Abstract
Increasing evidence suggests that regular moderate exercise increases neurogenesis in the dentate gyrus (DG) of the hippocampus and improves memory functions in both humans and animals. The DG is known to play a role in pattern separation, which is the ability to discriminate among similar experiences, a fundamental component of episodic memory. While long-term voluntary exercise improves pattern separation, there is little evidence of alterations in DG function after an acute exercise session. Our previous studies showing acute moderate exercise-enhanced DG activation in rats, and acute moderate exercise-enhanced prefrontal activation and executive function in humans, led us to postulate that acute moderate exercise may also activate the hippocampus, including more specifically the DG, thus improving pattern separation. We thus investigated the effects of a 10-min moderate exercise (50% V̇O2peak) session, the recommended intensity for health promotion, on mnemonic discrimination (a behavioral index of pattern separation) in young adults. An acute bout of moderate exercise improved mnemonic discrimination performance in high similarity lures. These results support our hypothesis that acute moderate exercise improves DG-mediated pattern separation in humans, proposing a useful human acute-exercise model for analyzing the neuronal substrate underlying acute and regular exercise-enhanced episodic memory based on the hippocampus.
Keywords: pattern separation, aerobic exercise, memory, hippocampus, dentate gyrus
Increasing evidence suggests that physical exercise of moderate intensity (moderate exercise) has beneficial effects not only on physical fitness, but also on brain function, especially memory function in the hippocampus. Indeed, a number of animal studies have revealed that regular exercise has a positive impact on neural plasticity, particularly in the dentate gyrus (DG), a subfield of the hippocampus, and improves learning and memory (van Praag et al., 1999, 2005; Lee et al., 2013). Similar effects have been observed in human studies. Erickson et al. (2011) showed increased hippocampal volume and improved spatial memory in older adults after 1 year of a moderate exercise intervention. Additionally, Pereira et al. (2007) showed increased DG cerebral blood volume, an indirect marker for neurogenesis, in young adults after 12 weeks of moderate exercise training.
In hippocampal memory formation, the DG plays an important role in pattern separation, which is the ability to discriminate among similar experiences (Yassa and Stark, 2011). Despite the notable effects of exercise on the DG, the impacts of aerobic exercise on the hippocampal memory system, including pattern separation, are not well understood. One study showed that long-term voluntary running in rodents enhances spatial pattern separation (measured via touchscreen) and that this enhancement was mediated at least in part by newborn granule cells (Creer et al., 2010). In humans, only one study has examined the relationship between aerobic fitness and pattern separation, and that study indicated that change in task performance is positively correlated with increased aerobic fitness in young adults (Déry et al., 2013).
While past work has focused on the impact of chronic interventions on DG pattern separation, thereby potentially reflecting the role of neurogenesis, acute effects of exercise have been less studied. Past work in our laboratory has indicated that neural activation increases during moderate exercise in the rodent DG (Soya et al., 2007b). In addition, a number of studies, including our current neuroimaging work, has shown that acute exercise modulates prefrontal activation and improves executive function, which could be due to the exercise-induced activation of the reticular-activating system (RAS), responsible for regulating ascending projections including to the hippocampus (Yanagisawa et al., 2010; Hyodo et al., 2012; Byun et al., 2014). These results led us to postulate that acute moderate exercise also activates the hippocampus and improves pattern separation.
In this study, we tested the effect of acute moderate exercise on pattern separation. Moderate intensity is the recommended intensity as an exercise prescription for general health, defined as 40–59% of the peak oxygen uptake (V̇O2peak) by the American College of Sports Medicine (ACSM) (Pescatello, 2014). We chose this level of intervention due to prior results documenting beneficial effects of chronic and acute moderate exercise on the hippocampus and its memory function (Pereira et al., 2007; Coles and Tomporowski, 2008; Erickson et al., 2011; Labban and Etnier, 2011). To assess pattern separation, we used a mnemonic discrimination task (Stark et al., 2013) that consists of an encoding study phase and a retrieval test phase. During the study phase, participants are shown pictures of everyday objects and asked to indicate for each picture if it is an indoor or an outdoor item. This is followed by a recognition memory test where participants are asked to identify each item as either “old” (targets, i.e., previously seen items), “similar” (lures that are similar but not identical to previously viewed images), or “new” (foils, i.e., new items not previously seen). To assess discrimination performance, we collapsed trials in which participants made “similar” responses for lures minus “similar” responses given for foils (to correct for response biases). This “lure discrimination index” (LDI) is strongly tied to age-related changes in input to the DG and the CA3 (Yassa et al., 2011b) and functional signals in the DG/CA3 (Yassa et al., 2011b), as well as other tests of hippocampal function such as word-list delayed recall (Yassa et al., 2011b). Thus, the test and its corresponding lure discrimination measure are appropriate for assessing hippocampal pattern separation. Here we test the hypothesis that a single bout of moderate exercise improves discrimination performance.
Twenty-one healthy young adults (mean age 20.5 ± 1.4 years, 10 females) participated in this study. No participants reported a history of neurological or psychiatric disorders, or had a disease requiring medical care. All participants had normal or corrected-to-normal vision and normal color vision. Written consent was obtained from all participants. Ethical clearance for the study was obtained from the Institutional Ethics Committee of the University of Tsukuba. The study conformed to the ethical requirements of the latest version of the Helsinki Declaration. Participants’ demographic and physiological characteristics are presented in Table 1.
TABLE 1.
Participant Demographic and Physiological Characteristics (mean ± SD)
| Measure | All | Male | Female |
|---|---|---|---|
| Sample size | 21 | 11 | 10 |
| Age (yr) | 20.5 (1.40) | 20.2 (1.25) | 20.8 (1.55) |
| Height (cm) | 164.8 (7.63) | 169.9 (4.99) | 158.5 (5.22) |
| Weight (kg) | 57.7 (6.28) | 60.5 (5.86) | 54.7 (5.48) |
| BMI (kg m−2) | 21.5 (1.94) | 21.0 (2.05) | 22.2 (1.66) |
| Graded exercise test | |||
| V̇O2peak (mL kg−1 min−1) | 41.0 (7.37) | 46.3 (5.42) | 35.2 (3.96) |
| HRpeak (bpm) | 180.0 (8.71) | 179.0 (9.79) | 181.1 (7.72) |
| RPEpeak | 19.4 (0.93) | 19.5 (0.52) | 19.3 (1.25) |
| WRpeak (W) | 211.0 (49.94) | 248.6 (34.79) | 169.6 (23.70) |
| Exercise session | |||
| HR (bpm) | 130.0 (7.17) | 131.4 (6.68) | 128.4 (7.72) |
| RPE | 12.7 (1.59) | 13.1 (1.51) | 12.3 (1.64) |
| WR (W) | 91.9 (26.15) | 112.1 (16.56) | 69.8 (13.20) |
Note: peak oxygen uptake; HR: heart rate; RPE: rating of perceived exertion; WR: work rate.
Prior to the experiments, the participants performed a cardiorespiratory fitness assessment test to estimate the appropriate individual exercise load corresponding to moderate intensity (50% V̇O2peak) using a recumbent ergometer (Strength-ergo 240, Mitsubishi Electric Corporation, Japan). After warming up for 3 min at 30 W, the work rate increased by 20 W (females: 15 W) per minute in a constant and continuous manner to exhaustion. The pedaling rate was kept at 60 rpm. Exhaled gas was analyzed using a gas analyzer (Aeromonitor AE280S, Minato Medical Science, Japan). Heart rate (HR) and rating of perceived exertion (RPE) were recorded every minute. V̇O2peak was determined when at least two of the following criteria were satisfied: (1) the respiratory exchange ratio (R) exceeded 1.05, (2) achievement of 90% of age-predicted peak HR (220—age), and (3) an RPE of 19 or 20.
At least 48 h after the fitness assessment test, all participants underwent randomized control (CTL) and exercise (EX) conditions on separate days (Fig. 1A). All experimental conditions were conducted at the same time of day for each participant. Two experimental conditions were separated by a minimum of 48 h. Participants were also asked to refrain from exercise and the consumption of alcohol and caffeine for at least 24 h prior to the experiment so as to control for outside factors that could affect cognitive function.
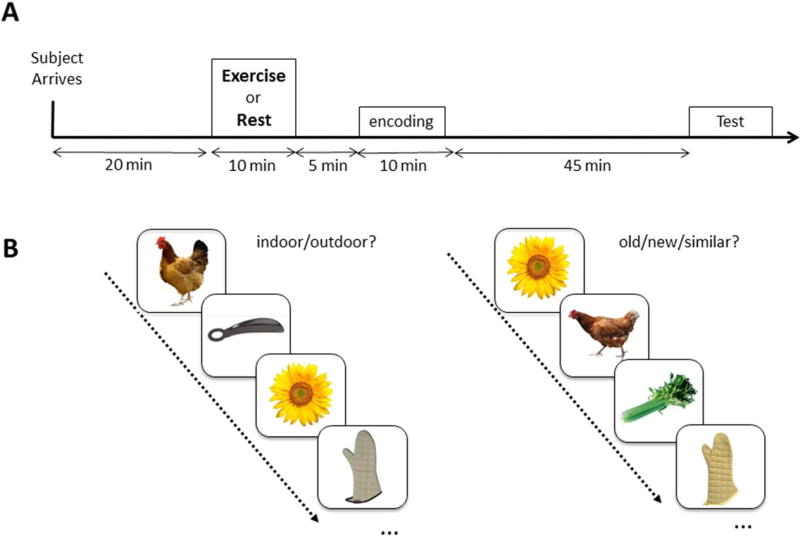
FIGURE 1.
(A) Outline of the experimental procedures. At 15 min after arrival, participants performed 10 min of exercise or resting control on different experimental days. After that, the encoding phase of the task was administered. Then participants waited ~45 min before performing the retrieval phase. (B) Mnemonic discrimination task. The encoding phase was an incidental indoor-outdoor judgment task. Recognition was tested using an old-similar-new judgment task using targets, foils and similar lures that hippocampal pattern separation is particularly sensitive to. [Color figure can be viewed at wileyonlinelibrary.com]
An outline of the experimental procedures is shown in Figure 1A. Twenty minutes after arrival, the participants performed 10 minutes of moderate exercise on the recumbent cycle ergometer, with an individualized load corresponding to 50% of a participant’s V̇O2peak in the EX condition. In prior work, we determined that a single 10-min bout of exercise enhances prefrontal activation and executive function in young and older adults (Yanagisawa et al., 2010; Hyodo et al., 2012; Byun et al., 2014), thus we opted to keep the same parameters for this experiment. HR (Polar RS800CX, Polar, Finland) and RPE (Borg, 1982) were monitored every minute during exercise. In the CTL condition, participants sat on the recumbent cycle ergometer for 10 min instead of performing exercise. Approximately 5 min after exercise or rest, the participants began the encoding phase of the discrimination task. After the encoding phase, the participants rested for 45 min while they watched a movie (low arousal stimulus) without sound to avoid sleeping. After rest, the participants performed the retrieval phase of the mnemonic discrimination task.
The task used in this study consisted of an encoding and a retrieval phase (Fig. 1B). In the encoding phase, the participants saw a series of 196 color photographs of everyday objects on a white background on a computer screen and were required to judge whether the presented picture was an indoor or outdoor object. In the retrieval phase, the participants saw a series of 256 items identified each item as “old,” “similar,” or “new” by pressing buttons. One-fourth of the stimuli in the retrieval phase were “old” or exact repetitions of stimuli presented in the encoding phase (64 targets); half of the stimuli were “similar” to those seen during the encoding phase, but not identical (128 lures); and one-fourth of the stimuli were “new” stimuli not previously seen (64 foils). In both phases, each picture was presented for 2 s with an inter-stimulus interval (ISI) of 0.5 s. All participants underwent a practice session (4 encoding items; 8 retrieval items) to ascertain their understanding of task instructions and procedures using different stimuli from experimental task sets.
The task measures discrimination performance for varying degrees of similarity of lures. The lure stimuli were sorted into three bins based on the degree of mnemonic similarity to the targets, with high, middle, and low similarity lures (Yassa et al., 2011a). The LDI was calculated as the probability of correctly responding “similar” to similar lure objects minus the probability of incorrectly responding “similar” to novel foil objects (p (“similar”|lure)—p (“similar”|new)) for each similarity bin. Subtraction was used to correct for any bias in responding “similar” overall.
First, we compared response proportions for each stimulus and response type in CTL and EX conditions using the paired t test. Next, we ran a repeated measures two-way ANOVA on LDI with condition (CTL, EX) × similarity (high, middle, low) followed by Bonferroni’s post hoc test. Descriptive data are presented as mean ± SE. Statistical significance was set a priori at P < 0.05 after multiple comparison correction. Statistical analyses were performed using SPSS Statistical Packages version 19 (SPSS, USA).
Mean HR at the end of the exercise was 129.3 ± 2.5 (beats per minute) and mean RPE at the end of the exercise was 12.9 ± 0.3 (points), both of which were within the range of moderate-intensity exercise according to the guidelines of the ACSM (Pescatello, 2014).
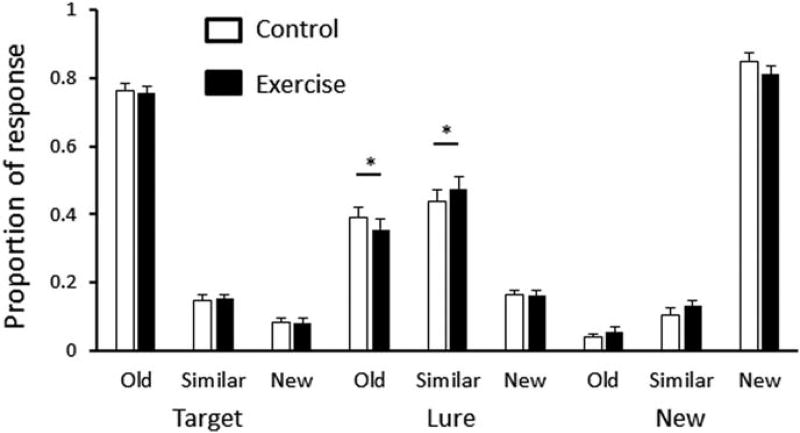
Response proportions for each stimulus and response type of are presented in Figure 2. The occurrence of lures identified as “old” was significantly lower in the EX condition (t (20) = 2.75, P = 0.012), while the occurrence of lures identified as “similar” was significantly higher in the EX condition (t (20) = 2.72, P = 0.013). There was no difference between the EX and CTL conditions for the occurrence of lures identified as “new” (t (20) = 0.53, P = 0.80). There were no significant response changes between target and new stimuli.
FIGURE 2.
Response proportions for each stimulus and response type of mnemonic discrimination task in CTL and EX conditions. The occurrence of lures identified as similar is higher in the EX condition. There is a clear trade-off between lures identified as similar and lure identified as old. Values are mean ±SE, *P < 0.05.
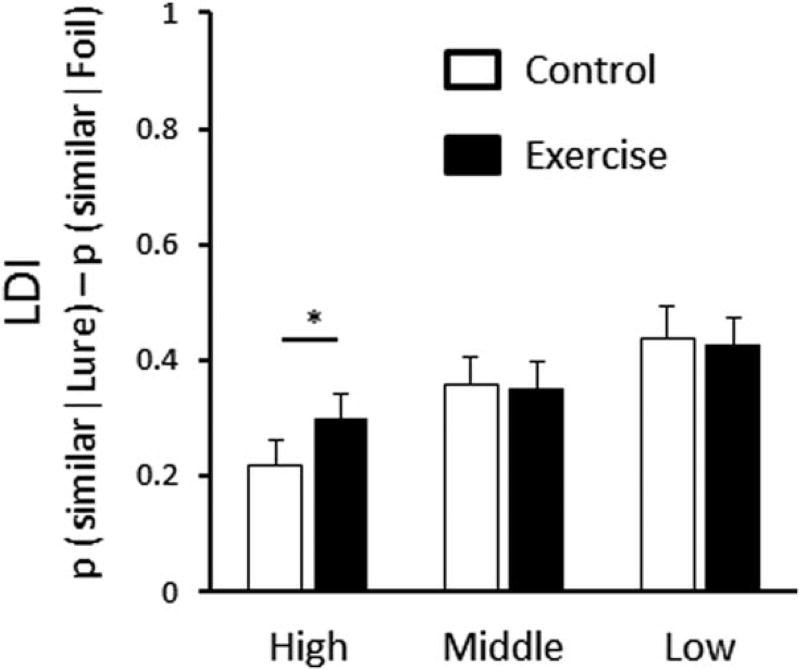
In the analysis of LDI, the repeated measures two-way ANOVA for condition and similarity exhibited no main effect of exercise (F (1, 20) = 1.07, P = 0.31), a significant main effect of similarity (F (2, 40) = 67.29, P < 0.001), and a significant interaction (F (2, 40) = 4.25, P = 0.021). A series of post hoc comparisons via Bonferroni’s test revealed that LDI in the EX condition was significantly higher for high similarity bins (F (1, 20) = 16.39, P = 0.001), and there was no difference for middle (F (1, 20) = 0.06, P = 0.82) and low similarity bins (F (1, 20) = 0.11, P = 0.743) (Fig. 3).
FIGURE 3.
Discrimination performance assessed by lure discrimination index (LDI). Lures were split into high, middle, and low mnemonic similarity bins. Moderate exercise improved LDI for high-similarity bins compare to the CTL condition. Values are mean ±SE, *P < 0.05.
The aim of the present study was to clarify whether mnemonic discrimination performance, an index of pattern separation, would improve after an acute bout of moderate intensity aerobic exercise. We found that the LDI for high-similarity lures was higher after 10 min of moderate aerobic exercise compared to resting control. This is the first study to suggest that a bout of acute aerobic exercise could improve pattern separation (thought to rely on the DG) in humans. Combined with our previous studies (Yanagisawa et al., 2010; Hyodo et al., 2012), these results suggest that acute moderate exercise may have beneficial effects on the hippocampus and the prefrontal cortex (PFC), both of which are jointly involved in memory and executive functions and play important roles in cognitive functions.
Performance overall on the discrimination task was comparable to prior studies (Stark et al., 2013; Borota et al., 2014). There is a clear trade-off between lures identified as “similar” and lures identified as “old,” that is participants correctly identified more lures as “similar” than as “old”. Looking at LDI for each similarity bin, participants correctly identified more items from the highly similar bin, which is to say that they were better able to discriminate between highly similar objects, after the EX condition compared to after the resting CTL condition. These data suggest that moderate aerobic exercise improves pattern separation. Several past studies have found acute effects of aerobic exercise on episodic memory performance using delayed recall tasks (Coles and Tomporowski, 2008; Labban and Etnier, 2011; Salas et al., 2011), vocabulary learning tasks (Winter et al., 2007), and face-name association (Griffin et al., 2011). Pattern separation is thought to be important for these memory tasks; however, examining pattern separation through a task designed to selectively assess its contributions has not been systematically accomplished in prior work. Thus, the current study fills an important gap in the literature and provides clues as to one potential mechanism for the memory enhancement previously shown with exercise interventions.
A similar transient modulation of pattern separation was previously shown in a study in which emotional arousal was elicited by viewing fearful stimuli, facilitating subsequent lure discrimination performance (Segal et al., 2012), and this was mediated by noradrenergic modulation. Thus, transient changes in discrimination performance may be attributable to neurotransmitter and/or neurotrophic factor concentration enhancement. Pattern separation is likely regulated by several neuromodulatory systems, including cholinergic (ACh) input from the medial septum (MS) (Hasselmo et al., 1995; Hasselmo and Giocomo, 2006), and exercise, even a mild intensity of which increases hippocampal ACh concentration (Nakajima et al., 2003). In addition to increased neurotransmitters, exercise-induced release of neurotrophic factors such as brainderived neurotrophic factor (BDNF) can also play a role (Soya et al., 2007b).
There are several limitations to this study. First, although the present study clearly shows that an acute bout of moderate exercise improves discrimination performance for high similarity lures, the neural substrates of this effect are unclear. While the DG/CA3 is thought to be important in discriminating highly similar experiences (Lacy et al., 2011), other brain regions including the prefrontal cortex also likely play a role in discrimination performance (Pidgeon and Morcom, 2016). Because the hippocampus cannot directly communicate with motor output regions, its output must be filtered and processed by the PFC. Thus, the involvement of PFC regions and, possibly, the positive impact of exercise on their processing are likely to have an influence on task performance. This would be consistent with prior reports from our research group showing that a similar exercise intervention can improve PFC processing and associated executive function (measured by the Stroop task; Byun et al., 2014). However, since the improvement in executive function would not be expected to vary with similarity, and the improvement observed here was selective to the high similarity lures (which require highly demanding pattern separation), we suggest that the key mechanism that mediates this effect is related to DG/CA3 processing. To explore this, BOLD signal changes in the DG/CA3 as well as the PFC regions should be examined using high-resolution fMRI after acute moderate exercise.
Second, we set a 45-min interval between the encoding and retrieval phases because moderate-exercise-induced neuroendocrine changes return to baseline levels within ~60 min (Hodgetts et al., 1991). Thus, the retrieval task was not affected by these changes. However, we cannot isolate the effect of exercise from that on retrieval phase as the exercise intervention took place before the encoding phase. Future studies should utilize (1) a post-study intervention design (e.g., Borota et al., 2014) to test the effect of exercise specifically on consolidation and (2) a preretrieval intervention design to test the effect of exercise specifically on enhancing retrieval processes. Both of these designs would likely require a 24-h delay between encoding and testing.
Third, the level of exercise intensity that maximally improves lure discrimination performance is unclear. This study examined only the effects of moderate exercise, which is around the lactate threshold (LT), the work rate at which the steady state of blood lactate accumulation breaks down. At this stage, hormonal and sympathetic nervous systems, including stress response, are activated to meet the increased physiological demands (Soya et al., 2007a). It is also important to note that the work rate used during the exercise session was tailored to each participant’s cardiorespiratory fitness (measured via V̇O2peak), thus exercise intensity for each participant was relatively equivalent. Dose ranging by examining mild (below LT) and high (above LT) intensity exercise interventions at the individual level is a critical avenue for future research and could aid in the development of exercise prescriptions that maximize cognitive enhancement.
In conclusion, the present study reveals that a single bout of moderate exercise improves pattern separation in young adults. While a number of animal studies have revealed the chronic effects of exercise on the DG and its function, the current study provides the first empirical evidence for the acute effect of exercise on hippocampal DG function in humans. Although future research is required to explore the underlying mechanism of this effect, the present research may shed light on how exercise impacts memory functions associated with the hippocampal network.
Acknowledgments
Grant sponsor: Education and Research of the Ministry of Education, Culture, Sports, Science and Technology (MEXT); Grant number: 1111501004 (to H.S.); Grant sponsor: the Japan Society for Promotion of Science (JSPS); Grant number: HFH27016 and 23240091 (to H.S); Grant sponsor: US National Institutes of Health; Grant number: R01 MH102392 and R21 AG049220, P50 AG16573 (to M.A.Y.).
References
- Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, Toscano JP, Yassa MA. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun K, Hyodo K, Suwabe K, Ochi G, Sakairi Y, Kato M, Dan I, Soya H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage. 2014;98:336–345. doi: 10.1016/j.neuroimage.2014.04.067. [DOI] [PubMed] [Google Scholar]
- Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. J Sport Sci. 2008;26:333–344. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déry N, Pilgrim M, Gibala M, Gillen J, Martin Wojtowicz J, MacQueen G, Becker S. Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30:133–135. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Hodgetts V, Coppack SW, Frayn KN, Hockaday TD. Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J Appl Physiol. 1991;71:445–451. doi: 10.1152/jappl.1991.71.2.445. [DOI] [PubMed] [Google Scholar]
- Hyodo K, Dan I, Suwabe K, Kyutoku Y, Yamada Y, Akahori M, Byun K, Kato M, Soya H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging. 2012;33:2621–2632. doi: 10.1016/j.neurobiolaging.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Labban JD, Etnier JL. Effects of acute exercise on long-term memory. Res Q Exerc Sport. 2011;82:712–721. doi: 10.1080/02701367.2011.10599808. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Inoue K, Okamoto M, Liu YF, Matsui T, Yook JS, Soya H. Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load-free running. Neurosci Lett. 2013;537:6–10. doi: 10.1016/j.neulet.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Uchida S, Suzuki A, Hotta H, Aikawa Y. The effect of walking on regional blood flow and acetylcholine in the hippocampus in conscious rats. Auton Neurosci Basic Clin. 2003;103:83–92. doi: 10.1016/s1566-0702(02)00263-1. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS. ACSM’s Guidelines for Exercise Testing and Prescription. Wolters Kluwer; The Netherlands: 2014. [DOI] [PubMed] [Google Scholar]
- Pidgeon LM, Morcom AM. Cortical pattern separation and item-specific memory encoding. Neuropsychologia. 2016;85:256–271. doi: 10.1016/j.neuropsychologia.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Salas CR, Minakata K, Kelemen WL. Walking before study enhances free recall but not judgement-of-learning magnitude. J Cogn Psychol. 2011;23:507–513. [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiol Learn Mem. 2012;97:465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya H, Mukai A, Deocaris CC, Ohiwa N, Chang H, Nishijima T, Fujikawa T, Togashi K, Saito T. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: Establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007a;58:341–348. doi: 10.1016/j.neures.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007b;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]