ABSTRACT
Prisma® Skin is a new pharmaceutical device developed by Mediolanum Farmaceutici S.p.a. It includes alginates, hyaluronic acid and mainly mesoglycan. The latter is a natural glycosaminoglycan preparation containing chondroitin sulfate, dermatan sulfate, heparan sulfate and heparin and it is used in the treatment of vascular disease. Glycosaminoglycans may contribute to the re-epithelialization in the skin wound healing, as components of the extracellular matrix. Here we describe, for the first time, the effects of Prisma® Skin in in vitro cultures of adult epidermal keratinocytes and dermal fibroblasts. Once confirmed the lack of cytotoxicity by mesoglycan and Prisma® Skin, we have shown the increase of S and G2 phases of fibroblasts cell cycle distribution. We further report the strong induction of cell migration rate and invasion capability on both cell lines, two key processes of wound repair. In support of these results, we found significant cytoskeletal reorganization, following the treatments with mesoglycan and Prisma® Skin, as confirmed by the formation of F-actin stress fibers. Additionally, together with a significant reduction of E-cadherin, keratinocytes showed an increase of CD44 expression and the translocation of ezrin to the plasma membrane, suggesting the involvement of CD44/ERM (ezrin-radixin-moesin) pathway in the induction of the analyzed processes. Furthermore, as showed by immunofluorescence assay, fibroblasts treated with mesoglycan and Prisma® Skin exhibited the increase of Fibroblast Activated Protein α and a remarkable change in shape and orientation, two common features of reactive stromal fibroblasts. In all experiments Prisma® Skin was slightly more potent than mesoglycan. In conclusion, based on these findings we suggest that Prisma® Skin may be able to accelerate the healing process in venous skin ulcers, principally enhancing re-epithelialization and granulation processes.
KEYWORDS: glycosaminoglycans, mesoglycan, Prisma® Skin, re-epithelialization, skin wound healing
Introduction
Glycosaminoglycans (GAGs) are unbranched polysaccharides with repetitive disaccharide units; they are structurally heterogeneous in terms of their carbohydrates composition, chain length, sulfation pattern and degree. GAGs can be classified into sulfated such as chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS) and heparin (HEP), and non-sulfated as hyaluronan (HA). The latter is formed by the regular repetition of a disaccharide unit (glucuronide-N-acetylglucosamine), and its chain is much longer than other GAGs. When these chains, except for HA, are covalently attached to a protein core, they form the proteoglycans (PGs).1
GAGs represent only the 0.1–0.3% of the total skin weight but, due to their large water-retaining capability, they define skin volume and elasticity. They are common constituents of cell surfaces and extracellular matrix (ECM) and, together with protein as fibrils of collagen, fibronectin, laminins, vitronectin, have various biological effects.2
ECM facilitates repair of the skin wound by both direct modulation of cell processes such as adhesion, migration, proliferation, and cell differentiation and by indirect regulation of extracellular protease secretion/activation or growth factor activity. Their content could effectively change inside the ECM during wound repair giving rise to the formation of a GAG-bed: this accumulation is required for granulation, tissue growth and closure.3,4
Skin wound healing evolves through a complex of cellular, physiologic, and biochemical events, such as inflammation, granulation, re-epithelialization and remodeling process. An abnormal regeneration process causes non-healing chronic wounds or abnormal scar and keloid formation. Many biological factors, such as growth factors and cytokines, are necessary for the multiplex steps of wound healing contributing to the migration of inflammatory cells, fibroblasts, keratinocytes, and endothelial precursor cells into the wound site.5
During the phases of the wound healing process, fibroblasts and other mesenchymal cells enter the wound inflammatory site in response to growth factors that are required for the stimulation of cell proliferation. In particular, fibroblasts are able to synthesize collagen and PGs and to actively secrete several GAGs allowing the formation of a hydrophilic matrix suitable for skin remodeling.6
Initially, HA is synthesized in large quantities by fibroblasts, followed by an increase in the levels of DS and CS.6 Gradually, when cell proliferation reaches a plateau, the levels of Heparan sulfate proteoglycan (HSPG) are higher in the wound. Heparan sulfate proteoglycan 2 (HSPG2) regulates the healing process by inducing angiogenesis7 and stimulates keratinocytes migration by binding to syndecan-1 and −4.7,8
DS is the most abundant GAG in human wound fluid and functionally active in the healing process thanks to its combination with Fibroblast Growth Factor 10 (FGF-10) which accelerates keratinocyte proliferation and migration. FGF-10 and FGF-2 are known to bind DS and this affinity is enhanced for DS containing more iduronic acid. Idunorate-enriched DS is responsible for mediating FGF responsiveness in the fibroblasts, indicating that this GAG is a required cofactor for maximal cellular responsiveness to FGF-10 during wound repair.9-12
DS is essential to modify CD44 and enables fibroblast migration in fibronectin/fibrin gel during the earliest phases of wound healing.13
As previously stated the migration of keratinocytes is essential for wound re-epithelialization and re-establishment of skin remodeling.14 Hyaluronic acid (HA) is involved in tissue repair, serves as an integral part of the ECM of basal keratinocytes in epidermis and promotes the proliferation and migration of keratinocytes in the re-epithelialization process.15
Mesoglycan is a natural GAG preparation extracted from porcine intestinal mucosa and is composed of HS (47.5%), DS (35.5%), slow-moving HEP (8.5%) and CS (8.5%). It is commercially available in some European countries, both in a parenteral and oral form, for the treatment of vascular disease with an associated thrombotic risk such as deep venous thrombosis and chronic venous insufficiency.16-19 Additionally, mesoglycan is known to have favorable actions on the fibrinolytic system and to restore the electronegativity of the vascular endothelium in case of damage.20 In the past, like the sulodexide, mesoglycan has been found able to potentiate the mitogenic activity of FGFs and restore defective fibrinolysis in patients affected by cutaneous necrotizing venulitis.21,22
It is commonly accepted that GAGs represent promising biomaterials to design new bioactive supplies for tissue repair and re-epithelialization;23,24 also the observations reported by literature demonstrate the potential for the local use of bioactive film containing GAGs. Using GAGs locally is easier nowadays due to the possible use of new kind of active biofilm containing a mixture of GAGs and placed directly on the wound bed.
Prisma® Skin is a water-soluble dressing with advanced-technology alginate on inert polyethylene terephthalate (PET) support material, including mainly mesoglycan and hyaluronic acid.25 Given the importance of GAGs in the wound environment and the uniqueness of the Prisma® Skin, the aim of this study was to carefully assess the effect of this new pharmaceutical device. We have investigated some important biological processes involved in the closure of skin lesion, as migration and invasion, on adult dermal fibroblasts and epidermal keratinocytes in vitro.
Results
Prisma® Skin and mesoglycan are not cytotoxic on human HaCaT keratinocytes and BJ fibroblasts
The lack of information about the use of mesoglycan on in vitro epithelial cells and, mainly, the complete absence of data about Prisma® Skin led us to investigate, first of all, their potential effects on viability of HaCaT and BJ cells. Our first approach included the evaluation of the cell viability through the MTT assay using a dose-response curve. This curve started from 0.1 mg/ml until 0.5 mg/ml of sodium mesoglycan, including the middle concentration (0.3 mg/ml) as indicated by Mediolanum Farmaceutici company. As shown in Fig. 1A and B, neither mesoglycan nor Prisma® Skin showed cytotoxic effects on tested cell lines. Furthermore, on BJ cells we observed a weak increase of proliferation at 24 and 48 hours in presence of mesoglycan and particularly of Prisma® Skin (Fig. 1B). The absence of cytotoxic effects was also shown through hemocytometer-cell counting (Fig. S1, panels A and B).
Figure 1.
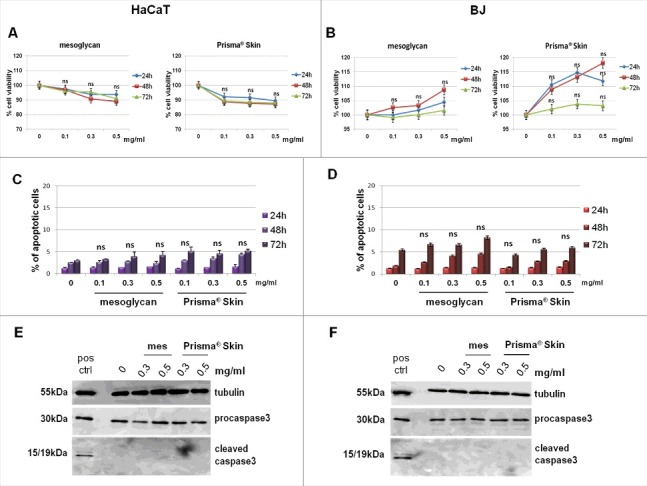
Viability assays on HaCaT and BJ cells treated or not with mesoglycan and Prisma® Skin. Cell viability was evaluated by MTT colorimetric assay on keratinocytes (A) and on fibroblasts (B). Absorbance relative to controls was used to determine the percentage of cells treated with varying concentrations of sodium mesoglycan and Prisma® Skin at 24, 48 and 72 h. The values reported in the graphs are the mean ± SEM from 5 independent experiments performed in triplicates, results appeared not significant, thus they have been indicated as ns (p > 0.05). Analysis of apoptotic cells by cytofluorimetric assay of the effect of 0.1, 0.3 and 0.5 mg/ml of mesoglycan and Prisma® Skin at 24, 48 and 72 h on HaCaT (C) and on BJ (D). The data are the mean of 5 experiments with similar results, ns p > 0.05 based on Student's t-test, assuming a 2-tailed distribution and unequal variance. Western blot for pro- and cleaved caspase-3 of keratinocytes (E) and fibroblasts (F). Both of cell lines were treated or not with mesoglycan and Prisma® Skin for 48 h and a positive control of apoptotic cells was used. Protein bands were normalized on tubulin levels. Image is representative of 3 independent experiments.
To further confirm the HaCaT and BJ viability, we evaluated the possible induction of apoptosis. As reported in Fig 1C and D, there were no significant changes in response to the mesoglycan and Prisma® Skin. These data was also confirmed by the lack of activation of caspase-3, typical hallmark of apoptosis and indispensable for apoptotic chromatin condensation and DNA fragmentation, which appears only in its not-cleaved form (Fig. 1E and F).
Prisma® Skin and mesoglycan increased fibroblasts proliferation
Based on results on cell viability, we performed a cell cycle analysis on both HaCaT and BJ cells. Confirming the MTT data, HaCaT, which were characterized by a notable cell division rate, did not show any differences on cell cycle phases when treated with mesoglycan and Prisma® Skin at 24, 48 and 72 hours (Figure S2, panels A-F). On the other hand, BJ cells showed a strong and significant increase of S phase at 24 hours (Fig. 2A) and of G2 one at 48 hours of treatment with mesoglycan and Prisma® Skin (Fig. 2B). As we expected, at 72 hours from substances administration, a rescue verified and cells came back to their normal cycle with a prevalent G1 phase and an absent G2 one (Fig. 2C). In Fig. 2D, E and F an example of analysis performed by ModFit LT software with mesoglycan and Prisma® Skin 0.3 mg/ml at 24, 48, and 72 hours is shown.
Figure 2.
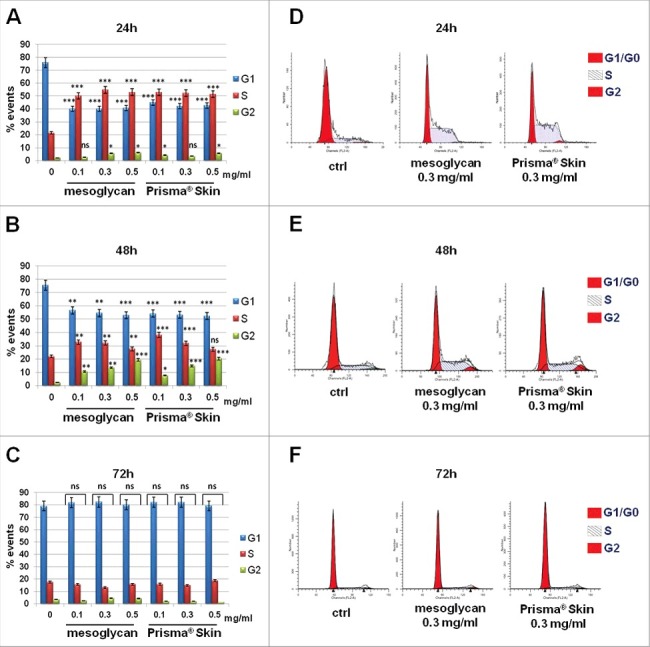
BJ cycle analysis with PI staining. The histograms are representative of (A) 24 h, (B) 48 h and (C) 72 h of culture, after 24 h serum starvation. Cell cycle phase distributions of fibroblasts analyzed by ModFit LT software at (D) 24 h, (E) 48 h and (F) 72 h after treatment with sodium mesoglycan and Prisma® Skin. The data are representative as a mean of 3 experiments with similar results, statistical analyses for significance of results were performed using Student's t-test, assuming a 2-tailed distribution and unequal variance. *p < 0.05, **p < 0.01, ***p < 0.001, ns p > 0.05.
The migration rate of HaCaT and BJ cells increased in the presence of Prisma® Skin and mesoglycan
Several studies gave insights on the significant relationship between cell migration during wound repair and GAGs, mainly as ECM components, but also as capable to regulate cytokines release and growth factor activity.26 Based on these evidences, we performed a functional assay of in vitro wound healing, as reported in Materials and Methods section, to assess the migration ability of HaCaT and BJ cells. During 24 hours a 38% increase of the covered distance for HaCaT treated with mesoglycan 0.3 mg/ml was observed if compared with non-treated cells and a 47% increase when the same cells were treated with Prisma® Skin at the same concentration (Fig. 3A for representative images and 3C). Very similar results were obtained with BJ cells which showed a 53% increase of distance covered in presence of mesoglycan and a 60% increase when treated with Prisma® Skin, with respect to non treated control (Fig. 3B for representative images and 3D).
Figure 3.
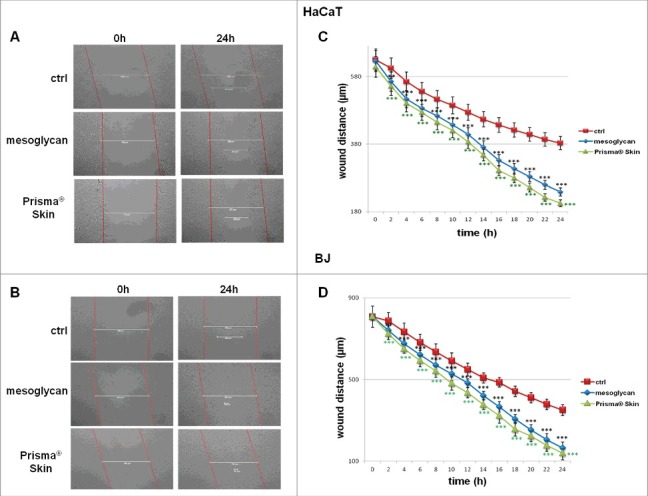
Evaluation of migration rate of human keratinocytes and fibroblasts in presence of mesoglycan and Prisma® Skin. Representative images of Wound Healing assay on HaCaT (A) and BJ (B) cells treated or not with sodium mesoglycan and Prisma® Skin 0.3 mg/ml. Results of Wound Healing assay analysis for HaCat (C) and BJ (D) cells. The migration rate was determined by measuring the wound closure by individual cells from the initial time to the selected time-points (bar of distance tool, Leica ASF software). The data represent a mean of 3 independent experiments ± SEM, their statistical significance were evaluated using Student's t-test, assuming a 2-tailed distribution and unequal variance. **p < 0.01; ***p < 0.001.
Prisma® Skin and mesoglycan enhanced the invasion ability of HaCaT and BJ
To investigate the effects of Prisma® Skin and mesoglycan, as its major reference element, on cell invasiveness ability, we performed functional assays of invasion through trans-wells. The chambers were coated by matrigel for BJ cell line and type IV collagen for HaCaT cells, since it represents the better matrix to support keratinocytes in vitro migration.27
As shown in Fig. 4A, HaCaT showed a great raise of their invasion rate both in presence of mesoglycan 0.3 mg/ml (154,6% more) and better with Prisma® Skin (166,9% more), as further reported in Fig. 4C representing bright field pictures. Similarly, the invasion rate of BJ cells increased in a significant manner with the same trend as HaCaT cells in presence of sodium mesoglycan (178,4% more) and of Prisma® Skin (192,6% more) (Fig. 4B and D). In support of the results obtained by invasion assay, we performed gel zymography to analyze the activity of gelatinolytic enzymes secreted by cells. Therefore, the enhanced degrading activity of BJ cells has been reported in Fig. 4E, where it is shown gel degradation by metalloproteinases 9 and 2 (MMP9 and MMP2) at 90 and 75 kDa, respectively. Based on the absence of MMP9 and MMP2 signal in non-treated cells, the activity of both MMPs was significantly increased in presence of mesoglycan and Prisma® Skin particularly at 24 and 48 hours of treatment to lightly decrease at 72 hours, as further confirmed in the histogram in Fig. 4F. Additionally, we performed zimography assay on HaCaT cells by which we showed a slight activation of MMP2 at 24 and 48 hours of treatments with sodium mesoglycan and Prisma® Skin (see figure S3 A and B).
Figure 4.
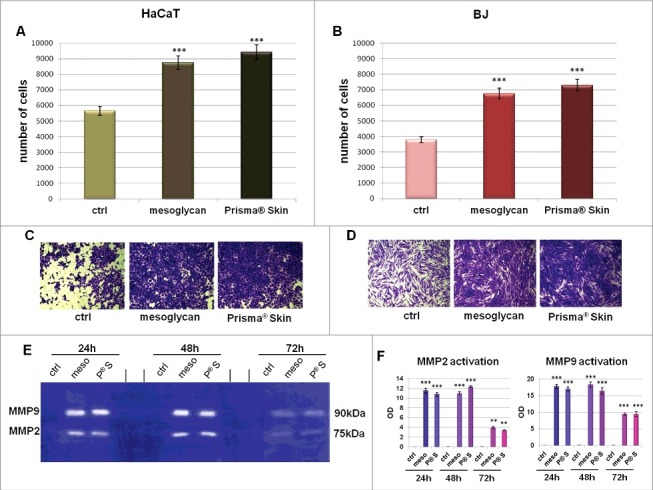
Analysis of invasion speed of HaCaT (A) and BJ (B) cells with mesoglycan and Prisma® Skin. Data represent the mean cell counts of 12 separate fields per well ± SEM of 5 experiments with similar results. *** p < 0.001 vs non treated control. This mean derived from cell counts of 12 separate fields per well ± SEM of each experiments. Representative images of analyzed fields of invasion assay on HaCaT (C) and BJ (D) cells. Magnification 20x. Bar = 150 μm. (E) Gelatin zymography showing increased gelatinolytic activity of MMP-9 and MMP-2 of BJ cells. Zymography was performed using 0.1% gelatin gel as described in Materials and Methods followed by Coomassie blue staining. (F) Densitometry analysis of the intensity of lanes calculated respect the sample volume which was the same for every experimental point. The results represent the mean ± SEM of 4 independent experiments, ** p < 0.01; *** p < 0.001 calculated by Student's t-test, assuming a 2-tailed distribution and unequal variance.
Prisma® Skin and mesoglycan affected the expression of E-cadherin, CD44 and the cytoskeletal organization in human HaCaT keratinocytes
E-cadherin plays a critical role in epithelial cell polarization and morphogenesis and its trafficking is required for normal cell-cell adhesions and junction stability.28 Since E-cadherin can control a switch from an adhesive to a migratory keratinocyte phenotype, we investigated its expression and localization in HaCaT cell line following treatment with sodium mesoglycan 0.3 mg/ml and with Prisma® Skin at the same concentration of mesoglycan. The expression of this protein decreased in a time-dependent manner in either case, if compared with non-treated cells (Fig. 5A representing one of the 3 performed Western blots showing similar results). We confirmed this aspect also by immunofluorescence assay, in Fig. 5B (panels a, b, c). In Fig. 5E it is possible to observe a strong reduction of E-cadherin signal from its membrane localization above all when cells were treated with Prisma® Skin.
Figure 5.
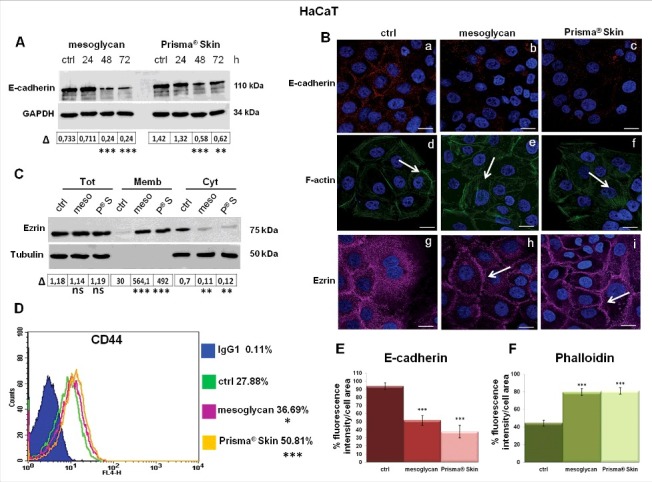
Molecular characterization of cytoskeletal rearrangement on keratinocytes. (A) Western blots showing E-cadherin and GAPDH expression in HaCaT cells treated or not with 0.3 mg/ml of sodium mesoglycan and Prisma® Skin for 24, 48 and 72 h. The blots were exposed and analyzed to Las4000 (GE Healthcare Life Sciences). Quantitative analysis (OD) of E-cadherin expression has been performed on GAPDH levels for each experimental point, by ImageJ (NIH) software. (B) Immunofluorescence analysis to detect E-cadherin (panels a, b, c), F-actin (panels d, e, f) and ezrin (panels g, h, i) treated with mesoglycan and Prisma® Skin for 48 h. Nuclei were stained with DAPI. Magnification 63 × 1.4 NA. Bar = 10 µm. (C) Whole (Tot), membrane (Memb) and cytosol (Cyt) expression of ezrin on HaCaT cells analyzed by Western blot in presence or not of mesoglycan and Prisma® Skin 0.3 mg/ml for 48 h. Cellular compartments were obtained as described in Materials and Methods section. Quantitative analysis of ezrin expression has been performed on tubulin levels for Tot and Cyt extracts and on µg/µl (50γ) of protein sample for Memb ones, **p < 0.01; ***p < 0.001. (D) Cell surface expression of CD44 was analyzed by flow cytometry. The blue areas in the plots are relative to human IgG1; CD44 signals are showed in green for ctrl HaCaT, in purple for HaCaT in presence of mesoglycan and in yellow for cells with Prisma® Skin, at 24, 48 and 72 h. The results are representative of the mean ± SEM of 3 analyzed experiments. Quantification of (E) E-cadherin and (F) phalloidin as percent of fluorescence intensity per cell area from corresponding panels in figure 5B. Statistical analyses for significance of results were performed using Student's t-test, assuming a 2-tailed distribution and unequal variance. *p < 0.05, **p < 0.01, **p < 0.001.
Several experimental evidences reported that the upregulation of Epithelial to Mesenchymal Transition (EMT)-related genes is associated with an increased motility and also keratinocytes, when switched to a mesenchymal phenotype, could invade the surrounding normal tissues.29 For this reason, we investigated also the expression of vimentin, important mesenchymal marker, usually absent in epidermal keratinocytes.30, 31 In our in vitro model, vimentin did not appear either in presence of Prisma® Skin or of mesoglycan (data not shown).
To migrate over the wound site, keratinocytes must disengage cell-cell junctions and reorganize their cytoskeleton increasing the contractility forces, which are essential for motility.29 In this scenario, actin is typically considered a mechanical regulator of cell migration and, particularly, F-actin polymerization is essential for the protrusion of lamellipodia.32 Therefore, we analyzed the HaCaT F-actin organization by phalloidin staining highlighting a cortical arrangement at the border of not-treated cells (Fig. 5B, panel d, white arrow). On the contrary, in case of administration of mesoglycan and especially of Prisma® Skin, F-actin polymerization in stress fibers became evident also in cells which were localized in the middle area of the typical islets characterizing the growth manner of epithelial cells. This result highlights that the tensile forces generated by F-actin stress fibers involved the majority of population of HaCaT cells to facilitate cell contractility (Fig. 5B, panels e and f, white arrows; Fig. 5F).
At the same time, we investigated also the distribution pattern of ezrin, a link protein of cell membrane and cytoskeleton, that can anchor actin in specific membrane, maintain cell polarity, take part in cell shape regulation and cell movement, interact with a variety of cell surface molecules, including CD44.33 When treated for 48 hours with Prisma® Skin, and to a lesser extent with mesoglycan, ezrin translocated to plasma membrane starting from a general cytosol distribution in non treated cells. We highlighted this aspect by immunofluorescence assay and by Western blot on compartmentalized protein extracts (Fig. 5B, panels g, h, I, white arrows; 5C).
Keratinocytes contain the additional exons v3 to v10 inserted into the CD44 standard form transcripts. CD44 play an important role in multiple cellular functions.34,35 In Fig. 5D, the green, the purple and the yellow lines refer to the CD44 expression in non-treated, treated with mesoglycan and with Prisma® Skin HaCaT cells, respectively; the blue area refers to the APC-conjugated human IgG1 used as technical control. In this way we observed an increase of the receptor expression at 48 hours of treatment.
Prisma® Skin and mesoglycan conditioned the stress fibers formation and the activation of human BJ fibroblasts
Fibroblasts represent a cell population capable to migrate, proliferate and carry out several key activities during skin wound healing.36 These cells are known to not express several adhesion molecules such as E-cadherin,28 as we confirmed by immunofluorescence assay (Fig. 6A, panels a, b, c; Fig. 6B). On the other hand, BJ cell line showed high levels of vimentin, which were not affected by either mesoglycan or Prisma® Skin (Fig. 6A, panels d, e, f; 6C). Based on the increased migration and invasion rates, our analyses on BJ cells continued focusing on the cytoskeletal organization. Previous studies have suggested how the actin stress fibers generate force driving cell movement.37 In our experimental model, we found a significant increase of well-organized stress fibers, as seen through staining with phalloidin for immunofluorescence assay (Fig. 6A, panels g, h, i; 6D).
Figure 6.
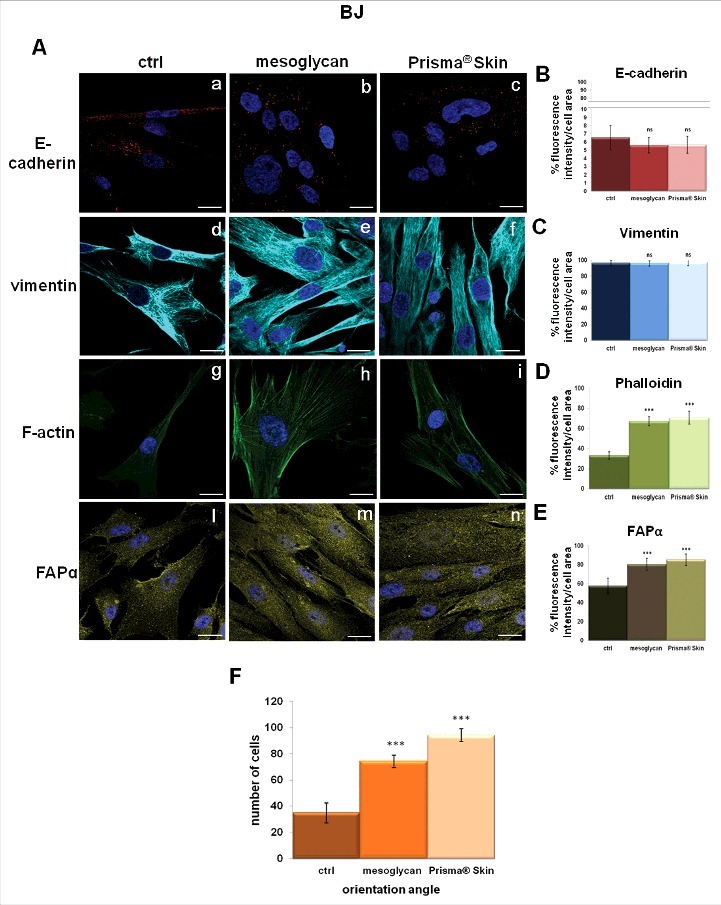
Characterization of cytoskeletal organization and activation of fibroblasts. (A) Immunofluorescence analysis to detect E-cadherin (panels a, b, c), vimentin (panels d, e, f), F-actin (panels g, h, i), FAPα (panels l, m, n) treated with mesoglycan and Prisma® Skin for 48 h. Quantification of (B) E-cadherin (a, b, c), (C) vimentin (d, e, f), (D) phalloidin (g, h, i) and (E) FAPα (l, m, n) as percent of fluorescence intensity per cell area. (F) Cell distribution was determined by calculating the percentage of cells arranged in parallel for each acquired region ( ± 100 of the mode angle). Nuclei were stained with DAPI. Magnification 63 × 1.4 NA. Bar = 10 µm. All the results are representative ± SEM of 3 experiments based on Student's t-test, assuming a 2-tailed distribution and unequal variance. ***p < 0.001, ns p > 0.05.
Another important marker we tested specifically on BJ cell line was represented by Fibroblast Activated Protein α (FAPα), a member of the group II integral serine proteases, whose expression is a common attribute of reactive stromal fibroblasts.38,39 It has been reported that this protein increased in keloid fibroblasts compared with normal skin fibroblasts playing an important role in degrading ECM thereby and facilitating cell invasion.40 BJ cells showed a notable increase of levels of FAPα expression when treated with sodium mesoglycan and mainly with Prisma® Skin (Fig. 6A, panels l, m, n; 6E). Moreover the compounds induced different morphological features compared with non-treated cells: i.e. treated cells appeared elongated into an enhanced spindled shape characterized by a parallel pattern. This precise directionality, with cells orientated within ± 10° of the mode angle, reached quite the 100% of the seeded population, after 48 hours from Prisma® Skin administration (Fig. 6A, panel n; 6F). The revealed percentage were about 70% for cells incubated with sodium mesoglycan at the same experimental time (Fig. 6A, panel m; 6F), with respect to non-treated cells which were disorganized and orientated in a casual manner (Fig. 6A, panel l; 6F).
Discussion
The wound healing in human skin begins immediately after a cutaneous injury and it appears correlated with a general event of tissue growth and regeneration. This dynamic and orchestrated process includes a series of sequential yet overlapping stages with the aim to realize an efficient and functional re-epithelialization.41,42 In this scenario, beyond cells of immune system and endothelium, fibroblasts and keratinocytes appear to be important cellular components.
Particularly, to re-establish the protection of the wounded tissue, keratinocytes proliferate, migrate and differentiate, activities primarily governed by the ECM components through signal transduction via cell membrane receptors.43 On the other hand, the generation of mechanical tension and the concomitant increase of proliferation of dermal fibroblasts represent a key process in the granulation tissue formation.44
In this work, we choose an in vitro system based on the culture of human epidermal keratinocytes and dermal fibroblasts, to provide answers to several open questions about the use of GAGs in the formation of the epidermal tongue. It has been reported that, during several weeks after wound damage, fibroblasts synthesize collagen and GAGs-PGs.45,46 Most of the information about this process, however, are related to the involvement of these macromolecules as angiogenesis modulators.47 Moreover, in regulating ECM organization and metabolism, only HA has been described as a key molecule in each phase of wound healing.48,49
The aim of the present study was to examine the effects of the new biomedical device Prisma® Skin on human BJ fibroblasts and HaCaT keratinocytes, recommended for the treatment of skin lesions and developed by Mediolanum Farmaceutici S.p.a. This device, which appears as a sterile gauze, includes mesoglycan, HA and well-characterized alginates polymers, favoring the skin wound healing.50,51
Once verified the lack of toxicity of Prisma® Skin and its content of mesoglycan, we studied BJ cell cycle since the regulation of cell proliferation represents an important early event in response to injury.52 In our case, GAGs induced an augment of S and G2 phases in cell cycle distribution associated with a modest, but not significant, increase of proliferation. Previous studies showed that 6-O-sulfation of N-acetylglucosamine seems to be one of the key structural features in the HS hexasaccharide, which determines optimal binding of FGF1 and FGF2 to the FGF receptor.53 Moreover, it has been shown that the functional characterization of the whole complex of DS together with its derivative PGs and FGFs check human dermal fibroblast proliferation.54
However, beyond the proliferation process, re-epithelialization of the wound requires keratinocytes and fibroblasts migration. To test the efficacy of Prisma® Skin in this important phase of skin lesions repair, we mimed the wound healing in vitro and observed a strong increase of the migration rate, following the stimulation by both Prisma® Skin and mesoglycan. The stronger effect of the whole biomedical device could be attributed to the additional presence of HA and alginates.
The restoration of normal tissue architecture requires also that cells bind and release the ECM in a dynamic manner to drive migration. Notably, after migrating into the provisional wound matrix, epithelial cells are able to degrade provisional matrix while depositing collagen and other ECM components: cell-matrix and cell-cell adhesions need to be broken, possibly through a MMPs-mediated proteolysis, or disassembled to allow cells to move freely forward.55 This ability, evaluated through our invasion assay, showed a significant increase when both HaCaT and BJ cells were treated with mesoglycan, an increase that was more pronounced in cells treated with Prisma® Skin. This data were also confirmed by the activation of the two metallogelatinase MMP2 and MMP9 which are released mainly by fibroblasts and play an important role in cell migration and re-epithelialization.56
Cell migration is a dynamic interplay between cells and ECM through organization of cytoskeletal actin to form focal adhesion points. Keratinocyte migration is triggered by loss of contact inhibition and cell adhesion structures, which activates signals for reorganization of cytoskeleton driving migration.57 HaCaT cell line showed a significant decrease of E-cadherin levels and a strong F-actin reorganization when treated with mesoglycan and particularly with Prisma® Skin. Moreover, the actin cytoskeleton appeared massed in the cellular cortical zone to become well organized in treated HaCaT cells, which are ready to detach their partners and start the motility process, leading to acquisition of an invasive phenotype.57
Other protein complexes involved in cell growth, survival and differentiation in ECM include CD44 and its associated partners. In vitro interactions of CD44 with HA, as well as other GAGs, collagen, laminin and fibronectin seem to promote matrix-dependent migration.58 It was shown that CD44 interacts with CS and derived PGs, mediating cell motility, particularly about leukocytes rolling. Moreover, GAG-modified CD44 has been shown to bind components of the ECM, as well as certain chemokines and growth factors, and can promote cell migration.59,60 It has been further reported that treatment of human keratinocytes with IL-1β enhances the association of CD44 with ezrin and, thereby, with the actin cytoskeleton.61 According to previous studies we observed an increase of the CD44 receptor by treatment with mesoglycan, but it appeared stronger when HaCaT cells were treated with Prisma® Skin. Interestingly, C-terminal domain of CD44 is linked to actin filaments through ERM (ezrin-radixin-moesin) demonstrating the essential role of physical or functional interaction of proteins at every level of cellular processes.62 Principally, ezrin helps maintain cell shape and motility, binds to adhesion molecules, participates in the regulation of intracellular signal transduction and, mainly in keratinocytes, serves as a cross linking molecule between the membranes and cytoskeleton.63,64 It was shown that the phosphorylation in threonine residue in the C-terminus of ezrin induces the translocation to plasma membrane and causes conformational changes through unmasking of its binding sites.65,66
Also fibroblasts play their central role in the formation of the granulation tissue through migrating from the nearby dermis to the wound in response to cytokines and growth factors.67 Their movement requires notable morphological changes such as cell protrusions formation like lamellipodia or filopodia, adhesion to the underlying substrate, translocation of the cellular contents, and retraction of the cell at the trailing edge.68 We confirmed in BJ cells the retention of high levels of vimentin, protein of intermediate filaments and involved in tissue repair. Vimentin has been found in mesenchymal repair cells to regulate the collective movement of the epithelium in response to injury.69 Moreover, although F-actin expression is present in basal conditions, BJ cells are deficient of a well-defined cytoskeleton, proving the lack of migratory stimulus. On the contrary, when treated with mesoglycan and Prisma® Skin, BJ appear rich of lamellipodial protrusions where F-actin is well assembled and oriented toward the membrane. It was shown that the bFGF is able to increase the migratory activity of mesenchymal cells through activation of the Akt/protein kinase B (PKB) pathway. As previously discussed, the stabilization of bFGF on the receptor induced by GAGs enhanced its chemo-attractant function mediating the dynamic organization of actin filaments in parallelized pattern.70
Moreover, it might also be speculated that the local use of bioactive film containing GAGs could exert similar effects as syndecans, a small family of HSPGs. They are type I trans-membrane glycoproteins, that possess HS and CS/DS chains which enable direct interactions with many growth factors, cytokines and extracellular matrix (ECM) macromolecules and are involved in cell proliferation, migration and invasion.71
It was reported that FAPα overexpression is typical of pathological conditions such as fibrosis, tumorigenicity, inflammation, and keloid scars formation.72 It might be a key protease involved in promoting ECM degradation, tissue remodeling, and fibrosis and may form transient adhesive bonds with collagen and other ECM components.73 Therefore, this protein is often used as a robust and selective marker of differentiated fibroblasts.74,75 In our in vitro model, FAPα is still expressed by BJ cells, but its expression notable increases in presence of mesoglycan and especially with Prisma® Skin. Probably, in a more complex system, the induction of FAPα expression could drive fibroblasts to the formation of a more solid matrix through which cells can orient themselves to easily migrate, as we found through immunofluorescence assay.
In conclusion, the findings of this study reveal the notable effects of the medical device Prisma® Skin on in vitro systems like human dermal HaCaT keratinocytes and BJ fibroblasts. We found the marked induction of cell migration and invasion rate, supported by profound cytoskeletal rearrangement and fibroblast activation. Finally, our results suggest that the local use of Prisma® Skin, containing mixture of GAGs, might accelerate the healing process in venous skin ulcers principally enhancing re-epithelialization and granulation processes.
Materials and methods
Cell cultures
HaCaT cell line (Human immortalized keratinocytes) was purchased from CLS Cell Lines Service GmbH (Germany) and was maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) following the instructions reported in ref. 76 BJ cell line (Human immortalized fibroblast) was purchased from ATCC (ATCC® CRL2522™) and cultured in Eagle's Minimum Essential Medium (MEM) with 10% FBS, 1% L-glutamine, 1% Sodium Pyruvate, 1% NEAA. All the media were supplemented with antibiotics (10000 U/ml penicillin and 10 mg/ml streptomycin), cells were stained at 37°C in 5% CO2 −95% air humidified atmosphere and were serially passed at 70–80% confluence.
Preparation and seeding of mesoglycan and Prisma® Skin
Powder of sodium mesoglycan is composed by HEP (40% low molecular weight from 6500 to 10500 Da and 60% less than 12000 Da, sulfation degree 2.2–2.6), HS (UFH -unfranctioned heparin- from 12000 to 18000 Da up to 40000 Da; sulfation degree 2.6), DS, deriving from epimerization of glucuronic acid of CS (molecular weight 18000–30000 Da, sulfation degree 1.3) with a total sulfation degree of 9.1. It was provided by Mediolanum Farmaceutici S.p.a. (Milan, Italy) and dissolved in cell medium at an initial concentration of 1 mg/ml. Prisma® Skin medical device is composed by sodium alginate, sodium hyaluronan, microcrystalline cellulose, vegetable proteins, sorbitol, glycerin, polysorbate, polyvinylpyrrolidone, sodium mesoglycan on an inert polyethylene terephthalate (PET) support material. To properly administrate Prisma® Skin to the cells for the experimental execution, sheets were cut with a sterile scalpel in a laminar flow safety hood and dissolved in cell medium. The inert component was discarded. Based on the total content of mesoglycan in each sheet, its concentration was obtained by putting the appropriate film amount in the medium to gain an initial concentration of 1 mg/ml of sodium mesoglycan.
MTT assay
After treatment with mesoglycan and Prisma® Skin at 0.1; 0.3; 0.5 mg/ml HaCaT and BJ cells were harvested at the indicated times (24, 48 and 72 hours) and cell viability was calculated as described previously.77 The optical density (OD) of each well was measured with a spectrophotometer (Titertek Multiskan MCC/340) equipped with a 620 nm filter.
Apoptosis detection
The effect of sodium mesoglycan and Prisma® Skin on cell death was checked by propidium iodide (PI) (Sigma-Aldrich) staining and flow cytometry at 24, 48 or 72 hours from the administration. The percentage of the cells in the hypodiploid nuclei was analyzed and calculated with FACScan cytometer (Becton-Dickinson) by Cell Quest program.
Western blotting
Protein expression was examined by SDS-PAGE, as described previously.78 Briefly, total intracellular proteins were extracted from the cells by freeze/thawing in lysis buffer containing protease inhibitors. Protein content was estimated according to Biorad protein assay (BIO-RAD). Samples (20 µg protein) were loaded onto 10% denaturing-polyacrylamide gel and separated by SDS-PAGE. The separated proteins were then transferred electrophoretically to nitrocellulose membranes (Immobilon-NC, Millipore). Membranes were blocked with 5% non-fat dry milk in TBS-Tween 20 (0.1% v/v) and then incubated overnight at 4°C with primary polyclonal antibody against pro-caspase-3 (rabbit polyclonal; #9662; 1:1000; Cell Signaling) and cleaved caspase-3 (rabbit polyclonal; #9664; 1:1000; Cell Signaling) and with mouse monoclonal α-tubulin (clone DM1A; 1:1000; Sigma-Aldrich), E-cadherin (clone 36; 1:1000, Becton Dickinson Labware), GAPDH (clone G-9; 1:1000 Santa Cruz Biotechnologies), ezrin (clone sc-71082, 1:500, Santa Cruz Biotechnologies) and then at room temperature with an appropriate secondary rabbit or mouse antibody (1:5000; Sigma-Aldrich). Immunoreactive protein bands were detected using enhanced chemioluminescence reagents (ECL; Amersham), the blots were exposed and analyzed to Las4000 (GE Healthcare Life Sciences).
Cell cycle analysis
Cell cycle was analyzed as reported in ref. 79 Briefly, after 24 hours serum starvation, HaCaT and BJ cells were grown in complemented medium for 24, 48 and 72 hours with or without sodium mesoglycan and Prisma® Skin 0.1, 0.3 and 0.5 mg/ml. Cell cycle profiles were evaluated by DNA staining with PI solution using a FACScan cytometer (Becton Dickinson) using Cell Quest evaluation program. The distinct cell cycle phases were determinate using ModFit LT analysis software.
In vitro wound-healing
HaCaT and BJ cells were seeded in a 12-well plastic plate at 5 × 105 and 10 × 105 cells, respectively, per well. After 24 hours incubation, cells reached 100% confluency and a wound was produced at the center of the monolayer by gently scraping the cells with a sterile plastic p10 pipette tip to create a wound area of about 500µm. After removing incubation medium and washing with PBS, cell cultures were incubated in the presence of sodium mesoglycan 0.3 mg/ml, Prisma® Skin 0.3 mg/ml or in growth medium as control. All experimental points were further treated with mitomycin C (10 μg/ml, Sigma Aldrich) to ensure the block of mitosis. The wounded cells were then incubated at 37°C in a humidified and equilibrated (5% v/v CO2) incubation chamber of an Integrated Live Cell Workstation Leica AF-6000 LX. A 10x phase contrast objective was used to record cell movements with a frequency of acquisition of 10 minutes on at least 10 different positions for each experimental condition. The migration rate of individual cells was determined by measuring the wound closure from the initial time to the selected time-points (bar of distance tool, Leica ASF software). For each wound 5 different positions were registered, and for each position 10 different cells were randomly selected to measure the migration distances.
Invasion assay
Cell invasiveness was analyzed as reported in.77 Briefly, trans-well Cell Culture (12 mm diameter, 8.0-fim pore size) purchased form Corning Inc. (USA). The chambers were coated with type IV collagen (Sigma Aldrich) for HaCat cell line, and Matrigel (Becton Dickinson Labware) for BJ cells, that were diluted with 3 volumes of medium serum-free and stored at 37°C until its gelation. Cells were plated in 350 µl of medium serum-free at several 5 × 104/insert in the upper chamber of the trans-well. 1,4 ml of DMEM or MEM with FBS and with or without sodium mesoglycan or Prisma® Skin were put in the lower chamber and the trans-well was left for 24 hours at 37°C in 5% CO2 −95% air humidified atmosphere. After that, the medium was aspirated, the filters were washed twice with PBS 1x and fixed with 4% p-formaldehyde for 10 minutes, then with 100% methanol for 20 minutes. The filters so fixed, were stained with 0,5% crystal violet prepared from stock crystal violet (powder, Merck Chemicals) by distilled water and 20% methanol for 15 minutes. After that, the filters were washed again in PBS 1x and cleaned with a cotton bud. All experimental points were further treated with mitomycin C (10 μg/ml, Sigma Aldrich) to ensure the block of mitosis. The number of cells that had migrated to the lower surface was counted in 12 random fields using EVOS light microscope (10X) (Life technologies Corporation).
Cytosol and membrane extracts
Compartmentalized protein extracts were obtained as reported in ref. 77 Briefly, HaCaT cells were washed twice with PBS, detached with trypsin-EDTA 1x in PBS (Euroclone), harvested in PBS and centrifuged for 5 minutes at 600 × g at 4°C. After that, cells were lysed in 4 ml of buffer A (Tris HCl 20 mM, pH 7, 4; sucrose 250 mM; DTT 1 mM; protease inhibitors, EDTA 1 mM in water), sonicated (5 seconds pulse – 9 seconds pause for 2 minutes, amplitude 42%) and then centrifuged at 4°C for 10 minutes, at 5000 × g. The resulting supernatants were ultra-centrifuged for 1 hour at 100000 × g at 4°C, until to obtain new supernatants corresponding to cytosol extracts. Each resultant pellet was dissolved in 4 ml of buffer A and ultra-centrifuged for 1 hour at 100000 × g at 4°C. The pellets were then resuspended in 250 μl of buffer B (Tris HCl 20 mM, pH 7, 4; DTT 1 mM; EDTA 1 mM; Triton X-100 1%, in water) and left overnight on orbital shaker at 4°C. Next, the solution was centrifuged for 30 minutes at 50000 × g at 4°C: the supernatants represent membrane extracts.
Gelatin gel zymography
Gelatinolytic activity was detected by SDS-PAGE zymography, as reported in ref. 80 Briefly, serum-free supernatant samples were analyzed under non-reducing conditions without boiling, through a 10% SDS-polyacrylamide gel co-polymerized in the presence of gelatin 0,1% (Sigma-Aldrich). After the electrophoresis run, performed at 125 V, the proteins in the gel were renatured in a 2.5% Triton X-100 solution for 1 h. The gel was then incubated with 50 mM Tris–HCl, pH 7.8, 200 mM NaCl, 5 mM CaCl2 and 5 μM ZnCl2 at 37°C for 48 h, which allows substrate degradation. Finally, the gels were stained with 0.5% Coomassie Brillant Blue R-250. Proteolytic bands were visualized by destaining with 10% methanol and 5% acetic acid.
Flow cytometry
HaCaT and BJ cells were harvested at several 1 × 106 and analyzed for CD44 protein Pellets were incubated on ice for 30 minutes in 100 µl of PBS containing APC-conjugated CD44 anti-human antibody (mouse monoclonal, clone G44–26; BD Pharmigen), APC-conjugated human IgG1 (BD Pharmigen) was used as scrambled. The cells were analyzed with Becton Dickinson FACScan flow cytometer using the Cells Quest program.
Confocal microscopy
After the specific time of incubation, HaCaT and BJ cells were fixed in p-formaldehyde (4% v/v with PBS) for 5 minutes, permeabilized in Triton X-100 (0.5% v/v in PBS) for 5 minutes, and then incubated in goat serum (20% v/v PBS) for 30 minutes. Then cells were incubated with anti-E-cadherin antibody (mouse monoclonal; clone 36; 1:500, Becton Dickinson Labware), anti-vimentin (mouse monoclonal, clone E-5; 1:500; Santa Cruz Biotechnologies), anti-ezrin (mouse monoclonal, clone sc-71082, 1:100, Santa Cruz Biotechnologies), anti-FAPα (rabbit polyclonal, clone H-56; 1:250; Santa Cruz Biotechnologies) overnight at 4°C. After 2 washing steps, cells were incubated with anti-rabbit and/or anti-mouse AlexaFluor (488 and/or 555; 1:1000; Molecular Probes) for 2 hours at RT Other cells were incubated with FITC-conjugated anti-F-actin (5 µg/ml; Phalloidin-FITC, Sigma-Aldrich) for 30 minutes at RT in the dark. To detect nucleus, samples were excited with a 458 nm Ar laser. A 488 nm Ar or a 555 nm He-Ne laser was used to detect emission signals from target stains. Samples were vertically scanned from the bottom of the coverslip with a total depth of 5 μm and a 63X (1.40 NA) Plan-Apochromat oil-immersion objective. Images and scale bars were generated with Zeiss ZEN Confocal Software (Carl Zeiss MicroImaging GmbH). For immunofluorescence analysis and quantification, final images were generated using Adobe Photoshop CS4, version 11.0. Quantifications were performed from multichannel images obtained using a 63 × objective using ImageJ, marking either the cell perimeter or the nucleus as the region of interest and calculating integrated densities per area from the appropriate channel. A minimum of 50 cells were analyzed for each data set. The obtained mean value was used to compare experimental groups. For the calculation of BJ angle orientation, cell distributions corresponding to each experimental setting were determined by percentage of cells that were oriented at 100 variation angles from the identified modes.
Statistical analysis
All results are the mean ± SEM (Standard Error of Mean) of at least 3 experiments performed in triplicate. The optical density of the protein bands detected by Western blotting was normalized against tubulin and GAPDH levels. Statistical comparisons between groups were made using 2-tailed t-test comparing 2 variables. Differences were considered significant if p < 0.05, p < 0.01 and p < 0.001.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge Mediolanum Farmaceutici S.p.a for financial support.
References
- [1].Sisu E, Flangea C, Serb A, Zamfir AD. Modern developments in mass spectrometry of chondroitin and dermatan sulfate glycosaminoglycans. Amino Acids 2011; 41:235-56; PMID:20632047; https://doi.org/ 10.1007/s00726-010-0682-4 [DOI] [PubMed] [Google Scholar]
- [2].Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, Simon JC, Hofbauer LC. Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med 2012; 90:625-35; PMID:22187113; https://doi.org/ 10.1007/s00109-011-0843-2 [DOI] [PubMed] [Google Scholar]
- [3].Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Misra S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol 2015; 2015:834893; PMID:26448760; https://doi.org/ 10.1155/2015/834893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olczyk P, Komosinska-Vassev K, Winsz-Szczotka K, Stojko J, Klimek K, Kozma EM. Propolis induces chondroitin/dermatan sulphate and hyaluronic Acid accumulation in the skin of burned wound. Evid Based Complement Alternat Med 2013; 2013:290675; PMID:23533471; https://doi.org/ 10.1155/2013/290675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Martin P. Wound healing-aiming for perfect skin regeneration. Science 1997; 276:75-81; PMID:9082989; https://doi.org/ 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- [6].Kosir MA, Quinn CC, Wang W, Tromp G. Matrix glycosaminoglycans in the growth phase of fibroblasts: more of the story in wound healing. J Surg Res 2000; 92:45-52; PMID:10864481; https://doi.org/ 10.1006/jsre.2000.5840 [DOI] [PubMed] [Google Scholar]
- [7].Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res 2004; 64:4699-702; PMID:15256433; https://doi.org/ 10.1158/0008-5472.CAN-04-0810 [DOI] [PubMed] [Google Scholar]
- [8].Elenius K, Vainio S, Laato M, Salmivirta M, Thesleff I, Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol 1991; 114:585-95; PMID:1860887; https://doi.org/ 10.1083/jcb.114.3.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Radek KA, Taylor KR, Gallo RL. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen 2009; 17:118-26; PMID:19152659; https://doi.org/ 10.1111/j.1524-475X.2008.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem 1998; 273:28116-21; PMID:9774430; https://doi.org/ 10.1074/jbc.273.43.28116 [DOI] [PubMed] [Google Scholar]
- [11].Taylor KR, Rudisill JA, Gallo RL. Structural and sequence motifs in dermatan sulfate for promoting fibroblast growth factor-2 (FGF-2) and FGF-7 activity. J Biol Chem 2005; 280:5300-6; PMID:15563459; https://doi.org/ 10.1074/jbc.M410412200 [DOI] [PubMed] [Google Scholar]
- [12].Plichta JK, Radek KA. Sugar-coating wound repair: a review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J Burn Care Res 2012; 33:299-310; PMID:22561305; https://doi.org/ 10.1097/BCR.0b013e318240540a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clark RA, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol 2004; 122:266-77; PMID:15009704; https://doi.org/ 10.1046/j.0022-202X.2004.22205.x [DOI] [PubMed] [Google Scholar]
- [14].Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin 2007; 25:643-53, x; PMID:17903623; https://doi.org/ 10.1016/j.det.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol 2007; 127:687-97; PMID:17082783; https://doi.org/ 10.1038/sj.jid.5700614 [DOI] [PubMed] [Google Scholar]
- [16].Olczyk P, Ł Mencner, Komosinska-Vassev K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Biomed Res Int 2015; 2015:549417; PMID:26236728; https://doi.org/ 10.1155/2015/549417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arosio E, Ferrari G, Santoro L, Gianese F, Coccheri S. Mesoglycan Venous Insufficiency Group. A placebo-controlled, double-blind study of mesoglycan in the treatment of chronic venous ulcers. Eur J Vasc Endovasc Surg 2001; 22:365-72 [DOI] [PubMed] [Google Scholar]
- [18].Andreozzi GM. Effectiveness of mesoglycan in patients with previous deep venous thrombosis and chronic venous insufficiency. Minerva Cardioangiol 2007; 55:741-53; PMID:18091643 [PubMed] [Google Scholar]
- [19].Maresca L, Foggia C, Leonardo G. Restoring microvascular efficiency with mesoglycan in women affected by moderate chronic venous disease. Minerva Cardioangiol 2015; 63:105-11; PMID:25711836 [PubMed] [Google Scholar]
- [20].Tufano A, Arturo C, Cimino E, Di Minno MN, Di Capua M, Cerbone AM, Di Minno G. Mesoglycan: clinical evidences for use in vascular diseases. Int J Vasc Med 2010; 2010:390643; PMID:21152191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tardieu M, Bourin MC, Desgranges P, Barbier P, Barritault D, Caruelle JP. Mesoglycan and sulodexide act as stabilizers and protectors of fibroblast growth factors (FGFs). Growth Factors 1994; 11:291-300; PMID:7540022; https://doi.org/ 10.3109/08977199409011002 [DOI] [PubMed] [Google Scholar]
- [22].Lotti T, Celasco G, Tsampau D, Teofoli P, Benci M, Dahm M, Comacchi C, Ghersetich I, Matucci-Cerinic M. Mesoglycan treatment restores defective fibrinolytic potential in cutaneous necrotizing venulitis. Int J Dermatol 1993; 32:368-71; PMID:8505166; https://doi.org/ 10.1111/j.1365-4362.1993.tb01477.x [DOI] [PubMed] [Google Scholar]
- [23].Schnabelrauch M, Scharnweber D, Schiller J. Sulfated glycosaminoglycans as promising artificial extracellular matrix components to improve the regeneration of tissues. Curr Med Chem 2013; 20:2501-23; PMID:23521682; https://doi.org/ 10.2174/0929867311320200001 [DOI] [PubMed] [Google Scholar]
- [24].Scharnweber D, Hübner L, Rother S, Hempel U, Anderegg U, Samsonov SA, Pisabarro MT, Hofbauer L, Schnabelrauch M, Franz S, et al.. Glycosaminoglycan derivatives: promising candidates for the design of functional biomaterials. J Mater Sci Mater Med 2015; 26:232; PMID:26358319; https://doi.org/ 10.1007/s10856-015-5563-7 [DOI] [PubMed] [Google Scholar]
- [25]. WO 2014/013413, PCT/IB2013/055807 patent publication registered by Mediolanum Farmaceutici S.p.a. [Google Scholar]
- [26].van der Smissen A, Hintze V, Scharnweber D, Moeller S, Schnabelrauch M, Majok A, Simon JC, Anderegg U. Growth promoting substrates for human dermal fibroblasts provided by artificial extracellular matrices composed of collagen I and sulfated glycosaminoglycans. Biomaterials 2011; 32:8938-46; PMID:21875749; https://doi.org/ 10.1016/j.biomaterials.2011.08.025 [DOI] [PubMed] [Google Scholar]
- [27].O'Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol 2001; 26:525-30; PMID:11678882; https://doi.org/ 10.1046/j.1365-2230.2001.00891.x [DOI] [PubMed] [Google Scholar]
- [28].Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006; 20:3199-214; PMID:17158740; https://doi.org/ 10.1101/gad.1486806 [DOI] [PubMed] [Google Scholar]
- [29].Yan L, Cao R, Liu Y, Wang L, Pan B, Lv X, Jiao H, Zhuang Q, Sun X, Xiao R. MiR-21-5p Links Epithelial-Mesenchymal Transition Phenotype with Stem-Like Cell Signatures via AKT Signaling in Keloid Keratinocytes. Sci Rep 2016; 6:28281; PMID:27596120; https://doi.org/ 10.1038/srep28281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheng F, Shen Y, Mohanasundaram P, Lindström M, Ivaska J, Ny T, Eriksson JE. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci U S A 2016; 113:E4320-7; PMID:27466403; https://doi.org/ 10.1073/pnas.1519197113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol 2011; 194:669-78; PMID:21893596; https://doi.org/ 10.1083/jcb.201008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science 2004; 305:1782-6; PMID:15375270; https://doi.org/ 10.1126/science.1100533 [DOI] [PubMed] [Google Scholar]
- [33].Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol 2003; 46:165-86; PMID:12711360; https://doi.org/ 10.1016/S1040-8428(02)00172-5 [DOI] [PubMed] [Google Scholar]
- [34].Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem 2002; 277:4589-92; PMID:11717317; https://doi.org/ 10.1074/jbc.R100038200 [DOI] [PubMed] [Google Scholar]
- [35].Bourguignon LY. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol 2014; 184:1912-9; PMID:24819962; https://doi.org/ 10.1016/j.ajpath.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 2013; 22:407-8, 410-12; PMID:23924840; https://doi.org/ 10.12968/jowc.2013.22.8.407 [DOI] [PubMed] [Google Scholar]
- [37].Burnette DT, Shao L, Ott C, Pasapera AM, Fischer RS, Baird MA, Der Loughian C, Delanoe-Ayari H, Paszek MJ, Davidson MW, et al.. A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J Cell Biol 2014; 205:83-96; PMID:24711500; https://doi.org/ 10.1083/jcb.201311104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O'Brien P, O'Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta 2008; 1784:1130-45; PMID:18262497; https://doi.org/ 10.1016/j.bbapap.2008.01.006 [DOI] [PubMed] [Google Scholar]
- [39].Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ, Rettig WJ. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A 1994; 91:5657-61; PMID:7911242; https://doi.org/ 10.1073/pnas.91.12.5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dienus K, Bayat A, Gilmore BF, Seifert O. Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res 2010; 302:725-31; PMID:20872224; https://doi.org/ 10.1007/s00403-010-1084-x [DOI] [PubMed] [Google Scholar]
- [41].Strodtbeck F. Physiology of wound healing. Newborn Infant Nursing Rev 2001; 1:43-52; https://doi.org/ 10.1053/nbin.2001.23176 [DOI] [Google Scholar]
- [42].Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 2012; 49:35-43; PMID:22797712; https://doi.org/ 10.1159/000339613 [DOI] [PubMed] [Google Scholar]
- [43].Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 2013; 62:1-21; PMID:23490414; https://doi.org/ 10.1016/j.cyto.2013.02.015 [DOI] [PubMed] [Google Scholar]
- [44].Cáceres M, Oyarzun A, Smith PC. Defective Wound-healing in Aging Gingival Tissue. J Dent Res 2014; 93:691-7; PMID:24776985; https://doi.org/ 10.1177/0022034514533126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Laterra J, Ansbacher R, Culp LA. Glycosaminoglycans that bind cold-insoluble globulin in cell-substratum adhesion sites of murine fibroblasts. Proc Natl Acad Sci U S A 1980; 77:6662-6; PMID:6256752; https://doi.org/ 10.1073/pnas.77.11.6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li JP, Kusche-Gullberg M. Heparan Sulfate: Biosynthesis, Structure, and Function. Int Rev Cell Mol Biol 2016; 325:215-73; PMID:27241222 [DOI] [PubMed] [Google Scholar]
- [47].Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006; 20:9-22; PMID:16394262; https://doi.org/ 10.1096/fj.05-4682rev [DOI] [PubMed] [Google Scholar]
- [48].Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan's role in fibrosis: A pathogenic factor or a passive player? Biomed Res Int 2015; 2015:790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Myers SR, Partha VN, Soranzo C, Price RD, Navsaria HA. Hyalomatrix: a temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng 2007; 13:2733-41; PMID:17880270; https://doi.org/ 10.1089/ten.2007.0109 [DOI] [PubMed] [Google Scholar]
- [50].Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl 2014; 48:651-62; PMID:25579968; https://doi.org/ 10.1016/j.msec.2014.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang T, Gu Q, Zhao J, Mei J, Shao M, Pan Y, Zhang J, Wu H, Zhang Z, Liu F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int J Clin Exp Pathol 2015; 8:6636-45; PMID:26261545 [PMC free article] [PubMed] [Google Scholar]
- [52].Das S, Baker AB. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng Biotechnol 2016; 4:82; PMID:27843895; https://doi.org/ 10.3389/fbioe.2016.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 2000; 407:1029-34; PMID:11069186; https://doi.org/ 10.1038/35039551 [DOI] [PubMed] [Google Scholar]
- [54].Denholm EM, Cauchon E, Poulin C, Silver PJ. Inhibition of human dermal fibroblast proliferation by removal of dermatan sulfate. Eur J Pharmacol 2000; 400:145-53; PMID:10988328; https://doi.org/ 10.1016/S0014-2999(00)00381-2 [DOI] [PubMed] [Google Scholar]
- [55].Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol 2015; 44–46:113-21; PMID:25770908; https://doi.org/ 10.1016/j.matbio.2015.03.002 [DOI] [PubMed] [Google Scholar]
- [56].Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol 2001; 3:E117-23; PMID:11331897; https://doi.org/ 10.1038/35074643 [DOI] [PubMed] [Google Scholar]
- [57].Knirsh R, Ben-Dror I, Spangler B, Matthews GD, Kuphal S, Bosserhoff AK, Vardimon L. Loss of E-cadherin-mediated cell-cell contacts activates a novel mechanism for up-regulation of the proto-oncogene c-Jun. Mol Biol Cell 2009; 20:2121-9; PMID:19193763; https://doi.org/ 10.1091/mbc.E08-12-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4:33-45; PMID:12511867; https://doi.org/ 10.1038/nrm1004 [DOI] [PubMed] [Google Scholar]
- [59].Faassen AE, Schrager JA, Klein DJ, Oegema TR, Couchman JR, McCarthy JB. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol 1992; 116:521-31; PMID:1730766; https://doi.org/ 10.1083/jcb.116.2.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Delcommenne M, Kannagi R, Johnson P. TNF-alpha increases the carbohydrate sulfation of CD44: induction of 6-sulfo N-acetyl lactosamine on N- and O-linked glycans. Glycobiology 2002; 12:613-22; PMID:12244074; https://doi.org/ 10.1093/glycob/cwf080 [DOI] [PubMed] [Google Scholar]
- [61].Jokela T, Oikari S, Takabe P, Rilla K, Kärnä R, Tammi M, Tammi R. Interleukin-1β-induced reduction of CD44 Ser-325 phosphorylation in human epidermal keratinocytes promotes CD44 homomeric complexes, binding to Ezrin, and Extended, monocyte-adhesive hyaluronan coats. J Biol Chem 2015; 290:12379-93; PMID:25809479; https://doi.org/ 10.1074/jbc.M114.620864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pokharel D, Padula MP, Lu JF, Jaiswal R, Djordjevic SP, Bebawy M. The role of CD44 and ERM proteins in expression and functionality of P-glycoprotein in breast cancer cells. Molecules 2016; 21:290; PMID:26938523; https://doi.org/ 10.3390/molecules21030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 2004; 6:855-64; PMID:15177033; https://doi.org/ 10.1016/j.devcel.2004.05.007 [DOI] [PubMed] [Google Scholar]
- [64].Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol 2004; 5:272-9; PMID:14758359; https://doi.org/ 10.1038/ni1039 [DOI] [PubMed] [Google Scholar]
- [65].Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 1998; 140:647-57; PMID:9456324; https://doi.org/ 10.1083/jcb.140.3.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu B, Li J, Huang D, Wang W, Chen Y, Liao Y, Tang X, Xie H, Tang F. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through Ezrin in A431 cells. BMC Cancer 2011; 11:527; PMID:22204275; https://doi.org/ 10.1186/1471-2407-11-527 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [67].Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17:153-62; PMID:19320882; https://doi.org/ 10.1111/j.1524-475X.2009.00466.x [DOI] [PubMed] [Google Scholar]
- [68].Tschumperlin DJ. Fibroblasts and the ground they walk on. Physiology (Bethesda) 2013; 28:380-90; PMID:24186933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cheng F, Shen Y, Mohanasundaram P, Lindström M, Ivaska J, Ny T, Eriksson JE. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci U S A 2016; 113:E4320-7; PMID:27466403; https://doi.org/ 10.1073/pnas.1519197113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schmidt A, Ladage D, Schinköthe T, Klausmann U, Ulrichs C, Klinz FJ, Brixius K, Arnhold S, Desai B, Mehlhorn U, et al.. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells 2006; 24:1750-8; PMID:16822883; https://doi.org/ 10.1634/stemcells.2005-0191 [DOI] [PubMed] [Google Scholar]
- [71].Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem 2014; 83:129-57; PMID:24606135; https://doi.org/ 10.1146/annurev-biochem-060713-035314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kelly T, Huang Y, Simms AE, Mazur A. Fibroblast activation protein-α: a key modulator of the microenvironment in multiple pathologies. Int Rev Cell Mol Biol 2012; 297:83-116; PMID:22608558 [DOI] [PubMed] [Google Scholar]
- [73].Ghersi G, Dong H, Goldstein LA, Yeh Y, Hakkinen L, Larjava HS, Chen WT. Regulation of fibroblast migration on collagenous matrix by a cell surface peptidase complex. J Biol Chem 2002; 277:29231-41; PMID:12023964; https://doi.org/ 10.1074/jbc.M202770200 [DOI] [PubMed] [Google Scholar]
- [74].Jacob M, Chang L, Puré E. Fibroblast activation protein in remodeling tissues. Curr Mol Med 2012; 12:1220-43; PMID:22834826; https://doi.org/ 10.2174/156652412803833607 [DOI] [PubMed] [Google Scholar]
- [75].Chung H, Multhaupt HA, Oh ES, Couchman JR. Minireview: Syndecans and their crucial roles during tissue regeneration. FEBS Lett 2016; 2590:2408-17; https://doi.org/ 10.1002/1873-3468.12280 [DOI] [PubMed] [Google Scholar]
- [76].Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106:761-71; PMID:2450098; https://doi.org/ 10.1083/jcb.106.3.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bizzarro V, Belvedere R, Milone MR, Pucci B, Lombardi R, Bruzzese F, Popolo A, Parente L, Budillon A, Petrella A. Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget 2015; 6:25076-92; PMID:26312765; https://doi.org/ 10.18632/oncotarget.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Belvedere R, Bizzarro V, Popolo A, Dal Piaz F, Vasaturo M, Picardi P, Parente L, Petrella A. Role of intracellular and extracellular annexin A1 in migration and invasion of human pancreatic carcinoma cells. BMC Cancer 2014; 14:961; PMID:25510623; https://doi.org/ 10.1186/1471-2407-14-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Belvedere R, Bizzarro V, Forte G, Dal Piaz F, Parente L, Petrella A. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of formyl peptide receptor pathway. Sci Rep 2016; 6:29660; PMID:27412958; https://doi.org/ 10.1038/srep29660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pagliara V, Adornetto A, Mammì M, Masullo M, Sarnataro D, Pietropaolo C, Arcone R. Protease Nexin-1 affects the migration and invasion of C6 glioma cells through the regulation of urokinase plasminogen activator and matrix metalloproteinase-9/2. Biochim Biophys Acta 2014; 1843:2631-44; PMID:25072751; https://doi.org/ 10.1016/j.bbamcr.2014.07.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


