Abstract
Membrane biology seeks to understand how lipids and proteins within bilayers assemble into large structures such as organelles and the plasma membranes. Historically, lipids were thought to merely provide structural support for bilayer formation and membrane protein function. Research has now revealed that phospholipid metabolism regulates nearly all cellular processes. Sophisticated techniques helped identify >10,000 lipid species suggesting that lipids support many biological processes. Here, we highlight the synthesis of the most abundant glycerophospholipid classes and their distribution in organelles. We review vesicular and nonvesicular transport pathways shuttling lipids between organelles and discuss lipid regulators of membrane trafficking and second messengers in eukaryotic cells.
Keywords: glycerophospholipid, phospholipase, phospholipid metabolism, membrane trafficking, organelle, membrane contact sites, nonvesicular transport, vesicular transport, phosphatidylinositol, sphingolipid, flippase, scramblase, phospholipids, lipid transfer proteins
Lipid components of eukaryotic membranes
Glycerophospholipids (GPL),5 sphingolipids, and sterols are the three major classes of lipids found in eukaryotic membranes. This review will focus primarily on the GPLs, including the biophysical nature of these molecules, biosynthetic pathways and the role of lipases in vesicular transport pathways, and the generation of critical signaling molecules. Additionally, we will highlight the distribution of GPLs between various organelles, their transbilayer distribution, and the role of nonvesicular transport pathways to shuttle lipids between organelles.
Glycerophospholipids
Phosphatidylcholine (PtdCho), phosphatidylethanolamine (PtdEtn), phosphatidylserine (PtdSer), and phosphatidylinositol (PtdIns) make up the majority of the glycerol backbone-containing phospholipids and are prominent components of most organellar membranes (Fig. 1) (1). Because of their amphiphilic nature, GPLs are energetically favored to self-assemble to form a continuous bilayer with the headgroups facing outward and the hydrophobic tail lining the interior (2). PtdCho is the most abundant phospholipid in the majority of organelles, ranging from 41 to 57 mol % of the total GPL (Fig. 1). The cylindrical shape of PtdCho allows it to spontaneously organize into planar bilayers, and its propensity to contain at least one unsaturated fatty acyl chain means that the bilayers possess significant fluidity at 37 °C (3). PtdEtn is the second most abundant phospholipid in eukaryotic membranes, which comprises 17–38 mol % of total phospholipid (Fig. 1). PtdEtn differs from PtdCho by the absence of the three methyl groups compared with the choline moiety (4). As a result, PtdEtn has a smaller headgroup that results in a more conical shape compared with PtdCho. Additionally, PtdEtn can arrange as a hexagonal phase, unlike most other GPLs (5). The addition of the nonbilayer forming PtdEtn to PtdCho facilitates the generation of spontaneous curvature that in the context of the cell is vital for membrane bending and tubulation that is necessary to support the fission and fusion steps in vesicular transport (6). In mammalian cells, PtdEtn and PtdCho also serve as substrates for the production of PtdSer via enzyme-mediated base-exchange reactions (7).
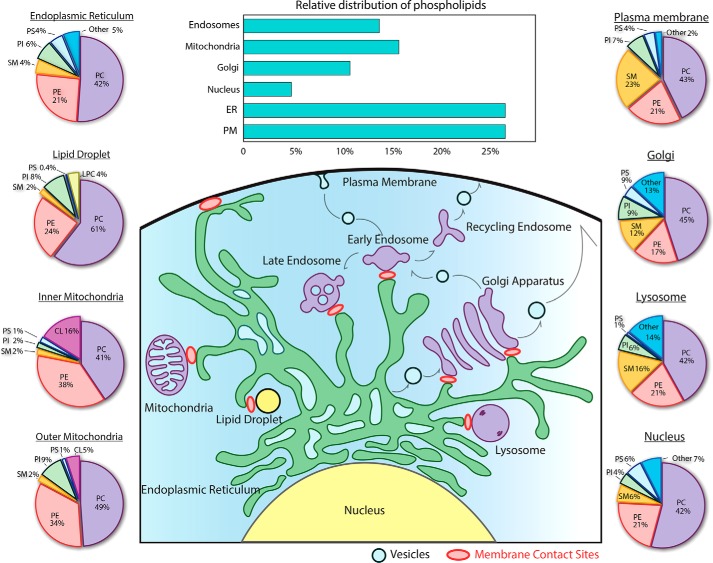
Figure 1.
Glycerophospholipid composition of organelles. The bar graph highlights the subcellular distribution of glycerophospholipids (GPLs) between the different organelles in baby hamster kidney cells (128). The pie charts display the relative abundance of each class of GPL in organelles based on composite data from rat hepatocytes (1) and for lipid droplets from murine hepatocytes (129). Except for the mitochondrial PtdGly (PG) and CL, the other GPLs are present in all organelle membranes but display heterogeneity. PtdCho (PC) is the most abundant, comprising 45–55 mol % of the GPL in the cell. PtdEtn (PE) is the second most abundant GPL and is mainly enriched in inner membranes of mitochondria (∼35–40 mol %), although it is less prominent in other organelles (∼17–25 mol %). PtdSer (PS) is a precursor for the mitochondrial PE, and as a result of repaid consumption, its abundance is very low in the inner mitochondrial membranes (1 mol %). PS is also a minor component of lysosomes (∼1 mol %), the endoplasmic reticulum (∼4 mol %), nucleus (∼6 mol %), Golgi (∼4 mol %), and plasma membrane (∼4%). Post-Golgi apparatus organelles are enriched in sphingomyelin (SM) with it being most abundant (∼23%) in the PM. The designation Other includes essential precursors and signaling lipids such as PA, DAG, and lysolipids. The schematic highlights the vesicular and nonvesicular pathways responsible for the intracellular trafficking of lipids. The endoplasmic reticulum (ER) is the principal site of synthesis for most lipid species. The extensively branched reticular network of the ER facilitates the establishment of MCS with other organelles, including the Golgi apparatus, mitochondria, endosomes, lysosomes, lipid droplets, and plasma membrane. These MCS bring donor and acceptor membranes in proximity (∼30 nm) where the exchange of GPLs and cholesterol can occur. Most organelles are interconnected via the vesicular transport pathways. GPLs are essential for the formation of vesicles that transport transmembrane and lumenal proteins throughout the cell. Thus, a by-product of vesicular transport is the movement of GPLs and other lipids.
PtdSer is, by comparison, a relatively minor component of eukaryotic membranes (comprising 1–6 mol % of total phospholipids) (Fig. 1), and it plays a crucial role in providing a negative surface charge to membranes due to the acidic nature of its headgroup (8). PtdSer is enriched in the inner leaflet of the plasma membrane despite the fact that it is synthesized in the ER and consumed in the mitochondria (4). A significant fraction of the newly synthesized PtdSer can be transported from the ER to the mitochondria (28). There it serves as a substrate to produce a mitochondrial pool of PtdEtn that is essential for mitochondrial function (29, 30). The mechanism by which PtdSer is accumulated in the PM and enriched in the inner leaflet of the PM is discussed below.
Similarly, PtdIns is also negatively charged, comprising 2–9% of total phospholipids of organelle membranes (Fig. 1). However, its arguably more important feature is the fact that the inositol ring can be phosphorylated on the 3-, 4-, and 5-OH groups to produce phosphoinositides (discussed below). Furthermore, PtdIns is also used to covalently link peripheral proteins to the outer leaflet of the plasma membrane. Collectively, these proteins are referred to as glycosylphosphatidylinositol-linked proteins (9). Thus, in addition to being critical for the structure of biological membranes, GPLs also play critical roles in protein targeting and signaling.
Other GPLs, such as phosphatidic acid (PA), phosphatidylglycerol (PtdGly), and cardiolipin (CL), typically constitute a relatively minor portion of the total cellular GPLs. PA comprises a relatively minor 1 mol % of total phospholipids of organelle membranes but is a critical intermediate in biosynthetic pathways and as a signaling molecule (Fig. 2). PtdGly is predominantly found in the mitochondria where it is an intermediate in the biosynthesis of CL. However, PtdGly synthase activity has been found in the ER (10), although there is the possibility that this is due to the contamination of mitochondrial membranes during the cellular fractionation. CL is also primarily restricted to the mitochondria where it mainly supports functions into the mitochondria, including protein translocation, and respiratory chain function (11–13).
Figure 2.
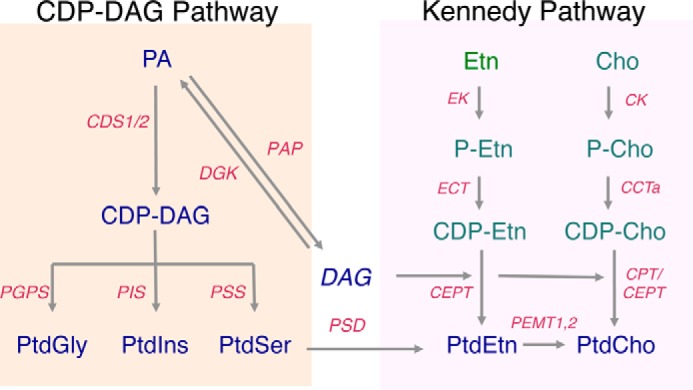
CDP-DAG and Kennedy pathways. The CDP-DAG pathway begins with the consumption of phosphatidate (PA) on the cytosolic leaflet of the endoplasmic reticulum (ER) by either CDP-DAG synthase (CDS) 1 or 2 to produce cytidine diphosphate diacylglycerol (CDP-DAG). The CDP-DAG is then used as a substrate by the phosphatidylinositol synthase enzyme (PIS) to catalyze the production of phosphatidylinositol (PtdIns) from CDP-DAG. CDP-DAG is also used in the mitochondria by the phosphatidylglycerophosphate synthase (PGPS) to produce phosphatidylglycerol (PtdGly)-phosphate, which is, in turn, dephosphorylated to produce PtdGly. Phosphatidylserine (PtdSer) synthesis is catalyzed by PtdSer synthase 1 (PSS1). A significant fraction of the newly synthesized PtdSer is transported from the ER to the mitochondria. There it serves as a substrate for the enzyme PtdSer decarboxylase (PSD) to produce a mitochondrial pool of phosphatidylethanolamine (PtdEtn). In the Kennedy pathway, choline (Cho) and ethanolamine (Etn) are first activated for phosphorylation by choline kinase (CK) and ethanolamine kinase (ETNK), respectively. Next, the phosphobase serves as substrates for the rate-limiting step of the pathway catalyzed by CTP:phosphocholine cytidyltransferase (CCT) and CTP:phosphoethanolamine cytidyltransferase (ECT), respectively, yielding CDP-Cho and CDP-Etn. The final step of the pathway is catalyzed by two homologous proteins, the phosphatidylcholine (PtdCho)-specific CPT and the PtdCho/PtdEtn producing CEPT. Finally, PtdEtn can be converted to PtdCho by three successive methylation reactions catalyzed by PtdEtn methyltransferases, PEMT1 and -2, in the liver of mammals. PAP, phosphatidate phosphatase.
Sphingolipids and sterols
Sphingolipids, which consist of a serine backbone as opposed to the glycerol backbone, are critical components of the exofacial leaflet of the plasma membrane with sphingomyelin (SM) comprising 23 mol % of the total phospholipid (Fig. 1). Sphingolipids display an extensive range of headgroup size; they can possess merely a hydroxyl group, as in the case of ceramide, an intermediate-sized phosphocholine headgroup as seen in the highly abundant sphingomyelin, or the more complex sugar-modified headgroups collectively referred to as glycosphingolipids (14).
The third class of lipid molecules essential for eukaryotes is classified as steroid alcohols or simply sterols. Cholesterol is the predominant sterol found in all mammalian cells. Cholesterol acts to modulate the membrane fluidity and permeability via interacting with neighboring lipids, such as PtdCho and SM (15, 16). Because of the importance of cholesterol in cell biology and the development of atherosclerosis, many aspects of its synthesis, transport, and functions have been extensively studied.
An overview of glycerophospholipid biosynthetic pathways
The ER is the predominant site of GPL biosynthesis, including PtdCho, PtdEtn, PtdIns, and PtdSer (13), whereas the mitochondria produce two other GPLs, PtdGly and CL (14). The condensation of the glycerol backbone and two acyl chains represents the initial steps in GPL synthesis. Typically, glycerol 3-phosphate, a key intermediate in the glycolysis pathway, is dually acylated to form PA that in turn can be dephosphorylated to form diacylglycerol (DAG) (15–17). Together, DAG and PA are essential substrates for the further production of GPLs by the CDP-DAG and Kennedy pathways (Fig. 2).
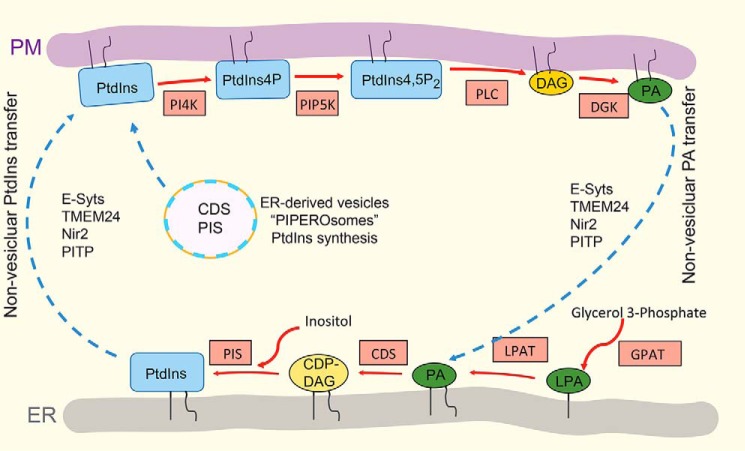
The CDP-DAG pathway begins with the consumption of PA and cytidine triphosphate (CTP) on the cytosolic leaflet of the ER to produce CDP-DAG and pyrophosphate (17). The CDP-DAG is then used by a variety of enzymes such as PtdIns synthase together with myo-inositol to produce PtdIns (Fig. 3) (18). A recent study identified highly mobile ER-derived PtdIns synthase containing vesicles in the cytoplasm as a potential site of PtdIns synthesis (Fig. 3) (19). These small vesicles were termed PIPEROsomes for “PtdIns Producing ER-derived Organelle.” The importance and regulation of the PIPEROsome are not understood, but it is hypothesized that it represents an intermediate between the ER and PM to supply the PM with PtdIns. Whether this is through direct fusion, hemifusion, or requires a PtdIns transfer protein is still unknown. Within the plasma membrane, PtdIns serves as a substrate for kinases and the generation of phosphorylated forms of PtdIns, in particular, PtdIns(4,5)P2, a lipid that regulates a multitude of cellular processes. See below for more on the PI cycle.
Figure 3.
Phosphatidylinositol cycle. The synthesis of PtdIns occurs in the ER or possibly in ER-derived vesicles termed PIPEROsomes. First, glycerol 3-phosphate (G3P) is dually acylated by the actions of acyltransferases glycerol-3-phosphate O-acyltransferase (GPAT) and 1-acylglycerol-3-phosphate O-acyltransferase (LPAT), which forms lysophosphatidic acid (LPA) and produces phosphatidic acid (PA), respectively. Next, PA in the ER or the PIPEROsomes is converted to CDP-diacylglycerol (CDP-DAG) by the enzyme CDP-DAG synthases (CDS). Phosphatidylinositol synthase (PIS) in the ER of PIPEROsome catalyzes the coupling of CDP-DAG to myo-inositol to form PtdIns. Once synthesized in the ER or the ER-derived vesicles, PtdIns is delivered to the PM by the secretory pathway (not depicted) or by the actions of either nonselective (TMEM24, E-Syts) or PtdIns (Nir2, PITP). The PtdIns serves as a substrate for generating the plasmalemmal phosphoinositides. PI4,5P2 is vital to facilitate many of the plasmalemmal transactions such as signaling in response to growth factors, exocytosis, endocytosis, and the polymerization of cortical actin. The activation of PLC isoforms converts PI4,5P2 into DAG, which can then be converted back to PA by one of 10 DAG kinases. To prevent the accumulation of PA and to replenish the plasmalemmal PtdIns pool, PA is transferred back to the ER via nonvesicular lipid transport proteins. Red arrows represent metabolic reactions; blue arrows represent intracellular transport process; enzymes. DGK, diacylglycerol kinase.
The next pathway responsible for synthesizing much of the PtdCho and PtdEtn is named after Dr. Eugene Kennedy who described the pathway (Fig. 2) (20–22). The final step of the pathway is catalyzed by two homologous proteins, the PtdCho-specific choline phosphotransferase (CPT) and the PtdCho/PtdEtn-producing choline/ethanolamine phosphotransferase (CEPT). Experimental evidence suggests that CEPT resides in the ER, whereas CPT is enriched in the Golgi apparatus. The presence of CPT in the Golgi is important as it will consume DAG and thereby temper vesicular transport emanating from the Golgi (25). The Kennedy pathway both produces PtdEtn and PtdCho, whereas PtdEtn can be converted to PtdCho in yeast and in the liver of mammals via the concerted actions of two PtdEtn methyltransferases that triply methylate PtdEtn (26, 27).
Glycerophospholipid topology and topogenesis
As constituents of an amphiphilic bilayer, GPLs display lateral diffusion and rotation about their longitudinal axis (23, 24). However, the spontaneous transversal diffusion of the polar headgroups through the hydrophobic core of the bilayer is restricted due to the high-energy barrier (25). Estimates suggest that within a bilayer GPLs laterally diffuse ∼109 faster than they spontaneously translocate (or flip-flop) between leaflets, which occurs on the order of hours per molecules (Fig. 4) (25, 26). These observations suggest that in the absence of any facilitated movement the transbilayer distribution of lipids should remain virtually indelible once a membrane or vesicle is formed. At the cellular level for GPLs to transverse a bilayer, proteins must lower the energy barrier of the movement.
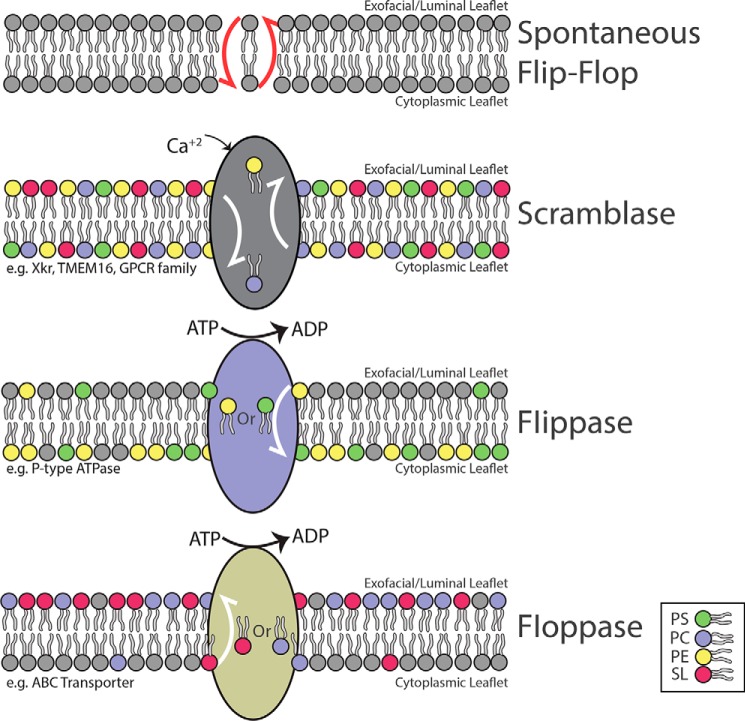
Figure 4.
Phospholipid translocases in lipid bilayers. The spontaneous flip-flop of GPLs between leaflets of a bilayer is energetically unfavorable. Scramblase is the term used to describe a variety of proteins (i.e. TMEM16F, Xlr4, select GPCRs) that can in an energy-independent manner mediate the bidirectional transfer of GPLs between leaflets thereby collapsing the symmetry of the PM. Conversely, Flippase (inward movement) and Floppase (outward movement) are energy-dependent proteins that couple the consumption of ATP with the movement of lipids across the bilayer.
GPLs, with perhaps the exception of PtdSer (27, 28), are symmetrically distributed between both leaflets of the bilayer in the ER. Progression through the secretory pathway is accompanied by the appearance of asymmetry in the trans-Golgi and is apparent in the PM with PtdEtn and PtdSer being enriched in the cytosolic leaflet, and PtdCho and sphingolipids present mainly in the exofacial leaflet (29–31). The majority of the active sites responsible for the synthesis of sphingomyelin and other sphingolipids are found on the lumenal side of the Golgi (32–34), and thus, their asymmetry can be explained by a lack of flip-flop in post-Golgi membranes. The topological distribution of the GPLs is controlled by the actions of proteins referred to as scramblases, floppases, and flippases (see Fig. 4) (35). Overall, the marked asymmetry of the PM generates two monolayers that are chemically distinct. The abundance of the anionic PtdSer in the cytosolic leaflet contributes to a negative surface charge that accommodates ionic interactions of polybasic proteins with the inner layer of the PM (36). This negative surface charge can also influence the activity of integral membrane proteins by interacting with N- or C-terminal tails and cytosolic loops of these proteins. The importance of asymmetry of the PM is evident, and remarkably little is known about the topological distribution of GPLs in other organelles such as endosomes and lysosomes (37). As the cellular functions of flippases are examined in greater detail, we suspect that the role of asymmetry or at least phospholipid flipping will become more apparent. Indeed, the loss of the flippase Tat-1 in Caenorhabditis elegans is associated with a loss of PtdSer asymmetry in endosomal compartments and defective sorting and recycling pathways (38).
Scramblase-mediated expansion of the ER
The ER resident enzymes responsible for the synthesis of GPLs are oriented in such a manner that their active sites are exclusively facing the cytosol (39). Immediately, one should recognize that having an utterly asymmetric synthesis of GPLs would pose a problem for the cell if not dealt with rapidly: having lipid synthesis confined to one leaflet would lead to the rapid expansion of the cytosolic leaflet while the luminal leaflet remained constant. It is suggested that a phospholipid transporter known as scramblase allows for the rapid flip-flop of GPLs between leaflets of the bilayer. Collectively, scramblases facilitate the bidirectional translocation of GPLs in an energy-independent manner (40). In the case of the ER with on-going lipid synthesis, scramblase activity supports not only symmetry between the two leaflets but also to couple growth of the two leaflets (41). The protein(s) responsible for scramblase activity in the ER had remained elusive for many years, but recent results suggest that select G-protein–coupled receptors (GPCRs) might provide this activity. Two GPCRs, the apoprotein opsin and holo-rhodopsin, are proposed to equilibrate GPLs across the photoreceptor disc membranes in the retina (42, 43). Additionally, it was demonstrated that the β2-adrenergic and the adenosine A2A receptors could scramble lipids in vitro (42). Furthermore, molecular dynamics simulations of using opsin suggest that the headgroup of the phospholipids pass through a hydrophilic cavity created by transmembrane helices 6 and 7 (44). To date, the notion that select GPCRs could act as scramblases is based on in vitro reconstitution experiments and has not yet been examined in living cells. The obvious question that arises from these studies is as follows: why do GPCRs act as scramblases in the ER but have no apparent activity in the PM? One possibility is that the local lipid environment influences the ability of the GPCRs to scramble phospholipids. For instance, when compared with the PM, the ER contains more unsaturated acyl chains, less cholesterol, and little sphingolipids. Additional biophysical differences exist as the ER is not as thick as the PM (45), and although the cytosolic leaflet of the PM is negatively charged, the ER is near neutral (36). Undoubtedly, this area needs further investigations to help clarify the role of GPCRs as scramblases and to determine whether a specific subset of GPCRs provide scramblase activity to the ER, during transit to other subcellular compartments, or whether another unknown ER-resident scramblase exists.
Scramblase activation and the disruption of plasmalemmal asymmetry
Constitutively active scramblases support the growth of the ER. However, the activity of plasmalemmal scramblases is often less apparent. Two classic examples of scramblase activation are present in mammalian cells. The first example is the exposure of PtdSer on the surface of platelets in response to activation that is required for blood coagulation (46, 47). The second example is the exposure of PtdSer by apoptotic cells with the exposed PtdSer serving as an “eat-me” signal for neighboring cells and macrophages (48, 49). The protein identity of these scramblase activities remained elusive for many years. However, recent seminal findings have identified the proteins responsible for these activities. The importance of scramblase activity in platelets is exemplified in Scott syndrome, a rare bleeding disorder where PtdSer fails to translocate to the extracellular leaflet (50, 51). This syndrome is due to inactivating mutations in the protein referred to as transmembrane protein (TMEM) 16 family of proteins F (TMEM16F) (52). The TMEM16 family of proteins has been characterized as calcium-gated chloride channels, but a growing body of literature suggests that TMEM16F and possibly TMEM16C, -D, -F, -G, and -J have evolved the ability to scramble phospholipids in response to elevated cytosolic calcium (53, 54). The manifestation of Scott syndrome appears to be due to the predominance of TMEM16F in platelets. The cellular roles for this family of scramblases in other cell types are less clear. However, TMEM16E has recently been shown to be necessary for the motility of mouse sperm (55).
Platelets from Scott syndrome patients still expose PtdSer in response to apoptotic signals (56). Additionally, apoptotic platelets can expose PtdSer in the absence of calcium, consistent with the notion that another type of scramblases is present in the PM (56, 57). This second class of scramblase responsible for PtdSer exposure during apoptotic cell death was identified as XK family protein 8 (Xkr-8) (58). Additional studies using Xkr8-deficient murine cells and human cancer cell lines with low levels of Xkr-8 demonstrated that these cells fail to expose PtdSer during apoptosis (58). Importantly, in all cases, the impaired exposure of PtdSer resulted in the inefficient clearance by phagocytic cells. Expression of two related proteins Xkr-4 and -9 also rescued apoptotic scrambling in Xkr8-deficient cells (59) demonstrating that, similar to the TMEM16 family, multiple members of the Xk family possess scramblase activity. PtdSer exposure is a critical step in the execution of apoptotic cell death and clearance by phagocytes. Instead of being stimulated by cytosolic calcium, Xkr-8 is activated following proteolysis by active caspase-3 (58). This regulation ensures that PtdSer is only exposed after a sufficient level of caspase-3 activation has been reached. The exposure of PtdSer on the cell surface is further potentiated by the caspase-3–mediated cleavage of ATP11C, a PtdSer flippase (60).
Flippases and floppases, generators of membrane asymmetry
The asymmetric distribution of GPLs in the PM is a hallmark of eukaryotes. Flippases are aminophospholipid translocases that consume ATP to move PtdSer and PtdEtn from the lumenal and exofacial leaflets to the cytosolic leaflet (61). A flippase activity had initially been purified from red blood cells, chromaffin granules, and synaptic vesicles with the gene encoding the chromaffin granule resident flippase being cloned in the mid-1990s (62). Sequence comparisons determined that this gene encoded an unrecognized P-type ATPase with a homolog in yeast named Drs2. The original study found that drs2 cells were deficient in the flipping of fluorescently-tagged NBD-PtdSer across the PM, and later studies suggest that the Drs2 functions primarily in the Golgi (63) and that its homologs, Dnf1 and Dnf2, provide the plasmalemmal flippase activity (64). P4-ATPases are absent in prokaryotes but are present as multiple members in eukaryotes. For example, the human genome contains 14 putative flippases and the yeast Saccharomyces cerevisiae genome encodes five. Several of the P4-ATPases form heterodimers with the noncatalytic β-subunit Cdc50 that contains two transmembrane domains and a glycosylated ectodomain (65). Although Cdc50 is noncatalytic, association with P4-ATPase is vital for export from the ER and flippase activity (66).
Conceptually, aminophospholipid flipping and the generation of membrane asymmetry are straightforward. Perhaps a less obvious by-product of flipping is the generation of membrane curvature. Studies in yeast have revealed that flippases–and likely the generation of membrane curvature–can support the formation of secretory vesicles or endocytic carriers (64, 67). In mammalian cells, the first appearance of bilayer asymmetry is found in the trans-Golgi cisternae (TGN) (27), supporting the notion that flippase activity could be supporting vesiculation of these membranes. Flippases have also been shown to support endocytic recycling (68), although in this compartment the identity of the substrate is less clear. Overall, mutations in the mammalian flippases have been described to be associated with a wide range of pathophysiological conditions impacting nearly every system in the body (69). This is likely due in part to the difference in the tissue distribution of the various flippases. However, it is unclear whether the defects arise from a disruption in asymmetry or impairment in vesicular transport pathway(s).
In contrast, floppases mediate the translocation of lipids in the opposite direction: from cytosolic leaflet to exofacial leaflet (70, 71). Known members of the floppase family include the ATP-binding cassette (ABC) transporter superfamily of transporters that were initially identified as multidrug-resistant pumps (71). In contrast to the somewhat promiscuous drug-effluxing ABC transporters, the lipid floppases ABCA1, ABCB1, ABCG1, ABCB4, and ABCC4 have evolved more specialized function to transport sterols, PtdCho, SM, and PtdSer. For instance, ABCA1 and ABCG1 are known to be critical for the ability of macrophage to efflux cholesterol to high-density lipoprotein (72). However, in hepatocytes ABCG4 catalyzes the movement of PtdCho to the exofacial leaflet of the canalicular membrane to allow excretion of PtdCho into the bile (73).
Inter-organellar transport of lipids
As highlighted in Fig. 1, organelles possess their lipid compositions to go along with unique proteomes. However, the PM and endosomes do not possess a significant capacity to synthesize lipids, especially GPLs. Instead, these organelles acquire their GPLs from the other organelles, especially the ER. Eukaryotic cells rely on the following two modes of transport to move lipids: vesicular and nonvesicular trafficking (see Fig. 1). Vesicular transport has long been studied and is essential for the formation of secretory vesicles, endocytosis, and the inter-organellar transport of luminal and integral membrane proteins. For instance, COPII vesicles assemble at ER exit sites and contain proteins destined for the Golgi apparatus, and at the TGN, vesicles destined for the PM or endosomes are formed. Vesicles, like organelles, are delineated by a membrane bilayer, and consequently, this mode of protein trafficking inevitably includes bulk lipid transport. The action of TGN resident flippases together with the segregation of cholesterol and sphingolipids supports the formation of these carriers (67, 74, 75). As shown in Fig. 1, most organelles participate in vesicular trafficking either by giving rise to or accepting vesicles. However, as the rates of cell growth and vesicular transport can vary significantly by cell type, the precise estimates of the bulk vesicular movement of lipids are lacking.
An alternative to the formation of transport vesicles is the use of soluble proteins to shuttle the hydrophobic lipids from one organelle to the other. The existence of this nonvesicular lipid transport was alluded to many years ago as lipid transport could still be observed under conditions where vesicular trafficking is inhibited (76–78). Membrane contact sites (MCS) have been implicated in this mode of lipid transport (79). These regions are ≈30 nm apart between two adjacent organelles and likely help to facilitate the inter-organellar exchange of lipids through the local enrichment of specific enzymes and proteins (80, 81). The ER typically creates MCS with other organelles, including Golgi, mitochondria, endosomes, peroxisomes, lipid droplets, and PM. In vitro studies have demonstrated that spontaneous exchange of lipids between vesicles can occur, but this process is exceedingly slow and would likely have little importance within the cell (82, 83). Thus, merely bringing membranes close together would have little benefit. To deal with this challenge, cells have evolved soluble lipid-transport proteins (LTPs), which contain a hydrophobic binding pocket that can extract lipids and facilitate transport through an aqueous environment (84, 85). In vitro, these LTPs can extract lipid and then either reinsert them in the same “donor” liposome or transport the lipid through an aqueous buffer and deposit it in a “recipient” liposome. However, most LTPs have modest transfer rates in vitro, considering the vast quantities of cellular lipids such as cholesterol and PtdSer. This has raised the question whether these molecules function as simple transfer proteins or whether they have specialized context-dependent functions (86, 87).
The number of putative LTPs has grown immensely in the last 2 decades. In general, LTPs are found as multigene families with varying substrate specificities. For instance, sterol transfer is mediated by a subset of the oxysterol-binding protein (OSBP)-related proteins (ORPs) (88), and the steroidogenic acute regulatory protein (StAR)-related lipid-transfer (StART) domain proteins (89). Conversely, the transfer of PtdIns is mediated by PtdIns-transfer proteins (PITP) and Nir2 (19, 90). Recently, ORP5/8 and TMEM24 have been implicated in PtdSer and PtdIns transfer, respectively, at ER-PM MCS (Fig. 1) (91, 92). Because of their modular nature, several of the LTPs can interact with both target donor and acceptor membranes. For example, OSBP has a pleckstrin homology domain that interacts with the phosphoinositides, PtdIns4P, and active ADP-ribosylation factor (Arf)1 (93). Additionally, the presence of an FFAT (two phenylalanines in acidic tract) motif within OSBP allows binding to the ER resident integral membrane protein, vesicle-associated membrane protein-associated protein (VAP), thereby associating OSBP with ER membranes (94, 95). This dual targeting to two proximal membranes creates a narrow bridge that is thought to support the transfer of sterols from ER to Golgi. Alternatively, a few LTPs are integral proteins that contain transmembrane domains to allow them to be anchored to organelles, including the ER and late endosomes. This includes the late endosome-anchored cholesterol transfer protein STARD3, the ER tail-anchored PtdSer–PI4P exchange proteins ORP5 and ORP8, and the nonselective ER-tethered extended synaptojanins (E-Syts). For more information about LTPs and MCS sites, we refer readers to review articles 79, 85.
Roles of GPLs as second messengers and molecular beacons
Phospholipases and cell signaling
In addition to being a critical structural building block of membrane bilayers, GPLs also serve as a reservoir for lipid second messengers. Indeed, many of low abundant lipids, such as lyso-PtdCho (LPC), lyso-PA (LPA), PA, and DAG, can act as signaling molecules with a variety of targets. Examples of this include the ability of LPA to act as an autocrine or paracrine signal through activation of a specific -protein–coupled receptor (e.g. LPA) (96). Within the cell, DAG and PA can influence the recruitment and activation of cytosolic proteins (97, 98). Some of these lipid molecules are intermediates in the de novo biosynthetic pathway, whereas the actions of phospholipases can locally generate individual species.
Phospholipase is a general term used to describe an enzyme that hydrolyzes phospholipids. However, phospholipases constitute several families of enzymes with unique activities, substrate preferences, and regulation. As such, phospholipases are categorized into four major classes, termed A, B, C, and D, according to the type of reaction they catalyze (99). Phospholipase A (PLA) enzymes cleave either the sn-1 acyl chain (designated PLA1) or the sn-2 acyl chain (PLA2), whereas the phospholipase B enzymes cleave at both the sn-1 and sn-2 positions. The PLA2 family is extensive and impacts numerous biological processes. The family of PLA2 enzymes can be divided into five distinct categories, namely secreted PLA2, cytosolic PLA2, Ca2+-independent PLA2, platelet-activating factor acetylhydrolase, and lysosomal PLA2s. The PLA2 superfamily of enzymes varies in catalytic mechanism, function, localization, and structural features. As this is a Minireview with limited space, we refer readers to other reviews for greater detail on PLA2 enzymes (100, 101). Typically, PLA2 uses GPLs as a substrate to release polyunsaturated fatty acids, such as arachidonic acid (AA), from the sn-2 position. AA can be used by a variety of enzymes to produce compounds called eicosanoids, which include prostaglandins and leukotrienes (102). Eicosanoids act to participate in a wide range of physiological and pathological processes, including immune response, inflammation, sleep regulation, and pain perception, by activating specific GPCRs (103). The lysophospholipids can be used as a precursor for LPA and platelet-activating factor (PAF), which are lipid mediators. LPAs play a role in cell proliferation, survival, and migration (104); PAF is vital to the processes of inflammation (105). Many of these molecules serve as ligands for GPCRs and can serve as autocrine, paracrine, or endocrine signaling molecules.
In addition to the breaking down of GPLs and the generation of signaling molecules, concerted deacylation-reacylation reactions also remodel the acyl chains of GPLs. This process, referred to as the Lands cycle, involves the generation of lysophospholipids by the actions of PLA enzymes together with specific LPAATs (106, 107). This cycle serves critical cellular roles, including the replacement of oxidized fatty acids and helping to provide diversity in acyl chain compositions (108). Additionally, the generation and consumption of lysophospholipids by the Lands cycle has essential implications in vesiculation and transport especially within the Golgi apparatus (107). The inverted conical shape of lysophospholipids supports vesiculation of membranes and vesicle fusion (109, 110). In the Golgi apparatus, the actions of at least four PLAs enzymes contribute to the structure of the Golgi cisternae as well as fusion and fission of transport carriers (107). The actions of these enzymes are counteracted by the actions of at least one enzyme, lysophosphatidic acid acyltransferase 3, as overexpression of this protein prevents Golgi tubulation while its genetic silencing promotes fragmentation (111). How these enzymes are coordinated and regulated is still not well-understood but again highlights the importance of lipids as components of biological membranes.
Remodeling of CL has been described to occur in the mitochondria of yeast and mammals. Improper acyl chain remodeling of CL in humans results in an inherited cardiomyopathy termed Barth syndrome (112). Newly synthesized CL in yeast contains saturated acyl chains (113) that are replaced with oleic acid, whereas hepatocytes initially synthesize tetraoleoyl-CL that is remodeled to tetralinoleoyl-CL (114). In yeast, a cardiolipin-specific phospholipase A-like enzyme designated Cld1 (115) has been characterized that works upstream of Taz1, the homolog of the human enzyme Taffazzin, which possess monolyso-CL transacylation activity and is the causative agent in Barth syndrome (116, 117). To our knowledge, the functional human equivalent of Cld1 has not been identified. Regardless, the failure to adequately remodel the acyl chain composition of CL or possibly to prevent the accumulation of monolyso-CL is associated with a variety of mitochondrial defects, including protein import, oxidative phosphorylation, and fission and fusion.
The mammalian phosphoinositide-specific phospholipase C (PLC) enzymes are classified into six isotypes (β, γ, δ, ϵ, ζ, and η) and play an essential role in signal transduction (118). Upon isoform-specific activation, PLCs act to generate the secondary messengers, inositol 1,4,5-triphosphate (IP3) and DAG, by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) (119). The released IP3 can then bind and activate IP3-gated calcium channels in the ER leading to increased cytosolic calcium. This increase in cytosolic calcium can stimulate secretion via SNARE-mediated exocytosis and further signaling by conventional protein kinase C isoforms. Importantly, the DAG generated by PLCs can activate both conventional and novel isoforms of protein kinase C (as well as other proteins) that further potentiate signals (120).
Phospholipase D (PLD) is the final type of phospholipase. PtdCho is the natural substrate of the mammalian PLD1 and PLD2 (121). The hydrolysis of the phosphodiester bond of PtdCho by PLD results in the production of free choline and phosphatidic acid. PLD1 is found in a variety of subcellular compartments, including the Golgi apparatus, endosomes, and lysosomes, whereas PLD2 is found primarily at the PM. The activation of these enzymes leads to the localized production of PA. The small headgroup of PA promotes membrane curvature, and PLD activation has been reported to be required for optimal clathrin-mediated endocytosis (122, 123). Additionally, as PA is negatively charged, it could also contribute to the activation or recruitment of proteins with polybasic regions such as Rac1. Finally, a variety of proteins have been described to bind PA in a “lock-and-key” manner, including mammalian target of rapamycin (mTOR), its downstream activation of S6 kinase, and Raf1 kinase (97).
Phosphoinositides
Among GPLs, the phosphorylated derivatives of PtdIns often referred to as “phosphoinositides” or “PIPs” are especially important in signal transduction and protein targeting. As described above, the metabolism of the seven PIP species is controlled by the actions of 19 phosphoinositide kinases and 28 phosphoinositide phosphatases, for information, please see the extensive review by Balla (124). Each of the seven PIPs displays its intracellular distribution with variable overall abundance. The PIPs display a highly-relative turnover rate compared with PtdIns, which likely helps control the magnitude of the signaling transduction or the coordination of vesicular trafficking. For instance, a small fraction of the plasmalemmal PtdIns(4,5)P2 can be converted to phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) by the actions of PI 3-kinase. In turn, PtdIns(3,4,5)P3 leads to the recruitment and activation of Akt to promote cell survival and protein synthesis. PtdIns(3,4,5)P3 is also rapidly dephosphorylated at the 3- or 5-hydroxyl positions thereby terminating and in some circumstances prolonging its signaling, respectively (125, 126). Beyond their role in signal transduction, individual PIP species support many cellular functions. This includes but is not limited to the following: (i) contributing to the establishment of organelle identity; (ii) recruiting small GTPase to support actin polymerization, organelle, and vesicular transport; and (iii) serving as a ligand for cytosolic proteins thereby recruiting or regulating their function. Collectively, phosphoinositides can mediate a large variety of effects, including survival, differentiation, proliferation, migration, endocytosis, and endosomal maturation (127).
Conclusion
Lipid metabolism and transport continue to be significant and intriguing areas of biochemistry and cell biology. To date, nearly all of the enzymes involved in the synthesis and catabolism of lipids have been identified. The next phase of lipid research will build on this wealth of knowledge generated by the research community and seek to investigate the more exquisite details of the biology of lipids and the proteins that bind and transport them. How control and integration of the lipidome are achieved and its relation to the broader metabolome will also be critical for understanding health and pathophysiology.
Acknowledgment
We thank Jay Chun for assistance in generating Figs. 1 and 4.
This work was supported in part by Operating Grant MOP-133656 from the Canadian Institutes of Health Research and a Discovery Grant from the Natural Sciences and Engineering Council of Canada (to G. D. F.). The authors declare that they have no conflicts of interest with the contents of this article.
- GPL
- glycerophospholipid
- PIP
- phosphoinositide
- CPT
- choline phosphotransferase
- CEPT
- choline/ethanolamine phosphotransferase
- ER
- endoplasmic reticulum
- DAG
- diacylglycerol
- PtdCho
- phosphatidylcholine
- PtdEtn
- phosphatidylethanolamine
- PtdIns
- phosphatidylinositol
- PtdSer
- phosphatidylserine
- PtdGly
- phosphatidylglycerol
- PA
- phosphatidic acid
- CL
- cardiolipin
- PM
- plasma membrane
- TMEM
- transmembrane protein
- LTP
- lipid-transport protein
- OSBP
- oxysterol-binding protein
- ORP
- OSBP-related protein
- PITP
- PtdIns-transfer protein
- E-Syts
- ER-tethered extended synaptojanin
- MCS
- membrane contact site
- PLA
- phospholipase A
- AA
- arachidonic acid
- PAF
- platelet-activating factor
- GPCR
- G-protein–coupled receptor
- PLC
- phospholipase C
- IP3
- inositol 1,4,5-triphosphate
- SM
- sphingomyelin
- CTP
- cytidine triphosphate
- TGN
- trans-Golgi network
- LPA
- lysophosphatidic acid
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PLD
- phospholipase D
- PIP
- phosphoinositide
- PtdIns(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate.
References
- 1. Vance J. E. (2015) Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18 10.1111/tra.12230 [DOI] [PubMed] [Google Scholar]
- 2. Tanford C. (1987) Amphiphile orientation: physical chemistry and biological function. Biochem. Soc. Trans. 15, Suppl, 1–7 [PubMed] [Google Scholar]
- 3. Thewalt J. L., and Bloom M. (1992) Phosphatidylcholine: cholesterol phase diagrams. Biophys. J. 63, 1176–1181 10.1016/S0006-3495(92)81681-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vance J. E. (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49, 1377–1387 10.1194/jlr.R700020-JLR200 [DOI] [PubMed] [Google Scholar]
- 5. Mantsch H. H., Martin A., and Cameron D. G. (1981) Characterization by infrared spectroscopy of the bilayer to nonbilayer phase transition of phosphatidylethanolamines. Biochemistry 20, 3138–3145 10.1021/bi00514a024 [DOI] [PubMed] [Google Scholar]
- 6. Epand R. M., and Epand R. F. (1994) Calorimetric detection of curvature strain in phospholipid bilayers. Biophys. J. 66, 1450–1456 10.1016/S0006-3495(94)80935-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuge O., Nishijima M., and Akamatsu Y. (1991) A Chinese hamster cDNA encoding a protein essential for phosphatidylserine synthase I activity. J. Biol. Chem. 266, 24184–24189 [PubMed] [Google Scholar]
- 8. Yeung T., Heit B., Dubuisson J. F., Fairn G. D., Chiu B., Inman R., Kapus A., Swanson M., and Grinstein S. (2009) Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. J. Cell Biol. 185, 917–928 10.1083/jcb.200903020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nosjean O., Briolay A., and Roux B. (1997) Mammalian GPI proteins: sorting, membrane residence and functions. Biochim. Biophys. Acta 1331, 153–186 10.1016/S0304-4157(97)00005-1 [DOI] [PubMed] [Google Scholar]
- 10. Batenburg J. J., Klazinga W., and van Golde L. M. (1985) Regulation and location of phosphatidylglycerol and phosphatidylinositol synthesis in type II cells isolated from fetal rat lung. Biochim. Biophys. Acta 833, 17–24 10.1016/0005-2760(85)90248-6 [DOI] [PubMed] [Google Scholar]
- 11. Zhang M., Mileykovskaya E., and Dowhan W. (2005) Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 280, 29403–29408 10.1074/jbc.M504955200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKenzie M., Lazarou M., Thorburn D. R., and Ryan M. T. (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J. Mol. Biol. 361, 462–469 10.1016/j.jmb.2006.06.057 [DOI] [PubMed] [Google Scholar]
- 13. Gebert N., Joshi A. S., Kutik S., Becker T., McKenzie M., Guan X. L., Mooga V. P., Stroud D. A., Kulkarni G., Wenk M. R., Rehling P., Meisinger C., Ryan M. T., Wiedemann N., Greenberg M. L., and Pfanner N. (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr. Biol. 19, 2133–2139 10.1016/j.cub.2009.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Airola M. V., and Hannun Y. A. (2013) Sphingolipid metabolism and neutral sphingomyelinases. Handb. Exp. Pharmacol. 2013, 57–76 10.1007/978-3-7091-1368-4_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang J., and Feigenson G. W. (1999) A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76, 2142–2157 10.1016/S0006-3495(99)77369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krause M. R., and Regen S. L. (2014) The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc. Chem. Res. 47, 3512–3521 10.1021/ar500260t [DOI] [PubMed] [Google Scholar]
- 17. Carter J. R., and Kennedy E. P. (1966) Enzymatic synthesis of cytidine diphosphate diglyceride. J. Lipid Res. 7, 678–683 [PubMed] [Google Scholar]
- 18. Takenawa T., and Egawa K. (1977) CDP-diglyceride:inositol transferase from rat liver. Purification and properties. J. Biol. Chem. 252, 5419–5423 [PubMed] [Google Scholar]
- 19. Kim Y. J., Guzman-Hernandez M. L., Wisniewski E., Echeverria N., and Balla T. (2016) Phosphatidylinositol and phosphatidic acid transport between the ER and plasma membrane during PLC activation requires the Nir2 protein. Biochem. Soc. Trans. 44, 197–201 10.1042/BST20150187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy E. P. (1956) The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J. Biol. Chem. 222, 185–191 [PubMed] [Google Scholar]
- 21. Weiss S. B., Smith S. W., and Kennedy E. P. (1958) The enzymatic formation of lecithin from cytidine diphosphate choline and d-1,2-diglyceride. J. Biol. Chem. 231, 53–64 [PubMed] [Google Scholar]
- 22. Kent C. (1995) Eukaryotic phospholipid biosynthesis. Annu. Rev. Biochem. 64, 315–343 10.1146/annurev.bi.64.070195.001531 [DOI] [PubMed] [Google Scholar]
- 23. Kornberg R. D., and McConnell H. M. (1971) Lateral diffusion of phospholipids in a vesicle membrane. Proc. Natl. Acad. Sci. U.S.A. 68, 2564–2568 10.1073/pnas.68.10.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devaux P., and McConnell H. M. (1972) Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J. Am. Chem. Soc. 94, 4475–4481 10.1021/ja00768a600 [DOI] [PubMed] [Google Scholar]
- 25. Kornberg R. D., and McConnell H. M. (1971) Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry 10, 1111–1120 10.1021/bi00783a003 [DOI] [PubMed] [Google Scholar]
- 26. Bai J., and Pagano R. E. (1997) Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry 36, 8840–8848 10.1021/bi970145r [DOI] [PubMed] [Google Scholar]
- 27. Fairn G. D., Schieber N. L., Ariotti N., Murphy S., Kuerschner L., Webb R. I., Grinstein S., and Parton R. G. (2011) High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 194, 257–275 10.1083/jcb.201012028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins J. A., and Dawson R. M. (1977) Asymmetry of the phospholipid bilayer of rat liver endoplasmic reticulum. Biochim. Biophys. Acta 470, 342–356 10.1016/0005-2736(77)90126-2 [DOI] [PubMed] [Google Scholar]
- 29. Devaux P. F., and Morris R. (2004) Transmembrane asymmetry and lateral domains in biological membranes. Traffic 5, 241–246 10.1111/j.1600-0854.2004.0170.x [DOI] [PubMed] [Google Scholar]
- 30. Daleke D. L. (2007) Phospholipid flippases. J. Biol. Chem. 282, 821–825 10.1074/jbc.R600035200 [DOI] [PubMed] [Google Scholar]
- 31. van Meer G., Voelker D. R., and Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lannert H., Bünning C., Jeckel D., and Wieland F. T. (1994) Lactosylceramide is synthesized in the lumen of the Golgi apparatus. FEBS Lett. 342, 91–96 10.1016/0014-5793(94)80591-1 [DOI] [PubMed] [Google Scholar]
- 33. Jeckel D., Karrenbauer A., Burger K. N., van Meer G., and Wieland F. (1992) Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell Biol. 117, 259–267 10.1083/jcb.117.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwarzmann G., and Sandhoff K. (1990) Metabolism and intracellular transport of glycosphingolipids. Biochemistry 29, 10865–10871 10.1021/bi00501a001 [DOI] [PubMed] [Google Scholar]
- 35. Pomorski T. G., and Menon A. K. (2016) Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. Prog Lipid Res. 64, 69–84 10.1016/j.plipres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A., and Grinstein S. (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- 37. Vance J. E., and Tasseva G. (2013) Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554 10.1016/j.bbalip.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 38. Chen B., Jiang Y., Zeng S., Yan J., Li X., Zhang Y., Zou W., and Wang X. (2010) Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet. 6, e1001235 10.1371/journal.pgen.1001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bell R. M., Ballas L. M., and Coleman R. A. (1981) Lipid topogenesis. J. Lipid Res. 22, 391–403 [PubMed] [Google Scholar]
- 40. Bevers E. M., Comfurius P., Dekkers D. W., Harmsma M., and Zwaal R. F. (1998) Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus 7, Suppl. 2, 126–131 [DOI] [PubMed] [Google Scholar]
- 41. Bishop W. R., and Bell R. M. (1988) Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu. Rev. Cell Biol. 4, 579–610 10.1146/annurev.cb.04.110188.003051 [DOI] [PubMed] [Google Scholar]
- 42. Goren M. A., Morizumi T., Menon I., Joseph J. S., Dittman J. S., Cherezov V., Stevens R. C., Ernst O. P., and Menon A. K. (2014) Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat. Commun. 5, 5115 10.1038/ncomms6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon I., Huber T., Sanyal S., Banerjee S., Barré P., Canis S., Warren J. D., Hwa J., Sakmar T. P., and Menon A. K. (2011) Opsin is a phospholipid flippase. Curr. Biol. 21, 149–153 10.1016/j.cub.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morra G., Razavi A. M., Pandey K., Weinstein H., Menon A. K., and Khelashvili G. (2018) Mechanisms of lipid scrambling by the G protein-coupled receptor opsin. Structure 26, 356–367 10.1016/j.str.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sharpe H. J., Stevens T. J., and Munro S. (2010) A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169 10.1016/j.cell.2010.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bevers E. M., Tilly R. H., Senden J. M., Comfurius P., and Zwaal R. F. (1989) Exposure of endogenous phosphatidylserine at the outer surface of stimulated platelets is reversed by restoration of aminophospholipid translocase activity. Biochemistry 28, 2382–2387 10.1021/bi00432a007 [DOI] [PubMed] [Google Scholar]
- 47. Zwaal R. F., Comfurius P., and Bevers E. M. (1998) Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta 1376, 433–453 10.1016/S0304-4157(98)00018-5 [DOI] [PubMed] [Google Scholar]
- 48. Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., and Henson P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 [PubMed] [Google Scholar]
- 49. Fadok V. A., de Cathelineau A., Daleke D. L., Henson P. M., and Bratton D. L. (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276, 1071–1077 10.1074/jbc.M003649200 [DOI] [PubMed] [Google Scholar]
- 50. Dekkers D. W., Comfurius P., Vuist W. M., Billheimer J. T., Dicker I., Weiss H. J., Zwaal R. F., and Bevers E. M. (1998) Impaired Ca2+-induced tyrosine phosphorylation and defective lipid scrambling in erythrocytes from a patient with Scott syndrome: a study using an inhibitor for scramblase that mimics the defect in Scott syndrome. Blood 91, 2133–2138 [PubMed] [Google Scholar]
- 51. Zwaal R. F., Comfurius P., and Bevers E. M. (2004) Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta 1636, 119–128 10.1016/j.bbalip.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 52. Suzuki J., Umeda M., Sims P. J., and Nagata S. (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- 53. Suzuki J., Fujii T., Imao T., Ishihara K., Kuba H., and Nagata S. (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J. Biol. Chem. 288, 13305–13316 10.1074/jbc.M113.457937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gyobu S., Ishihara K., Suzuki J., Segawa K., and Nagata S. (2017) Characterization of the scrambling domain of the TMEM16 family. Proc. Natl. Acad. Sci. U.S.A. 114, 6274–6279 10.1073/pnas.1703391114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gyobu S., Miyata H., Ikawa M., Yamazaki D., Takeshima H., Suzuki J., and Nagata S. (2016) A role of TMEM16E carrying a scrambling domain in sperm motility. Mol. Cell. Biol. 36, 645–659 10.1128/MCB.00919-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martínez M. C., and Freyssinet J. M. (2001) Deciphering the plasma membrane hallmarks of apoptotic cells: phosphatidylserine transverse redistribution and calcium entry. BMC Cell Biol. 2, 20 10.1186/1471-2121-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bevers E. M., and Williamson P. L. (2010) Phospholipid scramblase: an update. FEBS Lett. 584, 2724–2730 10.1016/j.febslet.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 58. Suzuki J., Denning D. P., Imanishi E., Horvitz H. R., and Nagata S. (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki J., Imanishi E., and Nagata S. (2014) Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J. Biol. Chem. 289, 30257–30267 10.1074/jbc.M114.583419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Segawa K., Kurata S., Yanagihashi Y., Brummelkamp T. R., Matsuda F., and Nagata S. (2014) Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 10.1126/science.1252809 [DOI] [PubMed] [Google Scholar]
- 61. Seigneuret M., and Devaux P. F. (1984) ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. U.S.A. 81, 3751–3755 10.1073/pnas.81.12.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang X., Halleck M. S., Schlegel R. A., and Williamson P. (1996) A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272, 1495–1497 10.1126/science.272.5267.1495 [DOI] [PubMed] [Google Scholar]
- 63. Natarajan P., Wang J., Hua Z., and Graham T. R. (2004) Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. U.S.A. 101, 10614–10619 10.1073/pnas.0404146101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., and Holthuis J. C. (2003) Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240–1254 10.1091/mbc.E02-08-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., and Tanaka K. (2004) Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 3418–3432 10.1091/mbc.E03-11-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paulusma C. C., Folmer D. E., Ho-Mok K. S., de Waart D. R., Hilarius P. M., Verhoeven A. J., and Oude Elferink R. P. (2008) ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology 47, 268–278 [DOI] [PubMed] [Google Scholar]
- 67. Gall W. E., Geething N. C., Hua Z., Ingram M. F., Liu K., Chen S. I., and Graham T. R. (2002) Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623–1627 10.1016/S0960-9822(02)01148-X [DOI] [PubMed] [Google Scholar]
- 68. Lee S., Uchida Y., Wang J., Matsudaira T., Nakagawa T., Kishimoto T., Mukai K., Inaba T., Kobayashi T., Molday R. S., Taguchi T., and Arai H. (2015) Transport through recycling endosomes requires EHD1 recruitment by a phosphatidylserine translocase. EMBO J. 34, 669–688 10.15252/embj.201489703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van der Mark V. A., Elferink R. P., and Paulusma C. C. (2013) P4 ATPases: flippases in health and disease. Int. J. Mol. Sci. 14, 7897–7922 10.3390/ijms14047897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hankins H. M., Baldridge R. D., Xu P., and Graham T. R. (2015) Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 16, 35–47 10.1111/tra.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Daleke D. L. (2003) Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 44, 233–242 10.1194/jlr.R200019-JLR200 [DOI] [PubMed] [Google Scholar]
- 72. Vaughan A. M., and Oram J. F. (2006) ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 47, 2433–2443 10.1194/jlr.M600218-JLR200 [DOI] [PubMed] [Google Scholar]
- 73. Crawford A. R., Smith A. J., Hatch V. C., Oude Elferink R. P., Borst P., and Crawford J. M. (1997) Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy. J. Clin. Invest. 100, 2562–2567 10.1172/JCI119799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Klemm R. W., Ejsing C. S., Surma M. A., Kaiser H. J., Gerl M. J., Sampaio J. L., de Robillard Q., Ferguson C., Proszynski T. J., Shevchenko A., and Simons K. (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J. Cell Biol. 185, 601–612 10.1083/jcb.200901145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Deng Y., Rivera-Molina F. E., Toomre D. K., and Burd C. G. (2016) Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. U.S.A. 113, 6677–6682 10.1073/pnas.1602875113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaplan M. R., and Simoni R. D. (1985) Intracellular transport of phosphatidylcholine to the plasma membrane. J. Cell Biol. 101, 441–445 10.1083/jcb.101.2.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sleight R. G., and Pagano R. E. (1983) Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J. Biol. Chem. 258, 9050–9058 [PubMed] [Google Scholar]
- 78. Vance J. E., Aasman E. J., and Szarka R. (1991) Brefeldin A does not inhibit the movement of phosphatidylethanolamine from its sites for synthesis to the cell surface. J. Biol. Chem. 266, 8241–8247 [PubMed] [Google Scholar]
- 79. Holthuis J. C., and Menon A. K. (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48–57 10.1038/nature13474 [DOI] [PubMed] [Google Scholar]
- 80. Levine T. (2004) Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol. 14, 483–490 10.1016/j.tcb.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 81. Dittman J. S., and Menon A. K. (2017) Speed limits for nonvesicular intracellular sterol transport. Trends Biochem. Sci. 42, 90–97 10.1016/j.tibs.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McLean L. R., and Phillips M. C. (1981) Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry 20, 2893–2900 10.1021/bi00513a028 [DOI] [PubMed] [Google Scholar]
- 83. Merklinger E., Schloetel J. G., Spitta L., Thiele C., and Lang T. (2016) No evidence for spontaneous lipid transfer at ER-PM membrane contact sites. J. Membr. Biol. 249, 41–56 10.1007/s00232-015-9845-2 [DOI] [PubMed] [Google Scholar]
- 84. Nichols J. W. (1988) Kinetics of fluorescent-labeled phosphatidylcholine transfer between nonspecific lipid transfer protein and phospholipid vesicles. Biochemistry 27, 1889–1896 10.1021/bi00406a014 [DOI] [PubMed] [Google Scholar]
- 85. Wong L. H., Čopič A., and Levine T. P. (2017) Advances on the transfer of lipids by lipid transfer proteins. Trends Biochem. Sci. 42, 516–530 10.1016/j.tibs.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Olkkonen V. M., and Li S. (2013) Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog. Lipid Res. 52, 529–538 10.1016/j.plipres.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 87. Beh C. T., McMaster C. R., Kozminski K. G., and Menon A. K. (2012) A detour for yeast oxysterol binding proteins. J. Biol. Chem. 287, 11481–11488 10.1074/jbc.R111.338400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kentala H., Weber-Boyvat M., and Olkkonen V. M. (2016) OSBP-related protein family: mediators of lipid transport and signaling at membrane contact sites. Int. Rev. Cell Mol. Biol. 321, 299–340 10.1016/bs.ircmb.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 89. Alpy F., and Tomasetto C. (2014) START ships lipids across interorganelle space. Biochimie 96, 85–95 10.1016/j.biochi.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 90. Cockcroft S., and Garner K. (2013) Potential role for phosphatidylinositol transfer protein (PITP) family in lipid transfer during phospholipase C signalling. Adv. Biol. Regul 53, 280–291 10.1016/j.jbior.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 91. Chung J., Torta F., Masai K., Lucast L., Czapla H., Tanner L. B., Narayanaswamy P., Wenk M. R., Nakatsu F., and De Camilli P. (2015) Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428–432 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lees J. A., Messa M., Sun E. W., Wheeler H., Torta F., Wenk M. R., De Camilli P., and Reinisch K. M. (2017) Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science 355, eaah6171 10.1126/science.aah6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levine T. P., and Munro S. (2002) Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12, 695–704 10.1016/S0960-9822(02)00779-0 [DOI] [PubMed] [Google Scholar]
- 94. Loewen C. J., Roy A., and Levine T. P. (2003) A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 22, 2025–2035 10.1093/emboj/cdg201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wyles J. P., McMaster C. R., and Ridgway N. D. (2002) Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J. Biol. Chem. 277, 29908–29918 10.1074/jbc.M201191200 [DOI] [PubMed] [Google Scholar]
- 96. Luquain C., Sciorra V. A., and Morris A. J. (2003) Lysophosphatidic acid signaling: how a small lipid does big things. Trends Biochem. Sci. 28, 377–383 10.1016/S0968-0004(03)00139-7 [DOI] [PubMed] [Google Scholar]
- 97. Wang X., Devaiah S. P., Zhang W., and Welti R. (2006) Signaling functions of phosphatidic acid. Prog. Lipid Res. 45, 250–278 10.1016/j.plipres.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 98. Eichmann T. O., and Lass A. (2015) DAG tales: the multiple faces of diacylglycerol–stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 72, 3931–3952 10.1007/s00018-015-1982-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Aloulou A., Ali Y. B., Bezzine S., Gargouri Y., and Gelb M. H. (2012) Phospholipases: an overview. Methods Mol. Biol. 861, 63–85 10.1007/978-1-61779-600-5_4 [DOI] [PubMed] [Google Scholar]
- 100. Vasquez A. M., Mouchlis V. D., and Dennis E. A. (2018) Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 67, 212–218 10.1016/j.jbior.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mouchlis V. D., and Dennis E. A. (2016) Membrane and inhibitor interactions of intracellular phospholipases A2. Adv. Biol. Regul. 61, 17–24 10.1016/j.jbior.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 10.1126/science.294.5548.1871 [DOI] [PubMed] [Google Scholar]
- 103. Famaey J. P. (1982) Phospholipases, eicosanoid production and inflammation. Clin. Rheumatol. 1, 84–94 10.1007/BF02275597 [DOI] [PubMed] [Google Scholar]
- 104. Moolenaar W. H., van Meeteren L. A., and Giepmans B. N. (2004) The ins and outs of lysophosphatidic acid signaling. Bioessays 26, 870–881 10.1002/bies.20081 [DOI] [PubMed] [Google Scholar]
- 105. Prescott S. M., Zimmerman G. A., Stafforini D. M., and McIntyre T. M. (2000) Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69, 419–445 10.1146/annurev.biochem.69.1.419 [DOI] [PubMed] [Google Scholar]
- 106. Lands W. E., and Hart P. (1965) Metabolism of glycerolipids. Vi. Specificities of acyl coenzyme a: phospholipid acyltransferases. J. Biol. Chem. 240, 1905–1911 [PubMed] [Google Scholar]
- 107. Ha K. D., Clarke B. A., and Brown W. J. (2012) Regulation of the Golgi complex by phospholipid remodeling enzymes. Biochim. Biophys. Acta 1821, 1078–1088 10.1016/j.bbalip.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. van den Berg J. J., Op den Kamp J. A., Lubin B. H., and Kuypers F. A. (1993) Conformational changes in oxidized phospholipids and their preferential hydrolysis by phospholipase A2: a monolayer study. Biochemistry 32, 4962–4967 10.1021/bi00069a035 [DOI] [PubMed] [Google Scholar]
- 109. de Figueiredo P., Drecktrah D., Katzenellenbogen J. A., Strang M., and Brown W. J. (1998) Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl. Acad. Sci. U.S.A. 95, 8642–8647 10.1073/pnas.95.15.8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kozlov M. M., Leikin S. L., Chernomordik L. V., Markin V. S., and Chizmadzhev Y. A. (1989) Stalk mechanism of vesicle fusion. Intermixing of aqueous contents. Eur. Biophys. J. 17, 121–129 [DOI] [PubMed] [Google Scholar]
- 111. Schmidt J. A., and Brown W. J. (2009) Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell Biol. 186, 211–218 10.1083/jcb.200904147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vreken P., Valianpour F., Nijtmans L. G., Grivell L. A., Plecko B., Wanders R. J., and Barth P. G. (2000) Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279, 378–382 10.1006/bbrc.2000.3952 [DOI] [PubMed] [Google Scholar]
- 113. Gu Z., Valianpour F., Chen S., Vaz F. M., Hakkaart G. A., Wanders R. J., and Greenberg M. L. (2004) Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol. 51, 149–158 [DOI] [PubMed] [Google Scholar]
- 114. Xu Y., Kelley R. I., Blanck T. J., and Schlame M. (2003) Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278, 51380–51385 10.1074/jbc.M307382200 [DOI] [PubMed] [Google Scholar]
- 115. Beranek A., Rechberger G., Knauer H., Wolinski H., Kohlwein S. D., and Leber R. (2009) Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J. Biol. Chem. 284, 11572–11578 10.1074/jbc.M805511200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vaz F. M., Houtkooper R. H., Valianpour F., Barth P. G., and Wanders R. J. (2003) Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J. Biol. Chem. 278, 43089–43094 10.1074/jbc.M305956200 [DOI] [PubMed] [Google Scholar]
- 117. Xu Y., Malhotra A., Ren M., and Schlame M. (2006) The enzymatic function of tafazzin. J. Biol. Chem. 281, 39217–39224 10.1074/jbc.M606100200 [DOI] [PubMed] [Google Scholar]
- 118. Vines C. M. (2012) Phospholipase C. Adv. Exp. Med. Biol. 740, 235–254 10.1007/978-94-007-2888-2_10 [DOI] [PubMed] [Google Scholar]
- 119. Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., and Irvine R. F. (1983) Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem. J. 212, 473–482 10.1042/bj2120473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bell R. M., Hannun Y. A., and Loomis C. R. (1986) Mechanism of regulation of protein kinase C by lipid second messengers. Symp. Fundam. Cancer Res. 39, 145–156 [PubMed] [Google Scholar]
- 121. Exton J. H. (1994) Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1212, 26–42 10.1016/0005-2760(94)90186-4 [DOI] [PubMed] [Google Scholar]
- 122. Kooijman E. E., Chupin V., de Kruijff B., and Burger K. N. (2003) Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4, 162–174 10.1034/j.1600-0854.2003.00086.x [DOI] [PubMed] [Google Scholar]
- 123. Antonescu C. N., Danuser G., and Schmid S. L. (2010) Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol. Biol. Cell 21, 2944–2952 10.1091/mbc.E10-05-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Balla T. (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun H., Lesche R., Li D. M., Liliental J., Zhang H., Gao J., Gavrilova N., Mueller B., Liu X., and Wu H. (1999) PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 6199–6204 10.1073/pnas.96.11.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Scharenberg A. M., El-Hillal O., Fruman D. A., Beitz L. O., Li Z., Lin S., Gout I., Cantley L. C., Rawlings D. J., and Kinet J. P. (1998) Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 17, 1961–1972 10.1093/emboj/17.7.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Di Paolo G., and De Camilli P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- 128. Allan D. (1996) Mapping the lipid distribution in the membranes of BHK cells (mini-review). Mol. Membr. Biol. 13, 81–84 10.3109/09687689609160580 [DOI] [PubMed] [Google Scholar]
- 129. Chitraju C., Trötzmüller M., Hartler J., Wolinski H., Thallinger G. G., Lass A., Zechner R., Zimmermann R., Köfeler H. C., and Spener F. (2012) Lipidomic analysis of lipid droplets from murine hepatocytes reveals distinct signatures for nutritional stress. J. Lipid Res. 53, 2141–2152 10.1194/jlr.M028902 [DOI] [PMC free article] [PubMed] [Google Scholar]