Abstract
At present, there are no clinical options for preventing neointima-caused (re)stenosis after open surgery such as bypass surgery for treating flow-limiting vascular disease. Perivascular drug delivery is a promising strategy, but in translational research, it remains a major challenge to achieve long-term (e.g., > 3 months) anti(re)stenotic efficacy. In this study, we engineered a unique drug delivery system consisting of durable unimolecular micelles, formed by single multiarm star amphiphilic block copolymers with only covalent bonds, and a thermosensitive hydrogel formed by a poly(lactide-co-glycolide)–poly(ethylene glycol)–poly(lactide-co-glycolide) triblock copolymer (abbreviated as triblock gel) that is stable for about 4 weeks in vitro. The drug-containing unimolecular micelles (UMs) suspended in Triblock gel were able to sustain rapamycin release for over 4 months. Remarkably, even 3 months after perivascular application of the rapamycin-loaded micelles in Triblock gel in the rat model, the intimal/medial area ratio (a restenosis measure) was still 80% inhibited compared to the control treated with empty micelle/gel (no drug). This could not be achieved by applying rapamycin in Triblock gel alone, which reduced the intimal/medial ratio only by 27%. In summary, we created a new UM/Triblock gel hybrid system for perivascular drug delivery, which produced a rare feat of 3-month restenosis inhibition in animal tests. This system exhibits a real potential for further translation into an anti(re)stenotic application with open surgery.
Graphical Abstract
1. INTRODUCTION
Each year, millions of people are treated worldwide for flow-limiting atherosclerosis or for hemodialysis access. Although initially successful, a large proportion of these vascular interventions eventually fail due to (neo)intimal hyperplasia (IH).1,2 IH is a complex process that involves hyperplastic phenotype changes of smooth muscle cells (SMCs), the building blocks of the tunica media, and loss or dysfunction of endothelial cells (ECs) that form the protective inner lining of blood vessels.3 IH leads to the formation of neointimal lesion on the inner vessel wall, which narrows the lumen of the native artery or bypass conduit. This postintervention recurrent disease is termed restenosis or stenosis depending on the intervention.4,5
The clinical management technique used to prevent postangioplasty restenosis is the endovascular placement of a drug-eluting stent that delivers IH-inhibiting drugs such as rapamycin.1 Though effective in reducing restenosis rates,6,7 this endovascular delivery method cannot be applied with coronary and lower extremity bypass, carotid endarterectomy, or dialysis access, herein collectively termed open surgery.2 Thus, there is a great clinical need for drug delivery methods that can be applied concomitantly with these open surgical procedures,8 as the treated artery or bypass conduit is readily accessible at this time, making perivascular drug administration achievable.2
Despite the apparent simplicity of the perivascular (vs endovascular) approach, there remains a conspicuous absence of an available clinical option, likely because of the lack of a viable drug-releasing platform for perivascular deployment.2,9 In the past decade, various materials and platforms have been tried for perivascular drug delivery and shown efficacy in animal models,9–16 but none has reached clinical application.2,8,17 (Re)stenosis in humans is progressive; thus, it demands sustained drug release for treatment. Unfortunately, the majority of the animal tests on perivascular drug delivery were short-term (2–4 weeks).2 To our knowledge, long-term tests extending to 3 months or longer have been extremely rare.
In a previous proof-of-concept study,18 we tested a perivascular delivery platform featuring a combination of PLGA nanoparticles (NPs, 265 nm in diameter) and a hydrogel (pluronic) that prolonged drug release for 4 weeks. Rapamycin delivery in pluronic gel alone inhibited IH for 2 weeks; however, by 4 weeks, IH had recurred.18 Alternatively, prolonged rapamycin release achieved by PLGA NPs suspended in pluronic gel resulted in sustained inhibition of IH that persisted throughout the 4 weeks. This paradigm for prolonging drug efficacy was further supported by a recent report in which combining PLGA microparticles (15 μm in diameter) with a cross-linked hyaluronic acid gel markedly extended drug release and inhibition of IH in a mouse model for 4 weeks.13 These studies indicate that prolonged drug release produces durable efficacy. Thus, a perivascular delivery system capable of long-term drug release is highly desirable for developing effective treatments for IH.
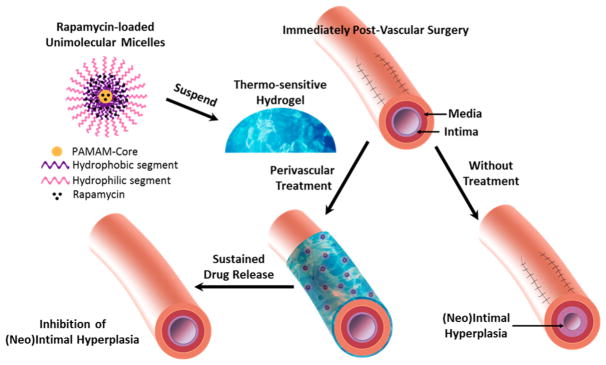
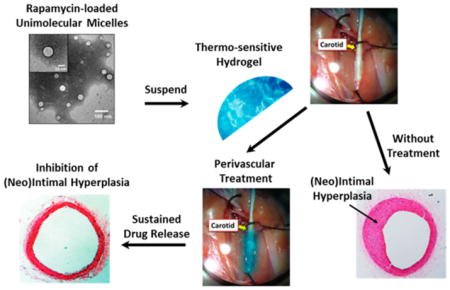
There are, however, several limitations with the PLGA/ pluronic gel perivascular delivery system. First of all, the pluronic gel was only stable in vivo for about 3 days. Second, the biodegradable PLGA NPs exhibited significant degradation after 2 weeks and complete drug release by 4 weeks. Enlightened by this proof-of-concept study, in the current study, we engineered a new NP/gel hybrid perivascular delivery system that can provide much longer and steadier drug release, and we have investigated its preclinical effectiveness for IH inhibition (Figure 1). We employed a unique type of NPs, namely, unimolecular micelles (abbreviated as UMs, shown in Figure 1), formed by a single multiarm star amphiphilic block copolymer containing only covalent bonds.19,20 In particular, we chose to use polyvalerolactone (PVL) as the hydrophobic segment of the amphiphilic arms as it offers much better durability than PLGA while still being biodegradable. We also synthesized a thermosensitive hydrogel made of a PLGA–PEG–PLGA triblock copolymer (hereafter referred as triblock gel), which is biodegradable yet much more durable than pluronic gel in vivo.21,22 The combination of UMs and triblock gel extended drug release over 4 months in vitro and kept IH from recurring for at least 3 months after perivascular application in a rat model. This new perivascular delivery system represents a useful template for future optimization and further translational development.
Figure 1.
Schematic illustration of a rapamycin-loaded unimolecular micelle (UM)/gel hybrid system for perivascular drug delivery to inhibit intimal hyperplasia. UM was formed by a single multiarm star amphiphilic block copolymer poly(amidoamine)–polyvalerolactone–poly(ethylene glycol) (PAMAM–PVL–PEG).
2. MATERIALS AND METHODS
2.1. Materials
Rapamycin was purchased from LC Laboratories (Woburn, MA). Poly(amidoamine) (PAMAM; fourth generation) dendrimer was purchased from NanoSynthons, LLC (Mt. Pleasant, MI, USA). Dimethyl sulfoxide (DMSO), valerolactone (VL), lactide (LA), glycolide (GA), Tween 80, paraformaldehyde, and stannous (II) octoate (Sn(Oct)2) were purchased from Sigma-Aldrich (St. Louis, MO). HOOC–PEG–OCH3 (Mn = 5 kDa) was acquired from JenKem Technology (Allen, TX, USA). Kolliphor P407 (a poly-(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) tri-block copolymer) was kindly provided by BASF Corporation (Tarrytown, NY) and was used to prepare the pluronic gel (30 wt % of polymers in DI water). OH–PEG–OH (Mn = 1 kDa) and all other reagents were purchased from ThermoFisher Scientific (Fitchburg, WI, USA) unless otherwise specified.
2.2. Synthesis of PLGA–PEG–PLGA Triblock Copolymer (for Triblock Gel Preparation)
OH–PEG–OH (2.4 g) was dried in a three-necked flask under vacuum at 120 °C for 2 h. LA (5.0 g) and GA (1.2 g) were added to the flask and dried under vacuum at 70 °C for 30 min. After complete melting of the mixture, a catalyst amount of Sn(Oct)2 ([Sn(Oct)2]/[LA+GA] = 1:500 mol/mol) was added to start the polymerization. The reaction was carried out at 150 °C for 8 h. The mixture was then dissolved in cold water (4 °C), and the resulting solution was heated to 80 °C to precipitate the triblock copolymers and remove other impurities. The precipitation process was repeated three times to purify the polymers. The final product was dried by lyophilization. Triblock gel was prepared by dissolving the PLGA–PEG–PLGA polymer in water (23% by weight) and kept at 4 °C.
2.3. Synthesis of PAMAM–PVL–PEG
PAMAM–PVL–PEG was synthesized following methods outlined in our previous report.19 Briefly, PAMAM–PVL was first prepared via ring-opening polymerization using PAMAM–OH (G4) and VL as the macroinitiator and monomers, respectively. HOOC–PEG–OCH3 was conjugated to the PAMAM–PVL via an esterification reaction to form PAMAM–PVL–PEG.
2.4. Preparation of Rapamycin-Loaded Unimolecular Micelles (Rapa-UMs)
PAMAM–PVL–PEG and rapamycin were dissolved in 3 mL of DMF. DI water (9 mL) was added dropwise into the above solution. Thereafter, the solution was dialyzed against DI water by a cellulose membrane (molecular weight cutoff, 15 kDa) for 48 h to remove DMF and free drug. The final product was dried by lyophilization.
2.5. Characterization
The chemical structures and molecular weights of all polymer products were determined using 1H NMR spectroscopy and gel permeation chromatography (GPC), respectively.19 The rapamycin loading level and its release rate in the Rapa-UMs were measured by high-performance liquid chromatography (HPLC) using ultraviolet (UV) detection at 278 nm. The morphologies of the UMs were determined by dynamic light scattering (DLS, ZetaSizer Nano ZS90, Malvern Instrument) and transmission electron microscopy (TEM, FEI Tecnai G2 F30 TWIN 300 kV, E.A. Fischione Instruments, Inc.).
2.6. In Vitro Rapamycin Release Study
We followed our published methods18 for in vitro release assays with modifications. The in vitro rapamycin release profiles from Rapa-UMs, Rapa-UMs dispersed in pluronic gel (Rapa-UM/pluronic gel), Rapa-UMs dispersed in Triblock gel (Rapa-UM/triblock gel), or free rapamycin dispersed in triblock gel (Rapa/triblock gel) were studied in PBS (pH 7.4). Briefly, 600 μg of rapamycin in 15 μL of DMSO/H2O (v/v = 9/ 1) or 3 mg of Rapa-UMs (drug loading level: 20 wt %, equivalent to 600 μg of rapamycin) was dispersed in 300 μL of PLGA–PEG–PLGA triblock copolymer solution (to form triblock gel), PEG–PPG–PEG polymer solution (to form pluronic gel), or PBS. After solidification at 37 °C, gels were transferred into a dialysis bag (molecular weight cutoff, 15 kDa) containing 3 mL of PBS. The dialysis bags were immersed in 25 mL of PBS (containing 0.2% of Tween 8018). At the indicated time points, 6 mL of release media was collected and replaced by fresh media. The rapamycin concentration in the collected media was then analyzed by HPLC as described earlier.
2.7. Animals
All animal procedures conformed to the NIH guide for the ethical care and use of laboratory animals. Animal protocols were approved by the Institutional Animal Care and Use Committee of University of Wisconsin-Madison. All surgeries were performed under isoflurane anesthesia (inhalation at 2 mL/min flow rate). Animals were euthanized in a chamber gradually filled with CO2.
2.8. Rat Carotid Artery Balloon Angioplasty and in Vivo Drug Delivery
For in vivo evaluation, carotid artery balloon angioplasty was performed in male Sprague–Dawley rats (300–350 g) as we previously reported.18 This model features both exposure of the vessel and its surgical injury that induces IH, thus mimicking open surgery. Rats were then treated with the following five groups: (1) Rapa-UM/triblock gel, (2) Rapa/triblock gel, (3) triblock gel alone, (4) UM/triblock gel, or (5) PBS (injury alone control). On the basis of our previous studies,18,23 a rapamycin dosage of 2 mg/kg was used in all treatment groups. Briefly, rapamycin (in DMSO stock), Rapa-UMs, or UMs were dispersed uniformly in 300 μL of PLGA–PEG–PLGA triblock gel solution on ice. The solutions were then applied around the outside of the injured segment of carotid artery. The gel solidified immediately after exposure to body temperature. The neck incision was then closed using a suture. For the injury alone control, 300 μL of PBS buffer was applied containing an equal amount of DMSO that was used in the Rapa/triblock gel group.
2.9. Morphometric Analysis of Intimal Hyperplasia
Three months after balloon injury, animals were sacrificed and perfused with 4% paraformaldehyde at a physiological pressure of 100 mmHg. Balloon-injured artery segments were collected and processed for paraffin embedding and sectioning. Serial cross-sections were collected at 50 μm intervals and stained with hematoxylin–eosin (HE) for morphometric analysis. Images were acquired using a Nikon Ti–U Eclipse microscope equipped with Nikon Elements software. The following morphometric parameters were calculated using ImageJ software: area inside external elastic lamina (EEL area), area inside internal elastic lamina (IEL), lumen area, intimal area (i.e., IEL area–lumen area), and medial area (i.e., EEL area – IEL area). Neointimal hyperplasia (IH) was assessed with the area ratio of intima versus media (I/M ratio). Data from 5–7 sections were pooled to generate the mean values for each rat. The means from all of the animals in each group were averaged, and the standard errors (SE) were calculated.
2.10. Immunostaining for in Vivo Assessment of Re-endothelialization
To assess re-endothelialization, immunostaining of CD31 (an endothelial cell marker) was performed, as we previously reported,24 using a goat anti-CD31 primary antibody (R&D Systems; 1:150 dilution) and a biotinylated rabbit–antigoat secondary antibody (Vector Laboratories; 1:200 dilution). Staining of CD31 was visualized using streptavidin-HRP and 3,3-diaminobenzidine. For quantification, the luminal perimeter and the percentage of this perimeter that stained for CD31 on each section were measured using ImageJ software. The percentage of re-endothelialization was scored from 1–5 (1: < 20%, 2:20–40%, 3:40–60%, 4:60–80%, and 5:80–100%), as we previously reported.18
2.11. Statistical Analysis
The required sample sizes in animal experiments were calculated based on estimates of mean differences, variances, and power. Data were analyzed using ANOVA (OriginLab, Northampton, MA). Significance was set at P < 0.05. In all bar graph representations, the error bars indicate standard error of the mean (SE).
3. RESULTS
3.1. Preparation and Thermogelling Properties of the PLGA–PEG–PLGA Triblock Copolymer
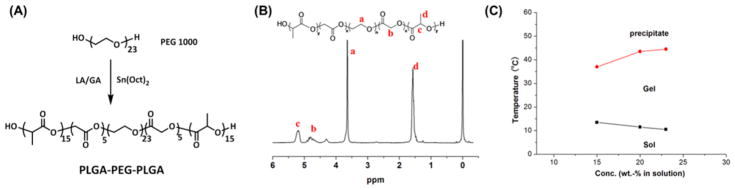
The LA and GA were copolymerized onto the hydroxyl-terminated PEG via ring opening polymerization (Figure 2A), which resulted in the formation of the PLGA–PEG–PLGA triblock copolymer. The structure of the PLGA–PEG–PLGA was confirmed by 1H NMR (Figure 2B), with a molar ratio of LA/GA of 3:1. The molecular weight was determined to be 3680 g/mol.
Figure 2.
(A) Synthesis of PLGA–PEG–PLGA triblock copolymer. (B) 1H NMR spectrum of the PLGA–PEG–PLGA triblock copolymer. (C) Phase transition diagram of the PLGA–PEG–PLGA in aqueous solutions.
Figure 2C shows the phase transition diagram of the PLGA–PEG–PLGA triblock copolymers in aqueous solutions. At low temperatures, the polymer/water system formed a free-flowing liquid. As the temperature increased, the system underwent a reversible sol–gel transition. At higher temperatures, another transition of gel to polymer precipitation in the aqueous solution occurred. The phase transitions were dependent on the polymer concentration. Triblock gel formed by 23 wt % of the triblock copolymer solution was selected for the following experiments since, at a low temperature of 10 °C, the polymer solution is a free-flowing liquid, and once applied in vivo (37 °C), it can rapidly turn into gel. The thermosensitivity of the triblock gel, along with good biocompatibility and biodegradability, makes it a suitable injectable material.
3.2. Preparation and Properties of UMs
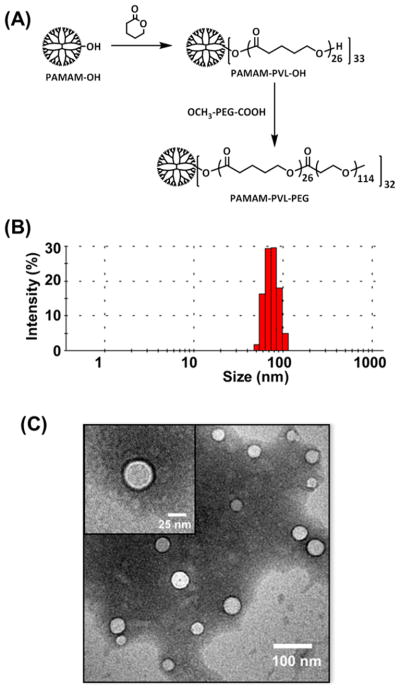
The multiarm star amphiphilic block copolymers PAMAM–PVL–PEG were synthesized following our previous report.19 The synthetic scheme is shown in Figure 3A. All of the intermediate and final products were fully characterized by 1H NMR (Figure S1) and GPC.
Figure 3.
(A) Synthesis of multiarm star amphiphilic block copolymer PAMAM–PVL–PEG, (B) DLS analysis of the UMs, and (C) TEM images of the UMs.
The PAMAM–PVL–PEG polymer can form a stable UM in an aqueous solution due to its large number of amphiphilic arms, with proper hydrophilic (PEG) to hydrophobic (PVL) ratios, as well as its globular architecture (Figure 1). The hydrodynamic diameter of the UMs ranged from 50–110 nm, with an average of 65 nm, as measured by DLS (Figure 3A). TEM images of the UMs (Figure 3C) show a spherical morphology with an average diameter of around 35 nm. The differences in sizes as measured by DLS and TEM were due to the fact that DLS measures the hydrodynamic diameters of the UMs with the hydrophilic PEG segments freely extending into the aqueous solution, while TEM characterized the size of dried samples.
3.3. Suspending Rapamycin-Loaded UMs in Triblock Gel Prolongs Drug Release in Vitro
Our previous pilot study indicated that suspending rapamycin-loaded PLGA NPs in a pluronic gel resulted in sustained drug release for 4 weeks leading to high antirestenotic efficacy for at least 4 weeks.18 In the current study, the hydrophobic drug, rapamycin, was encapsulated into the UMs through hydrophobic interactions as well as hydrogen bonding. The drug loading level was measured to be 20 wt % by HPLC. The drug-loaded UMs were then mixed with either triblock gel or pluronic gel. We determined the drug release profiles of four rapamycin-containing formulations in vitro in PBS buffer including UMs, triblock gel, UMs in pluronic gel, and UMs in triblock gel (Figure 4). UMs alone showed fast initial release of rapamycin, likely due to the rapamycin present on or near the NP surface, followed by a steady release for up to 2 months. Curiously, suspending UMs in pluronic gel resulted in a somewhat faster release that lasted for about 1.5 months. A possible explanation for this is that once the pluronic gel dissolved into the release media (~3 days), the pluronic gel had a surfactant effect on rapamycin solubilization that accelerated rapamycin release from the UMs.25,26 In the Rapa/triblock groups, during the first 4 weeks, they showed a gradual release of ~23% of the loaded rapamycin (Day 28). However, the remaining drug was abruptly lost due to a burst release. These data suggest that triblock gel was only stable for about 4 weeks, and the dissolution of triblock gel led to a burst release of the remaining drug. Similarly, we observed a gradual drug release during the first 4 weeks followed by a relatively fast release for about a week (from Day 28 to Day 36) for the hybrid Rapa-UM/ triblock gel system. However, beyond Day 36, a steady drug release was observed that lasted for another three months, apparently from the stable UMs. These results demonstrate that, compared to the first three formulations, the UMs/ triblock gel combination produced superior steady and prolonged rapamycin release kinetics for 4 months, which is highly desirable for in vivo application.
Figure 4.
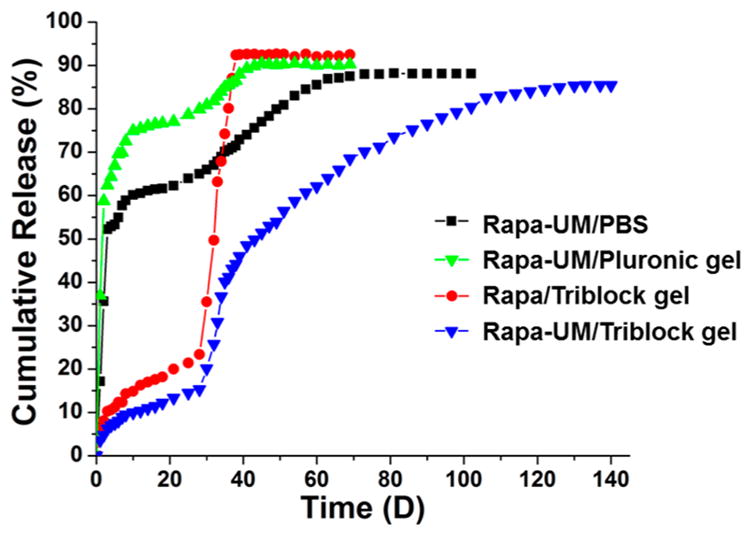
In vitro cumulative rapamycin release profiles from Rapa-UM, Rapa-UM/pluronic gel, Rapa/triblock gel, and Rapa-UM/triblock gel.
3.4. Periadventitial Administration of Rapa-UMs/Tri-block Gel Produces Sustained Inhibition of Intimal Hyperplasia (IH)
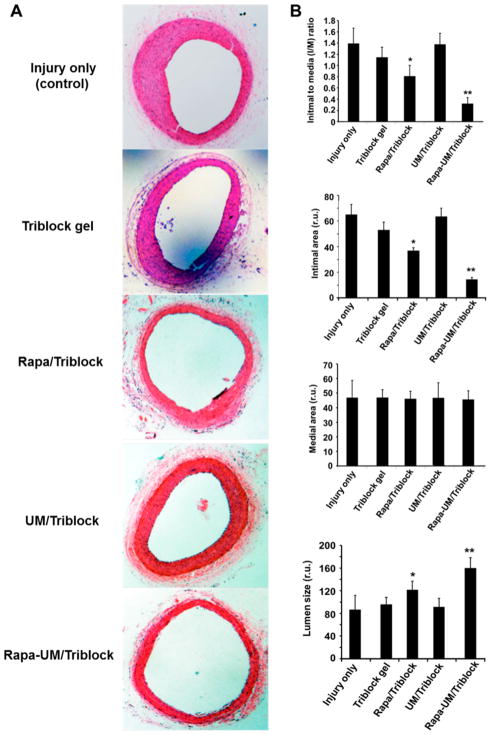
For in vivo evaluation, we performed the rat balloon angioplasty model that mimics open surgery because of the exposure of the vessel and its surgical injury that induces IH. On the basis of the 4-month prolonged rapamycin release achieved in vitro with the UM/triblock gel hybrid formulation, we performed morphometric analysis 3 months after perivascular drug delivery (Figure 5). As also observed in our previous study, balloon injury induced severe IH in the arteries without applying a drug or a carrier (I/M = 1.4).18 Compared to this negative control, neither of the two drug-free carriers, that is, UM/triblock gel and triblock gel alone, showed an effect on IH. However, Rapa/triblock gel treatment reduced IH by 27% compared to its respective control of gel alone without drug. Remarkably, the hybrid system of rapamycin-loaded UMs and triblock gel (Rapa-UM/triblock gel) inhibited IH by 80% compared to its respective drug-free control. Consequently, the lumen area was enlarged by 60%.
Figure 5.
Hybrid UM/triblock gel perivascular drug delivery system inhibited IH much more effectively than all control groups including the gel alone 3-month after the administration. To mimic open vascular surgery, a rat neck incision was made to expose the common carotid artery, which was then injured with a balloon catheter to induce IH, as described in the Materials and Methods section. Carotid arteries were retrieved 3 months after surgery for paraffin section preparation and HE staining. (A) Representative microscopic images of cross-sections from the indicated treatment groups. (B) Morphometric quantification of intimal-to-medial area ratio (I/M), intimal area, medial area, and lumen size. Data are presented as mean ± SE from 5–6 animals in each treatment group. There is no statistically significant difference among the three control conditions: injury alone, triblock gel (no drug) and UM/triblock (no drug). There is no significant difference in medial area among the five treatment groups. *, P < 0.05 compared between triblock gel (no drug) and Rapa/triblock; **, P < 0.01 compared between UM/triblock (no drug) and Rapa-UM/triblock.
As the integrity of the endothelium is important not only for inhibition of IH, but also for the prevention of thrombosis,27 we also evaluated the recovery of angioplasty-denuded endothelium (termed re-endothelialization) by immunohistological analysis of CD31 coverage. As shown in Figure 6, re-endothelialization scores throughout all treatment groups showed no significant differences, suggesting that endothelium recovery was not affected.
Figure 6.
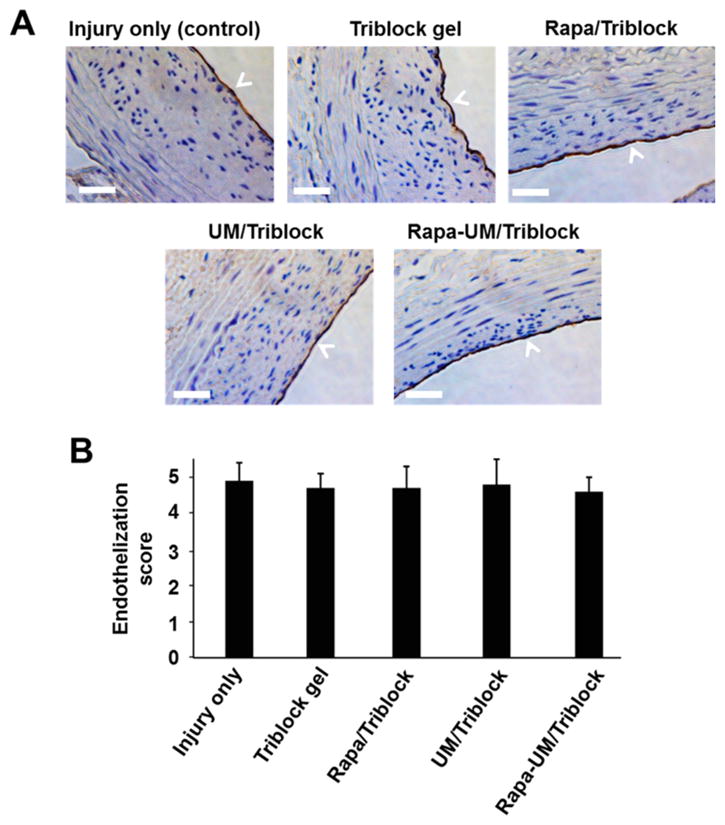
Recovery of the balloon-denuded endothelium (re-endothelialization) was not affected by the drug-containing UM/gel hybrid system and other formulations. (A) Representative microscopic images of CD31-immunostained cross-sections from the indicated treatment groups. Scale bar: 50 μm. (B) Quantification of re-endothelialization (CD31 positive versus total perimeter). Data are presented as mean ± SE from 5 animals in each group. No significant difference was found among the five treatment groups.
4. DISCUSSION
In this study, we developed a hybrid perivascular drug delivery system featuring drug-loaded long-lasting UMs suspended in a durable triblock hydrogel. This perivascular drug delivery system demonstrated long-term (3 months) efficacy in mitigating neointimal growth in an IH-producing rat model. To date, there are no clinical options for preventing the (re)stenoses that develops following millions of open vascular reconstructions performed each year.2,28 Consequently, failure rates can reach ~40–80% within ten years. Therefore, developing a perivascular delivery strategy, which is suitable for open surgery, is both urgent and impactful. In spite of many trials in animals with a variety of perivascular delivery devices, long-term drug delivery and efficacy remain challenging.2 The UM/triblock gel hybrid system developed in this study can effectively prolong drug release and preclinical efficacy and thus provide a unique platform for the prevention of postopen surgery (re)stenosis.
Current stent-mediated endovascular drug delivery methods cannot be applied in open surgery. In contrast, perivascular delivery promises multiple advantages and suitability for this cohort of patients.2 First, since a native vessel or graft is exposed during open surgery, it is convenient to deliver a drug in its carrier perivascularly. Second, the perivascular space allows for deployment of drug carriers (e.g., hydrogels) of substantial volumes, which would otherwise obstruct blood flow if administered endovascularly. Third, perivascularly delivered drugs do not directly enter the circulation and hence are not subject to rapid metabolic decomposition. Fourth, high local drug concentrations could be attained at the targeted vessel area. Moreover, while the endothelium is vulnerable to thrombogenic risks including drug toxicity,27,29 perivascular delivery generates a gradient of drug, which could reach the endothelial inner layer yet with nontoxic low concentrations. Therefore, an appropriate perivascular delivery system applied during open surgery could capitalize on these advantages for optimal therapeutic outcomes to prevent postopen surgery (re)stenosis.2
A number of materials in various forms have been tried for perivascular drug delivery in animal models. While results are promising, limitations remain, thereby hampering further translation.2 For example, pluronic gel dissolves within 3 days, thereby providing a short burst of drug release without sustainability.2 Drug release from PLGA or PLLA polymer wraps is more durable. However, after in vivo deployment, these wraps became stiff and confining. This imposed physical stress on the vessel wall induces IH and thrombosis.30 A unique layered system composed of PCL/PLGA wraps and hyaluronic acid gel could be used for directional perivascular drug delivery, although anti-IH efficacy has not been reported.11 Since PLGA stiffens in vivo,30 whether this complex structure would stress the vessel and induce thrombosis remains unclear. A PCL cuff has been used for perivascular drug delivery, but the cuff itself can induce IH,12 thus limiting its clinical application. Hence, there remains the need for more innovative approaches to perivascular drug delivery.
Advantages of UMs include excellent in vivo stability because each micelle is formed by a single multiarm amphiphilic block copolymer molecule containing only covalent bonds.20,31–37 UM provides a high drug loading capacity and sustained release while protecting the drug from premature in vivo degradation. Moreover, it is easy to apply these micelles in vivo due to their excellent solubility in an aqueous solution as provided by the hydrophilic PEG outer shell. Importantly, their chemical structures can be conveniently engineered to achieve a desirable drug release kinetics. In addition, these micelles can be tailored for in vivo tracking; for example, fluorescent dye or biotin tagging. All of these favorable traits are combined in one micelle molecule, rendering it superior to traditional NPs (e.g., PLGA) or other polymeric nanoplatforms formed by self-assembly of a large number of polymer molecules. However, because of their small sizes and high solubility, these micelles may quickly diffuse into surrounding tissues and the capillary bed or be cleared by immune cells such as macrophages. To circumvent this problem, a plausible solution is a micelle/ hydrogel hybrid system. In this study, prior to perivascular application, we suspended the UMs in a triblock hydrogel. The thermosensitive triblock gel is liquid at low temperatures and convenient for perivascular application, yet quickly transforms into a semisolid at body temperature, thus preventing UMs from flowing away.
Suspending UMs in triblock gel substantially prolonged rapamycin release in vitro (over 4 months), compared to either UMs or triblock gel alone. This long-term drug release profile was achieved not only because of the improved release profiles of UMs and triblock gel each alone over traditional PLGA NPs and pluronic gel, respectively, but also because of their combination. While pluronic gel was only stable for about 3 days,18 triblock gel was stable for about 1 month. Compared to pluronic gel, from which rapamycin was almost completely released within only 3 days,18 the 4-week rapamycin release profile of triblock gel is much more favorable. Furthermore, while the biodegradable PLGA (LA:GA = 50:50) NPs were only stable for about 2–4 weeks,18,38,39 biodegradable UMs made of PAMAM–PVL–PEG were stable chemically for at least 4 months as evidenced by their drug release profiles.40,41 Importantly, the combination of UMs and triblock gel enabled steady rapamycin release for 4 months. The gel matrix that encapsulated the micelles likely functioned as an extra barrier to slow rapamycin diffusion out of the micelles when the gel matrix was stable. Apparently, the properties of the gel had a strong influence on the release kinetics of the UM/gel hybrid. For example, combining UMs with pluronic gel did not extend, but rather accelerated, rapamycin release from UMs, possibly due to a surfactant effect of pluronic gel once it dissolved in the media (~3 days).25,26 This underscores the importance of the appropriate matching of the properties of UMs and triblock gel, and thus optimization of this pair warrants future investigations.
The long-term drug release achieved by the UM/triblock gel hybrid system is a critically important trait for translation. Unlike in animal models where growth of the neointimal lesion may dwindle over time, IH in human patients is progressive and persists until a native vessel or a graft is completely occluded.2 While the combinations of nanoparticles (or microparticles) with a hydrogel are promising in extending drug release, thus far, few studies have achieved long-term preclinical effectiveness over 3 months.24 Using rapamycin-loaded microspheres in pluronic gel, Rajathurai et al. demonstrated the inhibition of IH for 4 weeks in a pig vein graft model.14 Our pilot study showed the combination of PLGA NP/pluronic gel was effective for 4 weeks in keeping IH at a low level.18 More recently, Mylonaki et al. reported prolonged atorvastatin release by dispersing drug-loaded PLGA microparticles in a hyaluronic gel. This formulation was shown to reduce IH by 68% at 4 weeks in a mouse carotid artery ligation model.13
In the current study, using the UM/triblock gel hybrid system, we observed 80% inhibition of rat carotid IH, even 3 months after perivascular application. This prominently sustained neointimal inhibition suggests a real potential to achieve a longer efficacy of well beyond 3 months. Of note, we observed full recovery of the endothelium, which could have been compromised due to the unfavorable properties of a drug carrier that could have generated endothelium-damaging hemodynamics (e.g., a rigid PLGA wrap).23 Both IH and compromised endothelia are basic contributors to (re)stenosis pathologies.3,27
5. CONCLUSIONS
The lack of a viable perivascular drug delivery system capable of long-term drug release is a major barrier for translating treatment methods to mitigate (re)stenosis concomitantly with open surgery.2,8 We synthesized a UM and a thermosensitive triblock hydrogel, and the combination of UMs and triblock gel resulted in substantially prolonged steady rapamycin release over 4 months. The perivascular application of this hybrid system in rats produced a pronounced IH-inhibitory effect that persisted for at least 3 months, providing a foundation for further optimization. As IH is the primary cause of failure for a variety of open surgical procedures,2 this hybrid perivascular delivery system is amenable to broad translational opportunities.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant Nos. R01 HL129785 (to K.C.K., S.G., and L.-W.G.), R01HL-068673 (to K.C.K.), NIH R01 HL133665 (to L.-W.G.), R01 EY022678 (to L.-W.G.), NIH K25CA166178 (to S.G.), the State of Wisconsin Partnership Program New Investigator Award, the Wisconsin Alumni Research Foundation (WARF) Accelerator Award (to L.-W.G.), and an AHA Predoctoral Award 16PRE30160010 (to B.W.). We would also like to thank Drew Alan Roenneburg for cross-section preparation and HE staining.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bio-mac.7b00617.
1H NMR spectrum of PAMAM–PVL–PEG (PDF)
References
- 1.Jukema JW, Ahmed TA, Verschuren JJ, Quax PH. Restenosis after PCI. Part 2: prevention and therapy. Nat Rev Cardiol. 2011;9(2):79–90. doi: 10.1038/nrcardio.2011.148. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary MA, Guo LW, Shi X, Chen G, Gong S, Liu B, Kent KC. Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery. J Controlled Release. 2016;233:174–180. doi: 10.1016/j.jconrel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9(1):53–62. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 4.Suwanabol PA, Kent KC, Liu B. TGF-beta and restenosis revisited: a Smad link. J Surg Res. 2011;167(2):287–97. doi: 10.1016/j.jss.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 6.Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schomig A, Kastrati A. Randomized trial of paclitaxel-versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol. 2010;55(24):2710–6. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Curcio A, Torella D, Indolfi C. Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy. Circ J. 2011;75(6):1287–96. doi: 10.1253/circj.cj-11-0366. [DOI] [PubMed] [Google Scholar]
- 8.Mylonaki I, Allemann E, Saucy F, Haefliger JA, Delie F, Jordan O. Perivascular medical devices and drug delivery systems: Making the right choices. Biomaterials. 2017;128:56–68. doi: 10.1016/j.biomaterials.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Gregory EK, Webb AR, Vercammen JM, Flynn ME, Ameer GA, Kibbe MR. Periadventitial atRA citrate-based polyester membranes reduce neointimal hyperplasia and restenosis after carotid injury in rats. Am J Physiol. 2014;307(10):H1419–29. doi: 10.1152/ajpheart.00914.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lance KD, Chatterjee A, Wu B, Mottola G, Nuhn H, Lee PP, Sansbury BE, Spite M, Desai TA, Conte MS. Unidirectional and sustained delivery of the proresolving lipid mediator resolvin D1 from a biodegradable thin film device. J Biomed Mater Res, Part A. 2017;105(1):31–41. doi: 10.1002/jbm.a.35861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Controlled Release. 2012;161(1):81–9. doi: 10.1016/j.jconrel.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pires NM, van der Hoeven BL, de Vries MR, Havekes LM, van Vlijmen BJ, Hennink WE, Quax PH, Jukema JW. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly(epsilon-caprolactone) stent cuff. Biomaterials. 2005;26(26):5386–94. doi: 10.1016/j.biomaterials.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 13.Mylonaki I, Strano F, Deglise S, Allemann E, Alonso F, Corpataux JM, Dubuis C, Haefliger JA, Jordan O, Saucy F, Delie F. Perivascular sustained release of atorvastatin from a hydrogel-microparticle delivery system decreases intimal hyperplasia. J Controlled Release. 2016;232:93–102. doi: 10.1016/j.jconrel.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Rajathurai T, Rizvi SI, Lin H, Angelini GD, Newby AC, Murphy GJ. Periadventitial rapamycin-eluting microbeads promote vein graft disease in long-term pig vein-into-artery interposition grafts. Circ: Cardiovasc Interventions. 2010;3(2):157–65. doi: 10.1161/CIRCINTERVENTIONS.109.864660. [DOI] [PubMed] [Google Scholar]
- 15.Skalsky I, Szarszoi O, Filova E, Parizek M, Lytvynets A, Maluskova J, Lodererova A, Brynda E, Lisa V, Burdikova Z, Capek M, Pirk J, Bacakova L. A perivascular system releasing sirolimus prevented intimal hyperplasia in a rabbit model in a medium-term study. Int J Pharm. 2012;427(2):311–9. doi: 10.1016/j.ijpharm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Terry CM, Li L, Li H, Zhuplatov I, Blumenthal DK, Kim SE, Owen SC, Kholmovski EG, Fowers KD, Rathi R, Cheung AK. In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis. J Controlled Release. 2012;160(3):459–67. doi: 10.1016/j.jconrel.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boire TC, Balikov DA, Lee Y, Guth CM, Cheung-Flynn J, Sung HJ. Biomaterial-Based Approaches to Address Vein Graft and Hemodialysis Access Failures. Macromol Rapid Commun. 2016;37(23):1860–1880. doi: 10.1002/marc.201600412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi X, Chen G, Guo L-W, Si Y, Zhu M, Pilla S, Liu B, Gong S, Kent KC. Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis. PLoS One. 2014;9(2):e89227. doi: 10.1371/journal.pone.0089227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Chen G, Li J, Fu Y, Mavlyutov TA, Yao A, Nickells RW, Gong S, Guo LW. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J Controlled Release. 2017;247:153–166. doi: 10.1016/j.jconrel.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkman AM, Chen G, Wang Y, Hedman CJ, Sherer NM, Havighurst TC, Gong S, Xu W. Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials. 2016;101:20–31. doi: 10.1016/j.biomaterials.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong B, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res. 2000;50(2):171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA–PEG–PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1):103–112. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Takayama T, Goel SA, Shi X, Zhou Y, Kent KC, Murphy WL, Guo LW. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J Controlled Release. 2014;191:47–53. doi: 10.1016/j.jconrel.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Takayama T, Wang B, Kent A, Zhang M, Binder BY, Urabe G, Shi Y, DiRenzo D, Goel SA, Zhou Y, Little C, Roenneburg DA, Shi XD, Li L, Murphy WL, Kent KC, Ke J, Guo LW. Restenosis Inhibition and Re-differentiation of TGFbeta/Smad3-activated Smooth Muscle Cells by Resveratrol. Sci Rep. 2017;7:41916. doi: 10.1038/srep41916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antunes FE, Gentile L, Oliviero Rossi C, Tavano L, Ranieri GA. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf, B. 2011;87(1):42–48. doi: 10.1016/j.colsurfb.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Chávez JJ, López-Cervantes M, Naik A, Kalia Y, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9(3):339–58. [PubMed] [Google Scholar]
- 27.Pendyala LK, Yin X, Li J, Chen JP, Chronos N, Hou D. The first-generation drug-eluting stents and coronary endothelial dysfunction. J Am Coll Cardiol. 2009;2(12):1169–77. doi: 10.1016/j.jcin.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Jim J, Owens PL, Sanchez LA, Rubin BG. Population-based analysis of inpatient vascular procedures and predicting future workload and implications for training. J Vasc Surg. 2012;55(5):1394–9. doi: 10.1016/j.jvs.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Goel SA, Guo LW, Wang B, Guo S, Roenneburg D, Ananiev GE, Hoffmann FM, Kent KC. High-throughput screening identifies idarubicin as a preferential inhibitor of smooth muscle versus endothelial cell proliferation. PLoS One. 2014;9(2):e89349. doi: 10.1371/journal.pone.0089349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X, Takayama T, Goel SA, Shi X, Zhou Y, Kent KC, Murphy WL, Guo LW. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J Controlled Release. 2014;191:47–53. doi: 10.1016/j.jconrel.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Jaskula-Sztul R, Harrison A, Dammalapati A, Xu W, Cheng Y, Chen H, Gong S. KE108-Conjugated Unimolecular Micelles Loaded with a Novel HDAC Inhibitor Thailandepsin-A for Targeted Neuroendocrine Cancer Therapy. Biomaterials. 2016;97:22–33. doi: 10.1016/j.biomaterials.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery. Biomaterials. 2009;30(16):3009–19. doi: 10.1016/j.biomaterials.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Wang L, Cordie T, Vokoun C, Eliceiri KW, Gong S. Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials. 2015;47:41–50. doi: 10.1016/j.biomaterials.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y, Theuer CP, Barnhart TE, Cai W, Gong S. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials. 2013;34(33):8323–32. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, Gong S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34(21):5244–53. doi: 10.1016/j.biomaterials.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaskula-Sztul R, Xu W, Chen G, Harrison A, Dammalapati A, Nair R, Cheng Y, Gong S, Chen H. Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy. Biomaterials. 2016;91:1–10. doi: 10.1016/j.biomaterials.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao W, Zhou J, Mann A, Wang Y, Zhu L. Folate-functionalized unimolecular micelles based on a degradable amphiphilic dendrimer-like star polymer for cancer cell-targeted drug delivery. Biomacromolecules. 2011;12(7):2697–2707. doi: 10.1021/bm200487h. [DOI] [PubMed] [Google Scholar]
- 38.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolide) nano-and microparticles. J Controlled Release. 2003;92(1):173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Garcia CA, Mikos AG. In vitro degradation of thin poly (DL-lactic-co-glycolic acid) films. J Biomed Mater Res. 1999;46(2):236–244. doi: 10.1002/(sici)1097-4636(199908)46:2<236::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 40.Lin S-Y, Chen K-S, Teng H-H, Li M-J. In vitro degradation and dissolution behaviours of microspheres prepared by three low molecular weight polyesters. J Microencapsulation. 2000;17(5):577–586. doi: 10.1080/026520400417630. [DOI] [PubMed] [Google Scholar]
- 41.Lin WJ, Wang CL, Juang LW. Characterization and comparison of diblock and triblock amphiphilic copolymers of poly (δ-valerolactone) J Appl Polym Sci. 2006;100(3):1836–1841. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.