Abstract
The gene encoding the precursor to stinging nettle (Urtica dioica L.) isolectin I was introduced into tobacco (Nicotiana tabacum). In transgenic plants this precursor was processed to mature-sized lectin. The mature isolectin is deposited intracellularly, most likely in the vacuoles. A gene construct lacking the C-terminal 25 amino acids was also introduced in tobacco to study the role of the C terminus in subcellular trafficking. In tobacco plants that expressed this construct, the mutant precursor was correctly processed and the mature isolectin was targeted to the intercellular space. These results indicate the presence of a C-terminal signal for intracellular retention of stinging nettle lectin and most likely for sorting of the lectin to the vacuoles. In addition, correct processing of this lectin did not depend on vacuolar deposition. Isolectin I purified from tobacco displayed identical biological activities as isolectin I isolated from stinging nettle. In vitro antifungal assays on germinated spores of the fungi Botrytis cinerea, Trichoderma viride, and Colletotrichum lindemuthianum revealed that growth inhibition by stinging nettle isolectin I occurs at a specific phase of fungal growth and is temporal, suggesting that the fungi had an adaptation mechanism.
UDA is a single-chain peptide found in roots and rhizomes of stinging nettle (Urtica dioica L.) (Peumans et al., 1984; Van Damme and Peumans, 1987). In most ecotypes UDA is present as a mixture of isolectins with similar chitin-binding and agglutination activities (Van Damme and Peumans, 1987; Van Damme et al., 1988). Recently, one acidic and five basic UDA isolectins were identified in the Weerselo ecotype of the stinging nettle (Does et al., 1999). In in vitro assays, UDA showed antifungal activity toward various plant pathogenic fungi containing chitin in their cell walls (Broekaert et al., 1989).
UDA consists of two Cys-rich chitin-binding domains (Beintema and Peumans, 1992). These domains are homologous to hevein, a small chitin-binding peptide of 43 amino acids from the lutoid bodies of rubber tree latex (Archer, 1960; Walujono et al., 1975). Mature UDA is processed from a precursor that comprises an N-terminal signal peptide, the two chitin-binding or hevein domains, a small hinge region, and a C-terminal chitinase domain (Lerner and Raikhel, 1992; Does et al., 1999). Genes encoding precursors to UDA isolectins contain two introns in the chitinase-encoding region located at the same positions as those in class I, II, IV, and VI chitinase genes (Does et al., 1999). Because of their homology with other plant chitinases and the presence of two hevein domains, UDA precursors are classified as class V or Chia5 chitinases (Neuhaus et al., 1996).
Many hevein domain-containing proteins are localized in vacuoles. The precursors to these proteins are synthesized on the rough ER and translocated into its lumen (Blobel, 1980; Gomord and Faye, 1996). For targeting to the vacuoles, soluble proteins that pass the Golgi apparatus require additional information (Dorel et al., 1989). Such vacuole-sorting determinants reside within the C-terminal or N-terminal propeptides of the precursor proteins or within the mature protein sequence (Chrispeels and Raikhel, 1992; Neuhaus, 1996). In precursors to vacuolar hevein domain-containing proteins, these targeting sequences have been shown to be located on the C terminus. Mature vacuolar tobacco (Nicotiana tabacum) class I chitinase contains one hevein domain fused to the catalytic domain (Mauch and Staehelin, 1989; Shinshi et al., 1990).
The targeting determinant of prochitinase is contained within a C-terminal propeptide of seven amino acids and has been shown to be necessary and sufficient for vacuolar sorting (Neuhaus et al., 1991). Without this C-terminal extension the protein is secreted (Neuhaus et al., 1991; Melchers et al., 1993). The vacuolar lectins wheat germ agglutinin, rice lectin, and barley lectin are each composed of four hevein-like domains (Mishkind et al., 1983; for review, see Raikhel and Lerner, 1991) and are all processed from precursors that contain a small C-terminal propeptide (Stinissen et al., 1984; Mansfield et al., 1988; Lerner and Raikhel, 1989; Wilkins and Raikhel, 1989). Like the chitinase propeptide, the C-terminal propeptide of the barley prolectin is required for correct vacuolar deposition of the mature lectin and is able to redirect an extracellular cucumber chitinase to the vacuoles (Bednarek et al., 1990; Bednarek and Raikhel, 1991; Neuhaus et al., 1991).
Hevein is localized in vacuole-derived lutoid bodies and, similarly to UDA, is processed from a precursor with a large C-terminal domain (Archer, 1960; Lee et al., 1991). In contrast to the UDA precursor, this C-terminal domain does not show homology to chitinases but rather to tobacco PR4-type (pathogenesis-related) proteins (Broekaert et al., 1990; Linthorst et al., 1991). A putative vacuole-targeting signal has been shown to be cleaved off from the C terminus of the PR4-like domain of the hevein precursor (Soedjanaatmadja et al., 1995). Other known C-terminal propeptides containing essential vacuole-sorting determinants are present in precursors to tobacco class I β-1,3-glucanase (Shinshi et al., 1988; Sticher et al., 1992; Melchers et al., 1993), vacuolar PR5 proteins (Singh et al., 1989; Melchers et al., 1993), Brazil nut 2S albumin (Harris et al., 1993; Saalbach et al., 1996), and kiwifruit proactinidin (Paul et al., 1995).
In the present study we show that the precursor to UDA is processed to mature-sized isolectin in transgenic tobacco and that this isolectin is localized intracellularly, most likely in the vacuoles. Removal of the C-terminal 25 amino acids of the precursor results in the secretion of UDA. Extracellularly targeted UDA is correctly processed and displays similar agglutination, chitin-binding, and antifungal activities as the same isolectin from stinging nettle. The growth-inhibiting effect of UDA on B. cinerea and T. viride appears to be temporal, and adaptation of both fungi coincides with a phase of accelerated hyphal growth. C. lindemuthianum did not adapt to UDA within 2 d after addition and showed a severely stunted and inhibited growth.
MATERIALS AND METHODS
Cloning Procedures
A genomic sequence encoding the precursor to stinging nettle (Urtica dioica L.) UDA isolectin I was isolated previously (Does et al., 1999). The sequence was cloned into the vector pMOG181 (clone 3N11; Does et al., 1999), which contains an expression cassette consisting of the cauliflower mosaic virus 35S promoter with a double enhancer followed by a unique BamHI site and the nopaline synthase transcription terminator on an EcoRI-HindIII fragment. The expression unit containing the wild-type UDA-precursor construct was cloned into the binary vector pMOG402 (Jongedijk et al., 1995) and is referred to as the wild-type construct.
We used site-directed mutagenesis by PCR to introduce a stop codon in the targeting mutant construct. PCR was performed on 0.1 ng of clone 3N11 plasmid DNA, as described previously (Does et al., 1999), using the 5′-terminal primer UD1 (Does et al., 1999) and the mutagenesis primer UD14 (5′-ACTTCAAGTCATGTCAATAGCTCAC-3′). The PCR fragment obtained was cloned, together with the ScaI-HindIII fragment from clone 3N11, into pMOG181, thereby restoring the ScaI restriction site. To exclude a PCR amplification misreading by the polymerase, PCR sequences were exchanged with clone 3N11 sequences or sequenced (Sanger et al., 1977). The presence of the introduced stop codon was verified by sequencing. The expression unit containing the targeting mutant construct was cloned into pMOG402.
Both binary constructs were transferred from Escherichia coli DH5α into Agrobacterium tumefaciens strain MOG101 (Hood et al., 1993) by triparental mating (Pen et al., 1992). Standard recombinant DNA procedures were performed as described previously (Sambrook et al., 1989).
Tobacco Transformation and Leaf Extraction
Transgenic tobacco (Nicotiana tabacum L. cv Samsun NN) plants were obtained by the standard leaf disc transformation method using kanamycin selection (100 mg L−1) (Horsch et al., 1985). Leaf discs were prepared from the top leaves of axenically grown tobacco plants.
Leaf samples were ground in 0.1 n HCl to obtain the total extract. Extracellular washing fluid was isolated using 0.1 n HCl according to a procedure described previously (De Wit and Spikman, 1982). After the extracellular washing fluid was isolated, the remnant extract (the remains of the leaves) was obtained by grinding in 0.1 n HCl. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Sigma-Aldrich).
Protoplast and Vacuole Isolation
Protoplasts and vacuoles were purified from mature tobacco leaves as described previously (Dombrowski et al., 1994). The purity of protoplast and vacuole preparations was determined by light microscopy, because vacuoles could be visualized using neutral red dye (Dombrowski et al., 1994). Proteins in the vacuole preparations that were clear of other cell material were precipitated with acetone and subjected to western analysis.
Western Analysis
After 20% Tricine-SDS-PAGE (Schägger and Von Jagow, 1987), proteins were blotted onto nitrocellulose membranes by the semidry blotting method described previously (Does et al., 1999). For immunological detection of UDA, α-UDA antibodies that had been raised by Eurogentec (Seraing, Belgium) against a synthetic peptide of 15 amino acids (WSGERSDHRCGAAVG), corresponding to amino acids 40 to 54 of UDA isolectin I, were used. Blots were treated as described by Does et al. (1999). A goat-anti-rabbit peroxidase-linked antibody (rabbit IgG-horseradish peroxidase, Zymed Laboratories, South San Francisco, CA) was used for the secondary antibody. Detection was performed using an enhanced chemiluminescence western-blotting detection system (ECL, Amersham Life Science, ‘s-Hertogenbosch, The Netherlands) according to the manufacturer’s protocol. Antibodies that had been raised against the bacteria-produced chitinase domain of the UDA precursor were obtained from Dr. Beatrice Iseli (Botanisches Institut, Basel, Switzerland). The gels were silver stained with a kit (Bio-Rad) according to the manufacturer's protocol.
UDA Isolation from Transgenic Tobacco
The purification of UDA isolectin I from tobacco leaves was based on a procedure described by Peumans et al. (1984). Extracellular washing fluid was isolated using 0.1 n HCl as described above. Total extract was prepared with the following procedure. The main leaf veins were removed from mature leaves. After 1 teaspoon of charcoal was added to the sample, 100 g of leaf material was homogenized in 200 mL of ice-cold extraction buffer (0.1 n HCl and 0.1% [w/v] ascorbic acid) using a blender. The material was squeezed through three layers of gauze, and the extract was kept on ice. Pulp material was again homogenized with 100 mL of extraction buffer and squeezed through three layers of gauze. The extract was centrifuged at 3,200g for 30 min at 4°C. The supernatant was filtered through eight layers of gauze and centrifuged for 60 min at 20,000g at 4°C. The subsequent supernatant was again filtered through eight layers of gauze, and the extract obtained is referred to as the total extract. From this point on, the extracellular washing fluid and the total extract were treated equally.
The extract was brought to pH 3.8 with 2 n NaOH and kept on ice for at least 1 h. Subsequently, the extract was centrifuged at 20,000g for 60 min at 4°C. The resulting supernatant was applied at the rate of 3.5 mL min−1 to a chitin-affinity column (±100 mL) that was prepared as described previously (Peumans et al., 1984) and equilibrated with sodium acetate buffer (50 mm sodium acetate and 0.1 m NaCl, pH 3.8). The column was washed at the rate of 0.8 mL min−1 with 1 L of the same buffer. Bound protein was eluted with 0.5 n acetic acid at the rate of 2 mL min−1. Fractions were tested for the presence of UDA by western analysis. UDA-containing fractions were pooled and brought to pH 3.8 with 2 n NaOH and 0.1 m NaCl was added.
The solution was kept on ice for at least 12 h and then centrifuged at 20,000g for 60 min at 4°C. The volume of the supernatant was reduced to approximately 30 mL through an ultrafiltration membrane (YM1 Diaflo, Amicon, Beverly, MA) at 4°C using a 400-mL stirred cell (Amicon). To this concentrate was added 250 mL of 50 mm sodium acetate buffer. The solution was subjected to cation-exchange chromatography using a column (HiLoad SP Sepharose Fast-Flow column 26/10, Pharmacia) equilibrated with sodium acetate buffer. The column was washed with the same buffer and protein was eluted using a 1000-mL gradient of 0.1 to 0.5 m NaCl in buffer. Fractions containing UDA were pooled, concentrated over an ultrafiltration membrane, dialyzed against PBS, and concentrated to 2.5 mL by ultrafiltration.
The concentrate was applied to a gel-filtration column (Biogel P10, 16/90, Bio-Rad) at the rate of 0.2 mL min−1. The pure UDA peak eluted after 24 h. Fractions were concentrated, dialyzed thoroughly against water, and concentrated by ultrafiltration, and then a final concentration was obtained with a concentrator (Centricon-3, Amicon).
For antifungal assays the UDA solution was filter-sterilized over a 0.22-μm filter. The concentration of the purified UDA was calculated from A280 using the calculated molar-extinction coefficient of UDA isolectin I (ε280 = 29 mm−1 cm−1).
UDA Isolation
UDA isolectin I was further purified from a fraction containing UDA isolectin I and II that was obtained earlier (Does et al., 1999). The fraction was 50-fold diluted with sodium acetate buffer and subjected to cation-exchange chromatography. Isolectins were separated using a 700-mL gradient of 0.1 to 0.3 m NaCl. Only the fractions containing isolectin I were pooled, concentrated, thoroughly dialyzed against water, and concentrated as described for the tobacco isolectin. Uniformity of the isolectin was examined by ESI-MS. For antifungal assays, the UDA isolectin preparation was filter-sterilized. MALDI-MS and ESI-MS were performed as described by Does et al. (1999).
Agglutination Assays
Agglutination by crude leaf extracts was performed on microscope glasses with squashed leaves to which trypsin-treated rabbit erythrocytes (Peumans et al., 1984) and PBS were added. After agglutination had occurred the soluble contents were transferred to a 96-well microtiter dish and photographed.
To test for agglutination activity, different concentrations of purified UDA in PBS were added to 30 μL of trypsin-treated rabbit erythrocytes in small glass test tubes. PBS was added to a final volume of 60 μL. Agglutination was scored after 30 min of incubation at room temperature.
In the agglutination-inhibition assay, 0.3 μg of UDA or 0.5 μg of concanavalin A was added to 30 μL of trypsin-treated rabbit erythrocytes to which 5 μL of a chitin suspension of 1 mg mL−1 was added. PBS was added to a final volume of 60 μL. After agglutination had occurred the mixtures were transferred to a 96-well microtiter dish and photographed.
In Vitro Antifungal Assay
Botrytis cinerea and Trichoderma viride strains were obtained from Zeneca Mogen (Leiden, The Netherlands). The Colletotrichum lindemuthianum UPS9 strain was obtained from Dr. M. Dufresne (Laboratoire de Phytopathologie Moléculaire, Université Paris Sud). B. cinerea and T. viride were kept on potato dextrose agar (Difco Laboratories, Detroit, MI) and cultured in the dark at 20°C and 23°C, respectively. To obtain fresh spores of C. lindemuthianum, 2 × 105 spores were plated onto potato dextrose agar and cultured at 23°C in the dark.
Spores were harvested after 5 weeks (B. cinerea), 2 weeks (T. viride), or 3 weeks (C. lindemuthianum) by flooding the agar plates with sterile water and filtering through three layers of Miracloth (Calbiochem). Spores were stored at −80°C at a concentration of 1.8 × 105 spores mL−1 in 15% glycerol. In germination tests, 99% of the B. cinerea spores, 25% of the T. viride spores, and 50% of the C. lindemuthianum spores appeared to germinate. Before antifungal assays, spores were diluted to a concentration of 1.2 × 104 spores mL−1 (B. cinerea and C. lindemuthianum) or 4.8 × 104 spores mL−1 (T. viride).
Assays were performed in 48-well microtiter dishes as described previously (Woloshuk et al., 1991). Wells were filled with 125 μL of potato dextrose agar, on top of which 25 μL of the spore suspension was layered. For pregermination of the B. cinerea spores, the microtiter dish was kept in the dark on ice in a 4°C cold room for 12 h and then incubated at 23°C for 3.5 to 4 h in the dark. UDA was added when ≥95% of the spores were germinated and the length of the germ tubes was 1 to 4 times the size of the spores. Pregermination of T. viride spores occurred at 23°C in the dark after 16 h. UDA was added when germ tube emergence was observed. Pregermination of C. lindemuthianum spores occurred after 12 to 14 h overnight at 14°C. For further germination, the microtiter dish was kept at 23°C in the dark for another 2.5 h. UDA was added when 10% of the spores showed germ tube emergence.
UDA (2.5, 5.0, and 22.5 μg) and BSA (22.5 μg) were added to the germinated spores as 50-μL solutions in water to obtain the final UDA concentrations of 33, 67, and 300 μg mL−1, respectively, and 300 μg BSA mL−1. Images of fungal growth at 0, 2, 4, 6, 24, 48, and 72 h were acquired with an inverted light microscope (model IMT-2, Olympus) equipped with a cooled CCD (charge-coupled device) camera (model DDE/3200, Astromed, Cambridge, UK). The image-processing package ScilImage (Ten Kate et al., 1990) was used to measure hyphal length. Overall fungal-growth inhibition was estimated by two persons independently.
RESULTS
Processing and Localization of UDA in Tobacco
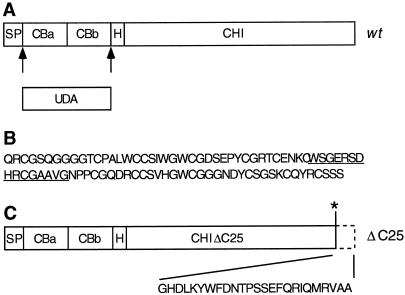
We previously isolated a gene encoding the precursor to UDA isolectin I from the stinging nettle ecotype Weerselo (Does et al., 1999). Mature UDA was processed from the precursor by cleavage between the signal peptide and the first chitin-binding domain and by an unknown processing event located C-terminally of the second chitin-binding domain (Fig. 1A). To study the subcellular localization of mature UDA, the gene was placed behind the cauliflower mosaic virus 35S promoter (the wild-type construct) and transformed into tobacco.
Figure 1.
A, Model for processing of the wild-type (wt) UDA precursor to mature UDA. SP, Signal peptide; CBa and CBb, chitin-binding domains a and b; H, hinge region; CHI, chitinase domain. B, Amino acid sequence of UDA isolectin I from stinging nettle rhizomes. The sequence of the synthetic peptide used for antibody production is underlined. C, The C-terminal 25 amino acids are deleted from the targeting mutant precursor ΔC25 by introduction of a stop codon (*) in the encoding DNA sequence.
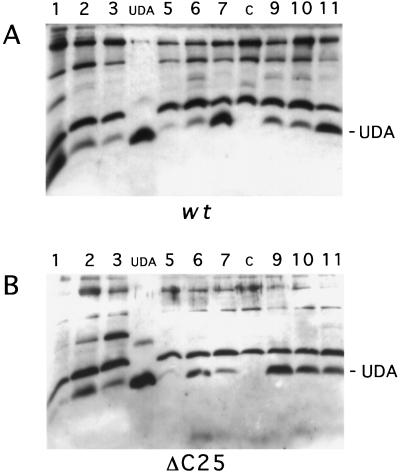
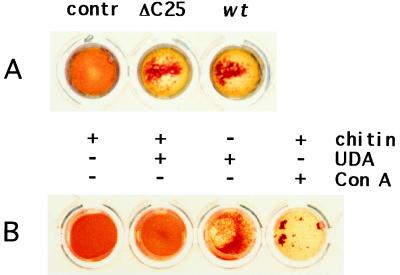
For detection of UDA in transgenic tobacco, α-UDA antibodies were raised against a synthetic peptide corresponding to 15 amino acids of mature isolectin I (Fig. 1B). These antibodies were used to detect UDA migrating at the predicted size in total leaf extracts from different tobacco lines by western analysis (Fig. 2A). Some cross-reactivity with larger proteins was found. In nontransformed control plants, α-UDA antibodies cross-reacted with the larger proteins only, and proteins with a size corresponding to that of UDA were not detected. Crude extracts from several transgenic lines showed agglutination activity on trypsin-treated rabbit erythrocytes (Fig. 3A). Agglutination was not observed in the control extract.
Figure 2.
Detection of mature-sized UDA by western analysis of 2.55 μg of protein from the total extracts of leaves from different tobacco lines expressing the wild-type (wt) (A) or the ΔC25 mutant precursor construct (B) using α-UDA antibodies. Lanes 1 to 3, 5 to 7, and 9 to 11, Transgenic lines; lanes c, control total extract of nontransformed tobacco; and lanes UDA, 50 ng of UDA-isolectin mixture from stinging nettle rhizomes.
Figure 3.
Tobacco-produced UDA agglutinates erythrocytes and binds to chitin. A, Agglutination of trypsin-treated rabbit erythrocytes by crude leaf extracts of tobacco expressing a wild-type (wt) or a ΔC25 mutant precursor construct but not by a crude extract of nontransformed tobacco (contr). B, Agglutination of trypsin-treated rabbit erythrocytes by 0.3 μg of UDA purified from tobacco was inhibited by the addition of chitin; agglutination by 0.5 μg of concanavalin A (Con A) was not inhibited.
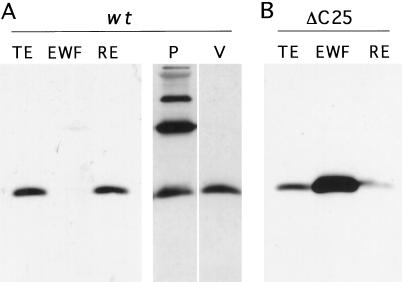
Intracellular localization of UDA was examined by comparison of the total extract, the extracellular washing fluid, and the remnant extract of leaves of different tobacco lines expressing UDA. Equal amounts of proteins from the total extract, the extracellular washing fluid, and the remnant extract were used in SDS-PAGE. The presence of UDA was determined immunologically. The results obtained with a high expressor are shown in Figure 4A. In the extracellular washing fluid of this plant, a very low signal for UDA was detected compared with the total extract and the remnant extract. Similar results were obtained for other plants. These results indicate that plants transformed with the wild-type construct retain most of the produced UDA intracellularly.
Figure 4.
Subcellular localization of UDA in tobacco expressing the wild-type (wt) (A) or the ΔC25 mutant precursor construct (B) by western analysis using α-UDA antibodies. A, Analysis of 9 μg of protein from the total extract (TE), extracellular washing fluid (EWF), and remnant extract (RE): 1.8 × 103 protoplasts (P) and proteins of 1.8 × 103 vacuoles (V) of a wild-type precursor tobacco line. B, Analysis of 9 μg of protein from the total extract, extracellular washing fluid, and remnant extract of a ΔC25 mutant precursor line.
The subcellular localization of UDA in mature tobacco leaves was further assessed by the isolation of protoplasts and vacuoles. Similar amounts of UDA were detected in an equal number of purified leaf mesophyll protoplasts and vacuoles (Fig. 4A). These results suggest a vacuolar localization of UDA in plants producing the wild-type precursor.
Extracellular Targeting of UDA
To study the presence of a vacuole-targeting determinant at the C terminus of the UDA precursor, targeting mutant construct ΔC25 was transformed into tobacco. This mutant construct, which encodes a precursor lacking the C-terminal 25 amino acids, was created by introduction of a stop codon in the encoding DNA (Fig. 1C). Extracts of tobacco leaves expressing the mutant construct revealed the presence of mature-sized UDA (Fig. 2B) and agglutination activity took place on the rabbit erythrocytes (Fig. 3A). The total extract, extracellular washing fluid, and remnant extract were also examined from a high-expressor ΔC25 line (Fig. 4B). Compared with the total extract, the extracellular washing fluid fraction was enriched in isolectin. In contrast, only a small amount of UDA was detected in the remnant extract. Evidently the removal of the C-terminal 25 amino acids of the UDA precursor caused the secretion of UDA into the intercellular space. The mutant precursor appeared to be processed to the mature UDA size.
Correct Processing of Extracellularly Targeted UDA in Tobacco
Closely related UDA isolectins can be identified accurately by mass determination using MALDI-MS or ESI-MS as described previously (Does et al., 1999). This technique was therefore used to investigate the processing of UDA isolectin I in tobacco. The isolectin was purified from the extracellular washing fluid of leaves of the homozygous high-expressor line ΔC25–33.14. Mass determination by MALDI-MS showed that the molecular mass of the tobacco extracellular washing fluid isolectin was identical to the theoretical mass (Table I). Since the isolation of UDA from extracellular washing fluid was inefficient, the isolectin was purified from a total extract of the ΔC25–33.14 line in subsequent experiments. At low temperature (4°C), purified UDA appeared to precipitate at concentrations higher than 0.7 μg μL−1. We also observed a decrease in the agglutination activity of the resolved purified isolectin after lyophilization. Therefore, unlyophilized UDA was used and stored at concentrations of ≤0.6 μg μL−1 water.
Table I.
Molecular mass determination of UDA isolectin I purified from stinging nettle and transgenic tobacco
| UDA Isolectin I Source | Molecular Mass
|
||
|---|---|---|---|
| Theoreticala | MALDIb | ESIc | |
| D | |||
| Stinging nettle | 9382.5 | NDd | 9382.1 ± 1.3 |
| 9380.7 ± 0.4 | |||
| 9382.3 ± 1.4 | |||
| Tobacco | |||
| Extracellular washing fluid | 9382.5 | 9382.6 | ND |
| Total extract | 9382.5 | ND | 9382.1 ± 0.8 |
| 9382.0 ± 0.7 | |||
Theoretical average mass of UDA isolectin I has been corrected for the N-terminal pyroglutamate (Chapot et al., 1986) and for the presence of disulfide bridges.
MALDI masses were corrected for protonization of the proteins during the experiment (–1 D). MALDI was internally calibrated.
Results of individual experiments.
ND, Not determined.
Purity of the isolectin was examined by western analysis and SDS-PAGE, followed by silver staining (not shown). Mass determination again showed a pure, homogeneous, and correctly processed isolectin (Table I). The same isolectin was purified from stinging nettle and homogeneity was verified using ESI-MS (Table I). The results showed the presence of a single molecule in samples of isolectin I purified from stinging nettle and tobacco (data not shown). The absence of molecules with “ragged ends” indicates that extracellularly targeted UDA was correctly processed at its C terminus in transgenic tobacco. UDA contains 16 Cys residues, which are involved in eight disulfide bridges (Beintema, 1994). Formation of one disulfide bridge causes a 2-D decrease in mass because of the loss of one hydrogen atom for each Cys residue involved. The determined mass differed from the theoretical mass by only 0.1 D (Table I), which suggests that all of the disulfide bridges were present in the tobacco-derived UDA.
Agglutination and Chitin-Binding Activity of Purified UDA
Agglutination activity of the purified tobacco isolectin was similar to that of the genuine isolectin I. Trypsin-treated rabbit erythrocytes agglutinated best with isolectin concentrations of 5 μg mL−1 or more. Little agglutination was observed at a concentration of 2.5 μg mL−1. The UDA purified from tobacco was still bound to chitin, since the addition of chitin inhibited agglutination (Fig. 3B). Chitin did not directly inhibit the ability of the erythrocytes to agglutinate, since agglutination by the nonchitin-binding concanavalin A was still visible in the presence of chitin (Fig. 3B).
Antifungal Activity of UDA Isolectin I
UDA displays antifungal activity on several plant pathogenic fungi that contain chitin in their cell walls (Broekaert et al., 1989). We compared the antifungal activity of different amounts of tobacco UDA with the same isolectin from stinging nettle in assays on germinated spores of B. cinerea, T. viride, and C. lindemuthianum. Growth of the fungi was examined by inverted light microscopy, and images were obtained using a CCD camera at several times after the addition of the isolectin. BSA was used as a control. No differences in growth inhibition were detected between the tobacco-produced and the authentic UDA isolectin I from stinging nettle. The distinct sensitivity of the three fungi to UDA appeared to be time and concentration dependent, as shown in Table II.
Table II.
In vitro antifungal activity of UDA on different plant-pathogenic fungi containing chitin in their cell walls
| UDA Isolectin I | BSA | Time after Addition | Growth Inhibition
|
||
|---|---|---|---|---|---|
| B. cinerea | T. viride | C. lindemuthianum | |||
| μg mL−1 | h | % | |||
| 0 | 0 | 0 | 0 | 0 | |
| 33 | 0 | 2 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | ||
| 6 | 0 | 10–20 | 0 | ||
| 24 | 0 | 0 | 0 | ||
| 48 | NDa | ND | 0 | ||
| 67 | 0 | 2 | 20 | 0 | 0 |
| 4 | 5–10 | 0 | 0 | ||
| 6 | 0 | 20–30 | 0 | ||
| 24 | 0 | 0 | 10–30 | ||
| 48 | ND | ND | 30 | ||
| 300 | 0 | 2 | 50–60 | 0 | 0 |
| 4 | 50 | 10–20 | 0 | ||
| 6 | 30–40 | 40–50 | 0 | ||
| 24 | 0 | 30–40 | 50–60 | ||
| 48 | ND | 0 | 80–90 | ||
| 0 | 300 | 2 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | ||
| 6 | 0 | 0 | 0 | ||
| 24 | 0 | 0 | 20 | ||
| 48 | ND | 0 | 30 | ||
Different amounts of UDA isolectin I purified from the total extract of a ΔC25 tobacco line or from stinging nettle were added to pregerminated spores of B. cinerea, T. viride, and C. lindemuthianum. Fungal growth was monitored by inverted light microscopy. BSA was used as a control. The final concentrations of UDA and BSA in water are shown. Growth inhibition is the percentage of growth compared with the water control (no UDA or BSA added).
ND, Not determined.
Antifungal Activity on B. cinerea
Upon germination, B. cinerea grew fast and reached a length of 8 to 15 times that of the spores within 4 h and 20 to 30 times greater within 6 h. Two hours after the addition of UDA, growth was inhibited approximately 20% by a concentration of 67 μg mL−1. Compared with the control, growth inhibition of 50% to 60% was observed upon the addition of 300 μg mL−1 isolectin. However, inhibition declined after 4 h; after 6 h, some growth inhibition was still detectable only at the highest concentration. No differences from the control could be observed after 24 h. BSA did not visibly affect fungal growth.
Antifungal Activity on T. viride
Hyphae of T. viride grew to 5 to 8 times the size of the spores within 4 h and 20 to 30 times greater within 6 h. Compared with B. cinerea, T. viride appeared to be more sensitive to UDA but had a later response time. After 6 h, a growth inhibition of 10% to 20% and 20% to 30% was observed for 33 and 67 μg mL−1 isolectin, respectively. A concentration of 300 μg mL−1 isolectin inhibited growth within 4 h. In contrast to B. cinerea, growth inhibition increased after 2 h to between 40% and 50%. The effect of 300 μg mL−1 isolectin I was still visible after 24 h, because the surface of the agar was less covered with fungi than that in the control well. BSA did not affect the growth of T. viride.
Antifungal Activity on C. lindemuthianum
The most dramatic effects of UDA were observed for C. lindemuthianum. In contrast to B. cinerea and T. viride, C. lindemuthianum grew slowly and its hyphae reached a length of only 5 to 7 times the size of the spores within 6 h. No growth inhibition at all was detected before this time. By 24 h, 67 and 300 μg mL−1 isolectin had caused inhibited, altered hyphal growth. The addition of 300 μg mL−1 UDA caused severely stunted fungal growth. Compared with the control, a similar number of branches had been created but at much shorter distances from each other. Within 48 h, growth inhibition increased. However, in contrast to the other two fungi, C. lindemuthianum also showed some inhibited growth in the presence of 300 μg mL−1 BSA, with phenotypic differences similar to those shown for 67 μg mL−1 isolectin I. This indicates that the fungus might be sensitive to proteinous agents. However, it is clear that most (approximately 75%) of the observed growth inhibition of C. lindemuthianum was caused by UDA. Because of aerial growth and spore formation, we were not able to detect differences in growth after 72 h.
DISCUSSION
Correct Processing of UDA in Transgenic Tobacco
Heterologous plant systems have been used successfully in the past for protein production and vacuole-targeting studies. Several seed-specific storage proteins from different plant species are correctly processed, assembled, and transported to protein storage vacuoles or deposited invacuole-derived protein bodies in tobacco seed (Greenwood and Chrispeels, 1985; Bäumlein et al., 1987; Sturm et al., 1988; Altenbach et al., 1989; De Clercq et al., 1990; Saalbach et al., 1991; Holwerda et al., 1992). In tobacco leaves, certain heterologous seed-specific proteins have been shown to be targeted to vacuoles but are rapidly degraded or incorrectly processed (Beachy et al., 1986; Saalbach et al., 1996). Barley lectin, patatin, and sporamin and kiwifruit actinidin are specifically produced in roots, tubers, tuberous roots, and fruit, respectively (Racusen and Foote, 1980; Mishkind et al., 1983; Hattori et al., 1985; Preakelt et al., 1988). These proteins are targeted to the vacuoles in tobacco leaves as well and, with the exception of sporamin, processed to their mature sizes (Sonnewald et al., 1989; Matsuoka et al., 1990; Wilkins et al., 1990; Paul et al., 1995). Sporamin is processed to a protein that is three amino acids longer at its N terminus than authentic sporamin (Matsuoka et al., 1990).
We used tobacco plants to study the targeting of UDA, which is normally present in the roots and rhizomes of stinging nettle. Constructs containing the gene encoding the UDA-isolectin I precursor were expressed in tobacco plants under the control of the cauliflower mosaic virus 35S promoter. The results show that the UDA precursor is processed to its mature size. The determined molecular mass of UDA isolectin I isolated from transgenic ΔC25 tobacco leaves matches the theoretical mass and the mass of authentic isolectin I from stinging nettle. Therefore, we conclude that UDA is processed correctly in transgenic tobacco.
It is unknown whether cleavage of the UDA precursor occurs with a single cleavage near the hinge region or in a stepwise fashion, removing parts from the C-terminal end of the precursor. In contrast to mature UDA, the UDA precursor and the C-terminal chitinase domain could not be detected on immunoblots of extracts from tobacco leaves using α-UDA antibodies or antibodies that had been raised against the bacteria-produced chitinase domain of the UDA precursor (not shown). This could be explained by a rapid processing of the UDA precursor and subsequent degradation of the chitinase domain. Processing of the UDA precursor might be similar to processing of the hevein precursor. Soedjanaatmadja and coworkers (1995) showed that the hevein precursor is processed to mature hevein by the removal of a C-terminal propeptide followed by the removal of the 14-kD PR4-like domain. In the lutoid-body fraction of rubber latex, both the hevein precursor and the PR4-like domain have been found in a molar ratio of 1:30 relative to hevein, which indicates rapid degradation of the PR4-like domain (Soedjanaatmadja et al., 1995). However, it is possible that the extraction buffer used for leaf extraction was unsuitable for isolation of the UDA precursor and the chitinase domain.
Vacuolar and Extracellular Targeting of UDA
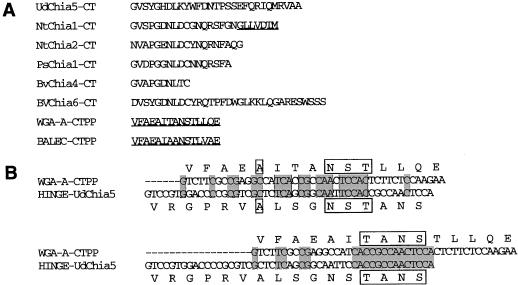
In tobacco transformed with the wild-type construct, UDA is detected intracellularly, most likely in the vacuoles, suggesting a vacuolar localization of this isolectin in stinging nettle roots and rhizomes. Unlike the N-terminal part of the chitinase domains of the UDA precursor and plant chitinases, the sequence of the C-terminal 25 amino acids of the UDA precursor is not similar to the C termini of any known plant chitinase (Fig. 5A). We imagined that this C-terminal region of the UDA precursor might be involved in the vacuolar targeting of UDA. To examine the presence of a vacuole-targeting signal in the C terminus of the UDA precursor, ΔC25, a mutant encoding a precursor lacking the C-terminal 25 amino acids, was constructed.
Figure 5.
Alignments of C-terminal (CT) protein sequences of the UDA precursor (UdChia5) with C-terminal sequences of several (pro)chitinases and prolectins (A), and homology of the hinge region of the UDA-precursor with the C-terminal propeptide (CTPP) of wheat germ agglutinin A (WGA-A) at the DNA level and protein level (B). Two possible alignments are given. Sequences derived from published data are: UdChia5, Does et al. (1999); NtChia1, Shinshi et al. (1990); wheat germ agglutinin A, Smith and Raikhel (1989); barley lectin (BALEC), Lerner and Raikhel (1989). Other sequences were derived from the EMBL nucleotide database under the following accession numbers: NtChia2 from tobacco, M29869; PsChia1 from pea, X63899; and BvChia4 and BVChia6 from beet, L25826 and X79301, respectively. Known propeptide sequences are underlined, homologous nucleotide sequences are shaded, and homologous amino acid sequences are boxed.
In plants transformed with the ΔC25 construct, UDA was present in the extracellular washing fluid. In contrast, in the extracellular washing fluid of tobacco plants producing the wild-type precursor, UDA was not observed. Thus, within the C-terminal segment of 25 amino acids of the UDA precursor, a signal is present that retains UDA intracellularly, presumably in the vacuoles. However, in both protoplasts and vacuoles isolated from leaves of a ΔC25 line, low amounts of UDA were reproducibly detected (not shown). It is possible that these protoplast and vacuole fractions were contaminated with extracellular UDA. Another possibility is that the deletion of the C-terminal sorting signal causes only partial secretion of the isolectin, which would suggest the presence of additional vacuole-targeting determinants within the precursor. A possible candidate harboring such a determinant is the hinge region, which is located C-terminally of the mature UDA sequence.
This hinge region shows similarity to the vacuole-targeting propeptides located C-terminally of mature wheat germ agglutinin (Fig. 5B) and mature barley lectin. The homologous amino acid sequences might cause partial sorting of UDA to the vacuoles. It is possible that the hinge region of the UDA precursor is evolutionarily related to the C-terminal vacuole-targeting propeptides of the Gramineae prolectins. UDA and the four hevein-domain lectins have been hypothesized to be products of duplications of an ancestral single-hevein domain (Wright et al., 1991). Shinshi et al. (1990) postulated that a hevein domain containing chitinases and prohevein-like proteins arose by transposition of the hevein domain in front of an ancestral chitinase sequence. In accordance with these hypotheses, the hinge region might have been introduced by translocation together with UDA into the UDA precursor genes during evolution.
In contrast to N-terminal propeptides, C-terminal propeptides do not share homology, a common motif, or a specific conformation (Dombrowski et al., 1993; Nakamura and Matsuoka, 1993). The exact sequence harboring the C-terminal vacuole-targeting determinant of the UDA precursor and the question of whether this sequence is sufficient for proper vacuolar targeting of UDA remains to be elucidated.
Both vacuolar and extracellular targeted UDA were correctly processed, indicating that the C-terminal vacuole-sorting information on the UDA precursor is not required for correct processing to mature UDA. In plant cells, proteins that are destined for secretion and soluble vacuolar proteins that harbor positive targeting information are transported from the ER to the Golgi apparatus (for review, see Neuhaus and Rogers, 1998). In the Golgi, vacuolar proteins are sorted from the secretory proteins by means of their targeting information. Subsequently, secretory proteins and vacuolar proteins are further transported in specific vesicles (for review, see Neuhaus and Rogers, 1998). In general, processing of vacuolar proteins occurs after their arrival in vacuoles or prevacuolar compartments other than the Golgi or ER (for review, see Müntz, 1998). Both the wild type and the mutant UDA precursor might be processed in the Golgi apparatus after they have been sorted into the different transport vesicles. If so, both the vesicles carrying the secretory UDA and the vesicles carrying the vacuolar UDA should also contain active proteases.
Some vacuolar storage proteins have been reported to be transported with their specific maturation proteases in the same vesicle (for review, see Robinson et al., 1998). However, these proteases appeared to be in an inactive form until they had been deposited in the (pre)vacuoles (for review, see Robinson et al., 1998). Alternatively, the wild-type precursor and mutant precursor may be processed by proteases in the (pre)vacuoles and in extracellular space, respectively. In both cellular compartments the same type of proteases that are involved in the processing of vacuolar storage proteins or the degradation of pathogenesis-related proteins may be present, as has been shown for aspartic proteinases (Rodrigo et al., 1991; Runeberg-Roos et al., 1994; Hiraiwa et al., 1997). It is even possible that processing of the UDA precursors occurs by aspecific proteases, since the tertiary structure of the UDA molecule may protect mature UDA from further degradation. Additional experiments may elucidate the subcellular trafficking route and the processing of the UDA precursor.
Both Antifungal Activity of UDA and Adaptation of the Fungus Depend on the Phase of Fungal Growth
Purified UDA isolectin I from leaves of a ΔC25 tobacco line agglutinated red blood cells as effectively as purified UDA isolectin I from stinging nettle and bound to chitin in an agglutination-inhibition assay. In in vitro antifungal assays, UDA from transgenic tobacco and from stinging nettle displayed identical antifungal activities on germlings of the chitin-containing plant pathogenic fungi B. cinerea, T. viride, and C. lindemuthianum.
Little is known about the mechanism of fungus-growth inhibition by UDA. Unlike plant chitinases, mature UDA does not display hydrolase activity and is not able to lyse the cell walls of fungi that contain chitin (Schlumbaum et al., 1986; Mauch et al., 1988; Broekaert et al., 1989; Sela-Buurlage et al., 1993). It has been suggested that instead, because of its small size, UDA penetrates the cell wall and affects cell wall synthesis (Broekaert et al., 1989; Van Parijs et al., 1992).
We have presented evidence that inhibition of hyphal growth by UDA is only temporal and depends on the phase of accelerated growth. B. cinerea showed fast hyphal growth within the first 2 h after germination, and maximal growth inhibition by UDA had probably occurred within this period, since it declined within the next few hours. Similarly, T. viride showed accelerated growth and maximal growth inhibition by UDA between 4 and 6 h after germination. C. lindemuthianum grew the most slowly. No effect of the addition of UDA was detected in the first few hours, whereas maximal growth inhibition occurred at approximately 48 h. After 24 h and after 48 h, hyphal growth of C. lindemuthianum showed a phenotype of a severely stunted hyphae. Because of the time that is available to penetrate the cell wall and bind to chitin, a slow grower such as C. lindemuthianum is probably more affected by UDA. However, the growth inhibition of C. lindemuthianum is not only because of the biological action of UDA; it also appears to be sensitive to the addition of large amounts of protein; a concentration of 300 μg BSA mL−1 caused phenotypic differences similar to those caused by 67 μg UDA mL−1.
It is not clear why the fungi showed increased sensitivity to UDA only at a specific phase of fast growth. The temporality of the inhibition might be explained by the adaptation of the fungal cell wall. Fungal adaptation to a chitin-binding protein has been demonstrated previously (Sela-Buurlage, 1996). Lysis of Fusarium solani by tobacco chitinase I appears to depend on the phase of germination of the macroconidia. Neither the first added chitinase, which remained active during the assays, nor a second boost of chitinase has been shown to be able to lyse germ tubes if the first amount was added to the macroconidia at an early stage of pregermination. These results indicate that the macroconidia were able to adapt to chitinase (Sela-Buurlage, 1996). It is possible that UDA alters the hyphal cell wall architecture of B. cinerea, T. viride, and C. lindemuthianum, as exemplified in Phycomyces blakesleeanus germlings treated with UDA (Van Parijs et al., 1992). A change in cell wall composition might result in the resistance of fungi to UDA.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Rick Ghauharali for his assistance with microscopic camera work, Jaap Fontijn for taking care of our plants in the greenhouse, and Simon van Mechelen for photographic work. We also thank Zeneca Mogen for providing us with expression vectors, primers, bacterial strains, and B. cinerea and T. viride strains used in this work, Prof. Willy Peumans for the stinging nettle UDA used as a positive control in western analysis of transgenic plants, Dr. Marie Dufresne for the gift of the C. lindemuthianum strain, and Dr. Beatrice Iseli for the chitinase domain antiserum. We are grateful to Dr. Ton Muijsers for helpful discussions concerning MS and to Dr. Maarten Stuiver for critically reading this manuscript.
Abbreviations:
- ESI
electrospray ionization
- MALDI
matrix-assisted laser desorption ionization
- UDA
Urtica dioica (stinging nettle) agglutinin
LITERATURE CITED
- Altenbach SB, Pearson KW, Meeker G, Staraci LC, Sun SSM. Enhancement of the methionine content of seed proteins by the expression of a chimeric gene encoding a methionine-rich protein in transgenic plants. Plant Mol Biol. 1989;13:513–522. doi: 10.1007/BF00027311. [DOI] [PubMed] [Google Scholar]
- Archer BL. The proteins of Hevea brasiliensis latex: isolation and characterization of crystalline hevein. Biochem J. 1960;75:236–240. doi: 10.1042/bj0750236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumlein H, Müller A, Schiemann J, Helbing D, Manteuffel R, Wobus U. A legumin B gene of Vicia faba is expressed in developing seeds of transgenic tobacco. Biol Zentbl. 1987;106:569–575. [Google Scholar]
- Beachy RN, Chen ZL, Bray E, Lawton M, Komeda Y, Nakamura I, Naito S, Anderson E, Dube P, Rogers SG, and others (1986) Expression of subunits of beta-conglycinin in transgenic tobacco and petunia plants. In L Shannon, M Chrispeels, eds, Molecular Biology of Seed Storage Proteins and Lectins. American Society of Plant Physiologists, Rockville, MD, pp 193–201
- Bednarek SY, Raikhel NV. The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell. 1991;3:1195–1206. doi: 10.1105/tpc.3.11.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Wilkins TA, Dombrowski JE, Raikhel NV. A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell. 1990;2:1145–1155. doi: 10.1105/tpc.2.12.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema JJ. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994;350:159–163. doi: 10.1016/0014-5793(94)00753-5. [DOI] [PubMed] [Google Scholar]
- Beintema JJ, Peumans WJ. The primary structure of stinging nettle (Urtica dioica) agglutinin: a two-domain member of the hevein family. FEBS Lett. 1992;299:131–134. doi: 10.1016/0014-5793(92)80231-5. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci USA. 1980;77:1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert W, Lee H-I, Kush A, Chua N-H, Raikhel N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis) Proc Natl Acad Sci USA. 1990;87:7633–7637. doi: 10.1073/pnas.87.19.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert WF, Van Parijs J, Leyns F, Joos H, Peumans WJ. A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science. 1989;245:1100–1102. doi: 10.1126/science.245.4922.1100. [DOI] [PubMed] [Google Scholar]
- Chapot MP, Peumans WJ, Strosberg AD. Extensive homologies between lectins from non-leguminous plants. FEBS Lett. 1986;195:231–234. [Google Scholar]
- Chrispeels MJ, Raikhel NV. Short peptide domains target proteins to plant vacuoles. Cell. 1992;68:613–616. doi: 10.1016/0092-8674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- De Clercq A, Vandewiele M, De Rycke R, Van Damme J, Van Montagu M, Krebbers E, Vandekerckhove J. Expression and processing of an Arabidopsis 2S albumin in transgenic tobacco. Plant Physiol. 1990;92:899–907. doi: 10.1104/pp.92.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit PJGM, Spikman G. Evidence for the occurrence of race and cultivar-specific elicitors of necrosis in intercellular fluids of compatible interactions of Cladosporium fulvum and tomato. Physiol Plant Pathol. 1982;21:1–11. [Google Scholar]
- Does MP, Ng DK, Dekker HL, Peumans WJ, Houterman PM, Van Damme EJM, Cornelissen BJC. Characterization of Urtica dioica agglutinin isolectins and the encoding gene family. Plant Mol Biol. 1999;39:335–347. doi: 10.1023/a:1006134932290. [DOI] [PubMed] [Google Scholar]
- Dombrowski JE, Gomez L, Chrispeels MJ, Raikhel NV. Targeting of proteins to the vacuole. In: Gelvin S, Schilperoort R, editors. Plant Molecular Biology Manual, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–29. [Google Scholar]
- Dombrowski JE, Schroeder MR, Bednarek SY, Raikhel NV. Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell. 1993;5:587–596. doi: 10.1105/tpc.5.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C, Voelker TA, Herman EM, Chrispeels MJ. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol. 1989;108:327–337. doi: 10.1083/jcb.108.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord V, Faye L. Signals and mechanisms involved in intracellular transport of secreted proteins in plants. Plant Physiol Biochem. 1996;34:165–181. [Google Scholar]
- Greenwood JS, Chrispeels MJ. Correct targeting of the bean storage protein phaseolin in the seeds of transformed tobacco. Plant Physiol. 1985;79:65–71. doi: 10.1104/pp.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N, Henderson SJ, Abbot J, Mulcrone J, Davies JT. Seed development and structure. Proc Phytochem Soc Eur. 1993;35:3–21. [Google Scholar]
- Hattori T, Nakagawa T, Maeshima M, Nakamura K, Asahi T. Molecular cloning and nucleotide sequence of cDNA for sporamin, the major soluble protein of sweet potato tuberous roots. Plant Mol Biol. 1985;5:313–320. doi: 10.1007/BF00020629. [DOI] [PubMed] [Google Scholar]
- Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I. An aspartic endopeptidase is involved in the breakdown of propeptides of storage proteins in protein-storage vacuoles of plants. Eur J Biochem. 1997;246:133–141. doi: 10.1111/j.1432-1033.1997.00133.x. [DOI] [PubMed] [Google Scholar]
- Holwerda BC, Padgett HS, Rogers JC. Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell. 1992;4:307–318. doi: 10.1105/tpc.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2:208–218. [Google Scholar]
- Horsch RB, Fry JE, Hofmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jongedijk E, Tigelaar H, Van Roekel JSC, Bres-Vloemans SA, Dekker I, Van den Elzen PJM, Cornelissen BJC, Melchers LS. Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica. 1995;85:173–180. [Google Scholar]
- Lee H-I, Broekaert WF, Raikhel NV. Co- and post-translational processing of the hevein preproprotein of latex of the rubber tree (Hevea brasiliensis) J Biol Chem. 1991;266:15944–15948. [PubMed] [Google Scholar]
- Lerner DR, Raikhel NV. Cloning and characterization of root-specific barley lectin. Plant Physiol. 1989;91:124–129. doi: 10.1104/pp.91.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DR, Raikhel NV. The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. J Biol Chem. 1992;267:11085–11091. [PubMed] [Google Scholar]
- Linthorst HJM, Danhash N, Brederode FT, Van Kan JAL, De Wit PJGM, Bol JF. Microbe Interact. 1991;4:586–592. doi: 10.1094/mpmi-4-586. [DOI] [PubMed] [Google Scholar]
- Mansfield MA, Peumans WJ, Raikhel NV. Wheat-germ agglutinin is synthesized as a glycosylated precursor. Planta. 1988;173:482–489. doi: 10.1007/BF00958961. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Matsumoto S, Hattori T, Machida Y, Nakamura K. Vacuolar targeting and posttranslational processing of the precursor to the sweet potato tuberous root storage protein in heterologous plant cells. J Biol Chem. 1990;265:19750–19757. [PubMed] [Google Scholar]
- Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F, Staehelin LA. Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell. 1989;1:447–457. doi: 10.1105/tpc.1.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers LS, Sela-Buurlage MB, Vloemans SA, Woloshuk CP, Van Roekel JSC, Pen J, Van den Elzen PJM, Cornelissen BJC. Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and β-1,3-glucanase in transgenic plants. Plant Mol Biol. 1993;21:583–593. doi: 10.1007/BF00014542. [DOI] [PubMed] [Google Scholar]
- Mishkind ML, Palevitz BA, Raikhel NV, Keegstra K. Localization of wheat germ agglutinin-like lectins in various species of the Gramineae. Science. 1983;220:1290–1292. doi: 10.1126/science.220.4603.1290. [DOI] [PubMed] [Google Scholar]
- Müntz K. Deposition of storage proteins. Plant Mol Biol. 1998;38:77–99. [PubMed] [Google Scholar]
- Nakamura K, Matsuoka K. Protein targeting to the vacuole in plant cells. Plant Physiol. 1993;101:1–5. doi: 10.1104/pp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J-M. Protein targeting to the plant vacuole. Plant Physiol Biochem. 1996;34:217–221. [Google Scholar]
- Neuhaus J-M, Fritig B, Linthorst HJM, Meins F, Mikkelsen JD, Ryals J. A revised nomenclature for chitinase genes. Plant Mol Biol Rep. 1996;14:102–104. [Google Scholar]
- Neuhaus J-M, Rogers JC. Sorting of proteins to vacuoles in plant cells. Plant Mol Biol. 1998;38:127–144. [PubMed] [Google Scholar]
- Neuhaus J-M, Sticher L, Meins F, Boller T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA. 1991;88:10362–10366. doi: 10.1073/pnas.88.22.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W, Amiss J, Try R, Praekelt U, Scott R, Smith H. Correct processing of the kiwifruit protease actinidin in transgenic tobacco requires the presence of the C-terminal propeptide. Plant Physiol. 1995;108:261–268. doi: 10.1104/pp.108.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pen J, Molendijk L, Quax WJ, Sijmons PC, Van Ooyen AJJ, Van den Elzen PJM, Rietveld K, Hoekema A. Production of active Bacillus licheniformis alpha-amylase in tobacco and its application in starch liquefaction. Bio/Technology. 1992;10:292–296. doi: 10.1038/nbt0392-292. [DOI] [PubMed] [Google Scholar]
- Peumans WJ, De Ley M, Broekaert WF. An unusual lectin from stinging nettle (Urtica dioica) rhizomes. FEBS Lett. 1984;177:99–103. [Google Scholar]
- Praekelt UM, McKee A, Smith H. Molecular analysis of actinidin, the cysteine protease of Actinidia chinensis. Plant Mol Biol. 1988;10:193–202. doi: 10.1007/BF00027396. [DOI] [PubMed] [Google Scholar]
- Racusen D, Foote M. A major soluble glycoprotein of potato tubers. J Food Biochem. 1980;4:43–52. [Google Scholar]
- Raikhel NV, Lerner DR. Expression and regulation of lectin genes in cereals and rice. Dev Genet. 1991;12:255–260. doi: 10.1002/dvg.1020120402. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hinz G, Holstein SEH. The molecular characterization of transport vesicles. Plant Mol Biol. 1998;38:49–76. [PubMed] [Google Scholar]
- Rodrigo I, Vera P, Van Loon LC, Conejero V. Degradation of tobacco pathogenesis-related proteins. Plant Physiol. 1991;95:616–622. doi: 10.1104/pp.95.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeberg-Roos P, Kervinen J, Kovaleva V, Raikhel NV, Gal S. The aspartic proteinase of barley is a vacuolar enzyme that processes probarley lectin in vitro. Plant Physiol. 1994;105:321–329. doi: 10.1104/pp.105.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G, Jung R, Kunze G, Saalbach I, Adler K, Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991;3:695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G, Rosso M, Schumann U. The vacuolar targeting signal of the 2S albumin from brazil nut resides at the C terminus and involves the C-terminal propeptide as an essential element. Plant Physiol. 1996;112:975–985. doi: 10.1104/pp.112.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schlumbaum A, Mauch F, Vögeli U, Boller T. Plant chitinases are potent inhibitors of fungal growth. Nature. 1986;324:365–367. [Google Scholar]
- Sela-Buurlage MB (1996) In vitro sensitivity and tolerance of Fusarium solani towards chitinases and β-1,3-glucanases. PhD thesis. Landbouwuniversiteit Wageningen, Wageningen, The Netherlands
- Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, Van den Elzen PJM, Cornelissen BJC. Only specific tobacco (Nicotiana tabacum) chitinases and β-1,3-glucanases exhibit antifungal activity. Plant Physiol. 1993;101:857–863. doi: 10.1104/pp.101.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H, Neuhaus J-M, Ryals J, Meins F. Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol Biol. 1990;14:357–368. doi: 10.1007/BF00028772. [DOI] [PubMed] [Google Scholar]
- Shinshi H, Wenzler H, Neuhaus J-M, Felix G, Hofsteenge J, Meins F. Evidence for N- and C-terminal processing of a plant defense-related enzyme: primary structure of tobacco prepro-β-1,3-glucanase. Proc Natl Acad Sci USA. 1988;85:5541–5545. doi: 10.1073/pnas.85.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Nelson DE, Kuhn D, Hasegawa PM, Bressan RA. Molecular cloning of osmotin and regulation of its expression by ABA and adaptation to low water potential. Plant Physiol. 1989;90:1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Raikhel NV. Nucleotide sequences of cDNA clones encoding wheat germ agglutinin isolectins A and D. Plant Mol Biol. 1989;13:601–603. doi: 10.1007/BF00027321. [DOI] [PubMed] [Google Scholar]
- Soedjanaatmadja UMS, Subroto T, Beintema JJ. Processed products of the hevein precursor in the latex of the rubber tree (Hevea brasiliensis) FEBS Lett. 1995;363:211–213. doi: 10.1016/0014-5793(95)00309-w. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Sturm A, Chrispeels MJ, Willmitzer L. Targeting and glycosylation of patatin the major potato tuber protein in leaves of transgenic tobacco. Planta. 1989;179:171–180. doi: 10.1007/BF00393687. [DOI] [PubMed] [Google Scholar]
- Sticher L, Hinz U, Meyer AD, Meins F. Intracellular transport and processing of a tobacco vacuolar β-1,3-glucanase. Planta. 1992;188:559–565. doi: 10.1007/BF00197049. [DOI] [PubMed] [Google Scholar]
- Stinissen HM, Peumans WJ, Chrispeels MJ. Subcellular site of lectin synthesis in developing rice embryos. EMBO J. 1984;3:1979–1985. doi: 10.1002/j.1460-2075.1984.tb02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Voelker TA, Herman EM, Chrispeels MJ. Correct glycosylation, Golgi-processing, and targeting to protein bodies of the vacuolar protein phytohemagglutinin in transgenic tobacco. Planta. 1988;175:170–183. doi: 10.1007/BF00392425. [DOI] [PubMed] [Google Scholar]
- Ten Kate TK, Van Balen R, Smeulders AWM, Groen FCA, Den Boer GA. SCILIAM, a multi-level interactive image processing environment. Pattern Recogn Lett. 1990;11:429–441. [Google Scholar]
- Van Damme EJM, Broekaert WF, Peumans WJ. The Urtica dioica agglutinin is a complex mixture of isolectins. Plant Physiol. 1988;86:598–601. doi: 10.1104/pp.86.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme EJM, Peumans WJ. Isolectin composition of individual clones of Urtica dioica: evidence for phenotypic differences. Physiol Plant. 1987;71:328–334. [Google Scholar]
- Van Parijs J, Joosen HM, Peumans WJ, Geuns JM, Van Laere AJ. Effect of the Urtica dioica agglutinin on germination and cell wall formation of Phycomyces blakesleeanus Burgeff. Arch Microbiol. 1992;158:19–25. [Google Scholar]
- Walujono K, Scholma RA, Beintema JJ, Mariono A, Hahn AM (1975) Amino acid sequence of hevein. In Proceedings of the International Rubber Conference, Vol 2. Rubber Research Institute of Malays, Kuala Lumpur, Malaysia, pp 518–531
- Wilkins TA, Bednarek SY, Raikhel NV. Role of propeptide glycan in post-translational processing and transport of barley lectin to vacuoles in transgenic tobacco. Plant Cell. 1990;2:301–313. doi: 10.1105/tpc.2.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins TA, Raikhel NV. Expression of rice lectin is governed by two temporally and spatially regulated mRNAs in developing embryos. Plant Cell. 1989;1:541–549. doi: 10.1105/tpc.1.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk CP, Meulenhoff EJS, Sela-Buurlage MB, Van den Elzen PJM, Cornelissen BJC. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3:619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HT, Sandrasegaram G, Wright CS. Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. J Mol Evol. 1991;33:283–294. doi: 10.1007/BF02100680. [DOI] [PubMed] [Google Scholar]