Abstract
RNA topoisomerase activity has recently been detected in multiple Type IA DNA topoisomerases from all three domains of life: bacteria, archaea, and eukarya. Many, but not all, Type IA topoisomerases are found to possess activities for not only DNA, but also RNA, suggesting that they may solve topological problems for both types of nucleic acids. Here we describe a detailed assay used by our group to detect RNA topoisomerase activity for many Type IA topoisomerases. We discuss the strategy, experimental procedures, troubleshooting, and limitations for this assay.
Keywords: Topoisomerase, Top3β, Circular RNA, Knot
1 Introduction
Topoisomerases solve essential problems produced during metabolic reactions on DNA, such as replication, transcription, recombination, repair, and segregation [1, 2]. These enzymes have a unique strand passage activity that can alter the topological states of DNA. Type I topoisomerases can create a transient break on one strand of DNA duplex, whereas type II topoisomerases can produce breaks on both strands. The topoisomerase then allows the other strand to pass through the break, and rejoin the broken ends. As a result, supercoils created during DNA metabolism can be removed, interlocked DNA rings can be separated (decatenation), and knots can be introduced into or removed from DNA circles. The importance of topoisomerases can be reflected by the fact that they are broadly present in all species [3], and that their inactivation can often lead to slow growth, lethality, and diseases [1, 4–6].
While DNA topoisomerases have been extensively characterized during the past four and half decades, RNA topoisomerases have been largely ignored. E.coli Topoisomerase III (EcoTop3), a Type IA topoisomerase, was the first enzyme that was shown to be capable to catalyze strand passage reactions using circular RNA as substrates in 1996 [7]. However, no follow-up studies have been reported, so that the biological relevance of the observed RNA topoisomerase activity is unclear. The RNA topoisomerase has finally attracted some attention after the discovery that human Top3β is an RNA topoisomerase that works with an RNA-binding protein, FMRP, to bind mRNAs, associate with translation machinery, and to promote neurodevelopment; and its deletion is linked to schizophrenia and intellectual disability [4, 8]. To date, RNA topoisomerase activity has been detected in many Type IA topoisomerases from all domains of life, bacteria, archaea, and eukarya. In particular, some of the most-commonly studied topoisomerases, including Top1 (EcoTop1) and Top3 (EcoTop3) of E.coli, Top3 of yeast, Top3β of human (humTop3β) and Drosophila, have all been shown to possess RNA topoisomerase activity [7–10]. Moreover, Top3β from several animal species (human, chicken, and Drosophila), have been shown to associate with mRNA translation machinery [4, 8, 9], suggesting that these enzymes may solve RNA topological problems during mRNA translation. Furthermore, a bona fide RNA-binding domain, RGG-box, has been identified in Top3β, but not its paralog, Top3α, in Type IA enzymes from animals [8]. Deletion of this domain diminishes the RNA topoisomerase activity of Top3β. These data provide structural basis for why the former, but not the latter, possesses RNA topoisomerase activity.
Here we describe a detailed assay used by our group to detect the RNA topoisomerase activity for Type IA topoisomerases from all three domains of life. We believe that this assay can be broadly applied to examine RNA topoisomerase activity for this family of topoisomerases.
1.1 Strategy
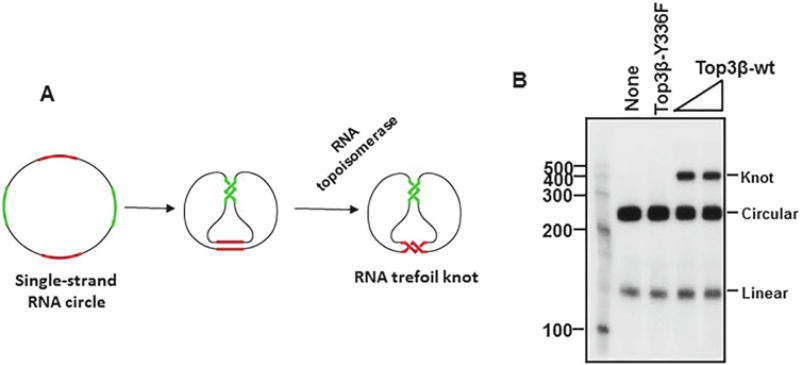
The original assays used to detect RNA topoisomerase activity for EcoTop3 were designed elegantly by Seeman and his colleagues [7]. They engineered a circular single-stranded RNA substrate consisting two pairs of complementary regions of 10 bp each, which are separated by four intervening spacers, which are also 10 bp each (Fig. 1a) [7]. The two complementary regions are thermodynamically favored to form two short duplexes. However, only through a strand-passage reaction catalyzed by a topoisomerase, can both regions form normal duplexes. After the strand-passage reaction, the circular RNA substrate will be converted to a trefoil with a single knot (Fig. 1a). The trefoil knot product can be distinguished from its circular substrate by denaturing polyacrylamide electrophoresis (PAGE) analysis (Fig. 1b). Using this assay, RNA topoisomerase activity was detected for EcoTop3, but not EcoTop1 [7].
Fig. 1.
A trefoil knot formation assay to show that human Top3β protein has RNA topoisomerase activity. (a) Schematic representation of an RNA topoisomerase assay that is based on conversion of an RNA circle to a trefoil knot. Two complementary regions are marked by red and green colors, whereas the spacer regions are marked by black. The circle substrate and the knot are indicated. (b) An autoradiograph shows that wild type human Top3β, but not the catalytic mutant Y336F, has RNA topoisomerase activity that can convert the RNA circle substrate to a trefoil knot product. 5 nM of protein was used in the assay
We used the same strategy designed by Seeman and colleagues, but modified their circular RNA substrate by increasing the complementary regions from 10 to 12 bps [8]. We reasoned that the increased length should further stabilize the duplex regions, and make the reaction thermodynamically more favorable. Indeed, using our substrate, we found that EcoTop1 does have robust RNA topoisomerase activity, and this activity is comparable to that of hum Top3β [9]. Because the new substrate allowed us to detect the RNA topoisomerase activity in EcoTop1, whereas that used by Seeman and colleagues did not, we conclude that the new substrate makes the assay more sensitive. We have since used the new substrate to detect RNA topoisomerase activity for many type IA topoisomerases.
2 Materials
2.1 Preparation of Radiolabeled Linear RNA Substrate
MEGAshortscript™T7 Kit (Ambion) for in vitro transcription reactions.
The synthetic DNA oligo sequences needed to make the circular RNA substrate are listed in Table 1. A single-strand RNA (GGGAGAUUUUUUUUUUUUUUUUUUUUGUCAGACGGAUCUUUUUUUUUUUUUUUUUUUUUCUCCCGACUGGUUUUUUUUUUUUUUUUUUUUGAUCCGUCUGACUUUUUUUUUUUUUUUUUUUUCCAGUC) was transcribed by MEGAshortscript™T7 Kit (Ambion) from an annealed DNA template consisting of synthetic oligos K128f and K128r (Table 1).
All purification steps for RNA topoisomerases should be performed in ice cold buffers. All solutions for RNA topoisomerase reactions should be prepared in RNase-free, DNase-free, and DEPC-treated molecular biology grade water. Work place and pipettes should be wiped with RNaseZAp (Ambion) solution to avoid RNase contamination. It is advised to use autoclaved RNase free microcentrifuge tubes. RNA substrates should be kept on Ice (unless the temperature is specified for particular reaction) while working, and stored at −80° freezers for long-term storage.
RNase free-DNase: Turbo DNase (Ambion).
6% TBE-Urea polyacrylamide gel (Invitrogen).
Molecular weight marker mixture of 100–500 bases (Ambion).
6% TBE-Urea polyacrylamide gel containing a single well.
Surgical blade.
D-Tube dialyser (EMD Millipore) filled with TE.
TBE.
Phenol–chloroform–isopropanol mixture (Invitrogen).
Cold absolute ethanol.
NanoDrop to determine the concentration of the RNA preps.
50–60 µCi of Υ-p32-ATP, 6000 Ci/mmol, 10 mCi/ml (PerkinElmer).
Kinase Max™ kit (Ambion).
NucAway spin column (Ambion).
Geiger counter.
Table 1.
A list of DNA oligo sequences used for making the circular RNA substrate
| Oligos used in the RNA topoisomerase assay | |
|---|---|
| DNA oligos | Sequence |
| K128f | ACTTCGAAATTAATACGACTCACTATAGGGAGATTTTTTTTTTTTTTTTTTTTGTCAGACGGATCTTTTTTTTTTTTTTTTTTTTTCTCCCGACTGGTTTTTTTTTTTTTTTTTTTTGATCCGTCTGACTTTT TTTTTTTTTTTTTTTTCCAGTC |
| K128r | GACTGGAAAAAAAAAAAAAAAAAAAAGTCAGACGGATCAAAAAAAAAAAAAAAAAAAACCAGTCGGGAGAAAAAAAAAAAAAAAAAAAAAGATCCGTCTGACAAAAAAAAAAAAAAA AAAAATCTCCCTATAGTGAGTCGTATTAATTTCGAAGT |
| K128link | GATCCGTCTGACAAAAAAAAAAAAAAAAAAAATCTCCCGACTGGAAAAA |
This table has been taken from Supplemental Fig. 5 of a previous publication for the convenience of readers [8]
2.2 Circularization of the Radiolabeled Linear RNA
10× annealing buffer: 100 mM Tris pH 7.5, 1 mM NaCl.
Heat block.
T4 RNA ligase with buffer (Ambion).
RNaseout (Invitrogen).
Gel Loading Buffer II (Provided with the MEGAshortscript T7™ kit).
15% TBE-Urea polyacrylamide gel (Invitrogen).
RNase-free water.
2.3 The RNA Strand Passage Reaction
5× reaction buffer: 100 mM Tris–HCl pH 7.5, 500 mM NaCl, 50 mM MgCl2, 0.5 mg/ml BSA, 25% glycerol. DTT (2 mM final concentration) and PEG400 (10% final concentration or 1 µl in 10 µl reaction) are added just before setting up the reaction.
Purified type 1A topoisomerase.
5× stop buffer: 1 mg/ml proteinase K, 2.5% SDS, 100 mM EDTA.
Gel Loading Buffer II (Provided with the MEGAshortscript T7™ kit).
15% TBE-Urea polyacrylamide gel (Invitrogen).
3 Methods
3.1 Preparation of Radiolabeled Linear RNA Substrate
Oligos K128f and K128r (Table 1) were annealed to produce the DNA template for in vitro transcription to produce a linear RNA. For the annealing reaction, 500 ng of each oligo was mixed with 1 µl of 10× transcription buffer from the MEGA-shortscript™ T7 Kit and appropriate amount of H2O, so that the final volume is 10 µl.
The tube containing the reaction mixture was first incubated at 95 °C for 5 min, and then placed into boiling water in a beaker to let it slowly cool down to room temperature. This will take about 2 h. The tube was centrifuged for 10 s when temperature reaches to about 60 °C, to re-collect the liquid that was evaporated and condensed on the cap of the microcentrifuge tube. The annealed template will be used for in vitro transcription to produce linear RNA using the MEGAshortscript™ kit from Ambion as described below.
Take 9 µl of the annealed mixture and add 2 µl of each ribonucleotide (75 µM rATP, rUTP, rCTP and rGTP), 1.0 µl 10× transcription buffer (0.9 µl is already present in the annealing mix), 2 µl of T7 polymerase, and water to make the final reaction volume at 20 µl. Incubate the reaction mixture at 37 °C for 2 h. Afterward, add 2 µl of turbo DNase (Ambion) and incubate the mixture at 37 °C for 15 min to degrade the DNA template.
The mixture was added with equal volume of Gel Loading Buffer II (Provided with the MEGAshortscript T7™ kit). The RNA product was purified by electrophoresis on a 6% TBE–Urea polyacrylamide gel (Invitrogen) at 180 V for 40–50 min. A molecular weight marker mixture of 100–500 bases (Ambion) was included during electrophoresis. The gel needs a prerun for 30 min at 180 V before being used for purification of RNA.
We noticed that the linear RNA product can sometimes form irreversible dimers or multimers when it was boiled in the loading buffer. It is possible that the linear RNA molecules become tangled between each other to form knots and other structures that can no longer be separated on denaturing gels. To avoid this to happen, the RNA mixture (40 µl) can be added with 500 µl of Loading buffer to reduce the RNA concentration. After heat denaturation, the mixture can be purified using a preparative 6% TBE-Urea gel containing a single wide well (a special gel comb is needed to make such a gel).
After electrophoresis, the RNA product (128 base) was visualized by UV shadowing, excised from the gel using a surgical blade, and transferred to a D-Tube dialyzer (EMD Millipore) filled with TE.
The RNA product was then eluted by electroelution against 1X TBE at 120 V for 1–1.5 h. It was purified by extraction with equal volume of phenol–chloroform–isopropanol mixture (In Vitrogen) once. The aqueous layer containing the RNA product was collected. The RNA was then precipitated by adding 2.5 volumes of 100% ethanol and incubated at −20 °C for overnight. On the next day, the RNA was collected by centrifugation using a tabletop centrifuge at 13,500 rpm (14000 × g) for 10 min at 4 °C. The pellet was then washed once with cold 80% ethanol, centrifuged again, air-dried for 5 min, and dissolved in RNase-free water. The RNA should not be over-dried, which will make it difficult to dissolve. The concentration of RNA was measured using NanoDrop. Its concentration was adjusted to 2 µg/µl using water.
The linear RNA of 20–40 µg (2 µg/µl) was labeled with Υ-p32-ATP at its 5′ end using the Kinase Max ™ kit as per the manufacturer protocol (Ambion). Briefly, 20 µl of RNA was added to 3 µl 10× phosphatase buffer, 3 µl calf intestine alkaline phosphatase, and water to final volume to 30 µl. The mixture was incubated for 1 h at 37 °C to remove the 5-′-phosphate on the linear RNA. Equal volume of phosphatase removal buffer was added to the mix and incubated at room temperature for 3 min with intermittent shaking. The mixture was then centrifuged at 13,000 rpm (13000 × g) for 30 s. Carefully remove the supernatant that was now approximately 40–45 µl. The mixture was then added with 6 µl 10× kinase buffer, 50–60 µCi of Υ-p32-ATP (6000 Ci/mmol 10 mCi/ml, PerkinElmer), and 6 µl of T4 polynucleotide kinase enzyme, and appropriate amount of water to adjust the reaction volume to 60 µl. The mixture was then incubated at 37 °C for 1 h. The kinase reaction was terminated by heating at 95 °C for 2 min. The free radioactive nucleotides were removed using NucAway spin column (Ambion). The success of the radiolabeling reaction can be verified by measuring the radioactivity in the linear RNA using a Geiger counter or a scintillation counter (see Note 1).
3.2 Circularization of the Radiolabeled Linear RNA
The p32-labeled linear RNA was annealed with the DNA linker oligo K128link (Table 1). This linker oligo has sequence complementary to both ends of the linear RNA. When both ends of RNA are annealed to the same DNA linker, the linear RNA becomes circularized, although its 5′ and 3′ ends are not covalently linked. An RNA ligase will then be added to ligate the two free ends.
For the annealing reaction, a mixture of 15 µl was made by mixing 12 µl of p32-labelled linear RNA, 1.5 µl of K128 linker oligo (100 µM), and 1.5 µl of 10× annealing buffer (100 mM Tris pH 7.5, and 1 M NaCl). We found that the 10× RNA ligase buffer (Ambion) can also be used. The annealing mixture was heated at 95 °C in heat block for 5 min, and transferred to a water bath at 70 °C. The water bath was then turned off, so that the mixture will be cooled down gradually until the water temperature reaches to the room temperature. The liquid condensed on the cap of the tube can be collected by centrifugation.
For ligation: the 15 µl mixture was added with 2 µl of 10× T4-RNA ligation buffer, 2 µl T4 RNA ligase (Ambion), 0.5 µl of RNaseout (Invitrogen), and water to make the final volume to 20 µl. The mixture was incubated for 3 h at 37 °C. If the yield of circular RNA is unsatisfactory, the mixture can be incubated at 16 °C overnight.
To remove the DNA linker oligo after RNA circularization, 2 µl of DNase (5 U/µl) was added, and the mixture was incubated for 15 min at 37 °C. The reaction was terminated by adding equal volume of Gel Loading Buffer II. The RNA-containing mixture was then denatured at 95 °C for 5 min, loaded on 15% TBE-Urea gel (Invitrogen)(which should be no more than 20 days old), and resolved by electrophoresis at 150 V for 10–11 h. The radiolabeled RNA products were detected by X-ray autoradiography. To avoid contamination of radioactivity, the gel should be wrapped carefully with a saran wrap before being exposed to the X-ray film for 0.5–1 h.
The circular product can be distinguished from its linear and other forms (such as trefoil knots) based on its mobility variation on different percentages of polyacrylamide gels (Fig. 2a–c). Comparing to known molecular weight markers (Ambion), the circular RNA exhibits variation in its mobility on different percentage of gels, whereas the linear RNA does not. The relative position of the knot versus the circle also varies: the knot has slower mobility than the circle on high percentage gels, but has equal or faster mobility on low percentage gels. The conversion from a circle to a knot can be inhibited when the self-annealing of the two complementary regions of the circle is blocked by adding a competing oligo that is complementary to one of the regions.
The gel slice containing the circular RNA was excised, extracted by electroelution described above, precipitated by adding 1/10th volume of 3 M sodium acetate pH 5.0, and 2.5 volume of 100% ethanol, and incubated overnight at −20 °C. The RNA was collected by centrifugation, and dissolved in RNase-free water (40 µl). The radioactivity of RNA was measured by scintillation counter. We typically obtain radioactivity of about 8000–12,000 CPM/µl.
Fig. 2.
Distinguishing the circular, linear, and knot forms of RNA by electrophoresis on polyacrylamide gels of different concentrations. (a) An autoradiograph shows that the RNA circle and knot have mobility similar to that of the 200 base marker on a 6% denaturing gel, whereas the corresponding linear substrate has mobility comparable to that of its expected size of 128 bases. Ligation refers to the RNA mixture in which the linear RNA substrate has been ligated using T4 ligase. (b) An autoradiograph shows that the RNA circle and knot display mobility similar to that of the 300 base marker on a 10% polyacrylamide–urea gel, while the linear substrate runs at its expected size of 128 bases. (c) An autoradiograph shows that the RNA knot has mobility similar to that of the 500 base marker, and the RNA circle runs at mobility similar to that of the 300 base marker, whereas the linear substrate runs at its expected size of 128 bases. Overall, the circular and knot forms of RNA have reduced mobility in contrast to their linear counterpart
3.3 The RNA Strand Passage Reaction that Converts the RNA Circle to Trefoil Knot
Make 5× reaction buffer as follows: 100 mM Tris–HCl, pH 7.5; 500 mM NaCl, 50 mM MgCl2, 0.5 mg/ml BSA, 25% glycerol. DTT (2 mM final concentration) and PEG400 (10% final concentration or 1 µl in 10 µl reaction) should be added freshly just before setting up the reaction. We often add 4 units RNaseOut (Invitrogen) to the 1× reaction mixture to minimize RNA degradation.
The strand passage reaction was set up by mixing the following components: the purified Type IA topoisomerase (such as hum Top3β or EcoTop1), the p32-labeled circular RNA substrate (2500–3000 CPM), 5× reaction buffer, DTT, PEG400, and water. The final volume is 10 µl.
The concentration of the topoisomerase used in the reaction needs to be determined empirically (see Note 2). This is because too much topoisomerase in the reaction can inhibit the strand passage reaction. This may be because that if the complementary regions of the substrate are fully coated by the enzymes, they will no longer be able to form duplexes, which are the driving force for the strand-passage reaction that converts the circle to knot. For humTop3β, its optimal concentration is about 1–4 nM concentration.
The optimum reaction temperature for some topoisomerases, particularly those from thermo-resistant bacteria and archaea, may be higher than 37 °C. We often use 50 °C for enzymes from these species.
The mixture was incubated at 37 °C for 90 min. The reaction was terminated adding 2 µl 5× stop buffer (1 mg/ml Proteinase K, 2.5% SDS, 100 mM EDTA). The mixture was incubated at 50 °C for 30 min to allow degradation of proteins by the Proteinase K. The mixture was then extracted by phenol–-chloroform and precipitated with ethanol.
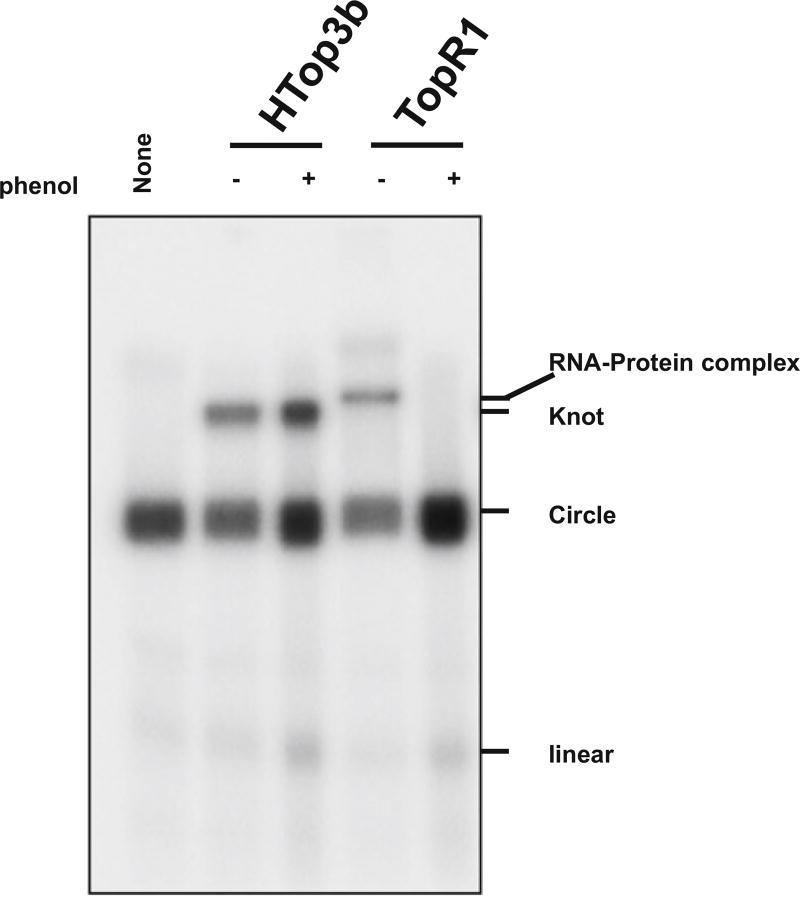
We found that for many topoisomerases, such as hum Top3β and EcoTop1, the step of phenol–chloroform extraction can be omitted, as proteinase K digestion is sufficient to degrade these enzymes. However, for other topoisomerases (such as yeast Top3), this step is necessary. These topoisomerases can form strong RNA–protein complexes that are resistant to Proteinase K digestion and heat-denaturation. The complexes can produce gel shift on denaturing gels that has similar mobility as the trefoil knot (Fig. 3). Phenol–chloroform extraction can efficiently disrupt these RNA–protein complexes, remove the protein, and avoid the confusion of mistaking these gel shifts as trefoil knots.
After ethanol precipitation, the RNA mixture was redissolved with 5 µl water and 5 µl Gel loading dye II, denatured by heat at 95 °C for 3–5 min, and fractionated on a 15% TBE–urea gel (Invitrogen). Gels that are more than 3 weeks old should not be used because they often have poor resolution (see Note 3). After electrophoresis at 150 V for about 5–6 h, the gel was analyzed by Storm 860 Molecular Imager (Molecular Dynamics).
To improve the image quality, the radiolabeled RNA in the gel can be first transferred to a Nylon membrane by electrophoresis (similar to Western blotting), and then analyzed by Storm 860 Molecular Imager. Because the membrane is much thinner than the gel, the radiolabeled RNA becomes more concentrated after the transfer, and thus produces a much sharper signal on the Imager.
Fig. 3.
Phenol–chloroform extraction distinguishes the RNA knot from a gel shift caused by stable RNA–protein complexes. An autoradiograph shows that the RNA knot produced by humTop3β RNA topoisomerase (4 nM) does not disappear after phenol chloroform extraction (Lane 2 and 3). In contrast, the product produced by a reverse gyrase from an archaea species, TopR1 (4 nM) of Sulfolobus solfataricus, was disrupted by phenol chloroform extraction, indicating that the latter reaction did not produce the RNA knot. Instead, the latter reaction generated a highly stably RNA–protein complex containing the circular RNA substrate and the enzyme, which results in the gel shift. Yeast topoisomerase 3 can also form a gel shift when incubated with the circular RNA substrate at high protein concentrations (data not shown)
3.4 RNA Substrate for Type 1A Topoisomerase Assays
The trefoil knot formation assay described here has been used to detect RNA topoisomerase activity for many Type I enzymes from all three domains of life, which include humTop3β, yeast Top3, E. coli Top1, Nanoarchaeum equitans Top3, and Sulfolobus solfataricus Top3 [9]. However, it failed to detect RNA topoisomerase activity for Type IA enzymes (Top1) from Mycobacterium tuberculosis and Mycobacterium smegmatis. It is possible that the latter two enzymes lack RNA topoisomerase activity. However, it is equally possible that our assay may not be sensitive enough for detecting the RNA topoisomerase activity in all Type IA topoisomerases. One way to improve the sensitivity of the assay is to further increase the length of the complementary regions of the substrate to make the reaction thermodynamically more favorable. The length of the spacer region can also be optimized to favor the knot formation. New assays may be developed that are more sensitive and test different features of these topoisomerases. Indeed, Hsieh and colleagues have recently described a new RNA topoisomerase assay based on annealing of two complementary single-stranded RNA circles to produce a double-strand RNA circle [10]. Using the new assay, they also observed RNA topoisomerase activity in Type IA enzymes from E. coli, archaea, and Drosophila. All these data support the notion that RNA topoisomerase activity is broadly present in Type IA topoisomerases in all domains of life, which can solve topological problems for both DNA and RNA.
4 Notes
We found that it is important to have the p32-labeled circular substrate with high specific radioactivity. If specific radioactivity of the substrate is too low, the conversion from the circle to knot will be less efficient.
It is essential to make good preparations of topoisomerases.We found the RNA topoisomerase activity of humTop3β varies between different preparations. Moreover, careful titration of the topoisomerase is also needed to find the optimal concentration of the enzyme for the reaction. Too little or two much enzymes may give poor conversion of the RNA circle to knot.
One should avoid using old gels, because their resolution is poor that they may not efficiently separate the RNA circle from the knot.
Acknowledgments
The experimental protocols described here have been previously reported in less detail in two previous publications of our group [8, 9]. This work is supported in part by the Intramural Research Program of the National Institute on Aging (Z01 AG000657-08), National Institutes of Health; the National Basic Research Program of China (2013CB911002); and National Natural Science Foundation of China (31271435).
References
- 1.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 2.Pommier Y, Sun Y, Huang SN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol. 2016;17(11):703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forterre P, Gribaldo S, Gadelle D, Serre MC. Origin and evolution of DNA topoisomerases. Biochimie. 2007;89:427–446. doi: 10.1016/j.biochi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Stoll G, Pietilainen OP, Linder B, Suvisaari J, Brosi C, Hennah W, Leppa V, Torniainen M, Ripatti S, Ala-Mello S, et al. Deletion of TOP3beta, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16:1228–1237. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan KY, Greenwald RJ, Mohanty S, Sharpe AH, Shaw AC, Wang JC. Development of autoimmunity in mice lacking DNA topoisomerase 3beta. Proc Natl Acad Sci U S A. 2007;104:9242–9247. doi: 10.1073/pnas.0703587104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan KY, Wang JC. Mice lacking DNA topoisomerase IIIbeta develop to maturity but show a reduced mean lifespan. Proc Natl Acad Sci U S A. 2001;98:5717–5721. doi: 10.1073/pnas.101132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Di Gate RJ, Seeman NC. An RNA topoisomerase. Proc Natl Acad Sci U S A. 1996;93:9477–9482. doi: 10.1073/pnas.93.18.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Shen W, Guo R, Xue Y, Peng W, Sima J, Yang J, Sharov A, Srikantan S, Fox D, 3rd, et al. Top3beta is an RNA topoisomerase that works with fragile × syndrome protein to promote synapse formation. Nat Neurosci. 2013;16:1238–1247. doi: 10.1038/nn.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad M, Xue Y, Lee SK, Martindale JL, Shen W, Li W, Zou S, Ciaramella M, Debat H, Nadal M, et al. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Res. 2016;44:6335–6349. doi: 10.1093/nar/gkw508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siaw GE, Liu IF, Lin PY, Been MD, Hsieh TS. DNA and RNA topoisomerase activities of Top3beta are promoted by mediator protein Tudor domain-containing protein 3. Proc Natl Acad Sci U S A. 2016;113(38):E5544–E5551. doi: 10.1073/pnas.1605517113. [DOI] [PMC free article] [PubMed] [Google Scholar]