Abstract
Xenograft models are transforming our understanding of the output capabilities of primitive human hematopoietic cells in vivo. However, many variables that affect posttransplantation reconstitution dynamics remain poorly understood. Here, we show that an equivalent level of human chimerism can be regenerated from human CD34+ cord blood cells transplanted intravenously either with or without additional radiation-inactivated cells into 2- to 6-month-old NOD-Rag1−/−-IL2Rγc−/− (NRG) mice given a more radioprotective conditioning regimen than is possible in conventionally used, repair-deficient NOD-Prkdcscid/scid-IL2Rγc−/−(NSG) hosts. Comparison of sublethally irradiated and non-irradiated NRG mice and W41/W41 derivatives showed superior chimerism in the W41-deficient recipients, with some differential effects on different lineage outputs. Consistently superior outputs were observed in female recipients regardless of their genotype, age, or pretransplantation conditioning, with greater differences apparent later after transplantation. These results define key parameters for optimizing the sensitivity and minimizing the intraexperimental variability of human hematopoietic xenografts generated in increasingly supportive immunodeficient host mice.
The discovery more than two decades ago that primitive normal human hematopoietic cells can engraft and regenerate hematopoiesis in CB17-scid mice proved to be a turning point in the analysis of the earliest stages of human hematopoiesis [1]. Subsequently, both empirically characterized and directly introduced genetic modifications have produced dramatic improvements in the diversity, level, and duration of human hematopoietic cell chimerism that can be obtained in xenografted mouse models [2–4]. These improvements have led to the identification of subsets of human CD34+cells that recapitulate the concept, now well advanced in the mouse system, of multiple biologically distinct hematopoietic cell populations that produce different types of mature progeny over time in transplanted recipients [5–8].
Early studies of nongenetic variables that affect chimerism levels attained in nonobese diabetic–severe combined immunodeficient (NOD.CB17-Prkdcscid/J, NS) mice included evidence of strong effects of the age of the mouse transplanted [9], whether other cells were coinjected [10], and the conditioning regimen used [11]. Most hematopoietic xenotransplantation studies now make use of NS mice into which a null mutation of the gamma chain of the interleukin 2 (IL2) receptor has been introduced (referred to as NOD-Prkdcscid/scid-IL2Rγc−/− [NSG] mice). This modification has the advantage of eliminating the generation of the fatal thymic lymphomas that appear universally in NS mice at between 3 and 6 months of age. NSG mice thus have a normal lifespan and the dynamics of transplanted human hematopoietic cells can be followed reproducibly for up to a year. NSG mice also lack natural killer (NK) cells, in contrast to NS mice, in which the levels of NK cells produced are still sufficient to suppress selectively the ability of most CD34+CD38+ human hematopoietic cells to engraft the bone marrow (BM) and thus fail to display their innate short-term repopulating activity [6,12]. Evidence that the sex of the recipient can affect the level of chimerism obtained from either syngeneic (mouse) [13,14] or xenogeneic (human) transplantations [15] has also been reported.
More recently, NSG mice with various mutant c-kit alleles have been created and shown to be even more permissive than NSG mice in terms of their support of human hematopoietic cells obtained from transplantations of human cord blood (CB) cells [16–18]. Of particular interest are c-kit-deficient NSG mice expressing a homozygous W41 allele because these remain fertile. In C57BL/6(B6)-W41/W41 mice, maintenance of the hematopoietic stem cell (HSC) and downstream erythroid progenitor compartments is sufficiently compromised to enable their repopulation by transplanted B6-+/+ HSCs without being subjected to a lethally myeloablative treatment [19–21]. Key findings from assessments of previously described “NSG-W41” mice transplanted with human hematopoietic cells include an increase in the levels of human chimerism that they support compared with NSG mice, including the production of human erythroid precursors and platelets detectable even in the absence of any prior host conditioning [16–18].
However, for many future studies, particularly those with the objective of assessing treatments of engrafted human malignant cells, the scid mutation (that encodes a defective DNA repair protein [22]) is undesirable because it sensitizes all of the host tissues markedly to many radiomimetic drugs that would be likely candidates for inclusion in test treatment protocols. Therefore, we initiated an examination of a radio-resistant alternative to NSG-W41 mice and evaluated variables that might affect the level and duration of human hematopoietic chimerism that would be supported.
Here, we report the relevance of a number of variables in mice genetically identical to NSG mice but with a homozygous Rag1null genotype and wild-type Prkdc genotype to retain the same level of immunodeficiency but a normal DNA repair capacity. We then introduced the W41/W41 genotype into NOD-Rag1−/−-IL2Rγc−/− (NRG) mice to generate a derivative strain. We refer to the latter as “SRG-W41” mice because they have been selected in the F2 generation to be homozygous for the Sirpα [23], Rag1-null, and IL2rγc-null alleles of the NRG mouse, as well as the introduced W41 allele on an otherwise mixed NOD-B6 genetic background.
Materials and methods
Mice
Breeding colonies of NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ (NRG) and B6-KitW–41/W–41 (B6-W41/W41) mice originally obtained from The Jackson Laboratory have been established for many years in the Animal Resource Centre of the British Columbia Cancer Agency. Mice referred to here as “SRG-W41” were F2 mice generated by intercrossing the F1 progeny of NRG with B6-W41/W41 matings and then selecting the progeny that were homozygous for the Sirpa allele, the null Rag1 and Il2rg alleles of the NRG mouse, and the W41 mutant Kit allele of the B6-W41/W41 mouse to obtain all of these alleles on an otherwise mixed segregating NODxB6 (STOCK) background. Genotyping of ear notch samples was performed by polymerase chain reaction (PCR) gel electrophoresis, except for determining the presence of two, one, or no copies of the single nucleotide mutation that encodes the W41 allele, which were detected by Sanger sequencing of amplified PCR products. All animal breeding and experiments were performed according to regulations approved by the Animal Care Committee of the University of British Columbia.
Human cells
CD34+-enriched suspensions of human low-density CB cells (>80% purities) were obtained using an EasySep kit (STEM-CELL Technologies, Vancouver, Canada) from cryopreserved cells originally acquired and processed as described previously [5]. Human BM cells were obtained as discarded anonymized autologous transplantation harvests from deceased patients. All cells were obtained and used according to procedures approved by the University of British Columbia Research Ethics Board.
Transplantation
Cells were injected intravenously into 7- to 12- or 21- to 25-week-old male and female mice that had either not been irradiated at all or had been irradiated with 137Cs γ-rays or 250 kvp X-rays, as indicated. All acute exposures involved irradiating mice at a dose rate of 100–150 cGy/minute (for both types of ionizing radiation). All NRG mice used as transplantation recipients were irradiated with 900 cGy of 137Cs γ-rays given over a 3 hour period (5 cGy/min) to allow significant repair of DNA damage in nonhematopoietic cell types that have such capacity (e.g., intestinal epithelial cells [24]). In mice given transplantations accompanied by coinjected accessory cells, the latter consisted of 106 irradiated (15 Gy) human BM cells. Mice were maintained under specific pathogen-free conditions using protocols approved by the Animal Care Committee of the University of British Columbia.
Flow cytometry
Absolute numbers of human CD34+ cells were determined using appropriate antibodies and AccuCheck counting beads (Life Technologies, Carlsbad, CA) as described previously [25]. Human CD45+, glycophorin A (GPA) erythroid, CD45+CD33/15+ granulocyte-macrophage (GM), CD45+CD19+ B-cells, and FSClowCD41+CD61+ platelets were also quantified using previously described antibodies and their absolute numbers determined in recipient peripheral blood (PB) samples using AccuCheck counting beads (Life Technologies) [5,25].
Statistical analysis
Data analysis and graph generation made use of R version 3.2.3 [26]. Data shown are geometric means ± SEM, with reconstitution data log transformed to improve fit to assumptions of normal distribution and equal variance. Kaplan–Meier curves were produced using the R “survival” package. Pairwise differences were examined using the Student t test. Multiple group comparisons were examined using one-way ANOVA with post hoc Tukey’s honest significant difference analysis.
Results
NRG mice can support similar levels of human hematopoietic cell chimerism as NSG mice
Given the different radiation sensitivities of NRG and NSG mice [27], we first undertook experiments to develop a conditioning regimen for NRG mice that would exploit the selectively enhanced repair capacity of many of their nonhematopoietic tissues. As expected, acute exposure (150 cGy/min) to increasing doses of X-rays showed 750 cGy caused 100% mortality within 3 weeks, whereas 600 cGy was the maximum dose that allowed the full survival of all mice in that test group. However, an estimated equivalent split dose protocol (two acute exposures of 400 cGy separated by a 6-hour interval) also allowed all six mice tested to survive (Fig. 1). Subsequent studies showed that a similar result could be obtained with 900 cGy of 137Cs γ-rays spread over 3 hours (5 cGy/minute). This latter protocol was then adopted for all subsequent experiments.
Figure 1.
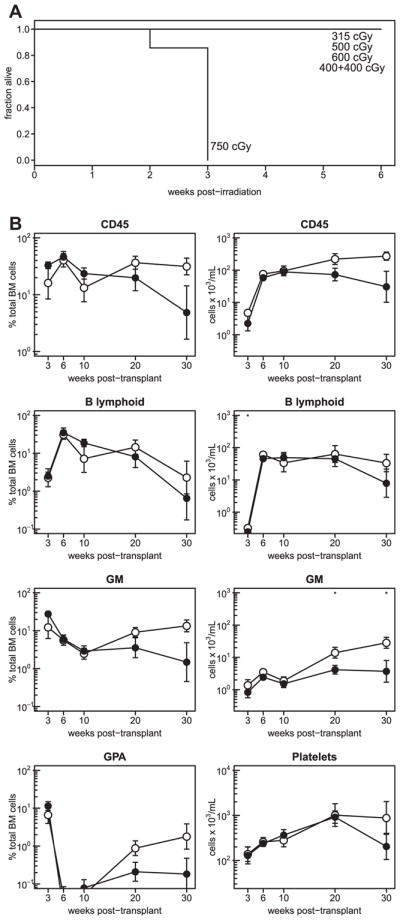
Similar human cell reconstitution of NSG and NRG mice. (A) Cohorts of 7- to 12-week-old NRG mice were X-irradiated at a high dose rate with 315 cGy (4 mice), 500 cGy (4 mice), 600 cGy (6 mice), 750 cGy (7 mice), or with two doses of 400 cGy separated by 6 hours (6 mice) and their survival was then tracked. (B) Kinetics of human CD45+, GM, B-lymphoid, and erythroid cell reconstitution of the BM (left column) and of numbers of human CD45+, GM, and B-lymphoid cells and platelets per milliliter of PB (right column) of NSG (open symbols) and NRG (filled symbols) mice after their transplantation with 2 × 104 human CD34+ CB cells plus 106 irradiated human BM cells. Data are pooled from three replicate experiments with a combined total of 11 mice per group. Asterisks indicate statistical significance (*p ≤ 0.05).
We then compared human CD34+ CB transplantation outcomes in young (8- to 12-week-old) NRG mice with those obtained in sex- and age-matched groups of NSG mice conditioned with a radiobiologically similar, single acute exposure (~100 cGy/min) to 315 cGy of 137Cs γ-rays (i.e., a near maximum sublethal dose that allows the full long-term survival of NSG mice [27]). The dynamics of human hematopoietic cell chimerism obtained from 2 × 104 human CD34+ CB cells (± coinjected 106 irradiated BM cells according to the experiment) in the BM and PB of these recipients was then tracked for up to 30 weeks. The results showed that, for at least 10 weeks, both strains thus conditioned supported similar outputs of total human CD45+, GM, B-lymphoid, and erythroid cells, as well as platelets, with a slight but insignificant favoring of the NSG host thereafter (Fig. 1B).
Differential effects of recipient sex, age, and use of coinjected irradiated human BM cells on the levels of human chimerism obtained in NRG mice
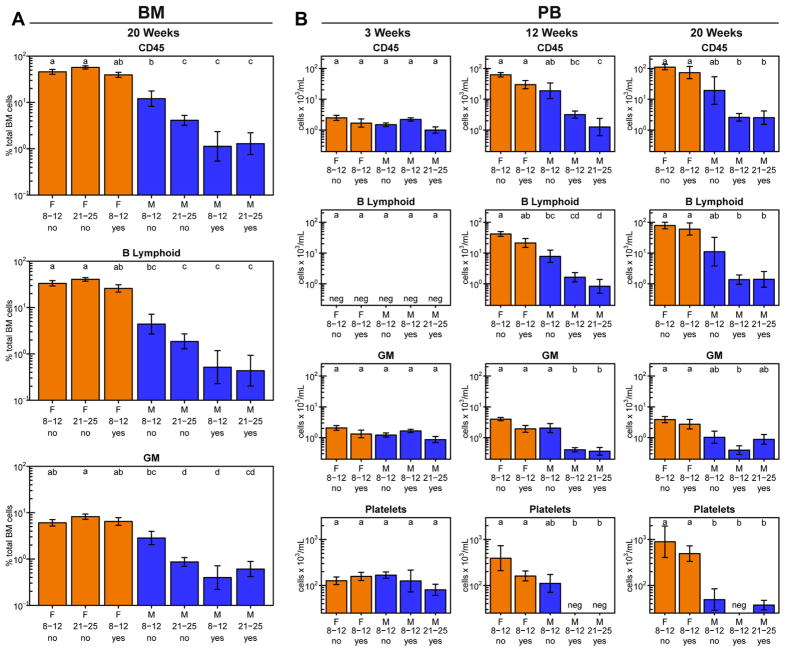
We next examined the role of the sex and age (up to 6 months) of the NRG host or a coinjected dose of 106 irradiated (1500 cGy) human BM cells on the ability of 2 × 104 purified human CD34+ CB cells to regenerate both rapid and sustained multilineage grafts in sublethally irradiated NRG mice. Consistent with earlier data [15], assessments of the chimerism seen in both the BM (Fig. 2A) and the PB (Fig. 2B) showed that female mice generally supported a greater output of all human lineages regardless of their age when transplanted or whether additional cells were injected. However, this greater supportive activity of the female hosts appeared slightly more pronounced on the production of human B-lymphoid cells than on the production of GM cells and the negative effect of using male recipients was more marked when these were older or were coinjected with irradiated cells.
Figure 2.
Female NRG mice support higher human cell outputs than male hosts. Female (orange bars) and male (blue bars) mice were each transplanted with 2 × 104 human CD34+ CB cells and assessed for percentage of viable human cells in the BM 20 weeks later (A), and numbers per milliliter of PB 3 (left column), 12 (middle column), and 20 (right column) weeks later (B). The X-axis labels indicate the recipient sex, recipient age at transplantation in weeks, and whether irradiated human BM cells were coinjected with the CB cells. Letters above the bars indicate groups that are not significantly different from each other. Total numbers of mice in each group (from two experiments) were as follows: female, 8–12 weeks and no accessory cells: 20 mice; female, 21–25 weeks and no accessory cells: 18 mice; female, 8–12 weeks and accessory cells: 5 mice; male, 8–12 weeks and no accessory cells: 24 mice; male, 21–25 weeks and no accessory cells: 20 mice; male, 8–12 weeks and accessory cells: 4 mice; male, 21–25 weeks and accessory cells: 4 mice.
Both irradiated and untreated SRG-W41 hosts support elevated human chimerism compared with NRG mice
We then compared the human multilineage hematopoietic cell outputs obtained from 2–5 × 104 human CB CD34+ cells transplanted into both sublethally irradiated or untreated male and female SRG-W41 and NRG mice. Previous studies had shown 400 cGy of 137Cs γ-rays (given at a high dose rate) to be an optimal sublethal conditioning regimen for preparing B6-W41/W41 mice as hosts of B6 cells [20]. However, NRG mice are considerably more radiosensitive than B6 mice, with an acute dose of 600 cGy X-rays on NRG mice (Fig. 1) being approximately equal to the effect of an acute dose of 900 137Cs γ-rays (~800 cGy X-rays) on B6 mice. We thus estimated (and subsequently found) that a single dose of 150 cGy of 137Cs γ-rays given at the high dose rate would be a conservatively sublethal conditioning dose for SRG-W41 mice.
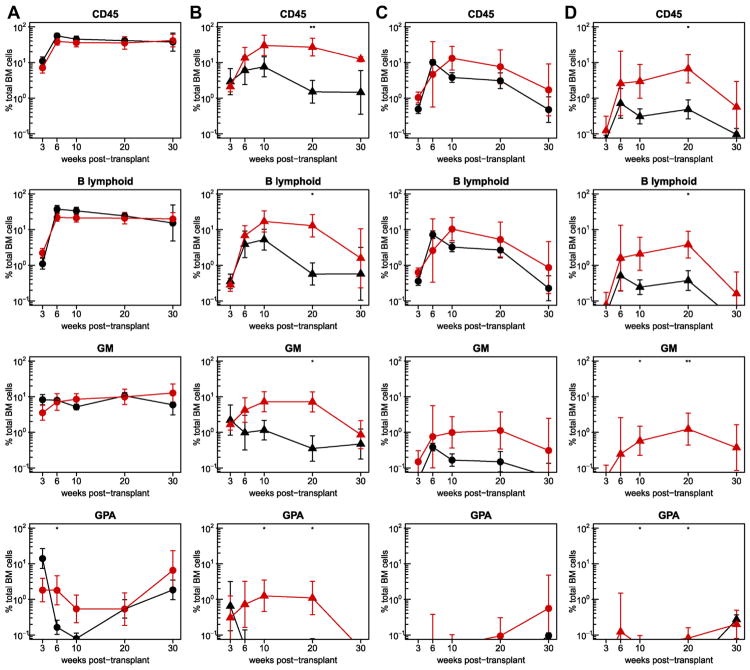
Analysis of sequentially harvested BM aspirate cells from the four different groups of sublethally irradiated recipients again showed generally higher levels of human chimerism in female versus male mice of the same genotype, particularly at later time points after transplantation (Fig. 3A and B). In the male hosts, the outputs of human cells in the SRG-W41 mice were consistently enhanced compared with the NRG mice. In the female hosts, the levels obtained in the NRG mice were initially already very high, except in the erythroid lineage, so, not surprisingly, there was little evidence of increased chimerism in the SRG-W41 hosts.
Figure 3.
Enhanced human cell chimerism in the BM of SRG-W41 versus NRG mice. Shown are the kinetics of human cell reconstitution of the BM of irradiated female (A), irradiated male (B), non-irradiated female (C), and non-irradiated male (D) mice (all 7–12 weeks of age at the time of the transplantation). Results in SRG-W41 hosts are shown by the red symbols and for the parental NRG hosts in black. Mice were transplanted with 2–5 × 104 human CD34+ CB cells and the percentages of total human CD45+, GM, B-lymphoid, and erythroid cells in the BM were assessed in serial aspirates obtained 3, 6, 10, 20, and 30 weeks later. Circles and triangles indicate female and male mice, respectively. Results are pooled from three experiments with a combined total of six to 10 mice per group. Asterisks indicate statistical significance (*p ≤ 0.05; **p ≤ 0.01).
The BM content of human hematopoietic cells obtained from the same CD34+ CB cells injected into male and female SRG-W41 and NRG mice that had received no prior treatment are shown in Figure 3C and D. Readily detectable multilineage human cell chimerism was evident in both the non-irradiated male and female SRG-W41 mice for the entire 30 weeks of follow-up. Unexpectedly, parallel analyses of the non-irradiated female NRG recipients also revealed detectable, albeit much lower, levels of human GM and B-lymphoid cells in the BM. Even in the non-irradiated male NRG recipients, low and transient levels of human B-lymphoid cells were seen during the first 20 weeks.
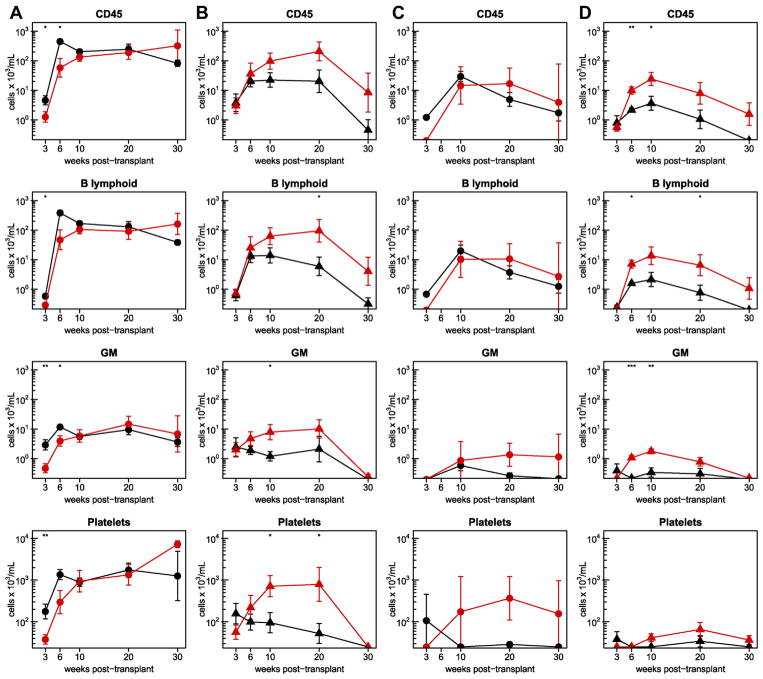
The results of concurrently assessed PB samples obtained from the same irradiated and untreated mice tracked in these experiments were similar to those obtained for the BM. Absolute counts of circulating human hematopoietic cells in both female genotypes were higher than in the corresponding males independent of their prior conditioning; furthermore, the results obtained in the SRG-W41 mice were consistently higher than in the matched groups of NRG mice (Fig. 4). Importantly, these differences extended to the output of human platelets, which was uniquely sustained for 30 weeks in untreated as well as irradiated female SRG-W41 hosts.
Figure 4.
Enhanced human cell numbers in the PB of transplanted SRG-W41 versus NRG mice. Kinetics of human cell numbers in the PB of the irradiated female (A), irradiated male (B), non-irradiated female (C), and non-irradiated male (D) mice described in Figure 3. Results in SRG-W41 mice are again shown by the red symbols and for the NRG mice in black. Numbers of human CD45+, GM, and B-lymphoid cells and platelets per milliliter of recipient blood were determined 3, 6, 10, 20, and 30 weeks after transplantation. Circles and triangles indicate female and male mice, respectively. Asterisks indicate statistical significance (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001).
Discussion
Much interest is now focused on defining the hematopoietic potential and activity of different types of primitive human hematopoietic cells, how these properties are intrinsically regulated, how they are elicited, and how they might be manipulated. This interest inevitably relies on combining molecular and genetic analyses of different phenotypes with robust readouts of the number and types of maturing progeny that they can generate under defined in vitro and in vivo conditions. The use of sublethally irradiated highly immunodeficient mice as hosts of intravenously transplantable human hematopoietic cells has proven an important platform for gaining insights into such issues and revealing the complexity of this system [2,3]. Despite many advances, however, species differences between humans and mice continue to pose challenges to optimizing the sensitivity and specificity of current assays that rely on transplantation readouts. For example, assessments of human repopulating cell activity that rely on measurements of total chimerism obtained in recipients of bulk transplantations are often used to infer HSC frequencies in the cell suspension being evaluated. Such surrogate approaches are valuable because they circumvent the cost and challenges of larger-scale limiting dilution transplantation assays. However, any alteration in the numbers or types of the cells produced, or their assessment under conditions in which inputs and outputs are nonlinear, can compromise interpretation of the results and the validity of using this approach to infer input frequency values. The recent and anticipated continuing creation of increasingly permissive recipient mice for such studies of normal and perturbed human xenografts underscores the continuing need to address these issues.
The Sirpa polymorphism and scid and IL2rg mutations of NSG mice contribute to enhancing their reconstitution by transplantations of human hematopoietic cells by restraining multiple components of the host mouse immune system. Substitution of an inactivating mutation of the Rag1 or Rag2 gene for the scid mutation results in a recipient that is immunologically similar, but less radiosensitive. Replacement of the c-kit gene with a mutant (e.g., W41) allele enables even higher levels of human chimerism to be obtained by impairing the competitiveness of the primitive hematopoietic cells of the recipient. This latter genetic alteration also enhances the output of human erythroid precursors selectively due to the important role of KIT in sustaining erythropoiesis in both mice and humans [28,29]. The ability of an added KIT deficiency to improve the chimerism obtained in NOD, BALB/c, and mixed NOD-B6 genotypes has been reported recently by several groups [16–18]. Although a number of mutant c-kit alleles have been shown to confer this effect, the W41 mutation has the advantage of not compromising the fertility of the host strain. Here, we extend the demonstration of its utility when combined with a Rag1null instead of a scid genotype, to enable less radiotoxic conditioning regimens to be used. This model should thus offer advantages in future studies designed to test the effects of cytotoxic therapies that might not be tolerated in c-kit-deficient NSG mice.
A systematic examination of variables historically reported to affect the level, durability, and lineage contributions to the human chimerism obtained in NS and NSG mice showed that concerns with the use of >12-week-old mice as recipients [9] and the need for coinjected human “carrier” cells for transplantations of small cell numbers [10] no longer pertain with NRG mice. In contrast, we found the previously reported superiority of female recipients [15] remains a critical influence in SRG-W41 and NRG recipients, regardless of whether they are irradiated before performing the transplantation, with the most pronounced effects seen at later time points up to 30 weeks after transplantation.
As predicted by previous studies with NSG-W41 mice [16–18], we found that SRG-W41 mice support greater human chimerism than parental NRG mice, although this difference was masked in the irradiated female recipients studied here by the high chimerism already achieved in the NRG mice by the number of human CD34+ CB cells injected. Of particular note was the marked effect obtained in female NRG and SRG-W41 mice in enhancing the number of circulating human platelets. This finding, together with the enhanced human erythropoiesis also seen, confirms the utility of immunodeficient mice with an additional W41 mutant genotype for studies of manipulations that cause or might correct the delayed platelet recovery characteristic of many human transplantations. Importantly, all of the experiments reported here made use of expanded F2 progeny generated from NRG × B6-W41 F1 mice selected exclusively for homozygosity for the four allelic differences between these two strains anticipated to be relevant to their utility as improved hosts of human cells. The consistency of the xenograft results obtained using them reinforces previous evidence that the Sirpa allele unique to NOD mice accounts for most, if not all, of the permissivity that it contributes [23,30]. Continued backcrossing to obtain more broadly homogeneous genotypes on both NOD and B6 backgrounds is ongoing and will eventually allow this inference to be tested more rigorously.
The extent to which any of the observed recipient sex, genotype, or conditioning regimen-associated differences in chimerism are driven by effects on the initial level of repopulating cell engraftment or stimulation obtained, as opposed to subsequent downstream effects on their output of differentiating cells, remains unresolved. The high frequency of clones derived from injections of highly purified human HSCs [8] argues in favor of the latter, at least for cells with long-term in vivo repopulating activity. However, this latter observation does not rule out a contribution from an increased seeding efficiency or initial activation of human cells in an environment where their endogenous mouse counterparts have a reduced responsiveness to the KIT ligand.
In summary, we have analyzed multiple factors that affect the level of repopulation of immunodeficient mice by intravenously transplanted human CD34+ CB cells. The results demonstrate variable effects early after transplantation, with maximal and readily detectable levels of human B-lineage cells, GM cells, erythroid precursors, and circulating platelets evident for at least 30 weeks in sublethally irradiated female SRG-W41 mice. These findings should facilitate future investigation of the mechanisms of this enhanced chimerism in both irradiated and unconditioned recipients and show the utility of these mice for improving human cell and gene therapy and in studies of human leukemic cell growth and treatment response.
Acknowledgments
The authors thank Margaret Hale and Glenn Edin for excellent technical support and the British Columbia Cancer Agency Stem Cell Assay Laboratory for assistance in processing and cryopreserving CB and BM samples.
This work was supported by a grant to C.J.E. from the Terry Fox Foundation. P.H.M. held a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Doctoral Scholarship. N.N. received a Mitacs Elevate Postdoctoral Fellowship. D.J.H.F.K. received a CIHR Vanier Scholarship. K.D. received a Stem Cell Network Training (co-op) award. L.D.S. was supported by National Institutes of Health Cancer Core Grant CA34196.
P.H.M., G.R., M.M., P.A.B., and L.D.S. assisted in the derivation of the SRG-W41 strains. G.R., with P.H.M. and D.J.H.F.K., performed sex, age, and accessory cell experiments on NRG mice. P.H.M., G.R., P.A.B., D.J.H.F.K., and N.N. optimized transplant analysis protocols. P.H.M., A.M.S.C., and K.D. performed NSG and NRG comparison experiments. P.H.M., R.K.H., and C.J.E. designed the experiments. P.H.M. and C.J.E. wrote the manuscript, which was reviewed and approved by all co-authors.
Footnotes
The authors declare no competing financial interests.
References
- 1.Kamel-Reid S, Dick JE. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242:1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 2.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood. 2015;125:2630–2640. doi: 10.1182/blood-2014-11-570218. [DOI] [PubMed] [Google Scholar]
- 4.Miller PH, Knapp DJ, Eaves CJ. Heterogeneity in hematopoietic stem cell populations: implications for transplantation. Curr Opin Hematol. 2013;20:257–264. doi: 10.1097/MOH.0b013e328360aaf6. [DOI] [PubMed] [Google Scholar]
- 5.Cheung AM, Leung D, Rostamirad S, et al. Distinct but phenotypically heterogeneous human cell populations produce rapid recovery of platelets and neutrophils after transplantation. Blood. 2012;119:3431–3439. doi: 10.1182/blood-2011-12-398024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glimm H, Eisterer W, Lee K, et al. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-β2 microglobulin-null mice. J Clin Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 9.Ballen KK, Valinski H, Greiner D, et al. Variables to predict engraftment of umbilical cord blood into immunodeficient mice: usefulness of the non-obese diabetic–severe combined immunodeficient assay. Br J Haematol. 2001;114:211–218. doi: 10.1046/j.1365-2141.2001.02904.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet D, Bhatia M, Wang JC, Kapp U, Dick JE. Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant. 1999;23:203–209. doi: 10.1038/sj.bmt.1701564. [DOI] [PubMed] [Google Scholar]
- 11.Fulop GM, Phillips RA. Full reconstitution of the immune deficiency in scid mice with normal stem cells requires low-dose irradiation of the recipients. J Immunol. 1986;136:4438–4443. [PubMed] [Google Scholar]
- 12.Shultz LD, Banuelos SJ, Leif J, et al. Regulation of human short-term repopulating cell (STRC) engraftment in NOD/SCID mice by host CD122+ cells. Exp Hematol. 2003;31:551–558. doi: 10.1016/s0301-472x(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg GL, Alpdogan O, Muriglan SJ, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 14.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115:3704–3707. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- 16.Cosgun KN, Rahmig S, Mende N, et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014;15:227–238. doi: 10.1016/j.stem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh BE, Brown ME, Duffin BM, et al. Nonirradiated NOD,B6.S-CID Il2rγ−/−KitW41/W41 (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurino A, Takenaka K, Yamauchi T, et al. Enhanced reconstitution of human erythropoiesis and thrombopoiesis in an immunodeficient mouse model with Kit(Wv) mutations. Stem Cell Reports. 2016;7:425–438. doi: 10.1016/j.stemcr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DE, Astle CM. Lymphoid and erythroid repopulation in B6 W-anemic mice: a new unirradiated recipient. Exp Hematol. 1991;19:374–377. [PubMed] [Google Scholar]
- 20.Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lymphomyeloid reconstituting ability. Proc Natl Acad Sci U S A. 1997;94:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida N, Dykstra B, Lyons KJ, Leung FY, Eaves CJ. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;31:1338–1347. doi: 10.1016/j.exphem.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 23.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 24.Thames HD, Withers HR, Peters LJ. Tissue repair capacity and repair kinetics deduced from multifractionated or continuous irradiation regimens with incomplete repair. Br J Cancer Suppl. 1984;6:263–269. [PMC free article] [PubMed] [Google Scholar]
- 25.Miller PH, Cheung AM, Beer PA, et al. Enhanced normal short-term human myelopoiesis in mice engineered to express human-specific myeloid growth factors. Blood. 2013;121:e1–e4. doi: 10.1182/blood-2012-09-456566. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2015. Available at: https://www.R-project.org/ [Google Scholar]
- 27.Shultz LD, Lang PA, Christianson SW, et al. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol. 2000;164:2496–2507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 28.Gregory CJ, Tepperman AD, McCulloch EA, Till JE. Erythropoietic progenitors capable of colony formation in culture: Response of normal and genetically anemic W/Wv mice to manipulations of the erythron. J Cell Physiol. 1974;84:1–12. doi: 10.1002/jcp.1040840102. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Luger SM, DeRiel K, Abrahm J, Calabretta B, Gewirtz AM. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci U S A. 1992;89:1710–1714. doi: 10.1073/pnas.89.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strowig T, Rongvaux A, Rathinam C, et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]