Abstract.
We conducted a cross sectional study to assess 1) the association between access to basic sanitation and fecal contamination of sentinel toy balls and 2) if other sanitation factors such as shared use and cleanliness are associated with fecal contamination of sentinel toy balls. We assessed sanitation facilities in 454 households with a child aged 6–24 months in rural Bangladesh. We defined “basic” sanitation as access to improved sanitation facilities (pit latrine with a slab or better) not shared with other households. In each household, an identical toy ball was given to the target child. After 24 hours, the balls were rinsed to enumerate fecal coliforms as an indicator of household fecal contamination. Households with basic sanitation had lower fecal coliform contamination than households with no access to basic sanitation (adjusted difference in means: −0.31 log10 colony forming units [CFU]/toy ball; 95% confidence interval [CI]: −0.61, −0.01). Shared sanitation facilities of otherwise improved type were more likely to have visible feces on the latrine slab compared with private facilities. Among households with access to improved sanitation, households with no visible feces on the latrine slab had less toy ball contamination than households with visible feces on the latrine slab (adjusted difference in means: −0.38 log10 CFU/toy ball; 95% CI: −0.77, 0.02). Access to basic sanitation may prevent fecal contamination of the household environment. An Improved sanitation facility used by an individual household may be better in preventing household fecal contamination compared with improved facilities shared with other households.
INTRODUCTION
Inadequate sanitation is an important risk factor for poor health especially in low and middle income countries.1–4 In addition to its link with diarrhea morbidity5–8 and mortality,8–10 inadequate sanitation is associated with the risk of trachoma,11,12 helminthiasis,8,13,14 and schistosomiasis.3
The WHO/UNICEF Joint Monitoring Program (JMP) for water supply and sanitation categorizes sanitation as improved or unimproved for international monitoring. The sanitation categories that are considered as improved for international estimates include pit latrine with a slab, ventilated improved pit latrine, or flush/pour-flush latrine (Table 1).15–17 To monitor the progress toward the post-2015 Sustainable Development Goals (SDG) related to sanitation “basic sanitation” is defined as access to improved sanitation facilities not shared with other households.18 Since the Millennium Development Goal (MDG) period, shared facilities of otherwise improved technology were not considered as basic because of concerns regarding cleanliness, maintenance, and access.15,19 However, the implications of using a shared facility are likely to be different in urban and rural contexts. In crowded, urban areas of most low income countries, shared facilities might be the only viable option to avoid open defecation, and in rural areas households with family ties often share a facility to keep costs down.15 To monitor the progress toward sanitation for the post-2015 SDG, the technical working group on sanitation proposed that sanitation facilities shared by up to five households who know each other should be considered as basic. However, the JMP decided to continue excluding shared facilities from basic sanitation and report shared facilities separately as a different rung (limited sanitation) in the sanitation ladder because of concerns including lack of country level data on limited sharing and lack of privacy and security of the shared facilities.16,18,20 There is very limited empirical evidence to judge the extent to which basic sanitation facilities achieve their purpose in separating feces from the environment.6,7,21
Table 1.
Household characteristics (N = 454)
| Variable (N) | n* | Percent or mean |
|---|---|---|
| Mean number of household residents | 454 | 5.6 |
| Mean number of children age < 5 years | 454 | 1.3 |
| Mother with no formal education | 78 | 17% |
| Father’s occupation | ||
| Farmer | 103 | 23% |
| Day labor, rickshaw puller | 100 | 22% |
| House construction | ||
| Cement floor† | 73 | 16% |
| Brick walls† | 69 | 15% |
| Household with electric connection† | 309 | 68% |
| Proportion who owned | ||
| House† | 430 | 95% |
| Mobile phone† | 378 | 83% |
| Color television† | 109 | 24% |
| Refrigerator† | 44 | 10% |
| Mean acres of agricultural land† | 453 | 0.52 |
| Mean acres of non-agricultural land† | 451 | 0.13 |
| Owned any domestic animal | 375 | 83% |
| Access to improved water source for drinking | 454 | 100% |
| Have access to a latrine | 431 | 95% |
| Have access to a shared latrine | 230 | 53% |
| Mean number of household sharing a latrine facility | 431 | 1.99 |
| Mean number of individuals sharing a latrine facility | 431 | 7.6 |
| Ownership of latrine (N = 437) | ||
| Individual ownership | 247 | 57% |
| Shared ownership | 114 | 26% |
If sample size is different it is presented next to the variable in the table.
Number with presented category.
Included to calculate wealth quintile.
Few studies have explored the effect on health of access to different levels of the sanitation ladder as classified by the JMP.9,21,22 Findings from Indonesia suggested that lack of basic sanitation was associated with higher reported diarrhea (odds ratio = 1.23, 95% confidence interval [CI] = 1.18–1.29).9 A recent systematic review identified 21 studies that compared health outcomes associated with shared versus individual household latrines.21 However, as noted by the authors of the systematic review, most of these studies did not adequately address potential confounding and did not allow the effect of different types of shared sanitation to be distinguished. An analysis of Demographic and Health Surveys from 51 countries found access to limited sanitation facilities to be associated with adverse health outcomes (compared with basic sanitation access).22 However, this finding was not consistent across all countries, suggesting that the environmental, social, and economic contexts are also important. There is evidence from a small number of observational and intervention studies that access to flush or pour flush latrines connected to a piped sewer system or septic tank or pit and composting latrines is associated with a lower risk of diarrhea.23–28 However, from these studies it is not known whether pit latrines with a slab (improved, as defined by JMP) provide similar protection from diarrhea.
There is limited evidence on the impact of basic sanitation access on household fecal contamination.29–32 The current approaches to measure levels of household fecal contamination include sampling for contamination in drinking water,29–31,33–35 on hands,31 and on household surfaces.29,32 Toys used by young children may have a high level of fecal contamination and may play an important role in diarrheal disease transmission.36–39 Children are more likely to be exposed to contamination on toys than other surfaces and fomites. Hands may be a more direct indicator of levels of contamination that the child may come across. However, hand contamination data are likely to be highly variable because of variation in handwashing practices.40 Compared with hands, toy balls are less subject to washing. Toy balls might be more directly in contact with the household environment than stored water. As a result, the contamination levels on a toy ball (a sentinel toy) might be a useful indicator of a child’s exposure to household fecal contamination.41 The sentinel toy method has been used in previous studies of sanitation in Bangladesh and India.41,42 Whereas these studies demonstrated the feasibility of using toy balls as a measure of household fecal contamination, from these studies we do not know if access to basic sanitation is associated with household fecal contamination.
A study conducted in Peru found that basic sanitation was associated with lower levels of fecal indicator bacteria (Escherichia coli) on toys compared with households that lacked basic sanitation.43 A second study conducted in Honduras also found that households with basic sanitation had lower levels of total coliforms on toys compared with households that lacked basic sanitation.44 However, these studies had limited sample sizes, so could not assess the effect of a range of confounding variables that may affect the association between fecal contamination and sanitation access.
In this study, we assessed the association between sanitation facility type and microbial fecal contamination of “sentinel toy” balls (as an indicator of household environmental contamination). An identical toy ball (the “sentinel toy”) was given to a child in each participating household, and microbial contamination of the balls was subsequently measured. The primary objective of the study was to assess the association between access to basic sanitation and fecal contamination of sentinel toy balls. The secondary objective was to determine whether sanitation factors, such as shared sanitation facility use, presence of flush or pour flush technology, and cleanliness of the sanitation facility were associated with fecal contamination of sentinel toy balls.
METHODS
We conducted an observational, cross sectional study between September and October 2013, in rural areas of Mymenshingh and Narshingdi districts of Bangladesh.
Study context.
We conducted the study in villages that were participating in the Sanitation, Hygiene Education, and Water supply in Bangladesh (SHEWA-B) health impact study described elsewhere.45 As part of the SHEWA-B program, selection of intervention subdistricts was determined by the perceived need and the absence of other active programs addressing water, sanitation, and hygiene. In Bangladesh, subdistricts are subdivided into unions, the smallest administrative unit. A representative sample of unions from the target subdistricts was selected with the probability of selection proportional to the population of the union. Once a union was selected, one village from the union was selected at random.
Household selection.
We first collected a list of study villages enrolled in the SHEWA-B health impact study situated in Mymensingh and Narshingdi districts. Using the list, we selected a simple random sample of 46 villages using the random number generator in Microsoft Excel. Fieldworkers identified 10 eligible households in each village. A household was considered eligible if it included a child aged between 6 and 24 months residing at the house (target child) on the day of the visit, had no more than one latrine, and was more than 50 m from any other selected household. Field workers entered the village and identified the beginning of its main road by asking the local inhabitants. From the starting point they searched for the closest eligible household. After selecting the first study household they looked for the next eligible household which had to be at least 50 m from the first study household. The distance between households was measured using a handheld global positioning system unit “Garmin Etrex legend H” (GARMIN).46
Data and sample collection.
Data were collected using a questionnaire survey and environmental spot-check. To assess fecal contamination of household environments, the enumerators also collected microbiological samples. These methods are outlined below.
Questionnaire survey.
The enumerators used a verbally administered, structured questionnaire survey to collect information about household possessions, parental education, water, sanitation, and hygiene behavior. The respondents were the primary caregivers (usually the mothers) of the target children.
Environmental spot-check.
The environmental check included a visual inspection of the house and compound. A compound in rural Bangladesh is comprised of a few households, often owned by members of an extended family who usually share a yard and water and sanitation facilities. The enumerators conducted visual inspections of water, sanitation, and hygiene related infrastructure using a checklist. The enumerators recorded the features related to infrastructure and cleanliness at the time of visit. They also visually checked around the house and compound for presence of animal and human feces and recorded the number and type of feces observed. The field workers inspected the hands and nails of the target child for visible dirt.
Microbiological sample collection.
Sample collection.
Fieldworkers, trained in collection and handling of microbiological samples, supplied an identical, sterile, rubber toy ball with a 20 cm circumference (Picture 1) to the target child in every study household. The primary caregiver was told that the child should be allowed to play with the toy ball in his/her usual play sites and with his/her usual playmates. The fieldworkers returned to the households approximately 23–25 hours after supplying the toy balls. They rinsed the balls in a Whirl-pak bag (19 × 38 cm) filled with 200 mL of Ringer’s solution for 30 seconds.41 The field workers transported the samples to the Environmental Microbiology Laboratory of icddr,b maintaining a temperature of 4–10°C in a cool box within 15–18 hours of collection.
Picture 1.

Sentinel toy. This figure appears in color at www.ajtmh.org.
Enumeration of fecal coliforms.
We used fecal coliforms as the indicator to assess fecal contamination of toys based on the previously detected association between sanitation and the presence of fecal colifroms on toys in two small scale observational studies conducted in Bangladesh.30,47 Although E. coli is more fecal specific than fecal coliforms and recommended as an indicator for recent, human and animal fecal contamination of water,48–51 there is limited evidence for presence of E. coli on toys and hands being associated with sanitation.31,52 Enterococci are more fecal specific and a better predictor of diarrheal disease risk than fecal streptococci and fecal coliforms.48,53–57 However, there is a lack of evidence that enterococci presence on hands or toys is associated with sanitation. Moreover in the current study setting there was no capacity to detect enterococci.
The samples were stored at 2–8°C and analyzed to detect fecal coliforms within 24 hours of collection. The toy rinse samples were processed using the membrane filtration technique. Because the method of enumerating fecal indicator bacteria from rubber toy balls was used in two previous studies in similar settings, we did not conduct a validation of the method.41,52 However, we conducted a pilot study to assess the feasibility of sample collection and processing.
Five milliliters (mL) of the recovery medium that bathed each toy ball was collected and filtered through a 0.22 μm Millipore (Billerica, MA) membrane filter. The membrane filter was then placed onto modified fecal coliform (mFC) agar plates. The plates were incubated at 44.5 ± 0.2°C for 22–24 hours, and the blue and greenish-blue colored colonies on the mFC agar were recorded as fecal coliforms after standard procedures.58,59
A previous study conducted by Pope et al.60 found that if samples are held below 10°C and are allowed not to freeze, most surface water samples analyzed by using commonly used methods (e.g., membrane filtration) up to 48 hours after sample collection, can generate E. coli data comparable to those generated within 8 hours of sample collection. In our study, for samples with no colonies or more than 500 colony forming units (CFU) per plate were recultured after 24 hours. If no colonies were found from the initial sample, 50 mL of recovery media was filtered on the following day from the stored sample and the culturing process was repeated. If the characteristic colony counts from the initial sample were more than 500 per plate, 5 mL of 10 times diluted sample was taken and the filtration and culturing processes was repeated. We also inoculated 100 μL of original, 10 times diluted and 100 times diluted samples on to mFC media after the drop plate technique and used this to quantify samples where the colonies on the membrane filters from the second day were again too numerous to count.61,62 The results were expressed as CFU per 200 mL of recovered medium that bathed the toy ball.
Human subject protection.
The study protocol was approved by the Ethical Review Committee of icddr,b and the London School of Hygiene and Tropical Medicine, United Kingdom. Written, informed consent was taken from the primary caregiver of the child.
Sample size calculation.
Results of a pilot study conducted in 20 households found that in households with access to basic sanitation the mean fecal coliform count was 2.33 log10 CFU per toy ball. We expected the ratio of households without access to basic sanitation to households with basic sanitation to be 1.5 in the sample selected regardless of the latrine access status based on an earlier study in a similar setting.45 In the SHEWA-B impact assessment study, we calculated a design effect of 2.5 considering diarrhea as outcome. So we considered a design effect of 2 assuming a similar intraclass correlation of environmental contamination as diarrhea because we did not have enough data to calculate design effect in this setting. We did not know how much reduction in fecal contamination on the toy ball will be policy relevant. So rather than calculating a sample size based on how much reduction is expected, we calculated the minimum detectable difference in mean CFU if we take a sample size that we could manage with our budget. Assuming a design effect of 2, comparing 180 households with basic latrines and 270 household without basic latrines with at least 80% power, we estimated to be able to detect a minimum difference of −0.65 mean log10 CFU of fecal coliforms per sentinel toy ball. Allowing for a 2% loss to follow up, we estimated the necessary sample size to be 460 households.
Data analysis.
We first categorized sanitation facilities using the sanitation ladder proposed by the JMP as: 1) basic (use of improved sanitation facilities which are not shared with other households, 2) limited (use of improved sanitation facilities shared between two or more households), 3) unimproved (use of pit latrines without slabs, hanging latrines, or flush/pour flush latrines connected to open water bodies), and 4) open defecation (no facility).20,63 We then categorized sanitation access as a binary variable referred to as basic or lack of access to basic sanitation (the latter being the combination of limited, unimproved, and no facility).
To assess the household wealth, we used principal component analysis with 23 household characteristics64,65 (Table 1), excluding water and sanitation. We calculated the means, frequencies, and score coefficients and used the correlation matrix of the 23 variables to calculate sample weights.64,66,67
During the questionnaire survey, if the enumerators observed no visible dirt on the hands and nails of the target child, the child was considered to have clean hands. We considered a household to have a clean latrine if the enumerators found no visible feces on the slab/floor or pan of the latrine during the spot-check. Reported disposal of feces of children under 3 years of age was categorized as safe (defecation into a latrine, disposal of stool into a latrine or buried) and unsafe as proposed by the JMP.68
If the fecal coliform concentration was zero per toy ball, we replaced the zero value with 0.5 and then transformed the fecal coliform concentrations using logarithm to the base of 10. We calculated the difference in log10 transformed arithmetic mean CFU of fecal coliforms comparing households with different types of sanitation using a linear regression model. To account for clustering at the village level, we used a generalized least squares random-effects model explicitly allowing the average outcome to vary between village clusters.
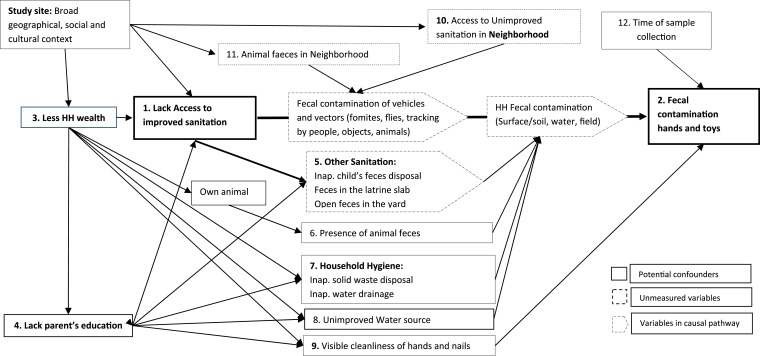
We conducted univariable analyses to estimate the crude effect of the primary exposure variables and potential confounding variables on the main outcome (fecal coliform count) adjusting for the effect of village level clustering. We then conducted multivariable analysis that included the primary exposure, primary outcome and potential confounders. A causal diagram was developed to decide which variables should be included as a potential confounder, excluding variables on the same causal pathway as the exposure variables (Figure 1).69,70 All of the potential variables that were associated (P value < 0.1) with the exposure and the outcome in the univariable analysis were included in the final multivariable model. For a variable to be considered associated with the outcome in the univariable analysis, we used P < 0.1 as cut off instead of the conventional P < 0.05 so as to be more inclusive in our effort to include all potential confounders. However, for associations based in adjusted analyses, based on multivariable models we did not consider a specific threshold P value.69 Rather we considered the smaller the P value the stronger the evidence against the null hypothesis. The models were tested for normality of residuals and homoscedasticity. We implemented separate models to understand the sanitation factors associated with fecal contamination among a subgroup of households that had access to an improved sanitation.
Figure 1.
Directed acyclic graph showing the variables that were measured and included in the multivariable analysis.
RESULTS
Descriptive household characteristics.
Of 468 households visited, eight were excluded for having more than one latrine. Of 460 households enrolled for the study we could not sample sentinel toys from six households. As a result, we present data from 454 (99%) households.
Among the 454 households there were on average 5.6 persons per household with on average 1.3 children under the age of 5 years. The majority of households (83%) owned domestic animals. Most of the households (95%) reported having access to a latrine. Among them, 53% (N = 230) reported sharing the latrine with at least one other household. On average, a latrine was used by two households or 7.6 individuals (Table 1).
Only 22% of households reported disposing of feces of children under 3 years of age safely. Enumerators observed human feces around the house in 13% of the households. Of the 409 (90%) households with access to a latrine with a slab, enumerators classified 35% of the latrines as clean. The most common type of latrine was a pit latrine with a slab but no water seal (N = 189, 42%). About half (51%) of the 230 households that reported using a shared latrine reported sharing the facility with only one other household. Only eight households shared a latrine among more than five households (data not shown). Twenty five percent of the households visited had access to basic sanitation (Table 2).
Table 2.
Relationship between water sanitation and hygiene related variables and log10 transformed fecal coliform colony forming units per toy ball (N = 454)
| Variables | Descriptive | Univariable† | Multivariable†‡ | |||||
|---|---|---|---|---|---|---|---|---|
| n* (%) | Mean | Standard deviation | Median | Difference in mean (95% CI) | P value | Diff. mean (95% CI) | P value† | |
| Sanitation type (SDG) | ||||||||
| Access to basic sanitation | 113 (25) | 1.84 | 1.23 | 1.60 | −0.36 (−0.65, −0.07) | 0.02 | −0.31 (−0.61, −0.01) | 0.04 |
| No access to basic sanitation | 341 (75) | 2.17 | 1.41 | 2.20 | – | – | – | – |
| Presence of any goat feces | 103 (23) | 2.36 | 1.46 | 2.38 | 0.36 (0.06, 0.67) | 0.02 | 0.31 (0.02, 0.61) | 0.04 |
| Presence of cow dung | ||||||||
| No cow dung | 264 (58) | 2.04 | 1.38 | 1.90 | – | – | – | – |
| Up to 10 piles | 136 (30) | 2.08 | 1.37 | 2.20 | 0.04 (−0.25, 0.32) | 0.79 | 0.08 (−0.21, 0.36) | 0.60 |
| More than 10 piles | 54 (12) | 2.37 | 1.35 | 2.45 | 0.36 (−0.05, 0.77) | 0.08 | 0.40 (0.00, 0.79) | 0.05 |
| Presence of appropriate water drainage | 261 (57) | 1.99 | 1.36 | 1.90 | −0.24 (−0.50, 0.01) | 0.06 | −0.32 (−0.58, −0.06) | 0.02 |
| Hands and nails looked visibly clean | 71 (16) | 1.81 | 1.34 | 1.90 | −0.35 (−0.69, −0.01) | 0.05 | −0.26 (−0.06, 0.09) | 0.15 |
| Household belongs to upper wealth quintile | 90 (20) | 1.81 | 1.22 | 1.70 | −0.41 (−0.72, −0.09) | 0.01 | −0.18 (−0.52, 0.16) | 0.31 |
| Mother with any formal education | 376 (83) | 2.03 | 1.36 | 1.90 | −0.33 (−0.66, 0.00) | 0.05 | −0.30 (−0.64, 0.04) | 0.08 |
| Change in time of data collection by hour as the day progress | – | – | – | – | −0.17 (−0.27, −0.06) | 0.02 | −0.16 (−0.27, −0.06) | < 0.01 |
| Study site | ||||||||
| Narshingdi district | 238 (52) | 2.26 | 1.38 | 2.20 | 0.36 (0.07, 0.65) | 0.01 | 0.52 (0.25, 0.78) | < 0.01 |
| Mymenshingh district | 216 (48) | 1.90 | 1.34 | 1.90 | – | – | – | – |
CI = confidence interval.
Throughout the table, if P value is less than 0.1 the p values have been formatted as bold.
Number with presented category.
Adjusting for clustering at village.
Adjusting for all other variable in the table.
Household characteristics and fecal contamination of toy balls.
Among the 454 sentinel toys sampled, 37% of the samples were repeated on a second day because either zero or more than 500 CFU were detected on the first day. The proportion of samples repeated from households with improved sanitation was similar (37%) to those from household with unimproved sanitation (27%). The lower detection limit per 200 mL of recovery media was (50/200 × 1 = 4) and the upper detection limit was ([200 × 1,000 × 100]/100 × 500 = 100,000,000). After repeating the samples after 24 hours, 49 (11%) of the samples were below the lower detection limit for fecal coliforms. On average there were 2.09 (standard deviation = 1.37) log10 CFU/toy ball of fecal coliforms with a median of 2.08 log10 CFU/toy.
The levels of fecal coliforms in samples collected from Narshingdi district were higher than those collected from Mymenshingh district (adjusted difference in means = 0.52 log10 CFU/toy ball; 95% CI: 0.25, 0.78; P < 0.01). The toy rinse samples were collected between 9.00 am and 2.00 pm. With each 1 hour increase in time of day of the sample collection, there was a 0.16 log10 decrease in the level of fecal contamination (adjusted, 95% CI: −0.27, −0.06; P < 0.01) (Table 2). Samples collected from households where the mother had some formal education had lower levels of fecal contamination compared with those households where mother had no formal education (adjusted difference in means = −0.30 log10 CFU/toy ball, 95% CI: −0.64, 0.04), but the statistical evidence was weak (P = 0.08) (Table 2).
Households where enumerators observed more than 10 piles of cow dung on the household premises had more contamination with fecal coliforms than those with no cow dung at the time of visit (adjusted difference in means = 0.40 log10 CFU/toy ball; 95% CI: 0.00; 0.79; P = 0.05) (Table 2). Toy ball samples collected from households with a water drainage system had less contamination than those without (adjusted difference in means = −0.32 log10 CFU/toy ball; 95% CI: −0.58, −0.06; P = 0.02).
Basic sanitation and fecal contamination.
Toy rinse samples from households with basic sanitation (MDG definition) had less contamination with fecal coliforms than households without access to basic sanitation (difference in means = −0.36 log10 CFU/toy ball; 95% CI: −0.65, −0.07; P = 0.02) (Table 2). After adjusting for potential confounders the difference in means was reduced to 0.31 log10 CFU/toy ball (95% CI: −0.61, −0.01), and the strength of statistical association became weaker (P value = 0.04) (Table 2).
Other sanitation characteristics and fecal contamination (sub-group analysis).
Households with improved flush/pour flush latrines had less fecal toy contamination than those with improved but nonflush technologies (Difference in means −0.45; 95% CI: −0.81, −0.09). In the adjusted analysis the difference in means was reduced and the statistical evidence weakened considerably (difference in means −0.27; 95% CI: −0.67, 0.13; P value = 0.19), suggesting that the difference observed could be because of chance alone (Table 3).
Table 3.
Relationship between features of sanitation facilities and log10 transformed fecal coliform colony forming units per toy ball, among households with access to improved sanitation as defined by Joint Monitoring Program (JMP) (N = 205) (subgroup analysis)
| Sanitation characteristics among household with improved sanitation technology | Descriptive | Univariable† | Multivariable†‡ | |||||
|---|---|---|---|---|---|---|---|---|
| n* (%) | Mean | Standard deviation | Median | Difference in mean (95% CI) | P value | Difference in mean (95% CI) | P value | |
| Sanitation technologies (N = 205) | ||||||||
| Flush/pour-flush | 97 (47) | 1.83 | 1.27 | 1.60 | −0.45 (0.81, −0.09) | 0.02 | −0.27 (−0.67, 0.13) | 0.19 |
| Non flush/pour-flush§ | 108 (53) | 2.27 | 1.34 | 2.20 | – | – | – | – |
| Sharing status (N = 205) | ||||||||
| Private/individual | 113 (55) | 1.84 | 1.23 | 1.60 | – | – | – | – |
| Shared by 2–5 HH | 92 (45) | 2.33 | 1.39 | 2.30 | 0.49 (0.13, 0.85) | 0.01 | 0.35 (−0.05, 0.75) | 0.08 |
| < 3 Child’s feces disposal practices | ||||||||
| Safe | 54 (26) | 2.03 | 1.27 | 1.90 | −0.05 (−0.46, 0.36) | 0.82 | 0.19 (−0.23, 0.61) | 0.37 |
| Unsafe | 151 (74) | 2.07 | 1.35 | 1.90 | – | – | – | – |
| Cleanliness of latrine | ||||||||
| Clean | 92 (45) | 1.87 | 1.08 | 1.90 | −0.36 (−0.73, −0.00) | 0.05 | −0.38 (−0.77, 0.02) | 0.06 |
| Dirty | 113 (55) | 2.22 | 1.48 | 2.08 | – | – | – | – |
| Presence of open feces in and around HH | ||||||||
| Open feces | 20 (10) | 2.21 | 1.49 | 1.90 | 0.15 (−0.46, 0.77) | 0.62 | 0.10 (−0.53, 0.72) | 0.76 |
| No open feces | 185 (90) | 2.05 | 1.31 | 1.90 | – | – | – | – |
CI = confidence interval.
Throughout the table, if P value is less than 0.1 the p values have been formatted as bold.
Number with presented category.
Adjusting for clustering at village.
Adjusting for all other variable in the table as well as presence of cow/goat, visible cleanliness of hands, wealth, mothers education and study site/district, time of sample collection, water waste disposal.
This includes pit latrine without slab which is considered improved according to JMP.
Toy ball samples collected from households with private improved sanitation had less fecal contamination than those with access to improved sanitation shared by two to five households (difference in means −0.49 log10 CFU/toy ball; 95% CI: 0.13, 0.85; P = 0.01). In the adjusted analysis the difference in means was somewhat smaller and the strength of association became weaker (difference in means −0.45 log10 CFU/toy ball; 95% CI: −0.05, 0.75; P = 0.08) (Table 3).
Toy ball samples from households with access to improved and clean latrines had less fecal contamination (difference in mean −0.36 log10 CFU/toy ball; 95% CI: −0.73, −0.00; P = 0.05) compared with improved but dirty latrines. In the adjusted analysis, the difference in fecal coliform contamination changed slightly with slightly weaker strength of association (difference in means −0.38 log10 CFU/toy ball; 95% CI: −0.77, 0.02; P = 0.06) (Table 3).
DISCUSSION
In this observational study, we assessed the association between sanitation type and microbiological fecal contamination. We found that access to basic sanitation was associated with lower levels of fecal contamination compared with households with no access to basic sanitation after adjusting for potential confounding factors. Although some of the difference in fecal contamination observed in this study was due to confounding, there were still differences in levels of household fecal contamination that could be due to the protective effect of access to basic sanitation.
In this study, sanitation was measured before fecal contamination of sentinel toys was measured. The association of basic sanitation with fecal contamination in the unadjusted analysis is consistent with findings from earlier studies conducted in Honduras,44 Peru,43 and Bangladesh.30,41 Although by contrast, in a study conducted in Tanzania, basic sanitation was not associated with fecal indicator bacteria level on hand-contact surfaces in latrines.32 However, the geographical context was different and most importantly the exposure pathway and the indicator bacteria measured were different. Studies conducted in Tanzania and Indonesia that attempted to adjust for the effect of several confounding factors found basic sanitation to be associated with lower levels of both fecal indicator bacteria31 and diarrhea.9 However, in our observational study, we cannot establish causality as many unmeasured household and child characteristics may have influenced fecal contamination. In our study, lower fecal contamination of the toy ball was also associated with the absence of animal feces, mother’s education, and presence of appropriate water drainage and study site. It is possible that fecal contamination of the household environment is actually influenced by underlying, unmeasured, broader, social, cultural, and environmental differences.22,71 It is possible that access to an improved latrine and absence of animal feces, mother’s education, and presence of appropriate water drainage are all proxy measures of these unmeasured differences and hence associated with fecal contamination. An experimental study would help better understand this issue.
Our data suggest that the observed differences in indicators of fecal contamination on sentinel toys between households with access to basic sanitation and no access to basic sanitation may be attributed to factors related to use rather than the sanitation infrastructure. There could be several possible explanations to support this assumption.
First, it is possible that the sanitation facilities considered as improved by the JMP are not more effective in confining feces than the facilities considered as unimproved. The main infrastructural difference between improved and unimproved sanitation facilities is the presence of a slab. Even in the presence of a slab, flies can still act as a vector to transmit organisms originating in the feces and contaminate household environment.72 In our study in the subgroup analysis, improved sanitation with a water seal was associated with greater reduction in fecal contamination compared with improved sanitation with a slab but without a water seal. The presence of a water seal may prevent flies breeding within the latrine and may reduce fly numbers and, thereby, provide protection from one route of fecal contamination of household environment. Our findings are in line with findings from previous studies conducted in Ghana, where households with a dry pit latrine or no latrine had higher odds of having E. coli contamination of stored water relative to those with a water seal latrine.35 There is also evidence from other studies that access to improved sanitation with a water seal is associated with lower diarrhea morbidity, compared with improved sanitation with no water seal.23,26,27 This may suggest that access to sanitation facilities with a water seal provides better protection from fecal contamination compared with nonflush latrines.
Second, in these settings a household could be exposed to fecal contamination even in the presence of a sanitation facility that successfully separates human feces from the environment because of other routes of household fecal contamination such as unsafe disposal of child feces,32,73 lack of exclusive use of sanitation facilities, lack of improved sanitation facilities in the neighborhood, and the presence of animal feces. Moreover, lack of optimal management of fecal sludge from onsite sanitation facilities may also contribute to household fecal contamination that access to household sanitation cannot prevent. Thus, improving household sanitation infrastructure alone may be insufficient to reduce household fecal contamination.
When we conducted subgroup analysis among households with access to improved sanitation, we found that sharing sanitation was associated with higher levels of fecal contamination, although with a small sample size in the subgroup the statistical evidence was weak. Previous studies have also reported adverse health outcomes associated with shared sanitation facilities.21,22 In contrast, shared sanitation was found to be protective against fecal contamination of hand-contact surfaces within a latrine in rural Tanzania.32 However, in this study the mechanism by which sharing a latrine prevented fecal contamination is unclear. The findings related to the effects of shared sanitation in previous studies are inconsistent and context specific.22
Shared sanitation facilities may not be as effective in separating feces from the environment as individual latrines for the following two reasons. First, shared facilities may be dirtier and may wear out or break more quickly than private latrines because of higher use rates. In our study shared facilities were more likely to have feces present on the latrine floor (data not shown). However our data suggest that sharing may lead to higher fecal contamination as measured by sentinel toys independent of latrine cleanliness suggesting that other mechanisms may also play an important role. Second, people who use shared facilities are likely to be poorer and headed by people who lack formal education.74 Socioeconomic status and lack of parents formal education has been linked with higher level of fecal contamination in this study and in a previous study.41 Although, in our study sharing was associated with higher fecal contamination independent of wealth status and mother’s education there may be residual confounding because of unmeasured social, environmental, and cultural factors that may influence fecal contamination in this context. The mechanism of how shared sanitation increases health risk needs to be understood in more detail in future research.
Our estimated minimum detectable difference in mean fecal coliform counts used for the power calculation was higher than the difference we found from our data. This suggests that our study had low statistical power. Nevertheless, the fact that access to basic sanitation is associated with lower levels of fecal contamination even after adjusting for common confounding, which is also consistent with findings from previous studies, may point to an independent link between sanitation type and fecal contamination.
In this study there was a trend of reduction of fecal coliforms as the day progressed which may, have been due to increasing sunlight causing sunlight-induced die-off of pathogens in the environment as well as on the toy ball.75 The decrease in level of fecal contamination with increase in time of day was observed over multiple days. However, we did not include the day of data collection in the multivariable analysis; but we did adjust for clustering at village level. We collected data from all households from a village cluster on the same day. So any confounding due to collecting data on a given date will be accounted for by adjusting for the village cluster. Moreover, adding the date of data collection in the multivariable model did not change our effect estimate. Because it was more important to adjust for village cluster than date of data collection, we have decided not to include date of data collection in our multivariable model.
An important limitation of this study is that the use of fecal indicator bacteria used to assess fecal contamination were not human-specific. This random measurement error can introduce bias because of misclassification of outcome. As a consequence, the confidence intervals of the estimates presented are likely to be wide making the results less likely to be statistically significant even if in reality a difference exists.76 Further study with a larger sample size could increase our understanding of the role of improved sanitation in reducing household fecal contamination.76 Using molecular markers of human specific pathogens as indicators of fecal coliform could help reduce this bias in future studies. Presence of fecal indicator bacteria does not necessarily mean health risks. However, there is evidence to suggest that the presence of fecal coliforms in environmental samples may be associated with diarrheal illness.36–38,77–80 In our study, the presence of fecal coliforms was associated with sanitation type after adjusting for the effect of presence of animal feces, consistent with findings from similar settings.30,41
Another important limitation of the study is use of a proxy measure to assess household fecal contamination. We sampled sentinel toys after 24 hours and on only one occasion. This may not constitute a comprehensive measure of child exposure to average household fecal contamination. Nonetheless, contamination on toys may be more representative of exposure to fecal contamination from the household environment than water, hands, or surfaces. Existing household toys might be better proxies than experimentally introduced sentinel toys. However, in previous studies contamination of existing household toys was found well correlated with sentinel toys.41 Moreover, in previous studies sanitation was found to be associated with both existing household toys and experimentally introduced toys.44 Previous studies have left toys for longer periods, up to 10 days before sampling, but in our study we left the ball only for a day. However, considering the survival times of fecal indicator bacteria in the environment and the recovery efficiency, it seems likely that the indicator bacteria measured in these studies may be from recent contamination.42 Previous studies have also found that two toy samples introduced at the same time and few days apart had similar coefficients of variation, suggesting that fecal contamination of a toy left for only 24 hours is still comparable to contamination on toys already existing in the household. Future studies assessing the reliability of these measurements in the same setting and over time would also be helpful to understand how well a single measurement might capture exposure.
The findings from this observational study suggest that basic sanitation used by individual households may be better in reducing household fecal environmental contamination compared with shared facilities. Addition of a water seal may also improve the environmental protection afforded to a household by a latrine compared with latrines with a slab alone. However, further studies with sufficient statistical power and an experimental design are required to understand if this association is causal. In addition to sanitation infrastructure, cleanliness of latrines should be considered an important indicator to monitor sanitation. Even in the context of rural areas where sanitation facilities are shared by acquaintances, the facilities may be dirtier than individual latrines. We recommend public health interventions to improve and monitor latrine cleanliness, particularly for shared sanitation. Shared facilities may pose health risks because of many factors other than just cleanliness. We need further studies to better understand the mechanism by which shared facilities pose health risks.
Acknowledgments:
International Centre for Diarrhoeal Disease Research, Bangladesh is grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support. No funding bodies had any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge the contributions of the study participants, icddr,b admin, and field and laboratory research staff. We specially thank Dr. Leanne Unicomb from icddr,b for her guidance during data collection and Astrid Dier for her support with English. We also thank the research advisory committee of the environmental health group at LSHTM.
REFERENCES
- 1.Bartram J, Cairncross S, 2010. Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med 7: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mara D, Lane J, Scott B, Trouba D, 2010. Sanitation and health. PLoS Med 7: e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pruss-Ustun A, et al. 2014. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health 19: 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM, Jr, 2005. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 6.Clasen TF, Bostoen K, Schmidt W-P, Boisson S, Fung ICH, Jenkins MW, Scott B, Sugden S, Cairncross S, 2010. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev 6: CD007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf J, et al. 2014. Assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: systematic review and meta-regression. Trop Med Int Health 19: 928–942. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung ICH, Schmidt W-P, 2010. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 39 (Suppl 1): i193–i205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semba RD, Kraemer K, Sun K, de Pee S, Akhter N, Moench-Pfanner R, Rah JH, Campbell AA, Badham J, Bloem MW, 2011. Relationship of the presence of a household improved latrine with diarrhea and under-five child mortality in Indonesia. Am J Trop Med Hyg 84: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AM, Fuchs SC, Moreira LB, Gigante LP, Barros FC, 1988. Water supply, sanitation and housing in relation to the risk of infant mortality from diarrhoea. Int J Epidemiol 17: 651–654. [DOI] [PubMed] [Google Scholar]
- 11.Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, Freeman MC, 2014. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med 11: e1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerson PM, et al. 2004. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 363: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 13.Ziegelbauer K, Speich B, Mausezahl D, Bos R, Keiser J, Utzinger J, 2012. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albonico M, Montresor A, Crompton DW, Savioli L, 2006. Intervention for the control of soil-transmitted helminthiasis in the community. Adv Parasitol 61: 311–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO/UNICEF , 2012. Progress on Drinking Water and Sanitation 2012 Update. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 16.WHO/UNICEF JMP , 2014. Progress on Sanitation and Drinking-Water—2014 Update. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 17.JMP , 2016. WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation Available at: http://www.wssinfo.org/about-the-jmp/introduction/. Accessed June 20, 2016.
- 18.JMP , 2015. JMP Green Paper: Global Monitoring of Water, Sanitation and Hygiene Post-2015. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation.
- 19.WHO/UNICEF , 2013. Progress on Sanitation and Drinking-Water—2013 Update. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 20.JMP , 2016. WASH in the 2030 Agenda Available at: https://www.wssinfo.org/fileadmin/user_upload/user_upload/JMP_WASH_in_the_2030_Agenda_factsheet.pdf. Accessed June 29, 2017.
- 21.Heijnen M, Cumming O, Peletz R, Chan GK, Brown J, Baker K, Clasen T, 2014. Shared sanitation versus individual household latrines: a systematic review of health outcomes. PLoS One 9: e93300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller JA, Clasen T, Heijnen M, Eisenberg JN, 2014. Shared sanitation and the prevalence of diarrhea in young children: evidence from 51 countries, 2001–2011. Am J Trop Med Hyg 91: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink G, Gunther I, Hill K, 2011. The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. Int J Epidemiol 40: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 24.Fana VY-M, Mahalb A, 2011. What prevents child diarrhoea? The impacts of water supply, toilets, and hand-washing in rural India. J Dev Effect 3: 340–370. [Google Scholar]
- 25.WHO , 2009. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 26.Bose R, 2009. The Impact of Water Supply and Sanitation Interventions on Child Health: Evidence from DHS Surveys. Bi-annual Conference on Impact Evaluation, Colombo, Sri Lanka, April 22–23, 2009. [Google Scholar]
- 27.Capuno JJ, Tan CA, Jr, Fabella VM, 2011. Do piped water and flush toilets prevent child diarrhea in rural Philippines? Asia Pac J Public Health 27: NP2122–NP2132. [DOI] [PubMed] [Google Scholar]
- 28.Barreto ML, et al. 2007. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet 370: 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering AJ, Julian TR, Marks SJ, Mattioli MC, Boehm AB, Schwab KJ, Davis J, 2012. Fecal contamination and diarrheal pathogens on surfaces and in soils among Tanzanian households with and without improved sanitation. Environ Sci Technol 46: 5736–5743. [DOI] [PubMed] [Google Scholar]
- 30.Ram PK, Luby SP, Halder AK, Islam MS, Granger DS, 2010. Improving Measures of Handwashing Behavior Water and Sanitation Program Technical Paper. Washington, DC: World Bank.
- 31.Pickering AJ, et al. 2010. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol 44: 3267–3272. [DOI] [PubMed] [Google Scholar]
- 32.Exley JL, Liseka B, Cumming O, Ensink JH, 2015. The sanitation ladder, what constitutes an improved form of sanitation? Environ Sci Technol 49: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 33.Henry FJ, Rahim Z, 1990. Transmission of diarrhoea in two crowded areas with different sanitary facilities in Dhaka, Bangladesh. J Trop Med Hyg 93: 121–126. [PubMed] [Google Scholar]
- 34.Copeland CC, Beers BB, Thompson MR, Fitzgerald RP, Barrett LJ, Sevilleja JE, Alencar S, Lima AA, Guerrant RL, 2009. Faecal contamination of drinking water in a Brazilian shanty town: importance of household storage and new human faecal marker testing. J Water Health 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGarvey ST, Buszin J, Reed H, Smith DC, Rahman Z, Andrzejewski C, Awusabo-Asare K, White MJ, 2008. Community and household determinants of water quality in coastal Ghana. J Water Health 6: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekanem EE, Dupont HL, Pickering LK, Selwyn BJ, Hawkins CM, 1983. Transmission dynamics of enteric bacteria in day-care centers. Am J Epidemiol 118: 562–572. [DOI] [PubMed] [Google Scholar]
- 37.Van R, Morrow AL, Reves RR, Pickering LK, 1991. Environmental contamination in child day-care centers. Am J Epidemiol 133: 460–470. [DOI] [PubMed] [Google Scholar]
- 38.Laborde DJ, Weigle KA, Weber DJ, Kotch JB, 1993. Effect of fecal contamination on diarrheal illness rates in day-care centers. Am J Epidemiol 138: 243–255. [DOI] [PubMed] [Google Scholar]
- 39.Jiang X, Dai X, Goldblatt S, Buescher C, Cusack TM, Matson DO, Pickering LK, 1998. Pathogen transmission in child care settings studied by using a cauliflower virus DNA as a surrogate marker. J Infect Dis 177: 881–888. [DOI] [PubMed] [Google Scholar]
- 40.Ram PK, Jahid I, Halder AK, Nygren B, Islam MS, Granger SP, Molyneaux JW, Luby SP, 2011. Variability in hand contamination based on serial measurements: implications for assessment of hand-cleansing behavior and disease risk. Am J Trop Med Hyg 84: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vujcic J, Ram PK, Hussain F, Unicomb L, Gope PS, Abedin J, Mahmud ZH, Islam MS, Luby SP, 2014. Toys and toilets: cross-sectional study using children’s toys to evaluate environmental faecal contamination in rural Bangladeshi households with different sanitation facilities and practices. Trop Med Int Health 19: 528–536. [DOI] [PubMed] [Google Scholar]
- 42.Torondel B, Gyekye-Aboagye Y, Routray P, Boisson S, Schimdt W, Clasen T, 2015. Laboratory development and field testing of sentinel toys to assess environmental faecal exposure of young children in rural India. Trans R Soc Trop Med Hyg 109: 386–392. [DOI] [PubMed] [Google Scholar]
- 43.Galiani S, Orsola-Vidal A, 2010. Scaling Up Handwashing Behavior: Findings from the Impact Evaluation Baseline Survey in Peru. Technical Paper. Washington DC: Water and Sanitation Programme (WSP), World Bank. [Google Scholar]
- 44.Stauber CE, Walters A, Fabiszewski de Aceituno AM, Sobsey MD, 2013. Bacterial contamination on household toys and association with water, sanitation and hygiene conditions in Honduras. Int J Environ Res Public Health 10: 1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP, 2012. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Soc Sci Med 75: 604–611. [DOI] [PubMed] [Google Scholar]
- 46.Garmin eTrex Legend® H. Available at: https://buy.garmin.com/en-GB/GB/outdoor/discontinued/etrex-legend-h/prod30120.html. Accessed June 29, 2017.
- 47.Vujcic J, Luby SP, 2011. WASH Benefits Sentinel Toy Study. Dhaka, Bangladesh: International Centre for Diarrhoeal Disease Research, Bangladesh.
- 48.Scott TM, Rose JB, Jenkins TM, Farrah SR, Lukasik J, 2002. Microbial source tracking: current methodology and future directions. Appl Environ Microbiol 68: 5796–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO , 2011. Guidelines for Drinking-Water Quality Available at: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf. Accessed January 14, 2013.
- 50.Leclerc H, Mossel DA, Edberg SC, Struijk CB, 2001. Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annu Rev Microbiol 55: 201–234. [DOI] [PubMed] [Google Scholar]
- 51.Tallon P, Magajna B, Lofranco C, Leung K, 2005. Microbial indicators of faecal contamination in water: a current perspective. Water Air Soil Pollut 166: 139–166. [Google Scholar]
- 52.DiVita MA, Halder AK, JI K, Islam M, Sobsey MD, Luby SP, Ram PK, 2008. The Utility of Common Household Objects as Markers of Home Hygiene in the Context of Access to Improved Sanitation. ISEE 20th Annual Conference, Pasadena, CA, October 12–16, 2008. [Google Scholar]
- 53.Bitton G, 2005. Microbial Indicators of Fecal Contamination: Application to Microbial Source Tracking. Tallahassee, FL: Florida Stormwater Association. [Google Scholar]
- 54.Moe CL, Sobsey MD, Samsa GP, Mesolo V, 1991. Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ 69: 305–317. [PMC free article] [PubMed] [Google Scholar]
- 55.Borchardt MCP, Devries E, Belongia E, 2003. Septic system density and infectious diarrhoea in a defined population of children. Environ Health Perspect 111: 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabelli VJ, Dufour AP, McCabe LJ, Levin MA, 1982. Swimming-associated gastroenteritis and water quality. Am J Epidemiol 115: 606–616. [DOI] [PubMed] [Google Scholar]
- 57.Wade TJ, Pai N, Eisenberg JN, Colford JM, Jr, 2003. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect 111: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.CDC , 2010. Microbiological Indicator Testing in Developing Countries: A Fact Sheet for the Field Practitioner Available at: http://sanitationupdates.files.wordpress.com/2010/11/microbiology2020.pdf. Accessed September 12, 2013.
- 59.USEPA , 2002. Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium). Washington DC: US Environmental Protection Agency. [Google Scholar]
- 60.Pope ML, et al. 2003. Assessment of the effects of holding time and temperature on Escherichia coli densities in surface water samples. Appl Environ Microbiol 69: 6201–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miles AA, Misra SS, Irwin JO, 1938. The estimation of the bactericidal power of the blood. J Hyg (Lond) 38: 732–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hedges AJ, 2002. Estimating the precision of serial dilutions and viable bacterial counts. Int J Food Microbiol 76: 207–214. [DOI] [PubMed] [Google Scholar]
- 63.WHO/UNICEF JMP , 2015. 25 years Progress on Sanitation and Drinking-Water—2015 Update and MDG Assessment. Geneva, Switzerland: WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. [Google Scholar]
- 64.Luby SP, Halder AK, Huda T, Unicomb L, Johnston RB, 2011. The effect of handwashing at recommended times with water alone and with soap on child diarrhea in rural Bangladesh: an observational study. PLoS Med 8: e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vyas S, Kumaranayake L, 2006. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 21: 459–468. [DOI] [PubMed] [Google Scholar]
- 66.Barros AJ, Victora CG, 2005. A nationwide wealth score based on the 2000 Brazilian demographic census. Rev Saude Publica 39: 523–529. [DOI] [PubMed] [Google Scholar]
- 67.Houweling TA, Kunst AE, Mackenbach JP, 2003. Measuring health inequality among children in developing countries: does the choice of the indicator of economic status matter? Int J Equity Health 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.WHO/UNICEF , 2006. Core Questions on Drinking-Water and Sanitation for Household Surveys Available at: http://www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf. Accessed September 12, 2013.
- 69.Kirkwood BR, Sterne JAC, 2003. Essential Medical Statistics. Oxford, UK: Blackwell Science Ltd. [Google Scholar]
- 70.Greenland S, Pearl J, Robins JM, 1999. Causal diagrams for epidemiologic research. Epidemiology 10: 37–48. [PubMed] [Google Scholar]
- 71.Fuller JA, Westphal JA, Kenney B, Eisenberg JNS, 2015. The joint effects of water and sanitation on diarrhoeal disease: a multicountry analysis of the Demographic and Health Surveys. Trop Med Int Health 20: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galal S, Sundaram C, Hassan N, Salem K, Lashin S, 2001. Infections in children under 5 years old and latrine cleanliness. Int J Environ Health Res 11: 337–341. [DOI] [PubMed] [Google Scholar]
- 73.Majorin F, Freeman MC, Barnard S, Routray P, Boisson S, Clasen T, 2014. Child feces disposal practices in rural Orissa: a cross sectional study. PLoS One 9: e89551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heijnen M, Rosa G, Fuller J, Eisenberg JN, Clasen T, 2014. The geographic and demographic scope of shared sanitation: an analysis of national survey data from low- and middle-income countries. Trop Med Int Health 19: 1334–1345. [DOI] [PubMed] [Google Scholar]
- 75.Fujioka RS, Narikawa OT, 1982. Effect of sunlight on enumeration of indicator bacteria under field conditions. Appl Environ Microbiol 44: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hutcheon JA, Chiolero A, Hanley JA, 2010. Random measurement error and regression dilution bias. BMJ 340: c2289. [DOI] [PubMed] [Google Scholar]
- 77.Abu Amr SS, Yassin MM, 2008. Microbial contamination of the drinking water distribution system and its impact on human health in Khan Yunis Governorate, Gaza Strip: seven years of monitoring (2000–2006). Public Health 122: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 78.Moe CL, Sobsey MD, Samsa GP, Mesolo V, 1991. Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ 69: 305–317. [PMC free article] [PubMed] [Google Scholar]
- 79.Risebro HL, Breton L, Aird H, Hooper A, Hunter PR, 2012. Contaminated small drinking water supplies and risk of infectious intestinal disease: a prospective cohort study. PLoS One 7: e42762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luby SP, Agboatwalla M, Billhimer W, Hoekstra RM, 2007. Field trial of a low cost method to evaluate hand cleanliness. Trop Med Int Health 12: 765–771. [DOI] [PubMed] [Google Scholar]