Abstract
Sclerostin is a 190 amino acid protein secreted primarily by osteocytes. It was initially identified due to mutations in the SOST gene associated with high bone mass phenotypes. Much recent work has sought to determine the importance of sclerostin across an array of conditions which affect the human skeleton. However, accurate measurement of sclerostin from serum and plasma sources remains a significant impediment, with currently available commercial assays showing marked differences in measured sclerostin values. Accordingly, sclerostin assay standardization remains an important but unmet need before sclerostin measurements can be used for the clinical management of bone disease. Here we characterize a novel automated chemiluminescent sclerostin assay (LIAISON®, DiaSorin) which overcomes many of these limitations. Important assay characteristics include: a wide dynamic range (50–6500 pg/mL); high intra- (<2.5%) and inter- (<5%) assay precision; matched serum and plasma equivalence (<10% difference); specificity for the intact sclerostin molecule; and rapid assay results. Serum sclerostin levels measured with the LIAISON® assay in a population-based sample of adult men (n=278) and women (n=348) demonstrated that sclerostin levels were significantly higher in men as compared to women and were positively associated with age in both sexes, consistent with previously published work. In postmenopausal women, serum sclerostin levels measured with the LIAISON® assay were reduced in response to treatment with either estrogen or teriparatide, again consistent with previous findings. Collectively, the above data demonstrate that the LIAISON® sclerostin assay provides a reliable tool for more confident assessment of emergent mechanisms wherein sclerostin may impact a number of bone related pathologies.
Keywords: sclerostin, SOST, bone, standardization, intact, parathyroid hormone, estrogen
Introduction
Sclerostin is a soluble glycoprotein secreted primarily by osteocytes [1 ]. Mutations in SOST, the gene encoding sclerostin, have been identified as the causative defect in patients with the rare high bone mass disorder sclerosteosis [2, 3]. Following these initial findings, understanding of sclerostin biology has evolved dramatically. The canonical osteoanabolic Wnt/β-catenin pathway underlies nearly all facets of osteoblast biology including osteoblast differentiation, proliferation, survival, and ultimately activity. There is now good evidence that sclerostin serves as a central mediator of skeletal anabolism due to its function as an endogenous soluble antagonist of Wnt/β-catenin signaling.
Despite recognition of sclerostin’s integral role in skeletal metabolism, significant questions remain about the function of sclerostin both in normal skeletal physiology and in various pathologic conditions which affect the skeleton. Some of this uncertainty regarding the utility of circulating sclerostin as a biomarker reflects current deficits in our knowledge of normal biologic variables that likely effect sclerostin levels, including whether circadian or seasonal effects impact sclerostin levels, the mechanism by which sclerostin is cleared from the circulation, and the relationship between total body bone mineral content and sclerostin levels. An additional source of uncertainty reflects the belief that sclerostin functions primarily locally to integrate paracrine and possibly autocrine factors, although recent evidence indicates that circulating sclerostin may regulate peripheral fat depots and therefore function as a systemic hormone [4]. Nearly all reports in humans have examined circulating (serum and plasma) sclerostin levels in efforts to better understand the role of sclerostin in human biology. In support of this approach, there is evidence in humans that circulating sclerostin levels are highly correlated with bone marrow plasma sclerostin levels [5]. Given this strong correlation, it appears appropriate to consider circulating sclerostin levels as a reasonable surrogate for sclerostin levels in the bone microenvironment, keeping in mind that particularly the intact molecule may also have systemic hormonal effects [4].
To this end, several commercial immunoassays have been developed. To date, three have dominated the published literature. These include an ELISA from BioMedica (the most commonly reported assay), an ELISA from TECOmedical, and an electrochemiluminescence immunoassay produced by Meso Scale Discovery. More recently, an ELISA from R&D Systems was described [6]. However, while accurate measurement of sclerostin levels may ultimately be of significant value for the diagnosis of disorders of bone modeling and remodeling, including the response to therapeutic intervention, substantial discordance in reported sclerostin values between the various commercially available assays currently precludes the ability to compare results across studies [6–11], and confounds understanding of the clinical implications of sclerostin value measurement.
Here we describe a novel, rapid sclerostin assay (hereafter referred to as LIAISON sclerostin CLIA) which overcomes many limitations inherent to earlier assays, including specifically both the lack of matched serum and plasma equivalence and the measurement of sclerostin fragments in addition to intact sclerostin. Assay validation was performed in an age-stratified adult population-based sample, as well as in direct human interventional studies in which circulating sclerostin levels were shown to decline in response to treatment with estrogen and teriparatide.
Materials and Methods
Methods
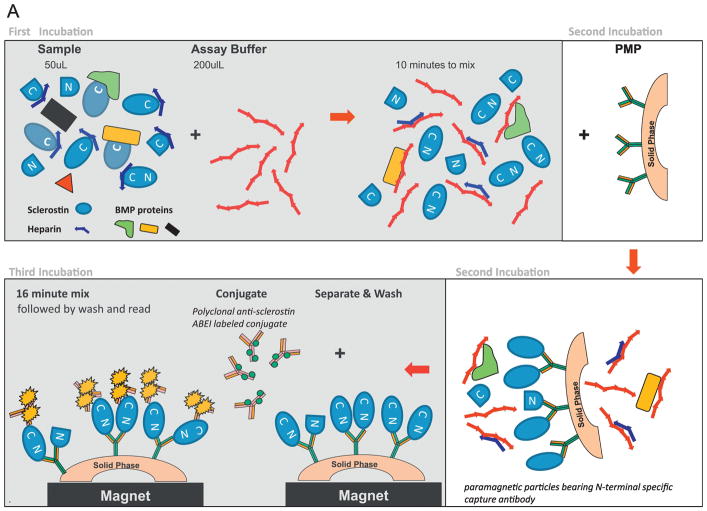
The LIAISON Sclerostin CLIA (Figure 1A) is a recently developed assay from DiaSorin. The first incubation step (10 minutes) allows for the equilibration of patient sample (50 μL of either plasma or serum) with an assay buffer containing a basic polymer to dislodge anionic binding partners like heparin and BMP proteins that can occlude sclerostin’s carboxyl-terminal surface in order to make it more amenable to conjugate recognition. In a second ten minute incubation step, an amino-terminal specific murine monoclonal capture antibody coupled to paramagnetic particles is added. On binding to sclerostin, this capture antibody orients sclerostin such that the carboxyl-terminal portion of sclerostin is distal to the capture particle’s surface. Potential interferants (like complexed BMP2, BMP-4 and/or C-terminal fragments of sclerostin, for example) are then removed with a sequential magnetic separation and wash step. A third sixteen minute incubation involves formation of a sandwich with addition of an ABEI (N-(4-aminobutyl)-N-ethyl-isoluminol)-labeled polyclonal goat functionally anti-sclerostin carboxyl-terminal tail specific conjugate (Figures 1B and 1C). After a final sequential separation and wash cycle, starter reagents are added and emitted light photons proportionate to the sample’s sclerostin levels are converted to assigned concentration values based on a stored master curve positioned dependent upon local/ambient conditions. First results are available at 65 minutes.
Figure 1. 1A. LIAISON sclerostin CLIA.
First incubation 1: 50 μl patient sample incubated with an assay buffer containing a basic polymer to dislodge anionic binding partners like heparin and BMP proteins than can occlude sclerostin’s C-terminal surface, making it more accessible to conjugate recognition. Second incubation: N-terminal specific murine monoclonal capture antibody coupled to paramagnetic particles is added. Upon binding sclerostin, it orients sclerostin such that the C-terminal portion of sclerostin is distal to the capture particle’s surface. A sequential separation and wash cycle is then performed to remove unbound sclerostin fragments and other non-specific serum/plasma proteins. Third incubation: Sandwich formation is achieved via addition of an ABEI-labelled polyclonal goat functionally anti-sclerostin C-terminal tail specific conjugate. Following a final separation and wash cycle, developer is added and emitted light photons [relative light units (RLU)] are measured and converted to pg/mL via a master standard curve. Total time to results is approximately 65 minutes.
1B. Specificity of the LIAISON sclerostin CLIA components overlaid upon a Clustal O(1.2.4) alignment of sclerostin and SOSTDC1 primary sequences. The capture monoclonal antibody is specific to a 16-mer located within the N-terminal tail (Gln1 to Ser56) of sclerostin. The tracer polyclonal antibody is conjugated to ABEI. Its functional C-terminal tail specificity is inferred from, and based upon, three observations: 1) The distal orientation of sclerostin’s C-terminal portion consequent to N-terminal capture specificity of the PMP; 2) SOSTDC1’s C-terminal portion (Gly117 to Ser206) that aligns with the C-terminal thrombin peptide of sclerostin (Gly99 to Tyr190) is twice as basic as its N-terminal portion (2.33fold [21.7%/9.2%] which is similar to sclerostin, whose C-terminal fragment is 2.23 fold [25%/11.2%] more basic than its N-terminal fragment; and 3) SOSTDC1, which has 47.1% direct homology to sclerostin’s post-thrombin digest C-terminal Loop2-Loop3 fragment domain (Gly99 to Arg149) and only 19.5% homology to the post-thrombin digest C-terminal tail domain (Phe150 to Tyr190), does not inhibit the assay when added to the third incubation step. Collectively these observations make it more likely that the conjugate’s specificity is predominantly towards the C-terminal tail.
1C. Primary attributes of sclerostin and SOSTDC1. Intact molecule: sclerostin (Gln1 to Tyr190) has 35.9% direct homology with SOSTDC1 (M1 to Ser206). Gly99 – Arg149 and Phe150 – Tyr190 comprise the Loop2–Loop3 and C-terminal portions of the C-terminal thrombin fragment, respectively. Percent basic (arginine/lysine) residue content of sclerostin secondary structural elements (i.e. Loops 1, 2 and 3) along with the N- and C-terminal thrombin fragments (and the aligned portions from SOSTDC1) demonstrate a similar relative basic aspect to the C-terminal portion of each molecule.
Sensitivity, limit of quantitation (LOQ), was assessed according to Clinical and Laboratory Standards Institute Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures, 2nd Edition (CLSI EP17-A2). Twelve replicates of nine samples with values near the LOQ were measured for three days and percent coefficents of variation (CVs) determined. Thirty paired fresh serum and EDTA plasma samples and thirty paired fresh serum and SST plasma samples spanning the assay’s dynamic range were run to assess sample equivalence.
Assay accuracy was both intentionally fabricated and assessed as follows. Sclerostin kit controls and calibrators were prepared with the addition of purified recombinant human sclerostin (rh-sclerostin; catalogue 1406-ST, R&D Systems, Minneapolis Minnesota) expressed from a murine cell line into a human serum matrix which had been charcoal stripped for delipidation. Determination of relative light unit (RLU) placement for true zero sclerostin levels was made possible by evaluation of serum samples obtained from three subjects with sclerosteosis. Cross-reactivity was assessed by taking serum samples containing 500 pg/mL and 1000 pg/mL of rh-sclerostin and spiking them with 90,000 pg/mL of potential cross-reactants. RLU were then compared to spikes of comparable volume from the vehicle (diluent) controls. Determinations were done in duplicate. Common interferents were spiked into the same 500 pg/mL and 1000 pg/mL serum samples at concentrations dictated by CLSI-07-A2 guidelines and assayed. Substances are classified as interferents when spiked responses differed from vehicle control by > 10%. In some experiments, samples were also measured with the BioMedica, MesoScale and/or R&D Systems sclerostin assays for comparative purposes according to the manufacturers’ respective instructions for use (IFU).
Fragment interference was assessed experimentally by digesting rh-sclerostin with thrombin, assessing degradation of the full-length rh-sclerostin molecule and corresponding fragment generation by gel electrophoresis, and measuring the digests by the LIAISON sclerostin CLIA. Briefly, 250 μg/mL rh-sclerostin samples were digested using immobilized thrombin-agarose from Sigma-Aldrich per Thrombin CleanCleave™ Kit instructions. At 0, 4, 8, and 24 hour time-points, 40 μL of reaction mixture were removed (corresponding to 10 μg of initial rh-sclerostin). Half of digested rh-sclerostin from each time point were run on a SDS-PAGE gradient gel (4–12%), under either reducing conditions (β-mercaptoethanol and boiling) or non-reducing conditions, followed by quantitation of band intensities on a ChemiDoc™Touch Imager. The other half of the samples were diluted with charcoal stripped serum to multiple concentrations within the measuring range of the assay, based on initial rh-sclerostin concentrations, and assayed by the LIAISON sclerostin CLIA. Expected values were calculated from the % intact rh-sclerostin remaining after the digest in the non-reducing conditions as assessed by the imager.
Study subjects
Fasting serum samples were from study subjects previously recruited from an age-stratified random sample of Rochester, MN, residents selected using the medical records linkage of the Rochester Epidemiology Project between the ages of 21 and 97 years and included 348 women and 278 men, as previously reported [12]. The response of sclerostin values to estrogen treatment was measured in serum samples from 34 early postmenopausal women between the ages of 40 to 65 years who were randomized to receive 17β-estradiol (17β-E2, 100 μg/day) by cutaneous patches or no treatment for 4 weeks, as previously described [13]. Alteration of sclerostin levels in response to teriparatide treatment was assessed from fasting peripheral serum samples collected from 40 postmenopausal women between the ages of 55 and 85 years treated once-daily with PTH 1–34 for 14 days, as previously described [5]. Non-fasting serum samples were obtained from 3 South African patients with sclerosteosis, as previously described [23]. Serum samples from all cohorts were frozen and stored at −80°C, and only previously unthawed samples were used for all analyses in this study. All studies were approved by the Mayo Clinic Institutional Review Board, or the Medical Ethical Committee of the Leiden University Medical Center, and written (Rochester) or oral (Leiden) informed consent was obtained from all subjects prior to evaluation.
Statistical analyses
Sclerostin levels were summarized as means ± SEM. Two-sample t tests were used for comparisons between groups. Sclerostin level changes associated with aging were based on predicted values from a linear model. For comparison between sclerostin assays, linear relationships were evaluated by Spearman’s correlation. P values of < 0.05 were considered significant. All analyses were performed using the Statistical Package for the Social Sciences for Windows, Version 22.0 (SPSS, Chicago, IL).
Results
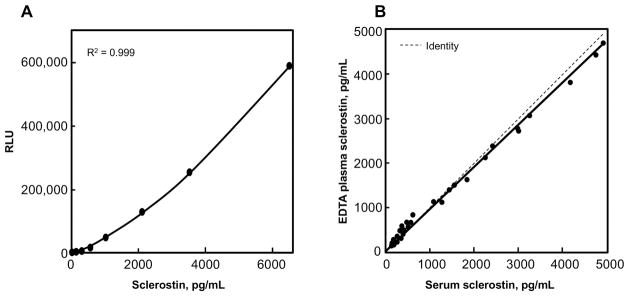
The LIAISON sclerostin CLIA, performed on the LIAISON® XL automated, random access analyzer, delivers precise measurements with intra- and inter- assay imprecision of < 2.5 %CV and < 5.0 % CV, respectively (Table 1), with measurements across a very broad dynamic range (50 to 6500 pg/mL, Figure 2A) while having a sensitivity, or LOQ of 39.2 pg/mL but conservatively reported as ≤50 pg/mL (as determined per CLSI EP17-A2 guidelines). In addition, serum (SST) and plasma-EDTA equivalence is tightly controlled and shown to be within 10% (Figure 2B).
Table 1.
Comparison of commercially available human sclerostin assays*.
| Assay Metric | DiaSorin | BioMedica | TECO | R&D | MesoScale [23, 24] | |
|---|---|---|---|---|---|---|
|
| ||||||
| Range | 50 to 6500 pg/mL | 170 to 5455pg/mL | 58 to 3000 pg/mL | 31.3 to 2000 pg/mL | 1 to 10,000 pg/mL | |
|
| ||||||
| Reference Range | Serum | 124 to 853 pg/mL | 248 to 652 pg/mL | 67.0 to 300 pg/mL | 12.4 to 68.19 pg/mL | |
| EDTA Plasma | 148 to 955 pg/mL | 386 to 857 pg/mL | 249 to 1153 pg/mL | 200 to 712 pg/mL | ||
| Heparin Plasma | 222 to 805 pg/mL | |||||
|
| ||||||
| Precision | Intra | <2.5% | <7% | <4.2% | <2.1% | <6% |
| Inter | <5% | <10% | <4.8% | <10.8% | <10% | |
|
| ||||||
| LOQ | ≤ 50 pg/mL | 170 pg/mL | 58 pg/mL | ND | ND | |
| LOD | ≤ 20 pg/mL | 73 pg/mL | 9 pg/mL | 3.8 pg/mL | 1pg/mL | |
| LOB | ≤ 4 pg/mL | 5.9 pg/mL | 8 pg/mL | 1.74 pg/mL MDD | ND | |
|
| ||||||
| Linearity | 100.3% across entire range | 110% mean @ 1:1, 1:3, 1:7 | 100.3% across entire range | 102% | ||
|
| ||||||
| Recovery | 94% | 94% | 98% | 86 to 112% | ||
|
| ||||||
| TTFR | 65 minutes | 18 hours | 4.5 hours | 4.5 hours | 4.5 hours | |
|
| ||||||
| Sample Volume | 50 μL | 20 μL | 25 μL | 50 μL | 25 μL | |
|
| ||||||
| Sample Type | Serum EDTA Plasma |
Serum EDTA/heparin Plasma |
Serum EDTA/heparin Plasma |
Serum EDTA/heparin Plasma Cell culture supernatants |
Serum EDTA Plasma |
|
|
| ||||||
| Sample Equivalence | EDTA Plasma = 0.94 × (fresh serum) + 57.69 Fresh SST = 1.00 × (fresh serum) − 19.63 |
Serum = 0.8438 × (heparin plasma): R=0.9815 Serum = 0.8509 × (EDTA plasma): R=0.9755 |
||||
|
| ||||||
| Specificity | <0.1% reactivity Noggin, SOSTDC1, BMPs 2,4,5,6,7,8a | No reactivity to Noggin or SOSTDC1 | <0.1% cross reactivity Cerbeus, DAN, Gremlin, LRP4,5,6, BMP 2,4,5,6,7 BMPRIA |
|||
|
| ||||||
| Fragments Detected | NO | YES | INFERRED [10] | No detection reported£ | ||
|
| ||||||
| Interference | ||||||
| Bilirubin. | 15 mg/dL | 40mg/dL | ||||
| Hemoglobin. | 300 mg/dL | 500 mg/dL | ||||
| Triglycerides. | 3000 mg/dL | 3000 mg/dL | ||||
| Glucose. | 300 mg/dL | 1200 mg/dL | ||||
| Cholesterol. | 302 mg/dL | 500 mg/dL | ||||
| Albumin. | 12000mg/dL | 6000mg/dL | ||||
| Gamma globulin. | 12000 mg/dL | 6000 mg/dL | ||||
Values tabulated for the BioMedica, TECO, R&D and MesoScale assays come from the respective manufacturer’s IFUs.
Reported specificity [23] of capture (2 epitopes: one to the N-terminus [aa1 to aa50] and another to the distal C-terminus [aa150 to aa191]) and tracer (3 epitopes: to the N-terminus, to the third loop [Cys111 to Lys143] and to the C-terminus) antibodies for the MesoScale assay are inconsistent with the assay’s reported lack of detection of sclerostin fragments. Although one would expect the reported lack of detection against the three fragments cited [23] comprising {1. a Loop1-2-3 fragment without N- and C- termini ( ie aa51 to aa149), 2. Loop 1 [Cys57 to Cys82] and Loop 3 [Cys111 to Lys143] without N- and C- termini, respectively, and 3. Loop 2 [Cys86 to Arg110]}, one would expect more realistic natural fragments, like those derived from endogenous thrombin action, to be detected, since the capture Ab of the MesoScale assay has both N- and C-terminal reactivity.
Figure 2. LIAISON sclerostin CLIA characteristics.
(A) The dynamic range of the LIAISON sclerostin CLIA spans from 50 to 6500 pg/mL. (B) Matched plasma and serum equivalence: Serum (SST) and plasma (EDTA) equivalence was determined on 28 samples. Measurements showed the 2 matrices to be highly correlated.
Measurement of circulating sclerostin levels in three subjects with sclerosteosis showed RLU values between 600 and 750, all of which were well below the 0 pg/mL threshhold RLU of ~1000–1200 (Table 2) and logically reported as sclerostin serum levels <50 pg/mL. Notably, when these same three samples were assayed with the BioMedica ELISA, the same three samples generated values ranging from 49 to 560 pg/mL (Table 2). Importantly, no significant cross-reactivity was detected (<0.1%) for the eight most likely potential serum proteins (Table 3A). Further, no significant deviations were observed for the eleven most common serum-based interferents (Table 3B).
Table 2.
Sclerostin levels in patients with sclerosteosis determined by the LIAISON sclerostin CLIA and BioMedica assays.
| Sample | LIAISON® XL RLU* |
LIAISON® XL Sclerostin, pg/mL |
BioMedica OD 450nm |
BioMedica Sclerostin, pg/mL |
|---|---|---|---|---|
| Cal matrix | 740 | <0 pg/mL | ND | ND |
| Sclerostosis 1 | 631 | <0 pg/mL | 0.0120 | 77.9 |
| Sclerostosis 2 | 616 | <0 pg/mL | 0.0710 | 560.0 |
| Sclerostosis 3 | 708 | <0 pg/mL | 0.0115 | 49.1 |
RLU for blanks range from 1000 to 1200 RLU.
Table 3A. LIAISON sclerostin CLIA cross-reactivity to potential confounders.
Cross-reactivity was tested with the substances listed. Sclerostin samples were spiked with each substance and compared to the vehicle control. % Cross-reactivity was calculated.
| Substance | Spiked Substance, pg/mL | % Cross reactivity |
|---|---|---|
| SOST DC | 90,000 | <0.1% |
| Noggin | 90,000 | <0.1% |
| BMP 2 | 90,000 | <0.1% |
| BMP 4 | 90,000 | <0.1% |
| BMP 5 | 90,000 | <0.1% |
| BMP 6 | 90,000 | <0.1% |
| BMP 7 | 90,000 | <0.1% |
| BMP 8A | 90,000 | <0.1% |
Table 3B. LIAISON sclerostin CLIA susceptibility to common serum-based interferents.
Each interferent substance was spiked into the samples at the concentration listed according to CLSI-07-A2 guidelines. Interference threshold was defined as greater than 10% difference from the control.
| Substance | Threshold |
|---|---|
| Bilirubin unconjugated | ≤15 mg/dL |
| Bilirubin conjugated | ≤15 mg/dL |
| Hemoglobin | ≤300 mg/dL |
| Triglycerides | ≤3000 mg/dL |
| Cholesterol | ≤302 mg/dL |
| Total protein human IgG | ≤12 g/dL |
| Total protein HSA | ≤12 g/dL |
| Rheumatoid Factor | ≤1500 IU/mL |
| HAMA | ≤3000 ng/mL |
| Uric acid | ≤20 mg/dL |
| Glucose | ≤300 mg/dL |
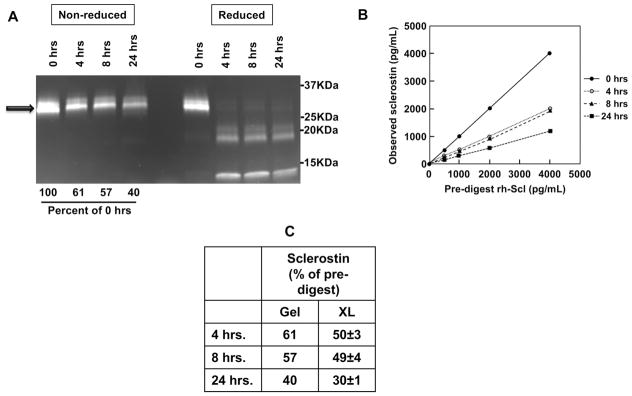
The LIAISON sclerostin CLIA was designed to recognize intact sclerostin only. This was confirmed in an experiment that fractionated rh-sclerostin by thrombin digestion into two fragments (thrombin cleavage site between Arg98 and Gly99 of the primary sequence). Using gel electophoresis to separate intact versus fragments, the remaining intact sclerostin was estimated in the non-denatured samples (Figure 3A). Fragments were not visible under non-denaturing conditions, possibly due to aggregate formation and low penetrance into the gel, but were clearly visible under denaturing conditions. LIAISON sclerostin CLIA measurements of the digests showed decreasing levels of whole sclerostin with increasing time of digest (Figure 3B) with observed rh-sclerostin concentrations reflecting decrements nearly equivalent to the integrated band areas observed from the gradient SDS-PAGE gels (Figure 3C).
Figure 3. The LIAISON sclerostin CLIA does not detect sclerostin fragments.
(A) rh-Sclerostin samples were digested using thrombin for 0, 4, 8, 24 hour time-points. Half of the digests from each time point were run on a SDS-PAGE gradient gel (4–12%), under either non-reducing conditions or reducing conditions (β-mercaptoethanol and boiling), followed by quantitation of band intensities on an imager. Arrow denotes full-length rh-sclerostin prior to thrombin digestion. (B) Digests were diluted to multiple concentrations spanning the measuring range of the assay, based on initial rh-sclerostin concentrations, using charcoal stripped serum, and assayed by the LIAISON sclerostin CLIA. (C) Gel assessments of intact rh-sclerostin and LIAISON sclerostin CLIA measurements are compared and expressed as percent of pre-digested rh-sclerostin.
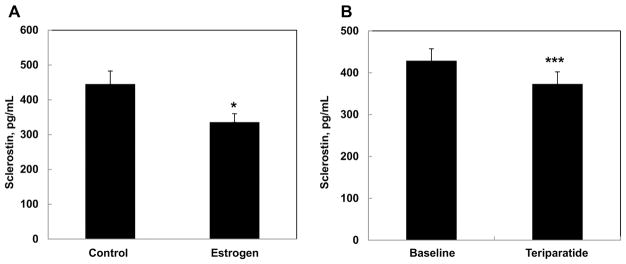
Clinically, trends observed in earlier studies from our group were confirmed using the novel LIAISON sclerostin CLIA: serum sclerostin levels increased with age in both women (Figure 4A) and men (Figure 4B), and were higher in men relative to women. Observed normal ranges in 265 women with the LIAISON sclerostin CLIA were 141 pg/mL to 807 pg/mL (mean 401±8.2), in contrast to the range observed previously with the BioMedica assay (0 to 2050 pg/mL) [12]. Similarly, observed normal serum sclerostin ranges in 271 adult men were between 186 pg/mL to 1160 pg/mL (mean=530±12.1) with the LIAISON sclerostin CLIA, but between 0 to 2000 pg/mL when assessed with the BioMedica assay [12]. In both women (R=0.561, P<0.001) and men (R=0.624, P<0.001), sclerostin values signficantly correlated with age. Further, consistent with our earlier reported findings, treatment of postmenopausal women with either estradiol (Figure 5A) [13] or teriparatide (Figure 5B) [5] significantly reduced circulating sclerostin levels. In agreement with the above trends, measured sclerostin levels tended to be lower when assessed with the DiaSorin as compared to the BioMedica assay, likely due to the lack of fragment detection by the LIAISON sclerostin CLIA.
Figure 4. LIAISON sclerostin CLIA measurements in a population-based sample of (A) women and (B) men from Olmsted County, MN.
Sclerostin levels were significantly higher in men as compared to women, and were positively associated with age in both sexes. These findings are consistent with previous work from our group [12].
Figure 5. Serum sclerostin levels in postmenopausal women were reduced in response to estrogen (A) or teriparatide (B) treatment.
These findings are consistent with previous studies from our group [5, 13]. *P < 0.05 versus control; ***P < 0.001 versus baseline.
Finally, enhanced sensitivity was observed when the LIAISON sclerostin CLIA was directly compared to the MesoScale and BioMedica sclerostin assays, demonstrating important differences with respect to low response (Mesoscale) and marked imprecision (BioMedica), respectively (Figure 6). Notably, this low end range circumscribes values for the vast majority of subjects represented in Figure 4. Further comparisons of existing assay characteristics are shown in Table 1.
Figure 6. Sclerostin assays perform differently at the low end of the measuring range.
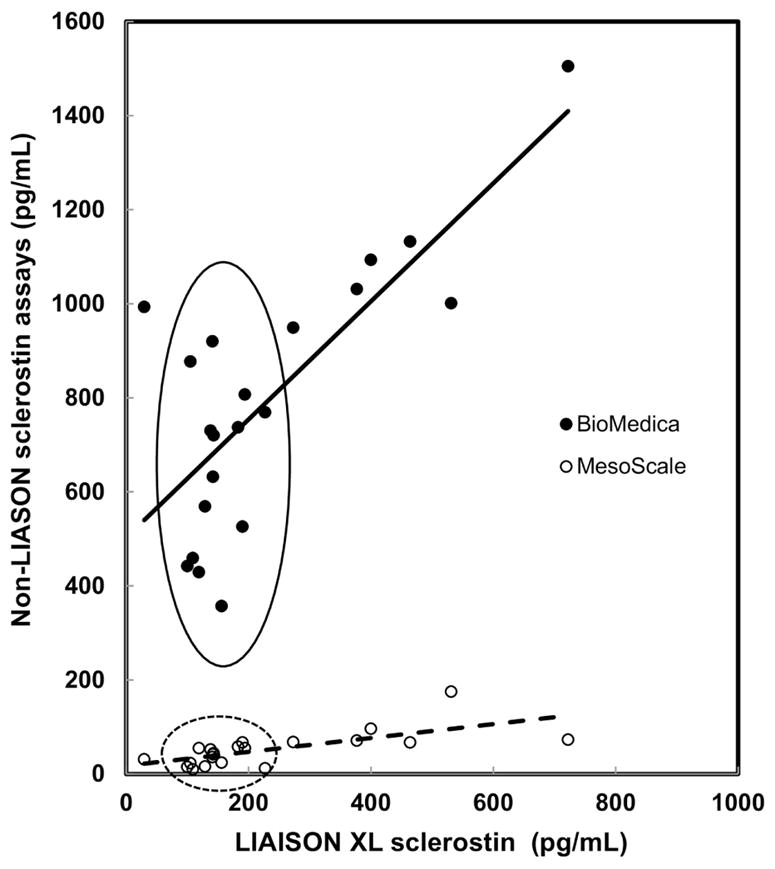
BioMedica and MesoScale assays were compared to the LIAISON sclerostin CLIA in healthy adult subjects.
Discussion
Here we describe characteristics and validation of the novel, rapid LIAISON sclerostin CLIA for measurement of human sclerostin from DiaSorin, which is characterized by a wide dynamic range, high intra- (<2.5%) and inter- (<5%) assay precision, equivalence for the measurement of serum and plasma values, and specificity for the intact sclerostin molecule as evidenced by the lack of detectable circulating sclerostin from sera of subjects with sclerosteosis, as well as the diminished detection of intact rh-sclerostin proportionate to thrombin digestion time. Consistent with earlier findings from our group evaluating sclerostin levels across a wide age range, sclerostin levels increased with age in both sexes and were comparatively higher in men than women at each age point. In keeping with our earlier findings, when measured with the LIAISON sclerostin CLIA, sclerostin levels in postmenopausal women were decreased in response to treatment with either estrogen of teriparatide.
As well detailed by several groups [6, 7, 9, 10], substantial differences in circulating human sclerostin levels have been found when each of the currently available assays (as manufactured by BioMedica, TECOmedical, Meso Scale Discovery, and R&D) have been directly compared. Reasons for such discrepant measurements presumably vary, but likely reflect a wide array of etiologies including: 1) differences in the abilities of the assays to detect interfering substances [9]; 2) differences in detection of sclerostin fragments; 3) differences in each assay’s architecture (sequence of reagent additions with and without wash steps); 4) differences in the primary and secondary antibodies used and corresponding sclerostin epitopes recognized [11]; 5) differences in the ability to detect dimeric or other sclerostin-associated protein complexes; 6) differences in calibration schema between the assays; and 7) differences in measured sclerostin values between serum and plasma, and perhaps other as yet unrecognized confounders. Collectively, such fundamental differences between the assays have strongly hindered commutability of results obtained with the different assays across both normal and pathologic conditions in humans.
The LOQ of ≤ 50 pg/mL, limit of detection of ≤20 pg/mL, and limit of blank of ≤4 pg/mL [14] provide evidence for the precision of the assay. This is most apparent when the low end of the assay between 0 and 682 pg/mL is examined: as an example, when compared to the BioMedica assay, signal responsiveness is nearly double at those concentrations (32888 RLU / 1058 RLU = 31 fold vs. 0.096 OD450 / 0.0055 OD450 = 17 fold, respectively). Given these widespread limitations, the sensitivity, precision and accuracy of the LIAISON sclerostin CLIA decribed herein should enable access to more reliable clinical inference.
A recent area of burgeoning interest in sclerostin is that of its role as a ‘biomarker’ in vascular calcification [15], particularly in patients with chronic kidney disease-mineral bone disorder [16, 17]. Increased sclerostin levels have been detected with declining renal function, with at least some studies suggesting a protective survival benefit of elevated sclerostin levels against vascular calcification [18, 19]. Further, it has been reported that the elevated levels of sclerostin in chronic renal failure do not result from failure of renal elimination [20]. These findings raise the clinically relevant question(s) as to whether the elevated sclerostin levels that have been described reflect detection of sclerostin fragments [21], and/or perhaps whether sclerostin levels increase due to sclerostin expression from non-skeletal sources such as the vasculature [22]. In this setting, the LIAISON® assay we have validated might be of particular use given that it does not detect sclerostin fragments, therefore eliminating this as a potential confounder of efforts to understand the relationship between circulating sclerostin levels and renal disease/vascular health.
Despite the advances in circulating sclerostin measurement described herein, it is important to recognize that other aspects of sclerostin biology which remain only poorly explored are also likely to be of fundamental importance to our future understanding of the role that sclerostin may play as a biomarker for the assessment of skeletal or other clinically relevant issues. Thus, while data to date show that both age and sex clearly appear to affect circulating sclerostin levels, future efforts must be directed at understanding and documenting how a myriad of additional biological factors impact circulating sclerostin levels. Some such factors are likely to include the impact of circadian or seasonal effects on sclerostin levels; potential differences in the proportion of osteocyte-derived sclerostin released into the circulation; the mechanism(s) involved in sclerostin clearance from the circulation (i.e. do changes in renal or hepatic function impact circulating levels of either intact or fragmented sclerostin); the impact of oral intake or physical activity on measured sclerostin levels; whether correction for whole body bone mineral content in order to ‘normalize’ sclerostin levels is necessary to assess for differences across studied conditions; and the impact of skeletal pharmacologic (i.e. anti-resorptive versus anabolic) interventions on circulating sclerostin levels. A more refined knowledge of such factors will almost certainly be needed before any measurement of sclerostin levels is likely be have true utility as a biomarker.
We have reached a critical time in our understanding of the role of sclerostin in human biology. While much has been done, there is a great need for sclerostin assay standardization so that studies in which sclerostin values are assessed can be appropriately compared. This need will be even greater if sclerostin measurements are to be brought into routine clinical practice. The assay described here is a significant step in the right direction due to its broad dynamic range, matched plasma and serum equivalence, demonstrated ability to detect only intact sclerostin, and rapid turnaround time.
Highlights.
Accurate sclerostin measurements in humans remain challenging with currently available assays.
The novel sclerostin assay described overcomes many of current issues and permits the rapid measurement of intact sclerostin with high precision across a wide dynamic range.
Acknowledgments
This work was supported by National Institutes of Health grants AG004875, AR027065, and UL1 TR000135, and by the European Commission grant HEALTH-F2-2008-20199, TALOS.
Footnotes
Conflict of Interest: Jennifer Fenske, Frank A. Blocki, and Claudia Zierold are employees of DiaSorin Inc., the in vitro diagnostics company that manufactured and supplied the sclerostin assay.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drake MT, Khosla S. Hormonal and systemic regulation of sclerostin. Bone. 2017;96:8–17. doi: 10.1016/j.bone.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–89. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, Wein MN, Divieti Pajevic P. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res. 2017;32(2):373–384. doi: 10.1002/jbmr.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5056–62. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piec I, Washbourne C, Tang J, Fisher E, Greeves J, Jackson S, Fraser WD. How Accurate is Your Sclerostin Measurement? Comparison Between Three Commercially Available Sclerostin ELISA Kits. Calcif Tissue Int. 2016;98(6):546–55. doi: 10.1007/s00223-015-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNulty M, Singh RJ, Li X, Bergstralh EJ, Kumar R. Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. J Clin Endocrinol Metab. 2011;96(7):E1159–62. doi: 10.1210/jc.2011-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke BL, Drake MT. Clinical utility of serum sclerostin measurements. Bonekey Rep. 2013;2:361. doi: 10.1038/bonekey.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa AG, Cremers S, Dworakowski E, Lazaretti-Castro M, Bilezikian JP. Comparison of two commercially available ELISAs for circulating sclerostin. Osteoporos Int. 2014;25(5):1547–54. doi: 10.1007/s00198-014-2635-3. [DOI] [PubMed] [Google Scholar]
- 10.Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R. Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab. 2013;98(9):3873–83. doi: 10.1210/jc.2013-2113. [DOI] [PubMed] [Google Scholar]
- 11.Costa AG, Cremers S, Bilezikian JP. Sclerostin measurement in human disease: Validity and current limitations. Bone. 2017;96:24–28. doi: 10.1016/j.bone.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26(2):373–9. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26(1):27–34. doi: 10.1002/jbmr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiaSorin. Packaging Insert/ Instructions for Use. 2015. LIAISON® Sclerostin Assay. [Google Scholar]
- 15.Kanbay M, Solak Y, Siriopol D, Aslan G, Afsar B, Yazici D, Covic A. Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis. Int Urol Nephrol. 2016;48(12):2029–2042. doi: 10.1007/s11255-016-1387-8. [DOI] [PubMed] [Google Scholar]
- 16.Graciolli FG, Neves KR, Barreto F, Barreto DV, Dos Reis LM, Canziani ME, Sabbagh Y, Carvalho AB, Jorgetti V, Elias RM, Schiavi S, Moyses RM. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017 doi: 10.1016/j.kint.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Brandenburg VM, D’Haese P, Deck A, Mekahli D, Meijers B, Neven E, Evenepoel P. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol. 2016;31(2):195–206. doi: 10.1007/s00467-015-3069-7. [DOI] [PubMed] [Google Scholar]
- 18.Viaene L, Behets GJ, Claes K, Meijers B, Blocki F, Brandenburg V, Evenepoel P, D’Haese PC. Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant. 2013;28(12):3024–30. doi: 10.1093/ndt/gft039. [DOI] [PubMed] [Google Scholar]
- 19.Evenepoel P, Goffin E, Meijers B, Kanaan N, Bammens B, Coche E, Claes K, Jadoul M. Sclerostin Serum Levels and Vascular Calcification Progression in Prevalent Renal Transplant Recipients. J Clin Endocrinol Metab. 2015;100(12):4669–76. doi: 10.1210/jc.2015-3056. [DOI] [PubMed] [Google Scholar]
- 20.Cejka D, Marculescu R, Kozakowski N, Plischke M, Reiter T, Gessl A, Haas M. Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab. 2014;99(1):248–55. doi: 10.1210/jc.2013-2786. [DOI] [PubMed] [Google Scholar]
- 21.Moyses RM, Jamal SA, Graciolli FG, dos Reis LM, Elias RM. Can we compare serum sclerostin results obtained with different assays in hemodialysis patients? Int Urol Nephrol. 2015;47(5):847–50. doi: 10.1007/s11255-015-0971-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Yang M, Li M, Cui L. Radial artery sclerostin expression in chronic kidney disease stage 5 predialysis patients: a cross-sectional observational study. Int Urol Nephrol. 2017 doi: 10.1007/s11255-017-1604-0. [DOI] [PubMed] [Google Scholar]
- 23.van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011;26(12):2804–11. doi: 10.1002/jbmr.474. [DOI] [PubMed] [Google Scholar]
- 24.van Lierop AH, Witteveen JE, Hamdy NA, Papapoulos SE. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol. 2010;163(5):833–7. doi: 10.1530/EJE-10-0699. [DOI] [PubMed] [Google Scholar]