Abstract
Protein carbamylation by cyanate is a post-translational modification associated with several (patho)physiological conditions, including cardiovascular disorders. However, the biochemical pathways leading to protein carbamylation are incompletely characterized. This work demonstrates that the heme protein myeloperoxidase (MPO), which is secreted at high concentrations at inflammatory sites from stimulated neutrophils and monocytes, is able to catalyze the two-electron oxidation of cyanide to cyanate and promote the carbamylation of taurine, lysine, and low-density lipoproteins. We probed the role of cyanide as both electron donor and low-spin ligand by pre-steady-state and steady-state kinetic analyses and analyzed reaction products by MS. Moreover, we present two further pathways of carbamylation that involve reaction products of MPO, namely oxidation of cyanide by hypochlorous acid and reaction of thiocyanate with chloramines. Finally, using an in vivo approach with mice on a high-fat diet and carrying the human MPO gene, we found that during chronic exposure to cyanide, mimicking exposure to pollution and smoking, MPO promotes protein-bound accumulation of carbamyllysine (homocitrulline) in atheroma plaque, demonstrating a link between cyanide exposure and atheroma. In summary, our findings indicate that cyanide is a substrate for MPO and suggest an additional pathway for in vivo cyanate formation and protein carbamylation that involves MPO either directly or via its reaction products hypochlorous acid or chloramines. They also suggest that chronic cyanide exposure could promote the accumulation of carbamylated proteins in atherosclerotic plaques.
Keywords: atherosclerosis, cardiovascular disease, lipoprotein, myeloperoxidase, post-translational modification (PTM), cyanide, cyanate
Introduction
Carbamylation is a post-translational modification of biological molecules bearing an amino group. It occurs in several physio- and/or pathophysiological conditions. For example, it is well known that the ϵ-amino group of lysine residues of proteins can be converted to carbamyllysine (1–4), also named homocitrulline (Hcit),6 and that chronic renal insufficiency correlates with enhanced carbamylation mediated by urea-derived cyanate (−O–C N). In patients with renal disease, carbamylation of low-density lipoproteins (LDLs) is considered as a nontraditional risk factor for cardiovascular insufficiency. It manifests all of the biological effects relevant to atherosclerosis, including endothelial cell injuries, expression of adhesion molecules, and vascular smooth muscle cell proliferation (5). Carbamylation of type I collagen has been shown to induce conformational changes and reduce its ability to activate human polymorphonuclear neutrophils involved in the immune response (6).
N). In patients with renal disease, carbamylation of low-density lipoproteins (LDLs) is considered as a nontraditional risk factor for cardiovascular insufficiency. It manifests all of the biological effects relevant to atherosclerosis, including endothelial cell injuries, expression of adhesion molecules, and vascular smooth muscle cell proliferation (5). Carbamylation of type I collagen has been shown to induce conformational changes and reduce its ability to activate human polymorphonuclear neutrophils involved in the immune response (6).
Recently, myeloperoxidase (MPO) has been demonstrated to be indirectly involved in protein carbamylation (7). This enzyme, a member of the peroxidase-cyclooxygenase superfamily (8), is secreted at inflammatory sites from stimulated neutrophils and also from monocytes (9). It catalyzes the two-electron oxidation of thiocyanate (−S–C N), thereby producing hypothiocyanous acid (HO–S–C
N), thereby producing hypothiocyanous acid (HO–S–C N) and cyanate as a minor by-product (7). Protein carbamylation may therefore be driven not only by uremia but also by inflammation. Upon exposure of LDL to either MPO/H2O2/−SCN or the potassium salt of −O–C
N) and cyanate as a minor by-product (7). Protein carbamylation may therefore be driven not only by uremia but also by inflammation. Upon exposure of LDL to either MPO/H2O2/−SCN or the potassium salt of −O–C N, protein recognition by LDL receptor was attenuated, whereas its interaction with the scavenger receptor SR-A1 was enhanced. A clear relationship between the plasma levels of protein-bound ϵ-carbamyllysine (Hcit) and an increased risk of cardiovascular disease could be established (7).
N, protein recognition by LDL receptor was attenuated, whereas its interaction with the scavenger receptor SR-A1 was enhanced. A clear relationship between the plasma levels of protein-bound ϵ-carbamyllysine (Hcit) and an increased risk of cardiovascular disease could be established (7).
We hereafter demonstrate the existence of a new alternative mechanism for cyanate formation and potential protein carbamylation at sites of inflammation, according to the following reaction, which involves the hydrogen peroxide–mediated oxidation of cyanide (−C N) catalyzed by MPO (Reaction 1).
N) catalyzed by MPO (Reaction 1).
Cyanide is well known as a product of organic material combustion (10) (atmospheric pollution, tobacco smoking) or from intake of cyanogenic glycosides (almond) (11). In vivo, it is a universal ligand able to bind to and inhibit heme enzymes. Exposure to high concentrations results in death within seconds to minutes by impairment of dioxygen (O2) distribution within the body and inhibition of the respiratory electron transport chain in the mitochondria (12). Five-coordinated ferric high-spin heme proteins (e.g. cytochrome c oxidase, methemoglobin, metmyoglobin, catalase, peroxidases, etc.) are its preferential targets. Cyanide, which at most biological pH levels is protonated (pKa = 9.14), binds as an anion to Fe(III) and transforms the corresponding heme protein into its six-coordinated low-spin (LS) form, thereby impeding the interaction with natural substrates (e.g. O2 or H2O2). The binding rates and complex equilibria involved are very variable. Typically, eukaryotic peroxidases (including MPO) and catalases bind cyanide rapidly (1.1 × 105 m−1 s−1 to 1.3 × 106 m−1 s−1) (13–17), taking up both the anion and a proton (18). So far, this LS complex formation has been thought to effectively block the redox chemistry of all heme enzymes without any exceptions (KD = 1–10 μm). However, the present study unequivocally demonstrates that cyanide can act both as ligand of MPO and electron donor to compound I of MPO.
Myeloperoxidase is a unique oxidoreductase in human physiology. The reconstructed phylogeny of mammalian peroxidases indeed demonstrates that MPOs form a well-separated clade within the vertebrate peroxidases (i.e. Family 1 of the peroxidase-cyclooxygenase superfamily) (8). This superfamily also includes peroxidasins, peroxinectins, cyclooxygenases, and dual oxidases (8). Fig. 1A depicts the family of vertebrate peroxidases, with the clades of myeloperoxidases and eosinophil peroxidases (EPOs) being well separated from the other branches. Segregation of MPO is well reflected by its unique biophysical and biochemical properties (19) that are closely related to its physiological role and the nature of its substrates. It participates in innate immune defense mechanisms through formation of microbicidal-reactive oxidants and diffusible radical species. Its unique substrate is chloride that is oxidized to antimicrobial hypochlorous acid (HOCl). Chloride oxidation is a thermodynamically challenging reaction that requires the formation of strong oxidizing redox intermediates (i.e. the so-called “compound I” formed by oxidation of ferric MPO by hydrogen peroxide) (Fig. 2A) (20). The standard reduction potential of the couple compound I/MPO(III) (E°′ = 1.16 V) provides the driving force of this reaction (Fig. 2A). Moreover, as will be demonstrated below, it also uniquely enables MPO to oxidize cyanide to cyanate, thereby contributing to a so far unknown route for protein carbamylation. The presented data also suggest multiple pathways of cyanate formation and protein carbamylation that involve MPO either directly or its reaction products hypochlorous acid and/or chloramine. Experiments in mouse model demonstrate the relevance of these pathways in vivo as cyanide is directly metabolized and promotes accumulation of protein-bound Hcit in atheroma plaque.
Figure 1.
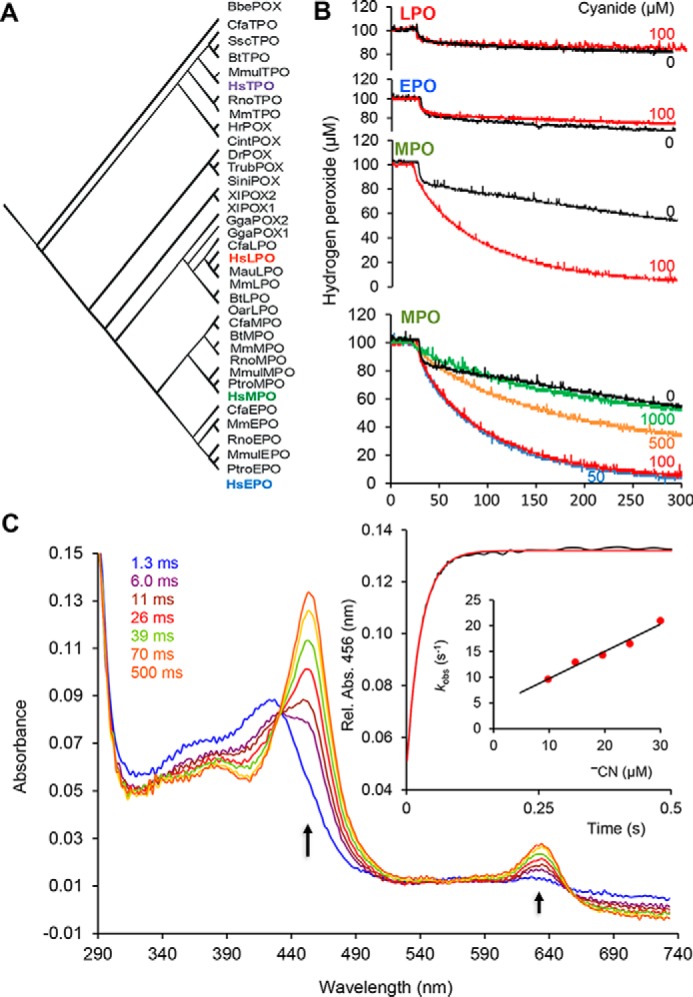
A, reconstructed phylogenetic tree showing the family of vertebrate peroxidases, including the mammalian enzymes MPO, EPO, LPO, and thyroid peroxidase (TPO). B, amperometric measurements (650 mV) of the H2O2 consumption in the presence of 0.5 μm peroxidases (LPO, EPO, or MPO), 100 μm H2O2, and −CN at various concentrations. C, reaction of 2 μm MPO compound I with 50 μm cyanide followed in the sequential-mixing stopped-flow mode. Conditions were as follows: 100 mm phosphate buffer, pH 7.0, 25 °C. Arrows, wavelength with absorbance changes. The first spectrum was taken 1.3 ms after mixing. Inset, typical time trace followed at 456 nm (maximum absorbance of MPO(III)-cyanide complex) and plot of the pseudo-first-order rate constants, kobs, versus cyanide concentrations.
Figure 2.
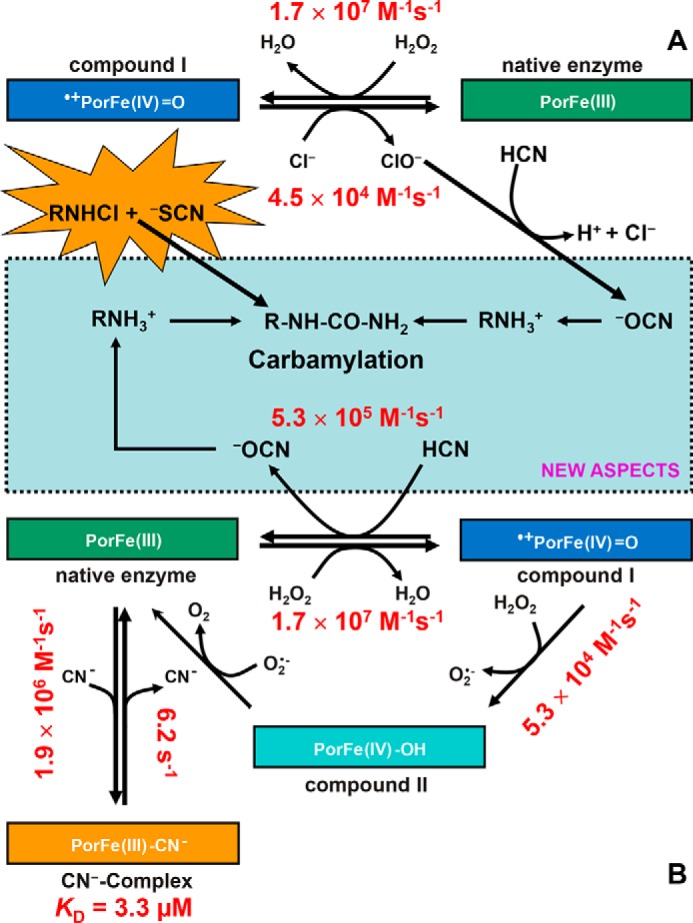
Carbamylation promoted by the system MPO/H2O2/−CN system and MPO-typical reaction products. A, MPO-typical oxidation of chloride to hypochlorous acid (i.e. chlorination cycle). Ferric MPO (PorFe(III)) is oxidized to compound I (•+PorFe(IV)=O; oxoiron(IV) porphyrin radical), which is directly reduced to the resting state by chloride, thereby releasing hypochlorous acid (HOCl/−OCl). Hypochlorous acid mediates the oxidation of cyanide to cyanate and the formation of chloramines, which may be also involved in carbamylation by direct reaction with thiocyanate (NEW ASPECTS). B, two-electron oxidation of cyanide to cyanate by MPO compound I, thereby restoring the ferric protein and releasing cyanate (5.3 × 105 m−1 s−1; NEW ASPECTS). Ferric MPO binds cyanide, forming the low-spin complex (PorFe(III)-CN−, KD = 3.3 μm). Further MPO-typical reactions include the one-electron reduction of compound I to compound II by H2O2 (14) or by other one-electron donors followed by compound II reduction to ferric MPO. Kinetic constants were calculated from current experiments (see “Experimental procedures”) or from the literature when already known (13–17).
Results
Hydrogen peroxide consumption mediated by cyanide
In a preliminary study, the consumption of hydrogen peroxide by MPO, promoted by cyanide, was probed by amperometric monitoring. Fig. 1B clearly shows that 0.5 μm MPO consumes 100 μm H2O2 within 300 s in the presence of 100 μm cyanide, whereas no effect is observed under identical conditions with related enzymes, such as lactoperoxidase (LPO) and EPO. At lower cyanide concentrations (50–100 μm), MPO rapidly consumes H2O2, whereas the inhibitory effect is evident at higher concentrations (500–1000 μm; Fig. 1B). The slow H2O2 consumption observed in control experiments (absence of cyanide) is due to the well-known low (pseudo)catalase activity of mammalian peroxidases (20).
Cyanide acts as electron donor for MPO compound I
Myeloperoxidase catalyzes one- and two-electron oxidation reactions (Fig. 2A). The relevant redox intermediate involved in both reactions (compound I; i.e. an oxoiron(IV) porphyrin radical cation) is formed via a two-electron oxidation of the ferric enzyme (PorFe(III)) by H2O2.
In two-electron transitions, compound I (about 50% hypochromicity of Soret band compared with ferric MPO) is directly reduced to ferric MPO (Soret band at 428 nm), whereas in one-electron transitions, compound II (Soret band at 456 nm) is formed. These reactions can easily be probed by sequential stopped-flow spectroscopy. Fig. 1C shows a representative series of spectra obtained by mixing 4 μm MPO compound I with 100 μm cyanide. The reaction is monophasic and linearly dependent on cyanide concentration. It rapidly forms a MPO species that had its Soret band at 456 nm, which could derive from either compound II or the LS complex between ferric MPO and cyanide (19). The determined apparent bimolecular rate constant is (5.3 ± 0.5) × 105 m−1 s−1 at pH 7.0 and 25 °C. It is unlikely that cyanide mediates the reduction of compound I to compound II, because this reaction is thermodynamically unfavorable (see below). Moreover, the reaction of preformed compound II with cyanide probed in the sequential stopped-flow mode clearly demonstrated that compound II does not participate in the turnover of the MPO/H2O2/−CN system. By contrast, the observed spectral transition suggests the cyanide-mediated direct reduction of compound I to ferric MPO. The latter rapidly binds excess cyanide, forming the LS complex with Soret maximum at 456 nm (see also Figs. S4 and S5).
For comparison, the reaction of cyanide with preformed compound I of LPO and EPO as well as horseradish peroxidase (a model plant-type peroxidase) was also investigated under identical conditions. With LPO and EPO, compound I is slowly transformed to compound II (2 s−1), which is a well-known phenomenon of intramolecular electron transport in the absence of exogenous electron donors (21–23) (see Fig. 1A). Horseradish peroxidase compound I is stable in the presence of cyanide (see Fig. S1B).
MPO-mediated cyanide oxidation generates cyanate
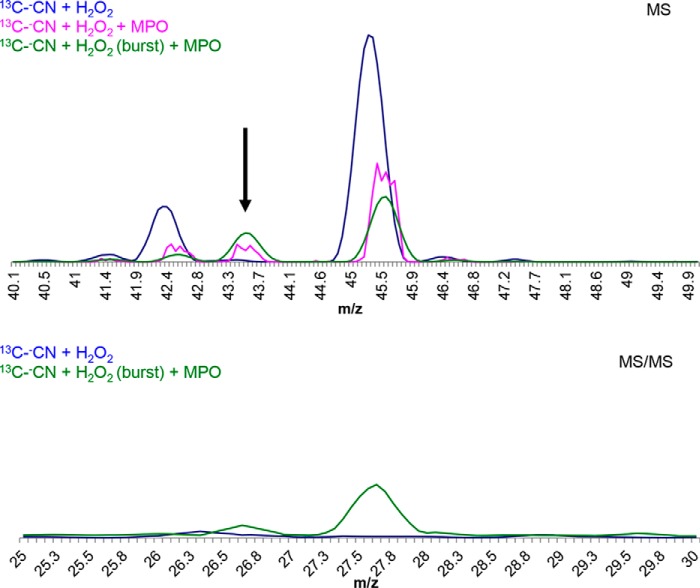
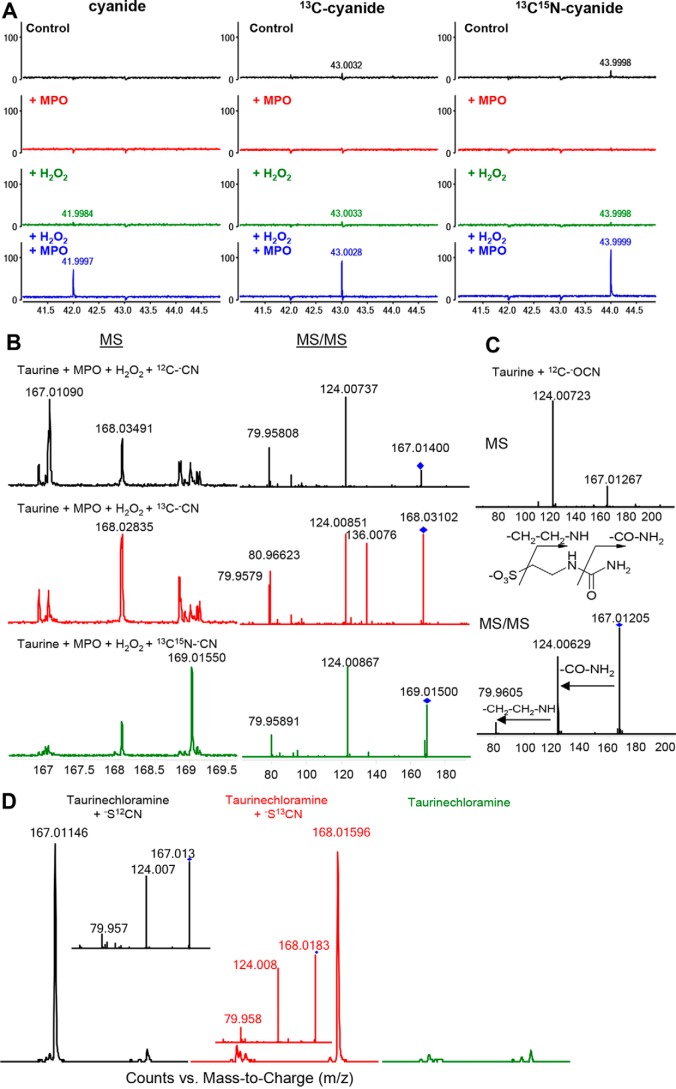
To prove direct reduction of compound I to ferric MPO and concomitant two-electron oxidation of cyanide to −OCN, MPO was incubated with both H2O2 and the isotopomers −CN, [13C]−CN, or [13C,15N]−CN. The reaction products were analyzed by MS. Fig. 3 shows the formation of a reaction product with an appropriate m/z (i.e. m/z = 43) and a fragment ion at m/z = 27. Fig. 4A illustrates the formation of cyanate using the three isotopomers. Upon using −CN, [13C]−CN, or [13C,15N]−CN, peaks at m/z values of 41.999, 43.002, or 43.999 were found, clearly demonstrating the formation of the isotopomers −OCN, [13C]−OCN or [13C,15N]−OCN, respectively. In the absence of cyanide or hydrogen peroxide or MPO, no cyanate was produced except for hydrogen peroxide with cyanide, where a low cyanate production was detected (around 1%). Furthermore, using a calibration curve of cyanate, we were able to calculate that around 13% of cyanide is transformed to cyanate (n = 3) after 1 h (see supporting data for detailed results). These findings clearly indicate that the system MPO/H2O2/cyanide is able to produce cyanate and that the conversion of cyanide to cyanate is incomplete (Fig. 4A).
Figure 3.
Tandem MS/MS determination of cyanate production. MS spectra show a peak around 43 m/z corresponding to 13C-cyanate, when MPO (1 μm) was added to a mixture of [13C]cyanide (2 mm) and H2O2 (200 μm) (pink solid line). Results obtained upon the repeated addition of H2O2 (three times) are depicted by the green solid line. MS/MS spectra show a peak around 27 m/z corresponding to the fragmentation of [13C]cyanate into [13C]cyanide (green solid line).
Figure 4.
Mass spectrometric analyses. A, oxidation of cyanide by the MPO/H2O2 system. The isotopomers of −CN (final concentration = 1 mm) were mixed with H2O2 (1 mm) in 10 mm ammonium acetate buffer, pH 7.4, and the reaction was started with 0.3 μm human recombinant MPO at 37 °C with a reaction time of 1 h. −OCN, −O13CN, and −O13C15N peaks (at 41.9997, 43.0030, and 43.9999 m/z, respectively) could be detected, which correspond to the oxidation products of cyanide isotopes. Spectra have been generated by subtracting spectra of a blank (buffer alone). B, taurine carbamylation. Taurine (1 mm) was mixed with 0.3 μm human recombinant MPO and isotopic cyanide (1 mm) for 12 h (37 °C) in 10 mm ammonium acetate buffer, pH 7.4, and the reaction was started with 1 mm H2O2. A peak was detected at m/z of 167.0109, which corresponds to [12C]carbamyltaurine and MS/MS spectra fit with those of the control. In similar experiments with isotopomers, peaks were detected at m/z = 168.0284 and 169.0155, which correspond to 13C- and 13C,15N-labeled carbamyltaurine. C, MS and MS/MS spectra of carbamyltaurine obtained after mixing taurine (1 mm) and cyanate (1 mm) in the acetate buffer and overnight incubation. D, MS and MS/MS spectra for the reaction of taurine chloramines (formed by mixing of 0.5 mm HOCl and 1 mm taurine) with 12C- and 13C-labeled thiocyanate after a reaction time of 12 h. Peaks detected at m/z values 167.0115 and 168.0160 correspond to 12C- and 13C-labeled carbamyltaurine (absent in the taurine chloramine MS spectra). Their identity was confirmed by MS/MS experiments (D, inset).
Carbamylation of taurine, lysine, and LDL
Furthermore, cyanate produced by MPO-mediated cyanide oxidation was probed in its reaction with nucleophilic amino groups in both taurine and lysine. Taurine is a low-molecular weight amine abundant in millimolar concentration in the cytosol of neutrophils (24). In this study, taurine was used as a chemical model of monoamine because it is stable and its chlorination is well documented. Taurine (1 mm) was incubated with MPO (0.3 μm), hydrogen peroxide (1 mm) and [12C]−CN, [13C]−CN, or [13C,15N]−CN (1 mm) in ammonium acetate buffer, pH 7.4, and the reaction mixture was analyzed by electrospray ionization (ESI)-quadrupole TOF (QTOF) MS in negative mode. Peaks at expected m/z values of 167.0109, 168.0284, and 169.0155 corresponding to 12C-, 13C-, and 13C,15N-carbamylated taurine were found (Fig. 4B). To further confirm the formation of carbamylated taurine, the isolated peaks were fragmented, leading to peaks with m/z values of 124.0074 and 79.9581, respectively, that fully matched with fragments obtained from standard carbamylated taurine (Fig. 4C). Similar experiments performed with lysine demonstrated the formation of Hcit (Fig. S2). In the absence of either hydrogen peroxide or MPO, carbamylation of taurine or lysine was negligible or diminished.
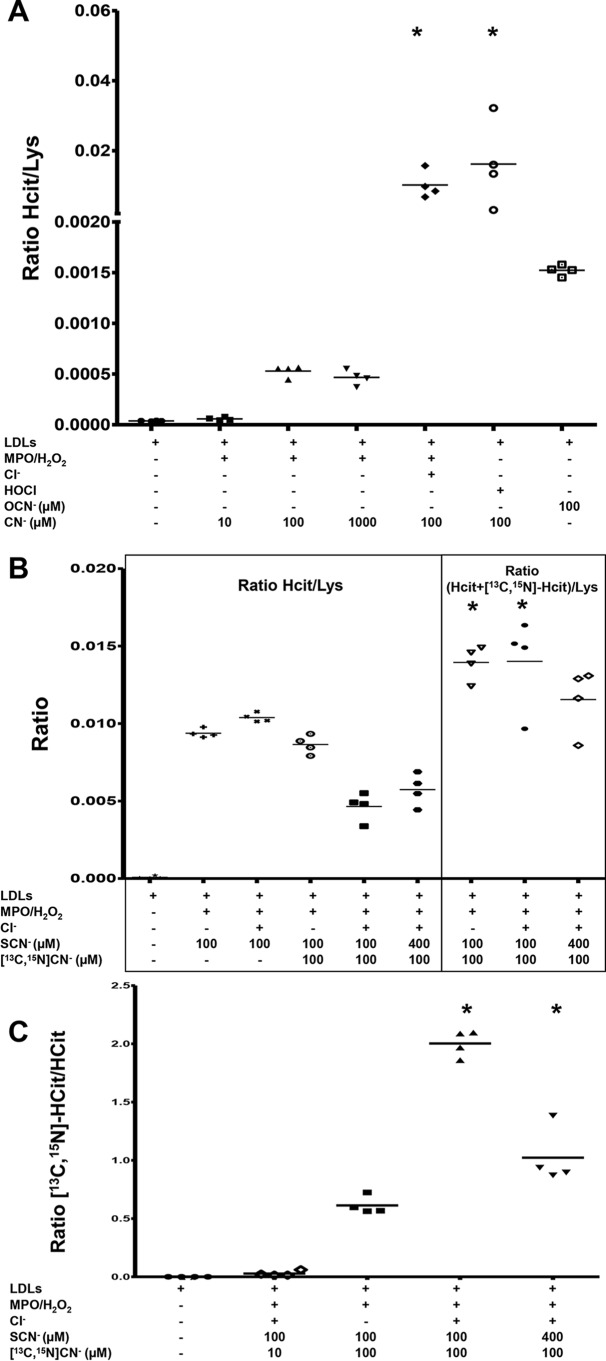
Another tested target for carbamylation was LDL. LDLs were incubated with the MPO/H2O2/cyanide system before being delipidated and hydrolyzed. Subsequently, the formation of protein-bound Hcit was analyzed by MS and correlated with the abundance of protein-bound lysine by monitoring the ratio Hcit/Lys. A low amount of Hcit was detected in native LDLs. The incubation of LDLs with MPO, H2O2, and increasing concentrations of cyanide led to increasing Hcit/Lys ratios (1.5- and 12-fold increases; Fig. 5A). The addition of chloride (150 mm) to the MPO/H2O2 system in the presence of 100 μm cyanide even increased the extent of carbamylation. Similar levels of carbamylation were observed when LDLs were incubated with HOCl and cyanide (100 μm) (Fig. 5A). In the absence of MPO, the Hcit concentration was almost identical to that of native LDLs (data not shown). Furthermore, a positive control with 100 μm of cyanate (−OCN) was analyzed. This concentration was used supposing the full conversion of cyanide to cyanate. Surprisingly, this experiment showed a lower carbamylation compared with the MPO/H2O2/cyanide/chloride system but a 3 times higher carbamylation when compared with the MPO/H2O2/cyanide system (Fig. 5A).
Figure 5.
Formation of carbamylated LDL. A, box-whisker plots (5th to 95th percentile) showing the ratios between Hcit and Lys residues (ratio Hcit/Lys), including conditions using only cyanide as substrate. B, box-whisker plots (5th to 95th percentile) showing the ratios between Hcit and Lys residues (ratio Hcit/Lys), including conditions using thiocyanate (SCN−) and labeled cyanide ([13C,15N]CN−). Left, only monoisotopic Hcit (unlabeled) is considered; right, total Hcit is considered (Hcit + [13C,15N]Hcit). C, box-whisker plots showing the ratios between labeled homocitrulline ([13C,15N]Hcit) and Hcit (ratio [13C,15N]Hcit/Hcit). Native LDLs were incubated overnight at 37 °C in buffer (10 mm phosphate, pH 7.4) in the presence of MPO (300 nm), H2O2 (1 mm), and/or chloride anions (150 mm) and/or HOCl (1 mm) and/or thiocyanate (SCN−, 100 or 400 μm) and/or cyanide (CN−) and/or [13C,15N]cyanide ([13C,15N]CN−). LDLs were then delipidated, hydrolyzed by microwave under acidic conditions. Amino acid residues were labeled and, finally, analyzed using LC-MS/MS. Each condition was performed in quadruplicate (n = 4). *, statistically significant difference with the native LDL condition (Kruskal–Wallis, p < 0.01).
The quantitative approach of Hcit formation enabled us to observe that approximately 0.7% of thiocyanate (100 μm) mediated the formation of Hcit in the presence of MPO and H2O2. When cyanide was added in the same concentration as thiocyanate (100 μm), the thiocyanate-mediated formation of Hcit decreased to 0.35%; however, the cyanide-mediated formation of Hcit increased to 0.7%. If the thiocyanate concentration was increased to 400 μm, the thiocyanate-mediated formation of Hcit increased to 0.4%, whereas the cyanide-mediated formation decreased to 0.4%. These results highlight the importance of generated HOCl for the Hcit formation mediated by cyanide. Finally, the positive control using cyanate to carbamylate LDLs at 100 μm revealed that only 0.1% of cyanate was converted into Hcit under the conditions used.
Finally, we tested the contribution of each anion (thiocyanate, cyanide, and chloride) in the carbamylation process. Upon the addition of cyanide to the system MPO/H2O2/thiocyanate, the contribution of thiocyanate in the carbamylation process decreased (Fig. 5B, left). However, this was compensated by cyanide-mediated carbamylation, as illustrated by an increase of labeled and total Hcit content (labeled and not labeled; Fig. 5, B (right) and C). Interestingly, when chloride (150 mm) was added in addition to cyanide to the MPO/H2O2/thiocyanate system, there was a drop in the formation of Hcit from thiocyanate (Fig. 5B, left). However, the total carbamylation content increased (total Hcit; Fig. 5B, right) as cyanide-dependent formation of Hcit (Fig. 5C) increased.
Role of hypochlorous acid and chloramines in carbamylation pathways
Hypochlorous acid (HOCl) is an important reaction product of MPO deriving from the two-electron oxidation of chloride by compound I. It is well known that HOCl reacts with amines forming chloramines (e.g. taurine chloramines) that in addition contribute to the antimicrobial activity of MPO (25). We could demonstrate that HOCl but not monochloramine is another oxidant of cyanide. Upon mixing hypochlorous acid and cyanide with taurine, carbamylation of taurine is observed (Fig. S3). Actually, HOCl promotes the formation of cyanate from cyanide (Fig. S3). As demonstrated above, formation of Hcit is observed upon incubation of LDL with HOCl and cyanide (Fig. 5A).
Additionally, carbamylation can derive from the reaction between thiocyanate and monochloramine. Fig. 4D shows the formation of carbamylated adducts with m/z values of 167.0115 and 168.0160 by using 12C- or 13C-labeled thiocyanate and taurine mono-chloramine. Incubating HOCl-oxidized LDL with thiocyanate increases the Hcit/lysine ratio (Fig. 5B).
Cyanide exposure promotes protein-bound carbamylation in atheroma plaque
To confirm that those reactions participate in formation of carbamylated proteins in vivo, we used humanized MPO mice transgenic for the intact human MPO gene (−463G allele) (hMPO-TG) with native promoter elements. The rationale for using the hMPO-TG mice is that the human MPO gene is expressed in macrophages in atherosclerotic plaques, whereas the mouse MPO gene is not expressed. This is thought to be due in part to a primate-specific Alu element with binding sites for SP1 and nuclear receptors, including PPARγ (26). Prior studies show MPO presence in atherosclerotic plaques in the MPOG-LDLR−/− model correlating with increased atherosclerotic plaque and hyperlipidemia (27). This cross was used in an earlier study that found increased protein-bound homocitrulline content in atheroplaque laden aorta in huMPOG-LDLR−/− mice as compared with LDL receptor–deficient LDLR−/− controls (7). In this experiment, control mice, human MPO transgenics (−463G allele) (hMPO-TG) mice, and hMPO-TG crossed to the LDLR−/− mice (all on C57BL/6 background) were treated with [13C,15N]cyanide (0.4 mg/kg everyday, ∼5 μg/day) or PBS as negative control for 8 weeks (see “Experimental procedures”). Furthermore, mice were exposed to acute inflammation before sacrifice using intraperitoneal injection of zymosan as in prior studies (Wang et al. (7) with slight modifications). Protein-bound [13C,15N]Hcit was analyzed in plasma, in atheroma plaques, and in the supernatant and cell pellets of peritoneal lavages. Fig. 6A shows that hMPO-TG-LDLR−/− mice treated with [13C,15N]cyanide have an increased ratio of [13C,15N]Hcit/Hcit (0.011 ± 0.003) in the atheroma plaques compared with animals treated with PBS (ratio of 0.0009 ± 0.0005, p < 0.01). Labeled cyanide was used because carbamylation naturally occurs in vivo. Labeling with [13C,15N]Hcit allowed us to demonstrate that chronic exposure to cyanide causes carbamylation via MPO activity. In this context, the ratio of [13C,15N]Hcit/Hcit was the preferred readout to express carbamylation. On the other hand, [13C,15N]Hcit was too low in plasma, cell pellets, and supernatant of peritoneal lavages and was not detected. Nevertheless, it is worth noting that Hcit levels in atheroma plaques were the same in the cyanide hMPO-TG-LDLR−/− mice and PBS control hMPO-TG-LDLR−/− mice (Fig. 6B), showing that it is the chronic exposure to cyanide that led to increased carbamylation by MPO-dependent processes.
Figure 6.
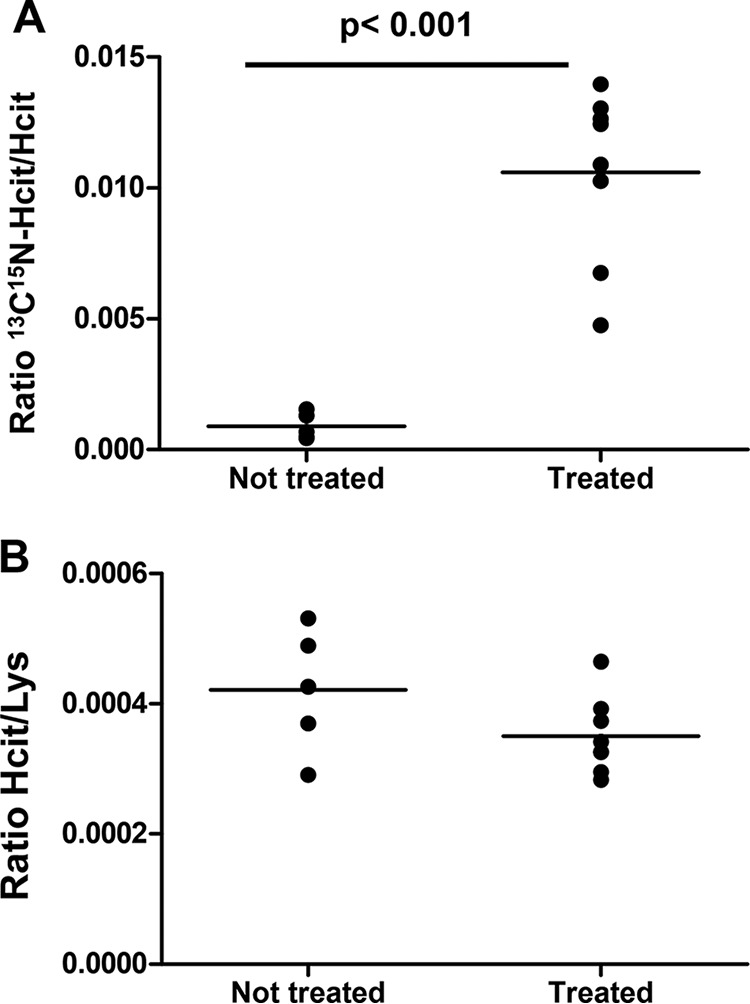
In vivo effect of cyanide. Exposure of hMPO-TG-LDLR−/− mice to [13C,15N]cyanide (0.4 mg/kg/day) led to protein-bound [13C,15N]Hcit formation in atheroma plaque. Mice were exposed for 8 weeks to isotope-labeled cyanide (n = 8) or to PBS (control, n = 5). Protein-bound Lys, Hcit, and [13C,15N]Hcit were monitored, and [13C,15N]Hcit/Hcit (A) and Hcit/Lys (B) ratios were calculated. A statistically significant difference was observed between groups (t test, p < 0.001).
Discussion
Mounting evidence for the involvement of MPO in atherogenesis has emerged for the past decades. It is now accepted that MPO catalyzes oxidation reactions via the release of reactive halogenating and nitrating species that render LDL atherogenic and high-density lipoprotein dysfunctional (28–33). In mice, Wang et al. (7) showed a marked increase in the content of protein-bound homocitrulline at atherosclerotic plaque-laden aortas of human MPO-transgenic mice as compared with nontransgenic counterparts. In both clinical and experimental in vivo situations, MPO seems to be a major catalytic component.
The enhanced consumption of hydrogen peroxide in the presence of low cyanide concentrations and the strict concentration dependence of compound I reduction unequivocally demonstrate the role of cyanide as electron donor for MPO. The inability of cyanide to promote the transition of compound I into compound II fits with the high one-electron reduction potential of the •CN/−CN couple (1.9 V) (20) that is significantly higher than that of the redox couple compound I/compound II (1.35 V) (34).
The observed spectral transition (Fig. 1C) can be explained by the following reaction sequence that involves cyanide as two-electron donor of compound I and as LS ligand for ferric MPO.
The calculated biomolecular rate of the reaction between cyanide and MPO(III) was determined to be 1.9 × 106 m−1 s−1 at pH 7.0 and 25 °C (k3, Reaction 4). Because k3 > k2, the measured rate constant of the transition compound I → MPO(III) → LS MPO-cyanide complex reflects k2 (Fig. 2). The two-electron oxidation of cyanide (k2) is fast (5.3 × 105 m−1 s−1 at pH 7.0 and 25 °C) and reflects the high oxidation capacity of MPO, which is the consequence of heme distortion due to unique heme-to-protein linkages, including an electron-withdrawing vinyl-sulfonium group (19). It is noteworthy that higher concentrations of cyanide favor the MPO-CN− complex formation (k3), limiting the MPO activation by reaction with H2O2 (k1). Catalysis of the two-electron oxidation of cyanide by MPO is unique because related human peroxidases like LPO and EPO did not consume hydrogen peroxide in the presence of cyanide.
Mass spectrometric analyses have confirmed the production of cyanate derived from the two-electron oxidation of cyanide by compound I (see Fig. 2). This was supported by consecutive carbamylation reactions of produced cyanate with ϵ-amino groups of taurine and of free or LDL-bound lysine (Figs. 3–5). However, quantitative MS analyses also demonstrated that only 13% of cyanide was converted to cyanate by the MPO/H2O2/cyanide system (Fig. 4A). Nevertheless, the incubation of cyanide with HOCl (same concentration as hydrogen peroxide; 1 mm) converted only 20% of the cyanide to cyanate. It is also noteworthy that higher concentrations of cyanide (e.g. 1 mm) did not increase LDL carbamylation, which corroborates the kinetic studies (Fig. 5A).
In addition, the present work suggests further new routes of carbamylation, namely cyanate formation by HOCl-mediated cyanide oxidation and/or by reaction of stable chloramines with thiocyanate (35, 36). Both pathways follow reactions that only indirectly depend on MPO. Chloramines are relatively stable derivatives and are produced by the reaction of amines with HOCl released from MPO at sites of inflammation (37). In addition to thiocyanate oxidation (7), our findings show that MPO can promote carbamylation in three additional ways: (i) by direct oxidation of cyanide, (ii) by reaction of hypochlorous acid with cyanide, or (iii) by reaction of chloramines with thiocyanate.
In vivo, several anions compete with cyanide for reaction with MPO compound I, including chloride (150 mm) and thiocyanate (10–500 μm). We observed that in the presence of chloride, thiocyanate, and cyanide, carbamylation of lysine residues was mediated by oxidation of both thiocyanate and cyanide (Fig. 5B). In the presence of 100 μm concentrations of both thiocyanate and cyanide, about one-third of carbamylation derived from thiocyanate and two-thirds from cyanide oxidation (Fig. 5, B and C). Furthermore, higher concentration of thiocyanate (400 μm) decreased the carbamylation mediated by cyanide, but this was compensated by carbamylation mediated by thiocyanate. Under these conditions, the production of HOCl by MPO is lower than the production of HOSCN. However, the quantitative determination of Hcit demonstrated that only ∼0.4% of thiocyanate and cyanide were involved in Hcit formation. Nevertheless, the carbamylation observed in LDLs is about 6 times higher compared with the addition of cyanate only (Fig. 5, A and B). Altogether, these data show a synergic effect of thiocyanate, cyanide, and chloride in the carbamylation process. This synergy might reflect previous reports about the reaction of cyanide with hypothiocyanate to a dicyanosulfide intermediate (36).
It remains to be discussed whether these reactions are relevant in vivo. MPO and −CN do exist in soluble forms in blood, and the concentration ratios between these molecules used in the above experiments match those reported in human blood (e.g. [−CN]/[MPO] ratio ∼400:800, although the relative concentrations may vary depending on physiopathological conditions (e.g. chronic or acute inflammation, diet, and smoking) (38). Indeed, each cigarette can lead to exposure to 18–1500 μg of cyanide (10). However, the concentration measured in plasma even of smokers remains between 0.1 and 1.8 μm (39). But this can be explained by the rapid complexation and thiocyanate transformation (see also below). This led us to investigate the impact of chronic exposure to cyanide in a mouse model that expresses human MPO. The results of these experiments demonstrate that the postulated pathways are relevant in vivo. Protein-bound [13C,15N]homocitrulline was indeed increased in hMPO-TG-LDLR−/− mice that were exposed to labeled cyanide. Of importance, this increase was observed in atheroma plaque, where MPO and chronic inflammation are present (27), but not in plasma or in the peritoneal lavages (cell pellets and protein supernatant) during the acute inflammation process. These results show that cyanide is involved in chronic inflammatory processes by promoting the accumulation of protein-bound Hcit in atheroma plaque but is definitely not the preferred pathway in acute processes. Moreover, the absence of protein-bound [13C,15N]homocitrulline in plasma could reflect a dilution effect, making this marker undetectable. Moreover, cyanide oxidation by MPO in plasma may be affected by natural MPO inhibitors, such as the plasma metalloenzyme ceruloplasmin, anti-MPO Igs, and components of the complement system (40). Finally, it is important to highlight that the experiments have been carried out based on a theoretical cyanide exposure of 5 μg/day for 8 weeks, which could lead to submicromolar concentration of cyanide in blood. A better monitoring of cyanide exposure correlated to blood concentrations should help in understanding the impact of cyanide exposure on cardiovascular diseases.
On the other hand, cyanide is known to be rapidly metabolized by three main routes (41): (i) production of 2-amino-4-thiazoline carboxylic acid from cysteine and −CN, (ii) synthesis of cyanocobalamin (CN-B12) via binding to vitamin B12 analogues (e.g. hydroxycobalamin or methylcobalamin), and (iii) transformation into thiocyanate by a thiosulfate sulfurtransferase in the presence of a sulfur donor, such as thiosulfate (42). All of these reactions hamper the quantification of MPO-mediated cyanide oxidation in vivo as well as of HOCl-mediated cyanide oxidation. However, they have no impact on carbamylation mediated by chloroamines and thiocyanate. The plasma concentration of the latter is known to be modulated by diet and smoking (7).
Summing up, this is the first work describing cyanide as a substrate for a peroxidase, thereby changing the perception of heme protein–cyanide interaction. The presented data also suggest new pathways of cyanate formation and protein carbamylation in vivo that involve MPO either directly or its reaction products hypochlorous acid and/or chloramines. The in vivo experiments show that chronic exposure to cyanide promotes the accumulation of carbamylated protein by multiple chemical pathways in atheroma plaque in a mouse model that mimics cardiovascular risk. Chronic exposure to air pollutants produced by combustion of organic materials (e.g. industrial and motor combustions) is reported to increase physiological levels of cyanide (10, 43–45), and recent data confirm the link between air pollution and cardiovascular risk (46–48). Future studies should explore the impact of MPO on oxidation of cyanide derived from pollution or tobacco smoke in human cardiovascular diseases.
Experimental procedures
Materials and reagents
Experiments were performed using both leukocyte and recombinant MPO for which no differences in kinetics of transitions were observed within experimental errors (49). Highly purified MPO of a purity index (A430/A280) of at least 0.85 was purchased from Planta Natural Products. Transfection of recombinant plasmids into Chinese hamster ovary cells, selection, culture procedures, and protein purification protocols were described previously (50). The recombinant protein had a purity index (A430/A280) of about 0.7. Generally, concentration of MPO was calculated using ϵ430 nm = 91 mm−1 cm−1 as described previously (51). Eosinophil peroxidase was purified as described (52). Bovine lactoperoxidase and HRP were from Sigma-Aldrich Handels GmbH (Vienna, Austria). Hydrogen peroxide (H2O2) concentration was determined spectrophometrically at 240 nm (ϵ = 39.4 m−1 cm−1) (53).
LDLs were isolated from human plasma by ultracentrifugation according to Havel et al. (54). This study conforms with the Declaration of Helsinki, and its protocol was approved by the Ethics Committee of “CHU de Charleroi.” Finally, all subjects gave their written informed consent. Before modification, the LDL fraction (1.019 < d < 1.067 g/ml) was desalted by two consecutive passages through PD10 gel-filtration columns (Amersham Biosciences) using phosphate buffer (10 mm, pH 7.4). The different steps were carried out in the dark, and the protein concentration was measured by an ELISA kit for apoB-100 measurement.
Amperometric analysis of hydrogen peroxide consumption
Hydrogen peroxide consumption by MPO was measured by amperometry using a combined platinum/reference electrode (covered with a hydrophilic membrane) fitted to an Amperometric Biosensor Detector 3001 (Universal Sensors, Inc.). The applied electrode potential at pH 7.4 was 650 mV, and the H2O2 electrode-filling solution was freshly prepared twice daily. The electrode was calibrated against known concentrations of H2O2. Consumption of H2O2 (100 μm) in 100 mm phosphate buffer, pH 7.4, was followed (25 °C), and reactions were started by the addition of either 500 nm MPO, EPO, or LPO.
Transient-state experiments
Multimixing stopped-flow measurements were performed with an SX-18MV spectrofluorometer (Applied Photophysics). As soon as 100 μl were shot into a flow cell having a 1-cm light path, the fastest time for mixing the solution and recording the first data point was 1.3 ms. The reactions were followed both at fixed wavelength and by using a diode-array detector. At least three determinations (2000 data points) of pseudo-first-order rate constants (kobs) were performed for each substrate concentration (pH 7.0, 25 °C). The mean value was used in the calculation of the second-order rate constants obtained from the slope of the plot of kobs versus substrate concentration. Concentrations of substrates were at least 10 times in excess as compared with that of the enzyme.
Due to the inherent instability of compound I of MPO, the sequential stopped-flow (multimixing) technique was used for determination of rates of reaction of compound I with cyanide. Typically, MPO (8 μm) was premixed with a 10-fold excess of H2O2 in the aging loop for 30 ms (100 mm phosphate buffer, pH 7.0). LPO and HRP (8 μm) were premixed with an equimolar concentration of hydrogen peroxide for 100 and 1200 ms, respectively. Finally, compound I was allowed to react with varying concentrations of cyanide. Cyanide oxidation was followed by monitoring the absorbance change at 456 nm (MPO), 430 nm (LPO), and at 403 nm (HRP), respectively.
All reactions were also investigated using the diode-array detector (Applied Photophysics PD.1) attached to the stopped-flow machine. Normal data sets were analyzed using the Pro-K simulation program from Applied Photophysics, which allowed the synthesis of artificial sets of time-dependent spectra as well as spectral analysis of enzyme intermediates.
Carbamylation assays, product analysis, and Hcit quantitation by MS
Cyanate production was carried out at 37 °C in a final volume of 1.0 ml. The reaction mixture contained the following reagents at the final concentrations indicated in parentheses: ammonium acetate buffer, pH 7.4 (10 mm), H2O2 (1 mm), and cyanide (1 mm). The reaction was started by the addition of MPO (300 nm). Alternatively, cyanate was obtained by mixing isotopomers of cyanide (1 mm) and HOCl (1 mm) in the same buffer. Cyanate concentration was calculated to a calibration curve from 50 to 1000 μm KOCN. After incubation for 1 h, the solution was analyzed by MS using an infusion system within an Agilent (Palo Alto, CA) 6520 ESI source QTOF mass spectrometer in negative mode with a source temperature at 325 °C, a VCap at 4500 V, a drying gas at 5 liters/min, a nebulizer gas at 10 p.s.i.g., a fragmentor at 150 V, and a skimmer at 80 V. Spectra were accumulated for 2 min.
Tandem MS/MS analysis of the cyanate production was carried out by infusion on a Quattro premier XE (Waters, Milford, MA) using an electrospray in negative mode with a source temperature at 120 °C, a desolvation temperature at 400 °C, a gas flow at 503 liters/h, a capillary voltage at 2.5 kV, and a cone voltage at 50 V. The fragmentation was allowed with collision energy at 90 eV. The spectra are the results of 30 accumulated scans.
Reactions of carbamylation were performed in triplicate in 10 mm ammonium acetate buffer, pH 7.4, at 37 °C with recombinant MPO. Usually a 50:50 methanol/solution mixture was directly infused (0.3 ml/min) into the Agilent QTOF described above, and spectra were accumulated for 2 min.
Carbamylation of taurine or lysine was performed using a mixture of taurine (1 mm) or lysine (1 mm), MPO (300 nm), and cyanide (1 mm) in ammonium acetate buffer, pH 7.4 (37 °C), and the reaction was started by the addition of H2O2 (1 mm).
Chlorotaurine was produced by mixing taurine (1 mm) with HOCl (500 μm) at 37 °C. After 4 h, cyanide (1 mm) or thiocyanate (1 mm) was added. After 12 h, carbamyltaurine and homocitrulline formation was analyzed in negative mode on the ESI-QTOF described above with a source temperature at 325 °C, a VCap at 4000 V, a drying gas at 5 liters/min, a nebulizer gas at 10 p.s.i.g., a fragmentor at 165 V, and a skimmer at 80 V. MS/MS spectra were obtained by monitoring the collision energy at 1 V. Analysis of carbamyllysine was performed with the same specifications but in positive mode. Standard solutions were obtained by mixing in taurine (1 mm) or lysine (1 mm) with cyanate (1 mm) at 37 °C under identical conditions (incubation time of 12 h).
LDLs (1 mg/ml) were isolated by standard protocol (54) and were incubated overnight in phosphate buffer (10 mm), pH 7.4 (37 °C), with MPO (300 nm) and cyanide (10, 100, or 1000 μm) with or without chloride (150 mm). The reaction was started by the addition of H2O2 (1 mm). A control condition without MPO but with H2O2 (1 mm) and cyanide (100 μm) was also performed. LDLs (1 mg/ml) were also incubated with phosphate buffer (10 mm), pH 7.4 (37 °C), and cyanide (100 μm) before the addition of HOCl (1 mm). A positive control where LDLs (1 mg/ml) were incubated overnight in the same buffer in the presence of cyanate (100 μm) at 37 °C was performed. LDLs (1 mg/ml) were also incubated for 4 h (37 °C) with HOCl (1 mm) in phosphate buffer (10 mm), pH 7.4 (37 °C), before the addition of thiocyanate (100 μm) and overnight incubation. Finally, LDLs (1 mg/ml) were incubated overnight (37 °C) in phosphate buffer (10 mm), pH 7.4 (37 °C), with MPO (300 nm), chloride anions (150 mm), thiocyanate (100 or 400 μm), and [13C,15N] cyanide (10 or 100 μm).
Native LDL and carbamyl-LDL were precipitated with TCA (10%) and centrifuged for 10 min at 16,000 × g. Supernatant was discarded, and the process was repeated one more time. The precipitate was then mixed with a solution of water/methanol/diethylic ether (1:3:7) to remove lipids. The mixture was centrifuged 10 min at 16,000 × g, the supernatant was discarded, and the process was repeated. The obtained pellets were dried down using a vacuum centrifuge before acid hydrolysis, derivatization, and MS/MS analysis. The latter was carried out as described below.
Carbamylation was monitored by LC-MS/MS after acidic hydrolysis as described previously (55). [13C,15N]Lysine (3.4 μm, final concentration) was added as an internal standard. Acid hydrolysis was carried out at 110 °C using a microwave oven; resulting amino acid residues were labeled, and samples were cleaned up as described previously (55). At the end of the process, samples were reconstituted in 0.1% formic acid and injected into a 1290 Infinity series UHPLC system (Agilent Technologies, Palo Alto, CA). Amino acid residues were resolved on a Poroshell 120 EC-C18 column (2.1 × 100 mm, 2.7 μm) (Agilent Technologies) using a gradient of 0.2% formic acid and methanol. Lys, Hcit, and [13C,15N]Hcit residues were quantified by tandem MS on a 6490 series ESI-triple quadrupole mass spectrometer using a JetStream source (Agilent Technologies, Palo Alto, CA). Parameters were as follows: positive mode; dynamic MRM; gas temperature of 250 °C; drying gas of 14 liters/min; nebulizer pressure of 20 p.s.i.g.; sheath gas heater at 350 °C; sheath gas flow of 7 liters/min; capillary voltage of 3000 V; high-pressure RF of 150 V; low pressure RF of 60 V; fragmentor at 380 V. Fixed collision energies (CEs) were optimized for each residue and for each product ion and were as follows: Hcit, precursor ion m/z of 246.2, quantifier product ion = 229.1 using CE = 9 V, qualifier product ion = 84.1 using CE = 25 V; [13C,15N]Hcit: precursor ion m/z of 248.2, quantifier product ion = 230.1 using CE = 9 V, qualifier product ion = 84.1 using CE = 25 V; Lys: precursor ion m/z of 203.2, quantifier product ion = 186.2 using CE = 9 V, qualifier product ion = 84.1 using CE = 13 V. The precursor ions correspond to the butanolic esters of the amino acid residues. Precursor isolation mode was in unit mode for Lys and enhanced mode for Hcit and [13C,15N]Hcit. For the quantitation of Hcit in samples, a standard curve of Hcit was performed from 1 to 10,000 nm, and [13C,15N]lysine was used as an internal standard. Data were acquired using MassHunter Acquisition® software and analyzed by MassHunter Quantitative Analysis® software (version B.07, Agilent Technologies).
Animals
Age- and sex-matched C57BL/6, human MPO transgenics (−463G allele) (hMPO-TG), and hMPO-TG crossed to the LDLR−/− mice on the C57BL/6 background were used in these studies. All animal experiments adhered to the Guide for the Care and Use of Laboratory Animals (56) and were performed using approved protocols from the Animal Research Committee at Sanford Burnham Prebys Medical Discovery Institute.
Potassium cyanide exposure of mice
Mice were fed a high-fat atherogenic diet (42% kcal from fat) (Envigo, TD 88137) for 12 weeks. After 4 weeks on a high-fat diet, the mice were randomly allocated to treated or control groups. Mice in the first group were injected intraperitoneally with potassium [13C,15N]cyanide (0.4 mg/kg, ∼5 μg/day) in PBS every day for 8 weeks. Control mice were injected with PBS. Blood collection at sacrifice was by cardiac puncture under Avertin® anesthetic. Serum was prepared by centrifugation and stored at −80 °C until analyzed. The mice were then perfused with ice-cold PBS through the left ventricle, and proximal aortae were carefully dissected to remove adventitial fat. The region of the aorta containing the plaque was removed and frozen at −80 °C until analyzed. The brain, liver, and kidney were also collected and stored at −80 °C until analyzed.
Mouse model of inflammation
The procedure for inflammation was performed essentially as described by Wang et al. (7) except that in this study, we injected potassium [13C,15N]cyanide. Briefly, animals were injected intraperitoneally with 1 ml of 4% thioglycollate broth. Twenty hours later, mice were injected with zymosan (250 mg/kg) and potassium [13C,15N]cyanide (1.6 mg/kg). Peritoneal lavage was performed 4 h later with PBS containing 100 μm butylated hydroxytoluene and 100 μm diethylenetriaminepentaacetic acid. The samples were centrifuged at 1000 rpm for 5 min at 4 °C to separate the lavage fluid from the peritoneal cells. The samples were frozen and stored at −80 °C until analyzed.
Statistics
Data were analyzed using SigmaPlot® version 12.0 software. Mean values with S.D. were reported because the data were normally distributed (normality tests, Shapiro-Wilk passed). Parametric t tests were the most appropriate for our study design. Outcomes were considered as statistically significant with a two-tailed p < 0.05. Based on six preliminary mice (treated versus nontreated, difference on mean and S.D.), we determined the number of mice to obtain a power of performed two-tailed test (with α = 0,050) to 0.8. No mice were excluded from the study.
The operator was blinded, as each sample was allocated to a code number, and the corresponding group was revealed after LC-MS data analyses and before statistical analysis.
Author contributions
P. V. A., C. D., P. G. F., R. A. M., W. F. R., V. N., M. D., B. R., A. R., C. N., D. D., F. R., C. C., and M. S. performed experiments; P. V. A., C. D., K. Z. B., W. F. R., R. A. M., P. G. F., L. V., and C. O. designed experiments and analyzed data; C. D., P. V. A., K. Z. B., P. G. F., R. A. M., W. F. R., M. R., N. M., M. V., J. D., J. N. and C. O. wrote the manuscript; K. Z. B., B. R., W. F. R., R. A. M., and P. V. A. provided reagents, notably recombinant MPO, materials, and analysis tools.
Supplementary Material
Acknowledgment
We thank Dr. Kjell Mortier (Waters, Zeelik, Belgium) for helpful advice on the Quattro Premier XE instrument.
This study was supported by Austrian Science Funds Grant FWF P 20664 (to P. G. F.) and doctoral program BioToP Grant W1224 (to M. S.), Belgian National Fund for Scientific Research (FRS) Grants 3.4553.08 and T.0136.13 PDR, Université Libre de Bruxelles Grant FER-207, and Department of International Relationships of the Université Libre de Bruxelles Grant BRIC SJ/AL/BRIC/130. This study was also supported in part by National Institutes of Health Grants RO1-NS074303 (to W. F. R.) and R43 AG040935 (to R. A. M.). This work was also supported by the Institute of Pathology and Genetics (IRSPG, Loverval, Belgium) and the “CHU of Charleroi.” Additional funds to support this work were provided by Sanford Burnham Prebys Medical Discovery Institute. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S5.
- Hcit
- homocitrulline
- LDL
- low-density lipoprotein
- MPO
- myeloperoxidase
- LS
- low-spin
- EPO
- eosinophil peroxidase
- LPO
- lactoperoxidase
- ESI
- electrospray ionization
- QTOF
- quadrupole TOF
- LDLR
- LDL receptor
- HRP
- horseradish peroxidase
- CE
- collision energy.
References
- 1. Zeng J. (1991) Lysine modification of metallothionein by carbamylation and guanidination. Methods Enzymol. 205, 433–437 10.1016/0076-6879(91)05127-H [DOI] [PubMed] [Google Scholar]
- 2. Golemi D., Maveyraud L., Vakulenko S., Samama J. P., and Mobashery S. (2001) Critical involvement of a carbamylated lysine in catalytic function of class D β-lactamases. Proc. Natl. Acad. Sci. U.S.A. 98, 14280–14285 10.1073/pnas.241442898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraus L. M., Jones M. R., and Kraus A. P. Jr. (1998) Essential carbamoyl-amino acids formed in vivo in patients with end-stage renal disease managed by continuous ambulatory peritoneal dialysis: isolation, identification, and quantitation. J. Lab. Clin. Med. 131, 425–431 10.1016/S0022-2143(98)90143-3 [DOI] [PubMed] [Google Scholar]
- 4. Kraus L. M., and Kraus A. P. Jr. (2001) Carbamoylation of amino acids and proteins in uremia. Kidney Int. Suppl. 78, S102–S107 10.1046/j.1523-1755.2001.59780102.x [DOI] [PubMed] [Google Scholar]
- 5. Ok E., Basnakian A. G., Apostolov E. O., Barri Y. M., and Shah S. V. (2005) Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int. 68, 173–178 10.1111/j.1523-1755.2005.00391.x [DOI] [PubMed] [Google Scholar]
- 6. Jaisson S., Lorimier S., Ricard-Blum S., Sockalingum G. D., Delevallée-Forte C., Kegelaer G., Manfait M., Garnotel R., and Gillery P. (2006) Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem. Biol. 13, 149–159 10.1016/j.chembiol.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 7. Wang Z., Nicholls S. J., Rodriguez E. R., Kummu O., Hörkkö S., Barnard J., Reynolds W. F., Topol E. J., DiDonato J. A., and Hazen S. L. (2007) Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 13, 1176–1184 10.1038/nm1637 [DOI] [PubMed] [Google Scholar]
- 8. Zamocky M., Jakopitsch C., Furtmüller P. G., Dunand C., and Obinger C. (2008) The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 72, 589–605 10.1002/prot.21950 [DOI] [PubMed] [Google Scholar]
- 9. Nicholls S. J., and Hazen S. L. (2005) Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 25, 1102–1111 10.1161/01.ATV.0000163262.83456.6d [DOI] [PubMed] [Google Scholar]
- 10. Mahernia S., Amanlou A., Kiaee G., and Amanlou M. (2015) Determination of hydrogen cyanide concentration in mainstream smoke of tobacco products by polarography. J. Environ. Health Sci. Eng. 13, 57 10.1186/s40201-015-0211-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham K., Buhrke T., and Lampen A. (2016) Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: a crossover study in humans. Arch. Toxicol. 90, 559–574 10.1007/s00204-015-1479-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper C. E., and Brown G. C. (2008) The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 40, 533–539 10.1007/s10863-008-9166-6 [DOI] [PubMed] [Google Scholar]
- 13. Marquez L. A., and Dunford H. B. (1989) Cyanide binding to canine myeloperoxidase. Biochem. Cell Biol. 67, 187–191 10.1139/o89-029 [DOI] [PubMed] [Google Scholar]
- 14. Bolscher B. G., and Wever R. (1984) A kinetic study of the reaction between human myeloperoxidase, hydroperoxides and cyanide: inhibition by chloride and thiocyanate. Biochim. Biophys. Acta 788, 1–10 10.1016/0167-4838(84)90290-5 [DOI] [PubMed] [Google Scholar]
- 15. Ikeda-Saito M. (1987) A study of ligand binding to spleen myeloperoxidase. Biochemistry 26, 4344–4349 10.1021/bi00388a024 [DOI] [PubMed] [Google Scholar]
- 16. Dolman D., Dunford H. B., Chowdhury D. M., and Morrison M. (1968) The kinetics of cyanide binding by lactoperoxidase. Biochemistry 7, 3991–3996 10.1021/bi00851a028 [DOI] [PubMed] [Google Scholar]
- 17. Palcic M. M., and Dunford H. B. (1981) The kinetics of cyanide binding by human erythrocyte catalase. Arch. Biochem. Biophys. 211, 245–252 10.1016/0003-9861(81)90451-3 [DOI] [PubMed] [Google Scholar]
- 18. Chance B. (1949) The reaction of catalase and cyanide. J. Biol. Chem. 179, 1299–1309 [PubMed] [Google Scholar]
- 19. Furtmüller P. G., Zederbauer M., Jantschko W., Helm J., Bogner M., Jakopitsch C., and Obinger C. (2006) Active site structure and catalytic mechanisms of human peroxidases. Arch. Biochem. Biophys. 445, 199–213 10.1016/j.abb.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 20. Arnhold J., Monzani E., Furtmüller P. G., Zederbauer M., Casella L., and Obinger C. (2006) Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur. J. Inorg. Chem. 2006, 3801–3811 10.1002/ejic.200600436 [DOI] [Google Scholar]
- 21. Jantschko W., Georg Furtmüller P., Zederbauer M., Lanz M., Jakopitsch C., and Obinger C. (2003) Direct conversion of ferrous myeloperoxidase to compound II by hydrogen peroxide: an anaerobic stopped-flow study. Biochem. Biophys. Res. Commun. 312, 292–298 10.1016/j.bbrc.2003.10.117 [DOI] [PubMed] [Google Scholar]
- 22. Furtmüller P. G., Burner U., Regelsberger G., and Obinger C. (2000) Spectral and kinetic studies on the formation of eosinophil peroxidase compound I and its reaction with halides and thiocyanate. Biochemistry 39, 15578–15584 10.1021/bi0020271 [DOI] [PubMed] [Google Scholar]
- 23. Furtmüller P. G., Jantschko W., Regelsberger G., Jakopitsch C., Arnhold J., and Obinger C. (2002) Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry 41, 11895–11900 10.1021/bi026326x [DOI] [PubMed] [Google Scholar]
- 24. Grisham M. B., Jefferson M. M., Melton D. F., and Thomas E. L. (1984) Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem. 259, 10404–10413 [PubMed] [Google Scholar]
- 25. Malle E., Marsche G., Arnhold J., and Davies M. J. (2006) Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim. Biophys. Acta 1761, 392–415 10.1016/j.bbalip.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 26. Piedrafita F. J., Molander R. B., Vansant G., Orlova E. A., Pfahl M., and Reynolds W. F. (1996) An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J. Biol. Chem. 271, 14412–14420 10.1074/jbc.271.24.14412 [DOI] [PubMed] [Google Scholar]
- 27. Castellani L. W., Chang J. J., Wang X., Lusis A. J., and Reynolds W. F. (2006) Transgenic mice express human MPO −463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in −463G males. J. Lipid Res. 47, 1366–1377 10.1194/jlr.M600005-JLR200 [DOI] [PubMed] [Google Scholar]
- 28. Abu-Soud H. M., and Hazen S. L. (2000) Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 275, 37524–37532 10.1074/jbc.275.48.37524 [DOI] [PubMed] [Google Scholar]
- 29. Eiserich J. P., Baldus S., Brennan M. L., Ma W., Zhang C., Tousson A., Castro L., Lusis A. J., Nauseef W. M., White C. R., and Freeman B. A. (2002) Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296, 2391–2394 10.1126/science.1106830 [DOI] [PubMed] [Google Scholar]
- 30. Baldus S., Heitzer T., Eiserich J. P., Lau D., Mollnau H., Ortak M., Petri S., Goldmann B., Duchstein H. J., Berger J., Helmchen U., Freeman B. A., Meinertz T., and Münzel T. (2004) Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic. Biol. Med. 37, 902–911 10.1016/j.freeradbiomed.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 31. Vita J. A., Brennan M. L., Gokce N., Mann S. A., Goormastic M., Shishehbor M. H., Penn M. S., Keaney J. F. Jr., and Hazen S. L. (2004) Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 110, 1134–1139 10.1161/01.CIR.0000140262.20831.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., Ischiropoulos H., Smith J. D., Kinter M., and Hazen S. L. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114, 529–541 10.1172/JCI200421109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holzer M., Gauster M., Pfeifer T., Wadsack C., Fauler G., Stiegler P., Koefeler H., Beubler E., Schuligoi R., Heinemann A., and Marsche G. (2011) Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signal. 14, 2337–2346 10.1089/ars.2010.3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berdnikov V. M., and Bazhin N. M. (1970) Oxidation-reduction potentials of certain inorganic radicals in aqueous solutions. Russ. J. Phys. Chem. 44, 395–398 [Google Scholar]
- 35. Xulu B. A., and Ashby M. T. (2010) Small molecular, macromolecular, and cellular chloramines react with thiocyanate to give the human defense factor hypothiocyanite. Biochemistry 49, 2068–2074 10.1021/bi902089w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemma K., and Ashby M. T. (2009) Reactive sulfur species: kinetics and mechanism of the reaction of hypothiocyanous acid with cyanide to give dicyanosulfide in aqueous solution. Chem. Res. Toxicol. 22, 1622–1628 10.1021/tx900212r [DOI] [PubMed] [Google Scholar]
- 37. Pattison D. I., Hawkins C. L., and Davies M. J. (2009) What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem. Res. Toxicol. 22, 807–817 10.1021/tx800372d [DOI] [PubMed] [Google Scholar]
- 38. Lundquist P., Rosling H., Sörbo B., and Tibbling L. (1987) Cyanide concentrations in blood after cigarette smoking, as determined by a sensitive fluorimetric method. Clin. Chem. 33, 1228–1230 [PubMed] [Google Scholar]
- 39. Cailleux A., Subra J. F., Riberi P., Tuchais E., Premel-Cabic A., and Allain P. (1988) Cyanide and thiocyanate blood levels in patients with renal failure or respiratory disease. J. Med. 19, 345–351 [PubMed] [Google Scholar]
- 40. Savenkova M. L., Mueller D. M., and Heinecke J. W. (1994) Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J. Biol. Chem. 269, 20394–20400 [PubMed] [Google Scholar]
- 41. Boxer G. E., and Rickards J. C. (1952) Studies on the metabolism of the carbon of cyanide and thiocyanate. Arch. Biochem. Biophys. 39, 7–26 10.1016/0003-9861(52)90256-7 [DOI] [PubMed] [Google Scholar]
- 42. Ansell M., and Lewis F. A. (1970) A review of cyanide concentrations found in human organs: a survey of literature concerning cyanide metabolism, “normal”, non-fatal, and fatal body cyanide levels. J. Forensic Med. 17, 148–155 [PubMed] [Google Scholar]
- 43. Matti Maricq M. (2007) Chemical characterization of particulate emissions from diesel engines: a review. J. Aerosol Sci. 38, 1079–1118 10.1016/j.jaerosci.2007.08.001 [DOI] [Google Scholar]
- 44. Baum M. M., Moss J. A., Pastel S. H., and Poskrebyshev G. A. (2007) Hydrogen cyanide exhaust emissions from in-use motor vehicles. Environ. Sci. Technol. 41, 857–862 10.1021/es061402v [DOI] [PubMed] [Google Scholar]
- 45. Moussa S., Leithead A., Li S.-M., Chan T., Wentzella J., Stroud C., Zhang J., Lee P., Lu G., Brook J., Hayden K., Narayan J., and Liggio J. (2016) Emissions of hydrogen cyanide from on-road gasoline and diesel vehicles. Atmos. Environ. 131, 185–195 10.1016/j.atmosenv.2016.01.050 [DOI] [Google Scholar]
- 46. Miller M. R., Shaw C. A., and Langrish J. P. (2012) From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 8, 577–602 10.2217/fca.12.43 [DOI] [PubMed] [Google Scholar]
- 47. Franchini M., Guida A., Tufano A., and Coppola A. (2012) Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms. J. Thromb. Haemost. 10, 2438–2451 10.1111/jth.12006 [DOI] [PubMed] [Google Scholar]
- 48. Lucking A. J., Lundbäck M., Barath S. L., Mills N. L., Sidhu M. K., Langrish J. P., Boon N. A., Pourazar J., Badimon J. J., Gerlofs-Nijland M. E., Cassee F. R., Boman C., Donaldson K., Sandstrom T., Newby D. E., and Blomberg A. (2011) Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 123, 1721–1728 10.1161/CIRCULATIONAHA.110.987263 [DOI] [PubMed] [Google Scholar]
- 49. Furtmüller P. G., Jantschko W., Regelsberger G., Jakopitsch C., Moguilevsky N., and Obinger C. (2001) A transient kinetic study on the reactivity of recombinant unprocessed monomeric myeloperoxidase. FEBS Lett. 503, 147–150 10.1016/S0014-5793(01)02725-9 [DOI] [PubMed] [Google Scholar]
- 50. Moguilevsky N., Garcia-Quintana L., Jacquet A., Tournay C., Fabry L., Piérard L., and Bollen A. (1991) Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur. J. Biochem. 197, 605–614 10.1111/j.1432-1033.1991.tb15950.x [DOI] [PubMed] [Google Scholar]
- 51. Furtmüller P. G., Burner U., and Obinger C. (1998) Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry 37, 17923–17930 10.1021/bi9818772 [DOI] [PubMed] [Google Scholar]
- 52. Furtmüller P. G., Jantschko W., Regelsberger G., and Obinger C. (2001) Spectral and kinetic studies on eosinophil peroxidase compounds I and II and their reaction with ascorbate and tyrosine. Biochim. Biophys. Acta 1548, 121–128 10.1016/S0167-4838(01)00230-8 [DOI] [PubMed] [Google Scholar]
- 53. Nelson D. P., and Kiesow L. A. (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 49, 474–478 10.1016/0003-2697(72)90451-4 [DOI] [PubMed] [Google Scholar]
- 54. Havel R. J., Eder H. A., and Bragdon J. H. (1955) The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345–1353 10.1172/JCI103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delporte C., Franck T., Noyon C., Dufour D., Rousseau A., Madhoun P., Desmet J. M., Serteyn D., Raes M., Nortier J., Vanhaeverbeek M., Moguilevsky N., Nève J., Vanhamme L., Van Antwerpen P., and Zouaoui Boudjeltia K. (2012) Simultaneous measurement of protein-bound 3-chlorotyrosine and homocitrulline by LC-MS/MS after hydrolysis assisted by microwave: application to the study of myeloperoxidase activity during hemodialysis. Talanta 99, 603–609 10.1016/j.talanta.2012.06.044 [DOI] [PubMed] [Google Scholar]
- 56. National Research Council of the National Academies (2011) Guide for the Care and Use of Laboratory Animals, 8th Ed., National Academies Press, Washington, D. C. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.