ABSTRACT
The accumulation of intratumoral CD8+ T cells is associated with the survival of high grade serous ovarian carcinoma patients, but it is unclear which CD8+ T cell subsets contribute to this effect and how they are affected by the peritoneal tumor microenvironment. Here, we provide evidence for a functional link between long relapse-free survival, accumulation of CD8+ effector memory T (TEM) cells in peritoneal effusion (ascites), and the level of the CD8+ TEM attracting chemokine CXCL9, produced by macrophages as a major source. We also propose a novel mechanism by which the tumor microenvironment could contribute to T cell dysfunction and shorter survival, i.e., diminished expression levels of essential signaling proteins, including STAT5B, PLCγ1 and NFATc2. CD8+ TEM cells in ascites, CXCL9 levels and the expression of crucial signal transduction proteins may therefore be important biomarkers to gauge the efficiency of immune therapies and potentially represent therapeutic targets.
KEYWORDS: CXCL9, ovarian carcinoma, T effector memory cells, tumor-associated macrophages
Introduction
Ovarian cancer is the most lethal gynecological malignancy with an overall 5-year survival rate of approximately 40%.1 The high grade serous carcinoma subtype represents the most common and aggressive subtype of ovarian cancer entities. As in many other malignancies, its tumor microenvironment (TME) plays a pivotal role in disease progression. A characteristic feature of the ovarian TME is its composition of anatomically and functionally different compartments, including tumor tissue, invaded host tissues (in particular the omentum) and the peritoneal fluid, which at advanced stages occurs as a malignancy-associated peritoneal effusion, termed ascites.2–5 Besides cytokines, growth factors, lipids and other soluble mediators this effusion contains large numbers of immune cells and tumor cell spheroids with “stem-like” properties that are likely to play a pivotal role in transcoelomic metastasis and chemotherapy failure.6 The most common cell types in ovarian cancer ascites are macrophages and CD8+ T cells that are immunosuppressed by the TME and programmed to exert pro-tumorigenic functions.2–5 Understanding the molecular mechanisms underlying these defects in immune surveillance is key to the development of efficient immune therapies targeting the pro-metastatic and resistance-promoting detached tumor cells and spheroids in the peritoneal fluid of ovarian cancer.
For more than a decade it has been known that increased accumulation of intra-tumoral T cells in ovarian carcinoma delays the recurrence of the disease and is beneficial for survival,3,5,7,8 thus indicating that T cells contribute to the eradication of tumor cells. Among infiltrating T cells, CD8+ T cells are associated with a better prognosis, while CD4+ T cells expressing the master transcription factor FOXP3 as a marker of T regulatory (Treg) cells suppress the beneficial effects of CD8+ T cells.9–14 In addition, the ovarian cancer environment impairs the anti-tumor function of CD8+ T cells through inhibitory signaling pathways, including checkpoints triggered by cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD1), lymphocyte activation gene 3 protein (LAG-3) and T-cell immunoglobulin and mucin-domain containing protein 3 (TIM-3).15–18 These suppressive signals are delivered by ligands (e.g., B7 for CTLA-4, PD-L1 for PD-1) expressed on antigen presenting cells tumor cells and/or tumor infiltrating immune cells. The induced suppression can be efficiently reversed by blocking antibodies, including anti-CTLA-4, anti-PD1 and anti-PD-L1, therefore referred to as immune checkpoint inhibitors.19,20 However, the precise contribution of immune checkpoints to ovarian cancer progression is unclear, since the checkpoint blockade by anti-CTLA4 antibodies (Ipilimumab)15 or anti-PD1 antibody (Nivolumab)17 showed limited clinical efficacy. The suppressive effect of ovarian cancer ascites also involves dampening of the T cell receptor (TCR) induced activation of transcription factors NFκB and NFAT, which are crucial for T cell activation, presumably resulting from an inhibition of signal transduction upstream of phospholipase C-γ (PLCγ).21 Nevertheless, the infiltrating CD8+ T cells appear to maintain their functionality to some extent as suggested by increased levels of IFN-γ in tumor tissue from patients with a favorable clinical outcome.7
Upon antigen encounter, naïve CD8+ T (TN) cells are activated and differentiate into multipotent memory stem cells, which progressively differentiate into memory T (TM)-cell subpopulations and eventually effector CD8+ T (TEFF) cells.22 This sequential differentiation process is accompanied by a step-wise loss of plasticity, proliferative potential and capacity for homing into lymphoid organs on the one hand, and the acquisition of cytotoxicity, production of the proinflammatory cytokines IFN-γ and TNF-α, tropism for inflamed tissues and eventually a senescent phenotype on the other.22,23 Subpopulations of TM cells with different homing and functional properties, termed central memory (TCM) and effector memory (TEM) cells, were defined by Sallusto.24 TEM cells express receptors, including CXCR3, for chemotaxis into inflamed tissue and rapidly produce the effector cytokine IFN-γ upon recall stimulation with antigen. In contrast, TCM cells express lymph node homing receptors, CCR7 and CD62L, produce less IFN-γ in response to antigen encounter and differentiated into TEM cells upon secondary stimulation.24 Besides these qualities the progressively differentiating cells differ in their dependence on aerobic glycolysis and oxidative phosphorylation as their energy source.22,25,26 While limiting glycolysis promotes CD8+ T cell maintenance and anti-tumor function, enhancing glycolysis limits cell survival.27,28 To be able to pharmacologically influence the anti-tumor properties of intra-tumorally accumulating CD8+ T cells in a therapeutically beneficial way, it is important to understand which CD8+ T cell subpopulations are present in the TME, how they are influenced by the TME and how they impinge on progression of the disease and patient survival. We have addressed these questions by a combination of flow cytometry-based immunophenotyping, transcriptome and proteome analyses, ex vivo functional assays and associations with relapse-free survival (RFS) of high grade serous ovarian carcinioma.
Results and discussion
To understand the local composition of CD4+ and CD8+ T cell subpopulations in high grade serous ovarian carcinoma patients we analyzed by flow cytometry tumor associated T cells (TAT) from ascites as well as T cells isolated from the peripheral blood (PB) of matched ovarian cancer patients and patients with non-malignant disease (myomatosis uteri or ovarian cysts; referred to as “normal”). Cells were stained for the expression of CD45RA and CCR7 (Figure S1), thus defining naive (TN), central memory (TCM), effector memory (TEM) and effector (TEFF) subpopulations, as previously described.26,29 As shown in Fig. 1A, the relative abundance of CD8+ TEM cells was significantly increased in ascites as compared to normal and ovarian cancer patient peripheral blood (PB), while CD8+ TN cells as well as TEFF were decreased. For CD4+ T cells, we observed a significantly decreased TN subpopulation in ascites relative to both normal and patient PB, while an increase in TEM was significant only in comparison to patient PB. In contrast, the relative abundance of CD4+ TCM and TEFF did not significantly differ between normal PB, patient PB and ascites (Fig. 1B), indicating that differences in the accumulation patterns of CD8+ and CD4+ T cell subpopulations are characteristic of ascites. We confirmed these significant redistributions of CD8+ T cell subpopulations between peripheral blood and ascites for each patient using pairwise comparisons (Figure S2). These observations complement previous findings showing that activated memory cells accumulate in ascites of patients with ovarian carcinoma using different markers for immunophenotyping.30
Figure 1.
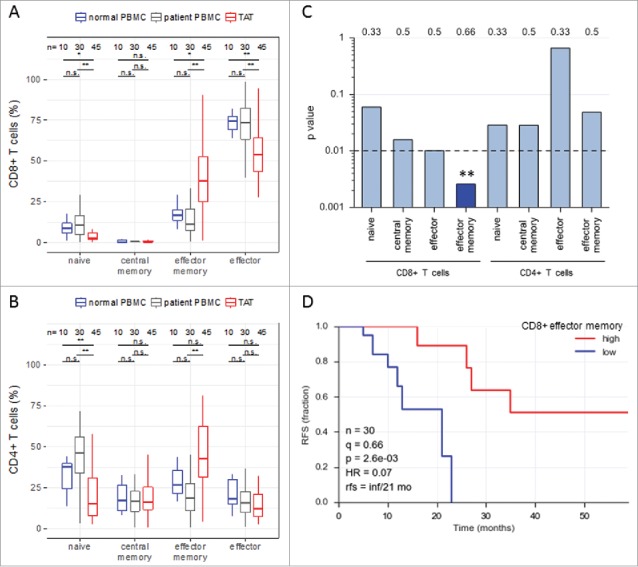
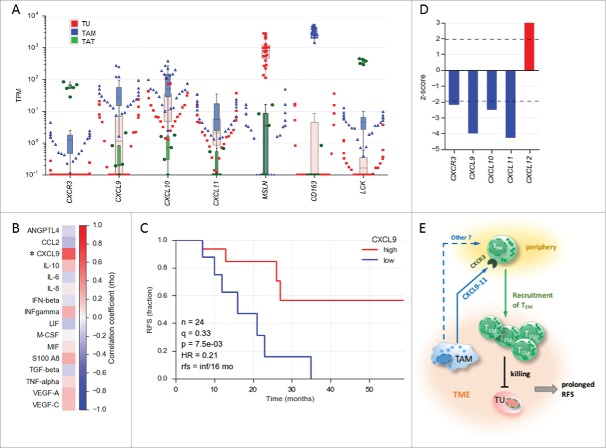
Abundance of subsets of CD8+ and CD4+ T cell subpopulations in human ovarian cancer ascites. (A) Flow cytometry analysis of CD8+ T cells from: blood of patients with benign disease (“normal blood”), blood of ovarian cancer patients (“patient blood”) and ascites of ovarian cancer patients (“ascites”). The gating for T cell subpopulations was performed based on the surface expression of CD45RA and CCR7 markers. Four T cell subpopulations were identified as follows: naïve (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), effector (CD45RA+CCR7−). The gating strategy is shown in Figure S1, and pairwise correlations in Figure S2 (B) Analysis of CD4+ T cells subpopulations as in panel A. Statistical analysis was performed by unpaired t test (**: p < 0.01; ns: not significant). Samples sizes: normal blood, n = 10; patient blood, n = 41; ascites, n = 49). (C) Association of the different CD4 and CD8 T cell populations with ovarian cancer RFS (logrank test). Best fit p values are shown. Due to the relatively small sample size the significance threshold was set to 0.01 (dashed line; **: p < 0.01). Numbers at the top show the quantiles (0.33, 0.5, 0.66) yielding the most significant dichotomization of the samples (best fit). Dark blue: significant p value (< 0.01) and hazard ratio <1; light grey blue: p > 0.01. (D) Kaplan-Meier analysis of the association of abundance of CD8+ TEM (% CD45RA−CCR7−) with ovarian cancer RFS. Samples were dichotomized at the upper tercile (q = 0.66). p: logrank p-value; HR: hazard ratio; rfs: RFS for high versus low levels; inf: infinite (> 56 months).
As T cells are crucial mediators of the anti-tumor response19 and have been linked to a more favorable outcome of ovarian carcinoma,5 we analyzed the association of different CD4+ and CD8+ T cell subpopulations in ascites with relapse-free survival (RFS) of ovarian cancer patients. As demonstrated by log-rank test (Fig. 1C) and Kaplan-Meier analysis (Fig. 1D), among all CD8+ T cell subpopulations only the abundance of CD8+ TEM cells in ascites showed a highly significant association (p<0.01) with a longer RFS (hazard ratio 0.07). The longer RFS of patients with a higher proportion of CD8+ TEM cells in ascites (median RFS >56 months versus 21 months; Fig. 1D) is consistent with the known properties of CD8+ TEM cells, including high cytotoxic activity and rapid effector cytokine production.29 Therefore, we focused on the mechanisms of CD8+ TEM cell migration and activation in ovarian carcinoma ascites.
TEM cells are known to rapidly migrate into inflamed tissue and the chemokine receptor CXCR3 has been described to contribute to this trafficking.24,29 Consistent with the accumulation of TEM cells in ovarian carcinoma ascites, transcriptome analysis revealed a high expression of CXCR3 in TAT (Fig. 2A). The main ligands for CXCR3 are CXCL9, CXCL10 and CXCL11.31 These chemokines are produced by activated CD103+ dendritic cells and to a lower extent by macrophages in an inflamed melanoma tumor model.32 Our transcriptome analysis identified tumor associated macrophages (TAM) in ovarian cancer ascites as predominant producers of CXCL9, CXCL10 and CXCL11 (Fig. 2A), suggesting that TAM play a role in attracting TEM cells into the ovarian cancer microenvironment. Consistent with this result, we found a significant correlation between the frequencies of TAMs (CD14+ cells) and the levels of CXCL9 in ascites (Fig. 2B), further supporting that TAMs are a major source of CXCL9 in ovarian carcinoma. If CXCL9 indeed contributes to the trafficking of TEM cells into ovarian cancer environment, then its abundance should have a beneficial effect on the clinical outcome. To test this hypothesis, we quantified CXCL9 in the ascites of ovarian carcinoma patients by ELISA. As shown in Fig. 2C patients with higher CXCL9 levels displayed a significantly longer RFS (Fig. 2C; hazard ratio: 0.21). This is in agreement with a recent publication reporting that CXCL9 expression in solid tumor tissue is associated with improved patient survival in advanced high grade ovarian carcinoma.33 To further substantiate these data, we searched the data base Prediction of Clinical Outcomes from Genomic Profiles (PRECOG)34 for correlation between patient overall survival (OS) and expression of CXCR3, CXCL9, CXCL10, CXCL11 in ovarian carcinoma. Consistent with our results, we found a significant positive correlation between the expression of these transcripts and patient OS (Fig. 2D). Finally, in chemotaxis assays in vitro we found increased homing of CXCR3+ TEM cells as well as CD8+ TATs into ascites (Figure S3), thus supporting the ex vivo data. Taken together, our data suggest a scenario where TAM produce CXCL9, CXCL10 and CXCL11 to attract CXCR3+ expressing TEM cells, probably in concert with other homing receptors expressing TEM cells, resulting in the prolongation of RFS in a subset of patients (Fig. 2E).
Figure 2.
Association of CXCL9 levels in ovarian cancer ascites with relapse-free survival (RFS). (A) Expression of the genes encoding CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in tumor cells (TU) depicted in red, tumor-associated macrophages (TAM) depicted in blue and tumor-associated T cells (TAT) depicted in green from ovarian cancer ascites (TPM values determined by RNA-Seq). (TU, n = 23; TAM, n = 28; TAT, n = 6). MSLN, CD163 and LCK served as cell-type-specific markers for TU, TAM and TAT, respectively. The highest value illustrated the highest expression level in each cell type. (B) Correlation of TAM numbers in ascites (CD14+ cells/ml) with the level of different soluble mediators in ascites determined by ELISA (Spearman rho; n = 17 patients). (C) Kaplan-Meier analysis of CXCL9 ascites levels as in panel A. Samples were dichotomized at the lower tercile (q = 0.3) as indicated. p: logrank p-value; HR: hazard ratio; rfs: RFS for high versus low levels; inf: infinite (> 56 months). (D) Association of the expression of CXCL and CXCR3 genes in tumor tissue with the overall survival (OS) of ovarian cancer patients. Data were retrieved from the PRECOG database (https://precog.stanford.edu). z-score <2 (blue): significant association with OS; z-score >2 (red): inverse association with OS; (E) Model depicting regulation of TEM migration into the ovarian cancer environment by TAM and association of TEM accumulation in ascites with prolonged RFS. TAM produce chemokines CXCL9, CXCL10 and CXCL11, which attract CXCR3 expressing TEM cells from the periphery, possible in concert with other mediators (dashed arrow). The TEM cells migrating into the TME contribute to tumor eradication and a longer survival.
To gain further insight into the functional properties of TAT in the ovarian carcinoma microenvironment we made use of our recently obtained proteomic data sets for different cell types in ovarian cancer ascites.35 Extended bioinformatic analyses of this data revealed proteins in TAT which could separate patients into two groups (high and low expression groups indicated in two different colors, purple and green; Fig. 3A). The expression of these proteins showed a striking correlation across TAT samples from different patients (Pearson r = 0.75-0.99; Fig. 3B), suggesting that the abundance of these proteins is mediated by concomitant signaling events, presumably triggered by the ovarian ascites microenvironment. PANTHER functional analysis36 indicated that these coexpressed proteins are associated with intracellular signal transduction, regulation of immune response, antigen receptor-mediated signaling and TCR signaling pathway (Fig. 3C, Figure S4), and in fact include several proteins associated with activation-induced signal transduction in T cells, i.e., NFATc2, PLCγ1, PIK3CD and STAT5B.37,38
Figure 3.
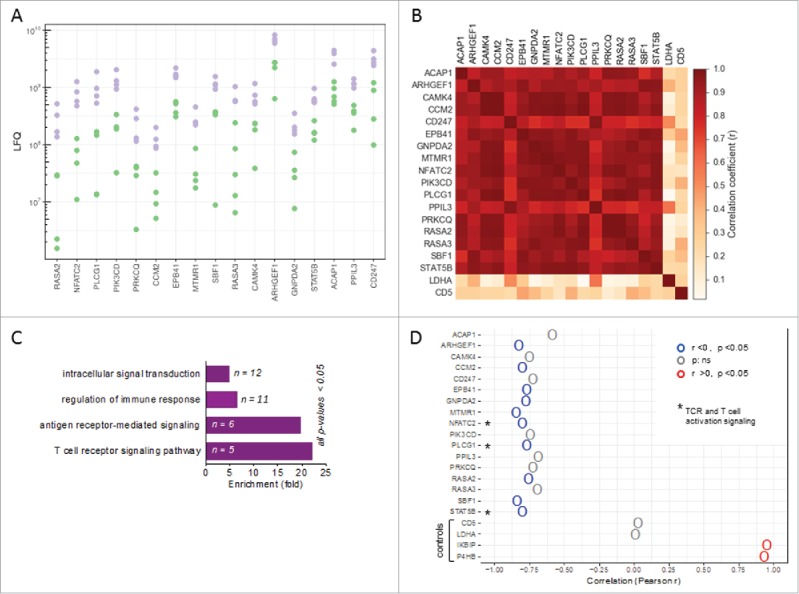
Patient-specific protein expression in TAT. (A) Proteins splitting ovarian cancer patients into high and low level expression groups (based on LFQ values in TAT determined by MS). Proteins with a > 2-fold difference between the maximum of the “low” group, depicted in green, and the minimum of the “high” group, depicted in purple, are displayed (descending order with the greatest difference on the left). (B) Heatmap showing the correlation (r-values) of expression (LFQ values) for the proteins in panel A. (C) PANTHER functional annotation (gene ontology enrichment analysis) of the proteins identified in panel A. n: number of genes in the respective group. List of the proteins is included in Figure S4. (D) Correlation (Pearson r) of the expression of these proteins in TAT with CD163 expression in TAM. High CD163 expression in TAM is a surrogate marker for a poor clinical outcome.32,37 Asterisks (*) indicate intracellular proteins associated with TCR signaling (“T cell receptor signaling pathway” in panel B). Lactate dehydrogenase A (LDHA) and CD5 served as controls for proteins showing a low fluctuation of expression. The IKBIP and the prolyl hydroxylase P4HB were included for comparison as proteins with a strong positive correlation with CD163 expression in TAM.
We have previously shown that the RFS of ovarian carcinoma patients is inversely associated with surface expression of CD163 on TAM,39 which allowed us to assess a potential linkage of the signaling proteins identified above with disease outcome. To this end, we used our proteome data to correlate the expression of CD163 by TAM with the abundance of the proteins identified above in TATs. This analysis revealed a strong inverse correlation for several proteins associated with TCR-signaling and intracellular signal transduction in T cells, including NFATc2, PLCγ1 and STAT5B (Fig. 3D), pointing to a link between a poor clinical outcome and downregulation of these proteins in TAT.
The regulation of molecules associated with TCR- and IL-2-signaling in TAT including NFATc2, PLCγ1 and STAT5B could potentially be caused by soluble factors in ovarian cancer ascites. We therefore analyzed the effects of cell-free ascites on the expression of these signal transduction molecules in TEM cells sorted from the peripheral blood of healthy donors. As shown in Figs. 4A and S5, the levels of both PLCγ1 and STAT5B were significantly downregulated by ascites relative to RPMI medium, indicating inhibitory effects on TCR and IL-2 signaling, respectively. This inhibition was observed with three ascites samples from different patients (different red symbols in Fig. 4A). The same ascites samples also strongly suppressed the TCR-mediated functional activation of TEM cells, as indicated by a low fraction of cells expressing the effector cytokine TNF-α (Figs. 4B and S6), and dampened their cytotoxic activity as assessed by a decreased expression of CD107a40 (Figs. 4C and S6). In contrast to TNF-α upregulation, induction of IFN-γ in activated TEM cells was observed only with cells from a subgroup of donors, but in these cases IFN-γ expression was strongly suppressed by ascites (Figure S6). This effect was specific as ascites did not influence apoptosis of TEM cells (Figure S7). However, the proliferation of TEM cells was partially affected by ascites (Figure S8), which could result from the suppression of PLCγ1 and STAT5B. These findings suggest that ovarian carcinoma ascites suppresses an anti-tumor T cell response at least partially by the downregulation of molecules associated with signaling pathways that are crucial for the recall of TEM cells.
Figure 4.
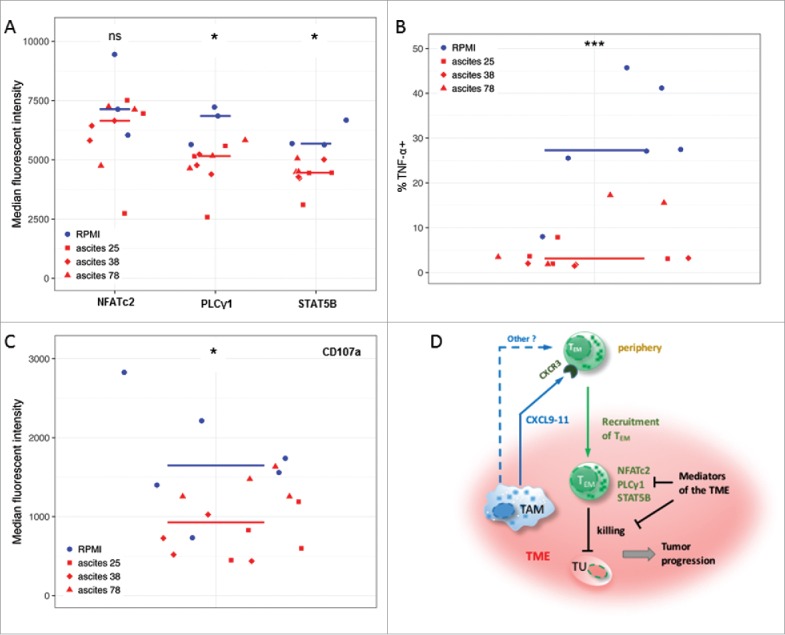
Ovarian carcinoma ascites suppresses function of CD8+ TEM cells. (A) Flow cytometry analysis of PLCγ1 and transcription factors STAT5B and NFATc2 associated with TCR- and IL-2-signaling in activated CD8+ TEM cells from healthy donors (n = 3). Median fluorescence intensity (MFI) is shown. The gating strategy and histograms are shown in Figure S5. (B, C) Flow cytometry analysis of intracellular TNF-α-positive cells (%) and CD107a (MFI) in activated CD8+ TEM cells from healthy donors (n = 3 per group). The gating strategy and histograms are shown in Figure S6. Statistical analysis by t-test: ***: p < 0.001, **: p < 0.01: *p < 0.05, n.s.: not significant (ascites groups considered as one group with n = 9). (D) A model integrating diverse effects of the ovarian cancer environment on the accumulation and function of CD8+ TEM cells. TAM (and to some extent other cell types) attract CXCR3-expressing TEM cells from the periphery via secretion of CXCL9, CXCL10 and CXCL11 chemokines (and possibly other mediators). However, once migrated into the tumor microenvironment (TME) the activation and function of these TEM cells is suppressed by mediators of tumor environment, thus causing shortened RFS of patients. This is in part mediated by lowering the expression levels of signal transduction proteins crucial for T cell activation.
Our findings shed light on the relationships between clinical outcome and TATs in the peritoneal microenvironment of ovarian cancer and indicate that different aspects of T cell biology essential for an efficient anti-tumor response are affected in a patient-specific fashion. Thus, the accumulation of CD8+ TEM cells in ovarian cancer ascites differs vastly among patients (Fig. 1A) and is directly associated with a more favorable clinical course (Fig. 1D). Furthermore, the ascites concentration of CXCL9, a CD8+ TEM cell-attracting chemokine, is likewise associated with a prolonged RFS (Fig. 2C), suggesting a functional link between the production of CXCL9 by cells of the ovarian cancer microenvironment, the recruitment of peripheral CD8+ TEM cells and a T-cell-mediated immune response. The cause(s) of the observed differences in CXCL9 levels in ascites are currently unclear. However, we show that TAMs in ascites are major producers of the all CXCR3 ligands, i.e., CXCL9, CXCL10 and CXCL11, and that expression levels in TAM range over two orders of magnitude (Fig. 2A), providing a possible explanation. If so, a potential therapeutic approach could be the education of TAMs to produce CXCL9, CXCL10 and CXCL11. Of course, other causes of the observed differences in CXCL9 levels in ascites can be envisaged, such as sequestration of CXCL9 or its proteolytic cleavage, but such mechanisms could also be amenable to pharmacological intervention, potentially by individualized approaches.
Besides beneficial effects on the attraction of CD8+ TEM cells in a subgroup of patients, the ovarian cancer microenvironment clearly has a deleterious impact on the functional activation of T cells,5,21 as confirmed by the ascites-mediated inhibition of IFN-γ, TNF-α and CD107a expression in activated CD8+ TEM cells (Fig. 4B, C). Even though some inhibitory molecules have been found in ascites, including cytokines, lipids and extracellular vesicles,5,41,42 the molecular mechanisms impinging on signaling pathways essential for T cell activation remain largely elusive. In one study, inhibition of PLCγ activity by ascites (leading to decreased NFκB and NFAT activity) has been proposed to be critical in this context,21 but the underlying mechanisms have not been addressed. In the present study, we describe a novel mechanism that could contribute to compromising T cell activation in the ovarian cancer microenvironment. Two pieces of evidence suggest that the expression level of proteins that are crucial for TCR- and IL-2 induced signal transduction in T cells37,38 is regulated by cell-free ascites. First, the abundance of several of these proteins, including STAT5B, PLCγ1 and NFATc2, is lower in TAT from patients with a high expression of CD163 by TAM (Fig. 3D), a surrogate marker of an unfavorable clinical outcome. Second, two of these proteins are significantly downregulated in TEM cells form healthy donors after exposure to ascites, concomitant with suppression of their functional activation (Fig. 4A–C). These observations indicate that TAT from different patients quantitatively differ in their equipment with signal transduction proteins required for T cell activation. An ascites-induced low level of these proteins may affect anti-tumor responses in concert with other known suppressive mechanisms, mediated for instance by checkpoint ligands, cytokines or lipids. The expression of signal transduction proteins in TAT may therefore be an important parameter to gauge the efficiency of existing immune therapies19,43 in individual patients. Furthermore, elucidation of the mechanism regulating the abundance of these proteins may lead to new options to enhance the efficacy of immune therapeutic approaches.
A recent study of a single advanced ovarian carcinoma patient indicated that each of five sampled tumors displayed a unique immune microenvironment. Consistent with the current view on CD8+ T cell function in anti-tumour immunity,19 regressing metastases were characterized by T cell oligoclonal expansion, whereas T cells were excluded from progressive metastases. Interestingly, CD8+ T cells reacting against tumour neoantigens were detectable within patient PBMCs, indicating that a specific microenvironment was responsible for the heterogeneity among individual tumors.44 Furthermore, a direct comparison of the TCR repertoire between tumor infiltrating lymphocytes (TILs) and TATs in ovarian carcinoma by next-generation sequencing revealed some similarities, but differences were more pronounced.45 The key findings concerning CD8+ T cell infiltration, heightened CD8+/Treg ratio and positive survival prognosis seem to apply to both TATs8 and TILs.7,10 Similarly, our finding on a positive correlation between CXCL9 levels and patient survival also applies for TILs.33 However, the peritoneal effusion strongly differs from the tumor tissue-specific environment5 with respect to soluble factors, extracellular vesicles and cellular composition, which presumably impacts in a compartment-specific fashion on the enrichment and activity of T cell subpopulations. Based on the specific spatial distribution it is tempting to speculate that TILs versus tumour associated lymphocytes (TALs), including TATs in ascites, display non-redundant functions. In view of the major contribution of ascites to metastatic spread,5,42 TALs could be involved in the control of tumor cell dissemination, whereas the man function of TILs could be the local restriction of tumor growth.7 The peritoneal fluid plays an essential role in ovarian cancer biology at all stages of the disease as an essential compartment of the tumor microenvironment.5 The volume of peritoneal fluid increases in approximately 50% of the patients culminating in the production of large volumes of ascites. These facts support the clinical relevance of the cellular and molecular constituents of ovarian cancer ascites.
Our observations clearly indicate that ascites can have distinct effects on T cell biology, including both beneficial (i.e., recruitment of T cells into the tumor) and detrimental (i.e., inhibition of activation-associated signal transduction pathways) aspects (Fig. 4D). A deep understanding of these processes and a precise knowledge of the mechanisms responsible for their perturbation in individual patients will be key to the development of improved immune therapies for ovarian cancer patients.
Patients and methods
Patient samples
Ascites and peripheral blood were collected from untreated patients with high grade serous ovarian carcinoma prior to surgery at Marburg University Hospital (Table S1). Peripheral blood was obtained from patients with benign diseases (myomatosis uteri or ovarian cysts) as “normal” controls. Mononuclear CD14+ cells were isolated from ascites and peripheral blood by Lymphocyte Separation Medium 1077 (PromoCell) density gradient centrifugation. Cell-free ascites and plasma was cryo-preserved at −80 C. The collection and the analysis of human material were approved by the ethics committee at Philipps University (reference number 205/10). Donors provided written consent in accordance with the Declaration of Helsinki.
CXCL9 quantification
CXCL9 in ascites was quantified by ELISA (Thermo Fisher Scientific; catalog number EHCXCL9) according to the instructions of the manufacturer.
Immunophenotyping
Lymphocyte subsets were characterized using the following antibodies for surface expression in FACS analysis (FACSCanto II BD Biosciences): anti-human CD4-PE-Cy7 (Southern Biotech), CD8-APC (Miltenyi Biotec), CD45RA-FITC (Miltenyi Biotec), and CCR7-PE (BD Biosciences). Corresponding isotype-matched controls were purchased from Miltenyi Biotec and Southern Biotech. The gating for T cell subpopulations was performed based on the surface expression of CD45RA and CCR7 markers. Four T cell subpopulations were identified as follows: naïve (CD45RA+CCR7+), central memory: (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), effector (CD45RA+CCR7−).
CD8+ T cell purification and culture with tumor ascites
Lymphocytes were isolated from normal blood (buffy coat) by Lymphocyte Separation Medium 1077 density gradient centrifugation (PromoCell GmbH, Germany) and further purified by MACS CD8+ T Cell Isolation Kit (Miltenyi Biotec) according to manufacturers’ instructions. Purified CD8+ Lymphocytes were stained with anti-CD8-PE-Cy7 (eBioscience), anti-CCR7-PE (BD Biosciences), and anti-CD45RA-APC (eBioscience) and CD8+CCR7−CD45RA− Tem cells were sorted on a FACS Aria III. Sorting purity was typically >97% in post-sort analysis. Appropriate cells were activated via Dynabeads Human T-Activator CD3/CD28 (Gibco, Life Technologies) according to manufacturers’ protocol and cultered in RPMI with 5% human male AB serum (Sigma-Aldrich, Germany) or ascites of different patients for 16 hours, followed by a restimulation step with PMA (50 nM) and Ionomycin (750 nM) (Sigma-Aldrich, Germany) and treatment with GolgiPlug (BD Biosciences) according to manufacturers’ instructions for further 4 hours. Two of the ascites samples (#25 and #38) were from patients with very poor RFS and one sample from a patient with exceptionally long RFS (#78).
Staining of activated effector memory CD8+ T cells
Activated effector memory CD8+ T cells were stained for intracellular expression of IFN-γ and TNF-α using intracellular cytokine staining or PLCγ1, STAT5B and NFATc2 using staining for transcription factors. Following antibodies were used for for FACS analysis (FACSCanto II BD Biosciences): anti-human IFN-γ-FITC, TNF-α-PerCP-Cy5.5 (both eBioscience), PLCγ1-APC, STAT5B-Alexa Fluor 488, and NFATC2-PerCP (all R&D Bio-Techne) and for surface expression of CD107a using anti-human CD107a-PE antibody (eBioscience). Corresponding isotype-matched controls were purchased from Miltenyi Biotec, BD Biosciences, R&D Bio-Techne and eBioscience.
Transcriptome analysis
RNA sequencing (RNA-Seq) data for tumor cells, TAM and TAT samples were retrieved from.46 The following samples used in the present study are listed in Table S1. Transcripts per million (TPM) values were used as measure of mRNA expression for the analysis in Fig. 2C. RNA-Seq data are deposited at EBI ArrayExpress (accession numbers E-MTAB-3167, E-MTAB-4162, E-MTAB-5199, E-MTAB-5498).
Proteome analysis
OrbiTrap-based mass spectrometry of 7 matched TAT and TAM samples and data analysis by MaxQuant are described in detail elsewhere35 sample IDs 105, 108, 114, 117, 119, 120, 128, 133). Label-free quantification (LFQ) intensities were used as a measure of protein expression for the analyses in Fig. 3. Mass spectrometry data are deposited at PRIDE (accession No.: PXD006138).
Chemotaxis assays
CD8+ Lymphocytes were isolated from buffy coat of healthy donors as described above, activated via Dynabeads Human T-Activator CD3/CD28 (Gibco, Life Technologies) for 2 days and then expanded with recombinant human IL-2 (300 IU/ml).47 CD8+ TATs were purified via MACS from cryo-preserved ascites derived cells as described above and cultured overnight in ascites. Chemotaxis was assayed in 24-well plates and 3 µm pore size transwell inserts (Sarstedt). Media alone (RPMI 1640 + 1% BSA), media containing a mix of recombinant chemokines CXCL9 and CXCL11 (total 100 ng/ml; Peprotech) or ascites was placed at the bottom of wells. 5 × 105 cells were placed on the transwell insert and incubated at 37°C for 4 h. Cells in the bottom chamber were enumerated by flow cytometry. Data are reported as migration relative to media control.
Statistical analysis
Comparative data were statistically analyzed by Student's t-test (two-sided, equal variance; Figs. 1, 4, S1). Box pots depicting medians (line), upper and lower quartiles (box) range (whiskers) and outliers were constructed using ggplot2 (Figs. 1, S1) or the Seaborn boxplot function (Fig. 2A). Correlations were analyzed using the scipy.stat functions. Associations with relapse-free survival (logrank test), hazard ratio (HR) and median survival times were analyzed using the Python Lifelines KaplanMeierFitter and CoxPHFitter functions (Figs. 1C, 1D, 2C). Due to the relatively small sample size we chose a significance threshold of p < 0.01 for all survival analyses Patients suffering from a relapse during first-line chemotherapy (RFS ≤ 3 months) were excluded (n = 1). Functional annotation (Fig. 3B) was performed by PANTHER gene ontology (GO) enrichment analysis (http://www.http://geneontology.org).48
Supplementary Material
Funding Statement
This work was supported by grants from the Universitätsklinikum Giessen und Marburg (UKGM) to SL and SR and to MH and SR.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to J. Graumann and M. Konzer (Max-Planck-Institute for Heart and Lung Research, Bad Nauheim, Germany) for mass spectrometry analyses and to M. Alt for excellent technical assistance.
References
- 1.Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, Sessa C, Castiglione M, Group EGW. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v23-30. doi: 10.1093/annonc/mdq244. PMID: 20555088. [DOI] [PubMed] [Google Scholar]
- 2.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–64. doi: 10.2353/ajpath.2010.100105. PMID: 20651229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan S, Coward JI, Bast RC Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, et al.. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–25. doi: 10.1038/nrc3144. PMID: 21941283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–82. doi: 10.1038/nrc3432. PMID: 23426401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worzfeld T, Pogge von Strandmann E, Huber M, Adhikary T, Wagner U, Reinartz S, Muller R. The unique molecular and cellular microenvironment of ovarian Cancer. Front Oncol. 2017;7:24. doi: 10.3389/fonc.2017.00024. PMID: 28275576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogge von Strandmann E, Reinartz S, Wager U, Muller R. Tumor-host cell interactions in ovarian cancer: pathways to therapy failure. Trends Cancer. 2017;3(2):137–48. doi: 10.1016/j.trecan.2016.12.005. PMID: 28718444. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. PMID: 12529460. [DOI] [PubMed] [Google Scholar]
- 8.Giuntoli RL 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29(8):2875–84. http://www.ncbi.nlm.nih.gov/pubmed/19661290. PMID: 19661290. [PubMed] [Google Scholar]
- 9.Chang DK, Peterson E, Sun J, Goudie C, Drapkin RI, Liu JF, Matulonis U, Zhu Q, Marasco WA. Anti-CCR4 monoclonal antibody enhances antitumor immunity by modulating tumor-infiltrating Tregs in an ovarian cancer xenograft humanized mouse model. Oncoimmunology. 2016;5(3):e1090075. doi: 10.1080/2162402X.2015.1090075. PMID: 27141347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. PMID: 16344461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–44. doi: 10.1158/1078-0432.CCR-13-1877. PMID: 24190978. [DOI] [PubMed] [Google Scholar]
- 12.Komdeur FL, Wouters MC, Workel HH, Tijans AM, Terwindt AL, Brunekreeft KL, Plat A, Klip HG, Eggink FA, Leffers N, et al.. CD103+ intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRalphabeta+ CD8alphabeta+ T cells that can be targeted for cancer immunotherapy. Oncotarget 2016;7(46):75130–75144. doi: 10.18632/oncotarget.12077. PMID: 27650547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. PMID: 24244610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al.. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. PMID: 15322536. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, et al.. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105(8):3005–10. doi: 10.1073/pnas.0712237105. PMID: 18287062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al.. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–80. doi: 10.1073/pnas.1003345107. PMID: 20385810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al.. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–22. doi: 10.1200/JCO.2015.62.3397. PMID: 26351349. [DOI] [PubMed] [Google Scholar]
- 18.Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6(29):27359–77. doi: 10.18632/oncotarget.4751. PMID: 26318293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30. doi: 10.1038/nature21349. PMID: 28102259. [DOI] [PubMed] [Google Scholar]
- 20.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson-Abelson MR, Loyall JL, Lehman HK, Barnas JL, Minderman H, O'Loughlin KL, Wallace PK, George TC, Peng P, Kelleher RJ Jr., et al.. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14. http://www.ncbi.nlm.nih.gov/pubmed/23882159. PMID: 23882159. [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23(1):18–27. doi: 10.1038/nm.4241. PMID: 28060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–18. http://www.ncbi.nlm.nih.gov/pubmed/9348298. PMID: 9348298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. PMID: 10537110. [DOI] [PubMed] [Google Scholar]
- 25.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17(4):364–8. doi: 10.1038/ni.3415. PMID: 27002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14(10):1064–72. doi: 10.1038/ni.2687. PMID: 23955661. [DOI] [PubMed] [Google Scholar]
- 27.Sukumar M, Kishton RJ, Restifo NP. Metabolic reprograming of anti-tumor immunity. Curr Opin Immunol. 2017;46:14–22. doi: 10.1016/j.coi.2017.03.011. PMID: 28412583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al.. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123(10):4479–88. doi: 10.1172/JCI69589. PMID: 24091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809. doi: 10.1002/eji.201343751. PMID: 24258910. [DOI] [PubMed] [Google Scholar]
- 30.Landskron J, Helland O, Torgersen KM, Aandahl EM, Gjertsen BT, Bjorge L, Tasken K. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother. 2015;64(3):337–47. doi: 10.1007/s00262-014-1636-6. PMID: 25416072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vignali D, Kallikourdis M. Improving homing in T cell therapy. Cytokine Growth Factor Rev. 2017;36:107–16. doi: 10.1016/j.cytogfr.2017.06.009. PMID: 28690108. [DOI] [PubMed] [Google Scholar]
- 32.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–23 e714. doi: 10.1016/j.ccell.2017.04.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronger H, Singer J, Windmuller C, Reuning U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M, et al.. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer. 2016;115(5):553–63. doi: 10.1038/bjc.2016.172. PMID: 27490802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al.. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. doi: 10.1038/nm.3909. PMID: 26193342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worzfeld T, Finkernagel F, Reinartz S, Konzer A, Adhikary T, Nist A, Stiewe T, Wagner U, Looso M, Graumann J, et al.. Proteotranscriptomics reveal signaling networks in the ovarian cancer microenvironment. Mol Cell Proteomics. 2017. pii: mcp.RA117.000400. [Epub ahead of print]. doi: 10.1074/mcp.RA117.000400. PMID: 29141914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551–66. doi: 10.1038/nprot.2013.092. PMID: 23868073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16(3):149–63. doi: 10.1038/nri.2015.18. PMID: 26875830. [DOI] [PubMed] [Google Scholar]
- 38.Man K, Kallies A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol. 2015;15(9):574–84. doi: 10.1038/nri3874. PMID: 26272293. [DOI] [PubMed] [Google Scholar]
- 39.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, et al.. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134(1):32–42. doi: 10.1002/ijc.28335. PMID: 23784932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. PMID: 25765070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelleher RJ Jr., Balu-Iyer S, Loyall J, Sacca AJ, Shenoy GN, Peng P, Iyer V, Fathallah AM, Berenson CS, Wallace PK, et al.. Extracellular vesicles present in human ovarian tumor microenvironments induce a phosphatidylserine-dependent arrest in the T-cell signaling cascade. Cancer Immunol Res. 2015;3(11):1269–78. doi: 10.1158/2326-6066.CIR-15-0086. PMID: 26112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. PMID: 24093089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58. doi: 10.1038/nrclinonc.2015.209. PMID: 26598942. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, et al.. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170(5):927–38 e920. doi: 10.1016/j.cell.2017.07.025.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang M, Yew PY, Hasegawa K, Ikeda Y, Fujiwara K, Fleming GF, Nakamura Y, Park JH. Characterization of T cell repertoire of blood, tumor, and ascites in ovarian cancer patients using next generation sequencing. Oncoimmunology. 2015;4(11):e1030561. doi: 10.1080/2162402X.2015.1030561. PMID: 26451311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y, Nockher WA, Nist A, Stiewe T, Jansen JM, et al.. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016;17(1):108. doi: 10.1186/s13059-016-0956-6. PMID: 27215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, et al.. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458. doi: 10.1038/ncomms8458. PMID: 26109379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mi H, Dong Q, Muruganujan A, Gaudet P, Lewis S, Thomas PD. PANTHER version 7: improved phylogenetic trees, orthologs and collaboration with the gene ontology consortium. Nucleic Acids Res. 2010;38(Database issue):D204–10. doi: 10.1093/nar/gkp1019. PMID: 20015972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.