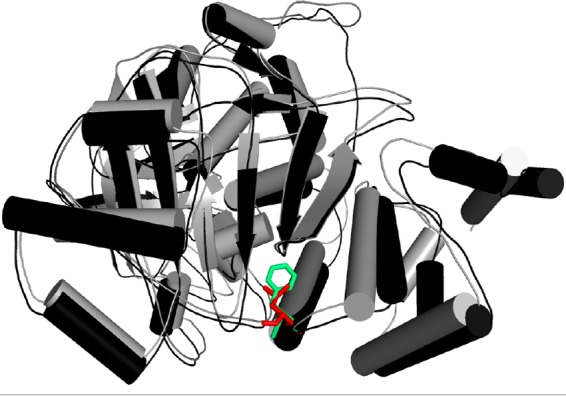
Threading representation in 3D structure of wild-type and mutant proteins in green and red, respectively. The amino acid change from phenylalanine to leucine distorts the protein structure.
DISCUSSION
A high prevalence (60%) of recurrent dermatophytosis was noted even in patients excluding tinea unguium. In recurrent dermatophytosis cases, self-medication or prior antifungal exposure was significantly more frequent, and T. interdigitale (66.1%) and T. rubrum (26.3%) were the most frequently isolated species; 15 (17%) T. interdigitale and 5 (14.3%) T. rubrum isolates had high MICs for terbinafine, the most effective systemic antidermatophytic agent. Majority of those isolates had cross-resistance to the other allylamine, naftifine. A T1189C transition in the open reading frame of the SE gene leading to a change of phenylalanine at the 397th position to leucine (Phe397Leu) was noted in four T. interdigitale and two T. rubrum isolates. In homology modeling, this mutation was indicated as the possible reason for failure of squalene epoxidase and antifungal interaction leading to antifungal drug resistance. In silico modeling accompanied by cloning would confirm the hypothesis.
Although all age groups are susceptible to dermatophyte infection, about half of our cases were in the younger age group (21 to 40 years), conforming with the earlier reported series (1, 10). In India, working young people are exposed to prolonged wet work and may acquire dermatophyte infection. Diabetes is considered an important underlying disease for dermatophytosis (11), but only 6.1% of our patients had diabetes as a comorbidity. The isolation rate (68.2%) of dermatophytes in the present study was comparable to those in other studies (1, 11, 12). However, in contrast to the majority of studies from India, T. interdigitale was the commonest isolate in the present study, rather than T. rubrum (1, 12–15). Few studies have reported T. mentagrophytes as the predominant etiologic agent (16). The present study may indicate a shift in causative agents of dermatophytosis in India, though the reason is not clear.
Triazole and the imidazole group of drugs are commonly used to treat dermatophytosis. Recently, azole resistance in dermatophytes has been reported in 19% of cases (17), notably acquired resistance to fluconazole among T. rubrum isolates (18). In the present study, 35.4% of our isolates had MICs of ≥8 μg/ml for fluconazole. In agreement with another study (30), we noted a high modal MIC, 32 μg/ml, for griseofulvin. Though ketoconazole, sertaconazole, clotrimazole, amorolfine, ciclopirox olamine, and luliconazole showed good in vitro activity, these drugs are not used in extensive lesions, for which terbinafine is preferred.
Terbinafine inhibits squalene epoxidase in a noncompetitive manner by blocking synthesis of 2,3-oxidosqualene, leading to accumulation of squalene and depletion of ergosterol, causing growth inhibition (20, 21). Relapse and treatment failure with terbinafine had been rarely reported until recent years (5), and similarly, resistance was rarely reported on the basis of in vitro susceptibility testing (see Table S1). Point mutations in the squalene epoxidase gene conferring Leu393Phe and Phe397Leu substitutions were implicated in terbinafine resistance in T. rubrum in the last 2 decades (5, 8). Recently, Yamada et al. (22) reported several point mutations leading to Leu393Phe, Leu393Ser, Phe397Ile, Phe397Leu, Phe397Val, 141 Phe415Val, and His440Tyr substitutions in 16 T. rubrum isolates and 1 T. interdigitale isolate. In the present study, we identified a T1189C mutation leading to amino acid substitution Phe397Leu in two of five T. rubrum isolates with high MICs for terbinafine. The absence of this mutation in sensitive T. rubrum isolates further emphasized its possible role in terbinafine resistance. However, we did not find other mutations described by Yamada et al. (22). The absence of the T1189C mutation in three of our isolates with high MICs indicates the possible existence of another mechanism conferring in vitro terbinafine resistance. We identified the T1189C mutation in four of nine T. interdigitale isolates with high terbinafine MICs. To date, only one T. interdigitale isolate from Japan is reported to harbor this mutation. The equivalent mutation responsible for terbinafine resistance has been reported for Aspergillus fumigatus, Aspergillus nidulans, and Saccharomyces cerevisiae isolates (23–25). All these reports suggest that point mutation in the squalene epoxidase gene can confer terbinafine resistance in different fungi.
Analysis of the effect of amino acid substitution resulting in drug resistance may help to understand drug and enzyme interactions. Nowosielski et al. (26) used atomic three-dimensional (3D) modeling of squalene epoxidase in S. cerevisiae isolates. They reported the strongest interaction between drug and enzyme at amino acids Phe402, Phe420, Phe417, Cys416, Val92, and Tyr90, which are localized in the C-terminal region of the squalene epoxidase. The residue Phe402 in squalene epoxidase corresponds to Phe397 in the T. rubrum squalene epoxidase gene (22). The alteration in the amino acid at this position significantly impacts drug-enzyme interactions. In this study, homology modeling revealed that enzyme from non-wild-type strains undergoes structural destabilization due to the Phe397Leu substitution. This structural destabilization affects the drug-enzyme binding. Yamada et al. successfully transformed terbinafine-sensitive Arthroderma vanbreuseghemeii with clones harboring the mutated SE gene alleles, leading to Leu393Phe, Leu393Ser, Phe397Ile, Phe397Leu, Phe397Val, Phe415Val or His440Tyr substitutions, which eventually confer resistance to terbinafine (22). The limitation of our study is that we could not confirm experimentally the impact of this mutation in conferring resistance due to difficulty in performing gene replacement study with this fungus.
It is not clear whether terbinafine resistance is primary or acquired after exposure of drug. Mukherjee et al. claimed it to be primary in T. rubrum after testing sequential isolates (5). In our 20 cases with high terbinafine MICs for Trichophyton isolates, only 3 cases had a definite history of terbinafine exposure, and one of those isolates exhibited T1189C mutation. However, half of our cases were grouped as relapse/recurrent/chronic dermatophytosis occurring in patients who were taking several over-the-counter medications. In the absence of sequential isolates, it is difficult to describe whether our isolates were primarily resistant or acquired resistance during the treatment. Further molecular evolutionary studies of sequential isolates from same patient may provide more insight in delineating the issue of primary and secondary resistance.
In conclusion, the present study shows that T. interdigitale is the commonest agent, responsible for the majority of recurrent cases of dermatophytosis at our center. In vitro high MICs for terbinafine and fluconazole may partially explain the recurrence. Though mutation in the squalene epoxidase enzyme is not a frequent phenomenon, the T1189C mutation in the SE gene leading to Phe397Leu substitution in one-quarter of the terbinafine isolates with high MICs and homology modeling explain the possible mechanism of resistance to terbinafine. A comprehensive larger cohort study on the host factors, environmental factors, and resistance profile including the impact of this mutation in the recurrent/relapsed cases may provide insight into the ongoing problem of treatment failure in dermatophytosis.
MATERIALS AND METHODS
Patients.
A total of 195 consecutive patients clinically diagnosed as having dermatophytosis at the outpatient department of our tertiary care institute during July 2014 through December 2014 were enrolled in the study. The study protocol was cleared by the Institute Ethics Committee. Demographic and clinical details of the cases were recorded after obtaining the patients' consent. Patients presenting only with tinea unguium were excluded from the study. For study purposes, the diagnosis of recurrent dermatophytosis was made where the patient had at least one episode of relapse within the last 6 months and after 4 weeks of stoppage of antifungal medication (27).
Isolation and identification of dermatophytes.
After preliminary clinical examination, skin scrapings were collected as per standard protocol (2). The samples were cultured on to Sabouraud's dextrose agar (SDA) containing chloramphenicol (0.05%) with and without cycloheximide (0.5%) and incubated at 37°C and 25°C for 6 weeks. Dermatophyte isolates were identified based on macroscopic and microscopic characteristics and physiological tests such as urease production, in vitro hair perforation, and nutritional requirement tests (2). Identities of the isolates were confirmed by sequencing the internal transcribed spacer (ITS) region of ribosomal DNA. Genomic DNA was extracted by the phenol-chloroform-isoamyl alcohol method (28). Amplification of the complete ITS region was performed using universal primer pair ITS1 and ITS4 (ITS1, 5′ TCCGTAGGTGAACCTTGCGG 3′, and ITS 4, 5′ TCCTCCGCTTATTGATATGC 3′). Sequencing PCR was performed for both of the strands using the above-mentioned primers and BigDye Terminator Cycle sequencing kit version 3.1 (Applied Biosystems, Foster City, CA). All the sequencing reaction products were purified and analyzed on an ABI 3130 genetic analyzer (Applied Biosystems). Sequences were compared with the GenBank DNA database using the BLAST tool, the ISHAM ITS database, and the CBS database (https://blast.ncbi.nlm.nih.gov, http://its.mycologylab.org/BioloMICSSequences.aspx, and http://www.westerdijkinstitute.nl/Collections/BioloMICSSequences.aspx).
Antifungal susceptibility testing.
A panel of 12 commonly used topical or systemic antifungal agents were tested by the broth microdilution technique of Clinical and Laboratory Standards Institute (CLSI) protocol M38-A2 (29), with minor modifications. Fluconazole, voriconazole, ketoconazole, sertaconazole, clotrimazole, itraconazole, terbinafine, naftifine, amorolfine, ciclopirox olamine, and griseofulvin (from Sigma-Aldrich, Bengaluru, India) were used for antifungal susceptibility testing. Fluconazole was dissolved in distilled water, while all other antifungals were dissolved in dimethyl sulfoxide. The final concentrations of the antifungals tested ranged from 0.0625 to 32 μg/ml for fluconazole, 0.0312 to 16 for ketoconazole, clotrimazole, ciclopirox olamine, luliconazole, and naftifine, 0.0078 to 4 μg/ml for voriconazole, amorolfine, and itraconazole, 0.0156 to 8 μg/ml for terbinafine and sertaconazole, and 0.25 to 128 μg/ml for griseofulvin. For terbinafine isolates with MICs of ≥8 μg/ml, further dilutions up to 64 μg/ml were tested.
Inoculum suspension and quantification were done as described by Adimi et al. (30) The plates were incubated at 28°C, and readings were taken after 5 days. Endpoints of MICs for azoles, griseofulvin, and amorolfine were considered when they showed prominent inhibition of growth (approximately 80%) compared to that in growth control wells, while for terbinafine, naftifine, luliconazole, and ciclopirox olamine, 100% growth inhibition was noted. Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), and Aspergillus flavus (ATCC 204304) were included as quality control strains.
Sequencing of the squalene epoxidase gene.
Three sets of primers were designed to amplify the squalene epoxidase gene. The primers were designed from the reference sequence of Trichophyton rubrum CBS 118892 using Clone manager software. The primers designed and used include SE1aF (5′ CAGAGATAATGCAGCCATCG 3′), SE1aR (5′ CCGGATTGATGTTCCTAGGT 3′), SE2aF (5′ CCACCAGCGGCGAATATAGA 3′), SE2aR (5′ AGTCCAGTGCCAGACTGATG 3′), SE3aF (5′ AGTCTGGCACTGGACTCCAA 3′), and SE3aR (5′ ATGATGCAGCGACGGTGACA 3′) (Integrated DNA Technologies, Gurgaon, India). Optimized annealing temperatures for amplification with different primers include 53.9°C for SE1a and 55.3°C for SE2a and SE3a. Steps for sequencing of amplicons using respective primers were similar to those described above. Consensus and concatenation of the sequences were done using Bionumerics software (Applied Maths, Ghent, Belgium). Sequences were aligned and amino acid sequences were depicted using the ExPASy online tool (https://web.expasy.org/translate/).
3D homology model and effect of point mutation.
The protein three-dimensional (3D) structure of squalene epoxidase was predicted using I-TASSER, which followed the threading approach for structural modeling. Only the modeled structures having the highest confidence scores were considered significant. Models were evaluated by plotting Ramachandran plots using RAMPAGE (http://molprobity.biochem.duke.edu/). Further refinement of models was performed using ModRefiner (31). The effect of point mutation (F397L) on overall structural stability of protein was inferred by calculation of ΔΔG (Gibbs free energy). For this purpose, the ERIS server was used, which allowed induction of point mutation and calculated the ΔΔG for the same. The mutation was considered destabilizing when the ΔΔG was >0 and vice versa.
Data availability.
Sequences have been deposited in GenBank under accession numbers MG587085 to MG587093.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge financial support from the Indian Council of Medical Research in conducting this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02522-17.
REFERENCES
- 1.Kaur R, Panda PS, Sardana K, Khan S. 2015. Mycological pattern of dermatomycoses in a tertiary care hospital. J Trop Med 2015:157828. doi: 10.1155/2015/157828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitzman I, Corporis T, Favosa T, Summerbell RC, Corporis T, Favosa T. 1995. The dermatophytes. Clin Microbiol Rev 8:240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dogra S, Uprety S. 2016. The menace of chronic and recurrent dermatophytosis in India: is the problem deeper than we perceive? Indian Dermatol Online J 7:73–76. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta AK, Kohli Y. 2003. Evaluation of in vitro resistance in patients with onychomycosis who fail antifungal therapy. Dermatology 207:375–380. doi: 10.1159/000074118. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. 2003. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother 47:82–86. doi: 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne CS, Leitner I, Favre B, Neil S, Ryder NS. 2005. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother 49:2840–2844. doi: 10.1128/AAC.49.7.2840-2844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarifakioglu E, Seçkin D, Demirbilek M, Can F. 2007. In vitro antifungal susceptibility patterns of dermatophyte strains causing tinea unguium. Clin Exp Dermatol 32:675–679. doi: 10.1111/j.1365-2230.2007.02480.x. [DOI] [PubMed] [Google Scholar]
- 8.Osborne CS, Leitner I, Hofbauer B, Fielding CA, Favre B, Ryder NS. 2006. Biological, biochemical, and molecular characterization of a new clinical Trichophyton rubrum isolate resistant to terbinafine. Antimicrob Agents Chemother 50:2234–2236. doi: 10.1128/AAC.01600-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley MC, Leidich S, Isham N, Elewski BE, Ghannoum MA. 1999. Antifungal susceptibilities and genetic relatedness of serial Trichophyton rubrum isolates from patients with onychomycosis of the toenail. Mycoses 42:105–110. doi: 10.1111/j.1439-0507.1999.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Vinod S, Grover S, Dash K, Singh G. 2000. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol 66:238–240. [PubMed] [Google Scholar]
- 11.Mahalakshmi R, Apoorva R, Joshua J. 2017. Dermatophytosis: clinical profile and association between socio-demographic factors and duration of infection. Int J Res Dermatol 3:282–285. doi: 10.18203/issn.2455-4529.IntJResDermatol20172212. [DOI] [Google Scholar]
- 12.Bhagra S, Ganju S, Kanga A, Sharma N, Guleria R. 2014. Mycological pattern of dermatophytosis in and around Shimla hills. Indian J Dermatol 59:268. doi: 10.4103/0019-5154.131392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poluri L, Indugula J, Kondapaneni S. 2015. Clinicomycological study of dermatophytosis in South India. J Lab Physicians 7:84. doi: 10.4103/0974-2727.163135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Beena PM. 2003. Profile of dermatophyte infections in Baroda. Indian J Dermatol Venereol Leprol 69:281–283. [PubMed] [Google Scholar]
- 15.Surendran KAK, Bhat RM, Boloor R, Nandakishore B, Sukumar D. 2014. A clinical and mycological study of dermatophytic infections. Indian J Dermatol 59:262. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. 2017. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J Dermatol Venereol Leprol 83:436–440. doi: 10.4103/ijdvl.IJDVL_519_16. [DOI] [PubMed] [Google Scholar]
- 17.Ghannoum M. 2015. Azole resistance in dermatophytes: prevalence and mechanism of action. J Am Podiatr Med Assoc 106:79–86. doi: 10.7547/14-109. [DOI] [PubMed] [Google Scholar]
- 18.Hryncewicz-Gwóźdź A, Kalinowska K, Plomer-Niezgoda E, Bielecki J, Jagielski T. 2013. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 176:49–55. doi: 10.1007/s11046-013-9655-y. [DOI] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Favre B, Ryder NS. 1996. Characterization of squalene epoxidase activity from the dermatophyte Trichophyton rubrum and its inhibition by terbinafine and other antimycotic agents. Antimicrob Agents Chemother 40:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryder NS. 1992. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol 126(Suppl):2–7. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, Salamin K, Fratti M, Monod M. 2017. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother 61:e00115-17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha EMF, Gardiner RE, Park S, Martinez-Rossi NM, Perlin DS. 2006. A Phe389Leu substitution in ergA confers terbinafine resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 50:2533–2536. doi: 10.1128/AAC.00187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graminha MAS, Rocha EMF, Prade RA, Martinez-Rossi NM. 2004. Terbinafine resistance mediated by salicylate 1-monooxygenase in Aspergillus nidulans. Antimicrob Agents Chemother 48:3530–3535. doi: 10.1128/AAC.48.9.3530-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leber R, Fuchsbichler S, Klobucníková V, Schweighofer N, Pitters E, Wohlfarter K, Lederer M, Landl K, Ruckenstuhl C, Hapala I, Turnowsky F. 2003. Molecular mechanism of terbinafine resistance in Saccharomyces cerevisiae. Antimicrob Agents Chemother 47:3890–3900. doi: 10.1128/AAC.47.12.3890-3900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowosielski M, Hoffmann M, Wyrwicz LS, Stepniak P, Plewczynski DM, Lazniewski M, Ginalski K, Rychlewski L. 2011. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Infect Model 51:455–462. doi: 10.1021/ci100403b. [DOI] [PubMed] [Google Scholar]
- 27.Hay RJ, Ashbee HR. 2010. Mycology, p 1–93. InRook's textbook of dermatology. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 28.Baghela A, Thungapathra M, Shivaprakash MR, Chakrabarti A. 2010. Multilocus microsatellite typing for Rhizopus oryzae. J Med Microbiol 59:1449–1455. doi: 10.1099/jmm.0.023002-0. [DOI] [PubMed] [Google Scholar]
- 29.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. CLSI document M38-A2. CLSI, Wayne, PA. [Google Scholar]
- 30.Adimi P, Hashemi SJ, Mahmoudi M, Mirhendi H, Shidfar MR, Emmami M, Rezaei-Matehkolaei A, Gramishoar M, Kordbacheh P. 2013. In-vitro activity of 10 antifungal agents against 320 dermatophyte strains using microdilution method in Tehran. Iran J Pharm Res 12:537–545. [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Zhang Y. 2011. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J 101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences have been deposited in GenBank under accession numbers MG587085 to MG587093.