Abstract
In a similar manner to erectile dysfunction (ED), osteocalcin (OC) is also said to be associated with cardiovascular disease (CVD); however, the effect of OC in ED is unclear. This study was conducted based on the Fangchenggang Area Male Health and Examination Survey (FAMHES) project that ran between September and December 2009. ED was evaluated using the International Index of Erectile Function (IIEF-5). OC was shown to be associated with mild (unadjusted: OR = 0.647; P = 0.016) or moderate (unadjusted: OR = 0.453; P = 0.007) ED. Meanwhile, higher OC levels were more prominently associated with ED (unadjusted: OR = 0.702; P = 0.014). When subdividing the groups by age, the correlation between OC and ED presented in those aged 40–49 years, even in the multi-adjusted model, for those with moderate (OR = 0.255, P = 0.044) and severe (OR = 0.065, P = 0.005) ED. The relationship between OC and ED was also associated with a high level of testosterone, non-obesity, drinking, and non-metabolic syndrome. In summary, OC may play a protective role in middle-aged (40–49 years) men with moderate-severe ED, especially those with a high level of testosterone, non-obesity, drinking, and non-metabolic syndrome.
Introduction
Osteocalcin (OC) is a non-collagenous protein derived from osteoblasts1, which can be γ-carboxylated (Gla) at one or more glutamic acid (Glu) residues2,3. It was first identified as a marker of bone formation4. In 1996, OC-deficient mice (Osn−/−) were shown to have increased visceral fat and insulin resistance5. This phenomenon was explained in 2007 when OC was shown to affect energy metabolism by favoring β-cell proliferation, insulin secretion, and insulin sensitivity6. In 2011, the same research group also showed that OC could influence the reproductive system and testosterone level7. In addition, it was recently suggested that OC could be associated with cardiovascular disease (CVD) and reduce the risk of CVD in older men8. Moreover, in 2016, Ling et al.9 identified a potential correlation between OC gene polymorphisms and blood pressure.
As one of the common male urogenital diseases, erectile dysfunction (ED) is defined as the inability to acquire and maintain a sufficient erection for satisfying sexual intercourse. Among its various etiologies, the hormone and vascular components are dominant10,11. Moreover, one study also reported that ED may be a marker of CVD12; however, the association between OC and ED has not been discussed until now. This study was conducted to remedy this gap in the literature using data from the Fangchenggang Area Male Health and Examination Survey (FAMHES) project.
Results
The essential characteristics of subjects with and without ED
A total of 4303 participants (aged 18–88 years) were recruited to the FAMHES project between September and December 2009, of whom 3593 responded to further interviews. Rigorous criteria were applied to investigate the association between OC and ED, and a total of 1567 eligible samples were selected for the analysis. The IIEF-5 system defined two groups: 746 individuals with ED (IIEF-5 ≤ 21) and 821 individuals without ED (IIEF-5 > 21). The baseline results suggested that those with ED were significantly older than those without ED (ED vs. non-ED: 38.14 ± 1.82 vs. 33.99 ± 8.10 years; P < 0.001). In addition, abdominal obesity was also more serious in the ED patients (ED vs. non-ED: 0.89 ± 0.06 vs. 0.88 ± 0.06; PWHR = 0.012; P% = 0.044). Interestingly, the level of OC was lower in those with ED than those without ED (ED vs. non-ED: 24.38 ± 8.21 vs. 25.56 ± 8.64 ng/ml; P = 0.003) (Table 1).
Table 1.
The baseline analysis for the eligible participants in this study.
| ED ≤ 21 | No-ED > 21 | P | |
|---|---|---|---|
| NO. | 746 | 821 | |
| Age, years | 38.14 ± 1.82 | 33.99 ± 8.10 | <0.001 |
| Osteocalcin, ng/ml | 24.38 ± 8.21 | 25.56 ± 8.64 | 0.003 |
| Testosterone, ng/ml | 6.34 ± 1.85 | 6.23 ± 1.93 | 0.233 |
| BMI, kg/m2 | 23.42 ± 3.26 | 23.30 ± 3.45 | 0.488 |
| <24 | 436 (58.45%) | 500 (60.90%) | |
| <28, ≥24 | 249 (33.38%) | 244 (29.72%) | |
| ≥28 | 61 (8.17%) | 77 (9.38%) | 0.259 |
| WHR, % | 0.89 ± 0.06 | 0.88 ± 0.06 | 0.012 |
| ≤0.9 | 454 (60.86%) | 540 (65.77%) | |
| >0.9 | 292 (39.14%) | 281 (34.23%) | 0.044 |
| Smoking, % | |||
| Yes | 410 (54.96%) | 455 (55.42%) | |
| No | 336 (45.04%) | 366 (44.58%) | 0.855 |
| Drinking, % | |||
| Yes | 636 (85.25%) | 726 (88.43%) | |
| No | 110 (14.75%) | 95 (11.57%) | 0.063 |
| MetS, % | |||
| Yes | 65 (8.71%) | 68 (8.28%) | |
| No | 681 (91.29%) | 753 (91.72%) | 0.760 |
| MetS score, % | |||
| 0 | 292 (39.14%) | 347 (42.27%) | 0.217 |
| 1 | 232 (31.10%) | 239 (29.11%) | 0.341 |
| 2 | 131 (17.56%) | 142 (17.30%) | 0.894 |
| 3 | 66 (8.85%) | 65 (7.92%) | 0.523 |
| ≥4 | 25 (3.35%) | 28 (3.40%) | 1.000 |
*The ED is defined with the threshold of IIEF-5 of 22 score.
*Quantitative traits represented as mean ± SD are tested with student’s t test. Qualitative traits are tested with chi-square test.
*ED: erectile dysfunction; BMI: body mass index; WHR: waist hip rate; SD: standard deviation.
Potential protective function of OC in ED
In the next analysis, regression analyses, including linear and binary regressions, were applied. Although no significant association between OC and ED risk was identified in the linear regression analysis, OC was significantly associated with ED risk in the binary logistic regression analysis (unadjusted: OR = 0.618, 95% CI = 0.449–0.850, P = 0.003) (Table 2). To further confirm this association, ED was divided into four groups: mild, moderate, severe, or no dysfunction. The multinomial logistic regression analysis was conducted using the no dysfunction group as the reference. The results suggested that OC may be predominantly related to the mild (IIEF = 17–21; unadjusted: OR = 0.647, 95% CI = 0.454–0.921, P = 0.016) and moderate (IIEF = 12–16; unadjusted: OR = 0.453, 95% CI = 0.255–0.804, P = 0.007) forms of ED (Table 3). Following these analyses, the OC level was divided into quartiles (Q1 < 25%, 25% ≤ Q2 ≤ 50%, 50% < Q3 ≤ 75%, Q4 > 75%). The results of the multinomial logistic regression analysis suggested that higher OC levels were more prominently associated with ED (Q4, unadjusted: OR = 0.702, 95% CI = 0.530–0.930, P = 0.014) (Table 4).
Table 2.
The linear and binary logistic regression analyses for the association between osteocalcin, testosterone and ED.
| Unadjusted | Age-adjusted | Multi-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BETA/OR | 95%CI | P | BETA/OR | 95%CI | P | BETA/OR | 95%CI | P | |
| IIEF-5 score | |||||||||
| osteocalcin | 0.566 | −0.105, 1.237 | 0.098 | −0.330 | −1.030, 0.371 | 0.356 | −0.131 | −0.856, 0.593 | 0.722 |
| testosterone | −0.625 | −1.313, 0.063 | 0.075 | −1.004 | −1.686, −0.323 | 0.004 | −0.815 | −1.591, −0.038 | 0.040 |
| ED | |||||||||
| osteocalcin | 0.618 | 0.449–0.850 | 0.003 | 0.979 | 0.693–1.383 | 0.905 | 0.936 | 0.655–1.339 | 0.718 |
| testosterone | 1.272 | 0.919–1.762 | 0.147 | 1.596 | 1.138–2.240 | 0.007 | 1.648 | 1.121–2.424 | 0.011 |
*In the linear regression analysis, as a continuous variable the IIEF-5 score is treated as the dependent factor. In the binary logistic regression analysis, ED (dichotomous variable: ED or without ED) is treated as the dependent factor.
*ED: erectile dysfunction; BMI: body mass index; WHR: waist hip rate; OR: odd ratio; 95%CI: 95% confidence interval.
*Multi-adjusted: age, BMI, WHR, smoke and drink.
Table 3.
The multinomial logistic regression analysis for the association between ED and osteocalcin.
| Unadjusted | Age-adjusted | Multi-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| ED | |||||||||
| None (22–25) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mild (17–21) | 0.647 | 0.454–0.921 | 0.016 | 0.935 | 0.641–1.365 | 0.728 | 0.919 | 0.621–1.358 | 0.671 |
| Moderate (12–16) | 0.453 | 0.255–0.804 | 0.007 | 0.930 | 0.503–1.721 | 0.818 | 0.871 | 0.461–1.647 | 0.672 |
| Severe (5–11) | 0.795 | 0.379–1.665 | 0.543 | 1.359 | 0.621–2.970 | 0.443 | 1.133 | 0.502–2.557 | 0.764 |
*ED is divided into four groups according to the IIEF-5 scores: None = 22–25 scores, Mild = 17–21 scores, Moderate = 12–16 scores and Severe = 5–11 scores. None ED is treated as the reference.
*ED: erectile dysfunction; BMI: body mass index; WHR: waist hip rate; OR: odd ratio; 95%CI: 95% confidence interval.
Table 4.
Results of multinomial logistic regression analysis for the association between ED risk and osteocalcin level.
| Q1 | Q2 | Q3 | Q4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | ||
| Unadjusted | 1 | 0.912 | 0.690–1.207 | 0.521 | 0.782 | 0.590–1.035 | 0.086 | 0.702 | 0.530–0.930 | 0.014 |
| Age-adjusted | 1 | 1.036 | 0.775–1.385 | 0.811 | 0.975 | 0.728–1.307 | 0.866 | 1.018 | 0.752–1.377 | 0.909 |
| Multi-adjusted | 1 | 1.024 | 0.765–1.370 | 0.874 | 0.952 | 0.709–1.280 | 0.747 | 0.988 | 0.723–1.350 | 0.940 |
*The levels of osteocalcin is divided into quartile (Q1 < levels of 25%, 25% ≤ Q2 ≤ 50%, 50% < Q3 ≤ 75%, Q4 > 75%).
*Multi-adjusted: adjust for age, smoking status, alcoholic drinking, BMI, WHR.
*ED = erectile dysfunction; BMI = Body Mass Index; WHR = waist hip rate.
OC is significantly related to moderate–severe ED in middle-aged (40–49 years) men
In the regression analyses mentioned above, the significant association between OC and ED risk disappeared after adjusting for age. Correlation analyses were subsequently performed, and age was negatively associated with the OC level (r = −0.336, P < 0.001) and IIEF score (r = −0.188, P < 0.001). Therefore age may influence the relationship between OC and ED. Considering this, a subgroup analysis of age was conducted. Previous work had highlighted that 40 years of age may be the joint point for the increasing morbidity of ED13. Therefore the participants were divided into four age groups: <40, 40–49, 50–59, and ≥60 years. No obvious associations were discovered in the <40, 50–59, or ≥60 groups; however, the results suggested that OC may be associated with ED in the 40–49 age group, even in the multi-adjusted model (IIEF-5: β = 2.480, 95% CI = 0.995–3.965, P = 0.001; ED: OR = 0.368, 95% CI = 0.168–0.807, P = 0.013). In addition, significant correlations were mainly present in the moderate (multi-adjusted: OR = 0.255, 95% CI = 0.067–0.965, P = 0.044) and severe (unadjusted: OR = 0.061, 95% CI = 0.009–0.394, P = 0.003; age-adjusted: OR = 0.060, 95% CI = 0.009–0.397, P = 0.003; multi-adjusted: OR = 0.065, 95% CI = 0.009–0.440, P = 0.005) ED groups (Table 5).
Table 5.
Association between ED and osteocalcin level in four ages groups (<40, 40–49, 50–59 and ≥60 years).
| No. ED | No. Non-ED | Unadjusted | Age-adjusted | Multi-adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BETA/OR | 95%CI | P | BETA/OR | 95%CI | P | BETA/OR | 95%CI | P | ||||
| Ages <40 | ||||||||||||
| IIEF-5 | 444 | 635 | −0.903 | −1.690, −0.117 | 0.024 | −0.520 | −1.368, 0.328 | 0.229 | −0.467 | −1.346, 0.411 | 0.297 | |
| ED | 1.048 | 0.709–1.548 | 0.814 | 1.142 | 0.748–1.742 | 0.538 | 1.097 | 0.708–1.698 | 0.679 | |||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Mild | 0.912 | 0.592–1.404 | 0.675 | 1.122 | 0.704–1.788 | 0.628 | 1.072 | 0.662–1.736 | 0.778 | |||
| Moderate | 0.740 | 0.328–1.668 | 0.468 | 0.739 | 0.309–1.764 | 0.495 | 0.696 | 0.284–1.709 | 0.430 | |||
| Severe | 4.846 | 1.864–12.600 | 0.001 | 2.003 | 0.690–5.810 | 0.201 | 2.037 | 0.669–6.204 | 0.211 | |||
| 40–49 | 189 | 154 | ||||||||||
| IIEF-5 | 2.412 | 0.952, 3.873 | 0.001 | 2.385 | 0.930, 3.840 | 0.001 | 2.480 | 0.995, 3.965 | 0.001 | |||
| ED | 0.363 | 0.167–0.785 | 0.010 | 0.366 | 0.169–0.791 | 0.011 | 0.368 | 0.168–0.807 | 0.013 | |||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Mild | 0.460 | 0.199–1.061 | 0.069 | 0.463 | 0.201–1.068 | 0.071 | 0.488 | 0.209–1.143 | 0.099 | |||
| Moderate | 0.308 | 0.085–1.114 | 0.073 | 0.311 | 0.086–1.124 | 0.075 | 0.255 | 0.067–0.965 | 0.044 | |||
| Severe | 0.061 | 0.009–0.394 | 0.003 | 0.060 | 0.009–0.397 | 0.003 | 0.065 | 0.009–0.440 | 0.005 | |||
| 50–59 | 74 | 26 | ||||||||||
| IIEF-5 | 0.418 | −2.815, 3.651 | 0.798 | 0.350 | −2.905, 3.604 | 0.832 | 0.929 | −2.509, 4.366 | 0.593 | |||
| ED | 0.708 | 0.135–3.720 | 0.684 | 0.700 | 0.132–3.705 | 0.675 | 0.623 | 0.106–3.655 | 0.600 | |||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Mild | 0.551 | 0.087–3.473 | 0.526 | 0.557 | 0.089–3.480 | 0.531 | 0.497 | 0.071–3.480 | 0.482 | |||
| Moderate | 1.622 | 0.182–14.433 | 0.665 | 1.503 | 0.167–13.502 | 0.716 | 1.898 | 0.183–19.662 | 0.591 | |||
| Severe | 0.369 | 0.031–4.347 | 0.428 | 0.394 | 0.032–4.844 | 0.467 | 0.205 | 0.012–3.403 | 0.269 | |||
| ≥60 | 39 | 6 | ||||||||||
| IIEF-5 | 2.024 | −1.726, 5.775 | 0.282 | 1.769 | −1.981, 5.519 | 0.347 | 1.530 | −2.366, 5.425 | 0.432 | |||
| ED | 0.631 | 0.071–5.583 | 0.631 | 0.551 | 0.057–5.352 | 0.608 | 0.237 | 0.016–3.570 | 0.298 | |||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Mild | 0.814 | 0.071–9.313 | 0.869 | 0.623 | 0.045–8.643 | 0.724 | 0.187 | 0.008–4.289 | 0.294 | |||
| Moderate | 0.792 | 0.069–9.108 | 0.851 | 0.741 | 0.058–9.478 | 0.818 | 0.276 | 0.013–5.714 | 0.405 | |||
| Severe | 0.194 | 0.008–4.596 | 0.310 | 0.199 | 0.009–4.544 | 0.312 | 0.150 | 0.005–4.484 | 0.273 | |||
*Linear regression analysis for the IIEF-5 scores; binary regression analysis for ED; multinomial logistic regression for the severity of symptom of ED.
*ED is divided into four groups according to the IIEF-5 scores: None = 22–25 scores, Mild = 17–21 scores, Moderate = 12–16 scores and Severe = 5–11 scores. None ED is treated as the reference in the multinomial logistic regression.
*ED: erectile dysfunction; BMI: body mass index; WHR: waist hip rate; OR: odd ratio; 95%CI: 95% confidence interval.
*Multi-adjusted: age, BMI, WHR, smoke and drink.
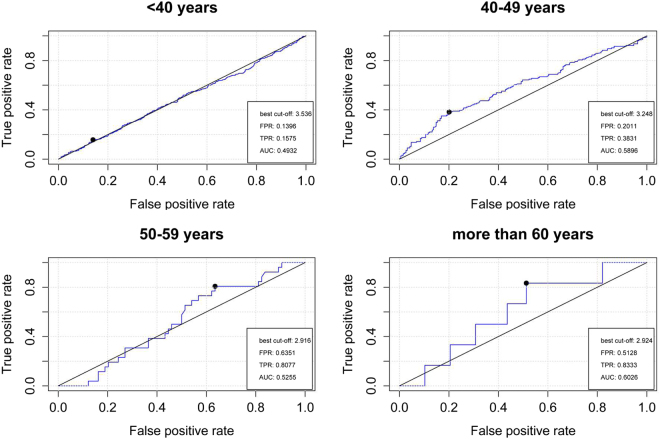
In the receiver operating characteristic (ROC) analysis, OC was also shown to predict ED in the 40–49 age group with a better area under the curve (AUC = 0.5896, P = 0.004, best cutoff lnOC = 3.248) compared to the other age groups (Fig. 1).
Figure 1.
Relative operating characteristic (ROC) curve analyses of osteocalcin in predicting the ED risk in four different age groups (<40, 40–49, 50–59 and ≥60 years). The Best cut-off, area under the curve (AUC), False Positive Rate (FPR) and True Positive Rate (TPR) are shown in the figures.
Correlation of OC and ED with higher testosterone levels
In the baseline analysis, no difference in testosterone level was detected between the ED (6.34 ± 1.85 ng/ml) and non-ED (6.23 ± 1.93 ng/ml, P = 0.233) groups (Table 1). Previous studies suggested that testosterone can influence the OC level and the development of ED10, and the level of testosterone was positively correlated with OC (r = 0.249, P < 0.001). To discover the influence of testosterone in the association between OC and ED, subgroup analyses were conducted after subdividing the testosterone levels into quartiles (Table 6). The 50–59 and ≥60 age groups were combined in this analysis due to the limited number of participants aged >60 years old. Once again, a significant association was only shown in the 40–49 age group. In the testosterone subgroups, the correlation between OC and ED was more prominent in those with higher testosterone levels [Q3 (unadjusted: OR = 0.077, 95% CI = 0.011–0.546, P = 0.010; age-adjusted: OR = 0.079, 95% CI = 0.011–0.578, P = 0.012; multi-adjusted: OR = 0.068, 95% CI = 0.008–0.555, P = 0.012) and Q4 (multi-adjusted: OR = 0.184, 95% CI = 0.034–0.985, P = 0.048)].
Table 6.
The logistic regression analysis for the association between ED and osteocalcin in various groups.
| No. | Osteocalcin, ng/ml | Unadjusted | Age-adjusted | Multi-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ED | non-ED | ED | non-ED | OR/95%CI | P | OR/95%CI | P | OR/95%CI | P | ||
| Age < 40 | |||||||||||
| testosterone | Q1 | 98 | 168 | 24.27 ± 7.41 | 23.18 ± 7.17 | 1.746/0.731–4.168 | 0.209 | 1.835/0.735–4.580 | 0.193 | 1.836/0.716–4.711 | 0.206 |
| Q2 | 114 | 160 | 24.23 ± 7.52 | 25.36 ± 8.21 | 0.627/0.276–1.428 | 0.266 | 0.985/0.402–2.417 | 0.974 | 1.044/0.415–2.628 | 0.927 | |
| Q3 | 108 | 163 | 26.73 ± 8.88 | 27.24 ± 9.29 | 0.794/0.362–1.742 | 0.565 | 0.808/0.343–1.901 | 0.625 | 0.788/0.319–1.948 | 0.606 | |
| Q4 | 124 | 144 | 29.19 ± 9.04 | 29.42 ± 9.60 | 0.989/0.451–2.168 | 0.978 | 0.878/0.383–2.014 | 0.759 | 0.917/0.391–2.153 | 0.843 | |
| BMI | <24 | 272 | 403 | 28.13 ± 9.12 | 27.39 ± 9.26 | 1.351/0.830–2.199 | 0.226 | 1.531/0.896–2.616 | 0.119 | 1.477/0.850–2.568 | 0.166 |
| <28, ≥24 | 146 | 173 | 23.19 ± 6.59 | 24.65 ± 8.14 | 0.508/0.229–1.126 | 0.095 | 0.500/0.219–1.143 | 0.100 | 0.488/0.212–1.125 | 0.092 | |
| ≥28 | 26 | 59 | 23.44 ± 5.43 | 22.53 ± 6.14 | 2.122/0.346–13.015 | 0.416 | 2.526/0.394–16.202 | 0.328 | 1.489/0.200–11.079 | 0.697 | |
| WHR | ≤0.9 | 312 | 448 | 27.53 ± 9.02 | 27.54 ± 9.33 | 1.012/0.638–1.605 | 0.961 | 1.164/0.708–1.912 | 0.550 | 1.194/0.716–1.990 | 0.498 |
| >0.9 | 132 | 187 | 23.17 ± 6.20 | 22.95 ± 6.62 | 1.213/0.532–2.764 | 0.647 | 1.138/0.484–2.676 | 0.768 | 1.002/0.420–2.389 | 0.997 | |
| Smoking | No | 191 | 277 | 26.51 ± 8.17 | 27.10 ± 9.69 | 0.901/0.501–1.620 | 0.727 | 0.896/0.465–1.729 | 0.744 | 0.970/0.494–1.903 | 0.929 |
| Yes | 253 | 358 | 26.02 ± 8.78 | 25.48 ± 8.11 | 1.189/0.703–2.012 | 0.518 | 1.341/0.768–2.341 | 0.302 | 1.158/0.646–2.073 | 0.623 | |
| Drinking | No | 55 | 68 | 26.53 ± 6.87 | 26.97 ± 11.12 | 1.238/0.402–3.809 | 0.710 | 1.351/0.404–4.513 | 0.625 | 0.946/0.247–3.627 | 0.936 |
| Yes | 389 | 567 | 26.19 ± 8.73 | 26.10 ± 8.56 | 1.020/0.672–1.547 | 0.926 | 1.108/0.705–1.740 | 0.658 | 1.079/0.677–1.719 | 0.748 | |
| MetS | No | 410 | 575 | 26.57 ± 8.65 | 26.59 ± 8.72 | 1.003/0.664–1.513 | 0.990 | 1.103/0.705–1.725 | 0.667 | 1.126/0.710–1.786 | 0.613 |
| Yes | 34 | 60 | 22.10 ± 5.31 | 22.38 ± 9.37 | 1.176/0.281–4.927 | 0.825 | 1.197/0.281–5.096 | 0.808 | 0.983/0.217–4.463 | 0.982 | |
| Age 40–49 | |||||||||||
| testosterone | Q1 | 44 | 41 | 21.16 ± 7.18 | 21.66 ± 6.75 | 0.787/0.204–3.031 | 0.727 | 0.778/0.201–3.007 | 0.716 | 0.955/0.224–4.077 | 0.951 |
| Q2 | 39 | 47 | 22.02 ± 5.26 | 23.61 ± 6.87 | 0.453/0.091–2.258 | 0.334 | 0.443/0.087–2.245 | 0.325 | 0.404/0.073–2.244 | 0.300 | |
| Q3 | 60 | 26 | 21.43 ± 5.76 | 25.24 ± 6.22 | 0.077/0.011–0.546 | 0.010 | 0.079/0.011–0.578 | 0.012 | 0.068/0.008–0.555 | 0.012 | |
| Q4 | 46 | 40 | 21.81 ± 4.46 | 25.14 ± 10.40 | 0.259/0.050–1.340 | 0.107 | 0.251/0.049–1.294 | 0.098 | 0.184/0.034–0.985 | 0.048 | |
| BMI | <24 | 102 | 82 | 22.02 ± 5.79 | 25.34 ± 8.70 | 0.221/0.075–0.650 | 0.006 | 0.232/0.079–0.684 | 0.008 | 0.199/0.064–0.614 | 0.005 |
| <28, ≥24 | 67 | 58 | 21.19 ± 5.97 | 22.24 ± 6.41 | 0.491/0.130–1.858 | 0.294 | 0.490/0.129–1.857 | 0.294 | 0.564/0.145–2.194 | 0.409 | |
| ≥28 | 20 | 14 | 20.63 ± 4.31 | 20.81 ± 6.60 | 1.449/0.134–15.630 | 0.760 | 1.545/0.137–17.420 | 0.725 | 1.669/0.141–19.744 | 0.684 | |
| WHR | ≤0.9 | 93 | 80 | 22.00 ± 5.61 | 25.20 ± 6.71 | 0.141/0.042–0.479 | 0.002 | 0.148/0.043–0.506 | 0.002 | 0.122/0.034–0.438 | 0.001 |
| >0.9 | 96 | 74 | 21.17 ± 5.81 | 22.21 ± 8.75 | 0.756/0.272–2.096 | 0.590 | 0.746/0.269–2.071 | 0.574 | 0.890/0.312–2.544 | 0.828 | |
| Smoking | No | 79 | 70 | 21.85 ± 5.76 | 24.06 ± 7.07 | 0.315/0.094–1.051 | 0.060 | 0.339/0.101–1.145 | 0.082 | 0.290/0.083–1.017 | 0.053 |
| Yes | 110 | 84 | 21.39 ± 5.70 | 23.52 ± 8.53 | 0.408/0.149–1.115 | 0.080 | 0.398/0.145–1.087 | 0.072 | 0.424/0.153–1.177 | 0.100 | |
| Drinking | No | 27 | 21 | 20.55 ± 4.33 | 23.87 ± 7.19 | 0.134/0.012–1.504 | 0.103 | 0.096/0.007–1.251 | 0.074 | 0.125/0.009–1.685 | 0.117 |
| Yes | 162 | 133 | 21.75 ± 5.91 | 23.75 ± 8.01 | 0.411/0.182–0.930 | 0.033 | 0.420/0.186–0.951 | 0.037 | 0.405/0.177–0.931 | 0.033 | |
| MetS | No | 157 | 127 | 22.07 ± 5.66 | 24.02 ± 7.01 | 0.387/0.162–0.925 | 0.033 | 0.394/0.164–0.943 | 0.036 | 0.400/0.164–0.975 | 0.044 |
| Yes | 32 | 27 | 19.17 ± 5.47 | 22.56 ± 11.19 | 0.220/0.033–1.471 | 0.118 | 0.220/0.033–1.486 | 0.120 | 0.203/0.027–1.551 | 0.124 | |
| Age ≥ 50 | |||||||||||
| testosterone | Q1 | 28 | 9 | 19.33 ± 7.13 | 19.68 ± 4.40 | 0.569/0.058–5.597 | 0.629 | 0.549/0.056–5.354 | 0.606 | 0.539/0.057–5.053 | 0.588 |
| Q2 | 30 | 5 | 21.45 ± 5.89 | 22.74 ± 3.62 | 0.256/0.005–12.138 | 0.489 | 0.418/0.006–27.641 | 0.684 | 0.148/0.001–22.152 | 0.455 | |
| Q3 | 25 | 12 | 25.50 ± 13.52 | 23.07 ± 5.02 | 1.391/0.169–11.484 | 0.759 | 1.396/0.166–11.774 | 0.759 | 0.996/0.057–17.420 | 0.998 | |
| Q4 | 30 | 6 | 21.29 ± 5.66 | 21.66 ± 5.00 | 0.648/0.017–24.019 | 0.814 | 0.789/0.017–36.854 | 0.904 | 1.174/0.013–108.554 | 0.945 | |
| BMI | <24 | 62 | 15 | 22.22 ± 6.60 | 22.64 ± 5.61 | 0.667/0.090–4.964 | 0.692 | 0.737/0.092–5.915 | 0.774 | 0.961/0.097–9.548 | 0.973 |
| <28, ≥24 | 36 | 13 | 21.58 ± 11.76 | 22.39 ± 2.87 | 0.368/0.052–2.598 | 0.316 | 0.363/0.050–2.634 | 0.316 | 0.304/0.042–2.191 | 0.237 | |
| ≥28 | 15 | 4 | 20.42 ± 6.95 | 16.73 ± 2.26 | 20.079/0.051–7888.492 | 0.325 | 13.892/0.033–5856.222 | 0.394 | NA /NA | NA | |
| WHR | ≤0.9 | 49 | 12 | 22.06 ± 6.53 | 22.43 ± 4.78 | 0.629/0.064–6.237 | 0.692 | 0.643/0.061–6.761 | 0.713 | 0.956/0.056–16.282 | 0.975 |
| >0.9 | 64 | 20 | 21.56 ± 9.89 | 21.42 ± 4.67 | 0.676/0.146–3.133 | 0.617 | 0.704/0.149–3.322 | 0.657 | 0.584/0.102–3.360 | 0.547 | |
| Smoking | No | 66 | 19 | 22.01 ± 9.62 | 21.29 ± 4.51 | 0.918/0.167–5.027 | 0.921 | 0.896/0.162–4.948 | 0.899 | 0.891/0.152–5.206 | 0.898 |
| Yes | 47 | 13 | 21.45 ± 6.90 | 22.55 ± 4.95 | 0.440/0.055–3.494 | 0.438 | 0.434/0.049–3.810 | 0.451 | 0.232/0.017–3.186 | 0.274 | |
| Drinking | No | 28 | 6 | 24.71 ± 13.30 | 20.25 ± 4.91 | 3.924/0.163–94.649 | 0.400 | 5.666/0.126–254.731 | 0.372 | 4.168/0.080–216.801 | 0.479 |
| Yes | 85 | 26 | 20.81 ± 6.10 | 22.16 ± 4.63 | 0.326/0.062–1.704 | 0.184 | 0.330/0.062–1.758 | 0.194 | 0.342/0.063–1.860 | 0.215 | |
| MetS | No | 88 | 26 | 21.97 ± 8.94 | 21.91 ± 4.78 | 0.706/0.171–2.908 | 0.630 | 0.730/0.174–3.065 | 0.667 | 0.743/0.159–3.483 | 0.707 |
| Yes | 25 | 6 | 21.11 ± 7.20 | 21.30 ± 4.48 | 0.614/0.032–11.748 | 0.746 | 0.635/0.030–13.423 | 0.771 | 0.626/0.019–20.596 | 0.793 | |
*Testosterone levels are divided into quartile (Q1 < levels of 25%, 25% ≤Q2 ≤ 50%, 50% <Q3 ≤75%, Q4 > 75%) in different ages.
*BMI is categorized into three groups (normal weight <24.0 kg/m2, overweight 24.0–27.9 kg/m2, obese 28.0 kg/m2). Meanwhile, the waist-hip ratio (WHR) is categorized as normal weight (WHR ≤ 0.9) and obese (WHR > 0.9).
*Smoking, drinking and metabolic syndrome (MetS) are considered as dichotomous variable (Yes/No).
*ED is considered as dichotomous variable (ED/without ED). Then, binary logistic regression analyses are conducted for every subgroup, in which ED is treated as the dependent factor. And the level of osteocalcin is treated as covariate.
*The level of osteocalcin (ng/ml) is presented as mean ± SD in the ED and non-ED groups.
*ED: erectile dysfunction; BMI: body mass index; WHR: waist hip rate; OR: odd ratio; 95%CI: 95% confidence interval; MetS: metabolic syndrome.
*Multi-adjusted: age, BMI, WHR, smoking and drink.
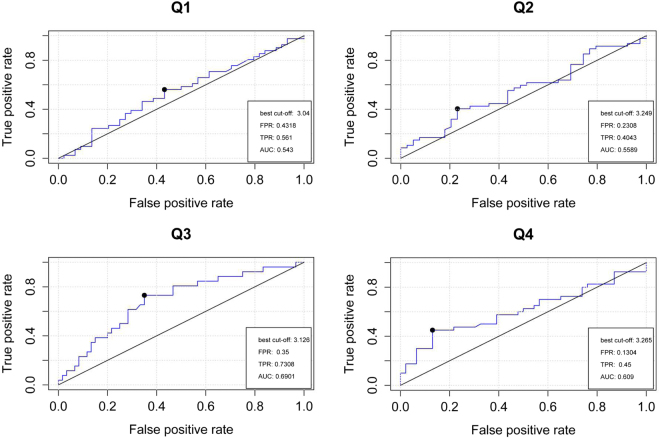
ROC analysis also indicated that OC presented an excellent predictive function for ED in those with high levels of testosterone among the 40–49 age group, with the AUC increasing to 0.6901 (P = 0.005). The best cut-off of lnOC was 3.126 (Fig. 2).
Figure 2.
Relative operating characteristic (ROC) curve analyses of osteocalcin in predicting the ED risk in four different groups (Q1 < levels of 25%, 25% ≤ Q2 ≤ 50%, 50% < Q3 ≤ 75%, Q4 > 75%) on the basis of testosterone levels among 40–49 years. The Best cut-off, area under the curve (AUC), False Positive Rate (FPR) and True Positive Rate (TPR) are shown in the figures.
OC is related to ED in the non-obese population
This study also aimed to identify the influence of obesity in the association between OC and ED. Obesity was defined using BMI (normal weight: <24.0 kg/m2, overweight: 24.0–27.9 kg/m2, obese: ≥28.0 kg/m2) and WHR (normal weight: WHR ≤ 0.9 and obese: WHR > 0.9). This analysis also confirmed the association between OC and the development of ED. In addition, consistent conclusions were reached that showed that the correlations were more prominent in the non-obese population [BMI < 24 kg/m2: (unadjusted: OR = 0.221, 95% CI = 0.075–0.650, P = 0.006; age-adjusted: OR = 0.232, 95% CI = 0.079–0.684, P = 0.008; multi-adjusted: OR = 0.199, 95% CI = 0.064–0.614, P = 0.005); WHR < 0.9: (unadjusted: OR = 0.141, 95% CI = 0.042–0.479, P = 0.002; age-adjusted: OR = 0.148, 95% CI = 0.043–0.506, P = 0.002; multi-adjusted: OR = 0.122, 95% CI = 0.034–0.438, P = 0.001)] (Table 6).
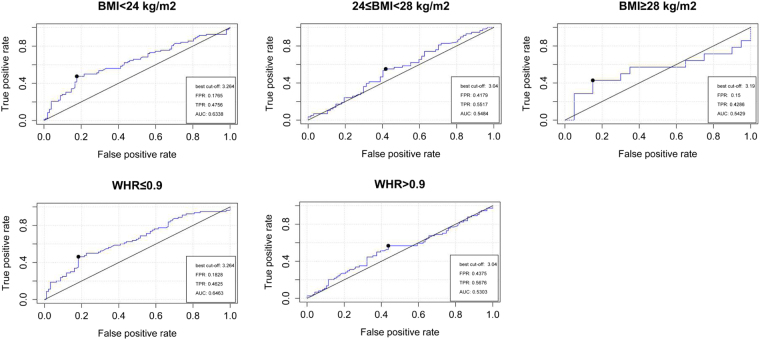
The results of the ROC analysis also indicated that better prediction occurred in the non-obese group (BMI < 24 kg/m2: AUC = 0.6338, P = 0.002; WHR < 0.9: AUC = 0.6463, P = 0.001), with the same best cutoff lnOC of 3.264 (Fig. 3).
Figure 3.
Relative operating characteristic (ROC) curve analyses of osteocalcin in predicting the ED risk for the obesity assessed by BMI and WHR. The Best cut-off, area under the curve (AUC), False Positive Rate (FPR) and True Positive Rate (TPR) are shown in the figures.
Drinking alcohol and the absence of metabolic syndrome (MetS) were associated with the potential protection of OC in ED
Among the 40–49 age group, the correlation between OC and ED was also shown in those that drank alcohol (unadjusted: OR = 0.411, 95% CI = 0.182–0.930, P = 0.033; age-adjusted: OR = 0.420, 95% CI = 0.186–0.951, P = 0.037; multi-adjusted: OR = 0.405, 95% CI = 0.177–0.931, P = 0.033) compared to those that did not drink. Interestingly, men without MetS seemed to be associated with the potential protection of OC (unadjusted: OR = 0.387, 95% CI = 0.162–0.925, P = 0.033; age-adjusted: OR = 0.394, 95% CI = 0.164–0.943, P = 0.036; multi-adjusted: OR = 0.400, 95% CI = 0.164–0.975, P = 0.044) comparing to those with MetS (Table 6).
Discussion
The results from this study combined with previous evidence suggest that OC may be significantly associated with ED to some extent. The positive correlation between OC and ED mainly presented in middle-aged men (40–49 years) with moderate–severe ED. To explain the potential mechanisms, data on other medical factors including testosterone levels, obesity, smoking, drinking alcohol, and MetS were also collected. Further analyses revealed that the correlation between OC and ED was only significant in those individuals with higher testosterone levels who were not obese, drank alcohol, and did not have MetS.
OC is a 5.6 kDa secreted protein, containing 46–50 amino acids, that is produced primarily by osteoblasts14. It was first isolated from bovine and human bone by Price et al.3, and for a long time it was thought that OC manifested its effects in bone. However, OC was recently shown to play important roles in energy metabolism, fertility, and the development of CVD6–8. As a complex disease, ED is also associated with CVD8. Moreover, ED is also suggested to be a marker of subclinical CVD15. Previous studies also hinted that ED may be associated with OC. In 2010, one study focused on OC-positive endothelial progenitor cells in patients affected by ED and cavernous atherosclerosis, and the results suggested that the levels of OC-positive endothelial progenitor cells could be a predictive marker for subsequent coronary artery disease in ED patients16. Although no direct evidence has explained the association between ED and OC levels, fragmentary signals could also identify the potential correlations between them.
In 2005, experimental animal studies proposed that androgen deprivation caused stromal progenitor cells to turn into adipocytes, which was one of the most frequent causes of ED17,18. Meanwhile, it was also suggested that testosterone could open smooth muscle potassium channels in human penile cavernous bodies to influence the erection19. In 2012, data from the FAMHES project revealed that testosterone was associated with ED10. Moreover, in 2014, a meta-analysis showed that testosterone replacement therapy could lead to an 18% reduction in the risk of ED20. Another well-known factor, nitric oxide, was also shown to regulate the erection of the corpus cavernosum, and the expression of nitric oxide was also shown to be affected by androgens21. Penile nitric oxide synthase (NOS) decreased following castration but was rectified with testosterone therapy22. These results suggest that testosterone could protect from ED to some extent. In addition, one previous study showed that OC could favor male fertility by enhancing testosterone production7. In accordance with previous studies23,24, this study also showed that the OC level was positively associated with testosterone (r = 0.249, P < 0.001). Although it was reasonable to assume that OC may be associated with ED, the significance only seemed to be interesting for middle-aged (40–49 years) men. It was previously reported that testosterone decreased as rapidly as 0.4–2% per year after age 3025; however, the function of testosterone was special. When the level of testosterone was lower than the threshold, it was associated with a worse sexual function. Upon reaching the threshold, the effect plateaud26. Therefore, this study suggested that the testosterone concentration in middle-aged men was predominantly lower than the threshold. On the other hand, testosterone levels were high enough to take effect in the <40 age group. This phenomenon could be consistent with the morbidity of ED. As shown previously, the occurrence rate of ED increased in men aged >40 years13, therefore the protective effect of testosterone was obvious in those aged ≥40 years. Similarly, osteoporosis, metabolic abnormalities, and hormonal disorders in older men induced reduced OC and testosterone levels, which weakened the association with ED to some extent.
Another interesting result from this study was that the positive correlation between OC and ED was mainly observed in the non-obese population. An association between obesity and sex hormones was recently established, with lower levels of free and bioavailable testosterone being significantly associated with obesity27. In 2012, Vaidya et al.28 confirmed that testosterone was negatively associated with WHR. Yamacake et al.29 also discovered that men with a higher BMI had lower serum androgen levels. In animal experiments, testosterone levels declined in obese rats fed a high-fat diet30. Possible mechanisms behind this phenomenon could be obesity-associated insulin resistance, hypothalamus–pituitary–testicular axis function, or the transformation and secretion of glucocorticoids31,32. The inverse correlation between OC and obesity in our analysis was consistent with the results from previous studies33,34. Therefore the levels of OC and testosterone increased in the non-obese population, which would have enhanced the protective effect against the development of ED.
This analysis also revealed that OC was associated with ED in individuals without MetS compared to those with MetS. This phenomenon could be explained using data from other studies. In 2013, researchers discovered lower OC levels in individuals with MetS35. This conclusion was later confirmed in the MINOS cohort36. In a population study, long-term drinkers showed significantly higher levels of OC than social drinkers37, which was also in line with the results obtained here. These studies suggest that OC levels are higher in those without MetS and those who drink. As a regulator, this would lead to increased testosterone levels, which would subsequently protect from ED using the mechanism described above.
Limitations
This study mainly focused on the association between OC and ED; however, some issues should not be ignored. Firstly, this study was a cross-sectional analysis and therefore it only suggests a potential protective effect of OC in the development of ED. Additional longitudinal studies are needed to confirm this conclusion. Secondly, other hormones such as luteinizing hormone and follicle-stimulating hormone may also influence the association between OC and ED. Therefore further studies should also consider these hormones more comprehensively. Thirdly, although testosterone could reflect the functional status of the testis to some extent, other factors such as semen quality were not included.
Conclusions
The results suggest that OC may have a protective role against moderate–severe ED in middle-aged (40–49 years) men, especially those with a high level of testosterone, those that are not obese, those that drink alcohol, and those without MetS. Further studies are needed to confirm the association and the potential mechanisms.
Methods and Materials
Population
FAMHES was a population-based project that mainly focused on environmental and genetic factors as well as their interrelations. A total of 4303 men participated in a routine physical examination at the medical center in Fangchenggang First People’s Hospital between September and December 2009. Data were collected from 3593 participants using interviews. The response rate was 83.5%13. All participants signed written informed consent and provided a whole blood sample. The study was approved by the medical ethics committee at Guangxi Medical University. All methods were performed in accordance with their relevant guidelines and regulations.
ED definition and sample screening
ED was defined using the International Index of Erectile Function (IIEF-5) system38. This system asks five questions that are mainly focused on erection confidence, erection firmness, maintenance ability, maintenance frequency, and satisfaction, with the total score ranging from 0 to 25. Each question has six answers, which score 0–5 points. Lower scores indicate a reduced sexual function and an increased severity of ED. As with the previous standard39, the population can be divided into four categories: no dysfunction (IIEF-5 score: 22–25), mild dysfunction (17–21), moderate dysfunction (12–16), and severe dysfunction (5–11). Moreover, an IIEF-5 score of 22 can be used to subdivide the population into those with ED (IIEF-5 ≤ 21; mild, moderate and severe dysfunction) and those without ED (IIEF-5 > 21).
Subjects were excluded from the analysis if: (i) there was no data available regarding their serum OC level (e.g., they failed to contribute a blood sample or the instrument failed to detect a value); (ii) incomplete information was available on the individual; (iii) they provided incomplete answers to the ED questions; (iv) they suffered from a disease (i.e., myocardial infarction, congestive heart failure, stroke, hyperthyroidism, rheumatoid arthritis, acquired immune deficiency syndrome, or any kind of cancer); had a history of pelvic or urinary tract trauma, surgery, inflammation or chronic hepatitis, etc.; or were taking medications (i.e., psychotropic drugs, nonsteroidal anti-inflammatory drugs, antibiotics, spironolactone, cimetidine, glucocorticoids or other steroidal drugs, etc.) which may influence the level of serum OC and the normal status. Finally, 1567 eligible samples were involved in the subsequent analyses.
Interview and physical examination
Essential information (e.g., age, sex, smoking, drinking, etc.) was collected from eligible individuals during a face-to-face interview, and a complete physical examination (e.g., height, weight, waistline, hipline, etc.) was also performed. Smoking status and alcohol consumption were divided into “smoking/drinking” or “non-smoking/non-drinking” groups. As for the physical examination, body mass index (BMI) was calculated using body weight (measured when wearing thin clothing) and height (measured without shoes). BMI was categorized into three groups according to the Chinese standard: normal weight (<24.0 kg/m2), overweight (24.0–27.9 kg/m2), and obese (≥28.0 kg/m2). Meanwhile, the waist–hip ratio (WHR) was categorized as normal weight (WHR ≤ 0.9) or obese (WHR > 0.9)40. The waist circumference was measured at the midpoint between the inferior costal margin and the superior iliac crest on the mid-axillary line, and the hipline was defined as the maximum circumference over the buttocks.
Metabolic syndrome (MetS) and testosterone
MetS was previously shown to be significantly associated with ED and OC41,42. Meanwhile, as an important hormone in men, testosterone was believed to influence the levels of OC and the development of ED9,23. Therefore MetS and testosterone were also included in this study.
According to the updated National Cholesterol Education Program Adult Treatment Panel III for Asian Americans43, MetS was defined as having three or more of the following components: (1) waist circumference ≥ 90 cm, (2) triglycerides ≥ 1.7 mmol/L, (3) HDL-C < 1.03 mmol/L, (4) blood pressure ≥ 130/85 mmHg or requiring the use of antihypertensive medications, and (5) fasting glucose ≥ 5.6 mmol/L, being previously diagnosed with type 2 diabetes mellitus, or using oral antidiabetic agents or insulin.
Serum examination
Blood samples were transported to the Department of Clinical Laboratory at the First Affiliated Hospital of Guangxi Medical University in Nanning within 2–3 h, where they were subsequently centrifuged within 15–25 min and stored at −80 °C. The serum OC and testosterone levels were measured using an electrochemiluminescence immunoassay on a COBAS 6000 system E601 (Elecsys module) immunoassay analyzer (Roche Diagnostics, GmbH, Mannheim, Germany). Triglyceride levels, HDL-C, and serum glucose were measured enzymatically on a Dimension-RxL Chemistry Analyzer (Dade Behring, Newark, DE).
Statistical analysis
Before the analyses, serum OC and testosterone levels were logarithmically transformed to achieve approximate Gaussian distribution. Based on their IIEF-5 scores, eligible samples were divided into those with ED (IIEF-5 ≤ 21) and those without ED (IIEF-5 > 21). Student’s t-tests and X2 tests were applied where appropriate. Following the baseline analysis, linear and binary regression analyses were applied to assess the association between OC and ED risk after adjusting for three different models of covariates: unadjusted, age-adjusted, and multivariate-adjusted models. In the multivariate-adjusted model, the covariates were age, smoking status, alcohol consumption, BMI, and WHR. To further detect a potential association, ED was subdivided into four groups: no dysfunction, mild dysfunction, moderate dysfunction, and severe dysfunction, and multinomial logistic regression analysis was conducted to evaluate the function of OC in the order of severity of ED. The OC level was subsequently divided into four groups (Q1 < 25%, 25% ≤ Q2 ≤ 50%, 50% < Q3 ≤ 75%, Q4 > 75%) and the lowest OC group was used as the reference. Previous studies suggested that 40 years may be the point at which the morbidity of ED increased12, therefore the participants were divided into four age groups: <40, 40–49, 50–59, and ≥60 years. The effect of age on the association between OC and ED risk was discussed using linear, binary, and multinomial logistic regression analyses. Besides age, testosterone, obesity, and MetS were also known to affect ED and OC to some extent9,23,41,42. Therefore subgroup analyses were also conducted for these influencing factors. Finally, ROC analysis was applied to assess the effects of OC in predicting the ED risk. All analyses were performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA) and R software. All statistical tests were two-tailed.
Acknowledgements
This work was supported by The Science and technology development plan of Guangxi (Guikegong 1598012-42).
Author Contributions
J.W. Cheng: Project development, Data collection, Manuscript writing. Y. Chen, J. Li: Data collection, Data analysis, Manuscript writing. J.L. Liao, Y.L. Hu, H.Y. Zhang, X.B. Yang, Q.Y. Wang, Z.N. Mo: Data collection, Data management.
Competing Interests
The authors declare no competing interests.
Footnotes
Yang Chen and Jie Li contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extraskeletal effects. J Cell Physiol. 2013;228:1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- 2.Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA. 1975;72:3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci USA. 1976;73:1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 5.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee NK, et al. Mauvais-Jarvis, P. Ducy, G. Karsenty, Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oury F, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holvik K, et al. Plasma osteocalcin levels as a predictor of cardiovascular disease in older men and women: a population-based cohort study. Eur J Endocrinol. 2014;171:161–170. doi: 10.1530/EJE-13-1044. [DOI] [PubMed] [Google Scholar]
- 9.Ling Y, et al. A common polymorphism rs1800247 in osteocalcin gene is associated with hypertension and diastolic blood pressure levels: the Shanghai Changfeng study. J Hum Hypertens. 2016;30:679–684. doi: 10.1038/jhh.2016.16. [DOI] [PubMed] [Google Scholar]
- 10.Liao M, et al. Testosterone is associated with erectile dysfunction: a cross-sectional study in Chinese men. PLoS One. 2012;7:e39234. doi: 10.1371/journal.pone.0039234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan ME, Keoghane SR, Miller MA. Vascular risk factors and erectile dysfunction. BJU Int. 2001;87:838–845. doi: 10.1046/j.1464-410x.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 12.Nehra A, et al. Diagnosis and treatment of erectile dysfunction for reduction of cardiovascular risk. J Urol. 2013;189:2031–2038. doi: 10.1016/j.juro.2012.12.107. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Immunization associated with erectile dysfunction based on cross-sectional and genetic analyses. PLoS One. 2014;9:e111269. doi: 10.1371/journal.pone.0111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price PA, Poser JW, Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci USA. 1976;73:3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quilter M, Hodges L, von Hurst P, Borman B, Coad J. Male Sexual Function in New Zealand: A Population-Based Cross-Sectional Survey of the Prevalence of Erectile Dysfunction in Men Aged 40-70 Years. J Sex Med. 2017;14:928–936. doi: 10.1016/j.jsxm.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Foresta C, et al. Increased levels of osteocalcin-positive endothelial progenitor cells in patients affected by erectile dysfunction and cavernous atherosclerosis. J Sex Med. 2010;7:751–757. doi: 10.1111/j.1743-6109.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- 17.Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen defciency. J Androl. 2005;26:242–248. doi: 10.1002/j.1939-4640.2005.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 18.Traish AM, Kim N. Weapons of penile smooth muscle destruction: androgen defciency promotes accumulation of adipocytes in the corpus cavernosum. Aging Male. 2005;8:141–146. doi: 10.1080/13685530500328183. [DOI] [PubMed] [Google Scholar]
- 19.Yildiz O, et al. Testosterone might cause relaxation of human corpus cavernosum by potassium channel opening action. Urology. 2009;74:229–232. doi: 10.1016/j.urology.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Corona G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11:1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 21.Traish A, Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med. 2005;2:759–770. doi: 10.1111/j.1743-6109.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Penson DF, Ng C, Cai L, Rajfer J, Gonzalez-Cadavid NF. Androgen and pituitary control of penile nitric oxide synthase and erectile function in the rat. Biol Reprod. 1996;55:567–574. doi: 10.1095/biolreprod55.3.567. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio S, et al. Trunk fat negatively influences skeletal and testicular functions in obese men: clinical implications for the aging male. Int J Endocrinol. 2013;2013:182753. doi: 10.1155/2013/182753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong N, et al. Positive Correlation between Serum Osteocalcin and Testosterone in Male Hyperthyroidism Patients with High Bone Turnover. Exp Clin Endocrinol Diabetes. 2016;124:452–456. doi: 10.1055/s-0042-107944. [DOI] [PubMed] [Google Scholar]
- 25.McBride JA, Carson CC, 3rd, Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8:47–60. doi: 10.1177/1756287215612961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor DB, et al. The relationships between sex hormones and sexual function in middle-aged and older European men. J Clin Endocrinol Metab. 2011;96:E1577–1587. doi: 10.1210/jc.2010-2216. [DOI] [PubMed] [Google Scholar]
- 27.Rohrmann S, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control. 2011;22:1141–1151. doi: 10.1007/s10552-011-9790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaidya D, et al. Association of baseline sex hormone levels with baseline and longitudinal changes in waist-to-hip ratio: Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond). 2012;36:1578–1584. doi: 10.1038/ijo.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamacake KG, et al. Impact of body mass index, age and varicocele on reproductive hormone profile from elderly men. Int Braz J Urol. 2016;42:365–372. doi: 10.1590/S1677-5538.IBJU.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigueras-Villaseñor RM, et al. Alterations in the spermatic function generated by obesity in rats. Acta Histochem. 2011;113:214–220. doi: 10.1016/j.acthis.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J, et al. Correlation between sex hormone levels and obesity in the elderly male. Aging Male. 2012;15:85–89. doi: 10.3109/13685538.2012.666585. [DOI] [PubMed] [Google Scholar]
- 33.De Pergola G, et al. Independent Relationship of Osteocalcin Circulating Levels with Obesity, Type 2 Diabetes, Hypertension, and HDL Cholesterol. Endocr Metab Immune Disord Drug Targets. 2016;16:270–275. doi: 10.2174/1871530317666170106150756. [DOI] [PubMed] [Google Scholar]
- 34.Bao Y, et al. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J Clin Endocrinol Metab. 2013;98:345–351. doi: 10.1210/jc.2012-2906. [DOI] [PubMed] [Google Scholar]
- 35.Bezerra dos Santos Magalhães K, et al. Metabolic syndrome and central fat distribution are related to lower serum osteocalcin concentrations. Ann Nutr Metab. 2013;62:183–188. doi: 10.1159/000342561. [DOI] [PubMed] [Google Scholar]
- 36.Confavreux CB, et al. Lower serum osteocalcin is associated with more severe metabolic syndrome in elderly men from the MINOS cohort. Eur J Endocrinol. 2014;171:275–283. doi: 10.1530/EJE-13-0567. [DOI] [PubMed] [Google Scholar]
- 37.Teraoka A. Association Between Long-term Alcohol Consumption and Undercarboxylated Osteocalcin. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2015;50:196–205. [PubMed] [Google Scholar]
- 38.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 39.Kupelian V, Araujo AB, Chiu GR, Rosen RC, McKinlay JB. Relative contributions of modifiable risk factors to erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. Prev Med. 2010;50:19–25. doi: 10.1016/j.ypmed.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Lu FC. Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17:Suppl, 1–36. [PubMed] [Google Scholar]
- 41.Chen K, et al. Metabolic syndrome: a potential and independent risk factor for erectile dysfunction in the Chinese male population. Urology. 2012;80:1287–1292. doi: 10.1016/j.urology.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Liu JJ, et al. Elevated undercarboxylated and reduced carboxylated osteocalcin are associated with metabolic syndrome in middle age Asian females. Exp Clin Endocrinol Diabetes. 2013;121:329–333. doi: 10.1055/s-0033-1336723. [DOI] [PubMed] [Google Scholar]
- 43.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]