Abstract
Background
Head injury is an increasing contributor to death and disability, particularly amongst the elderly. Older patients are less likely to be treated at trauma centers, and head injury is the most common severe injury treated at non-trauma centers. We hypothesized that patients initially triaged to trauma centers would have lower rates of mortality and higher rates of discharge home without services than those treated at non-trauma centers.
Study Design
We used the State Emergency Department and Inpatient Databases for six states, 2011-2012 to conduct a retrospective cohort study of patients with severe, isolated head injury. Combined, these databases capture all visits to non-federal EDs. We compared in-hospital mortality and discharge status for all adults and for the subgroup aged ≥ 65 who initially presented to either a trauma center or a neurosurgery-capable non-trauma center. To account for selection bias, we used differential distance from patients’ homes to a trauma center as an instrumental variable and performed a multivariable matched analysis.
Results
Of 62,198 patients presented with severe, isolated head injury, 44.2% presented to non-trauma centers and 55.8% to trauma centers. In multivariable matched instrumental variable analysis, initial presentation to a trauma center was associated with no significant difference in overall mortality (−1.06%; 95% CI −3.36%, 1.19%) but a 5.8% higher rate of discharge home (95% CI 1.7-10.0%). Among patients aged ≥ 65, initial presentation to a trauma center was associated with a 3.4% reduction in mortality (95% CI 0.0%-7.1%).
Conclusions
Patients with isolated, severe head injury have better outcomes if initially treated in designated trauma centers. As 40% of such patients were triaged to non-trauma centers, there are major opportunities for improving outcomes.
Keywords: head injury, trauma, trauma systems, undertriage
Background
Between 2001 and 2010, emergency department visits and hospitalizations for head injury increased from 521 to 824 per 100,000 people. Head injury contributes to more than 50,000 deaths annually in the United States.(1) More than 80,000 of these injuries occur in older adults, primarily due to falls.(2) While trauma centers reduce relative risk of death for seriously injured patients by 25%,(3,4) little is known about which injury diagnoses benefit most from trauma center care. Non-trauma center hospitals treat more than a third of severely injured patients.(5,6) Head injury is the most common type of severe injury treated at non-trauma centers, accounting for 40% of undertriaged patients.(7,8) Older adults are even less likely to reach a trauma center.(9) Although trauma centers have on-call neurosurgeons, neurosurgical intervention (such as craniotomy or invasive intracranial pressure monitoring) is performed in <5% of head injured patients.(10) However, other characteristics of trauma centers might benefit head injured patients, as they have been shown to benefit from greater intensity of care,(11) adherence to treatment guidelines,(12) and in higher volume centers.(13)
In an Ohio study, creation of a regionalized trauma system resulted in increased rates of primary triage to a Level 1 trauma center, increased rates of neurosurgical intervention and a concomitant 28% reduction in mortality after severe head injuries.(14) However, it remains unknown whether initial triage to trauma center vs. a non-trauma center improves outcomes for severe, isolated head injury. This is a critical knowledge gap, as severe head injuries are increasingly prevalent in older adults, and older age is associated both with undertriage (15) and with a lesser benefit from trauma center care.(3) We hypothesized that patients with severe, isolated head injuries would have lower rates of mortality and higher rates of favorable discharge (home without services as opposed to requiring nursing care or inpatient rehabilitation) if initially triaged to a trauma center compared to those initially triaged to non-trauma centers with neurosurgical capabilities. Absent the ability to conduct a randomized trial, we designed and conducted an observational study to compare outcomes of initial treatment at a trauma center vs. non-trauma center among patients with severe isolated head injuries. Selection bias leads patients who are more severely sick or injured to be preferentially triaged to trauma centers, making this problem particularly difficult to study, and motivating the novel approach we use here. While measures of injury severity and comorbidities can partially mitigate selection bias, it is likely that factors, such as initial physiology, response to therapy, and frailness, may be known to the patient or EMTs making triage decisions but go unrecorded in our data set. Because these factors may have a major impact on the patient’s outcome, adjusting for observed variables alone is not adequate.(16,17) Our observational design was chosen to specifically to address measured and unmeasured confounding and parallel the design of a randomized trial as closely as possible by using a matched instrumental variable approach.(17–20) Instrumental variables can control for the correlation of these unobserved factors with both the treatment and the outcome variables.(17,21)
Methods
Data source
The Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) includes two databases that together represent all emergency department visits. The State Emergency Department Database (SEDD) includes visits to the ED that do not result in hospital admission. The State Inpatient Database (SID) includes all hospital admissions, regardless of whether they originate in the ED. Combining these databases allows us to examine all ED visits to nonfederal hospitals, whether or not they result in hospital admission. This analysis utilizes the HCUP revisit variables visitlink and daystoevent, which allow tracking of visits for the same patient across years and hospitals within a single state, allowing us to track the outcomes of transferred patients. We included data from 2011 and 2012 in six states: New Jersey, North Carolina, Arizona, New York, Florida, and Utah. We selected these 6 states because they consistently reported variables important to our analysis in their state-level databases. In particular, these states reported patient zip code of residence, which was key to our geographic analysis; and hospital identifiers that allowed us to distinguish trauma centers from non-trauma centers. This study was deemed exempt from review by the University of Pennsylvania Institutional Review Board.
Population
We identified all visits for severe, isolated head injury as those an abbreviated injury score (AIS) of ≥ 3 for the head and neck body region; an ICD-9 diagnosis code specifying head injury; and AIS for all other body regions ≤ 2. We excluded visits coded as late effects of injury or as complications of injury. Patients were included if they presented to a Level I or II trauma centers or to non-trauma centers with neurosurgical capabilities. Trauma center designation was derived from the Trauma Information Exchange Program.(22) Non-trauma centers were classified as neurosurgery-capable if they had performed neurosurgery on any trauma patient admitted through the emergency department in the database (see Appendix 1). Because we sought to inform the initial triage decision-making, patients who initially presented to a non-trauma center and were later transferred to a trauma center were included as non-trauma center patients, as in an intention-to-treat analysis. Figure 1 shows patient selection.
Figure 1.
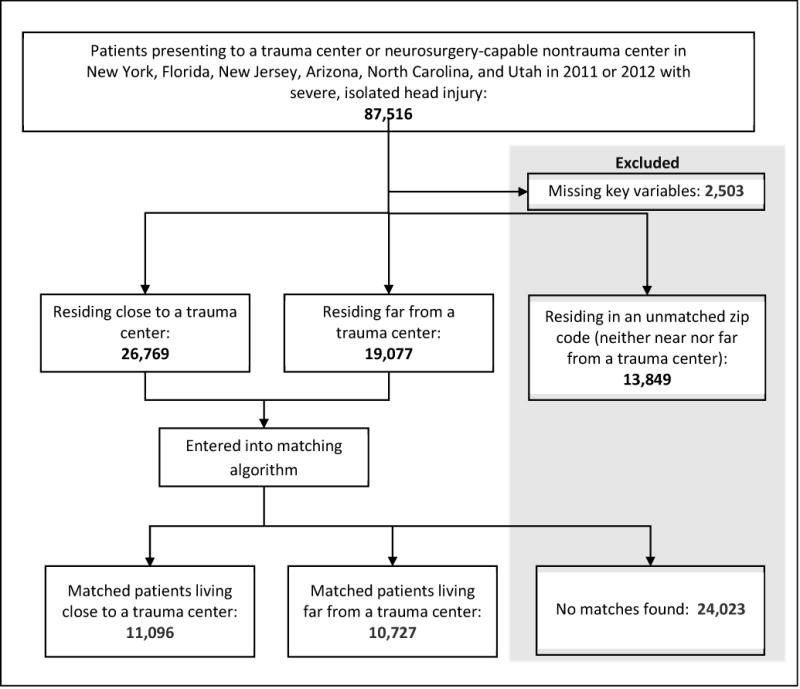
Patient selection.
Dependent Variables
The primary outcome variable was in-hospital mortality, including death after withdrawal of care. As variation in rates of withdrawal of care rates may reduce hospital-level mortality but increase the proportion discharged with poor functional status, we decided a priori to compare discharge status among survivors. The secondary outcome was favorable discharge, defined as discharge home without services (as opposed to discharge with nursing services or to a rehabilitation center, skilled nursing facility, or hospice). Analysis of the secondary outcome excluded patients whose point of origin prior to admission was a nursing home.
Independent variables
The primary predictor of interest was trauma center vs. non-trauma center treatment. Demographic covariates included patient age, sex and median household income of home zip code. Clinical covariates included the Elixhauser comorbidity index, a widely-used compendium of 30 comorbidities that are discoverable in administrative data and that have been shown to be associated with outcomes in hospitalized patients, (23) and type of head injury. Injury severity score (ISS) and body region abbreviated injury scores (AIS) were calculated from ICD-9 codes using validated methodology(24,25) and were incorporated into the model. We conducted a sensitivity analysis excluding patients with penetrating injuries.
Instrumental variable analysis
Standard methods for estimating causal effects, including regression and propensity score matching, rely on the assumption that there is no unmeasured confounding.(17,26,27) This assumption does not hold true in the case of triage of patients to trauma centers vs. non-trauma centers, as patients and field providers have information about patient condition that is not represented in administrative data, or even in clinical registries. In situations where there are important unmeasured confounders, instrumental variables (IV) can provide an unbiased estimate of treatment effect.(19,28) An IV is a pre-treatment variable that is associated with the treatment choice but is not otherwise associated with the outcome. Thus, the IV serves to approximate random assignment between treatment choices, and the estimate effect associated with the IV approximated the effect of treatment.
Geographic variation is a commonly-used instrumental variable, and has been validated in the trauma population.(16,29) Differential distance is strongly associated with hospital destination, but otherwise should not be related to injury severity, patient physiology, or any other determinant of outcome.(18,30) Based on prior research,(31) we approximate injury location by the patient’s home location, in turn approximated by the geographic centroid of his or her home zip code. We use the difference in road distance from this location to the nearest trauma center vs. to the nearest neurosurgery-capable non-trauma center as an instrumental variable. Driving distances were calculated in ArcMap (ESRI). We confirmed the validity of differential distance as an IV demonstrating the strong association initial presentation a trauma center but no correlation with important prognostic variables including age, sex, mechanism of injury, and injury severity.
To further minimize potential bias and ensure that observed clinical and demographic characteristics were well balanced according to treatment group, we used the optimal nonbipartite matching (32) algorithm to pair-match zip codes such that within each pair, zip codes were as similar as possible in terms of observed covariates, with one zip code located near to and the other far from a trauma center. Zip codes were divided into high, medium and low differential distance, cutting at the 30% point and 60% point and those with neither long nor short differential distance.(33) Optimal subset matching was used to pair the high and low zip codes on several types of covariates.(34) We tested the effect ratio using the method proposed by Hansen et al. for a clustered observational study.(35) We constructed confidence intervals by inverting the test, and point estimates correspond to the parameter value with highest p-value.(19) The final effect estimates capture the absolute risk difference in mortality of initial triage to a trauma center incorporating the potential benefit of trauma center care and any offsets to this benefit due to the longer transport distances to reach a trauma center. Statistical significance was based on 95 % confidence intervals, with a risk difference considered significant if the 95% confidence interval did not include 0.
Results
Among 62,198 patients with severe, isolated head injuries, 34,692 (55.8%) initially presented to trauma centers and 27,506 (44.2%) to neurosurgery-capable non-trauma centers. Trauma center patients were younger and less often white. They had more comorbidities on average and were more likely to live in urban areas. Mean ISS was 14 at both trauma centers and non-trauma centers. Patient characteristics are shown in Table 1.
Table 1.
Characteristics of Patients Presenting to Trauma Centers vs Non-Trauma Centers with Isolated, Severe Head Injury
| Characteristic | Non-trauma center, N= 26,829 | Trauma center, N= 34,022 | Standardized difference |
|---|---|---|---|
| Female | 12,580 (45.7) | 12,251 (35.32) | 0.21 |
| Age, y, median (IQR) | 74 (54-84) | 59 (39-78) | 0.46 |
| Ethnicity, n (%) | |||
| White | 19,953 (75.1) | 22,920 (68.3) | 0.15 |
| Black | 2,281 (8.6) | 4,272 (12.7) | 0.14 |
| Hispanic | 2,861 (10.8) | 3,735 (11.1) | 0.01 |
| Asian | 498 (1.9) | 720 (2.2) | 0.11 |
| Native American | 70 (0.3) | 437 (1.3) | −0.15 |
| Other | 890 (3.4) | 1,452 (4.3) | 0.05 |
| No. of Elixhauser comorbidities, median (IQR) | 2 (1-3) | 1 (1-3) | 0.21 |
| Insurance, n (%) | |||
| Medicare | 16,450 (61.4) | 13,466 (39.7) | 0.46 |
| Medicaid | 1,718 (6.4) | 3,582 (10.6) | 0.15 |
| Private | 5,152 (19.2) | 9,665 (28.5) | 0.22 |
| Uninsured/other | 3,481 (13.0) | 7,252 (21.4) | 0.22 |
| Quartile of median household income by zip code, median (IQR) | 2 (2-3) | 2 (1-4) | 0.04 |
| Injury severity score, median (IQR) | 14 (10-16) | 14 (10-17) | 0.01 |
| Head/neck AIS, median (IQR) | 4 (3-4) | 3 (3-4) | 0.18 |
| Type of injury, n (%) | |||
| Subarachnoid hemorrhage | 3,439 (12.8) | 4,948 (14.5) | 0.05 |
| Subdural hemorrhage | 9,579 (35.7) | 8,165 (24.0) | 0.26 |
| Epidural hemorrhage | 158 (0.6) | 245 (0.7) | 0.02 |
| Cerebral contusion or laceration | 2,766 (10.3) | 3,735 (11.0) | 0.02 |
| Other intracranial injury | 2,642 (9.9) | 2,206 (6.5) | 0.12 |
| Skull fracture | 6,518 (24.3) | 11,795 (34.7) | 0.23 |
| Multiple injuries | 1,727 (6.4) | 2,928 (8.6) | 0.03 |
| Cause of injury, n (%) | |||
| Blunt injury | 23,355 (99.2) | 29,189 (97.0) | 0.03 |
| Fall | 18,207 (66.2) | 17,266 (50.0) | |
| Penetrating injury | 185 (0.8) | 912 (3.0) | 0.15 |
| Open injury, n (%) | 94 (0.4) | 182 (0.5) | 0.03 |
| No. of head injuries in zip code, median (IQR) | 34 (20-51) | 30 (17-48) | 0.74 |
| Location, n (%) | |||
| Large metropolitan area | 16,113 (60.1) | 20,747 (61.0) | 0.02 |
| Small metropolitan area | 9,722 (36.2) | 10,326 (30.4) | 0.13 |
| Micropolitan area | 756 (2.8) | 2,064 (6.1) | 0.16 |
| Rural | 238 (0.9) | 885 (2.6) | 0.12 |
| State, n (%) | |||
| New York | 6,164 (23.0) | 9,571 (28.1) | 0.12 |
| Florida | 10,750 (40.1) | 8.201 (24.1) | 0.35 |
| New Jersey | 3,627 (13.5) | 5,037 (14.8) | 0.04 |
| Utah | 874 (3.3) | 739 (2.2) | 0.06 |
| North Carolina | 3,696 (13.8) | 6,427 (18.9) | 0.13 |
| Arizona | 1,718 (6.4) | 4,047 (11.9) | 0.19 |
AIS, abbreviated injury score; IQR, interquartile range.
Instrumental variable
Median differential distance was 4.1 miles (interquartile range 0.7 – 14.0 miles). Differential distance was strongly associated with presentation to a trauma center vs. non-trauma center: 75.9% of patients living closer to trauma centers presented to trauma centers, compared to 55.2% of those living closer to non-trauma centers. Key patient characteristics were similar across quartiles of the instrumental variable (Table 2). Zip codes according to differential distances from a trauma center are shown in Figure 2.
Table 2.
Patient Characteristics Based on Distance from Home Zip Code to the Nearest Trauma Center
| Characteristic | Quartiles of differential distance | |||
|---|---|---|---|---|
| 1, TC 22.6 miles closer to 0.7 miles further than NTC | 2, TC 0.7-4.1 miles further than NTC | 3, TC 4.1-14.0 miles further than NTC | 4, TC 14.0-259.4 miles further than NTC | |
| Female, % | 37 | 41 | 42 | 40 |
| Age.y, median (IQR) | 62 (42-80) | 67 (45-82) | 69 (47-82) | 66 (44-81) |
| Injury severity score, median (IQR) | 16 (10-16) | 16 (10-16) | 16 (10-16) | 16 (10-17) |
| Head/neck AIS, median (IQR) | 3 (3-4) | 3 (3-4) | 3 (3-4) | 3 (3-4) |
| Injured by fall, % | 57.4 | 60.2 | 61.0 | 55.3 |
AIS, abbreviated injury score; IQR, interquartile range, NTC, non-trauma center; TC, trauma center.
Figure 2.
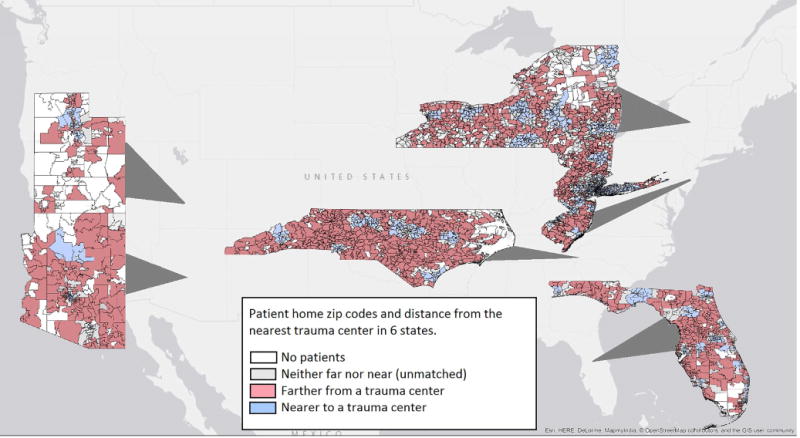
Home zip codes of patients with severe, isolated head injury presenting to a trauma center or neurosurgery-capable non-trauma center in 6 states, 2011-2012.
Optimal subset matching
Matches were found for 10,727 patients living near to a trauma center to 11,096 living far from a trauma center. We excluded 13,849 patients residing in 712 zip codes with intermediate differential distances, as well as 2,503 missing key variables and 24,023 for whom no match could be found. The multivariable match produced well-balanced treatment groups with standardized differences <0.1 for all variables (Table 3).
Table 3.
Covariate Balance after Multivariable Optimal Subset Matching
| Characteristic | Far from trauma center, N=10,727 | Close to trauma center, N=11,096 | Standardized difference after matching |
|---|---|---|---|
| Female, n (%) | 4,294 (40.0) | 4,671 (40.9) | 0.04 |
| Age, y, median (IQR) | 61 (55-69) | 62 (56-70) | 0.07 |
| Ethnicity, n (%) | |||
| White | 8,815 (82.0) | 9,045 (82.0) | 0.02 |
| Black | 677 (6.3) | 804 (7.3) | 0.05 |
| Hispanic | 845 (7.8) | 788 (6.7) | 0.06 |
| Asian | 126 (1.2) | 96 (1.0) | 0.06 |
| Native American | 40 (0.3) | 71 (0.6) | 0.03 |
| Other | 222 (2.0) | 290 (2.6) | 0.07 |
| No. of Elixhauser comorbidities, median (IQR) | 2 (1-3) | 2 (1-3) | 0.01 |
| Insurance, n (%) | |||
| Medicare | 5,042 (47.5) | 5,332 (48.5) | 0.04 |
| Medicaid | 741 (6.9) | 820 (7.4) | 0.03 |
| Private | 3,129 (29.2) | 3,224 (29.1) | 0.01 |
| Uninsured/other | 1,813 (16.9) | 1,718 (15.5) | 0.05 |
| Quartile of median household income by zip code, median (IQR) | 3 (2-4) | 3 (2-4) | 0.05 |
| Injury severity score, median (IQR) | 14 (13-15) | 14 (13-15) | 0.01 |
| Head/neck AIS, median (IQR) | 3 (3-4) | 3 (3-4) | 0.01 |
| Type of injury, n (%) | |||
| Subarachnoid hemorrhage | 1,482 (13.8) | 1,486 (13.4) | 0.02 |
| Subdural hemorrhage | 2,997 (27.9) | 3,180 (28.7) | 0.03 |
| Epidural hemorrhage | 33 (0.3) | 43 (0.4) | 0.03 |
| Cerebral contusion or laceration | 1,256 (11.7) | 1,224 (11.0) | 0.04 |
| Other intracranial injury | 863 (8.0) | 923 (8.3) | 0.02 |
| Skull fracture | 3,231 (30.6) | 3,354 (30.3) | 0.01 |
| Multiple injuries | 215 (2.0) | 333 (3.0) | 0.07 |
| Cause of injury, n (%) | |||
| Blunt injury | 9,581 (89.3) | 10,074 (90.8) | 0.09 |
| Falls | 6,217 (58.0) | 6,652 (59.9) | 0.07 |
| Penetrating injury | 198 (1.8) | 150 (1.4) | 0.06 |
| Open injury, n (%) | 28 (0.4) | 55 (0.5) | 0.03 |
| No. of head injuries in zipcode, median (IQR) | 11 (4-25) | 13 (5-27) | 0.03 |
| Location, n (%) | |||
| Large metropolitan area | 5,041 (47.0) | 5,174 (46.6) | 0.01 |
| Small metropolitan area | 5,319 (50.0) | 5,542 (49.9) | 0.01 |
| Micropolitan area | 227 (2.1) | 289 (2.6) | 0.02 |
| Rural | 140 (1.3) | 90 (0.8) | 0.02 |
| State, n (%) | |||
| New York | 3,418 (32.0) | 3,951 (36.0) | 0.04 |
| Florida | 3,087 (28.8) | 2,958 (26.7) | 0.05 |
| New Jersey | 1,761 (16.0) | 1,786 (16.0) | 0.00 |
| Utah | 279 (2.6) | 289 (2.6) | 0.00 |
| North Carolina | 1,657 (15.5) | 1,569 (14.1) | 0.00 |
| Arizona | 523 (4.9) | 541 (4.9) | 0.00 |
AIS, abbreviated injury score; IQR, interquartile range.
Unadjusted analysis
The overall mortality rate was 7.5% (95% CI 7.3-7.8%). Unadjusted morality was 8.7% (95% CI 8.4-9.0%), at a total of 89 trauma centers and 5.9% (95% CI 5.6-6.1%) at 213 non-trauma centers. When limited to the sample that could be matched, unadjusted mortality was 5.9% (95% CI 5.5-6.2%) in non-trauma centers and 8.8% (95% CI 8.4-9.1%) in trauma centers.
Matched, instrumental variable analysis
In matched, instrumental variable analysis there was no difference in mortality for patients presenting to a trauma center vs. neurosurgery-capable non-trauma (−1.1%; 95% CI −3.4%, 1.2%) (Table 4). However, patients presenting to trauma centers had a 5.8% higher rate of favorable discharge (95% CI 1.7-10.0%). These effects were similar for patients under age 65: there was no difference in mortality (0.9%; 95% CI −2.7, 4.6%) and a significant increase in favorable discharge (6.9%, 95% CI 0.8-13.2%). For patients 65 and older, trauma center presentation was associated with a significant 3.4% decrease in mortality (95% CI −7.1%, 0.0%), and a nonsignificant decrease in favorable discharge (−2.9%; 95% CI: −8.8, 3.2%). Results were similar when we excluded patients with penetrating injuries, with an overall mortality difference of 1.3% (95% CI −3.6, 0.9).
Table 4.
Impact of Initial Presentation to a Trauma Center vs Neurosurgery-Capable Non-Trauma Center on Mortality and Favorable Discharge
| Death | Discharge home without services | |||
|---|---|---|---|---|
| Absolute risk difference, % | 95% CI | Absolute risk difference, % | 95% CI | |
| Total population | −1.1 | −3.4, 1.2 | 5.8 | 1.7, 10.0 |
| Age < 65 y | 0.9 | −4.6, 2.7 | 6.9 | 0.8, 13.2 |
| Age ≥ 65 y | −3.4 | −7.1, 0.0 | − 2.9 | −8.8, 3.2 |
Discussion
This is the largest study on the comparative effectiveness of trauma center vs. non-trauma center care for patients with head injuries, including patients treated in 89 trauma centers and 213 non-trauma centers across 6 diverse states. Of 62,198 patients with severe, isolated head injuries, more than 2 in 5 initially presented to a non-trauma center. In this novel analysis designed to parallel a randomized trial, we matched patient cohorts based on relative distance from home residence to the closest trauma center vs. neurosurgery-capable non-trauma center to account for the selection bias that leads sicker patients to present to trauma centers. We identified a significant, 3.4% reduction in mortality in patients aged ≥ 65 triaged to trauma centers. This is particularly important as these patients are least likely to present to trauma centers. We found evidence of improved functional outcomes in patients under age 65, as overall rates of discharge home, rather than to skilled nursing facility or long-term care, were significantly better in the trauma center group, although this benefit was limited to patients under age 65. These advantages were found even though trauma centers had higher unadjusted mortality rates, likely indicating higher severity of illness and injury undetected by observed covariates such as ISS. As traumatic brain injury is a leading cause of long-term disability, our findings indicates a major opportunity to improve functional outcomes at the population level through the improved prehospital triage and transport of severely head injured younger adults to trauma centers. The instrumental variable estimate includes the potential benefit of trauma center care, and incorporates any offsets due to longer transport time. Therefore, our analysis closely parallels the outcome of real-world triage decision-making. Further, we expected our estimates of the benefit of triage to a trauma center to be conservative since we did not include patients triaged to non-trauma centers without neurosurgical capabilities.
The advantages offered by trauma center care may be related to intensity of treatment, experience, or coordination of care. Trauma centers have staff, resources, and protocols designed to care for injured patients. On arrival, patients are generally taken to the trauma bay where a team evaluates their injuries immediately and facilitates imaging and intervention.(36) At a non-trauma center, similar patients might be roomed in the ED and await routine evaluation. Although we compared trauma centers to non-trauma centers that performed neurosurgical procedures on trauma patients, it is likely that neurosurgical care was more readily available at trauma centers. Trauma center designation requires 24/7 neurosurgical coverage and regulates response times in most states. Trauma centers may also be better positioned to optimize care for head injury. For example, older adults are more likely to be on anticoagulants or antiplatelet agents, and trauma centers are more likely to have protocols for delivering blood products and medications to reverse coagulopathies.(37) While trauma centers may be more likely to move families toward early withdrawal of care for those with poor prognoses, this has not been associated with overall higher mortality after traumatic brain injury.(38)
Previous studies on trauma center vs. non-trauma center care in the U.S. have been limited to single states,(39) have been underpowered to detect differences by injury type,(3,6) or have compared different levels of trauma center designation.(16) Using emergency department and inpatient administrative data from multiple states remains one of the only ways to assemble a well-powered population-based cohort to assess the outcomes of trauma center vs. non-trauma center care. A significant strength of our study design is explicitly addressing unobserved confounding through an instrumental variable analysis while also limiting the analysis to a well-matched cohort. Using these methods allows for the closest estimate to a causal effect given the logistical and financial barriers to conducting a prospective randomized control trial.
Given that 2 in 5 patients severely head injured patients presented initially to non-trauma centers which offer inferior survival outcomes for older adults and inferior functional outcomes for younger adults, there is an opportunity to improve population-level outcomes through improving both prehospital triage and care at non-trauma centers. The Centers for Disease Control and Prevention and American College of Surgeons-Committee on Trauma field triage guidelines could be revised to recommend trauma center triage for anyone with an abnormal Glasgow Coma Scale (14 or less), rather than the current cutoff of 13. A recent study using older data on trauma center outcomes found that such a revision would increase overtriage of minor injuries to a cost-ineffective degree,(40) but our newer effect estimates might change this calculus. Novel means to identify head injuries in the field, such as point-of-care biomarkers, may improve triage.(41) Further study is needed to determine how to improve outcomes at non-trauma centers when triage to a trauma center is not feasible.
Geography may pose a barrier to trauma center access, and for 5% of patients, a trauma center was more than 50 miles further than a non-trauma center. Triaging these patients to a trauma center could result in unacceptable increases in prehospital time, a key contributor to mortality.(42) However, EMS transport favors the non-trauma center even when a trauma center is just 3 miles further.(29) At these short distances, the tradeoff in prehospital time is likely acceptable. In addition, EMS provider intuition play a major role in triage,(43) and head injury may be challenging to recognize in the field. Patient preference also contributes to triage destination, particularly for older patients.(44)
Mortality is the easiest outcome to identify but may not be the most important to patients with head injuries. Our secondary outcome was favorable discharge, and we found a significant improvement in this outcome for adults under 65 at trauma centers. For older adults, there was a nonsignificant decrease in favorable discharge. This suggests that some older adults who survived at a trauma center but might not have survived at a non-trauma center experienced diminished functional capacity post-injury. Hutchinson et al. studied decompressive craniectomy for intracranial hemorrhage and found improved survival associated with increases in patients with severe, moderate and mild disability.(45) We are unable to levels of impairment in the current study, or to identify patients’ ultimate functional recovery. Future research on head trauma should incorporate patient-centered outcomes such as functional status.
We acknowledge several limitations to this study. We cannot directly assess whether patients met trauma triage criteria and can only approximate this using post-hoc ISS. As with any study using administrative data, potential for miscoding exists, and trauma centers may be more attentive to coding of injury diagnoses. To construct our instrumental variable, we used patient home zip code, as location of injury was not available. Home is a good proxy for injury location, particularly in falls, the most common mechanism of injury here.(31) Moreover, trauma centers and hospitals are not evenly distributed around the country, and travel time per mile may differ by location. Patients and EMS teams on the ground likely have local knowledge that influences hospital choice. A spatial statistics approach, while beyond the scope of this manuscript, might yield additional insight into these issues. We matched zip codes by state, which attenuates this problem to the extent that traffic patterns are similar within any given state. Future research may benefit from measuring real-time travel conditions using mobile applications. We have used state-of-the-art analysis methods to control for selection bias in which patients are triaged to trauma centers vs. non-trauma centers. We see no evidence of a systematic difference in patients residing at various differential distances, but if this assumption were violated our results would be biased. Instrumental variable methods measure treatment effectiveness for a marginal population of patients whose treatment would have varied based on a change in the instrument. Some patients would have gone to a trauma center or non-trauma center regardless of distance, and may not experience the same treatment effect estimated here. We excluded from analysis patients residing in zip codes that were neither near nor far from trauma centers, in order to maintain a strong instrumental variable. (33) We also excluded patients residing in zip codes that could not be matched. These patients may have differed from the analyzed population, limiting generalizability. We included patients who were transferred from a non-trauma center to a trauma center in the non-trauma center group, in order to test the effect of initial triage. If these patients benefit from transfer, this may bias our results toward the null, yielding conservative results analogous to an intention-to-treat analysis. However, excluding transfers out of non-trauma centers could bias results in favor of non-trauma centers by excluding patients at high risk of mortality. The optimal role and timing of transfers in this patient population merits further research. With regard to outcomes measures, we rely on inpatient mortality and are unable to account for longer-term mortality. Likewise, we do not have any clear way of discerning the indication for nursing services at discharge. While injuries with AIS of ≤2 are unlikely to require nursing care, they might, and it is possible that this differs systematically between trauma centers and non-trauma centers, introducing bias.
Conclusions
More than 40% of patients with isolated, severe head injuries presented to non-trauma centers. Although patients ≥ 65 were least likely to present to a trauma center, they experienced decreased mortality at trauma centers. Furthermore, we found evidence of improved functional outcomes with initial treatment in trauma centers among adults < 65. Efforts to improve the care of patients with head injury should optimize protocols that allow field providers to identify patients who will benefit from trauma center care, but may also need to incorporate quality improvement at non-trauma centers.
Acknowledgments
Dr Holena was supported by the National Heart, Lung and Blood Institute award K08HL131995; Dr Delgado was supported by the National Institute on Aging award R03AG052117 and the National Institute for Child Health and Development award K23HD090272.
Appendix 1
Definition of neurosurgery-capable non-trauma centers
To identify those non-trauma centers capable of performing neurosurgery for trauma, we identified all patients admitted to a non-trauma center with a diagnosis of injury (ICD-9 CM codes 800-995). Within this population, we used ICD-9 CM procedure codes to identify those undergoing neurosurgical procedures (Table A-1). As hospitals might have neurosurgical services available for elective procedures that would not be available in cases of trauma, only trauma patients were included. As practitioners might code procedures done for trauma in a variety of ways, we made our list of procedures inclusive. Brain biopsies and excisions were excluded, as were placement and removal of neurostimulator leads.
| Procedure | Code |
|---|---|
| Intracranial pressure, oxygen or temperature monitoring | 01.10, 01.16, 01.17 |
| Cranial implantation or replacement of neurostimulator pulse generator | 01.20 |
| Incision and drainage of cranial sinus | 01.21 |
| Reopening of craniotomy site | 01.23 |
| Other craniotomy | 01.24 |
| Other craniectomy | 01.25 |
| Insertion of catheter(s) into cranial cavity or tissue | 01.26 |
| Removal of catheter(s) from cranial cavity or tissue | 01.27 |
| Placement of intracerebral catheter(s) via burr hole(s) | 01.28 |
| Other incision of brain | 01.39 |
| Elevation of skull fracture fragments | 02.02 |
| Formation of cranial bone flap | 02.03 |
| Bone graft to skull | 02.04 |
| Insertion of skull plate | 02.05 |
| Other cranial osteoplasty | 02.06 |
| Removal of skull plate | 02.07 |
| Simple suture of dura mater of brain | 02.11 |
| Other repair of cerebral meninges | 02.12 |
| Ligation of meningeal vessel | 02.13 |
| Insertion or replacement of external ventricular drain [EVD] | 02.21 |
| Ventricular shunt | 02.22, 02.31-02.39 |
| Revision or removal of shunt | 02.41-02.43 |
| Lysis of cortical adhesions | 02.91 |
| Repair of brain | 02.92 |
| Other operations on skull, brain and cerebral meninges | 02.99 |
Bipartite Matching
We used bipartite matching to strengthen the instrumental variable while balancing observed covariates. (1) Zip codes were divided into high, medium and low differential distance (i.e., instrument), cutting at the 30% point and 60% point. We discarded patients residing in zip codes with neither long nor short excess travel times (i.e., the medium group), consistent with the findings of Ertefaie et. al. that strengthening an instrument at the price of a reduced sample size leads to considerable efficiency gain.(2) We then created a distance matrix where each element in the matrix is calculated using the rank-based Mahalanobis distance. This distance quantifies the difference in patient covariates between each zip code categorized as high to each zip code categorized as low. For example, for L and M zip codes in high and low groups, respectively, we constructed an L × M distance matrix.
To improve the covariate balance between matched zip codes, we then discarded some zip codes that were excessively different from other zip codes (i.e., the corresponding distance value was high). We did this by adding some q columns to the constructed distance matrix with a constant distance value λ. We then used the pairmatch function of Hansen’s (2007) optmatch package in R to optimal match high and low zip codes using the constructed pseudo-distance matrix.(3) Zip codes that were matched to the inserted q columns represent zip codes that couldn’t be matched to the real zip codes. These unmatched zip codes were then discarded. This procedure involves two tuning parameters q and λ. We repeated the above procedure for different values of these tuning parameters in order to choose parameters that yielded the best matched pairs (i.e., pairs with standardized difference below 0.10 for all covariates).
Software implementation
We provide a generic R code for bipartite matching. The zip code level patient characteristics are denoted as a vector X. In our application, X includes all the covariates listed in Table 1. Variables “zip” and “diff_dist” denote the patient zip code and the excess travel distance, respectively. We first eliminated patients residing in zip codes with neither long nor short excess travel distances, as above, and constructed our binary instrumental variable (IV).
> q<-quantile(diff_dist, prob=c(.3,.6))
> IV<-cut(diff_dist, breaks=c(−1000, q[1], q[3]−0.001,1000), labels=c(0,2,1))
> IV[IV==1]<−1 # near trauma center
> IV[IV==3]<−0 # far from trauma center
> IV[IV==2]<-NA
Next, we calculated the distance matrix for zip codes that are either far or near a trauma center (i.e., ignore those with IV=2).
> library(optmatch)
> distmat <-match_on(IV ~ X, data = dat, method=“rank_mahalanobis”)
Next, we added the extra columns to the constructed distance matrix and perform pairmatching,
> extra.col<-matrix(lam, ncol=q, nrow=nrow(distmat2))
> distmat.new<-cbind(distmat2, extra.col)
> matchvec=pairmatch(distmat.new)
Finally, we discarded pairs in which a treated unit is matched with a column of matrix “extra.col”. The procedure described above performs the bipartite matching introduced in Yang et. al.(1)
- 1.Yang F, Zubizarreta JR, Small DS, et al. Dissonant Conclusions when testing the validity of an instrumental variable. Am Stat. 2014;68:253–263. [Google Scholar]
- 2.Ertefaie A, Small DS, Rosenbaum PR. Quantitative evaluation of the trade-off of strengthened instruments and sample size in observational studies. J Am Stat Assoc. 2017 in press. [Google Scholar]
- 3.Hansen BB, Fredrickson M, Buckner J, et al. Functions for optimal matching. Ann Arbor, MI: 2016. Available at: https://cran.r-project.org/web/packages/optmatch/index.html. Accessed January 30, 2018. [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Society for Academic Emergency Medicine, Orlando, FL, May 2017.
Disclosure Information: Nothing to disclose.
References
- 1.U.S. Centers for Disease Control and Prevention. TBI: Get the Facts. Atlanta, GA: Available at: http://www.cdc.gov/traumaticbraininjury/get_the_facts.html. Accessed May 5, 2016. [Google Scholar]
- 2.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: Epidemiology, Outcomes, and Future Implications. J Am Geriatr Soc. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKenzie EJ, Rivara EJ, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 4.Newgard CD, McConnell KJ, Hedges JR, Mullins RJ. The benefit of higher level of care transfer of injured patients from nontertiary hospital emergency departments. J Trauma Inj Infect Crit Care. 2007;63:965–971. doi: 10.1097/TA.0b013e31803c5665. [DOI] [PubMed] [Google Scholar]
- 5.Nathens AB, Maier RV, Brundage SI, et al. The effect of interfacility transfer on outcome in an urban trauma system. J Trauma Inj Infect Crit Care. 2003;55:444–449. doi: 10.1097/01.TA.0000047809.64699.59. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie EJ, Steinwachs DM, Ramzy AI. Evaluating performance of statewide regionalied systems of trauma care. J Trauma. 1990;30:681–688. doi: 10.1097/00005373-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Delgado MK, Yokell MA, Staudenmayer KL, et al. Factors associated with the disposition of severely injured patients initially seen at non–trauma center emergency departments: disparities by insurance status. JAMA Surg. 2014;149:422. doi: 10.1001/jamasurg.2013.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassar MJ, Holcroft JJ, Knudson MM, Kizer KW. Fractures in access to and assessment of trauma systems. J Am Coll Surg. 2003;197:717–725. doi: 10.1016/S1072-7515(03)00749-X. [DOI] [PubMed] [Google Scholar]
- 9.Xiang H, Wheeler KK, Groner JI, et al. Undertriage of major trauma patients in the US emergency departments. Am J Emerg Med. 2014;32:997–1004. doi: 10.1016/j.ajem.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Esposito TJ. Neurosurgeons, acute care surgeons or moms: who should care for the head injured? . Trauma Treat. 2012;6:137–142. [Google Scholar]
- 11.Thompson HJ, Rivara FP, Jurkovich GJ, et al. Evaluation of the effect of intensity of care on mortality after traumatic brain injury. Crit Care Med. 2008;36:282–290. doi: 10.1097/01.CCM.0000297884.86058.8A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber LM, Chiu Y-L, Carney N, et al. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119:1583–1590. doi: 10.3171/2013.8.JNS13276. [DOI] [PubMed] [Google Scholar]
- 13.Clement RC, Carr BG, Kallan MJ, et al. Volume-outcome relationship in neurotrauma care. J Neurosurg. 2013;118:687–693. doi: 10.3171/2012.10.JNS12682. [DOI] [PubMed] [Google Scholar]
- 14.Kelly ML, Banerjee A, Nowak M, et al. Decreased mortality in traumatic brain injury following regionalization across hospital systems. J Trauma Acute Care Surg. 2015;78:715–720. doi: 10.1097/TA.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 15.Gomez D, Haas B, Doumouras AG, et al. A population-based analysis of the discrepancy between potential and realized access to trauma center care. Ann Surg. 2013;257:160–165. doi: 10.1097/SLA.0b013e31827b9649. [DOI] [PubMed] [Google Scholar]
- 16.McConnell KJ, Newgard CD, Mullins RJ, et al. Mortality benefit of transfer to level I versus level II trauma centers for head-injured patients. Health Serv Res. 2005;40:435–457. doi: 10.1111/j.1475-6773.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agoritsas T, Merglen A, Shah ND, et al. Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA. 2017;317:748. doi: 10.1001/jama.2016.20029. [DOI] [PubMed] [Google Scholar]
- 18.Neuman MD, Rosenbaum PR, Ludwig JM, et al. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311:2508. doi: 10.1001/jama.2014.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–2340. doi: 10.1002/sim.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–278. doi: 10.1542/peds.2011-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29:722–729. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 22.Agency for Healthcare Research and Quality. HCUP Supplemental Variables for Revisit Analyses. Rockville, MD: Available at: https://www.hcup-us.ahrq.gov/toolssoftware/revisit/revisit.jsp. Accessed May 12, 22016. [Google Scholar]
- 23.American Trauma Society. Trauma Information Exchange Program (TIEP) Falls Church, VA: Available at: http://www.amtrauma.org/?page=TIEP. Accessed September 20, 2016. [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris RD, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Clark DE, Osler TM, Hahn DR. ICDPIC: Stata module to provide methods for translating International Classification of Diseases (Ninth Revision) diagnosis codes into standard injury categories and/or scores. Boston, MA: Available at: https://ideas.repec.org/c/boc/bocode/s457028.html. Accessed May 15, 2016. [Google Scholar]
- 26.Greene NH, Kernic MA, Vavilala MS, Rivara FP. Validation of ICDPIC software injury severity scores using a large regional trauma registry. Inj Prev. 2015;21:325–330. doi: 10.1136/injuryprev-2014-041524. [DOI] [PubMed] [Google Scholar]
- 27.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 29.Delgado MK, Nova J, DeLia D. Validation of travel distance as an instrumental variable for evaluating the effectiveness of regionalized trauma care. Acad Emerg Med. 2016;23:S7–276. [Google Scholar]
- 30.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas B, Doumouras AG, Gomez D, et al. Close to home: An analysis of the relationship between location of residence and location of injury. J Trauma Acute Care Surg. 2015;78:860–865. doi: 10.1097/TA.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baiocchi M, Small DS, Lorch S, Rosenbaum PR. Building a stronger instrument in an observational study of perinatal care for premature infants. J Am Stat Assoc. 2010;105:1285–1296. [Google Scholar]
- 33.Ertefaie A, Small DS, Rosenbaum PR. Quantitative evaluation of the trade-off of strengthened instruments and sample size in observational studies. J Am Stat Assoc. 2017 in press. [Google Scholar]
- 34.Rosenbaum PR. Optimal matching of an optimally chosen subset in observational studies. J Comput Graph Stat. 2012;21:57–71. [Google Scholar]
- 35.Hansen BB, Rosenbaum PR, Small DS. Clustered treatment assignments and sensitivity to unmeasured biases in observational studies. J Am Stat Assoc. 2014;109:133–144. [Google Scholar]
- 36.American College of Surgeons, editor. Advanced trauma life support: ATLS ; student course manual. 9. Chicago, Ill: American College of Surgeons; 2012. p. 366. [Google Scholar]
- 37.Nishijima DK, Gaona SD, Waechter T, et al. Out-of-hospital triage of older adults with head injury: a retrospective study of the effect of adding “anticoagulation or antiplatelet medication use” as a criterion. Ann Emerg Med. 2016;70:127–138, e6. doi: 10.1016/j.annemergmed.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCredie VA, Alali AS, Xiong W, et al. Timing of withdrawal of life-sustaining therapies in severe traumatic brain injury: impact on overall mortality. J Trauma Acute Care Surg. 2016;80:484–491. doi: 10.1097/TA.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 39.Staudenmayer K, Weiser TG, Maggio PM, et al. Trauma center care is associated with reduced readmissions after injury. J Trauma Acute Care Surg. 2016;80:412–418. doi: 10.1097/TA.0000000000000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newgard CD, Yang Z, Nishijima D, et al. Cost-effectiveness of field trauma triage among injured adults served by emergency medical services. J Am Coll Surg. 2016;222:1125–1137. doi: 10.1016/j.jamcollsurg.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hack D, Huff JS, Curley K, et al. Increased prognostic accuracy of TBI when a brain electrical activity biomarker is added to loss of consciousness (LOC) Am J Emerg Med. 2017 Feb 20; doi: 10.1016/j.ajem.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 42.Branas CC, MacKenzie EJ, Williams JC, et al. Access to trauma centers in the United States. JAMA. 2005;293:2626. doi: 10.1001/jama.293.21.2626. [DOI] [PubMed] [Google Scholar]
- 43.Newgard CD, Nelson MJ, Kampp M, et al. Out-of-hospital decision making and factors influencing the regional distribution of injured patients in a trauma system. J Trauma. 2011;70:1345–1353. doi: 10.1097/TA.0b013e3182191a1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newgard CD, Mann NC, Hsia RY, et al. Patient choice in the selection of hospitals by 9-1-1 emergency medical services providers in trauma systems. Acad Emerg Med. 2013;20:911–919. doi: 10.1111/acem.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–1130. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]


