Abstract
Neurological disorders – disorders of the brain, spine and associated nerves – are a leading contributor to global disease burden with a shockingly large associated economic cost. Various treatment approaches – pharmaceutical medication, device-based therapy, physiotherapy, surgical intervention, among others – have been explored to alleviate the resulting extent of human suffering. In recent years, gene therapy using viral vectors – encoding a therapeutic gene or inhibitory RNA into a “gutted” viral capsid and supplying it to the nervous system – has emerged as a clinically viable option for therapy of brain disorders. In this Review, we provide an overview of the current state and advances in the field of viral vector-mediated gene therapy for neurological disorders. Vector tools and delivery methods have evolved considerably over recent years, with the goal of providing greater and safer genetic access to the central nervous system. Better etiological understanding of brain disorders has concurrently led to identification of improved therapeutic targets. We focus on the vector technology, as well as preclinical and clinical progress made thus far for brain cancer and various neurodegenerative and neurometabolic disorders, and point out the challenges and limitations that accompany this new medical modality. Finally, we explore the directions that neurological gene therapy is likely to evolve towards in the future.
Keywords: Gene therapy, Viral vectors, Neurodegeneration, Brain tumors, Metabolic diseases, Lysosomal storage diseases
1. Introduction
Gene therapy is becoming a viable option for clinical intervention largely due to the success and safety of the current generation of virus-based vectors (Naldini, 2015; Maguire et al., 2014). In general viral vectors have proven more efficient at gene delivery in vivo than synthetic nanoparticle and liposome vectors, and in most diseases the goal is to transduce as many affected cells as possible. Within the nervous system, phase 1/2 clinical trials have shown benefit for several neurologic diseases involving replacement of defective genes. This includes: restoration of vision, at least for an extended period, in Leber’s congenital amaurosis using adeno-associated virus (AAV) vectors (Pierce and Bennett, 2015); curtailment of further brain neurodegeneration in the brain in metachromatic leukodystrophy (MLD) (Biffi et al., 2013) and adrenoleukodystrophy (ALD) (Cartier and Aubourg, 2010) using ex vivo genetic modification of hematopoietic stem cells with lentivirus vectors; and safety for oncolytic vectors in clinical trials (Piccioni and Kesari, 2013). Success has also been reported in blocking human immunodeficiency virus (HIV) infection in a single individual using ex vivo gene editing of autologous CD4 T cells to disrupt the viral receptor gene CCR5 (Tebas et al., 2014).
It is no wonder that viruses have led the field in gene therapy, as they have evolved to deliver their genes efficiently, in the form of RNA or DNA into mammalian cells. In many cases their aim is to replicate in and kill the host cell in the process, as in the case of adenovirus and herpes simplex virus (HSV). In other cases they may be non-pathogenic such as AAV, or may either become latent in the host cell for long periods (e.g. HSV in trigeminal neurons) or integrate into the host cell genome at different sites as with retrovirus and lentivirus. Viruses have evolved very efficient mechanisms to deliver their genome into cells (Fig. 1). In the case of HSV and retrovirus/lentivirus their genome is carried within a proteinaceous capsid surrounded by a lipid membrane envelope. This envelope can efficiently fuse with the plasma membrane of the cell delivering its contents directly into the cytoplasm with subsequent release of nucleic acids from the capsid, and either conversion to DNA (in the case of retro/lentivirus vectors) or delivery of DNA (in the case of HSV) into the cell nucleus. Other viruses, such as AAV and adenovirus, have a proteinaceous capsid that is taken up by endocytosis and have evolved ingenious means to escape the endosome/lysosome compartment to deliver the viral DNA to the nucleus. By “gutting” the virus of its own genes and just retaining the packaging signals, one can package viral vectors in culture using viral genes in trans for replication and virus structure and generate an efficient delivery vehicle for the transgene of interest with no viral genes.
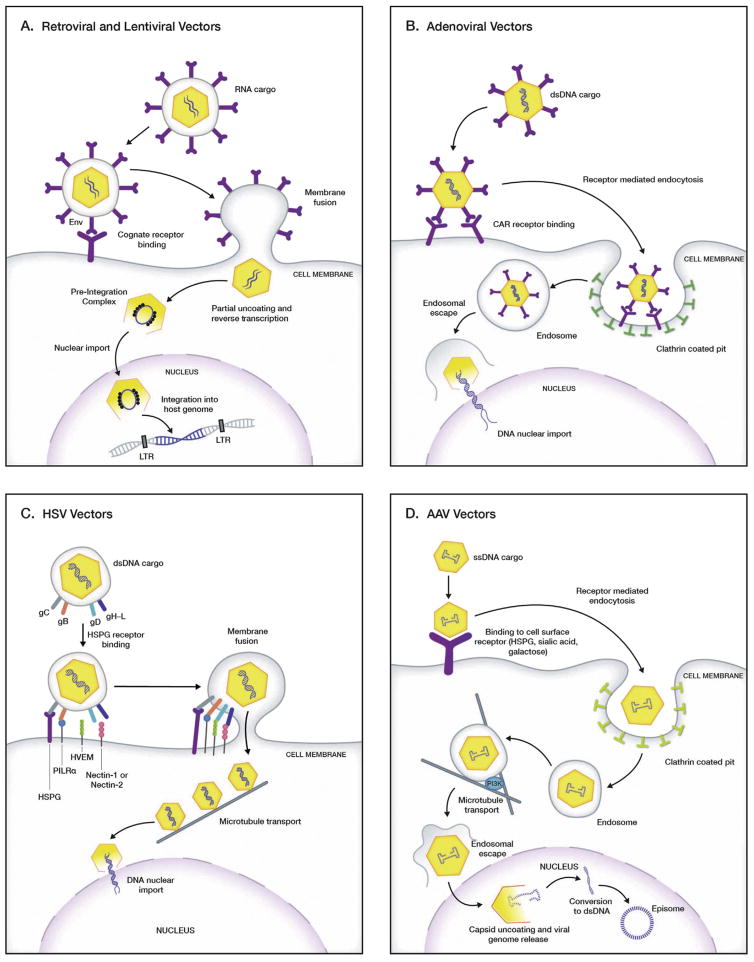
Fig. 1.
Mechanism of receptor-mediated host cell entry and subsequent gene transfer by viral vectors. A. Retroviral and lentiviral vectors. The viral attachment glycoproteins on the envelope surface of retroviral and lentiviral vectors bind to the cognate cell surface receptors and co-receptors and subsequently fuse to the cell membrane to enter the host cell. Once in the cytoplasm, the virion partially uncoats and the RNA transgene is reverse transcribed to the cDNA form. Nuclear entry is followed by integration of the viral cDNA into the host genome. B. Adenoviral vectors. These vectors bind to the CAR receptor on the host cell surface, through the fiber knob on the capsid. This facilitates cell entry by endocytosis through clathrin-coated pit pathway and subsequent conversion to an endosome. A reduction in pH in the endosome triggers the release of the degraded virion into the cytoplasm. The escaped virion releases its viral double stranded DNA into the nucleus through the nuclear pore. C. Herpes simplex virus (HSV) vectors. Glycoproteins gB and gC on the HSV virion envelope mediate the initial attachment of virus particles to HSPG on the host cell surface. gB binding to PILRα and gD binding to HVEM, nectin-1 or nectin-2 then triggers membrane fusion, followed by release of the capsids into the host-cell cytoplasm. Post cytoplasmic entry, the intact viral capsid travels to the nucleus via microtubule-dynein mediated transport, where the viral DNA enters the nucleus through the nuclear pore. D. Adeno associated virus (AAV) vectors. Receptor ligands on the surface of AAV capsids vary with serotype, and allow capsids to bind to a variety of glycan receptors on the host cell surface (HSPG, N- and O-linked sialic acids, galactose). Once bound, the virion gets internalized via clathrin-coated vesicles/endosomes and trafficked to the nuclear area while still associated with the endosome. Endosomal acidification leads to release of the damaged capsid, which is transported into the nucleus. Capsid uncoating in the nucleus is followed by conversion of the single-stranded vector genome to double-stranded DNA. The double-stranded vector genome persists as stable circular and/or concatemeric extrachromosomal episomes. Abbreviations: CAR – coxsackievirus and adenovirus receptor, Env - envelope glycoprotein, HSPG – heparin sulfate glycoprotein, HVEM – herpesvirus entry mediator, LTR – long terminal repeats, PI3K – phosphatidylinositol-3 kinase, PILRα – paired immunoglobulin-like type 2 receptor-α.
As in pharmacology, it is a therapeutic advantage if the cause of the disease (target) is known, as in monogenic hereditary diseases. For these diseases, gene therapy strategies using viral vectors include gene replacement by bringing in a normal copy of a defective gene using AAV vectors which establish themselves as stable “episomes” in the cell nucleus, and lentivirus vectors which integrate transgenes into the genome. Both AAV and lentivirus vectors are capable of infecting dividing and non-dividing cells, but the latter are a better choice for permanent modification of dividing cell populations as genomic integration ensures the progeny generated from the primary target cell is also genetically modified. These same vectors can be used to deliver siRNAs (shRNAs)/miRNAs that can downregulate a dominant-negative mutant mRNA/protein, sometimes also decreasing copies of the normal mRNA. This is combined with delivery of a replacement gene that contains “silent” mutations, making the mRNA resistant to cleavage by the RNAi molecule (Mao et al., 2012; Li et al., 2011a; Mueller et al., 2012). In the future with CRISPR or other gene editing technology (Mussolino and Cathomen, 2012) combined with viral vectors, it should be possible to correct gene defects in recessively inherited diseases and to eliminate mutant genes in dominantly inherited diseases in mammalian brain (Swiech et al., 2015). For diseases of unknown etiology, strategies have evolved to deliver neurotrophic factors, neurotransmitters, compensatory proteins, and selectively toxic proteins into the brain.
Some general principles of intervention are becoming clear. 1) Treat early. Excluding prenatal gene therapy, which is not considered ethical at this stage, and given that most neurologic diseases have progressive sequelae, it is best to begin treatment as early as possible. For example, for spinal muscular atrophy (SMA) in which motor neurons are lost leading to death within months for some babies, AAV gene replacement is being tested in babies 2–9 months of age (https://clinicaltrials.gov/ct2/show/NCT02122952). In MLD and ALD where gene therapy can arrest neurodegeneration, it is important to treat before extensive damage to the brain has already occurred (Biffi et al., 2013; Cartier et al., 2009). 2) Too much has to be okay. With the current generation of promoters, the levels of transgene expression cannot be regulated. You have the choice of using strong or weak, constitutive or cell specific promoters, but not of adjusting levels of expression or turning off expression after delivery. 3) Transgene size is limited. The two vectors for which we have the most clinical experience, AAV and lentivirus, have a limited transgene cassette capacity (includes regulatory sequences, promoter, cDNA, and polyA sequence, etc.), with a maximum size of about 4.5 kb for AAV and about 8.5 kb for lentivirus. For some hereditary diseases, such as ataxia telangiectasia or neurofibromatosis type 1 (NF1) with cDNAs of 9.6 kb and 8.4 kb respectively, this presents a difficult hurdle. In the case of dystrophin (11 kb), mutated in Duchenne’s muscular dystrophy, a condensed version of the gene (minigene) has been developed which lacks repeat sequences and fits into an AAV (Bengtsson et al., 2015). One approach to overcome this limitation is the dual AAV vector system, where an oversized transgene is split across two AAV vectors to reconstitute the full-length cDNA upon delivery (Yan et al., 2000). Full-length assembly can occur through recombination of homologous regions or/and trans-splicing (Chamberlain et al., 2016). Dual AAV vectors have shown therapeutic efficacy in mouse models of retinal disorders (Trapani et al., 2014). 4) Pick realistic outcome measures. It is difficult to monitor benefit if the natural history of the disease is variable and the phenotypic traits are not quantitative and are protracted over time. For example, neurotrophic therapy in Parkinson’s disease (PD) may work, but the outcome measures are subjective and the time to measureable clinical improvement may be unrealistically long (Bartus et al., 2014). Realistic outcome measures include, for example, metabolite levels, lesion size using a variety of imaging methods, and nerve conduction velocity, with improvements occurring in the scale of months. 5) You only get one shot. For now, given the immunogenicity of viral vectors, efficient delivery will only occur on the first exposure to the vector. Thus, it is a disservice to the patients to try non-therapeutic doses which may exclude the patient from future doses deemed therapeutic. This is a difficult problem as the increasing dose regime is an inherent part of the evaluation of toxicity in a phase 1 trial.
2. Which vector – why and when?
A number of different viruses have been explored for gene delivery or cancer oncolytic therapy. Here we will focus on the viruses most commonly used in clinical trials, including retrovirus vectors, lentivirus vectors, adenovirus vectors, herpes simplex virus type 1 (HSV-1) and AAV vectors covering transgene capacity, state in transduced cells, and expression properties (Table 1).
Table 1.
Viral vectors for gene therapy.
| Vector | MoMLV retroviral | Lentiviral | Adenoviral | Helper-dependent adenoviral | Recombinant HSV | HSV amplicon | AAV |
|---|---|---|---|---|---|---|---|
| Family | Retroviridae | Retroviridae | Adenoviridae | Adenoviridae | Herpesviridae | Herpesviridae | Parvoviridae |
| Particle size (nm) | 100 | 100 | 70–120 | 70–120 | 120–300 | 120–300 | 20–25 |
| Cargo | RNA | RNA | dsDNA | dsDNA | dsDNA | dsDNA | ssDNA |
| Packaging capacity (kB) | 7–8 | 7–9 | 8–10 | Up to 36 | 30–50 | Up to 150 | 4.8 |
| Vector yield (transducing units ml−1) | 1.00E+09 | 1.00E+09 | 1.00E+12 | 1.00E+12 | 1.00E+11 | 1.00E+08 | 1.00E+13 |
| Chromosomal integration? | Yes | Yes | No | No | No | No | No |
| Oncolytic? | No | No | Yes/no | No | Yes/no | No | No |
| Infects post-mitotic cells? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Risk of oncogene activation? | Yes | Yes | No | No | No | No | No |
Retroviral vectors derived from Moloney murine leukemia virus (MoMLV) were the first vectors to be used for FDA-approved clinical trials. While direct in vivo administration does not result in a therapeutic outcome (Kay et al., 1993), pseudotyped retroviral vectors have been used clinically for ex vivo correction in human progenitor cells, followed by autologous transplantation (Cavazzana-Calvo et al., 2000). Retrovirus vectors are generally not useful for neurological applications, as they cannot be used for gene transfer to non-dividing cells, such as neurons, although they can be used for lineage determination in embryos (Turner et al., 1990; Fields-Berry et al., 1992). While retroviral vectors have a modestly large packaging capacity (7–9 kb), they are produced at relatively poor titers and require proviral integration into the host genome for expression. The latter aspect is particularly concerning, as a trial for retroviral-mediated ex vivo gene therapy in children with X-linked severe combined immune deficiency (SCID) resulted in some patients developing leukemia as a result of activation of the LMO2 proto-oncogene due to proviral integration (Hacein-Bey-Abina et al., 2003). However, SCID was cured by the treatment in all cases, and the few patients who developed leukemia were treated and survived (Hacein-Bey-Abina et al., 2010). Several features of retrovirus integration, including tendency to integrate into promoter regions of genes combined with intact (long terminal repeat) LTR elements with enhancer activity in these early vectors have led to improvements in vector design (Naldini, 2015).
Lentiviral vectors derived from HIV-1 can mediate gene transfer to both dividing and non-dividing cells. Importantly, lentiviral vectors integrate preferentially in introns of transcriptionally active genes, and the use of self-inactivating designs that eliminate the promoter elements from LTRs in the provirus have reduced dramatically the oncogenic potential (Cattoglio et al., 2007; Montini et al., 2006). Furthermore, they can be pseudotyped with VSV-G (vesicular stomatitis virus G envelope protein) to allow purification to titers higher than MoMLV vectors, and have a similar transgene capacity as retroviral vectors. While lentiviral vectors can mediate long-term expression (years) in the non-human primate brain (Blömer et al., 1997; Brooks et al., 2002), their volumetric spread after direct infusion is limited to 500–700 μm from the site of infusion (Osten et al., 2006), presumably due to their large size preventing distribution through extracellular spaces in the brain and relatively few viral particles being injected per volume due to lower viral titers of lentiviral vectors (Cetin et al., 2006). They have mainly been used for ex vivo gene therapy applications. A particularly successful example has been the halt of neurodegeneration in infants and children afflicted with X-linked ALD (X-ALD) and MLD in clinical trials involving transplantation of lentivirally-corrected autologous CD34+ hematopoietic cells (Cartier et al., 2009; Biffi et al., 2013).
Adenoviral (Ad) vectors have some potential advantages over the abovementioned vectors: first, transgene packaging capacity can be up to 36 kb in high-capacity Ad vectors devoid of viral genes, albeit these “gutless” vectors are difficult to generate at high titers; second, the standard Ad vectors can be manufactured at titers up to 1000-fold higher than retro- and lentiviral vectors; and third, the transgene does not integrate into the host genome and instead remains episomal, leading to stable and sustained expression in brain for at least up to a year (Barcia et al., 2007). Direct infusion into brain parenchyma results in gene transfer to a broad range of cell populations, including neurons, astrocytes, microglia, oligodendrocytes and ependymal cells (Akli et al., 1993; Davidson et al., 1993). A major limitation, however, is the strong innate immune response to the Ad capsid itself, as well as continuing low level expression of viral genes in the standard vectors producing a secondary transgene/viral gene-dependent immune response, even after direct intraparenchymal infusion into the brain (Dubensky, 2002). While this antigenicity facilitates their use as an immunogenic adjuvant, in vivo delivery at high doses can result in severe inflammation and cytotoxicity, which resulted in the death of a patient in an ornithine transcarbamylase (OTC) deficiency clinical trial (Wilson, 2009). While this continues to be a very popular vector for clinical trials in cancer (Sheridan, 2011), the high levels of immunological response to the vector has precluded its use for neurological gene therapy, except in the case of brain tumors.
HSV vectors can efficiently infect neurons and other cell types in vivo, and are capable of both anterograde and retrograde transport within neurons (Chiocca et al., 1990; Diefenbach et al., 2008). This makes them attractive candidates for neurological gene therapy. HSV vectors can broadly be classified into two main categories: recombinant vectors, which have a packaging capacity of 50 kb, can be purified to high titers (up to 1011 particles per mL) and can persist as episomes post-infection; and plasmid-based HSV amplicons, which have a capacity of up to 150 kb, but are difficult to generate and can only be purified at 108 particles per mL (Spaete and Frenkel, 1982; Sena-Esteves et al., 2000). Recombinant HSV vectors, in turn, can be sub-divided into replication-competent and replication-defective vectors depending on whether they can undergo a lytic cycle, and both have found therapeutic use in a broad range of neurological disorders (for a list of disorders, see Costantini et al., 2000).
Adeno-associated virus (AAV) vectors are a relatively recent addition to this list of viral vectors, but have emerged as one of the most important ones for therapy of neurological disorders. It is currently the most frequently used viral vector for central nervous system (CNS) clinical trials (Table 2), and will thus largely be the focus of this review. AAV vectors are close to the ideal CNS gene therapy vector as they: a) can mediate gene transfer to both mitotic and post-mitotic cells, albeit the transgene is lost over time in dividing cells; b) are neurotropic after direct infusion into brain parenchyma (Davidson et al., 2000); c) can exist stably in an episomal state with a low rate of genomic integration (McCarty et al., 2004); d) exhibit no pathogenicity or cytotoxicity; e) have very mild immunogenicity, mostly humoral (Bessis et al., 2004); and f) can be manufactured at high titers (1013–1014 particles per mL, depending on the production method) and at high purity (Xiao et al., 1999; Urabe et al., 2002). AAV vectors have been shown to mediate stable transgene expression in the human brain for >10 years (Leone et al., 2012). However, a significant limitation of AAV vectors is their small packaging capacity (4.5 kb for single stranded vectors, 2.4 kb for self-complementary vectors), which places severe limitations on the therapeutic cargo size. Recently, vexosomes, which are hybrid viral vectors comprising AAV associated with extracellular vesicles, have been shown to allow enhanced transduction in cultured cells and anti-AAV antibody evasion in vivo in mice (Maguire et al., 2012; György et al., 2014). In addition to antibody evasion, recently vexosomes have been shown to achieve transduction efficiency rivaling conventional AAV9 vectors (Hudry et al., 2016).
Table 2.
Viral vector-mediated clinical trials for neurological disorders.
| Disease | Vector | Transgene | Phase | Trial code |
|---|---|---|---|---|
| Ex vivo | ||||
| Alzheimer’s disease | Retrovirus | NGF | I | US-0322 |
| Metachromatic leukodystrophy | Lentivirus | ARSA | I, II | Biffi et al., 2013 |
| Multiple sclerosis | Retrovirus | MBP | I, II | US-0851 |
| Wiskott-Aldrich syndrome | Lentivirus | WASP | I, II | Aiuti et al., 2013 |
| X-linked adrenoleukodystrophy | Lentivirus | ABCD1 | I, II | Cartier et al., 2009 |
| In vivo | ||||
| AADC deficiency | AAV | AADC | I, II | NCT01395641 |
| Alzheimer’s disease | AAV | NGF | I, II | NCT00087789, NCT00876863 |
| Batten disease | AAV | CLN2 | I | NCT00151216 |
| Batten disease | AAV | CLN2 | I, II | NCT01414985 |
| Canavan disease | AAV | ASPA | I | Leone et al., 2012 |
| Giant axonal neuropathy | AAV | GAN | I | NCT02362438 |
| Glioblastoma | Oncolytic poliovirus | – | I | NCT01491893 |
| Glioblastoma multiforme (GBM), other gliomas | Oncolytic adenovirus | – | I | NCT00805376, NCT01956734, NCT02197169 |
| Glioblastoma multiforme, other gliomas | Retrovirus | CD | I, II/III | NCT01470794, NCT02414165 |
| Glioblastoma, other gliomas | Oncolytic HSV | – | I | NCT02031965 |
| Glioblastoma, other gliomas | Oncolytic HSV | – | I | NCT00028158, NCT00157703 |
| Leber’s hereditary optic neuropathy | AAV | MT-ND4 | I | NCT02161380 |
| Metachromatic leukodystrophy | AAV | ARSA | I, II | NCT01801709 |
| MPS IIIA (Sanfilippo Disease Type A) | AAV | SGSH, SUMF1 | I, II | NCT01474343, NCT02053064 |
| Parkinson’s disease | AAV | GAD | I, II | NCT00195143, NCT00643890 |
| Parkinson’s disease | AAV | NTRN | I, II | NCT00252850, NCT00400634 |
| Parkinson’s disease | Lentivirus | TH, AADC, CH1 | I, II | NCT00627588, NCT01856439 |
| Parkinson’s disease | AAV | GDNF | I | NCT01621581 |
| Parkinson’s disease | AAV | AADC | I, II | NCT02418598 |
| Parkinson’s disease | AAV | AADC | I | NCT00229736 |
| Pompe disease | AAV | GAA | I, II | NCT00976352 |
| Pompe disease | AAV | GAA | I | NCT02240407 |
| Spinal muscular atrophy type 1 | AAV | SMN | I | NCT02122952 |
Other viral vectors that infect neurons and thus can potentially be used for CNS gene therapy applications include RNA viruses, such as poliovirus replicon vector (Bledsoe et al., 2000; Jackson et al., 2001), Semliki Forest virus (SFV) (Ehrengruber et al., 1999, 2001) and Sindbis virus vectors (Altman-Hamamdzic et al., 1997), as well as DNA viruses, such as SV40 (Cordelier et al., 2003; Louboutin et al., 2010).
3. Deliver the vector, the more the better (modes and sites of delivery)
Within the context of delivery to the brain, virus vectors can be injected directly into regions of pathology using strategies that either limit or extend the range of transduction, or through intrathecal (IT), intraventricular, or intravascular routes (Fig. 2). Of these, the intravascular route has historically been restrictive due to the blood-brain-barrier (BBB), but new serotypes of AAV, and modifications of AAV and hematopoietic stem cell-derived vehicles have now breached this barrier.
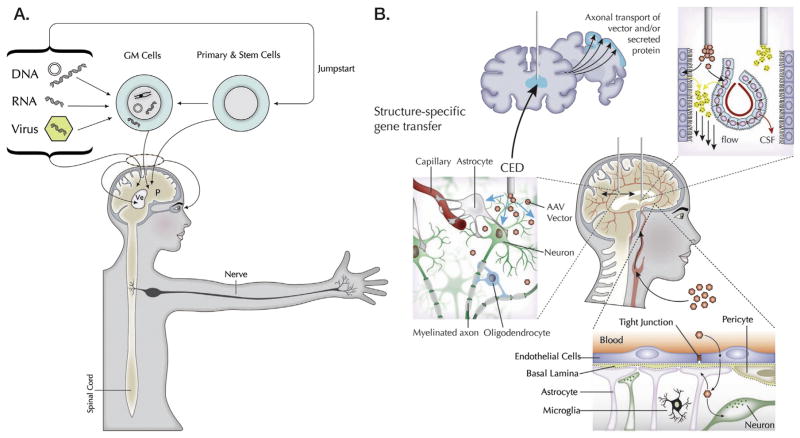
Fig. 2.
Modes of genetic modification and gene delivery in the nervous system. A: Genetic modification. Gene composition or expression in cells can be modified by introduction of DNA (e.g. expression plasmids), RNA oligonucleotides (e.g. siRNA, microRNA) and genes encoded in virus vectors. These are typically delivered by direct injection into the brain parenchyma (P) or ventricles (Ve), the spinal cord or the eye. These DNA/RNA sequences can also be used to genetically modify (GM) cells for delivery through direct injection or vascular infusion. These different vehicles: genes (G), vectors (V), oligonucleotides (O) and cells (C) can be combined in different modalities, including “jump starting” primary and stem cells to take on specific phenotypes through transcriptional regulation. B: Routes of gene delivery to the CNS. Vectors or cells can be introduced into a specific region of the brain by stereotactic surgery. In cases where more extensive vector distribution is desired, CED of viral vectors into the brain improves considerably their range in target structures by increasing transduction volumes. This technique can yield volumes of transduced cell distribution 3–3.5-fold larger than the infused volume, which is highly significant for human applications. Viral vectors or secreted transgene products (growth factors, lysosomal enzymes) can be further distributed from the primary target structure by axonal transport of vectors and/or by products and by release of products into the extracellular space. Infusion of vectors or oligonucleotides into the brain ventricular system, or intrathecal space of the spinal canal or subarachnoid space, leads to widespread CNS distribution via CSF flow. An alternative strategy is to use viral vectors to engineer ependymal cells lining the ventricles or choroid plexus cells to secrete therapeutic proteins into CSF. The BBB with its many constituents has thwarted most gene transfer vehicles from entering the brain from the vasculature. However some AAV serotypes, e.g. AAV9, AAVrh8 and AAVrh10, as well as vesicles containing AAV particles are able to transit this barrier quite efficiently, although the exact mechanism of that transit is not known. Fig. 2 and associated figure legend modified from Breakefield and Sena-Esteves (2010) and Bowers et al. (2011).
3.1. Direct injection into brain parenchyma
Most clinical trials to date have investigated the therapeutic efficacy of direct neurosurgical infusion of virus vectors into the brain parenchyma. This approach bypasses the need to cross the BBB and works for well for AAV vectors. Among AAV serotypes, AAV2, the first discovered serotype, was used in all first generation clinical trials as it is highly neurotropic, but its distribution out from the injection site is relatively poor. Newer serotypes of AAV capsids discovered in the intervening years have dramatically improved transduction efficiency and volumetric spread. Different AAV serotypes have different preferential patterns of cell transduction. For example, while AAV1, AAV8 and AAV9 and AAVrh10 are primarily neurotropic (Cearley and Wolfe, 2006), AAV4 preferentially infects astrocytes and ependymal cells (Liu et al., 2005), and AAV5 transduces both astrocytes and neurons (Davidson et al., 2000) but is predominantly neuronal (Burger et al., 2004; Klein et al., 2006). Part of this cell preference is probably due to differential binding of AAV serotypes to cell-surface receptors prior to internalization (Kaludov et al., 2001; Shen et al., 2011; Bell et al., 2011). AAV9 and AAVrh10 are the leading candidates for intraparenchymal infusion, not only because of their vector spread but also because they can be transported along axons at high efficiency (Cearley and Wolfe, 2007; Castle et al., 2014). Neurodegenerative disorders are often multifocal, affecting more than a single structure in the CNS (Wang et al., 2014). Therefore, global distribution of viral vectors, which is not limited to the site of injection, is desirable. Intracranial infusion of AAV vectors into axonally connected structures of the brain, such as the ventral tegmental area (VTA) or thalamus allows for widespread distribution of either AAV or encoded gene products that spread to distal projection sites by axonal transport (Cearley and Wolfe, 2007; Kells et al., 2009; Broekman et al., 2009). Use of pressurized convection-enhanced delivery (CED) and magnetic resonance imaging (MRI)-guided infusion can lead to significantly better distribution compared to single injections of smaller volumes (Bankiewicz et al., 2000). Facilitated access across the BBB with AAV has also been achieved using focused ultrasound and microbubbles (Wang et al., 2015).
3.2. Infusion into cerebrospinal fluid (CSF)
In addition to being spatially restricted (Vite et al., 2003), intraparenchymal infusion is invasive and carries risk of surgery-related side effects. To avoid these complications, an alternate less invasive route is administration into the CSF, where the viral vector can cross directly into the brain. This can be achieved by intracerebroventricular (ICV) or intrathecal (IT) injection of AAV vectors. Infusion into the lateral ventricles (ICV) in adult mice primarily results in transduction of the surrounding ependymal cell layer (Davidson et al., 2000) which can be extended to the brain parenchyma by intravenous co-administration of mannitol to raise osmolarity and thereby open up junctions between ependymal cells. ICV injection of AAV into neonatal mice provides a means of widespread gene delivery of many cell types throughout the brain and spinal cord (Kim et al., 2014; McLean et al., 2014). On the other hand, IT injection into the CSF, either at the subarachnoid space in the cisterna magna or into the intervertebral cistern in the lumbar spinal cord, is less invasive than ICV delivery and results in robust and widespread transduction of spinal cord motor neurons, as well as dorsal root ganglia (DRG) in mice (Snyder et al., 2011). In higher species such as pigs and non-human primates, the spread of gene transfer after IT injection is much wider, with multiple cell populations (including neurons) in the brain and brainstem being transduced at high efficiency (Federici et al., 2012; Samaranch et al., 2012; Gray et al., 2013). However, this route has some limitations. There are contradictory reports as to whether AAV administration via intra-CSF route shields against anti-AAV neutralizing antibodies (Gray et al., 2013; Samaranch et al., 2012). There is also some concern that the distribution results arise partly from AAV vector leaking from the subarachnoid CSF space into systemic circulation (Schuster et al., 2014).
3.3. Delivery via the vasculature
Systemic administration has the potential to mediate gene transfer to the entire CNS ubiquitously and non-invasively with every cell in the brain being a maximum of 40 microns from endothelial cells (Wong et al., 2013). AAV9 was the first AAV serotype to be shown to cross the BBB without pharmacological intervention (Foust et al., 2009), and remains the gold standard for CNS therapy by intravenous delivery. Other serotypes, such as AAVrh8 and AAVrh10, were subsequently demonstrated to lead to robust and sustained CNS transduction following intravascular administration, transducing both neuronal and non-neuronal cell populations (Zhang et al., 2011; Yang et al., 2014). The mechanism of this BBB circumvention is not yet known. It is possible that such a mechanism might be serotype-specific, as exemplified by the observation that while transient opening of BBB tight junctions by mannitol leads to increased AAV2 CNS gene transfer (Fu et al., 2003; McCarty et al., 2009), it does not enhance transport of AAV9 into the brain (Gray et al., 2011). There are conflicting reports on the extent of neuronal transduction upon systemic administration in adults, possibly due to variations in dosage and promoter usage (Foust et al., 2009; Gray et al., 2011). Several concerns for clinical development exist. The first is significant vector loss and concerns of toxicity (Grimm et al., 2006) due to high off target peripheral biodistribution, especially to the liver, which can be somewhat reduced by restricting blood flow by occlusion (Bevan et al., 2011) or by choosing to express a therapeutic gene under a neuronal-specific promoter. The other major concern is that the presence of neutralizing antibodies to AAV in monkeys and humans can severely limit the extent of gene transfer by systemic infusion (Gray et al., 2011; Samaranch et al., 2012). Recent efforts at capsid reengineering (Castle et al., 2016) have led to the generation of two AAV capsid variants, AAV-AS (Choudhury et al., 2015) and AAV-PHP.B (Deverman et al., 2016). Both vectors are superior to the current standard AAV9 at transducing the CNS, with high efficiency of gene transfer to neurons.
3.4. Age at time of infusion
The timing of AAV administration is one of the key factors that determine the extent of CNS transduction and the relative transduction of cell populations in the CNS. In general, transport across the BBB is higher in younger individuals (Hordeaux et al., 2015). Irrespective of the injection route, there are multiple reports suggesting that injection of AAV9 at P0 (post-natal day 0) in mice results in widespread neuronal transduction, while administration at older ages results primarily in glial and endothelial cell transduction (IV: Foust et al., 2009; Bevan et al., 2011; Samaranch et al., 2012; ICV: Chakrabarty et al., 2013). The switch away from neuronal transduction at older ages results in loss of therapeutic efficacy of AAV9-based approaches for some neurodegenerative diseases such as SMA where therapy is highly dependent on motor neuron transduction (Foust et al., 2010).
4. Different modalities for different diseases
4.1. Gene replacement or inhibition
Canavan disease
(CD) is a pediatric leukodystrophy, characterized by accumulation of N-acetyl aspartate (NAA) in the brain due to mutations in the aspartoacylase (ASPA) gene (Kaul et al., 1993). CD gene therapy has historical significance, as the disorder represents both the first clinical trials for a neurodegenerative disorder (ICV administration of lipid-encapsulated plasmid DNA (LPD) containing hASPA cDNA; Leone et al., 2000), and the first AAV-based FDA-approved clinical trial for a brain disorder (Janson et al., 2002 HGT 13: 1391–1412). This later Phase I/II clinical trial involved intraparenchymal infusion of AAV2-hASPA vector into the brain, and was based on pre-clinical proof-of-principle studies on a transgenic rat model of CD (McPhee et al., 2006). No toxicity or long-term adverse effects were evident. Importantly, a significant decline in the elevated NAA levels was observed in the brains of all treated patients, albeit only modest neurological improvement was reported (Leone et al., 2012). Although anti-capsid neutralizing antibodies could be detected, no correlation to clinical outcome was found. A recent preclinical study with intravenous delivery of AAV9 has shown seemingly complete phenotypic rescue of disease in a CD genetic mouse model (Ahmed et al., 2013).
Lysosomal storage disorders
Lysosomal storage disorders (LSDs) include over 50 rare individual genetic disorders resulting in defective metabolism and lysosomal accumulation, which collectively have a frequency of one per 7000–8000 live births (Wang et al., 2011). Enzyme replacement therapy is the most common clinical treatment for this group of disorders, but is less effective for the neurological symptoms due to the inability of the enzymes to cross the BBB. LSDs are a good target for gene replacement therapy, as correction of even 5–10% of normal enzyme levels is enough to stop disease progression, and genetically modified cells can cross-correct neighboring cells through enzyme secretion and uptake by the mannose-6-phosphate receptor pathway (Sands and Davidson, 2006). A Phase I/II clinical trial for MLD involving transplantation of autologous hematopoietic stem cells corrected ex vivo with a lentiviral vector expressing functional arylsulfatase A (ARSA) enzyme, reported stable ARSA gene replacement, no aberrant clonal cell expansion and no manifestation of disease 7–21 months after the predicted disease onset (Biffi et al., 2013). Gene therapy for LSDs involving in vivo AAV gene replacement have been successful in preclinical models, but less so in clinical trials. Numerous preclinical studies with gene replacement by in vivo intracranial AAV administration have shown partial to complete phenotypic correction in animal models of several LSDs. These include MPS I (Hinderer et al., 2014), MPS IIIA (Ruzo et al., 2012), MPS IIIB (Fu et al., 2011), MPS VII (Cearley and Wolfe, 2007), MLD (Miyake et al., 2014; Sevin et al., 2006), multiple sulfatase deficiency (MSD) (Spampanato et al., 2011), Niemann-Pick A disease (Passini et al., 2005), Pompe disease (Falk et al., 2013), GM1-gangliosidosis (Weismann et al., 2015), Tay Sachs disease (Cachón-González et al., 2006) and Sandhoff disease (Sargeant et al., 2011).
On the other hand, clinical trials have proved less effective. While intracranial administration of an AAV2-CLN2 vector in late-infantile Batten disease (late-infantile neuronal ceroid lipofuscinosis, LINCL) patients in a Phase I/II clinical trial was found to be safe, there was no significant clinical improvement (Worgall et al., 2008). Similar results were found in a Phase I/II trial for Sanfilippo syndrome type A (mucopolysaccharidosis IIIA, MPS IIIA), where intracranial administration of AAVrh10-SGSH-SUMF1 vector was well tolerated in the patients, but resulted in only minor improvement in clinical outcomes (Tardieu et al., 2014). Other clinical trials for LINCL (NCT01414985) and MPS IIIA (NCT02053064) based on the above two studies have been initiated. Clinical trials for Pompe disease (NCT02240407, NCT00976352), MLD (NCT01801709), giant axonal neuropathy (GAN) (NCT02362438) and a proposed trial for MPS IIIB, all based on gene replacement in vivo by AAV vectors, are currently being initiated. The modest outcomes in human trials may be related to the doses that have been tested which have yet to reach doses successfully tested in animal models. In addition the choice of delivery routes and target sites/structures which are effective to treat a small mouse brain may not be adequate to achieve widespread distribution of functional enzyme in the ~2000-fold larger human brain. Capsid selection can play a critical role in clinical outcomes. Most of the aforementioned preclinical efficacy has been achieved using improved AAV capsids such as AAV9, presumably due to their superior distribution properties. While AAV2 (NCT00151216) and AAV1 (NCT00976352) were used in the early clinical trials, more recent trials have utilized AAV capsids with superior gene delivery efficacy such as AAV9 (NCT02240407, NCT02362438) and AAVrh10 (NCT01414985, NCT01801709).
X-linked adrenoleukodystrophy
(X-ALD) is caused by mutations in the ABCD1 gene, which results in the accumulation of very long-chain fatty acids (VLCFA) in the body. The cerebral form of the disease is fatal. Hematopoietic stem cell transplantation of CD34+ cells corrected ex vivo by a lentiviral vector encoding ABCD1 gene into two X-ALD patients with cerebral demyelination resulted in reversal of demyelination and alleviation of associated neurological symptoms in both patients (Cartier et al., 2009). Furthermore, no clonal cell expansion or preference for oncogenic site of genome integration could be detected, highlighting the safety profile of such a therapy. A recent preclinical study in Abcd1 knockout mice showed that in vivo delivery of AAV9-ABCD1 could reduce VLCFA levels in the CNS (Gong et al., 2015).
Spinal muscular atrophy
(SMA) is a fatal infantile motor neuron disorder, resulting from loss of the SMN1 gene. Gene replacement by intravascular AAV9 administration has proved effective in preclinical studies in both mice and non-human primates (Foust et al., 2010; Bevan et al., 2010; Passini et al., 2014; Meyer et al., 2015; Duque et al., 2015). Based on these studies, a Phase I clinical trial (NCT02122952) with intravenous administration of AAV9-SMN1 has been initiated in babies 2–9 months of age (https://clinicaltrials.gov/ct2/show/NCT02122952).
Other disorders
Pre-clinical in vivo AAV gene therapy studies have been carried out for several other disorders involving both gene replacement in recessive disorders or inactivation of mutant genes in dominant disorders. These include Rett syndrome (delivery of MeCP2: Garg et al., 2013), amyotrophic lateral sclerosis (ALS) (shRNA targeting SOD1: Foust et al., 2013; delivery of ADAR2: Yamashita et al., 2013; delivery of single chain antibody targeting misfolded SOD1: Patel et al., 2014), Huntington’s disease (shRNA targeting HTT: Harper et al., 2005; artificial miRNA targeting HTT: McBride et al., 2008; Dufour et al., 2014) and Machado-Joseph disease (artificial miRNA targeting ATXN3: Rodríguez-Lebrón et al., 2013).
4.2. Trophic factor support and neurotransmitter modulation
4.2.1. Parkinson’s disease and AADC deficiency
Parkinson’s disease (PD) is an age-related neurodegenerative disorder that affects about one in every 100 people older than 60 years of age worldwide, and as many as one million people in the United States alone. PD is characterized by the loss of dopaminergic neurons in the substantia nigra with resulting loss of dopamine in the putamen (Samii et al., 2004). The current standard of care involves dopamine replacement by administration of levodopa and deep brain stimulation; however, levodopa becomes ineffective in advanced stages of the disease, probably because of the continuing loss of dopaminergic neurons.
Clinical intervention for PD by intraparenchymal delivery of viral vectors can broadly be divided into two strategies: over-expression of enzymes involved in neurotransmitter synthesis; and overexpression of neurotrophic factors that can promote neuron survival. One clinical trial was based on the first approach, where AAV2 encoding glutamic acid decarboxylase (GAD) was injected into the subthalamic nucleus of PD patients to generate the inhibitory transmitter gamma amino-butyric acid (GABA; Kaplitt et al., 2007). While this trial satisfied the Phase I safety criteria, a Phase II clinical trial, resulting from the findings of this study, showed only modest efficacy (LeWitt et al., 2011). Infusion of AAV encoding aromatic L-amino acid decarboxylase (AADC), which converts L-dopa to dopamine, in the putamenwas also shown to be safely tolerated in two Phase I clinical trials (Christine et al., 2009; Muramatsu et al., 2010). While long-term stable AADC expression could be detected by PET scanning, the initial clinical improvement deteriorated over 4 years even with continued administration of L-dopa (Mittermeyer et al., 2012). A new Phase I trial based on combined pressurized CED and MRI-guided infusion into the putamen is currently recruiting participants. A Phase I/II dose escalation study involving infusion of ProSavin, a tricistronic self-inactivating lentiviral vector encoding tyrosine hydroxylase (TH; the first and rate limiting enzyme in dopamine synthesis), AADC and GTP cyclohydrolase I (GCH1; which generates the biopterin cofactor for TH) was found to be well tolerated with no toxicity, and even showed improvement in motor function in combination with L-dopa administration in patients injected at the higher dose (Palfi et al., 2014). However, these interventions to alter firing patterns or increase dopamine levels in the brain may be less effective due to the continued degeneration of dopaminergic neurons.
The second approach is based on the expression of neurotrophic growth factors. The prime example is neurturin (NRTN), a natural analog of glia-derived neurotropic factor (GDNF), which has been shown to protect dopaminergic neurons in pre-clinical studies (Kordower et al., 2006; Gasmi et al., 2007a,b). Again, while Phase I clinical trials with CERE-120 AAV2-NRTN vectors showed no adverse effects (Marks et al., 2008), Phase II efficacy results were modest with no significant improvement in primary outcome measure at 1 year, albeit with some improvement at a later time (Marks et al., 2010). Injection into both putamen and substantia nigra in a Phase IIb clinical trial did not improve this disappointing clinical outcome (Herzog et al., 2013). A third emerging approach is to decrease levels of alpha-synuclein expression, which accumulates in the brains of PD patients, using an AAV vector encoding a short hairpin (sh) RNA targeting the mRNA for this protein (Zharikov et al., 2015), which has yet to be tested in patients.
While AAV2-AADC delivery to the putamen resulted in modest clinical outcomes in PD patients, a similar strategy used in a Phase I study to treat AADC deficiency (a fatal loss-of-function disorder with a high prevalence in Taiwan) showed highly promising results (Hwu et al., 2012). Treated children that are otherwise unable to move showed improved motor function and increased dopamine in the CSF, with no AAV-associated toxicity. This may have implications for PD therapy, as it could indicate that clinical intervention may become less effective at a later age.
4.3. Compensatory proteins – Alzheimer’s disease
Alzheimer’s disease (AD) is one of the main causes of dementia in the elderly, affecting 6% of individuals 65 years and older (Burns and Iliffe, 2009). The vast majority of patients suffer from the sporadic form of the disease, but numerous predisposing genes (Ballard et al., 2011), as well as environmental risk factors, such as traumatic brain injury (for review see Breunig et al., 2013), diabetes (for review see Bedse et al., 2015), stroke and hypertension (for review see Cechetto et al., 2008), have been identified over the years. The defining neuropathologic characteristics are accumulation of Aβ peptide, a cleaved product of amyloid precursor protein (APP), in toxic amyloid fibrils forming extracellular plaques, in association with abnormally hyperphosphorylated tau filaments composing intraneuronal neurofibrillary tangles. These hallmarks are associated with elevated levels of inflammatory cytokines, including interleukin1β and TNFα, disregulated calcium homeostasis, synapse collapse, neuronal dysfunction and death of neurons (especially of cholinergic neurons (Grothe et al., 2012)). The multiple etiologies of this disease make it hard to identify a therapeutic target, although the consensus seems to be that decreasing levels of Aβ peptides and/or preventing abnormal tau phosphorylation and aggregation is an important goal.
The devastating consequences of this disease have elicited a broad range of gene therapy strategies (reviewed in O’Connor and Boulis, 2015) with only a recent subset listed below. The most straightforward strategies have aimed at directly inhibiting the production of neurotoxic species of amyloid, either via viral vector expression of Aβ degrading enzymes, such as Neprilysin (Li et al., 2015; Lebson et al., 2010), or anti-Aβ single-chain antibodies to achieve passive immunization (Levites et al., 2015). Alternatively, targeting the cleavage of APP has been attempted, with genetic transfer of siRNA specific for β-secretase (Nawrot, 2004) or shRNA to knock-down the orphan G protein-coupled receptor that regulates activity of γ-secretase (Huang et al., 2015a), the first and second enzymes, respectively, controlling the proteolysis of APP to produce the neurotoxic amyloid peptides, respectively. Additionally, AAV-mediated expression of CD74, a chaperone that directly binds to the APP, also prevents the accumulation of Aβ in the hippocampus and improves cognitive deficits of AD mice (Kiyota et al., 2015).
Because of the complexity of the molecular mechanisms underlying AD neuropathological changes and the relatively low success of therapeutic strategies directly based on the “amyloid hypothesis” in clinical trials, other alternative approaches have emerged. Based on genetic risk studies, it was found that individuals who inherit the rare apolipoprotein E (APOE) ε2 allele have a markedly reduced risk of developing AD by about 50%. Expressing this specific isoform of APOE in the brains of a mouse model of AD after ICV infusion of an AAV4 vector reduced amyloid plaque formation and associated synapse loss (Hudry et al., 2013). Potentially, viral vectors could also be used to express anti-ApoE antibodies, an alternative immunotherapy approach that has proven effective in AD mouse models (Liao et al., 2014). Recently Murphy and colleagues showed that silencing expression of acylCoA-cholesterol acyltransferase, an important enzyme regulating lipid metabolism, as well as APP processing, using a vector encoding an artificial miRNA can alleviate both amyloid and tau pathology in a transgenic AD mouse model (Murphy et al., 2013). Finally, hippocampal delivery of an AAV vector encoding cholesterol-24S-hydroxylase, an enzyme that regulates neuronal cholesterol efflux, ameliorated cognitive deficits and spine defects associated with a murine model of tauopathy without impacting Tau hyperphosphorylation (Burlot et al., 2015). This approach was also shown to reduce dramatically the Aβ burden and neuropathology in the brain of APP23 mice (Hudry et al., 2010).
Other therapeutic approaches to AD gene therapy are more tangential, including AAV delivery of a FK506-binding protein (FKBP1b) that stabilizes Ca++ release from the endoplasmic reticulum in neurons (Gant et al., 2015) or delivery of growth factors that help neurons survive toxic injury, including delivery of cerebral dopamine neurotrophic factor (CDNF) (Kemppainen et al., 2015), insulin growth factor (Pascual-Lucas et al., 2014), brain-derived neurotrophic factor (BDNF; Nagahara et al., 2013) and nerve growth factor (NGF; Tuszynski et al., 2015) to the brain using different virus vectors. The latter has proceeded to promising clinical trials.
4.4. Tackling tumors
4.4.1. Tumor suppressor diseases – gene therapy for benign tumors in neurofibromatosis and tuberous sclerosis
It has proven challenging for viral vectors to eliminate malignant brain tumors (see below), although these vectors frequently lead to substantial tumor regression. The advantage of benign tumors for gene therapy is that curtailment of growth or regression of the tumors can be an effective therapy, as compromise of normal functions and pain are caused by nerve compression due to expanding tumor volume. In some cases inactivation of tumor suppressor genes can act as drivers of malignant tumors, as in retinoblastoma (Rb; Hutcheson et al., 2015). However, there are several hereditary conditions where individuals inherit a defective copy of a tumor suppressor gene, which can lead to a benign tumor when the second normal allele is lost due to somatic mutation. These diseases are inherited as autosomal dominant conditions with the disease phenotype varying stochastically depending on when in development/life and in which tissue the second hit takes place. Tumor suppressor diseases which affect the nervous system, as well as other tissues, include: 1) NF1 (incidence 1/3000) caused by loss of function of neurofibromin (negative regulator of the Ras signaling pathway) leading to learning disabilities, epilepsy, glial tumors, and neurofibromas; 2) neurofibromatosis type 2 (NF2; 1/40,000) caused by loss of merlin (a cytoskeletal protein), and characterized by painful/nerve compressing schwannomas (Lu-Emerson and Plotkin, 2009), acoustic neuromas (hearing loss), meningiomas, and ependymomas; and tuberous sclerosis complex type 1 and 2 (TSC1 and TSC2; 1/5500) with loss of hamartin and tuberin, respectively (which together regulate the mTOR growth pathway) causing autism, epilepsy, and overgrowths in many tissues, e.g. in the brain – cortical tubers, subependymal nodules and giant cell astrocytomas, and in the body – renal angiomyolipomas, cardiac rhabdomyoma, and lung lymphangioleiomyomatosis (Jülich and Sahin, 2014).
Gene therapy studies are at early stages in tackling these diseases, with the advantages that injections can be done directly into the tumors or systemically, leading to regression of one or more tumors and a decreased risk of benign tumors becoming malignant. In the case of NF2, an AAV vector encoding a cell death gene, caspase-1 under a Schwann cell specific promoter has been effective at reducing the growth and leading to regression of human schwannoma tumors implanted in the sciatic nerve of nude mice without causing nerve damage (Prabhakar et al., 2013), and may have a bystander effect via extracellular vesicles (Hall et al., in press). This targeted cell death approach has the advantage that it could be applied to a variety of benign tumor types accessible to injection by using different tumor-specific promoters. In the case of TSC1, a mouse bearing a conditional knock-out of the Tsc1 gene crossed to a transgenic mouse bearing Cre recombinase under a neuronal promoter leads to hamartin loss in essentially all neurons starting in embryonic development, with consequent enlargement of neurons and abnormal brain development (Meikle et al., 2007). Intraventricular (ICV) injection of an AAV vector encoding hamartin into the brain at P0 led to normalization of neuronal size and mTOR activity as well as extended lifespan in these mice (Prabhakar et al., 2015). This strategy of protein replacement should be effective for multiple lesions by systemic injections, for example with an AAV serotype that crosses the BBB, with a relatively rapid readout of benefit via tumor size reduction throughout the body and brain. Unfortunately for NF1 and TSC2 the sizes of the cDNAs encoding the missing proteins, neurofibromin and tuberin, respectively are too large to be accommodated in AAV vectors, so alternate vectors or strategic interventions need to be devised.
4.4.2. Brain tumors
Glioblastoma multiforme (GBM) is one of the most malignant forms of cancer for which effective treatment remains elusive (Grossman et al., 2010). Although vector design has improved to permit greater tumor-specific vector replication along with methods that improve initial vector distribution, lytic replication alone is unlikely to eliminate GBM. Recent data suggests that vector performance will be further advanced by the ability of oncolytic vectors to overcome aspects of the tumor micro-environment that negatively affect the induction of anti-tumor immunity. It is very likely that given the wide distribution of GBM tumor cells in the brain, anti-tumor immunity must be an essential component of the response to oncolytic vectors to promote a full anti-tumor response. Several viruses have been tested as oncolytic viruses (OVs).
The recombinant oncolytic poliovirus PVS-RIPO is a live attenuated, nonpathogenic virus containing the oral poliovirus Sabin type 1 in which the internal ribosomal entry site (IRES) is replaced with the IRES from human rhinovirus type 2 (HRV2) in order to abrogate vector replication in neurons (Dobrikova et al., 2012). PVS-RIPO infects glioma cells by recognition of its natural receptor Necl-5/CD155, where the virus replicates, causing tumor cell lysis. The data from the poliovirus (PVS-RIPO) clinical trial (NCT01491893) confirmed that OVs act to kill cancer cells by two mechanisms: direct oncolytic infection and death of the tumor cell, and a subsequent upregulation of an immune attack on the infected tumors (Brown and Gromeier, 2015). This Phase I dose-escalating study was designed to evaluate the safety of the vector and eventually its efficacy. There were 24 patients enrolled in the study, of which three are long-term survivors (still alive 22, 34, and 35 months after treatment), 12 have died and 9 received the treatment just in the past few months and they are under observation. Based on the preliminary data, high doses of PVS-RIPO caused massive swelling in the brain and cerebral edema that had to be treated. At the opposite end, the patients that successfully responded to the treatment received a lower dose of the virus and they were not heavily pretreated with steroids, suggesting that the efficacy may rely on the optimal activation of the immune system.
Toca 511 (vocimagene amiretrorepvec) is a nonlytic retroviral replicating vector (RRV) that encodes the transgene cytosine deaminase (Huang et al., 2013). This enzyme is used to catalyze the conversion of a novel oral extended-release prodrug 5-fluorocytosine (Toca FC) to the chemotherapeutically active 5-fluorouracil (5-FU). RRV’s replicate without killing the host cell and thus turn cancer cells into “factories” for generating RRV particles, which can then spread throughout the tumor. In a Phase I/II trial, 37 patients with recurrent high grade glioma had Toca 511 administered into the resection cavity wall at the time of surgery followed by cycles of orally administered Toca FC (https://clinicaltrials.gov/ct2/show/study/NCT01470794). Interim results showed a median overall survival of 53 weeks and survival rate of 93.8 percent at 6 months (42 weeks) in a study evaluating direct intratumoral delivery. Historical published data report median overall survival of 33.2 weeks and a six month survival rate of 59 percent, suggesting some benefit. Furthermore this study demonstrated a favorable safety profile.
The Delta-24-RGD (DNX-2401) is an oncolytic adenovirus deleted for 24 nucleotides in the E1A gene, enabling the virus to replicate selectively in cancer cells with defects in the Rb pathway, such as glioma cells (Jiang et al., 2009). Furthermore, the tropism of the virus has been modified through the insertion of an RGD motif which enables infection through integrins αvβ3 and αvβ5 both highly expressed on the surface of cancer cells. Patients with recurrent high grade gliomas were enrolled in one of two arms. One cohort of patients received a single intratumoral injection of DNX-2401, while a second cohort received the same treatment and 14 days later the tumorwas resected and the vector injected again into the tumor cavity (Lang et al., 2014). DNX-2401 was safe and three patients (12%) went into remission and are currently alive with no sign of disease. The overall median survival was 11 months for both cohorts. The three patients that responded had increased levels of interleukin-12p70 in the serum compared with all other patients. This suggests that DNX-2401 induced the polarization of T(h)0-cells to T(h)1-cells promoting anti-tumor cytotoxic immunity and it can be now consider as an in situ vaccine against glioma cells.
HSV has a number of important attributes that make it a desirable oncolytic vector that can be further engineered to express genes to convert the tumor micro-environment into one that supports cellular immune activities that participate in both innate and acquired anti-tumor immune responses. These features include its rapid replication cycle, exceptional high infectivity, large transgene capacity and tumor targeting properties (Uchida et al., 2013). The design of oncolytic herpes simplex virus (oHSV) has centered on the use of mutants that are deleted for neurovirulence determinants such as ICP34.5 (γ34.5), an antagonist of the cellular protein kinase R (PKR) pathway that normally blocks viral protein synthesis in neurons, and ICP6, the large subunit of ribonucleotide reductase that is required to create nucleotide pools in non-dividing cells (Grandi et al., 2009; Markert et al., 2000). Both of these functions are often complemented in GBM cells, although virus replication is still compromised compared to wild-type virus and tumors that do not inactivate the PKR system by mutational activation of the Ras pathway are highly resistant to vector propagation. Three different oncolytic vectors based on these mutations have been tested in GBM clinical trials: 1716 (deleted for γ34.5), G207 (deleted for γ34.5 and ICP6), and G47Delta (deleted for γ34.5, ICP6 and ICP47).
HSV1716 belongs to the first generation of engineered oHSV designed to selectively target and destroy cancer cells while leaving healthy cells unharmed. It is deleted only for γ34.5 and it has been safely tested in a total of 47 glioma patients (in 3 separate Phase I trials). This vector is now in clinical trials in pediatric/young adult patients with refractory or recurrent high grade glioma that cannot be removed by surgery (NCT02031965).
G207 has been tested in three different Phase I clinical trials for malignant glioma, all of which confirmed its safety and tolerability (Markert et al., 2000, 2009, 2014). In the latest clinical trial, Markert and collaborators reported that G207 (1 × 109 p.f.u. (particle forming unit)) given in combination with radiotherapy elicited reduction in tumor size in 6 out of 9 patients by MRI, as well as an increase in survival in 2 out of 9 patients.
The oncolytic virus G47delta represents the third generation of oncolytic vectors based on HSV since it is deleted for γ34.5, ICP6 and ICP47. The removal of ICP47 results in the presentation of viral and tumor antigens by infected cancer cells, which can lead to immune-mediated destruction of GBM cells. A clinical trial using this third generation, triple-mutated oncolytic HSV-1 (G47delta) is on-going in patients with recurrent GBM in Japan and preliminary data (http://www.asgct.org/the-vector/volume-1-issue-12-july-2014/meeting-center/international), suggested that this treatment is well tolerated with some patients are responding to the treatment.
More recently, attempts to create a more virulent form of the ICP34.5 mutant virus have employed the neuroprecursor/tumor-specific nestin promoter to control expression of ICP34.5, thereby providing more vigorous virus replication in GBM cells. This rQNestin34.5 vector showed strong oncolytic activity in rodent brain tumor models (Kambara et al., 2005) and an Investigational New Drug (IND) application has been submitted in order to test its safety in a Phase I clinical trial in high grade glioma patients.
4.5. Potential for genetic engineering in vivo
While lentiviral vectors have been used as the vector of choice for ex vivo gene transfer (Cartier et al., 2009), AAV vectors are emerging as the leading delivery vehicle for in vivo gene transfer, including for genetic engineering. This is largely due to high efficiency gene delivery to a large number of tissues including brain, excellent long-term safety profile in clinical trials (Gaudet et al., 2013; Nathwani et al., 2014), and low rates (~0.1%) of random chromosomal integration (McCarty et al., 2004). This last point may be somewhat controversial, as there may be some concerns of integration ‘hotspots’, particularly in unstable (Miller et al., 2005; Nakai et al., 2005) and transcriptionally active regions of the genome (Nakai et al., 2003). Most studies on AAV mediated genome editing have been based on homologous recombination (HR) between AAV-encoded transgenes and a specific chromosomal locus (Russell and Hirata, 1998). The transgene typically contains genomic sequences flanking the locus, allowing site-specific insertion via the canonical HR pathway. While the majority of the work using this method has been done in vitro to create modified cell lines (Kohli et al., 2004; Topaloglu et al., 2005), there have been several in vivo studies. These include correction of β glucuronidase for mucopolysaccharidosis VII (Miller et al., 2006), fumarylacetoacetate hydrolase (Fah) for hereditary tyrosinemia (Paulk et al., 2010), and factor IX (FIX) for hemophilia B (Barzel et al., 2015). The FIX study used a promoter-less editing strategy, relying on insertion of the transgene into the transcriptional unit of the albumin locus, which increases the safety of this approach (Barzel et al., 2015). However, the frequency of gene correction observed in these studies was extremely low (fraction of corrected cells ranging from 10−4 to 10−2). This frequency may be too low for clinical applications outside the liver, particularly in the CNS.
The field of genome engineering has been revolutionized by the discovery of targeted nucleases. These include zinc-finger nucleases (ZFNs), TALENS, and most recently CRISPR-Cas9 (Cox et al., 2015). These methods allow creation of site-specific double strand breaks in the genome, allowing for either disruption of gene function, or gene correction by homology repair at those sites using a normal oligonucleotide spanning the mutation. Therapeutic benefit from AAV-mediated in vivo genome editing has been demonstrated in both neonatal (Li et al., 2011b) and adult (Anguela et al., 2013) mouse models of hemophilia B, as well as in a mouse model of Huntington’s disease (Garriga-Canut et al., 2012). Genome editing by CRISPR-Cas9 represents the most recent advance in this field, and this technology has found use in many fields including generation of mouse models of human disease, cancer biology, drug development, etc (Hsu et al., 2014). AAV-mediated genome editing using CRISPR-Cas9 has been shown to be effective in liver (Ran et al., 2015; Yang et al., 2016), dystrophic muscle (Tabebordbar et al., 2016; Nelson et al., 2016), and more crucially in the context of this review, in brain (Swiech et al., 2015). Co-injection of two AAV vectors encoding SpCas9 and sgRNAs targeting the Mecp2 gene (mutated in Rett syndrome) into the visual cortex of mice resulted in effective gene editing at this locus and a resulting electrophysiological response (Swiech et al., 2015). Limitations of such therapy include the relatively large size of SpCas9 relative to the transgene capacity of AAV (hence the need for two vectors), the potential antigenic nature of this bacterial protein, and off-target effects in the genome that might accumulate with long term expression of these transgenes. The first can be resolved by using Cas9 from other bacterial species (Ran et al., 2015) and non-Cas9 CRISPR effectors are also being developed (Zetsche et al., 2015). Overall, this is a very promising field that is likely to foster the next generation of CNS gene therapy.
5. Barriers to delivery and safety issues
5.1. Barriers to gene delivery
Although the recent benefits in some gene therapy trials are very encouraging, it is paramount to continue improving the efficiency and targeting specificity of gene delivery, as well as the safety of viral vectors. Biologic barriers that decrease the efficiency of delivery in vivo can be divided into several categories, including immune interference, lack of entry receptors, intracellular trafficking dead-ends, off-target vector uptake and, for the nervous system, the difficulty in transiting the BBB.
It has been considered safest to use vectors derived from viruses that normally infect humans, but that comes with the price that the immune system may recognize them as pathogens and try to eliminate them. Both innate and adaptive immune mechanisms can limit vector uptake and transgene expression. Innate immunity is mediated by dendritic cells (DCs), macrophages, endothelial cells, natural killer T lymphocytes and Kuppfer cells in the liver which recognize conserved viral protein motifs via Toll-like receptors (TLRs) (Sack and Herzog, 2009). This leads to production of cytokines, which together with complement can promote phagocytosis of vectors in the biofluids before they reach the target cells. If the inflammatory signal induced by the virus/virus vector is sufficient, antigen presenting cells (APCs) such as DCs can induce a primary adaptive immune response by activating CD4+ T helper cells, resulting in antibody production by B cells and a cytolytic response by CD8+ T cells. These responses have the effect of removing transduced cells and limiting gene therapy efficacy (Zirger et al., 2012). The adaptive immune response can occur against both the virus structural proteins (McKelvey et al., 2004), as well as the transgene product itself, if it is not recognized as self (Yuasa et al., 2002). In some recessively inherited conditions, a stable mutant form of the protein may not be made, so the normal protein equivalent will be seen as foreign (Rogers et al., 2014). In the case of secreted proteins, stimulation of an immune response to the protein may limit future use of standard-of-care enzyme/protein replacement therapy. This antigenicity of the transgene product has been the major factor limiting clinical use of regulatable promoters that use engineered bacterial proteins for control, which would be seen as foreign proteins by the body (Ginhoux et al., 2004). Another important factor to consider in using virus vectors in vivo is preexisting immunity to wild-type viruses from which the vector is derived. This results in immune memory in the form of antibodies or cytotoxic T cells to the virus structural proteins and it can limit gene transfer efficiency and duration of transgene expression. It is important to mention that despite the barrier that the immune system poses there are preclinical (McIntosh et al., 2012) and clinical immunosuppressive strategies (Nathwani et al., 2014), which have shown promise at reducing the immune response to vector and stabilizing transgene expression.
Viruses typically bind to a receptor on the target cell surface, which mediates virus entry. In many cases these receptors are expressed on many cell types, such as the coxsackie and adenovirus receptor (CAR) for adenovirus, heparan sulfate proteoglycan for AAV2, and low density lipoprotein (LDL) receptor for VSV-G pseudotyped lentivirus (Finkelshtein et al., 2013). On the one hand this can lead to uptake by many different cell types, with most vectors being predominantly taken up by the liver after systemic injection. Many efforts have gone into modifying the binding ligands on virions to make them more cell type specific, but most of these are still in the pipeline and have not gone into clinical trials. In other cases, attempts have been made to expand the cellular targets and facilitate entry, e.g. incorporating VSV-G protein into the envelope of lentivirus to promote fusion with the host cell membrane. A “black hole” that vectors can fall into is intracellular degradation before ever reaching the nucleus to deliver their therapeutic cargo. Non-enveloped viruses, such as AAV and adenovirus typically enter cells by receptor-mediated endocytosis and escape the endosome–lysosome compartment by inducing changes in membrane integrity that allow them to escape into the cytoplasm. This is a relatively inefficient process and many virions may need to enter the cell for one to successfully deliver its DNA payload to the nucleus. For AAV, some of these critical “post-entry” steps towards successful transduction have been elucidated. These include, endosomal escape through activation of the capsid phospholipase domain upon acidification of the endosome (Stahnke et al., 2011), avoiding proteosomal and other degradation pathways (Berry and Asokan, 2016), capsid uncoating (Tenney et al., 2014), trafficking on microtubules towards the nucleus (Xiao and Samulski, 2012), and nuclear import of the capsid (Nicolson and Samulski, 2014). Still, there is much to be learned about the intracellular trafficking of virions to promote the efficiency of this process. Compared to most other cells in the body, neurons are further protected from virus infection by the robust BBB, which in turn presents a barrier to gene therapy. The variety of approaches that have been developed to overcome this barrier are discussed above.
5.2. Safety issues in clinical trials
As virus vectors are modified and new viruses are tested as vectors, it is important to keep safety concerns at the forefront of consideration. In prior clinical trials, many lessons have been learned that inform current strategies. Large amounts of virions, e.g. adenovirus, can elicit a strong inflammatory response with high cytokine levels leading to shock and organ failure, potentially leading to death (Wilson, 2009). Other vectors such as retrovirus can integrate into the promoter regions and thereby transactivate oncogenes (Hacein-Bey-Abina et al., 2003) and a huge effort has gone into decreasing promoter activity of vector sequences (Cesana et al., 2014) and trying to design vectors that will integrate randomly or at “safe harbors” in the genome (e.g. Papapetrou et al., 2011; Cortés et al., 2008). The virus preparations need to be optimized for a high ratio of full to empty capsids, integrity of the vector genomes, and lack of adventitious and potentially toxic contaminants.
There are other possible consequences of viral vector use that have not been seen to date, but must be considered in trials. One would be the potential genome modification of germ cells by virus vectors. This has been carefully monitored with vectors in use in clinical trials to date and never found. Another is the potential to activate or recombine with endogenous viruses in the body or environment. For example most humans harbor HSV-1 in latency in sensory neurons. Could infection with a HSV vector lead to activation of this wild-type virus with complications in an immune compromised person? In using replication competent viruses there is always the risk or recombining with similar viruses to create new epidemiologic agents. For example, could the replicating retrovirus used for cancer therapy (e.g. Huang et al., 2015b), recombine with human endogenous retrovirus sequences which are expressed in brain tumor cells (Balaj et al., 2011) and create a novel retrovirus that could replicate in humans and cause cancer, as these viruses do in other species (Mager and Stoye, 2015)? When replication-selective vectors are created by placing essential virus genes under tissue specific promoters, could that change the tropism of a replicating virus, e.g. what effect could adenovirus vectors in which essential genes are placed under prostate specific promoters have on functions of the normal prostate? Using viruses from other species, e.g. fowl pox, vaccinia-type virus or Newcastle disease virus presents the possibility that the virus may “jump” species and become a human pathogen. The viral sequences that restrict infectability to specific species can be small and mutable, and are frequently unknown. In considering the safety of virus vectors one not only has to consider potential consequences to the treated individual, but also the safety to other normal individuals who might be exposed to that vector.
6. Coming of age for gene therapy for neurologic diseases
Viral vector-mediated gene therapy holds great promise for treatment of neurological disorders. The current benchside efforts continue to be targeted towards generating better vectors, both at the virion level (e.g., developing capsids capable of crossing the BBB and targeting specific cell types) and at the transgene level (e.g., promoter and transgene design to improve efficacy in desired cell populations). Better non-invasive vector delivery routes for global and widespread CNS gene delivery are also being investigated for multi-focal neurological disorders. The result of this has been a slew of clinical trials for CNS disorders, which have been shown to be safe and non-toxic in Phase I, but few have been as efficacious in demonstrating therapeutic outcomes as perhaps may have been expected based on preclinical studies. These early trials have shed light on a myriad of challenges that must be surmounted for unequivocal clinical success. One of the major problems is the lack of good predictive animal models and inherent differences between species, which could be a primary cause of the failure of favorable preclinical outcomes to translate to the clinic. The potential of immune responses, both against the vector and expressed transgene, is often overlooked for neurological gene therapy, as the CNS is considered immune-privileged. This assumption has had to be revised in recent years as studies have demonstrated not only the generation of anti-capsid neutralizing antibodies, but also immune-mediated clearance of transduced antigen-presenting cells in the CNS (Ciesielska et al., 2013; Samaranch et al., 2014), which raises concerns for the next generation of CNS gene therapy clinical trials. More insight into the etiology of neurological disorders and particularly the development of biomarkers for early detection of disease will be of great help moving forward, as delivering therapies prior to onset of neurodegeneration will be key to improving efficacy of clinical intervention. Finally, recent advances in gene editing technologies, particularly CRISPR-Cas9, could lead to a move away from the current gene replacement therapies towards more permanent genome editing. The regulatory approval of Glybera, an AAV1 clinical vector for lipoprotein lipase deficiency, in the European Union, the US FDA regulatory approval for the oncolytic HSV-based T-VEC for therapy of advanced melanoma, and the recent encouraging results of Phase III clinical trials of AAV2-based SPK-RPE65 vector for inherited retinal dystrophies (IRDs) has raised hopes that similar success may follow for gene therapy of neurological disorders in the future.
Acknowledgments
We thank Ms. Suzanne McDavitt for skilled editorial assistance; and Ms. Emily Mills at Millstone Design for preparation of figures. This work was supported by National Institutes of Health/Cancer Institute (NIH/NCI) U19 CA179563 (supported by the NIH Common Fund through the Office of Strategic Coordination/Office of the NIH Director), and Department of Defense Army Grant W81XWH-13-1-0076 (XOB); National Institutes of Health NS066310, HD060576 (to MSE); American Brain Tumor Association Discovery Grant (CM); NIH: 1R01 1A175052-01, NIH P01 1P01 CA163205-01A1 (GAP).
Abbreviations
- AAV
adeno-associated virus
- Ad
adenoviral
- ALD
adrenoleukodystrophy
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ALS
amyotrophic lateral sclerosis
- APCs
antigen presenting cells
- APOE
apolipoprotein E
- AADC
aromatic L-amino acid decarboxylase
- ARSA
arylsulfatase A
- ASPA
aspartoacylase
- BBB
blood-brain-barrier
- BDNF
brain-derived neurotrophic factor
- CD
Canavan’s disease
- CAR
coxsackie and adenovirus receptor
- CNS
central nervous system
- CDNF
cerebral dopamine neurotrophic factor
- CSF
cerebrospinal fluid
- CED
convection-enhanced delivery
- CTL
cytotoxic T lymphocyte
- DCs
dendritic cells
- DRG
dorsal root ganglia
- FIX
factor IX
- Fah
fumarylacetoacetate hydrolase
- GABA
gamma amino-butyric acid
- GAN
giant axonal neuropathy
- GDNF
glia-derived neurotropic factor
- GBM
glioblastoma
- GAD
glutamic acid decarboxylase
- GCH1
GTP cyclohydrolase I
- HSV
herpes simplex virus
- HR
homology-directed recombination
- HIV
human immunodeficiency virus
- HRV2
human rhinovirus type 2
- IRDs
inherited retinal dystrophies
- ICV
intracerebroventricular
- IT
intrathecal
- IRES
internal ribosomal entry site
- IND
Investigational New Drug
- LINCL
late-infantile neuronal ceroid lipofuscinosis
- LPD
lipid-encapsulated plasmid DNA
- LTR
long terminal repeat
- LDL
low density lipoprotein
- LSDs
lysosomal storage disorders
- MRI
magnetic resonance imaging
- MLD
metachromatic leukodystrophy
- MoMLV
Moloney murine leukemia virus
- MSD
multiple sulfatase deficiency
- NAA
N-acetyl aspartate
- NGF
nerve growth factor
- NF1
neurofibromatosis type 1
- NF2
neurofibromatosis type 2
- NRTN
neurturin
- oHSV
oncolytic herpes simplex virus
- OV
oncolytic virus
- OTC
ornithine transcarbamylase
- PD
Parkinson’s disease
- Rb
retinoblastoma
- RRV
retroviral replicating vector
- SFV
Semliki Forest virus
- SCID
severe combined immune deficiency
- sh
short hairpin
- SMA
spinal muscular atrophy
- TLRs
Toll-like receptors
- TSC1
TSC2, tuberous sclerosis complex type 1 and 2
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- VLCFA
very long-chain fatty acids
- VSV-G
vesicular stomatitis virus G
- X-ALD
X-linked adrenoleukodystrophy
- ZFNs
zinc-finger nucleases
- 5-FC
5-fluorocytosine
- 5-FU
5-fluorouracil
References
- Ahmed SS, Li H, Cao C, Sikoglu EM, Denninger AR, Su Q, Eaton S, Liso Navarro AA, Xie J, Szucs S, Zhang H, Moore C, Kirschner DA, Seyfried TN, Flotte TR, Matalon R, Gao G. A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS gene therapy in Canavan mice. Mol Ther. 2013;21(12):2136–2147. doi: 10.1038/mt.2013.138. http://dx.doi.org/10.1038/mt.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, Bosticardo M, Evangelio C, Assanelli A, Casiraghi M, Di Nunzio S, Callegaro L, Benati C, Rizzardi P, Pellin D, Di Serio C, Schmidt M, Von Kalle C, Gardner J, Mehta N, Neduva V, Dow DJ, Galy A, Miniero R, Finocchi A, Metin A, Banerjee PP, Orange JS, Galimberti S, Valsecchi MG, Biffi A, Montini E, Villa A, Ciceri F, Roncarolo MG, Naldini L. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151. doi: 10.1126/science.1233151. http://dx.doi.org/10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3(3):224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Altman-Hamamdzic S, Groseclose C, Ma JX, Hamamdzic D, Vrindavanam NS, Middaugh LD, Parratto NP, Sallee FR. Expression of beta-galactosidase in mouse brain: utilization of a novel nonreplicative Sindbis virus vector as a neuronal gene delivery system. Gene Ther. 1997;4(8):815–822. doi: 10.1038/sj.gt.3300458. [DOI] [PubMed] [Google Scholar]
- Anguela XM, Sharma R, Doyon Y, Miller JC, Li H, Haurigot V, Rohde ME, Wong SY, Davidson RJ, Zhou S, Gregory PD, Holmes MC, High KA. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood. 2013;122(19):3283–3287. doi: 10.1182/blood-2013-04-497354. http://dx.doi.org/10.1182/blood-2013-04-497354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. http://dx.doi.org/10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. http://dx.doi.org/10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D, King GD, Johnson SA, Liu C, Xiong W, Candolfi M, Mondkar S, Ng P, Palmer D, Castro MG, Lowenstein PR. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol Ther. 2007;15(12):2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Weinberg MS, Samulski RJ. Parkinson’s disease gene therapy: success by design meets failure by efficacy. Mol Ther. 2014;22(3):487–497. doi: 10.1038/mt.2013.281. http://dx.doi.org/10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzel A, Paulk NK, Shi Y, Huang Y, Chu K, Zhang F, Valdmanis PN, Spector LP, Porteus MH, Gaensler KM, Kay MA. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature. 2015;517(7534):360–364. doi: 10.1038/nature13864. http://dx.doi.org/10.1038/nature13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci. 2015;9:204. doi: 10.3389/fnins.2015.00204. http://dx.doi.org/10.3389/fnins.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CL, Vandenberghe LH, Bell P, Limberis MP, Gao GP, Van Vliet K, Agbandje-McKenna M, Wilson JM. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J Clin Invest. 2011;121(6):2427–2435. doi: 10.1172/JCI57367. http://dx.doi.org/10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson NE, Seto JT, Hall JK, Chamberlain JS, Odom GL. Progress and prospects of gene therapy clinical trials for the muscular dystrophies. Hum Mol Genet. 2015 Oct 8; doi: 10.1093/hmg/ddv420. pii: ddv420 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry GE, Asokan A. Chemical modulation of endocytic sorting augments adeno-associated viral transduction. J Biol Chem. 2016;291(2):939–947. doi: 10.1074/jbc.M115.687657. http://dx.doi.org/10.1074/jbc.M115.687657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AH, Kaspar BK. Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet. 2010;19(20):3895–3905. doi: 10.1093/hmg/ddq300. http://dx.doi.org/10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, Porensky PN, Kolb SJ, Mendell JR, Burghes AHM, Kaspa BK. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19(11):1971–1980. doi: 10.1038/mt.2011.157. http://dx.doi.org/10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, Benedicenti F, Vallanti G, Biasco L, Leo S, Kabbara N, Zanetti G, Rizzo WB, Mehta NA, Cicalese MP, Casiraghi M, Boelens JJ, Del Carro U, Dow DJ, Schmidt M, Assanelli A, Neduva V, Di Serio C, Stupka E, Gardner J, von Kalle C, Bordignon C, Ciceri F, Rovelli A, Roncarolo MG, Aiuti A, Sessa M, Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158. http://dx.doi.org/10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Bledsoe AW, Jackson CA, McPherson S, Morrow CD. Cytokine production in motor neurons by poliovirus replicon vector gene delivery. Nat Biotechnol. 2000;18(9):964–969. doi: 10.1038/79455. [DOI] [PubMed] [Google Scholar]
- Blömer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71(9):6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Breakefield XO, Sena-Esteves M. Genetic therapy for the nervous system. Hum Mol Genet. 2011;20(R1):R28–R41. doi: 10.1093/hmg/ddr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield XO, Sena-Esteves M. Healing genes in the nervous system. Neuron. 2010;68(2):178–181. doi: 10.1016/j.neuron.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Guillot-Sestier MV, Town T. Brain injury, neuroinflammation and Alzheimer’s disease. Front Againg Neurosci. 2013;5:26. doi: 10.3389/fnagi.2013.00026. http://dx.doi.org/10.3389/fnagi.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman ML, Tierney LA, Benn C, Chawla P, Cha JH, Sena-Esteves M. Mechanisms of distribution of mouse beta-galactosidase in the adult GM1-gangliosidosis brain. Gene Ther. 2009;16(2):303–308. doi: 10.1038/gt.2008.149. http://dx.doi.org/10.1038/gt.2008.149. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Stein CS, Hughes SM, Heth J, McCray PM, Sauter SL, Johnston JC, Cory-Slechta DA, Federoff HJ, Davidson BL. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc Natl Acad Sci U S A. 2002;99(9):6216–6221. doi: 10.1073/pnas.082011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Gromeier M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr Opin Virol. 2015;13:81–85. doi: 10.1016/j.coviro.2015.05.007. http://dx.doi.org/10.1016/j.coviro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Burlot MA, Braudeau J, Michaelsen-Preusse K, Potier B, Ayciriex S, Varin J, Gautier B, Djelti F, Audrain M, Dauphinot L, Fernandez-Gomez FJ, Caillierez R, Laprévote O, Bièche I, Auzeil N, Potier MC, Dutar P, Korte M, Buée L, Blum D, Cartier N. Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like Tau pathology. Hum Mol Genet. 2015;24(21):5965–5976. doi: 10.1093/hmg/ddv268. http://dx.doi.org/10.1093/hmg/ [DOI] [PubMed] [Google Scholar]
- Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. http://dx.doi.org/10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- Cachón-González MB, Wang SZ, Lynch A, Ziegler R, Cheng SH, Cox TM. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc Natl Acad Sci U S A. 2006;103(27):10373–10378. doi: 10.1073/pnas.0603765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrère F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougnères P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. http://dx.doi.org/10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:857–862. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Perlson E, Holzbaur EL, Wolfe JH. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol Ther. 2014;22(3):554–566. doi: 10.1038/mt.2013.237. http://dx.doi.org/10.1038/mt.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Turunen HT, Vandenberghe LH, Wolfe JH. Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol Biol. 2016;1382:133–149. doi: 10.1007/978-1-4939-3271-9_10. http://dx.doi.org/10.1007/978-1-4939-3271-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B, Schmidt M, von Kalle C, Howe S, Thrasher AJ, Aiuti A, Ferrari G, Recchia A, Mavilio F. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood. 2007;110(6):1770–1778. doi: 10.1182/blood-2007-01-068759. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27(37):9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert Rev Neurother. 2008;8(5):743–750. doi: 10.1586/14737175.8.5.743. http://dx.doi.org/10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- Cesana D, Ranzani M, Volpin M, Bartholomae C, Duros C, Artus A, Merella S, Benedicenti F, Sergi Sergi L, Sanvito F, Brombin C, Nonis A, Serio CD, Doglioni C, von Kalle C, Schmidt M, Cohen-Haguenauer O, Naldini L, Montini E. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol Ther. 2014;22(4):774–785. doi: 10.1038/mt.2014.3. http://dx.doi.org/10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1(6):3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Rosario A, Cruz P, Siemienski Z, Ceballos-Diaz C, Crosby K, Jansen K, Borchelt DR, Kim JY, Jankowsky JL, Golde TE, Levites Y. Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS One. 2013;8(6):e67680. doi: 10.1371/journal.pone.0067680. http://dx.doi.org/10.1371/journal.pone.0067680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain K, Riyad JM, Weber T. Expressing transgenes that exceed the packaging capacity of AAV capsids. Hum Gene Ther Methods. 2016 Jan 12; doi: 10.1089/hgtb.2015.140. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, Choi BB, Cai W, DeLuca NA, Schaffer PA, DiFiglia M, Breakefield XO, Martuza RL. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus insertion mutants. New Biol. 1990;2:739–746. [PubMed] [Google Scholar]
- Choudhury SR, Harris AF, Cabral DJ, Keeler AM, Sapp E, Ferreira JS, Gray-Edwards HL, Johnson JA, Johnson AK, Su Q, Stoica L, DiFiglia M, Aronin N, Martin DR, Gao G, Sena-Esteves M. Widespread central nervous system gene transfer and silencing after systemic delivery of novel AAV-AS vector. Mol Ther. 2015 Dec 28; doi: 10.1038/mt.2015.231. http://dx.doi.org/10.1038/mt.2015.231 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. http://dx.doi.org/10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, Forsayeth J. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21(1):158–166. doi: 10.1038/mt.2012.167. http://dx.doi.org/10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelier P, Van Bockstaele E, Calarota SA, Strayer DS. Inhibiting AIDS in the central nervous system: gene delivery to protect neurons from HIV. Mol Ther. 2003;7(6):801–810. doi: 10.1016/s1525-0016(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Cortés ML, Oehmig A, Saydam O, Sanford JD, Perry KF, Fraefel C, Breakefield XO. Targeted integration of functional human ATM cDNA into genome mediated by HSV/AAV hybrid amplicon vector. Mol Ther. 2008;16(1):81–88. doi: 10.1038/sj.mt.6300338. [DOI] [PubMed] [Google Scholar]
- Costantini LC, Bakowska JC, Breakefield XO, Isacson O. Gene therapy in the CNS. Gene Ther. 2000;7(2):93–109. doi: 10.1038/sj.gt.3301119. [DOI] [PubMed] [Google Scholar]
- Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–131. doi: 10.1038/nm.3793. http://dx.doi.org/10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97(7):3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016 Feb 1; doi: 10.1038/nbt.3440. http://dx.doi.org/10.1038/nbt.3440 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18(1):35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- Dobrikova EY, Goetz C, Walters RW, Lawson SK, Peggins JO, Muszynski K, Ruppel S, Poole K, Giardina SL, Vela EM, Estep JE, Gromeier M. Attenuation of neurovirulence, biodistribution, and shedding of a poliovirus: rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis. J Virol. 2012;86(5):2750–2759. doi: 10.1128/JVI.06427-11. http://dx.doi.org/10.1128/JVI.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky TW. (Re-)Engineering tumor cell-selective replicating adenoviruses: a step in the right direction toward systemic therapy for metastatic disease. Cancer Cell. 2002;1(4):307–309. doi: 10.1016/s1535-6108(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Dufour BD, Smith CA, Clark RL, Walker TR, McBride JL. Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington’s disease mice. Mol Ther. 2014;22(4):797–810. doi: 10.1038/mt.2013.289. http://dx.doi.org/10.1038/mt.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque SI, Arnold WD, Odermatt P, Li X, Porensky PN, Schmelzer L, Meyer K, Kolb SJ, Schümperli D, Kaspar BK, Burghes AH. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol. 2015;77(3):399–414. doi: 10.1002/ana.24332. http://dx.doi.org/10.1002/ana.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gähwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1999;96(12):7041–7046. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber MU, Hennou S, Büeler H, Naim HY, Déglon N, Lundstrom K. Gene transfer into neurons from hippocampal slices: comparison of recombinant Semliki Forest Virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol Cell Neurosci. 2001;17(5):855–871. doi: 10.1006/mcne.2001.0982. [DOI] [PubMed] [Google Scholar]
- Falk DJ, Mah CS, Soustek MS, Lee KZ, Elmallah MK, Cloutier DA, Fuller DD, Byrne BJ. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther. 2013;21(9):1661–1667. doi: 10.1038/mt.2013.96. http://dx.doi.org/10.1038/mt.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, Handy CR, Passini MA, Samulski RJ, Boulis NM. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19(8):852–859. doi: 10.1038/gt.2011.130. http://dx.doi.org/10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- Fields-Berry SC, Halliday AL, Cepko CL. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci U S A. 1992;89(2):693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110(18):7306–7311. doi: 10.1073/pnas.1214441110. http://dx.doi.org/10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. http://dx.doi.org/10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–274. doi: 10.1038/nbt.1610. http://dx.doi.org/10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Foust KD, Salazar DL, Likhite S, Ferraiuolo L, Ditsworth D, Ilieva H, Meyer K, Schmelzer L, Braun L, Cleveland DW, Kaspar BK. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol Ther. 2013;21(12):2148–2159. doi: 10.1038/mt.2013.211. http://dx.doi.org/10.1038/mt.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, McCarty DM. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8(6):911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19(6):1025–1033. doi: 10.1038/mt.2011.34. http://dx.doi.org/10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Chen KC, Kadish I, Blalock EM, Thibault O, Porter NM, Landfield PW. Reversal of aging-related neuronal Ca2+ dysregulation and cognitive impairment by delivery of a transgene encoding FK506-binding protein 12.6/1b to the hippocampus. J Neurosci. 2015;35(30):10878–10887. doi: 10.1523/JNEUROSCI.1248-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.1248-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, Foust KD, Kaspar BK, Bird A, Mandel G. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J Neurosci. 2013;33(34):13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga-Canut M, Agustín-Pavón C, Herrmann F, Sánchez A, Dierssen M, Fillat C, Isalan M. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci U S A. 2012;109(45):E3136–E3145. doi: 10.1073/pnas.1206506109. http://dx.doi.org/10.1073/pnas.1206506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27(1):67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, Ketchum ET, Bartus RT. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol Ther. 2007b;15(1):62–68. doi: 10.1038/sj.mt.6300010. [DOI] [PubMed] [Google Scholar]
- Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361–369. doi: 10.1038/gt.2012.43. http://dx.doi.org/10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Turbant S, Gross DA, Poupiot J, Marais T, Lone Y, Lemonnier FA, Firat H, Perez N, Danos O, Davoust J. HLA-A*0201-restricted cytolytic responses to the rtTA transactivator dominant and cryptic epitopes compromise transgene expression induced by the tetracycline on system. Mol Ther. 2004;10(2):279–289. doi: 10.1016/j.ymthe.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Gong Y, Mu D, Prabhakar S, Moser A, Musolino P, Ren J, Breakefield XO, Maguire CA, Eichler FS. Adenoassociated virus seroytpe 9-mediated gene therapy for X-linked adrenoleukodystrophy. Mol Ther. 2015;23(5):824–834. doi: 10.1038/mt.2015.6. http://dx.doi.org/10.1038/mt.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9(4):505–517. doi: 10.1586/ern.09.9. http://dx.doi.org/10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19(6):1058–1069. doi: 10.1038/mt.2011.72. http://dx.doi.org/10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20(4):450–459. doi: 10.1038/gt.2012.101. http://dx.doi.org/10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, NABTT CC. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. http://dx.doi.org/10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic Basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry. 2012;71(9):805–813. doi: 10.1016/j.biopsych.2011.06.019. http://dx.doi.org/10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B, Fitzpatrick Z, Crommentuijn MH, Mu D, Maguire CA. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials. 2014;35(26):7598–7609. doi: 10.1016/j.biomaterials.2014.05.032. http://dx.doi.org/10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH, Leiva L, Sorensen R, Debré M, Casanova JL, Blanche S, Durandy A, Bushman FD, Fischer A, Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–364. doi: 10.1056/NEJMoa1000164. http://dx.doi.org/10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO. Review and potential treatments for Parkinson’s disease, glioma and schwannoma. J Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0309-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102(16):5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CD, Brown L, Kruegel BR, Wilson A, Tansey MG, Gage FH, Johnson EMJ, Bartus RT. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120) Neurobiol Dis. 2013;58:38–48. doi: 10.1016/j.nbd.2013.04.011. http://dx.doi.org/10.1016/j.nbd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin JP, Zhu Y, Bagel J, O’Donnell P, Sikora T, Ruane T, Wang P, Haskins ME, Wilson JM. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther. 2014;22(12):2018–2027. doi: 10.1038/mt.2014.135. http://dx.doi.org/10.1038/mt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Dubreil L, Deniaud J, Iacobelli F, Moreau S, Ledevin M, Le Guiner C, Blouin V, Le Duff J, Mendes-Madeira A, Rolling F, Cherel Y, Moullier P, Colle MA. Efficient central nervous system AAVrh10-mediated intrathecal gene transfer in adult and neonate rats. Gene Ther. 2015;22(4):316–324. doi: 10.1038/gt.2014.121. http://dx.doi.org/10.1038/gt.2014.121. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. http://dx.doi.org/10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Hlavaty J, Ostertag D, Espinoza FL, Martin B, Petznek H, Rodriguez-Aguirre M, Ibanez CE, Kasahara N, Gunzburg W, Gruber HE, Pertschuk D, Jolly DJ, Robbins JM. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20(10):544–551. doi: 10.1038/cgt.2013.51. http://dx.doi.org/10.1038/cgt.2013.51. [DOI] [PubMed] [Google Scholar]
- Huang Y, Skwarek-Maruszewska A, Horré K, Vandewyer E, Wolfs L, Snellinx A, Saito T, Radaelli E, Corthout N, Colombelli J, Lo AC, Van Aerschot L, Callaerts-Vegh Z, Trabzuni D, Bossers K, Verhaagen J, Ryten M, Munck S, D’Hooge R, Swaab DF, Hardy J, Saido TC, De Strooper B, Thathiah A. Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer’s disease mouse models. Sci Transl Med. 2015a;7(309):309ra164. doi: 10.1126/scitranslmed.aab3492. http://dx.doi.org/10.1126/scitranslmed.aab3492. [DOI] [PubMed] [Google Scholar]
- Huang TT, Parab S, Burnett R, Diago O, Ostertag D, Hofman FM, Espinoza FL, Martin B, Ibañez CE, Kasahara N, Gruber HE, Pertschuk D, Jolly DJ, Robbins JM. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015b;26(2):82–93. doi: 10.1089/hum.2014.100. http://dx.doi.org/10.1089/hum.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Van Dam D, Kulik W, De Deyn PP, Stet FS, Ahouansou O, Benraiss A, Delacourte A, Bougnères P, Aubourg P, Cartier N. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease. Mol Ther. 2010;18(1):44–53. doi: 10.1038/mt.2009.175. http://dx.doi.org/10.1038/mt.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T, Scheel M, Spires-Jones T, Arbel-Ornath M, Betensky R, Davidson BL, Hyman BT. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med. 2013;5(212):212ra161. doi: 10.1126/scitranslmed.3007000. http://dx.doi.org/10.1126/scitranslmed.3007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Martin C, Gandhi S, György B, Scheffer DI, Mu D, Merkel SF, Mingozzi F, Fitzpatrick Z, Dimant H, Masek M, Ragan T, Tan S, Brisson AR, Ramirez SH, Hyman BT, Maguire CA. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016 Feb 2; doi: 10.1038/gt.2016.11. http://dx.doi.org/10.1038/gt.2016.11 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Hutcheson J, Witkiewicz AK, Knudsen ES. The RB tumor suppressor at the intersection of proliferation and immunity: relevance to disease immune evasion and immunotherapy. Cell Cycle. 2015;14(24):3812–3819. doi: 10.1080/15384101.2015.1010922. http://dx.doi.org/10.1080/15384101.2015.1010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH, Snyder RO, Byrne BJ, Tai CH, Wu RM. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4(134):134ra61. doi: 10.1126/scitranslmed.3003640. http://dx.doi.org/10.1126/scitranslmed.3003640. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Cobbs C, Peduzzi JD, Novak M, Morrow CD. Repetitive intrathecal injections of poliovirus replicons result in gene expression in neurons of the central nervous system without pathogenesis. Hum Gene Ther. 2001;12(15):1827–1841. doi: 10.1089/104303401753153893. [DOI] [PubMed] [Google Scholar]
- Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, Wang DJ, Shera D, Hurh P, Rupin J, Saslow E, Goldfarb O, Goldberg M, Larijani G, Sharrar W, Liouterman L, Camp A, Kolodny E, Samulski J, Leone P. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002;13(11):1391–1412. doi: 10.1089/104303402760128612. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9(5):422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülich K, Sahin M. Mechanism-based treatment in tuberous sclerosis complex. Pediatr Neurol. 2014;50(4):290–296. doi: 10.1016/j.pediatrneurol.2013.12.002. http://dx.doi.org/10.1016/j.pediatrneurol.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75(15):6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65(7):2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kaul R, Gao GP, Balamurugan K, Matalon R. Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet. 1993;5(2):118–123. doi: 10.1038/ng1093-118. [DOI] [PubMed] [Google Scholar]
- Kay MA, Rothenberg S, Landen CN, Bellinger DA, Leland F, Toman C, Finegold M, Thompson AR, Read MS, Brinkhous KM, Woo SC. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, Bankiewicz KS. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci U S A. 2009;106(7):2407–2411. doi: 10.1073/pnas.0810682106. http://dx.doi.org/10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S, Lindholm P, Galli E, Lahtinen HM, Koivisto H, Hämäläinen E, Saarma M, Tanila H. Cerebral dopamine neurotrophic factor improves long-term memory in APP/PS1 transgenic mice modeling Alzheimer’s disease as well as in wild-type mice. Behav Brain Res. 2015;291:1–11. doi: 10.1016/j.bbr.2015.05.002. http://dx.doi.org/10.1016/j.bbr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Kim JY, Grunke SD, Levites Y, Golde TE, Jankowsky JL. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J Vis Exp. 2014;91:51863. doi: 10.3791/51863. http://dx.doi.org/10.3791/51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Zhang G, Morrison CM, Bosch ME, Weir RA, Lu Y, Dong W, Gendelman HE. AAV2/1 CD74 gene transfer reduces β-amyloidosis and improves learning and memory in a mouse model of Alzheimer’s disease. Mol Ther. 2015;23(11):1712–1721. doi: 10.1038/mt.2015.142. http://dx.doi.org/10.1038/mt.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13(3):517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli M, Rago C, Lengauer C, Kinzler KW, Vogelstein B. Facile methods for generating human somatic cell gene knockouts using recombinant adeno-associated viruses. Nucleic Acids Res. 2004;32(1):e3. doi: 10.1093/nar/gnh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, Gasmi M, Bartus R. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60(6):706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Lang FF, Conrad C, Gomez-Manzano C, Tufaro F, Yung W, Sawaya R, Weinberg J, Prabhu S, Fuller G, Aldape K, Fueyo J. First-in-human phase I clinical trial of oncolytic delta-24-RGD (DNX-2401) with biological endpoints: implications for viro-immunotherapy. Neuro Oncol. 2014;16(Suppl 3):iii39. http://dx.doi.org/10.1093/neuonc/nou208.61. [Google Scholar]
- Lebson L, Nash K, Kamath S, Herber D, Carty N, Lee DC, Li Q, Szekeres K, Jinwal U, Koren J, Dickey CA, Gottschall PE, Morgan D, Gordon MN. Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J Neurosci. 2010;30:9651–9658. doi: 10.1523/JNEUROSCI.0329-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone P, Janson CG, Bilaniuk L, Wang Z, Sorgi F, Huang L, Matalon R, Kaul R, Zeng Z, Freese A, McPhee SW, Mee E, During MJ. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol. 2000;48(1):27–38. doi: 10.1002/1531-8249(200007)48:1<27::aid-ana6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, Wang DJ, Assadi M, Goldfarb O, Goldman HW, Freese A, Young D, During MJ, Samulski RJ, Janson CG. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4(165):165ra163. doi: 10.1126/scitranslmed.3003454. http://dx.doi.org/10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levites Y, O’Nuallain B, Puligedda RD, Ondrejcak T, Adekar SP, Chen C, Cruz PE, Rosario AM, Macy S, Mably AJ, Walsh DM, Vidal R, Solomon A, Brown D, Rowan MJ, Golde TE, Dessain SK. A human monoclonal IgG that binds aβ assemblies and diverse amyloids exhibits anti-amyloid activities in vitro and in vivo. J Neurosci. 2015;35(16):6265–6276. doi: 10.1523/JNEUROSCI.5109-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.5109-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, Feigin A. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10(4):309–319. doi: 10.1016/S1474-4422(11)70039-4. http://dx.doi.org/10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao P, Gray SJ, Weinberg MS, Samulski RJ. Combination therapy utilizing shRNA knockdown and an optimized resistant transgene for rescue of diseases caused by misfolded proteins. Proc Natl Acad Sci U S A. 2011a;108(34):14258–14263. doi: 10.1073/pnas.1109522108. http://dx.doi.org/10.1073/pnas.1109522108, 475(7355), 217–221. doi:10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011b;475(7355):217–221. doi: 10.1038/nature10177. http://dx.doi.org/10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J, Zhang S, Liu Z. Neprilysin gene transfer: a promising therapeutic approach for Alzheimer’s disease. J Neurosci Res. 2015;93(9):1325–1329. doi: 10.1002/jnr.23564. http://dx.doi.org/10.1002/jnr.23564. [DOI] [PubMed] [Google Scholar]
- Liao F, Hori Y, Hudry E, Bauer AQ, Jiang H, Mahan TE, Lefton KB, Zhang TJ, Dearborn JT, Kim J, Culver JP, Betensky R, Wozniak DF, Hyman BT, Holtzman DM. Anti-ApoE antibody given after plaque onset decreases Aβ accumulation and improves brain function in a mouse model of Aβ amyloidosis. J Neurosci. 2014;34(21):7281–7292. doi: 10.1523/JNEUROSCI.0646-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.0646-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12(20):1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Chekmasova AA, Marusich E, Chowdhury JR, Strayer DS. Efficient CNS gene delivery by intravenous injection. Nat Methods. 2010;7(11):905–907. doi: 10.1038/nmeth.1518. http://dx.doi.org/10.1038/nmeth.1518. [DOI] [PubMed] [Google Scholar]
- Lu-Emerson C, Plotkin SR. The neurofibromatoses. Part 1: NF1. Rev Neurol Dis. 2009;6(2):E47–E53. [PubMed] [Google Scholar]
- Mager DL, Stoye JP. Mammalian endogenous retroviruses. Microbiol Spectr. 2015;3(1) doi: 10.1128/microbiolspec.MDNA3-0009-2014. http://dx.doi.org/10.1128/microbiolspec.MDNA3-0009-2014. MDNA3-0009-2014. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Balaj L, Sivaraman S, Crommentuijn M, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, Skog J. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20(5):960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire C, Ramirex SH, Merkel SF, Sena-Esteves M, Breakefield XO. Gene therapy for the nervous system: challenges and new strategies. Neurotherapeutics. 2014;11(4):817–839. doi: 10.1007/s13311-014-0299-5. http://dx.doi.org/10.1007/s13311-014-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther. 2012;23(4):356–366. doi: 10.1089/hum.2011.213. http://dx.doi.org/10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Gillespie GY. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. http://dx.doi.org/10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, Nabors LB, Markiewicz M, Agee BS, Coleman JM, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Weichselbaum RR, Fiveash JB, Gillespie GY. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22(5):1048–1055. doi: 10.1038/mt.2014.22. http://dx.doi.org/10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WJJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. http://dx.doi.org/10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Marks WJJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, Gilmore BL, Burstein H, Peluso RW, Polisky B, Carter BJ, Davidson BL. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci U S A. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Young SM, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- McCarty DM, DiRosario J, Gulaid K, Muenzer J, Fu H. Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther. 2009;16(11):1340–1352. doi: 10.1038/gt.2009.85. http://dx.doi.org/10.1038/gt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JH, Cochrane M, Cobbold S, Waldmann H, Nathwani SA, Davidoff AM, Nathwani AC. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene Ther. 2012;19(1):78–85. doi: 10.1038/gt.2011.64. http://dx.doi.org/10.1038/gt.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey T, Tang A, Bett AJ, Casimiro DR, Chastain M. T-cell response to adenovirus hexon and DNA-binding protein in mice. Gene Ther. 2004;11(9):791–796. doi: 10.1038/sj.gt.3302232. [DOI] [PubMed] [Google Scholar]
- McLean JR, Smith GA, Rocha EM, Hayes MA, Beagan JA, Hallett PJ, Isacson O. Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection. Neurosci Lett. 2014;576:73–78. doi: 10.1016/j.neulet.2014.05.044. http://dx.doi.org/10.1016/j.neulet.2014.05.044. [DOI] [PubMed] [Google Scholar]
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8(5):577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27(21):5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, Michels O, Govoni A, Fitzgerald J, Morales P, Foust KD, Mendell JR, Burghes AH, Kaspar BK. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 2015;23(3):477–487. doi: 10.1038/mt.2014.210. http://dx.doi.org/10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DG, Trobridge GD, Petek LM, Jacobs MA, Kaul R, Russell DW. Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol. 2005;79(17):11434–11442. doi: 10.1128/JVI.79.17.11434-11442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DG, Wang PR, Petek LM, Hirata RK, Sands MS, Russell DW. Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol. 2006;24(8):1022–1026. doi: 10.1038/nbt1231. [DOI] [PubMed] [Google Scholar]
- Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther. 2012;23(4):377–381. doi: 10.1089/hum.2011.220. http://dx.doi.org/10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N, Miyake K, Asakawa N, Yamamoto M, Shimada T. Long-term correction of biochemical and neurological abnormalities in MLD mice model by neonatal systemic injection of an AAV serotype 9 vector. Gene Ther. 2014;21(4):427–433. doi: 10.1038/gt.2014.17. http://dx.doi.org/10.1038/gt.2014.1. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviralvector integration. Nat Biotechnol. 2006;24(6):687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Mueller C, Tang Q, Gruntman A, Blomenkamp K, Teckman J, Song L, Zamore PD, Flotte TR. Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol Ther. 2012;20(3):590–600. doi: 10.1038/mt.2011.292. http://dx.doi.org/10.1038/mt.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther. 2010;18(9):1731–1735. doi: 10.1038/mt.2010.135. http://dx.doi.org/10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SR, Chang CC, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, Chang TY. Acat1 knockdown gene therapy decreases amyloid-β in a mouse model of Alzheimer’s disease. Mol Ther. 2013;21(8):1497–1506. doi: 10.1038/mt.2013.118. http://dx.doi.org/10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, Cathomen T. TALE nucleases: tailored genome engineering made easy. Curr Opin Biotechnol. 2012;23(5):644–650. doi: 10.1016/j.copbio.2012.01.013. http://dx.doi.org/10.1016/j.copbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, Koo EH, Masliah E, Tuszynski MH. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci. 2013;33(39):15596–15602. doi: 10.1523/JNEUROSCI.5195-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34(3):297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- Nakai H, Wu X, Fuess S, Storm TA, Munroe D, Montini E, Burgess SM, Grompe M, Kay MA. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol. 2005;79(6):3606–3614. doi: 10.1128/JVI.79.6.3606-3614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–360. doi: 10.1038/nature15818. http://dx.doi.org/10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A, Pie J, Rangarajan S, Bevan D, Recht M, Shen YM, Halka KG, Basner-Tschakarjan E, Mingozzi F, High KA, Allay J, Kay MA, Ng CY, Zhou J, Cancio M, Morton CL, Gray JT, Srivastava D, Nienhuis AW, Davidoff AM. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. http://dx.doi.org/10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot B. Targeting BACE with small inhibitory nucleic acids – a future for Alzheimer’s disease therapy? Acta Biochim Pol. 2004;51(2):431–444. [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, Asokan A, Zhang F, Duan D, Gersbach CA. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351(6271):403–407. doi: 10.1126/science.aad5143. http://dx.doi.org/10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson SC, Samulski RJ. Recombinant adeno-associated virus utilizes host cell nuclear import machinery to enter the nucleus. J Virol. 2014;88(8):4132–4144. doi: 10.1128/JVI.02660-13. http://dx.doi.org/10.1128/JVI.02660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DM, Boulis NM. Gene therapy for neurodegenerative diseases. Trends Mol Med. 2015;21(8):504–512. doi: 10.1016/j.molmed.2015.06.001. http://dx.doi.org/10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Osten P, Dittgen T, Licznerski P. Lentivirus-based genetic manipulations in neurons in vivo. In: Moss S, Kittler J, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. CRC Press/Taylor & Francis; Boca Raton, FL: 2006. pp. 249–259. (Chapter 13) [Google Scholar]
- Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. http://dx.doi.org/10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM, Kadota K, Roth SL, Giardina P, Viale A, Leslie C, Bushman FD, Studer L, Sadelain M. Genomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29(1):73–78. doi: 10.1038/nbt.1717. http://dx.doi.org/10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, González-Aseguinolaza G, Mulle C, Alberini CM, Cuadrado-Tejedor M, Garcia-Osta A. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol Med. 2014;6(10):1246–1262. doi: 10.15252/emmm.201404228. http://dx.doi.org/10.15252/emmm.201404228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini MA, Macauley SL, Huff MR, Taksir TV, Bu J, Wu IH, Piepenhagen PA, Dodge JC, Shihabuddin LS, O’Riordan CR, Schuchman EH, Stewart GR. AAV vector-mediated correction of brain pathology in a mouse model of Niemann-Pick A disease. Mol Ther. 2005;11(5):754–762. doi: 10.1016/j.ymthe.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Richards AM, Treleaven CM, Sullivan JA, O’Riordan CR, Scaria A, Kells AP, Samaranch L, San Sebastian W, Federici T, Fiandaca MS, Boulis NM, Bankiewicz KS, Shihabuddin LS, Cheng SH. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther. 2014;25:619–630. doi: 10.1089/hum.2014.011. [DOI] [PubMed] [Google Scholar]
- Patel P, Kriz J, Gravel M, Soucy G, Bareil C, Gravel C, Julien JP. Adeno-associated virus-mediated delivery of a recombinant single-chain antibody against misfolded superoxide dismutase for treatment of amyotrophic lateral sclerosis. Mol Ther. 2014;22(3):498–510. doi: 10.1038/mt.2013.239. http://dx.doi.org/10.1038/mt.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulk NK, Wursthorn K, Wang Z, Finegold MJ, Kay MA, Grompe M. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology. 2010;51(4):1200–1208. doi: 10.1002/hep.23481. http://dx.doi.org/10.1002/hep.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioni DE, Kesari S. Clinical trials of viral therapy for malignant gliomas. Expert Rev Anticancer Ther. 2013;13(11):1297–1305. doi: 10.1586/14737140.2013.851160. http://dx.doi.org/10.1586/14737140.2013.851160. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Bennett J. The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harb Perspect Med. 2015;5(9) doi: 10.1101/cshperspect.a017285. http://dx.doi.org/10.1101/cshperspect.a017285 pii: a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar S, Goto J, Zhang X, Sena-Esteves M, Bronson R, Brockmann J, Gianni D, Wojtkiewicz GR, Chen JW, Stemmer-Rachamimov A, Kwiatkowski DJ, Breakefield XO. Stochastic model of Tsc1 lesions in mouse brain. PLoS One. 2013;8(5):e64224. doi: 10.1371/journal.pone.0064224. http://dx.doi.org/10.1371/journal.pone.0064224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar S, Zhang X, Goto J, Han S, Lai C, Bronson R, Sena-Esteves M, Ramesh V, Stemmer-Rachamimov A, Kwiatkowski DJ, Breakefield XO. Survival benefit and phenotypic improvement by hamartin gene therapy in a tuberous sclerosis mouse brain model. Neurobiol Dis. 2015;82:22–31. doi: 10.1016/j.nbd.2015.04.018. http://dx.doi.org/10.1016/j.nbd.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–191. doi: 10.1038/nature14299. http://dx.doi.org/10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Lebrón E, do Costa MC, Luna-Cancalon K, Peron TM, Fischer S, Boudreau RL, Davidson BL, Paulson HL. Silencing mutant ATXN3 expression resolves molecular phenotypes in SCA3 transgenic mice. Mol Ther. 2013;21(10):1909–1918. doi: 10.1038/mt.2013.152. http://dx.doi.org/10.1038/mt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GL, Martino AT, Zolotukhin I, Ertl HC, Herzog RW. Role of the vector genome and underlying factor IX mutation in immune responses to AAV gene therapy for hemophilia B. J Transl Med. 2014;12:25. doi: 10.1186/1479-5876-12-25. http://dx.doi.org/10.1186/1479-5876-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Hirata RK. Human gene targeting by viral vectors. Nat Genet. 1998;18(4):325–330. doi: 10.1038/ng0498-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzo A, Marcó S, García M, Villacampa P, Ribera A, Ayuso E, Maggioni L, Mingozzi F, Haurigot V, Bosch F. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther. 2012;23(12):1237–1246. doi: 10.1089/hum.2012.029. http://dx.doi.org/10.1089/hum.2012.029. [DOI] [PubMed] [Google Scholar]
- Sack BK, Herzog RW. Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther. 2009;11(5):493–503. [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23(4):382–389. doi: 10.1089/hum.2011.200. http://dx.doi.org/10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, DeArmond S, Forsayeth J, Bankiewicz KS. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther. 2014;22(2):329–337. doi: 10.1038/mt.2013.266. http://dx.doi.org/10.1038/mt.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Sands MS, Davidson BL. Gene therapy for lysosomal storage diseases. Mol Ther. 2006;13(5):839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sargeant TJ, Wang S, Bradley J, Smith NJ, Raha AA, McNair R, Ziegler RJ, Cheng SH, Cox TM, Cachón-González MB. Adeno-associated virus-mediated expression of β-hexosaminidase prevents neuronal loss in the Sandhoff mouse brain. Hum Mol Genet. 2011;20(22):4371–4380. doi: 10.1093/hmg/ddr364. http://dx.doi.org/10.1093/hmg/ddr364. [DOI] [PubMed] [Google Scholar]
- Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, Elde RP, Fairbanks CA, Vulchanova L. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 2014;8:42. doi: 10.3389/fnana.2014.00042. http://dx.doi.org/10.3389/fnana.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena-Esteves M, Saeki Y, Fraefel C, Breakefield XO. HSV-1 amplicon vectors–simplicity and versatility. Mol Ther. 2000;2(1):9–15. doi: 10.1006/mthe.2000.0096. [DOI] [PubMed] [Google Scholar]
- Sevin C, Benraiss A, Van Dam D, Bonnin D, Nagels G, Verot L, Laurendeau I, Vidaud M, Gieselmann V, Vanier M, De Deyn PP, Aubourg P, Cartier N. Intracerebral adeno-associated virus-mediated gene transfer in rapidly progressive forms of metachromatic leukodystrophy. Hum Mol Genet. 2006;15(1):53–64. doi: 10.1093/hmg/ddi425. [DOI] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286(15):13532–13540. doi: 10.1074/jbc.M110.210922. http://dx.doi.org/10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29(2):121–128. doi: 10.1038/nbt.1769. http://dx.doi.org/10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, Boulis NM, Federici T. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther. 2011;22(9):1129–1135. doi: 10.1089/hum.2011.008. http://dx.doi.org/10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Spaete RR, Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Spampanato C, De Leonibus E, Dama P, Gargiulo A, Fraldi A, Sorrentino NC, Russo F, Nusco E, Auricchio A, Surace EM, Ballabio A. Efficacy of a combined intracerebral and systemic gene delivery approach for the treatment of a severe lysosomal storage disorder. Mol Ther. 2011;19(5):860–869. doi: 10.1038/mt.2010.299. http://dx.doi.org/10.1038/mt.2010.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnke S, Lux K, Uhrig S, Kreppel F, Hösel M, Coutelle O, Ogris M, Hallek M, Büning H. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology. 2011;409(1):77–83. doi: 10.1016/j.virol.2010.09.025. http://dx.doi.org/10.1016/j.virol.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33(1):102–106. doi: 10.1038/nbt.3055. http://dx.doi.org/10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, Cong L, Zhang F, Vandenberghe LH, Church GM, Wagers AJ. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407–411. doi: 10.1126/science.aad5177. http://dx.doi.org/10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M, Zérah M, Husson B, de Bournonville S, Deiva K, Adamsbaum C, Vincent F, Hocquemiller M, Broissand C, Furlan V, Ballabio A, Fraldi A, Crystal RG, Baugnon T, Roujeau T, Heard JM, Danos O. Intracerebral administration of adeno-associated viral vector serotype rh. 10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Hum Gene Ther. 2014;25(6):506–516. doi: 10.1089/hum.2013.238. http://dx.doi.org/10.1089/hum.2013.238. [DOI] [PubMed] [Google Scholar]
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. doi: 10.1056/NEJMoa1300662. http://dx.doi.org/10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney RM, Bell CL, Wilson JM. AAV8 capsid variable regions at the two-fold symmetry axis contribute to high liver transduction by mediating nuclear entry and capsid uncoating. Virology. 2014;454–455(454–455):227–236. doi: 10.1016/j.virol.2014.02.017. http://dx.doi.org/10.1016/j.virol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Topaloglu O, Hurley PJ, Yildirim O, Civin CI, Bunz F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 2005;33(18):e158. doi: 10.1093/nar/gni160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, Marrocco E, Rossi S, Giunti M, Palfi A, Farrar GJ, Polishchuk R, Auricchio A. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6(2):194–211. doi: 10.1002/emmm.201302948. http://dx.doi.org/10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4(6):833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Yang JH, Barba D, HSU, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, Nagahara AH. Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease. JAMA Neurol. 2015;72(10):1139–1147. doi: 10.1001/jamaneurol.2015.1807. http://dx.doi.org/10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Marzulli M, Nakano K, Goins WF, Chan J, Hong CS, Mazzacurati L, Yoo JY, Haseley A, Nakashima H, Baek H, Kwon H, Kumagai I, Kuroki M, Kaur B, Chiocca EA, Grandi P, Cohen JB, Glorioso JC. Effective treatment of an orthotopic xenograft model of human glioblastoma using an EGFR-retargeted oncolytic herpes simplex virus. Mol Ther. 2013;21(3):561–569. doi: 10.1038/mt.2012.211. http://dx.doi.org/10.1038/mt.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13(16):1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Vite CH, Passini MA, Haskins ME, Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10(22):1874–1881. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- Wang RY, Bodamer OA, Watson MS, Wilcox WR, ACMG W on Diagnostic Confirmation of Lysosomal Storage Diseases Group. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011;13(5):457–484. doi: 10.1097/GIM.0b013e318211a7e1. http://dx.doi.org/10.1097/GIM.0b013e318211a7e1. [DOI] [PubMed] [Google Scholar]
- Wang N, Gray M, Lu XH, Cantle JP, Holley SM, Greiner E, Gu X, Shirasaki D, Cepeda C, Li Y, Dong H, Levine MS, Yang XW. Neuronal targets for reducing mutant huntingtin expression to ameliorate disease in a mouse model of Huntington’s disease. Nat Med. 2014;20(5):536–541. doi: 10.1038/nm.3514. http://dx.doi.org/10.1038/nm.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015;22(1):104–110. doi: 10.1038/gt.2014.91. http://dx.doi.org/10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann CM, Ferreira J, Keeler AM, Su Q, Qui L, Shaffer SA, Xu Z, Gao G, Sena-Esteves M. Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan. Hum Mol Genet. 2015;24(15):4353–4364. doi: 10.1093/hmg/ddv168. http://dx.doi.org/10.1093/hmg/ddv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab. 2009;96(4):151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. http://dx.doi.org/10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19(5):463–474. doi: 10.1089/hum.2008.022. http://dx.doi.org/10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73(5):3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao PJ, Samulski RJ. Cytoplasmic trafficking, endosomal escape, and per-inuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J Virol. 2012;86(19) doi: 10.1128/JVI.00935-12. http://dx.doi.org/10.1128/JVI.00935-12, 10462–10471–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Chai HL, Teramoto S, Tsuji S, Shimazaki K, Muramatsu S, Kwak S. Rescue of amyotrophic lateral sclerosis phenotype in a mouse model by intravenous AAV9-ADAR2 delivery to motor neurons. EMBO Mol Med. 2013;5(11):1710–1719. doi: 10.1002/emmm.201302935. http://dx.doi.org/10.1002/emmm.201302935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang Y, Duan D, Engelhardt JF. Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci U S A. 2000;97(12):6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Li S, Wang H, Guo Y, Gessler DJ, Cao C, Su Q, Kramer J, Zhong L, Ahmed SS, Zhang H, He R, Desrosiers RC, Brown R, Xu Z, Gao G. Global CNS transduction of adult mice by intravenously delivered rAAVrh. 8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther. 2014;22(7):1299–1309. doi: 10.1038/mt.2014.68. http://dx.doi.org/10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, Yu H, Xu C, Morizono H, Musunuru K, Batshaw ML, Wilson JM. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 2016 Feb 1; doi: 10.1038/nbt.3469. http://dx.doi.org/10.1038/nbt.3469 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, Chamberlain JS, Xiao X, Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9(23):1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–771. doi: 10.1016/j.cell.2015.09.038. http://dx.doi.org/10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011;19(8):1440–1448. doi: 10.1038/mt.2011.98. http://dx.doi.org/10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG, Greenamyre JT, Burton EA. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest. 2015;125(7):2721–2735. doi: 10.1172/JCI64502. http://dx.doi.org/10.1172/JCI64502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirger JM, Puntel M, Bergeron J, Wibowo M, Moridzadeh R, Bondale N, Barcia C, Kroeger KM, Liu C, Castro MG, Lowenstein PR. Immune-mediated loss of transgene expression from virally transduced brain cells is irreversible, mediated by IFNγ, perforin, and TNFα, and due to the elimination of transduced cells. Mol Ther. 2012;20(4):808–819. doi: 10.1038/mt.2011.243. http://dx.doi.org/10.1038/mt.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]