Abstract.
An overwintering population of Aedes aegypti has been documented in the Capitol Hill neighborhood of Washington, DC, since 2011. Mitochondrial cytochrome oxidase I (mtCOI) sequence data presented in a previous study traced the origin to the New World. Here, we use microsatellite and 14,071 single nucleotide polymorphisms along with mitochondrial DNA (mtDNA) sequences on Washington Ae. aegypti samples and samples from potential sources to further narrow the origin of this population. Genetically, Washington Ae. aegypti are closest to populations in Florida, meaning this is the most likely source. Florida experienced the first mosquito-borne transmission of dengue in the United States after decades of absence of this disease, as well as local transmission of chikungunya and Zika in recent years. This suggests that the Capitol Hill, Washington, DC population of Ae. aegypti is capable of transmitting viruses such as dengue, chikungunya, and Zika in modern US city environments.
INTRODUCTION
Aedes aegypti was given the common name of “the yellow fever mosquito” because it was identified as the primary urban vector of that devastating disease in the early twentieth century. Today, it is a major public health concern as the main global vector of dengue, chikungunya, and Zika viruses. Human populations at risk for these diseases coincide with the distribution of Ae. aegypti, thus factors affecting the distribution of this mosquito are of considerable medical importance.
The distribution of Ae. aegypti is temperature restricted. The generally accepted dogma is that permanent year-round breeding populations cannot exist where the mean temperature of the coldest month is less than 10°C.1 In North America, this corresponds approximately to 33° N latitude. The report of a year-round breeding population in the Capitol Hill neighborhood of Washington, DC, was, therefore, unexpected.2 Washington, DC, is at about 39° N, with a mean January (minimum month) temperature of 6°C. Data from Lima et al.2 indicate that this population is temporally stable, suggesting an overwintering population. Although these data could also be consistent with an influx of large numbers of Ae. aegypti individuals from the same source to the same neighborhood in DC every year, this possibility is deemed unlikely. Evidence indicates that the population is overwintering, probably in subterranean refugia and engaging in limited breeding aboveground in summer and fall.2
Historical records of yellow fever in the northeastern United States indicate that temporary warm weather (summer/fall) introductions of Ae. aegypti have occurred for centuries;3 however, this Capitol Hill population is unique in overwintering. Given its distinctive appearance and major health importance, it is likely that the population would have been noted had it existed for some time, thus it is probably a recent introduction. The question remains as to where it came from. Lima et al.2 presented mitochondrial DNA (mtDNA) data consistent with the population originating within the Americas, but were not able to pinpoint a more precise location. Identifying the source of the Capitol Hill population can help to understand how Ae. aegypti may have arrived to Washington, DC, and to prevent similar introductions in the future, both to the area and to other naive regions. Furthermore, the ability of Aedes mosquitoes to transmit disease varies across geographic regions,4–10 as it does insecticide resistance.11–13 Determining the genetic affiliations of the Washington, DC, population of Ae. aegypti could guide control efforts and provide insights on the ability of these mosquitoes to transmit viruses with worldwide circulation.
Aedes aegypti populations are highly genetically differentiated across its range, displaying a strong and hierarchical genetic structure.14–17 This pattern of geographic differentiation can be used as population “genetic signatures” to track the source of novel introductions, when compared with a reference panel.15 In two previous cases, The Netherlands and California, we successfully used genetic approaches based on microsatellite markers to identify the origin of new introductions of Ae. aegypti.18,19 Here, we apply these methods, supplemented by 14,071 single nucleotide polymorphisms (SNPs) and additional mtDNA sequencing, to narrow down the likely origin of the overwintering Capitol Hill, Washington, DC, population of Ae. aegypti.
MATERIALS AND METHODS
Mosquito samples.
Collection information and specific locations for Ae. aegypti samples from the Capitol Hill neighborhood in Washington, DC, were previously described.2 DNA preparations representing a subset of individuals from that study collected during 2014 were included in microsatellite and SNP chip genotyping assays.
Most Ae. aegypti collections used as reference for microsatellites and the SNP genotypes for this analysis were reported previously.15,16,20–23 Collections not reported previously were obtained as eggs from oviposition traps or as adults sampled directly from the field. Eggs were hatched at Yale University and reared to adults for identification and preservation in 100% ethanol at −20°C. Collections included in this study are described in Table 1.
Table 1.
Aedes aegypti collections included in this study
| Population | Region | Year* | Nμ† | Reference | NSNP‡ | Reference |
|---|---|---|---|---|---|---|
| Dakar, SE | 2005 | Africa | 20 | 15 | – | – |
| Lunyo, UG | 2013 | Africa | 20 | 15 | – | – |
| Yaounde, CM | 2014 | Africa | 20 | 15 | – | – |
| Francesville, GA | 2014 | Africa | 20 | 15 | – | – |
| Johannesburg, ZA | 2015 | Africa | 20 | 15 | – | – |
| Bijagos, GW | 2009 | Africa | 20 | This study | – | – |
| Nairobi, KE | 2012 | Africa | 20 | 15 | – | – |
| Ho Chi Minh, VT | 2013 | Asia | 54 | 15 | 19 | This study |
| Hanoi, VT | 2013 | Asia | 54 | 15 | 22 | This study |
| Cebu City, PH | 2013 | Asia | 108 | 15 | 8 | This study |
| Pakistan, PK | 2010 | Asia | 49 | 15 | 17 | This study |
| Jeddah, SA | 2012 | Asia | 84 | 15 | 11 | This study |
| Rayong,TH | 2006 | Asia | 48 | 16 | – | – |
| Prachuabkhirikan, TH | 2009 | Asia | 47 | 16 | – | – |
| Townsville, AU | 2009 | Asia | 47 | 16 | – | – |
| Cairns, AU | 2009 | Asia | – | – | 12 | 20 |
| Cairns, AU | 2015 | Asia | 24 | 22 | – | – |
| Bangkok, TH | 2012 | Asia | 54 | This study | 11 | This study |
| Bangkok, TH | 2013 | Asia | – | – | – | – |
| Sri Lanka, SL | 2014 | Asia | 7 | 15 | 5 | This study |
| Patillas, PR | 2014 | Caribbean | 54 | 15 | 12 | 20 |
| Trinidad, TT | 2014 | Caribbean | 51 | 15 | 12 | This study |
| Tobago, TT | 2015 | Caribbean | – | – | 8 | This study |
| Saint Vincent, VC | 2015 | Caribbean | – | – | 12 | This study |
| Dominica, DM | 2009 | Caribbean | 95 | 15 | – | – |
| Dominica, DM | 2017 | Caribbean | – | – | 13 | This study |
| Siquirres, CR | 2014 | Central America | 51 | 15 | 6 | This study |
| Madeira, PO | 2012 | Europe | 66 | 15 | 6 | This study |
| Maricopa County, AZ, USA | 2013 | North America | 53 | 15 | – | – |
| Tucson, AZ, USA | 2012 | North America | – | – | 12 | This study |
| Tijuana, BC, MEX | 2013 | North America | 20 | 15 | 10 | This study |
| Madera, CA, USA | 2013 | North America | 50 | 15 | 24 | This study |
| Clovis, CA, USA | 2013 | North America | 60 | 15 | 11 | This study |
| Fresno, CA, USA | 2015 | North America | – | – | 12 | This study |
| Houston, TX, USA | 2011 | North America | 19 | 15 | 8 | 20 |
| Pijijiapan, CHP, MEX | 2008 | North America | 47 | 15 | – | – |
| Miami, FL, USA | 2011 | North America | 47 | 15 | – | – |
| Las Palomas, GRO, MEX | 2012 | North America | 54 | 15 | – | – |
| Lomas de Zapatero, GRO, MEX | 2012 | North America | 51 | 15 | – | – |
| Iguala, GRO, MEX | 2012 | North America | 54 | 15 | – | – |
| Mazatan, CHP, MEX | 2012 | North America | 45 | 15 | – | – |
| Columbus, GA, USA | 2012 | North America | 55 | 15 | 8 | This study |
| Tapachula Norte, CHP, MEX | 2012 | North America | 54 | 15 | 12 | 20 |
| San Mateo, CA, USA | 2013 | North America | 21 | 15 | – | – |
| Hermosillo, SON, MEX | 2013 | North America | 50 | 15 | – | – |
| Nogales, SON, MEX | 2013 | North America | 51 | 15 | 9 | This study |
| Chetumal, QRO, MEX | 2013 | North America | 54 | 15 | – | – |
| Rio, FL, USA | 2014 | North America | 51 | 15 | – | – |
| Conch Key, FL, USA | 2006 | North America | 42 | 15 | – | – |
| Palm Beach, FL, USA | 2006 | North America | 42 | 15 | – | – |
| Cameron, TX, USA | 2015 | North America | 60 | This study | – | – |
| Dallas, TX, USA | 2015 | North America | 60 | 23 | – | – |
| El Paso, TX, USA | 2015 | North America | – | – | 8 | This study |
| Las Cruces, TX, USA | 2015 | North America | 54 | This study | – | – |
| Vaca Key, FL, USA | 2015 | North America | 48 | 22 | – | – |
| Key West, FL, USA | 2016 | North America | 38 | 22 | 12 | 22 |
| New Orleans, LA, USA | 2015 | North America | 24 | 22 | 12 | 22 |
| Amacuzac, MOR, MEX | 2016 | North America | 52 | This study | 60 | This study |
| Washington, DC, USA | 2014 | North America | 27 | This study | 16 | This study |
| Hawaii, USA | 2009 | Pacific | 25 | This study | 6 | 20 |
| Hawaii, USA | 2016 | Pacific | – | – | 7 | This study |
| Tahiti, FP | 2010 | Pacific | 48 | 15 | 12 | 20 |
| Cali, CO | 2013 | South America | 80 | 15 | 12 | This study |
| Bolivar, VEN | 2004 | South America | 48 | 15 | – | – |
| Cachoeiro, BR | 2012 | South America | 47 | 15 | – | – |
| Zulia, VEN | 2004 | South America | 47 | 15 | – | – |
| Maraba, BR | 2010 | South America | 48 | 15 | 12 | This study |
| Natal, BR | 2010 | South America | 47 | 15 | – | – |
| Jacobina, BR | 2013 | South America | 94 | 15 | 40 | 20 |
| Rio de Janeiro, BR | 2014 | South America | 39 | 15 | 7 | This study |
| Total | 2,821 | – | 484 | – |
Year of collection.
No. of individuals genotyped with microsatellite loci.
No. of individuals genotyped with SNPs.
Nuclear DNA genotyping and analyses.
Total nucleic acids were extracted from individual mosquitoes with the DNeasy Blood and Tissue kit (Qiagen), according to manufacturer instructions, including an optional treatment with 4 μL of RNase A (Qiagen, Hilden, Germany). Samples were stored at −20°C until further analysis.
Microsatellite markers.
We used 12 highly variable microsatellites (∼10 alleles per locus, > 50% heterozygosity), validated in previous studies addressing the global genetic diversity of Ae. aegypti15,16,20 and used to identify the origin of introductions in other studies.18,19 Genotyping was performed as described in those publications.
SNP genotyping.
A custom designed SNP chip20 was used on a subset of the samples genotyped for microsatellites. Approximately 200 ng of genomic DNA from individual mosquitoes were sent to the Functional Genomics Core at the University of North Carolina, Chapel Hill, for hybridization with the Axiom aegypti1 SNP chip (Life Technologies Corporation CAT#550481) and production of genotypes. The Affymetrix Genotyping Console and the R package SNPolisher v1.4 (both from Affymetrix, Inc., Santa Clara, CA/now Life Technologies-Thermo Fisher Scientific, Waltham MA, USA) were used to generate and process genotype calls. While the SNP chip contains probes for > 27,000 well-validated biallelic SNPs (e.g., tested for Mendelian inheritance20), not all were variable in our samples. Additional pruning based on a linkage disequilibrium cutoff was performed using PLINK v.1.924 at a window size of 50 bp with a 10 bp window shift and considering a variance inflation factor threshold of 1.5, as recommended for small sample sizes.24 The final dataset containing 14,071 SNPs was the basis for our analyses.
Analytic methods.
The total number of alleles, average allele richness (AR), and private allele richness were calculated in HPRARE,25 which uses rarefaction to correct for unequal sample sizes. Average observed heterozygosities (Ho) were estimated using GenAlEx.26
Population structure and assignments of individuals from the Washington, DC, population to specific genetic clusters were performed for microsatellite markers via the Bayesian clustering method implemented by the software STRUCTURE v. 2.3.27 Each population was represented by 20 randomly chosen individuals. STRUCTURE identifies genetic clusters and assigns individuals to these clusters with no a priori information of the sample location. The most likely number of clusters (K) was determined by conducting 20 independent runs from K = 1 to 10 on 20 random individuals from each population. Each run assumed an admixture model and correlated allele frequencies using a burn-in value of 100,000 iterations followed by 500,000 repetitions. The optimal number of K clusters was determined following the guidelines of Pritchard et al.28 and the Delta K method.29,30 Results were plotted with the program DISTRUCT v.1.1.31
The program ADMIXTURE32 was used to explore the population structure and genetic cluster assignment with the SNP dataset. This program uses maximum likelihood (ML) to estimate ancestral allele frequencies of unrelated individuals, in a similar manner as STRUCTURE, but runs more efficiently when analyzing large numbers of markers, such as our SNP dataset, thanks to the implementation of an expectation-maximization algorithm. Clustering algorithms are known to be greatly influenced by uneven sampling,33,34 thus, each population was represented by 6–8 individuals (median = 8) chosen at random from the main dataset. This number of individuals has been shown to be sufficient to obtain accurate estimates of genetic diversity and differentiation when large numbers of SNPs are used (> 1,000 SNPs).35,36
GENECLASS237 was used to perform individual assignment tests on the Washington, DC, population against the reference population dataset that included all collections from America and the Caribbean listed in Table 1, using the Bayesian criteria for likelihood estimation to determine the population assignment ranking.38 The entire microsatellite dataset was run at once, but due to software constrains limiting the number of markers that can be analyzed, SNP-assignment tests were conducted as 10 independent runs of 4,000 randomly selected SNPs drawn from the same individuals. Self-assignment tests on the microsatellite reference dataset resulted in 76.2% of individuals assigned to the correct population. In addition, individual assignment tests of 1 or 2 random individuals from each of the reference populations were performed to further evaluate the accuracy of the assignment method. Based on population assignment ranking, 77% of the individuals were assigned to their population of origin, whereas for 91% of the individuals the population of origin was included within the top three highest assignment probabilities or scores, respectively. Self-assignment tests on the SNP reference dataset resulted in 97.1% ± 0.3% of the individuals assigned to the correct population, with most misassignments pointing to a geographically and genetically close population.
Pairwise genetic distances39 were calculated with the ADEGENET package in R v. 3.3.2.40,41 Principal component analysis (PCA) and construction of the Neighbor Joining tree on the genetic distance matrix generated, were performed in the same package.
PCA on the SNP dataset was conducted with the package LEA,42 available for the R software v. 3.3.2.41
Five individuals from each of the American and Caribbean populations were used to build a ML tree from the SNP data in RaxML v.843 using the GTRCAT model of rate heterogeneity, with ascertainment bias correction and midpoint rooting. Support for the branches was evaluated by running 1,000 independent bootstraps using the same package.
Mitochondrial cytochrome oxidase I (mtCOI) haplotype genotyping and analysis.
DNA samples representing randomly selected subsets of individuals (N = 10) from three locations in Florida (Key West, Miami, and Palm Beach), one location in western Georgia (Columbus), a southern Mexico population (Tapachula), and a population from Central America (Siquirres, Costa Rica) were subjected to mtCOI haplotype genotyping, as previously described.2 These populations were selected based on results from the microsatellite and SNP data analysis to narrow down the possible origin of the Washington, DC, population. Briefly, 710-bp mtCOI amplicons were obtained using 25-μL polymerase chain reaction (PCR) mixtures containing 1X Taq buffer (50 mM KCl, 10 mM Tris pH 9.0, 0.1% Triton X), 1.5 mM MgCl2, 200 μM each dNTP, 5 pmoles of each primer, 1 unit of Taq DNA polymerase, and 1 μL of DNA template (some samples were diluted from 1:10 to 1:100 in sterile water). PCR products were size fractionated by electrophoresis in 2% agarose gels stained with ethidium bromide and visualized under ultraviolet light. PCR products were purified using the E.Z.N.A. Cycle Pure Kit (Omega Bio-Tek, Norcross, GA) and sequenced using an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA). The same primer sequences were used for amplification and sequencing: LCO1490: GGTCAACAAATCATAAAGATATTGG and HCO2199: AAACTTCAGGGTGACCAAAAAATCA.44
Sequences were aligned in Clustal Omega v1.2.4.45,46 Clustal Omega. Available at: http://www.ebi.ac.uk). These sequences, along with a subset of Ae. aegypti mtCOI sequences from various geographic regions of the world used previously, were trimmed to a 497 base pair consensus and used to construct a phylogenetic tree in MEGA747 using a mtCOI sequence for Aedes albopictus (GenBank accession no. KC690960) as an outgroup.
Data availability.
Microsatellite and SNP genotypes were deposited in VectorBase48,49 PopBio projects: VBP0000201 (new data), and VBP0000138, VBP0000176-177 (previously published data). Sequencing data were deposited in NCBI under accession numbers MF371160–MF371174 and MG241351–MG241354.
RESULTS
Microsatellite markers.
Average AR of the Washington, DC, collection was similar to the average AR of the American and Caribbean samples across all loci (3.73 and 3.84 [range: 2.92–4.76], respectively). The average number of private alleles across all loci in Washington, DC, was 0.06, whereas the average across American and Caribbean samples was 0.03 (0–0.16). Observed heterozygosity (Ho) was 0.493 compared with an average Ho of 0.532 across the American and Caribbean populations. These data suggest that the Washington, DC, population has not undergone an extreme bottleneck, which would suggest the reestablishment of the population from a few founders every year. Instead, the observed levels of genetic diversity, similar to those of other field populations, are consistent with an overwintering population. AR values and heterozygosities for all collections are shown in Supplemental Table 1.
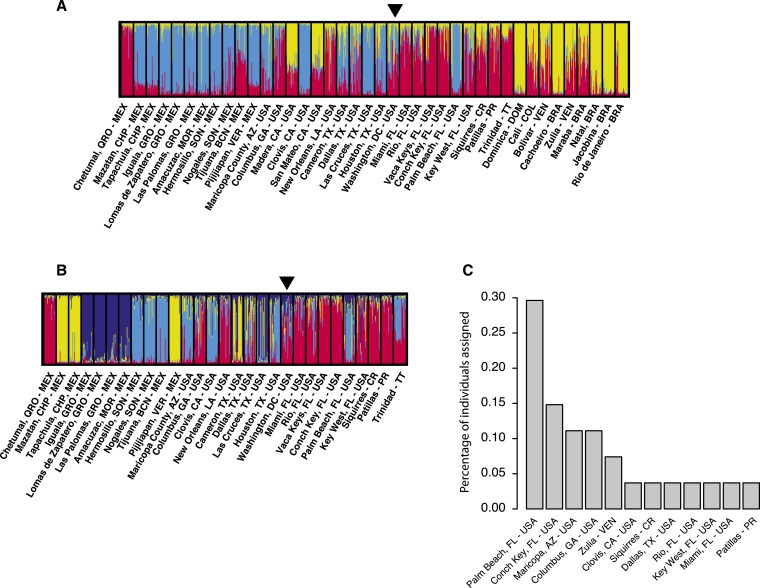
Bayesian clustering analysis on a subsample of 37 representative world populations confirmed that the Washington, DC, samples were of the Ae. aegypti aegypti type (Supplemental Figure 1A). Further analysis including 53 populations outside of Africa identified three main genetic clusters (Supplemental Figure 1B), consistent with previous work.15 Within these clusters, the Washington, DC, population was identified as an admixed population between the two clusters that included North and South America and the Caribbean (Supplemental Figure 1B). Subsequent hierarchical Bayesian clustering analyses suggest that the Washington, DC, population has a mixed ancestry, similar to populations from Georgia, Texas, and Florida (Figure 1A and B). PCA on genetic distances between the American populations was not conclusive (Supplemental Figure 2A and B). The neighbor-joining tree constructed from the same genetic distances positions the Washington, DC, population close to Georgia and Florida (Supplemental Figure 2C). Genetic assignment of the Washington, DC, individuals to the reference dataset of American populations (including the Caribbean) resulted in 13 out of 27 individuals assigned to a Florida population with the highest score, with the rest being mostly assigned to either Georgia or Arizona (Figure 1C, Supplemental Table 2).
Figure 1.
Analyses of the Aedes aegypti from Capitol Hill, Washington, DC, using 12 microsatellite markers. (A, B) STRUCTURE plots28 illustrating the genetic structure of Aedes aegypti in America. Each vertical bar represents an individual. The height of each bar is the probability of assignment to each of K genetic clusters (indicated by different colors), as determined using the Delta K method.29 K = 3 for America (A) and K = 4 for a subset of North American populations (B). An arrow at the top of the plot highlights the location of the Washington, DC, population. (C) Percentage of individuals from Washington, DC, assigned to each of the reference populations depicted in the Y-axis with the highest score. Only populations to which at least one individual was assigned are shown. Assignments were performed using Bayesian criteria for likelihood estimation with GENECLASS 2.0.37 This figure appears in color at www.ajtmh.org.
SNP chip.
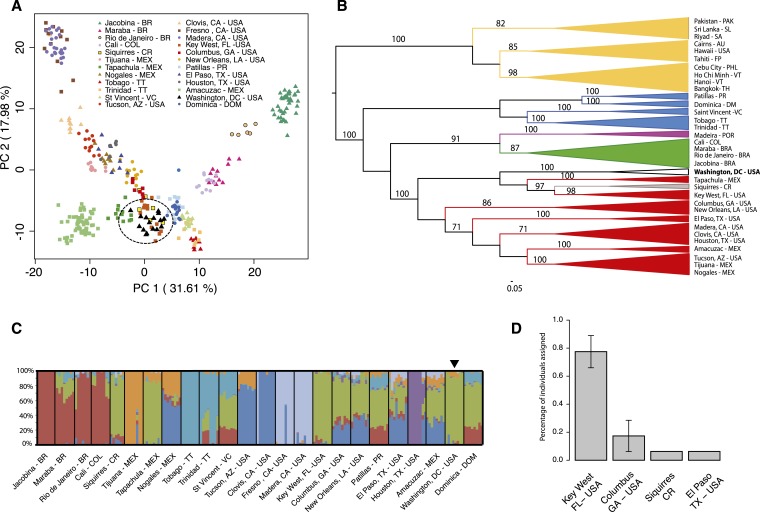
PCA on the American and Caribbean populations using the SNP markers positioned the Washington, DC, population nearby Tapachula, Mexico; Key West, FL; and Siquirres, Costa Rica (Figure 2A). This relationship is also evident by inspecting the ML tree in Figure 2B. In the ML tree, the Washington, DC, clade is sister to a weakly supported clade that includes samples from Siquirres, Costa Rica; Key West, FL; and Tapachula, Mexico. These two clades belong to a larger clade that includes the rest of the North American populations (Figure 2B). Hierarchical clustering analysis on the SNP dataset includes representative populations outside Africa and clusters Washington, DC, within America (American continent and the Caribbean; Supplemental Figure 3). Subsequent clustering within this group positions Washington, DC, with Siquirres, Costa Rica; Tapachula, Mexico; and Key West, FL (Figure 2C). Genetic assignment tests connected 77.5% ± 11.5% of the Washington individuals to Florida and 17.4% ± 11.2% to Georgia (Figure 2D, Supplemental Table 3).
Figure 2.
Analyses of the Aedes aegypti from Capitol Hill, Washington, DC, using 14,071 SNP markers. (A) Principal component analysis on allele frequencies. A dashed circle surrounds individuals from Washington, DC. (B) Midpoint rooted phylogenetic tree of Ae. aegypti populations outside Africa constructed using maximum likelihood in RAXML.43 Yellow: Asia, Blue: Caribbean, Purple: Europe, Green: South America, Grey: Central America, Red: North America, and Grey: Capitol Hill, Washington, DC. Numbers on branches indicate bootstrap values (1,000 replicates). Bootstrap values below 70% are not shown. (C) and (D) are same as Figure 1B and C except using 14,071 SNPs as markers. This figure appears in color at www.ajtmh.org.
Cytochrome oxidase I (COI) mtDNA.
We identified eight COI mtDNA haplotypes from 60 individuals collected at three locations in Florida, one location in Georgia, Costa Rica, and south Mexico (Table 2, Supplemental Figure 4); the three regions identified as possible origins of the Washington, DC, Ae. aegypti population by SNPs and microsatellite markers. Our sequence data for representative haplotypes identified at each location were submitted to GenBank under accession numbers MF371160–MF371174 and MG241351–MG241354. Both Washington, DC, haplotypes (A and B) were shared with Key West, Miami, and Palm Beach samples from Florida, but only haplotype A was present in the Columbus, GA; Siquirres, Costa Rica; and Tapachula, Mexico samples. None of the remaining seven haplotypes recovered (B–H) were represented at each of the six sample locations. Haplotype G was only found in Siquirres, Costa Rica, and Haplotype H only in Tapachula, Mexico. Phylogenetic comparisons with representative haplotype sequences of a larger group of mtCOI haplotypes from other geographical regions reported in Lima et al.2 are consistent with those results (Supplemental Figure 4).
Table 2.
Aedes aegypti mtDNA COI haplotypes present in Washington, DC, and various localities considered as potential sources
| A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|
| Washington, DC† | Washington, DC† | ||||||
| Miami, FL (2) | Miami, FL (1) | Miami, FL (7) | |||||
| Palm Beach, FL (3) | Palm Beach, FL (4) | Palm Beach, FL (1) | Palm Beach, FL (1) | Palm Beach, FL (1) | |||
| Key West, FL (4) | Key West, FL (4) | Key West, FL (2) | |||||
| Columbus, GA (2) | Columbus, GA (2) | Columbus, GA (3) | Columbus, GA (3) | ||||
| Siquirres, CR (7) | Siquirres, CR (2) | ||||||
| Tapachula, MEX (1) | Tapachula, MEX (6) | Tapachula, MEX (2) |
Haplotypes A–H were identified based on a 497 bp amplicon of the mitochondrial cytochrome oxidase I gene. The numbers in parentheses indicate the number of individuals with that haplotype observed per location.
Previously identified.2
Results summary.
Microsatellite and SNP data points to Florida, southern Mexico (Tapachula), or Costa Rica, as the likely source of the Washington, DC, population (Figures 1 and 2). Florida is the stronger candidate based on genetic assignment tests using SNPs and on complementary analysis of mitochondrial haplotypes. Both of the two mtDNA haplotypes present in Washington, DC, are found in a number of Florida populations (Table 2), whereas only one of them is shared with Georgia, south Mexico, or Costa Rica.
DISCUSSION
As summarized previously, our results from nuclear genetic markers and mtDNA consistently point to Florida as the most likely source of the Capitol Hill, Washington, DC, overwintering population of Ae. aegypti. Given the geographic locations and transportation connections, this is not surprising. Washington has two major airports, one ∼50 km from its center (Dulles) and one in the downtown area (Reagan) close to the Ae. aegypti population. Miami has 12 daily nonstop commercial flights to Reagan airport. In addition, a busy interstate highway (US1/I-95) originates in Key West and is a major shipping corridor along the East Coast of the US, passing through Washington. Washington and Miami are also directly connected by railroad with both passenger and freight traffic.
From a health perspective, it is relevant that in 2009, Key West, FL, was the location of the first transmitted cases of dengue fever in the US for > 60 years.50 The first time that chikungunya was reported transmitted in the US was in Florida in 2014 (ArboNET-CDC51). Likewise, in 2016, Miami Dade County, FL, was the first US mainland locality to report Zika virus transmission.52 This indicates that Florida populations of Ae. aegypti are capable of transmitting dengue, chikungunya, and Zika viruses in modern US urban environments. Washington, being an international center for visitors from around the world, routinely reports imported cases of Ae. aegypti-borne diseases. Since 2015, there have been 43 reported cases of imported Zika infections and 15 cases of dengue in Washington, DC, to date (CDC51 and USGS53 disease maps).
Lima et al.2 suggest that cryptic underground habitats, such as storm drains or tunnels, are the likely site of overwintering by the Capitol Hill Ae. aegypti population. Ae. aegypti using storm drains as a larval site has been documented elsewhere in Brazil,54 Mexico,55 California,56 and Arizona (K. Smith, personal communication; 2017). They have also been detected in septic tanks in Puerto Rico57 and Trinidad,58 a distinct subterranean larval site. However, all these subterranean breeding sites are in tropical or subtropical areas that also have year-round surface populations of this mosquito. The Washington, DC, population we studied is unique in being well-outside the normal year-round breeding range of Ae. aegypti.
Adult Ae. aegypti eclosing from septic tanks in Puerto Rico were found to be larger than those from surface larval habitats59 but septic tank populations are genetically indistinguishable from nearby surface populations.60 Thus, there is no evidence that subterranean breeding in Ae. aegypti has a genetic basis.
Is the establishment in Washington, DC, a harbinger of further expansion of the distribution of Ae. aegypti to higher latitudes? Will subterranean habitats become common overwintering sites? The epidemiological implications are immense. Should the northern distribution limits become today’s 6°C minimum isotherm, the additional number of human populations at risk for aegypti-borne diseases would expand greatly, most of which have never experienced these diseases and are thus immunologically naive.
Finally, we point out the importance of the publicly available genetic databases of the sort we used in this study. The microsatellite database for Ae. aegypti now comprises genotypes for ∼7,600 mosquitoes from 190 population samples taken in 40 countries in six continents; the SNP chip database has now ∼2,000 genotyped mosquitoes from over 95 populations in 31 countries in six continents (VectorBase48,49). Such databases are especially important for tracing new introductions, which, history tells us, are inevitable with mosquitoes such as Ae. aegypti. Knowing the origin allows inference of the mode of transportation of the introduction, assessment of the health threat the introduction poses (e.g., did it come from a region where it is actively transmitting a disease?), and may guide efforts to control the population (e.g., did it come from a region known to have resistance to certain insecticides?).
Supplementary Material
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.Christophers SR, 1960. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure. New York, NY: Cambridge University Press. [Google Scholar]
- 2.Lima A, Lovin DD, Hickner PV, Severson DW, 2015. Evidence for an overwintering population of Aedes aegypti in Capitol Hill neighborhood, Washington, DC. Am J Trop Med Hyg 94: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson KD, 1992. Yellow fever epidemics and mortality in the United States, 1693–1905. Soc Sci Med 34: 855–865. [DOI] [PubMed] [Google Scholar]
- 4.Tabachnick WJ, Wallis GP, Aitken THG, Miller BR, Amato GD, Lorenz L, Powell JR, Beaty BJ, 1985. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg 34: 1219–1224. [DOI] [PubMed] [Google Scholar]
- 5.Tesh RB, Gubler DJ, Rosen L, 1976. Variation among goegraphic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg 25: 326–335. [DOI] [PubMed] [Google Scholar]
- 6.Bennett KE, Olson KE, Munoz M de L, Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black WC, 4th, Beaty BJ, 2002. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg 67: 85–92. [DOI] [PubMed] [Google Scholar]
- 7.Failloux A-B, Vazeille M, Rodhain F, 2002. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J Mol Evol 55: 653–663. [DOI] [PubMed] [Google Scholar]
- 8.Gloria-Soria A, Armstrong PM, Powell JR, Turner PE, 2017. Infection rate of Aedes aegypti mosquitoes with dengue virus depends on the interaction between temperature and mosquito genotype. Proc R Soc B Biol Sci 284: 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, LourenÁo-de-Oliveira R, Failloux A-B, 2016. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis 10: e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge J-M, Lourenco-De-Oliveira R, Caro V, Lambrechts L, Failloux A-B, 2014. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc R Soc London B Biol Sci 281: 1792. 10.1098/rspb.2014.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deming R, Manrique-Saide P, Barreiro AM, Cardeña EUK, Che-Mendoza A, Jones B, Liebman K, Vizcaino L, Vazquez-Prokopec G, Lenhart A, 2016. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasit Vectors 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins AJ, Lima JBP, Peixoto AA, Valle D, 2009. Frequency of Val1016Ile mutation in the voltage‐gated sodium channel gene of Aedes aegypti Brazilian populations. Trop Med Int Health 14: 1351–1355. [DOI] [PubMed] [Google Scholar]
- 13.Linss JGB, Brito LP, Garcia GA, Araki AS, Bruno RV, Lima JP, Valle D, Martins AJ, 2014. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit Vectors 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell JR, Tabachnick WJ, Arnold J, 1980. Genetics and the origin of a vector population: Aedes aegypti, a case study. Science 208: 1385–1387. [DOI] [PubMed] [Google Scholar]
- 15.Gloria-Soria A, et al. 2016. Global genetic diversity of Aedes aegypti. Mol Ecol 25: 5377–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JE, et al. 2011. Worldwide patterns of genetic differentiation imply multiple “domestications” of Aedes aegypti, a major vector of human diseases. Proc Biol Sci 278: 2446–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloria-Soria A, Kellner DA, Brown JE, Gonzalez-Acosta C, Kamgang B, Lutwama J, Powell JR, 2016. Temporal genetic stability of Stegomyia aegypti (= Aedes aegypti) populations. Med Vet Entomol 30: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JE, Scholte E-J, Dik M, Den Hartog W, Beeuwkes J, Powell JR, 2011. Aedes aegypti mosquitoes imported into the Netherlands, 2010. Emerg Infect Dis 17: 2335–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloria-Soria A, Brown JE, Kramer V, Hardstone Yoshimizu M, Powell JR, 2014. Origin of the dengue fever mosquito, Aedes aegypti, in California. PLoS Negl Trop Dis 8: e3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans BR, Gloria-Soria A, Hou L, McBride C, Bonizzoni M, Zhao H, Powell JRA, 2015. Multipurpose, high-throughput single-nucleotide polymorphism chip for the dengue and yellow fever mosquito, Aedes aegypti. G3 (Bethesda) 5: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotsakiozi P, Gloria-Soria A, Caccone A, Evans B, Schama R, Martins AJ, Powell JR, 2017. Tracking the return of Aedes aegypti to Brazil, the major vector of the dengue, chikungunya and Zika viruses. PLoS Negl Trop Dis 11: e0005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saarman NP, Gloria‐Soria A, Anderson EC, Evans BR, Pless E, Cosme L V, Gonzalez‐Acosta C, Kamgang B, Wesson DM, Powell JR. Effective population sizes of a major vector of human diseases, Aedes aegypti. Evol Appl 10: 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pless E, Gloria-Soria A, Evans BR, Kramer V, Bolling BG, Tabachnick WJ, Powell JR, 2017. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLoS Negl Trop Dis 11: e0005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ, 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinowski ST, 2005. hp-rare 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5: 187–189. [Google Scholar]
- 26.Peakall ROD, Smouse PE, 2006. genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard JK, Wen W, Falush D, 2003. Documentation for structure software: version 2. Available at: https://web.standford.edu/pritchardlab/structure/release_versions/v2.3.4/structure_doc.pdf.
- 28.Pritchard JK, Stephens M, Donnelly P, 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evanno G, Regnaut S, Goudet J, 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 30.Earl DA, 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4: 359–361. [Google Scholar]
- 31.Rosenberg NA, 2004. DISTRUCT: a program for the graphical display of population structure. Mol Ecol Resour 4: 137–138. [Google Scholar]
- 32.Alexander DH, Lange K, 2011. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics 12: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puechmaille SJ, 2016. The program structure does not reliably recover the correct population structure when sampling is uneven: subsampling and new estimators alleviate the problem. Mol Ecol Resour 16: 608–627. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, 2017. The computer program STRUCTURE for assigning individuals to populations: easy to use but easier to misuse. Mol Ecol Resour 17: 981–990. [DOI] [PubMed] [Google Scholar]
- 35.Nazareno AG, Bemmels JB, Dick CW, Lohmann LG, 2017. Minimum sample sizes for population genomics: an empirical study from an Amazonian plant species. Mol Ecol Resour 17: 1136–1147. [DOI] [PubMed] [Google Scholar]
- 36.Willing EVA, Bentzen P, Van Oosterhout C, Hoffmann M, Cable J, Breden F, Weigel D, Dreyer C, 2010. Genome‐wide single nucleotide polymorphisms reveal population history and adaptive divergence in wild guppies. Mol Ecol 19: 968–984. [DOI] [PubMed] [Google Scholar]
- 37.Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A, 2004. GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95: 536–539. [DOI] [PubMed] [Google Scholar]
- 38.Rannala B, Mountain JL, 1997. Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94: 9197–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nei M, 1973. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70: 3321–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jombart T, 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team , 2017. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.r-project.org/. Accessed October 2017.
- 42.Frichot E, François O, 2015. LEA: an R package for landscape and ecological association studies. Methods Ecol Evol 6: 925–929. [Google Scholar]
- 43.Stamatakis A, 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vrijenhoek R, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3: 294–299. [PubMed] [Google Scholar]
- 45.Sievers F, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol Syst Biol 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R, 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res 38 (Web Server issue): W695–W699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Stecher G, Tamura K, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giraldo-Calderón GI, Emrich SJ, MacCallum RM, Maslen G, Dialynas E, Topalis P, Ho N, Gesing S, Madey G, Collins FH, 2014. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res 43: D707–D713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VectorBase National Institute of Allergy and Infectious Diseases (NIAID) Bioinformatics Resource Center (BRC). Available at: https://www.vectorbase.org/. Accessed October 2017.
- 50.Radke EG, et al. 2012. Dengue outbreak in Key West, Florida, USA, 2009. Emerg Infect Dis 18: 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CDC , 2017. Centers for Disease Control and Prevention (United States Government) Available at: https://www.cdc.gov/. Accessed July 26, 2017.
- 52.Beach M, River L, 2017. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 546: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Disease Maps Dynamic Map Viewer , 2015. United States Geological Survey (USGS). U.S. Department of Interior. Available at: https://diseasemaps.usgs.gov/. Accessed July 26, 2017.
- 54.Paploski IAD, Rodrigues MS, Mugabe VA, Kikuti M, Tavares AS, Reis MG, Kitron U, Ribeiro GS, 2016. Storm drains as larval development and adult resting sites for Aedes aegypti and Aedes albopictus in Salvador, Brazil. Parasit Vectors 9: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manrique-Saide P, Uc V, Prado C, Carmona C, Vadillo J, Chan R, Dzib-Florez S, Che-Mendoza A, Barrera-Perez M, Sanchez EC, 2012. Storm sewers as larval habitats for Aedes aegypti and Culex spp. in a neighborhood of Merida, Mexico. J Am Mosq Control Assoc 28: 255–257. [DOI] [PubMed] [Google Scholar]
- 56.Metzger ME, Hardstone Yoshimizu M, Padgett KA, Hu R, Kramer VL, 2017. Detection and establishment of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes in California, 2011–2015. J Med Entomol 54: 533–543. [DOI] [PubMed] [Google Scholar]
- 57.Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y, 2008. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol 22: 62–69. [DOI] [PubMed] [Google Scholar]
- 58.Chadee DD, Martinez R, 2016. Aedes aegypti (L.) in Latin American and Caribbean region: with growing evidence for vector adaptation to climate change? Acta Trop 156: 137–143. [DOI] [PubMed] [Google Scholar]
- 59.Mackay AJ, Amador M, Diaz A, Smith J, Barrera R, 2009. Dynamics of Aedes aegypti and Culex quinquefasciatus in septic tanks. J Am Mosq Control Assoc 25: 409–416. [DOI] [PubMed] [Google Scholar]
- 60.Somers G, Brown JE, Barrera R, Powell JR, 2011. Genetics and morphology of Aedes aegypti (Diptera: Culicidae) in septic tanks in Puerto Rico. J Med Entomol 48: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microsatellite and SNP genotypes were deposited in VectorBase48,49 PopBio projects: VBP0000201 (new data), and VBP0000138, VBP0000176-177 (previously published data). Sequencing data were deposited in NCBI under accession numbers MF371160–MF371174 and MG241351–MG241354.