Abstract
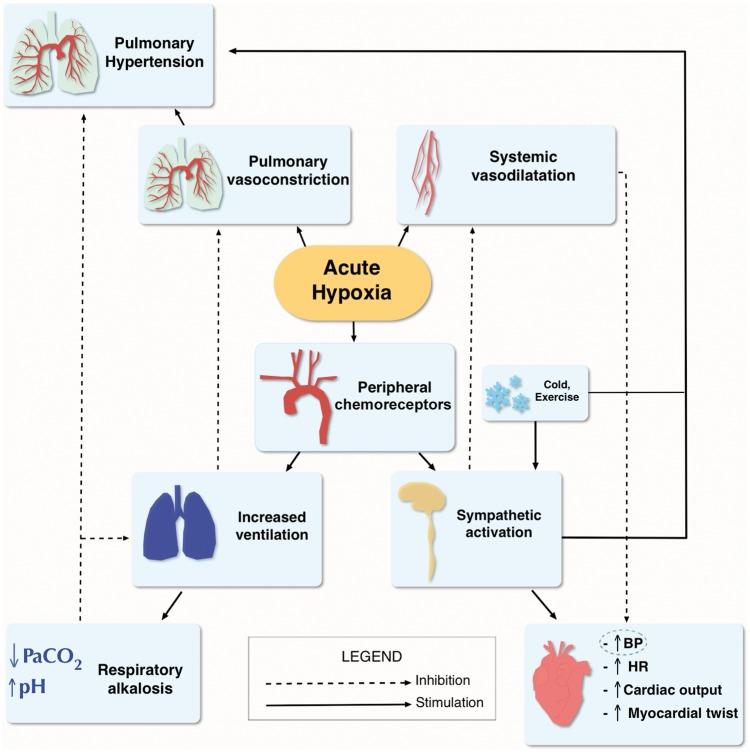
Take home figure.
Adapted from Bärtsch and Gibbs2 Physiological response to hypoxia. Life-sustaining oxygen delivery, in spite of a reduction in the partial pressure of inhaled oxygen between 25% and 60% (respectively at 2500 m and 8000 m), is ensured by an increase in pulmonary ventilation, an increase in cardiac output by increasing heart rate, changes in vascular tone, as well as an increase in haemoglobin concentration. BP, blood pressure; HR, heart rate; PaCO2, partial pressure of arterial carbon dioxide.
Introduction
The travelling options currently available allow an increasingly large number of individuals, including sedentary people, the elderly and diseased patients, to reach high altitude (HA) locations, defined as locations higher than 2500 m above sea level (asl),1S i.e. the altitude above which many of the physiological responses that represent challenges for the human body start developing. Physiological acclimatization mechanisms impose an increased workload on the cardiovascular system, but the actual risk of adverse cardiovascular events associated with HA exposure is still a debated issue.1–4
The aim of this article is to review the available evidence on the effects of HA in cardiovascular patients and to address their risk of developing clinically relevant events. This was done through multiple Medline searches on the PubMed database, with the main aim of promoting a generally safe access to mountains. Searched terms included a combination of either ‘high altitude’ or ‘hypobaric hypoxia’ plus each of the following: ‘physiology’, ‘maladaption’, ‘cardiovascular response’, ‘systemic hypertension’, ‘pulmonary hypertension’, ‘ischaemic heart disease’, ‘cardiac revascularisation’, ‘heart failure’, ‘congenital heart disease’, ‘arrhythmias’, ‘implantable cardiac devices’, ‘stroke’, ‘cerebral haemorrhage’, ‘exercise’, ‘sleep apnea’. Compared with a previous review article on this topic,2S we now include the most recent data on hypoxia-induced changes in left ventricular (LV) systolic and diastolic function, lung function and ventilation control, blood coagulation, and on the effects of pharmacological interventions. We also offer an update on the clinical and pathophysiological findings related to the exposure to altitude of patients with pre-existing cardiovascular conditions (ischaemic heart disease, heart failure, and arterial and pulmonary hypertension).
Physics and cardiovascular physiology at high altitude
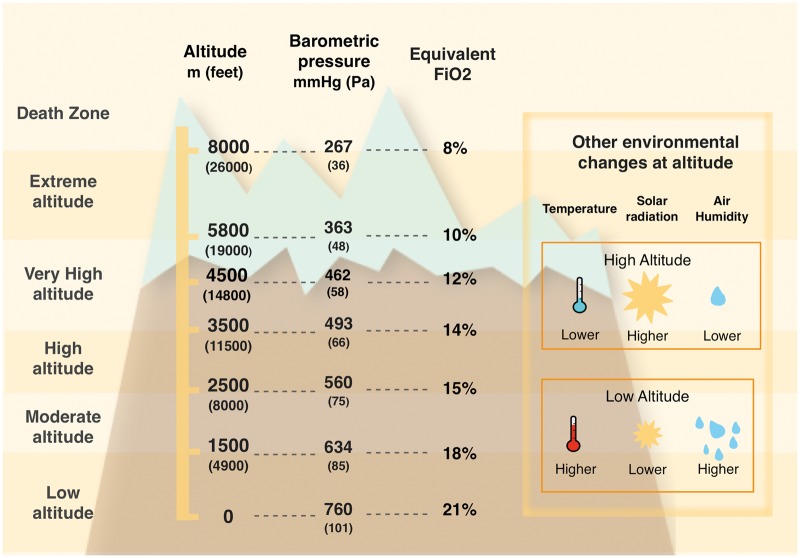
With increasing altitude, a progressive reduction in barometric pressure, air temperature and air humidity can be observed (Figure1).1S For the purpose of this article, we refer to Imray et al.’s1S classification of altitude ranges.
Figure 1.
Altitude classification (Imray et al.1S) (left column); corresponding barometric pressure and fraction in inspired oxygen for different simulated altitudes in a laboratory setting, according to the 1976 US standard Atmosphere by NASA.5S (central two columns); relationship between altitude2S and environmental characteristics (temperature, humidity, and solar radiation) (box on the right-hand side). We used the 1976 US standard atmosphere model by NASA to estimate barometric pressure at a given altitude, because the former is a function not only of altitude but also of latitude. For similar altitudes, barometric pressure (and consequently also partial pressure of arterial oxygen) is higher the closer we are to the equator line.
Barometric pressure directly determines the inspired oxygen (O2) partial pressure and, in combination with alveolar ventilation, sets the alveolar O2 partial pressure. Its reduction leads to a condition known as ‘hypobaric hypoxia’. In practice, at sea level, O2 constitutes 20.94% of total gas molecules in inspired air, which with a normal rate of alveolar ventilation leads to an alveolar partial O2 pressure of roughly 100 mmHg for a barometric pressure of roughly 760 mmHg.5S When breathing at 3000 m altitude asl, the same percentage of O2 in the inspired air, combined with a lower barometric pressure and higher rate of ventilation, results in an alveolar partial O2 pressure of roughly 67 mmHg, corresponding to what would occur breathing a hypoxic air mixture (fraction of inspired O2 0.14) at sea level (Figure1).5S A series of physiological responses help to maintain adequate tissue O2 delivery and supply at HA, through a process called ‘acclimatization’. Its efficacy depends on the duration of individual’s exposure to altitude, age, sea level partial pressure of oxygen in arterial blood (PaO2) and minute ventilation.2,3,4S,5S These crucial processes include increase in ventilation, cardiac output, red cell mass and blood O2 carrying capacity, and other metabolic modifications at the microvascular and cellular levels (Take home figure). Some of these mechanisms are activated almost immediately, whereas others need hours to days to attain full expression.2,4–6 A more extensive description of the effects of HA exposure on cardiovascular physiology is provided in the seminal papers by Bärtsch et al.2
Take home figure.
Adapted from Bärtsch and Gibbs2 Physiological response to hypoxia. Life-sustaining oxygen delivery, in spite of a reduction in the partial pressure of inhaled oxygen between 25% and 60% (respectively at 2500 m and 8000 m), is ensured by an increase in pulmonary ventilation, an increase in cardiac output by increasing heart rate, changes in vascular tone, as well as an increase in haemoglobin concentration. BP, blood pressure; HR, heart rate; PaCO2, partial pressure of arterial carbon dioxide.
Systemic blood pressure and heart rate
Acute exposure to hypoxia produces endothelium-dependent and endothelium-independent systemic vasodilation,6S–9S which may initially induce some degree of blood pressure (BP) reduction. After a few hours, this is counter-balanced, however, by a generalized altitude-dependent increase in sympathetically mediated vasoconstriction, caused primarily by arterial hypoxaemia through afferent signalling to the cardiovascular control regions of the mid-brain via the arterial peripheral chemoreceptors located in the carotid bodies.5,6,10S As a result, a significant and persistent arterial BP increase occurs shortly after the arrival at HA, proportional to the altitude reached and more evident at night.7 This leads to a reduced degree of the physiological blood pressure fall during sleep,8 which persists at least over the first 7 days of altitude exposure.9 This is accompanied by an increase in heart rate (HR) both at rest and during exercise,10,11,11S although maximal HR achieved during exercise at HA is lower compared to sea level (see Supplementary material online, Table S1).2,11–14
Ventilation
With acute exposure to altitude, the decrease in PaO2 stimulates peripheral chemoreceptors in the carotid bodies leading to sympathetic activation and to an increase in minute ventilation.2,14 Moreover, mild interstitial lung fluid accumulation15 can occur in physically active persons and decreases alveolar diffusing capacity.14 Hypoxia-induced hyperventilation leads to hypocapnia and respiratory alkalosis, which blunts the initial full hypoxic ventilatory response. The combination of hyperventilation and resulting hypocapnia, with increased peripheral chemosensitivity and abnormal loop gain in chemoreflex-induced respiratory regulation7S may lead to the appearance of nocturnal periodic breathing (PB). This is an abnormal ventilatory pattern, characterized by periods of central apnoea or hypopnoea alternating with periods of hyperventilation, mainly occurring during sleep.10,16,17,13S,18
With exposure over days to weeks, the sensitivity of the peripheral chemoreceptors to hypoxia increases, resulting in a further increase in sympathetic activity and enhancement of ventilation, despite the progressive increase in arterial blood O2 content and lower partial pressure of arterial carbon dioxide (PaCO2) (‘ventilatory acclimatization’, see Supplementary material online, Table S1).19,14S
Pulmonary arterial pressure
Alveolar hypoxia and arterial hypoxaemia (to a lesser degree) induce vasoconstriction in the pulmonary circulation, either directly and through sympathetic activation,14,18,19,14S,15S resulting in increased pulmonary vascular resistance and pulmonary artery pressure (hypoxic pulmonary vasoconstriction).14S Hypoxic pulmonary vasoconstriction is a protective mechanism during regional alveolar hypoxia (e.g. pneumonia) to shift blood flow to better ventilated lung regions, but at HA, where the hypoxic stimulus is ubiquitous throughout the lungs, global alveolar hypoxia leads to general pulmonary hypertension with the risk of pulmonary oedema or right ventricular failure in the extreme cases (see Supplementary material online, Table S1).16S We found only one study investigating pre-capillary pulmonary hypertension.17S Although the sample size was small (n = 14) and the exposure to simulated HA was short (at rest and after 20 min of mild exertion), non-invasive measures of the right heart function demonstrated a predictable rise in pulmonary arterial systolic pressure, not associated with a deterioration in the right heart function.
Left ventricular function
The left ventricle undergoes significant changes when exposed to HA.11,20,21,17S In particular, a reduction in both diastolic and systolic LV volumes and geometrical alterations (increase in the sphericity index) occur, the diastolic function worsens and the LV contractility and LV apex twist increase, the last change being similar to what is observed in cases of subendocardial LV fibre dysfunction.20 Moreover, after 2 weeks of exposure to very HA, LV mass decreases disproportionately when compared with the concomitant reduction in total body weight (11% vs. 3% reduction for LV mass vs. body mass, adjusted for body surface area, P < 0.05).21 Lung impairment via cardiopulmonary interaction (with arterial hypoxaemia made worse due to mild interstitial oedema), especially during the first 2 weeks, and the increased LV inotropic stimulation by an increased sympathetic activity are considered to play a role in the development of these changes, but probably they cannot completely explain the extent of the observed findings.
Recent evidence suggests that hypoxia itself can be the basis for LV alterations. 31P magnetic resonance spectroscopy performed before and after ascent to Mt. Everest in healthy individuals revealed a decrease in the cardiac creatine phosphate/adenosine triphosphate (PCr/ATP) ratio by 18% (P < 0.01), similarly to what is observed in patients with diseases associated with chronic hypoxia.21 All these reductions returned to pre-trek levels 6 months after return to the sea level. The authors concluded that a decrease in energy reserve may be a ‘universal response to periods of sustained low O2 availability, underlying hypoxia-induced cardiac dysfunction both in the healthy human heart and in patients with cardiopulmonary diseases’.
Other effects
Mild dehydration and a hypoxic-mediated diuresis were found to lead to an acute increase in haematocrit and haemoglobin concentration in the first several days at HA,18S after which the renal production of erythropoietin stimulates new red blood cell production to increase red cell mass, with a further rise in haemoglobin concentration. In the acute exposure phase, this can be associated with increased blood coagulability.22 Nevertheless, an increased thrombotic risk at HA has never been convincingly demonstrated, and the limited available data are conflicting with respect to a hypothetical prothrombotic state.22,23
Heart failure
Heart failure (HF) is often associated with co-morbidities, such as pulmonary hypertension, chronic obstructive pulmonary disease, chronic kidney disease, cardiac ischaemia, anaemia, and thrombophilia. This condition is also characterized by an increased chemosensitivity.20S All these conditions are likely to make HF patients more vulnerable to the HA environment. In spite of this, brief simulated HA exposure was found to be safe for HF patients,24 even when performing mild physical exercise. Agostoni et al.24 evaluated 38 patients with severe stable HF [New York Heart Association (NYHA) Class III–IV] undergoing cardiopulmonary exercise testing with a progressive reduction in inspired O2 from 21% (sea level) to 18%, 16%, and 14% (the last simulating 3000 m altitude). No episodes of angina, arrhythmias, or electrocardiographic (ECG) evidence of ischaemia occurred at any simulated altitude. The reduction in maximum work rate achieved at simulated altitude was progressively greater the more severe the exercise limitation at sea level. Finally, patients who showed the largest increase in the lung diffusion capacity for CO (DLCO) at sea level during moderate exercise were those who showed the lowest exercise capacity reduction at simulated altitude,20S linking HA exercise performance to gas exchange adaptability.
Schmid et al.25 evaluated stable HF patients (NYHA Class II) exercising during a short exposure to HA (3454 m, Jungfraujoch, Switzerland) and at sea level. During HA exercise, mean peak VO2 decreased by 22%, without causing arrhythmias or altering echocardiographic variables, with the exception of an increase in pulmonary artery systolic pressure (PAPs). Drug therapy of HF may also interfere with HA adaptation mechanisms. Critical HF drugs such as beta-blockers and angiotensin-converting enzyme (ACE) inhibitors act on the chemoreceptors and on haemodynamic responses through adrenergic receptors, which are also involved in the alveolar–capillary gas diffusion control (β2-receptors). Despite their importance in the care of patients with HF which is incontrovertible at sea level, ACE inhibitors and angiotensin receptor blockers (ARBs) do blunt the kidney’s ability to produce erythropoietin and could limit the compensatory rise in haematocrit and blood O2-carrying capacity that is important at HA.21S,22S HIGHCARE (HIGH altitude CArdiovascular REsearch) investigators reported that healthy subjects receiving the non-cardioselective β2 antagonist carvedilol reached lower peak exercise ventilation and lower VO2 peak at HA than those receiving the selective β1 receptor blocker nebivolol.13 Carvedilol also reduced hyperventilation during exercise, possibly by reducing peripheral chemoreceptor sensitivity. This effect may be favourable in normoxia, because the fall in arterial O2 saturation is quite small at sea level, but can be much greater and unfavourable at HA.20S,23S Moreover, administration of diuretics should be based on the balanced evaluation of signs of early dehydration or fluid gain. Among the diuretics, acetazolamide, a carbonic anhydrase inhibitor with mild diuretic effect, which is frequently used for mountain sickness prophylaxis and treatment, should be specifically considered.24S It should be emphasized that the concomitant administration of acetazolamide and other diuretics may increase the risk of dehydration and electrolyte imbalances at HA and should thus be carefully evaluated.
A final issue is related to periodic breathing. It can be present in HF patients at sea level, both during sleep and exercise, but likely it will worsen at HA.12S,25S,26 Whether it should be suppressed with acetazolamide remains an issue yet to be clarified.
Very little is known about the effects of HA in patients who underwent heart transplant. Living at moderate altitude seems not to be harmful, but no data are available on the effects of acute HA exposure.26S Recommendations for HF patients going to be exposed to HA are summarized in Table 1.
Table 1.
Recommendations for heart failure patients going to high altitude
| HF severity level | Recommendations | Class of evidence | Level of evidence | References |
|---|---|---|---|---|
| All HF patients | Carefully evaluate HF co-morbidities (e.g. pulmonary hypertension, anaemia, sleep apnoea) | I | C | |
| Carefully evaluate HF drugs (in particular diuretics, potassium supplementation, and β blockers). Whenever possible, β1 selective should be preferred to non-selective beta-blockers | I | B | 10,13,20S | |
| Slow ascent is recommended. Although we do not have precise data on advisable ascent rate, it is prudent not to exceed that recommended for healthy travellers (300–500 m/day when above 2500 m) | I | C | ||
| Stable NYHA I-II patients | May safely reach high altitude up to 3500 m | IIa | C | 25 |
| Once at altitude, not heavier than moderate physical activity is recommended | IIa | C | 25 | |
| Stable NYHA III patients | May safely reach high altitude up to 3000 m, if needed | IIa | C | 24 |
| Once at altitude, not heavier than light physical activity is recommended | IIa | C | 23,24 | |
| Unstable/NYHA IV patients | Avoid high altitude exposure | I | C |
The strength of these recommendations is to be weighted in the light of the limited evidence available.
HF, heart failure; NYHA, New York Heart Association.
Ischaemic heart disease
It has been suggested that living permanently at moderate altitude might be beneficial, reducing CV mortality.27S Acute exposure to HA, on the other hand, may represent a more challenging condition for the cardiovascular system. Given that total O2 demand is constant for a given workload and that myocardial O2 extraction is already very high at sea level, with acute HA exposure cardiac output must increase to maintain O2 delivery despite the reduced blood arterial O2 content.
In healthy subjects, epicardial coronary blood flow increases due to vasodilation during acute HA exposure and thus there may not be any significant impairment during exercise of coronary flow reserve, at least up to 4500 m.27 The actual risk of cardiac ischaemia associated with HA is indeed unclear. On the one hand, stress testing performed in healthy subjects above a simulated altitude of 8000 m27S did not induce ECG alterations; on the other hand, changes in arterial wall properties may reduce coronary O2 supply in diastole at HA, with possible clinical implications in subjects with silent coronary plaques. Evidence in this regard has been obtained through calculation of the subendocardial viability ratio (SEVR), defined as the ratio between diastolic pressure–time index (DPTI, an estimate of myocardial O2 supply based on both coronary artery driving pressure in diastole and diastolic time) and systolic pressure–time index (SPTI, an estimate of myocardial O2 consumption in systole). Subendocardial viability ratio, therefore, indirectly estimates the degree of myocardial perfusion and was found to be significantly reduced at 4559 m in healthy volunteers (from 1.63 ± 0.15 to 1.18 ± 0.17; P < 0.001).28 The administration of acetazolamide was associated with a smaller degree of SEVR reduction under hypobaric hypoxia exposure and with faster recovery after residing for 3 days at HA, suggesting that acetazolamide may offset the reduction in subendocardial O2 supply in these conditions.28 Similarly, at HA, also the O2 supply/demand ratio (SEVR-CaO2, i.e. SEVR corrected for the arterial O2 content) displayed significant reductions, which were more pronounced with placebo (from 29.6 ± 4.0 to 17.3 ± 3.0; P < 0.001), than with acetazolamide (from 32.1 ± 7.0 to 22.3 ± 4.6; P < 0.001), indicating a smaller reduction in subendocardial O2 supply/demand ratio with acetazolamide administration. The clinical relevance of a reduced SEVR at HA in patients with an increased cardiovascular risk is suggested by a case report of a middle-aged mild hypertensive subject with normal cardiopulmonary exercise testing (CPET) at sea level, in whom acute exposure to an altitude of 3340 m was responsible not only for a marked reduction in SEVR and in SEVR-CaO2 but also for the development of angina and ischaemic ECG changes when cardiopulmonary stress testing was repeated at HA. This occurred 2 days after a negative stress test at sea level, the only difference being the acute exposure to HA.29
Patients with coronary artery disease (CAD) may face more difficulties with HA exposure, because of the already increased basal coronary flow at sea level, impairment in arterial elastic properties induced by atheromatous lesions and microvascular dysfunction. However, there is little evidence available about HA-induced ischaemia in CAD patients. Schmid et al.30 did not find signs of ischaemia during CPET performed at 3454 m in 22 low-risk ischaemic patients with a normal CPET at sea level. The HA evaluation was performed 6–18 months after revascularization, either by percutaneous stenting or by surgical coronary artery bypass grafting (CABG), and after wash-out from beta-blockers. The same conclusion was reached by De Vries et al.12,31, who evaluated 8 low-risk patients with a history of acute myocardial infarction (MI) and 7 controls during the Dutch Heart Expedition 2007 to Aconcagua (4200 m). Furthermore, Messerli-Burgy et al.5 did not find any increased risk for cardiac arrhythmias in post-MI patients at 3546 m.
Heterogeneous definition of ‘low-risk’ individuals, limited information about ongoing treatment, previous performance of any revascularization procedure and time since acute MI occurrence reduce the ability to generalize these findings to a wider population.
Evidence for ‘medium-risk’ patients is even more limited. One study examined the simulated altitude effects on coronary flow in 8 medium-risk patients undergoing stress testing at a simulated altitude of 2500 m. The results showed an inability of their coronary blood flow to adapt after an increase in demand.30
Percutaneous revascularization
Only one study and a couple of case reports are available on this topic. In the above-mentioned study by Schmid etal.30, among the 22 low-risk, revascularized ischaemic patients, 15 had ST-elevation MI and 7 non-ST-elevation MI, undergoing CPET at HA. Among these patients, 15 had undergone percutaneous revascularization, and none showed signs of ischaemia during altitude exercise.30 Moreover, Wu et al.32 described the case of a 49-year-old male engineer with a history of CAD treated with 2 drug-eluting stents who, 2 years later, went to work on the Qinghai-Tibet railway (4905 m) for 10 days. ECG, echocardiography, and polysomnography did not show any signs of ischaemia or arrhythmia on multiple trips over 3 years. On the other hand, Basavarajaiah and O’Sullivan33 reported 2 cases of very late stent thrombosis in patients undergoing intense physical exertion at moderate altitude (3000 m and 1300 m, 16 and 48 months after drug-eluting stent implantation, respectively). However, no evidence supports a causal link between HA and stent thrombosis in these patients. In contrast, intense physical activity has been associated with late stent thrombosis at sea level.29S
Coronary artery bypass grafting
In the aforementioned study by Schmidt et al.,30 7 patients underwent CABG. A rapid ascent to HA (3454 m) and submaximal exercise were found to be safe 6 months after CABG.30S
Recommendations for ischaemic heart disease patients are reported in Table 2 and Supplementary material online, Table S2.
Table 2.
Recommendation for ischaemic patients ascending to high altitude
| Patient risk class | Recommendations | Class of evidence | Level of evidence | References |
|---|---|---|---|---|
| General recommendations for all cardiovascular patients | Patients should continue pre-existing medications at HA. All therapy changes, especially dual anti-antiplatelet therapy after drug-eluting stent implantation, must be discussed with a doctor before enacting. Individuals who do not engage in physical exertion at low altitude should not engage in physical activity at HA. | I | C | |
| Acetazolamide administration seems to reduce the risk of subendocardial ischaemia at HA in healthy subjects, and thus use of acetazolamide for AMS prevention might be helpful. No data are available, however, in patients with CAD. | IIa | C | 28 | |
| After AMI/CABG | Patients should wait at least 6 months after uncomplicated ACS episode as well as after revascularization before HA exposure. | I | C | 5,12,30 |
| After stenting | Patients should wait at least 6–12 months after coronary stenting before HA exposure. | IIa | C | 12,31,33,30S |
| Low risk (CCS 0-I) | May safely ascend to HA, up to 4200 m asl, and practice light-to-moderate physical exertion. | IIa | C | 12,31 |
| Moderate risk CAD (CCS II-III) | May carefully ascend up to 2500 m, but physical exercise heavier than light is contraindicated. | IIa | C | 22 |
| High risk (CCS IV) | Should not ascend to HA. | I | C |
The strength of these recommendations is to be weighted in the light of the limited evidence available.
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; HA, high altitude.
Systemic arterial hypertension
As described above, BP increases after a few hours at HA (especially at night) and remains virtually unchanged over the following days.34 Hypertensive patients may be more susceptible to HA due to an already elevated hypoxic peripheral and central chemoreflex sensitivity30S,32S and to alteration in calcium homeostasis.33S,34S
The HIGHCARE-Himalaya study found that the exposure of healthy volunteers to progressively higher altitudes (up to 5400 m) was associated with a progressive, marked increase in systolic and diastolic ambulatory BP, which was more evident than the corresponding increase in conventional BP measurements.7 This increase became evident a few hours after HA was reached, persisted during the prolonged altitude sojourn (12 days) and was more pronounced in older subjects (Figure 2).
Figure 2.
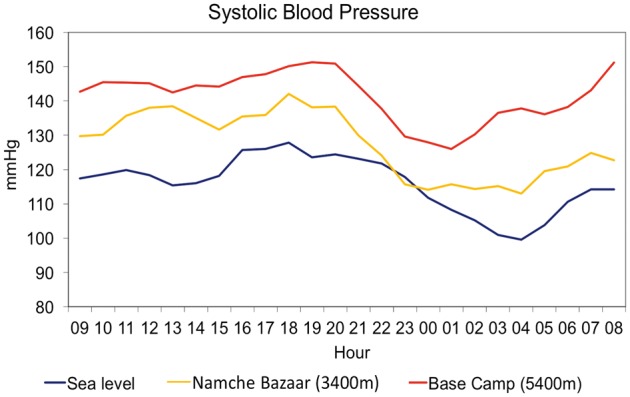
Systolic blood pressure profile for 24 h in a healthy volunteer at different altitudes. Blue line: sea level; yellow line: Namche Bazaar (3400 m); red line: Everest Base Camp 1 (5400 m).
The efficacy of a number of antihypertensive drugs at HA has been tested in volunteers, also through the various studies performed in the frame of the HIGHCARE projects. Non-selective beta-blockade with carvedilol in healthy subjects resulted in a significant reduction in the BP response to HA but was associated with reduced arterial haemoglobin O2 saturation and exercise tolerance. In contrast, a highly selective beta-1 adrenergic receptor blocker (nebivolol), while also effective in reducing the pressor response to HA, preserved the normal nocturnal BP dipping, and was associated with a lesser reduction in exercise tolerance at HA.8 Monotherapy with a long-acting ARB (telmisartan) demonstrated an impressive response on both daytime and night-time BP, but only up to an altitude of 3400 m. During acute exposure to a higher altitude (5400 m), the drug was ineffective, because of the concomitant suppression of the renin–angiotensin system.7 The mechanisms underlying the increase in BP above an altitude of 5000 m are indeed complex in nature, which could explain different responses obtained with different antihypertensive drugs. Interestingly, also acetazolamide was found to antagonize the BP rise induced by HA, as well as the parallel occurrence of central sleep apnoea.9,35
The effects of altitude exposure on hypertensive patients have also been specifically assessed. Wu et al.36 studied Chinese railroad workers and reported a greater increase in blood pressure at HA in hypertensive compared with healthy individuals. This observation was confirmed and expanded in the HIGHCARE-Andes Lowlanders Study,35 where acute exposure of hypertensive patients, permanently living at sea level, to an altitude of 3259 m, induced a further significant increase in 24 h BP above their already elevated pressures observed at sea level, with a mildly steeper rise during night-time than during daytime periods.
When considering the effects of antihypertensive treatment at altitude in patients suffering from high blood pressure included in the HIGHCARE ANDES study, the combination of a dihydropyridine calcium channel blocker and an angiotensin II receptor blocker [nifedipine GITS (Gastro Intestinal Therapeutic System) and telmisartan] was found to be effective in controlling BP at HA in hypertensive patients.35
In a large recent study by Keyes et al.,35S 672 trekkers, 60 with a prior diagnosis of hypertension, underwent conventional blood pressure measurement at rest at altitudes of 2860, 3400 and 4300 m. No significant differences in mean systolic BP (SBP) and diastolic BP (DBP) among altitudes were found either in normotensive or in hypertensive trekkers, probably because of the large interindividual variability in BP levels at different altitudes obtained with the method of BP measurement used in this study. At 3400 m, 60% of normotensive participants had a BP within 10 mmHg of their BP at 2860 m, while in 21% BP increased above and in 19% decreased below this threshold. Conventional BP decreased with altitude exposure in a greater proportion of hypertensive than of normotensives trekkers (36% vs. 21% at 3400 m, P = 0.01 and 30% vs. 15% at 4300 m, P = 0.05). These results should be interpreted on the background of the acknowledged limitations of conventional BP measurements.35S Indeed, conventional BP measurements at rest have been demonstrated to be poorly sensitive to environmental influences, in particular when assessing BP response to altitude.7,8 This limitation is emphasized by considering additional data reported by Keyes et al., obtained through the few good quality 24 h ambulatory BP recordings obtained in a very small subgroup of trekkers (2 hypertensive and 4 normotensive individuals). Severe hypertension was recorded in five participants, asymptomatic, regardless of their conventional BP values. Moreover, an increase in nocturnal SBP and DBP in hypertensive but not in normotensive participants was reported, although the authors did not show their mean 24 h, daytime, night-time SBP and DBP values. This study further emphasizes the limitations carried by focusing only on conventional BP measurements performed at rest, when exploring the environmental effects on BP at altitude.
Recommendations for hypertensive patients are summarized in Table 3.
Table 3.
Clinical and therapeutic recommendations for hypertensive patients planning to go to high altitude
| Patients | Recommendations | Class of evidence | Level of evidence | References |
|---|---|---|---|---|
| Patients with moderate–severe hypertension and hypertensive patients with moderate–high cardiovascular risk | Check BP values before and during HA sojourn. | IIa | B | 7,8,35,36 |
| Well-controlled hypertensive patients/mild hypertensive patients | May reach very HA (>4000 m) with adequate medical therapy. | I | C | 8,35,36 |
| Uncontrolled/severe hypertensive patients | Avoid HA exposure in order to prevent risk of organ damage. | I | C | 36S |
| Therapy | ||||
| Angiotensin II receptor blockade (tested with Telmisartan) lowers BP in healthy subjects up to 3400 m | I | B | 35 | |
| Acetazolamide administration lowers BP at HA while improving SaO2 and mountain sickness symptoms | I | B | 9 | |
| Combination of nifedipine/telmisartan effectively lowers BP in hypertensive patients at an altitude of 3300 m | I | B | 36 | |
| Nebivolol effectively controls HA-induced BP increase and preserves nocturnal BP dipping. Selective beta-1 adrenergic receptors blockade is associated with a lesser impairment of exercise performance when compared with the administration of non-selective beta-blockers | I | C | 13 | |
| When moderate–severe hypertensive patients and hypertensive patients at moderate–high cardiovascular risk plan exposure to HA, adequate modification of their antihypertensive therapy should be considered in co-operation with their physician | IIa | C | ||
The strength of these recommendations is to be weighted in the light of the limited evidence available.
BP, blood pressure; HA, high altitude.
Arrhythmias and implantable devices
Arrhythmias
The increased sympathetic activity5 and decreased SaO2 characterizing HA exposure, in addition to the increase in right ventricular work and in cellular transmembrane shifts of potassium, might be a favourable substrate for arrhythmias. However, to date, an increase in malignant arrhythmias at HA has never been systematically demonstrated. Kujanik et al. compared ECG Holter recordings of healthy volunteers obtained at HA and at sea level. They found increased ventricular and supraventricular ectopy already at mild-moderate altitude (1350 m), but these findings were not correlated with a significant increase in sustained ventricular arrhythmias.37 Gibelli et al. evaluated microvolt T-wave alternans (MTWA) and HR variability in eight healthy trained subjects at sea level and after a mountain climbing expedition (Pakistan, 8150 m), both at rest and during exercise. Despite enhanced sympathetic activity, MTWA testing during exercise at HA was negative in all participants.38 The authors concluded that there is a low risk of dangerous arrhythmias in healthy trained subjects during exercise under hypobaric hypoxia conditions. The possibility, however, of an increased arrhythmic risk in patients with heart diseases favouring arrhythmias, remains an issue yet to be properly addressed in future studies.37S During sleep at HA, bradycardia, bradyarrhythmias and cycling of heart rate with periodic breathing are common findings in young subjects.11S Recommendations are summarized in Supplementary material online, Table S3.
Pacing and implantable cardioverter devices
Little information is available. Brief exposure to a simulated altitude of 4000 m apparently does not affect ventricular stimulation thresholds39 and a recent observational study showed a low implantable cardioverter device (ICD) activation rate (4%) in patients living at altitude.38S Recommendations are summarized in Supplementary material online, Table S3.
Pulmonary hypertension
We found just one very recent study using flight simulation (exposure to a simulated altitude of 1700–2500 m) to determine the need for supplemental O2 during prolonged hypoxia in patients with pulmonary arterial hypertension. In this study, patients with PAH [World Health Organization (WHO) Group 1 with functional class (FC) III symptoms at diagnosis] on vasodilator treatment did not experience any acute deterioration in RV function during simulated mild altitude, at rest or following mild exertion.39S
The known physiological effects of hypoxia, however, suggest that in-flight O2 administration should be considered for patients with WHO-FC III and IV pulmonary hypertension and for those with PaO2 consistently <60 mmHg.40S–42S Similarly, such patients, as well as patients known to be affected by pulmonary hypertension, should consider the use of supplemental O2 when exposed to altitudes >1500–2000 m.43S Recommendations are summarized in Supplementary material online, Table S3.
Congenital heart disease
Adults with congenital heart disease should be stratified by specific haemodynamic and pathophysiological conditions to assess their fitness to HA exposure.40
Patients with concurrent pulmonary hypertension should be careful when going to HA. Indeed, the occurrence of an elevated pulmonary pressure due to a pre-existing clinical condition worsens the effects of HA exposure. This is exemplified by the fact that corrective surgery for Fontan patients, in whom maintaining low pulmonary resistance is essential, has a worse outcome when performed at HA41,44S, 45S than at sea level. On the other hand, after successful surgical correction, short-term HA exposure (at 3454 m) had no negative impact on pulmonary blood flow and exercise capacity when compared with controls and was clinically well tolerated.41
Patients with cyanotic heart conditions and right to left shunting are likely to develop more severe hypoxaemia than healthy individuals as the increase in pulmonary vascular resistance at HA can worsen the right-to-left shunt.46S,47S However, reduced blood O2 content is not dangerous by itself, because cardiac output and haematocrit increase sufficiently to maintain adequate systemic O2 delivery. Various trials carried out in a laboratory setting and during short-term exposure to hypobaric hypoxia at altitude suggest that some of those patients are able to maintain O2 delivery even with reductions in inspired pO2, by increasing their cardiac output along with their already higher haematocrit associated with their congenital cardiac abnormalities.42,43,44
Recommendations are summarized in Supplementary material online, Table S3 and can be applied also for air travel, although specific data in this regard are largely missing.
Cerebrovascular conditions
Ischaemic stroke
Even though evidence is limited, HA exposure seems to pose a risk of cerebral ischaemia for patients who already have suffered an ischaemic stroke,29,45,46 both because of the direct effect of hypoxia and due to a reduced cerebrovascular reactivity.47,48S
Whether the increase in haematocrit and the greater blood viscosity also contribute to a greater stroke risk at HA is unknown. There are, however, data, although somehow controversial,19 suggesting that highlanders and people working at altitude might have a higher risk of stroke compared to those living and working at low altitude.48,49
Although presently there is not enough evidence to provide any strong recommendation on this issue, it has been suggested only based on clinical experience that carotid ultrasound imaging, commonly performed in daily practice to check for the existence of complicated plaques or severe carotid stenosis, might help to assess the risk of new events during HA exposure after atherothrombotic stroke.
Haemorrhagic stroke
Arterial blood pressure elevation at HA increases the risk of rupture of cerebral aneurysms and arterial venous malformations as well as the theoretical risk of hypertension-related cerebral haemorrhage.50 Nevertheless, there is no evidence on the incidence of intracranial haemorrhage in alpine regions. We could not find any systematic study evaluating the incidence of intracranial bleeding at HA, nor any case report on this topic, although, admittedly, intracranial haemorrhage could be one of the causes of some of the sudden death cases reported in this condition.
Recommendations for cerebrovascular patients are summarized in Supplementary material online, Table S3.
Limitations
This article summarizes the available evidence on the effects of HA exposure in patients with a variety of cardiovascular problems, aimed at providing practical recommendations for preventive and therapeutic decision-making.
Some general limitations of our article, which apply to all sections, should be acknowledged (see Supplementary material online, Table S4). In the field of HA medicine only a limited number of well-conducted studies are available, which usually have small sample sizes, primarily evaluating young subjects and commonly with lack a randomized controlled design. Moreover, studies are often not fully comparable because they are performed at different altitude levels and are confounded by a number of different factors, such as the degree of baseline physical conditioning, extent of physical effort at HA, speed of ascent, climate changes, and latitude. Finally, different test settings have been used to investigate the cardiovascular effects of altitude exposure, either in the field or in an experimental laboratory with simulated altitude with either normobaric or hypobaric hypoxia exposure. All these limitations and the lack of a standardized data reporting system make it very difficult to verify the validity and strength of data from a given trial as well as the consistency of results among studies and to reproducibly and precisely assign the observed effects to different altitude levels.
Conclusions
Available studies, although only in a few instances being performed according to a randomized double-blind controlled design, provide indications that, in spite of their ‘soft’ nature, might nevertheless allow for a safer exposure to hypobaric hypoxia at altitude in patients with a variety of cardiovascular conditions, offering some practical advices and guidance for both patients and physicians. The relative lack of highly powered studies, and the type of data summarized in this article, emphasize the need for additional trials of suitable size to better address the cardiovascular implications of both acute and chronic HA exposure.
Acknowledgement
The authors thank the Department of Innovation, Research and University of the Autonomous Province of Bozen/Bolzano for covering the Open Access publication costs.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: P.A. reports personal fees from Menarini, grants from Daiichi Sankyo, personal fees from Novartis, personal fees from Boeringer, outside the submitted work. All other authors declared no conflicts of interest in relation to this work.
Supplementary Material
References
- For References [1S–48S], please refer to Supplementary material online.
- 1. Agostoni P. Considerations on safety and treatment of patients with chronic heart failure. High Alt Med Biol 2013;14:96–100. [DOI] [PubMed] [Google Scholar]
- 2. Bärtsch P, Gibbs JS.. Effects of altitude on the heart and the lungs. Circulation 2007;116:2191–2202. [DOI] [PubMed] [Google Scholar]
- 3. Donegani E, Hillebrandt D, Windsor J, Gieseler U, Rodway G, Schöffl V, Küpper T.. Pre-existing cardiovascular conditions and high-altitude travel. Consensus statement of the Medical Commission of the Union Internationale des Associations d’Alpinisme (UIAA MedCom). Travel Medicine and Infectious Disease. Travel Med Infect Dis 2014;12:237–252. [DOI] [PubMed] [Google Scholar]
- 4. Agostoni P, Swenson ER, Bussotti M, Revera M, Meriggi P, Faini A, Lombardi C, Bilo G, Giuliano A, Bonacina D, Modesti PA, Mancia G, Parati G.. High-altitude exposure of three weeks duration increases lung diffusing capacity in humans. J Appl Physiol (1985) 2011;110:1564–1571. [DOI] [PubMed] [Google Scholar]
- 5. Messerli-Burgy N, Meyer K, Steptoe A, Laederach-Hofmann K.. Autonomic and cardiovascular effects of acute high-altitude exposure after myocardial infarction and in normal volunteers. Circ J 2009;73:1485–1491. [DOI] [PubMed] [Google Scholar]
- 6. Heistad DD, Abboud FM.. Circulatory adjustments to hypoxia. Circulation 1980;61:463–470. [DOI] [PubMed] [Google Scholar]
- 7. Parati G, Bilo G, Faini A, Bilo B, Revera M, Giuliano A, Lombardi C, Caldara G, Gregorini F, Styczkiewicz K, Zambon A, Piperno A, Modesti PA, Agostoni P, Mancia G.. Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high-altitude exposure: a randomized clinical trial. Eur Heart J 2014;35:3113–3122. [DOI] [PubMed] [Google Scholar]
- 8. Bilo G, Caldara G, Styczkiewicz K, Revera M, Lombardi C, Giglio A, Zambon A, Corrao G, Faini A, Valentini M, Mancia G, Parati G.. Effects of selective and non- selective beta-blockade on 24-h ambulatory blood pressure under hypobaric hypoxia at altitude. J Hypertens 2011;29:380–387. [DOI] [PubMed] [Google Scholar]
- 9. Parati G, Revera M, Giuliano A, Faini A, Bilo G, Gregorini F, Lisi E, Salerno S, Lombardi C, Ramos Becerra CG, Mancia G, Salvi P.. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J 2013;34:759–766. [DOI] [PubMed] [Google Scholar]
- 10. Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS.. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol 1987;63:531–539. [DOI] [PubMed] [Google Scholar]
- 11. Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, Sainty JM, Jammes Y, Richalet JP.. Operation Everest III (Comex '97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med 2000;161:264–270. [DOI] [PubMed] [Google Scholar]
- 12. De Vries ST, Komdeur P, Aalbersberg S, van Enst GC, Breeman A, van’t Hof AW.. Effects of altitude on exercise level and heart rate in patients with coronary artery disease and healthy controls. Neth Heart J 2010;18:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valentini M, Revera M, Bilo G, Caldara G, Savia G, Styczkiewicz K, Parati S, Gregorini F, Faini A, Branzi G, Malfatto G, Magrì D, Agostoni P, Parati G.. Effects of beta-blockade on exercise performance at high altitude: a randomized, placebo-controlled trial comparing the efficacy of nebivolol versus carvedilol in healthy subjects. Cardiovasc Ther 2012;30:240–248. [DOI] [PubMed] [Google Scholar]
- 14. Agostoni P, Swenson ER, Fumagalli R, Salvioni E, Cattadori G, Farina S, Bussotti M, Tamplenizza M, Lombardi C, Bonacina D, Brioschi M, Caravita S, Modesti P, Revera M, Giuliano A, Meriggi P, Faini A, Bilo G, Banfi C, Parati G.. Acute high-altitude exposure reduces lung diffusion: data from the HIGHCARE Alps project. Respir Physiol Neurobiol 2013;188:223–228. [DOI] [PubMed] [Google Scholar]
- 15. Pratali L, Cavana M, Sicari R, Picano E.. Frequent subclinical high-altitude pulmonary edema detected by chest sonography as ultrasound lung comets in recreational climbers. Crit Care Med 2010;38:1818–1823. [DOI] [PubMed] [Google Scholar]
- 16. Caravita S, Faini A, Lombardi C, Valentini M, Gregorini F, Rossi J, Meriggi P, Di Rienzo M, Bilo G, Agostoni G, Parati G.. Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest 2015;147:120–131. [DOI] [PubMed] [Google Scholar]
- 17. Sutton JR, Houston CS, Mansell AL, McFadden MD, Hackett PM, Rigg JR, Powles AC.. Effect of acetazolamide on hypoxemia during sleep at high altitude. N Engl J Med 1979;301:1329–1331. [DOI] [PubMed] [Google Scholar]
- 18. Penaloza D, Arias-Stella J.. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 2007;115:1132–1146. [DOI] [PubMed] [Google Scholar]
- 19. Bailey DM, Dehnert C, Luks AM, Menold E, Castell C, Schendler G, Faoro V, Gutowski M, Evans KA, Taudorf S, James PE, McEneny J, Young IS, Swenson ER, Mairbäurl H, Bärtsch P, Berger MM.. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J Physiol 2010;588:4837–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osculati G, Revera M, Branzi G, Faini A, Malfatto G, Bilo G, Giuliano A, Gregorini F, Ciambellotti F, Lombardi C, Agostoni P, Mancia G, Parati G; on behalf of HIGHCARE Investigators. Effects of hypobaric hypoxia exposure at high altitude on left ventricular twist in healthy subjects: data from HIGHCARE study on Mount Everest. Eur Heart J Cardiovasc Imaging 2016;17:635–643. [DOI] [PubMed] [Google Scholar]
- 21. Holloway CJ, Montgomery HE, Murray AJ, Cochlin LE, Codreanu I, Hopwood N, Johnson AW, Rider OJ, Levett DZ, Tyler DJ, Francis JM, Neubauer S, Grocott MP, Clarke K; Caudwell Xtreme Everest Research Group. Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 2011;25:792–796. [DOI] [PubMed] [Google Scholar]
- 22. Martin DS, Pate JS, Vercueil A, Doyle PW, Mythen MG, Grocott MP; Caudwell Xtreme Everest Research Group. Reduced coagulation at high altitude identified by thromboelastography. Thromb Haemost 2012;107:1066–1071. [DOI] [PubMed] [Google Scholar]
- 23. Modesti PA, Rapi S, Paniccia R, Bilo G, Revera M, Agostoni P, Piperno A, Cambi GE, Rogolino A, Biggeri A, Mancia G, Gensini GF, Abbate R, Parati G.. Index measured at an intermediate altitude to predict impending acute mountain sickness. Med Sci Sports Exerc 2011;43:1811–1818. [DOI] [PubMed] [Google Scholar]
- 24. Agostoni P, Cattadori G, Guazzi M, Bussotti M, Conca C, Lomanto M, Marenzi G, Guazzi MD.. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med 2000;109:450–455. [DOI] [PubMed] [Google Scholar]
- 25. Schmid J, Nobel D, Brugger N, Novak J, Palau P, Trepp A, Wilhelm M, Saner H.. Short-term high altitude exposure at 3454 m is well tolerated in patients with stable heart failure. Eur J Heart Fail 2015;17:182–186. [DOI] [PubMed] [Google Scholar]
- 26. Lombardi C, Meriggi P, Agostoni PG, Faini A, Bilo G, Revera M, Caldara G, Di Rienzo M, Castiglioni P, Bussotti M, Gregorini F, Mancia G, Parati G.. High altitude hypoxia and periodic breathing during sleep: gender-related differences. J Sleep Res 2013;22:322–330. [DOI] [PubMed] [Google Scholar]
- 27. Wyss CA, Koepfli P, Fretz G, Seebauer M, Schirlo C, Kaufman PA.. Influence of altitude exposure on coronary flow reserve. Circulation 2003;108:1202–1207. [DOI] [PubMed] [Google Scholar]
- 28. Salvi P, Revera M, Faini A, Giuliano A, Gregorini F, Agostoni P, Becerra CG, Bilo G, Lombardi C, O’Rourke MF, Mancia G, Parati G.. Changes in subendocardial viability ratio with acute high-altitude exposure and protective role of acetazolamide. Hypertension 2013;61:793–799. [DOI] [PubMed] [Google Scholar]
- 29. Caravita S, Faini A, Bilo G, Revera M, Giuliano A, Gregorini F, Rossi J, Villafuerte FC, Salvi P, Agostoni P, Parati G.. Ischemic changes in exercise ECG in a hypertensive subject acutely exposed to high altitude. Possible role of a high-altitude induced imbalance in myocardial oxygen supply-demand. Int J Cardiol 2014;171:e100–e102. [DOI] [PubMed] [Google Scholar]
- 30. Schmid J-P, Noveanu M, Gaillet R, Hellige G, Wahl A, Saner H.. Safety and exercise tolerance of acute high altitude exposure (3454 m) among patients with coronary artery disease. Heart 2006;92:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Vries ST, Kleijn SA, van’t Hof AWJ, Snaak H, van Enst GC, Kamp O, Breeman A.. Impact of high altitude on echocardiographically determined cardiac morphology and function in patients with coronary artery disease and healthy controls. Eur J Echocardiogr 2010;11:446–450. [DOI] [PubMed] [Google Scholar]
- 32. Wu T, Ding S, Kayser B.. Work at high altitude after coronary stenting: safe? Wilderness Environ Med 2010;21:86–87. [DOI] [PubMed] [Google Scholar]
- 33. Basavarajaiah S, O’Sullivan M.. Physical exertion at high altitude-another risk factor for coronary stent thrombosis? J Invasive Cardiol 2013;25:E66–E68. [PubMed] [Google Scholar]
- 34. Liu Y, Zhang JH, Gao XB, Wu XJ, Yu J, Chen JF, Bian SZ, Ding XH, Huang L.. Correlation between blood pressure changes and AMS, sleeping quality and exercise upon high-altitude exposure in young Chinese men. Mil Med Res 2014;1:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bilo G, Villafuerte FC, Faini A, Anza-Ramírez C, Revera M, Giuliano A, Caravita S, Gregorini F, Lombardi C, Salvioni E, Macarlupu JL, Ossoli D, Landaveri L, Lang M, Agostoni P, Sosa JM, Mancia G, Parati G.. Ambulatory blood pressure in untreated and treated hypertensive patients at high altitude: the High Altitude Cardiovascular Research-Andes study. Hypertension 2015;65:1266–1272. [DOI] [PubMed] [Google Scholar]
- 36. Wu T-Y, Ding SQ, Liu JL, Yu MT, Jia JH, Chai ZC, Dai RC, Zhang SL, Li BY, Pan L, Liang BZ, Zhao JZ, Qi DT, Sun YF, Kayser B.. Who should not go high: chronic disease and work at altitude during construction of the Qinghai-Tibet railroad. High Alt Med Biol 2007;8:88–10 7. [DOI] [PubMed] [Google Scholar]
- 37. Kujaník S, Snincák M, Vokál J, Podracký J, Koval J.. Periodicity of arrhythmias in healthy elderly men at the moderate altitude. Physiol Res 2000;49:285–287. [PubMed] [Google Scholar]
- 38. Gibelli G, Fantoni C, Anzà C, Cattaneo P, Rossi A, Montenero A, Baravelli M.. Arrhythmic risk evaluation during exercise at high altitude in healthy subjects: role of microvolt T-wave alternans. Pacing Clin Electrophysiol 2008;31:1277–1283. [DOI] [PubMed] [Google Scholar]
- 39. Weilenmann D, Duru F, Schönbeck M, Schenk B, Zwicky P, Russi EW, Candinas R.. Influence of acute exposure to high altitude and hypoxemia on ventricular stimulation thresholds in pacemaker patients. Pacing Clin Electrophysiol 2000;23:512–515. [DOI] [PubMed] [Google Scholar]
- 40. Luks AM, Stout K, Swenson ER.. Evaluating the safety of high-altitude travel in patients with adult congenital heart disease. Congenit Heart Dis 2010;5:220–232. [DOI] [PubMed] [Google Scholar]
- 41. Staempfli R, Schmid JP, Schenker S, Eser P, Trachsel LD, Deluigi C, Wustmann K, Thomet C, Greutmann M, Tobler D, Stambach D, Wilhelm M, Schwerzmann M.. Cardiopulmonary adaptation to short-term high altitude exposure in adult Fontan patients. Heart 2016;102:1296–1301. [DOI] [PubMed] [Google Scholar]
- 42. Vargas E, Spielvogel H.. Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol 2006;7:138–149. [DOI] [PubMed] [Google Scholar]
- 43. Berman W Jr, Wood SC, Yabek SM, Dillon T, Fripp RR, Burstein R.. Systemic oxygen transport in patients with congenital heart disease. Circulation 1987;75:360–368. [DOI] [PubMed] [Google Scholar]
- 44. Broberg CS, Uebing A, Cuomo L, Thein SL, Papadopoulos MG, Gatzoulis MA.. Adult patients with Eisenmenger syndrome report flying safely on commercial airlines. Heart 2006;93:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clarke C. Acute mountain sickness: medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad Med J 2006;82:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Roux G, Larmignat P, Marchal M, Richalet J.. Haemostasis at high altitude. Int J Sports Med 1992;13:S49–S51. [DOI] [PubMed] [Google Scholar]
- 47. Van Osta A, Moraine J-J, Melot C, Mairbaurl H, Maggiorini M, Naeije R.. Effects of high altitude exposure on cerebral hemodynamics in normal subjects. Stroke 2005;36:557–560. [DOI] [PubMed] [Google Scholar]
- 48. Jha SK, Anand AC, Sharma V, Kumar N, Adya CM.. Stroke at high altitude: Indian experience. High Alt Med Biol 2002;3:21–27. [DOI] [PubMed] [Google Scholar]
- 49. Niaz A, Nayyar S.. Cerebrovascular stroke at high altitude. J Coll Physicians Surg Pak 2003;13:446–448. [PubMed] [Google Scholar]
- 50. Wilson MH, Newman S, Imray CH.. The cerebral effects of ascent to high altitudes. Lancet Neurol 2009;8:175–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.