Abstract
Early life stress has been associated with disrupted functional connectivity between the amygdala and medial prefrontal cortex (mPFC), but it is unknown how early in development stress-related differences in amygdala–mPFC connectivity emerge. In a resting-state functional connectivity (rs-FC) analysis with 79 four- to seven-year-old children, we found a significant correlation between more adverse experiences and weaker amygdala–mPFC rs-FC. We also found that weaker amygdala–mPFC rs-FC was associated with higher levels of aggressive behavior and attention problems. These findings suggest that the impact of stress on emotional circuitry is detectable in early childhood and that this impact is associated with mental health difficulties. Connectivity in this circuit may be useful as a marker for mental health risk and for tracking the efficacy of early interventions.
Keywords: stress, adversity, amygdala, medial prefrontal cortex, functional connectivity
Introduction
Childhood adversity increases the risk for mental health disorders (Shonkoff et al., 2009; Green et al., 2010; McLaughlin et al., 2012). One pathway by which adversity may confer mental health risk is through changes to the amygdala, a brain region central to threat detection and emotional salience (Phelps and LeDoux, 2005), and medial prefrontal cortex (mPFC), a brain region thought to modulate amygdala activity in support of emotion regulation (Quirk and Beer, 2006; Etkin et al., 2011; McLaughlin et al., 2014). Disruptions in frontoamygdala functional connectivity have been observed in a wide array of mental health disorders, including depression (Kaiser et al., 2015), anxiety (Kim et al., 2011; Hamm et al., 2014), post-traumatic stress disorder (Gilboa et al., 2004; Bryant et al., 2008), and personality and conduct disorders (Marsh et al., 2008). Thus, characterizing the link between early adversity and atypical development of the frontoamygdala network may provide insight into the emergence of psychopathology.
Adversity has been linked to amygdala volume (increases: Tottenham et al., 2010; Evans et al., 2016; decreases: Hanson et al., 2015), reduced cortical thickness and volume in mPFC (Hanson et al., 2010; Kelly et al., 2013), and increased amygdala reactivity during emotion processing and regulation tasks (McCrory et al., 2011; Dannlowski et al., 2012; Marusak et al., 2015; Swartz et al., 2015). Low socioeconomic status (SES), which elevates the risk of experiencing adversity (Brooks-Gunn and Duncan, 1997), is also associated with amygdala volume (Noble et al., 2012; Evans et al., 2016), mPFC structure (Noble et al., 2015) and amygdala reactivity (Kim et al., 2013).
In addition to its associations with the structure and function of the amygdala and mPFC, adversity has also been linked to the communication between these regions. Causal experimental designs in animals have shown that stress alters amygdala–PFC interactions (Sánchez et al., 2001; Milad et al., 2006). In correlational studies in humans, early life stress has been linked to altered amygdala–mPFC functional connectivity during emotional tasks (Gee et al., 2013; Javanbakht et al., 2015; Marusak et al., 2015; Herringa et al., 2016). Stress-related changes have also been observed in resting-state functional connectivity (rs-FC). Such changes are of particular interest because rs-FC is not biased by a task—it is thought to be a dynamic measure of the history of co-activation between brain regions (Guerra-Carrillo et al., 2014; Gabard-Durnam et al., 2016; Poldrack, 2017). In adults, exposure to childhood trauma is consistently negatively correlated with amygdala–mPFC rs-FC (Herringa et al., 2013; Birn et al., 2014; Fan et al., 2014). Early childhood cortisol levels also show a negative relationship with amygdala–mPFC rs-FC in adulthood (Burghy et al., 2012). Altered amygdala–mPFC rs-FC has been observed in older children and adolescents, between the ages of 9 and 15, but the directionality of the finding is reversed: two studies, one on negative life events (Pagliaccio et al., 2015) and one on trauma exposure (Thomason et al., 2015), observed a positive correlation between stress and amygdala–mPFC rs-FC. It is possible that stress interacts with ongoing developmental changes, leading to inconsistencies in correlation direction.
Amygdala–mPFC rs-FC not only reflects individual differences in early life stress exposure it also mediates the relationship between early life stress and mental health symptoms (Burghy et al., 2012; Herringa et al., 2013; Pagliaccio et al., 2015). These findings suggest that early life stress alters frontoamygdala circuitry, conferring risk for mental health problems (VanTieghem and Tottenham, 2017). However, because studies to date have focused on participants from late childhood through adulthood, it is unclear how early such alterations emerge. Little is known about the effects of stress on amygdala–mPFC functional connectivity in early childhood, a time during which the brain is perhaps most plastic and amenable to intervention (Fox et al., 2010). Further, because many previous studies have focused on severe cases of adversity and trauma, it is unknown whether more typical variation in early stress exposure relates to differences in amygdala–mPFC circuitry and mental health in early childhood.
In the current study, we investigated whether exposure to stressful life events, such as the death of a family member, parental conflict or a serious accident, is associated with weakened amygdala–mPFC rs-FC in children between the ages of 4 and 7 years old. We also tested whether weakened amygdala–mPFC rs-FC is related to mental health symptoms that reflect early developmental risk for broad adult deficits in emotion regulation (Holtmann et al., 2011; Bellani et al., 2012). We focused on the dysregulation profile of the Child Behavior Checklist, which includes the ‘AAA’ symptoms: aggressive behavior, attention problems and anxiety/depression. This profile was first identified by a meta-analysis on the mental health symptoms associated with bipolar disorder in childhood (Mick et al., 2003), and since has been associated with Axis I disorders more broadly (Biederman et al., 2012; Mbekou et al., 2014; Uchida et al., 2014). Longitudinal studies have found that elevated AAA symptoms in childhood predict impaired psychosocial functioning in adulthood, including mood and anxiety disorders, attention deficit hyperactivity disorder (ADHD), disruptive behavior disorders and substance abuse (Meyer et al., 2009; Althoff et al., 2010; Holtmann et al., 2011). Finally, because socioeconomic status (SES) has been linked to early life stress (Brooks-Gunn and Duncan, 1997), amygdala–mPFC connectivity (Javanbakht et al., 2015) and mental health symptoms (Lorant et al., 2003; Reiss, 2013), we recruited a socioeconomically diverse sample and conducted our analyses with and without controlling for SES. To our knowledge, this work represents the first investigation of the links between normative stress exposure, amygdala–mPFC functional connectivity and mental health symptoms in young children.
Methods
Participants
This study was approved by the Committee on the Use of Humans as Experimental Subjects at the Massachusetts Institute of Technology (MIT). All parents provided informed consent, and participants provided verbal assent. Participants were recruited from the Greater Boston Area as part of two larger studies, one study on executive function development, and one study on an intervention (only data prior to the intervention were included). Recruitment for the first study occurred through postings on parent forums, magazine ads, community family events, and Head Start programs. Recruitment for the second study occurred through schools. In an initial screening interview for both studies, parents were asked whether their child had a medical diagnosis of a neurological or psychiatric disorder. For the executive function study, children were excluded if they had a medical diagnosis. The parent of one child reported that their child may have ADHD, but was not taking medication or receiving other treatments, and so the child was included in this study. For the intervention study, children with diagnoses were included in the MRI portion of the study if their diagnoses did not prevent them from participating in the intervention. Two children with Autism Spectrum Disorder participated in this study, but their data were not included for the present analyses.
Resting-state scans were completed for 108 participants. Seventy-nine participants were included in the final sample. Demographic data of this final sample, which was 50.6% female, are summarized in Table 1. Participants were excluded for falling asleep during the scan (1 participant), missing the Life Events Scale for Children (3 participants) or a diagnosis of Autism Spectrum Disorder (2 participants). Participants were also excluded due to average head displacement (3 translations, 3 rotations) of >1 mm (23 participants). The participants who were excluded for motion (n = 23) had slightly, but not significantly, lower maternal education (Exc. median: 16, Inc. median: 16, t = 1.88, P = 0.06), family income (Exc. median: $63 K, Inc. median: $85.5 K, U = 690.0, P = 0.09) and were slightly younger (Exc. median: 6.0, Inc. median: 6.2, t = 1.9, P = 0.06) than included participants (n = 79). Excluded participants did not differ from included participants on exposure to stressful life events (Exc. median: 0, Inc. median: 0, U = 853.5, P = 0.89).
Table 1.
Descriptive statistics of key variables
| n | Mean (s.d.) | Median | Range | Shapiro–Wilk W (P) | |
|---|---|---|---|---|---|
| Age (years) | 79 | 6.06 (0.96) | 6.21 | 4.05–7.96 | 0.98 (0.16) |
| Family income (thousands of dollars) | 78 | 106.6 (78.8) | 85.5 | 5–350 | 0.93 (<0.001) |
| Maternal education (years) | 78 | 16.0 (2.8) | 16.0 | 12–20 | 0.99 (0.92) |
| Stressful life events (LES-C) | 79 | 2.8 (5.1) | 0 | 0–26 | 0.71 (<0.001) |
| Aggressive behavior | 38 | 53.5 (6.7) | 50 | 50–77 | 0.69 (<0.001) |
| Attention problems | 38 | 53.1 (5.1) | 51 | 50–67 | 0.71 (<0.001) |
| Anxious/depressed | 38 | 52.7 (4.8) | 50.5 | 50–72 | 0.70 (<0.001) |
| Somatic complaints | 38 | 53.0 (4.5) | 50.0 | 50–64 | 0.71 (<0.001) |
Note: Results from Shapiro–Wilk tests for normality are shown in the rightmost column, and reveal that only age and maternal education do not significantly deviate from a normal distribution.
Questionnaires
Parents were asked to report total annual income and maternal education. Income data was missing for one child, and maternal education data was missing for one child. Parents completed a modified form of the Life Events Scale for Young Children (LES-C) (Coddington, 1972), which asked parents to report whether specific events had happened to their child within the last 12 months and how stressful the child found these events, from 0 to 4. Examples of items include: ‘Your child had a serious accident or illness’, ‘A family member or close relative died’ and ‘You separated or got divorced from your partner’. LES-C score was calculated by summing the stress ratings for all events. In one of the studies (n = 38), parents also completed the Child Behavior Checklist (CBCL) to identify behavioral and emotional problems (Achenbach and Rescorla, 2000, 2001). Depending on the age of the participant, either the preschool (CBCL/1½–5) or the school-aged (CBCL/6–18) version was administered. Across the two versions of the CBCL, there were four subscales that were consistent: the AAA subscales (Aggressive Behavior, Attention Problems, Anxious/Depressed) and the Somatic Complaints subscale. As described earlier, there is mounting evidence that the dysregulation profile described by the AAA subscales is associated with later psychopathology (Mick et al., 2003; Meyer et al., 2009; Althoff et al., 2010; Holtmann et al., 2011; Bellani et al., 2012; Biederman et al., 2012; Mbekou et al., 2014; Uchida et al., 2014). However, because there has also been some research linking Somatic Complaints to anxiety, mood disorders and ADHD (Kasius et al., 1997; Egger et al., 1999), all four subscales were analyzed in relation to amygdala connectivity. Descriptive statistics on the LES-C and the four subscales of the CBCL are shown in Table 1. Correlations among these variables and demographic measures are presented in Table 2.
Table 2.
Correlations between demographic and questionnaire measures
| 1. Age | 2. Family Income | 3. Maternal Education | 4. LES-C | 5. Aggressive Behavior | 6. Attention Problems | 7. Anxious/ Depressed | 8. Somatic Complaints | |
|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | – | |||||||
| 2. Family income (thousands of dollars) | 0.23* | – | ||||||
| 0.05 | ||||||||
| 78 | ||||||||
| 3. Maternal education (years) | 0.11 | 0.66*** | – | |||||
| 0.33 | <0.001 | |||||||
| 78 | 77 | |||||||
| 4. Stressful life events (LES-C) | −0.09 | −0.38*** | −0.27* | – | ||||
| 0.43 | <0.001 | 0.02 | ||||||
| 79 | 78 | 78 | ||||||
| 5. Aggressive behavior | 0.12 | −0.34* | −0.07 | 0.29 | – | |||
| 0.49 | 0.04 | 0.66 | 0.08 | |||||
| 38 | 37 | 37 | 38 | |||||
| 6. Attention problems | 0.02 | −0.25 | −0.07 | 0.10 | 0.36* | – | ||
| 0.92 | 0.13 | 0.68 | 0.57 | 0.03 | ||||
| 38 | 37 | 37 | 38 | 38 | ||||
| 7. Anxious/depressed | 0.09 | −0.28 | 0.02 | 0.20 | 0.47** | 0.38* | – | |
| 0.61 | 0.09 | 0.89 | 0.24 | <0.001 | 0.02 | |||
| 38 | 37 | 37 | 38 | 38 | 38 | |||
| 8. Somatic complaints | 0.19 | −0.05 | 0.04 | <0.001 | 0.22 | 0.36* | 0.43** | – |
| 0.25 | 0.77 | 0.82 | 0.98 | 0.18 | 0.03 | 0.01 | ||
| 38 | 37 | 37 | 38 | 38 | 38 | 38 |
Note: Top values are Spearman rho statistics, middle values are P values, bottom values are ns.
P < 0.05,
P < 0.01,
P < 0.001.
Neuroimaging data acquisition
Imaging was performed at the Athinoula A. Martinos Imaging Center at MIT. Prior to the scanning session, participants were acclimated to the scanning environment with a mock scanner that simulates typical MRI noises. Participants practiced keeping still in the mock scanner, and were given feedback (paused video) if they moved their heads. During the MRI scan, a researcher stayed in the scanner room with the participant to reassure the child and to touch the child’s foot if the child moved.
Scanning was performed using a Siemens MAGNETOM Trio Tim 3 T MRI scanner with a 32 channel coil. A whole-brain, high-resolution, T1-weighted multi-echo structural scan (MPRAGE) was collected (acquisition parameters: TR = 2530 ms, TE = 1.64 ms/3.5 ms/5.36 ms/7.22 ms, flip angle = 7°, voxel size = 1 mm isotropic, matrix size = 192×192, 176 sagittal slices, FOV = 192 mm). This sequence was optimized for participants with high motion (Tisdall et al., 2012). A 5 minute T2*-weighted gradient echo resting-state scan was also collected (acquisition parameters: TR = 2500 ms, TE = 30 ms, flip angle = 90°, voxel size = 3.0×3.0×3.3 mm, matrix size = 64×64, 41 axial slices, 120 volumes, FOV = 192 mm) with a real-time motion correction technique called Prospective Acquisition Correction (PACE), which adjusts slice orientation and position as the volumes are collected (Thesen et al., 2000). The first four volumes of each scan were automatically discarded to allow time for scanner magnetization to reach equilibrium. Participants looked at a fixation cross throughout the scan.
Structural analyses
Structural data were used in registration (see below), to identify a bilateral amygdala seed, and to calculate bilateral amygdala volume. The structural images were examined and rated based on quality (1 = best quality to 4 = worst quality, mean = 2.20, s.d. = 0.68) by two researchers who were blind to any other data about the participants. Lower quality structural scans were associated with higher scores on the LES-C (rs(77) = 0.23, P = 0.046, 95% CI [0.03, 0.44]) and on the Aggressive Behavior subscale of the CBCL (rs(36) = 0.32, P = 0.03, 95% CI [0.07, 0.63]). No relations were found between scan quality and the Attention Problems (rs(36) = 0.22, P = 0.19, 95% CI [−0.07, 0.52]), Anxious/Depressed (rs(36) = 0.04, P = 0.81, 95% CI [−0.28, 0.35]) or Somatic Complaints (rs(36) = −0.17, P = 0.30, 95% CI [−0.47, 0.11]) subscales. Scan quality was included as a covariate in structural analyses, and controlled for in whole-brain resting-state analyses.
Cortical reconstruction and volumetric segmentation of the structural images were performed using the standard FreeSurfer processing stream (Dale et al., 1999). The surfaces were visually inspected for errors and manually edited by researchers who were blind to participant information. Cortical reconstructions were checked for accuracy after editing, and were deemed accurate by blinded researchers for all participants included in the functional connectivity analyses. The amygdala segmentations were also visually inspected for quality, and were found to be accurate for all participants.
Resting-state analyses
The functional imaging data were preprocessed using Nipype, a Python-based framework for flexibly integrating neuroimaging analysis tools (Gorgolewski et al., 2011). The software packages used in this preprocessing pipeline included FMRIB Software Library (FSL v5.0.8; Jenkinson et al., 2012), FreeSurfer (v5.3.0; Dale et al., 1999), Advanced Normalization Tools (ANTs v2.1.0; Avants et al., 2011) and Nipype’s implementation of Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect/).
Simultaneous realignment and slice timing correction was conducted using an algorithm implemented in Nipy (Roche, 2011). Outlier volumes in the functional data were defined using ART based on composite motion (>2 mm of head displacement between volumes) and global signal intensity (>3 s.d.s from the mean). Included participants had an average composite motion of <1 mm (mean = 0.35 mm, s.d. = 0.23 mm), and no participants had >15% outlier volumes (mean = 3.23%, s.d. = 3.69%).
The following confounds were regressed out of the functional data: 6 realignment parameters (3 translations, 3 rotations) and their first-order derivatives, outlier volumes flagged by ART (one nuisance regressor per outlier), composite motion, and linear and quadratic polynomials to detrend the data. Five principal components were also derived from segmentations of both cerebrospinal fluid (CSF) and white matter (WM), and regressed from the data, in order to help correct for physiological noise like heart rate and respiration (aCompCor; Behzadi et al., 2007). FMRIB’s Automated Segmentation Tool (FAST; Zhang et al., 2001) was used to generate the CSF and WM segmentations from the structural image.
The functional data were bandpass filtered (0.01–0.1 Hz), spatially smoothed with an isotropic 6 mm Gaussian kernel (FWHM), and normalized to the OASIS-30 Atropos template (in MNI152 2 mm space) in a two-step process. First, the median functional image was coregistered to the reconstructed surfaces using FreeSurfer’s bbregister (Greve and Fischl, 2009); next, the structural image was registered to the OASIS-30 Atropos MNI152 template using ANTs. The transformation matrices generated by these two steps were concatenated, allowing images to be transformed directly from functional to MNI space in a single interpolation step.
Bilateral amygdala was selected as the seed, and defined using FreeSurfer’s individually generated subcortical segmentations (Fischl et al., 2002). The average time series of the bilateral amygdala seed was extracted from the unsmoothed functional data, and FSL’s GLM tool was used to generate whole-brain subject-level connectivity maps. Because confounds related to motion and physiological noise were already filtered out of the functional data during the preprocessing stream, these subject-level GLMs only contained the seed time series as a regressor.
Group-level analyses were conducted with FMRIB’s Local Analysis of Mixed Effects tool (FSL’s FLAME 1), within a mask limited to voxels with unanimous coverage across subjects (which covered the cortex but not inferior cerebellum). The following group-level analyses were conducted: (i) positive and negative group average, (ii) positive and negative correlations with age, (iii) gender differences, (iv) positive and negative correlations with LES-C, and (v) positive and negative correlations with CBCL subscales. All analyses control for number of outliers. We controlled for age and gender unless these variables were the variables of interest. We controlled for dataset (executive function study or intervention study) in analyses 1–4. Analysis 5 only involved data from the executive function study. Residuals of the whole-brain GLMs were normally distributed.
Z-statistic maps were corrected for multiple comparisons with parametric clusterwise inference using FSL’s cluster tool (relies on Gaussian Random Field Theory) at a cluster-defining threshold of z = 3.1 (P < 0.001), neighborhood size of 26, and an FWE-corrected threshold of P < 0.05, based on evidence from Eklund et al. that false positives are not well controlled at a less stringent threshold (Eklund et al., 2016). Following recommendations for neuroimaging reporting (Poldrack et al., 2017), uncorrected statistical maps are available on NeuroVault (https://neurovault.org/collections/ZGBHJQOZ/). To determine whether reduced functional connectivity between the amygdala and mPFC is associated with mental health symptoms, parameter estimates were extracted from the whole-brain results and correlated with CBCL scores. All analyses were conducted in Stata 14.1. Confidence intervals were bootstrapped with 1000 repetitions.
Summary of motion considerations
Because young children are more likely to move their heads in the scanner, and because motion is especially problematic for resting-state fMRI (Satterthwaite et al., 2012; Power et al., 2015), we adapted our data collection procedures to minimize motion and controlled for motion in our analyses. Here, we summarize these steps. First, children practiced keeping still in a mock scanner while watching a video. The video paused if the child moved >1 mm. The child practiced in the mock scanner for ∼15 minutes. During the scan, a researcher who had previously worked with the child stayed in the scanner room to reassure the child and to remind the child to stay still. Motion correction of the resting-state data was performed both prospectively and retrospectively: prospectively by using real-time motion correction during data collection, and retrospectively by performing simultaneous motion and slice timing correction during preprocessing. Participants were excluded if average composite motion was >1 mm. Motion outlier volumes in the resting-state data were defined based on composite motion >2 mm between volumes. No included participants had >15% outlier volumes. At the single-subject level, we regressed out motion parameters including realignment parameters and their first-order derivatives, outlier volumes and composite motion. Finally, we controlled for number of outlier volumes in all group analyses. We did not observe correlations between number of outliers and age, family income, maternal education, amygdala–mPFC rs-FC parameter estimates or the CBCL measures (P > 0.05).
Results
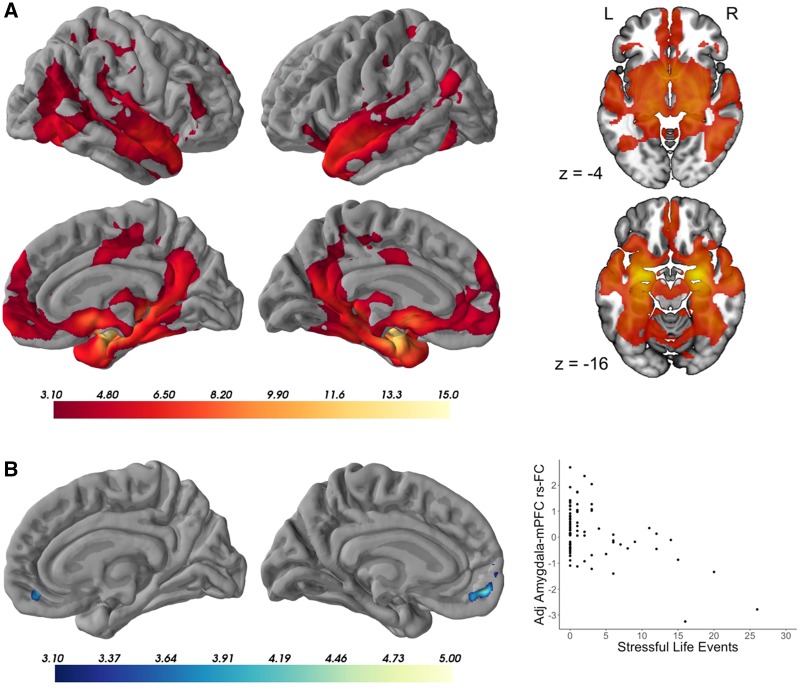
The amygdala showed widespread positive functional connectivity with mPFC, as well as with other hubs of the limbic and default mode networks (Yeo et al., 2011), and with subcortical regions including the hippocampus, striatum and thalamus (Figure 1A, n = 79, z = 3.1, P < 0.05). No regions showed significant negative connectivity with the amygdala. Additionally, there were no significant age-related changes or gender differences in amygdala functional connectivity.
Fig. 1.
(A) Amygdala functional connectivity in the group average. Model controls for age, gender, outliers and dataset, and is corrected for multiple comparisons at z = 3.1, P <0.05. N = 79. (B) Negative correlation between amygdala connectivity and stressful life events. Peak voxel coordinates (x, y, z, in MNI space): −10, 58, −6. Maximum z-statistic = 4.75, cluster volume = 699 voxels. Model controls for age, gender, outliers and dataset, and is corrected for multiple comparisons at z = 3.1, P <0.05. N = 79. Scatterplot shows relationship between extracted parameter estimates (adjusted for age, gender, outliers and dataset) and exposure to stressful life events (total score on Life Events Scale for Children). mPFC, medial prefrontal cortex; rs-FC, resting-state functional connectivity.
Greater exposure to stressful life events was significantly correlated with weaker functional connectivity between bilateral amygdala and bilateral mPFC (Figure 1B, n = 79, z = 3.1, P < 0.05). There were no significant positive correlations between LES-C and amygdala functional connectivity. Because greater exposure to stressful life events (LES-C) was related to lower family income (rs(76) = −0.38, P = 0.0007, 95% CI [−0.58, −0.20]), and less strongly to lower maternal education (rs(76) = −0.27, P = 0.02, 95% CI [−0.48, −0.07]) (Table 2), the analyses were repeated controlling for family income and maternal education, and the results were similar (Supplementary Figure S1). The results were also similar after controlling for structural scan quality (Supplementary Figure S2).
To better understand whether weaker amygdala–mPFC connectivity was related to susceptibility to mental health problems, we extracted parameter estimates from the whole-brain result. These parameter estimates did not significantly deviate from a normal distribution (W = 0.97, P = 0.09). Amygdala–mPFC rs-FC was not correlated with age (r(77) = −0.0005, P = 0.997, 95% CI [−0.24, 0.23]), did not differ by gender (t(77) = 1.38, P = 0.17, 95% CI [−0.23, 0.22]), did not differ between datasets (t(77) = 0.16, P = 0.87, 95% CI [−0.24, 0.23]), and was not related to outliers (rs(77) = −0.05, P = 0.68, 95% CI [−0.30, 0.19]). To be consistent with the whole-brain analyses, analyses of extracted parameter estimates controlled for age, gender and outliers.
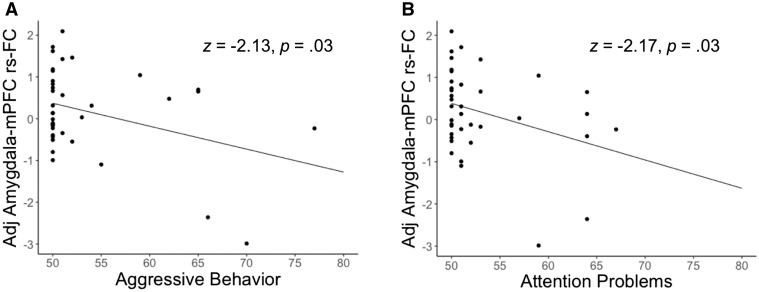
Amygdala–mPFC rs-FC was negatively related to Aggressive Behavior (n = 38, z = −2.13, P = 0.03, ϐ = −6.60, 95% CI [−12.67, −0.53], Figure 2A) and to Attention Problems (n = 38, z = −2.17, P = 0.03, ϐ = −4.63, 95% CI [−8.80, −0.46], Figure 2B). No relationships were observed between amygdala–mPFC rs-FC and the Anxious/Depressed subscale (n = 38, z = −0.15, P = 0.89, ϐ = −0.31, 95% CI [−4.36, 3.74]) or the Somatic Complaints subscale (n = 38, z = 1.22, P = 0.22, ϐ = 2.23, 95% CI [−1.36, 5.82]). At the whole-brain level, amygdala functional connectivity with ventral mPFC (orbital PFC) was related to Aggressive Behavior (Supplementary Figure S3). Whole-brain analyses linking amygdala functional connectivity to the other CBCL subscales were non-significant.
Fig. 2.
Negative relationships between amygdala–mPFC rs-FC and mental health symptoms. Scatterplots show parameter estimates extracted from result in Figure 1B, adjusted for age, gender and outliers. (A) Relationship between amygdala–medial prefrontal cortex (mPFC) resting-state functional connectivity (rs-FC) and the Aggressive Behavior subscale of the Child Behavior Checklist (CBCL) (n = 38). (B) Relationship between amygdala–mPFC rs-FC and the Attention Problems subscale of the CBCL (n = 38).
Amygdala–mPFC rs-FC was positively related to family income (n = 78, z = 3.09, P = 0.002, ϐ = 70.46, 95% CI [25.80, 115.13]), and slightly but not significantly related to maternal education (n = 78, z = 1.73, P = 0.08, ϐ = 1.48, 95% CI [−0.20, 3.16]). Controlling for family income and maternal education, the relationships between amygdala–mPFC rs-FC and CBCL subscales were similar in directionality but non-significant (n = 38, Aggressive Behavior: z = −1.44, P = 0.15, ϐ= −4.49, 95% CI [−10.60, 1.62]); Attention Problems: z = −1.89, P = 0.06, ϐ = −3.82, 95% CI [−7.78, 0.15]; Anxious/Depressed: z = 0.53, P = 0.60, ϐ = 1.17, 95% CI [−3.20, 5.55]; Somatic Complaints: z = 3.58, P = 0.06, ϐ = 3.58, 95% CI [−0.20, 7.36]).
Bilateral amygdala volume was not related to LES-C, family income or maternal education (P > 0.2). Amygdala volume additionally was not related to the Aggressive Behavior, Attention Problems, Anxious/Depressed or Somatic Complaints subscales (P > 0.2). Relationships were not changed by including intracranial volume as a covariate. The absence of significant relationships between variables of interest and amygdala volume suggests that differences in amygdala volume are unlikely to explain differences in amygdala functional connectivity. Further, controlling for bilateral amygdala volume in the whole-brain analysis with LES-C did not change the results (Supplementary Figure S4).
Discussion
The present study examined relationships between frontoamygdala circuitry, normative stressful experiences and mental health in early childhood. Exposure to more stressful life events was associated with decreased connectivity between bilateral amygdala and mPFC in young children between the ages of four and seven. Further, decreased connectivity between bilateral amygdala and mPFC was associated with increased levels of aggressive behavior and attention problems. These findings show that the relation between stressful life events and amygdala–mPFC functional connectivity is present early in development, and that this connectivity is associated with symptoms of poorer mental health. Further, whereas related prior findings observed altered frontoamygdala connectivity in older children following trauma, the present study shows that variation in connectivity is also related to variation in normative life stressors.
Weakened functional coupling may be a sign of less effective communication, and perhaps less powerful regulatory control of mPFC over the amygdala. Our results are consistent with previous studies in adults demonstrating that childhood adversity is related to weaker amygdala–mPFC functional coupling at rest (Herringa et al., 2013; Birn et al., 2014; Fan et al., 2014). Our findings complement work in older children and adolescents showing stress-related alterations to amygdala–mPFC rs-FC, although these results showed positive correlations between adversity and amygdala–mPFC rs-FC (Pagliaccio et al., 2015; Thomason et al., 2015). The directionality of the link between stress and frontoamygdala connectivity has varied in developmental studies, and the origin of this variability is unclear (Marusak et al., 2016). It could be due to methodological variability (Marusak et al., 2016) or could emerge from the interaction between stress and ongoing development. For example, there is evidence that stress is associated with faster maturation of frontoamygdala circuitry (Gee et al., 2013; Herringa et al., 2016; Thijssen et al., 2017). In the 3 year age range of our sample, we did not observe measurable age-related changes in connectivity in this circuit. Our finding of overall positive functional connectivity between amygdala and mPFC was, however, consistent with prior studies (Roy et al., 2009; Gabard-Durnam et al., 2014). We did not find evidence for negative amygdala functional connectivity with any regions of the brain. Although previous studies have shown negative connectivity between amygdala and regions like superior frontal gyrus, middle frontal gyrus, and the parietal and occipital lobes, these studies primarily included adolescents and adults (Roy et al., 2009; Gabard-Durnam et al., 2014). It may be that robust negative amygdala functional connectivity does not emerge until later in development. For example, a study examining age-related changes in the functional connectivity of mPFC found a shift from positive to negative connectivity with dorsolateral PFC between childhood and adolescence (Chai et al., 2014).
We also found that weaker amygdala–mPFC functional coupling was associated with more aggressive behavior and more attention problems, behaviors that predict risk for adult psychopathology (Meyer et al., 2009; Althoff et al., 2010; Holtmann et al., 2011; Bellani et al., 2012; Biederman et al., 2012; Mbekou et al., 2014; Uchida et al., 2014). We did not find a relationship between amygdala–mPFC rs-FC and somatic complaints, and, unlike in studies of older participants (Burghy et al., 2012; Herringa et al., 2013; Pagliaccio et al., 2015), we did not find a relationship with anxiety and depression. It is unclear whether our findings regarding symptoms of anxiety and depression differ from others because amygdala–mPFC connectivity is not related to symptoms of anxiety and depression in young children, or because parental report is less reliable than self-report on internalizing symptoms. Although our results add to the evidence that stress-related changes in amygdala connectivity confer risk of mental health problems, there is also evidence that such changes are protective (Gee et al., 2013; Herringa et al., 2016; Thijssen et al., 2017). It is as yet unclear as to when variation in frontoamygdala connectivity is protective or problematic for mental health.
Consistent with many other studies (Brooks-Gunn and Duncan, 1997), we found a positive correlation between SES, specifically family income, and stressful life events, suggesting that lower SES is associated with greater stress exposure. However, after controlling for SES, the relationship between stressful life events and amygdala–mPFC rs-FC remained similar. This suggests that it is stress, and not other associated features of lower SES, that is related to frontoamygdala circuitry (McLaughlin et al., 2014). Controlling for SES weakened relationships between amygdala–mPFC rs-FC and mental health symptoms, suggesting that other features of SES may be contributing to mental health. A larger sample size, perhaps with a weaker correlation between SES and adversity, is needed to fully understand how SES interacts with the ‘stress → amygdala–mPFC rs-FC → mental health’ pathway. In addition to changing the risk of stress exposure, SES could change the way in which stress affects the brain (e.g. via the presence or absence of social support) or could impact whether specific neural connectivity patterns give rise to mental health problems. For example, higher SES could be associated with greater compensatory activity in other brain networks.
Amygdala volume was not significantly related to stressful life events, SES, or mental health symptoms, suggesting that amygdala connectivity differences were unlikely to be driven by differences in volume. Previous studies have demonstrated both positive and negative associations between amygdala volume and early life stress (Tottenham et al., 2010; Lupien et al., 2011; Hanson et al., 2015). It may be that volumetric changes are specific to subregions of the amygdala that we could not identify at the resolution of our data.
This study has a number of limitations. First, our measure of stress exposure, the LES-C, is subjective. It requires an accurate memory for stressful events taking place within the previous year, as well as parent assessments of how stressful those events were for the child. Because the scale reflects differences in how children responded to the events, as reported by the parent, it is possible that weaker amygdala–mPFC connectivity confers a predisposition to finding events more stressful. Second, because the relationships between LES-C and the CBCL subscales were not significant in this sample, we were not able to test whether amygdala–mPFC rs-FC mediates the relationship between stress exposure and mental health, and therefore, we cannot rule out other relationships among stress, frontoamygdala circuitry and mental health. Third, our data are cross-sectional, so it cannot address questions about stress-induced changes in developmental trajectory. Longitudinal data from early childhood (or even infancy) into adolescence, with information on the occurrence, duration and severity of stressors, would be useful for further elucidating the mechanisms by which stress exposure shapes mental health. Finally, it is important to note that we cannot infer a causal role of stress from our correlational results. Factors such as genetics and prenatal exposures may covary with stress exposure, amygdala–mPFC rs-FC and mental health.
In sum, we demonstrated that relationships between normative stress exposure and frontoamygdala connectivity emerge in early childhood. Previous studies had conducted brain imaging years after the occurrence of early life stress, an approach with few practical applications for intervention. Our results suggest that abnormal amygdala functional connectivity in young children could be a potential marker of latent risk for poor emotion regulation capacity, and may manifest as clinically relevant symptoms later in life. However, our results also suggest that frontoamygdala connectivity could serve as a potential marker of early intervention efficacy. Early intervention programs, such as the PATHS program for socioemotional learning (Domitrovich et al., 2007; Fishbein et al., 2016), have shown promise for improving cognitive and emotional skills in children who have experienced adversity (Raver, 2012). However, there are still considerable individual differences in responsivity to such programs (Bradshaw et al., 2012). An important future direction is to understand whether neural measures, such as amygdala–mPFC connectivity, enhance the prediction of intervention outcomes in the short- and long-term (Gabrieli et al., 2015).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Acknowledgements
The authors thank the families and children for their participation in this research. They acknowledge the work of Sydney Robinson, Rachel Romeo, Arlette Reyes, Megumi Takada, Hannah Grotzinger and Ariella Yosafat on data collection, data entry and structural MRI data evaluation.
Funding
This work was supported by the National Institute of Child Health and Human Development (grant number 5F32HD079143) (A.P.M.), the Jacobs Foundation (A.P.M.), the University of Pennsylvania (A.T.P. and A.P.M.), the Walton Family Foundation (J.D.E.G.) and the Gates Foundation (J.D.E.G.).
References
- Achenbach T.M., Rescorla L.A. (2000). Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Achenbach T.M., Rescorla L.A. (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Althoff R.R., Verhulst F.C., Rettew D.C., Hudziak J.J., van der Ende J. (2010). Adult outcomes of childhood dysregulation: a 14-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(11), 1105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani M., Negri G.A.L., Brambilla P. (2012). The dysregulation profile in children and adolescents: a potential index for major psychopathology? Epidemiology and Psychiatric Sciences, 21(02), 155–9. [DOI] [PubMed] [Google Scholar]
- Biederman J., Petty C.R., Day H., et al. (2012). Severity of the aggression/anxiety-depression/attention child behavior checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics, 33(3), 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. (2014). Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depression and Anxiety, 31(10), 880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.P., Goldweber A., Fishbein D., Greenberg M.T. (2012). Infusing developmental neuroscience into school-based preventive interventions: implications and future directions. Journal of Adolescent Health, 51(2), S41–7. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Duncan G.J. (1997). The effects of poverty on children. Future of Children, 7(2), 55–71. [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., et al. (2008). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping, 29(5), 517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., et al. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. (2014). Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience, 26(3), 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington R.D. (1972). The significance of life events as etiologic factors in the diseases of children—II a study of a normal population. Journal of Psychosomatic Research, 16(3), 205–13. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–94. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., et al. (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–93. [DOI] [PubMed] [Google Scholar]
- Domitrovich C.E., Cortes R.C., Greenberg M.T. (2007). Improving young children’s social and emotional competence: a randomized trial of the preschool “PATHS” curriculum. Journal of Primary Prevention, 28(2), 67–91. [DOI] [PubMed] [Google Scholar]
- Egger H.L., Costello E.J., Erkanli A., Angold A. (1999). Somatic complaints and psychopathology in children and adolescents: stomach aches, musculoskeletal pains, and headaches. Journal of the American Academy of Child and Adolescent Psychiatry, 38(7), 852–60. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., Swain J.E., King A.P., et al. (2016). Childhood cumulative risk exposure and adult amygdala volume and function. Journal of Neuroscience Research, 94(6), 535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Herrera-Melendez A.L., Pestke K., et al. (2014). Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Human Brain Mapping, 35(10), 5328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- Fishbein D.H., Domitrovich C., Williams J., et al. (2016). Short-term intervention effects of the PATHS curriculum in young low-income children: capitalizing on plasticity. Journal of Primary Prevention, 37(6), 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1), 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., et al. (2014). The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage, 95, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Gee D.G., Goff B., et al. (2016). Stimulus-elicited connectivity influences resting-state connectivity years later in human development: a prospective study. Journal of Neuroscience, 36(17), 4771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Ghosh S.S., Whitfield-Gabrieli S. (2015). Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron, 85(1), 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A., Shalev A.Y., Laor L., et al. (2004). Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry, 55(3), 263–72. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., et al. (2011). Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Frontiers in Neuroinformatics, 5, 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., et al. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset. Of DSM-IV Disorders. Archives of General Psychiatry, 67(2), 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B., Mackey A.P., Bunge S.A. (2014). Resting-state fMRI: a window into human brain plasticity. Neuroscientist, 20(5), 522.. [DOI] [PubMed] [Google Scholar]
- Hamm L.L., Jacobs R.H., Johnson M.W., et al. (2014). Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of Mood & Anxiety Disorders, 4(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chung M.K., Avants B.B., et al. (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., et al. (2015). Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., et al. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America, 110(47), 19119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Burghy C.A., Stodola D.E., Fox M.E., Davidson R.J., Essex M.J. (2016). Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(4), 326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann M., Buchmann A.F., Esser G., Schmidt M.H., Banaschewski T., Laucht M. (2011). The Child Behavior Checklist-Dysregulation Profile predicts substance use, suicidality, and functional impairment: a longitudinal analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(2), 139–47. [DOI] [PubMed] [Google Scholar]
- Javanbakht A., King A.P., Evans G.W., et al. (2015). Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Frontiers in Behavioral Neuroscience, 9, 154.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). FSL. NeuroImage, 62(2), 782–90. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasius M.C., Ferdinand R.F., Berg H., Verhulst F.C. (1997). Associations between different diagnostic approaches for child and adolescent psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38(6), 625–32. [DOI] [PubMed] [Google Scholar]
- Kelly P.A., Viding E., Wallace G.L., et al. (2013). Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biological Psychiatry, 74(11), 845–52. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., et al. (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M., et al. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant V., Deliège D., Eaton W., Robert A., Philippot P., Ansseau M. (2003). Socioeconomic inequalities in depression: a meta-analysis. American Journal of Epidemiology, 157(2), 98–112. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Parent S., Evans A.C., et al. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 14324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G.V., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165(6), 712–20. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. (2015). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40(5), 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Thomason M.E., Peters C., Zundel C., Elrahal F., Rabinak C.A. (2016). You say “prefrontal cortex” and I say “anterior cingulate”: Meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Translational Psychiatry, 6(11), e944.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbekou V., Gignac M., MacNeil S., Mackay P., Renaud J. (2014). The CBCL dysregulated profile: an indicator of pediatric bipolar disorder or of psychopathology severity? Journal of Affective Disorders, 155, 299–302. [DOI] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., et al. (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21(23), R947–8. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Greif Green J., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69(11), 1151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S.E., Carlson G.A., Youngstrom E., et al. (2009). Long-term outcomes of youth who manifested the CBCL-Pediatric Bipolar Disorder phenotype during childhood and/or adolescence. Journal of Affective Disorders, 113(3), 227–35. [DOI] [PubMed] [Google Scholar]
- Mick E., Biederman J., Pandina G., Faraone S.V. (2003). A preliminary meta-analysis of the child behavior checklist in pediatric bipolar disorder. Biological Psychiatry, 53(11), 1021–7. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology, 73(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., et al. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., et al. (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. Journal of Abnormal Psychology, 124(4), 817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. (2017). Precision neuroscience: dense sampling of individual brains. Neuron, 95(4), 727–9. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Baker C.I., Durnez J., et al. (2017). Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18(2), 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16(6), 723–7. [DOI] [PubMed] [Google Scholar]
- Raver C.C. (2012). Low-income children’s self-regulation in the classroom: scientific inquiry for social change. American Psychologist, 67(8), 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Social Science & Medicine, 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Roche A. (2011). A four-dimensional registration algorithm with application to joint correction of motion and slice timing in fMRI. IEEE Transactions on Medical Imaging, 30(8), 1546–54. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., et al. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M.M., Ladd C.O., Plotsky P.M. (2001). Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology, 13(3), 419–49. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., et al. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P., Boyce W.T., McEwen B.S. (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA: Journal of the American Medical Association, 301(21), 2252–9. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Williamson D.E., Hariri A.R. (2015). Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. American Journal of Psychiatry, 172(3), 276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. (2000). Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine, 44(3), 457–65. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., et al. (2017). Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Development and Psychopathology, 29(02), 505–18. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience, 10(11), 1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall M.D., Hess A.T., Reuter M., Meintjes E.M., Fischl B., van der Kouwe A.J.W. (2012). Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine, 68(2), 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., et al. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M., Faraone S.V., Martelon M., et al. (2014). Further evidence that severe scores in the aggression/anxiety-depression/attention subscales of child behavior checklist (severe dysregulation profile) can screen for bipolar disorder symptomatology: a conditional probability analysis. Journal of Affective Disorders, 165, 81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M.R., Tottenham N. (2017). Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Current Topics in Behavioral Neurosciences. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.