Abstract
Background and Objective:
The current meta-analysis aims at evaluating whether the existing clinical evidence may ascertain the effects of growth hormone (GH) replacement therapy on cardiovascular risk, both in isolated GH deficien-cy (GHD) and in compensated panhypopituitarism including GH deficit.
Methods:
Original articles published from 1991 to 2015 were searched on Medline (Pubmed). Among an overall number of 181 potentially suitable studies, 24 fulfilled the selection criteria and were included in the analysis. Data aggregation was car-ried out through the calculation of the absolute risk reduction. The meta-analysis was then conducted by means of a fixed-effects model, according to the heteroge-neity test (Chi-square statistic).
Results:
Fat-free mass (FFM) increase and fat mass (FM) reduction were found, together with a C-LDL reduction, a wide variation in glycaemia and a neutral effect on glycated haemoglobin (HbA1c) and blood pressure. These effects were valid both for isolated GHD patients and for those with compensated panhypopituitarism. The global out-come D showed a nonsignificant reduction of the overall cardiovascular risk (0.53; 95% C.I. -1.23, 2.85).
Conclusion:
Our meta-analysis shows no signnificatly positive trend in cardiovascular risk after both short and long-term GH supplementation therapy in adult GHD patients. However, a reduction of LDL cholesterol levels has been found. No differences were found between isolated GHD participants and those affected by panhypopituitarism well compensated since at least 3 months.
Keywords: GH deficit, panhypopituitarism, GH supplementation, cardiovascular risk, body composition, cardiovascular risk factors
1. Introduction
In adults, the alteration of the growth hormone (GH)/ insulin-like growth factor 1 (IGF-1) axis is associated with an increased cardiovascular (CV) risk, both in GH deficit (GHD) and in GH excess (acromegaly). Physiologically GH/ IGF-I axis exerts relevant cardiovascular effects, regulating cardiac growth and myocardial contractility, and contributing to the maintenance of cardiac mass and function in normal adult [1]. In particular, isolated GHD subjects show a higher cardiovascular risk due to an atherosclerosis development acceleration, caused by the fat mass (FM) increase, mainly at the trunk level, together with the fat free mass (FFM) decrease. Moreover, the GH deficiency leads to metabolic changes, such as high levels of total cholesterol (C-TOT) and low-density lipoprotein cholesterol (C-LDL), low levels of high-density lipoprotein cholesterol (C-HDL), together with insulin resistance, and serum C-reactive protein (CRP) increase [2-4].
The effects of GH replacement therapy on CV risk have been explored using several single parameters so far. Some studies focused on metabolic aspects such as blood glucose, glycated hemoglobin (HbA1c), insulinemia, insulin resistance (IR), lipids levels, whereas other studies focused on markers of organ damage such as endothelial dysfunction, carotid intima-media thickness (IMT), biochemical mechanism of plaque formation, electric and mechanic heart functionality [5, 6]. As a consequence of this lack of uniformity, the available data on the effects of GH supplementation are fairly uneven and meta-analyses on the same issue show inconclusive results, which are still debated [7-9]. For example, GH substitution seems to reduce C-LDL levels, together with a worsening in the glucose homeostasis [2, 10]. In addition, the long-term effects (beyond 5 years) of the ultimate endpoints, demostrating the beneficial effects of GH supplementation on mortality, cardiovascular risk and fractures’ rate without any increase in cancer incidence, are still to be proved [9, 11, 12]. A recent review of the cardiovascular alteration in GHD adults highlighted the GH replacement effect at increasing cardiac size, improving cardiac performance, positively affecting body composition and lipid profile, and reducing IMT at common carotid arteries [9]. However, despite this wide evidence on the GH beneficial effects in this setting, the GH-mediated improvements in prognostic outcomes remains unclear [9].
The present meta-analysis aims at evaluating whether the effect on body composition and on CV risk factors of GH supplementation leads to a beneficial effect on the global CV risk in adult men with GHD. Moreover, in order to assess confounding variables such as other pituitary defects, we evaluated GH supplementation either in isolated GHD and in compensated panhypopituitarism at different timing of replacement therapy.
2. Materials and Methods
2.1. Data Collection
The meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Original articles, published from 1991 to 2015 inclusive, on the effects of GH supplementation therapy in adult patients (>18 years) affected by GHD were searched on Medline (PubMed) through the following search key “((GH) AND body composition) AND cardiovascular risk”.
Selection criteria were set before the literature search and are listed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies involving adult patients (both genders) Isolated GHD GHD in concomitance with panhypopituitarism only if compensated by hormonal therapy since at least 3 months Randomized-controlled clinical trials Placebo-controlled studies Open-label studies Non interventional follow-up studies Data from Registries (real world practice) |
Systematic reviews and meta-analysis Articles in language other than English Studies involving patient aged less than 18 GHD associated with other diseases that may require GH supplementation as a support therapy Uncompensated panhypopituitarism Obesity GHD in patients with previous acromegaly Adult survival of Leukemia in pediatric age Surveillance studies Cross-sectional studies GHD studies without data about replacement Unavailability of data of interest |
The following variables were extracted independently by two investigators (MC and VAG): age, blood pressure (BP), IGF-1, FM, FFM, glycemia, HbA1c, insulinemia, triglycerides, cholesterol (C-TOT; C-HDL; C-LDL) and IMT.
3. Statistical analysis
The results of the studies included in the analysis were first evaluated separately by identifying and extrapolating the relevant variables. The statistical significance of the mean differences calculated within each study was then verified through the t-test or Wilcoxon test as appropriate, according to the variables’ characteristics.
The variables included in the analysis were extremely inhomogeneous, both in terms of nature and of units of measurement. Moreover, a heterogeneity test was conducted in order to verify whether the percentage of variation across the studies was due to the degree of inconsistency between the studies or chance. Statistic heterogeneity was calculated by deriving the Chi-square, based on the following equation:
χ2 = ∑ ωj *(Ôj- Ô)2
where, the null hypothesis of homogeneity showed to be satisfied, and the Chi-square statistic approximately distributes with m-1 degrees of freedom. In this formula, Ôj represents the estimate of the effect in the j-n study, Ô is the pooled effect and ωj is the weight of the j-n studies.
The meta-analysis was performed by means of a fixed-effects model, which was the only possible model for our index made of different variables in such an heterogeneous context. The global outcome D representing the overall cardiovascular risk following exposure to GH administration was estimated in terms of weighted mean, according to the following general formula [13]:
D=∑ (ωi*di)/ ∑ ωi.
With the sum extended to n studies and ω representing the weight of each study, data aggregation was carried out through the calculation of the absolute risk reduction (d=(Oi/Ni)-(Os-Ns), with O representing the observed events, N the number of subject included in each study, i the treatment investigated and s the control condition weighted by the reciprocal of the related sample size (Wi=1/Ni). For avoiding possible null entries within the tables, each available value was added with a 0.5 correction. The analysis population was stratified in three subgroups, namely isolated GHD, GHD in panhypopituitarism compensated by pharmacological (hormonal) therapy since less than 1 year (minimum 3 months), and GHD in panhypopituitarism compensated by pharmacological (hormonal) therapy since 1 year and over. Studies involving a mixed population, or with unknown compensatory treatment period were excluded from our groups. In the absence of the relevant variable, studies were excluded from respective single analysis.
4. Study quality assessment
The risk of bias of included studies was assessed independently by both reviewers through the Cochrane Collaboration's tool for assessing risk of bias for the following aspects: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selecting reporting. For other bias, funding and authorship were assessed. Each domain was assigned with low, unclear or high risk of bias (15).
5. Results
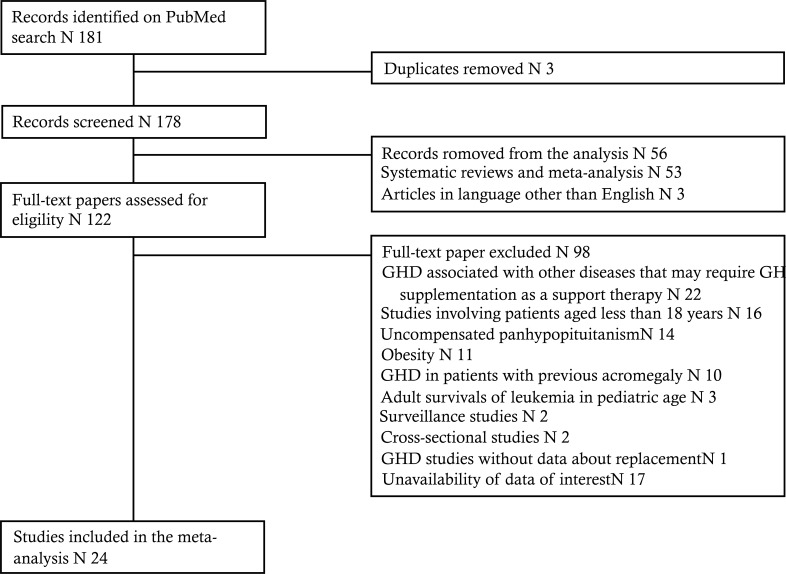
The search was carried out and cross-checked by two reviewers independently (MC and VAG). After removal of 3 duplicates, 178 potentially suitable studies were identified. Following eligibility assessment, 154 papers were excluded (Fig. 1) and 24 studies were included in the analysis (Tables 2 and 3). Overall meta-analysis results are summarized in Table 4.
Fig. (1).
Flow-chart of the study.
Table 2.
Characteristics of studies included in the meta-analysis.
| Study Ref. | Year | Patients (n) | Mean Age (Years) | GH Dose | Variables Considered | Study Design |
Study
Duration (Months) |
|---|---|---|---|---|---|---|---|
| Elbornsson M et al. [16] | 2013 | 156 | 50.5 | 0.40 mg/day | IGF-1. C-LDL. C-HDL. G. T. BP | OLS | 120 |
| Filipsson Nyström H et al. [10] | 2012 | 60 | 60.4 | 0.41 mg/day | C-LDL. C-HDL. G. I. FM. FFM. BP | RDB | 4 |
| Beauregard C et al. [17] | 2008 | 43 | 46.0 | 0.67 mg | C-LDL. C-HDL. G. I. FM. FFM | RCT | 6 |
| Cenci MC et al. [49] | 2008 | 32 | 48.0 | 0.20 mg/day | C-LDL. C-HDL. G. T | OLS | 24 |
| Fideleff HL et al. [18] | 2008 | 71 | 38.2 | 0.52 mg/day | C-LDL. C-HDL. G. FM. FFM. T. BP | RCT | 48 |
| Joaquin C et al. [19] | 2008 | 14 | 40.0 | 0.47 mg/day | IGF-1. I. HbA1c. FM. FFM. T. BP | OLS | 12 |
| Setola E et al. [33] | 2008 | 31 | 30.0 | 0.40 mg/day | IGF-1. C-LDL. C-HDL. G. I. FM. FFM. HbA1c. BP | OLS | 6 |
| Oliveira JL et al. [14] | 2007 | 20 | 46.0 | 0.35 mg/day | IGF-1 . C-LDL. C-HDL.G. I. HbA1c. IMT. BP | OLS | 6 |
| Yuen KC et al. [50] | 2006 | 16 | 49.5 | a) 0.10 mg/day b) 0.50 mg/day |
IGF-1. G. I. FM. FFM | FU | 12 |
| Chihara K et al. [51] | 2006 | 27 | 39.0 | 0.01 mg/kg/day | IGF-1 . C-LDL. C-HDL. FM. FFM | OLS | 12 |
| Bollerslev J et al. [15] | 2006 | 55 | 49.0 | a) 0.60 mg/day b) 0.40 mg/day |
C-LDL. C-HDL. FM. FFM | RDB | 9 |
| Yuen KC et al. [52] | 2005 | 25 | 47.0 | a) 0.10 mg/day b) 0.48 mg/day |
IGF-1 . C-LDL. C-HDL. G. I. FM. FFM | OLS | 12 |
| Hana V et al. [53] | 2004 | 17 | 40.9 | 0.31 mg/day | IGF-1 . C-LDL. C-HDL. G. I. HbA1c | OLS | 12 |
| Smith JC et al. [54] | 2002 | 32 | 43.0 | a) 0.45 mg/day b) 0.47 mg/day |
C-LDL. C-HDL. G. I | RCT | 3 |
| Borson-Chazot F et al. [2] | 1999 | 22 | 39.0 | 1 UI/day | IGF-1 . C-LDL. C-HDL. G. I. FM. FFM. HbA1c. IMT BP | OLS | 12 |
| O'Neal DN et al. [55] | 1999 | 22 | 42.0 | 0.24 IU/kg/week | IGF-1. C-LDL. C-HDL. G. I. FM. FFM. T | OLS | 24 |
| Rodriguez-Arnao J et al. [56] | 1999 | 35 | 39.8 | 0.12 IU/kg | FM. FFM. | RCT | 6 |
| Woodhouse LJ et al. [57] | 1999 | 28 | 39.5 | 0.012 mg/kg | FM. FFM. | RCT | 3 |
| Cuneo R et al. [58] | 1998 | 163 | 40.5 | 4.00 IU/day | IGF-1 . C-C-LDL. C-HDL. FM. FFM. HbA1c BP | OLS | 12 |
| Johansson JO et al. [59] | 1996 | 17 | 52.5 | 0.53 mg/day | IGF-1. C-HDL. G. I. FM. FFM. HbA1c | OLS | 24 |
| Nass R et al. [20] | 1995 | 20 | 44.2 | 0.0125 mg/kg | FM. FFM. | RCT | 6 |
| Weaver JU et al. [60] | 1995 | 22 | 45.0 | 0.20 IU/kg/week | IGF-1. FM. FFM. HbA1c | RCT | 6 |
| Jorgensen JO et al. [61] | 1994 | 10 | 24.7 | 2.00 IU / m2 | FM. FFM. BP. HbA1c | OLS | 36 |
| Whitehead HM et al. [62] | 1992 | 14 | 29.4 | 0.50 IU/kg/week | FM. FFM. HbA1c | RCT | 13 |
Fat mass (FM). fat free mass (FFM). glycaemia (G).glycated haemoglobin (HbA1c). insulin-like growth factor 1 (IGF-1). insulinaemia (I). triglycerides (T). blood pressure (BP). intimal medial thickness (IMT). Open label study (OLS). randomized controlled trial (RCT). randomized double-blind controlled trial (RDB). follow-up study (FU).
Table 3.
Significant parameters of the analysis.
| Study Refs. | Δ-FFM (%) | Δ-FM (%) | Δ-C-LDL (mmol/L) | HbA1c (%) | IGF-1 (μg/L) | I (μIU/mL) | T (mmol/L) |
|---|---|---|---|---|---|---|---|
| Elbornsson M. et al. [16] | - | - | -17.5%** | - | +226.59%** | - | - |
| Filipsson Nyström H. et al. [10] | - | +0.25% | -3.57% | +0.21%* | -33.46% | ||
| Beauregard C. et al. [17] | +0.03% | -14.17%* | -0.33% | - | - | +10.30% | -30.00% |
| Cenci MC. et al. [49] | - | - | - | - | - | -46.86% | -24.11% |
| Fideleff HL. et al.[18] | +2.49% | -4.97% | -5.97% | - | - | - | +6.39% |
| Joaquin C. et al. [19] | +1.35% | -3.91% | - | 0.00% | +432.67%** | +58.72%* | +41.70% |
| Setola E. et al.[33] | +5.36%** | -3.51% | -10.21%** | +8.16%* | +117.34%** | -21.11% | +24.14% |
| Oliveira JL. et al. [14] | - | - | decrease*(a) | -9.34% | +270.00%** | -10.69%* | -18.35% |
| Yuen KC. et al. [50]/ group 1 (Low GH dose) | decrease(a) | decrease*(a) | - | - | +229.70%** | - | - |
| Yuen KC. et al. [50]/ group 2 (Standard GH dose) | increase*(a) | decrease*(a) | - | - | +270.31%** | - | - |
| Chihara K. et al. [51] | +4.30*% | -7.85%* | -12.60%** | - | +410.97%** | - | - |
| Bollerslev J. et al. [15] | increase(a) | decrease(a) | -14.82%** | - | - | - | +22.70%* |
| Yuen KC. et al. [52] / group 1 (GH treatment 12 months) | -0.68% | -2.40% | 0.00% | - | +127.60%** | -0.20% | -0.30% |
| Yuen KC. et al. [52] / group 2 (Discontinuation 6 months) |
0.51% | -5.38% | +6.67% | - | +253.90%** | +0.10% | -0.20% |
| Hana V. et al. [53] | - | - | +4.10% | 0.00% | +137.57%** | 0.00% | -11.25% |
| Smith JC. et al. [54] | - | - | -16.67%* | - | - | - | - |
| Borson-Chazot F. et al. [2] | - | -12.04%* | -5.20% | - | +118.31%** | +17.35% | +4.60% |
| O'Neal DN. et al. [55] | - | +3.08% | -14.71%** | -1.92% | +163.26%** | +38.61%* | +15.38% |
| Cuneo RC. et al. [58]/group 1 (GH 12 months) | increase*(a) | decrease*(a) | -13.16%** | - | +191.00%** | - | 0.00% |
| Cuneo RC. et al. [58]/group 2 (Placebo 6-months + GH 6-months) |
increase*(a) | decrease*(a) | -7.69%* | - | +160.20%** | - | +5.00% |
| Johansson JO. et al. [59] | +5.61%* | -2.07% | - | - | +274.01%** | +38.55%** | +6.25% |
| Weaver JU. et al. [60] | -3.99% | +3.57% | - | - | - | - | - |
* = p<0.05; ** = p<0.001; (a)= absolute values not available in the source data; information derived from a diagram.
Fat mass (FM); fat free mass (FFM); glycated haemoglobin (HbA1c); insulin-like growth factor 1 (IGF-1); insulinaemia (I); triglycerides (T).
Table 4.
GH replacement therapy in GHD and cardiovascular risk: summary of main results.
| GHD(a) | GHD<12m(b) | GHD>12m(c) | ||
|---|---|---|---|---|
| Fat free mass | increased | increased | increased | p<0.05 |
| Fat mass | decreased | decreased | decreased | p<0.05 |
| Total cholesterol | neutral | neutral | neutral | |
| LDL cholesterol | decreased | decreased | decreased | p<0.05 |
| HDL cholesterol | neutral | neutral | neutral | |
| Glycemia | neutral | neutral | neutral | |
| HbA1c | neutral | neutral | neutral |
(a) Isolated GHD; (b) GHD in panhypopituitarism in stable compensation therapy since less than 12 months; (c) GHD in panhypopituitarism in stable compensation therapy since more than 12 months.
The age of participants ranged from 30.0 to 60.4 years, while GH administration was very different among the studies, varying both for dosage from 0.10 mg/day to 0.17 mg/kg/week and duration from 3 months to 10 years. A significant increase in FFM (around +3%) and decrease in FM levels (around -6%) was currently reported in most of the studies, both in GHD isolated patients and in panhypopituitary ones with stable compensation.
A significant decrease in C-LDL levels (around -7%) was commonly reported in almost all the analyzed studies, both in GHD isolated patients and in panhypopituitary ones with stable compensation. The available data did not allow to define the trend of C-TOT, C-HDL and T, which were therefore indicated as neutral (Table 4).
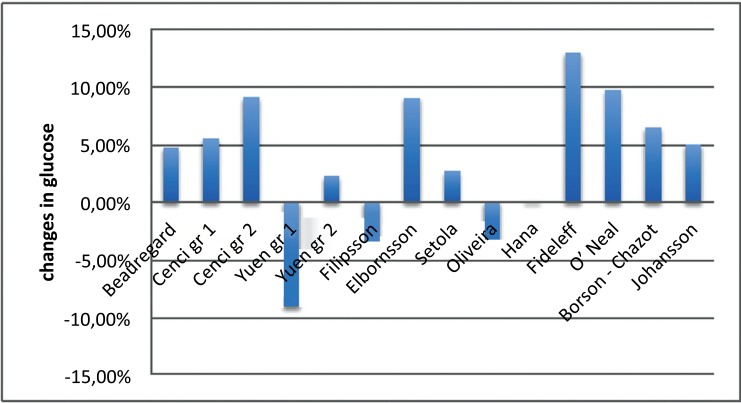
GH supplementation seemed to be ineffective on glucose metabolism. Data on glucose levels were often discordant mainly in the GHD panhypopituitary group with compensatory therapy for less than 12 months. Nevertheless, glycemic levels even when increased (around +4%, not statistically significant after meta-analysis), were always falling within the normal ranges and did never reach the diagnostic levels for impaired glucose tolerance (IGT) or diabetes (Fig. 2). Out of 10 studies including HbA1c, only 2 reported an increased level of this parameter after 6 months of treatment. This observation was not confirmed by the majority of the studies, despite the higher number of included patients and the wider range variety. Generally, the observed HbA1c increase was never pathological and was always found to be below 6% (Table 4).
Fig. (2).
Trends observed in glycaemia following GH replacement therapy.
No significant difference was found for BP (Supplementary Table 1 (40.3KB, pdf) ) and insulin levels (Table 3). The eligible studies containing data on IMT were only two: Borson-Chazot et al. [2] observed a significant decrease (around -10%); data extrapolated from diagrams provided by Oliveira et al. [14] showed a non-significant increase of the IMT. Information on IGF-1 reported in the analyzed studies was summarized in Table 3.
The homogeneity hypothesis could be accepted by virtue of the following results:
χ2 = ∑ ωi *(Ôj- Ô)2 = ∑ ωj (dj-D)2 = 0.038 < χ2 0.05;29 = 42.56 (significant for χ2 <50)
Overall, the administration of GH resulted to be associated with a general reduction of the cardiovascular risk, according to the following equation [13]:
D=∑ (ωi*di)/ ∑ ωi = 0. 53
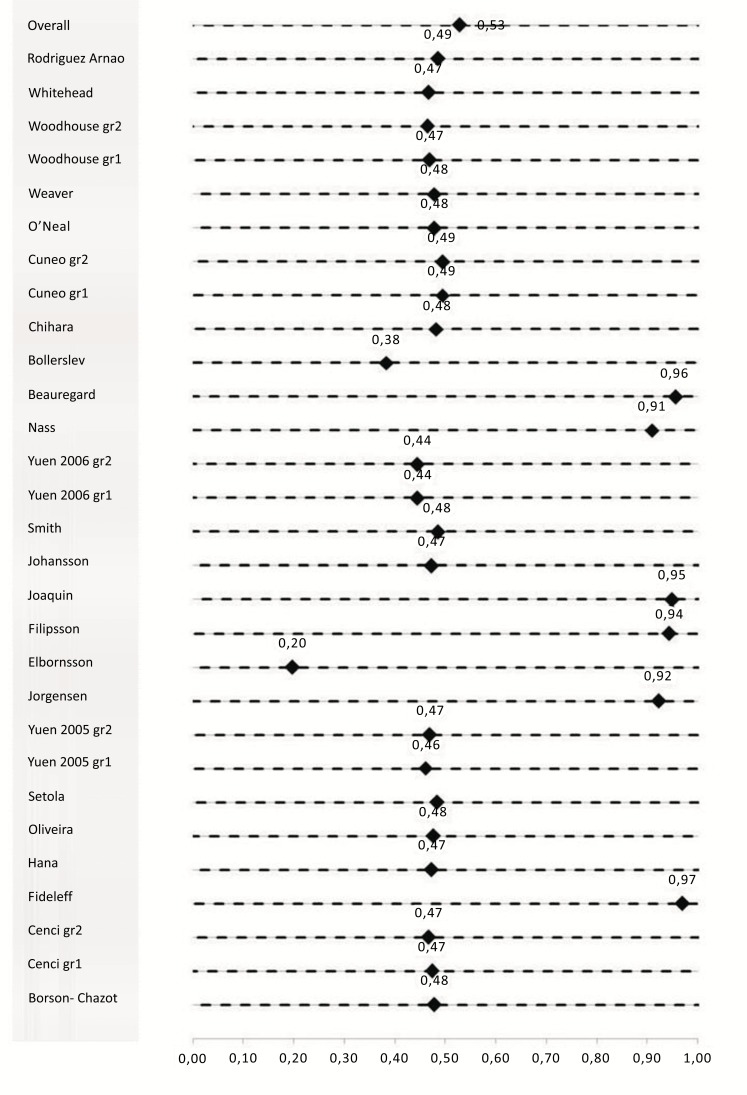
C.I (95%) = D± (1.96/√∑ ωi) = (-1.23; 2.85)
With D values close to or below zero identifying no improvement with respect of D values near or exceeding one show a probable relationship (Fig. 3). Considering each study individually, D value was very low for several studies [15, 16], and very high for others [10, 17-20].
Fig. (3).
Graphical representation of the meta-analysis results (forest plot).
As described in Table 2, the treatment duration was extremely variable among studies included in the analysis. For this reason, in order to exclude potential influence of such difference, the analysis was repeated after having stratified the studies in 2 subgroups (short studies < 1 year; medium/long studies > 1 year). The meta-analysis conducted according to the study duration did not provide any significant difference as compared to the overall investigation.
Results of the meta-analysis were applied only to the Short Studies (Table 2).
D=∑ (ωi*di)/ ∑ ωi = 0.56
C.I (95%) = D± (1.96/√∑ ωi) = (-2.28; 3 41)
Results of the meta-analysis applied only to the Medium/Long Studies (Table 2).
D=∑ (ωi*di)/ ∑ ωi = 0.51
C.I (95%) = D± (1 96/√∑ ωi) = (-1.72; 2.74)
6. Study quality assessment
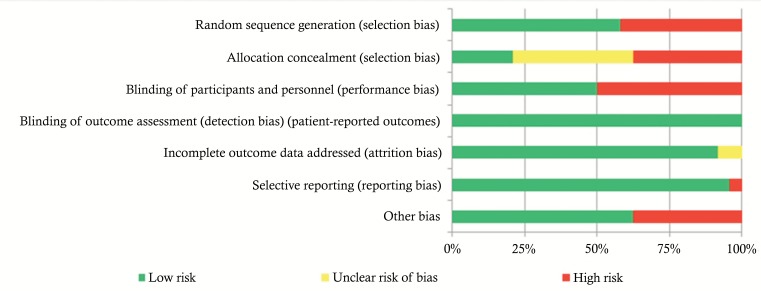
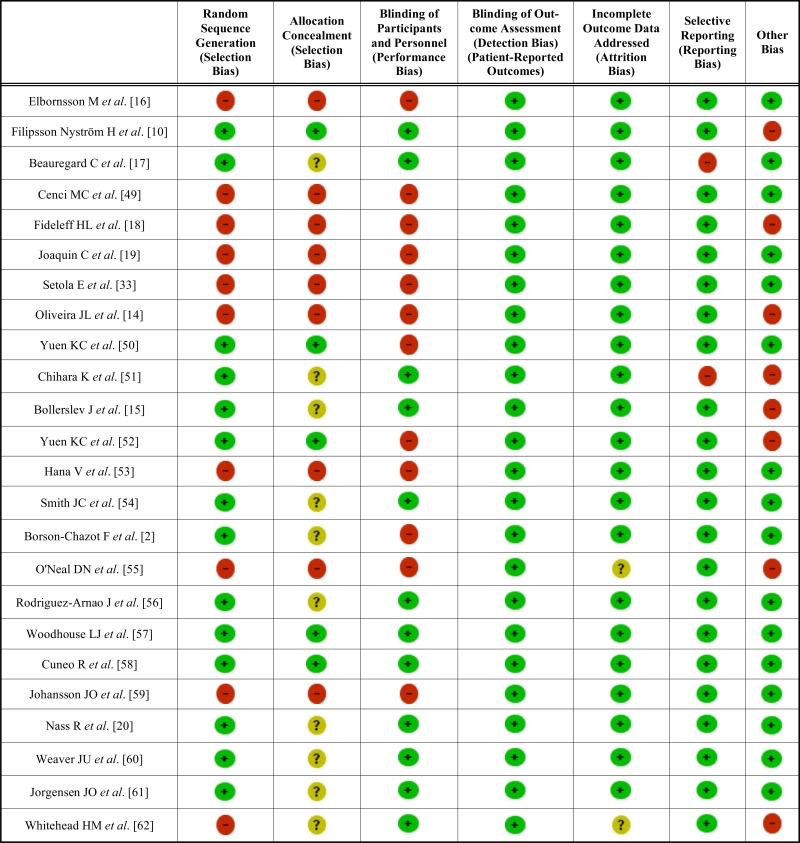
The risk of bias in the included studies is shown in Fig. (4) and Table 5. Risk of bias across studies was found for “random sequence generation”, “allocation concealment”, “blinding of participants and personnel” and “other bias” domains. Fourteen studies were not randomized [2, 14, 16, 19, 33, 49, 50-53, 55, 58, 59, 61]. A low risk of biased allocation was described only in five studies [10, 50, 52, 57, 58]. Twelve studies were open label [2, 14, 16, 18, 19, 33, 49, 50, 52, 53, 55, 59]. Six studies were funded by pharmaceutical industries [10, 15, 18, 51, 55, 62].
Fig. (4).
Risk of bias graph.
Table 5.
7. Discussion
This meta-analysis is focused on defining the GH supplementation effect on cardiovascular risk in adult GHD. Despite the rigorous literature search process, studies included in the analysis show a very high non-uniformity, not allowing us to perform at a quantitative analysis and limiting the interpretation of aggregate data trend. The evaluation of biases risk shows that this heterogeneity is mainly due to the patients’ characteristics, together with the small sample size. Thus, the subdivision in sub-group analyses does not reduce this non-homogeneity, as demonstrated in dividing studies according to treatment length. Future meta-analyses should provide this heterogeneity and should be addressed to consider different population characteristics, such as the age of GHD onset or the GH dosage used. The identification of significant quantitative differences was impeded by this high heterogeneity and only a slight nonsignificant reduction of parameters considered is found both in short-term and long-term studies. However, qualitative analyses of the studies included demonstrate: i) changes in body composition, with an increase of FFM and a reduction of FM; ii) reduction of C-LDL; iii) stable HbA1c and BP. Thus, despite the aggregated data-analysis confirms a non-significant reduction of overall cardiovascular risk, we could speculate that the GH administration could probably be effective in reducing cardiovascular adverse events, through a reduction of several parameters, such as C-LDL and not-impairing glucose metabolism. However, proper-designed and powered longitudinal trials are still needed, such as recently confirmed in a wide review of the literature [9].
Our analysis provided a papers’ research that was extended over a 25-year interval. Inclusion and exclusion criteria were defined prior to the literature search in order to avoid confounding factors such as uncompensated panhypopituitarism [21], obesity [22], metabolic syndrome and other factors commonly associated with acquired GHD (i.e. radiotherapy, craniopharyngioma, adrenal crisis, etc.) [23, 24]. Similarly, the population was subdivided into three groups: isolated GHD, GHD in panhypopituitarism pharmacologically compensated since at least 3 months, and GHD in panhypopituitarism pharmacologically compensated since at least one year. The results of the meta-analysis were homogeneous among the three study subgroups. Thus, these data suggest that a supplementation therapy lasting more than 3 months could be considered stable.
Despite the wide heterogeneity of this clinical condition, the qualitative evaluation of studies included well demonstrate that the GH supplementation effect in adult GHD patients mainly leads to FM decrease and FFM increase [25-27]. This effect is more evident in male rather than in female patients [28]. Moreover, GH replacement seems to reduce C-LDL levels, as well as demonstrated also by biochemical and in vitro studies. Indeed, GH acts on cholesterol regulating genes (such as the C-LDL receptor gene) by means of the sterol sensitive binding cis-element (sre-1). This effect is not depending on RNA expression, but it relates to the phosphorylation of SREBP-1a protein in human cultured hepatocytes [29]. Our data support the hypothesis that GH induces C-LDL reduction, representing one of the main reasons to use GH in GHD subjects. On the contrary, GH administration seems to not influence C-HDL levels. High doses of GH (aimed at maintaining serum IGF-1 levels between the median and upper reference limit) result in a significant C-HDL increase when compared to lower levels of replacement therapy [30]. It is worth noting that this could be influenced by the genotype of the patient, in particular by the 629C>A cholesterol ester transfer protein (CETP) promoter polymorphism [31]. Considering glucose metabolism, our qualitative analysis did not find a GH effect. However, it is well known that GH directly antagonizes insulin mediated glucose uptake in skeletal muscle. Its metabolic effects are either chronic diabetogenic or acute insulin-like effects, mediated by the cytosolic tyrosine kinase Janus kinase 2 (JAK2) upon GH-GH receptor interaction, resulting in insulin receptor substrate-1 (IRS-1) and IRS-2 activation via phosphatidylinositol-3 kinase (PI3-k)/Akt activation of glucose transporters (GLUT4) and increased glucose uptake. Simultaneously, JAK2 phosphorylates and activates the STAT-family transcription factors responsible for the diabetogenic effects of GH and blocking the IRS-proteins phosphorylation, which induces insulin-like effects of GH [32]. These mechanisms explain insulin-resistance, impaired glucose tolerance, impaired fasting glucose and diabetes development in GH excess, and are invoked by some authors for similar worsening of metabolic characteristics in GHD patients undergoing replacement therapy [11, 33]. According to the results of this extensive meta-analysis, neither plasma glucose levels nor HbA1c was significantly affected by GH supplementation even in the long term therapy. Hypopituitarism patients with GHD in stable compensatory treatment for less than 1 year showed very discordant results in terms of glycaemic levels. This may be linked to the effects of other pituitary hormones on glucose metabolism. On the contrary, data on the isolated GHD were more homogeneous. However, all the three subgroups showed a neutral effect of GH supplementation on glycemic levels, which constantly remain within the normal range. However, the results of our meta-analysis are in agreement with recent data from two population-based cohort studies consisting of 5100 children with idiopathic isolated GHD and above 104 subjects affected by Turner syndrome treated with GH therapy for many years. These results showed no difference in the risk of diabetes between GH treated patients compared with the reference population [34, 35]. Finally, blood pressure seems to be not affected by GH administration, either in the short and long term observation.
Results concerning the effects of GH supplementation on IMT in the literature are discordant [14, 36]. Several studies show an anti-atherogenic activity of GH itself and its derivatives [37], which is usually connected to the simultaneous decrease of the plasma cholesterol ester transfer protein (CETP) and C-LDL concentrations itself [38]. A second mechanism could be the reduction in asymmetric dimethyl arginine (ADMA), a key protein in atherosclerotic plaque formation, which has been described after 6 months of GH supplementation [33]. Some authors indicated IMT reduction as one of the main factors responsible for cardiovascular risk reduction after GH supplementation in GH deficient adult patients [39-42].
The result of our work are partially consistent with two previous meta-analyses. Maison et al. found that GH treatment in GHD significantly reduced C-LDL [-0.5 (SD 0.3) mmol/liter], C-TOT [-0.3 (SD 0.3) mmol/liter], fat mass [-3.1 (SD 3.3) kg], diastolic BP [-1.8 (SD 3.8) mmHg] and significantly increased FFM [+2.8 (SD 2.7) kg], fasting plasma glucose [+ 0.2 (SD 0.1) mM/L], and insulin [+8.7 (SD 7.0) pM/L] [7]. No variation was described on T, C-HDL, and systolic BP. Our meta-analysis failed in demonstrating a reduction in C-TOT which was expected because of reduced C-LDL with a neutral effect on C-HDL and T. This could probably be linked to the small number of studies included in the analysis. Another difference is represented by the effect on glucose metabolism since in Maison’s work only 6 out of 37 included studies had a duration of at least 12 months, while 6 out of 37 of no more than 3 months. Moreover, the author stated that the insulin-antagonistic effect was not maintained at 12 and 18 months follow-up and that mean blood glucose remained in the normal range [7]. Newman et al. pointed out that GH replacement therapy in GHD may induce increase in FFM [+2.61 (SD 6.8) kg], decrease in FM [-2.2 (SD 13.5) kg], C-TOT [-0.4 (1.1) mM/L], and C-LDL [-0.4 (1.8) mM/L] [43]. Furthermore, no difference was found in C-HDL and T levels [43].
Considering that GH replacement therapy can induce a reduction in CV risk manly through C-LDL, it could be suggested using statin treatment instead of GH substitution in GHD patients in order to reach the same goal. However, the use of GH is effective to improve body composition, well-being [44], cognitive performance [45], bone mineral density [46, 47], and cardiac function [7], and to reduce cancer risk [48]. Moreover, GH supplementation enhance the quality of life of GHD patients. Finally, considering the long-term (beyond 5 years) effects, despite some encouraging preliminary reports, the ultimate endpoint demonstrating beneficial effects of GH supplementation treatment on mortality, cardiovascular risk and fractures’ rate without any increase in cancer incidence is still to be demonstrated [9, 12].
Conclusion
This work supports the hypothesis that GH replacement therapy in GHD is safe and could probably lead to a significant cardiovascular risk reduction, although a quantitative demonstration has not been possible so far. However, the beneficial effect of GH substitution should stem from mainly the reduction in C-LDL levels, as well as from the absence of impairment of glucose metabolism and blood pressure. These results remain after long-term treatment, irrespective of the cause of GHD, both in panhypopituitaric and idiopathic forms. Nevertheless, it is worthy to underline that further studies, with a proper prospective design and with a large appropriate number of subjects, are needed to obtain conclusive evidence of GH beneficial effect on cardiovascular parameters in this setting, especially considering IMT and BP.
Acknowledgements
Medical writing assistance was provided by Fullcro s.r.l. Rome, Italy funded by Merck Serono S.p.A., Italy.
List of abbreviations
- BP
Blood pressure
- C-TOT; C-HDL;C-LDL
Cholesterol
- CV
Cardiovascular
- FFM
Fat-free mass
- FM
Fat mass
- FU
Follow-up study
- GHD
GH deficiency
- HbA1c
Glycated hemoglobin
- IGF-1
Insulin-like growth factor 1
- IMT
Intima-media thickness
- OLS
Open label study
- R
Retrospective study
- RCT
Randomized controlled trial
- RDB
Randomized double-blind controlled trial
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
Consent for Publication
Not applicable.
Conflict of interest
The authors take full responsibility for the content of the paper. All authors have read and approved the final version of the manuscript.
Raffaella Perrone is an employee of Merck Serono SpA, Italy. The other authors declare no conflicts of interest.
References
- 1.Isgaard J., Arcopinto M., Karason K., Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine. 2015;48(1):25–35. doi: 10.1007/s12020-014-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borson-Chazot F., Serusclat A., Kalfallah Y., Ducottet X., Sassolas G., Bernard S., Labrousse F., Pastene J., Sassolas A., Roux Y., Berthezène F. Decrease in carotid intima-media thickness after one year growth hormone (GH) treatment in adults with GH deficiency. J. Clin. Endocrinol. Metab. 1999;84(4):1329–1333. doi: 10.1210/jcem.84.4.5595. [DOI] [PubMed] [Google Scholar]
- 3.Allen D.B., Backeljauw P., Bidlingmaier M., Biller B.M., Boguszewski M., Burman P., Butler G., Chihara K., Christiansen J., Cianfarani S., Clayton P., Clemmons D., Cohen P., Darendeliler F., Deal C., Dunger D., Erfurth E.M., Fuqua J.S., Grimberg A., Haymond M., Higham C., Ho K., Hoffman A.R., Hokken-Koelega A., Johannsson G., Juul A., Kopchick J., Lee P., Pollak M., Radovick S., Robison L., Rosenfeld R., Ross R.J., Savendahl L., Saenger P., Toft Sorensen H., Stochholm K., Strasburger C., Swerdlow A., Thorner M. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur. J. Endocrinol. 2016;174(2):1–9. doi: 10.1530/EJE-15-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertsson-Wikland K., Martensson A., Savendahl L., Niklasson A., Bang P., Dahlgren J., Gustafsson J., Kriström B., Norgren S., Pehrsson N.G., Odén A. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J. Clin. Endocrinol. Metab. 2016;101(5):2149–2159. doi: 10.1210/jc.2015-3951. [DOI] [PubMed] [Google Scholar]
- 5.Vasan R.S., Sullivan L.M., D’Agostino R.B., Roubenoff R., Harris T., Sawyer D.B., Levy D., Wilson P.W. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann. Intern. Med. 2003;139(8):642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 6.Juul A., Scheike T., Davidsen M., Gyllenborg J., Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 7.Maison P., Griffin S., Nicoue-Beglah M., Haddad N., Balkau B., Chanson P. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J. Clin. Endocrinol. Metab. 2004;89(5):2192–2199. doi: 10.1210/jc.2003-030840. [DOI] [PubMed] [Google Scholar]
- 8.Widdowson W.M., Healy M.L., Sonksen P.H., Gibney J. The physiology of growth hormone and sport. Growth Horm. IGF Res. 2009;19(4):308–319. doi: 10.1016/j.ghir.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Di Somma C., Scarano E., Savastano S., Savanelli M.C., Pivonello R., Colao A. Cardiovascular alterations in adult GH deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31(1):25–34. doi: 10.1016/j.beem.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Filipsson Nystrom H., Barbosa E.J., Nilsson A.G., Norrman L.L., Ragnarsson O., Johannsson G. Discontinuing long-term GH replacement therapy--a randomized, placebo-controlled crossover trial in adult GH deficiency. J. Clin. Endocrinol. Metab. 2012;97(9):3185–3195. doi: 10.1210/jc.2012-2006. [DOI] [PubMed] [Google Scholar]
- 11.Christ E.R., Cummings M.H., Albany E., Umpleby A.M., Lumb P.J., Wierzbicki A.S., Naoumova R.P., Boroujerdi M.A., Sönksen P.H., Russell-Jones D.L. Effects of growth hormone (GH) replacement therapy on very low density lipoprotein apolipoprotein B100 kinetics in patients with adult GH deficiency: a stable isotope study. J. Clin. Endocrinol. Metab. 1999;84(1):307–316. doi: 10.1210/jcem.84.1.5365. [DOI] [PubMed] [Google Scholar]
- 12.Hoybye C., Christiansen J.S. Growth hormone replacement in adults - current standards and new perspectives. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29(1):115–123. doi: 10.1016/j.beem.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Leandro G. Meta-analysis in Medical Research. Blackwell Publishing; 2005. [Google Scholar]
- 14.Oliveira J.L., Aguiar-Oliveira M.H., D’Oliveira A., Jr, Pereira R.M., Oliveira C.R., Farias C.T., Barreto-Filho J.A., Anjos-Andrade F.D., Marques-Santos C., Nascimento-Junior A.C., Alves E.O., Oliveira F.T., Campos V.C., Ximenes R., Blackford A., Parmigiani G., Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis: effects of GH replacement in GH-naive adults. J. Clin. Endocrinol. Metab. 2007;92(12):4664–4670. doi: 10.1210/jc.2007-1636. [DOI] [PubMed] [Google Scholar]
- 15.Bollerslev J., Ueland T., Jorgensen A.P., Fougner K.J., Wergeland R., Schreiner T., Burman P. Positive effects of a physiological dose of GH on markers of atherogenesis: a placebo-controlled study in patients with adult-onset GH deficiency. Eur. J. Endocrinol. 2006;154(4):537–543. doi: 10.1530/eje.1.02125. [DOI] [PubMed] [Google Scholar]
- 16.Elbornsson M., Gotherstrom G., Bosaeus I., Bengtsson B.A., Johannsson G., Svensson J. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur. J. Endocrinol. 2013;168(5):745–753. doi: 10.1530/EJE-12-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauregard C., Utz A.L., Schaub A.E., Nachtigall L., Biller B.M., Miller K.K., Klibanski A. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 2008;93(6):2063–2071. doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fideleff H.L., Boquete H.R., Stalldecker G., Giaccio A.V., Sobrado P.G. Comparative results of a 4-year study on cardiovascular parameters, lipid metabolism, body composition and bone mass between untreated and treated adult growth hormone deficient patients. Growth Horm. IGF Res. 2008;18(4):318–324. doi: 10.1016/j.ghir.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Joaquin C., Aguilera E., Granada M.L., Pastor M.C., Salinas I., Alonso N., Sanmartí A. Effects of GH treatment in GH-deficient adults on adiponectin, leptin and pregnancy-associated plasma protein-A. Eur. J. Endocrinol. 2008;158(4):483–490. doi: 10.1530/EJE-07-0554. [DOI] [PubMed] [Google Scholar]
- 20.Nass R., Huber R.M., Klauss V., Muller O.A., Schopohl J., Strasburger C.J. Effect of growth hormone (hGH) replacement therapy on physical work capacity and cardiac and pulmonary function in patients with hGH deficiency acquired in adulthood. J. Clin. Endocrinol. Metab. 1995;80(2):552–557. doi: 10.1210/jcem.80.2.7852519. [DOI] [PubMed] [Google Scholar]
- 21.Topaloglu O., Gokay F., Koparal S.S., Akbaba G., Mete T., Arduc A., Tuna M.M., Yalcin Y., Yavuz H.C., Berker D., Guler S. Visceral fat measurement by ultrasound as a non-invasive method - can it be useful in evaluating subclinical atherosclerosis in male patients with hypopituitarism and growth hormone deficiency? Endokrynol. Pol. 2014;65(3):195–202. doi: 10.5603/EP.2014.0027. [DOI] [PubMed] [Google Scholar]
- 22.Savastano S., Di Somma C., Barrea L., Colao A. The complex relationship between obesity and the somatropic axis: the long and winding road. Growth Horm. IGF Res. 2014;24(6):221–226. doi: 10.1016/j.ghir.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Deodati A., Ferroli B.B., Cianfarani S. Association between growth hormone therapy and mortality, cancer and cardiovascular risk: systematic review and meta-analysis. Growth Horm. IGF Res. 2014;24(4):105–111. doi: 10.1016/j.ghir.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen J.S., Jorgensen J.O., Pedersen S.A., Muller J., Jorgensen J., Moller J., Heickendorf L., Skakkebaekm N.E. GH-replacement therapy in adults. Horm. Res. 1991;36(Suppl. 1):66–72. doi: 10.1159/000182192. [DOI] [PubMed] [Google Scholar]
- 25.Stochholm K., Berglund A., Juul S., Gravholt C.H., Christiansen J.S. Socioeconomic factors do not but GH treatment does affect mortality in adult-onset growth hormone deficiency. J. Clin. Endocrinol. Metab. 2014;99(11):4141–4148. doi: 10.1210/jc.2014-1814. [DOI] [PubMed] [Google Scholar]
- 26.van Bunderen C.C., van den Dries C.J., Heymans M.W., Franken A.A., Koppeschaar H.P., van der Lely A.J., Drent M.L. Effect of long-term GH replacement therapy on cardiovascular outcomes in isolated GH deficiency compared with multiple pituitary hormone deficiencies: a sub-analysis from the Dutch National Registry of Growth Hormone Treatment in Adults. Eur. J. Endocrinol. 2014;171(2):151–160. doi: 10.1530/EJE-14-0069. [DOI] [PubMed] [Google Scholar]
- 27.Gazzaruso C., Gola M., Karamouzis I., Giubbini R., Giustina A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH--an update. J. Clin. Endocrinol. Metab. 2014;99(1):18–29. doi: 10.1210/jc.2013-2394. [DOI] [PubMed] [Google Scholar]
- 28.Ezzat S., Fear S., Gaillard R.C., Gayle C., Landy H., Marcovitz S., Mattioni T., Nussey S., Rees A., Svanberg E. Gender-specific responses of lean body composition and non-gender-specific cardiac function improvement after GH replacement in GH-deficient adults. J. Clin. Endocrinol. Metab. 2002;87(6):2725–2733. doi: 10.1210/jcem.87.6.8542. [DOI] [PubMed] [Google Scholar]
- 29.Kotzka J., Knebel B., Avci H., Jacob S., Nitzgen U., Jockenhovel F., Heeren J., Haas J., Muller-Wieland D. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1a links growth hormone action to lipid metabolism in hepatocytes. Atherosclerosis. 2010;213(1):156–165. doi: 10.1016/j.atherosclerosis.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 30.Dal J., List E.O., Jorgensen J.O., Berryman D.E. Glucose and Fat Metabolism in Acromegaly: From Mice Models to Patient Care. Neuroendocrinology. 2016;103(1):96–105. doi: 10.1159/000430819. [DOI] [PubMed] [Google Scholar]
- 31.Dullaart R.P., van den Berg G., van der Knaap A.M., Dijck-Brouwer J., Dallinga-Thie G.M., Zelissen P.M., Sluiter W.J., van Beek A.P. HDL cholesterol response to GH replacement is associated with common cholesteryl ester transfer protein gene variation (-629C>A) and modified by glucocorticoid treatment. Eur. J. Endocrinol. 2010;162(2):227–234. doi: 10.1530/EJE-09-0742. [DOI] [PubMed] [Google Scholar]
- 32.Ridderstrale M. Signaling mechanism for the insulin-like effects of growth hormone--another example of a classical hormonal negative feedback loop. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2005;5(1):79–92. doi: 10.2174/1568008053174787. [DOI] [PubMed] [Google Scholar]
- 33.Setola E., Monti L.D., Lanzi R., Lucotti P., Losa M., Gatti E., Galluccio E., Oldani M., Fermo I., Giovannelli M., Bosi E., Piatti P. Effects of growth hormone treatment on arginine to asymmetric dimethylarginine ratio and endothelial function in patients with growth hormone deficiency. Metabolism. 2008;57(12):1685–1690. doi: 10.1016/j.metabol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Baronio F., Mazzanti L., Girtler Y., Tamburrino F., Lupi F., Longhi S., Fanolla A., Radetti G. The influence of GH treatment on glucose homeostasis in girls with Turner Syndrome: a 7 years study. J. Clin. Endocrinol. Metab. 2017;102(3):878–883. doi: 10.1210/jc.2016-3179. [DOI] [PubMed] [Google Scholar]
- 35.Poidvin A., Weill A., Ecosse E., Coste J., Carel J.C. Risk of diabetes treated in early adulthood following growth hormone treatment for short stature in childhood. J. Clin. Endocrinol. Metab. 2017;102(4):1291–1298. doi: 10.1210/jc.2016-3145. [DOI] [PubMed] [Google Scholar]
- 36.Colao A., Di Somma C., Spiezia S., Savastano S., Rota F., Savanelli M.C., Lombardi G. Growth hormone treatment on atherosclerosis: results of a 5-year open, prospective, controlled study in male patients with severe growth hormone deficiency. J. Clin. Endocrinol. Metab. 2008;93(9):3416–3424. doi: 10.1210/jc.2007-2810. [DOI] [PubMed] [Google Scholar]
- 37.Demers A., McNicoll N., Febbraio M., Servant M., Marleau S., Silverstein R., Ong H. Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem. J. 2004;382(Pt. 2):417–424. doi: 10.1042/BJ20040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrilho A.J., Cunha-Neto M.B., Nunes V.S., Lottenberg A.M., Medina W.L., Nakandakare E.R., Musolino N.R., Bronstein M.D., Quintão E.C. Plasma cholesteryl ester transfer protein and lipoprotein levels during treatment of growth hormone-deficient adult humans. Lipids. 2001;36(6):549–554. doi: 10.1007/s11745-001-0756-y. [DOI] [PubMed] [Google Scholar]
- 39.Khadilkar V., Ekbote V., Kajale N., Khadilkar A., Chiplonkar S., Kinare A. Effect of one-year growth hormone therapy on body composition and cardio-metabolic risk in Indian children with growth hormone deficiency. Endocr. Res. 2014;39(2):73–78. doi: 10.3109/07435800.2013.828742. [DOI] [PubMed] [Google Scholar]
- 40.Mesa J., Gomez J.M., Hernandez C., Pico A., Ulied A. Growth hormone deficiency in adults: effects of replacement therapy on body composition and health-related quality of life. Med. Clin. (Barc.) 2003;120(2):41–46. doi: 10.1016/s0025-7753(03)73599-4. [DOI] [PubMed] [Google Scholar]
- 41.Galoiu S., Jurcut R., Vladaia A., Florian A., Purice M., Popescu B.A., Ginghină C., Coculescu M. Structural and functional changes of carotid wall properties in patients with acromegaly are not restored after 1 year of GH/IGF1 normalization. Exp. Clin. Endocrinol. Diabetes. 2012;120(4):238–243. doi: 10.1055/s-0032-1304606. [DOI] [PubMed] [Google Scholar]
- 42.Murray R.D., Wieringa G., Lawrance J.A., Adams J.E., Shalet S.M. Partial growth hormone deficiency is associated with an adverse cardiovascular risk profile and increased carotid intima-medial thickness. Clin. Endocrinol. (Oxf.) 2010;73(4):508–515. doi: 10.1111/j.1365-2265.2009.03754.x. [DOI] [PubMed] [Google Scholar]
- 43.Newman C.B., Carmichael J.D., Kleinberg D.L. Effects of low dose versus high dose human growth hormone on body composition and lipids in adults with GH deficiency: a meta-analysis of placebo-controlled randomized trials. Pituitary. 2015;18(3):297–305. doi: 10.1007/s11102-014-0571-z. [DOI] [PubMed] [Google Scholar]
- 44.Deijen J.B., Arwert L.I., Witlox J., Drent M.L. Differential effect sizes of growth hormone replacement on Quality of Life, well-being and health status in growth hormone deficient patients: a meta-analysis. Health Qual. Life Outcomes. 2005;3:63. doi: 10.1186/1477-7525-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falleti M.G., Maruff P., Burman P., Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31(6):681–691. doi: 10.1016/j.psyneuen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Xue P., Wang Y., Yang J., Li Y. Effects of growth hormone replacement therapy on bone mineral density in growth hormone deficient adults: a meta-analysis. Int. J. Endocrinol. 2013;2013:216107. doi: 10.1155/2013/216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barake M., Klibanski A., Tritos N.A. Effects of recombinant human growth hormone therapy on bone mineral density in adults with growth hormone deficiency: a meta-analysis. J. Clin. Endocrinol. Metab. 2014;99(3):852–860. doi: 10.1210/jc.2013-3921. [DOI] [PubMed] [Google Scholar]
- 48.Li Z., Zhou Q., Li Y., Fu J., Huang X., Shen L. Growth hormone replacement therapy reduces risk of cancer in adult with growth hormone deficiency: A meta-analysis. Oncotarget. 2016;7(49):81862–81869. doi: 10.18632/oncotarget.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cenci M.C., Conceicao F.L., Soares D.V., Spina L.D., Brasil R.R., Lobo P.M., Michmacher E., Vaisman M. Impact of 5 years of growth hormone replacement therapy on cardiovascular risk factors in growth hormone-deficient adults. Metabolism. 2008;57(1):121–129. doi: 10.1016/j.metabol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Yuen K.C., Dunger D.B. Persisting effects on fasting glucose levels and insulin sensitivity after 6 months of discontinuation of a very low-dose GH therapy in adults with severe GH deficiency. Clin. Endocrinol. (Oxf.) 2006;64(5):549–555. doi: 10.1111/j.1365-2265.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- 51.Chihara K., Kato Y., Takano K., Shimatsu A., Kohno H., Tanaka T., Irie M. Effect of growth hormone treatment on trunk fat accumulation in adult GH-deficient Japanese patients: a randomised, placebo-controlled trial. Curr. Med. Res. Opin. 2006;22(10):1973–1979. doi: 10.1185/030079906X132460. [DOI] [PubMed] [Google Scholar]
- 52.Yuen K.C., Frystyk J., White D.K., Twickler T.B., Koppeschaar H.P., Harris P.E., Fryklund L., Murgatroyd P.R., Dunger D.B. Improvement in insulin sensitivity without concomitant changes in body composition and cardiovascular risk markers following fixed administration of a very low growth hormone (GH) dose in adults with severe GH deficiency. Clin. Endocrinol. (Oxf.) 2005;63(4):428–436. doi: 10.1111/j.1365-2265.2005.02359.x. [DOI] [PubMed] [Google Scholar]
- 53.Hana V., Silha J.V., Justova V., Lacinova Z., Stepan J.J., Murphy L.J. The effects of GH replacement in adult GH-deficient patients: changes in body composition without concomitant changes in the adipokines and insulin resistance. Clin. Endocrinol. (Oxf.) 2004;60(4):442–450. doi: 10.1111/j.1365-2265.2004.02000.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith J.C., Lang D., McEneny J., Evans L.M., Scanlon M.F., Young I., Davies J. Effects of GH on lipid peroxidation and neutrophil superoxide anion-generating capacity in hypopituitary adults with GH deficiency. Clin. Endocrinol. (Oxf.) 2002;56(4):449–455. doi: 10.1046/j.1365-2265.2002.01493.x. [DOI] [PubMed] [Google Scholar]
- 55.O’Neal D.N., Hew F.L., Best J.D., Alford F. The effect of 24 months recombinant human growth hormone (rh-GH) on LDL cholesterol, triglyceride-rich lipoproteins and apo [a] in hypopituitary adults previously treated with conventional replacement therapy. Growth Horm. IGF Res. 1999;9(3):165–173. doi: 10.1054/ghir.1999.0102. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Arnao J., Jabbar A., Fulcher K., Besser G.M., Ross R.J. Effects of growth hormone replacement on physical performance and body composition in GH deficient adults. Clin. Endocrinol. (Oxf.) 1999;51(1):53–60. doi: 10.1046/j.1365-2265.1999.00737.x. [DOI] [PubMed] [Google Scholar]
- 57.Woodhouse L.J., Asa S.L., Thomas S.G., Ezzat S. Measures of submaximal aerobic performance evaluate and predict functional response to growth hormone (GH) treatment in GH-deficient adults. J. Clin. Endocrinol. Metab. 1999;84(12):4570–4577. doi: 10.1210/jcem.84.12.6196. [DOI] [PubMed] [Google Scholar]
- 58.Cuneo R.C., Judd S., Wallace J.D., Perry-Keene D., Burger H., Lim-Tio S., Strauss B., Stockigt J., Topliss D., Alford F., Hew L., Bode H., Conway A., Handelsman D., Dunn S., Boyages S., Cheung N.W., Hurley D. The Australian Multicenter Trial of Growth Hormone (GH) Treatment in GH-Deficient Adults. J. Clin. Endocrinol. Metab. 1998;83(1):107–116. doi: 10.1210/jcem.83.1.4482. [DOI] [PubMed] [Google Scholar]
- 59.Johansson J.O., Landin K., Johannsson G., Tengborn L., Bengtsson B.A. Long-term treatment with growth hormone decreases plasminogen activator inhibitor-1 and tissue plasminogen activator in growth hormone-deficient adults. Thromb. Haemost. 1996;76(3):422–428. [PubMed] [Google Scholar]
- 60.Weaver J.U., Monson J.P., Noonan K., John W.G., Edwards A., Evans K.A., Cunningham J. The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J. Clin. Endocrinol. Metab. 1995;80(1):153–159. doi: 10.1210/jcem.80.1.7829604. [DOI] [PubMed] [Google Scholar]
- 61.Jorgensen J.O., Thuesen L., Muller J., Ovesen P., Skakkebaek N.E., Christiansen J.S. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur. J. Endocrinol. 1994;130(3):224–228. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- 62.Whitehead H.M., Boreham C., McIlrath E.M., Sheridan B., Kennedy L., Atkinson A.B., Hadden D.R. Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo controlled cross-over study. Clin. Endocrinol. (Oxf.) 1992;36(1):45–52. doi: 10.1111/j.1365-2265.1992.tb02901.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.